MINI LECTURE




LIPIDS
-SIMPLE LIPIDS ( triglycerol )
-COMPOUND LIPIDS ( phospholipid, sphingolypid, glycolipid )
-STEROIDS
-MISCELLANEOUS LIPIDS
SIMPLE LIPIDS




-Example of eicosanoids
: Thromboxanes
: Prostacyclins
: Prostaglandins
: Leukotrienes
-Eicosanoids do
: blood clotting
: increase in body temperature
: coordinate HCl and mucous secretion
: constriction of the bronchial tubes
: stimulate and attract white cells
: cause the white cells to disperse
: cause vasodilation and vasoconstriction
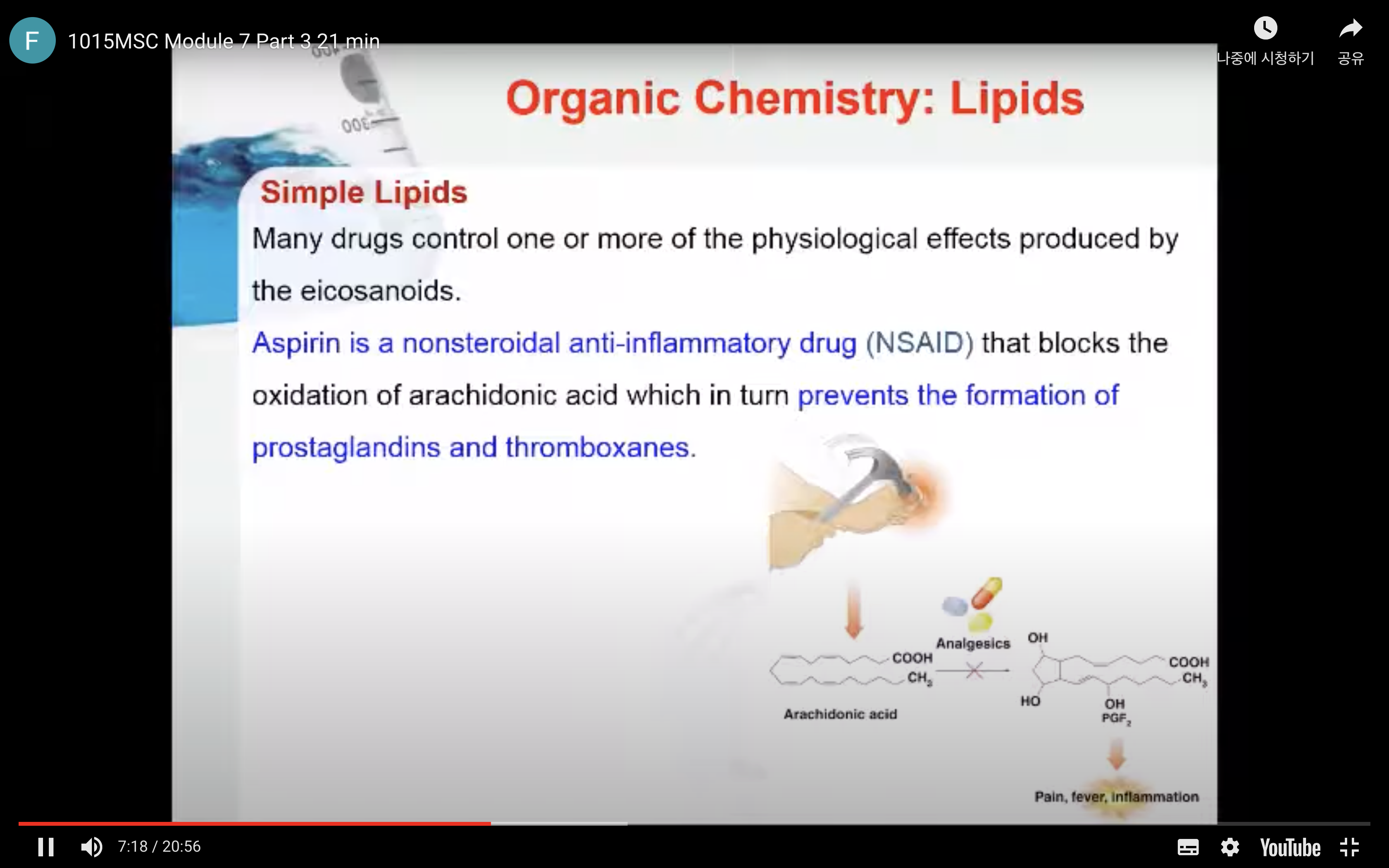
-Asprin is a nonsteroidal anti-inflammatory drug (NSAID) that blocks the oxidation of arachidonic acid which in turn prevents the formation of prostaglandins and thromboxanes




-Triglycerides : glycerol molecule + three fatty acid molecules
-Fatty acids have carbon chains with 14-18 carbon atoms and can be saturated or unsaturated
-Most metabolic energy is derived from Carbon oxidation.
(Carbohydrate glucose C6H12O6 and fatty acid C9H19COOH have similar molecular weights, however, fats are about 75% carbon compared to about 40% for carbohydrates)
(In addition, fat have a lower degree of carbon oxidation when compared to carbohydrates which results in higher energy yields when fats are oxidised)
COMPOUND LIPIDS - 1.2.3
1. PHOSPHOLIPIDS

2. SPINGOLIPIDS
3. GLYCOLIPIDS

STEROIDS




MISCELLANEOUS LIPIDS


Lipids: Hydrophobic Molecules
Lipids are water-insoluble substances that have several important biological functions. Lipids interact weakly with water molecules because they are composed primarily of nonpolar alkyl groups. Lipids are classified as hydrophobic (“water-fearing”) to designate their inability to interact effectively with water or their strong tendency to move away from water. Fatty acids are common components of lipids. As fatty acids get larger, the water solubility of the fatty acid decreases dramatically as shown below.
Classification of Lipids
Lipids molecules are relatively large and nonpolar. Yet, within this broad description, lipid structures vary markedly. Lipids can be classified in four categories which recognise major structural similarities.
- Simple lipids
- Compound lipids
- Steroids
- Miscellaneous lipids
Simple Lipids - (eicosanoids, triglyerols, waxes)
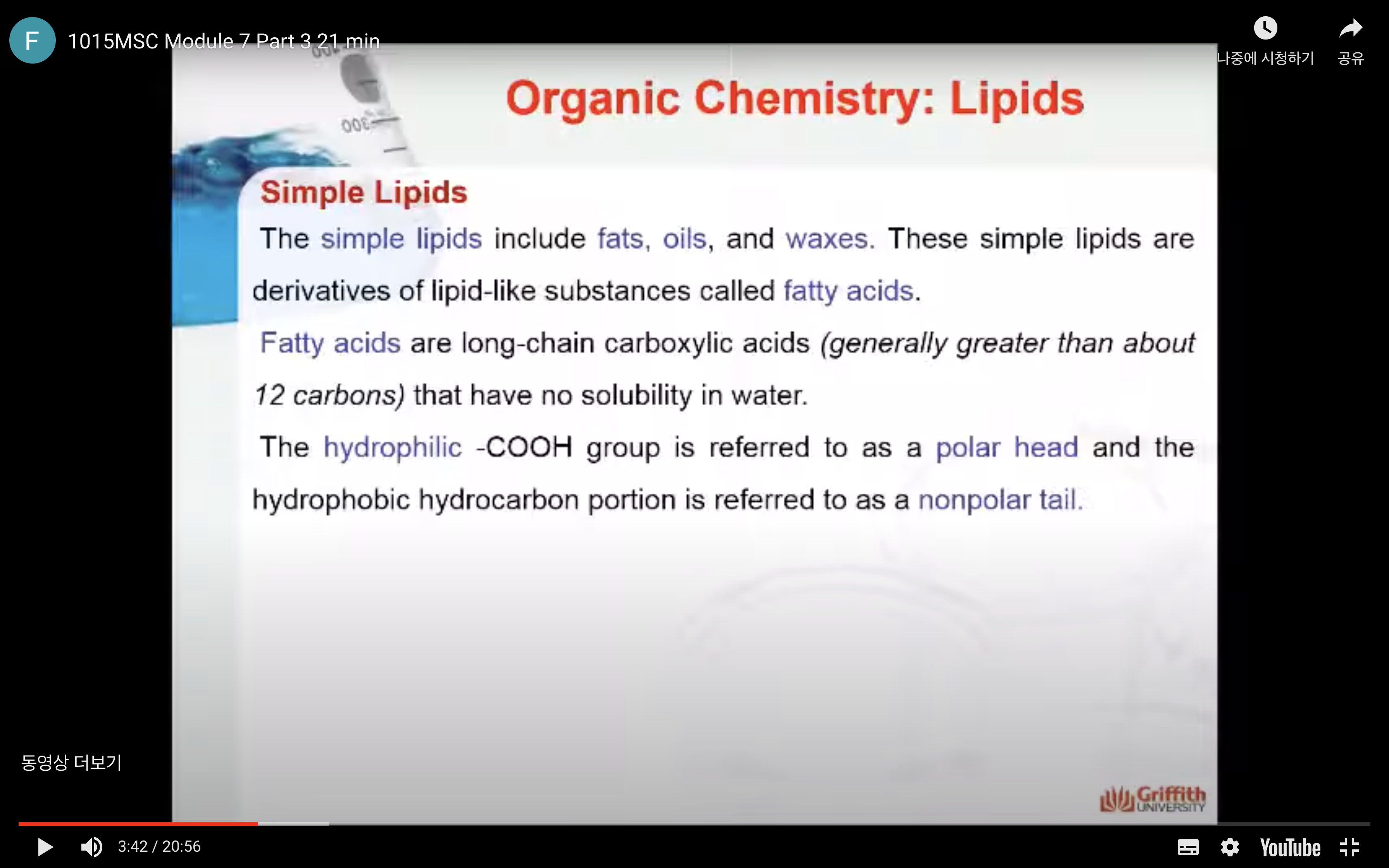
The simple lipids include fats, oils, and waxes. These simple lipids are derivatives of lipid–like substances called fatty acids. Fatty acids are long–chain carboxylic acids (generally greater than about 12 carbons) that have no solubility in water. The hydrophilic –COOH group is referred to as a polar head and the hydrophobic hydrocarbon portion is referred to as a nonpolar tail.
