Continue from previous post
~micromineral, metalloenzyme, metal in medicine
Microminerals
The microminerals
Many elements are essential to our bodies in trace amounts.
Trace elements (or trace minerals) are usually defined as minerals that are required in amounts between 1 and 100 mg/day by adults or make up less than 0.01% of the total body weight.
Some of these are metals.
Six of the essential metals are shown below.
Nickel and Vanadium are thought to probably be essential trace elements as well!


Why are most of the trace metals transition metals?
Transition metals are elements of the “d” block in the periodic table. All “d” block elements are metals and have empty d orbitals. These are shown in orange below in the periodic table

Metals have unique properties that make them ideal for participating in biological systems.
1) Metals can change their charge and therefore their properties
In aqueous solution, metal ions exist as positively charged species that can bind to negatively charged biological molecules.
Depending on what the metal is bonded to the overall charge of the complex can be changed to cationic, anionic or neutral - it's versatile!
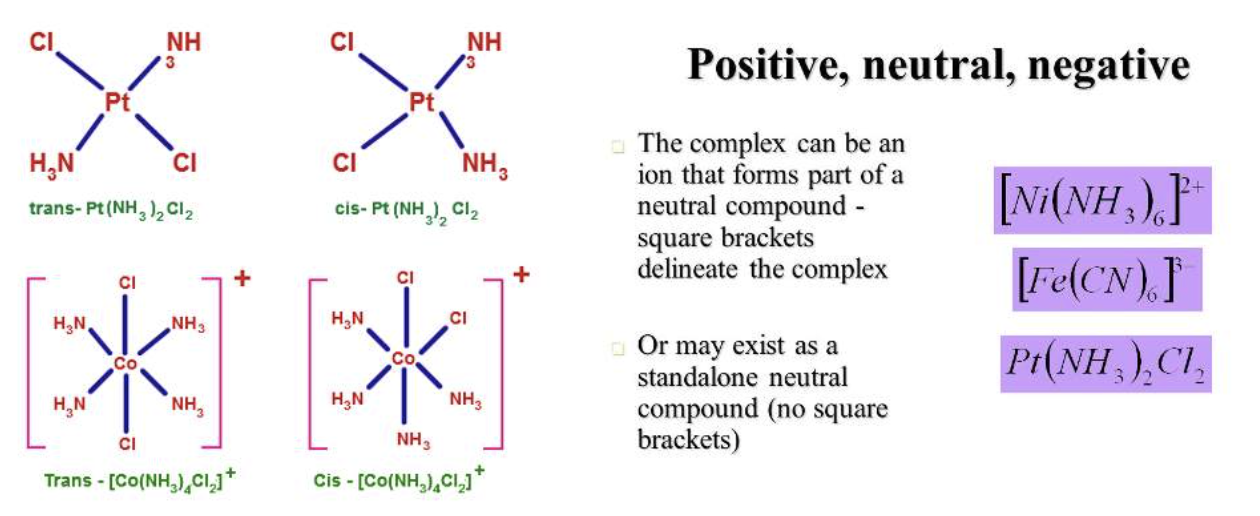
2) Metals can adopt different structures and bonding arrangements
Metal complexes can display a range of coordination geometries that give them unique shapes. The bond length, bond angle and coordination site vary depending on the metal and its oxidation state.
See an example of an octahedral Mg2+ below with 6 coordination bonds to the Mg
3) Metals can interact with ligans
Metal–ligand interaction: Different forms of metal–ligand interaction exist; however, these interactions usually lead to the formation of complexes that are unique from those of individual ligands or metals.
4) Metals act as Lewis acids
Metals have a high electron affinity and can easily polarize groups that are coordinated to them, and therefore can assist with their hydrolysis.
(A Lewis acid is an electron pair acceptor; because metal ions have one or more empty orbitals, especially d orbitals in the transition metals, they act as Lewis acids when coordinating ligands).
5) Metals have partially filled D shells
This means they readily accept electron pairs from ligands or substrates.
For transition metals, the variable number of electrons in the d shell or f shell (for lanthanides) influences the electronic and magnetic properties of transition metal complexes.
6) Metals can participate in redox reactions
Many transition metals have a tendency to undergo oxidation and reduction reactions.

IRON
Iron is an essential element for almost all living organisms.....
- required by enzymes that make amino acids, collagen, hormones and neurotransmitters;
- participates in a variety of metabolic processes, including oxygen transport and storage;
- can be oxidised and reduced and is therefore an important cofactor;
- participates in deoxyribonucleic acid (DNA) synthesis, and electron transport.
Iron exists in two oxidation states in the human body Fe2+ and Fe3+




Iron Distribution in the human body
In the human body, iron mainly exists as heme compounds (hemoglobin or myoglobin), heme enzymes, or non-heme compounds (flavin-iron enzymes, transferrin, and ferritin).
The body requires iron for the synthesis of its oxygen transport proteins, in particular hemoglobin and myoglobin, and for the formation of heme enzymes and other iron-containing enzymes involved in electron transfer and oxidation-reductions.
Fe distribution:
~ 60% hemoglobin on erythrocytes,
~ 25% iron storage (ferritin),
~ 15% is bound to myoglobin in muscle tissue & enzymes involved in the oxidative metabolism and many other cell functions.

Ferritin, the iron storer !
Ferritin is an intracellular protein that stores iron in the body.
- It acts as a buffer against iron deficiency because it stores iron until it is needed by our body and then delivers it where it is needed (= small amounts of serum ferritin)
- The small amount of ferritin that is released and circulates in the blood shows the total amount of iron stored in the body.
- Iron is stored in the centre of the ferritin protein as the non-toxic Fe3+ oxidation state.
- Iron is thought to enter as soluble Fe2+, then undergo oxidation by O2 in
channels or inside the cavity - Ferritin stores iron in an insoluble form and are present primarily in the liver, spleen, and bone marrow.

