https://www.youtube.com/watch?v=H_1ULIKzwfA


HOLOENZYME = enzyme + cofactor/coenzyme





ENZYMES - ARTICULATE SESSION
Enzymes are proteins that speed up biological reactions. Without enzymes the biological processes that support life would not be fast enough to sustain our life.
























What are enzymes?
Most enzymes are proteins (although some are RNAs are enzymes too!). Non-protein enzymes are called ribozymes. They are RNA molecules that have the ability to catalyze specific biochemical reactions. But in this module we are going to concentrate on protein enzymes.
Chemical reactions that make your cells function to support life are catalysed by enzymes:
- Enzymes speed up reactions in your cell (the typical biochemical reaction occurs more than a million times faster when catalyzed by an enzyme).
- Enzymes are are very specific for the particular molecules (called substrates) that they will transform in the catalytic reaction
- Enzymes are not changed in the reaction and therefore they can perform the particular molecular reaction over and over and over again (how efficient is that!).





Most reactions have an energy barrier that MUST be overcome before a reaction can begin. This is called an Activation Energy barrier.
If we cannot use temperature or pressure for our energy, then we can use a catalyst to speed up the reaction instead! So our catalyst will help us overcome this barrier by reducing the amount of energy required.



Enzymes speed up reactions by lowering the activation energy barrier to a reaction!
https://www.youtube.com/watch?v=ueup2PTkFW8
Enzymes are specific!
Below you will find three carbohydrates : maltose, sucrose and lactose. They are all quite similar because each contains at least one glucose and they all have 2 sugar rings. Even so, to split each of them into two sugars (break the glycosidic bond) our cells have to use different specific enzymes.



OXIDOREDUCTASE
Enzymes that catalyze the oxidation–reduction reactions between two substrates.
In this case L-lactate is oxidised to pyruvate and in this process NAD+ is reduced to NADH

TRANSFERASE
Enzymes that catalyze the transfer of a functional group between two substrates.
In this case the alpha-amino group of alanine is transferred to alpha-ketoglutarate to produce L-glutamate.
As a member of the aminotransferase family, ALT (alanine transaminase) catalyzes the reversible transfer of the amino group from glutamate to pyruvate while replacing the amino group of glutamate with a carbonyl group:

HYDROLASE
Enzymes that catalyze the hydrolysis of esters, carbohydrates, and proteins (polypeptides). Pyrophosphate has a phosphoester bond (P-O-P)

LYASE
Enzymes that catalyze the removal of groups from substrates by mechanisms other than hydrolysis

ISOMERASE
Enzymes that catalyze the interconversion of stereoisomers and structural isomers.

LIGASE
Enzymes that catalyze the linking of two compounds by breaking a phosphate anhydride bond in adenosine triphosphate (ATP).
In this case the ammonium ion NH4+ is linked to the carboxyl group of L-Glutamate


Over The HILL
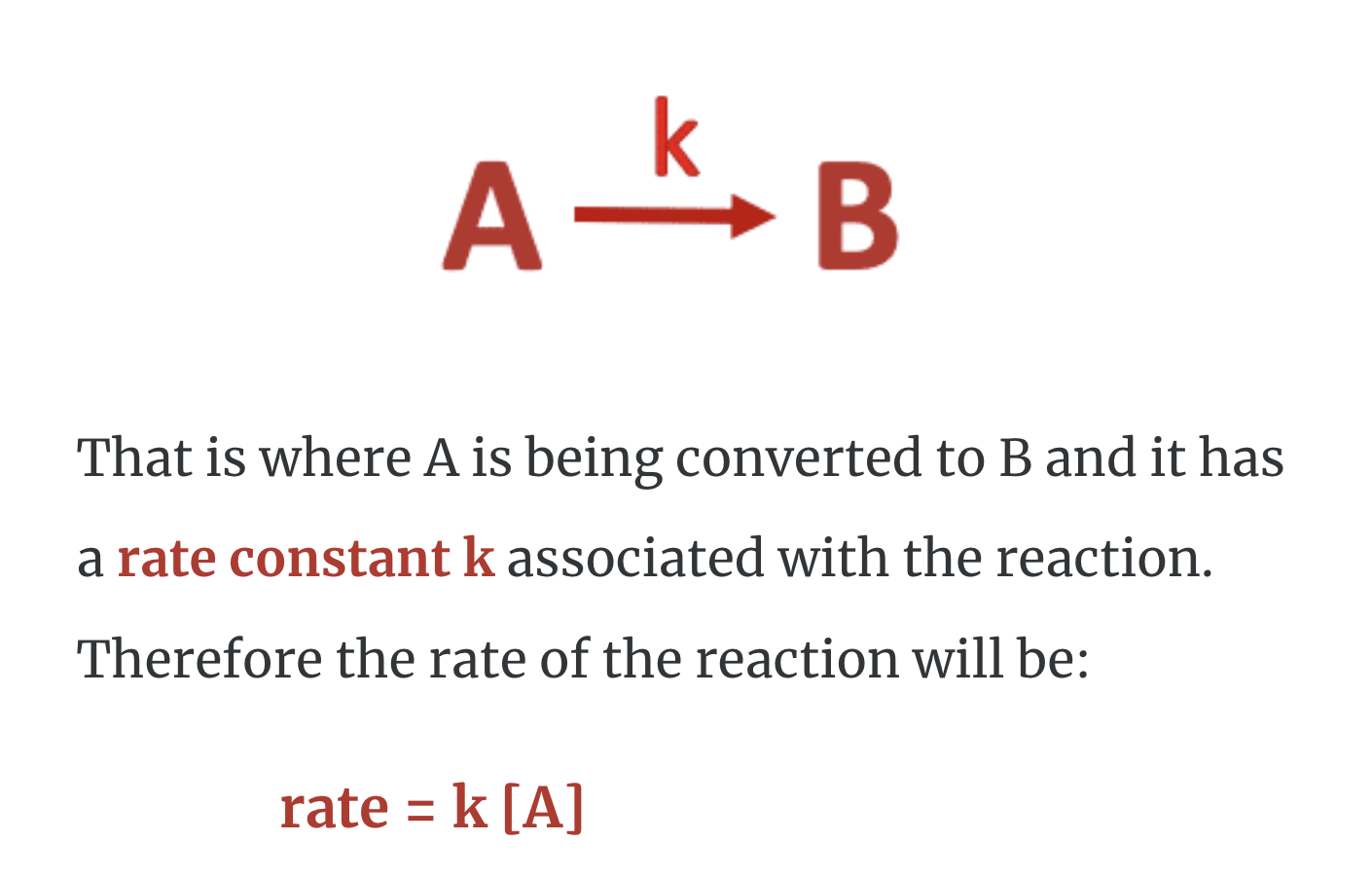
every chemical reaction starts with at least one reactant (A) and finishes with a minimum of one product (B).
As the reaction proceeds, the reactant concentration decreases (A) and the product concentration increases (B).
This reaction occurs at a specific rate and this is called the rate constant k and the rate of the reaction. It is also written like this...

In other words, k is the time it takes for molecule A to become molecule B. The value of this coefficient k will vary with conditions that affect the reaction rate, such as temperature, pressure, surface area, etc. But what you need to know is that a smaller rate constant indicates a slower reaction, while a larger rate constant indicates a faster reaction.

How can we change the rate of a reaction?
- Increasing the concentration of reactants (we've looked at this with our dancers)
- Increasing the reaction temperature
- Adding a catalyst (enzyme)
It must overcome the activation energy barrier and during this process it changes its shape and passes through a high energy transition state intermediate before being converted to product.
This high energy transition state intermediate is extremely unstable. What does it look like?


Free Energy and Free Energy Change—the Gibbs free energy, G, is used to describe the spontaneity of a process.
On the vertical Y-axis we have the Free energy (Go) of the reaction.
On the horizontal (X-axis) is how the reaction proceeds.
- Reactants = Substrate = dog the initial state. Notice that the Free energy (Go) at this point is GoA
- Transition state = dogcat. Notice that the Free energy (Go) is much higher than the reactants GoA or products GoB.
- Product = evil cat. Notice that the Free energy (Go) is lower than the initial state reactants.


I want you to notice that the transition state is DEFINITELY not a stable molecule (the carbon has 5 bonds for starters!). It is just a temporary intermediate. Just like with the catdog, it is a combination of what the initial reactants look like and what the products look like. In general, transition states tend to look a LITTLE bit more like the products than the initial states.
There are three ways to change the rate of a reaction (as we have already seen).
- Increasing the concentration of reactants (or pressure), as we looked at with our dancers
- Increasing the reaction temperature
- Adding a catalyst (enzyme)
Enzyme kinetics


Maud and Leonor!
They measured enzyme-catalyzed reaction rates as a function of substrate (reactant) concentration.
They observed that most enzyme-catalyzed reactions show an increasing rate with increasing substrate concentration, but ONLY to a specific maximum velocity, Vmax.

