


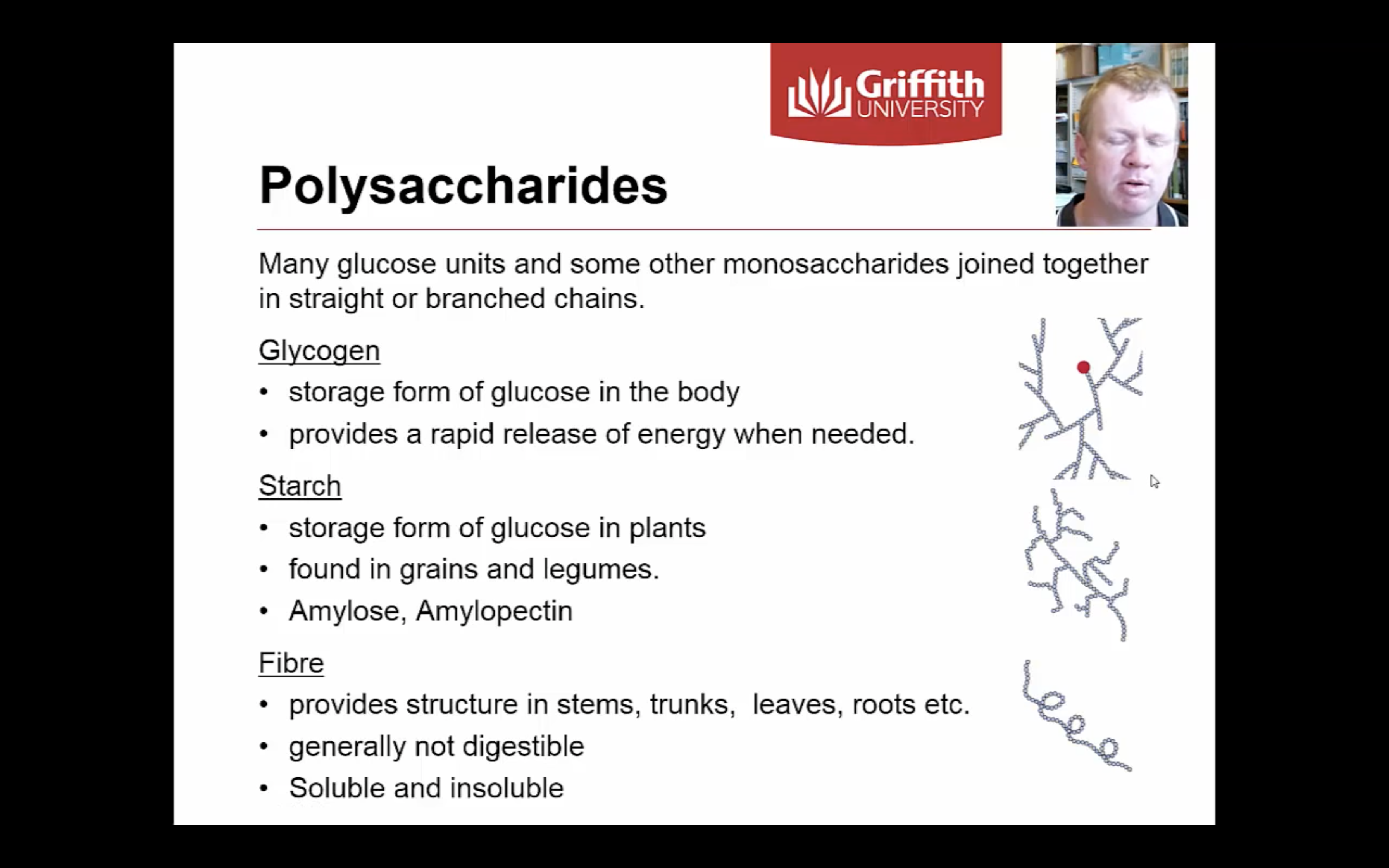
-Amilopectin -> 80% of starch / slightly branced
-Amilyose -> 20% of starch/ chain
-Glycogen -> highly branched
*Branched means it can produce energy easily
(Enzymes can only break down starch at the end of the chain = more branch = more place for enzyme to work on)
MINI LECTURE
PART1
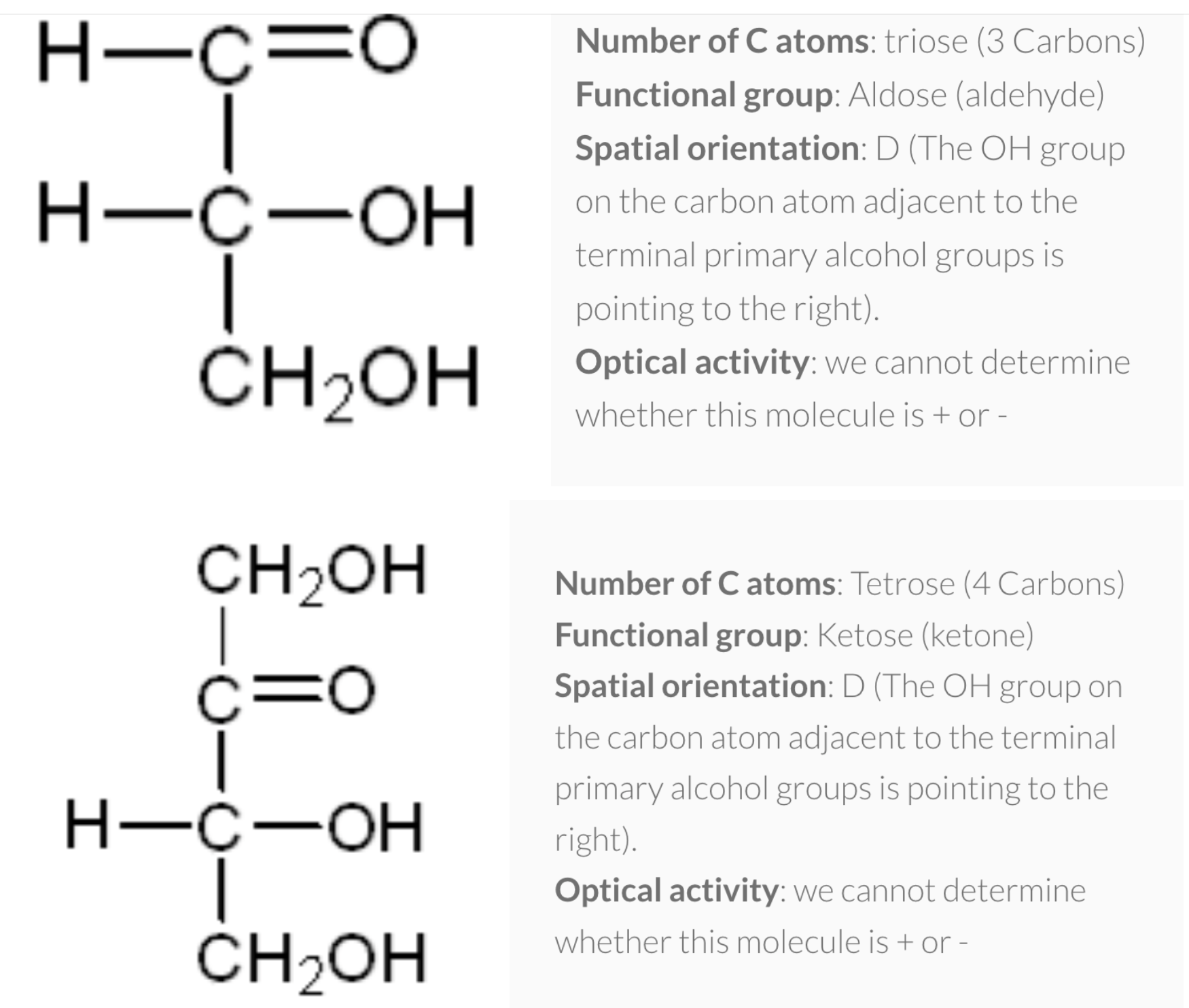
1) number of carbon atoms in the molecule
=> pentose(ribose/deoxyribose) , hexose (glucose/galactose/fructose)
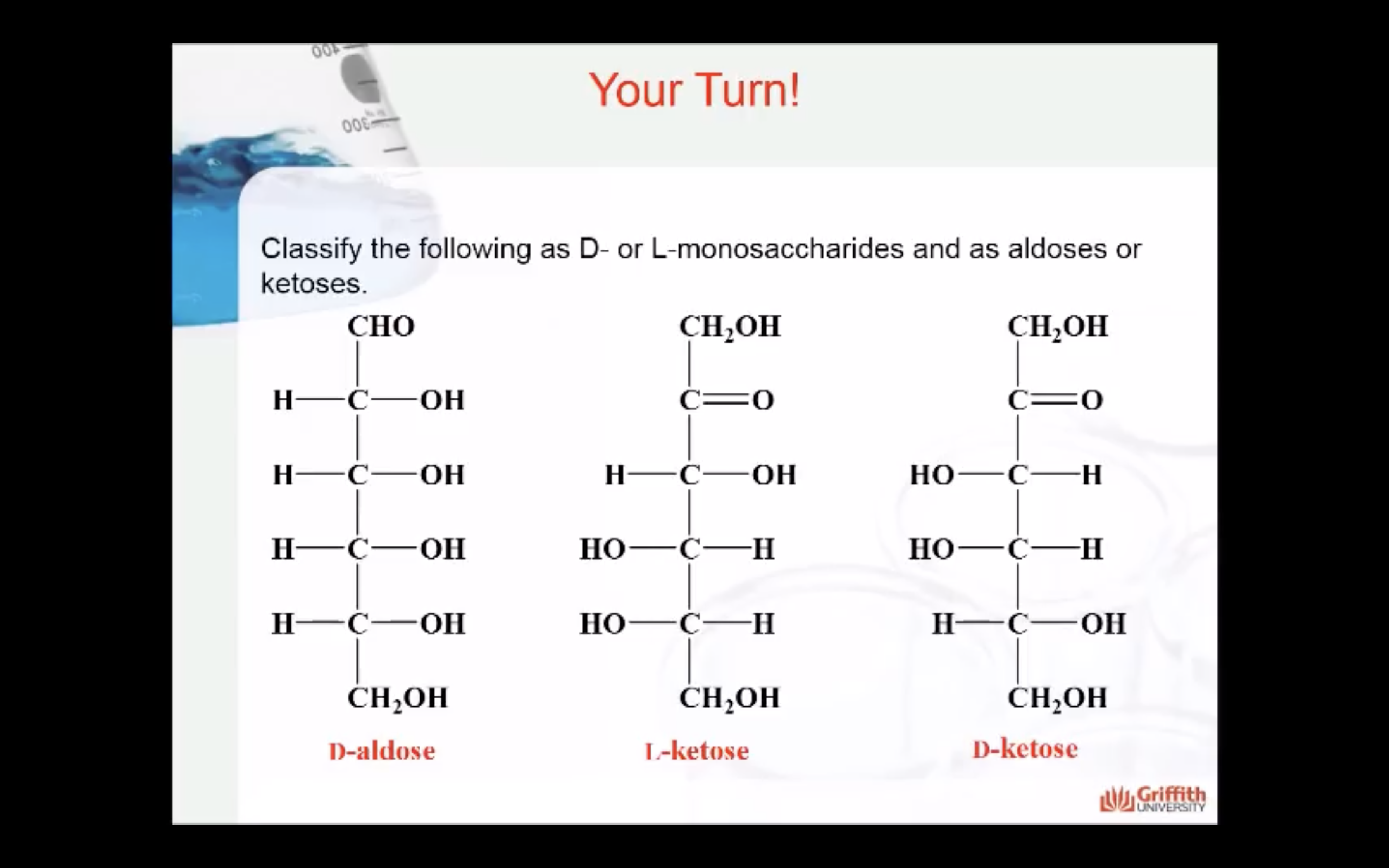
2) functional group present in the molecule
=> aldehyde (aldose) , ketose (ketone)
3) spatial orientation of the molecule
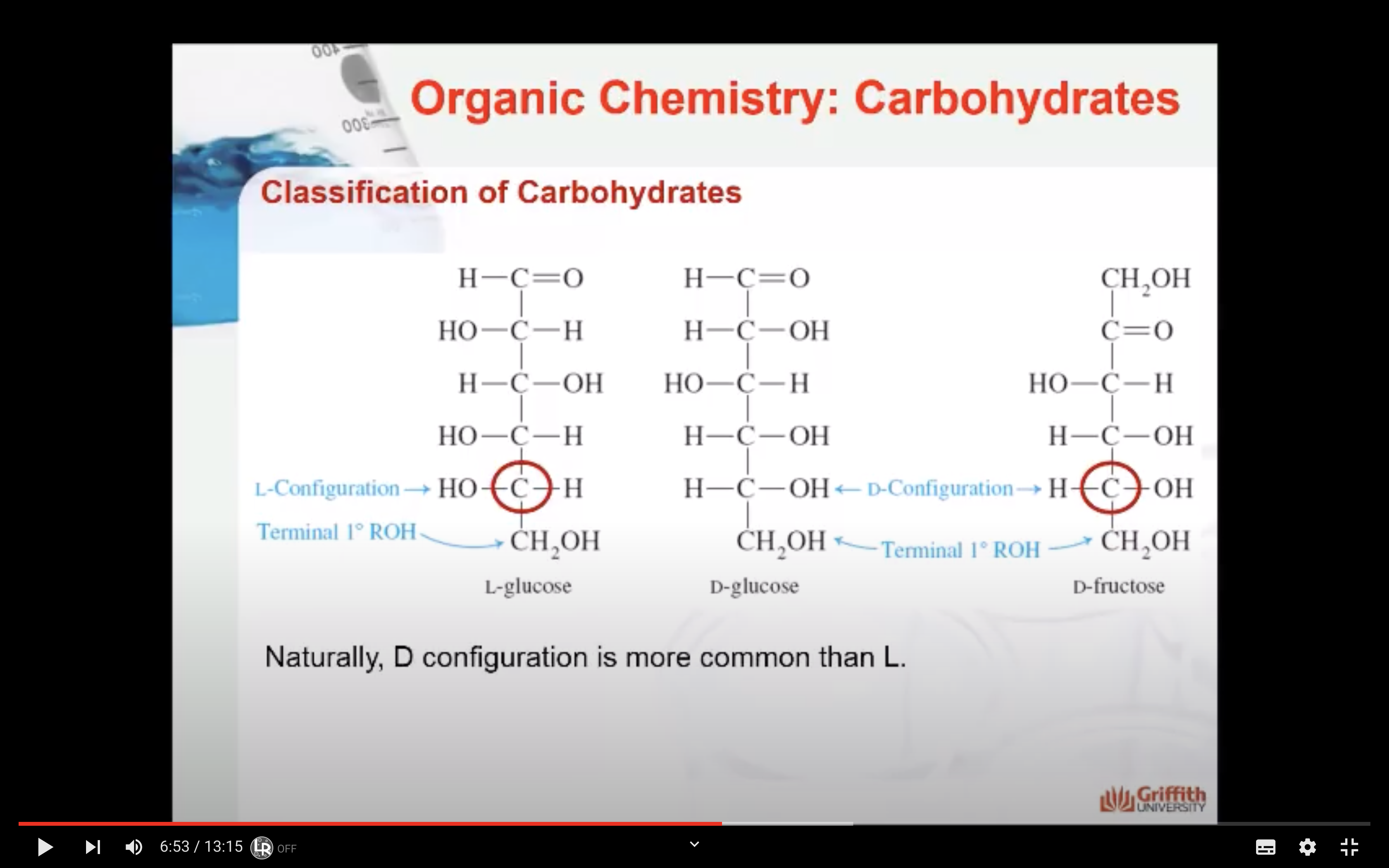
=> when -OH is in left side (L), Right side (D)
(For glucose, when Carbon #5 has OH group right side -> D)
4) optical activity of the molecule
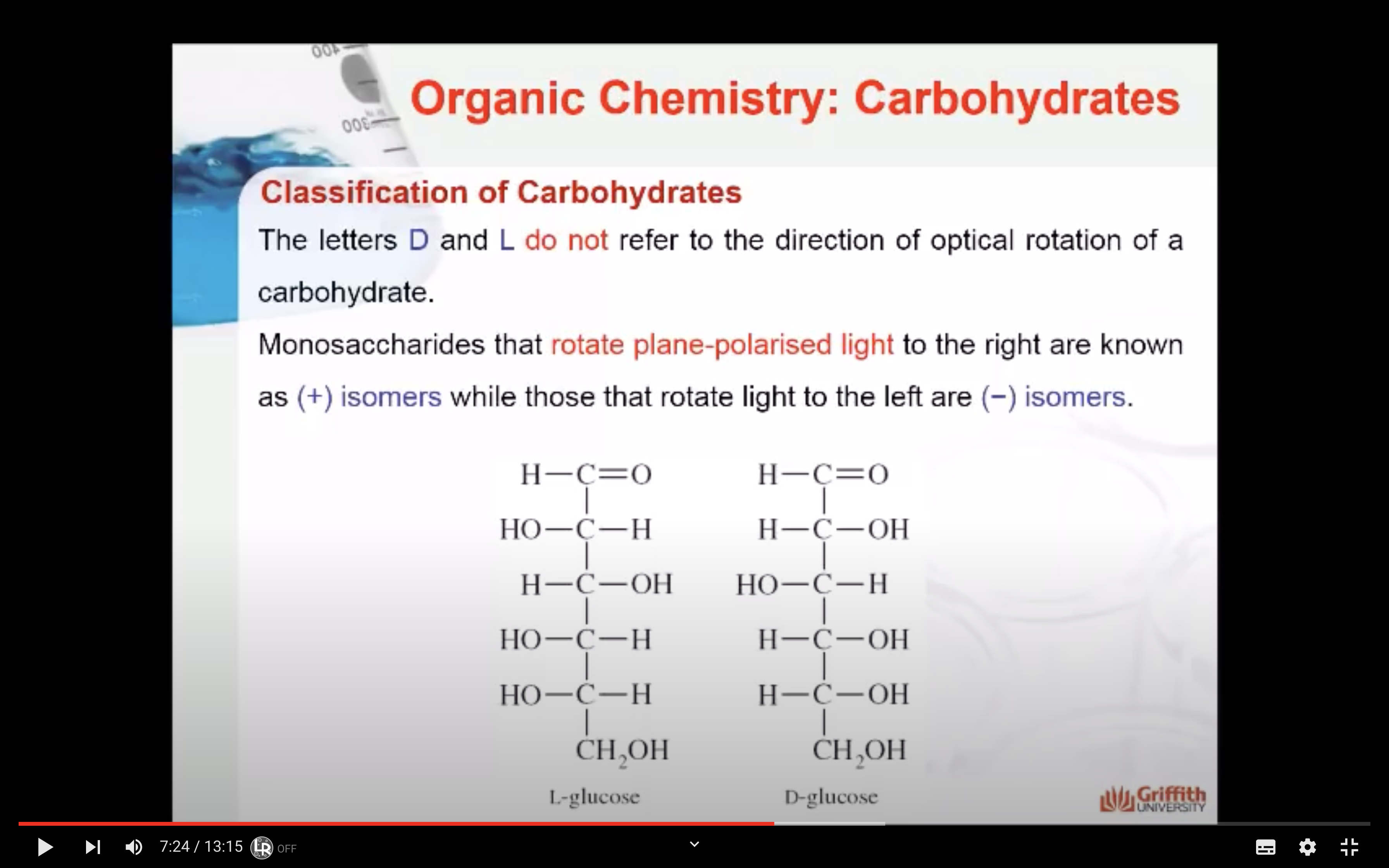
=> rotate plane polaised light to right (+) , left (-)
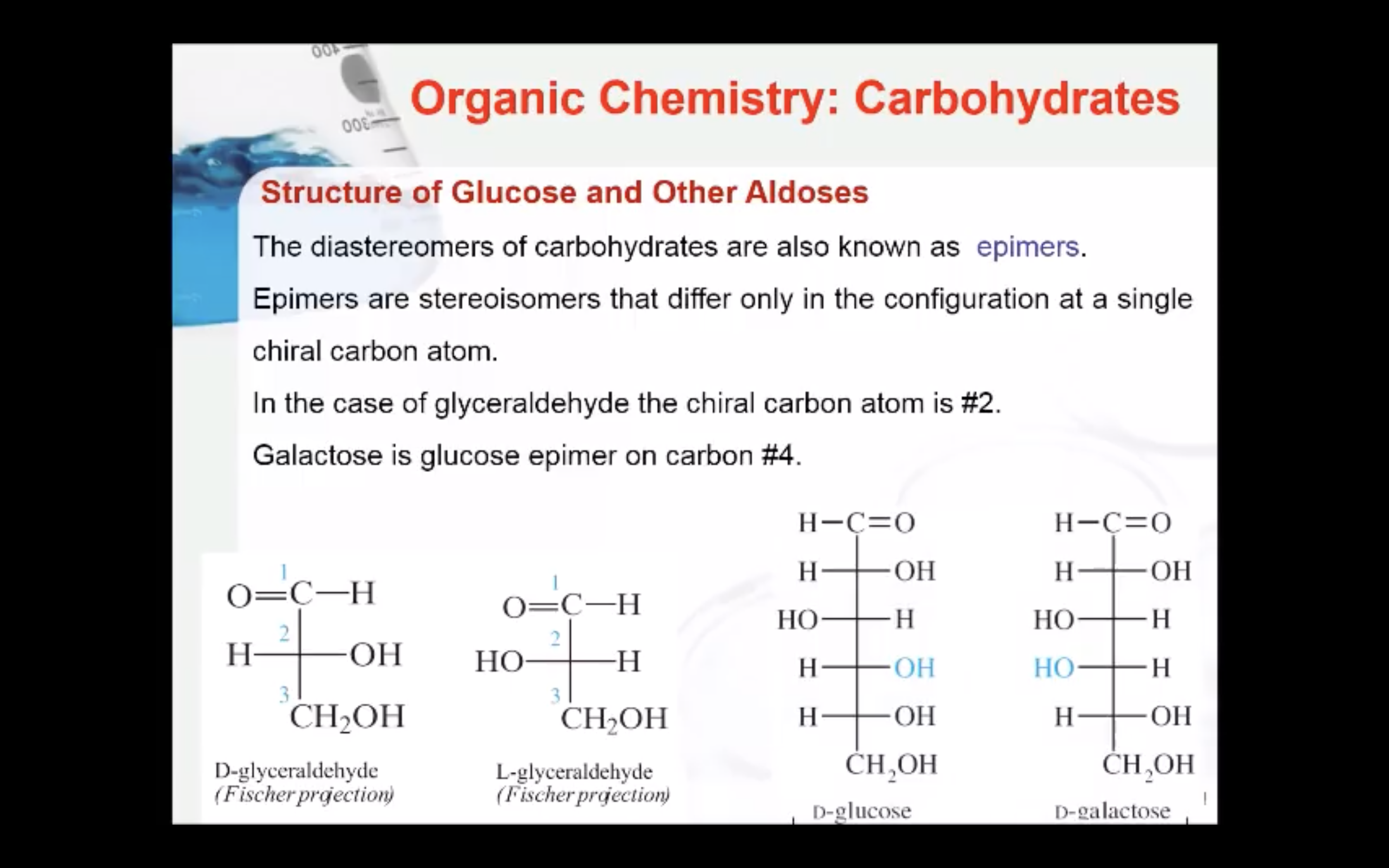
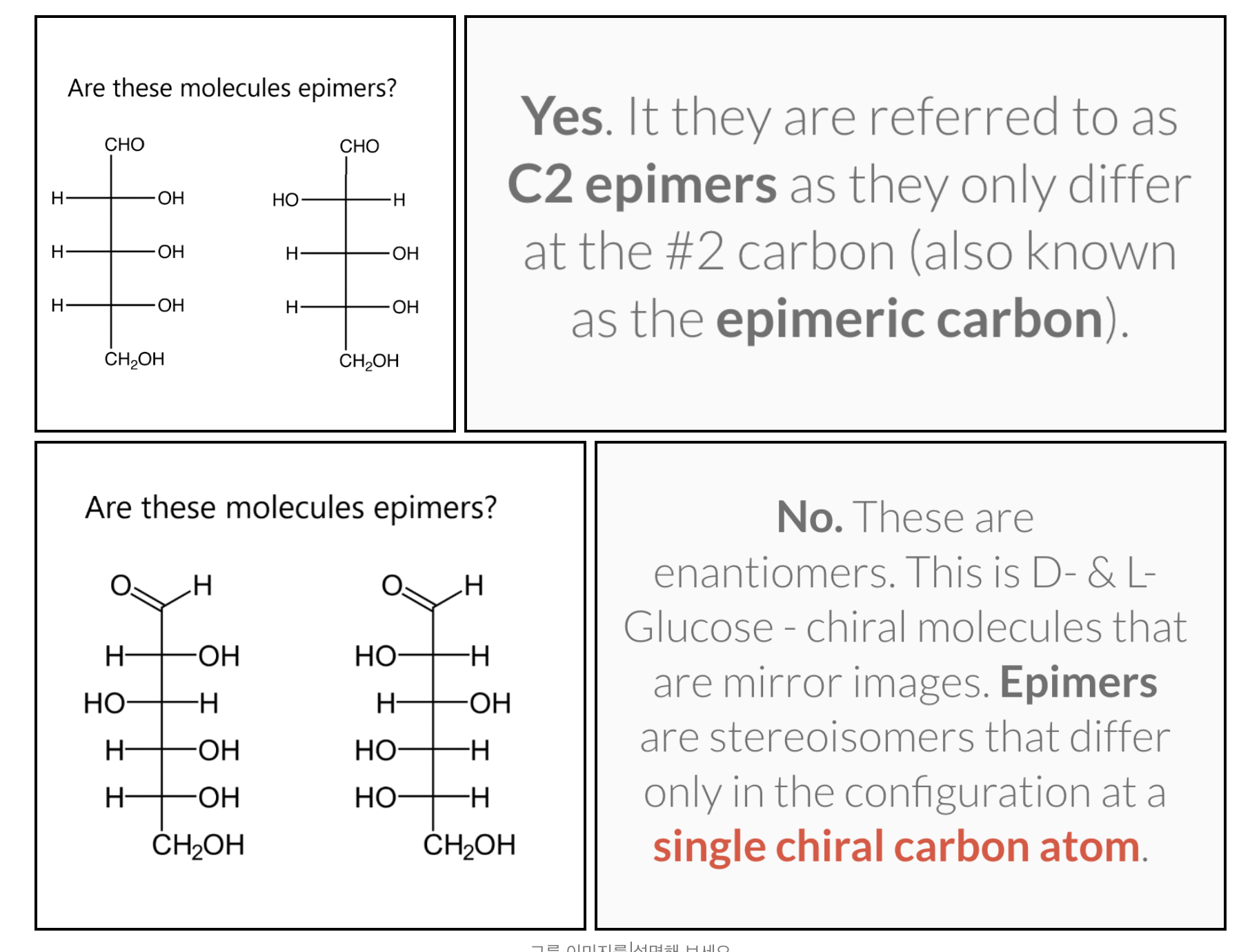
-The diastereomers of carbohydrates are also known as epimers
(Epimers are stereoisomers that differ only in the configuration at a single chiral carbon atom)
PART2
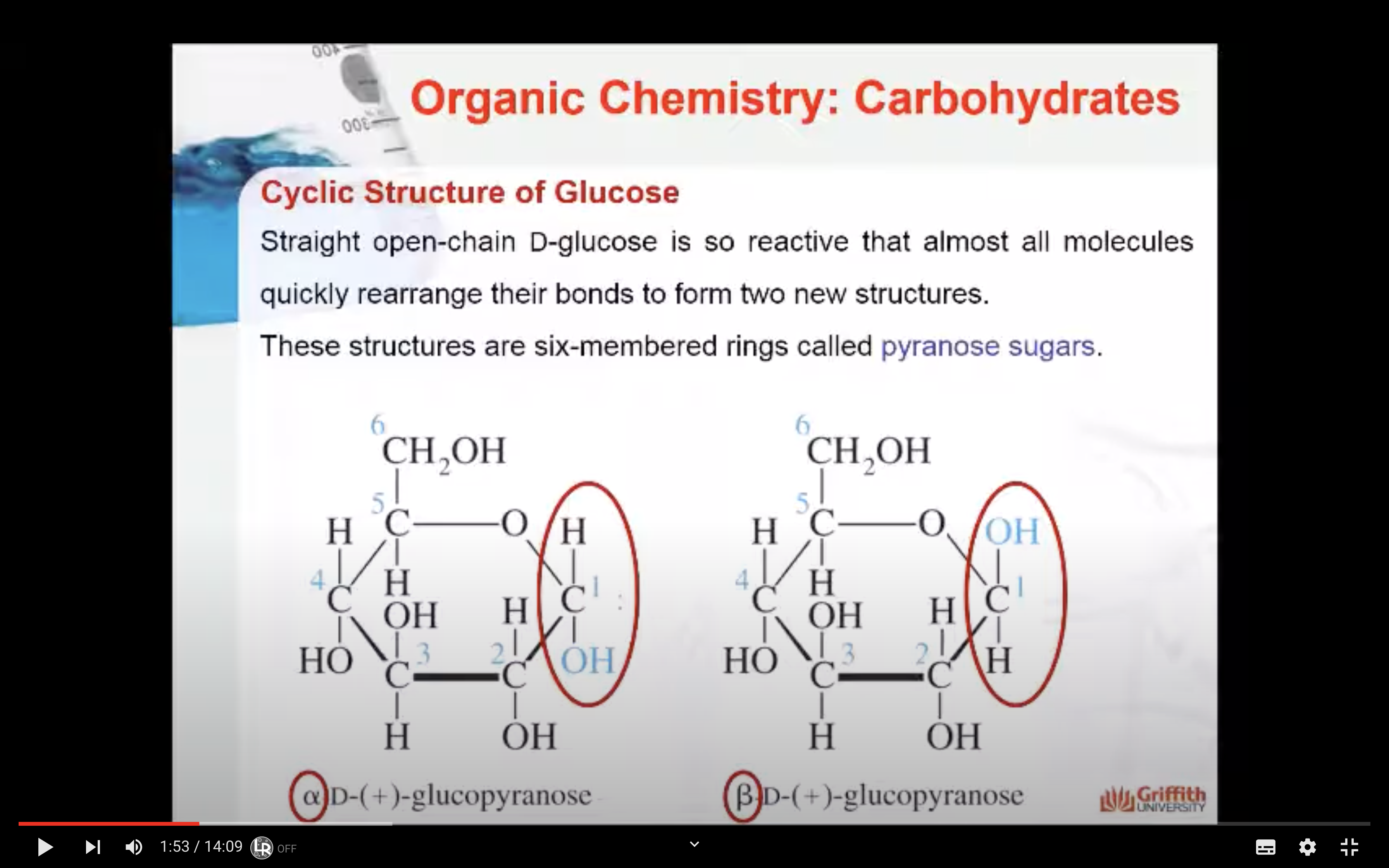
-Straight open-chain D-glucose is so reactive that almost all molecules quickly rearrange their bonds to from two new structures
-When OH attached to Carbon #1 is below -> alpha, OH attached to Carbon #2 is upward -> beta
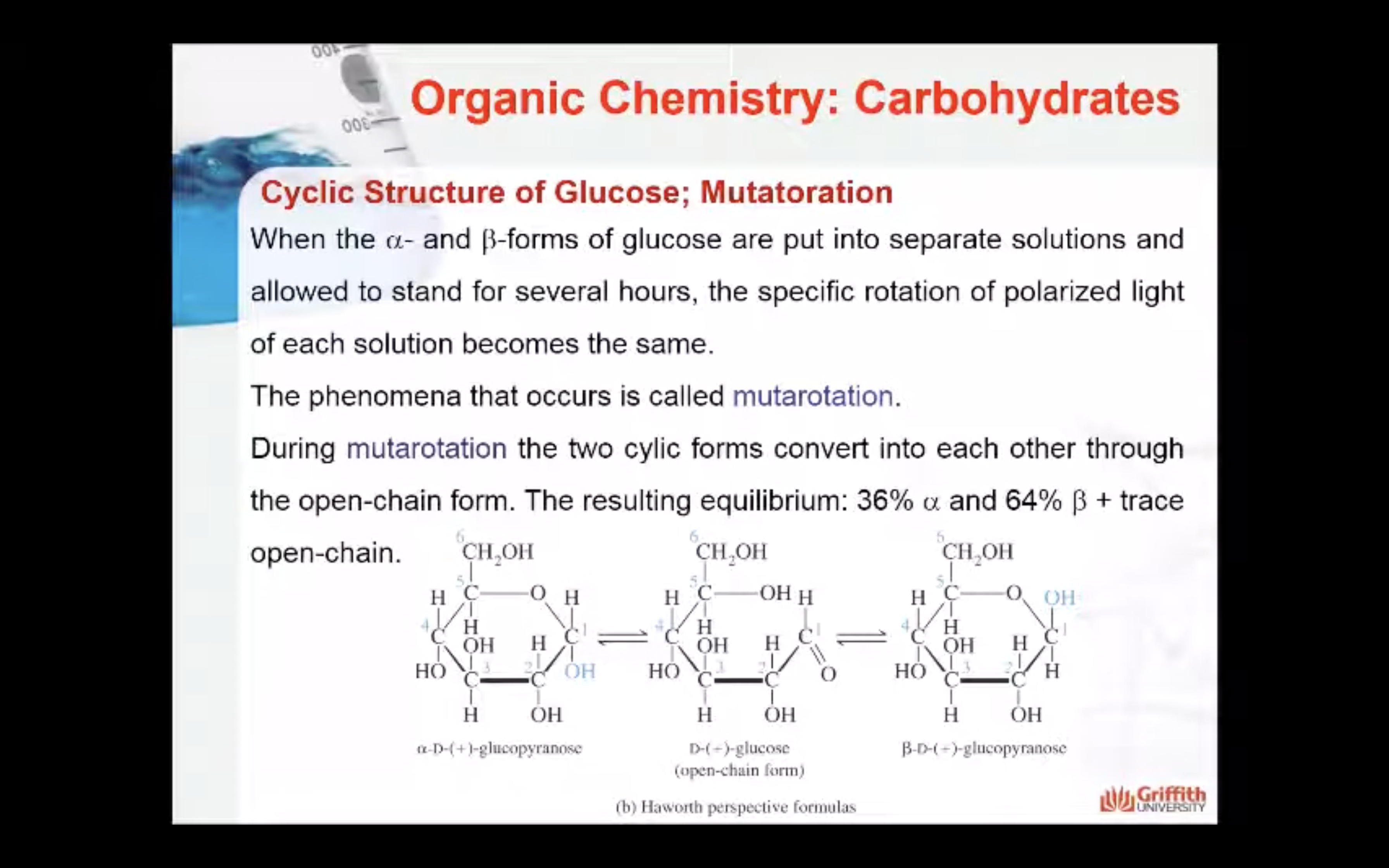
-Mutarotation : when the a- and b-forms of glucose are put into separate solutions, and the specific rotation of polarised light of each solution becomes the same
(Resulting equilibrium : 36% alpha, 64% beta)
-Below is drawn on RIGHT , Upward is drawn on LEFT
POLYSACCHRIDES
-Cellulose : used to construct cell walls in plants
=> b-1,4-glycosidic linkage , extensive hydrogen bondings
-Glycogen : energy storage in animals (liver and muscle tissues)
=>highly branched a-1,6-glycosidic linkages
-Starch : energy storage in plants
=> amylose (unbranded a-D-glucose joined by a-1,4-glycosidic linkage)
=> amylopectin (branched chain composed of a-1,4-glycosidic linkages mainchain and a-1,6-glycosidic linkage at branch points)
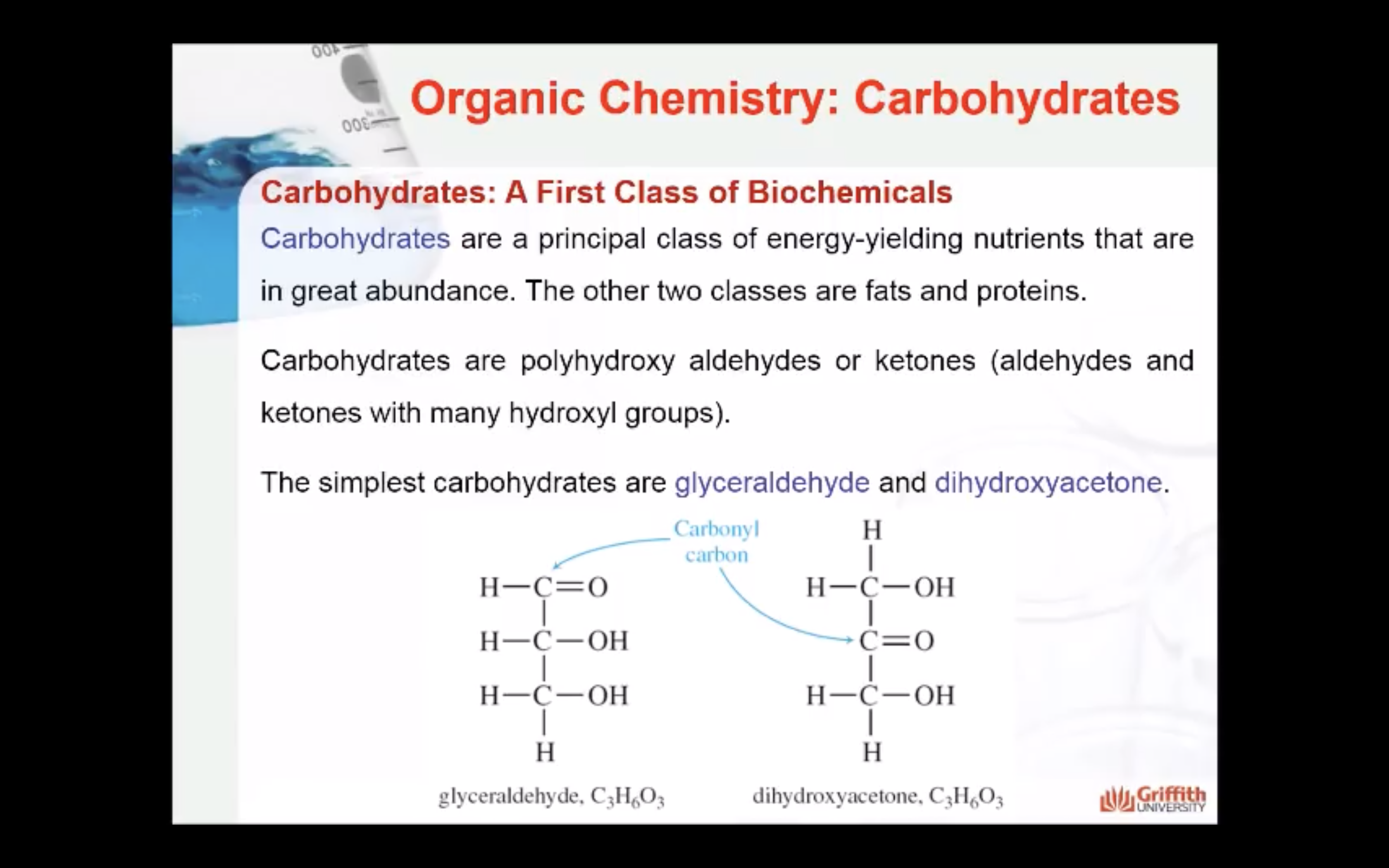
Carbohydrates: A First Class of Biochemicals
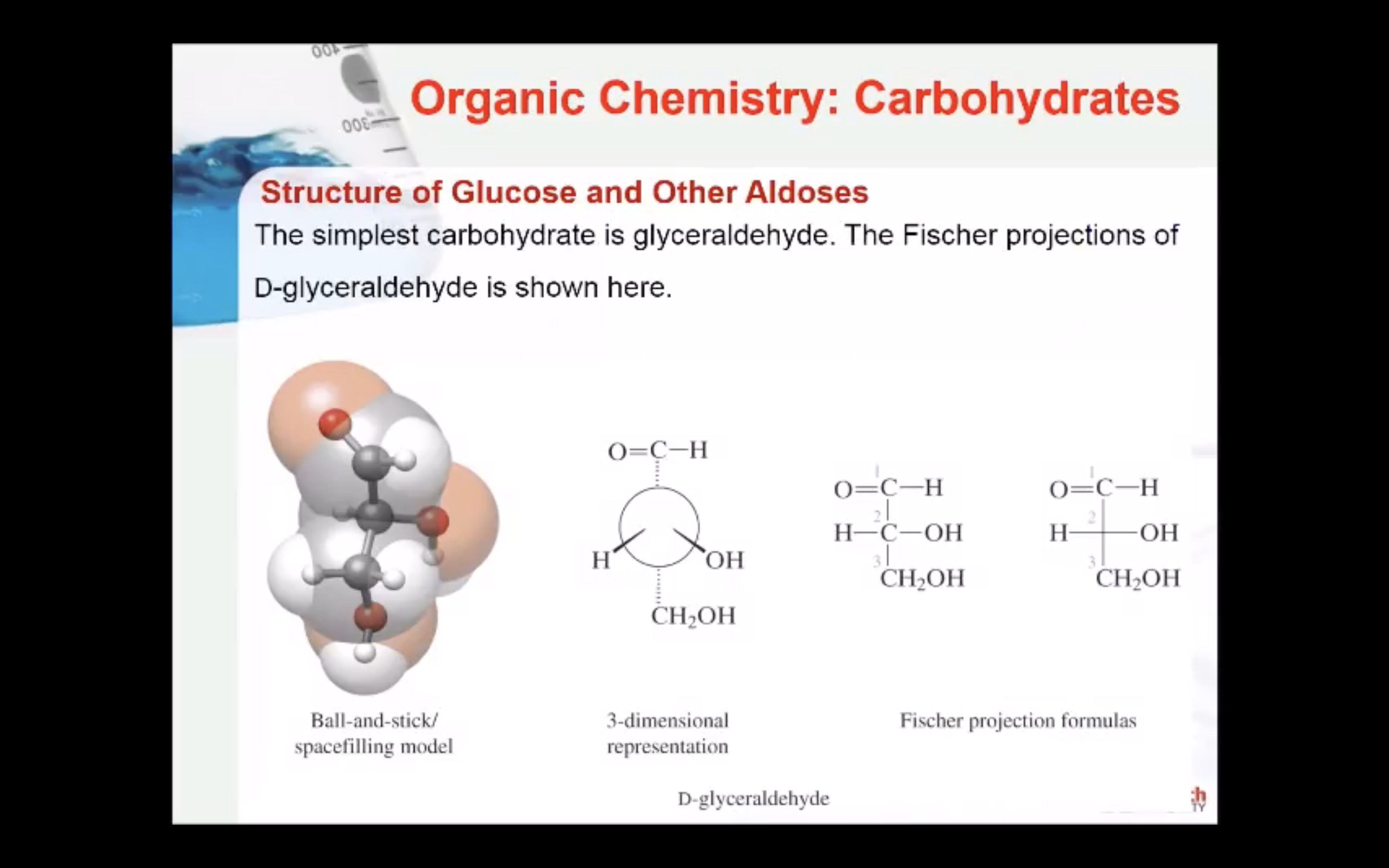
Carbohydrates are a principal class of energy–yielding nutrients that are in great abundance. Carbohydrates are polyhydroxy aldehydes or ketones (aldehydes and ketones with many hydroxyl groups). The simplest carbohydrates are glyceraldehyde and dihydroxyacetone.