Transferrin-the iron transporters
Transferrin is a protein that binds to iron and transports it in the blood plasma to transferrin receptors on the surface of cells primarily bone marrow.
Transferrin does not have a heme but instead the amino acid sidechains coordinate to two Fe3+ ions.
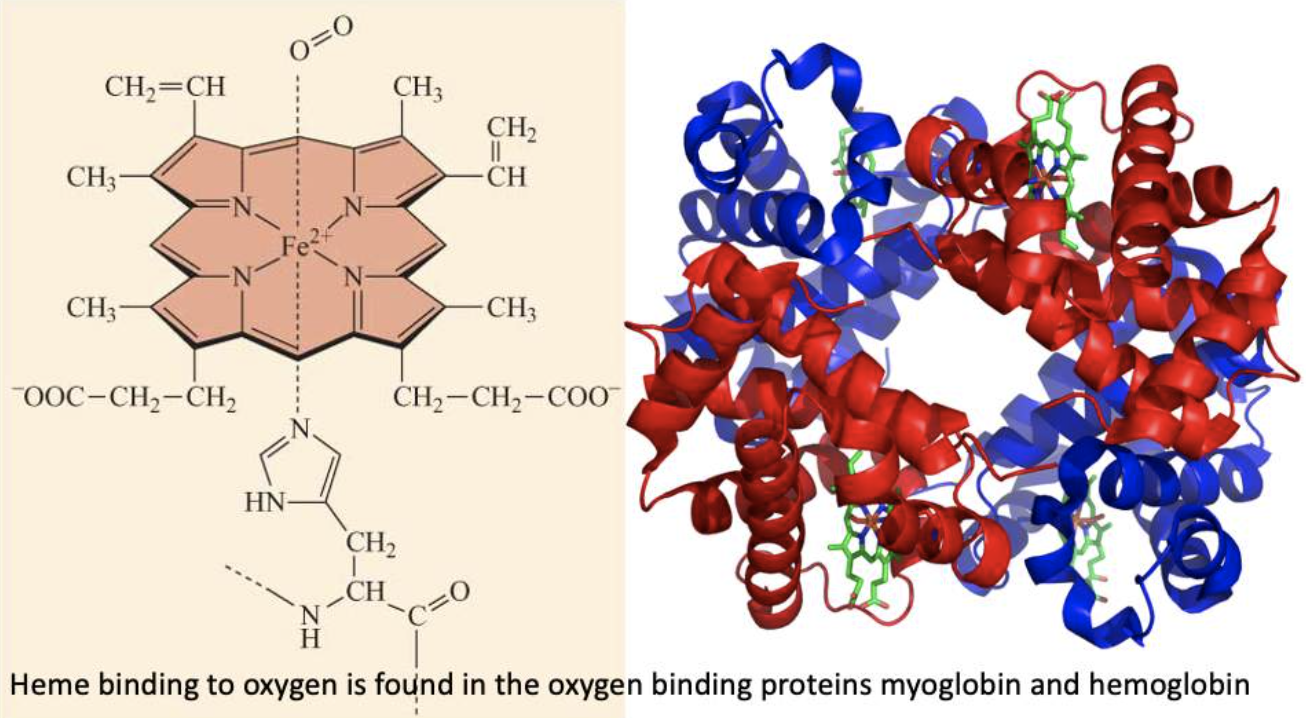
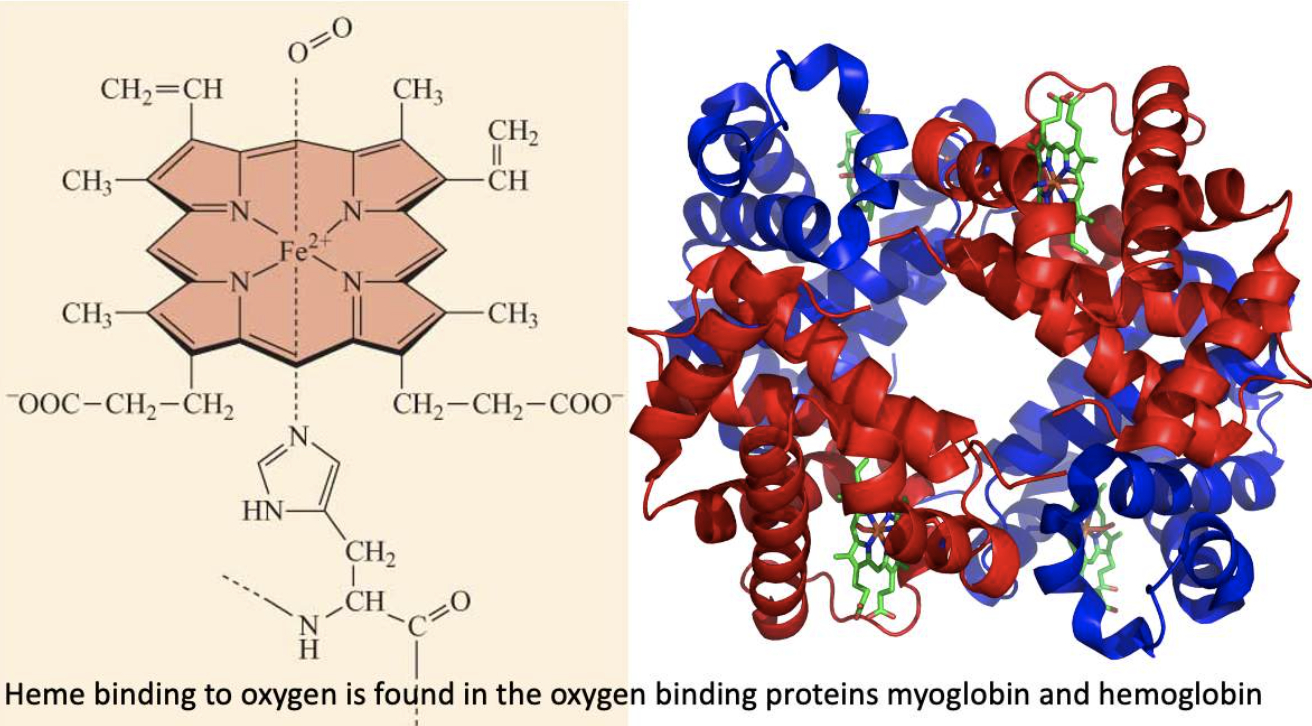
Hemoglobin and myoglobin - oxygen binders!
Hemoglobin transports oxygen from our lungs to our tissues.
In our tissues myoglobin stores oxygen and makes sure it is available to the mitochondria.
In both cases the iron that bonds to oxygen is in the Fe2+ oxidation state contained within a porphyrin ring = heme molecule

Too much or too little iron in the human body?
Apart from iron losses due to menstruation, other bleeding or pregnancy, iron is highly conserved and not readily lost from the body.
The average adult stores about 1-3 g of iron their body. A fine balance between dietary uptake and loss maintains this balance. The table below shows iron requirements at various ages. How is your iron?


ZINC
Zinc is a nutrient found throughout the body - in organs, tissues, bones, fluids, and cells.
Zinc helps the immune system and metabolism function.
Zinc is also important to wound healing and your sense of taste and smell.
Zinc stimulates the activity of at least 100 different enzymes.