the rate of catalysis doesn’t constantly continue to increase with substrate concentration (ie isn't linear) but instead it tails off, or plateaus, until it reaches a maximum (Vmax).
This curve is called a hyperbolic curve.
Why does the reaction reach a maximal velocity?
Think about the enzyme....remember that during the reaction, the concentration of the enzymes do not change and therefore the number of active sites available to convert a substrate to a product is fixed. The number of active sites do not change.
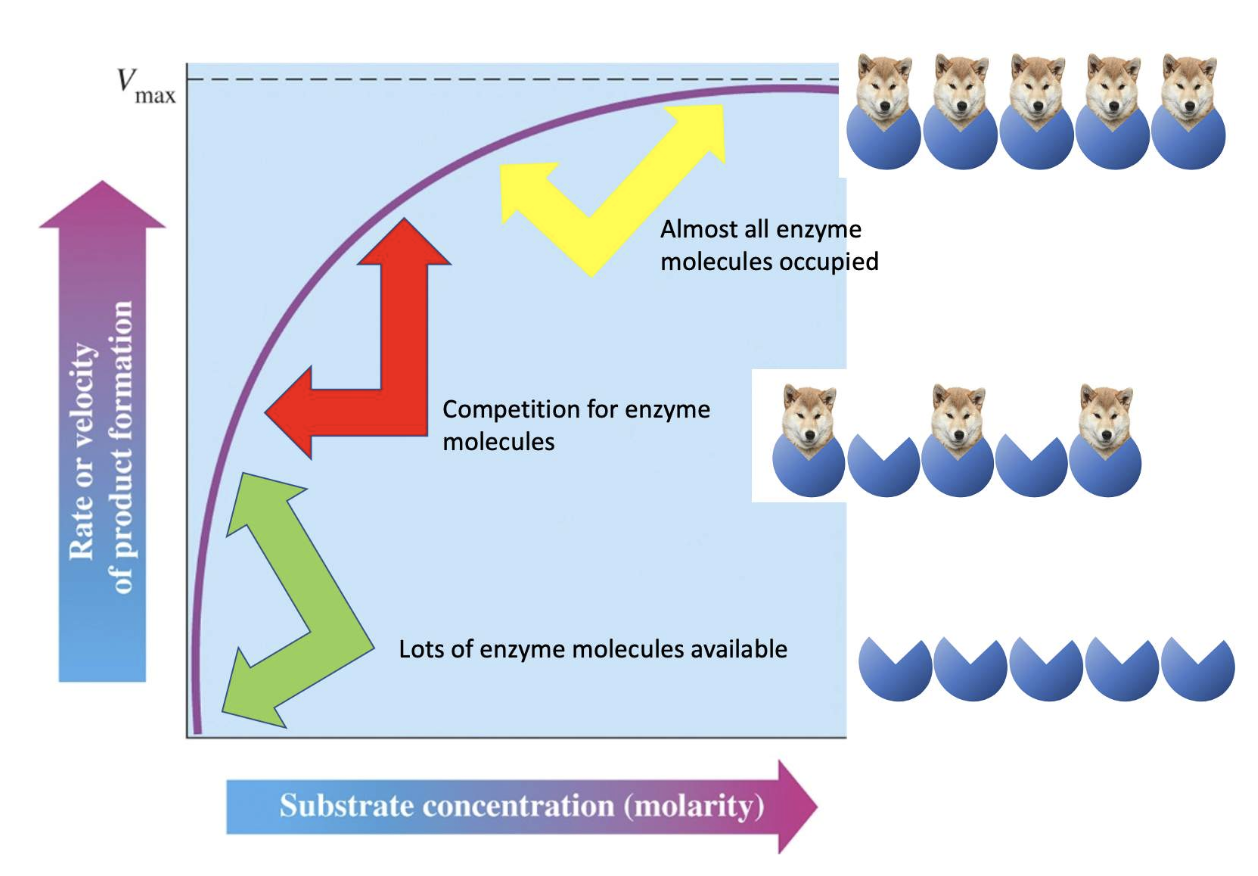
once the enzymes are all full, meaning all of them have substrates bound (this a situation that is never in reality reached), the potential new substrate dogs have to wait their turn to bind to the active site of the next available enzyme."
What did we mean by "it's never really reached". When an enzyme interacts with a substrate and converts it to a product, it then has to release the products before it can take up a new substrate.
In that moment, that enzyme is temporarily empty. Therefore Vmax is as close to being saturated as possible, but allowing momentary lapses where substrates are being exchanged.

Hexokinase is an enzyme that catalyses the reaction of Glucose to Glucose-6-phosphate in the first step of glycolysis. It is present in all cells except the liver and beta cells of the pancreas.

Just like hexokinase, glucokinase is an enzyme that also catalyses the reaction of Glucose to Glucose-6-phosphate. Only this time, Glucokinase is found in the liver and beta cells of the pancreas.
Glucokinase plays an important role in recognising how high the blood glucose is in the body. It acts as the “glucose sensor” for the pancreas, so that when the blood glucose rises, the amount of insulin produced, also increases. This means that the blood glucose does not become too high if glucokinase is functioning normally. It is also very important in glucose homeostasis.

As you can see the Hexokinase (green line) converts the glucose to glucose-6-phosphate at a much faster rate than Glucokinase (purple line)
This shows us clearly that Hexokinase has a stronger attraction for glucose than glucokinase.

Turnover number
An enzyme’s catalytic speed is also matched to an organism’s metabolic needs.
This catalytic speed is commonly measured as a turnover number which is the number of molecules an enzyme can convert, or “turn over,” in a given time span.
Turnover number is a convenient way to compare enzymes to each other or the effect of reaction conditions. Turnover number is also referred to as Kcat. That is K for the rate, and cat for catalysis. We will look at this in a video shortly.
Enzyme active site properties


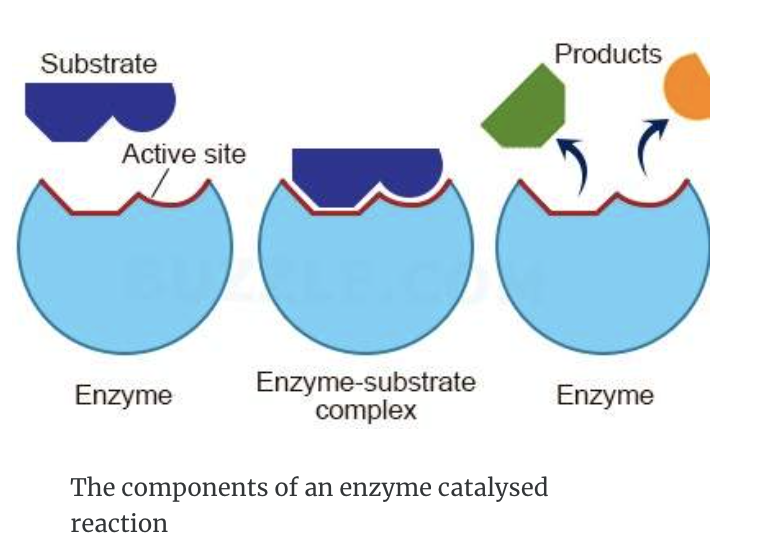
- Enzyme (E) and substrate (S) combine to form an enzyme–substrate complex (E–S)
- An high energy transition state is formed enzyme-product complex (E-P)
- The intermediate decomposes to give the product (P) and the enzyme which then leave the active site.
- the enzyme is the same at the start and end of the reaction - it's fully recyclable.
- the enzyme changes shape a bit to accommodate the substrate (like we saw for hexokinase/glucose E-S complex)
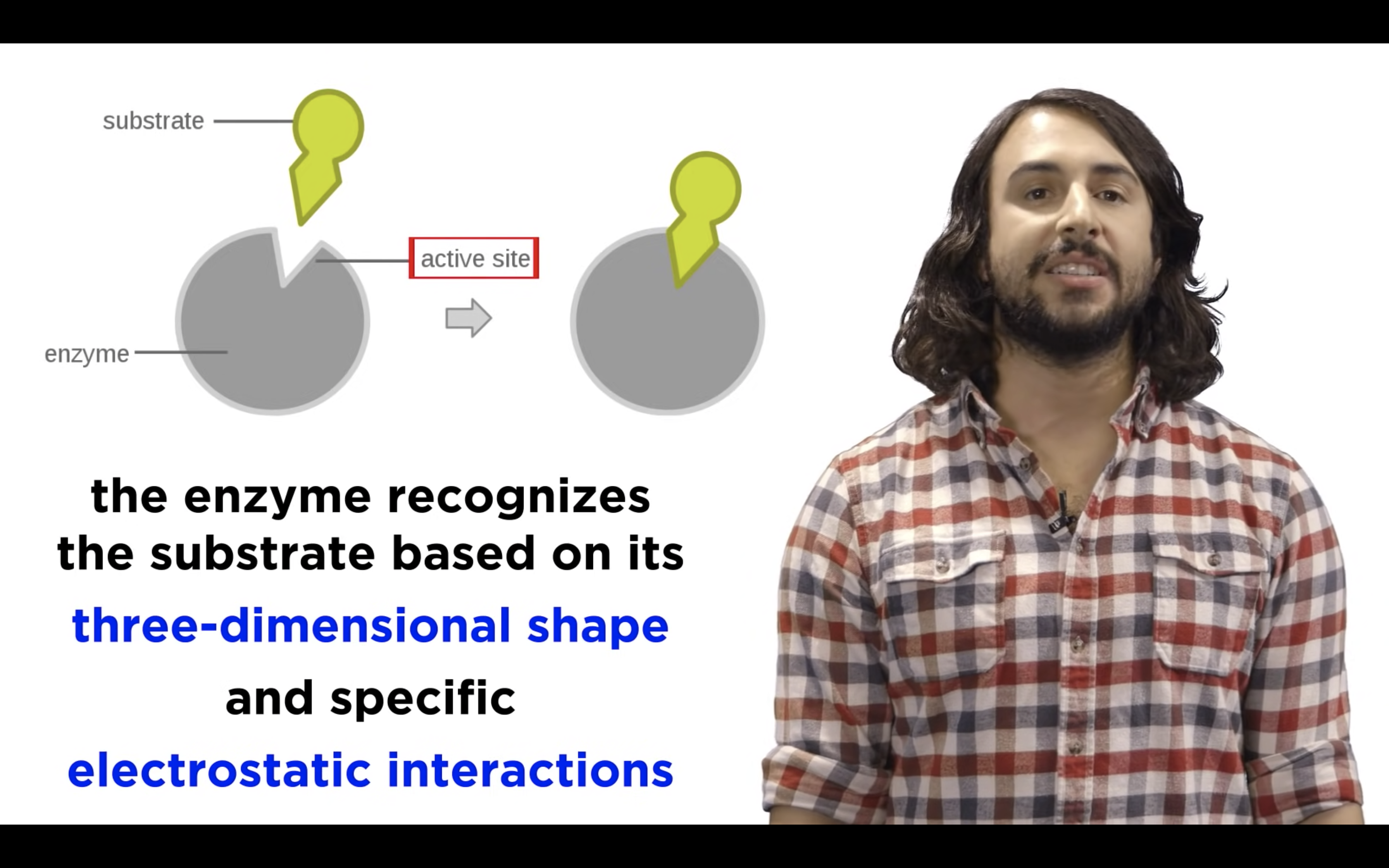
Each different enzyme has its own unique active site whose shape determines which substrates can bind.
- Enzymes are stereospecific - so an enzyme can be SO specific that it may only interact with only ONE specific isomer of a molecule!
- Each enzyme catalyzes reactions for only a limited number of different substrates.



