MINI LECTURES
Isomerism
-differ in how the atoms are connected => structural isomers
-differ in how the connected atoms are arranged in space => stereoisomers
*Stereoisomers
-cis or trans geometric isomers
-optical isomers (have the ability to rotate the plane of plane-polarised light)


-Optical activity: the ability of a substance to rotate plane-polarized light to the right or left
(Right: dextrorotatory / Left: levorotatory)
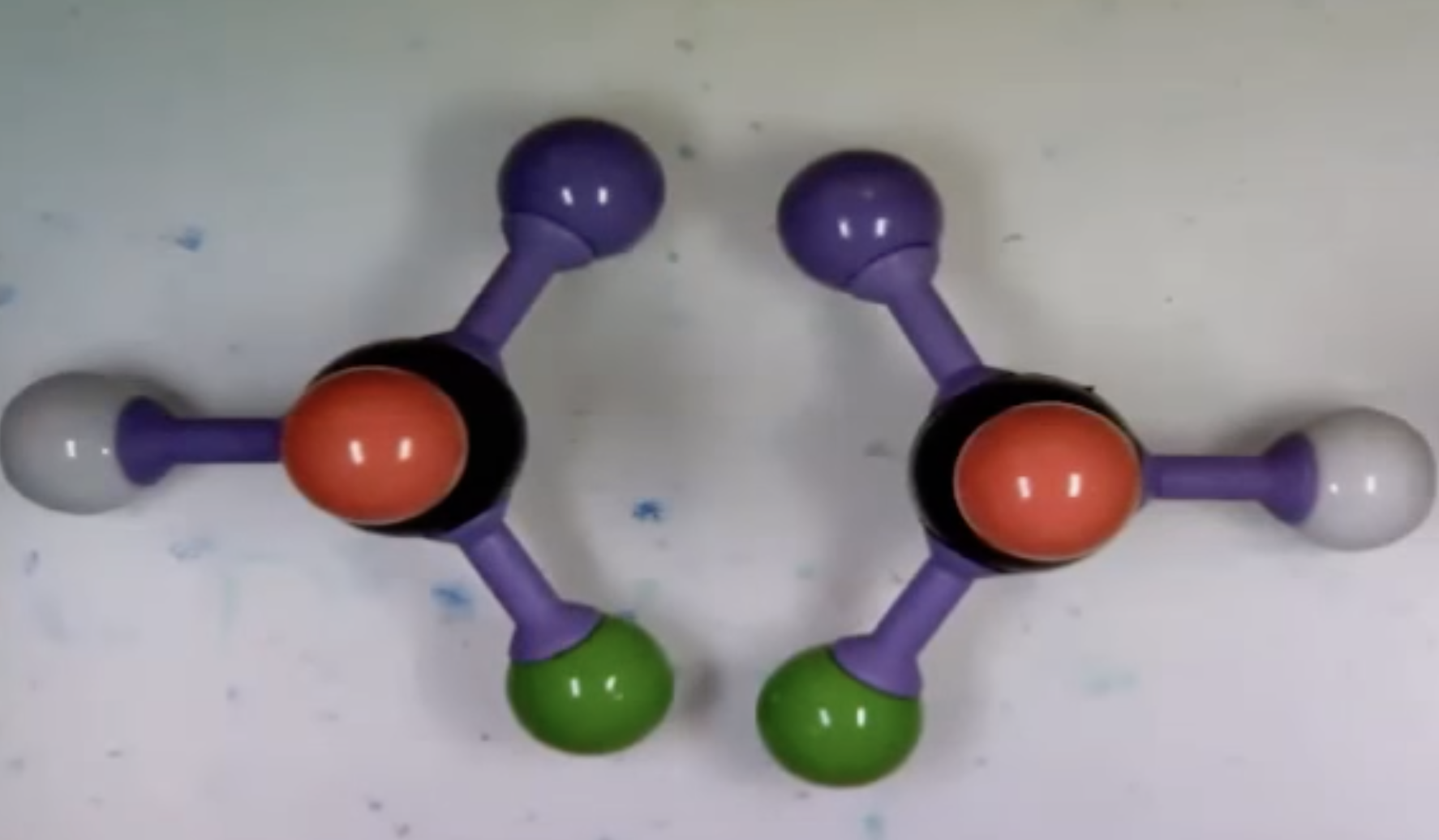
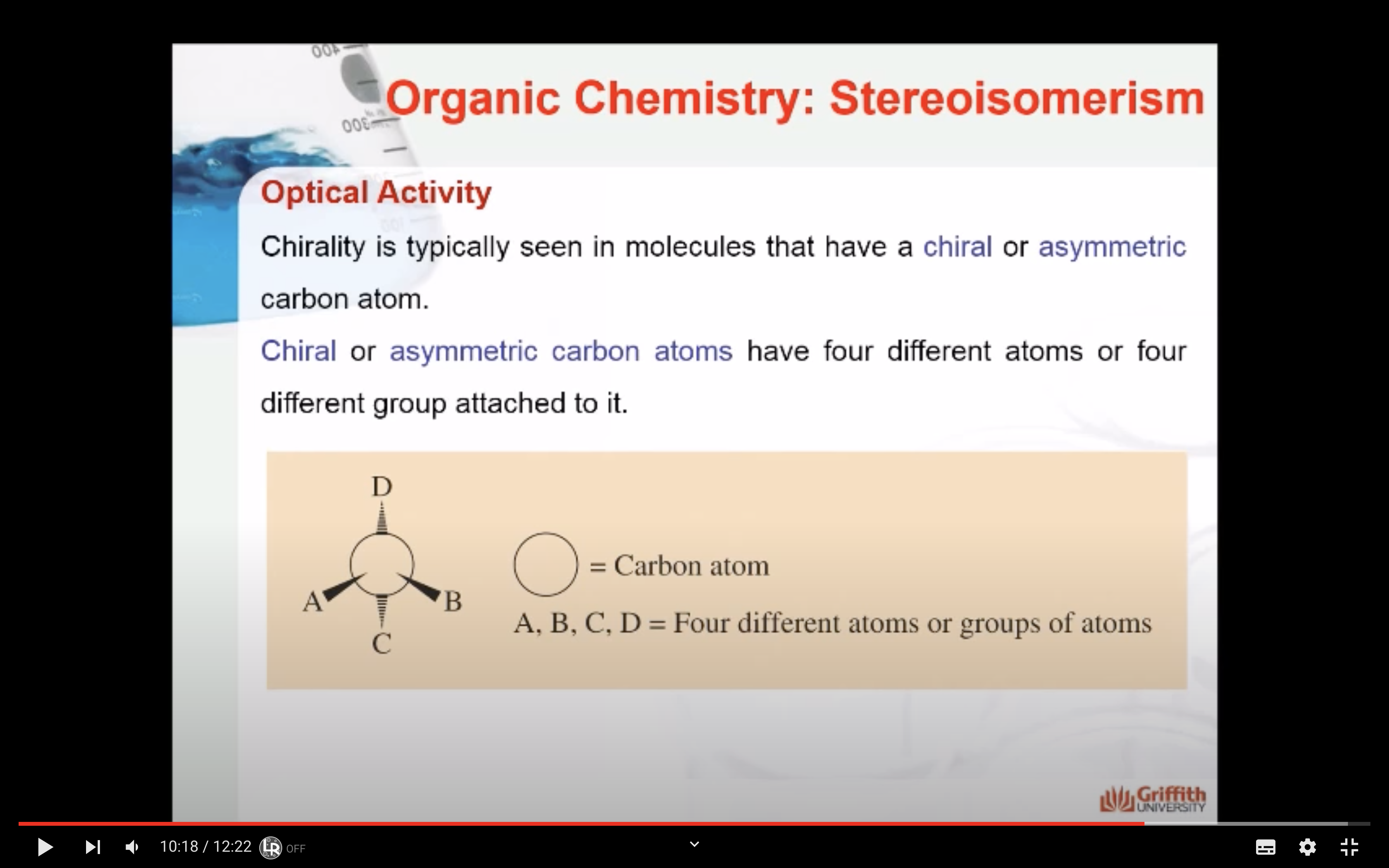
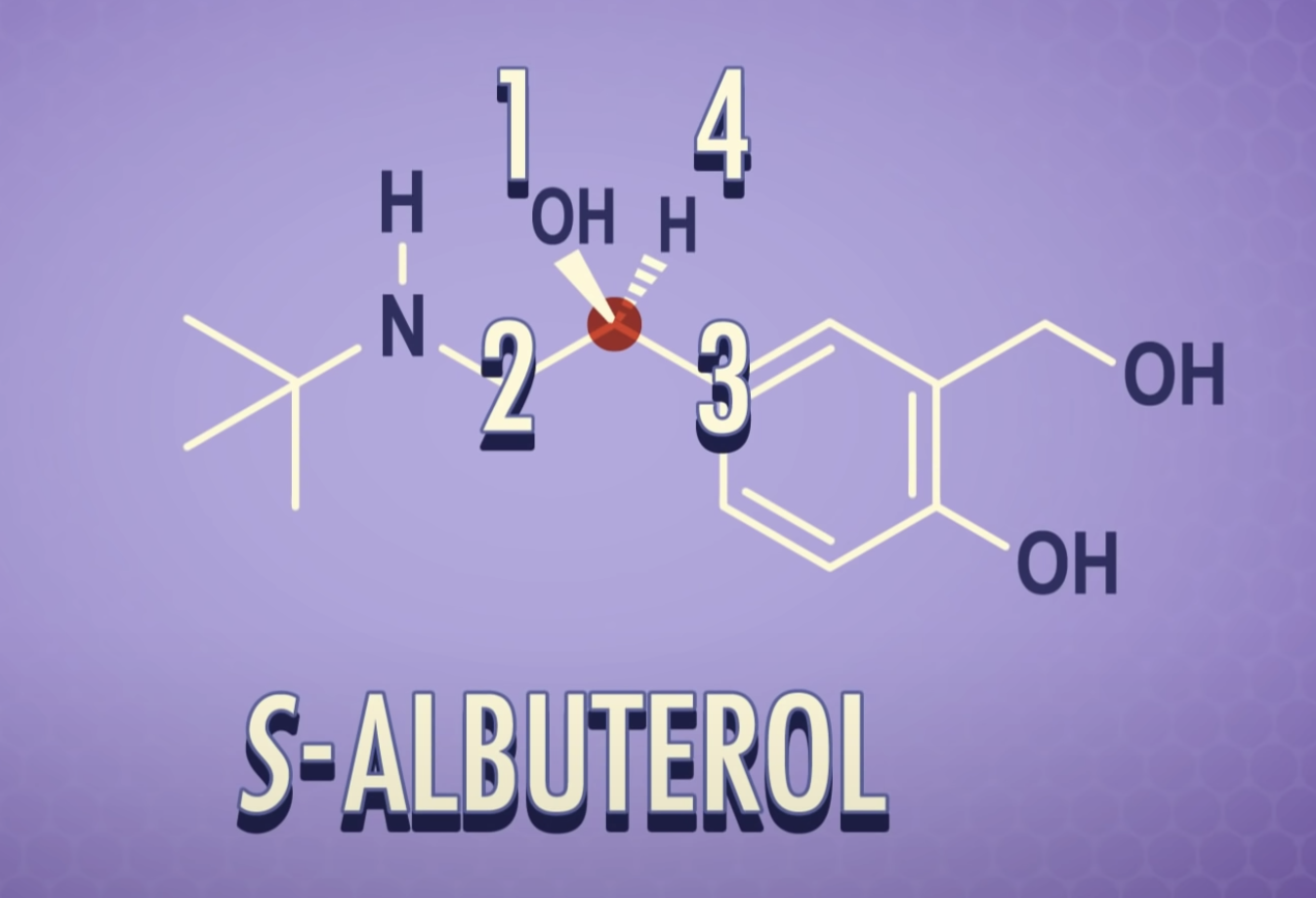
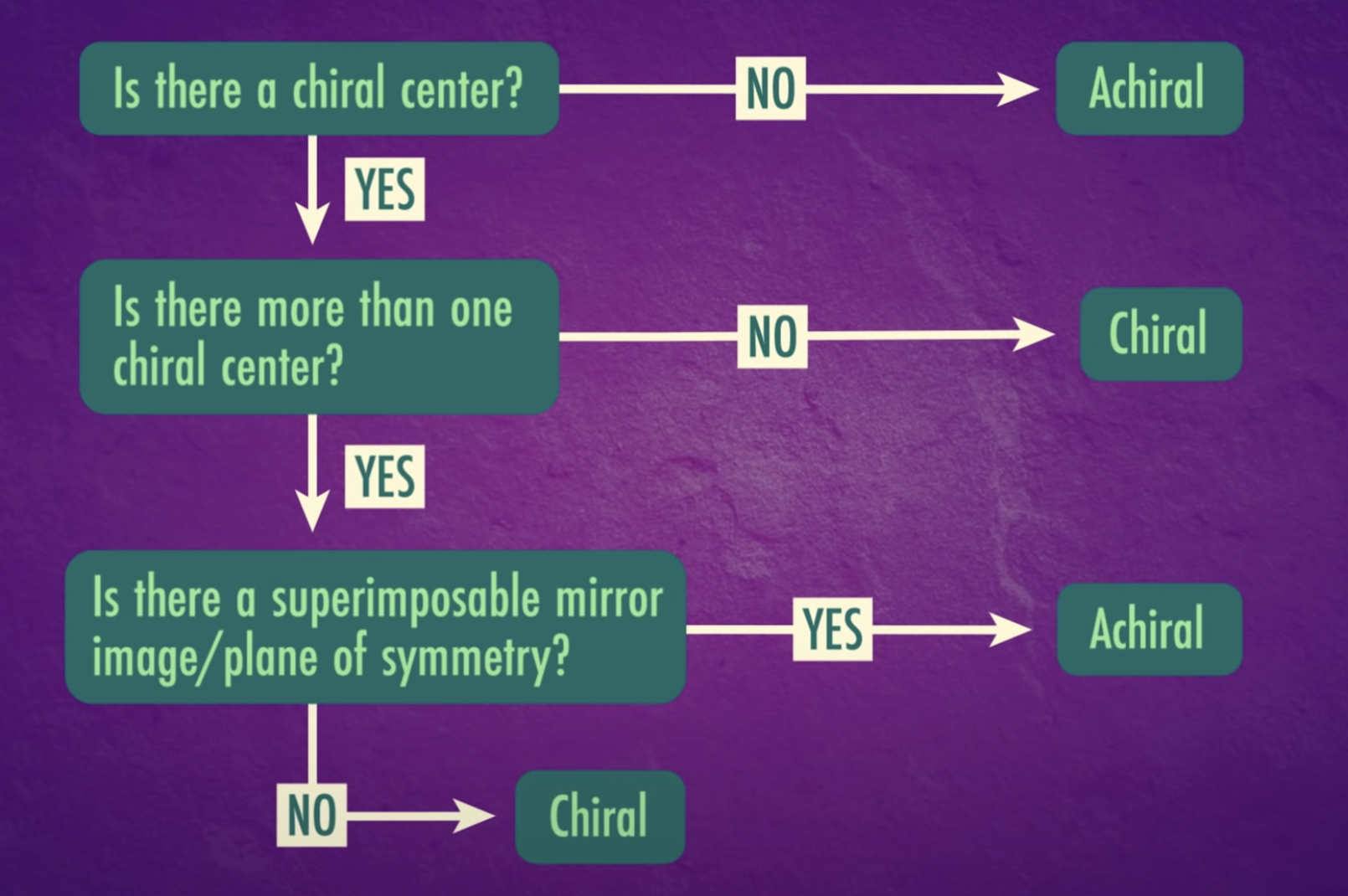
-Chirality : property present in an object that cannot be superimposed on its mirror image
(Chirla objects or chiral molecules do not have a plane of symmetry, they are asymmetric)
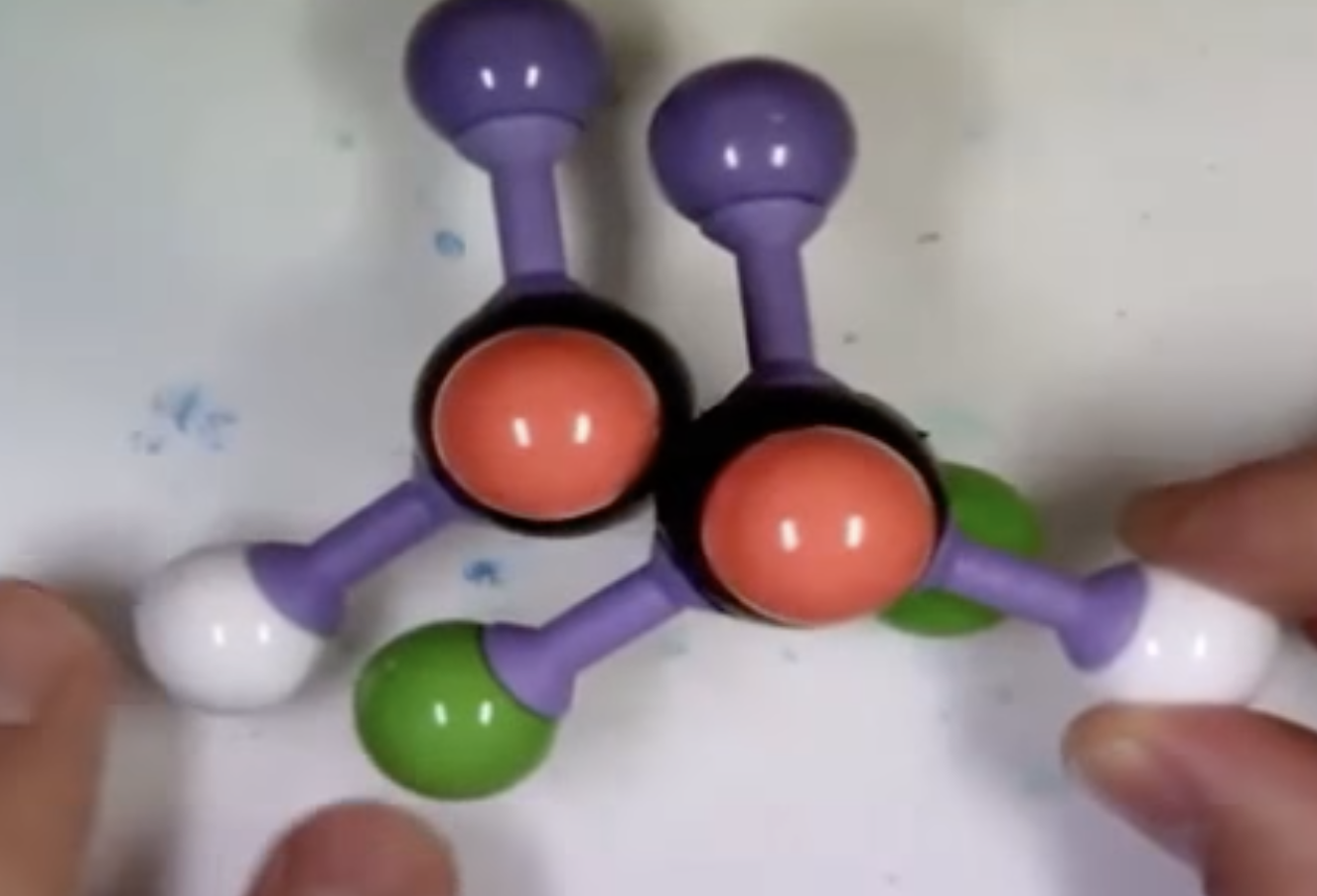


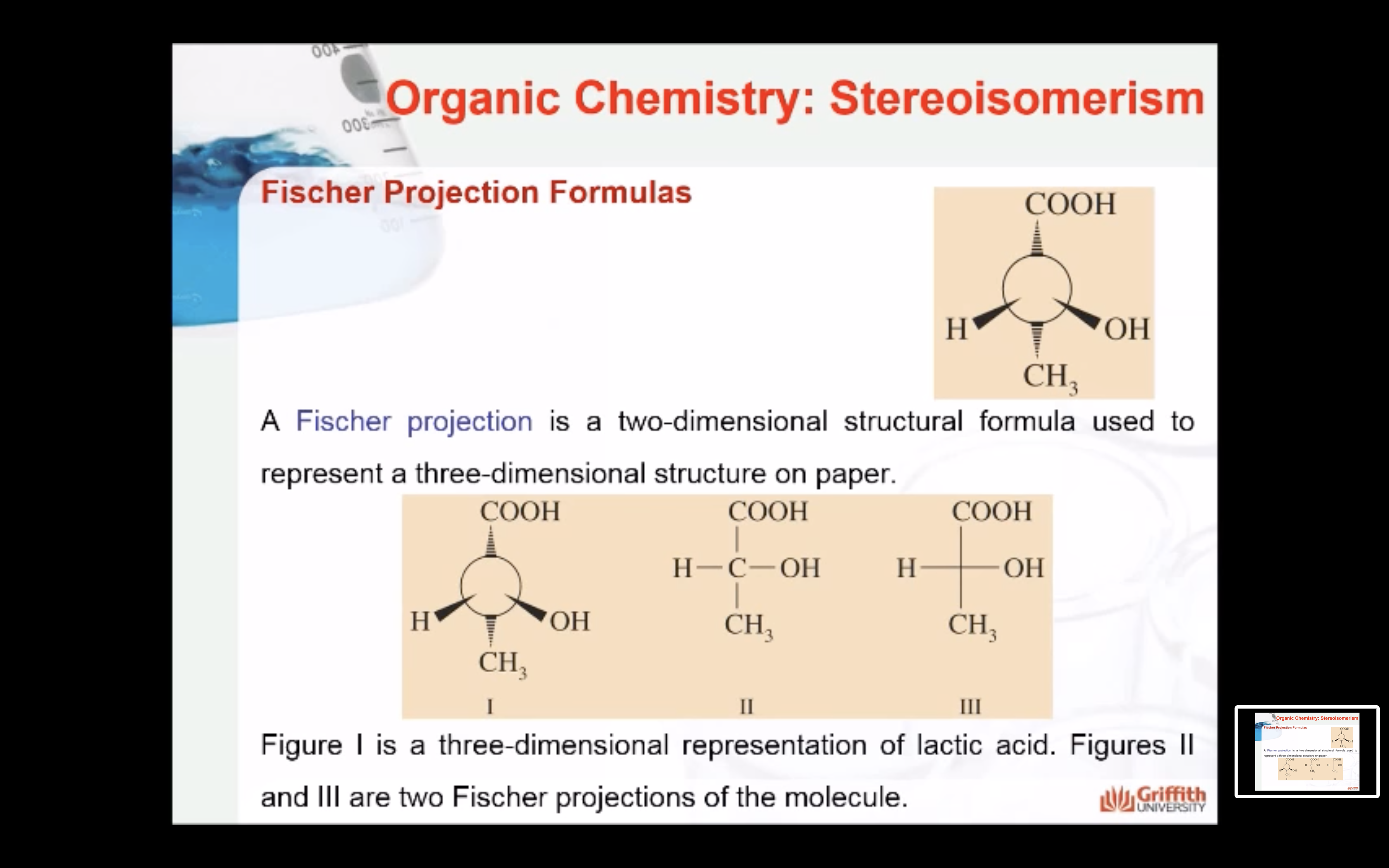
-Fischer projection
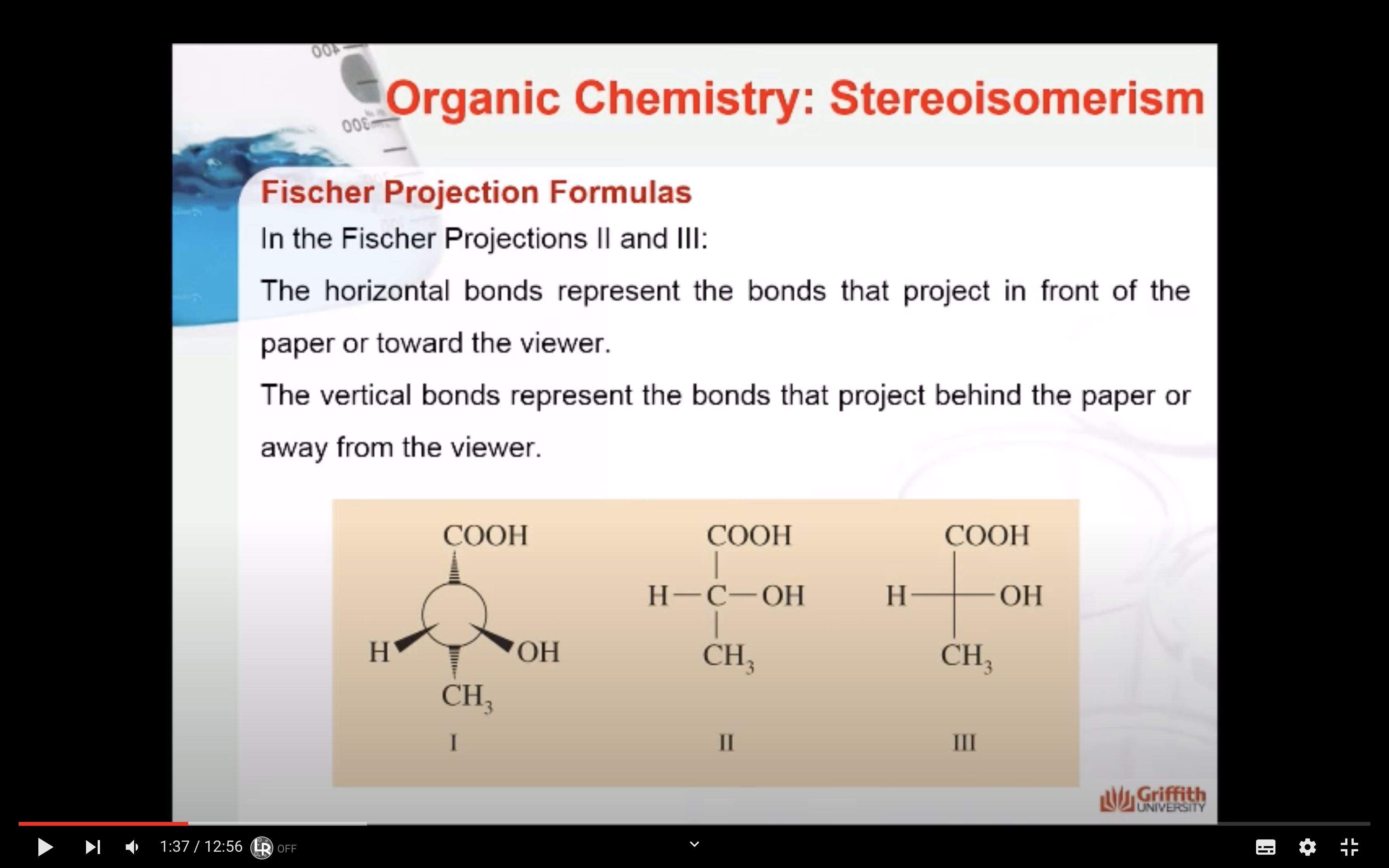
: horizontal bonds represent the bonds that project in front of the paper or towards the viewer
: vertical bonds represent the bonds that project behind the paper or away from the viewer