Zinc deficiency?
Currently, the recommended dietary allowance (RDA) for zinc in the United States is 8 milligrams (mg) a day for women and 11 mg a day for men.
Compared to adults, infants, children, adolescents, pregnant and lactating women have increased requirements for zinc so are at increased risk of zinc depletion. Zinc deficiency during growth periods results in growth failure.
Due to the multitude of basic biochemical functions of zinc in the cells of the human body, there is a broad range of physiological signs of zinc deficiency.
These signs vary depending on the severity of the condition.
Organs affected clinically by zinc deficiency include the epidermal, gastrointestinal, central nervous, immune, skeletal, and reproductive systems.
MANGANESE
Manganese is an essential trace mineral, naturally present in many foods and dietary supplements, for brain and nerve health and function.
Manganese is considered to be essential for the formation of healthy red blood cells, proper pituitary gland function, and the maintenance of good eyesight.
Manganese also plays a role in blood clotting and hemostasis in conjunction with vitamin K.
FUNCTIONS
-> brain function and health
-> blood clotting
-> eyesight
-> pituitary gland function
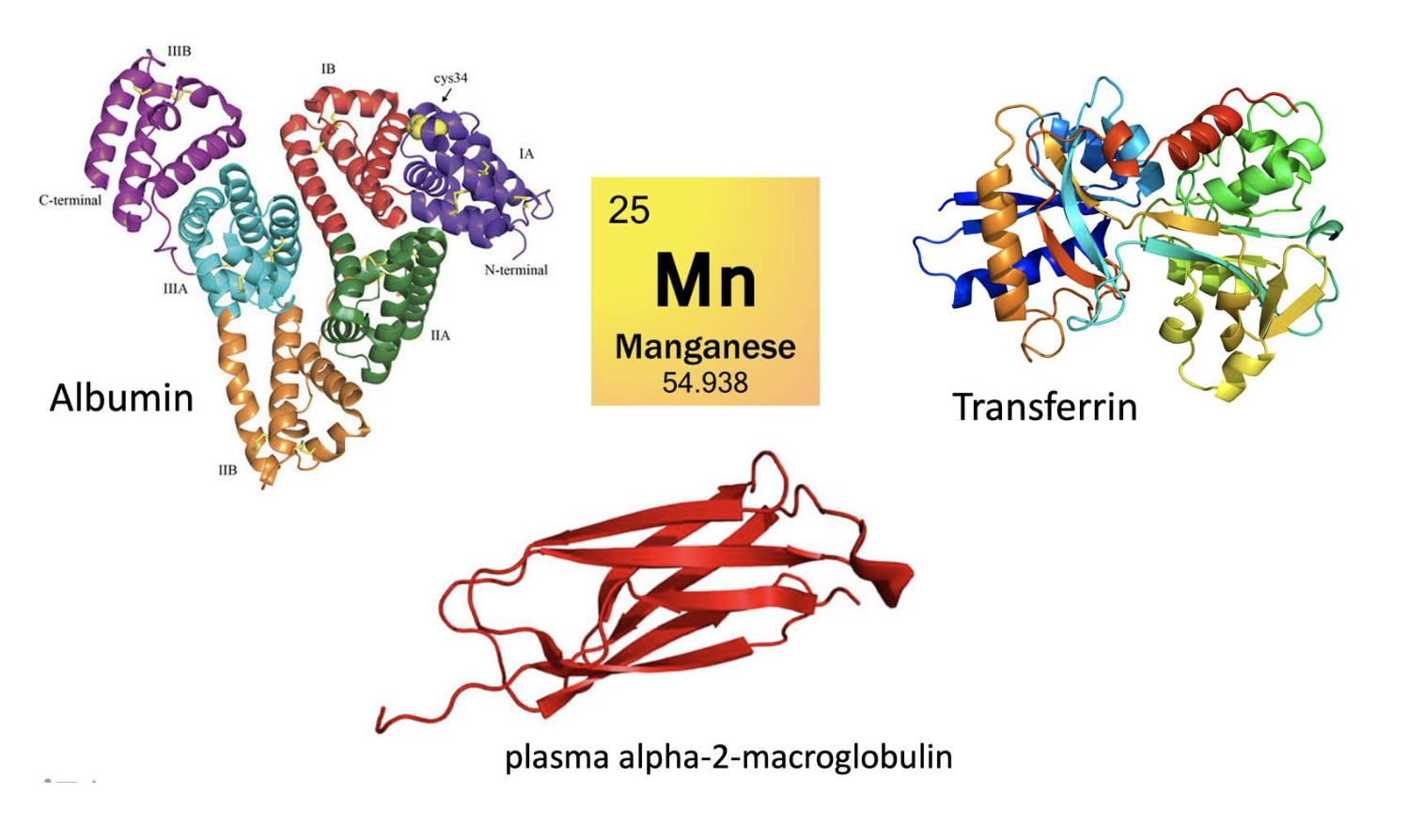
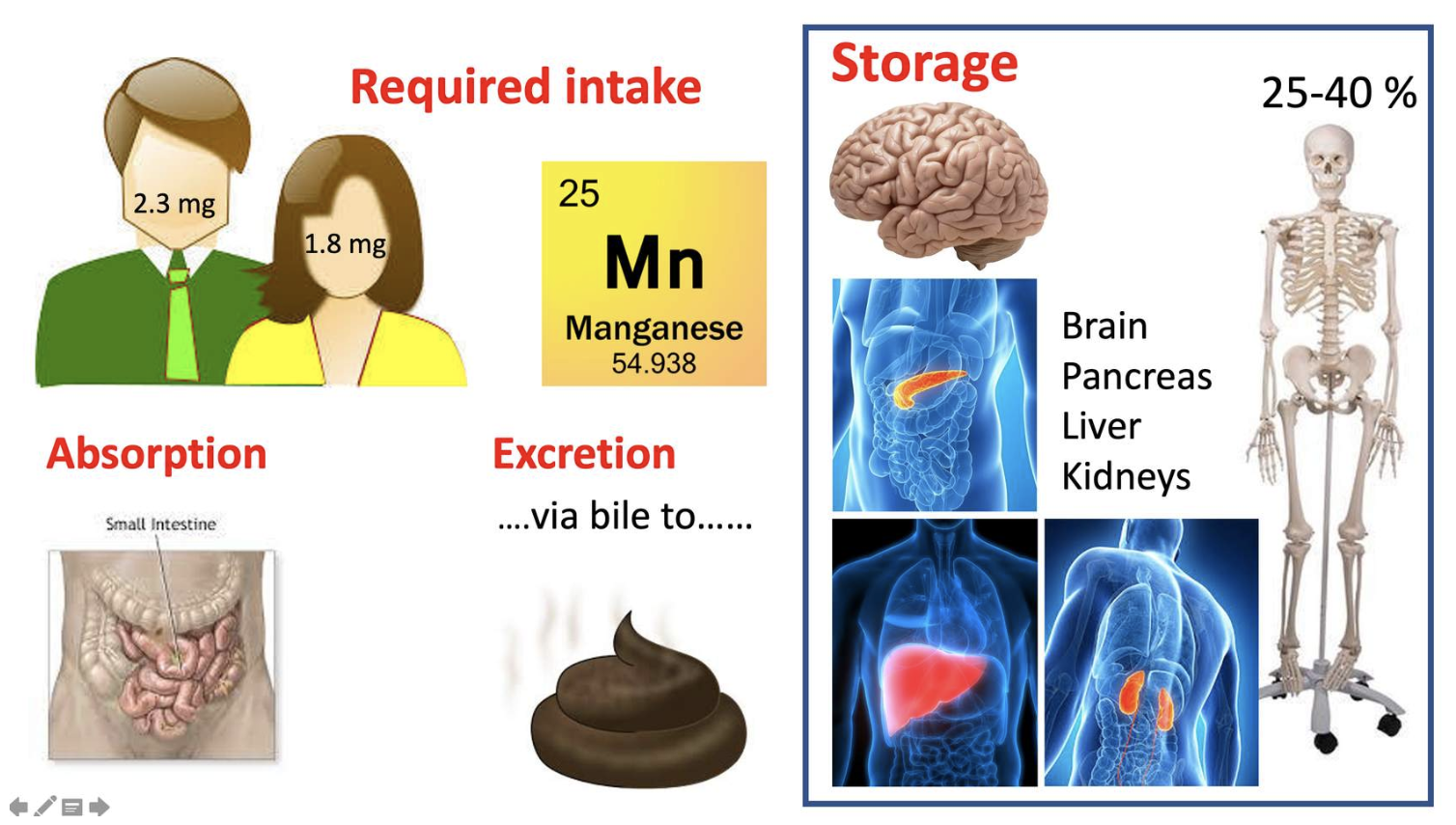
Manganese absorption, uptake and excretion...
Manganese is absorbed in the small intestine. After absorption, some manganese remains free, but most is bound to the proteins:
- transferrin,
- albumin, and
- plasma alpha-2-macroglobulin.
Manganese storage and excretion and excretion...
The human body contains about 10 to 20 mg manganese, of which 25% to 40% is in bone. The liver, pancreas, kidney, and brain also contain manganese.
MAINLY ABSORBED BY THE SMALL INTESTINE
The body maintains stable tissue manganese concentrations through regulatory control of manganese absorption and excretion.
More than 90% of absorbed manganese is excreted via bile into the faeces, and a small amount is reabsorbed.
Very little is excreted in urine.
STORED IN
-Bones, 25-40% liver pancreas, kidneys
Manganese is an important cofactor for enzymes...
Manganese is a cofactor for many enzymes, including:
- manganese superoxide dismutase
- arginase
- pyruvate carboxylase.
Through the action of these enzymes, manganese is involved in:
- amino acid, cholesterol, glucose, and carbohydrate metabolism;
- reactive oxygen species scavenging;
- bone formation;
- reproduction;
- the immune response.