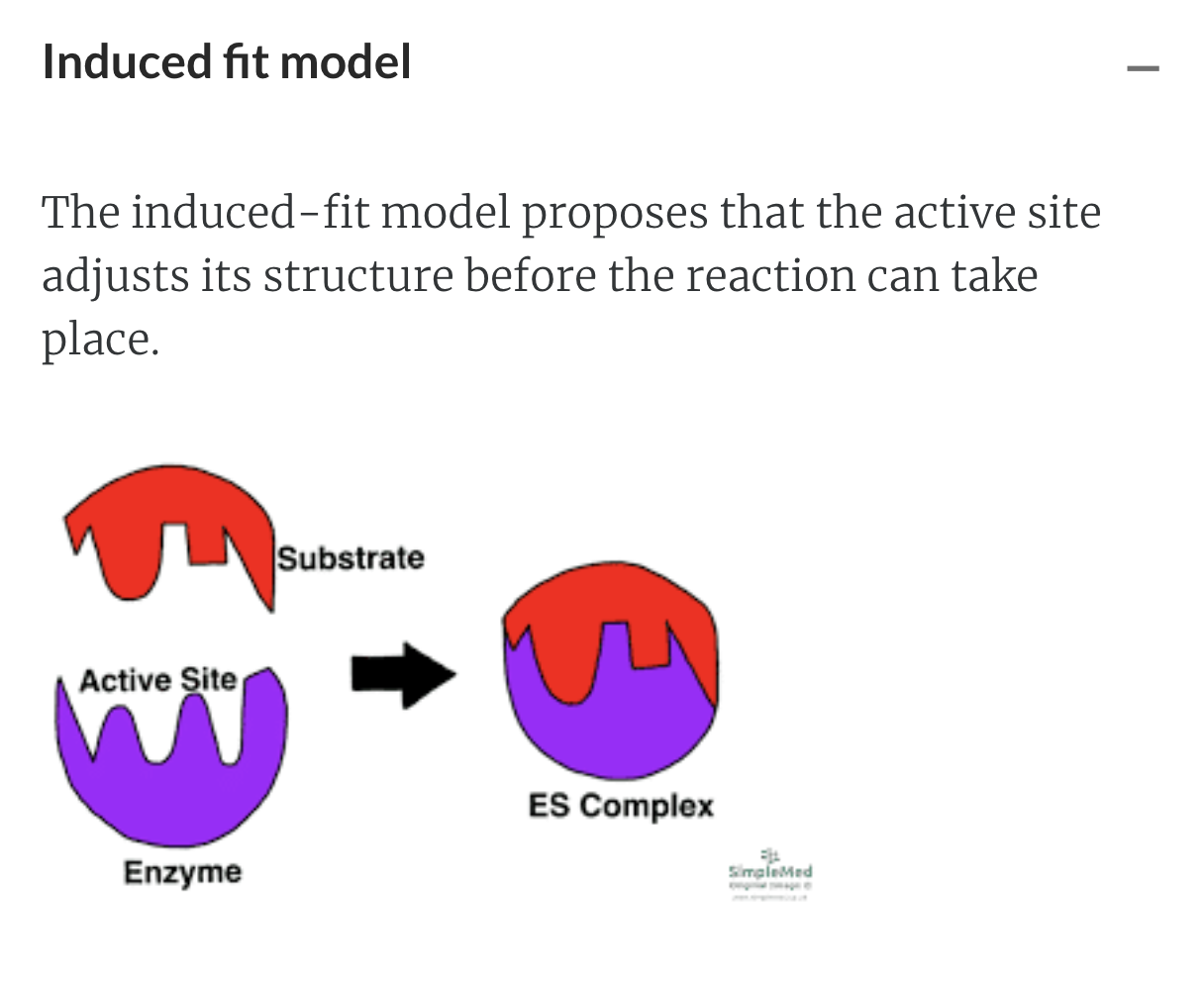

Amino acid existing in the active site will help the binding of the substrate with enzyme
Once substrate binds, the active site attains shape of substrate -> induced fit
Substrate + enzyme -> Product and release
Temperature and pH effects on enzyme reactions
Enzymes are proteins and so their catalytic function is effected by factors that denature them
Even slight changes in the pH can have profound effects on enzyme catalysis.


Optimal temperature
.....for an enzyme operating under physiological conditions is?
~37˚C
Optimal pH .....for an enzyme operating under physiological conditions is? ~7.4
METALS in biological systems- ARTICULATE SESSION




Metals
1) CATALYSE OXIDATION AND REDUCTION (Cu)
2) ACT AS LEWIS ACID IN HYDROLYTIC ENZYMES (Zn)
3) STRUCTURAL CO-FACTOR
Na+