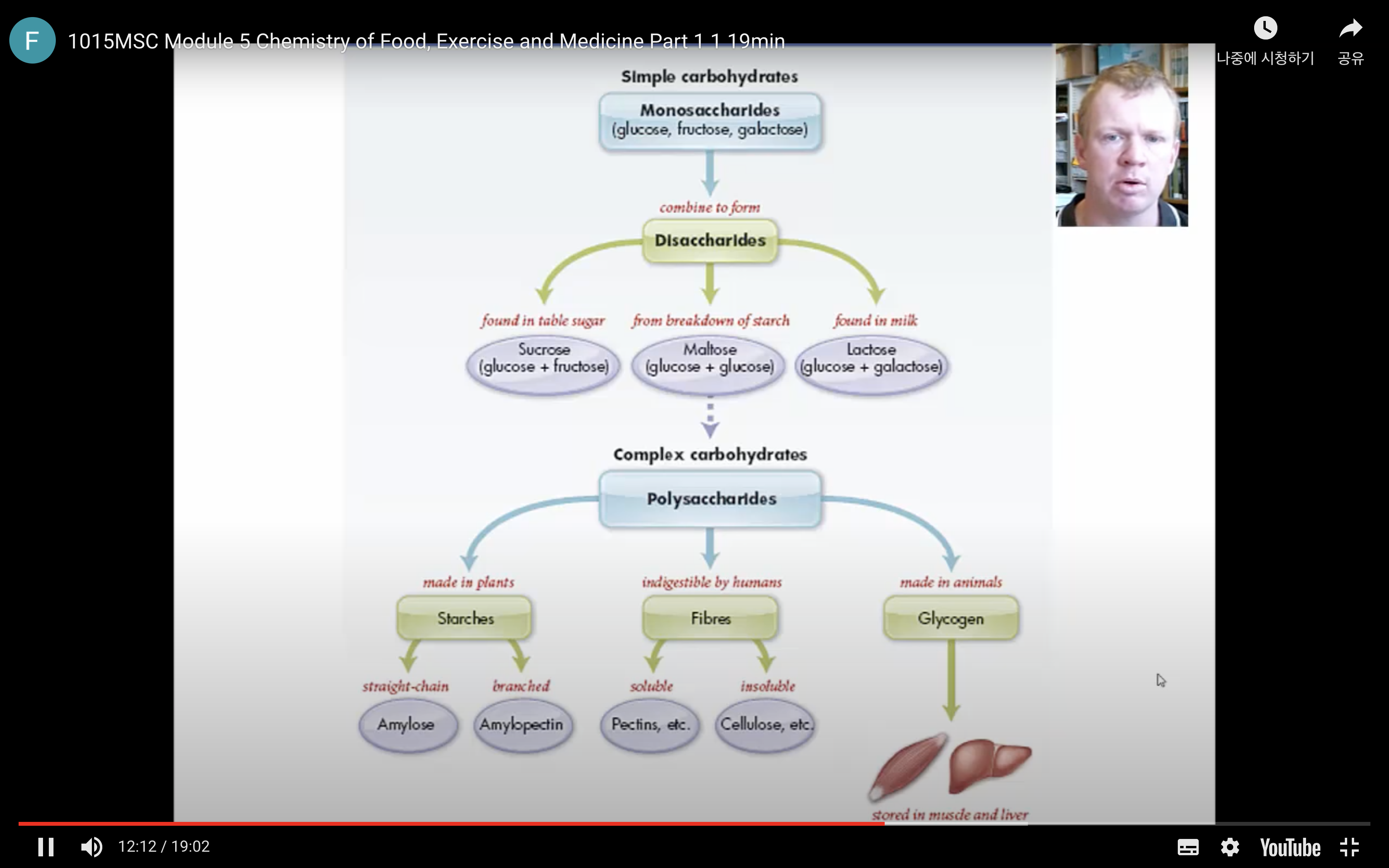
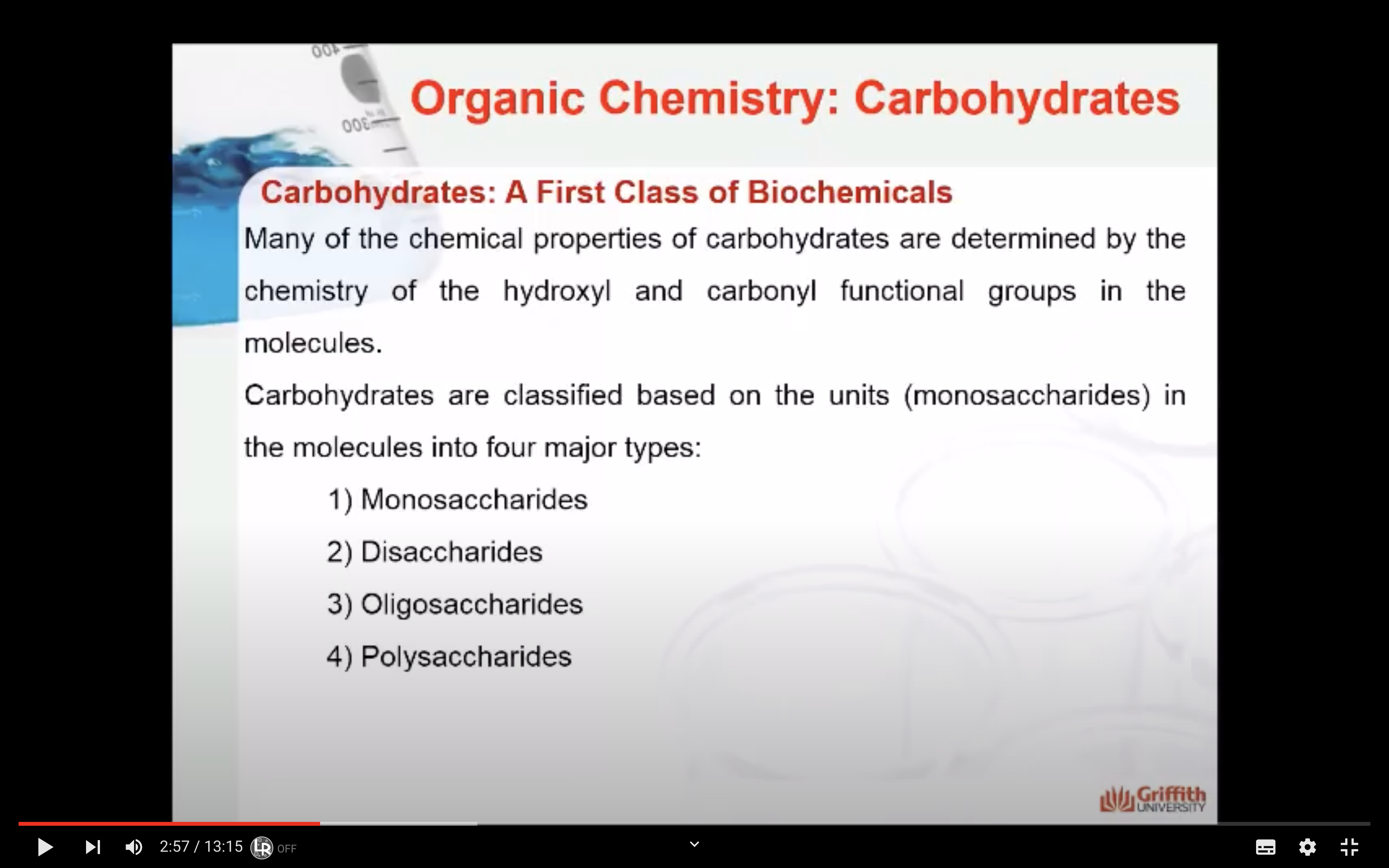
Many of the chemical properties of carbohydrates are determined by the chemistry of the hydroxyl and carbonyl functional groups in the molecules. The four major types of carbohydrates are:
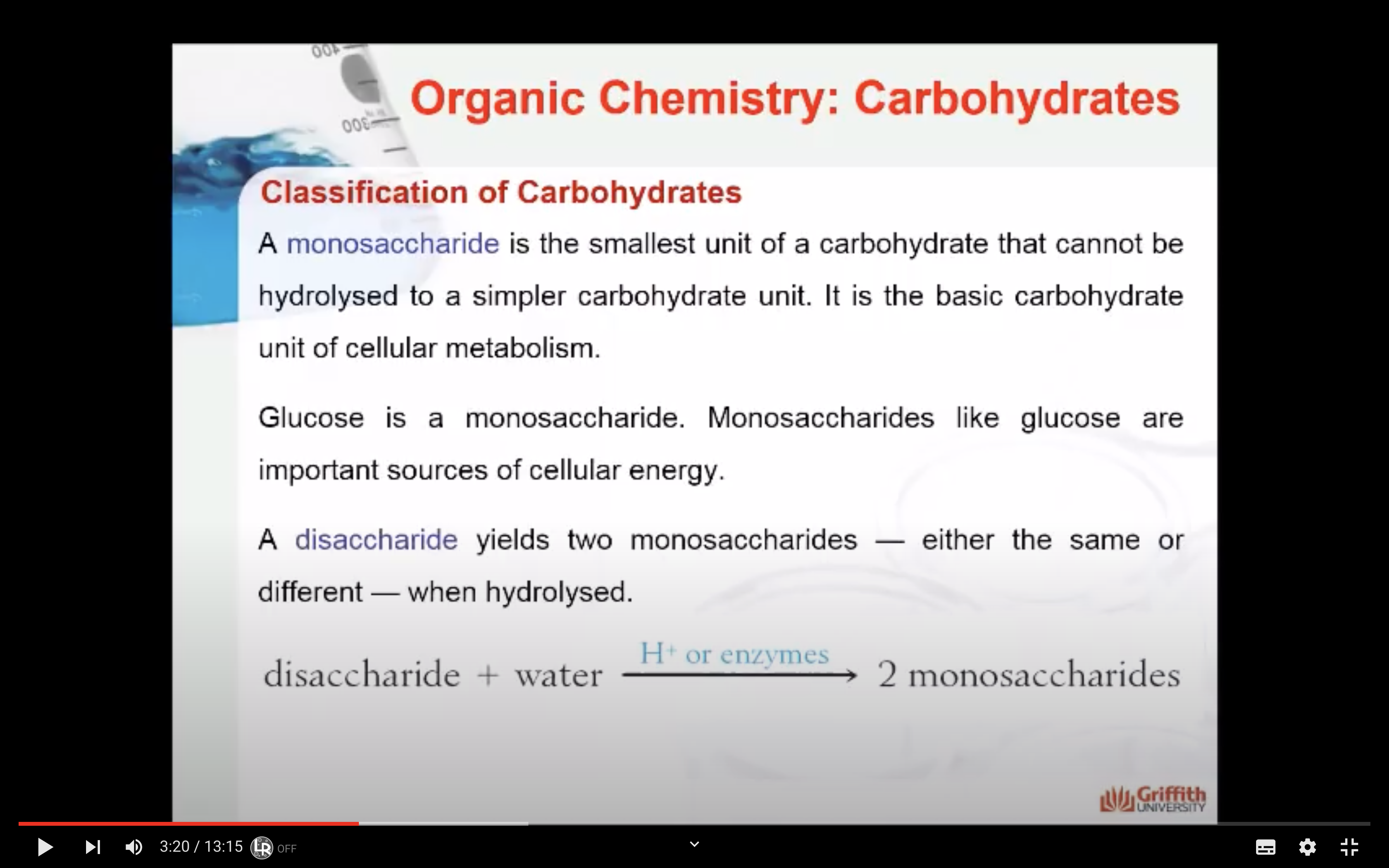
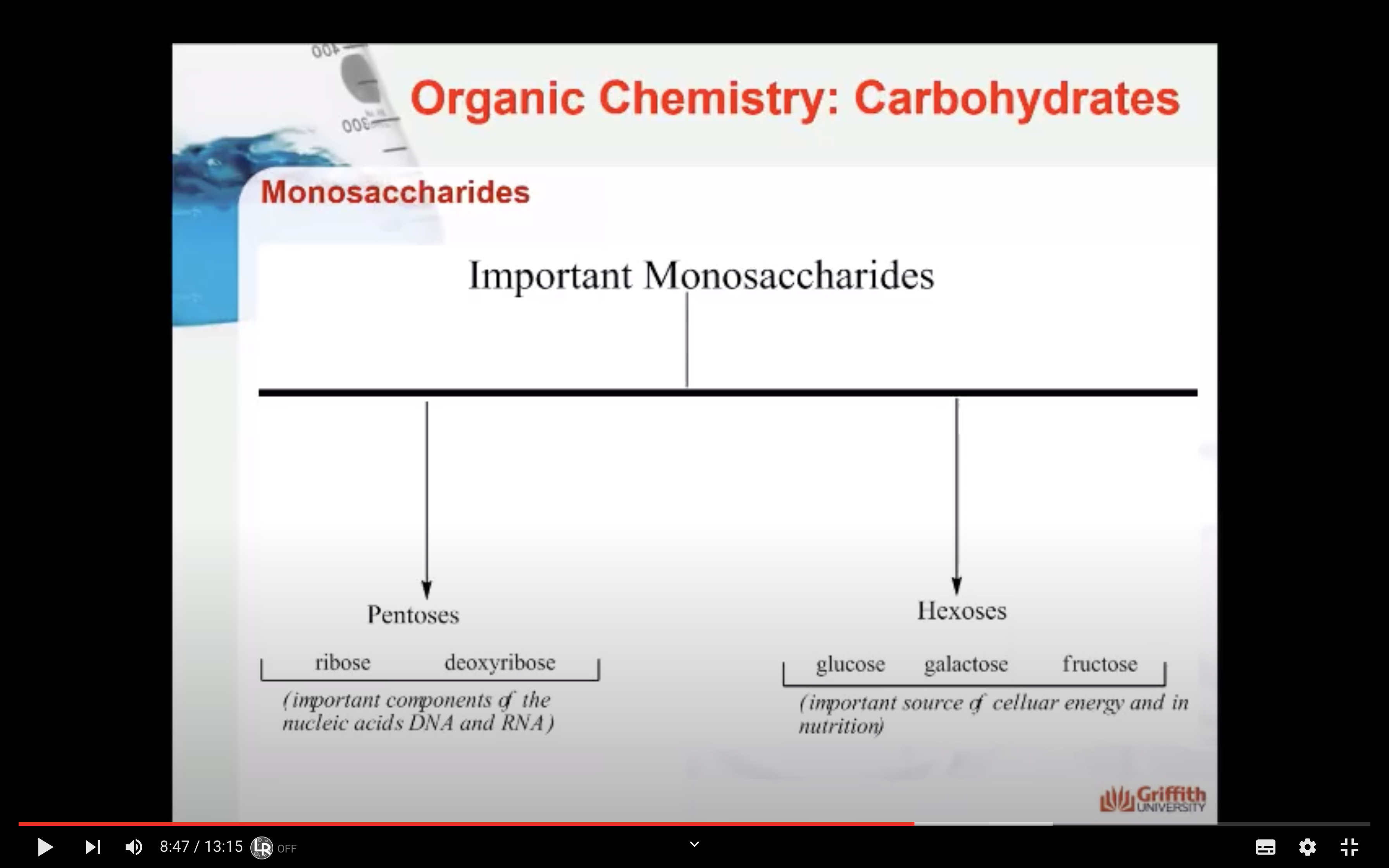
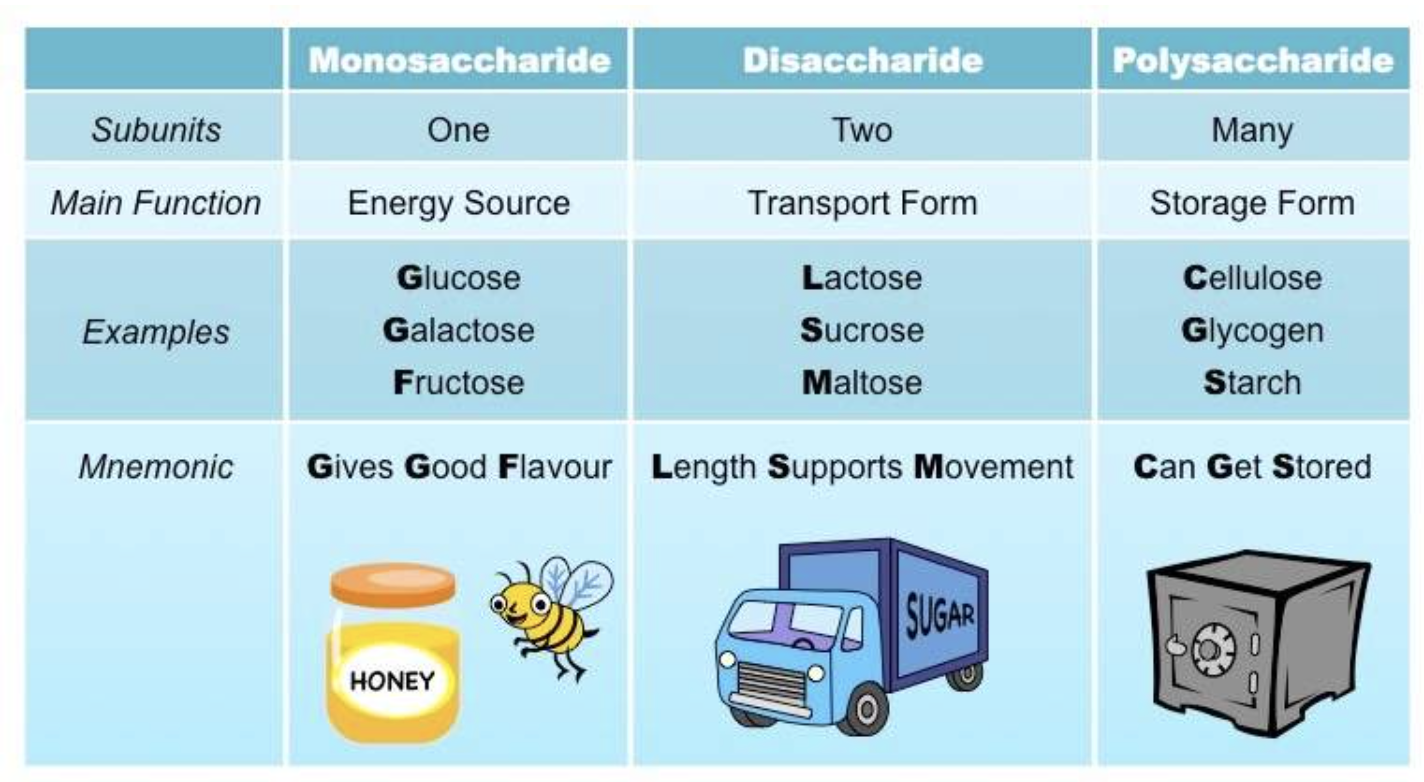
- Monosaccharides: the smallest unit of a carbohydrate that cannot be hydrolysed to a simpler carbohydrate unit.
- Disaccharides: yields two monosaccharides — either the same or different — when hydrolysed.
- Oligosaccharides: is a carbohydrate with at least two but not more than nine monosaccharide units linked together.
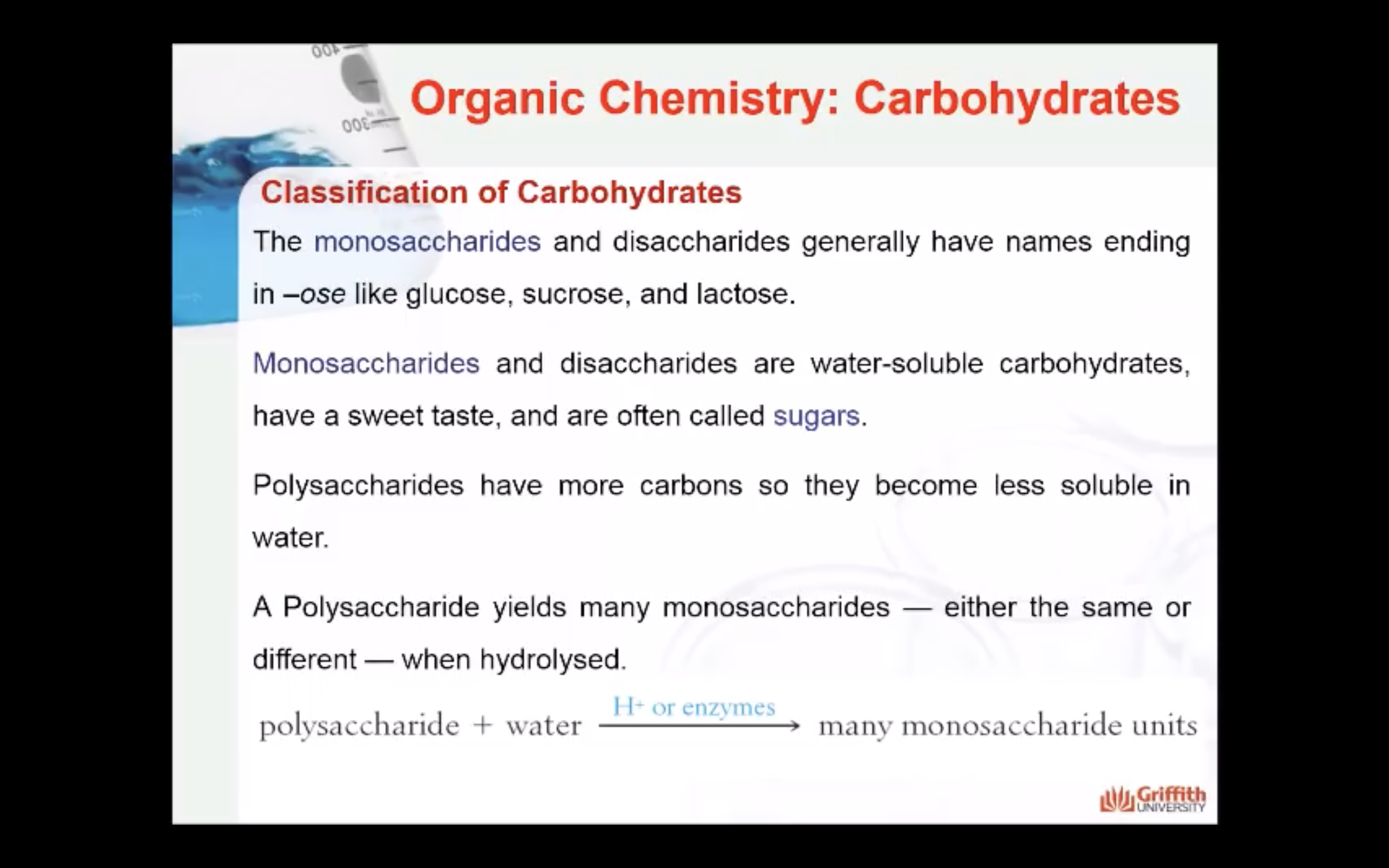
- Polysaccharides: is a macromolecular substance that can be hydrolysed to yield many monosaccharide units.
These classifications are based on the units (monosaccharides) in the molecules.
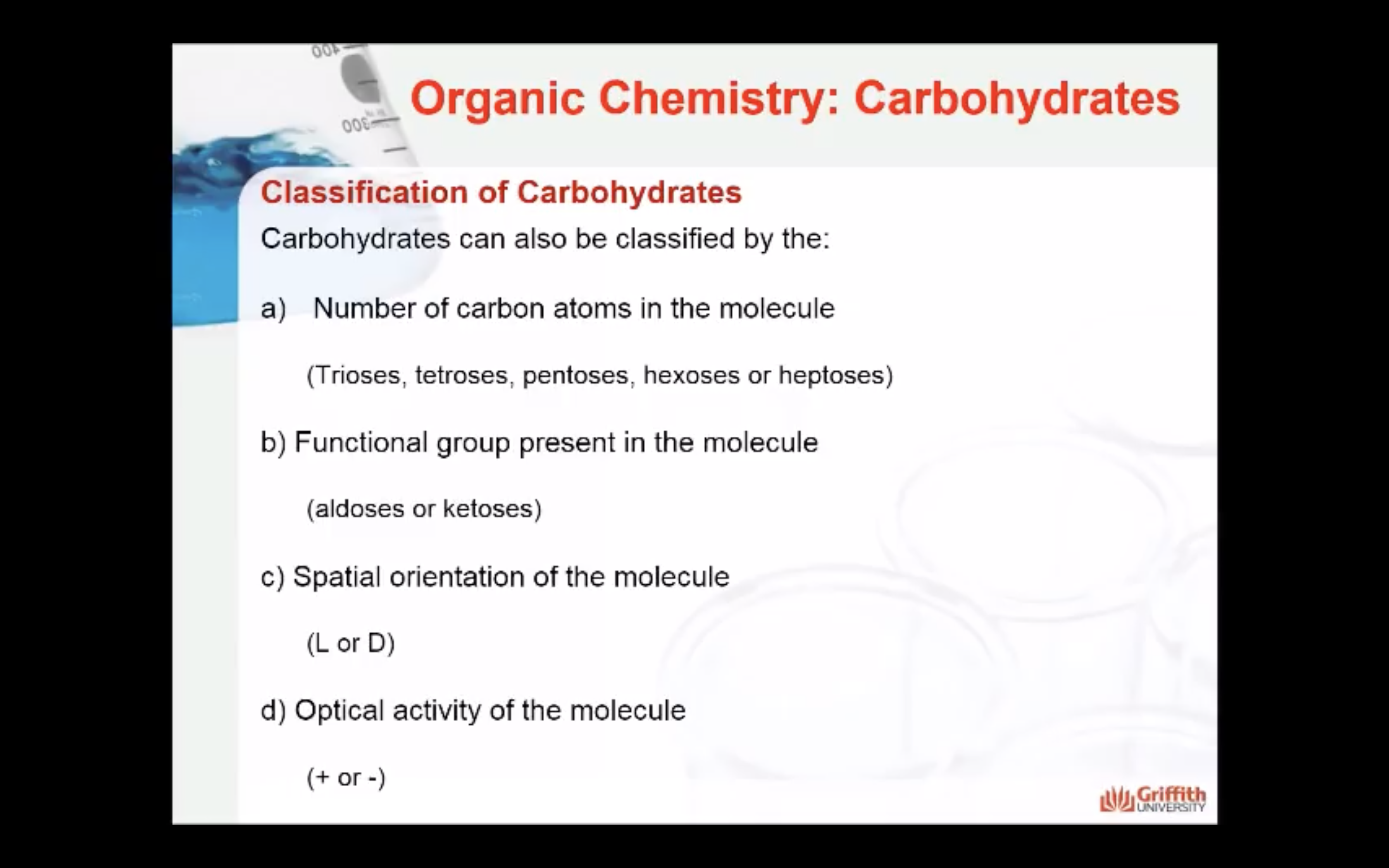
Carbohydrates can also be classified by the:
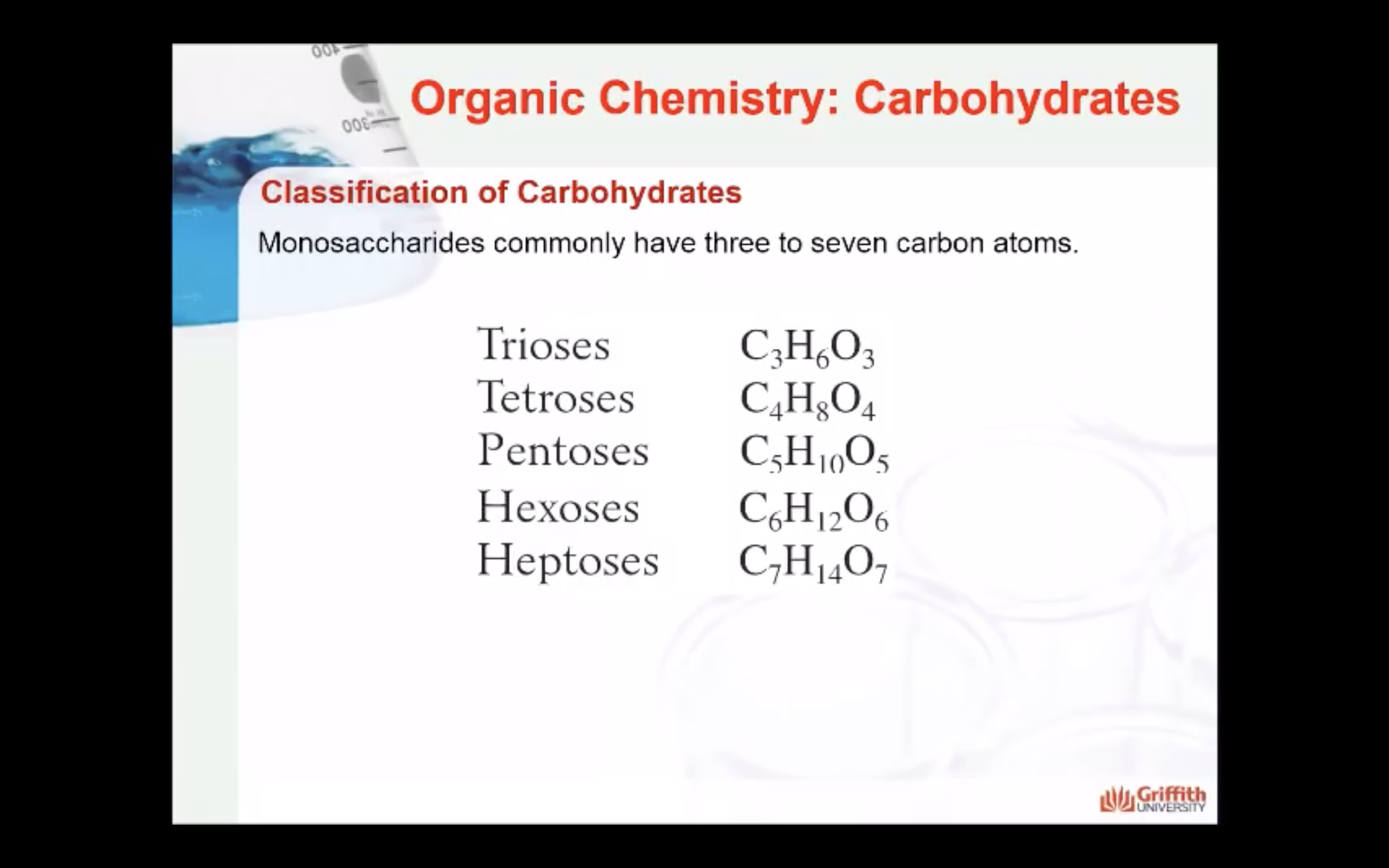
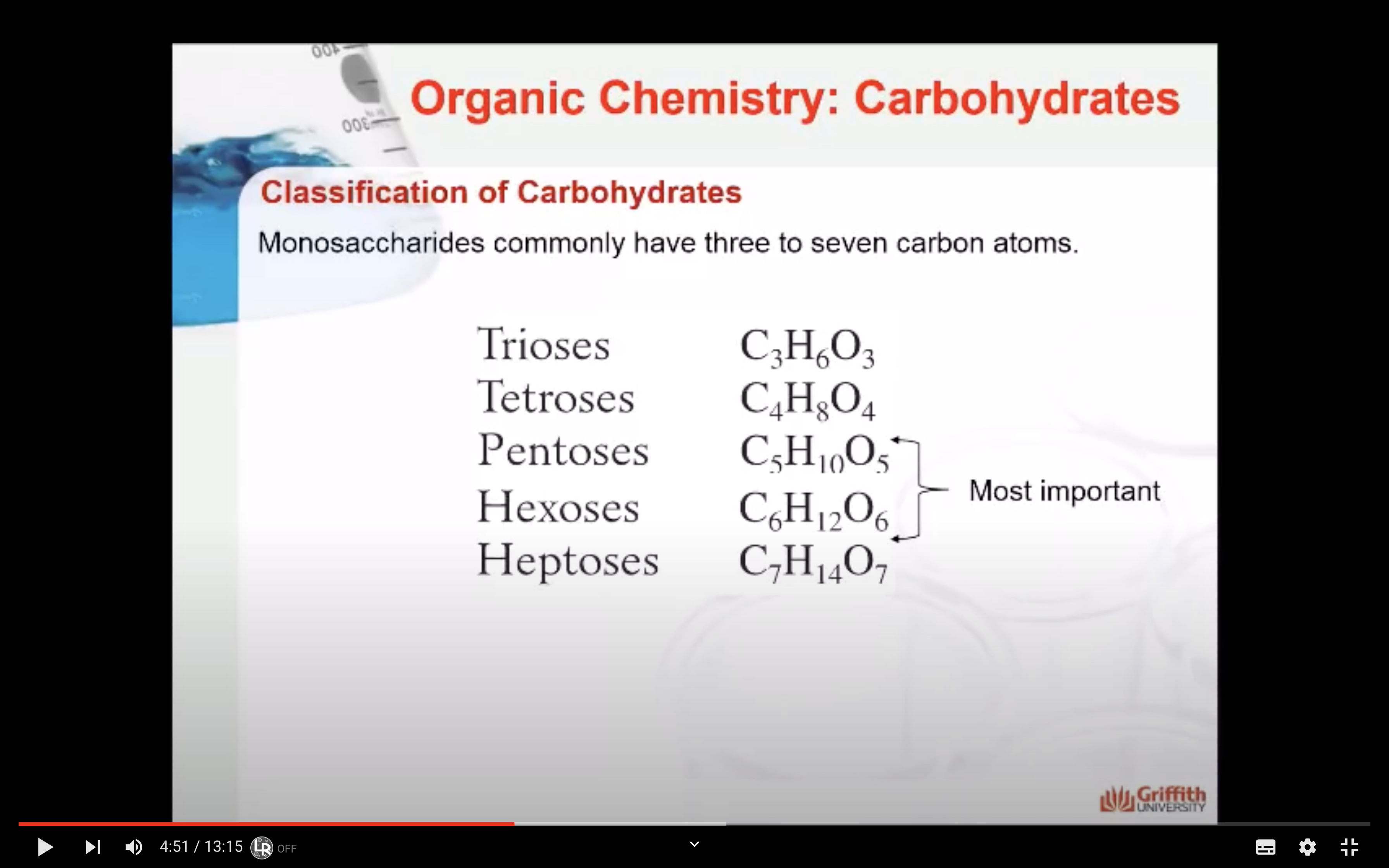
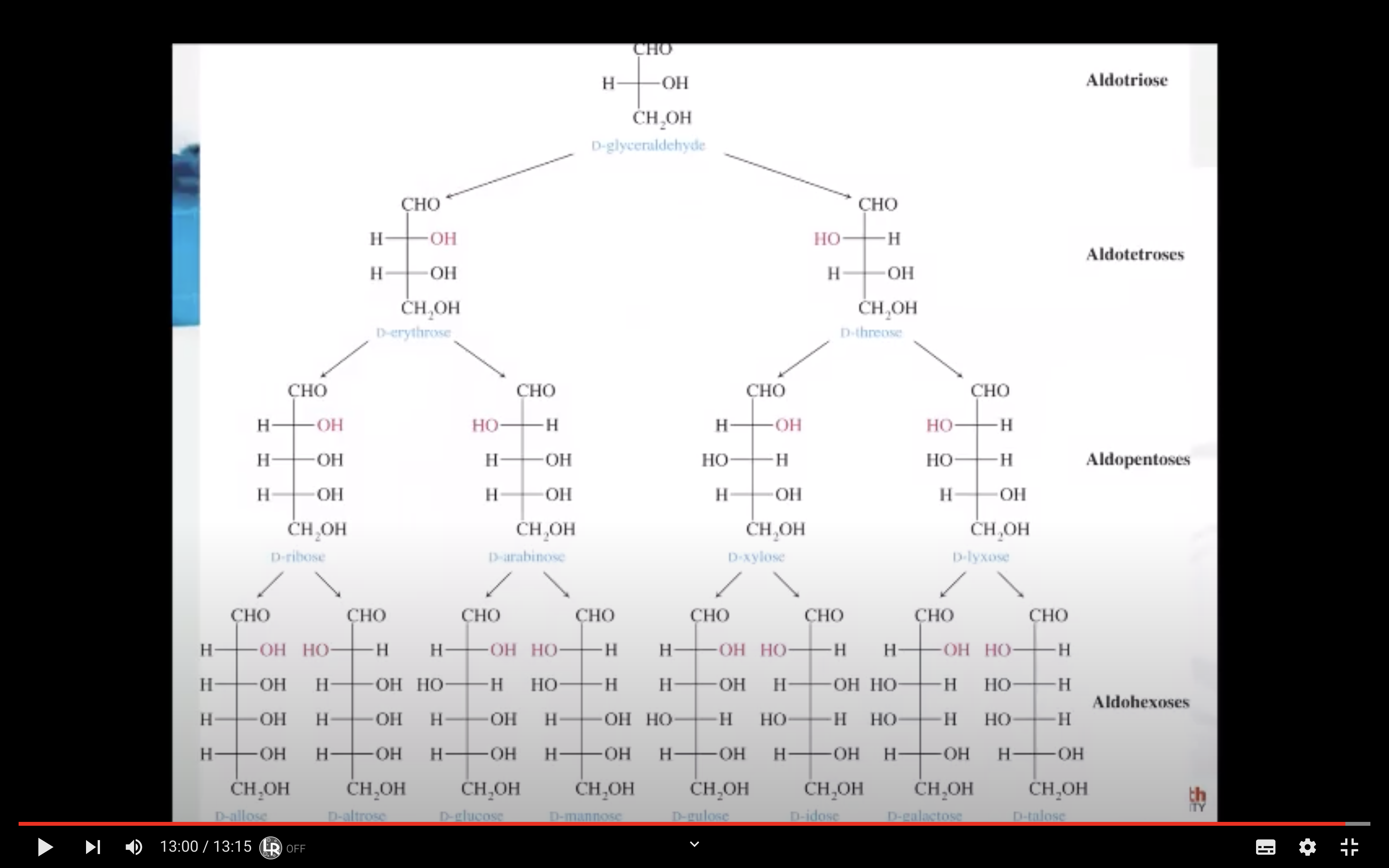
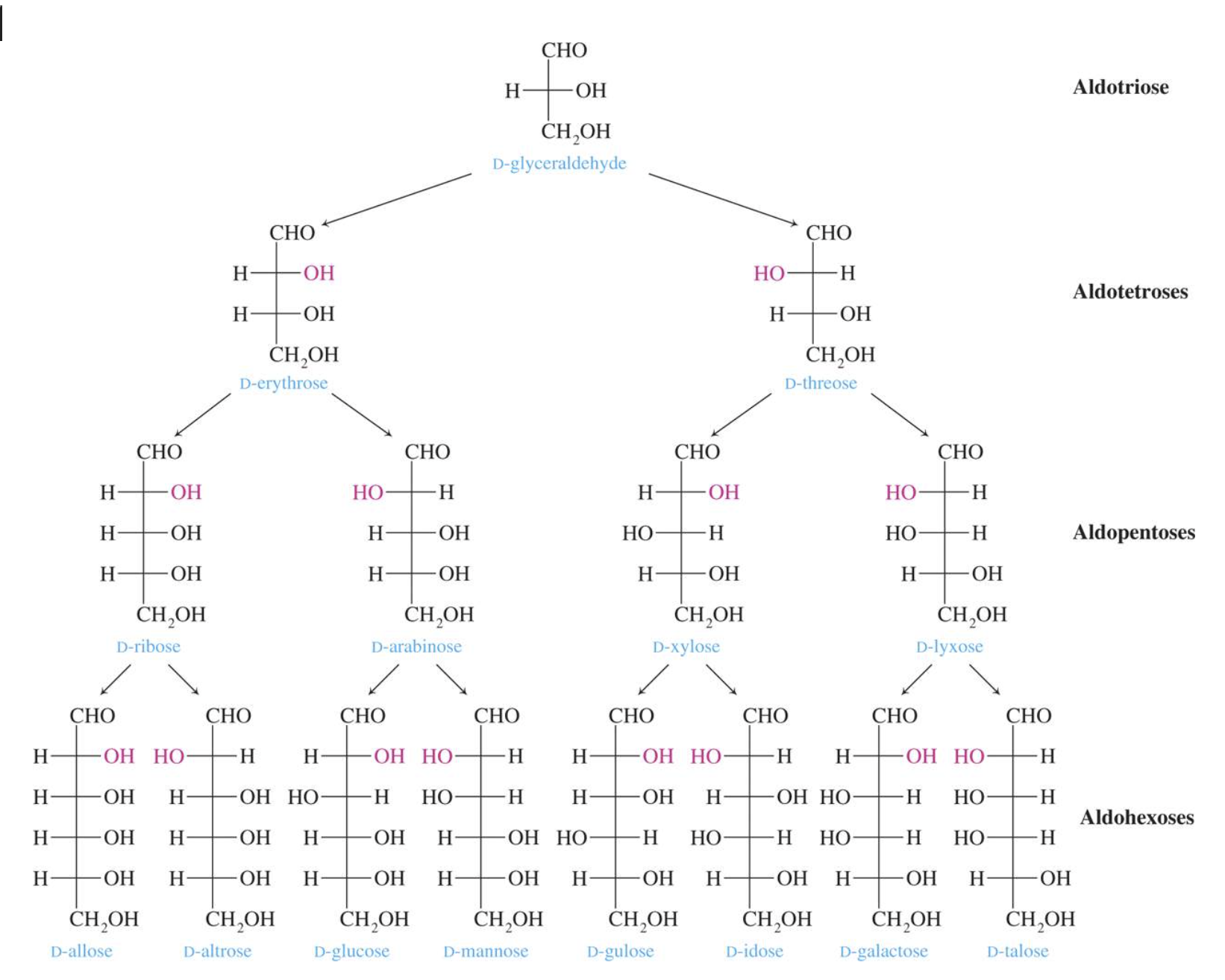
- Number of carbon atoms in the molecule: Monosaccharides commonly have three to seven carbon atoms. Some examples: Trioses C3H6O3, Tetroses C4H8O4, Pentoses C5H10O4, Hexoses C6H12O6
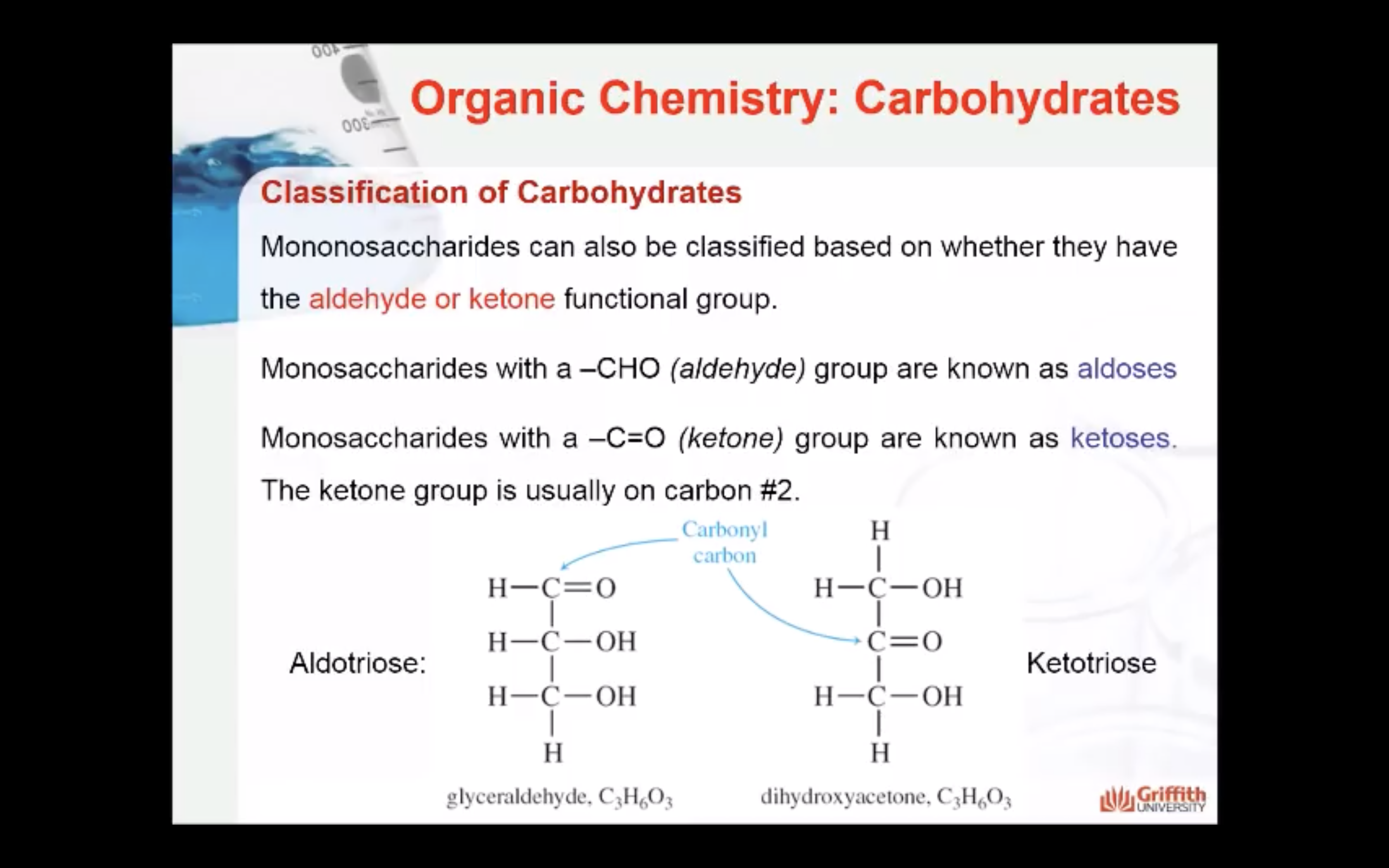
- Functional group present in the molecule: based on whether they have the aldehyde or ketone functional group. Monosaccharides with a –CHO (aldehyde) group are known as aldoses while those with a –C=O (ketone) group are known as ketoses. The ketone group is usually on carbon #2.
- Spatial orientation of the molecule (stereochemistry): explained next.
- Optical activity of the molecule: Monosaccharides that rotate plane–polarised light to the right are known as (+) isomers while those that rotate light to the left are (−) isomers. This classification can only be determined experimentally.
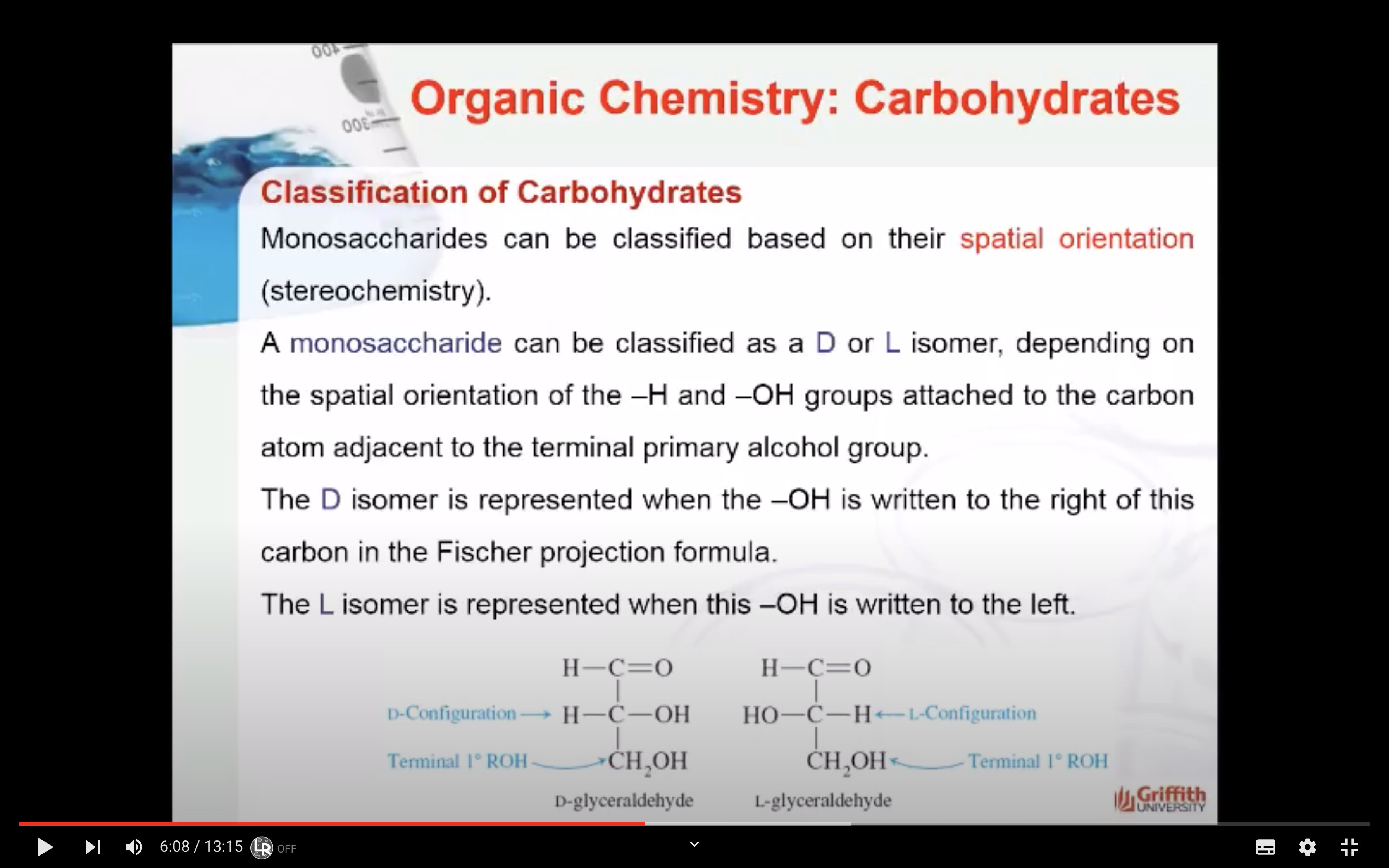
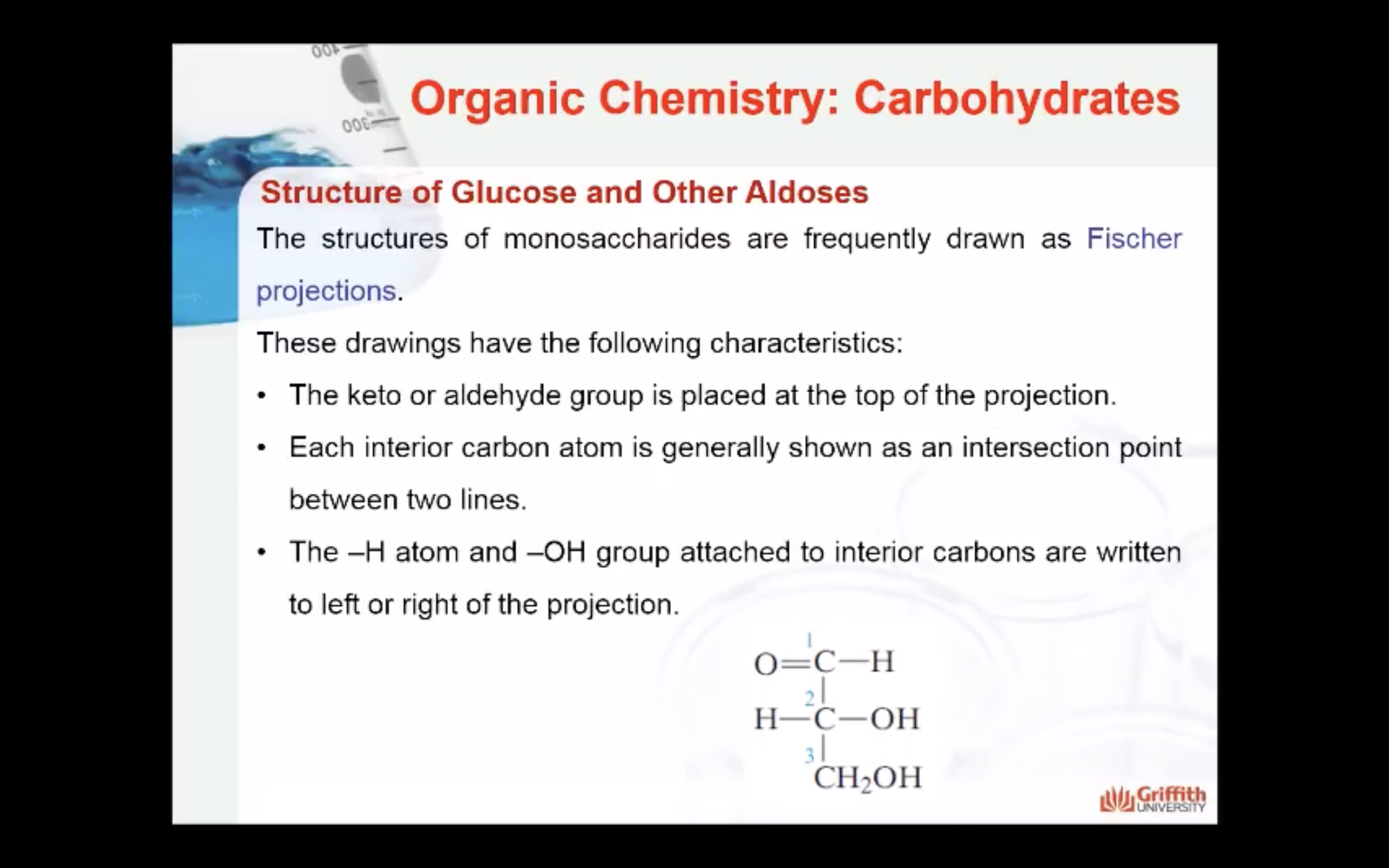
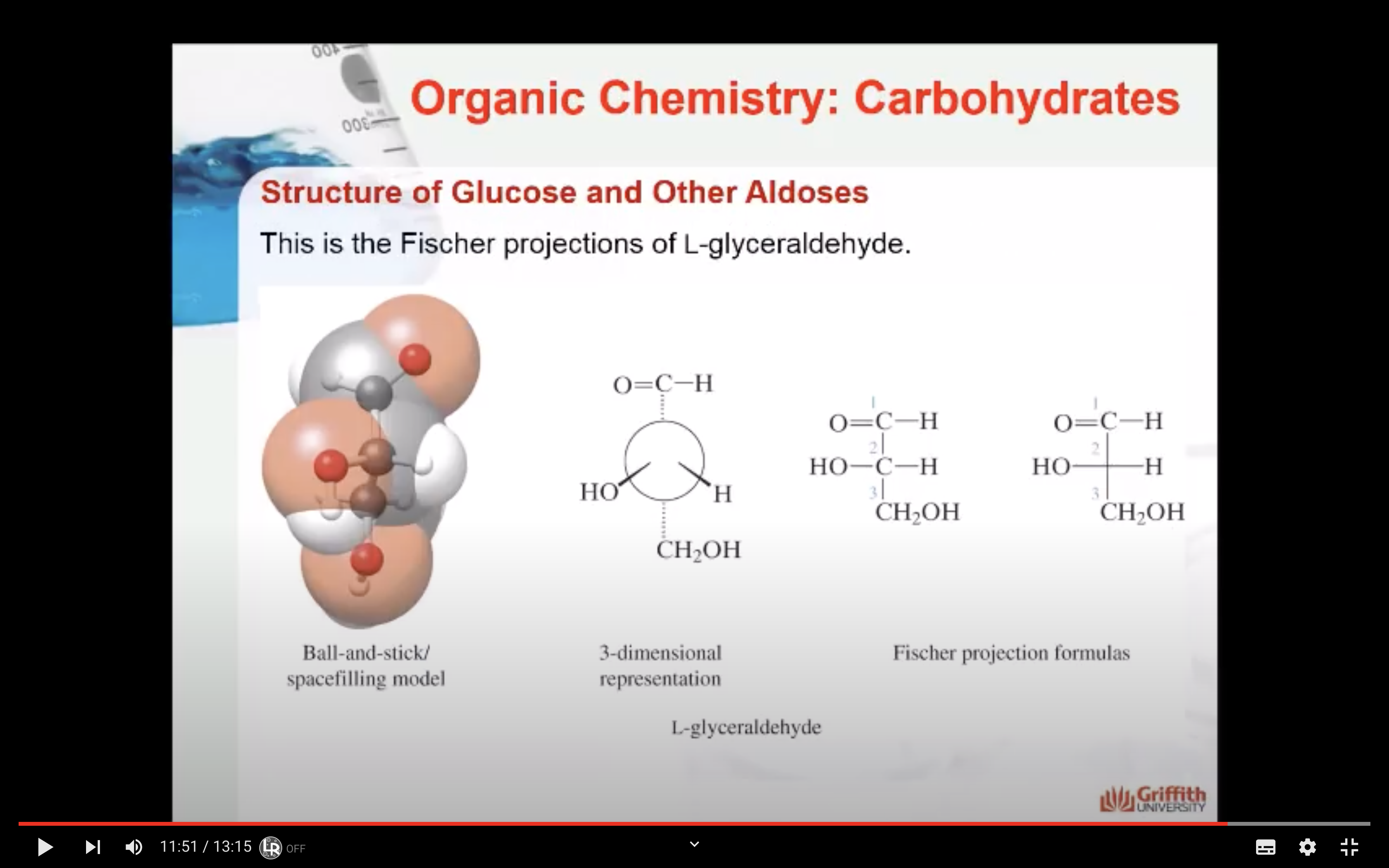
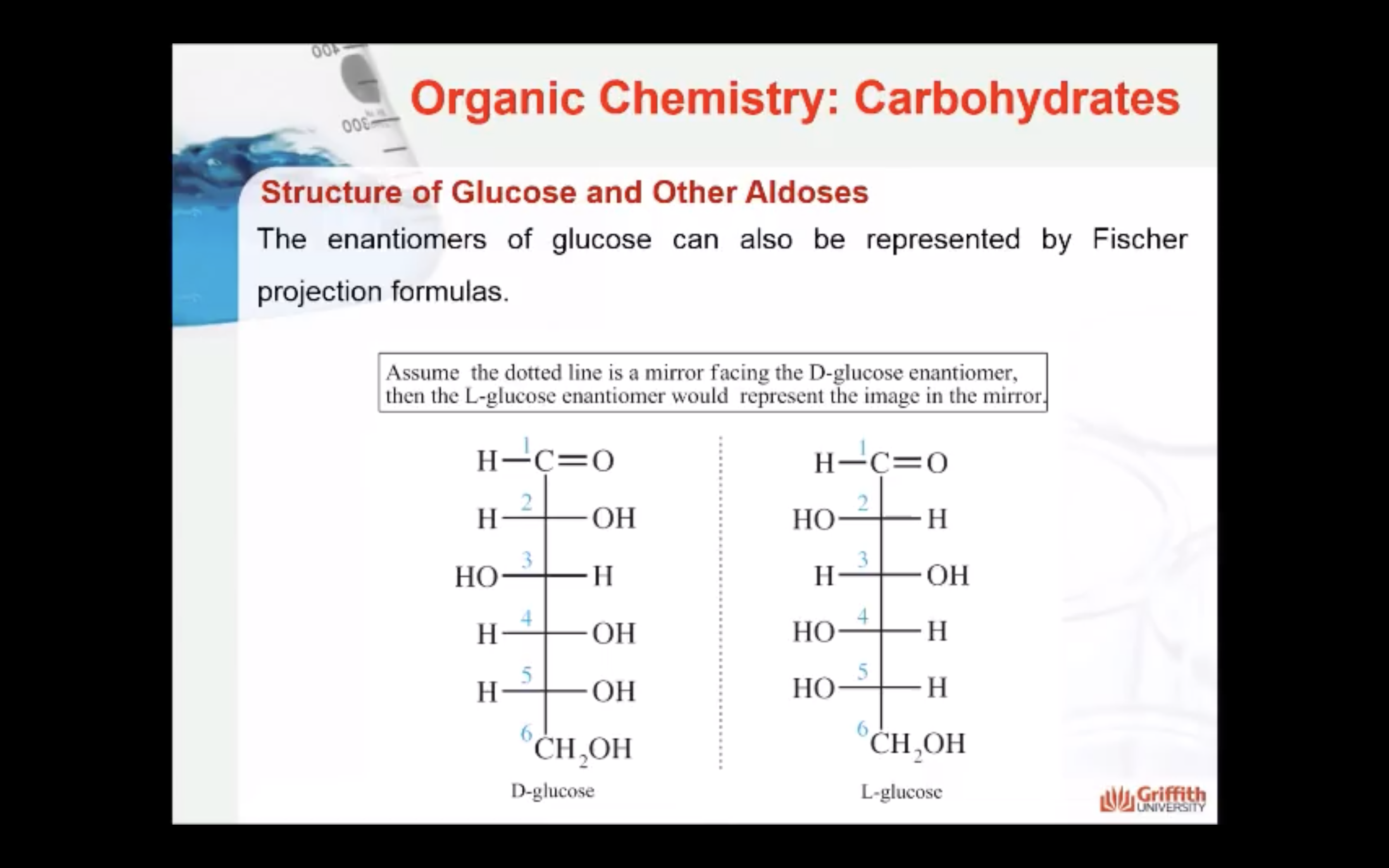
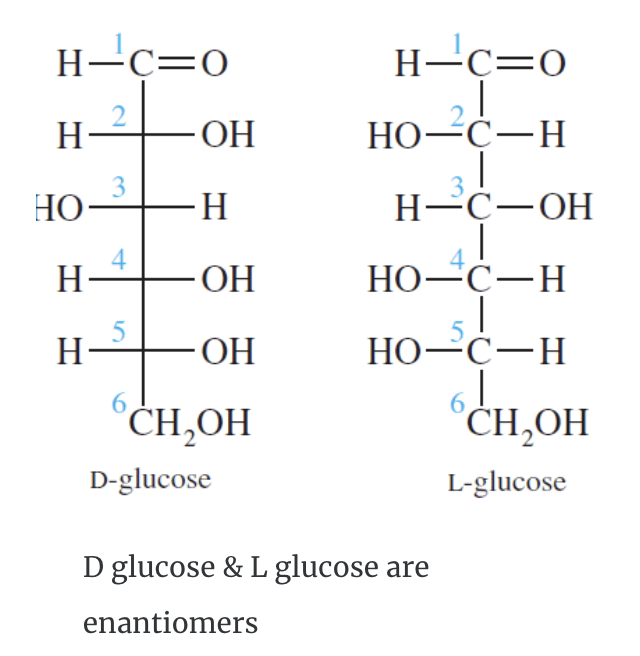
A monosaccharide can be classified as a D or L isomer, depending on the spatial orientation of the –H and –OH groups attached to the carbon atom adjacent to the terminal primary alcohol group. The D isomer is represented when the –OH is written to the right of this carbon in the Fischer projection formula. The L isomer is represented when this –OH is written to the left.
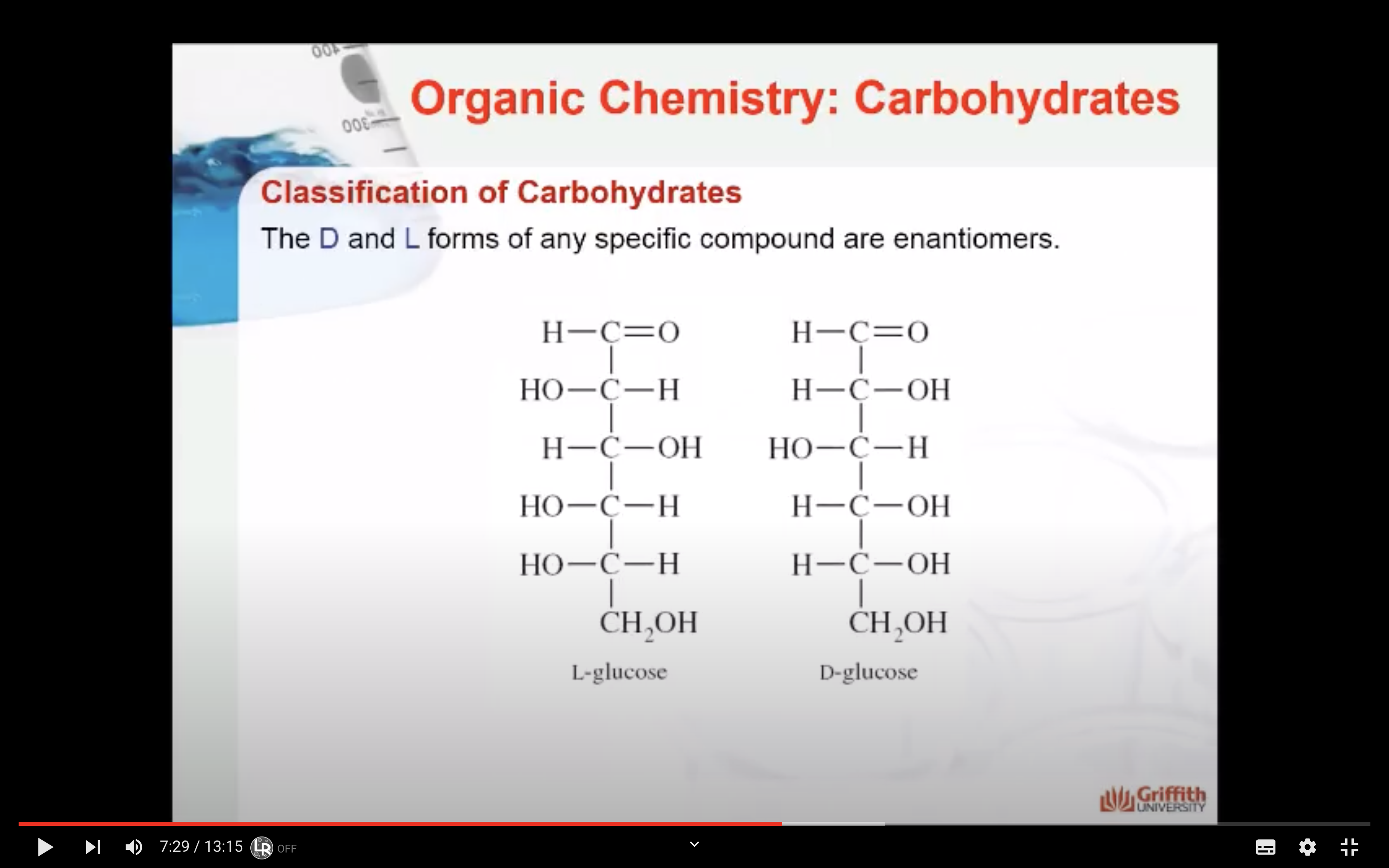
The D and L forms of any specific compound are enantiomers. Example: D–glucose and L–glucose are enantiomers (mirror image).
Take a close look at the diagram to the right to understand what you just read.