Fats and waxes are solids due to a higher composition of saturated fatty acids while oils are liquids due to a higher composition of unsaturated fatty acids.
Notice how unsaturated fatty acids generally have lower melting points than saturated fatty acids.
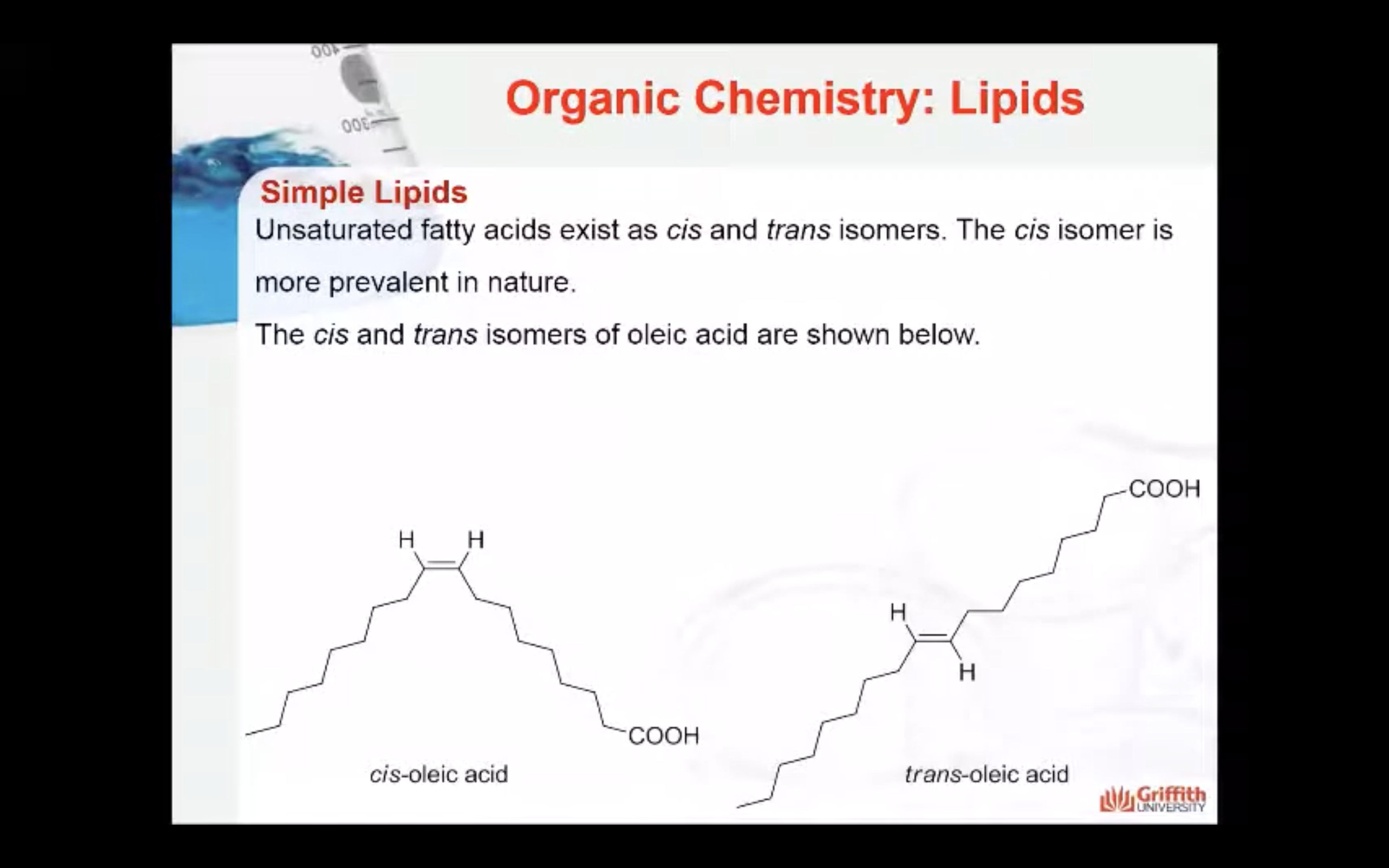
Unsaturated fatty acids exist as cis and trans isomers. The cis isomer is more prevalent in nature. The cis and trans isomers of oleic acid are shown below. Oleic acid is an unsaturated fatty acid. The cis isomer has a bent structure that prevents close stacking resulting in a compound that resists solidification (weaker intermolecular forces).
Melting point : saturated fatty acids > trans-saturated fatty acids > cis-unsaturated fatty acids
Lipids in our bodies
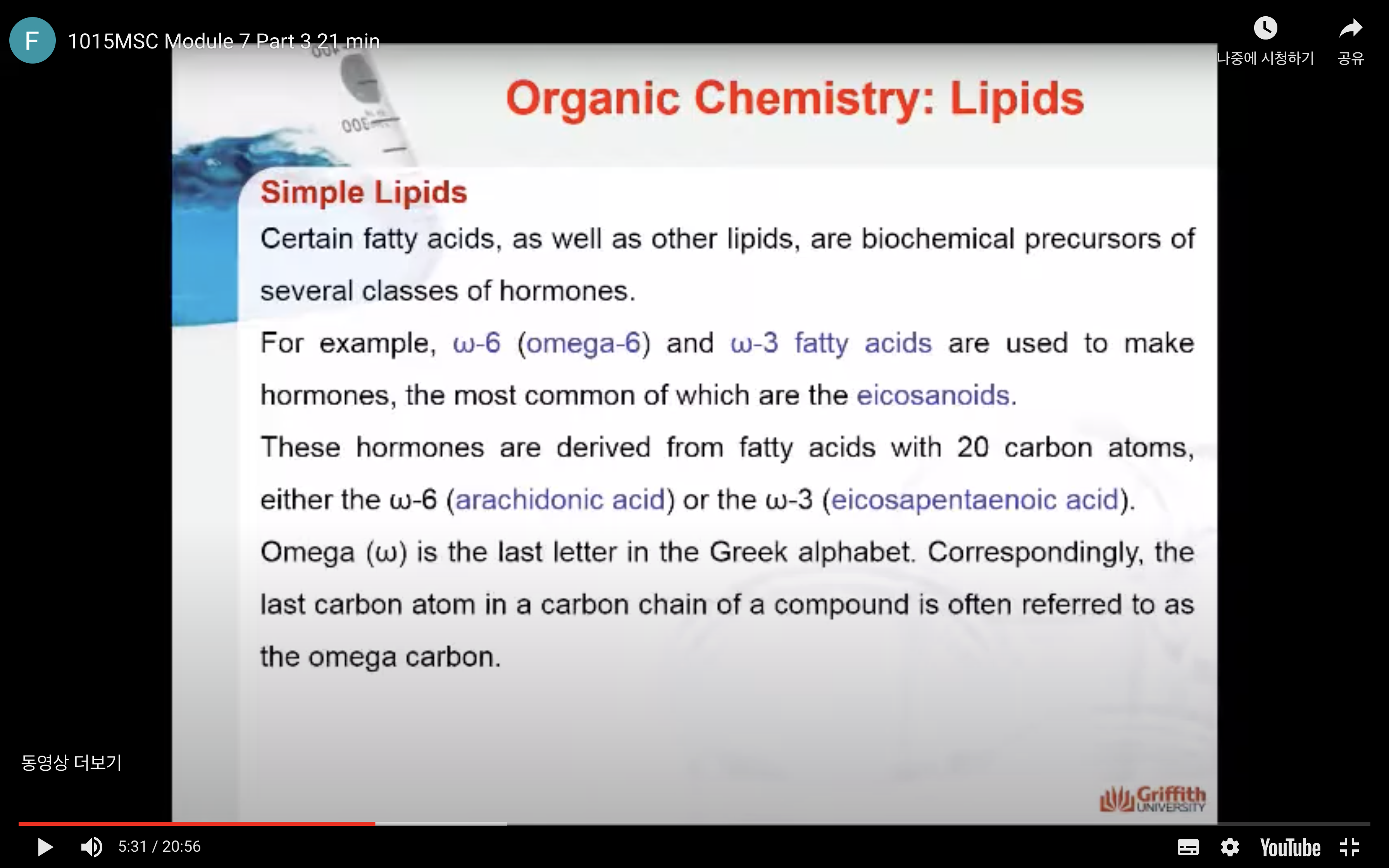
Certain fatty acids, as well as other lipids, are biochemical precursors of several classes of hormones. For example, ω–6 (omega–6) and ω–3 fatty acids are used to make hormones, the most common of which are the eicosanoids. Omega (ω) is the last letter in the Greek alphabet. Correspondingly, the last carbon atom in a carbon chain of a compound is often referred to as the omega carbon.