COPPER
Copper, an essential mineral, is naturally present in some foods and is available as a dietary supplement.
It is a cofactor for several enzymes involved in:
- energy production (cytochrome oxidase - see metalloenzymes section)
- iron metabolism (see below CP)
- neuropeptide activation
- connective tissue synthesis
- neurotransmitter synthesis.
One abundant copper-containing enzyme is ceruloplasmin (CP), which plays a role in iron metabolism and carries more than 95% of the total copper in healthy human plasma.
Copper is involved in many physiological processes, such as:
- angiogenesis;
- neurohormone homeostasis
- regulation of gene expression,
- brain development, pigmentation
- immune system functioning
- defense against oxidative damage depends mainly on the copper-containing superoxide dismutases
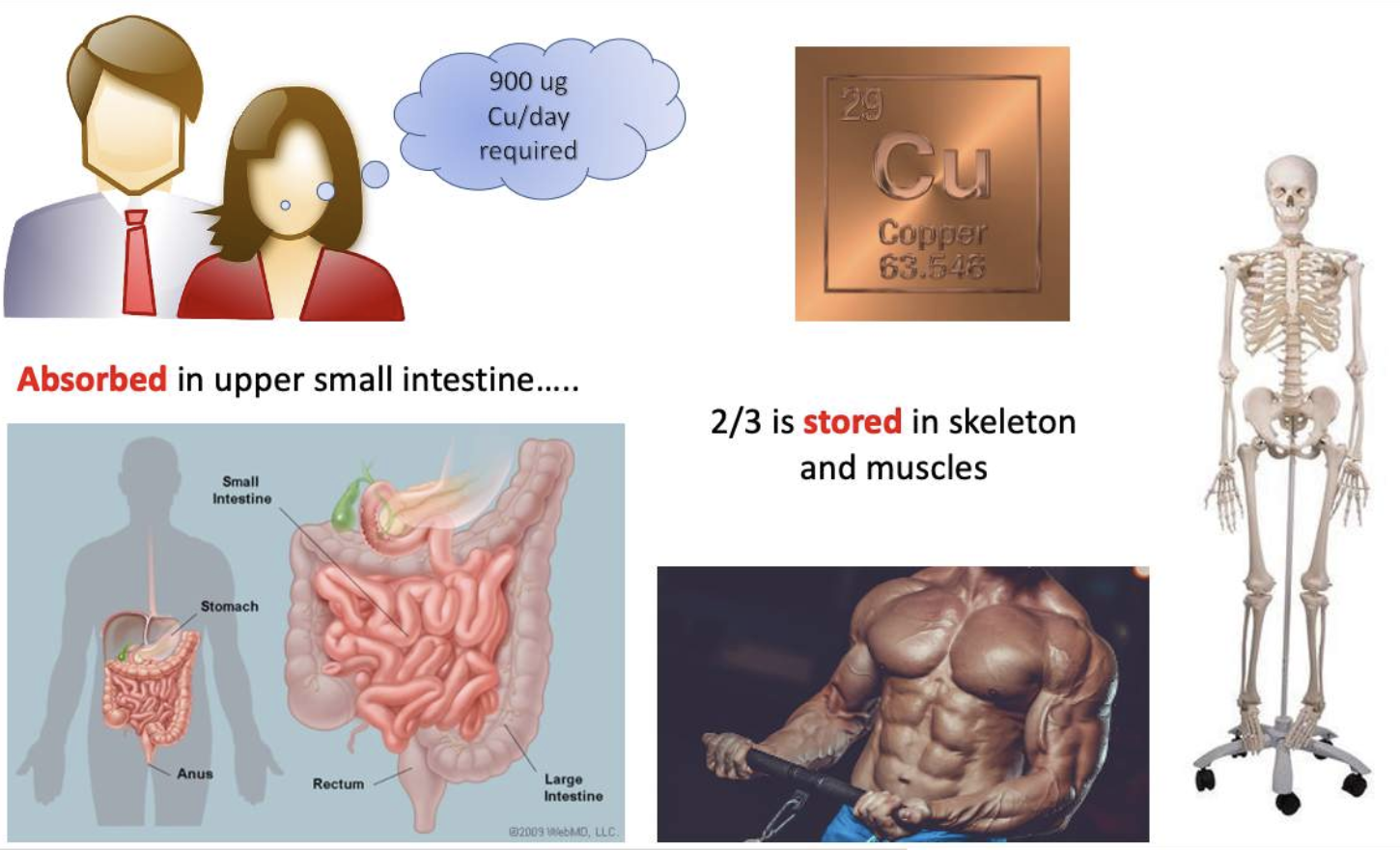
Copper requirements and diet?
The average human requires 900 ug/day that is primarily absorbed in the upper small intestine. (top of small intestine is where copper absorbed!!!)
Almost two-thirds of the body’s copper is located in the skeleton and muscle.
(95% of copper in serum is carried by ceruloplasmin protein!!!)
Food and copper
A wide variety of plant and animal foods contain copper including legumes, wholegrains and shell fish.

Severe copper deficiency?
Issues with copper metabolism can lead to Menkes and Wilson diseases.
Metalloenzymes
Metals in metalloproteins
Metalloenzymes are enzyme proteins that contain metal ions (metal cofactors), which are directly bound to the protein or to enzyme-bound nonprotein components (prosthetic groups).
The activity of these enzymes depends on a metal being bound at the active site of the enzyme.
Metals take part in electron transfer reactions.
They are versatile and occur frequently in proteins and enzymes.
(COFACTORS)
Metals act as cofactors to assist the enzyme's catalytic activity.
Examples of enzymes containing some important metals are given below:

(PROSTHETIC GROUPS)
A prosthetic group is a tightly bound, specific non-polypeptide molecule that assists with the biological function of some proteins.
- Vitamins, sugars, or lipids can be prosthetic groups, as can metal ions.
- Prosthetic groups bind tightly to proteins (cosubstrates are loosely bound).
- In enzymes, prosthetic groups are located in the active site, playing an important role in the functions of enzymes.
- Heme in hemoglobin and myoglobin is an example of a prosthetic group.
Besides enzymes, metalloproteins are involved in:
- non-enzyme electron transfer reactions (cytochromes);
- storage (e.g., ferritin for iron, myoglobin storage of oxygen); and
- transport proteins (e.g., hemoglobin transport of oxygen, transferrin for iron).
Examples of metalloenzymes
Metals are usually found in the active site of the enzyme.
The metals resemble protons (H+) in that they are electrophiles that are able to accept an electron pair to form a chemical bond.
Metals act as general acids to react with anionic and neutral ligands.
Below are examples of metalloenzymes where:
- Metals are part of a prosthetic group (cytochromes).
- Metals are built into the structure of the enzyme molecule and cannot be removed without destroying the structure of the enzyme (and therefore activity!) (cytochrome oxidase, phosphotransferases, alcohol dehydrogenase).
(Cytochromes-cytochrome C (Fe))
Cytochromes are integral membrane proteins.
They are present mostly in the inner mitochondrial membrane of eukaryotic organisms and Gram-positive and Gram-negative bacteria.
Cytochromes play crucial roles in electron transfer processes during energy generation in the electron transport chain.
For example:
Cytochrome C's function is to carry electrons from cytochrome bc to cytochrome oxidase of the electron transport chain during the production of ATP from food oxidation in metabolism.
Shown below is cytochrome C - it contains an iron bound to heme. The iron is reversibly oxidizable and serves as the electron acceptor for the cytochrome.
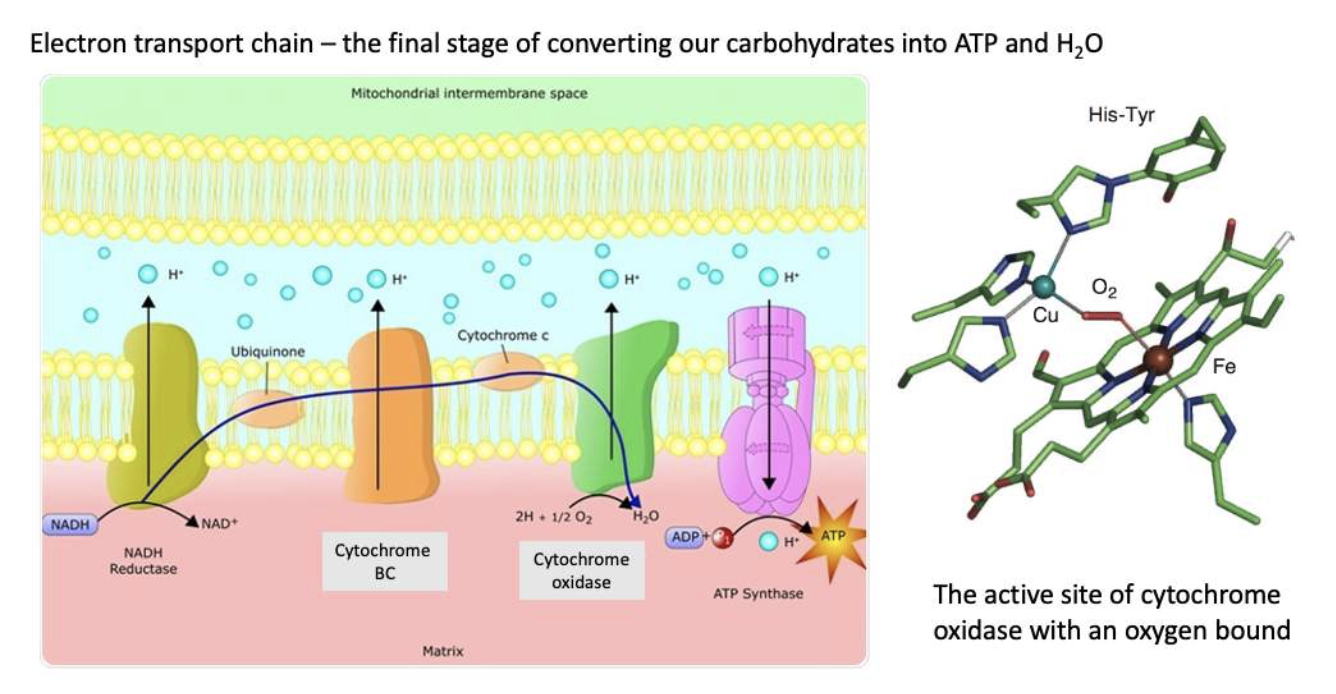
(Cytochromes-cytochrome oxidase (Cu and Fe in heme))
Cytochrome oxidase - controls the last step of food oxidation in metabolism.
- At this point, the atoms themselves have all been removed and all that is left are a few of the electrons from the food molecules.
- Cytochrome oxidase, takes these electrons and attaches them to an oxygen molecule.
- Then, hydrogen ions are added forming two water molecules.
Cytochrome oxidase contains copper ions that easily accommodate electrons removed from a substrate and readily transfer them to a molecule of oxygen.
Fe in the heme helps stabilise the oxygen at the binding site.
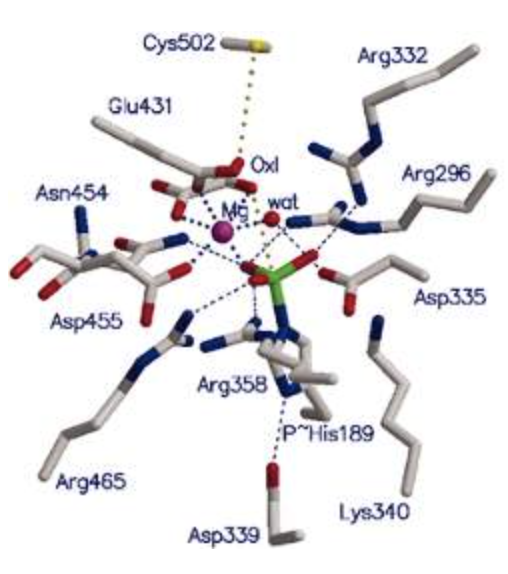
(Phosphotransferase Mg)
Phosphotransferases (or kinases) catalyse phosphorylation reactions and have Mg2+ built into the protein that participates in the catalytic reaction.
The phosphotransferase active site has three functions:
- the binding of the ATP (or GTP) phosphate donor as a complex with Mg2+
- the binding of the protein substrate
- the transfer of the γ-phosphate from ATP or GTP to the protein substrate.
In the figure below a histidine residue has been phosphorylated (shown in green/red). The Mg2+ ion is shown in purple.
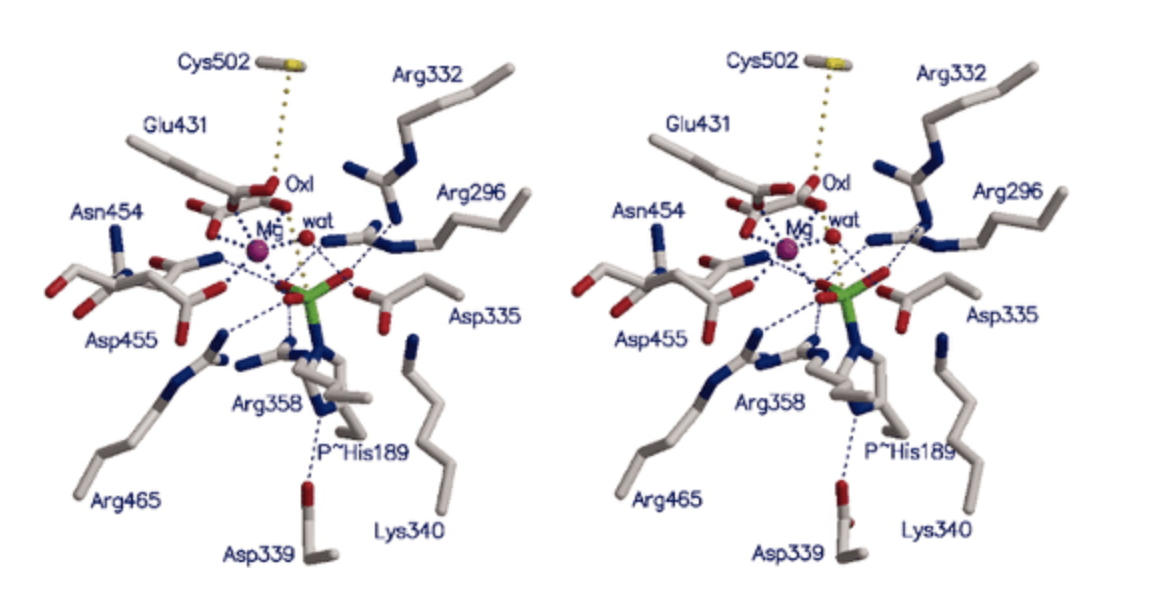
(Alcohol dehydrogenase Zn)
Alcohol dehydrogenase is a zinc metalloenzyme with broad specificity.
- They oxidize a range of aliphatic and aromatic alcohols to their corresponding aldehydes and ketones using NAD+ as a coenzyme
- Shown below is the active site of alcohol dehydrogenase with Zn in the active site bound to 2 Cys and 1 His and ethanol (substrate). NAD+ is also shown

Metals in Medicine
Some therapies were dubious....
For example mercury which we know is highly toxic to the human body.
Mercury was once used as a common elixir and topical medicine.
The ancient Persians and Greeks considered it a useful ointment.
From the Renaissance until the early 20th century, mercury was also used as a popular medicine for sexually transmitted diseases like syphilis.
Chinese alchemists of the 2nd century prized liquid mercury, or “quicksilver,” and red mercury sulfide for their supposed ability to increase lifespan and vitality:

-Metals used to treat anemia (Fe, Cu, Zn)
As we have seen humans need a range of metals at different concentrations to function normally. Anemia is caused by a lack of an essential metal and can result from a lack of sufficient nutrition or genetic defects. Examples:
- The most common deficiency causing anemia is Fe (iron) deficiency. This causes fatigue and can be treated by iron supplementation with tablets or an iron infusion (IV) or iron injection into muscle.
- Copper anemia in infants is also due to malnutrition and can cause heart disease. Copper deficiency anemia is treated with oral or intravenous copper replacement in the form of copper gluconate, copper sulfate, or copper chloride.
- Zinc anemia is due to poor nutrition and can result in growth retardation. Zn deficiency is treated with supplements.
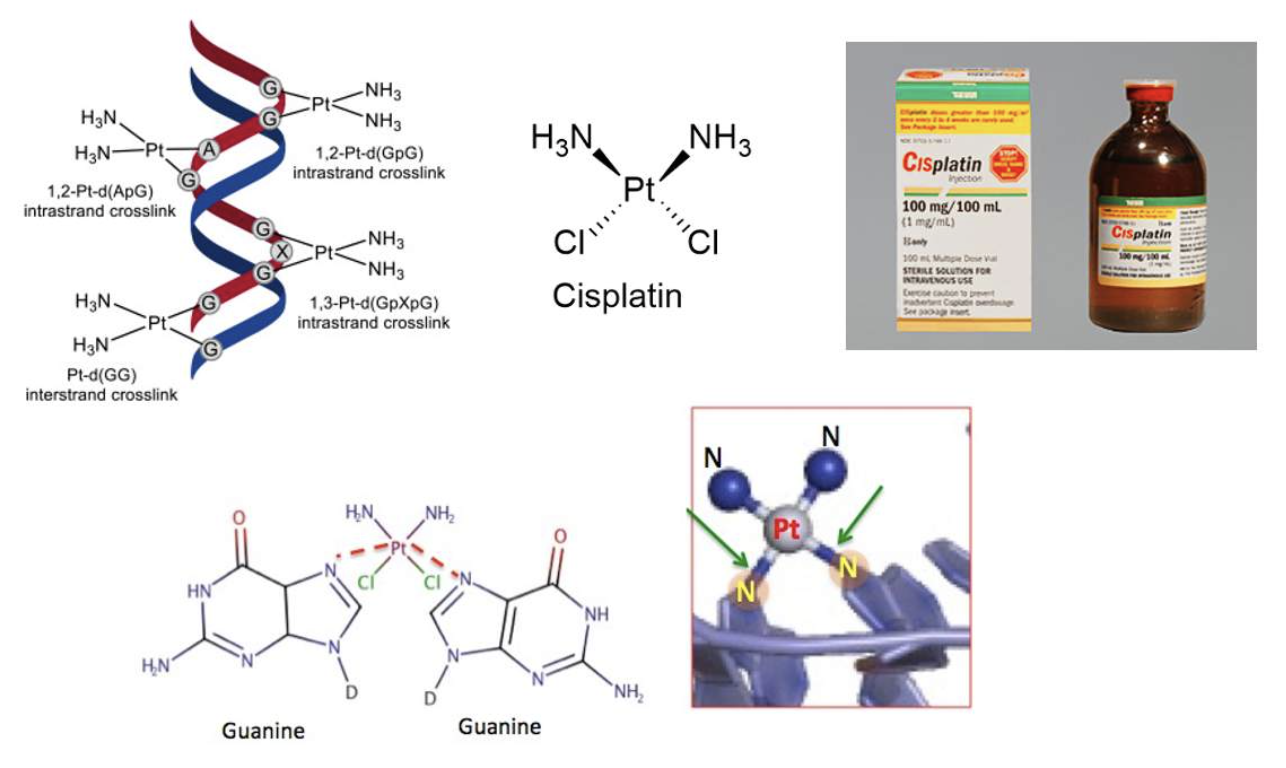
-Metals used to treat cancer
The most common and oldest modern cancer treatment is cis-platinum that was discovered in the 1960s.
Cis-platinum binds to DNA at the base pairs (especially guanine) and prevents its replication.
Many other metals have been used in cancer treatment, refer table 1 at this link:
-Metals used in diagnosis
Medical imaging relies heavily on metals as contrast agents to visually highlight regions of the body that are being examined.
Examples include:
- Gallium-68 is used as a positron emitting source in PET (Positron Emission Tomography)
- Technetium-99 is a radioisotope used commonly for imaging
- MRI Magnetic Resonance Imaging requires paramagnetic metals (with unpaired electrons) for contrast imaging. Gadolinium(III), Iron(III), and Manganese(II) are paramagnetic metals that are used for this.
-Metals used in medical implants-pacemakers catheters
A heart pacemaker is an implant that is designed to treat heart disorders with slow or irregular heartbeats by regulating your heartbeat!
The pacemaker is implanted in the body, usually below the collarbone, where it monitors the heart rhythm and triggers an electrical impulse if the heart is beating too slowly.
The pacemaker is composed of a small titanium encased pulse generator that contains a lithium battery and electrical circuitry attached to one, two, or three leads (wires) that are inserted into the heart.
- Pulse Generator - This small metal container houses a lithium battery and the electrical circuitry that regulates the rate of electrical pulses sent to your heart. The casing for the pulse generator components is usually made of titanium because titanium and two of its alloys, niobium and tantalum, are biocompatible and extremely resistant to corrosion and therefore durable.
- Leads & electrodes - One to three flexible, insulated wires are each placed in a chamber, or chambers, of your heart and deliver the electrical pulses to adjust your heart rate. Pacemakers usually contain at least two platinum-iridium electrodes, through which pulses of electricity are transmitted to stabilise the heartbeat. Platinum is also biocompatible, corrosion resistant and has good electrical connectivity.

-Metals used in prosthetics
A variety of metals are used for prosthetic limbs; Aluminum, Titanium, Magnesium, Copper, Steel as alloys (metal mixtures) or pure metals.
Titanium is favoured for making prosthetic limbs because it is lightweight, strong,
resistant to corrosion and biocompatible.
MINI LECTURE _ MEDICINE

ORIGINS OF DRUGS



-Plants and plant derivatives are the major sources of drugs, plant-based drugs account for approximately 30% of all pharamaceuticals
-A number of medicines (including tablets, injections, capsules, creams, mixtures and vaccines) contains animal products or are animal derived
-Microbes have made a phenomenal contribution to the health and well- being of people throughout the world
-In addition to producing many primary metabolites, such as amino acids, vitamins and nucleotides, they are capable of making secondary metabolites, which constitute half of the pharamaceuticals on the market today

-Using our understanding of chemistry, many of these drugs are structurally modified to produce safer (less toxic), more effective (more potent, increased half life) medicines for patients

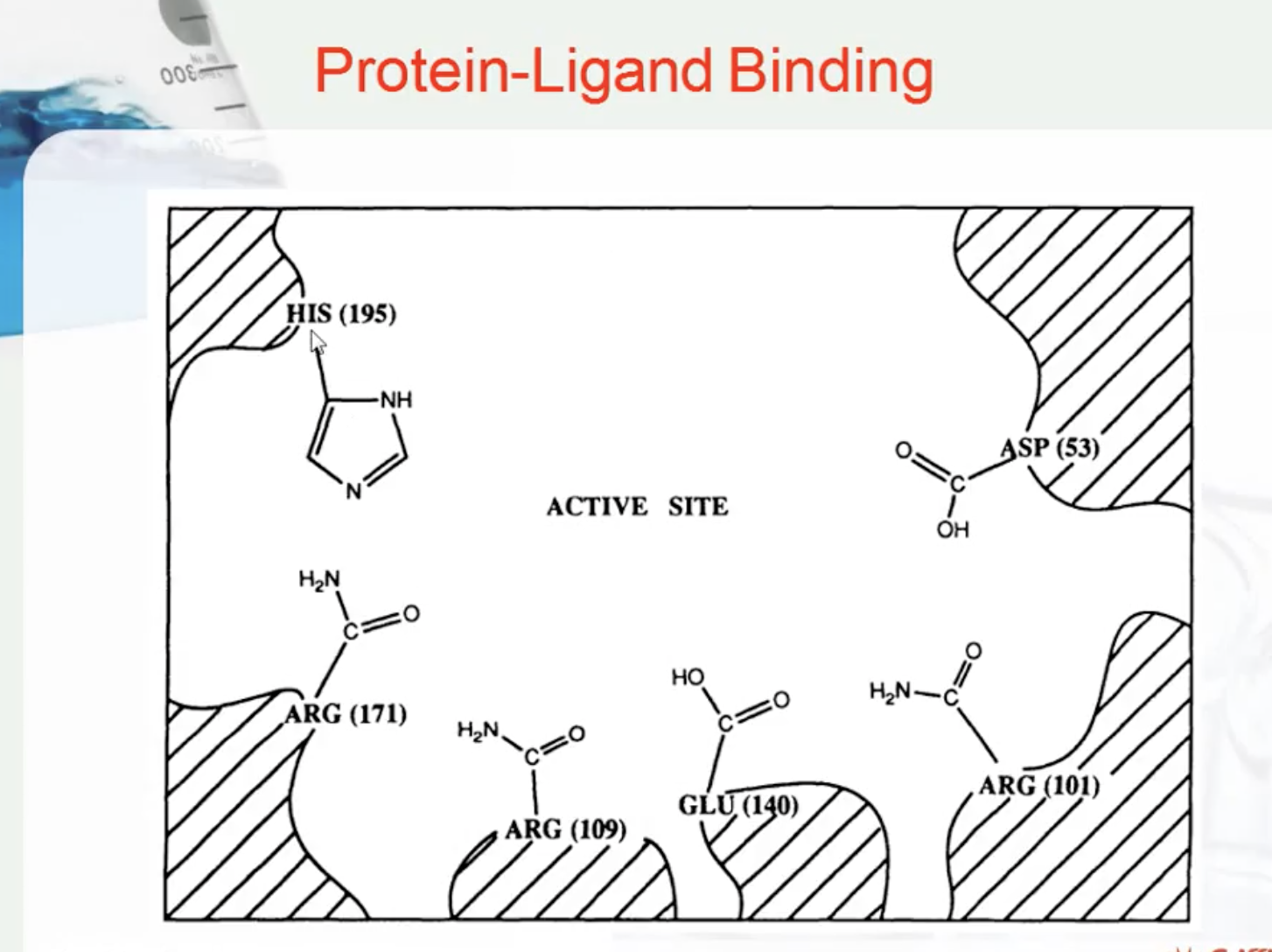
Protein target
receptor
enzyme
ionchannel
carrier
Active site -> substrate or ligand bind
right shape , chemical affinity to particular enxyme
PROTEIN-LIGAND BINDING




