Mini lectures
Carboxylic acid

















Esters





Why esters are non polar?????















Introduction of carboxylic acid









Physical properties of carboxylic acid
The relative high boiling points of carboxylic acids are due to intermolecular attractions resulting from hydrogen bonding. For example, the boiling point of ethanoic acid is 119 °C, higher than the boiling point of ethanol, 78 °C.


as the length of the carbon chain increases, melting point and boiling point increase, and solubility in water decreases.


FATTY ACIDS

In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28.
Unsaturated fatty acids normally contain a double bond in the cis configuration. Trans fatty acids are less common, but have been implicated in many health problems. Saturated fatty acids contain no carbon-carbon double bonds.
CARBOXYLIC ACID IN BIOCHEMISTRY

Carboxylic acids are released in the blood by the liver in an excessive amount during ketoacidosis which is a condition associated with uncontrolled diabetes.
Normal amounts of carboxylic acid can be controlled by blood buffers and by respiration rate and kidney function.
L-Dopa (levodopa) is a carboxylic acid that is a derivative of dopamine and is used in the treatment of Parkinson’s disease.
The major types of carboxylic acids are:
- Unsaturated carboxylic acids
- Aromatic carboxylic acids
- Hydroxy acids
- Amino acids
Unsaturated carboxylic acids
An unsaturated carboxylic acid contains one or more C=C bonds. The C=C bond affects the physical and chemical properties of the acid.
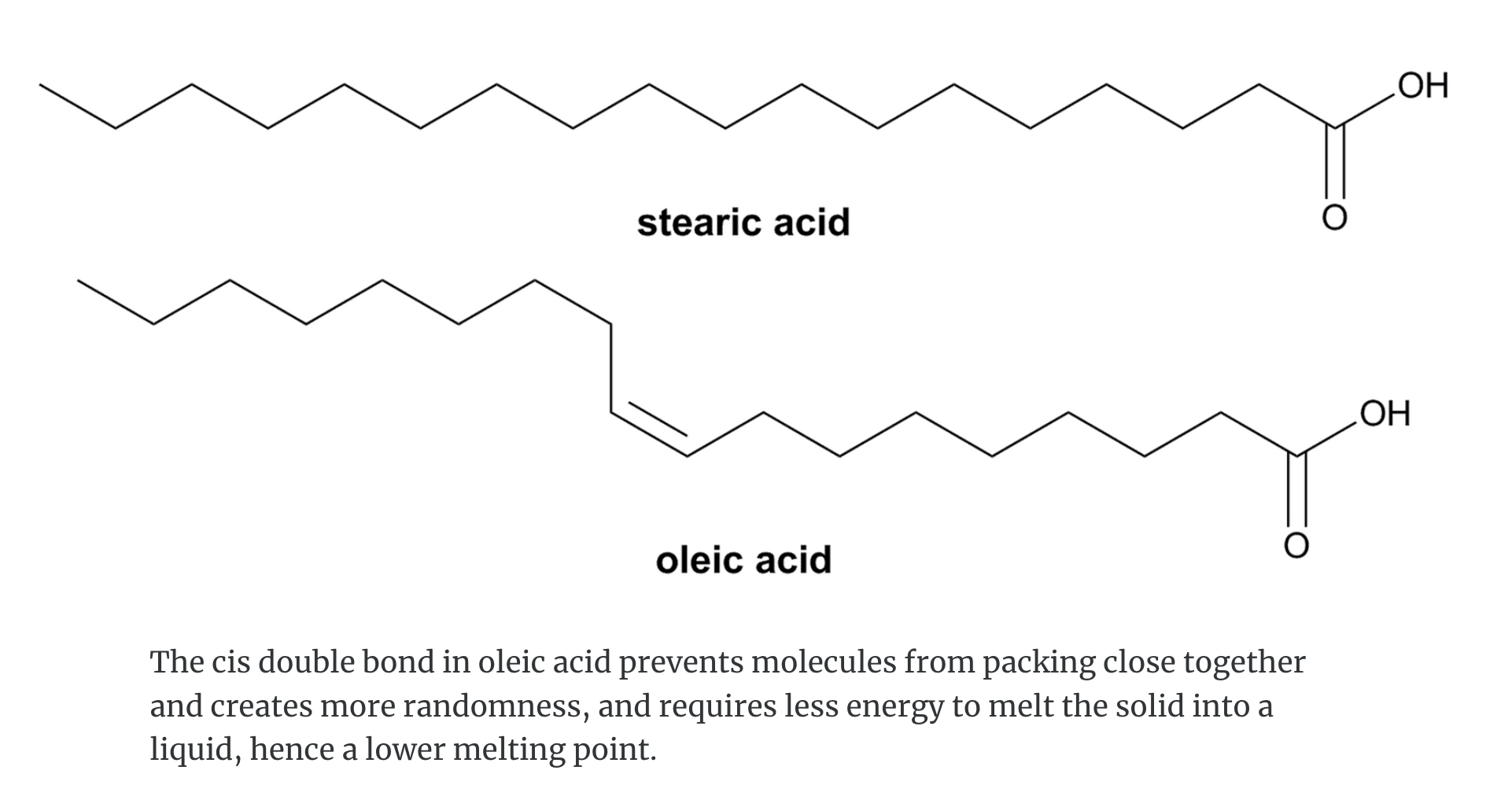
For example stearic acid, CH3(CH2)16COOH, and oleic acid, CH3(CH2)7CH=CH(CH2)7COOH, both contain 18 carbon atoms.
Stearic acid has no C=C bonds and melts at 70°C where as oleic acid, CH3(CH2)7CH=CH(CH2)7COOH, has one C=C bond and melts at 16°C.
Aromatic acids
Aromatic acids have a –COOH group bonded to a carbon in the aromatic ring. Benzoic acid and the three isomers of toluic acid are examples.