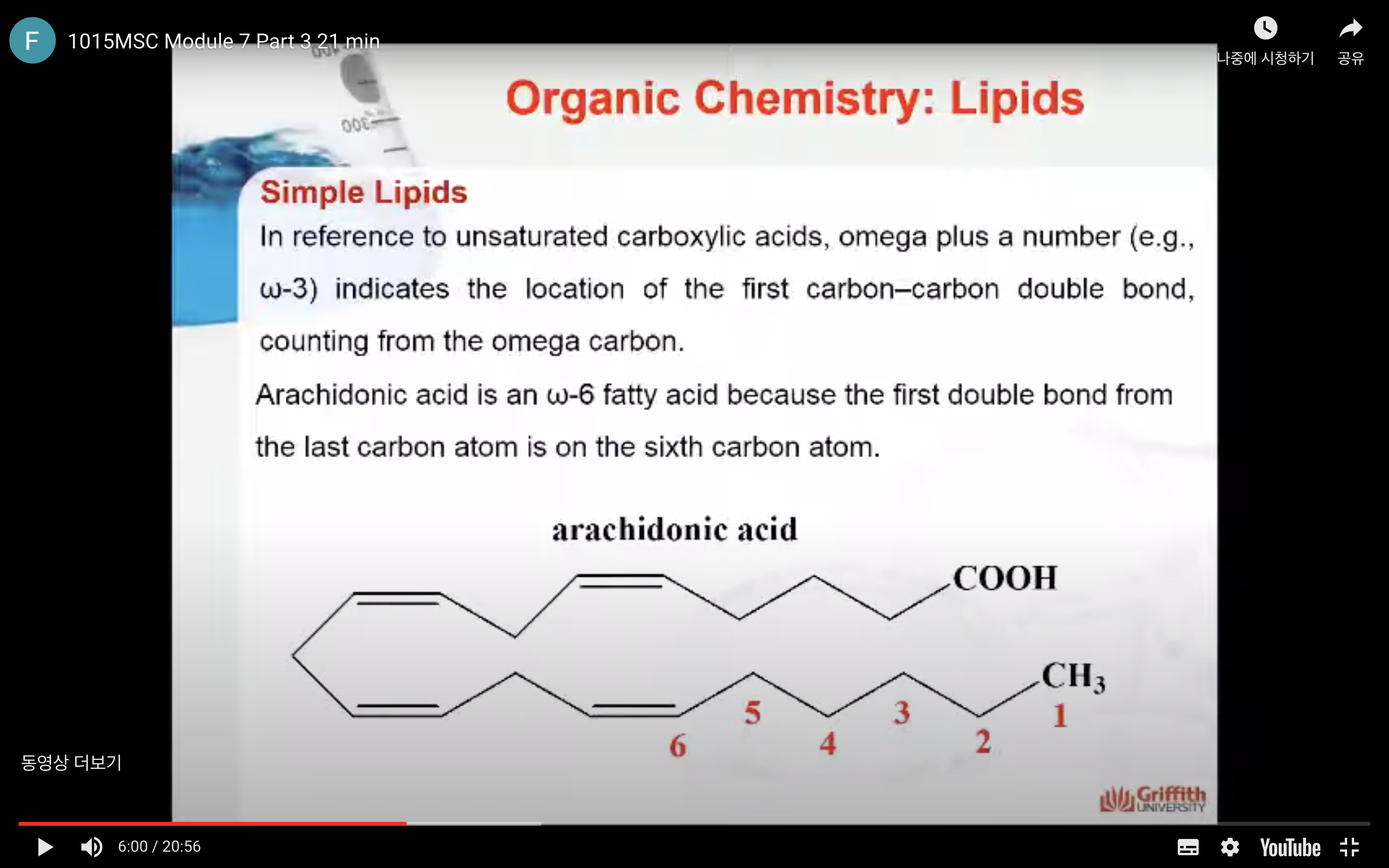
In reference to unsaturated carboxylic acids, omega plus a number (e.g., ω–3) indicates the location of the first carbon–carbon double bond, counting from the omega carbon. So for example, arachidonic acid is an ω–6 fatty acid because the first double bond from the last carbon atom is on the sixth carbon atom.


Eicosanoids
Eicosanoids are hormones and they coordinate various cellular responses. Some are involved with blood clotting as they can cause platelet aggregation while others trigger an increase in body temperature. Some eicosanoids coordinate HCl and mucous secretion by the stomach lining and constriction of the bronchial tubes in the lungs. Some eicosanoids stimulate and attract white cells, while other eicosanoids cause the white cells to disperse. Eicosanoids can also cause vasodilation as well as vasoconstriction.
Many drugs control one or more of the physiological effects produced by the eicosanoids. For example, aspirin is a nonsteroidal anti-inflammatory drug that blocks the oxidation of arachidonic acid which in turn prevents the formation of prostaglandins and thromboxanes.

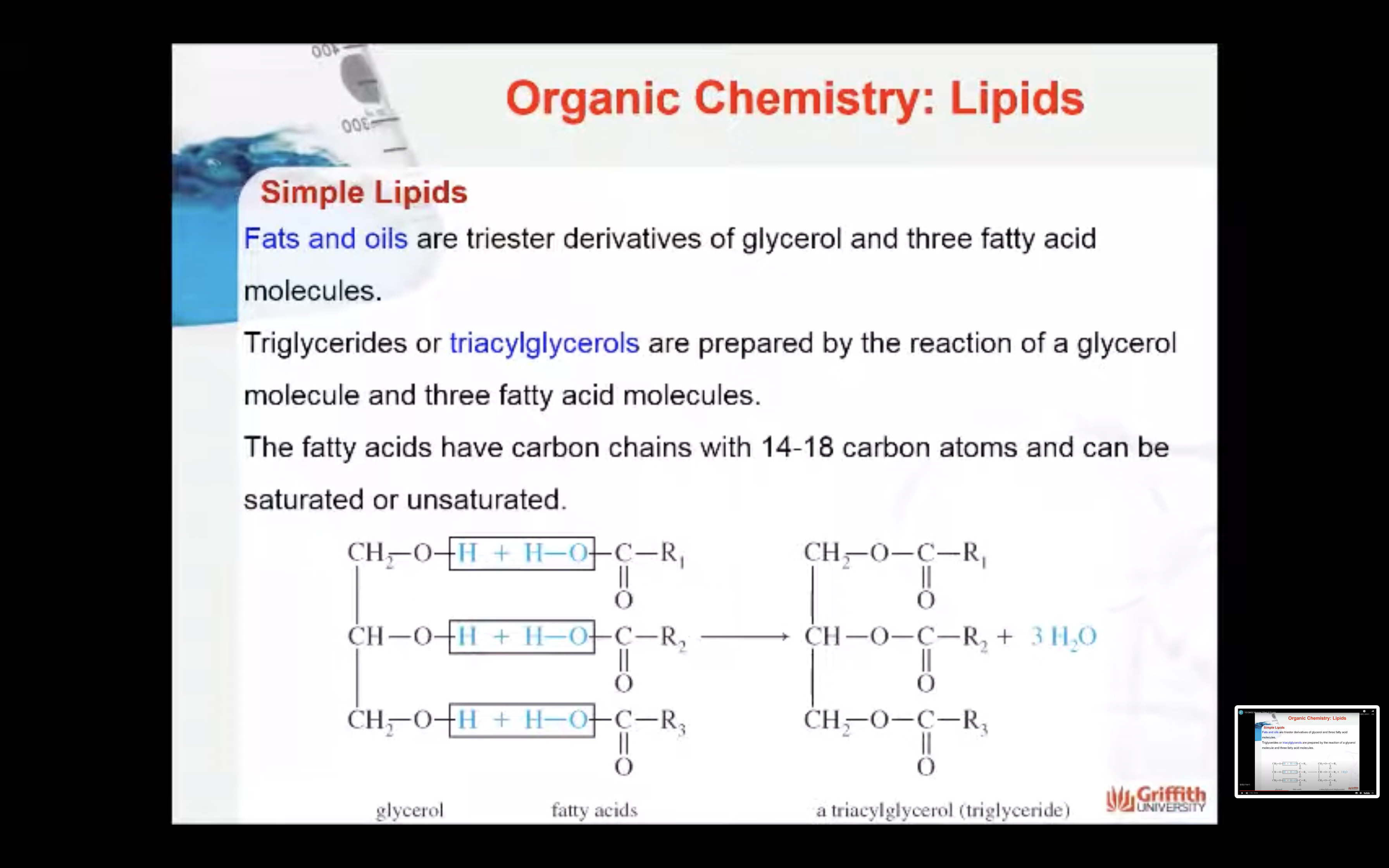
Triglycerides
Fats and oils are triester derivatives of glycerol and three fatty acid molecules as shown on the right. Because there are three ester groups per glycerol, these lipids are called triacylglycerols or triglycerides. The fatty acids have carbon chains with 14–18 carbon atoms. Triglycerides are prepared by the reaction of a glycerol molecule and three fatty acid molecules. The fatty acid molecules can be saturated or unsaturated.