K+






Mg2+, Fe2+, Fe3+, Zn, Mn,Cu .... Trace metals







Metalloenzyme








The composition of us....living organisms!
The macrominerals: elements found in large quantities in our body include:
- 99% of a mammal's mass comprises of the non-metals: carbon, nitrogen, oxygen, hydrogen, sulfur, phosphorus, chlorine and the metals: calcium, sodium, magnesium, and potassium.
- The non-metals: carbon, nitrogen, oxygen, hydrogen, sulfur, and phosphorus are components of important biomolecules such as proteins, lipids, carbohydrates, and the nucleic acids (especially carbon, oxygen, nitrogen and hydrogen).
The microminerals: Elements found in small quantities in our body include these two categories:
- There are seven elements found in small quantities: the metals: manganese, iron, cobalt, copper, zinc, molybdenum and the non-metal iodine.
- There are some elements only found in some species (not all):
the non-metals boron, fluorine, silicon, selenium and the metals: vanadium, chromium, selenium and tin.
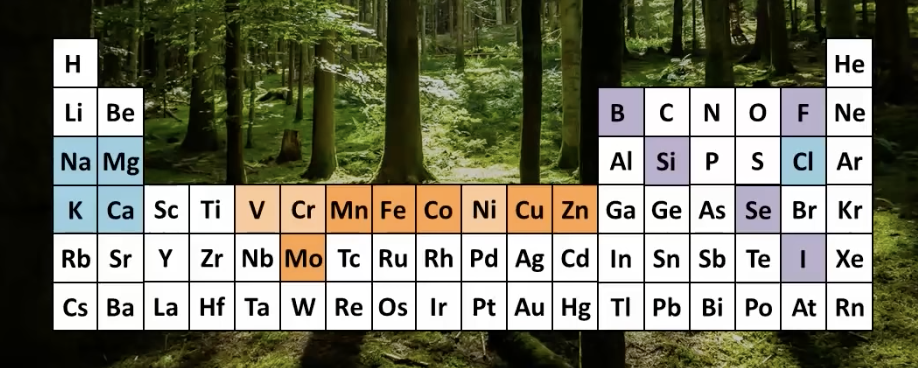
The essential metals for life:
These are the elements that are essential for life.
Alkali metals: Na, K
Alkaline earth metals: Mg, Ca
1st row transition metals: V, Cr, Mn, Fe, Co, Ni, Cu, Zn
Group VIB transition metals: Mo W
Important point
Just because trace elements or microminerals are found in trace quantities it does not mean that they are any less essential to our bodies than the macrominerals
Metals in sickness and in health
Metal ions are indispensable for living organisms but, of course, it is crucial that the metals ions are available at the correct concentrations.
Metal imbalances, where the concentration of the metal is either too high or too low, due to genetic or environmental sources, can cause a number of significant health issues.
In clinical medicine, the role of metal ions and metal-based drugs is important in three major areas:
- metal-related diseases;
- metal-based medicines (including drugs, imaging agents, and metal chelators);
- agents of metal-based toxicity.
The function of metals in biological processes
Metals play many roles through out our body.
- They can act to maintain ionic balance in cells: Na+ and K+ using the Na/K pump.
- They can participate in reactions such as redox (oxidation-reduction reactions) and general reactions: Dehydrogenation; catalyzed by Cu, Mo and Fe by changing their oxidation states
- They can act as a Lewis acids in hydrolytic enzymes: Zn2+ and Co2+ catalyzes the hydrolysis of phosphates by acting as Lewis acids (they can accept a pair of electrons)
- They can assist in the transport and storage of molecules (Fe2+ Hemoglobin and myoglobin, O2 transport and storage)
- They can act as structural cofactors stabilising protein structures (Ca2+, Mg2+, Mn2+, Zn2+)
- They can act as counterions for biological molecules (Mg2+ DNA)
- They can stimulate nerve impulses (Na+, K+) and Ca2+ is important in muscle contraction
- They can act as structural supports : Ca2+ in the mineral hydroxyapatite Ca(OH)2.3Ca3(PO4)2 a component of bones
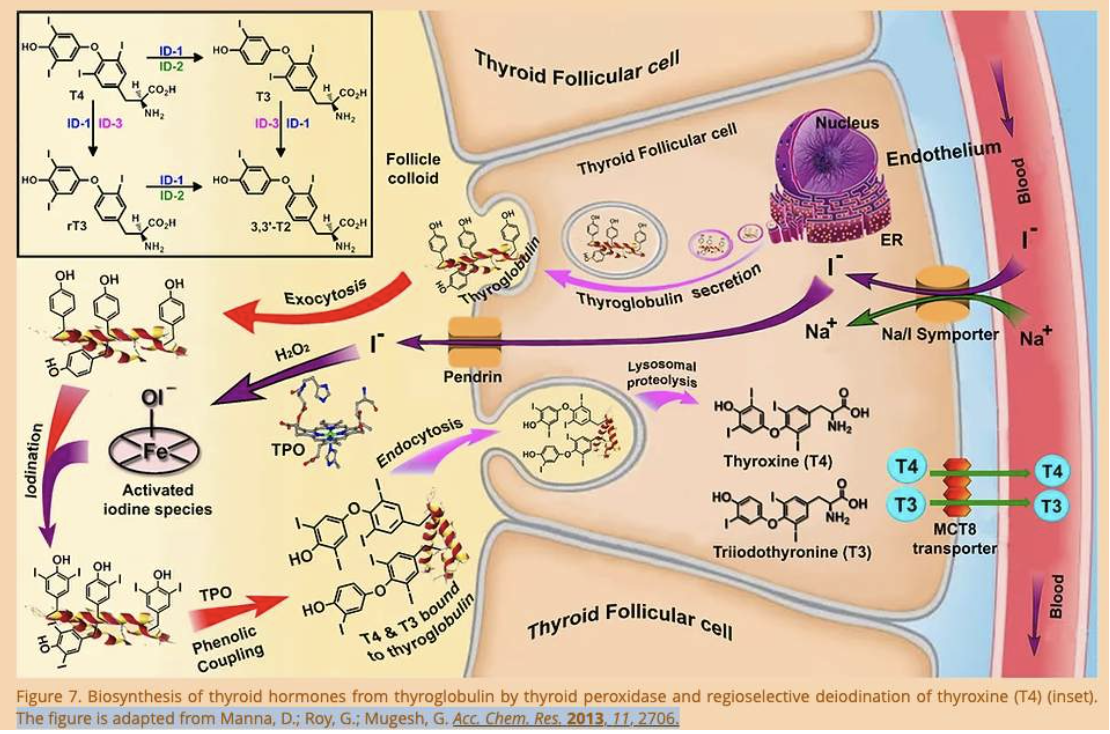
- They assist with transport across biological membranes (Na+ assists with the transport of Iodine into thyroid gland cells)
METALLOENZYMES
Metalloenzymes are enzyme proteins containing metal ions (metal cofactors), which are directly bound to the protein or to enzyme-bound nonprotein components (prosthetic groups such as ).
~40% of the known enzymes contain metals, especially the oxidoreductases (Fe, Cu, Mn, Mo, Ni, V) and hydrolases (e.g. peptidases, phosphatases: Zn, Mg, Ca and Fe
An example is catalase:
Catalase efficiently protects us from dangerous reactive oxidizing molecules such as hydrogen peroxide. Each catalase molecule can decompose millions of hydrogen peroxide molecules every second.
Catalase contains a heme group which is a porphyrin ring with an Fe bond (seen as the red/blue/grey balls below) and at this active site the hydrogen peroxide is degraded into harmless water and oxygen protecting our cells from oxidative stress.
NON-ENZYMATIC METALLOPROTEIN
Hemoglobin and myoglobin are proteins that bind to oxygen for transport and storage respectively.
These proteins also contain a porphyrin ring with an Fe2+ at its centre called heme that binds to oxygen.
Below right is the structure of hemoglobin. The heme groups are shown in green.

NATURAL PRODUCTS
An example of a naturally occurring small molecule that requires a metal to perform its function is chlorophyl (shown below) which is used by plants, algae and cyanobacteria for photosynthesis.
Its central structure is an aromatic porphyrin ring system (like catalase and myoglobin and hemoglobin) but this time with a magnesium atom bound.
CO-ENZYMES AND VITAMINS
An example of a metal containing vitamin is vitamin B12 which contains cobalt.
Cyanocobalamin (commonly known as Vitamin B12) is a highly complex, essential vitamin, containing cobalt. This vitamin is produced naturally by bacteria, and is necessary for DNA synthesis and cellular energy production. Several pharmaceutical forms of cyanocobalamin have been developed, including the tablet, injection, and nasal spray forms
NUCLEIC ACIDS
The nucleic acids are negatively charged due to the phosphate backbone of their structure. The electrostatic (ion-ion) repulsion of the negatively charged phosphates on the outside can make DNA potentially very unstable.
Magnesium ions (Mg2+) and positively charged proteins along with arginine and lysine residues interact with the negatively charged groups in the DNA and stabilize it.
The image below shows the interaction of one Mg2+ with two phosphate groups (red-orange) on the backbone of DNA

HORMONES
Metals can influence the activity of hormones:
- Some metals are essential for hormone function such as the essential metal micronutrients, such as copper, iron, zinc, and calcium, but....
- Some metals can negatively affect hormone function such as the disruptive nonessential metals, such as lead and cadmium.
An example:
Sodium is essential in the synthesis of the hormone thyroxine. Iodide ions are transported into the follicular cells of the thyroid gland by the sodium/iodide symporter (NIS) and for each iodide anion (I–), NIS transports two sodium cations (Na+) into the cell. This ensures there is enough Iodine in the cells of the thyroid gland to synthesize thyroxine.
ANTIBIOTICS
Valinomycin is a natural antibiotic from the Streptomyces bacteria.
- It is a cyclic peptide and acts as an ionophore (meaning that it increases the permeability of a cell membrane to ions).
- Valinomycin transports potassium K+ across cell membranes by forming a cage-like complex around the K+ protecting it from the lipid bilayer.
- Valinomycin can transport many K+ ions quickly and therefore destroy the ionic balance across the membrane.