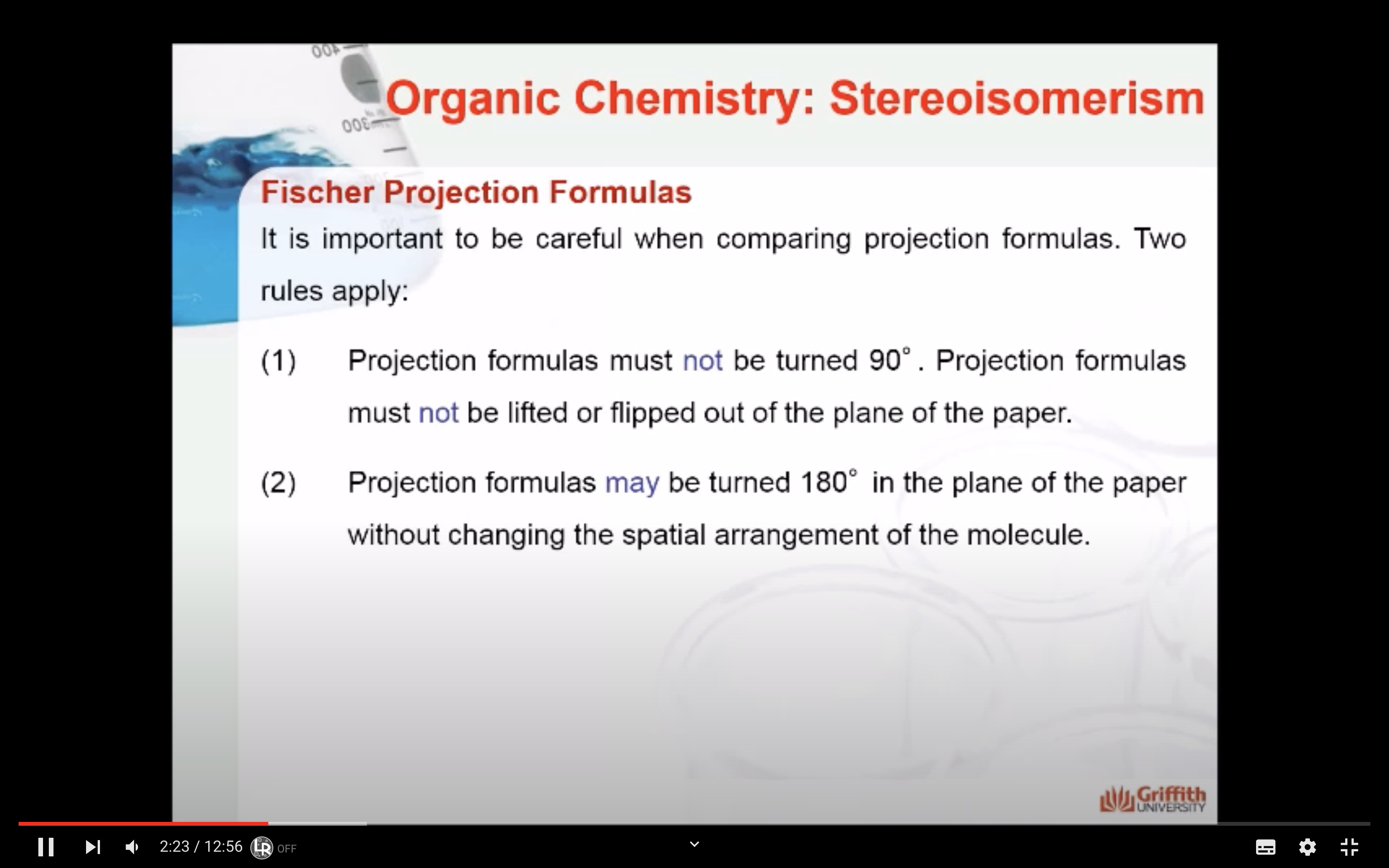
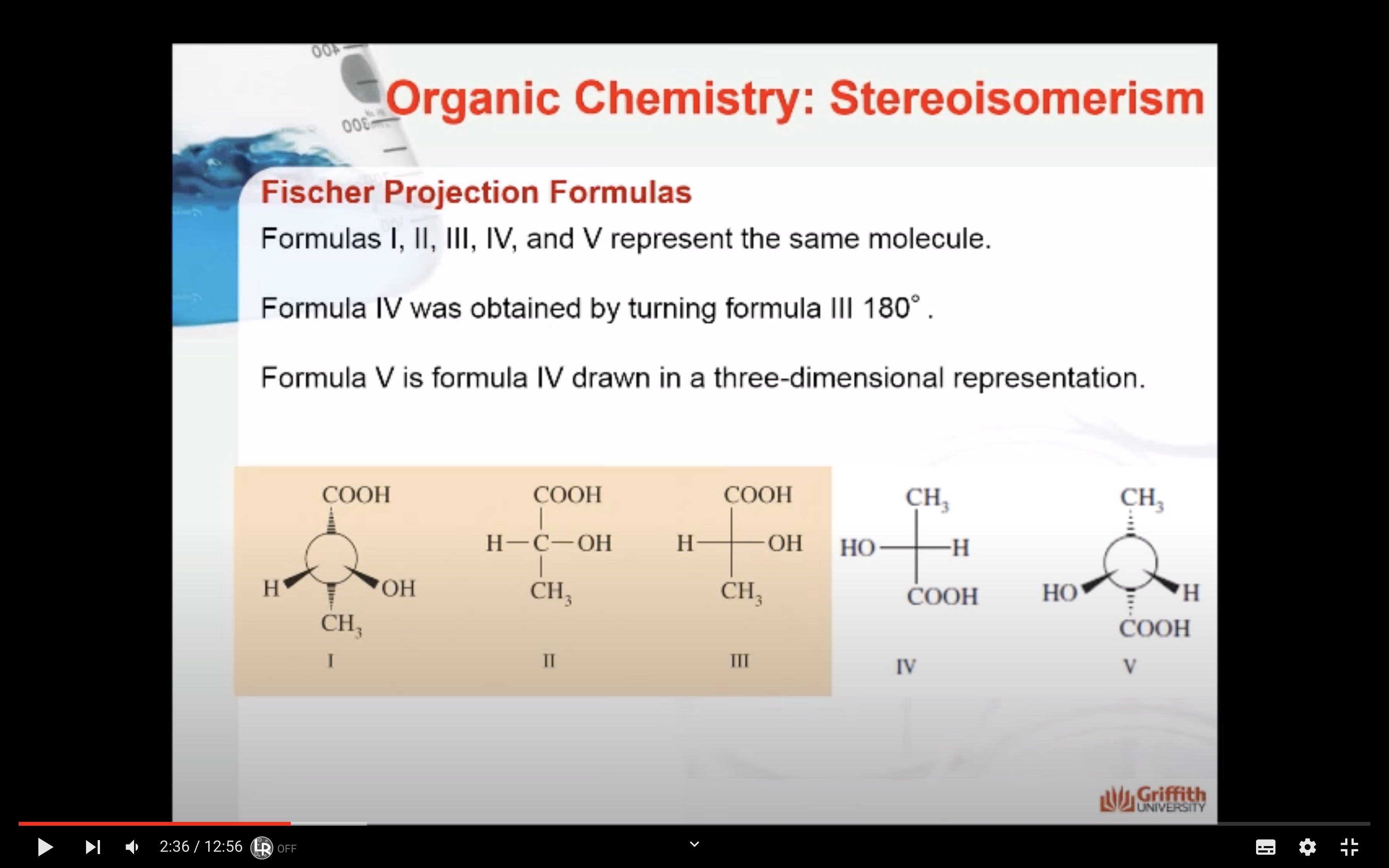
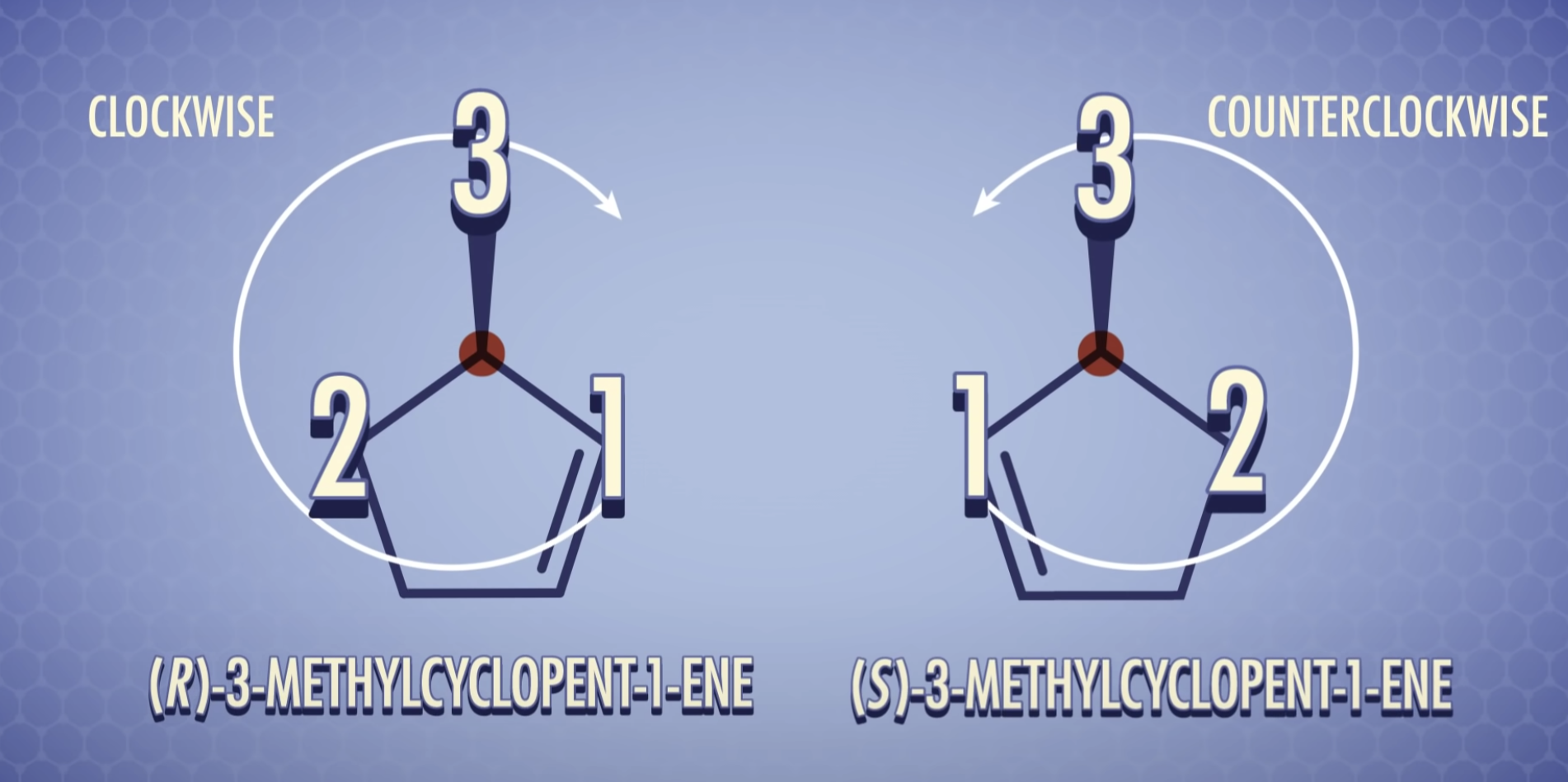
*Projection formulas must not be turned 90' (똑같은 분자를 표현하고자 할때는 딱 180'만 회전시켜야함)
*Projection formulas must not be lifted or flipped out of the plane of the paper
*Projection formulas may be turned 180' in the plane of the paper without changing the spatial arrangement of the molecule
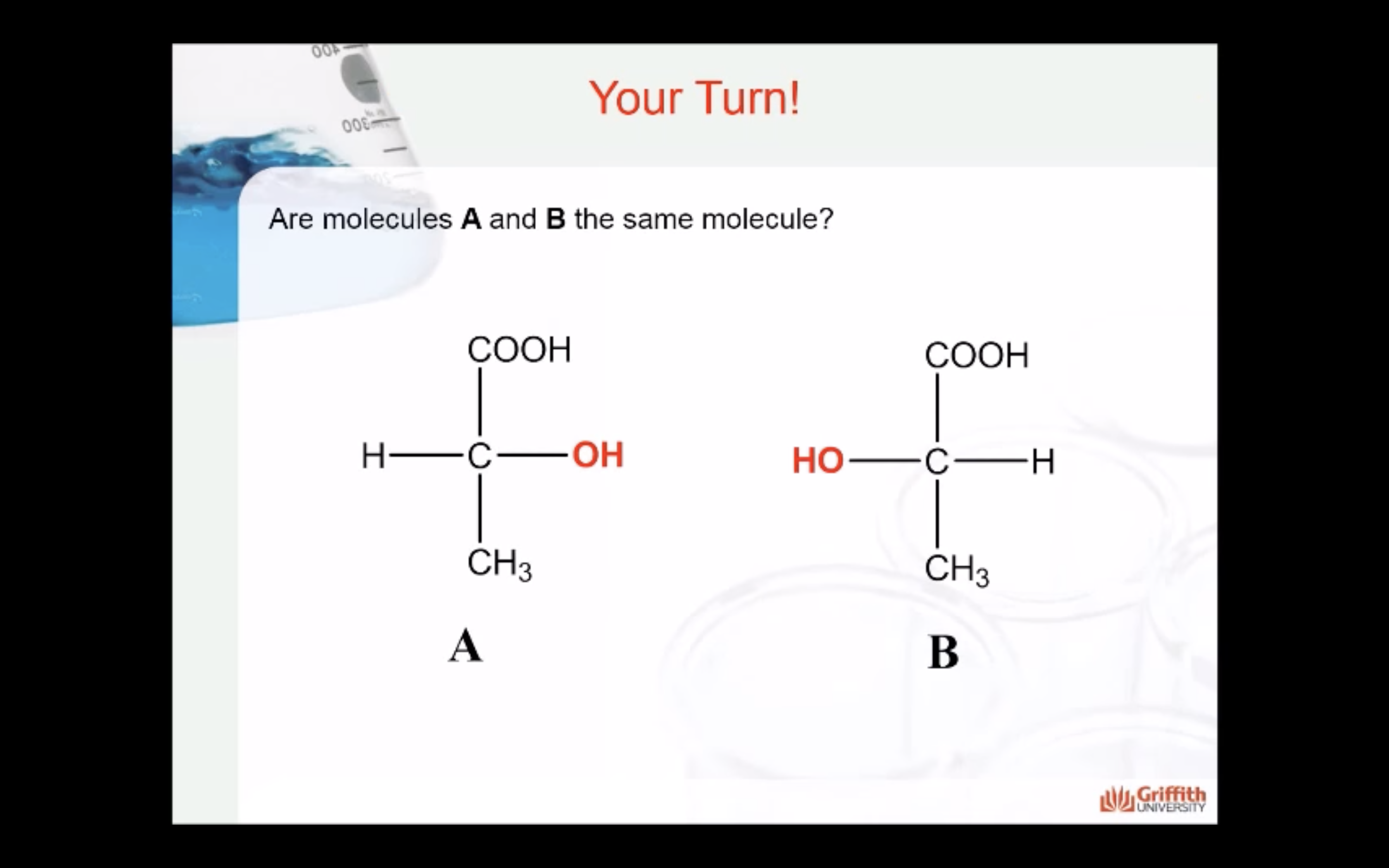
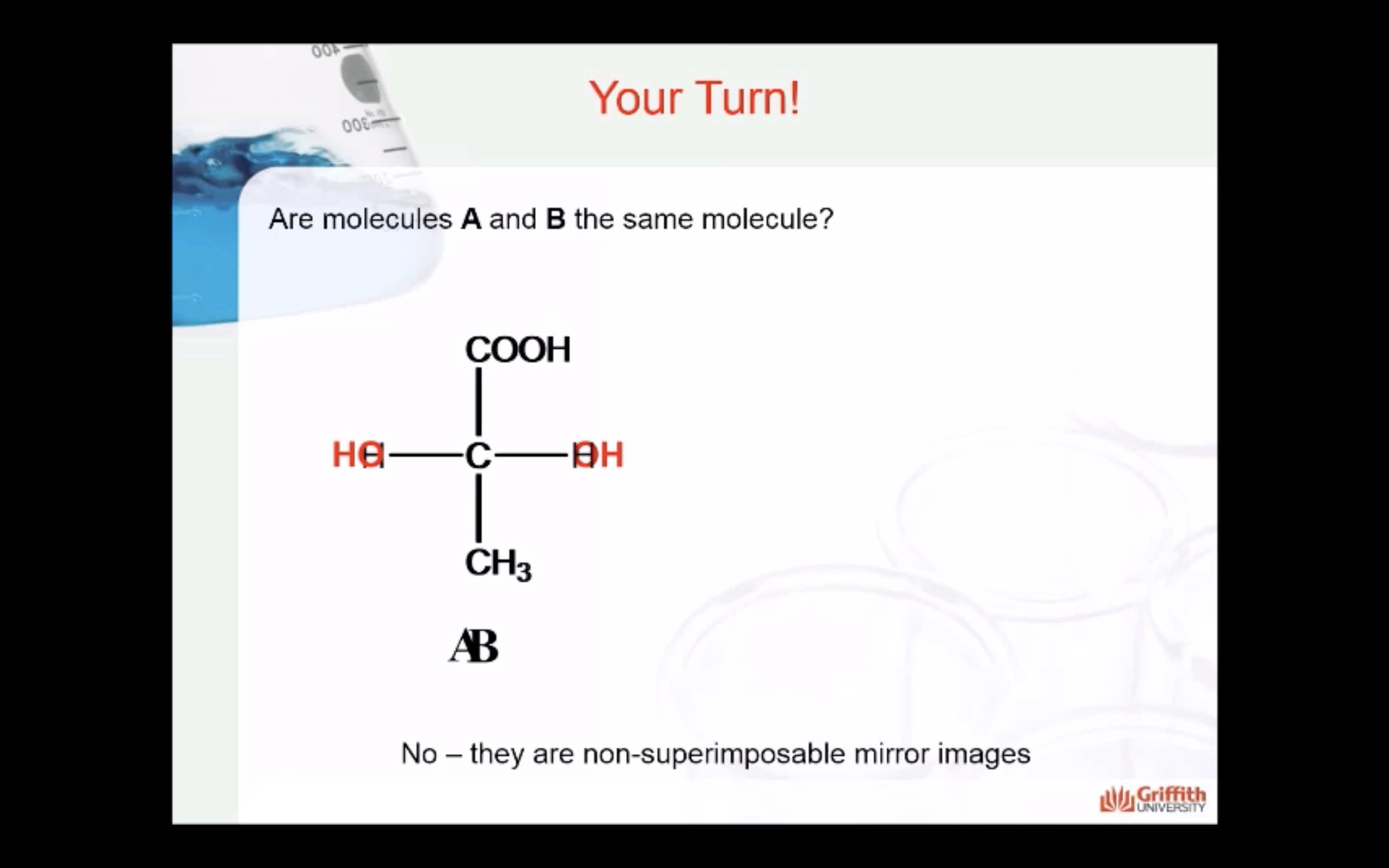

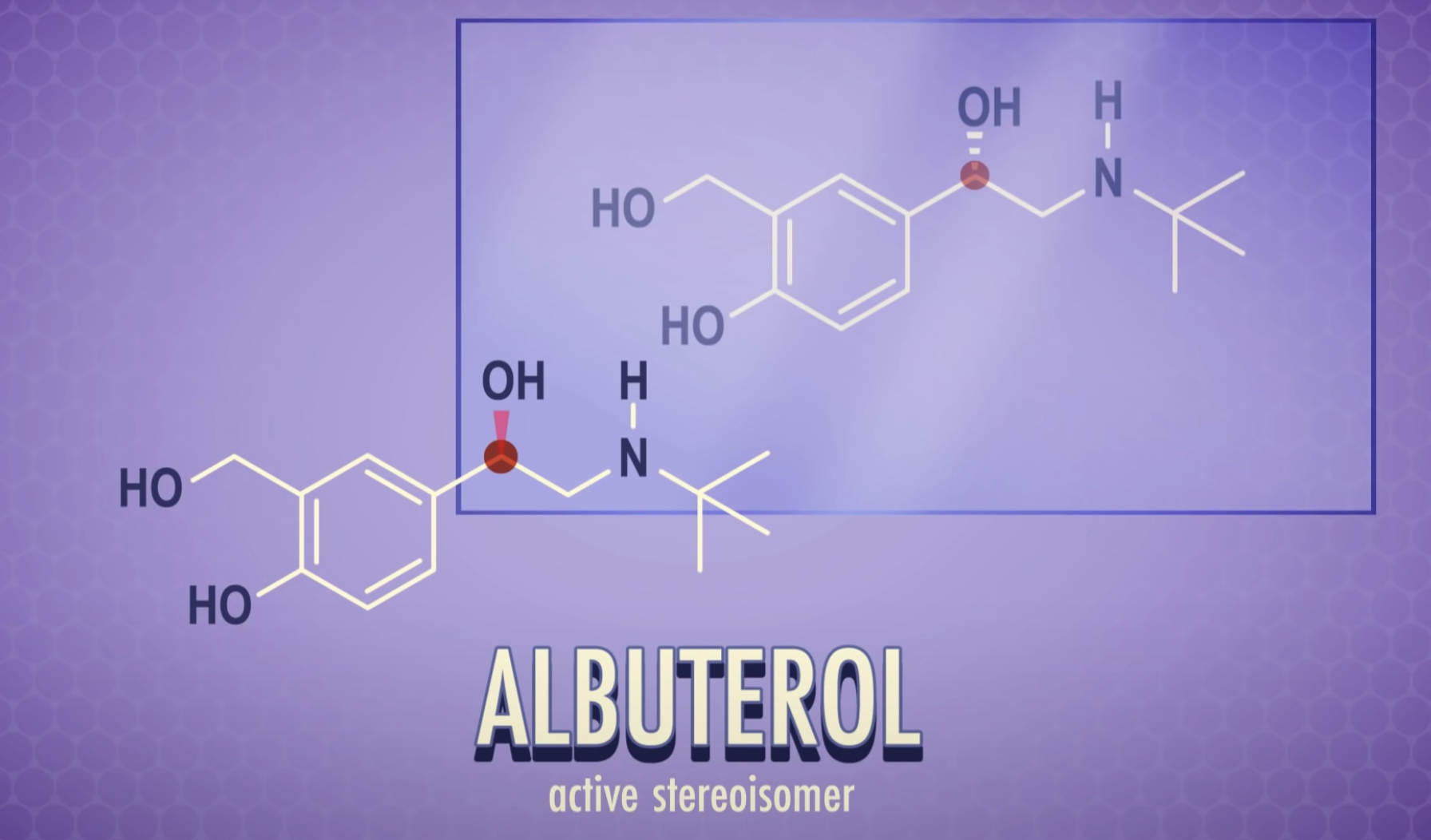
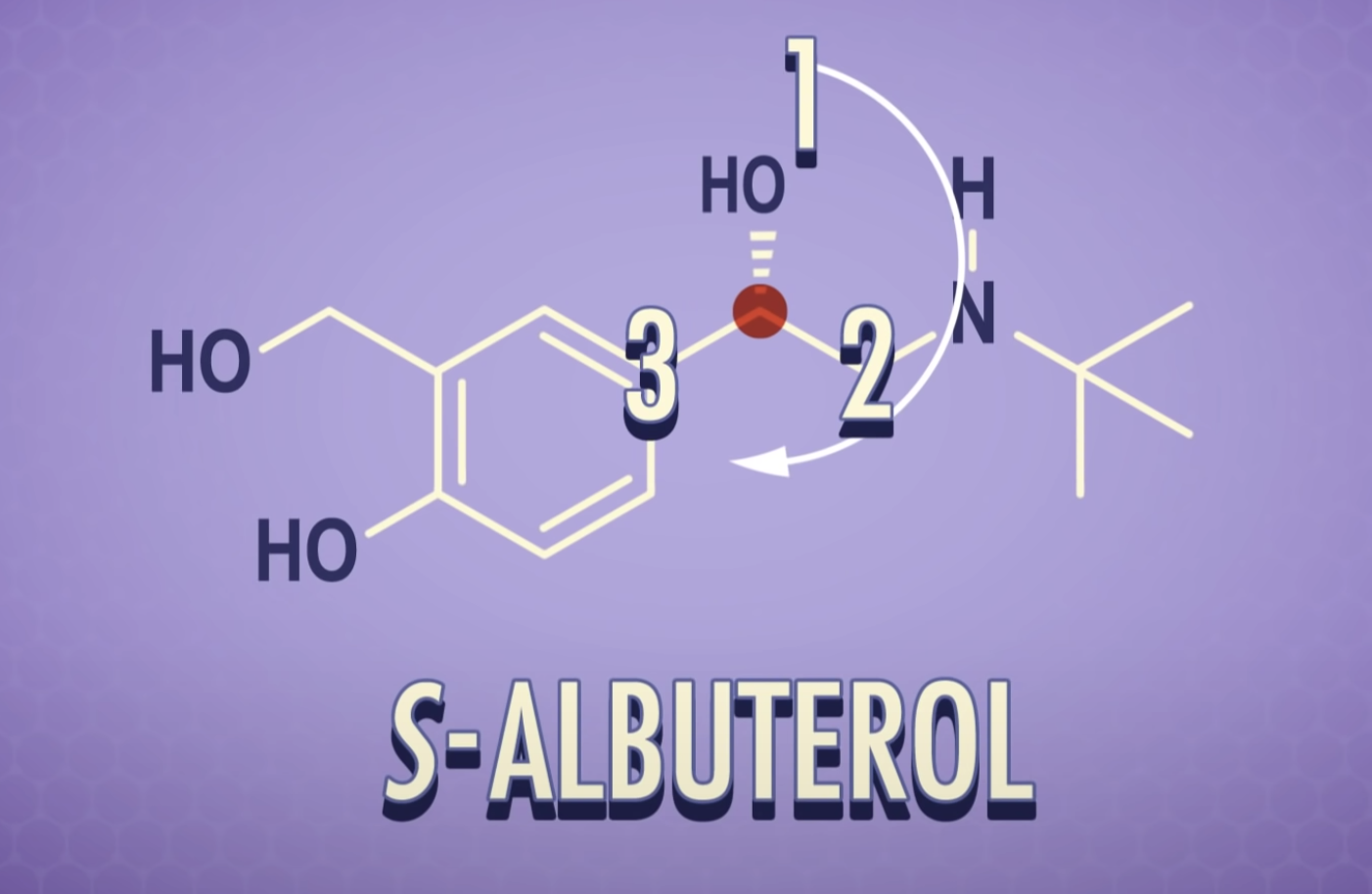
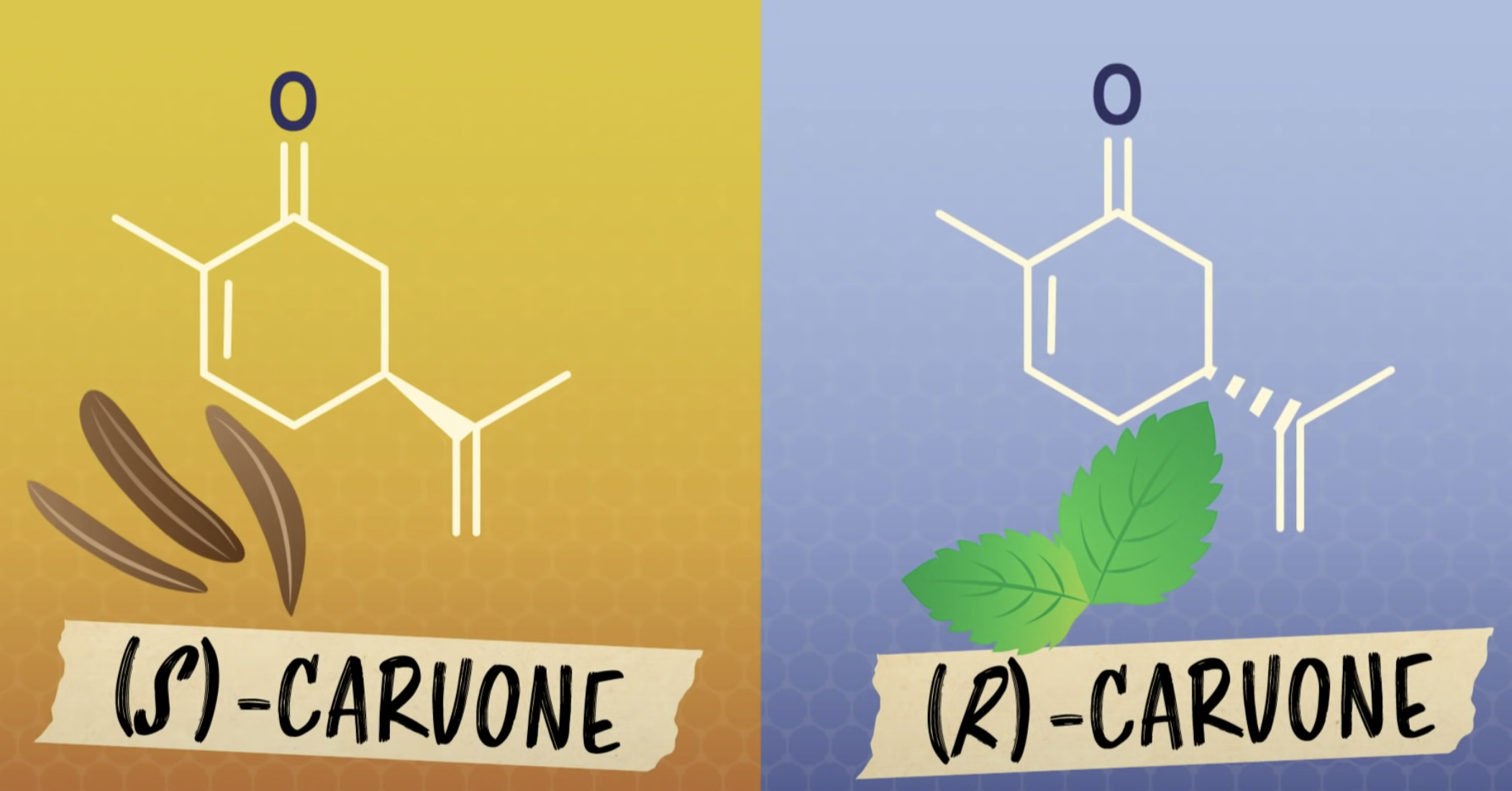
*Enantiomers: optically active, non-superimposable mirror image molecules that have the property of chirality
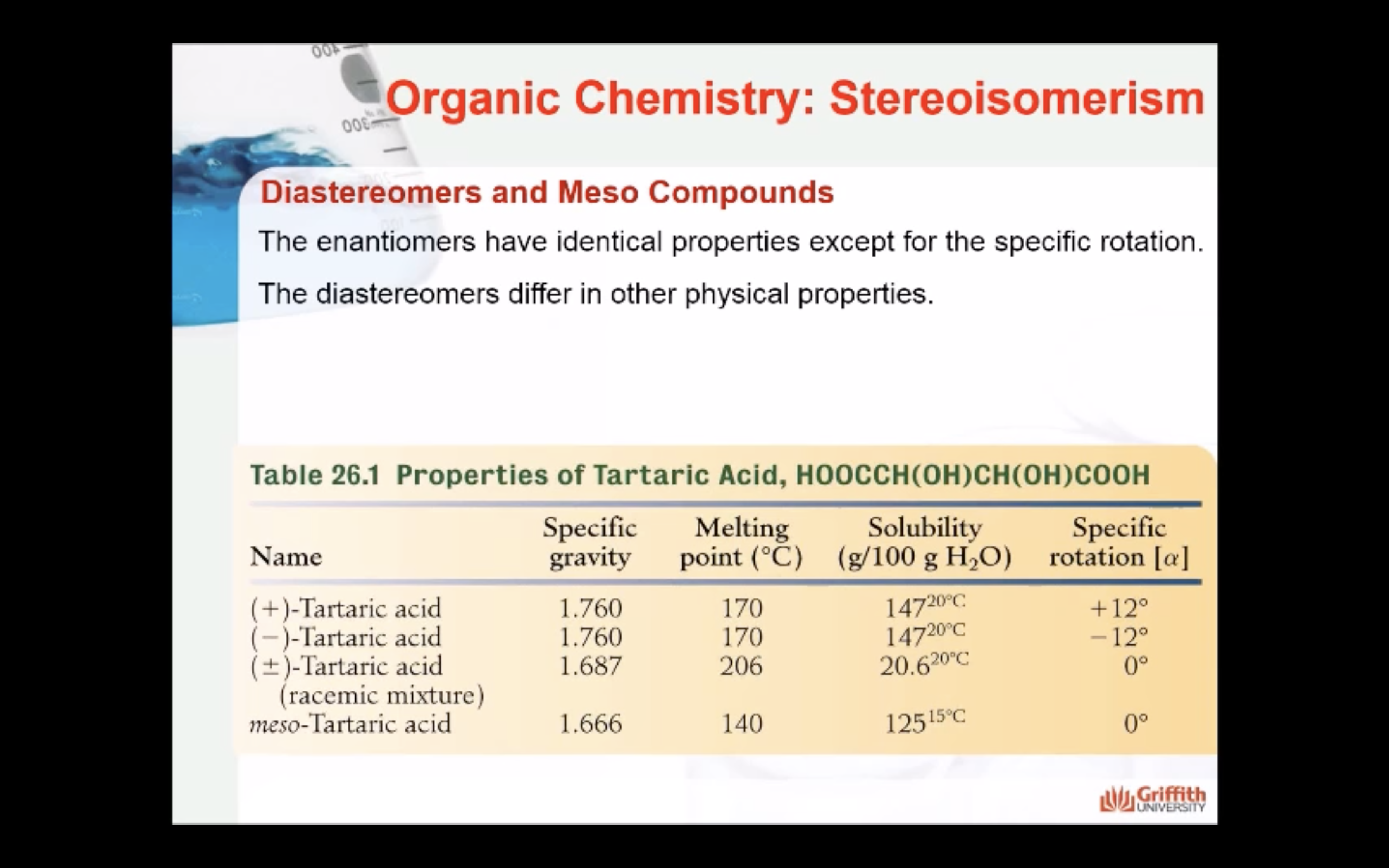
Same chemical properties
Same physical properties
Differ in biochemical properties
*Racemic mixture : a mixture containing equal amounts of a pair of enantiomers is known as a racemic mixture
(They are optically inactive as their each rotation cancels out opposite direction)
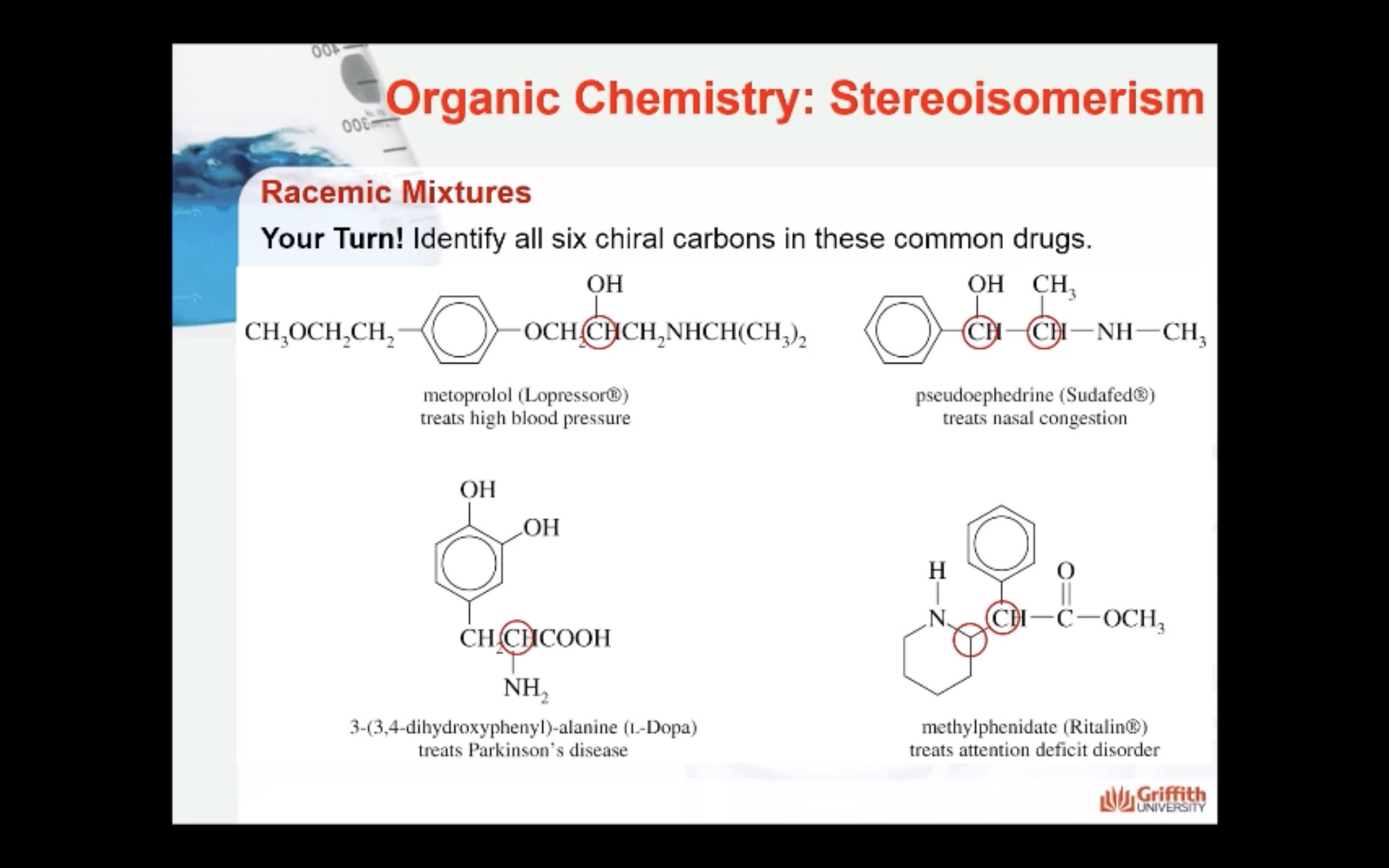
-Enantiomers : a compound containing chiral carbon