Hydroxy acids
Hydroxy acids have hydroxyl (–OH) and carboxyl (–COOH) functional groups. Two important α-hydroxy acids are lactic acid and salicylic acid.
Lactic acid is found in sour milk as well as in muscle tissue after strenuous exercise.
Salicyclic acid and its derivatives are found in analgesics like aspirin.
Amino acids
Amino acids have an amino group (–NH2) and a carboxylic acid group (–COOH). The –COOH group acts as an acid while the –NH2 acts as a base.
Amino acid units are the building blocks of proteins. Approximately 20 amino acids are biologically significant.
Most amino acids in nature have the –NH2 in the α-position as shown here.
Chemical properties of carboxylic acid
Preparation of Carboxylic acid

1) oxidation of primary alcohol or aldehydes

2) hydrolysis of esters
Preparation of arxylic Acids

-Oxidation of primary alcohols



-Oxidation of aldehydes


Acid-Base Properties
가벼운 칼복실산은 극성을 띠어서 물에 녹지만, 무거운 칼복실산은 R 그룹의 파워가 더 강해서 물에 녹지 않는다
하지만 강염기랑 반응한 이후에는 물에 녹는 상태가 된다
Low molar-mass carboxylic acids release H+ ions in solution and as a result have the following properties unique to acids in general.
- Sour taste
- Blue litmus paper changes to red in an acidic solution
- Forms solutions with a pH <7
- Reacts with bases in neutralisation reactions
These characteristics are not true of higher molar-mass carboxylic acids because the dominant nonpolar R group makes these higher-molar-mass carboxylic acids insoluble in water.
However, the higher molar mass carboxylic acids do react with strong bases like NaOH and KOH. The sodium and potassium salts of carboxylic acids are soluble in water.

Ester formation
Esters can be prepared by a reacting a carboxylic acid and an alcohol or a phenol in the presence of a strong acid catalyst.



CARBOXYLIC ACID SUMMARY


ESTERS




Physical Properties of Esters
The low polarity of ester molecules is illustrated by both their water solubility and boiling points are lower than those of either acids or alcohols of similar molar masses.
Low molar mass esters are volatile, colourless, generally nonpolar liquids at room temperature. For example esters such as ethyl acetate, butyl acetate, and isoamyl acetate are used extensively in paints, varnishes, and lacquers.
Low- and intermediate-molar-mass esters have characteristic fragrant or fruity odours. The difference in properties between an acid and its esters is remarkable.