BIOMINERALS
Our body contains biominerals that provide for support such as bones and teeth.
- Teeth are composed of calcium, phosphorus, and other minerals.
- Bones contain calcium, phosphorus, sodium and other minerals, but mostly consist of the protein collagen.
- Calcium fills in the space around the collagen framework and makes the bone strong enough to support the body's weight.
Macrominerals
Metals of abundance - the macrominerals
Our bodies require large amounts of certain alkali and alkaline metals.
These are:
- Sodium
- Potassium
- Calcium
- Magnesium
Metals of abundance
Na/K are alkali metals
Ca/Mg are alkaline metals
Sodium
Sodium is an essential nutrient involved in the maintenance of normal cellular homeostasis and in the regulation of fluid and electrolyte balance and blood pressure (BP) in our bodies.
Sodium is a positively charged ion that is found principally outside of cells - extracellular.
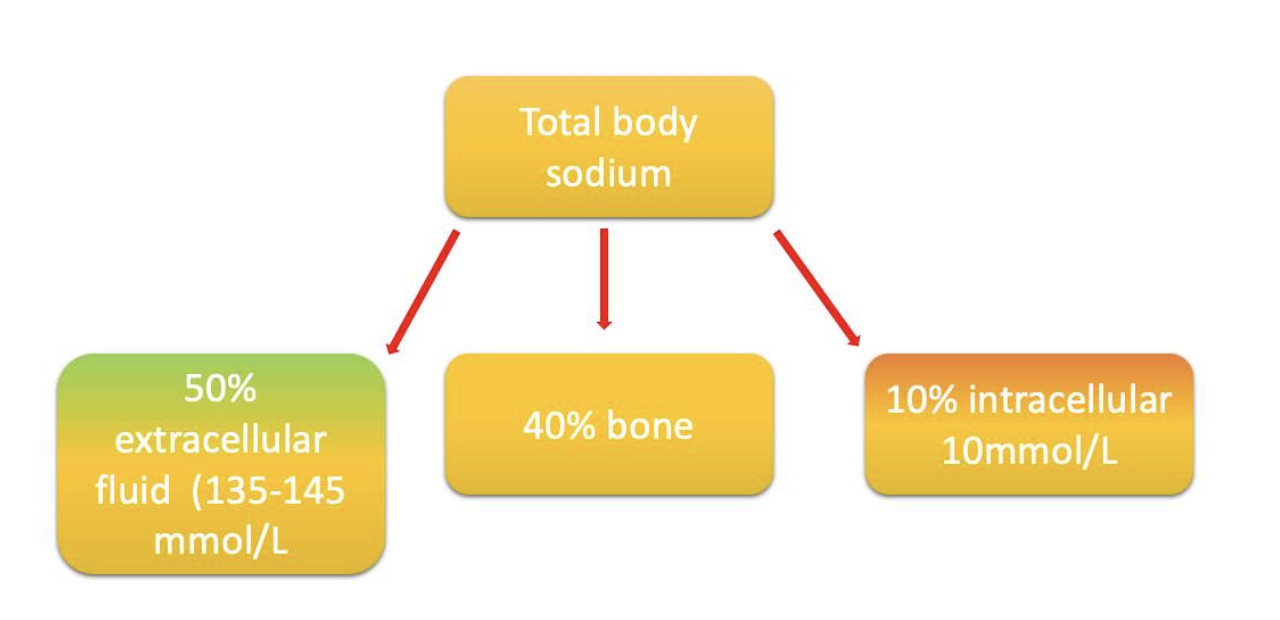
The body content of sodium in an adult male: ~92 g,
- half of which (46 g) is located in the extracellular fluid at a concentration of 135–145 mmol/L
- ∼11 g is found in the intracellular fluid at the concentration of ∼10 mmol/L
- ∼35 g is found in the skeleton.
How is the sodium concentration kept high in the extracellular fluid?
The concentration gradient of sodium between the extracellular and intracellular fluid is maintained by the sodium–potassium pump. This gradient is known as the resting potential.
The sodium-potassium pump transfers sodium from inside to outside of the cell; it transfers potassium from outside to inside the cell.
It needs lots of energy (ATP) to do this because the concentration of sodium outside the cell is so high compared to the inside (pumps against the concentration gradient, using the energy supplied by ATP).
What are the functions of sodium in our body?....
The functions of sodium in our body include:
- Sodium is the main cation found in extracellular fluid
- Sodium levels regulate the volume of extracellular fluid
- Sodium maintains acid/base balance
- Sodium is essential for the transmission of nerve impulses
- Sodium is essential for muscle contraction
You only have to know that sodium is essential for nerve transmission and muscle contraction
(NERVE)
The sodium-potassium pump maintains an electrical gradient across the plasma membrane of a neuron when it is not actively transmitting a nerve impulse.
This gradient is called the resting potential of the neuron.
Nerve impulses begin after exciting inputs (action potentials) are received by the nerve cell .
Once enough input signals have built up sodium will move into the cell and start start a nerve impulse at the axon initial segment. In response, potassium channels then open, so potassium ions flow out of the cell.
(MUSCLE)
What happens when muscles contract and how is sodium involved?
- The signal molecule for a muscle to contract is a neurotransmitter called acetylcholine.
- Acetylcholine binds to protein receptors on the outside of the muscle fiber membrane.
When acetylcholine reaches receptors on the membranes of muscle fibers, membrane channels open and the process that contracts a relaxed muscle fibers begins:
- Open membrane channels allow an influx of sodium ions into the cytoplasm of the muscle fiber.
- The sodium influx also sends a message within the muscle fiber to trigger the release of stored calcium ions.
- The calcium ions diffuse into the muscle fiber and cause changes to actin and muscle contraction occurs.
(TOO MUCH SODIUM)
Hypertension or high blood pressure:
When your heart beats, blood is pumped round your body to give it the energy and oxygen it needs. As the blood moves, it pushes against the sides of the blood vessels. The strength of this push on the blood vessels is your blood pressure.
- If your blood pressure is high, it means that there is too much strain on your blood vessels which can cause damage.
- Damage to blood vessels leading to your heart or the vessels in your brain may lead to heart attacks and strokes.
- A high salt diet disrupts the natural sodium balance in the body. This causes the body to retain water, which increases the pressure of the pushing of blood against the vessel walls.
Osteoporosis is a condition involving the thinning of bones (bone demineralisation), leaving them brittle and more susceptible to fracture.
- Most calcium is stored in the bones, so sufficient calcium is important in order to maintain strong bones
- Salt (and therefore sodium) is a major factor in controlling the amount of calcium in the urine and calcium lost from the bones. Because calcium is important for bone strength, too much salt can lead to bone weakening and therefore osteoporosis.
- High blood pressure caused by a high salt diet can also increase the risk of osteoporosis by increasing the rate at which calcium is lost from the bones.
- cardiovascular disease
- kidney disease
- stomach cancers
- water retention
- obesity
(NOT ENOUGH SODIUM)
Sodium deficiency can cause hyponatremia
- Common causes include excessive sweating, vomiting, diuretic use, diarrhea, heart failure, liver disease, renal disease, and the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
- Symptoms are neurological (due to an osmotic shift of water into brain cells causing edema), especially in acute hyponatremia, and include headache, confusion, and stupor; seizures and coma may occur.
Endurance athletes may suffer from hyponatremia caused by of loss of sodium and too much water intake leading to low levels of sodium in the blood.
Potassium
Potassium is an essential nutrient also involved in the maintenance of normal cellular homeostasis and in the regulation of fluid and electrolyte balance and blood pressure (BP) in our bodies.
K+ allows your nerves to respond to stimulation and muscles to contract including those in your heart.
K+ counteracts excessive sodium leading to increased blood pressure, and moves nutrients into cells and waste products out of cells......
Roughly 98% of the potassium in your body is found in your cells....
- 80% is found in your muscle cells,
- 20% can be found in your bones, liver and red blood cells.
Once inside your body, it functions as an electrolyte.
Potassium is a positively charged ion that is found principally inside of cells - intracellular.
Normal serum potassium levels are between 3.6 and 5.0 mmol/L.
Sufficient potassium is readily obtained from a healthy diet (love bananas)!

Functions of potassium
Potassium regulates the heartbeat, ensures proper function of the muscles and nerves, and is vital for synthesizing protein and metabolizing carbohydrates.
- Maintaining fluid and electrolyte balance
- Maintaining cell integrity, (correct shape and osmotic pressure)
- Potassium is essential for the transmission of nerve impulses
- Potassium is essential for muscle contraction, especially heart disease
- Correct potassium levels are necessary for a regular heartbeat
Not enough potassium?
Abnormally low levels of potassium in your body can cause the condition hypokalemia), you can feel weak and tired, get muscle cramps and become constipated.
Deficiency is characterized by a blood potassium level below 3.5 mmol /L.
An abnormal heart rhythm (arrhythmia) is the most worrying problem of low potassium levels (< 3.5 mmol/L), especially in someone with heart disease.
- Potassium helps regulate the heartbeat, and low levels may cause symptoms like heart palpitations.
- Palpitations may also be a symptom of arrhythmia, or irregular heartbeat, which may be a sign of a serious heart condition.
What can cause low potassium levels ?
- diuretic (water) pills
- vomiting or diarrhoea
- a very physically demanding job
- live in extremely hot climates
- being a professional athlete
- don’t get enough potassium from your diet (though this is very rare)
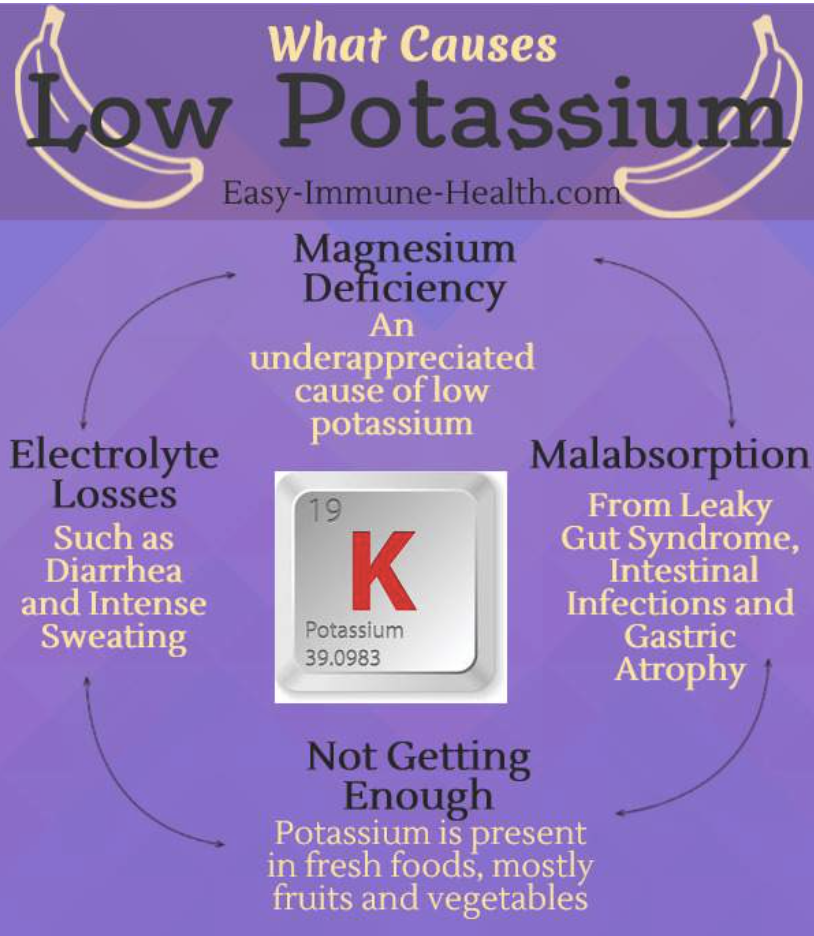
Potassium can counteract the effects of too much sodium
Potassium does not directly lower sodium, but getting adequate amounts of it can help to counteract the effect of sodium on blood pressure.
Look at the conditions on the cards below and flip them to read about their relationship to potassium.