-Enantinomers have mirror-image isomers which are in-superimposable
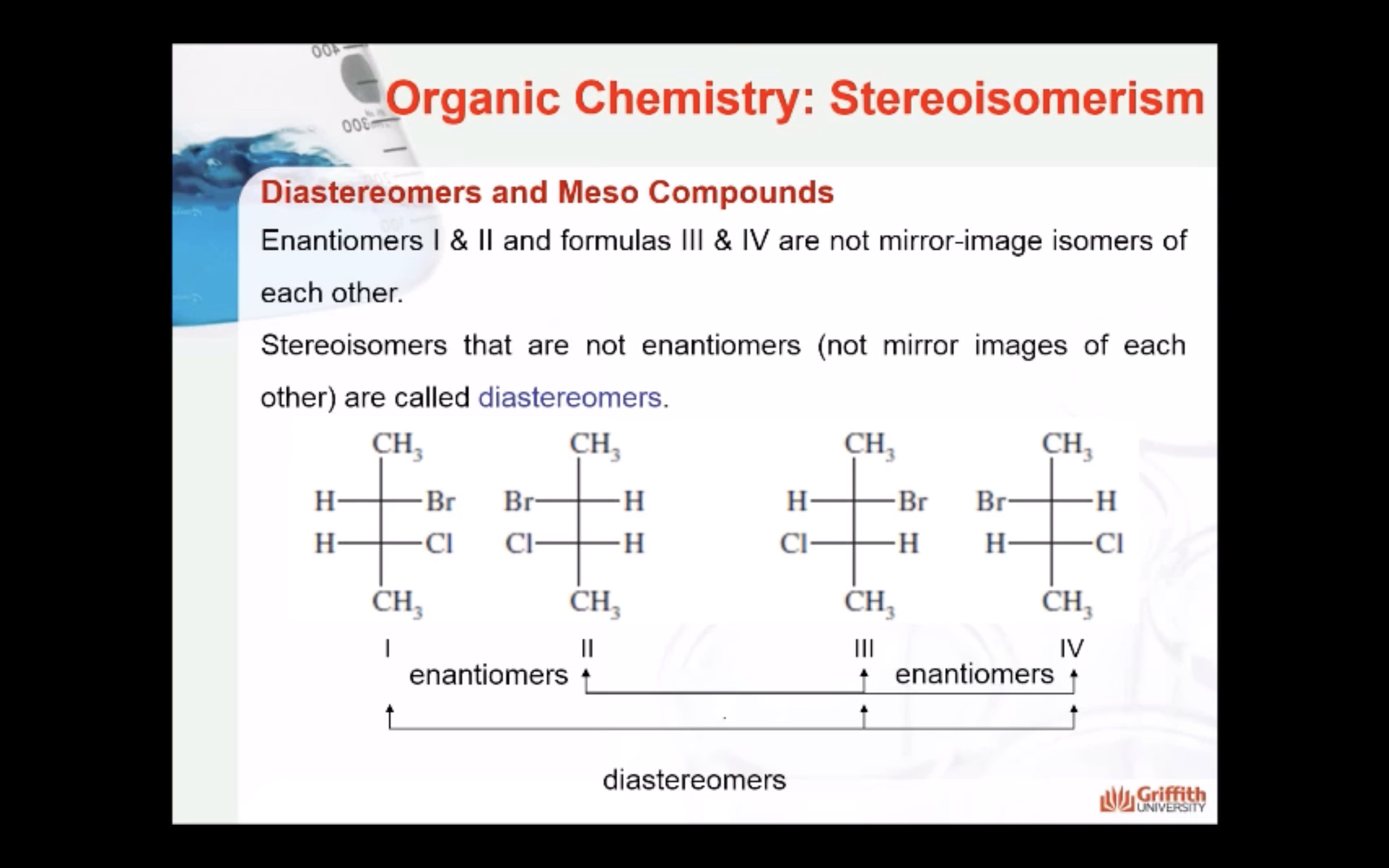
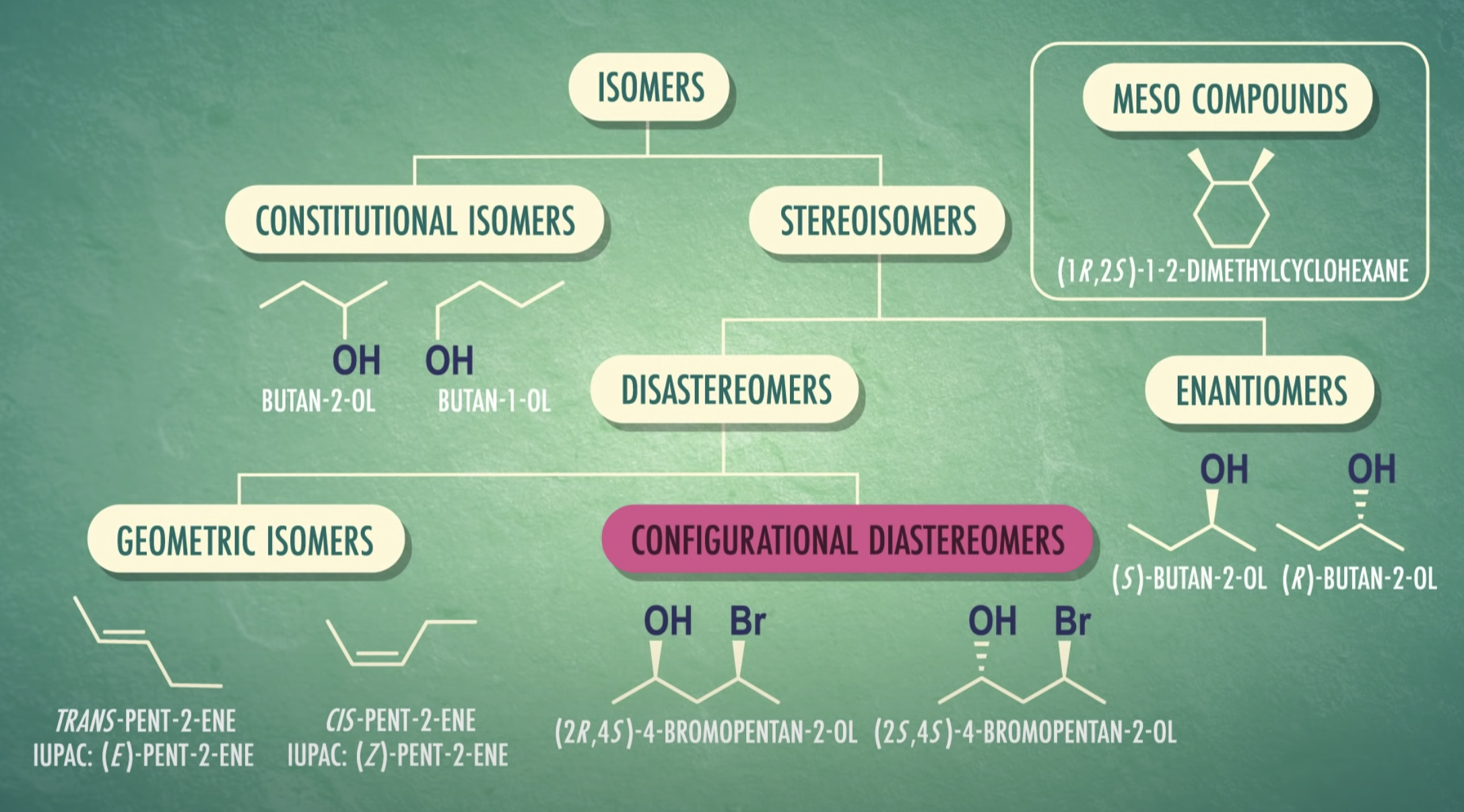
(Between enantinomers, if they are not mirror image together => they are diastereomers)
-Some of enantinomers have meso compounds which have chiral carbon atom but super-imposable on their own
=> meso structure/ meso compound (they are optically inactive)
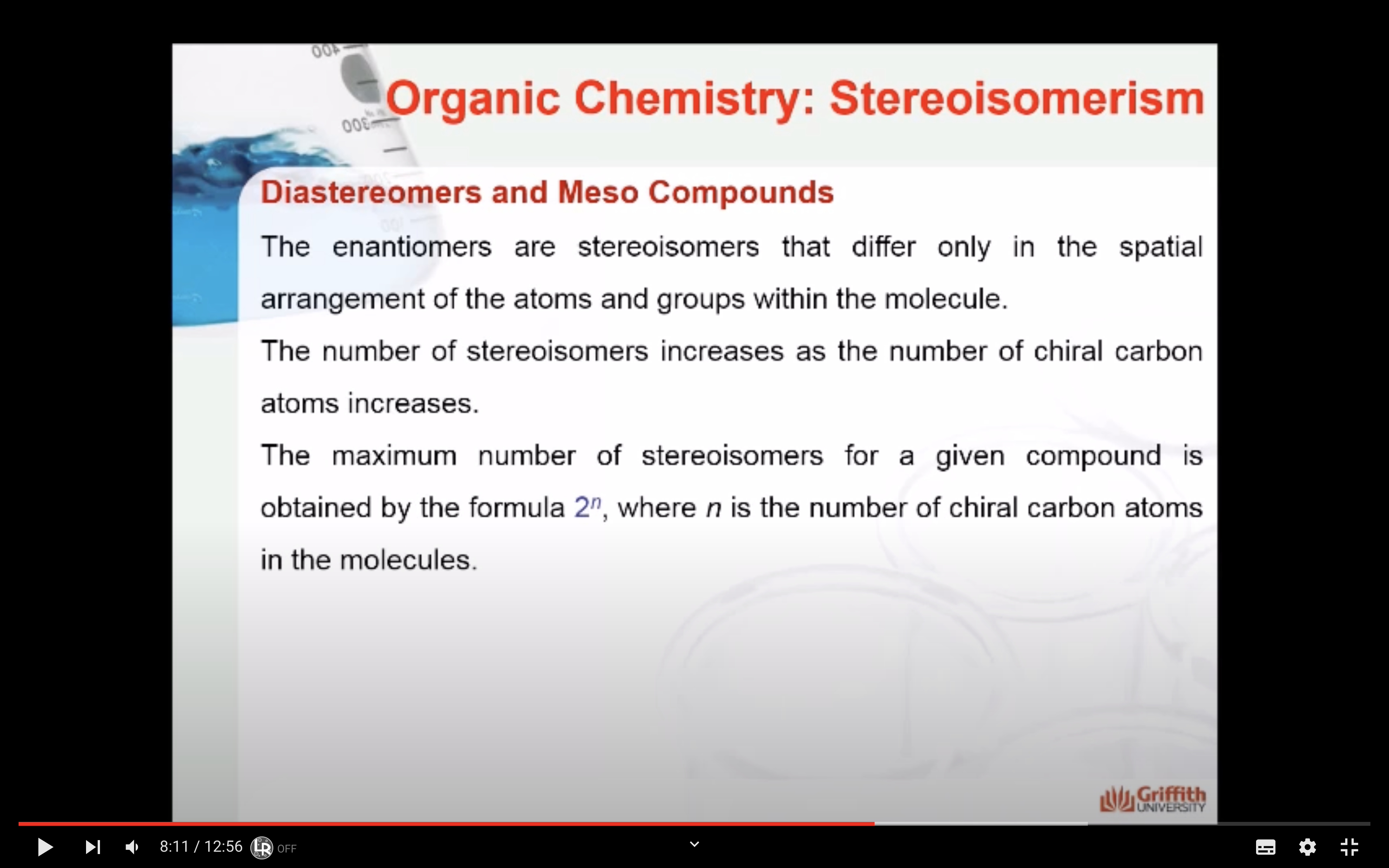
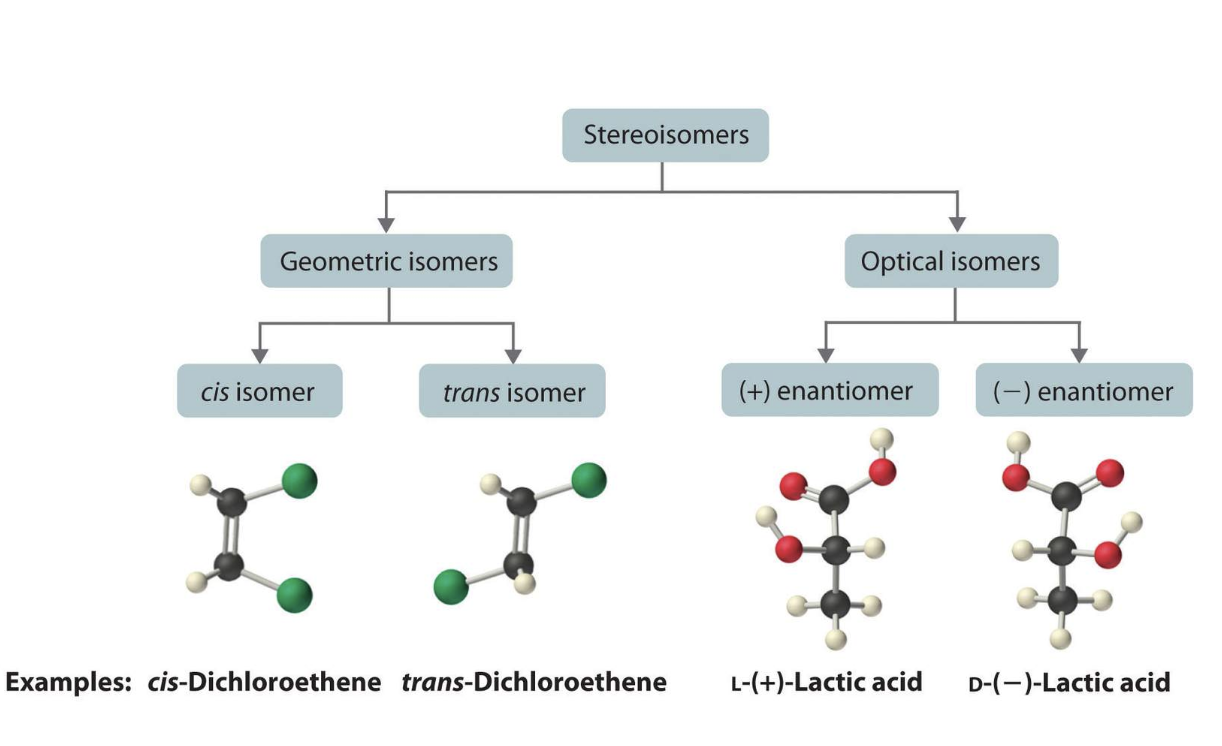
In stereochemistry, stereoisomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space.
Review of Isomerism
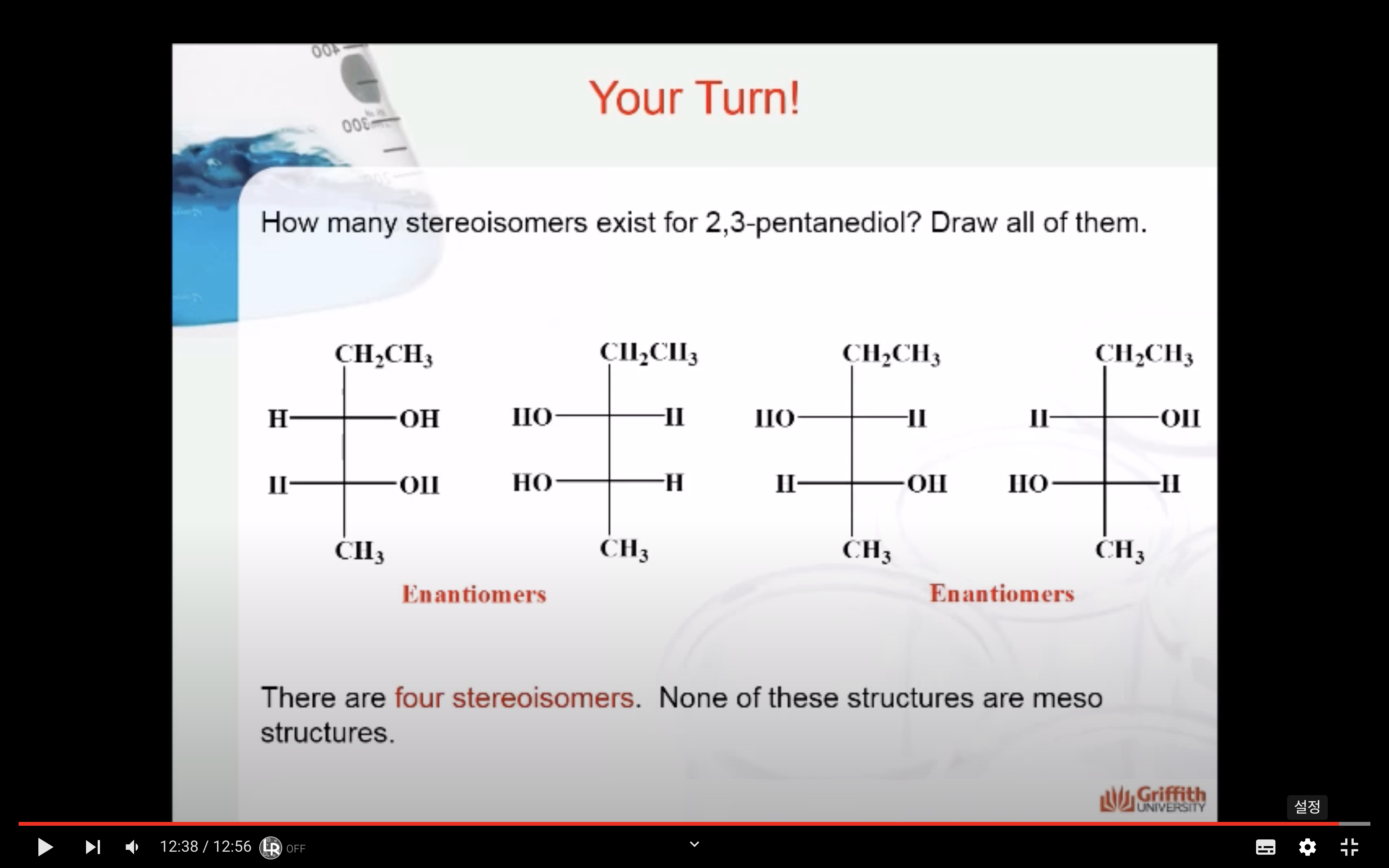
Isomers are molecules that have the same chemical formula but differ in either:
(a) how the atoms are connected or, -> structural isomer
(b) how the connected atoms are arranged in space. -> steroisomer
Two types of structural isomers
- Cis–trans or geometric isomers, which we have already considered.
- Optical isomers are the subject of this module.
One feature of optical isomers is that they can rotate the plane of plane-polarized light.