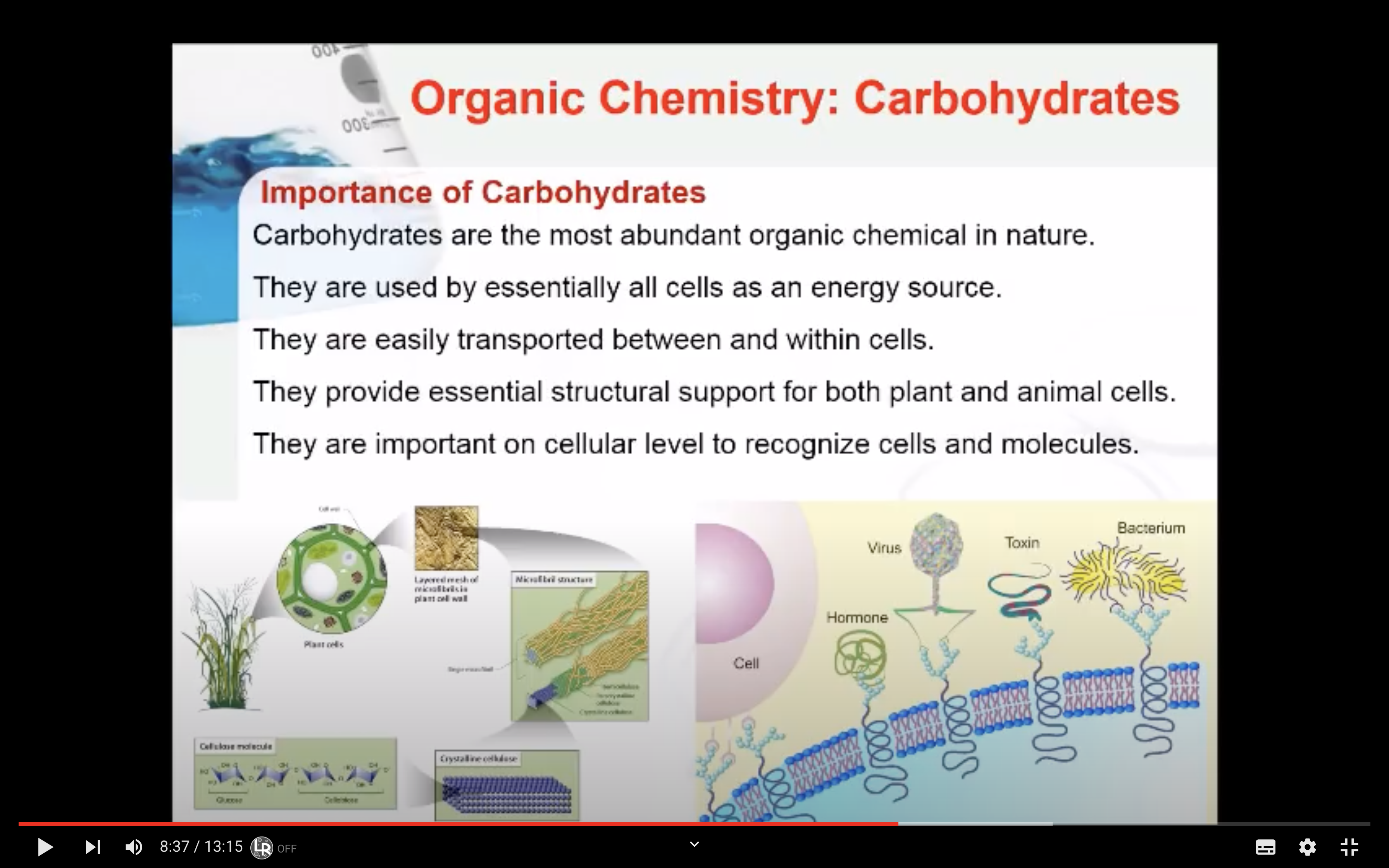
Carbohydrates are the most abundant organic chemical in nature. Why are they so important in biochemistry? They are used by essentially all cells as an energy source. They are easily transported between and within cells. They provide essential structural support for both plant and animal cells.
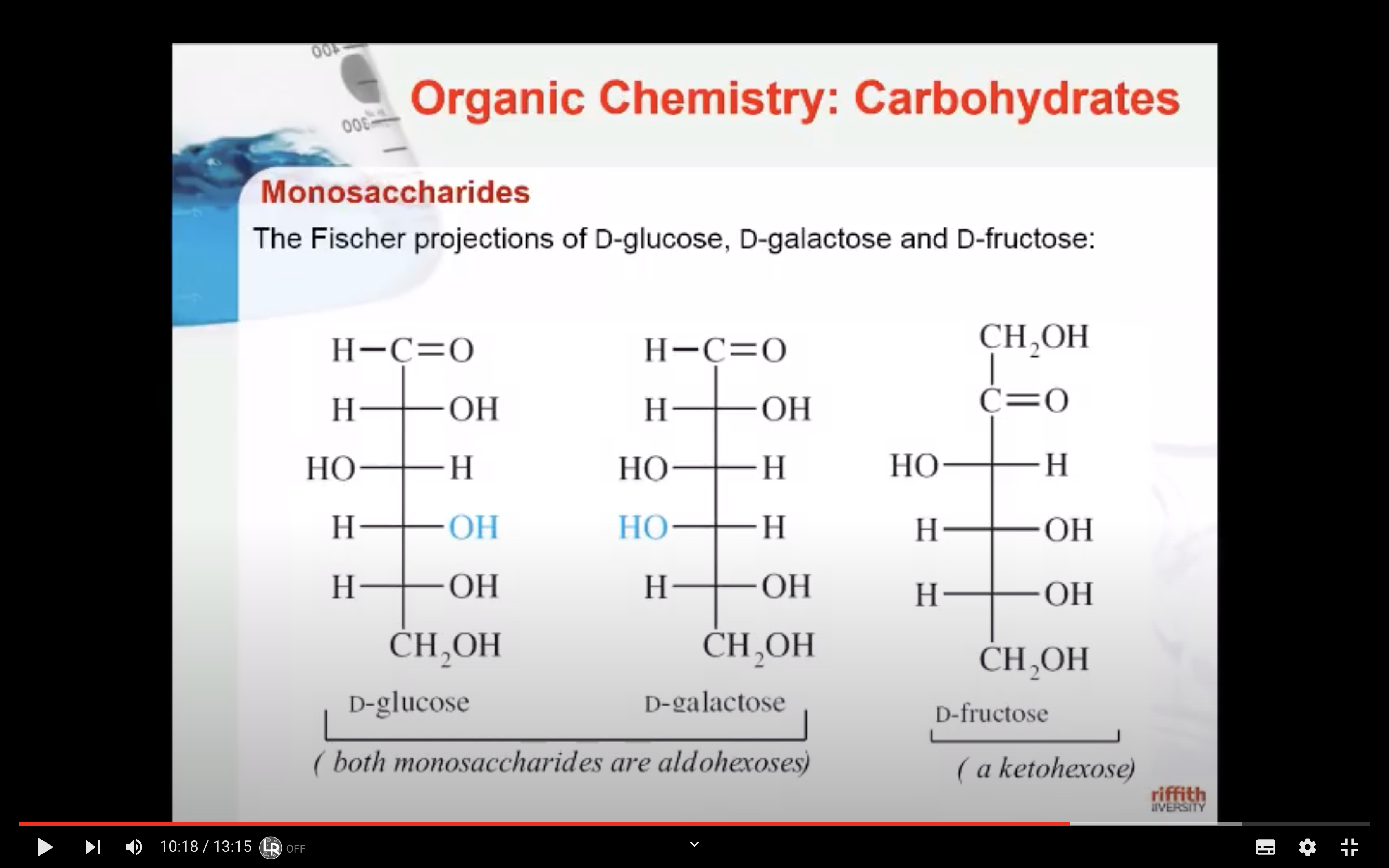
Monosaccharides - GLUCOSE,FRUCTOSE,GALACTOSE
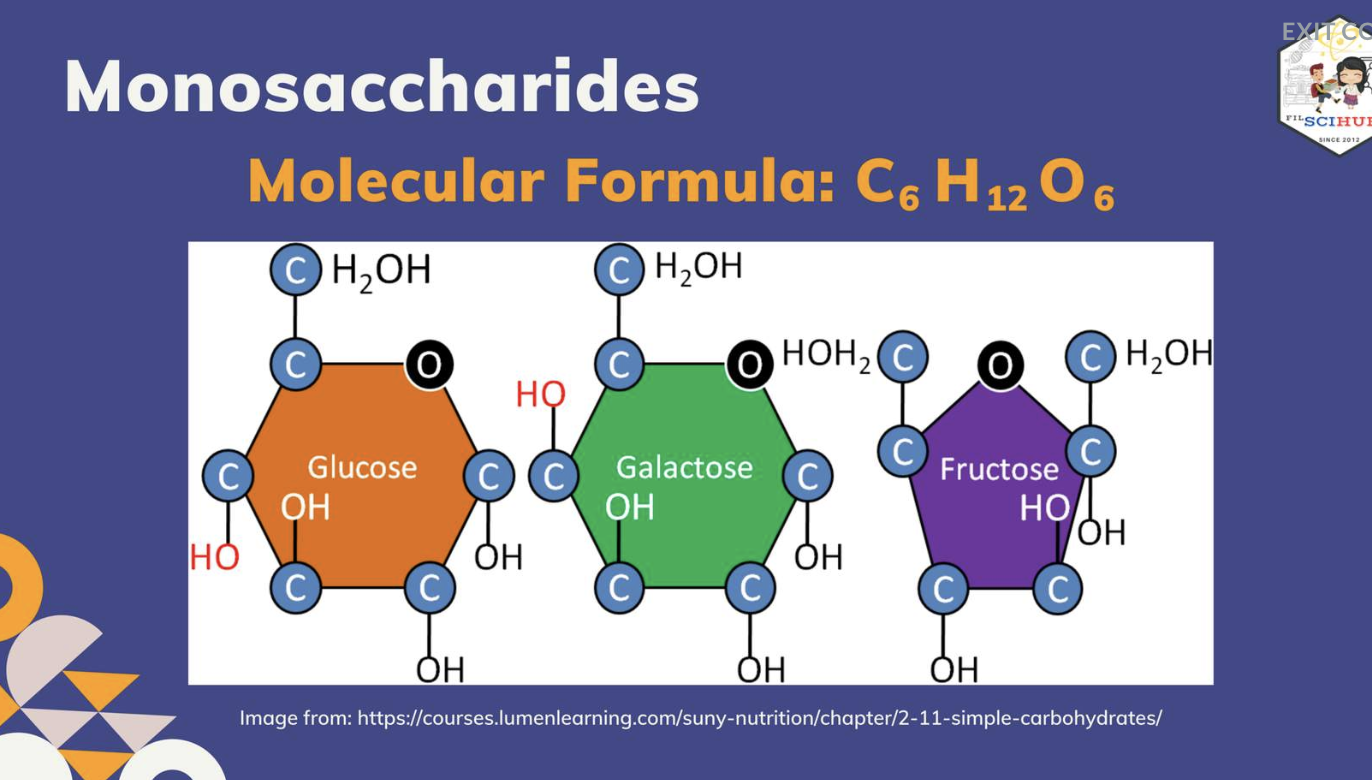
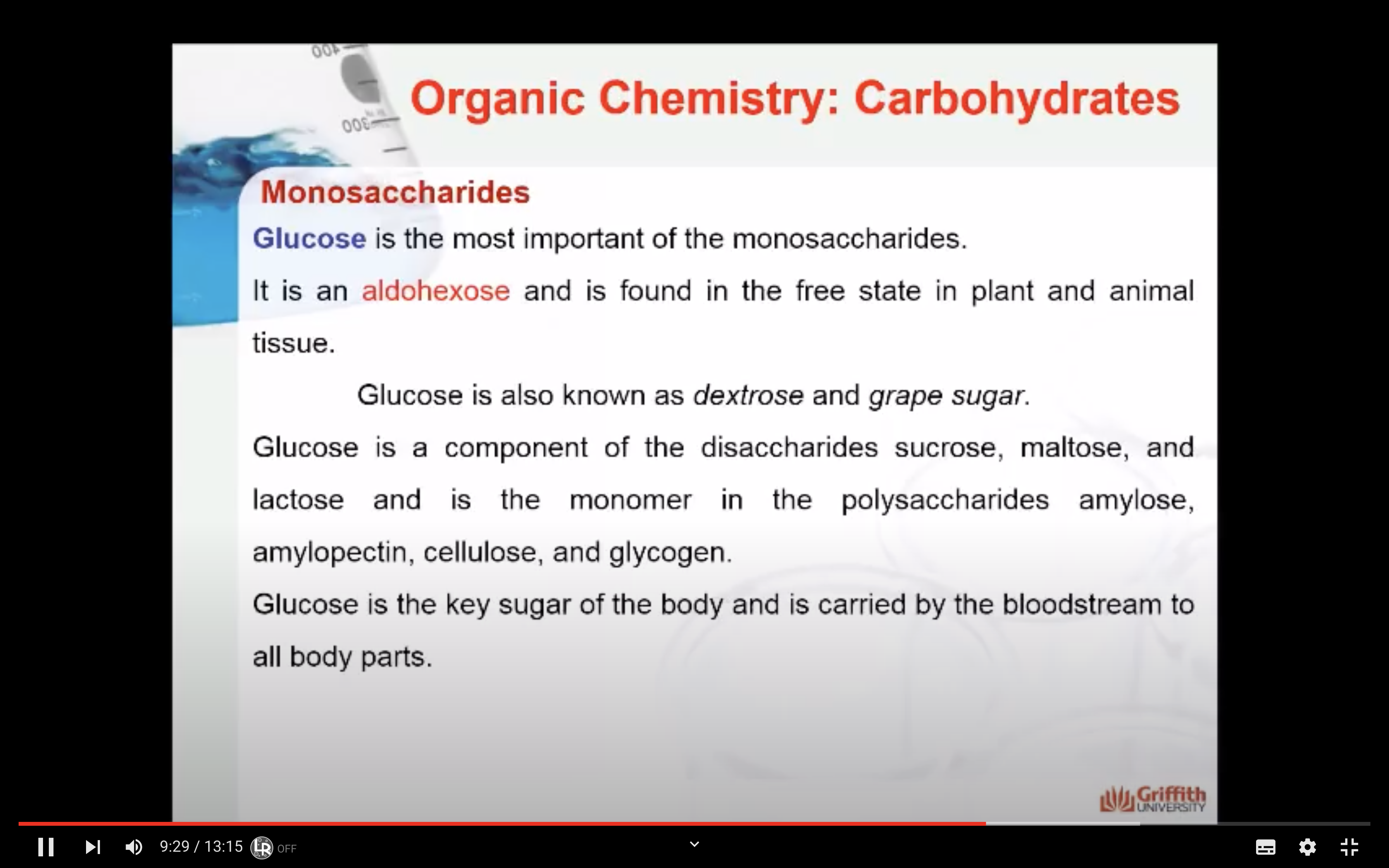
Glucose is the most important of the monosaccharides. It is an aldohexose and is found in the free state in plant and animal tissue. Glucose is also known as dextrose and grape sugar. Glucose is a component of the disaccharides sucrose, maltose, and lactose and is the monomer in the polysaccharides amylose, amylopectin, cellulose, and glycogen. Glucose is the key sugar of the body and is carried by the bloodstream to all body parts.
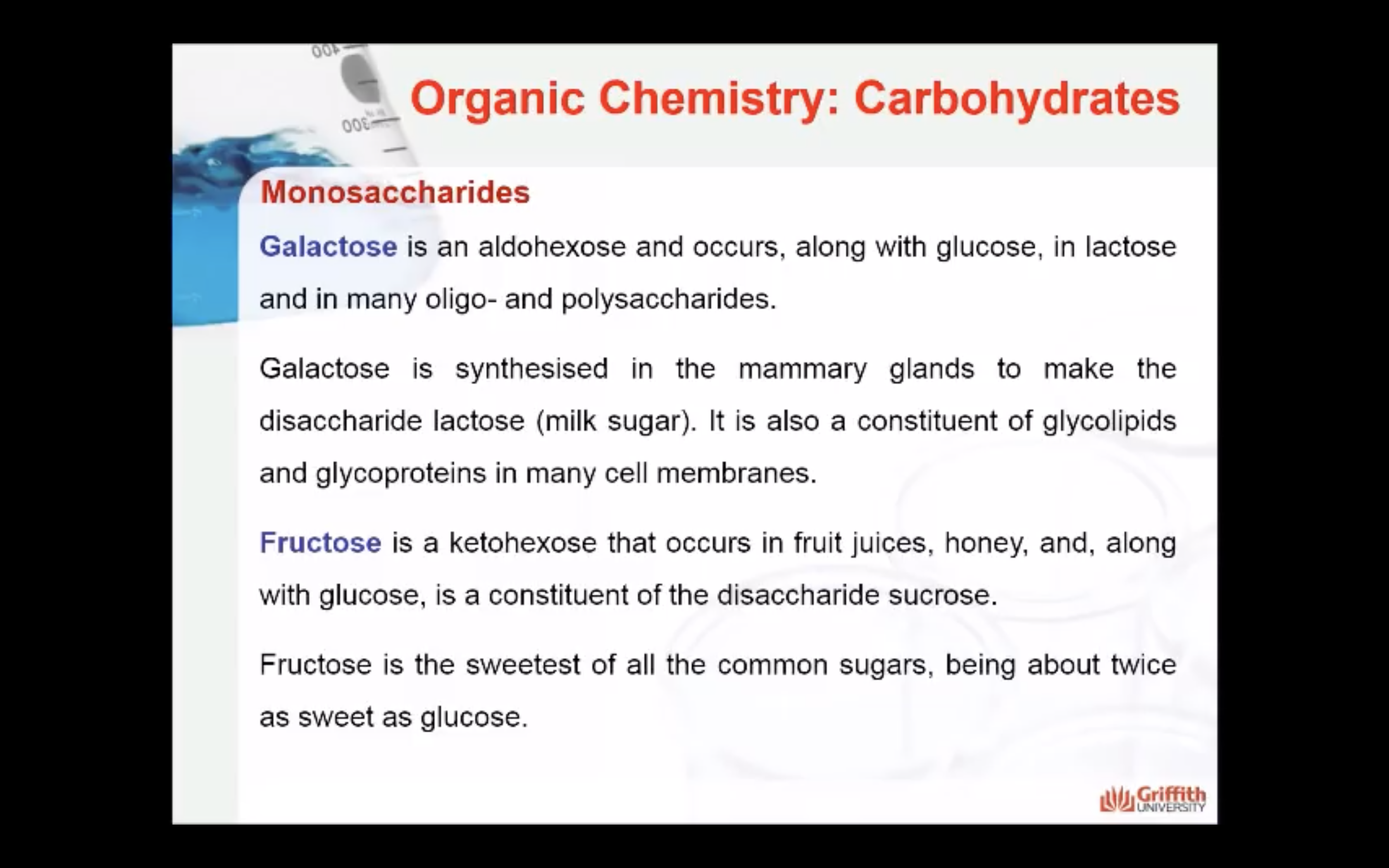
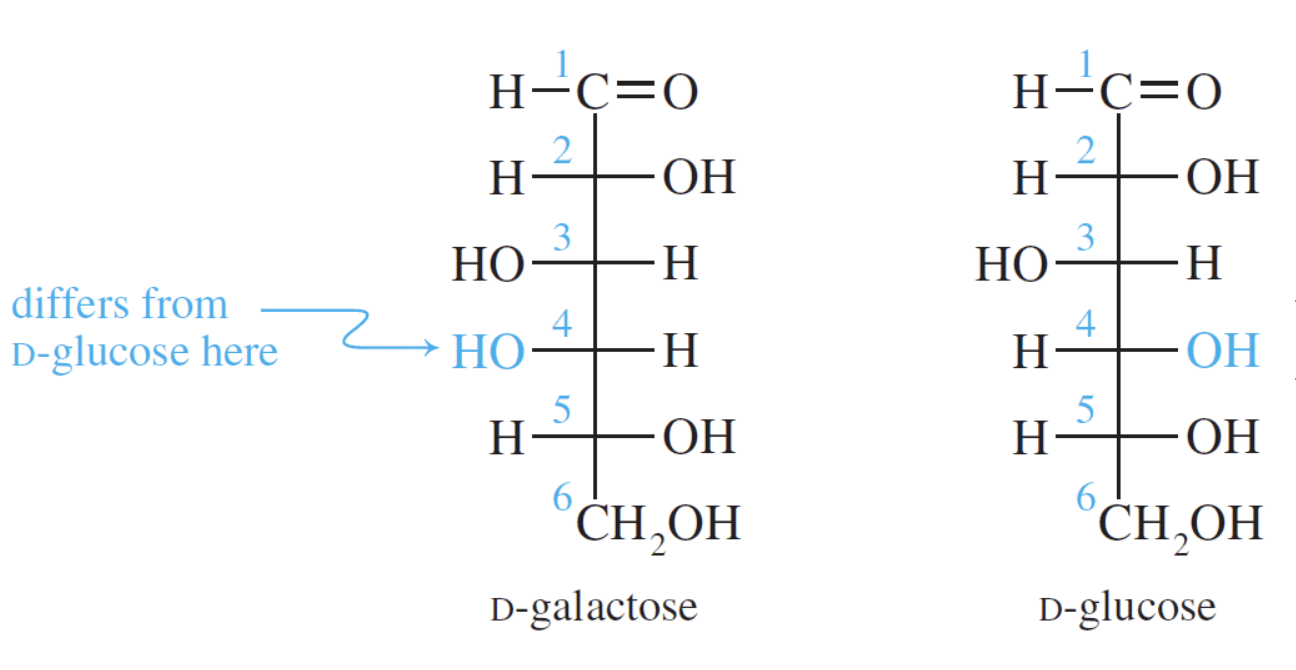
Galactose is an aldohexose like glucose and occurs, along with glucose, in lactose and in many oligo– and polysaccharides. Galactose is synthesised in the mammary glands to make the disaccharide lactose (milk sugar). It is also a constituent of glycolipids and glycoproteins in many cell membranes.
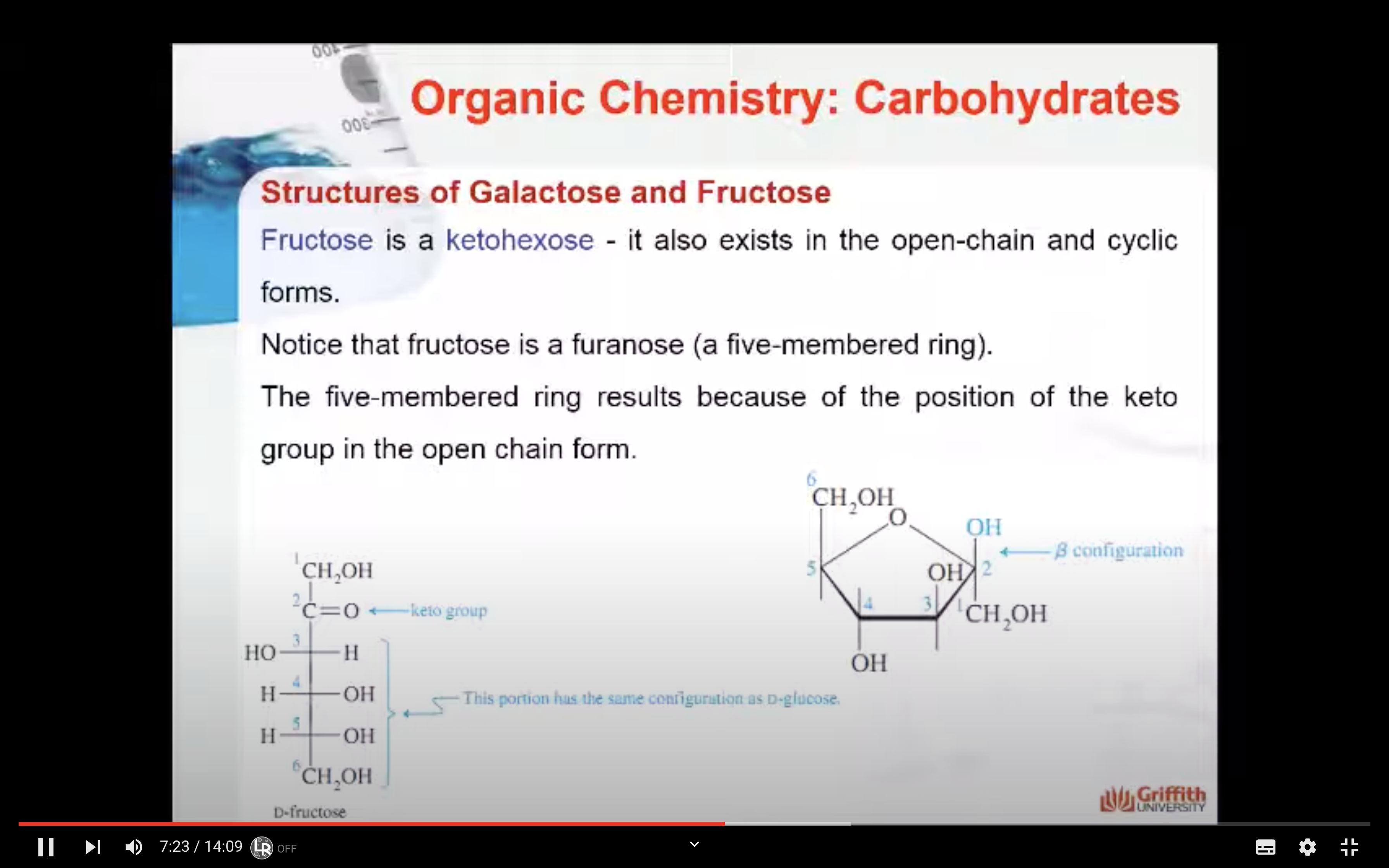
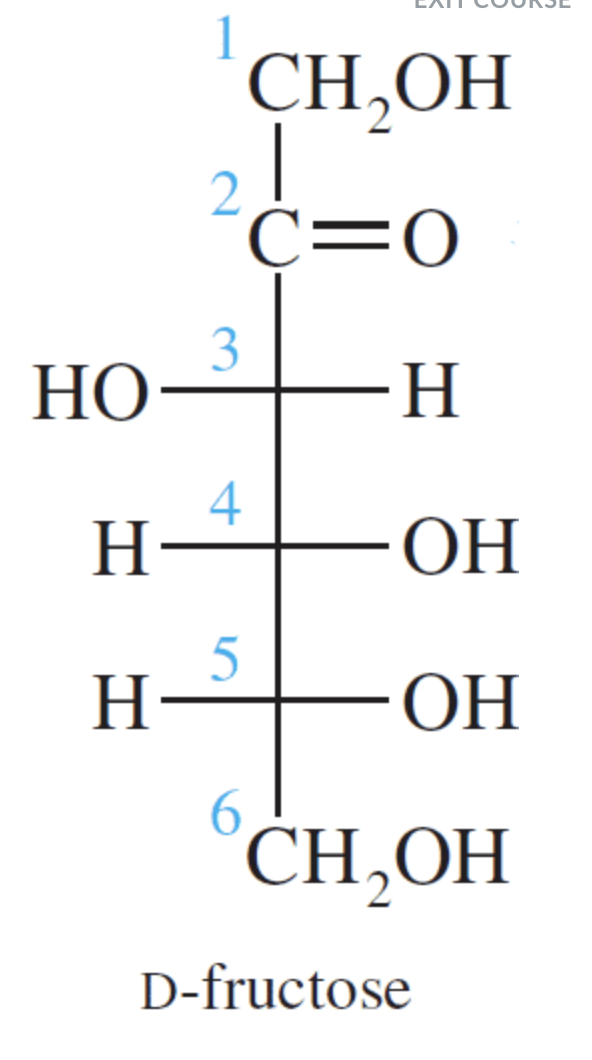
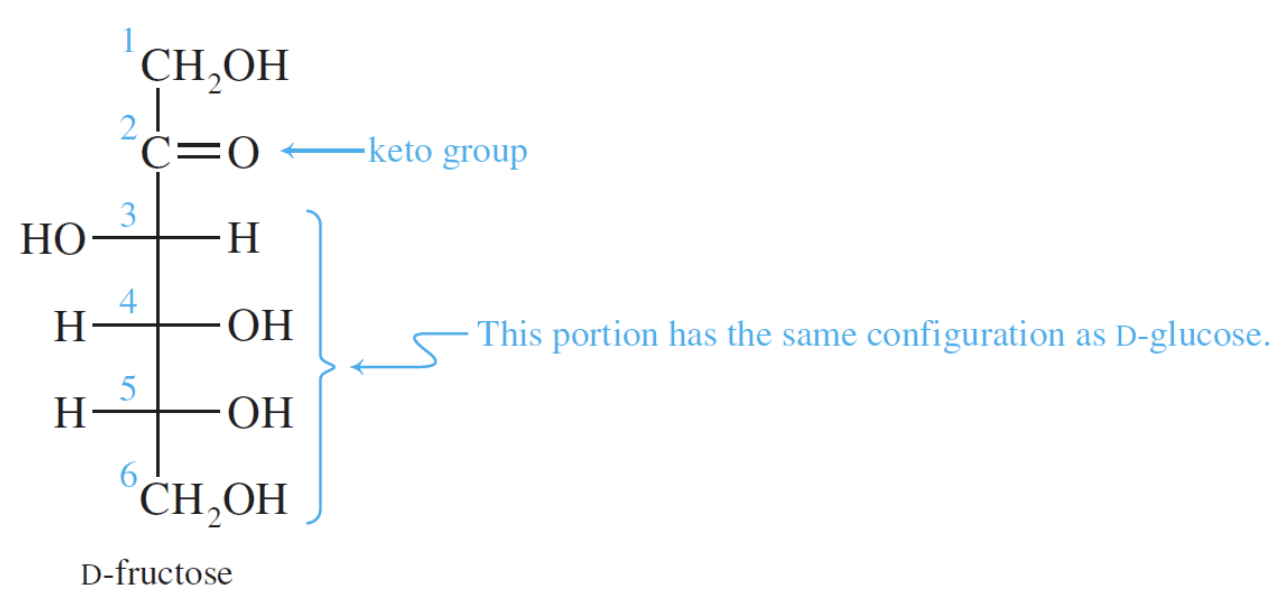
Fructose is a ketohexose that occurs in fruit juices, honey, and, along with glucose, is a constituent of the disaccharide sucrose. Fructose is the sweetest of all the common sugars, being about twice as sweet as glucose.
The enantiomers of glyceraldehyde are also known as epimers. Epimers are stereoisomers that differ only in the configuration at a single chiral carbon atom. In the case of glyceraldehyde the chiral carbon atom is #2.
The enantiomers of glucose can also be represented by Fischer projection formulas.
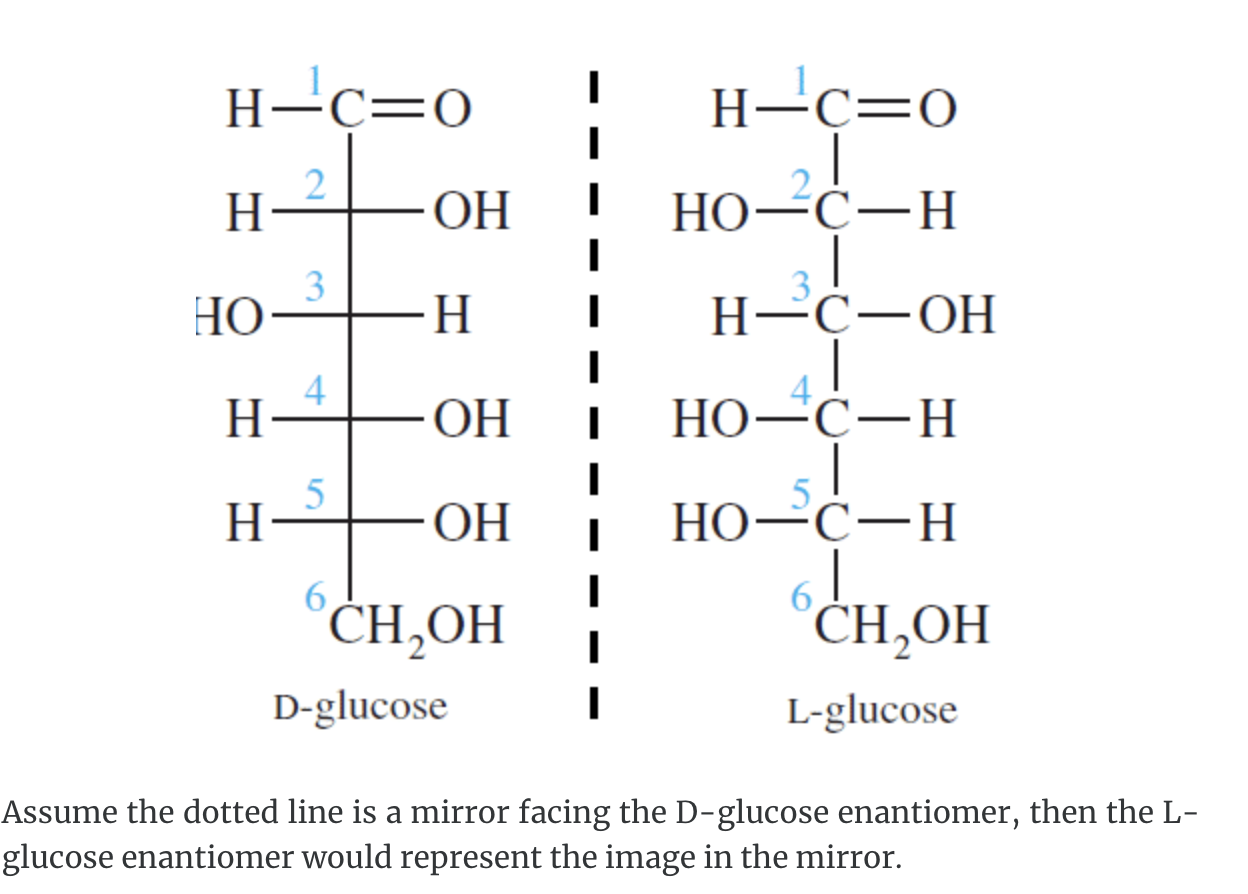
The structure called D-glucose is named because the –H and –OH on carbon 5 are in the same configuration of the –H and –OH on carbon 2 in D-glyceraldehyde.
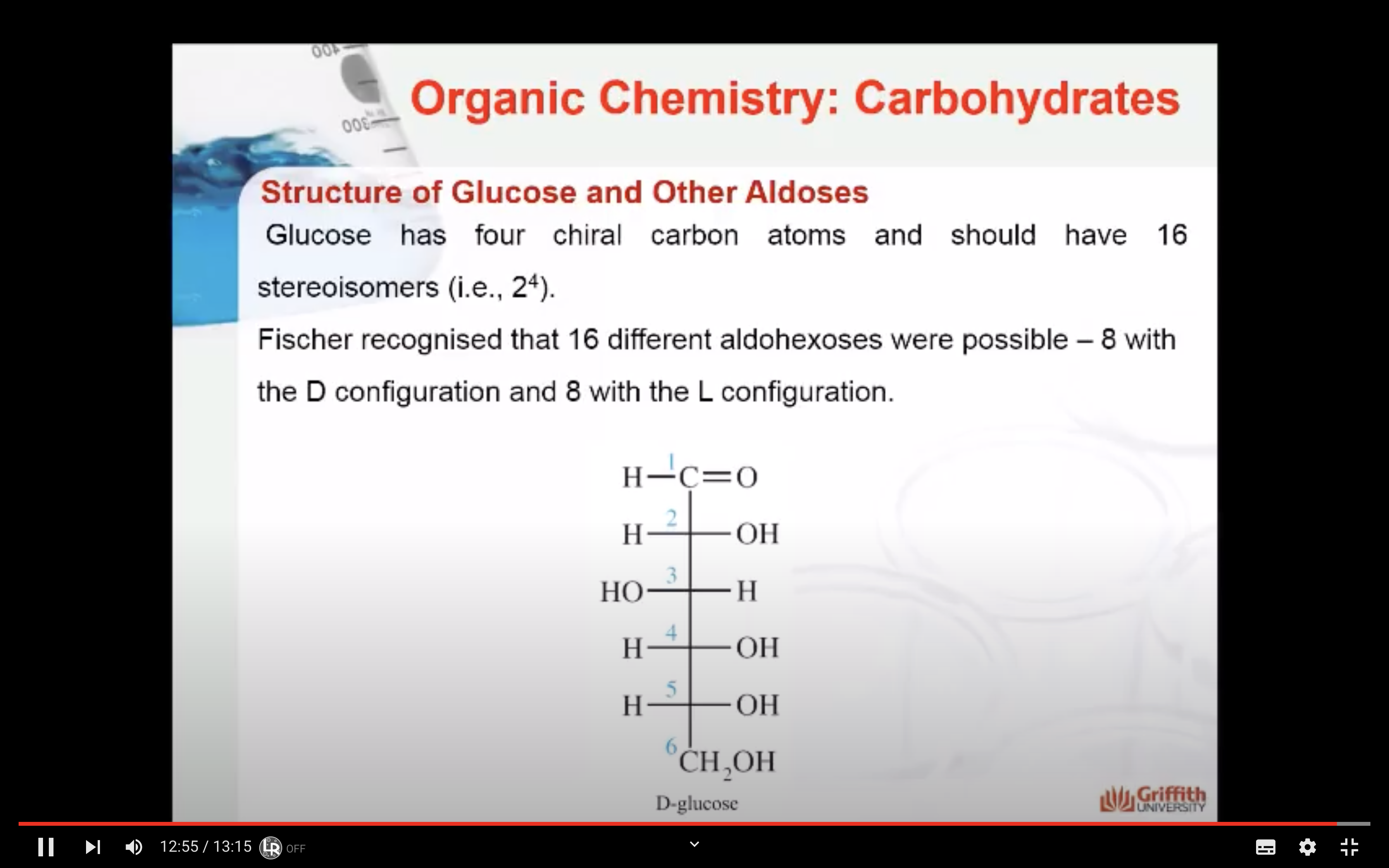
Fischer recognised that 16 different aldohexoses were possible – 8 with the D configuration and 8 with the L configuration. This follows the formula 2n for optical isomers. Glucose has four chiral carbon atoms and should therefore have 16 stereoisomers (i.e., 24).
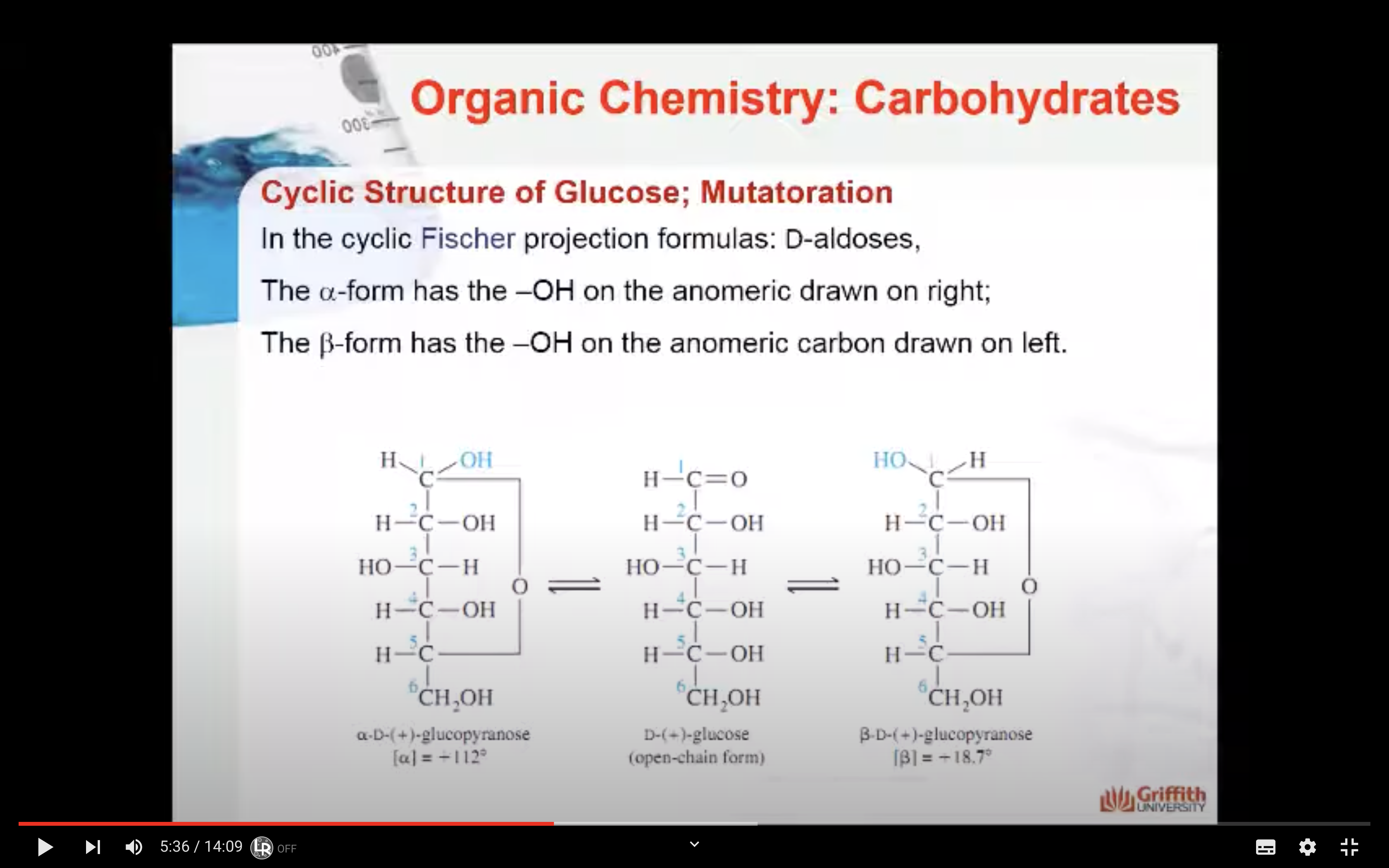
Cyclic Structure of Glucose; Mutarotation
Straight open-chain D–glucose is so reactive that almost all molecules quickly rearrange their bonds to form two new structures. These structures are six-membered rings called pyranose sugars.
When the α– and β–forms of glucose are put into separate solutions and allowed to stand for several hours, the phenomena that occurs is called mutarotation. During mutarotation, the two cyclic forms convert into each other through the open-chain form. The resulting equilibrium: 36% α and 64% β + trace amount of open–chain.
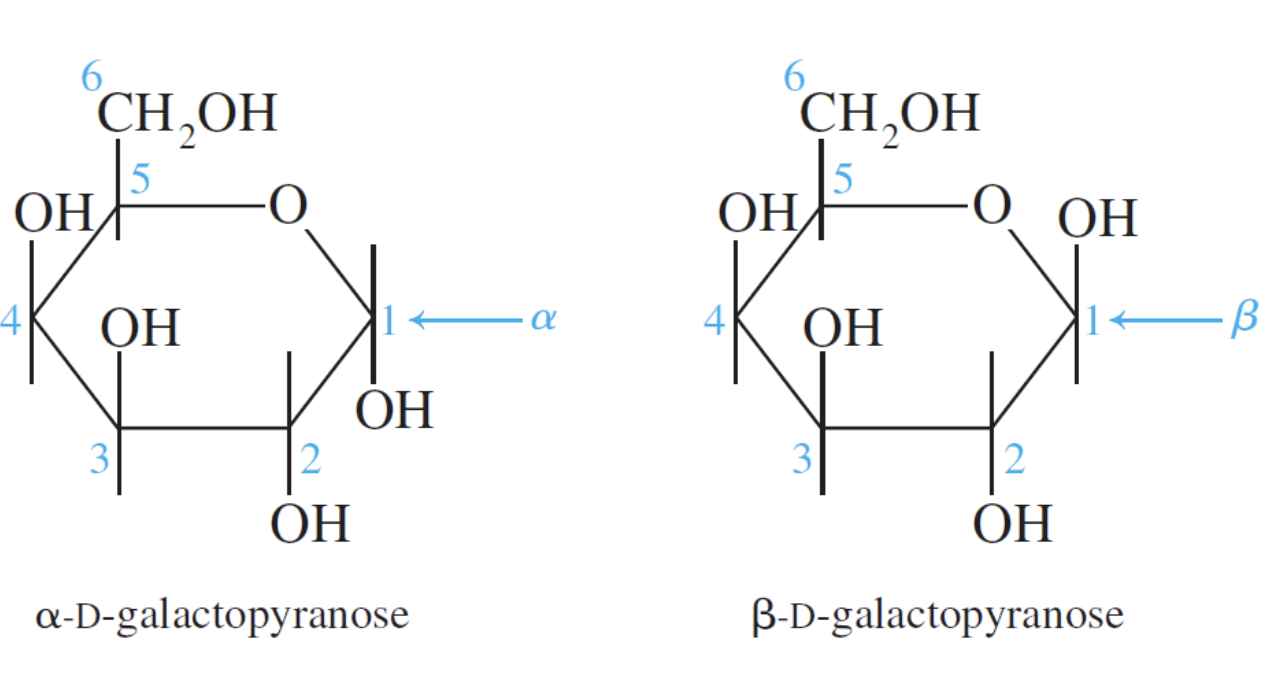
The cyclic forms only differ in the stereo arrangement of the carbon atom involved in the mutarotation (carbon #1). This carbon atom is called the anomeric carbon. Two cyclic isomers that differ only in their stereo arrangement about the carbon involved in mutarotation are called anomers. The α– and β– forms are anomers.