Calcium
Calcium is necessary for life. It builds our bones and keeps them healthy, and enables blood to clot, muscles to contract, and our heart to beat.
- About 99% of the calcium in our bodies is in our bones and teeth.
- Every day, calcium is lost through our skin, nails, hair, sweat, urine and feces.
- Our bodies cannot produce its own calcium.
- Calcium must be obtained from our diet.
When our bodies don’t get the calcium it needs from our diet, it is taken from our bones. This is fine once in a while, but if it happens too often, bones get weak and easier to break.
Sources of calcium include: diary, fish, leafy greens nuts and legumes

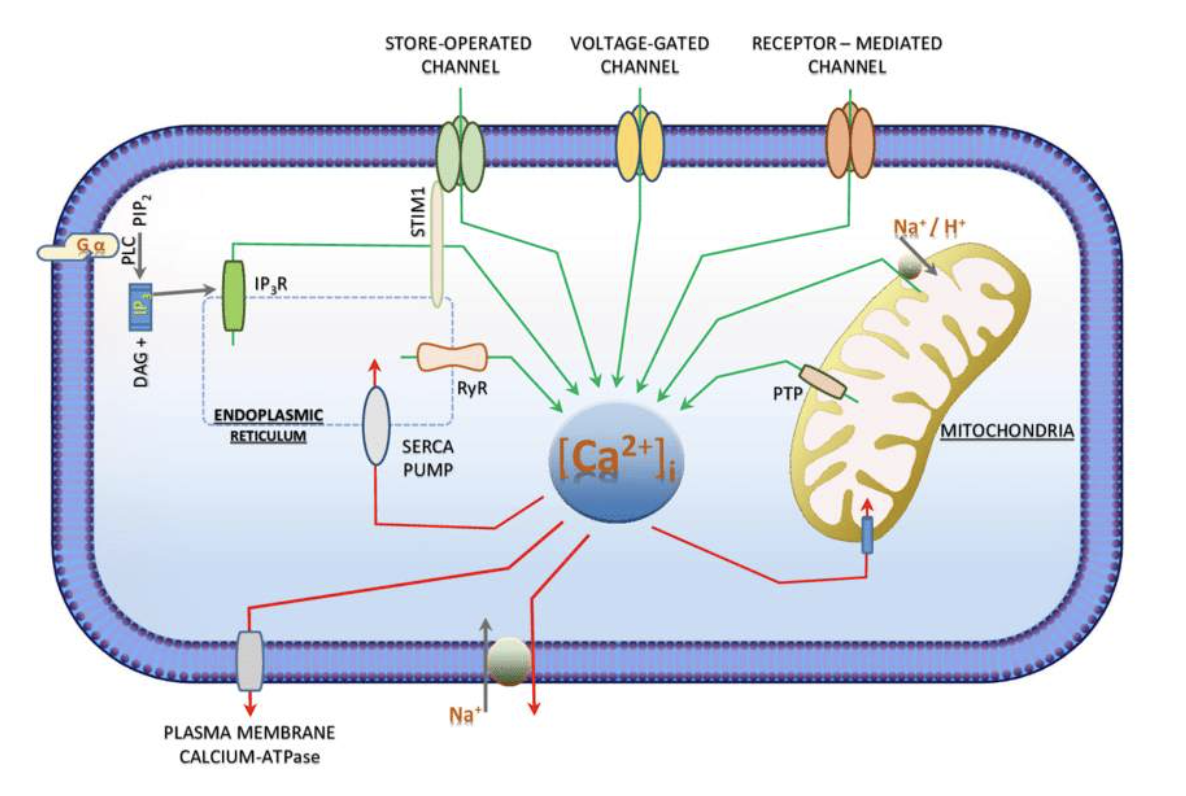
1. Intracellular calcium: Most calcium within cells is found in the mitochondria and the endoplasmic reticulum (ER).
- Intracellular free calcium concentrations range from 100 nM to greater than 1 uM,
- Calcium concentration fluctuations assist calcium's role in intracellular signalling, enzyme activation and muscle contractions.
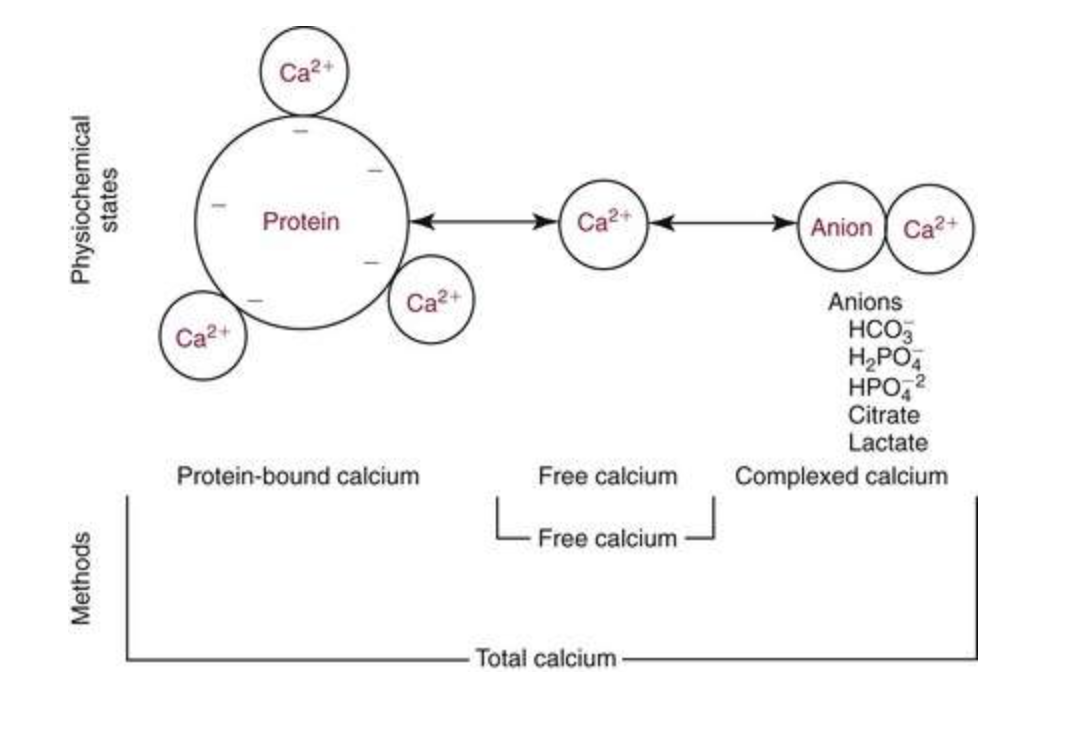
2. Calcium in blood and extracellular fluid: ~ 50% calcium in blood is bound to proteins.
- Ionized calcium (Ca2+) concentration is ~1 mM, or (10,000 x the concentration of free calcium in cells).
- In plasma
- 45% exists as Ca2+ the physiologically active "free" form,
- 45% is bound to proteins
- 10% is complexed with anions such as citrate, sulfate and phosphate
3.Bone calcium: Most calcium is in bone.
- Mainly as calcium hydroxyapatite (Ca10[PO4]6[OH]2)
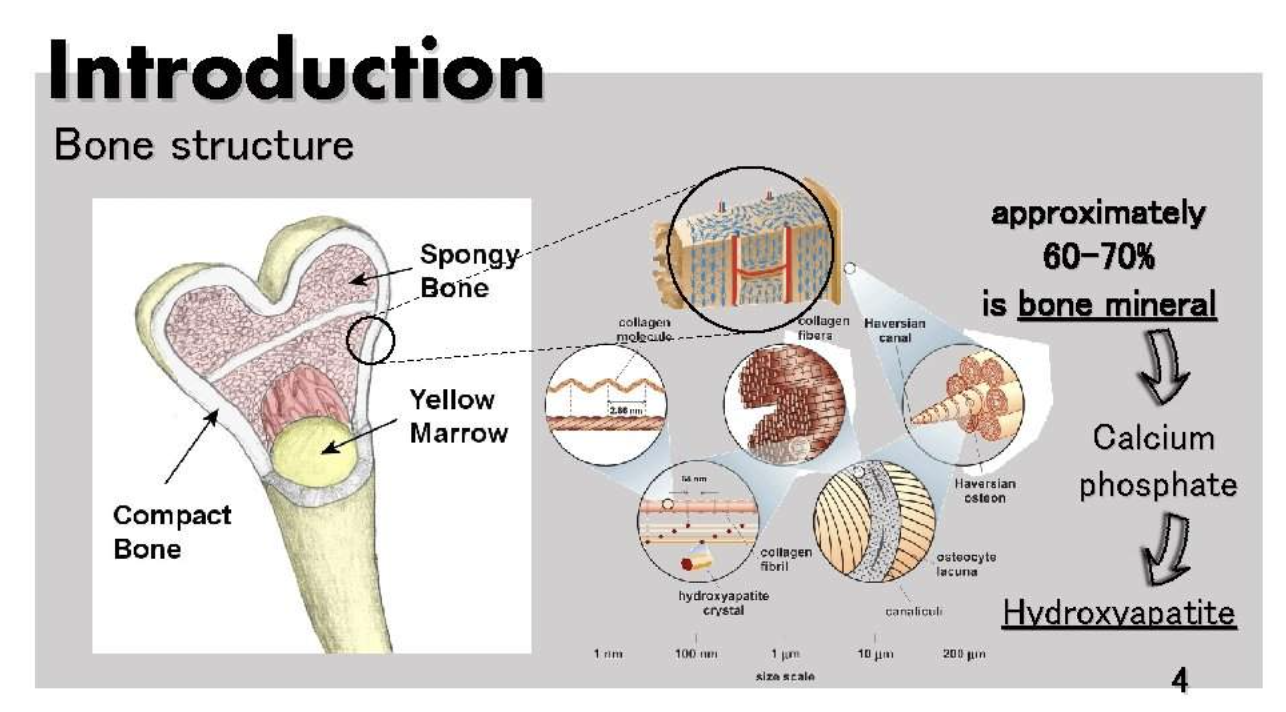
Over 99 percent of total body calcium is found as calcium hydroxyapatite (Ca10[PO4]6[OH]2) in bones and teeth, where it provides hard tissue with its strength.
Our bodies use the bones as a calcium bank - providing a source of Ca to body fluids if needed.
Approximately 500 mg of calcium is removed from the bones daily and replaced by an equal amount. Normally, the amount of calcium absorbed by the intestines is matched by urinary calcium excretion.
Despite this the levels of ionized calcium Ca2+ remain stable because of the tight control maintained by parathyroid hormone (PTH), vitamin D, and calcitonin through complex regulation.
- Parathyroid hormone (PTH) raises blood calcium levels when it is too low
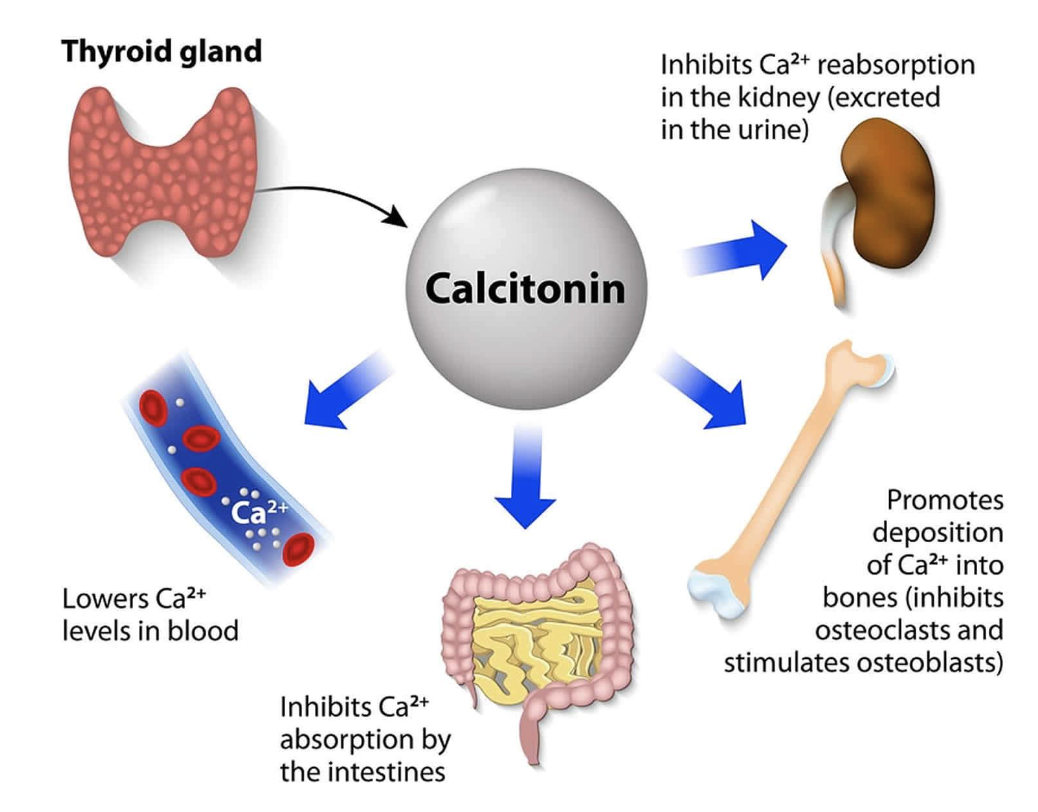
- Calcitonin lowers blood calcium levels when they are too high
- Vitamin D is stimulated by parathyroid hormone and inhibited by calcitonin
The secretion of both calcitonin and parathyroid hormone is determined by the level of calcium in the blood.
- Secreted from the para thyroid gland in response to high blood levels of calcium
- When levels of calcium in the blood increase, calcitonin is secreted in higher quantities.