<Plane-polarised light>
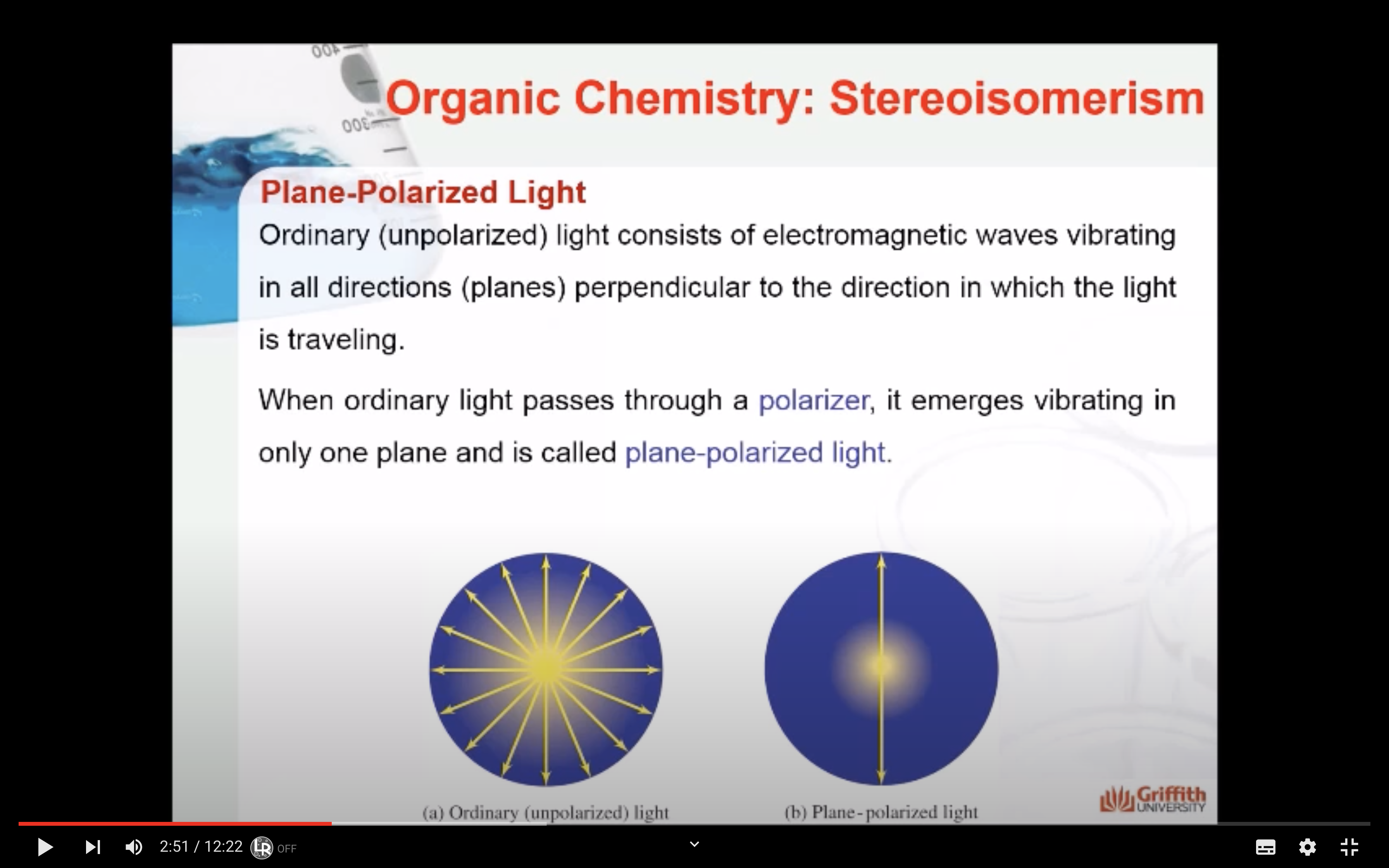
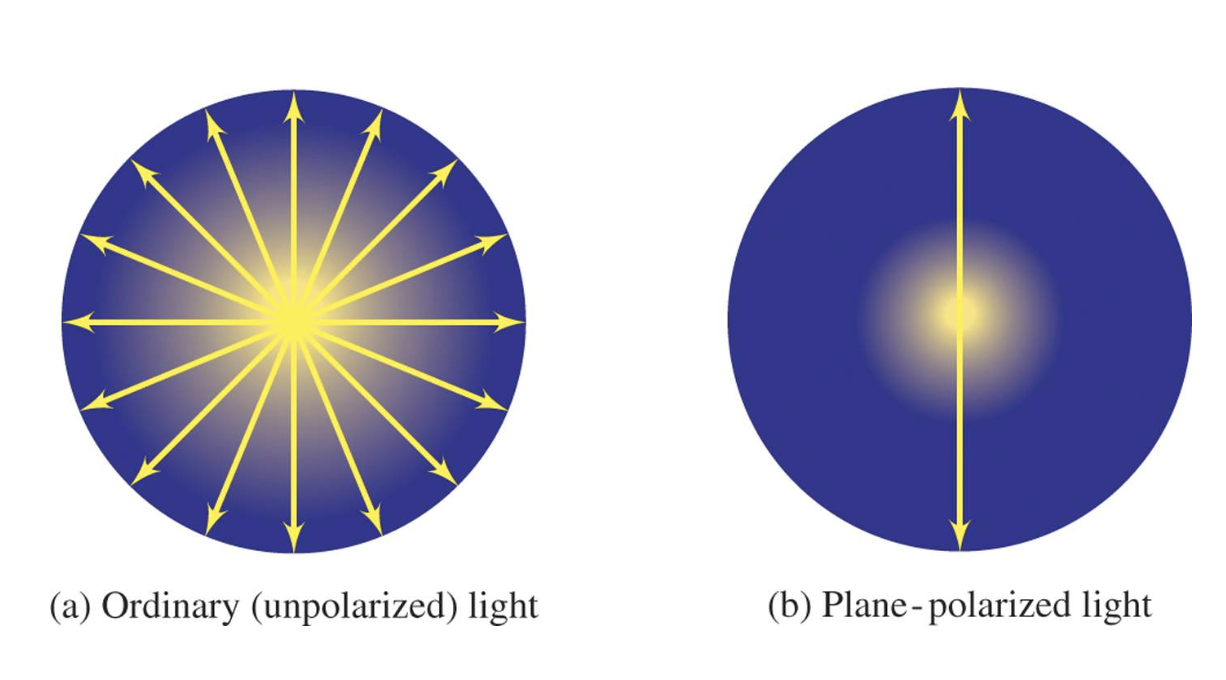
Ordinary (unpolarized) light consists of electromagnetic waves vibrating in all directions (planes) perpendicular to the direction in which the light is traveling.
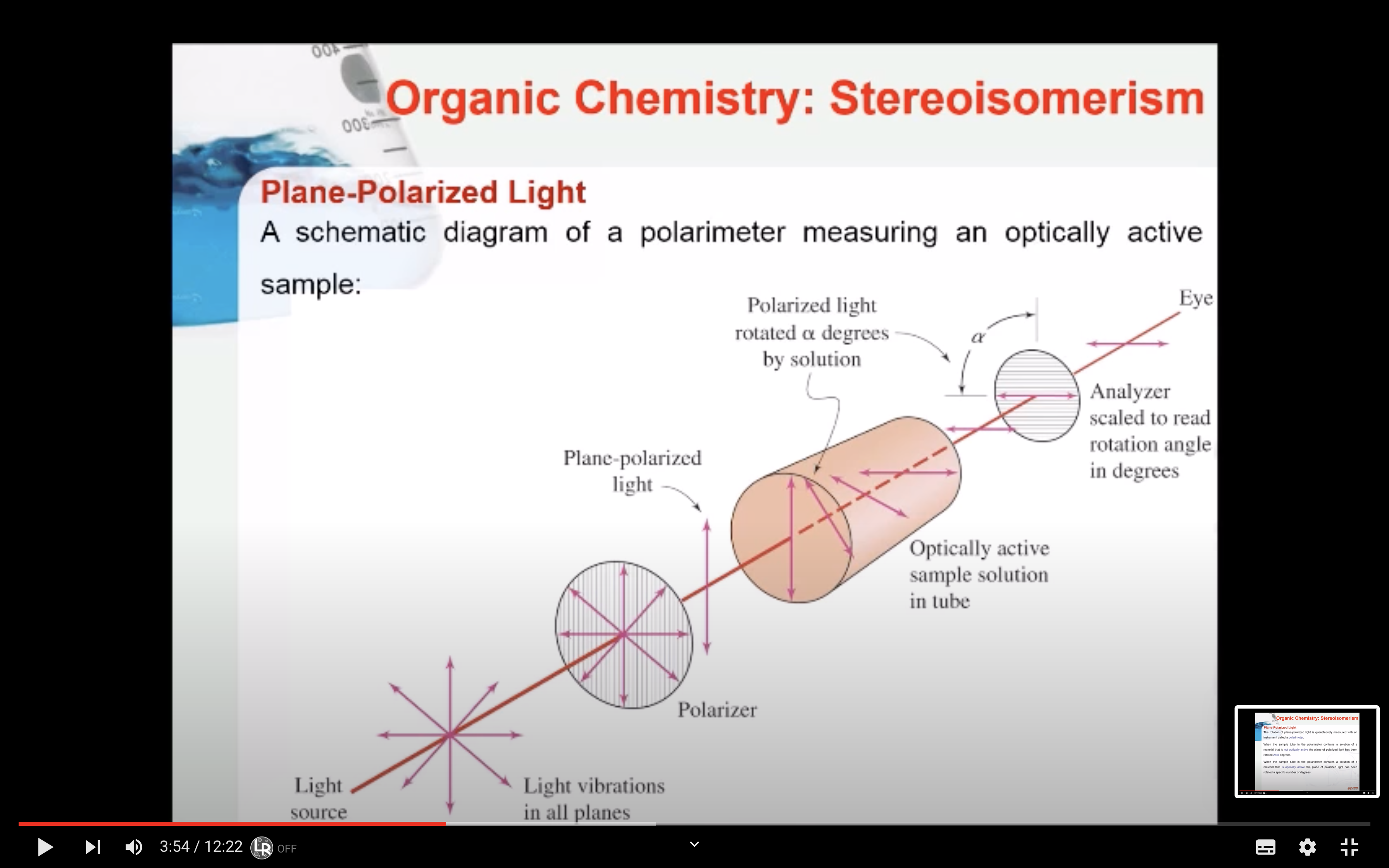
When ordinary light passes through a polarizer, it emerges vibrating in only one plane and is called plane-polarized light.

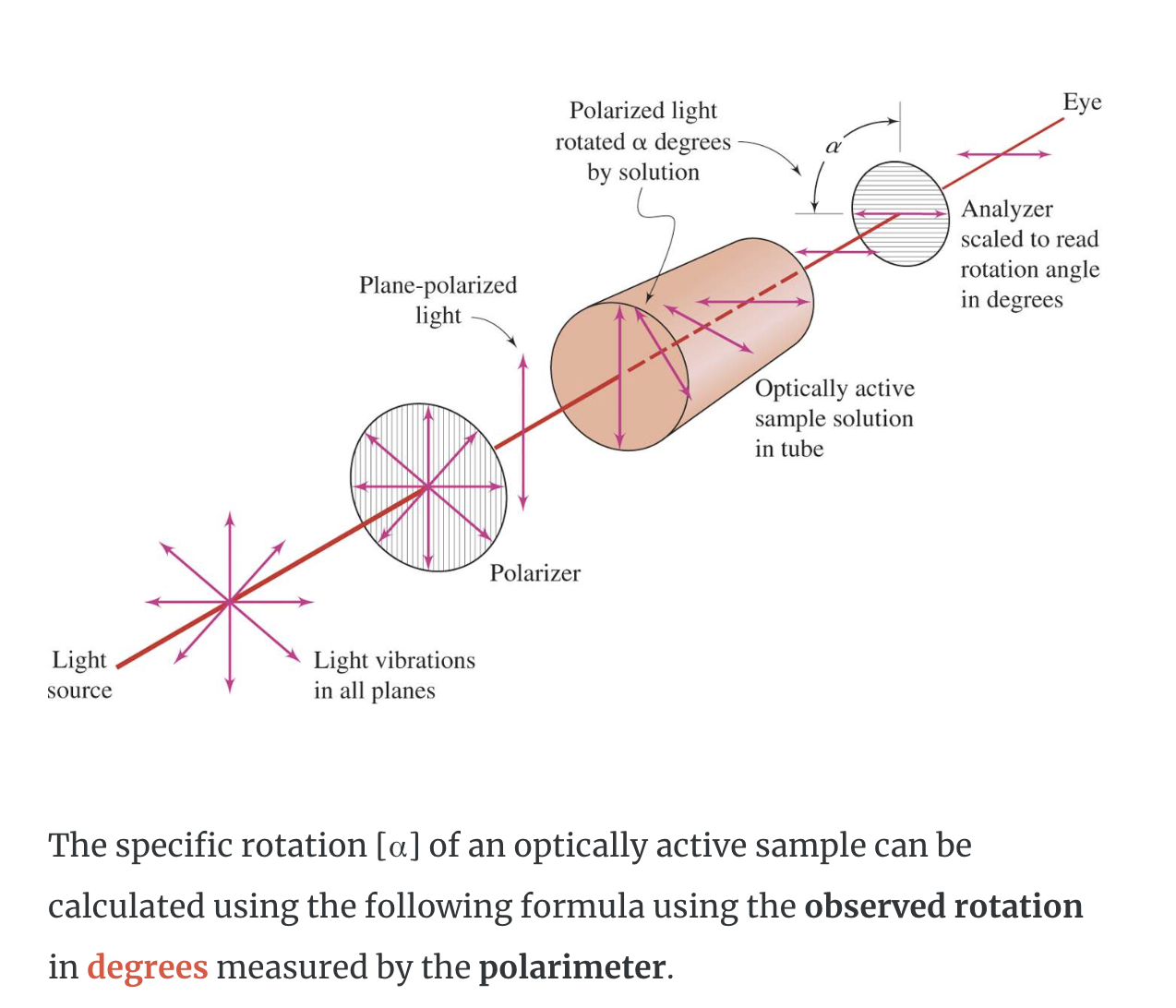
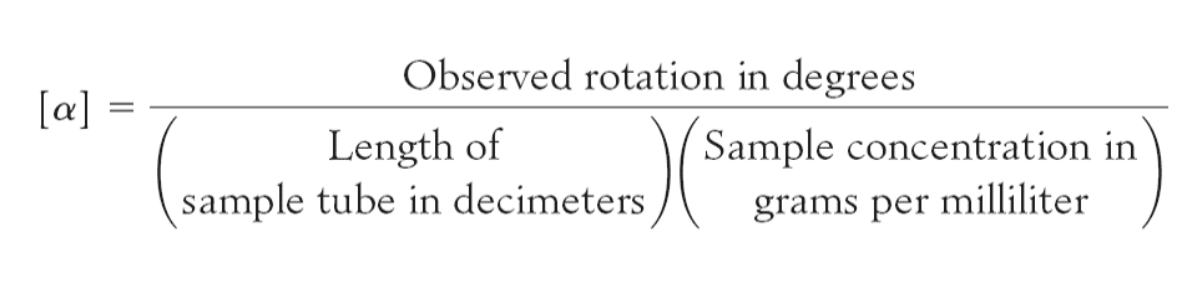
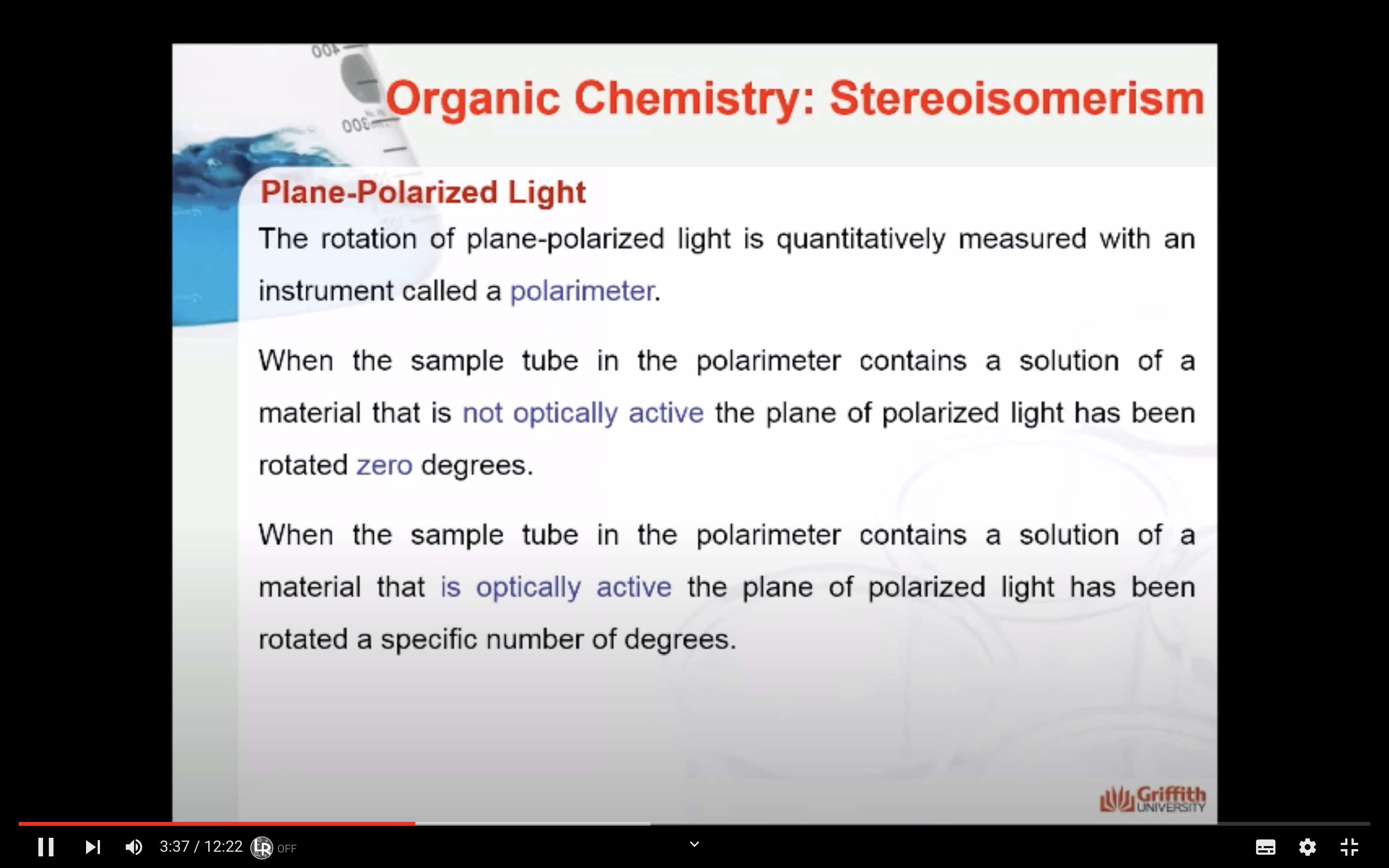
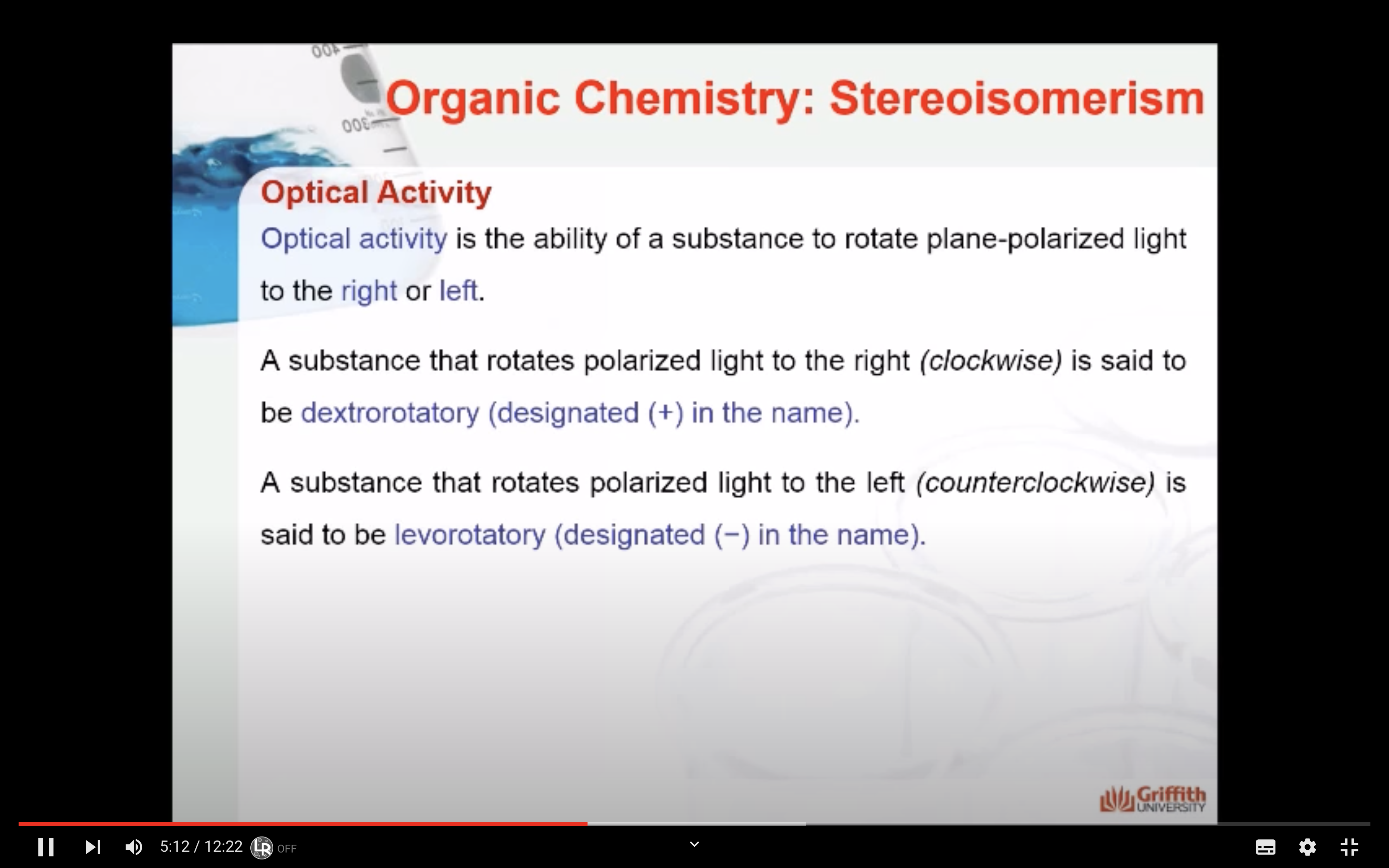
Optical activity is the ability of a substance to rotate plane-polarized light to the right or left.
A substance that rotates polarized light to the right (clockwise) is said to be dextrorotatory (designated (+) in the name).
A substance that rotates polarized light to the left (counterclockwise) is said to be levorotatory (designated (−) in the name).