Fats in Metabolism
Fats are an important food source for humans and normally account for about 25–50% of our caloric intake. Fats are an especially good source of metabolic energy. Most metabolic energy is derived from carbon oxidation. When oxidized to carbon dioxide and water, fats supply about 40 kJ per gram (9.5 kcal/g), which is more than twice the amount obtained from carbohydrates or proteins. Fats are what our bodies prefer when storing energy reserves. These reserves are in the form of triacylglycerols in fatty tissue. On average, this tissue stores about two to three weeks’ worth of energy.
Compound Lipids - (phosopholipids, sphingolipids, glycolipids)
- Phospholipids
- Sphingolipids
- Glycolipids
Phospholipids
The phospholipids are a group of compounds that yield one or more fatty acid molecules, a phosphate group, and usually a nitrogenous base upon hydrolysis. In contrast to the triacylglycerols, phospholipids have a hydrophilic end that interacts with water.
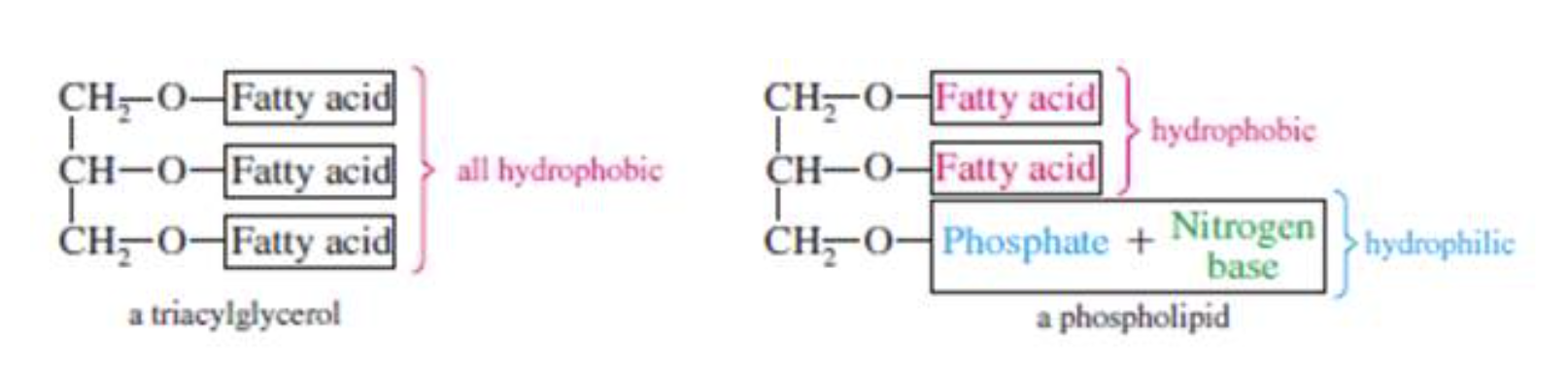
Phospholipids are one of the most important membrane components. They are also involved in the metabolism of other lipids and nonlipids.
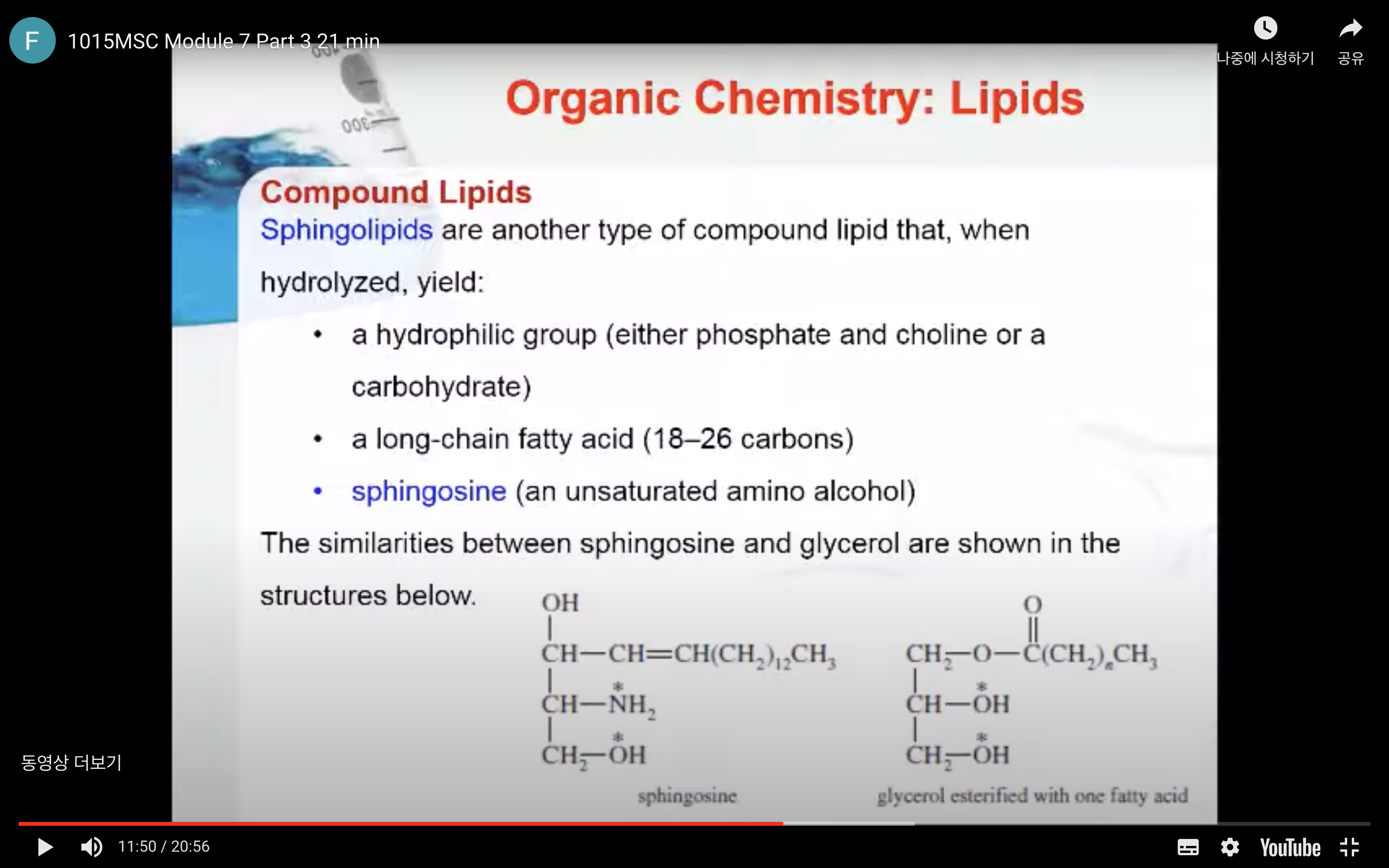
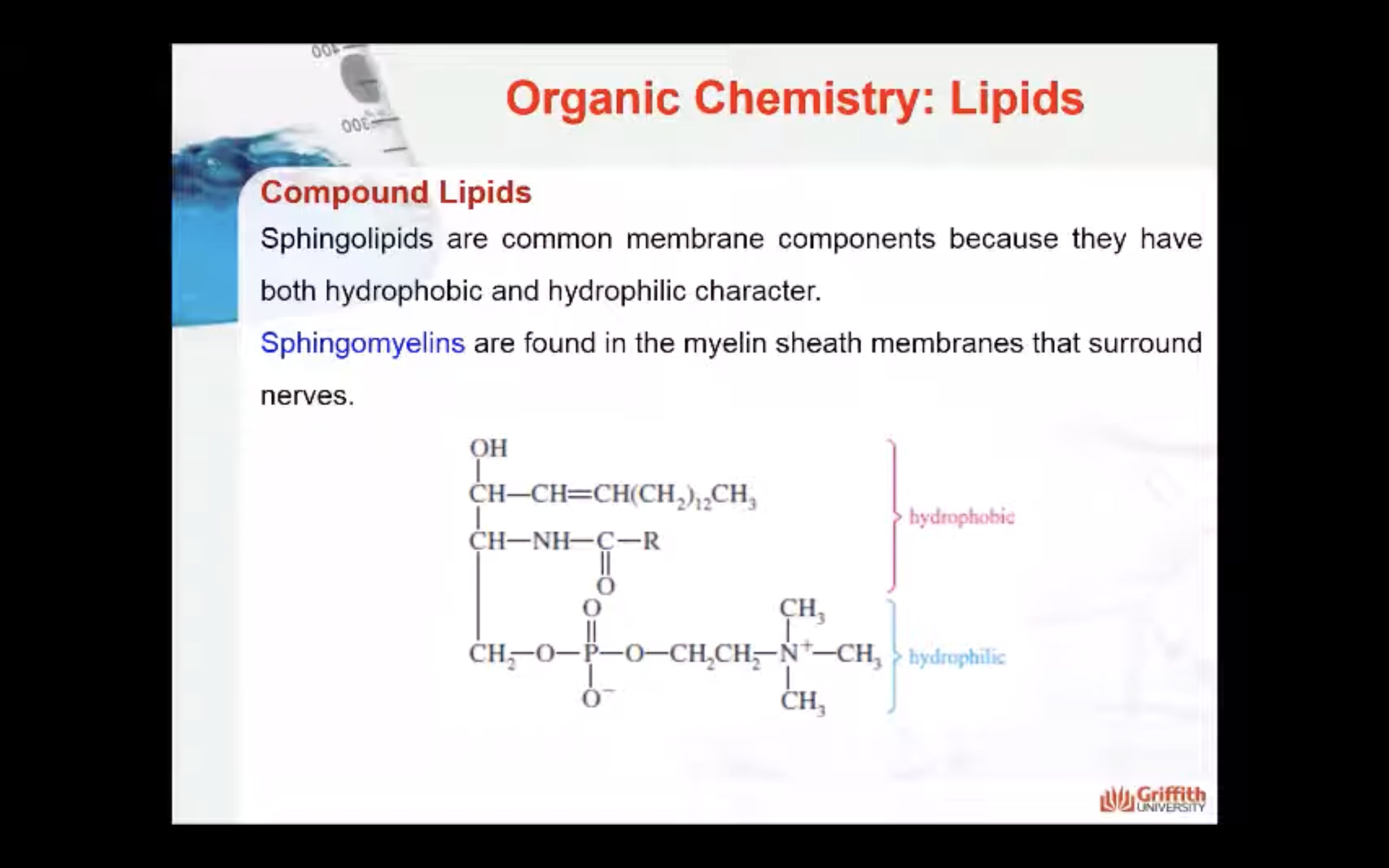
Sphingolipids
Sphingolipids are another type of compound lipid that, when hydrolysed, yield:
- a hydrophilic group (either phosphate and choline or a carbohydrate)
- a long–chain fatty acid (18–26 carbons)
- sphingosine (an unsaturated amino alcohol)