- When levels of calcium in the blood decrease, this causes the amount of calcitonin secreted to decrease too.
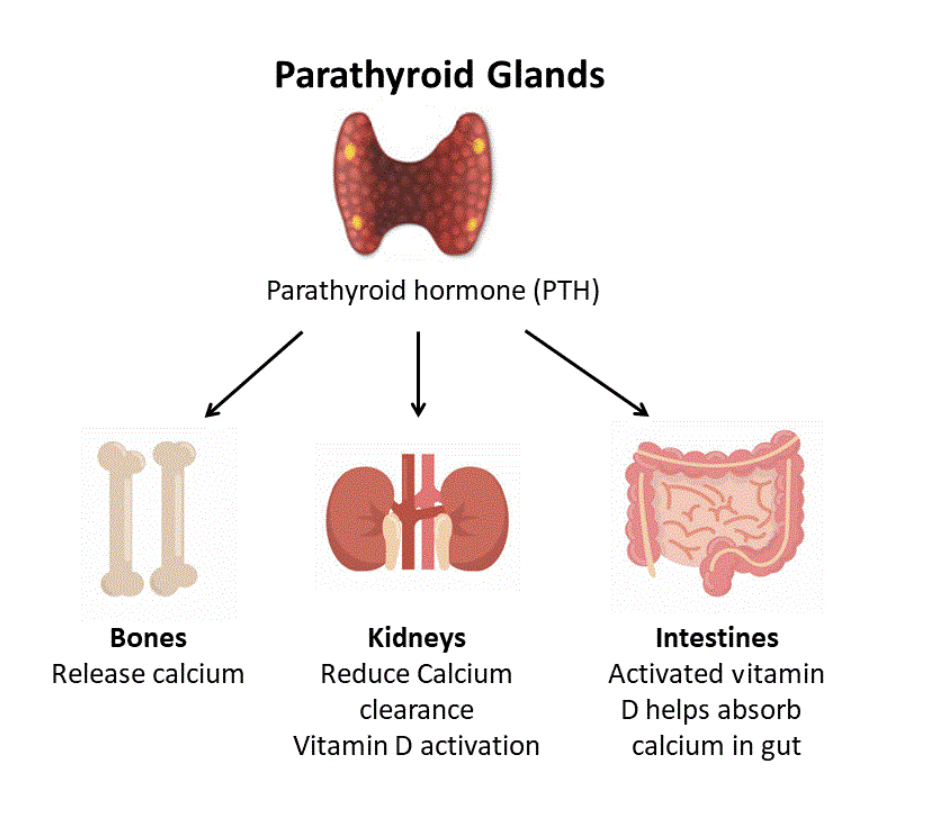
Parathyroid hormone
Parathyroid hormone (PTH) is secreted from parathyroid glands and regulates calcium levels in the blood, by increasing the levels when they are too low.
PTH acts on the kidneys, bones and intestine:
- Bones – PTH stimulates the release of calcium from bones into the bloodstream. This increases bone destruction and decreases the formation of new bone.
- Kidneys – PTH reduces loss of calcium in urine and also stimulates the production of active vitamin D in the kidneys.
- Intestine – PTH indirectly increases calcium absorption from food in the intestine, via its effects on vitamin D metabolism.
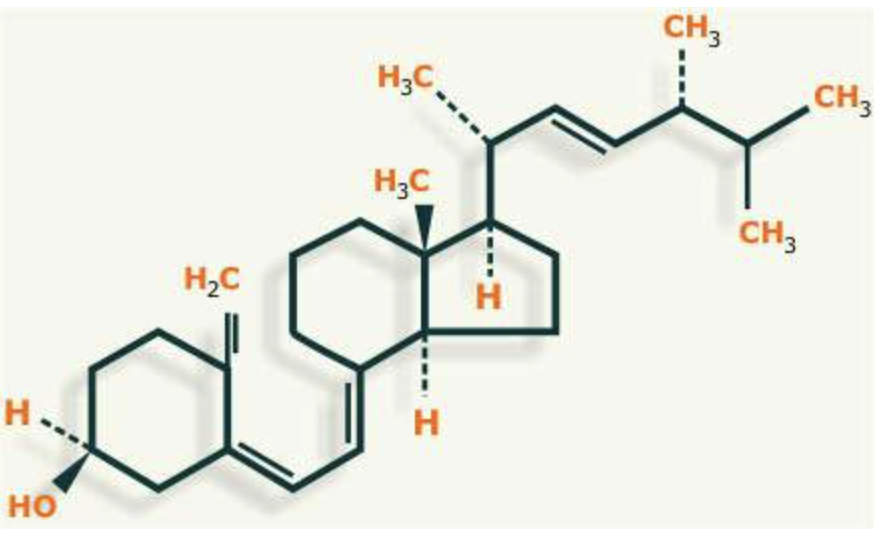
Vitamin D= calcitriol
Vitamin D protects bones, by helping your body absorb calcium and phosphorus.
The digestive system poorly absorbs calcium. Most people absorb only 15% to 20% of the calcium they eat in their diet.
Vitamin D is the hormone that helps the gut absorb more calcium.
Vitamin D protects teeth and bones from the effects of low calcium intake.
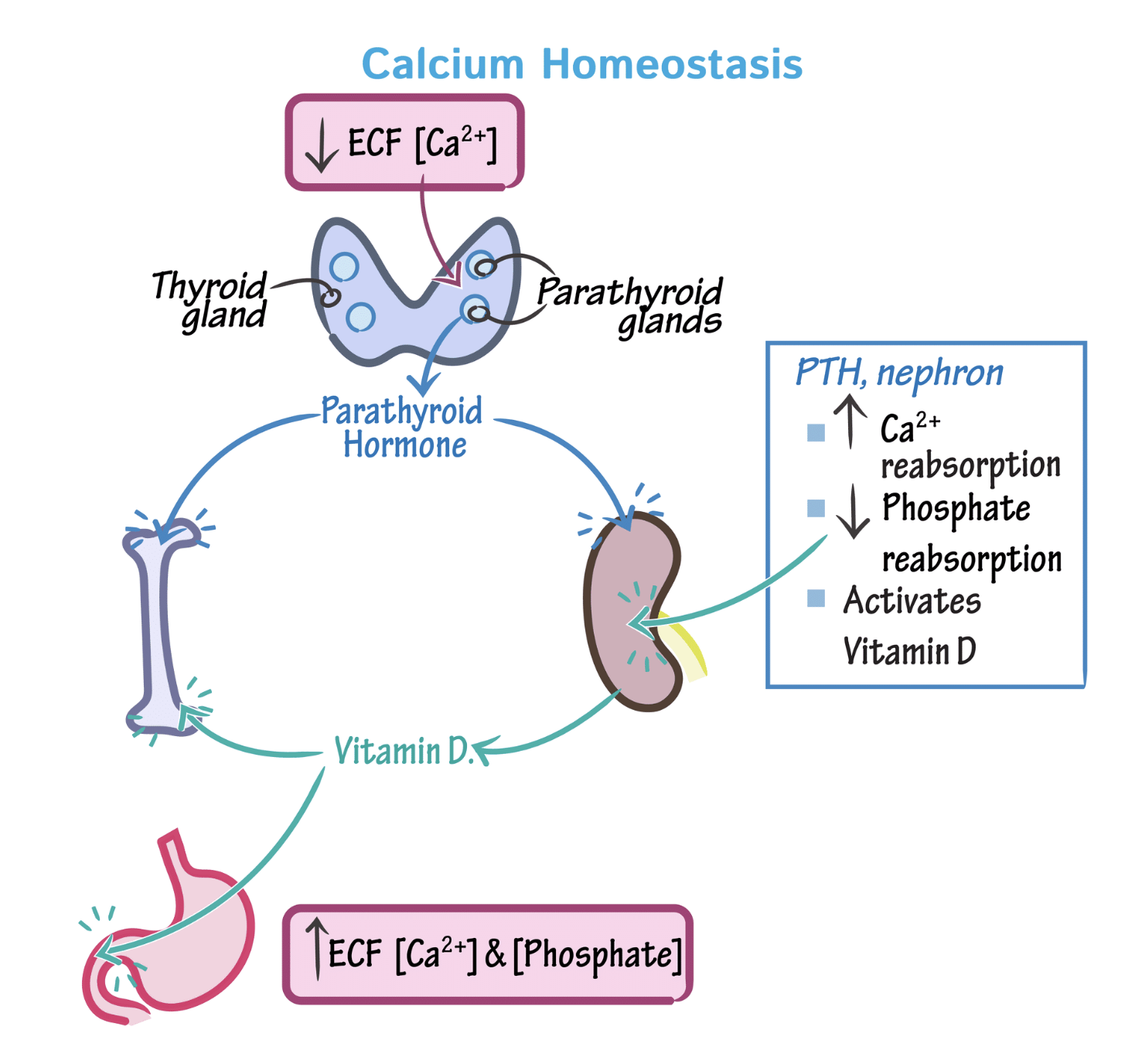
Calcium homeostasis PTH stimulates Vitamin D so more Calcium is absorbed.
The rest.....?
99% in bones so the remaining 1% Calcium [Ca2+]ECF circulates in extracellular fluids, intracellular structures and cell membranes and is essential to life.
Calcium homeostasis is regulation of the concentration of calcium ions in the extracellular fluid [Ca2+]ECF .
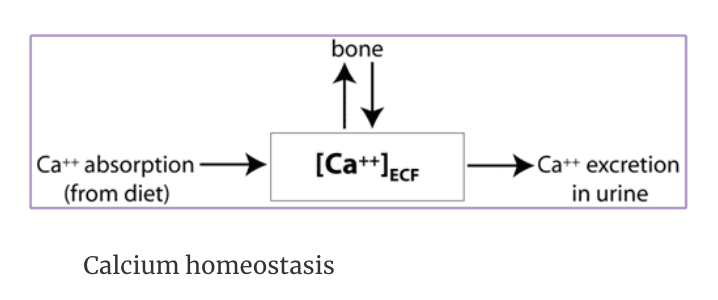
Ca2+ homeostasis
[Ca2+]ECF is influenced by dietary intake, Ca2+ absorption in the small intestine, and by excretion of Ca2+ in the urine.
Magnesium
Magnesium is a cofactor in more than 300 enzyme systems that regulate diverse biochemical reactions in the body, including protein synthesis, muscle and nerve function, blood glucose control and blood pressure regulation.
Most importantly magnesium has a major role as a catalyst in the reaction that adds a phosphate to ADP to produce the high energy compound ATP
Magnesium is required for energy production, oxidative phosphorylation, and glycolysis.
Magnesium contributes to the structural development of bone.
Magnesium is required for the synthesis of DNA, RNA, and the antioxidant glutathione.
Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, important to nerve impulse conduction, muscle contraction, and normal heart rhythm.
Sources of magnesium in our diet
Magnesium is widely distributed in plant and animal foods and in beverages.
- Green leafy vegetables, such as spinach, legumes, nuts, seeds, and whole grains.
- In general, foods containing dietary fibre provide magnesium.
- Magnesium is also added to some breakfast cereals and other fortified foods.