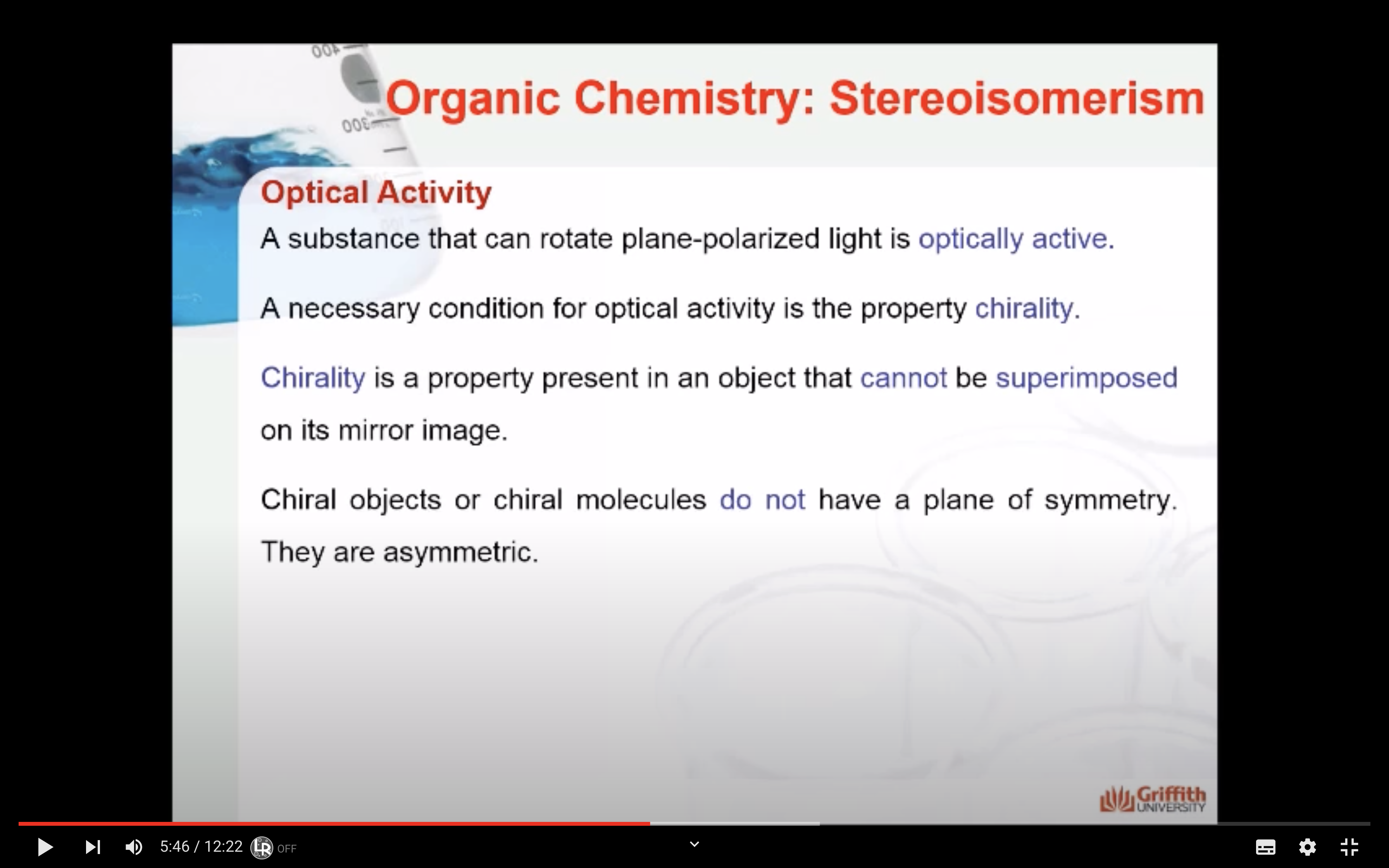
<Chirality>
-Chiral substance is optically active = able to rotate plane polarized light
-Chiral substance cannot be superimposed on it mirror image
-Chiral do not have a plane of symmetry (they are asymmetric)
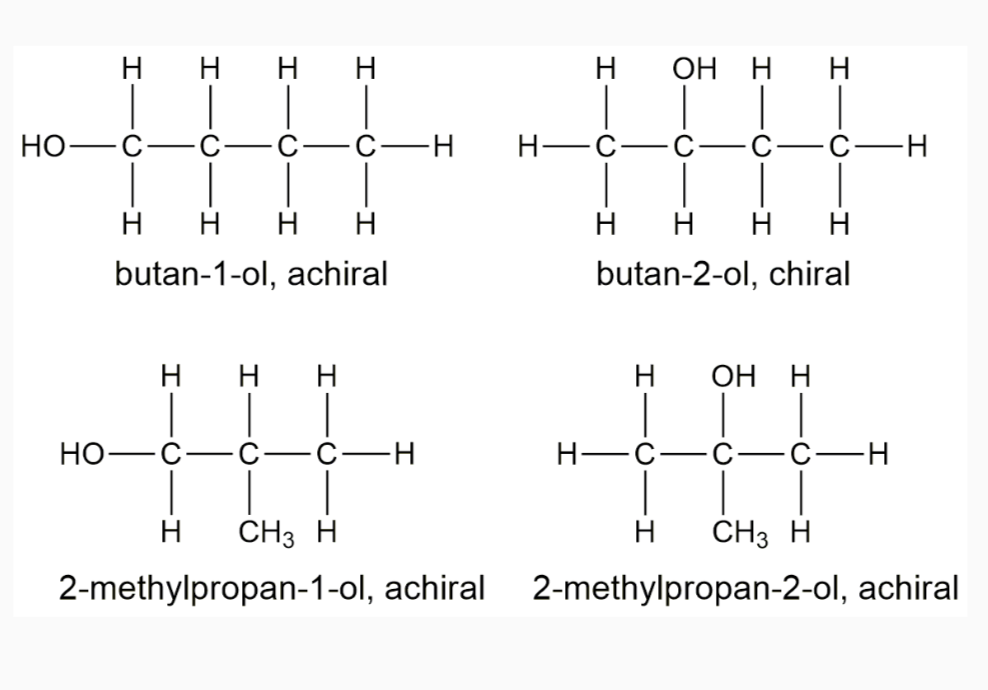
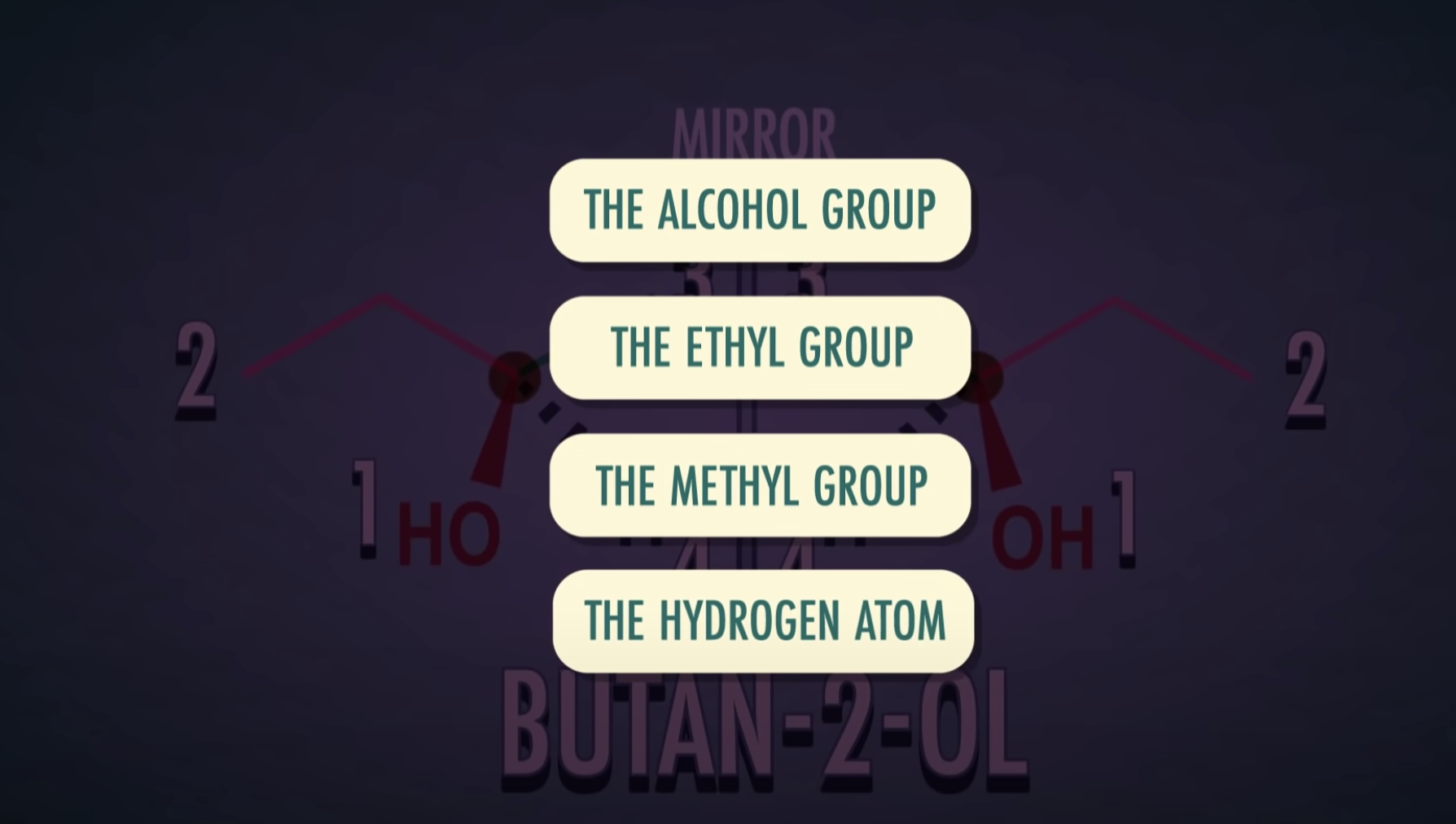
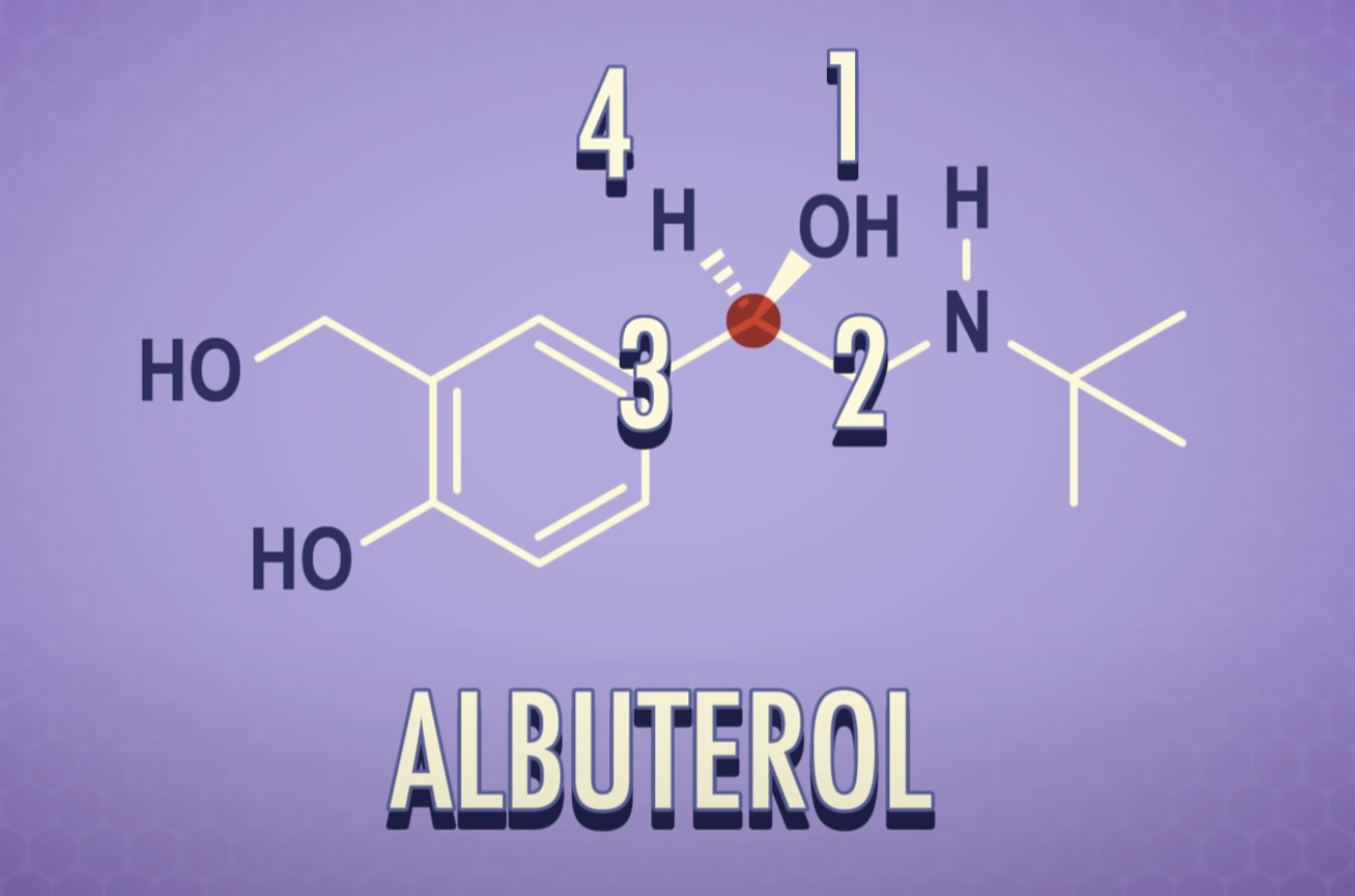
-Chiral or asymmetric carbon atoms have four different atoms or four different groups attached to it.
Your left hand and right hand are not superimposable. For example, when we lay one hand on top of the other, the thumbs are on opposite sides. Therefore your right hand and your left hand are chiral objects.
Superimposable means that, when we lay one object upon another, all parts of both objects coincide exactly
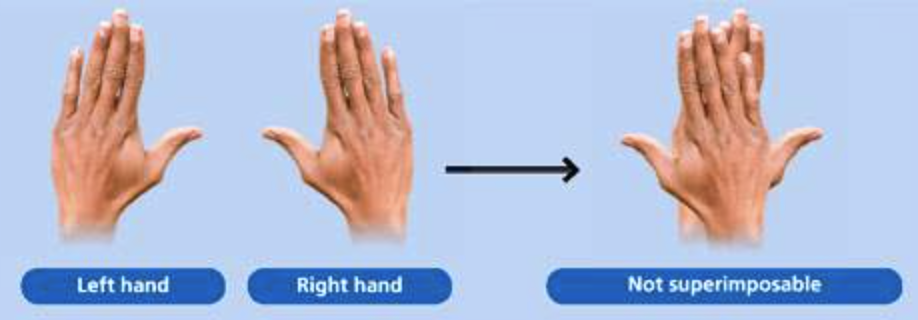
A molecule that is not superimposable on its mirror image is said to be chiral.
<Fischer projection formulas>
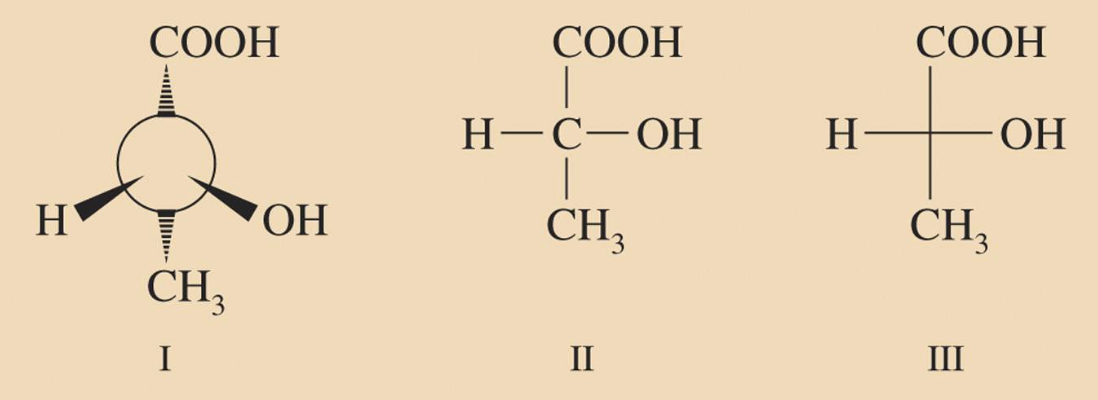

The horizontal bonds represent the bonds that project in front of the paper or toward the viewer. One way to remember this is like they are arms reaching out to hug you.
The vertical bonds represent the bonds that project behind the paper or away from the viewer.
*When we rotate 180', it becomes same molecule
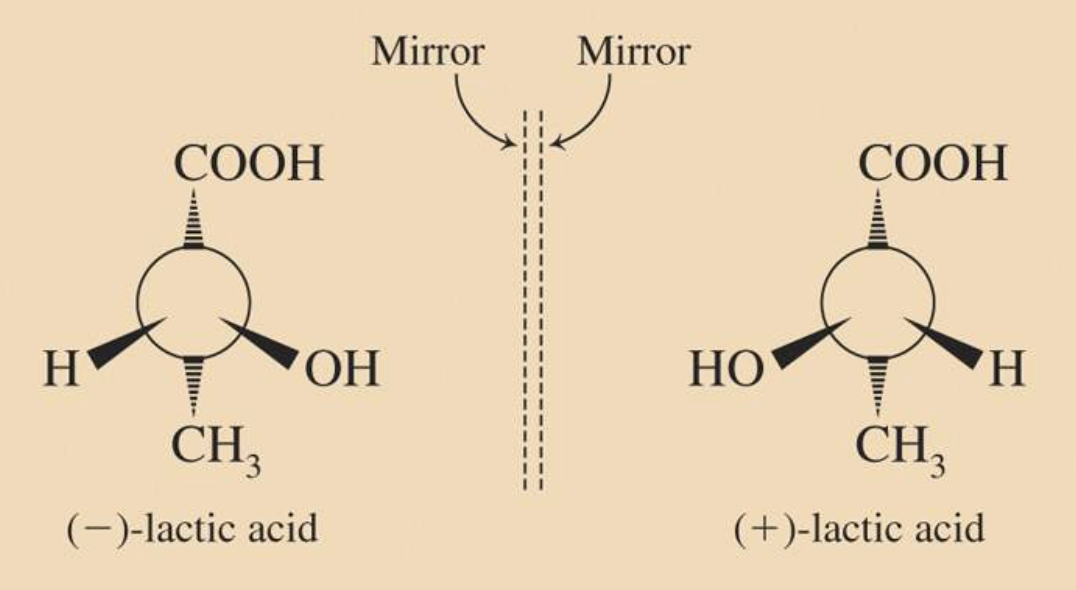
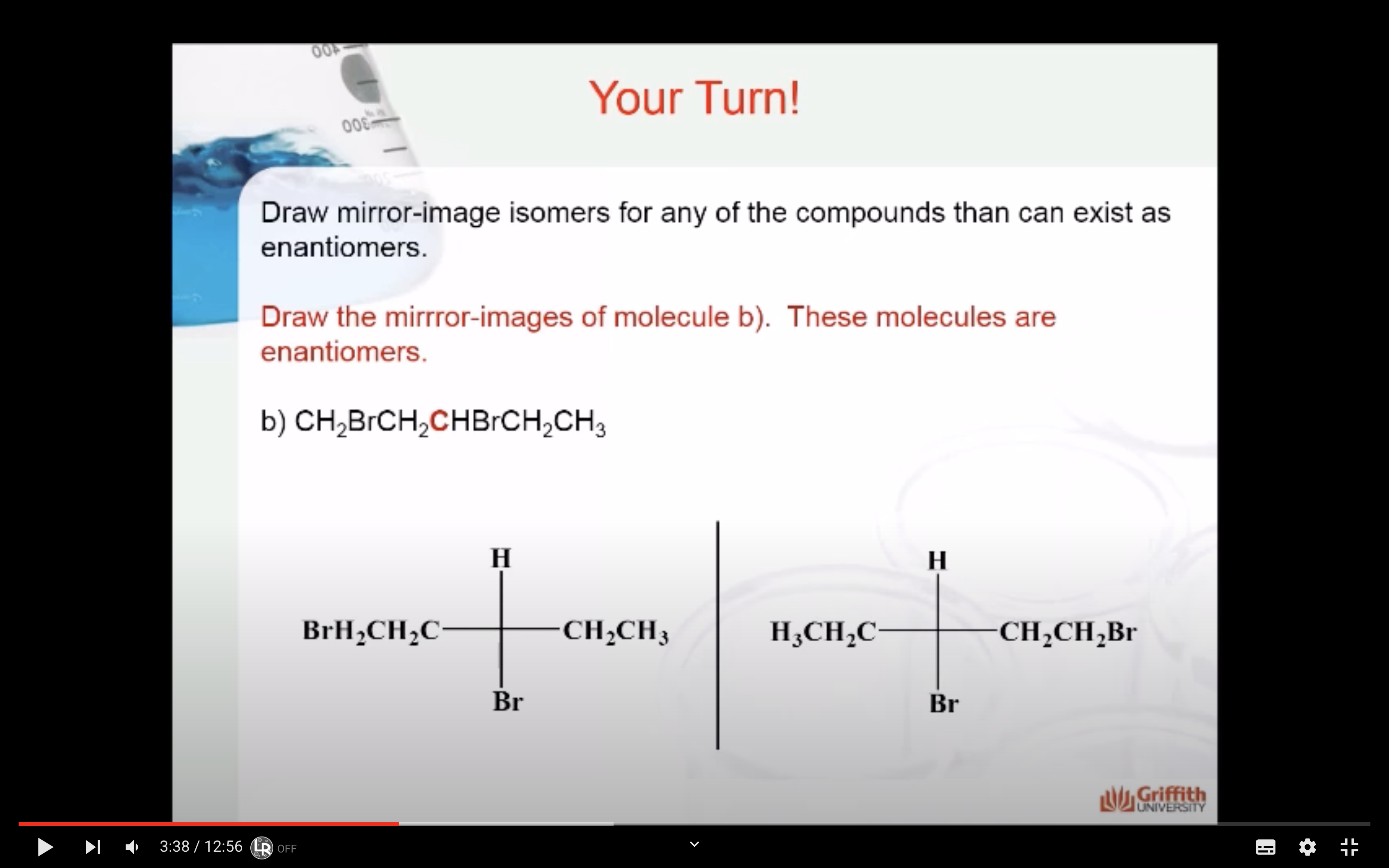
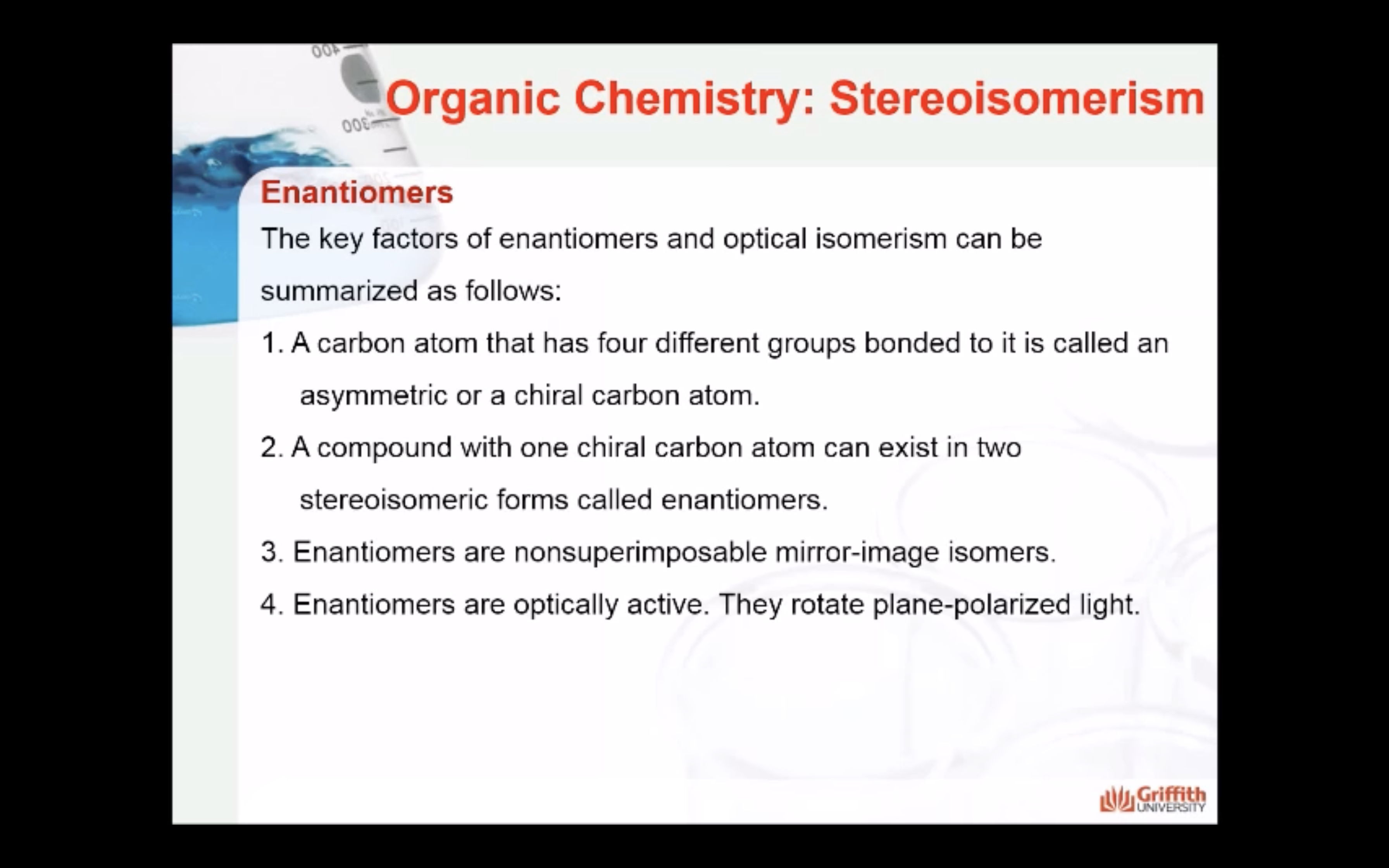
<Enantiomers>
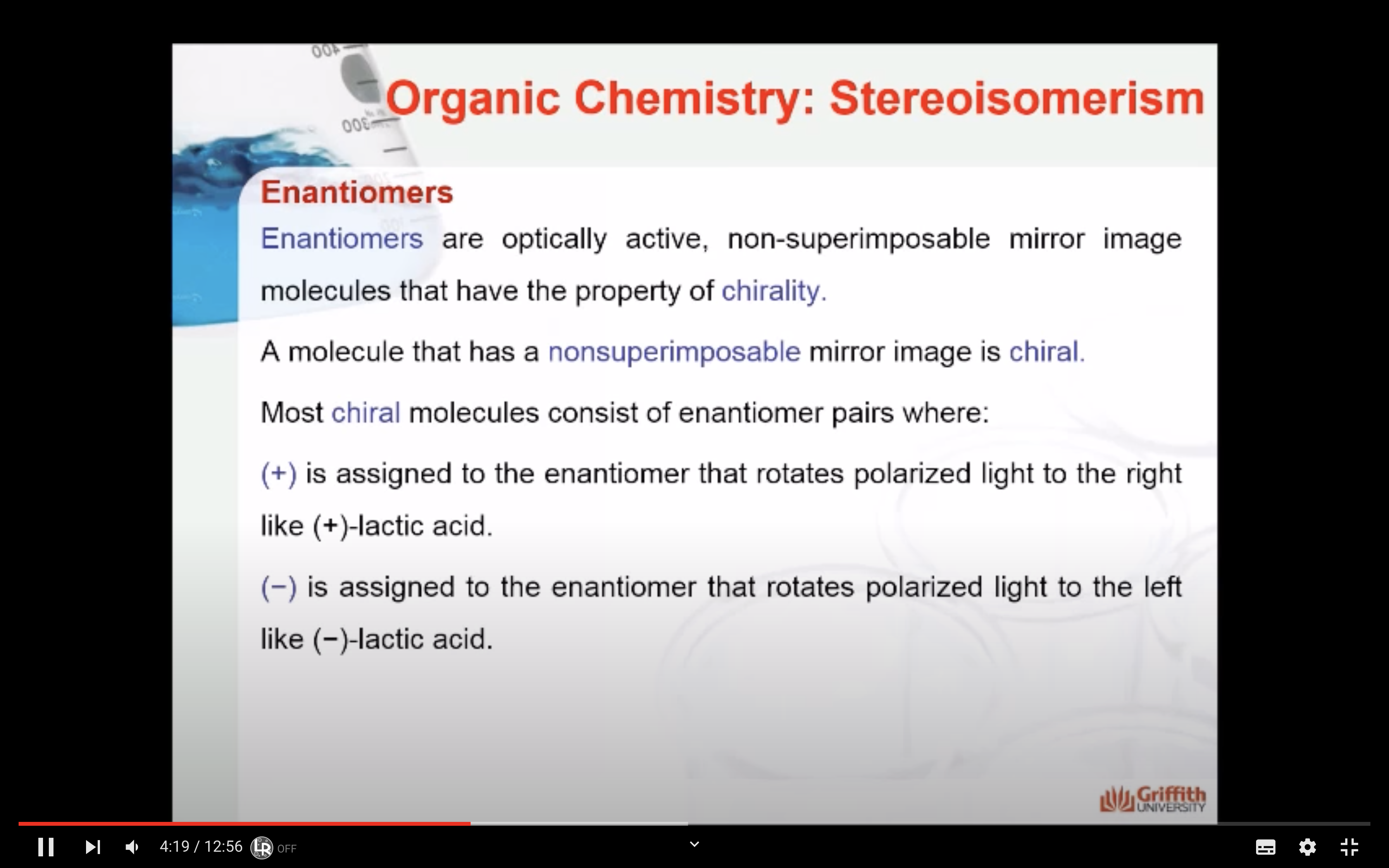
Enantiomers are optically active, non-superimposable mirror image molecules that have the property of chirality.
A molecule that has a non-superimposable mirror image is chiral.
Most chiral molecules consist of enantiomer pairs where:
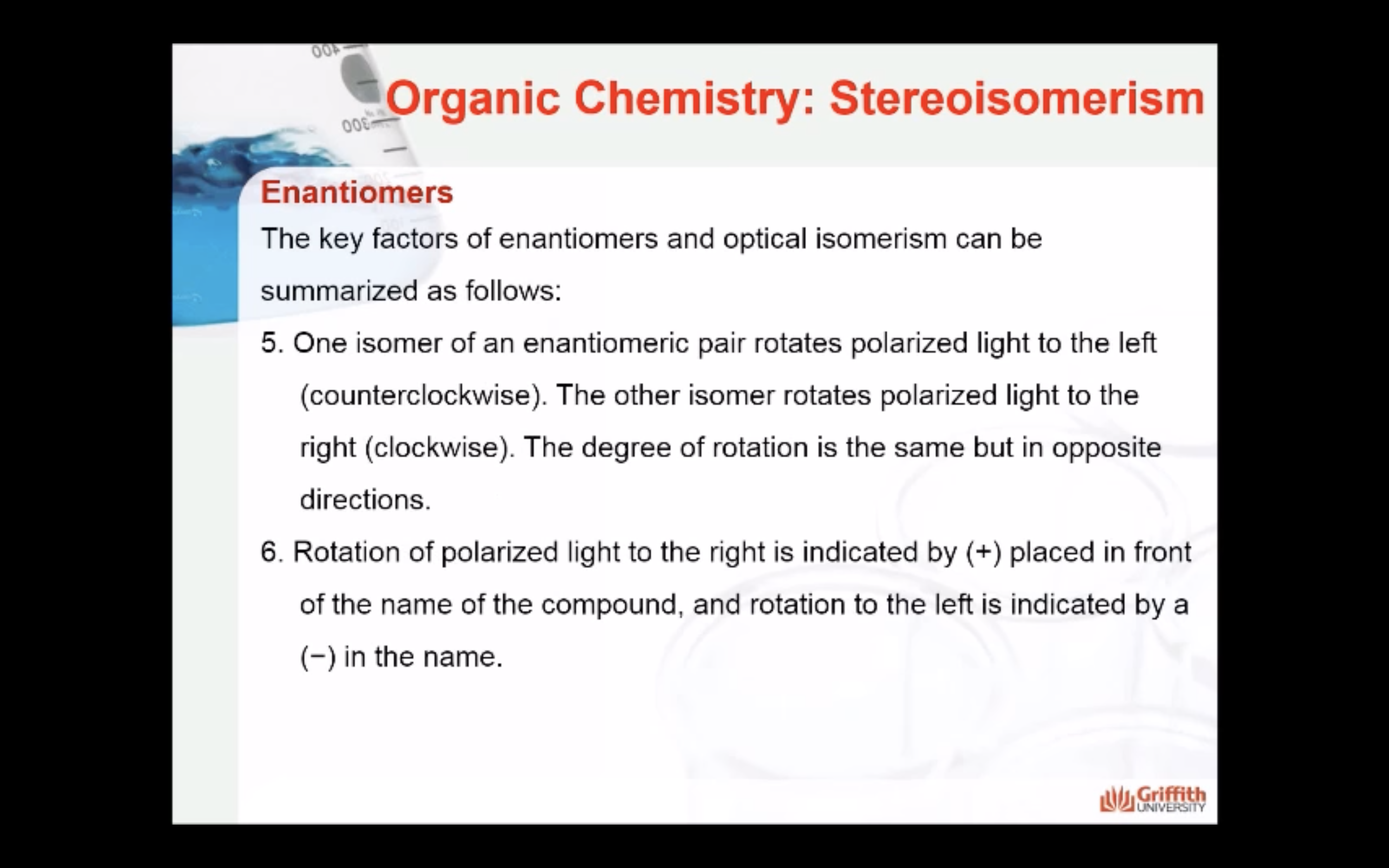
(+) is assigned to the enantiomer that rotates polarized light to the right like (+)-lactic acid.