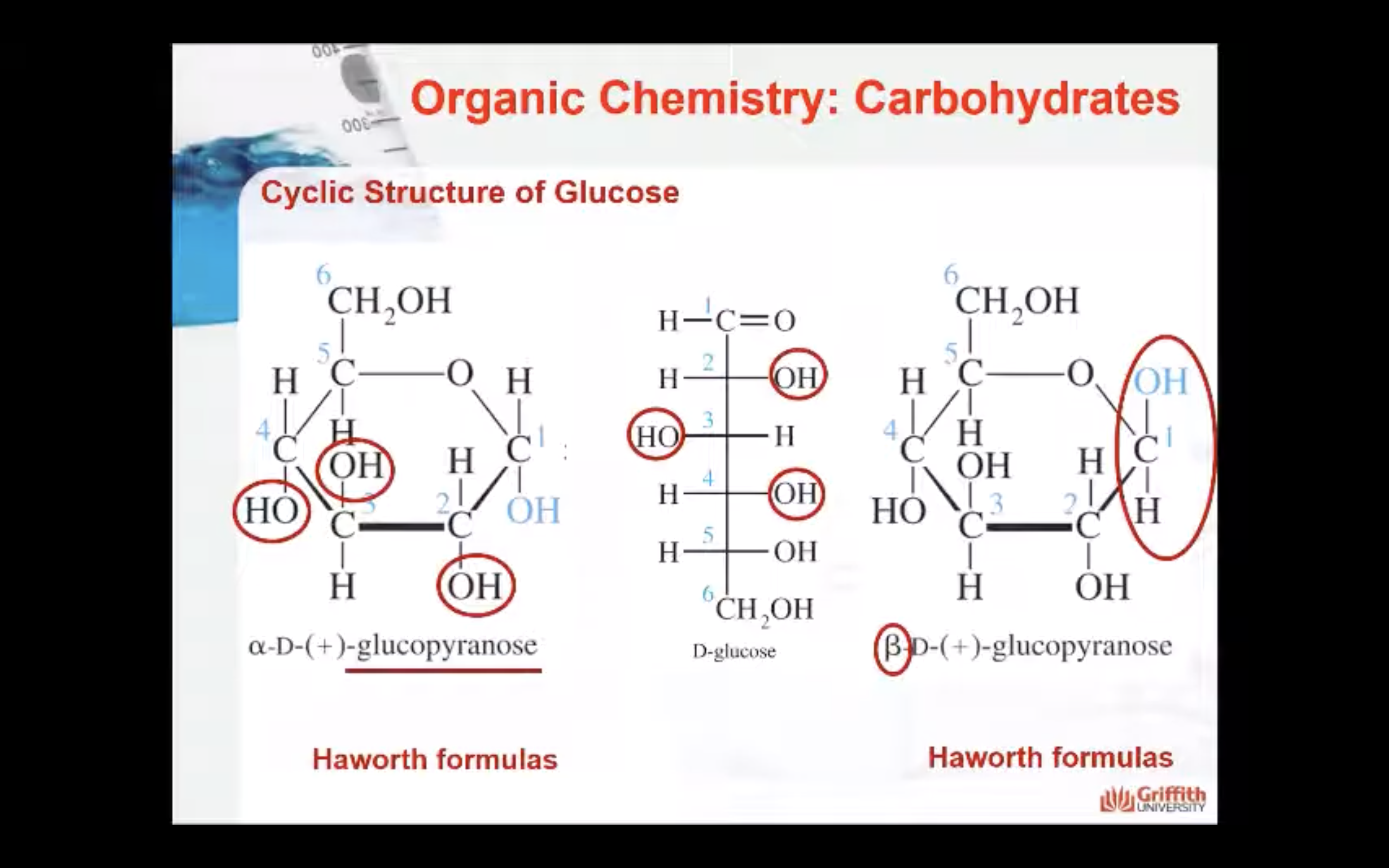
Haworth perspective formulas. The Haworth formula represents the molecule as a flat hexagon with the –H and –OH groups above and below the plane of the hexagon.
In α–anomers the hydroxyl group on the anomeric carbon is below the plane of the molecule (in an axial position).
In the β–anomers the hydroxyl group on the anomeric carbon is above the plane of the molecule (in an equatorial position).