You need more drug if it has low affinity
You need less drug if it has high affinity
Drugs that are more selective is generally safer to use, and target the specific body that we want
COX1 -> regulates gastrointestinal health
COX2-> associate inflammation
Influencing COX1 = having more side effects
Influencing COX2 = reducing inflammation
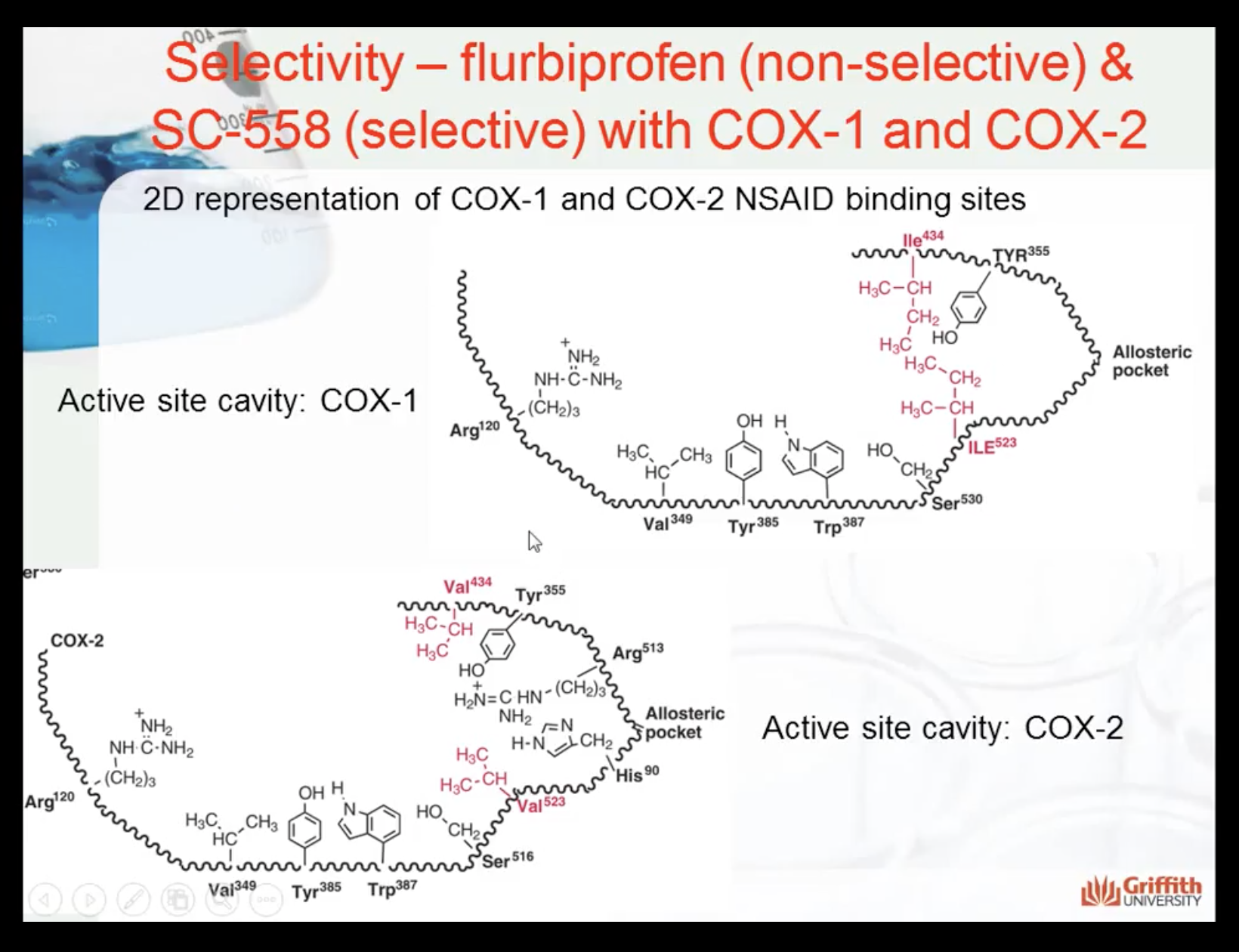
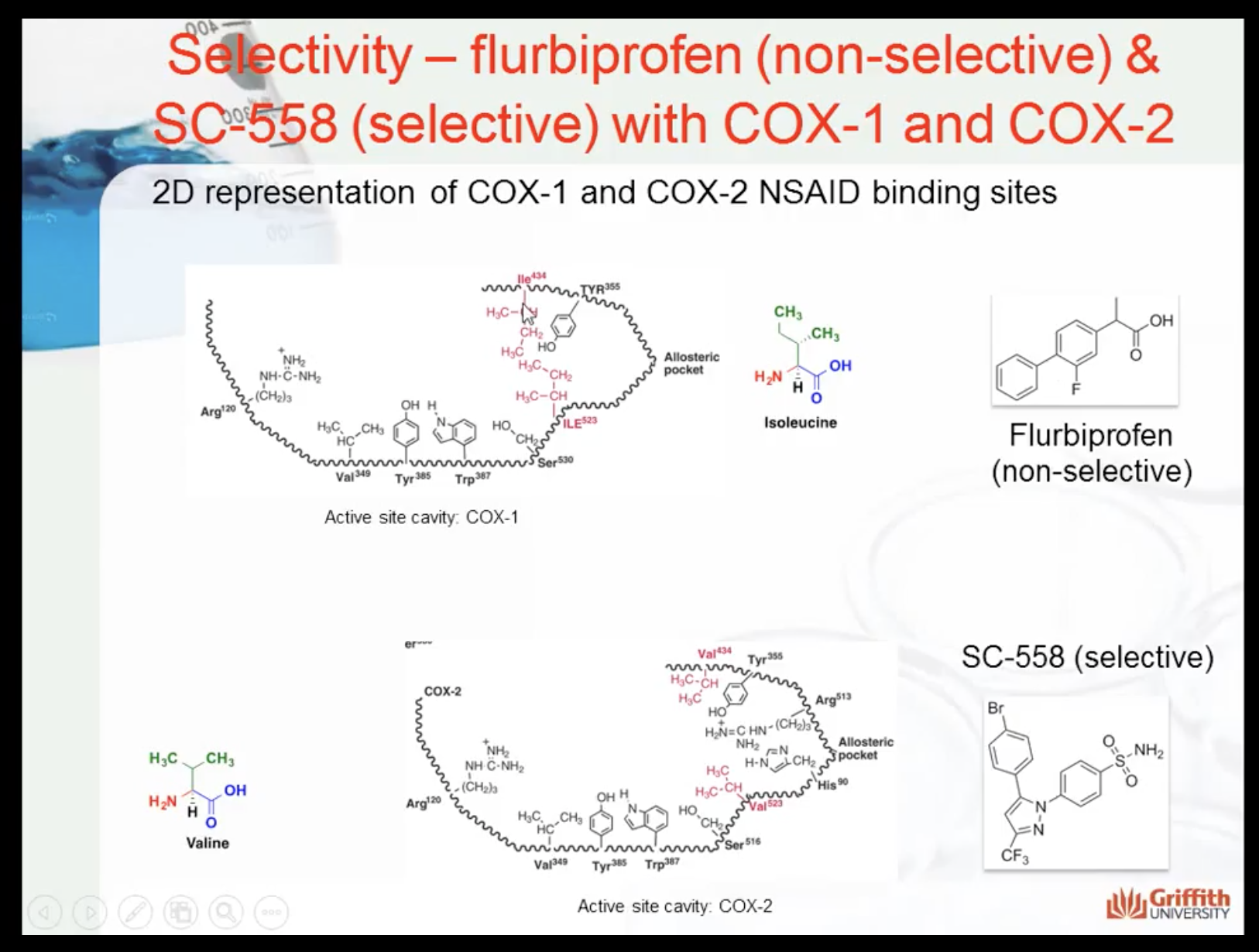
Shape and structure makes the selectivity of the drug to the enzyme
(SC-558 only affects to COX2)