(−) is assigned to the enantiomer that rotates polarized light to the left like (−)-lactic acid.
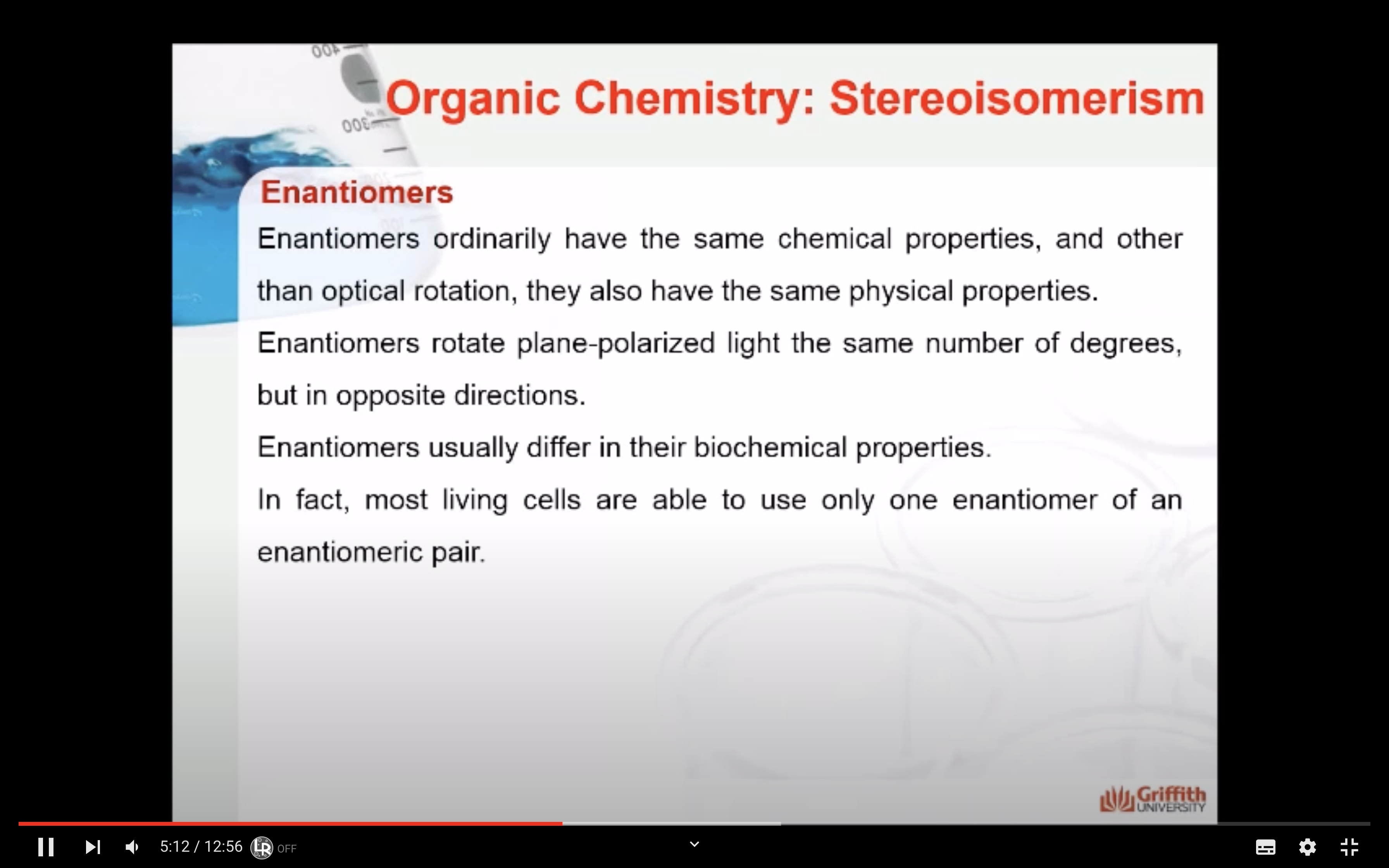
Enantiomers
-Same chemical properties
-Same physical properties
-different in optical rotation (rotate plane-polarized light the same number of degrees, but in opposite directions/ usually differ in their biochemical properties/ most living cells are able to use only one enantiomer of an enantiomer pair)
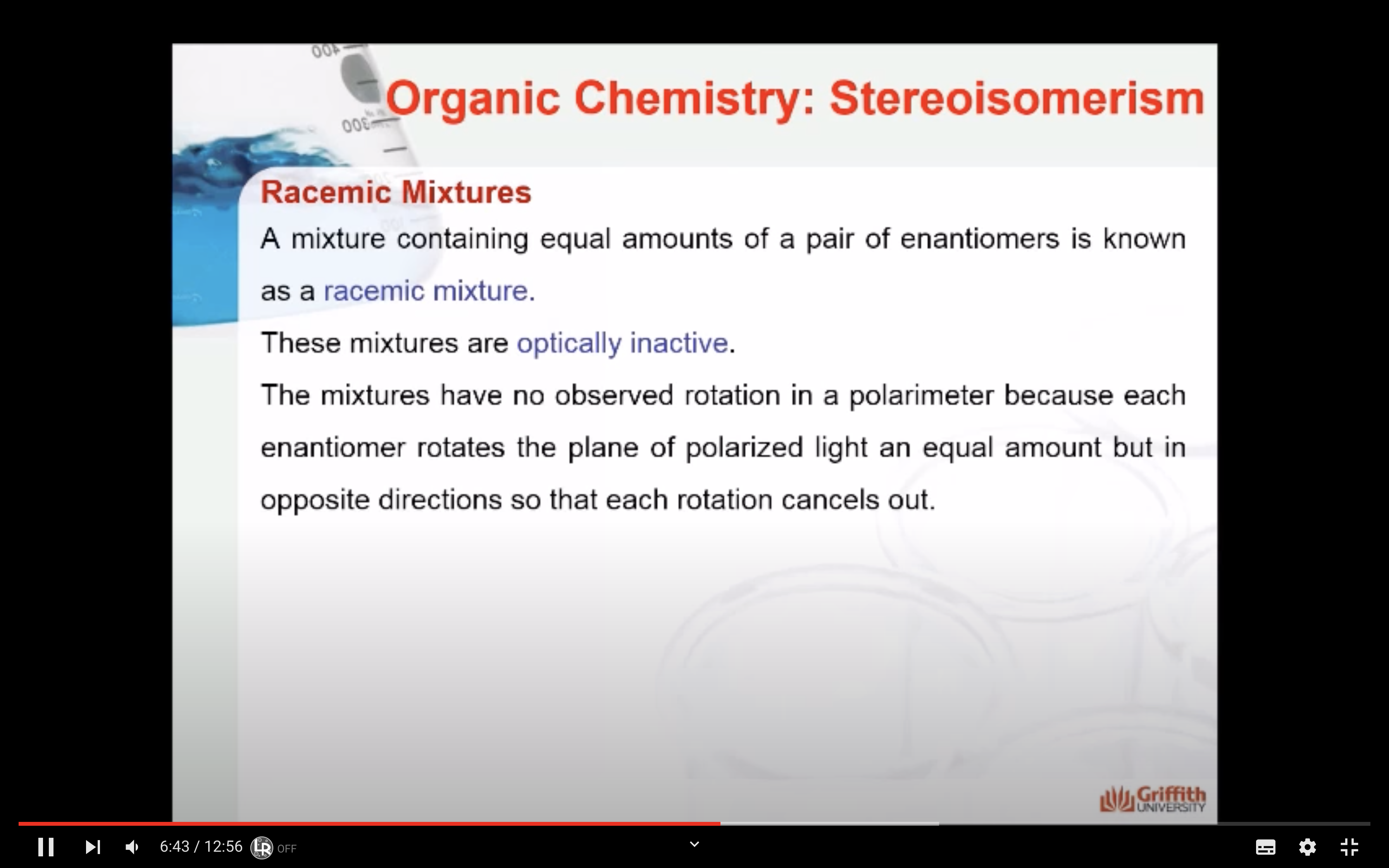
<Racemic Mixtures>
A mixture containing equal amounts of a pair of enantiomers is known as a racemic mixture.
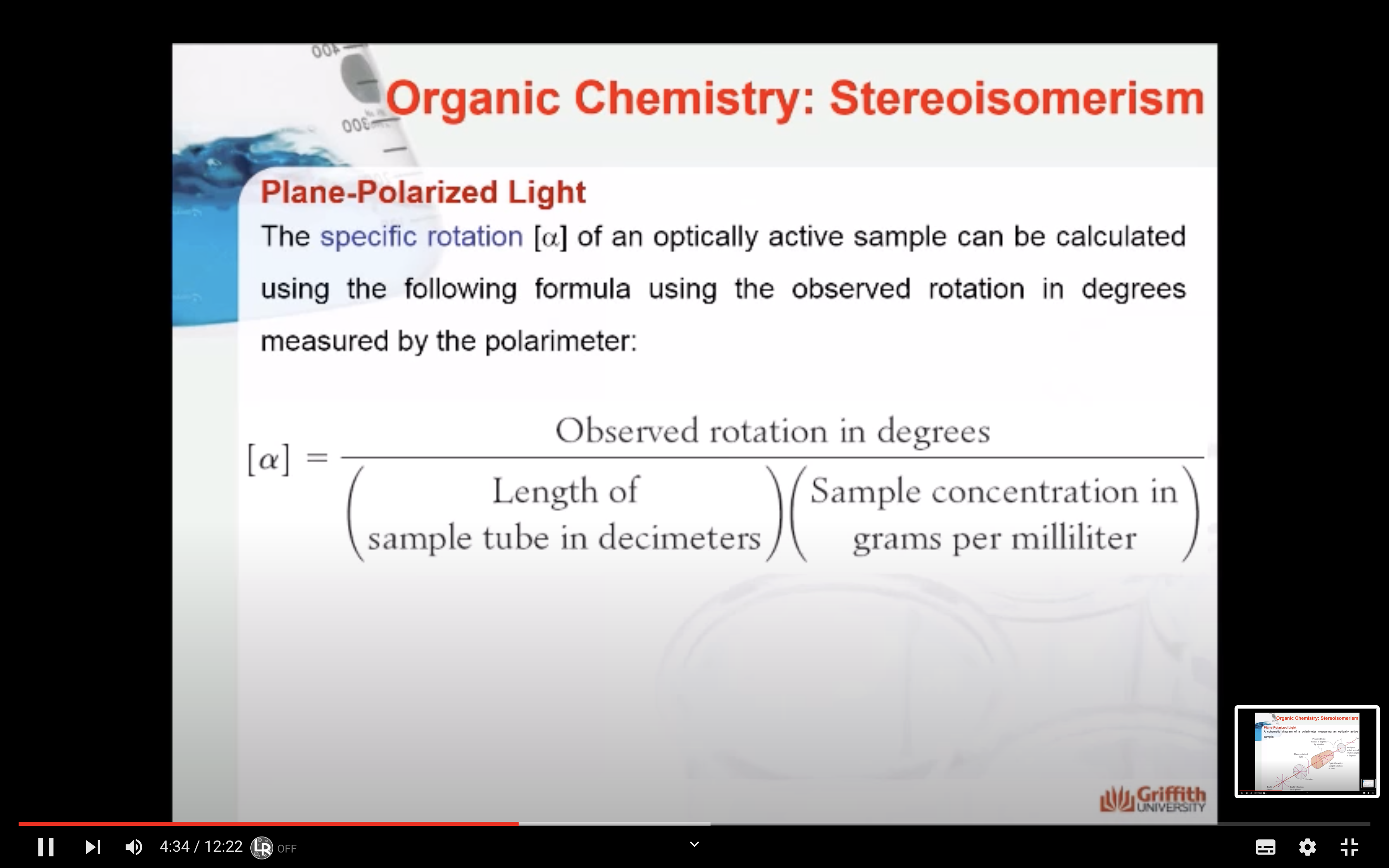
These mixtures are optically inactive. The mixtures have no observed rotation in a polarimeter because each enantiomer rotates the plane of polarized light an equal amount but in opposite directions so that each rotation cancels out.
The (±) symbol is often used to designate racemic mixtures.
As a general rule, only one of the isomers is produced in the biological synthesis of optically active compounds.
For example, only (+)-lactic acid is produced by reactions occurring in muscle tissue, and only (−)-lactic acid is produced by lactic acid bacteria in the souring of milk.
For a carbon to be chiral it must have four different groups attached to it.
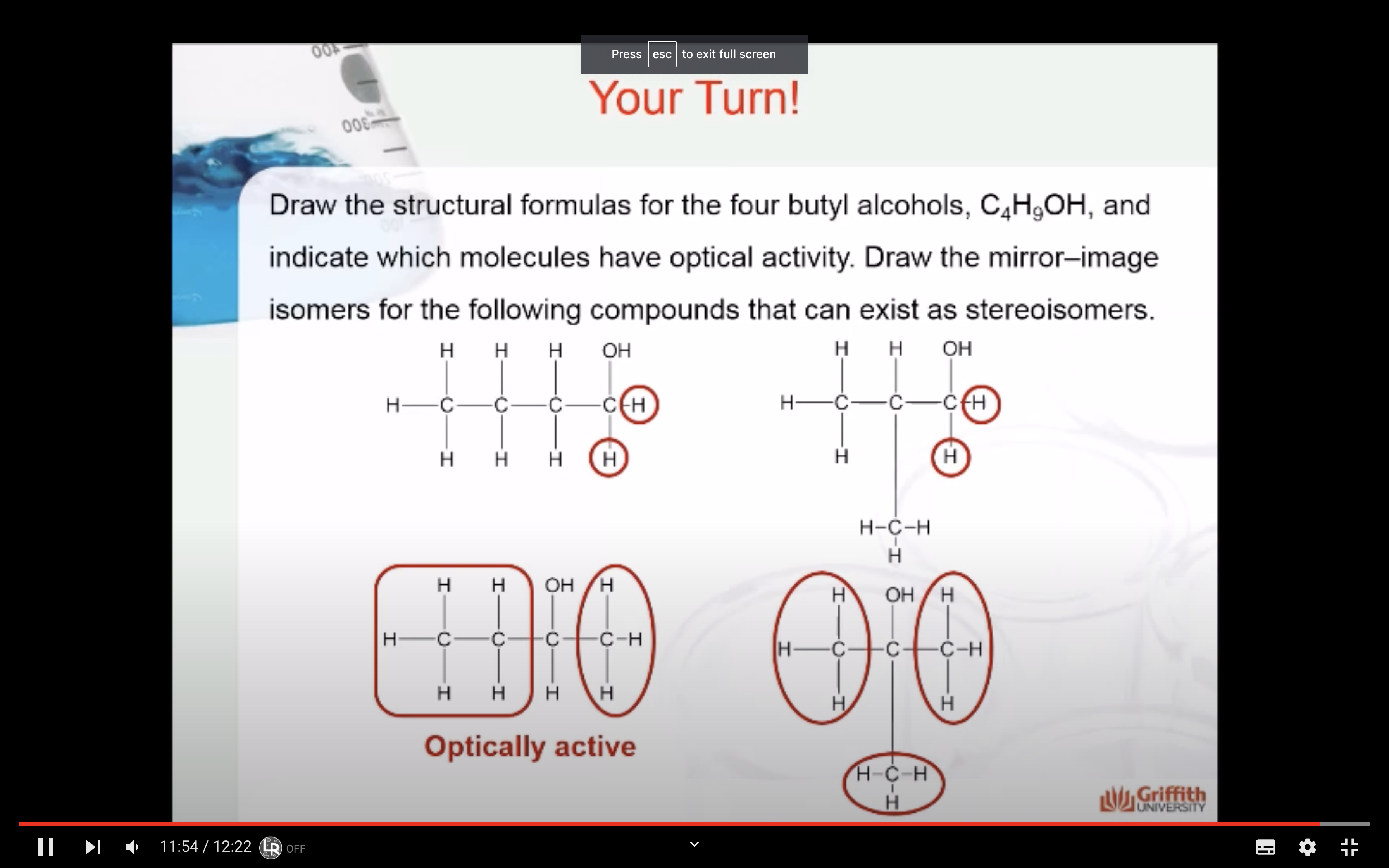
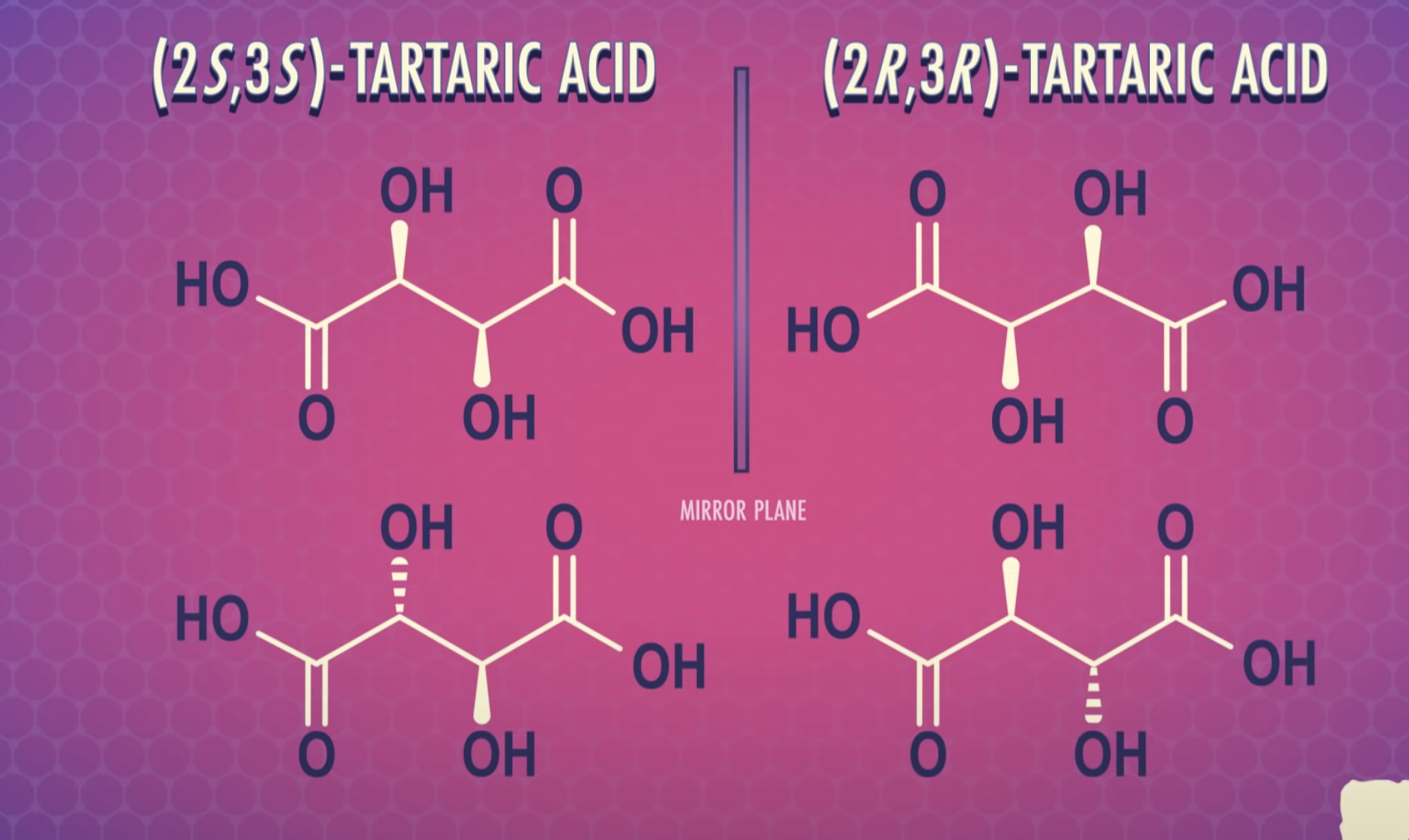
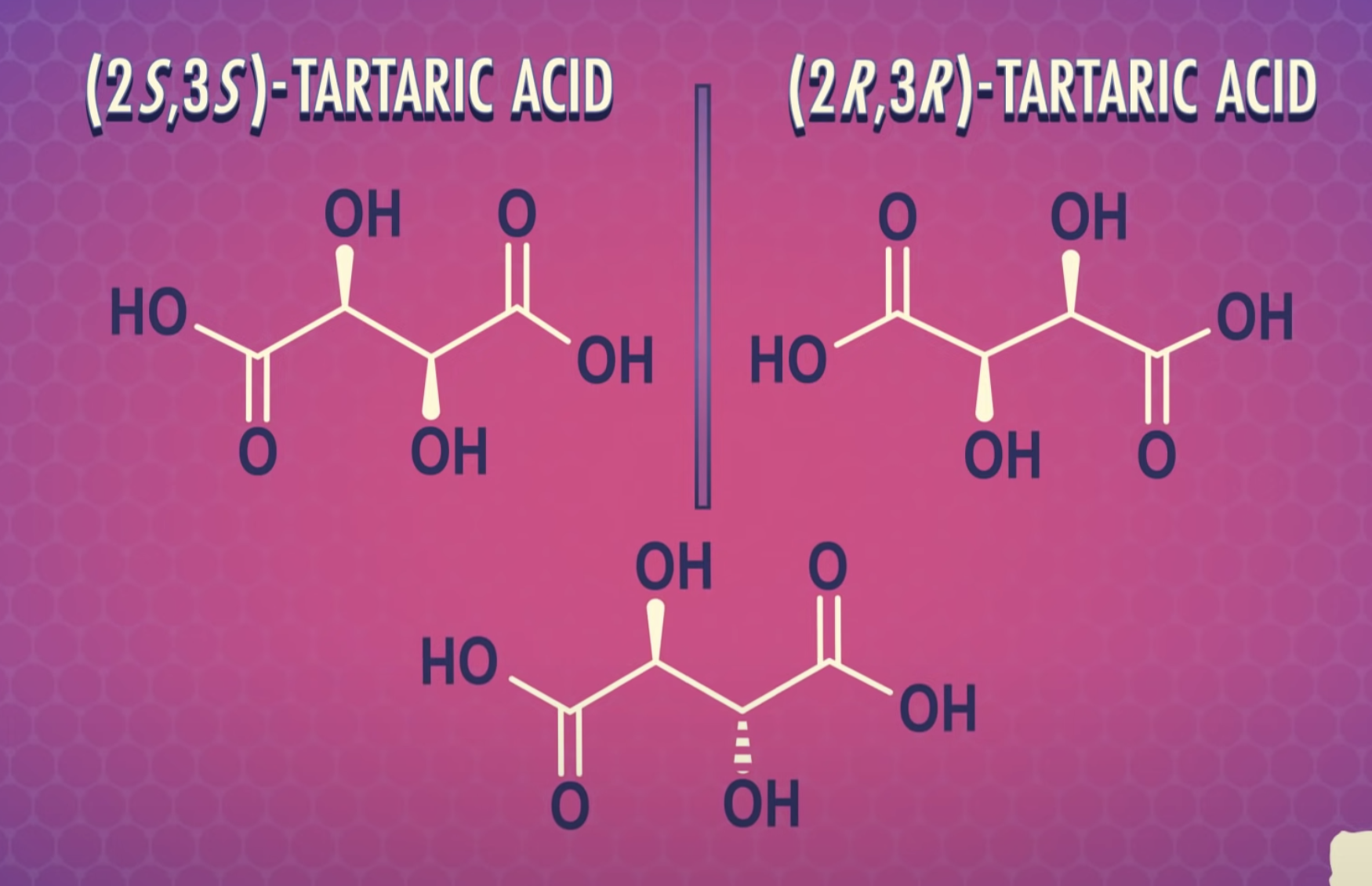
<Diasereomers and Meso compounds>
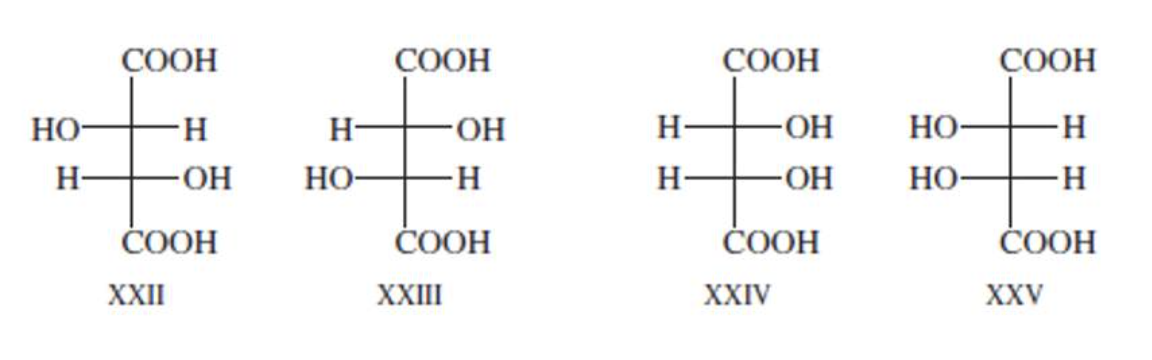
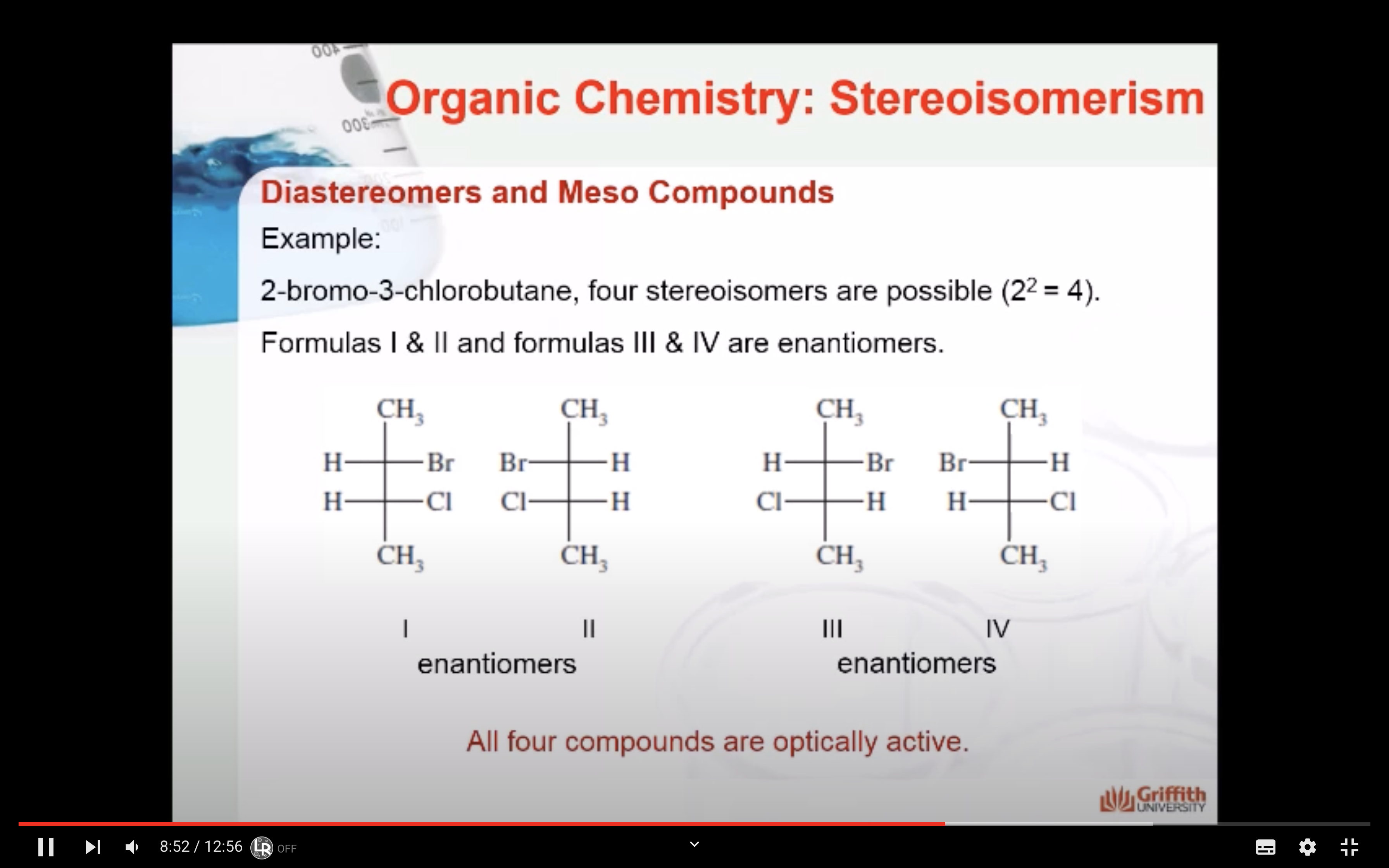
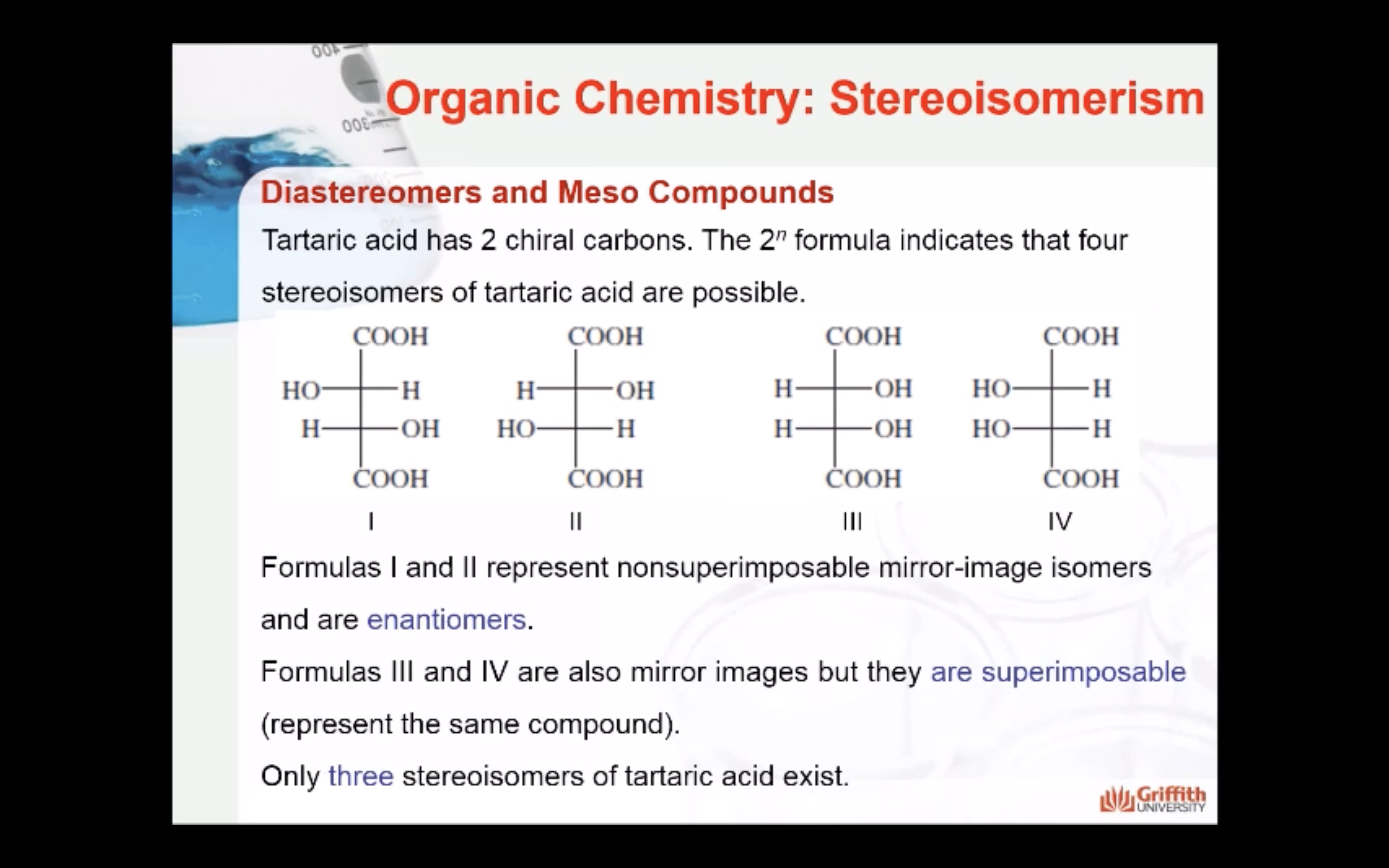
Formulas XXII and XXIII represent nonsuperimposable mirror-image isomers and are enantiomers.