In the cyclic Fischer projection formulas: D–aldoses, the α–form has the –OH on the anomeric drawn on right; in the β–form, the –OH on the anomeric carbon is on the left.

Modified Fischer projection formulas.
In α–anomers the hydroxyl group on the anomeric carbon is below the plane of the molecule (in an axial position).
In the β–anomers the hydroxyl group on the anomeric carbon is above the plane of the molecule (in an equatorial position).

In the cyclic Fischer projection formulas: D–aldoses, the α–form has the –OH on the anomeric drawn on right; in the β–form, the –OH on the anomeric carbon is on the left.

Modified Fischer projection formulas.
BELOW = ALPHA = RIGHT
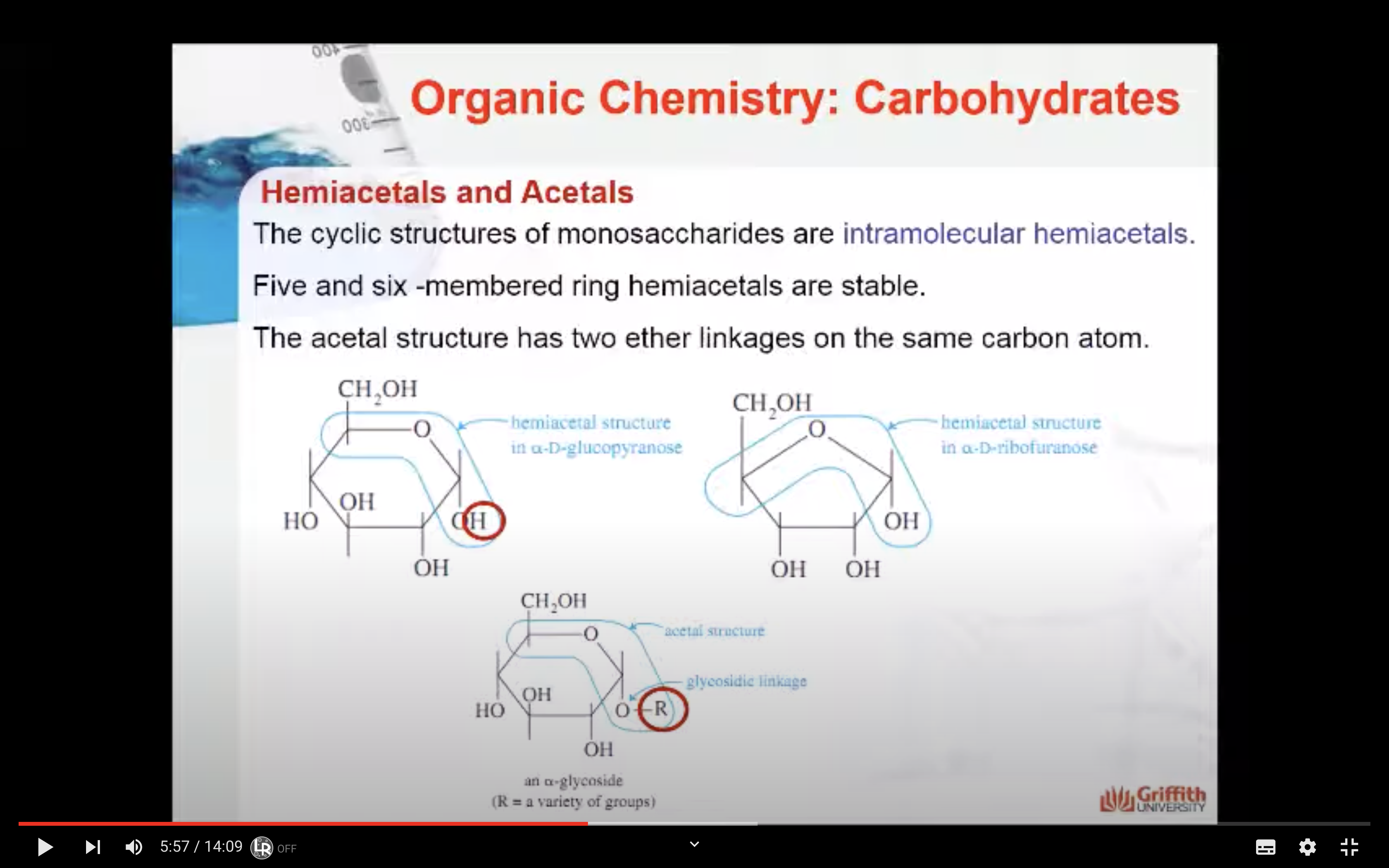
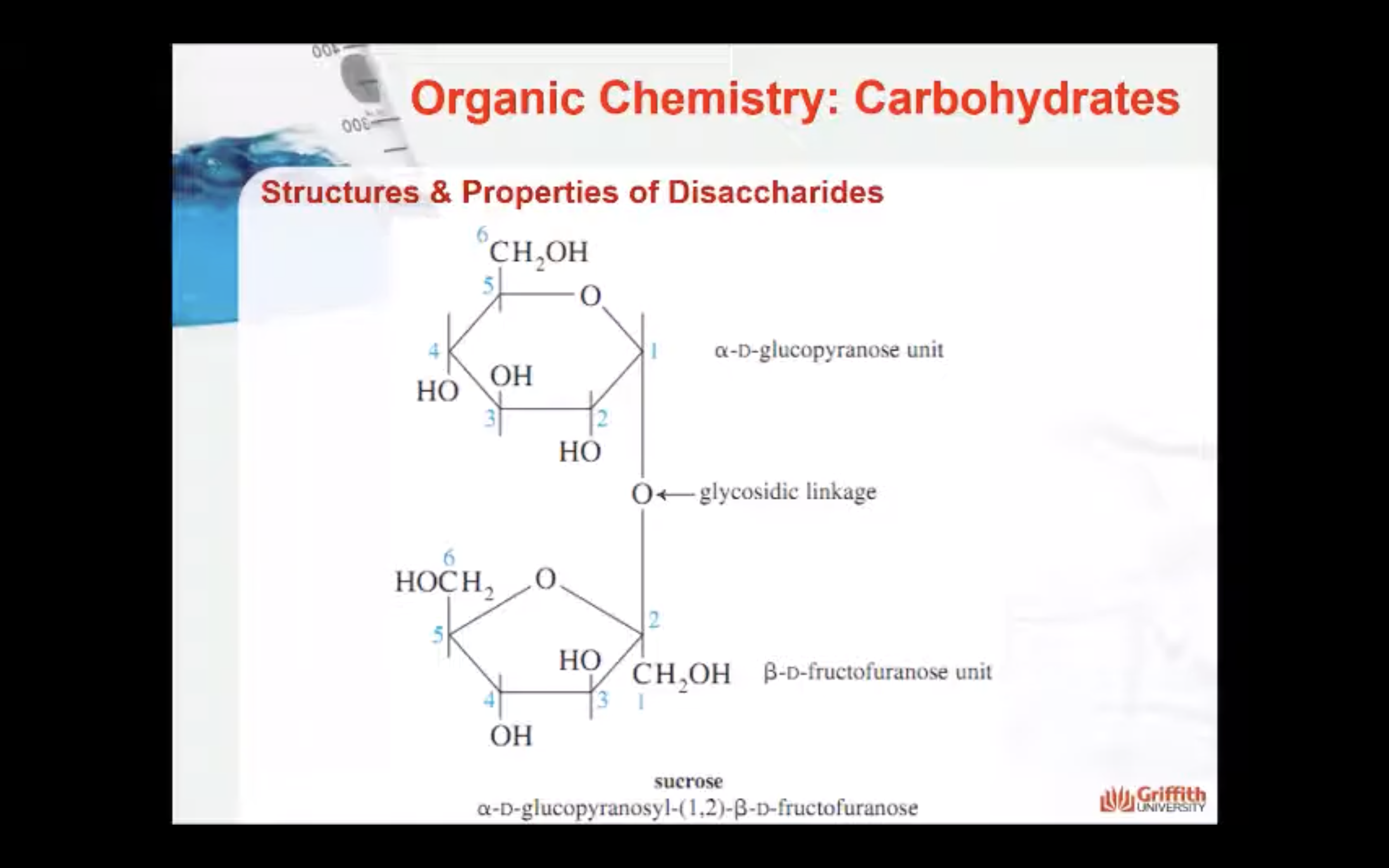
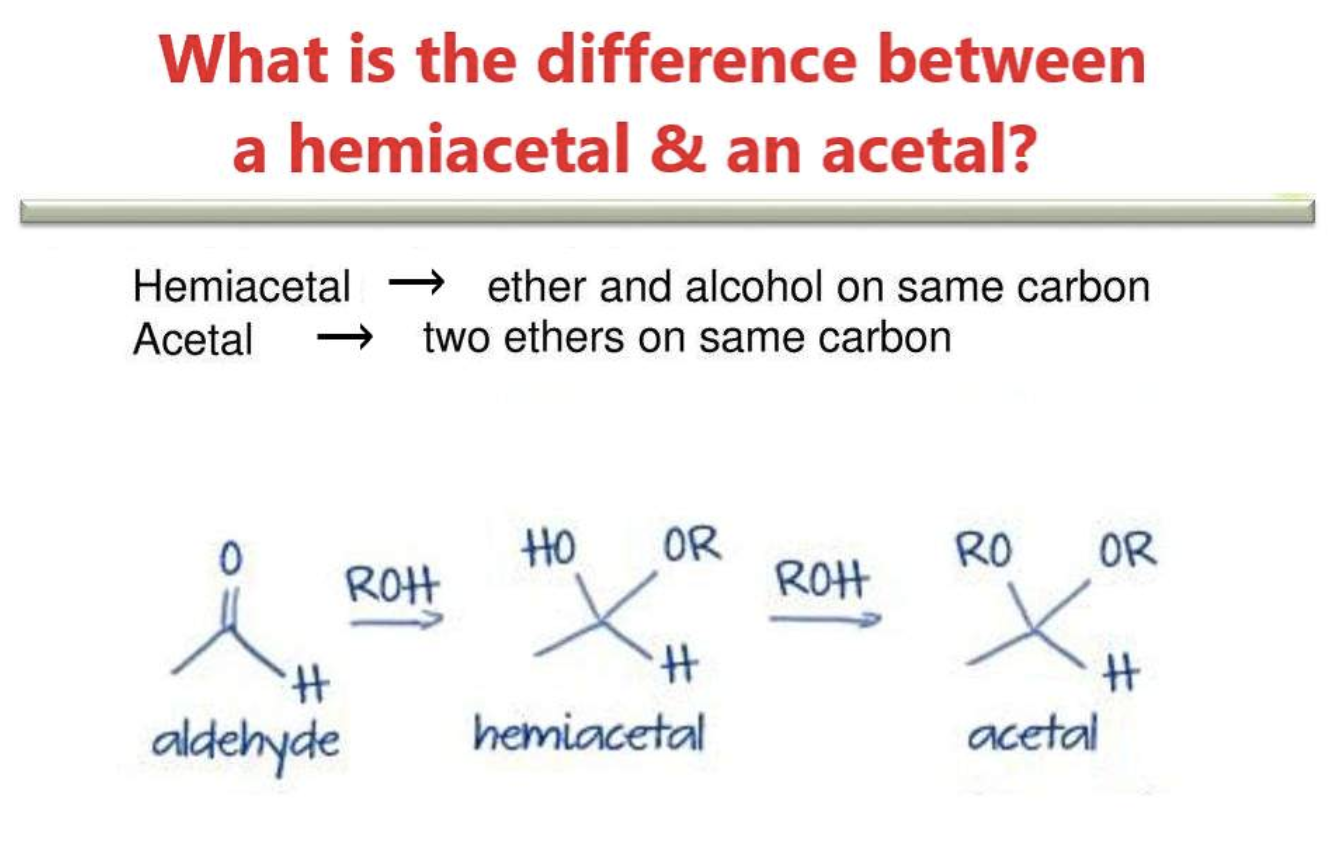
Hemiacetals and Acetals
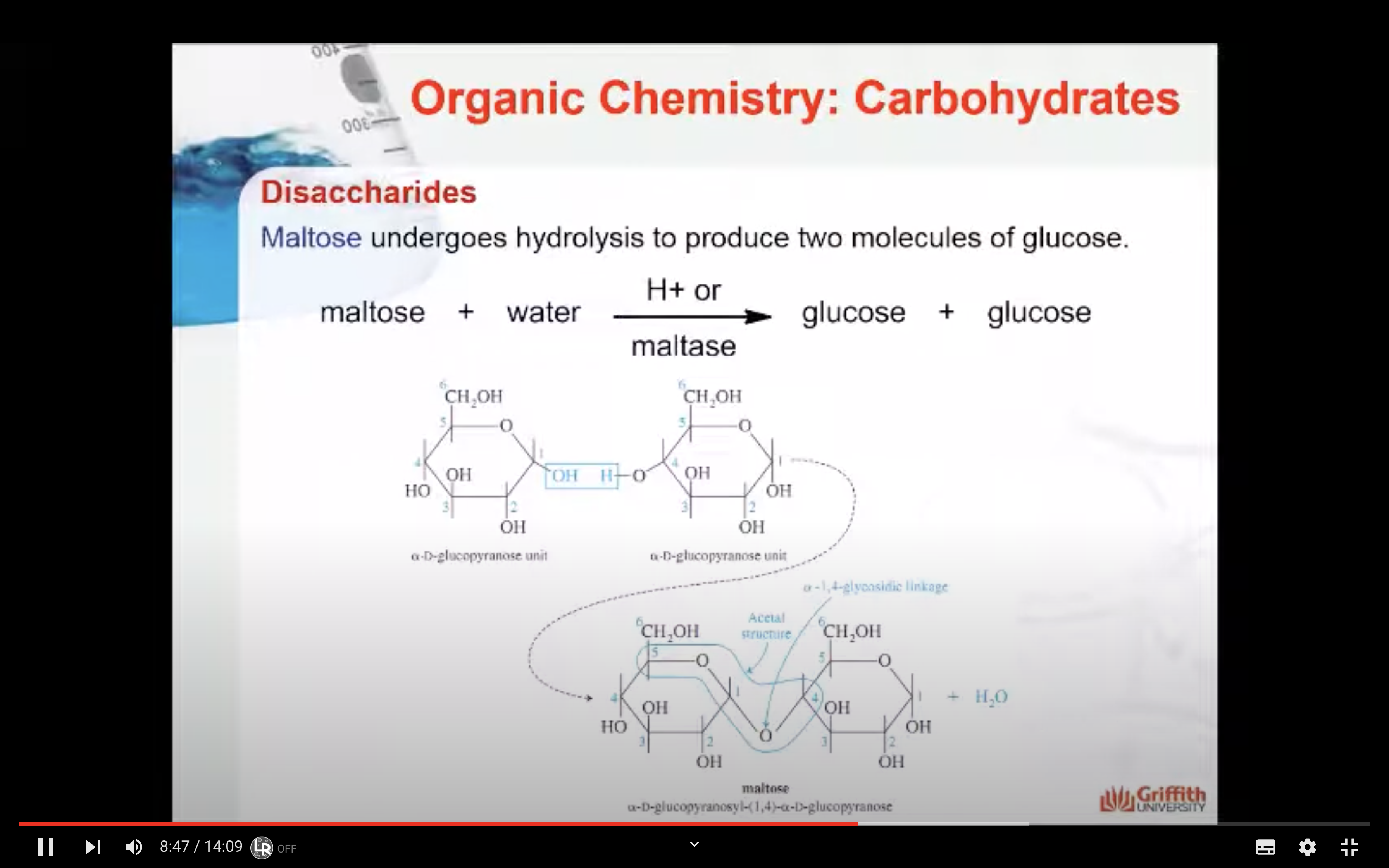
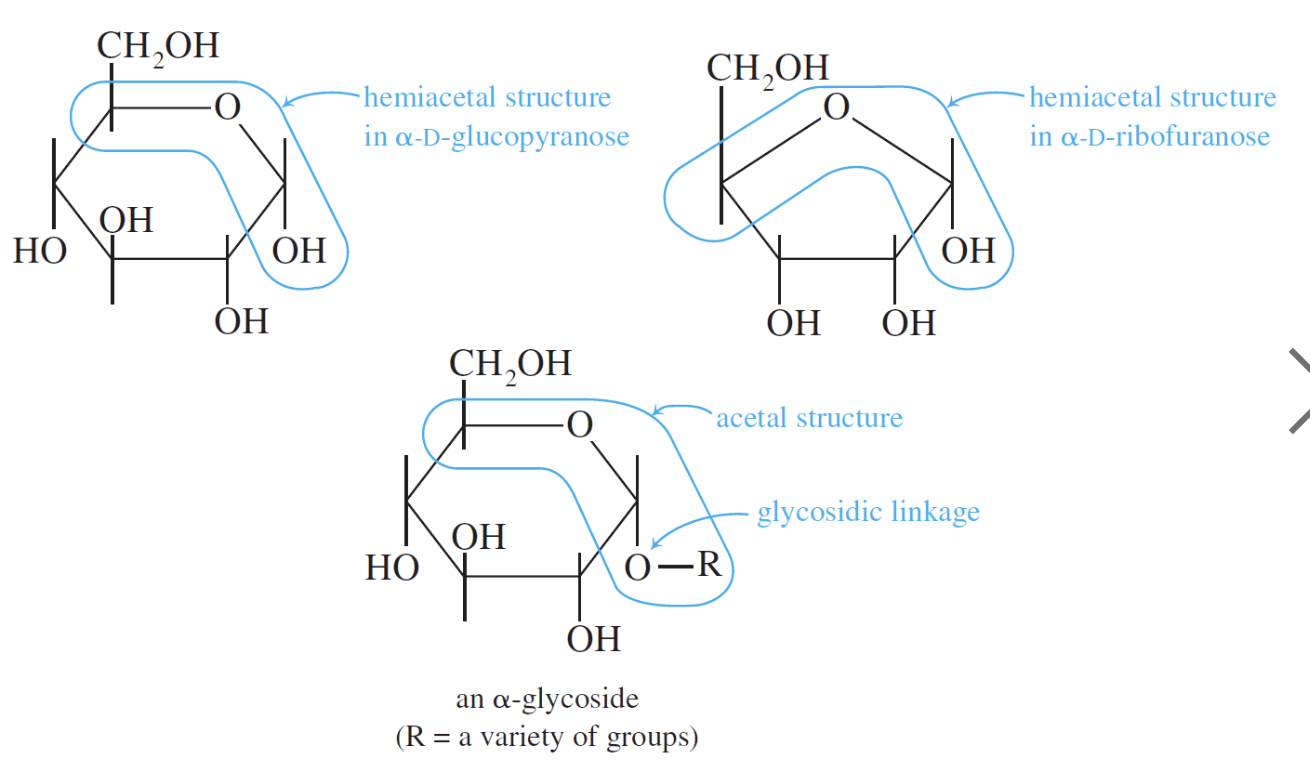
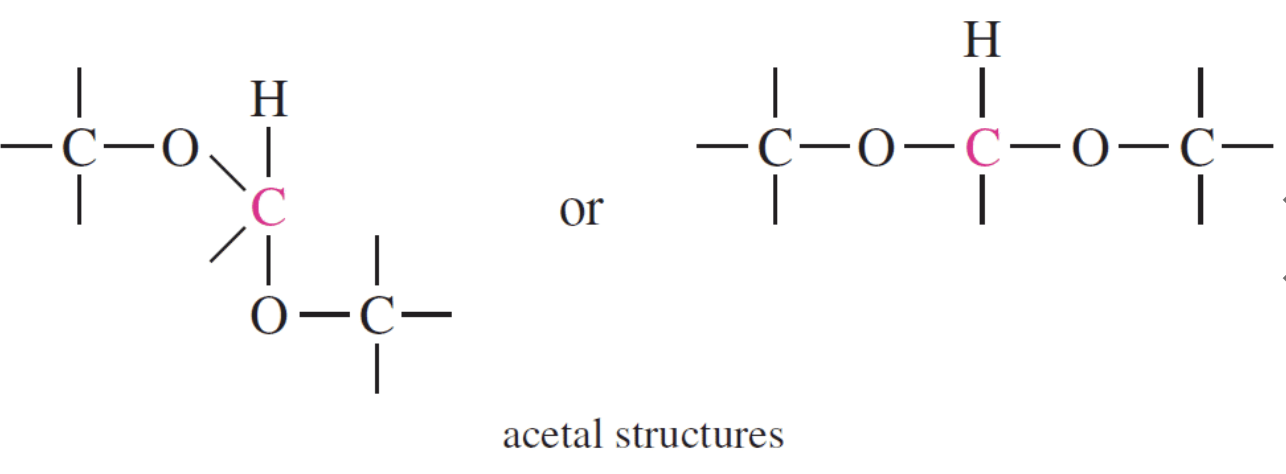
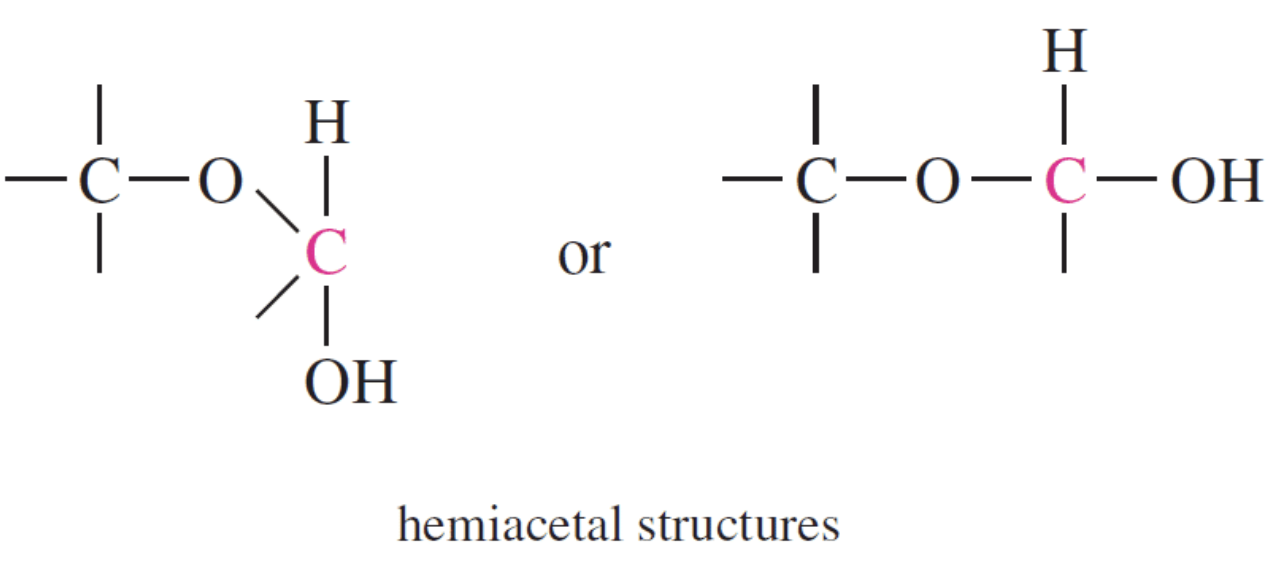
The acetal structure has two ether linkages on the same carbon atom (R-O-C-O-R`), whereas, the hemiacetal structure has one ether and one alcohol linkages on the same carbon atom (R-O-C-O-H). The cyclic structures of monosaccharides are intramolecular hemiacetals. Five and six –membered ring hemiacetals are stable.
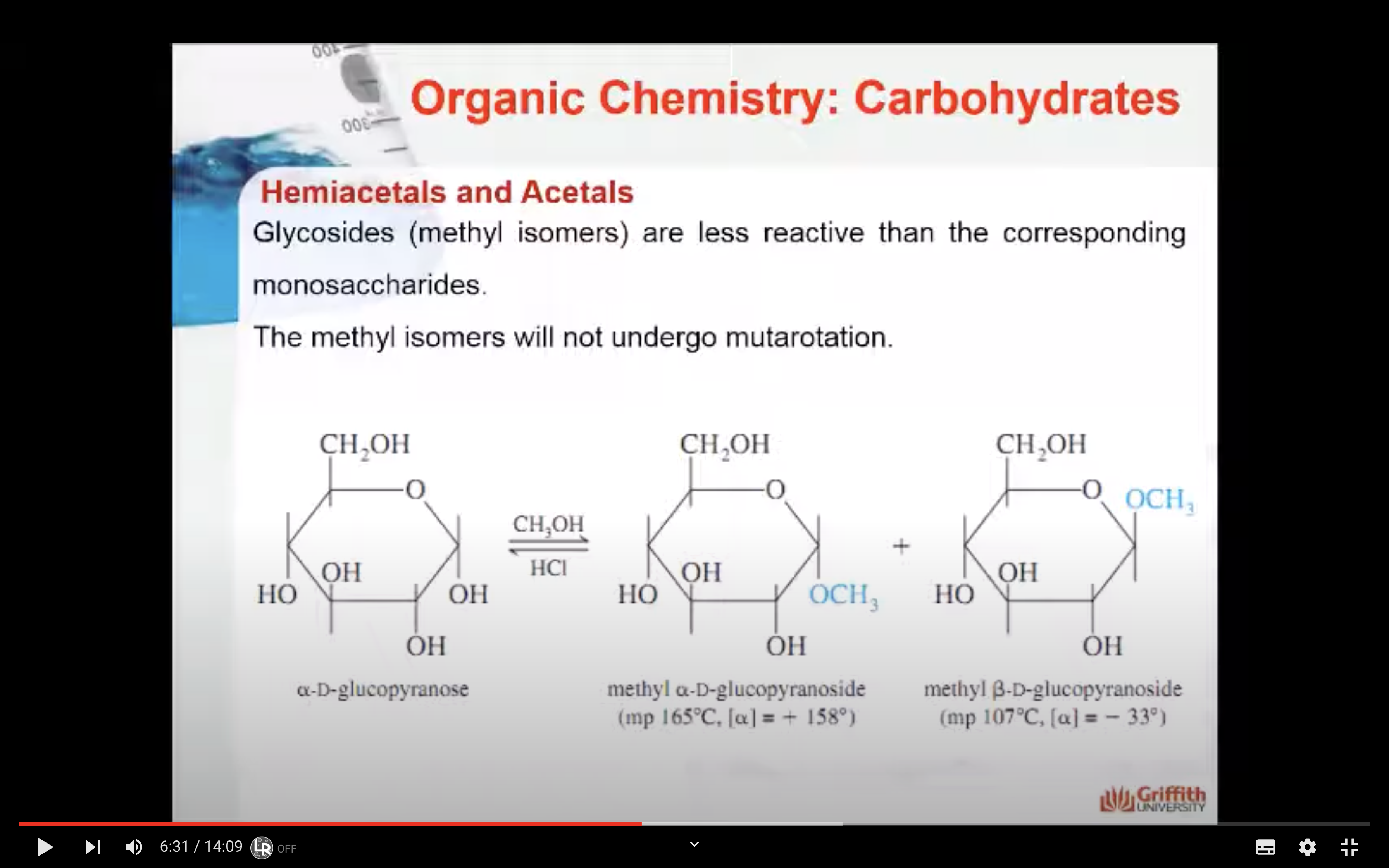
Glycosides, like the methyl isomers shown to the right, are less reactive than the corresponding monosaccharides. The methyl isomers shown here will not undergo mutarotation.