BINDING AFFINITY + SHAPE
INTERMOLECULAR FORCE

VAN DER WAALS FORCES
-Dispersion forces : very brief temporary dipole
-Dipole-Dipole bonding : between polar, permenant dipole, stronger than dispersion force
-Hydrogen bonds
IONIC BONDS : cations - anions
ION-DIPOLE BONDS
-Amines -> bases : accept H+
Ionic bonds are stronger than Van der waals
Important in Solubility of organic compounds in water !
VAN DER WAALS FORCES



IONIC BONDING / ION- DIPOLE BONDING


SOLUBILITY



Lipid media
-> examples of lipid media include water-immiscible solvents, cell membranes, blood vessel and GI track membranes, and the blood-brain barrier
-> van der Waal bonding is the predominant intermolecular bond
-> found in the aliphatic and aromatic portions of organic molecules (present to some extent in ALL organinc compounds)
Aqueous media
-> exmple of aqueous media include water, blood, and cellular cytosol
-> dipole-dipole bonding (hydrogen bonding) and ion-dipole bonding important
Chloramphenicol

-presence of oxygen and nitrogen containing functional groups enhances water solubility
-nonionizable hydrocarbon chains and ring systems enhances lipid solubility



1) Oxidation of Alcohols
-> dehydrogenase --> aldehyde, acid, ketone
2) Demethylation of Amines
-> eliminate methyl from amine and attach H+ to amine
3) Conjugation of Alcohols
->glucuronidation
=> All these reaction increase water solubility
=> hence increase the likelihood of excretion of these compounds
Polarity increase -> solubility increase
CHOLESTEROL BIOSYNTHESIS
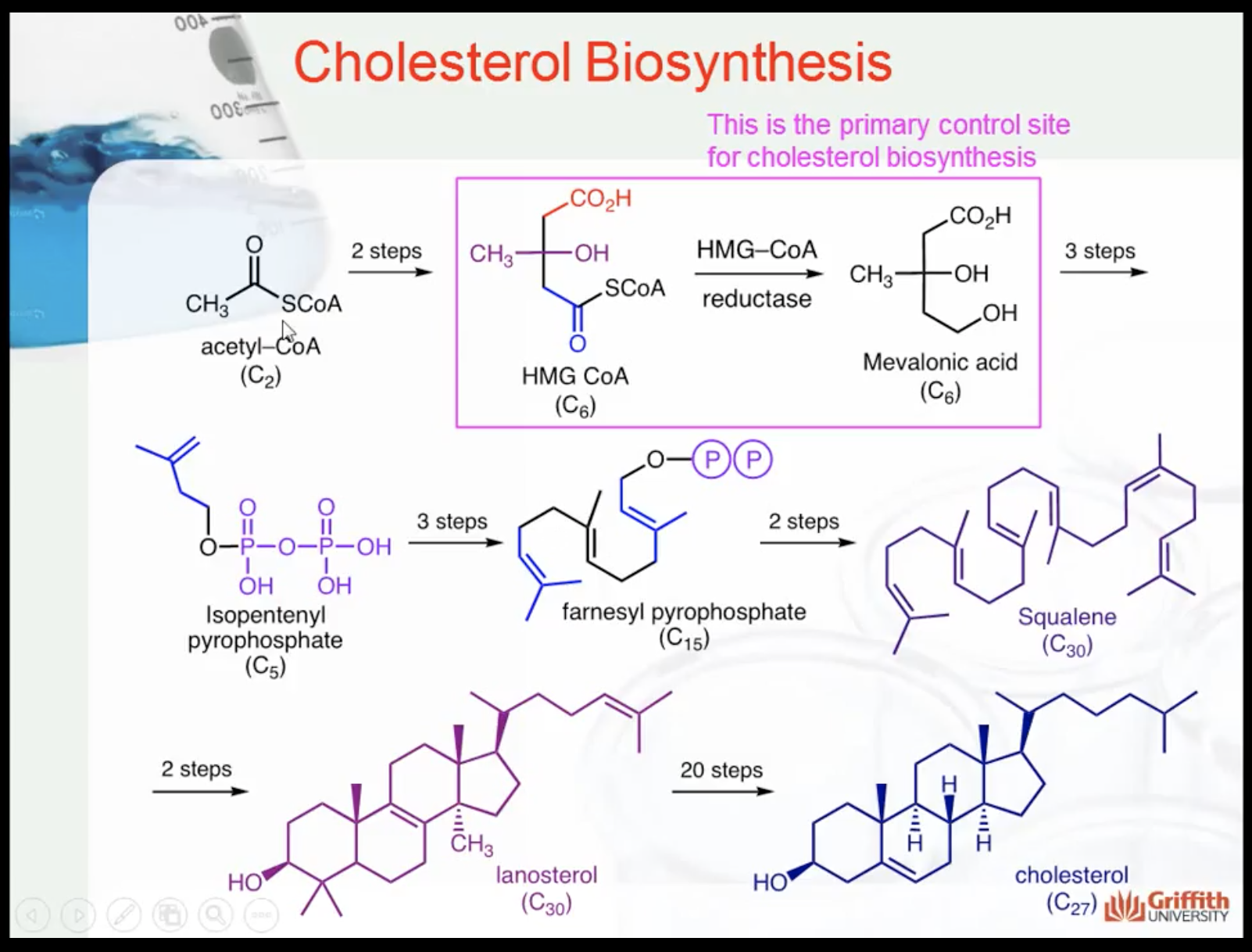







lovastatin -> pracastatin (increase solubility due to increased polarity + slightly increased acidity also)


'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK12] The Chemistry of Exercise (0) | 2023.05.26 |
|---|---|
| [WEEK11] Nucleic Acids (0) | 2023.05.16 |
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (0) | 2023.05.13 |
| [WEEK6]Chemistry of Food- Carbohydrates & Carbohydrates (0) | 2023.04.02 |
| [WEEK6] Stereoisomerism (0) | 2023.03.31 |