Formulas XXIV and XXV are also mirror images but they are superimposable.
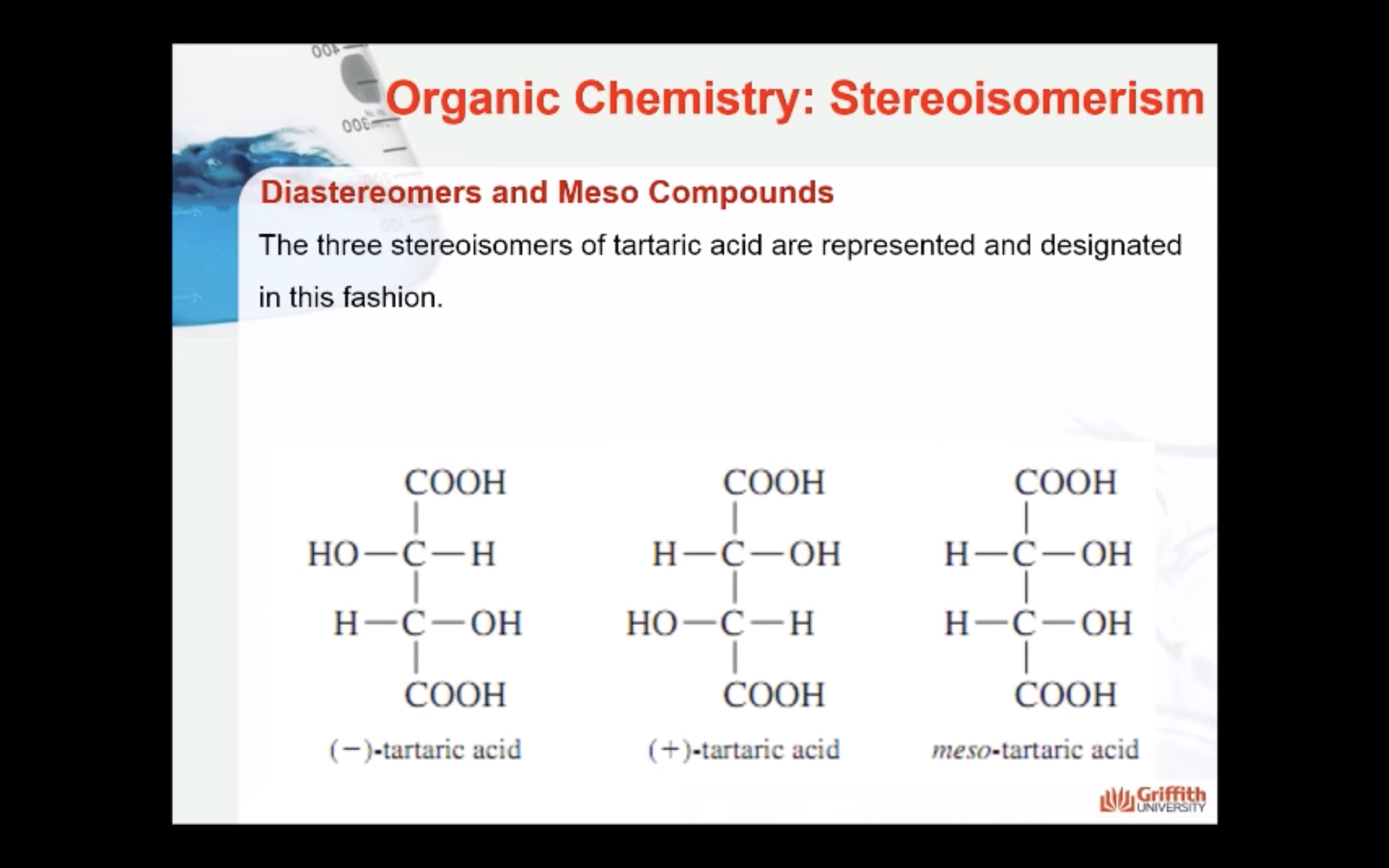
Formula XXIV and XXV represent the same compound. Only three stereoisomers of tartaric acid exist.
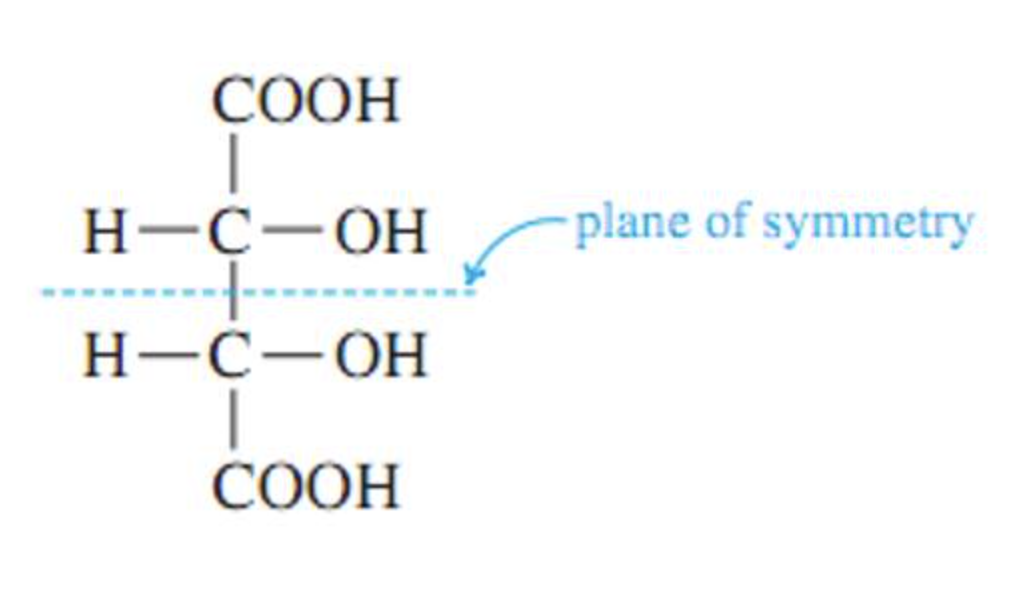
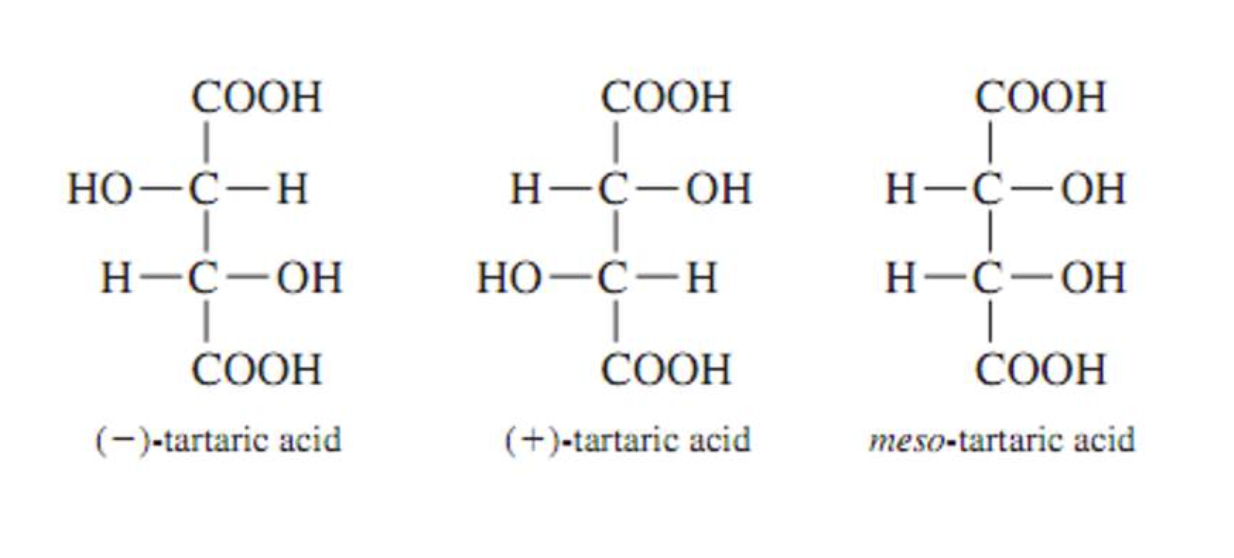
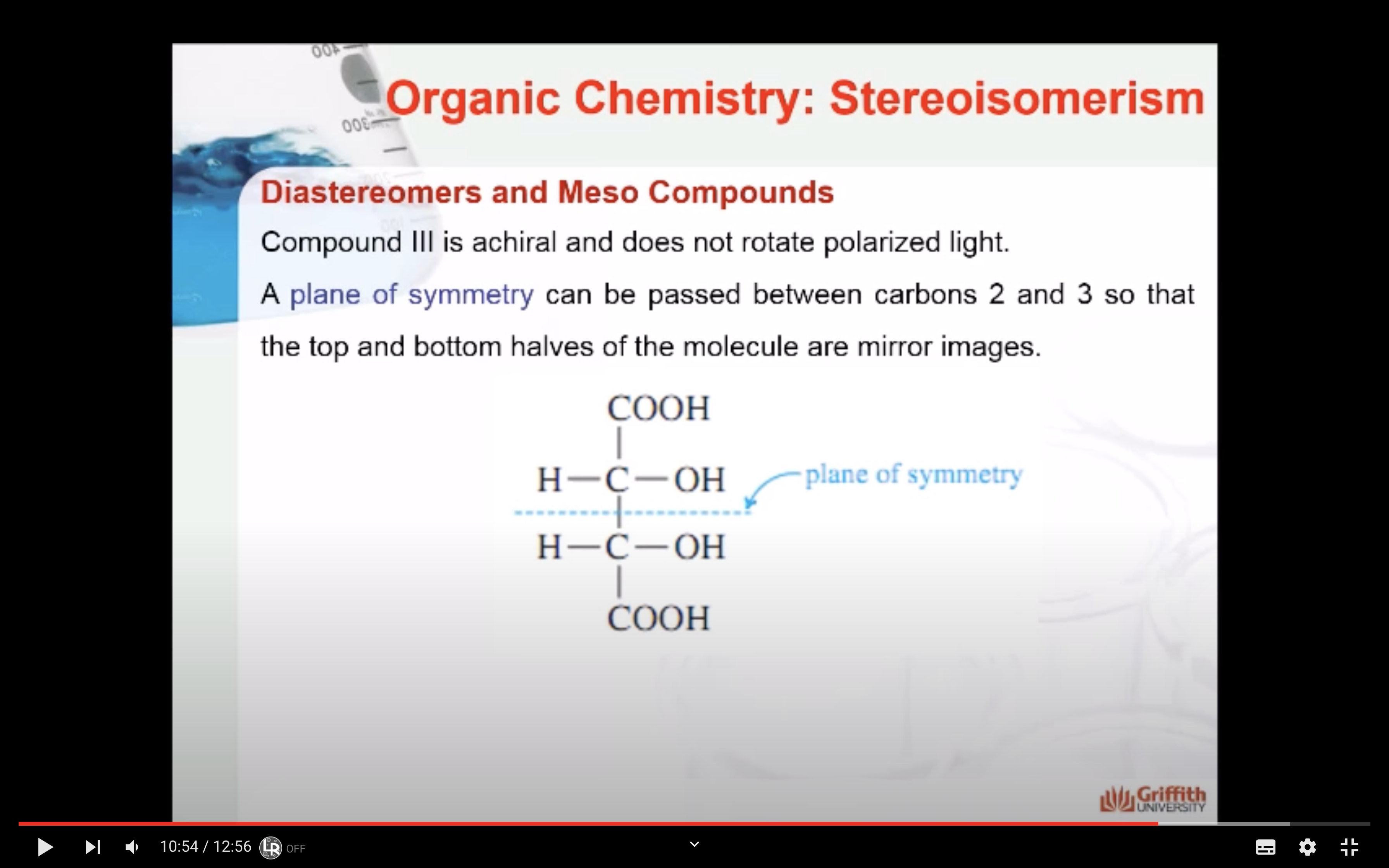
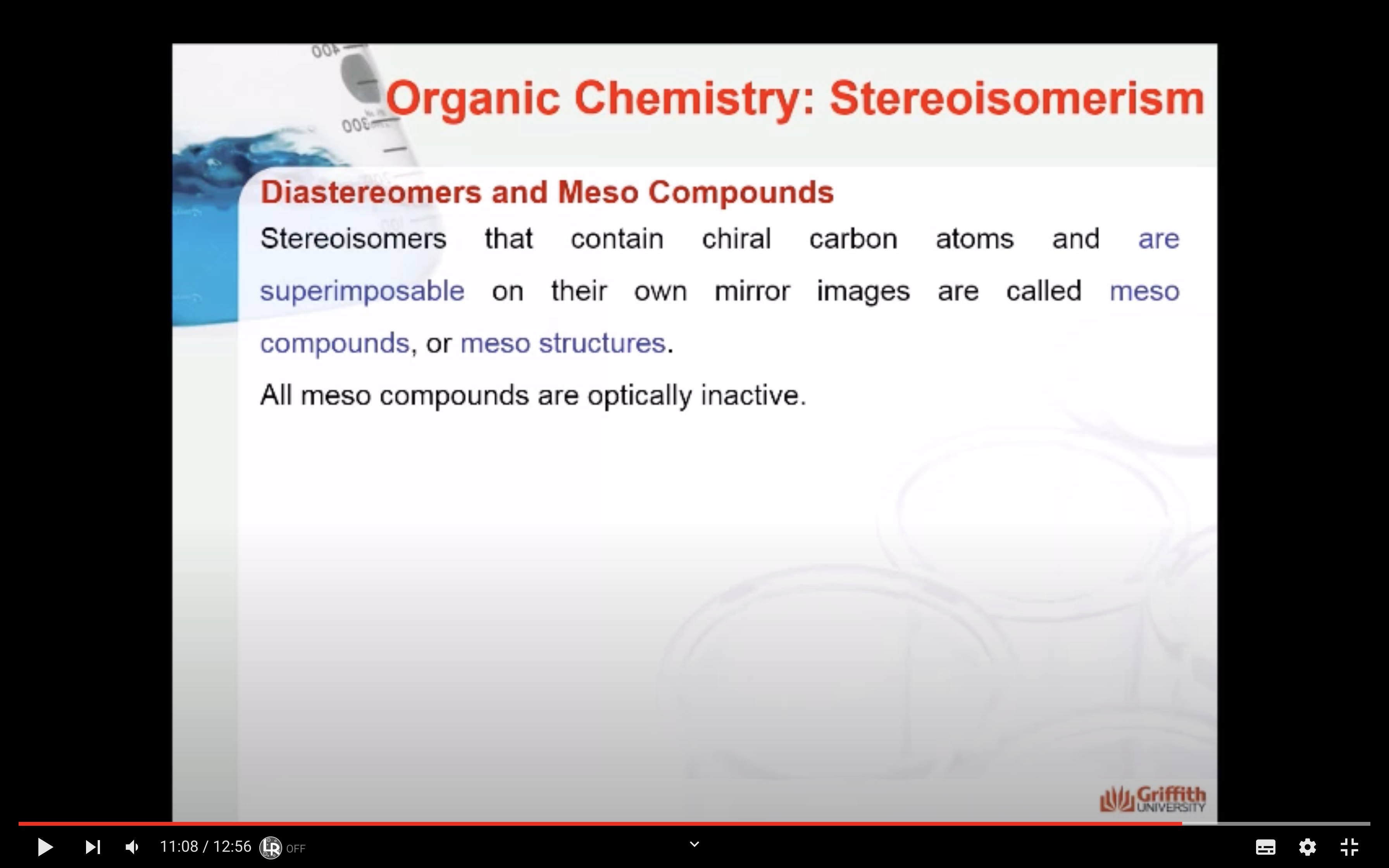
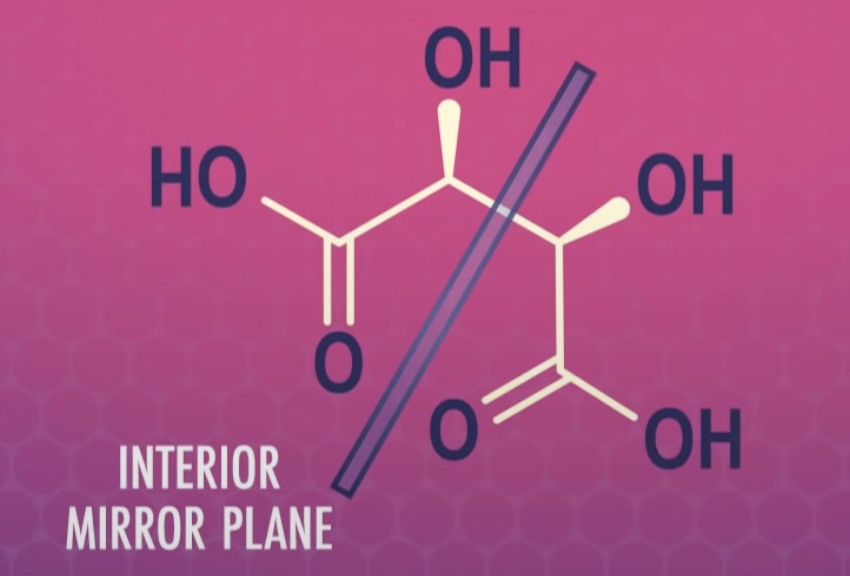
Stereoisomers that contain chiral carbon atoms and are superimposable on their own mirror images are called meso compounds, or meso structures.
All meso compounds are optically inactive.
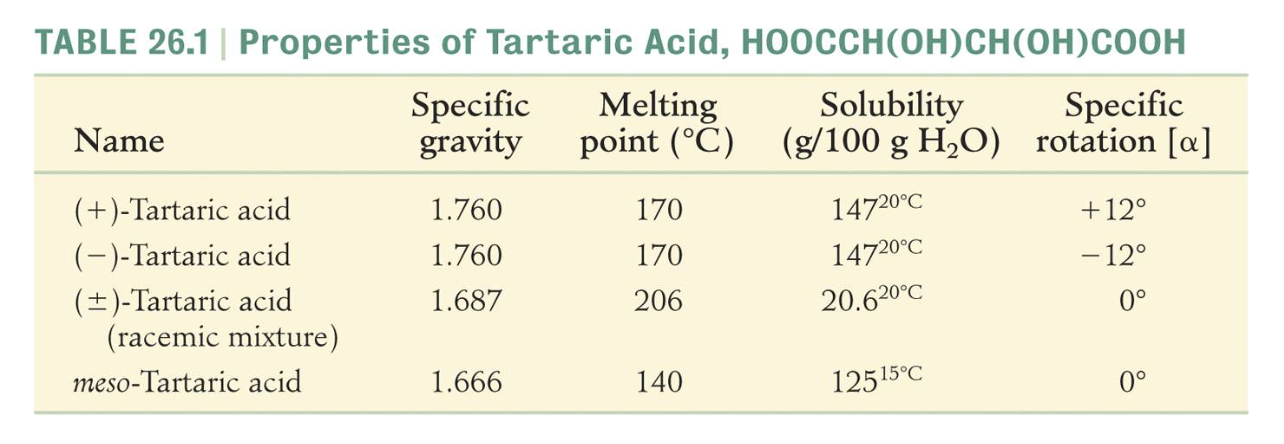
The three stereoisomers of tartaric acid are represented and designated in this fashion.