High molar mass esters are nonpolar solids at room temperature. Many of these esters are waxes.
Carnauba wax is an ester that can have a 28-carbon fatty acid chain and a 34-carbon alcohol chain.
High molar mass esters are used in furniture and automobile wax preparations.

Polyesters
A polyester is a polymer formed between an alcohol monomer and a carboxylic acid monomer.
Polyesters are classified as condensation polymers.

general polymerization reaction to produce a polyester

This is a polymerization reaction that produces the polyethylene terephthalate (PETE) polyester.

Chemical Properties of Esters
The principal reaction of esters is hydrolysis.
Ester hydrolysis is either acid-catalyzed (acid hydrolysis) or base-promoted (alkaline hydrolysis/saponification).
An ester hydrolyzed in the presence of an acid catalyst forms an alcohol and a carboxylic acid. The acid is a catalyst so it isn’t consumed during the reaction.
acid-catalyzed (acid hydrolysis)


base-promoted (alkaline hydrolysis/saponification)
The ester hydrolysis in the presence of a strong base to form an alcohol and a salt is called saponification.

The carboxylic acid may be obtained by reacting the salt obtained from the hydrolysis with a strong acid.





Glycerol Ester
Esters of glycerol (glycerol esters) are known as triacylglycerols and triglycerides.

Fats and oils are triacylglycerols.
Fats originating from animal sources are solids at room temperature because they have a higher percentage of saturated fatty acid derivatives in their triacylglycerol structure.
Oils originating from plant sources are liquids at room temperature because they have a higher percentage of unsaturated fatty acid derivatives in their triacylglycerol structure.
Some common unsaturated fatty acid derivatives found in oils include the corresponding parent fatty acids oleic acid, linoleic acid, and linolenic acid.


Triacylglycerols undergo two main types of reactions.
- Hydrogenation
- Acid and basic hydrolysis.
1) Hydrogenation of Glycerides
Oils can be partially hydrogenated using a metal catalyst and hydrogen gas to obtain a solid like the shortening found in Crisco

During partial hydrogenation some of the cis bonds change to trans bonds which is the basis for the term “trans fat”.
Research has shown these unnatural fats to be unhealthy and to increase the risk of heart disease.

2) Acid Hydrolysis
Fatty acids can be prepared by hydrolysing triacylglycerols with either enzymes or mineral acid catalysts.

2) Alkaline Hydrolysis (Saponification)
Soap (i.e. carboxylate salts) is prepared by the alkaline hydrolysis of triacylglycerols.



Soaps and Synthetic Detergents
Soaps are salts of long-chain fatty acids that are prepared by reacting fat or oil with sodium hydroxide.
fat or oil + NaOH → soap + glycerol
Sodium palmitate CH3(CH2)14COONa is a soap that is the sodium salt of palmitic acid CH3(CH2)14COOH.
The hydrocarbon tail of the carboxylate ion is nonpolar and dissolves the nonpolar grease. The hydrocarbon tail is hydrophobic (i.e. water-fearing).
The polar head of the carboxylate ion allows the ion to remain soluble in water which is also polar. The polar head is hydrophilic (i.e. water-loving).

The cleansing action of soaps in water with high mineral content is limited because the water contains ions that react with carboxylate ions to form insoluble salts (soap scum) as shown in the reaction below.
These ions include Ca2+, Fe3+, and Mg2+.



SUMMARY OF ESTER


'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK6]Chemistry of Food- Carbohydrates & Carbohydrates (0) | 2023.04.02 |
|---|---|
| [WEEK6] Stereoisomerism (0) | 2023.03.31 |
| [WEEK5] Lipids (0) | 2023.03.30 |
| [WEEK5] Chemistry of Food -Fatty Acids (0) | 2023.03.29 |
| REACTION NOTES (0) | 2023.03.28 |