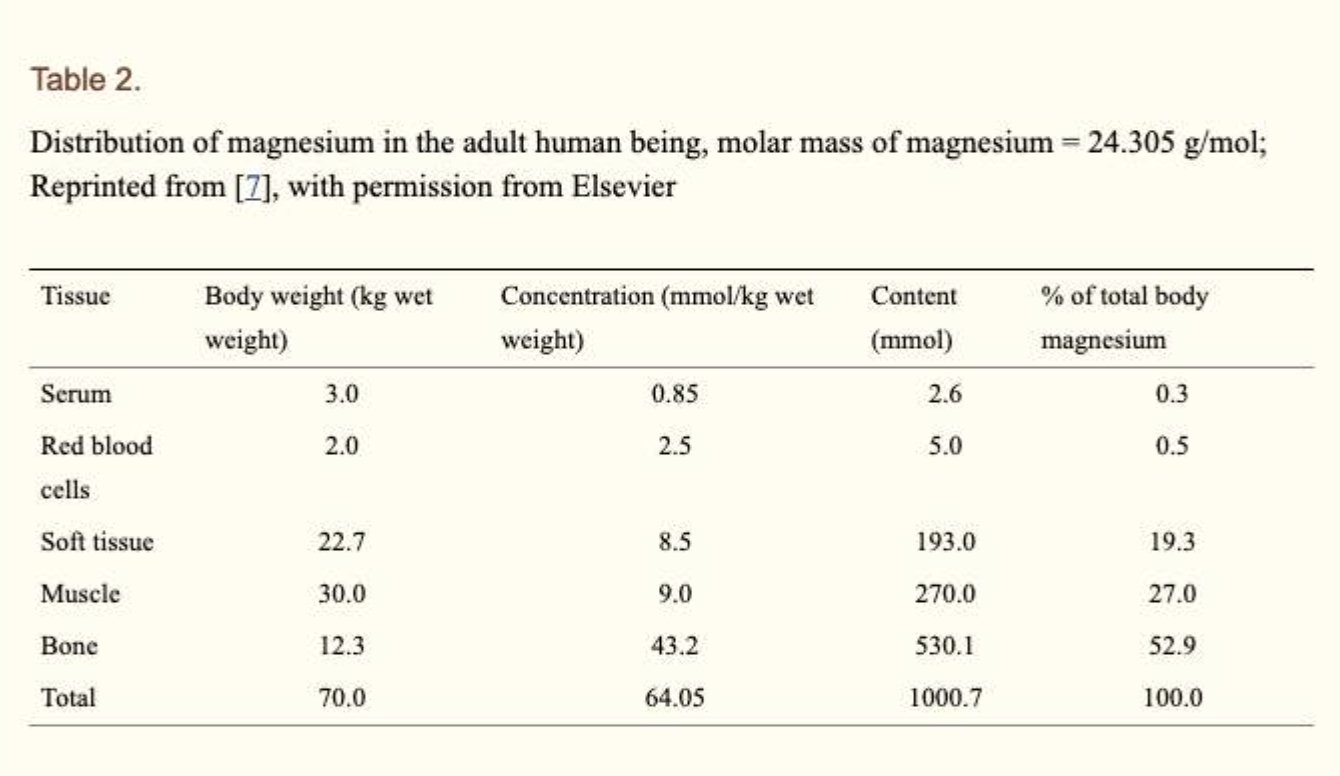
Distribution of magnesium in the body.
About 99% of total body magnesium is located in bone, muscles and non-muscular soft tissue:
- Approximately 50–60% of magnesium is on the surface of hydroxyapatite of bone
- Most of the remaining magnesium is contained in skeletal muscle and soft tissue
- Overall, one third of skeletal magnesium is exchangeable, serving as a "Mg bank"maintaining physiological extracellular magnesium levels
- Less than 1% of total magnesium is in blood serum, and these levels are kept under tight control.
Serum magnesium can—just like calcium—be categorized into three fractions:
- It is either free/ionized (active form)
- bound to protein
- complexed with anions such as phosphate, bicarbonate and citrate or sulphate.
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK11] Nucleic Acids (0) | 2023.05.16 |
|---|---|
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (1) | 2023.05.13 |
| [WEEK6]Chemistry of Food- Carbohydrates & Carbohydrates (0) | 2023.04.02 |
| [WEEK6] Stereoisomerism (0) | 2023.03.31 |
| [WEEK5] Carboxylic Acids, Esters (0) | 2023.03.30 |