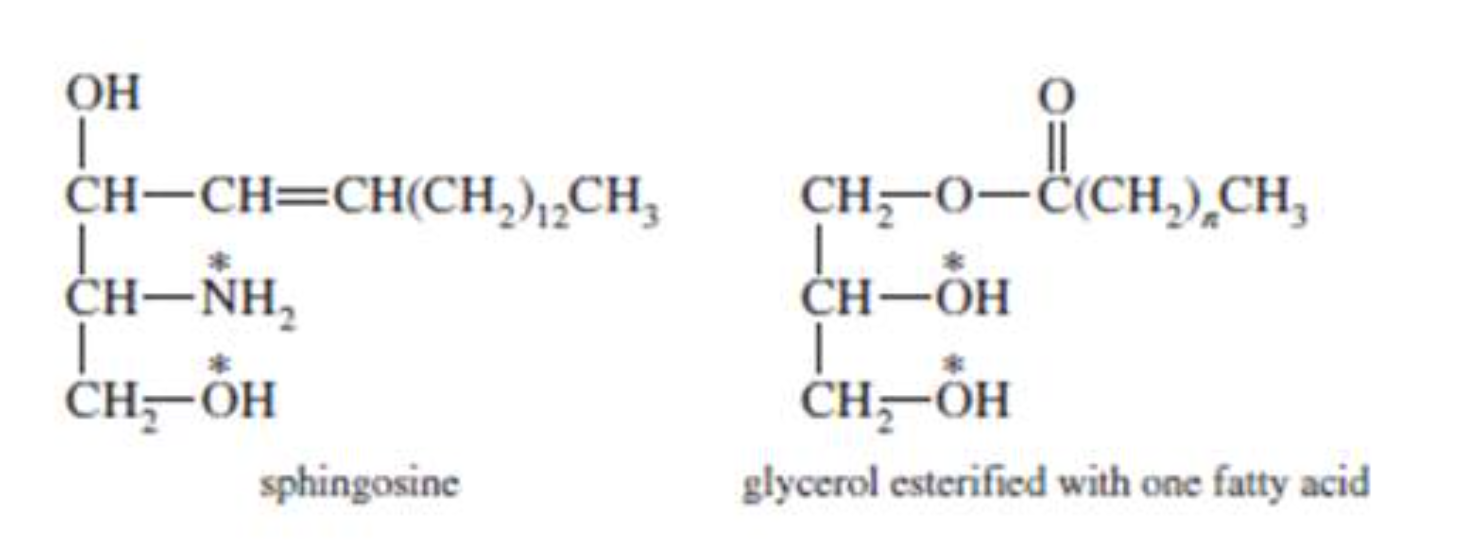
Sphingolipids are common membrane components because they have both hydrophobic and hydrophilic character. Sphingomyelins are found in the myelin sheath membranes that surround nerves.
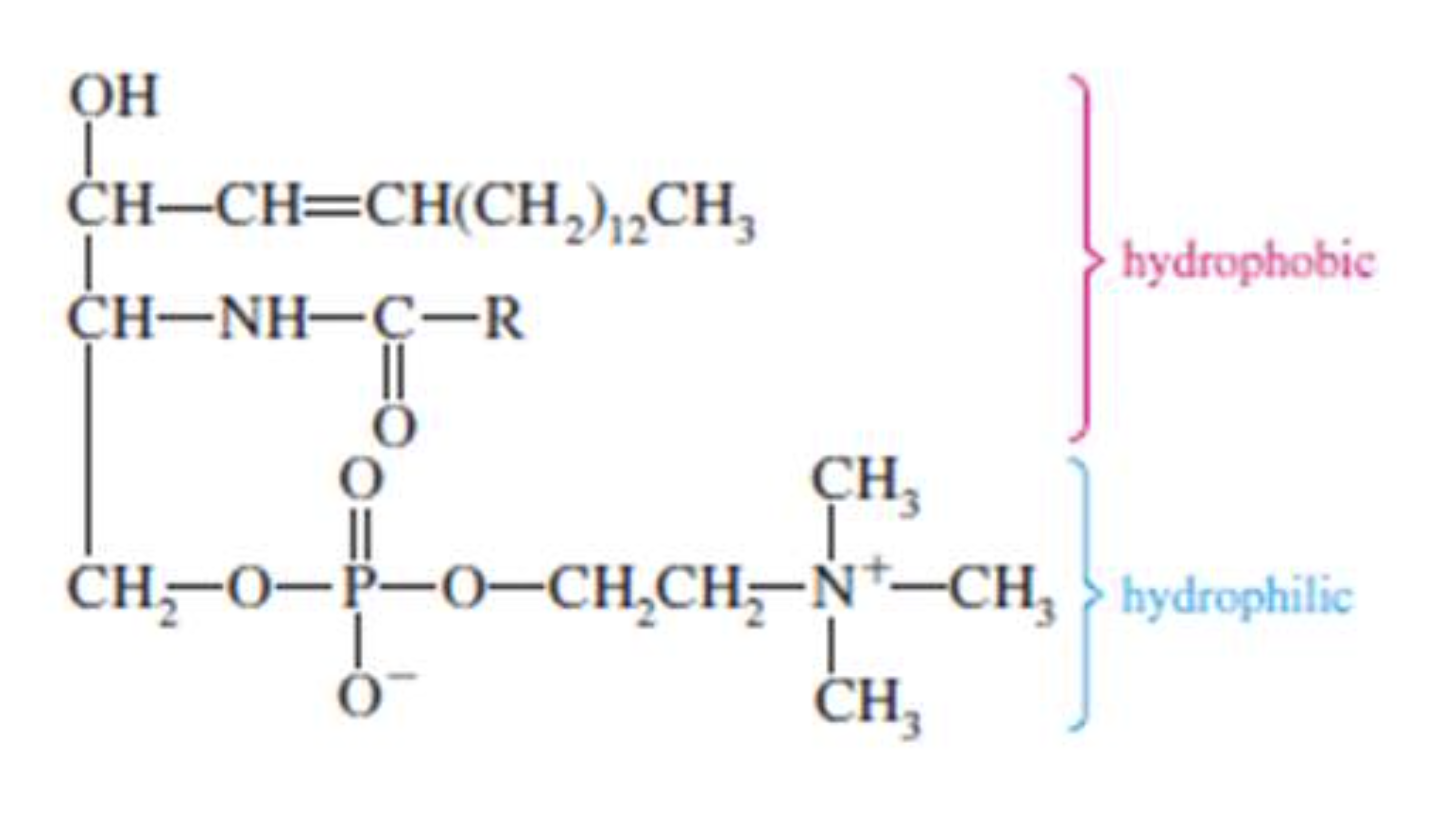
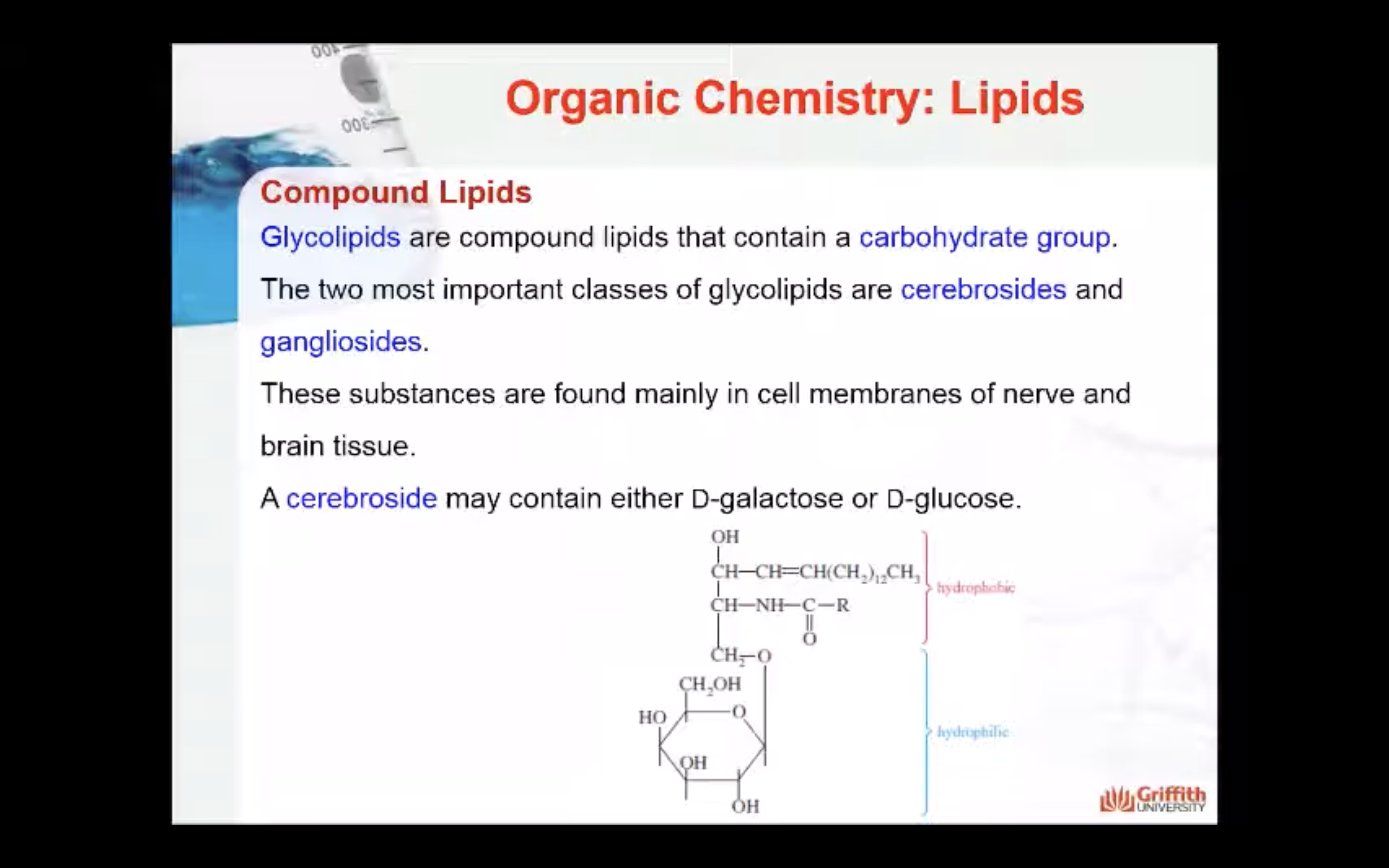
Glycolipids
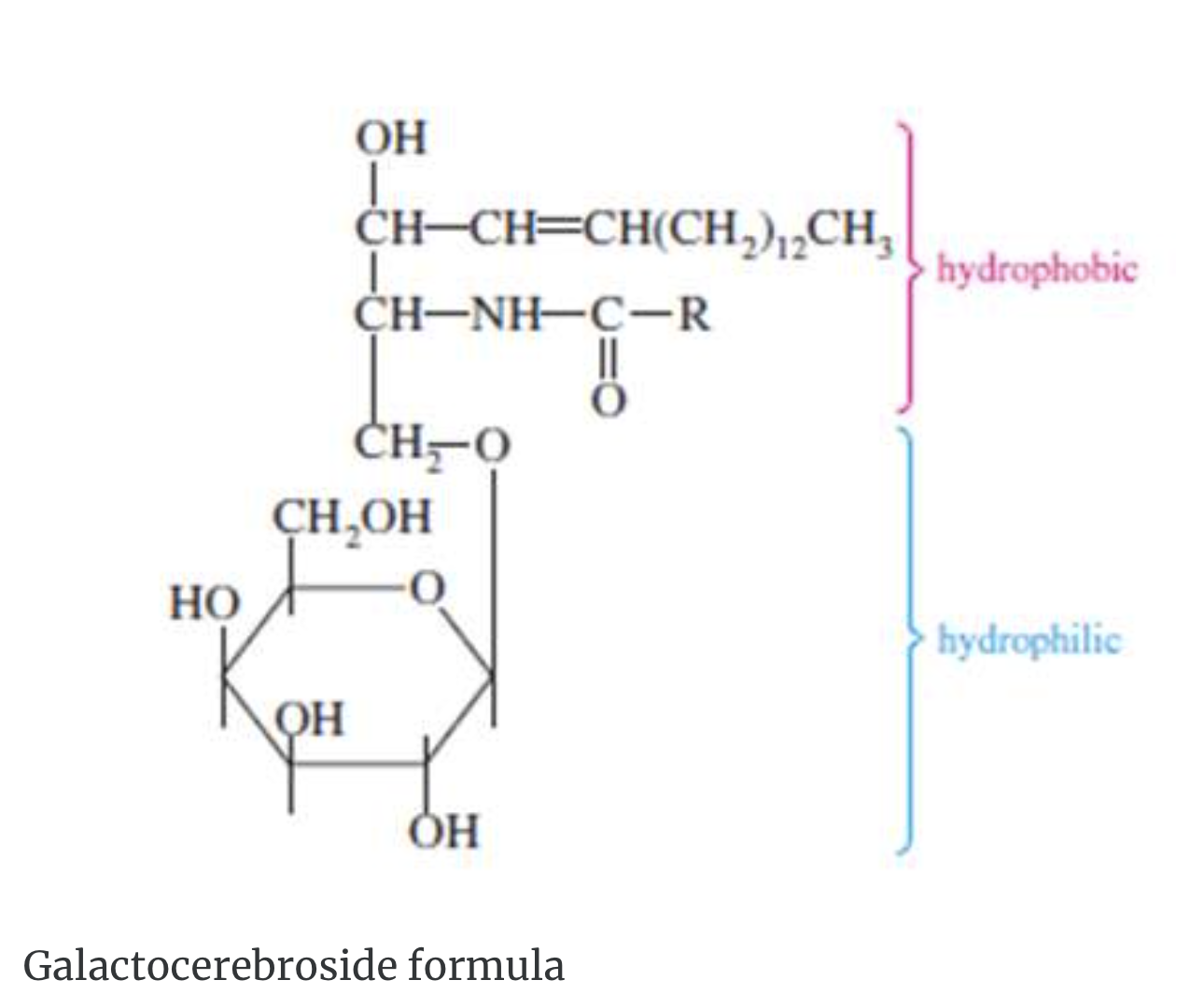
Glycolipids are a third type of compound lipid. These compounds contain a carbohydrate group. The two most important classes of glycolipids are cerebrosides and gangliosides. These substances are found mainly in cell membranes of nerve and brain tissue. A cerebroside may contain either D–galactose or D–glucose. The following formula of a galactocerebroside shows the typical structure of cerebrosides.