Glycosides, like the methyl isomers shown to the right, are less reactive than the corresponding monosaccharides. The methyl isomers shown here will not undergo mutarotation.
-Hemiacetal => mutarotation o
-Acetal => x
Structures of important monosaccharides
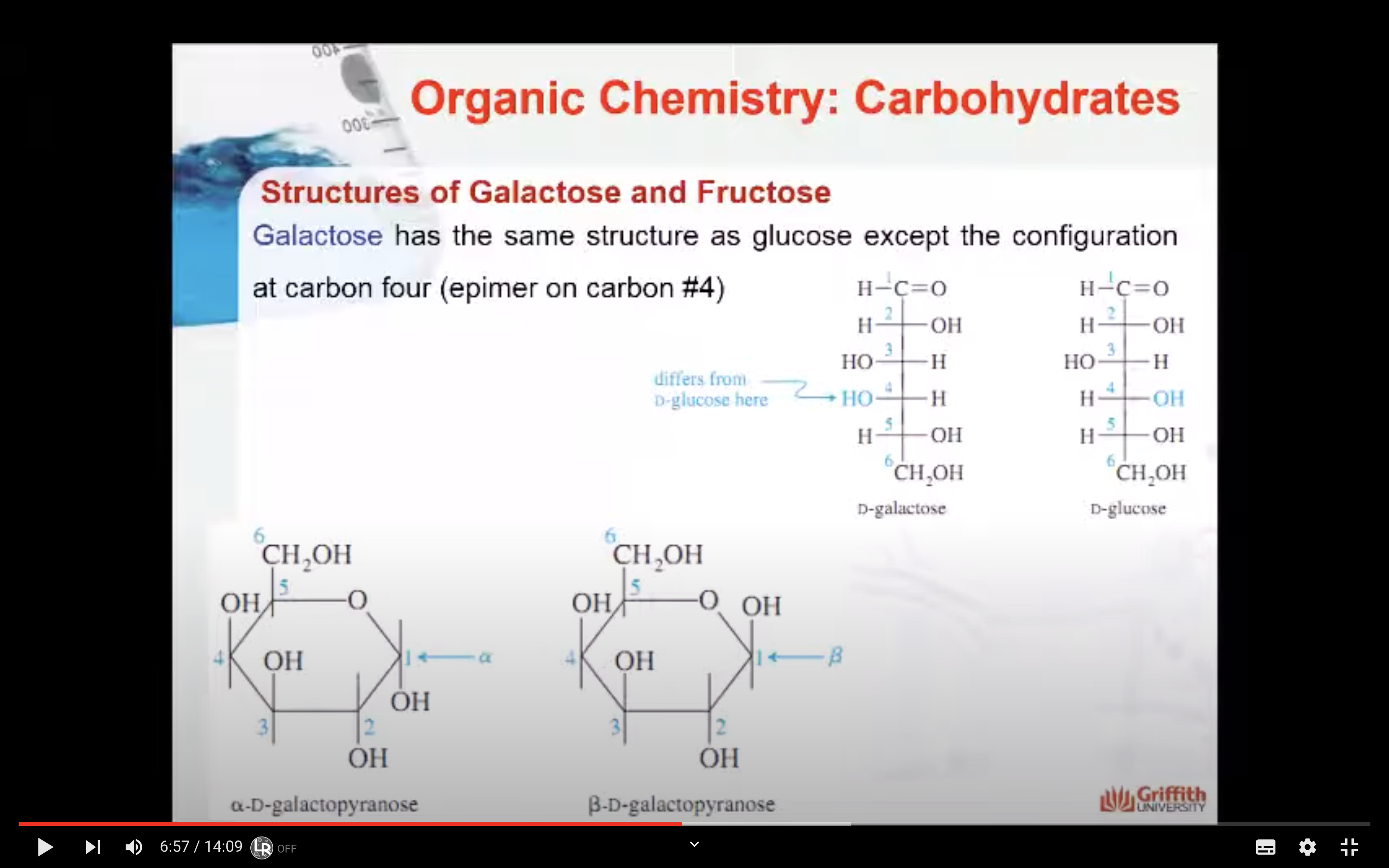
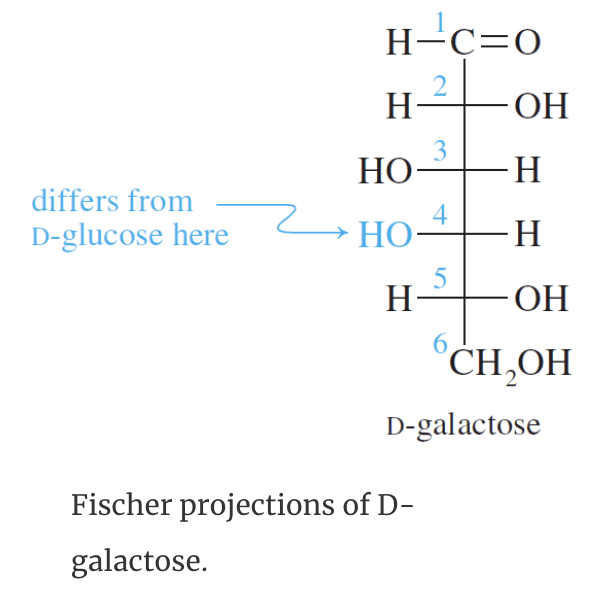
Galactose has the same structure as glucose except the configuration at carbon four is reversed.
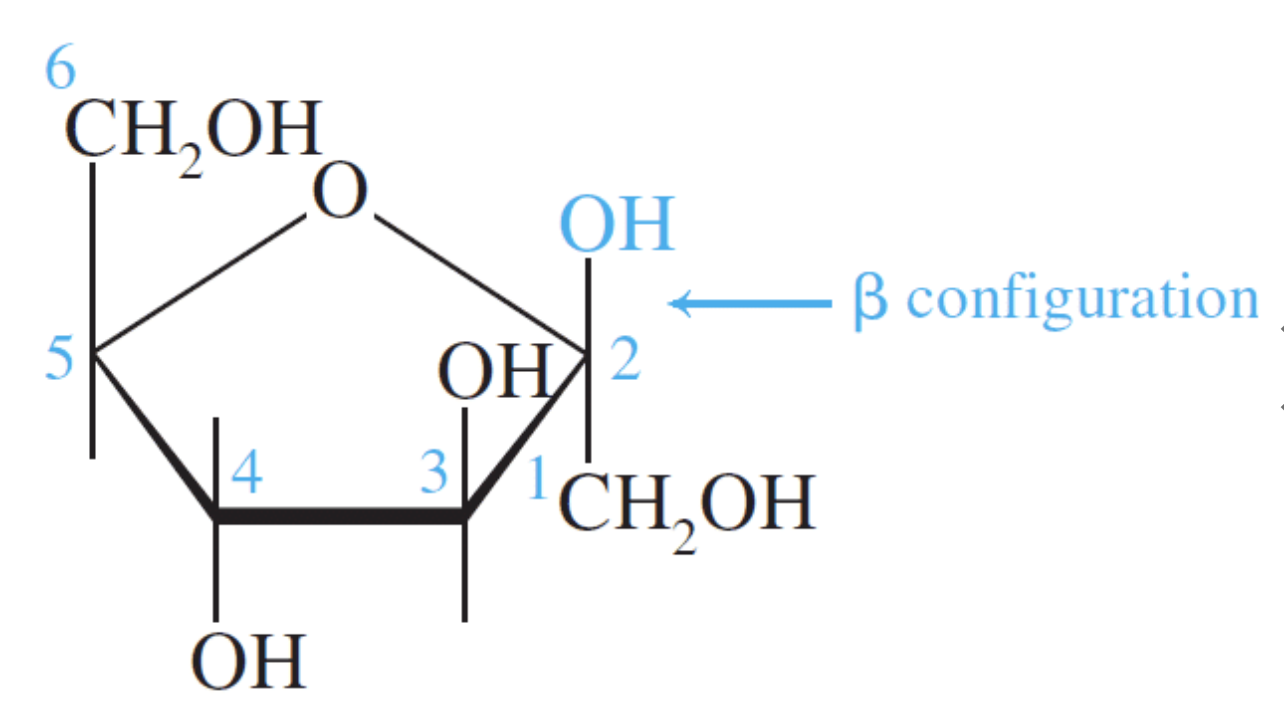
Fructose is a ketohexose and like glucose it also exists in the open-chain and cyclic forms. Notice that fructose is a furanose (a five-membered ring). The five-membered ring results because of the position of the keto group in the open-chain form.
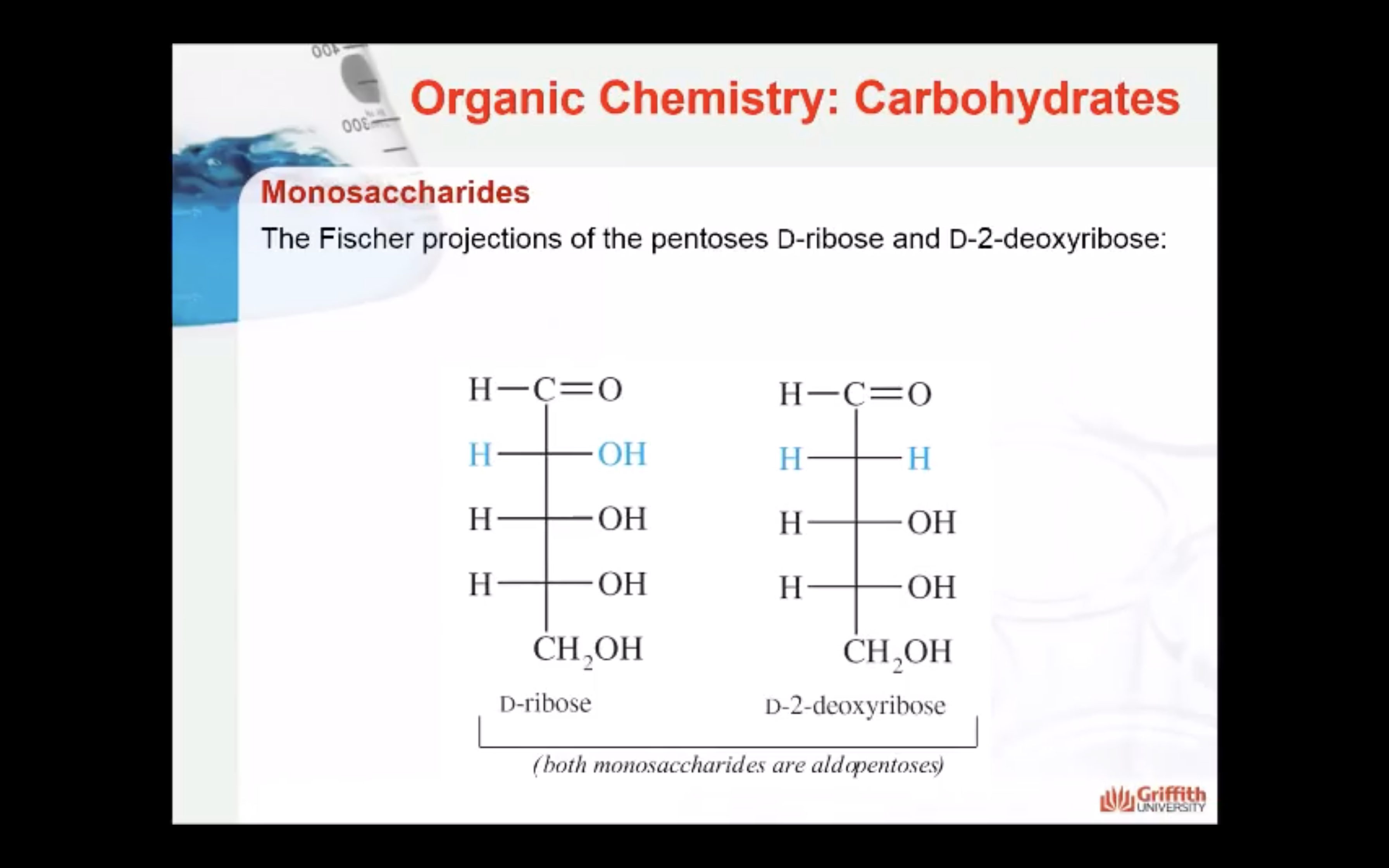
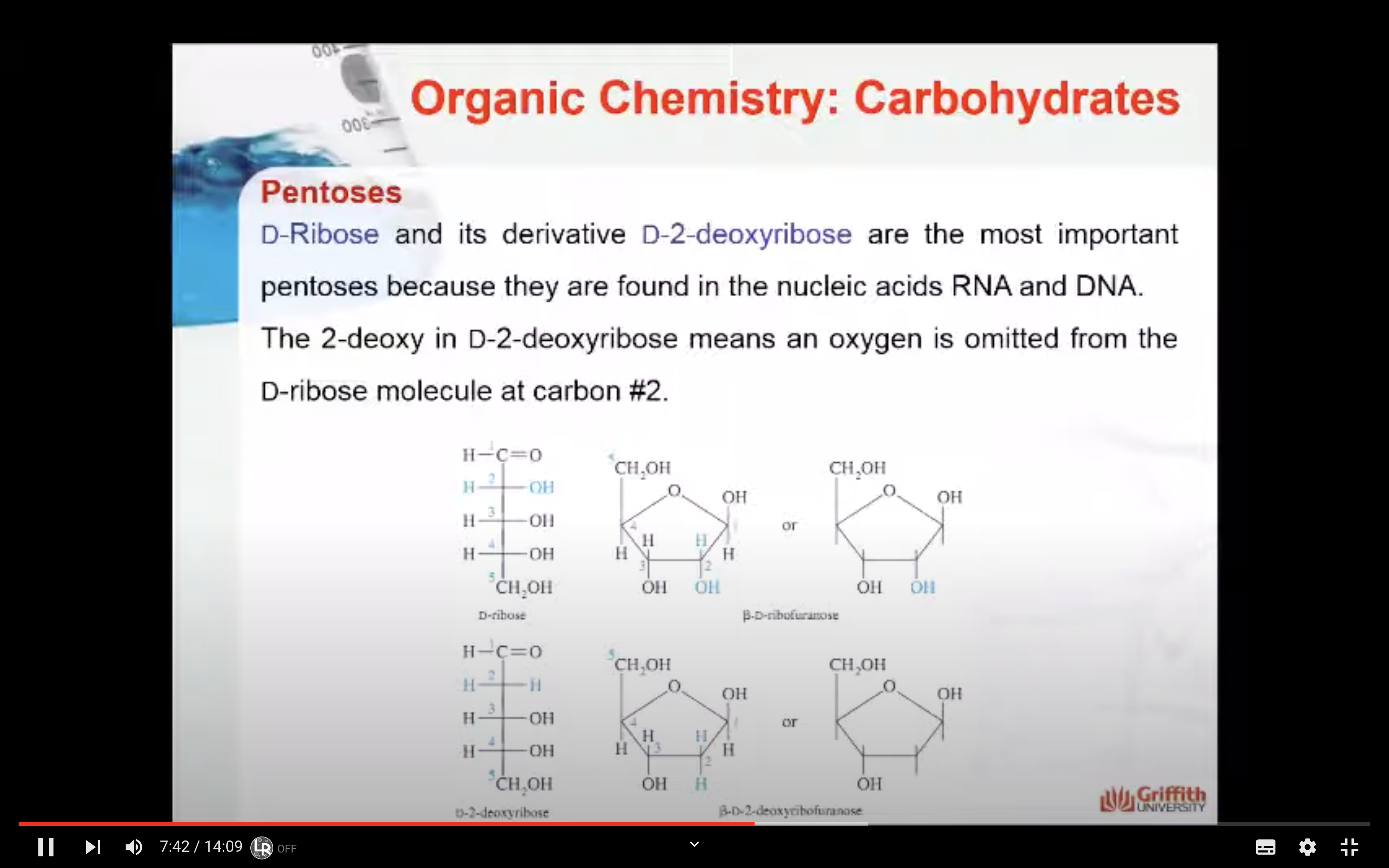
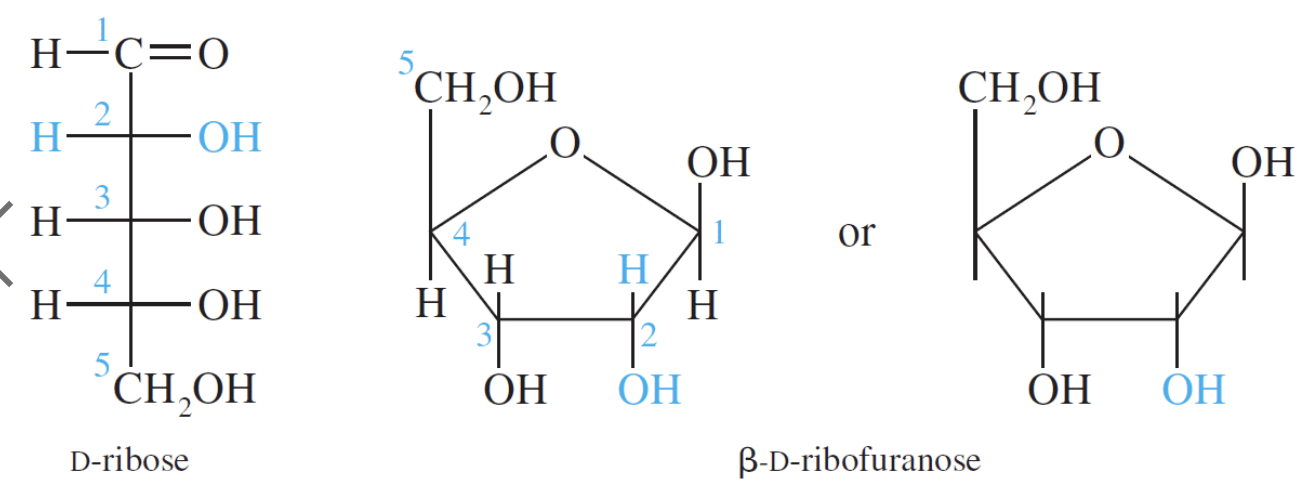
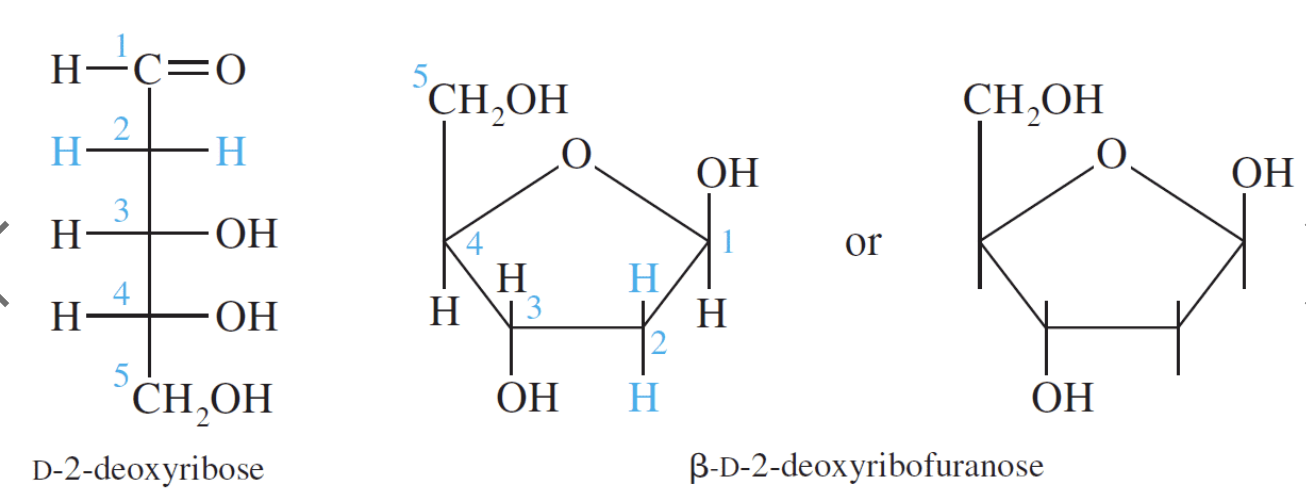
D–Ribose and its derivative D–2–deoxyribose are the most important pentoses because they are found in the nucleic acids RNA and DNA. The 2–deoxy in D–2–deoxyribose means an oxygen is omitted from the D–ribose molecule at carbon #2.
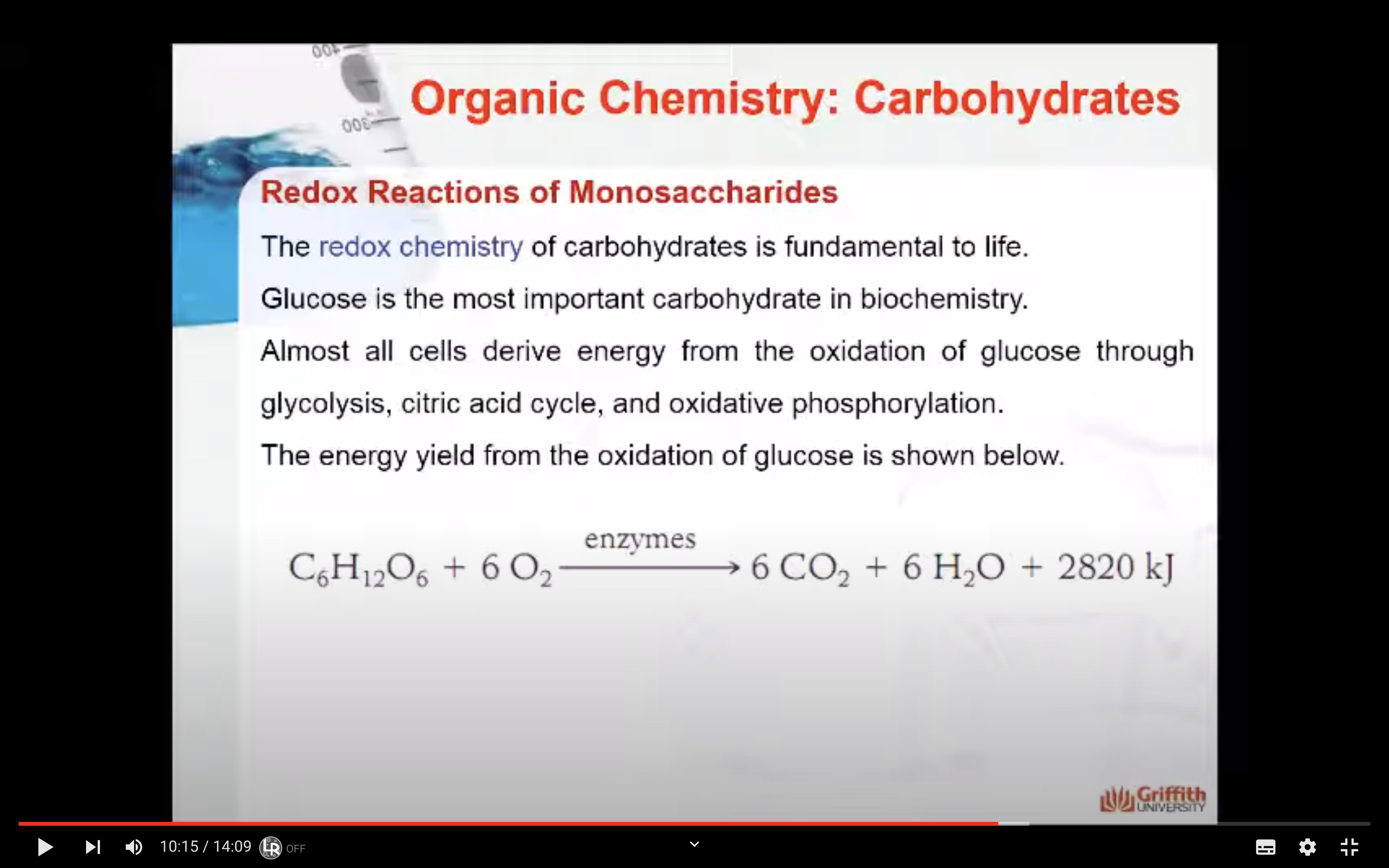
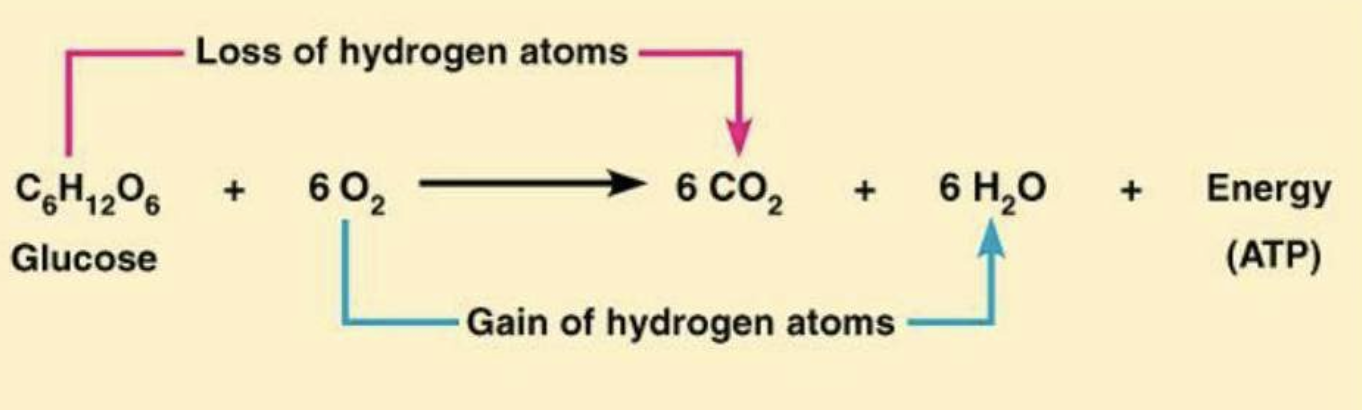
The redox chemistry of carbohydrates is fundamental to life. Glucose is the most important carbohydrate in biochemistry. Almost all cells derive energy from the oxidation of glucose through glycolysis, citric acid cycle, and oxidative phosphorylation. The energy yield from the oxidation of glucose is shown below:
C6H12O6 + 6 O2 ---enzymes---> 6 CO2 + 6H2O + 280 kJ (stored as ATP)
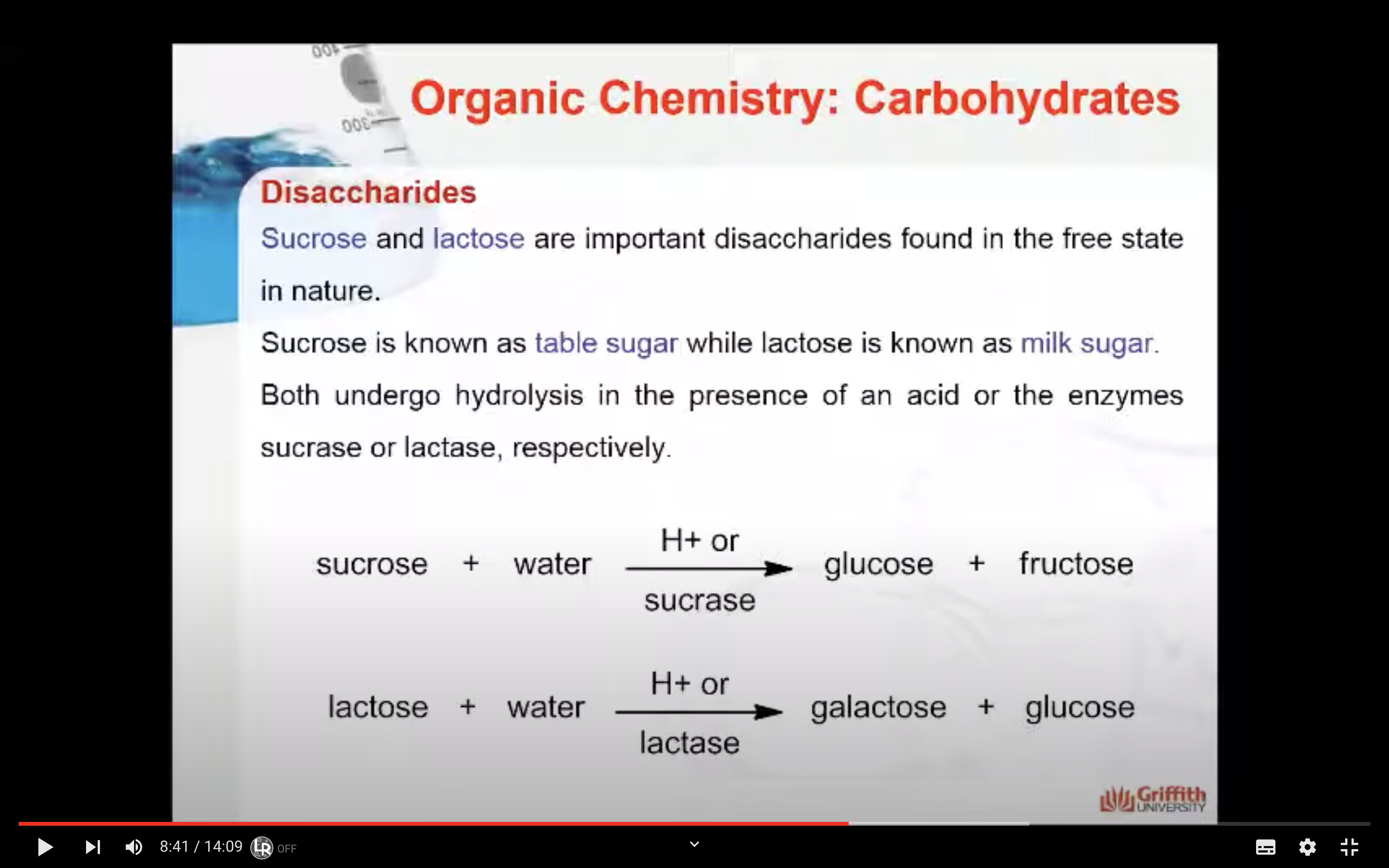
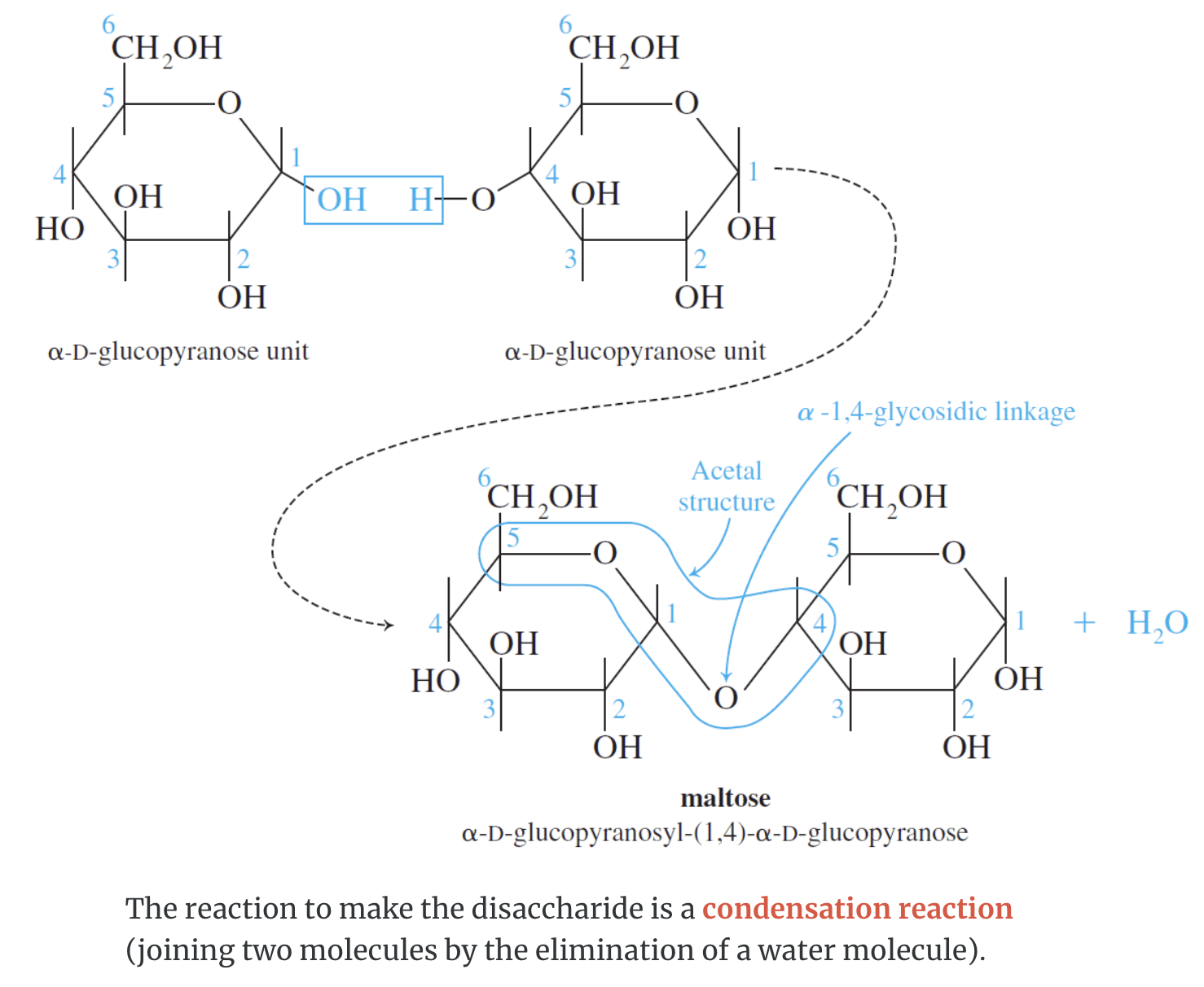
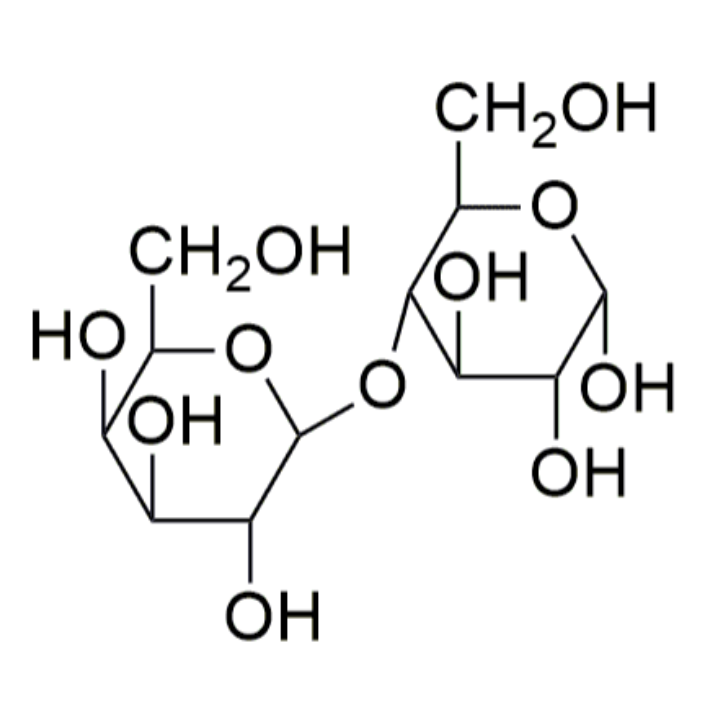
Disaccharides
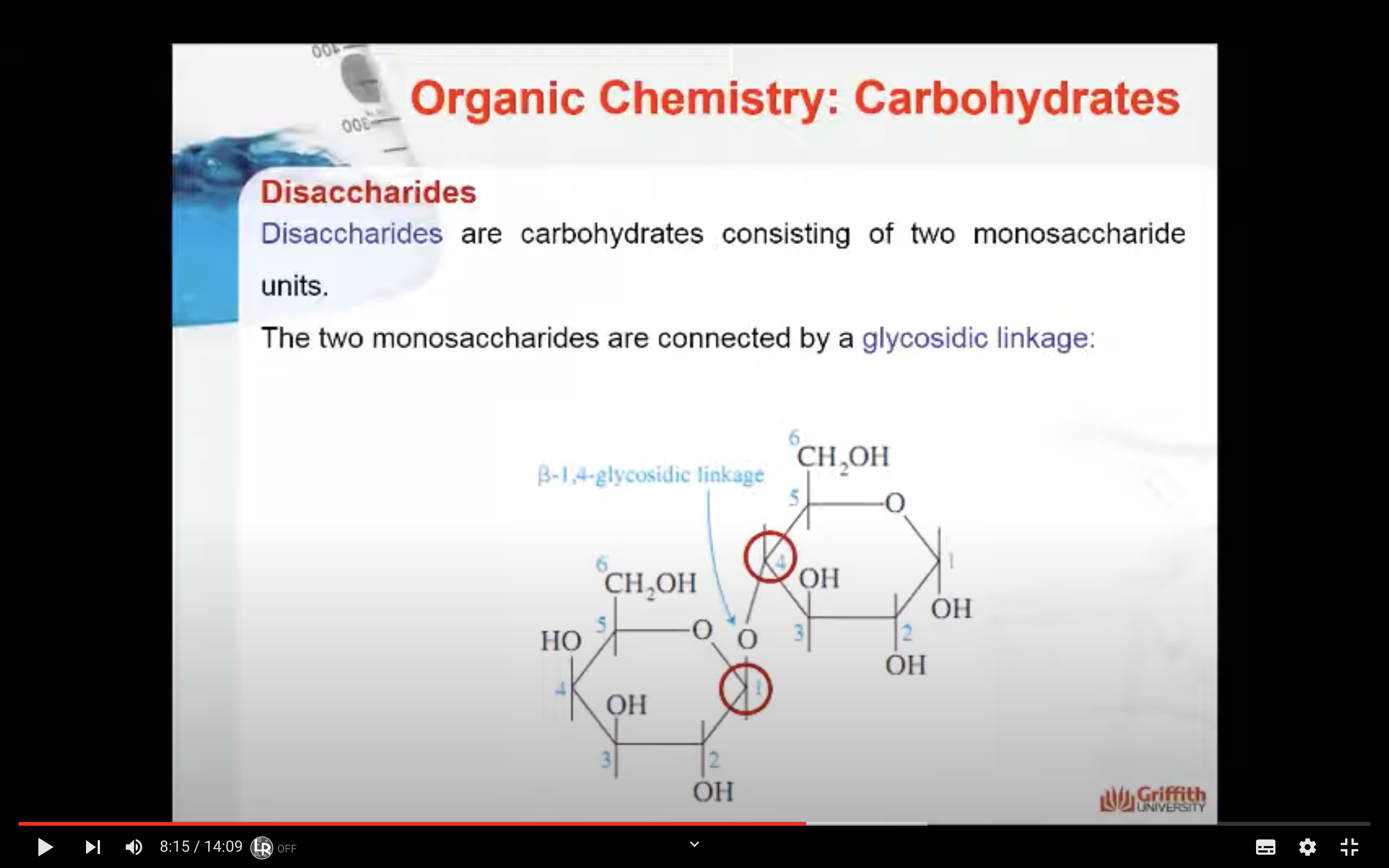
Disaccharides are carbohydrates consisting of two monosaccharide units. The two monosaccharides are connected by a glycosidic linkage as shown here for the disaccharide lactose.
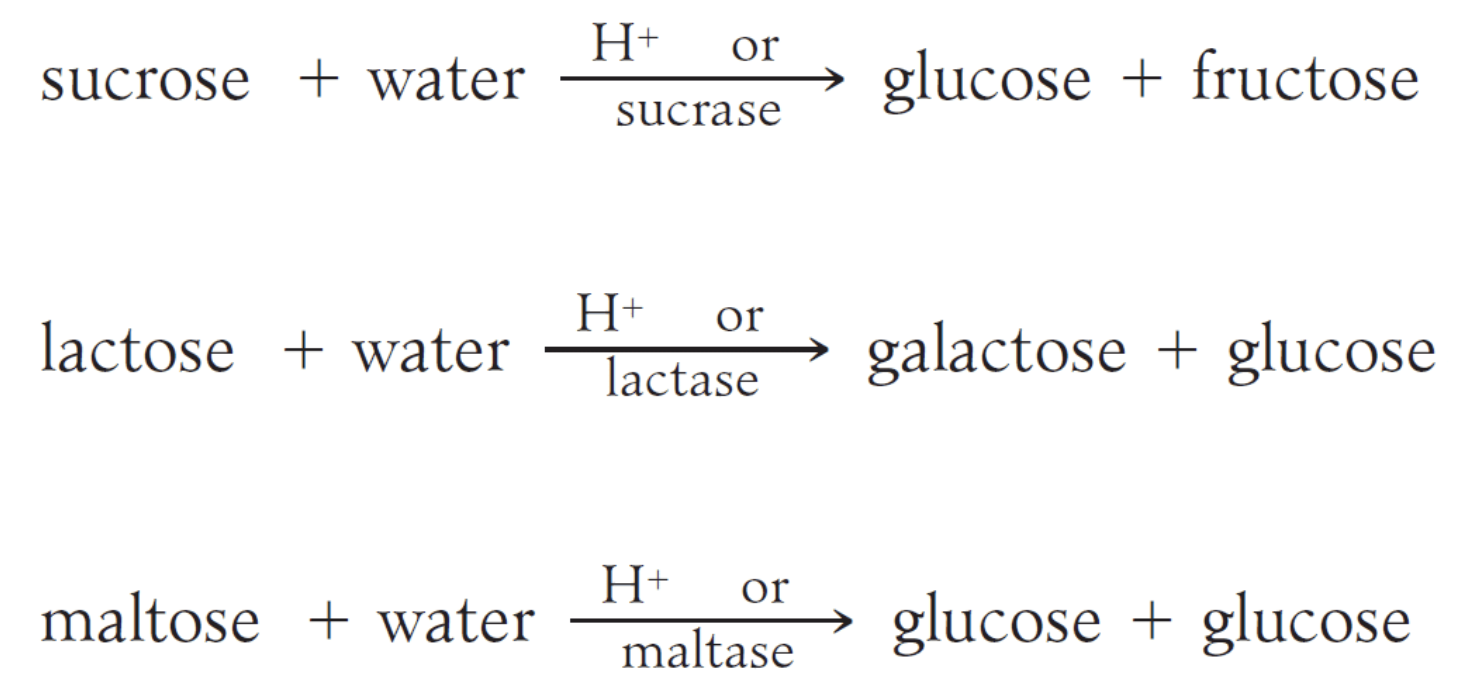
Sucrose and lactose are important disaccharides found in the free state in nature.
Sucrose is known as table sugar while lactose is known as milk sugar. Both undergo hydrolysis in the presence of an acid or the enzymes sucrase or lactase, respectively.