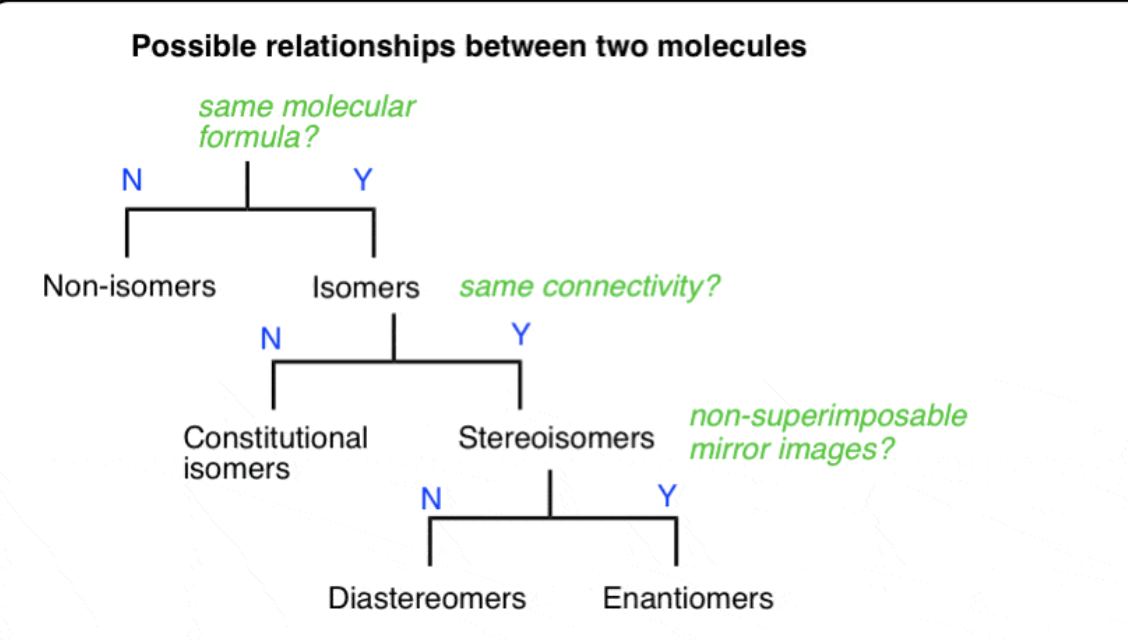

아직 밑에꺼 모르겟서

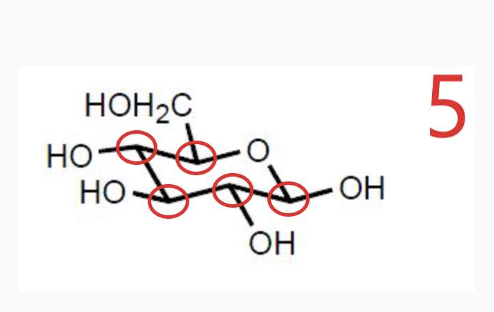
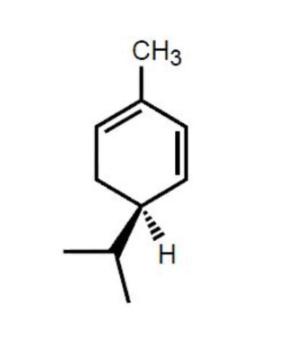
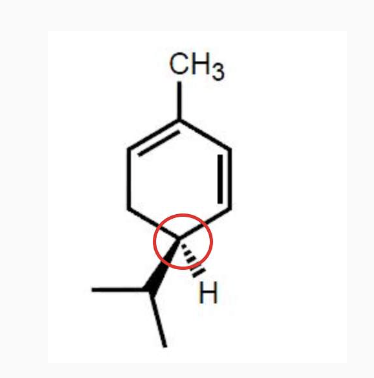

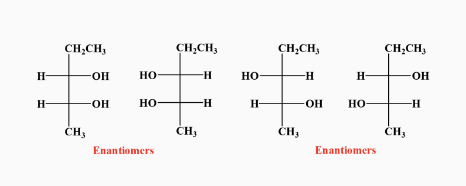
-Chirality : if its mirror images are non-superimposable, it has chairality
-Those mirror images are called enantiomers
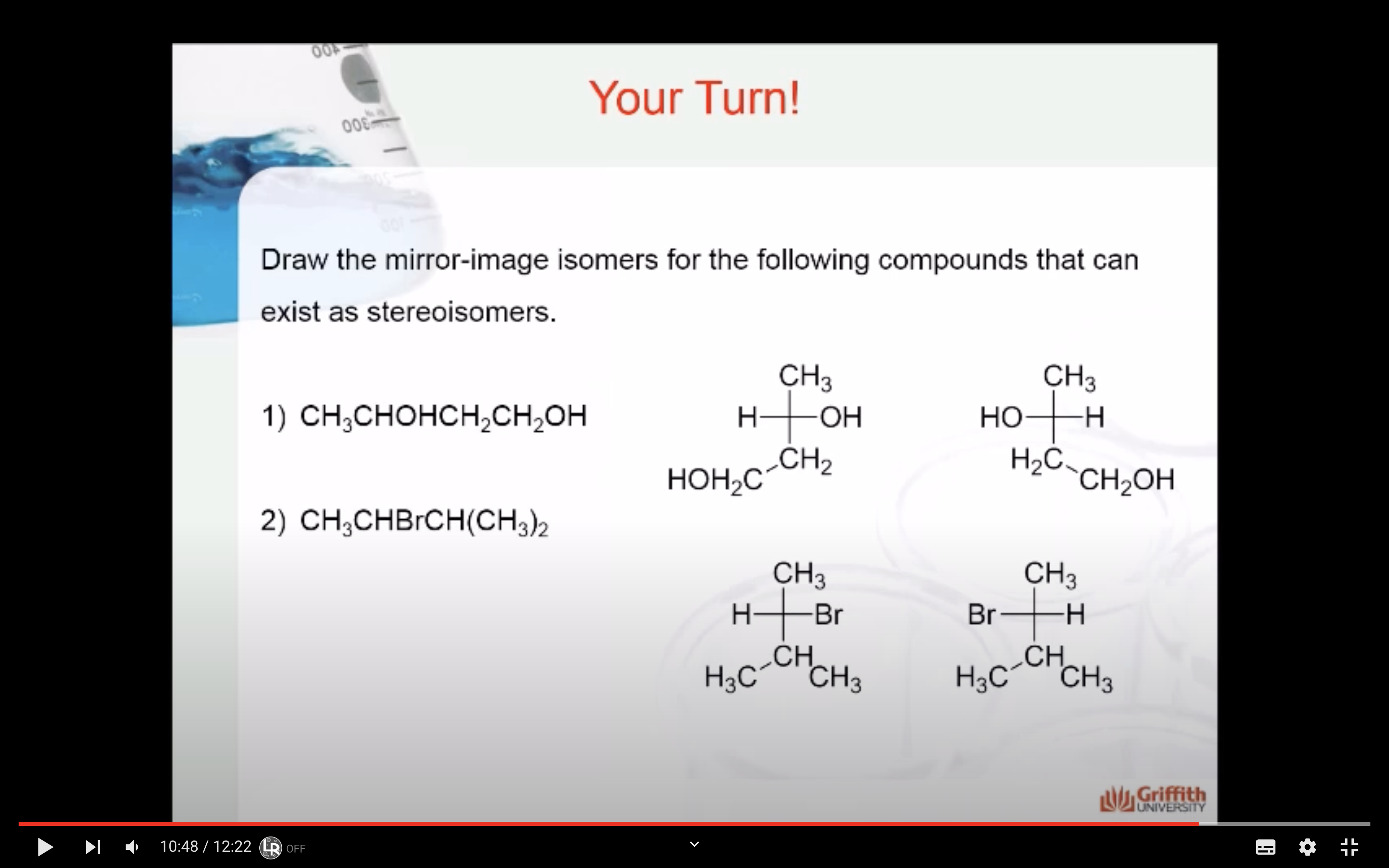
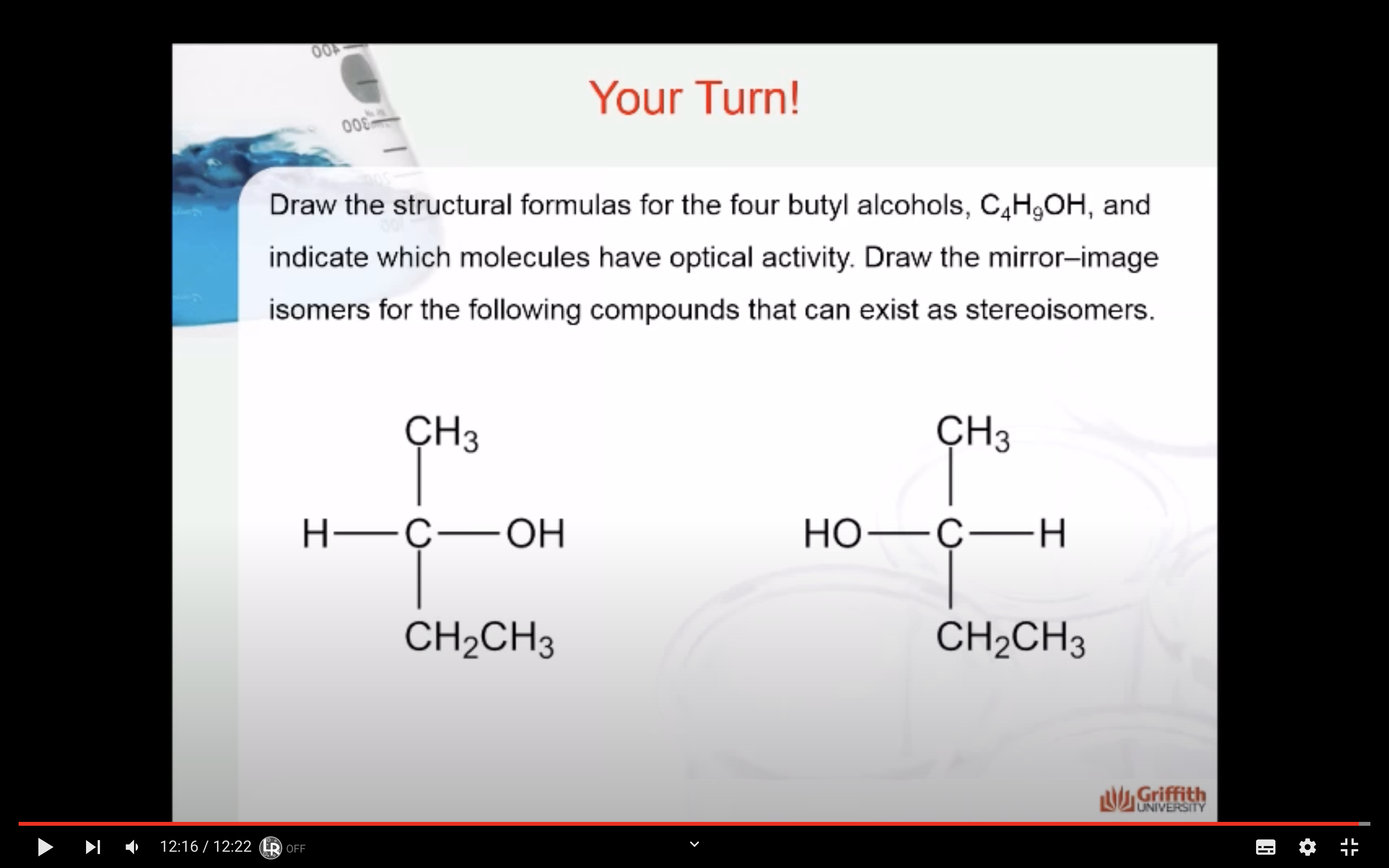
HOW TO DRAW MIRROR IMAGES
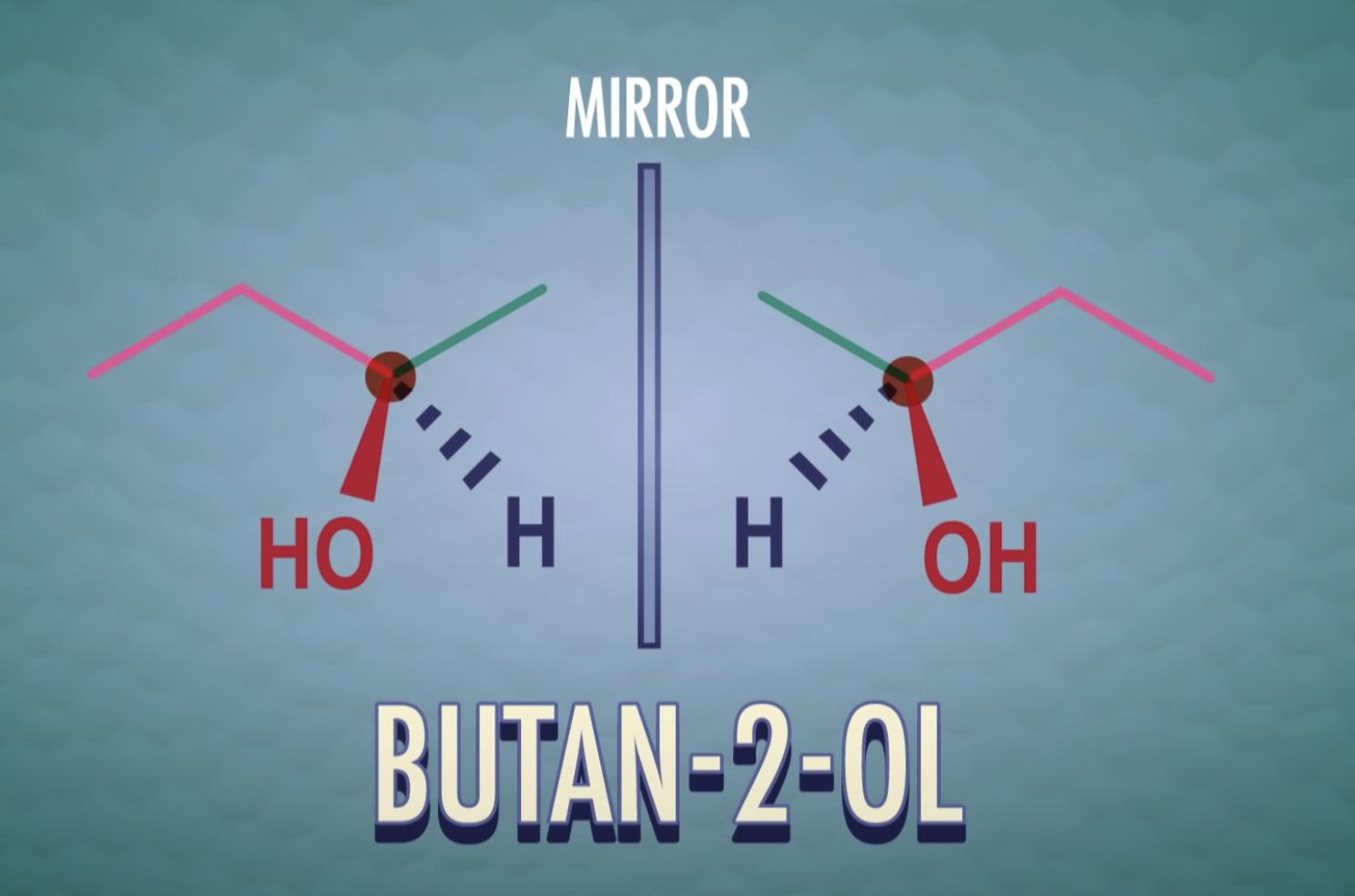

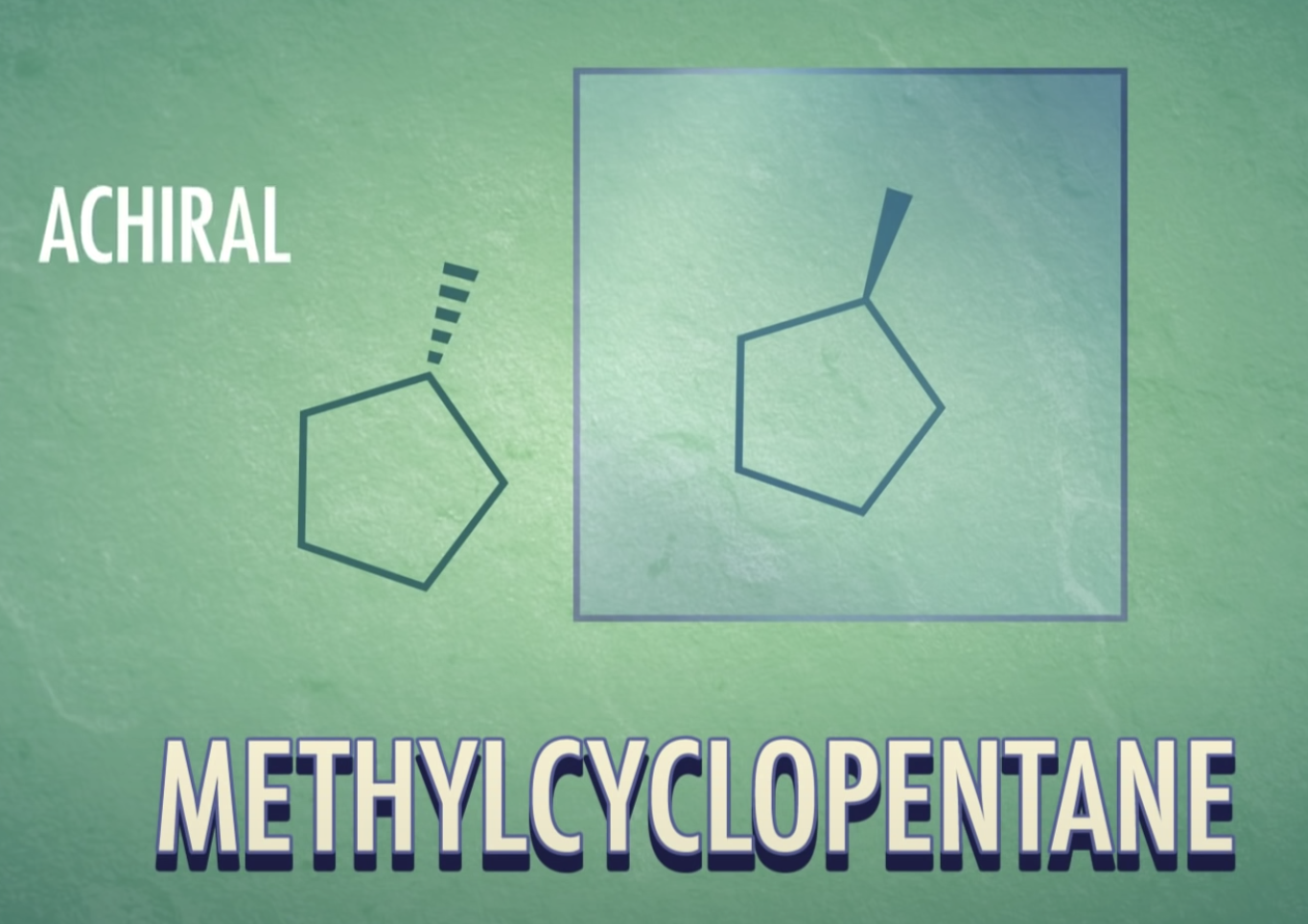
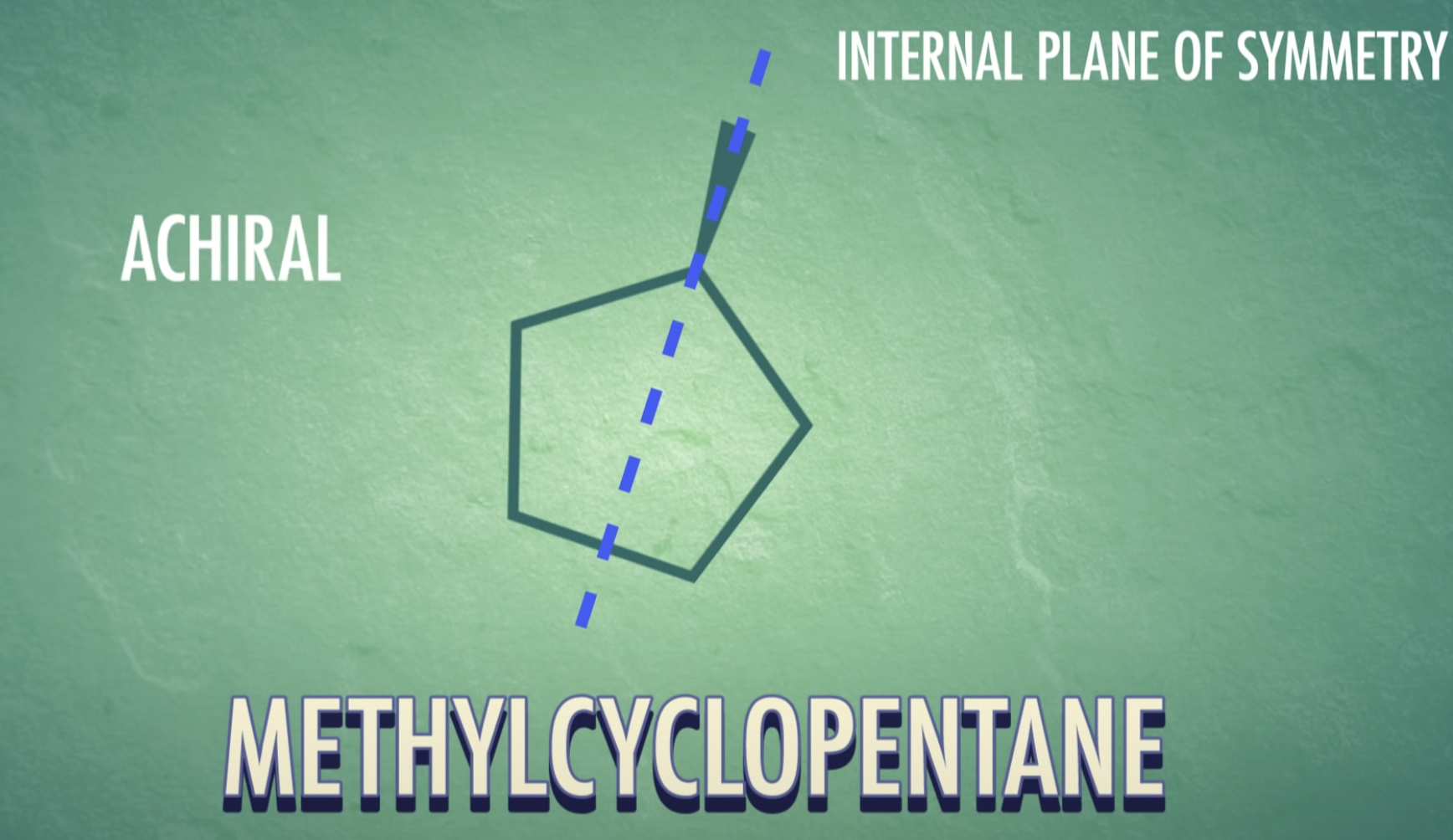
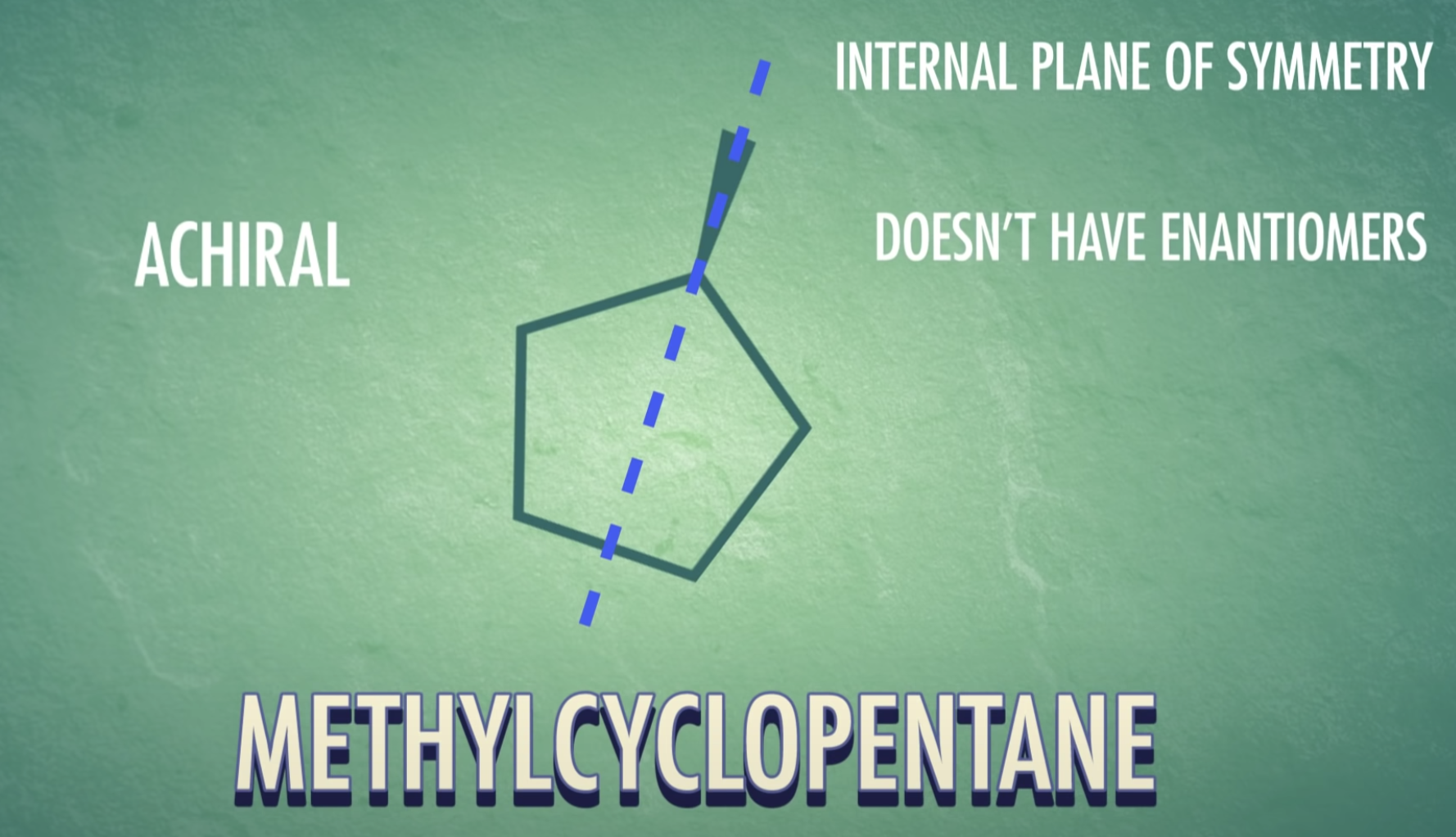
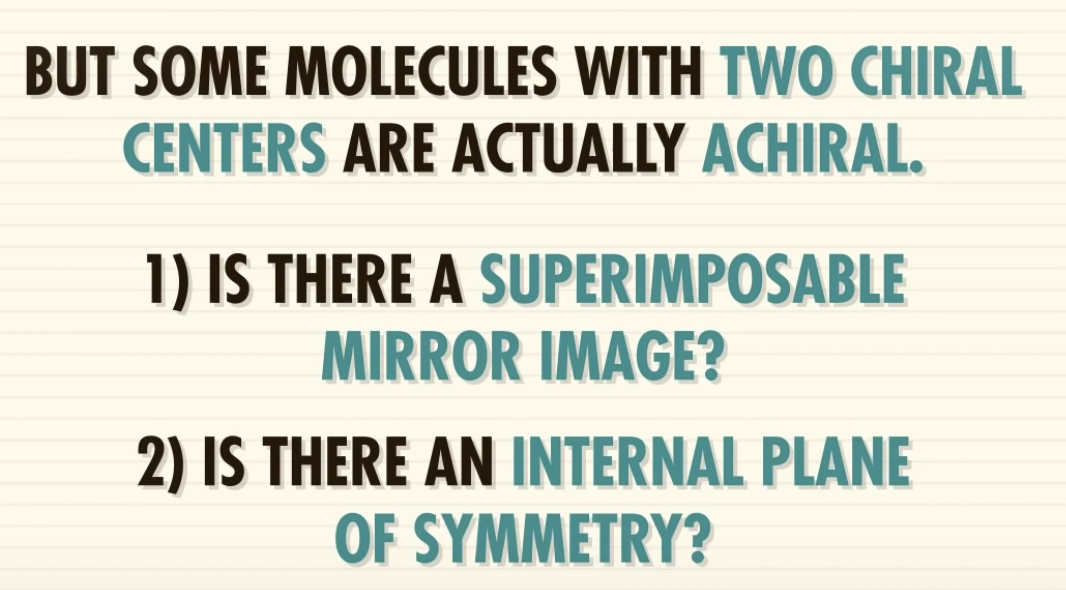
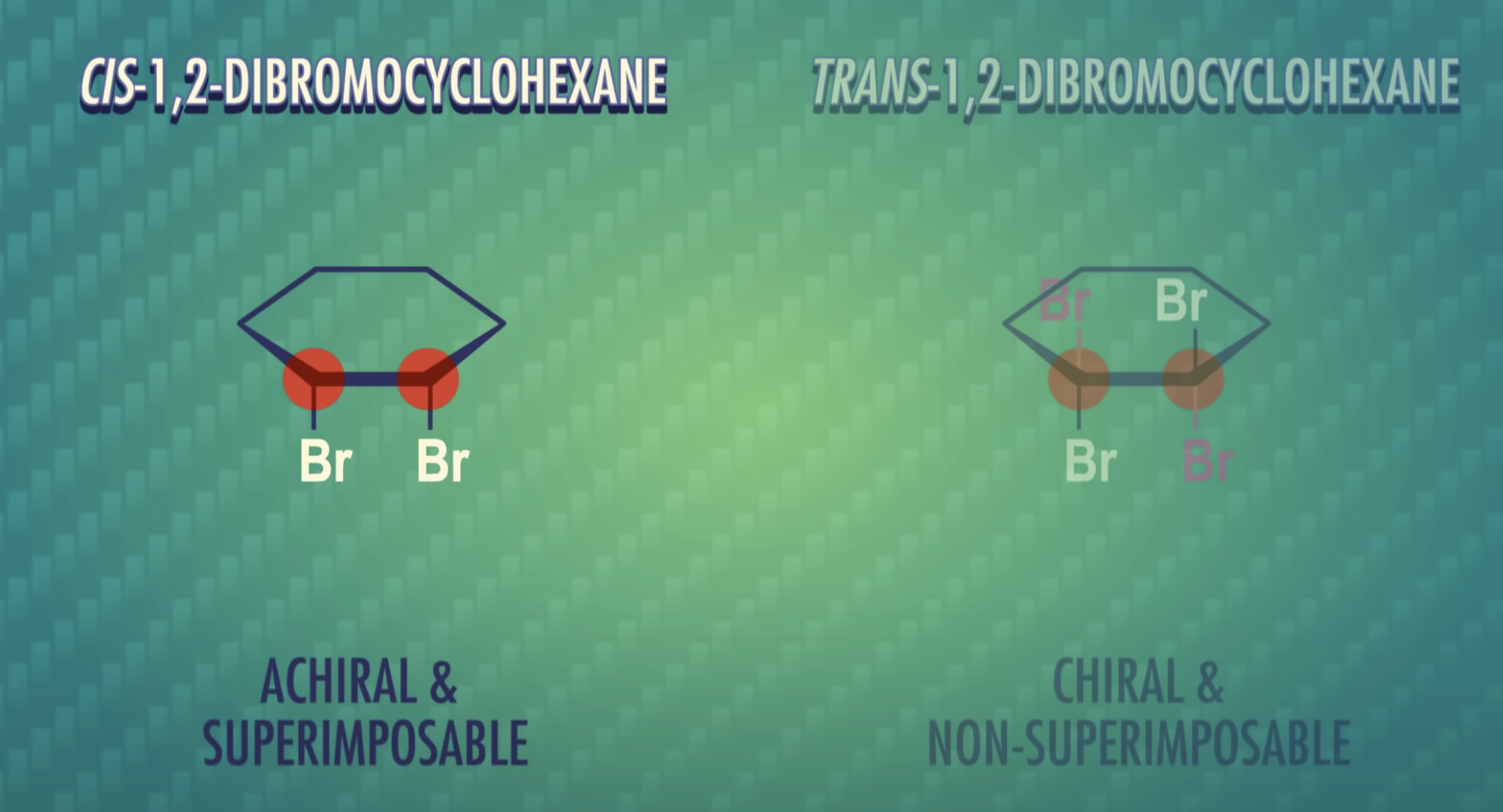
-meso-compound has internal plane of symmetry
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (0) | 2023.05.13 |
|---|---|
| [WEEK6]Chemistry of Food- Carbohydrates & Carbohydrates (0) | 2023.04.02 |
| [WEEK5] Carboxylic Acids, Esters (0) | 2023.03.30 |
| [WEEK5] Lipids (0) | 2023.03.30 |
| [WEEK5] Chemistry of Food -Fatty Acids (0) | 2023.03.29 |