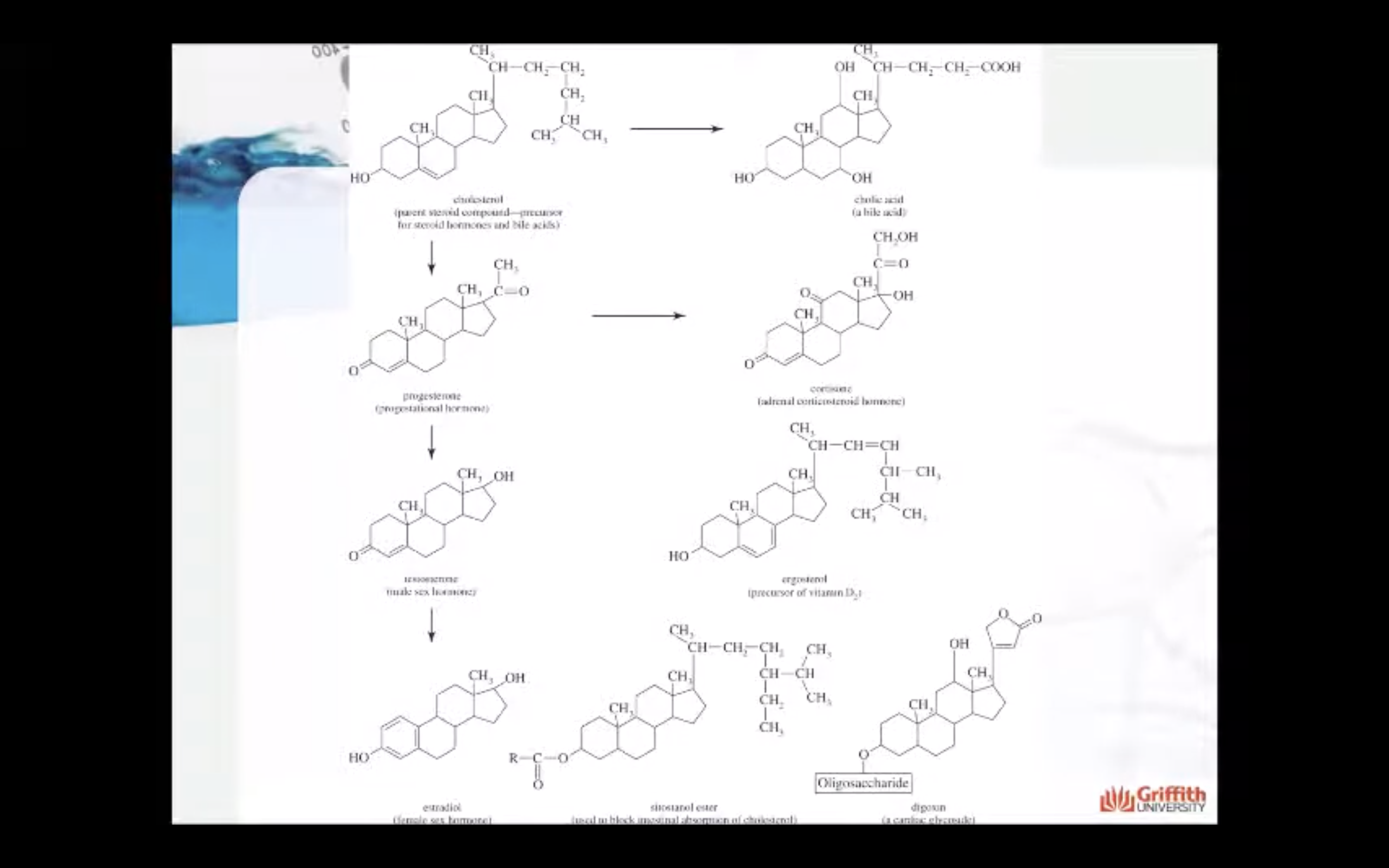
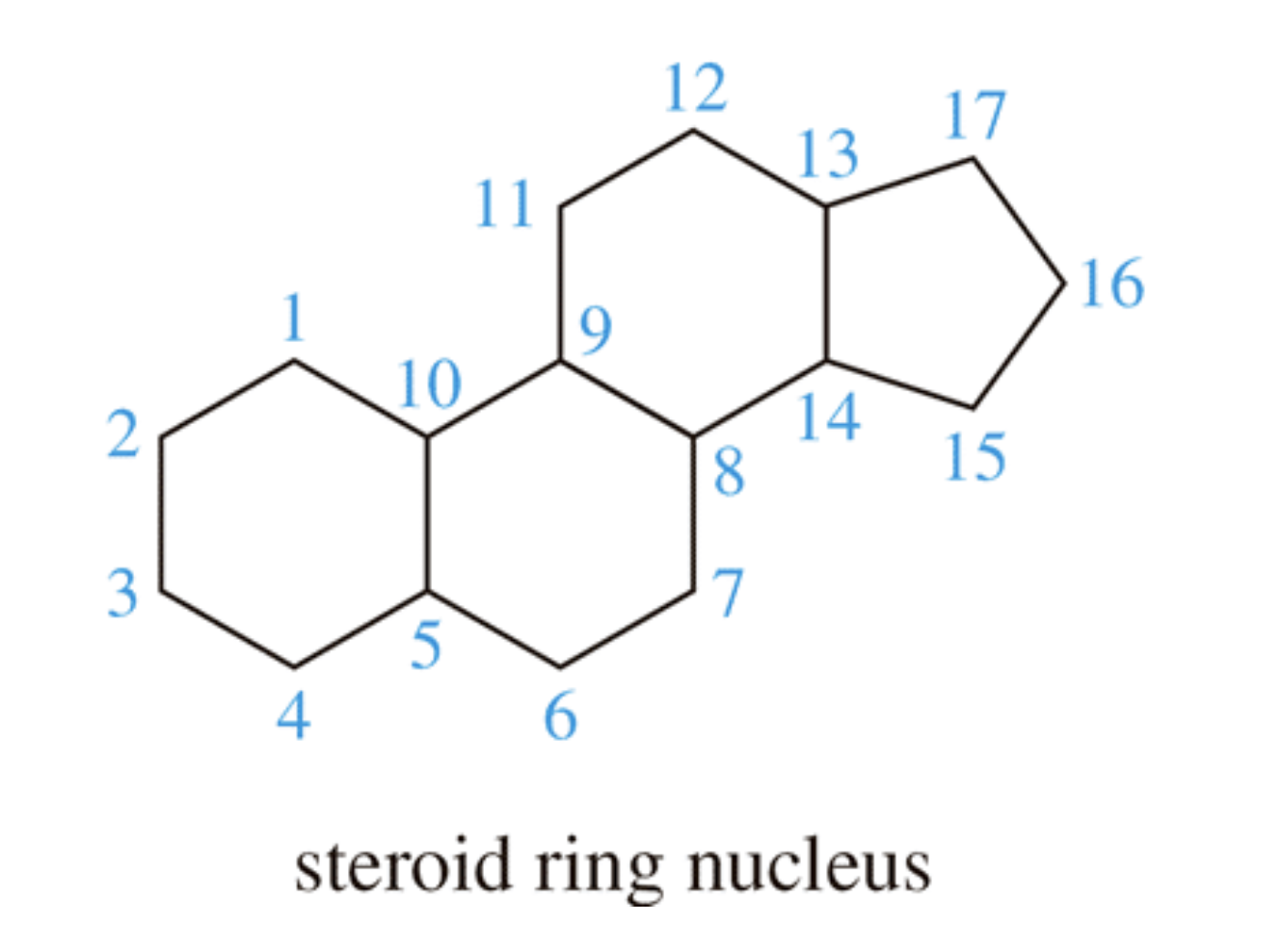
Steroids
Steroids are compounds that have the steroid nucleus, which consists of four fused carbocyclic rings. This nucleus contains 17 carbon atoms in one five–membered ring and three six–membered rings. Modifications of this nucleus that occur in the various steroid compounds include, for example, added side alkyl chains, hydroxyl groups, carbonyl groups, and ring double bonds.
Steroids are closely related in structure but are highly diverse in function. For example:
- Cholesterol, the most abundant steroid in the body, is widely distributed in all cells and serves as a major membrane component.
- Bile salts aid in the digestion of fats.
- Ergosterol, a yeast steroid, is converted to vitamin D by ultraviolet radiation.
- Digitalis and related substances called cardiac glycosides are potent heart drugs.
- The adrenal cortex hormones are involved in metabolism.
- The sex hormones.
Cholesterol
Cholesterol is the parent steroid compound from which the steroid hormones are synthesized. In this process, cholesterol is converted to progesterone, a compound that helps control the menstrual cycle and pregnancy.
Cholesterol is also the parent compound from which testosterone and the adrenal corticosteroids are produced. Cholesterol is also used to build cell membranes, many of which contain about 25% by mass of this steroid.