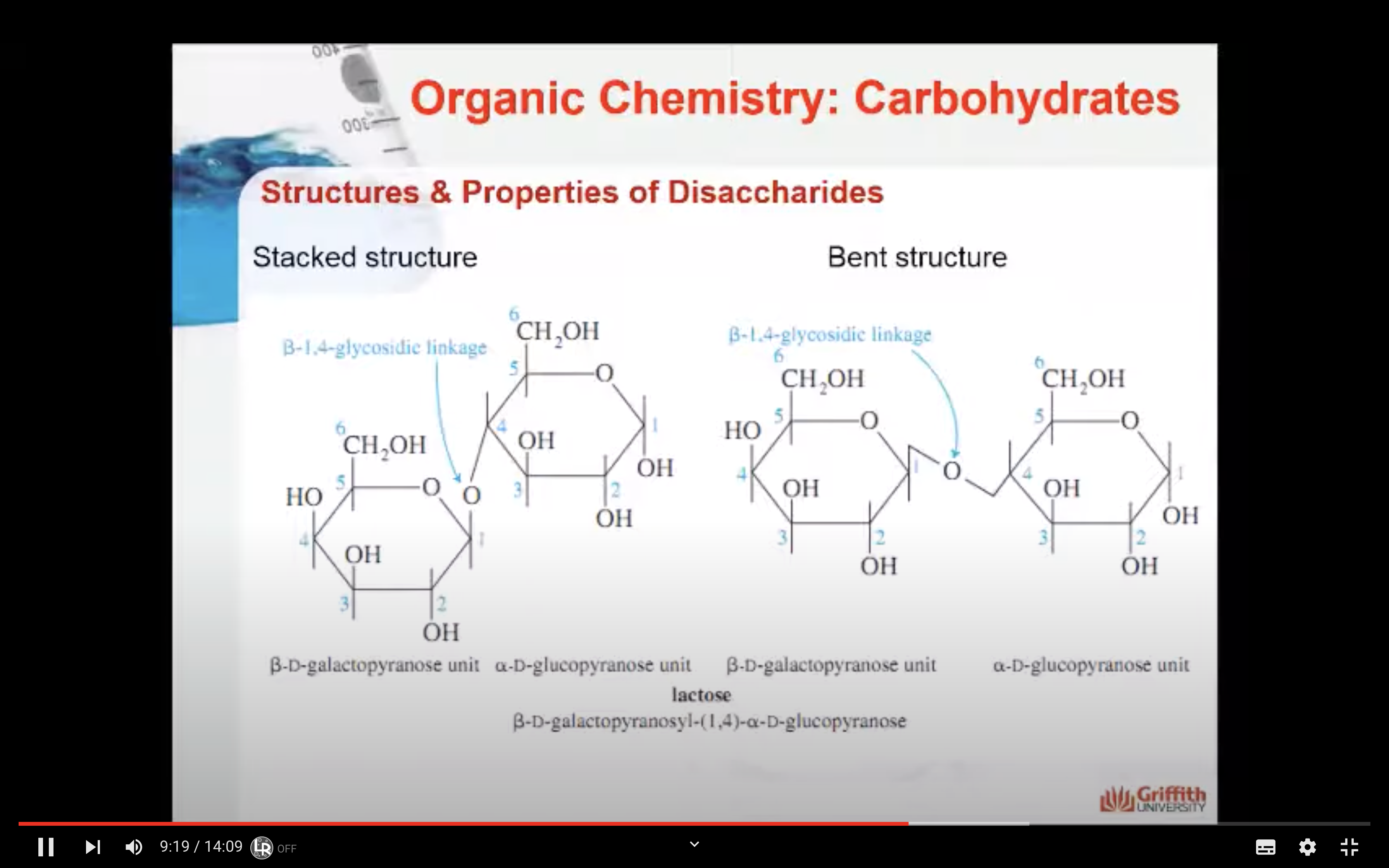
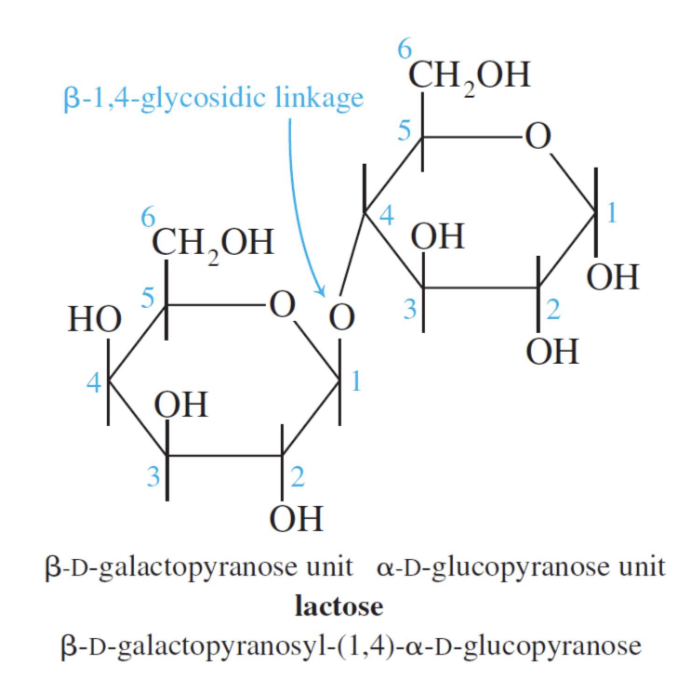
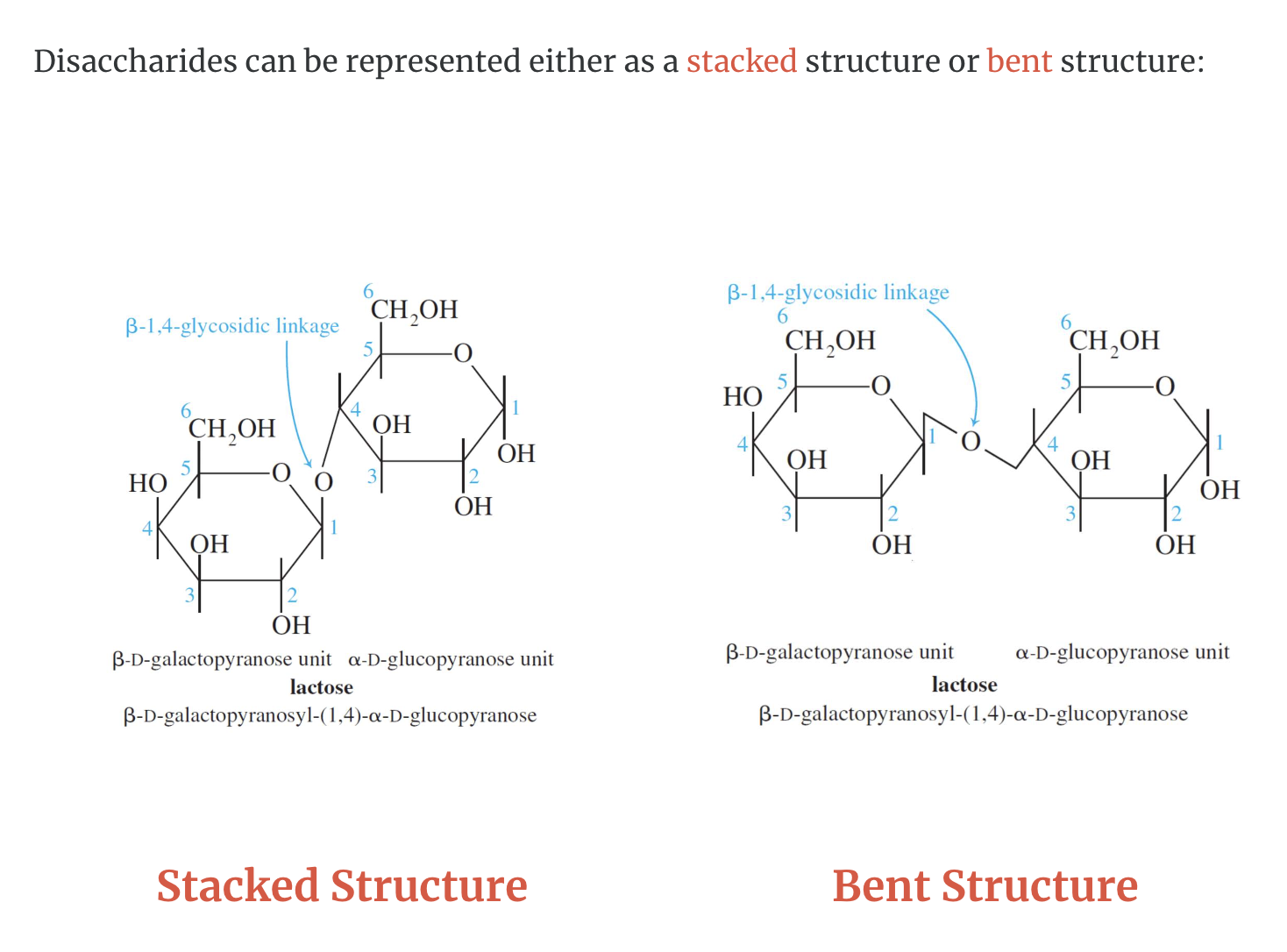
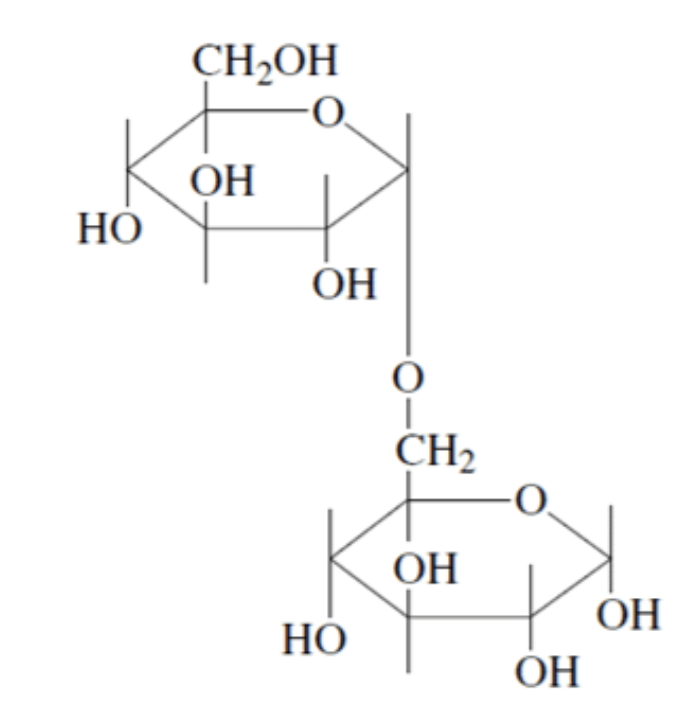
LACTOSE
Lactose consists of a β–D-galactopyranose unit (shown on the left) linked to an α–D-glucopyranose unit (on the right).
These are joined by a β–1,4-glycosidic linkage from carbon 1 on galactose to carbon 4 on glucose.
The systematic (IUPAC) name for lactose is β–D-galactopyranosyl-(1,4)-α–D-glucopyranose.
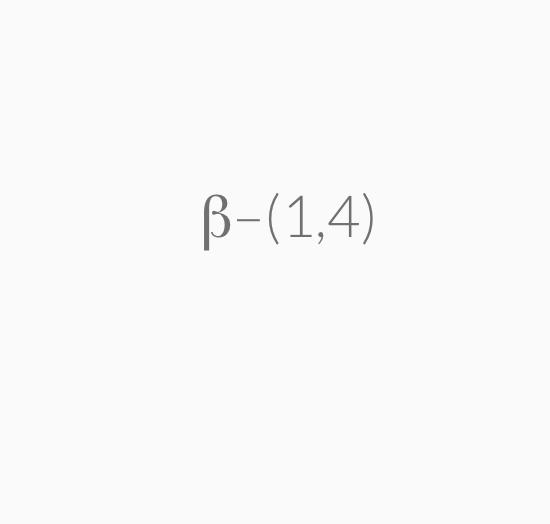
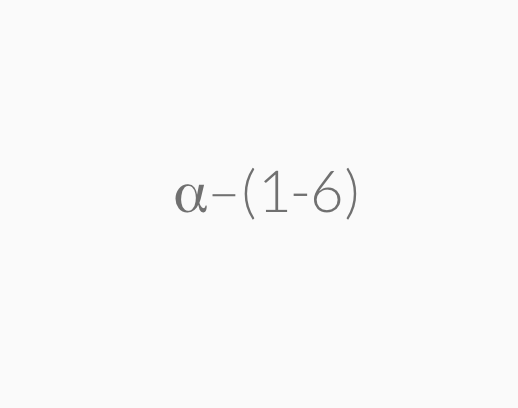
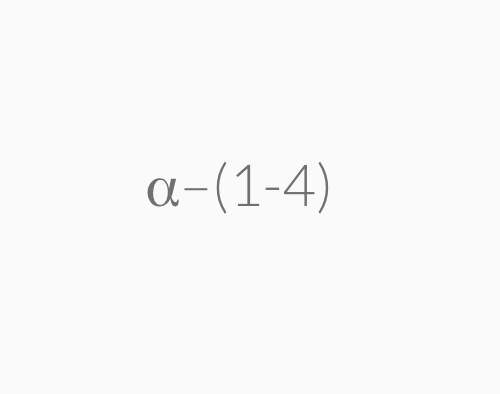
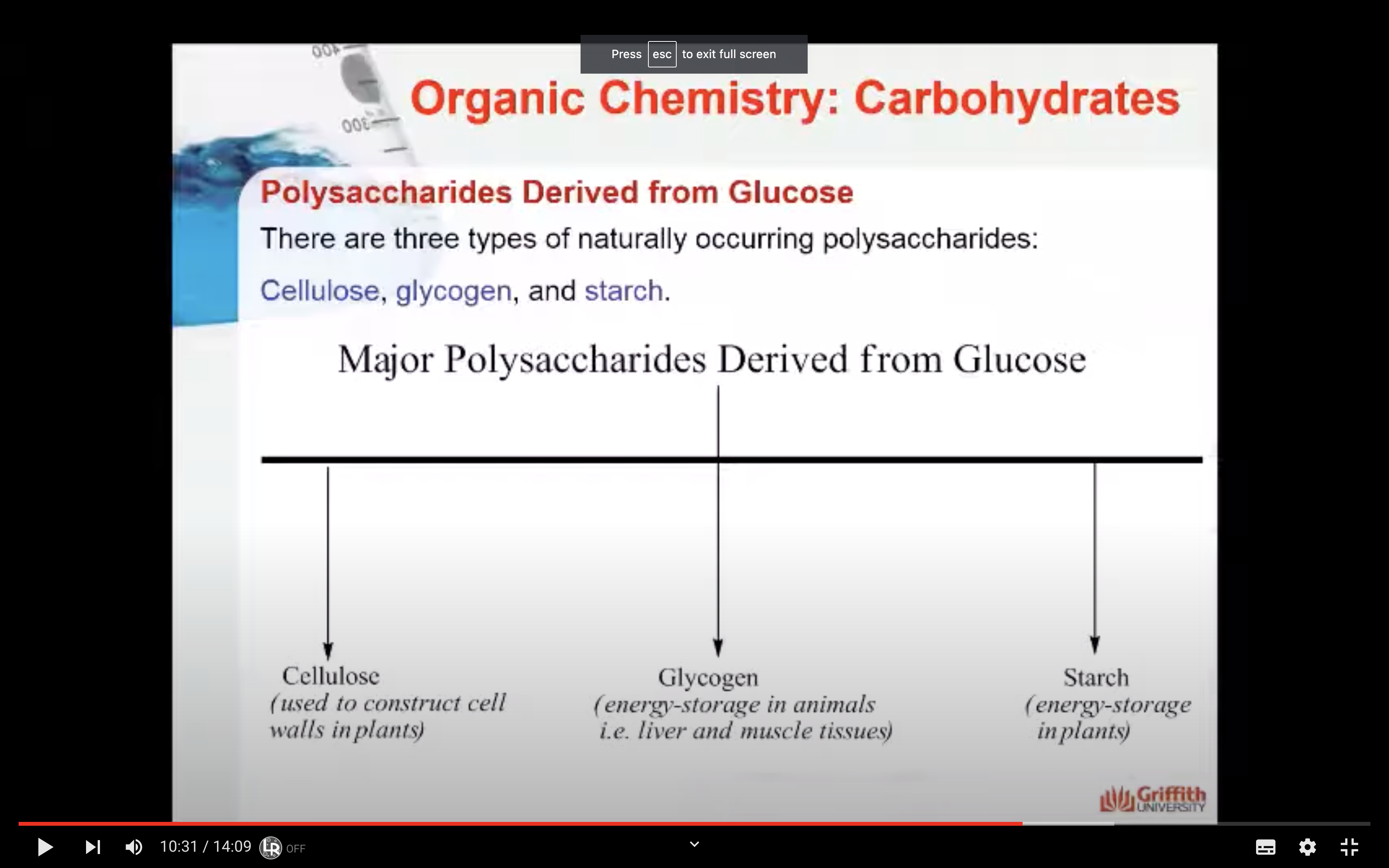
Polysaccharides & Complex Polysaccharides
There are three types of naturally occurring polysaccharides. They are starch, glycogen, and cellulose that are of major importance.

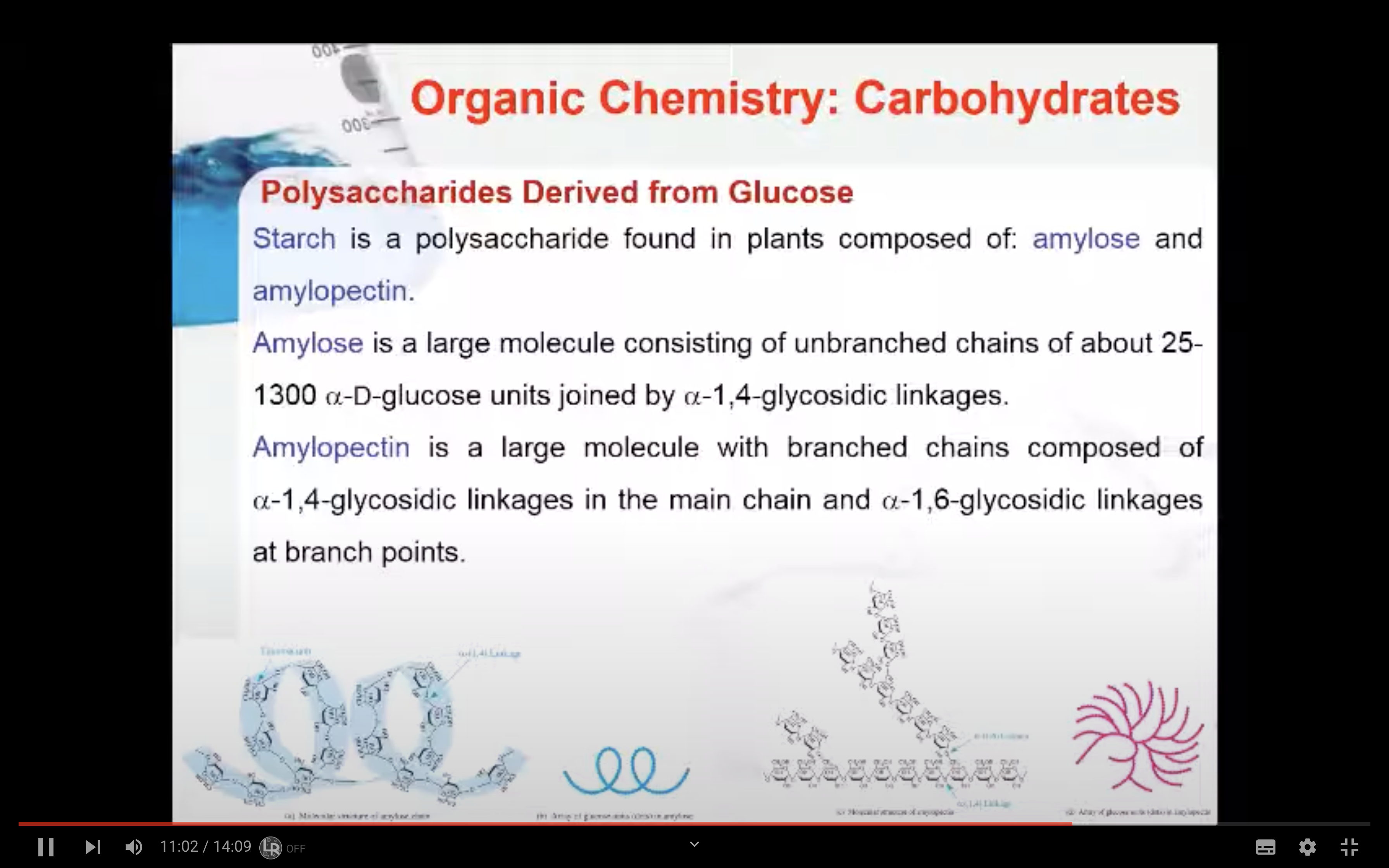
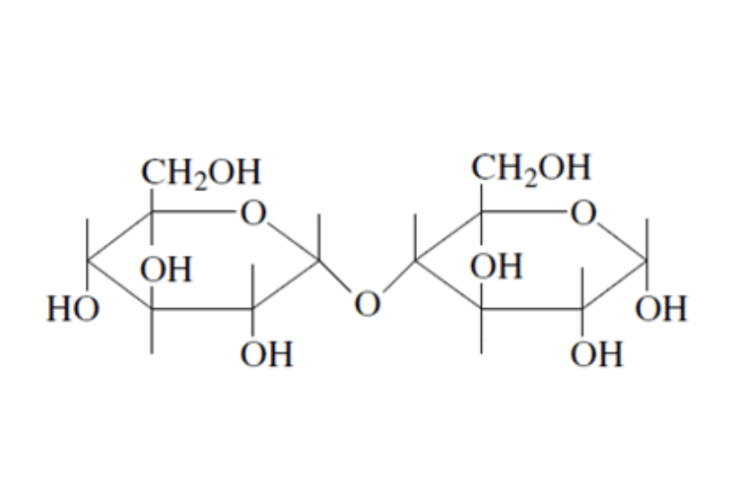
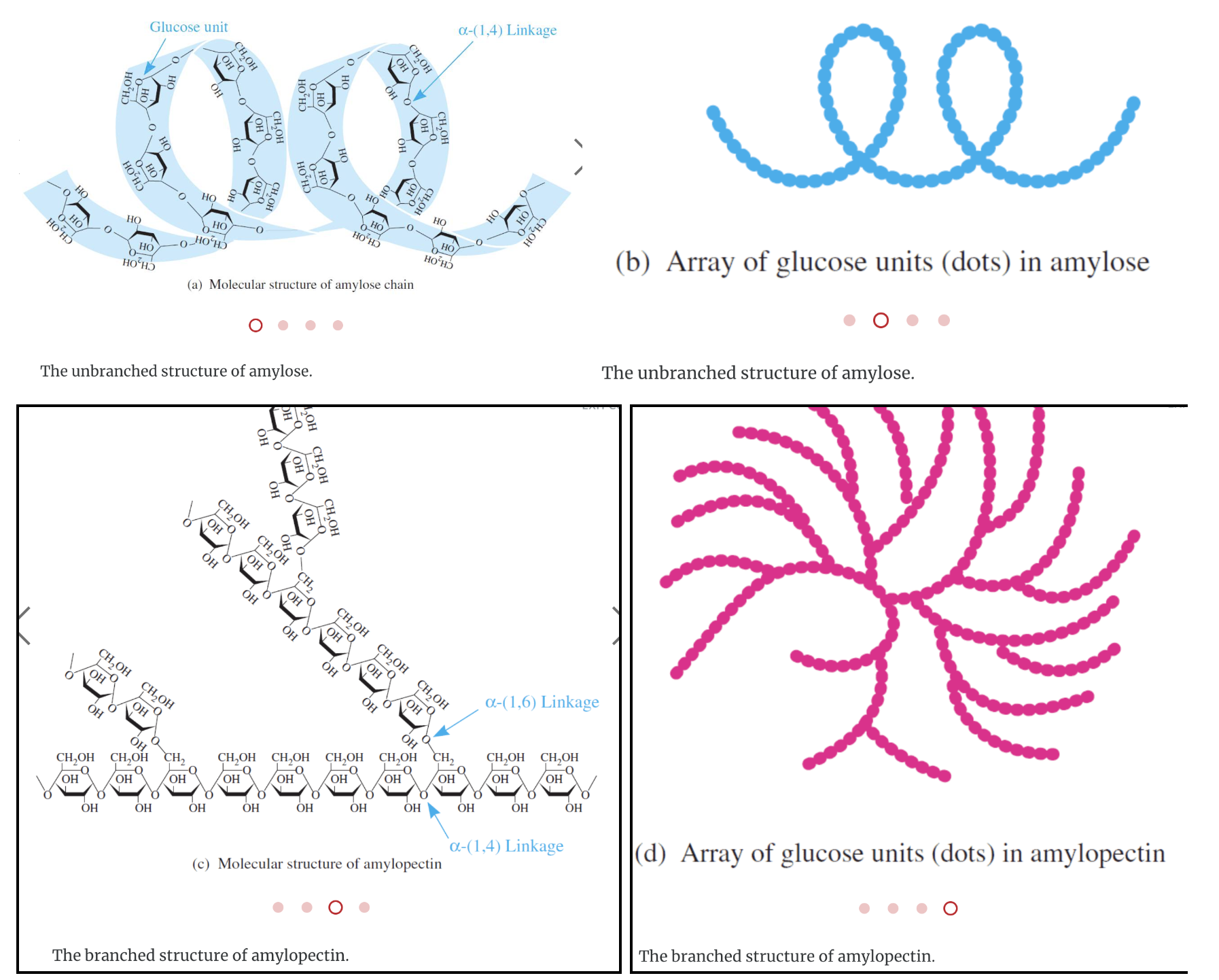
Starch is a polysaccharide found in plants to provide/store energy. Starch is composed of amylopectin and amylose.

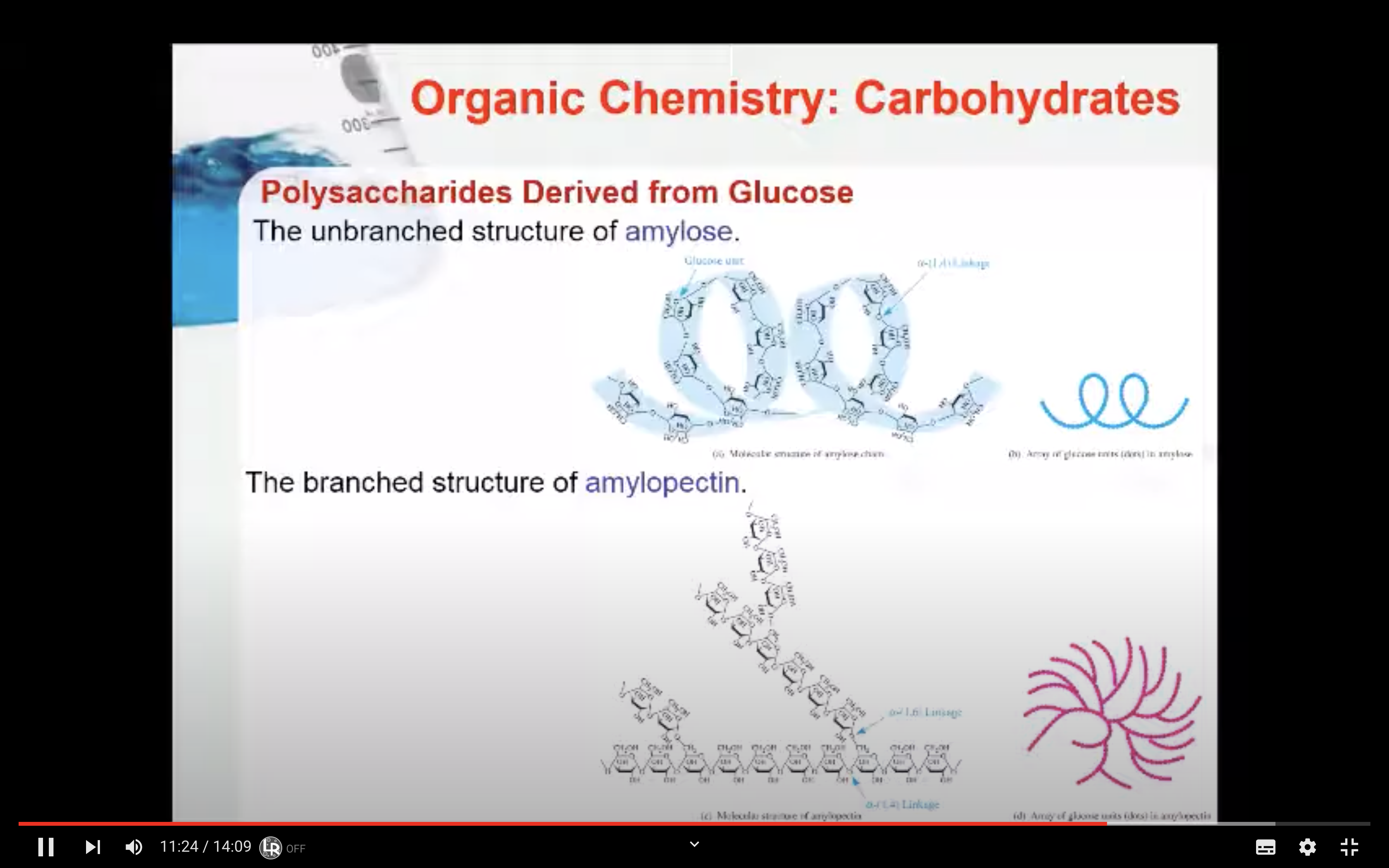
Amylopectin is a large molecule with branched chains composed of α–1,4–glycosidic linkages in the main chain and α–1,6–glycosidic linkages at branch points.
Amylose is a large molecule consisting of unbranched chains composed of about 25–1300 α‑D–glucose units joined by α–1,4–glycosidic linkages.
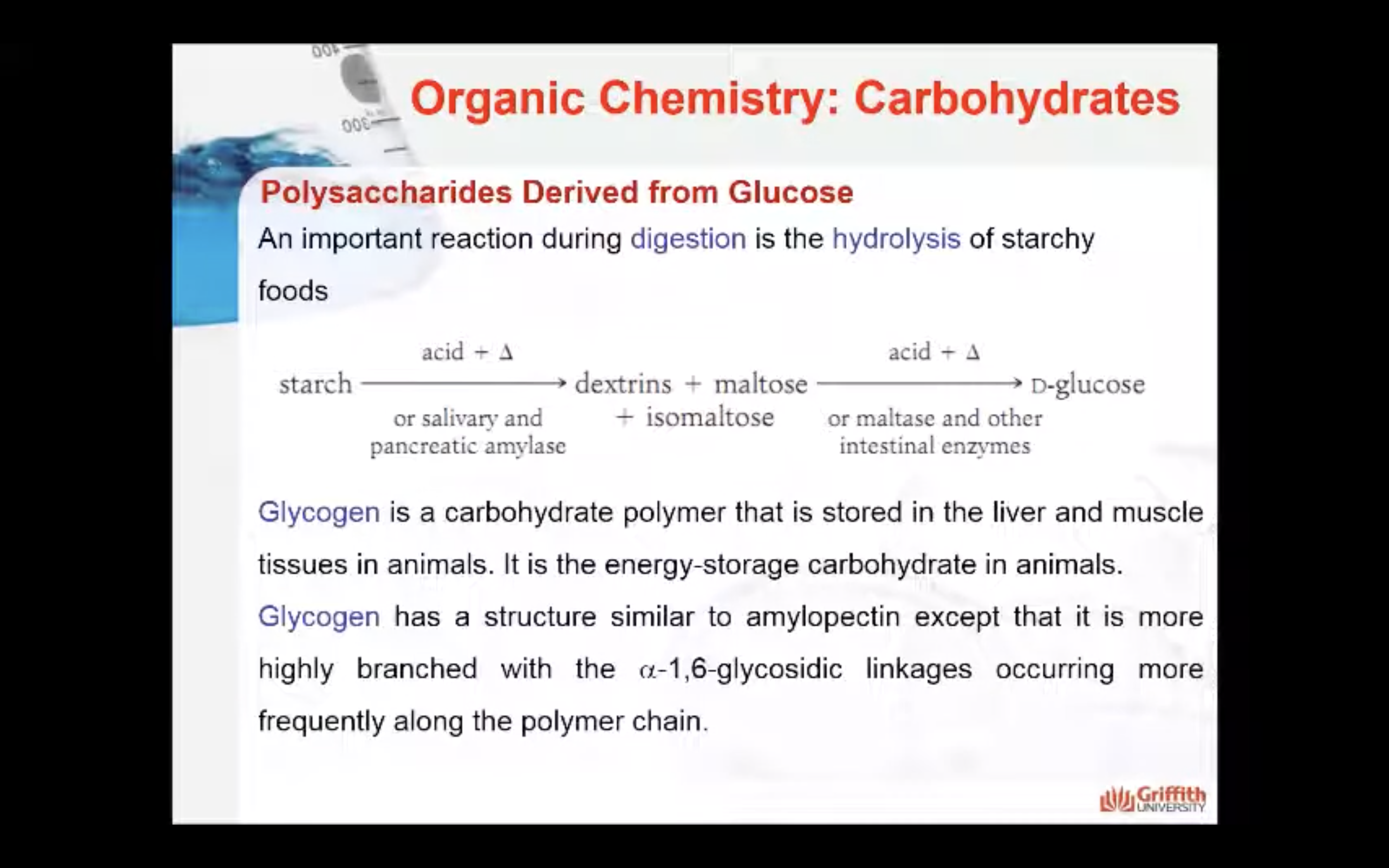
Starch hydrolysis
An important reaction during digestion is the hydrolysis of starchy foods. The partial hydrolysis of amylose yields the disaccharide maltose. The partial hydrolysis of amylopectin yields both maltose and related disaccharide isomaltose.

Glycogen
Glycogen is a carbohydrate polymer that is stored in the liver and muscle tissues in animals. It is the energy–storage carbohydrate in animals. Glycogen has a structure similar to amylopectin except that it is more highly branched with the α–1,6–glycosidic linkages occurring more frequently along the polymer chain.

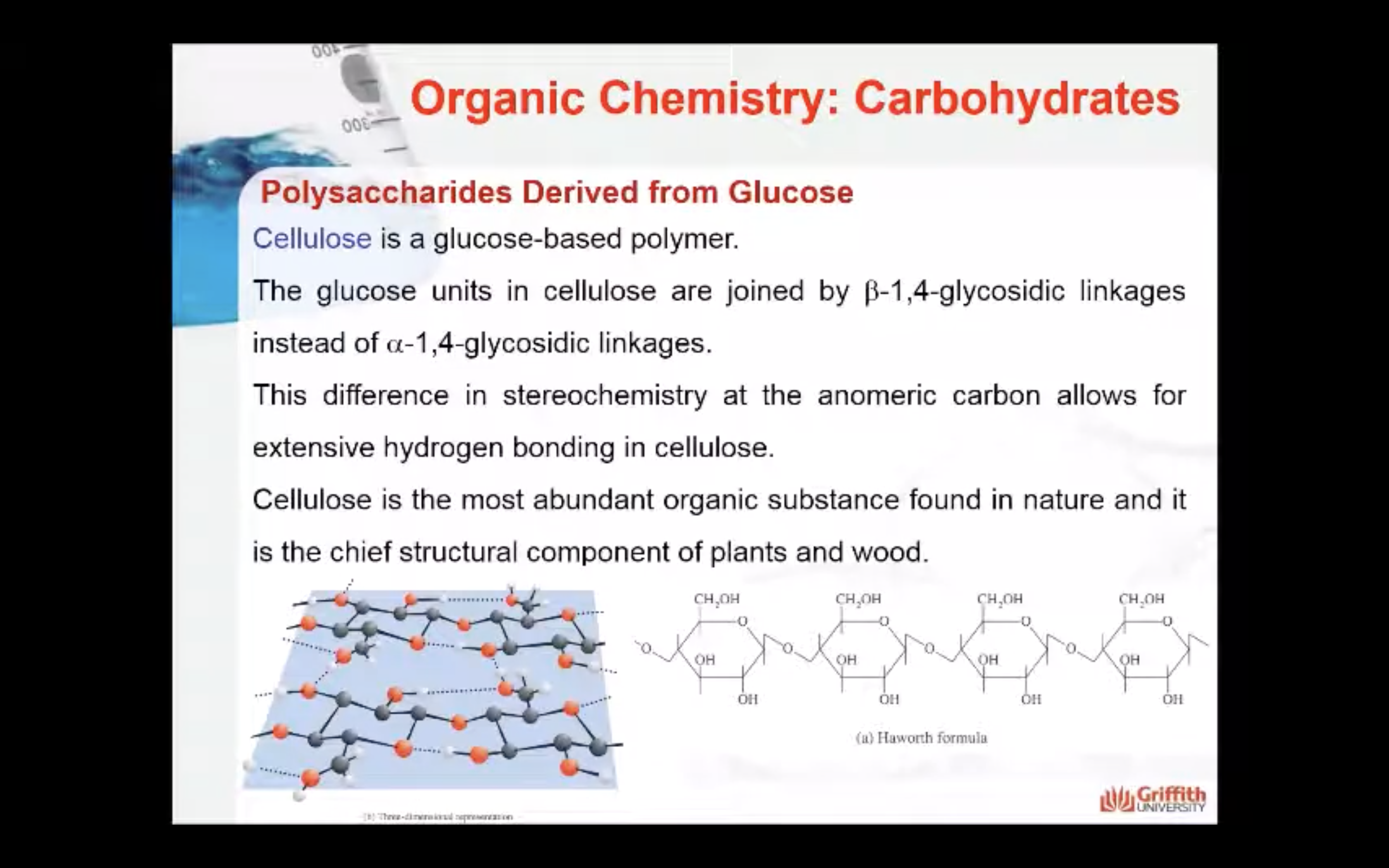
Cellulose
Cellulose, like starch and glycogen, is a glucose-based polymer. The glucose units in cellulose are joined by β–1,4–glycosidic linkages instead of α–1,4–glycosidic linkages. This difference in stereochemistry at the anomeric carbon allows for extensive hydrogen bonding in cellulose. Cellulose is the most abundant organic substance found in nature and it is the chief structural component of plants and wood.
Humans do not have the enzyme to break down the β–1,4 linkage so there is no energy associated with eating that type of carbohydrates. Cellulose is considered fibers.
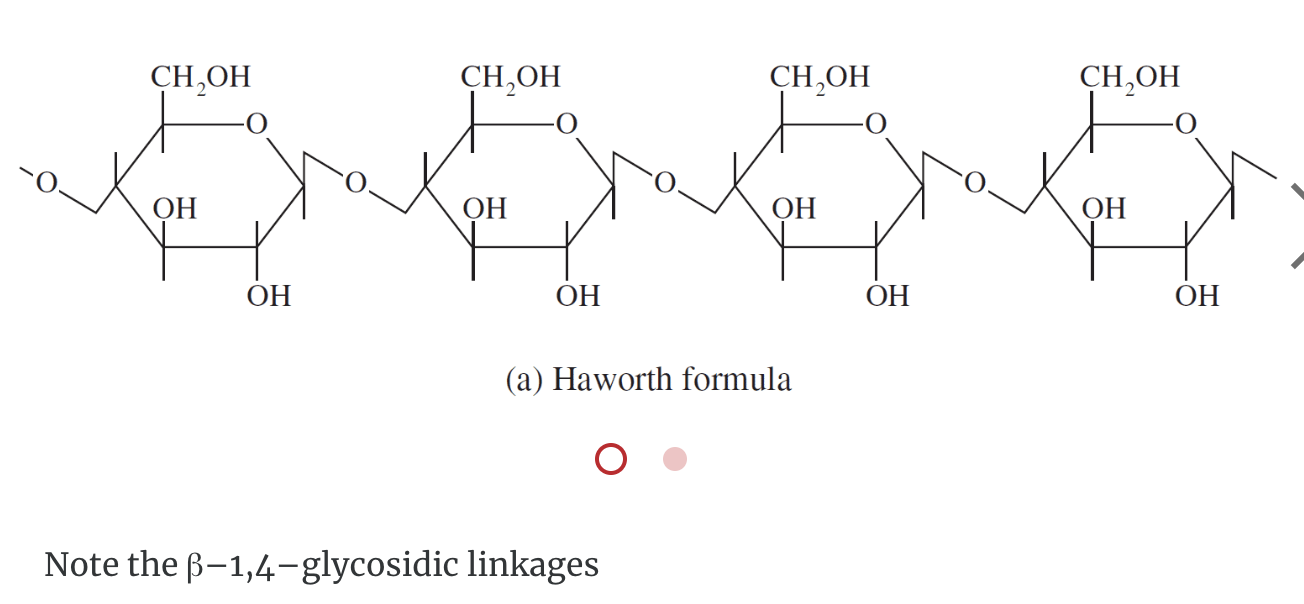
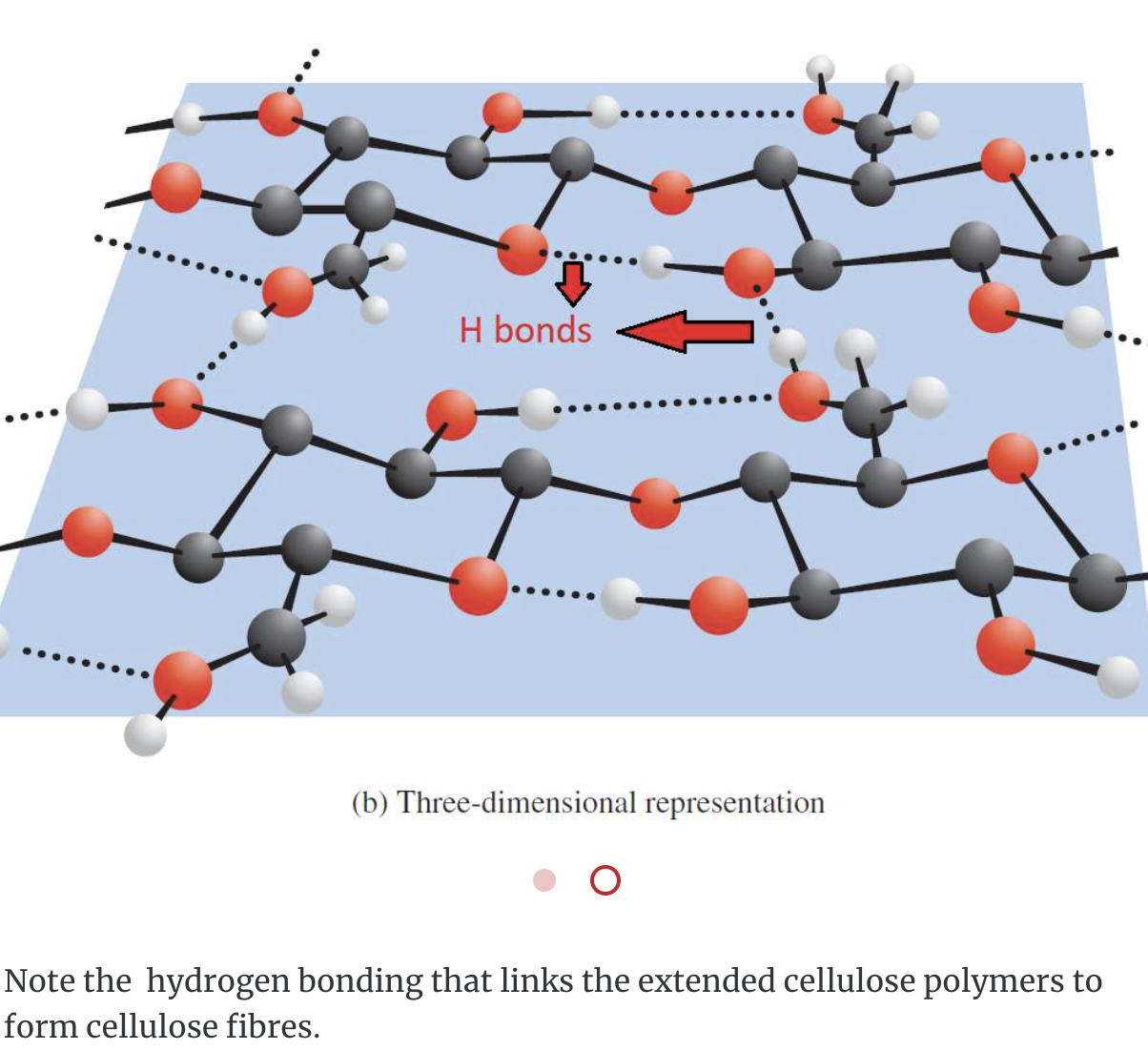
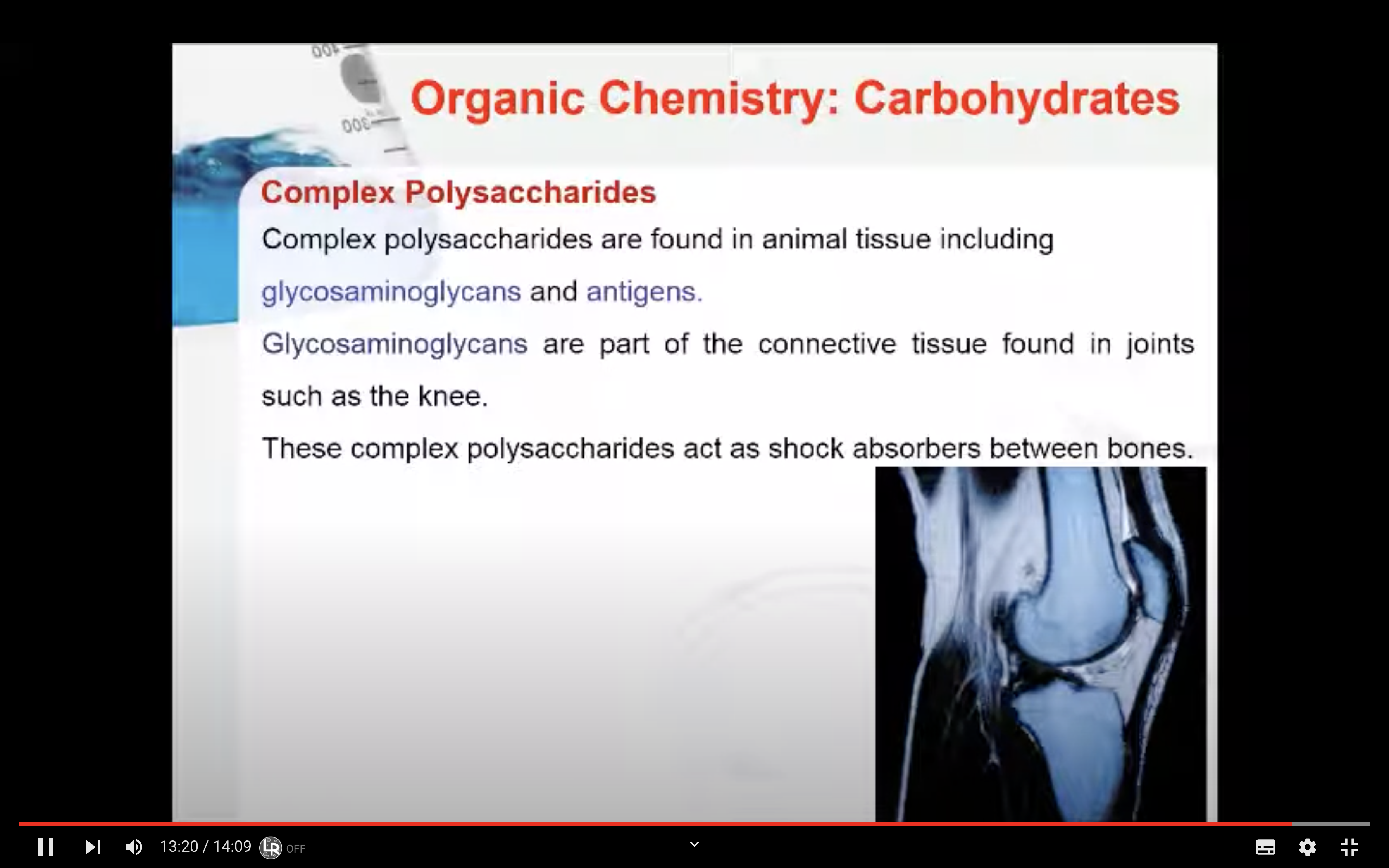
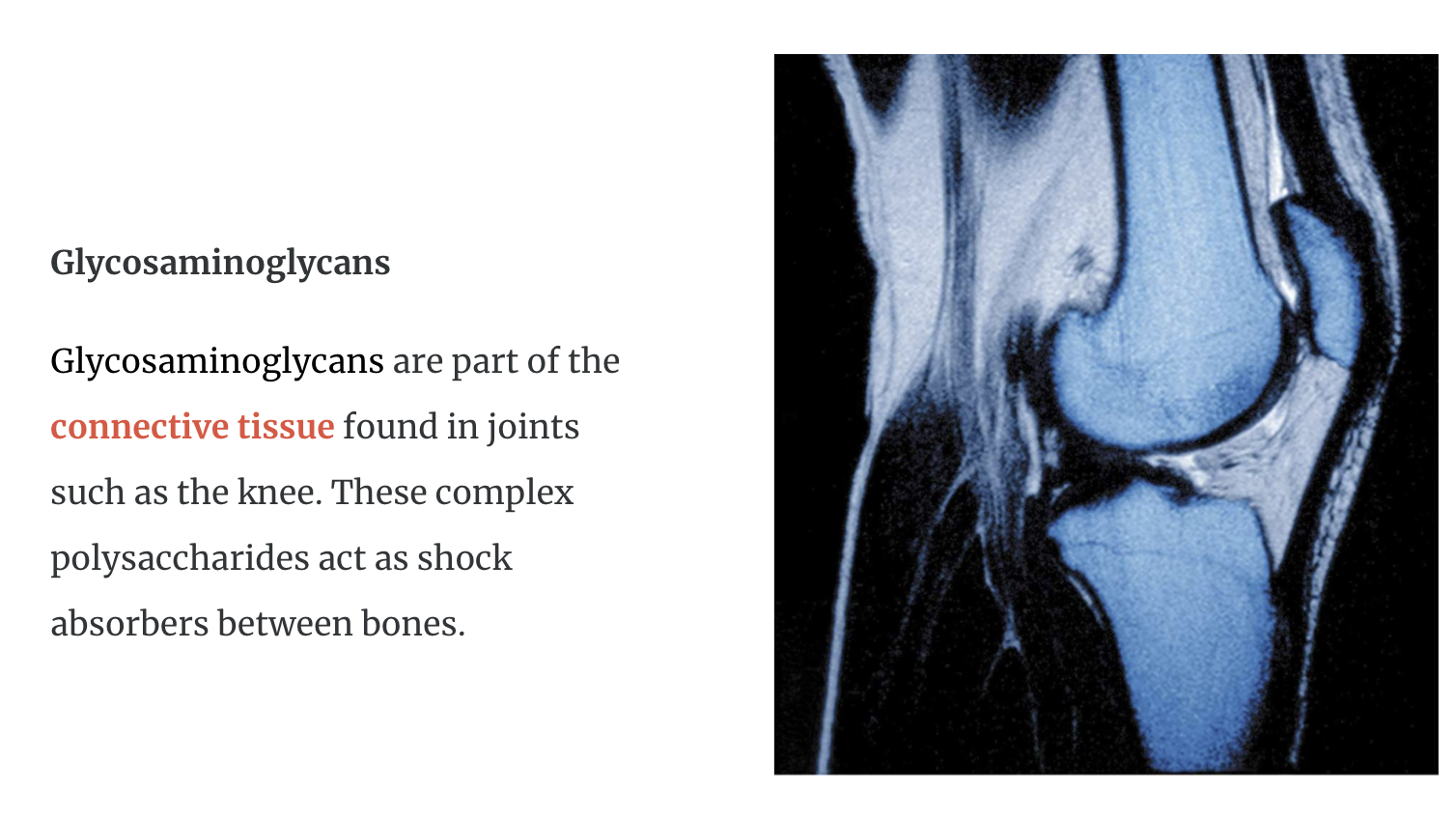
Complex Polysaccharides
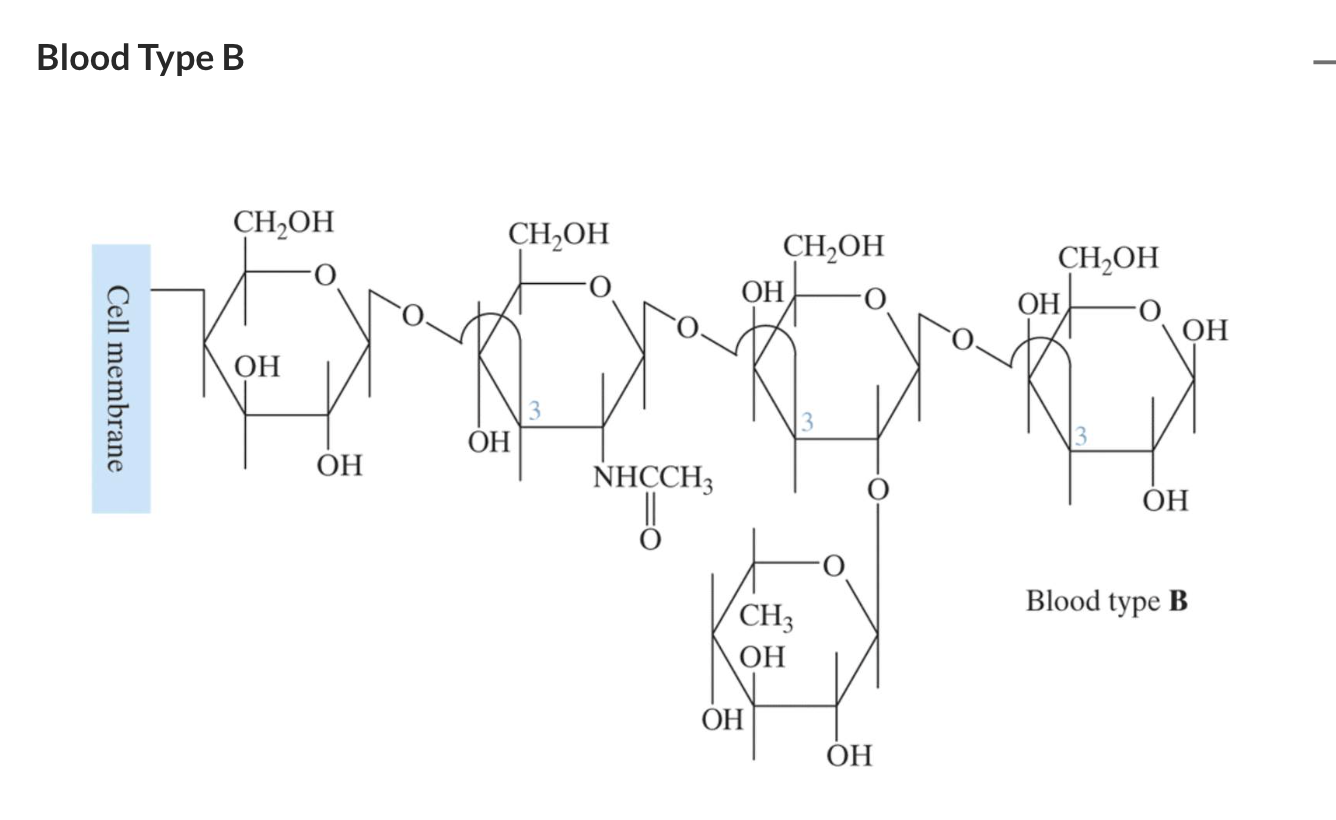
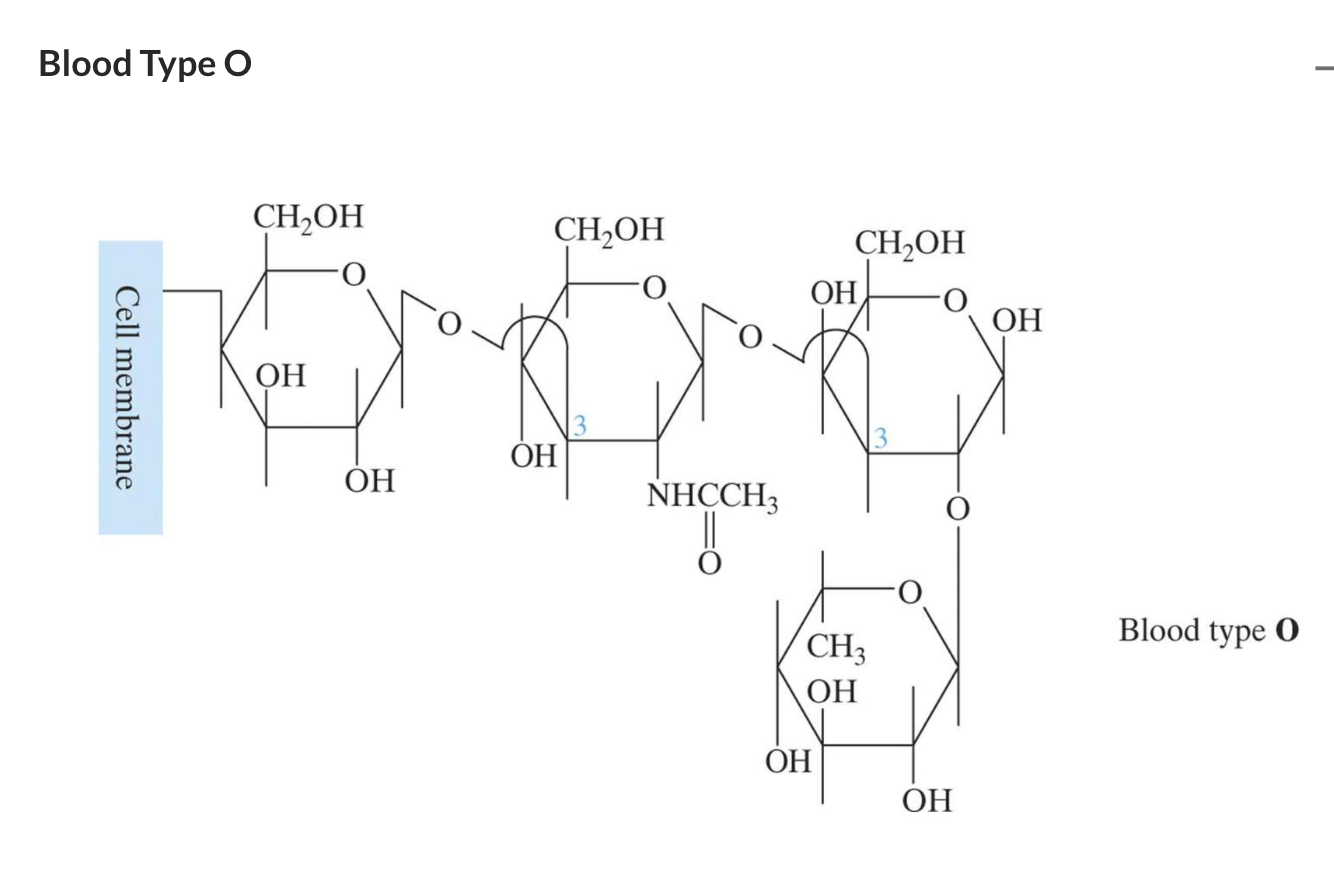
They are found in animal tissue including glycosaminoglycans and antigens.
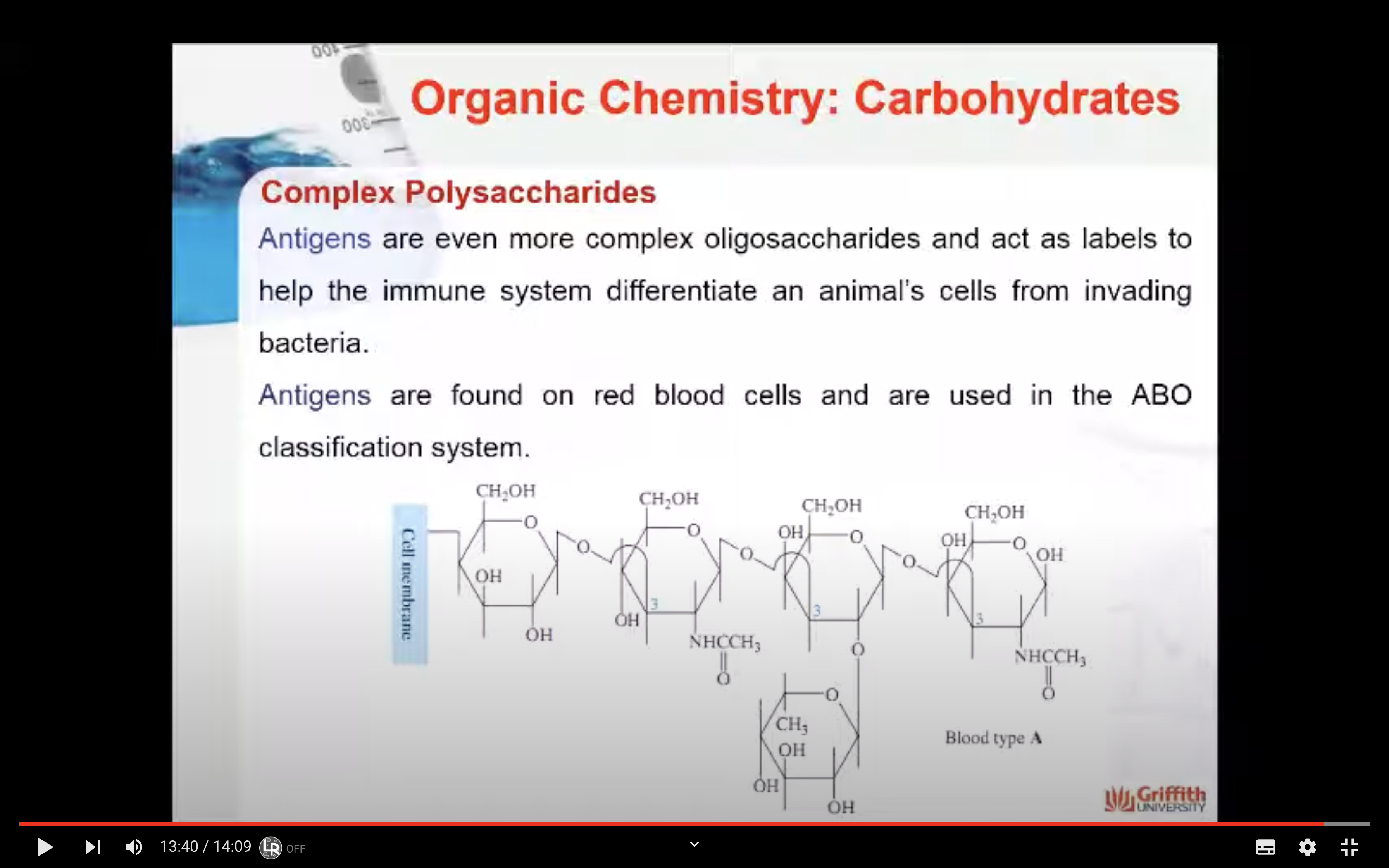
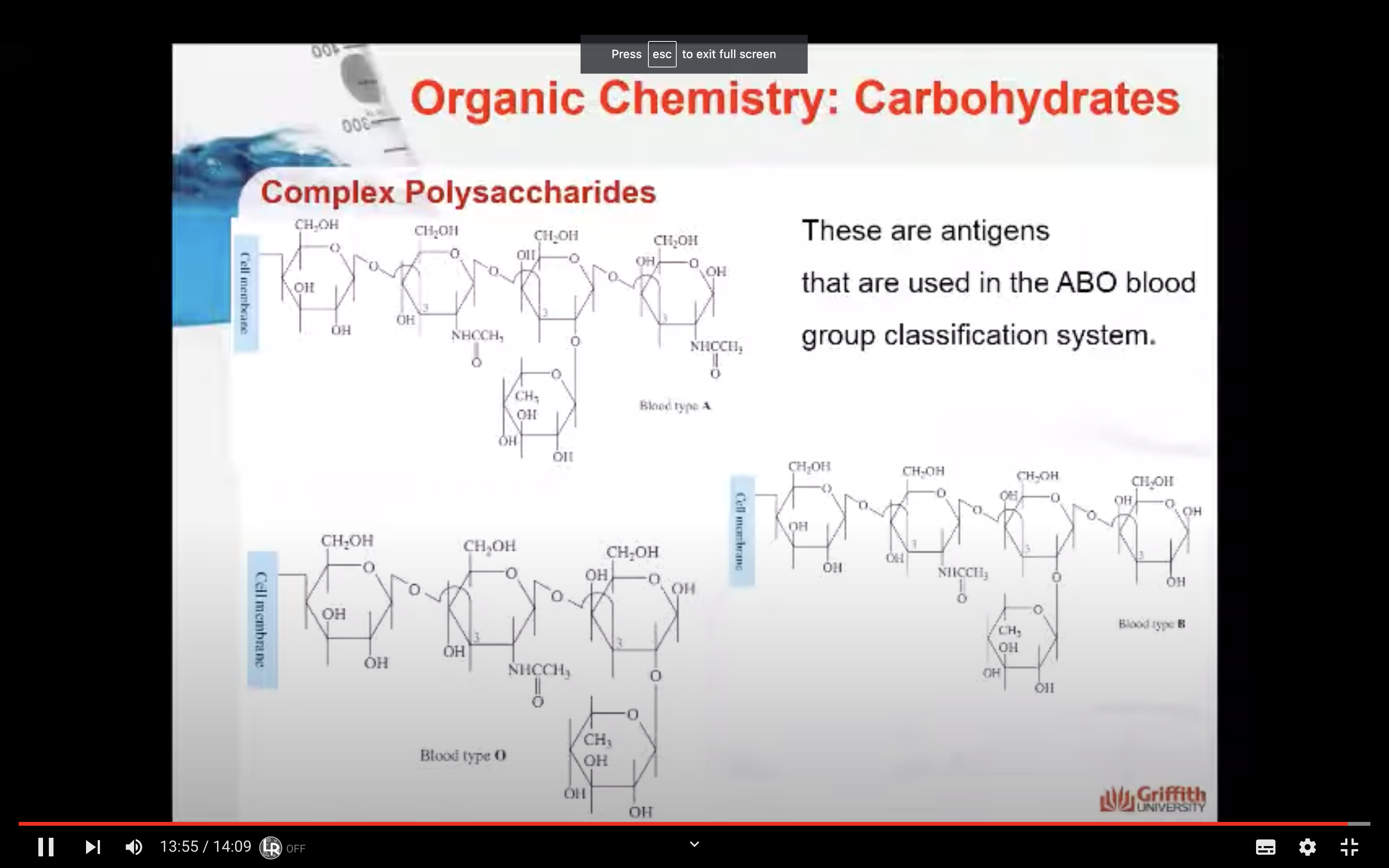
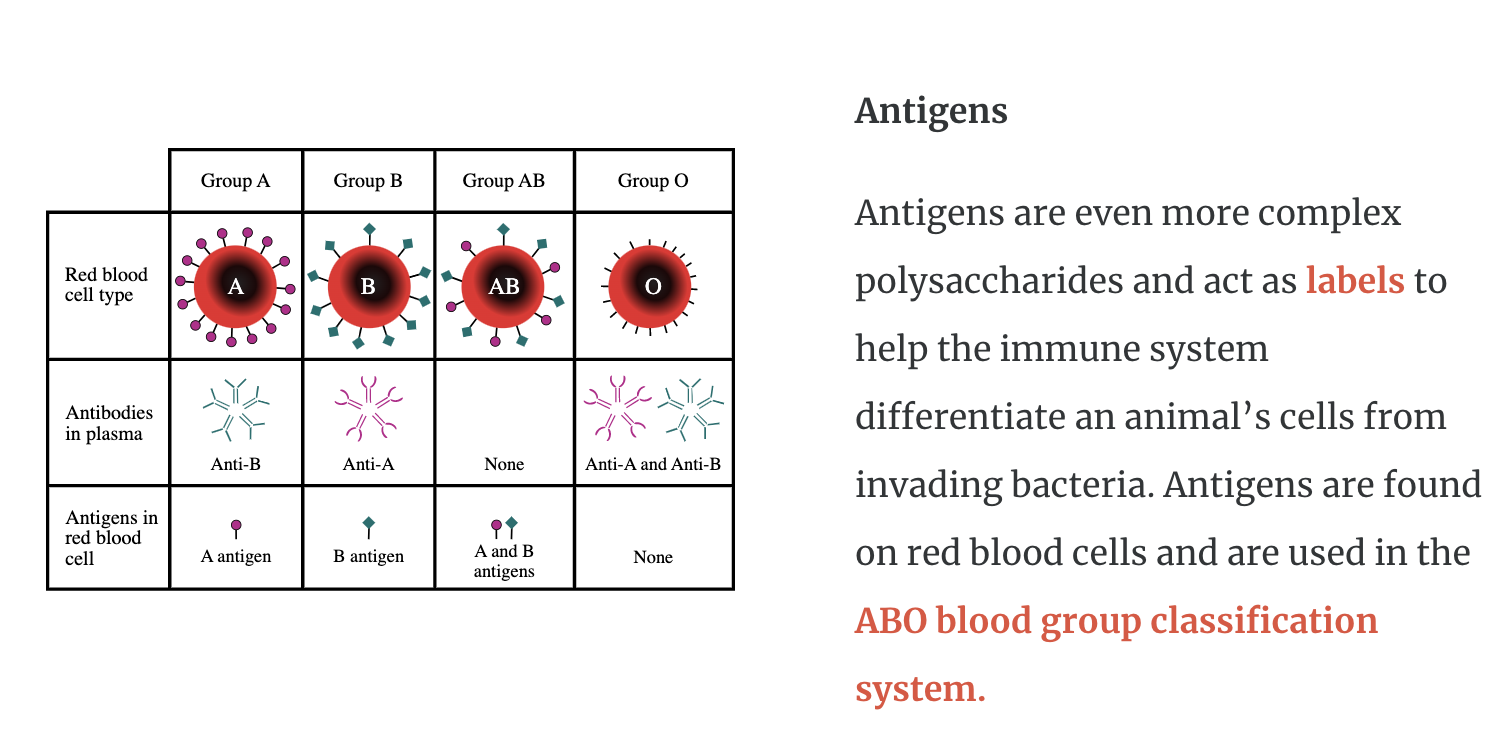
Antigens are even more complex polysaccharides and act as labels to help the immune system differentiate an animal’s cells from invading bacteria. Antigens are found on red blood cells and are used in the ABO blood group classification system.
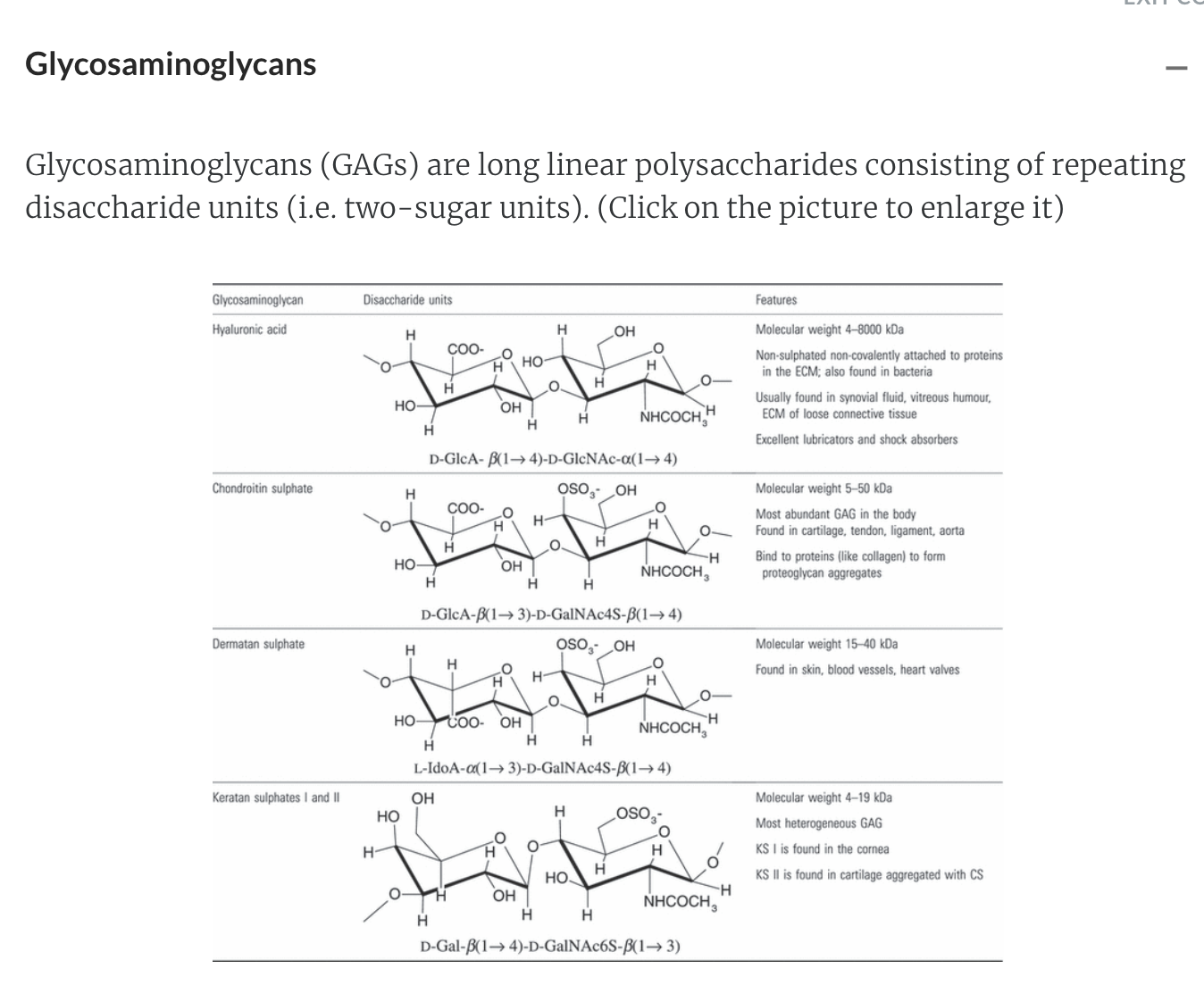

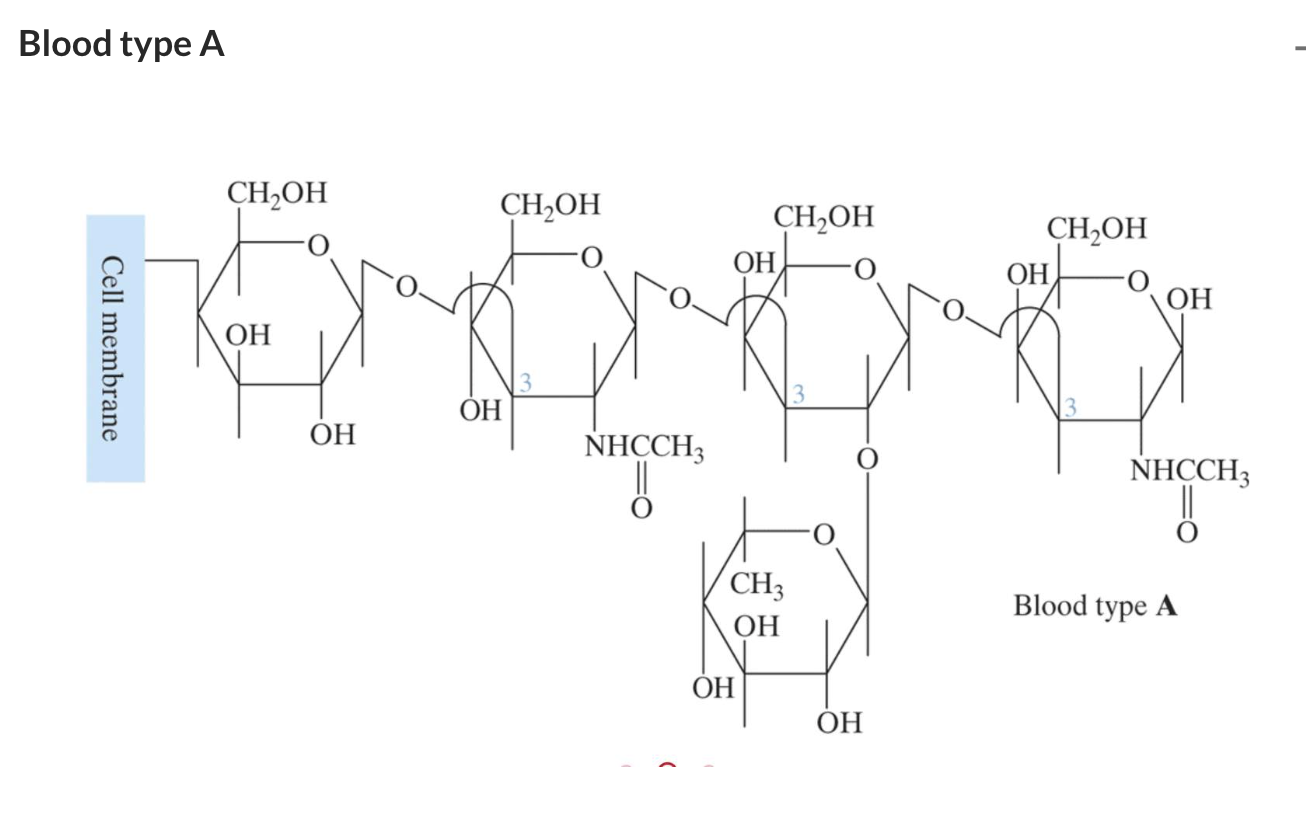
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (1) | 2023.05.13 |
|---|---|
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (0) | 2023.05.13 |
| [WEEK6] Stereoisomerism (0) | 2023.03.31 |
| [WEEK5] Carboxylic Acids, Esters (0) | 2023.03.30 |
| [WEEK5] Lipids (0) | 2023.03.30 |