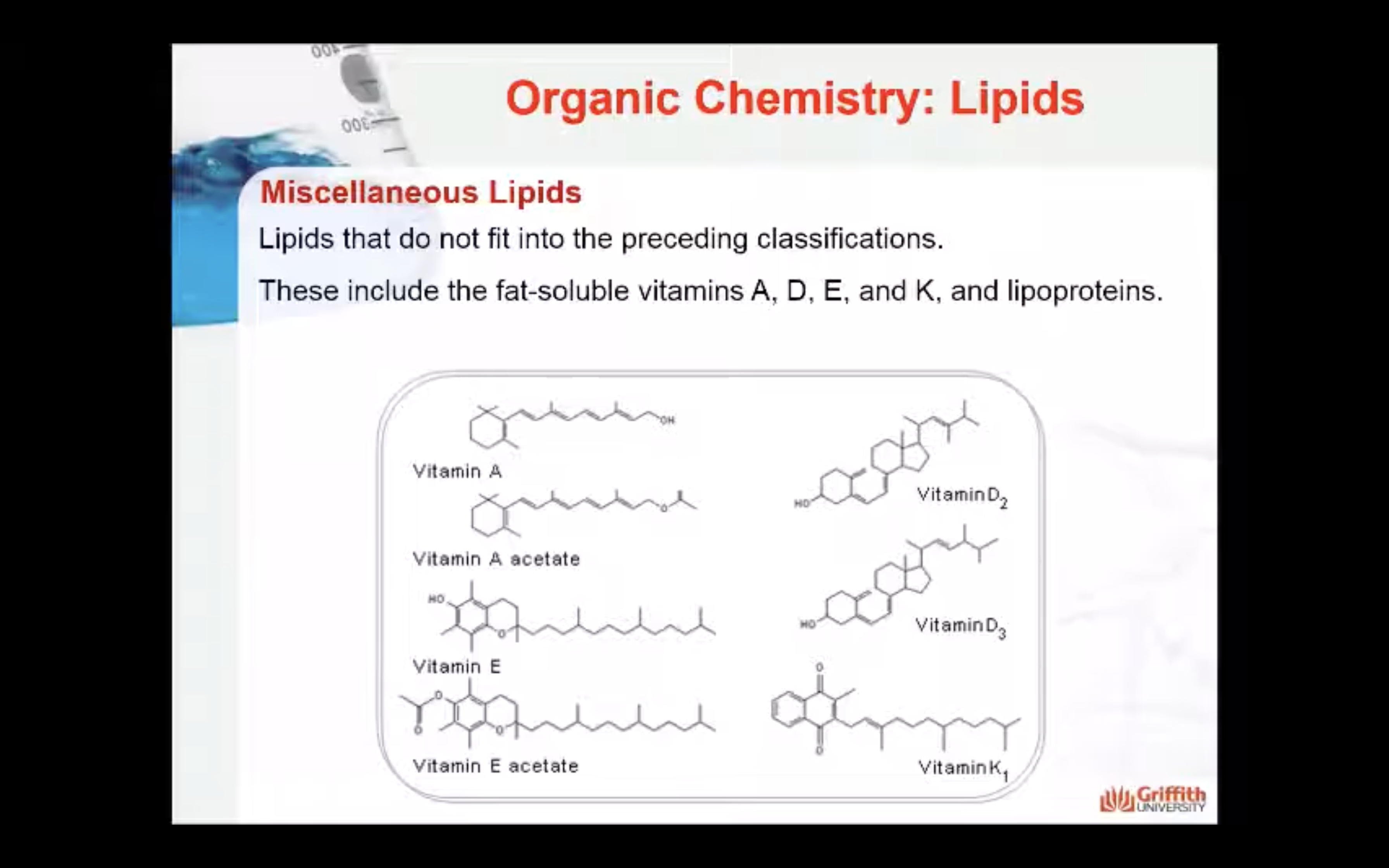
Miscellaneous Lipids
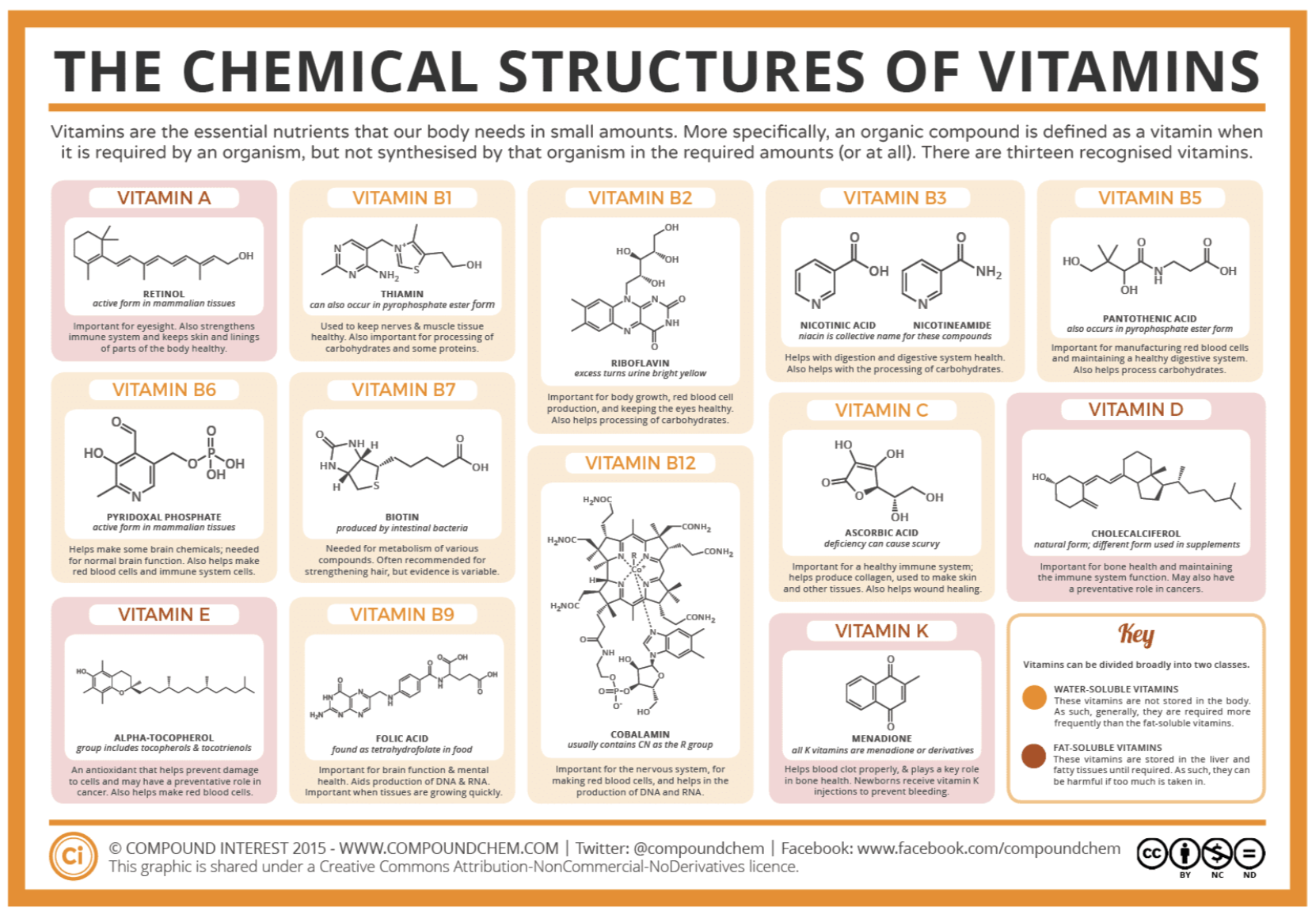
Miscellaneous lipids are lipids that do not fit into the preceding classifications. These include the fat-soluble vitamins A, D, E, and K, and lipoproteins.

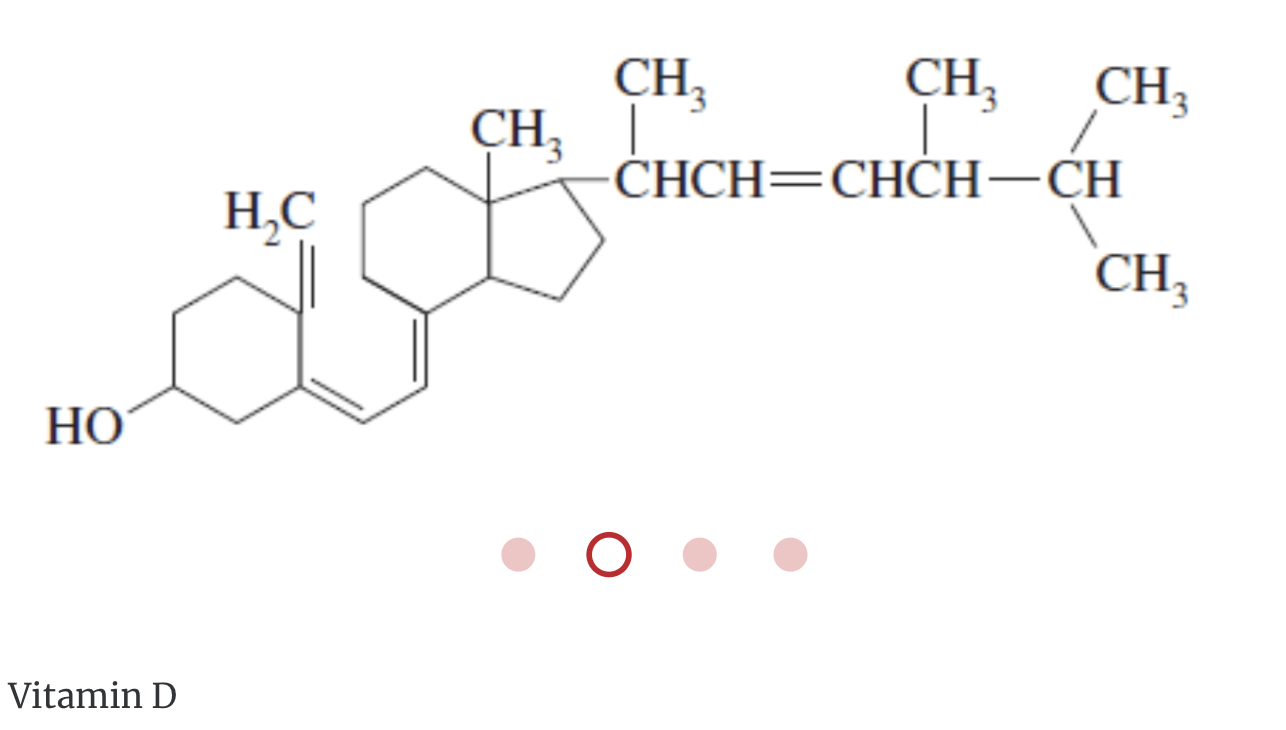
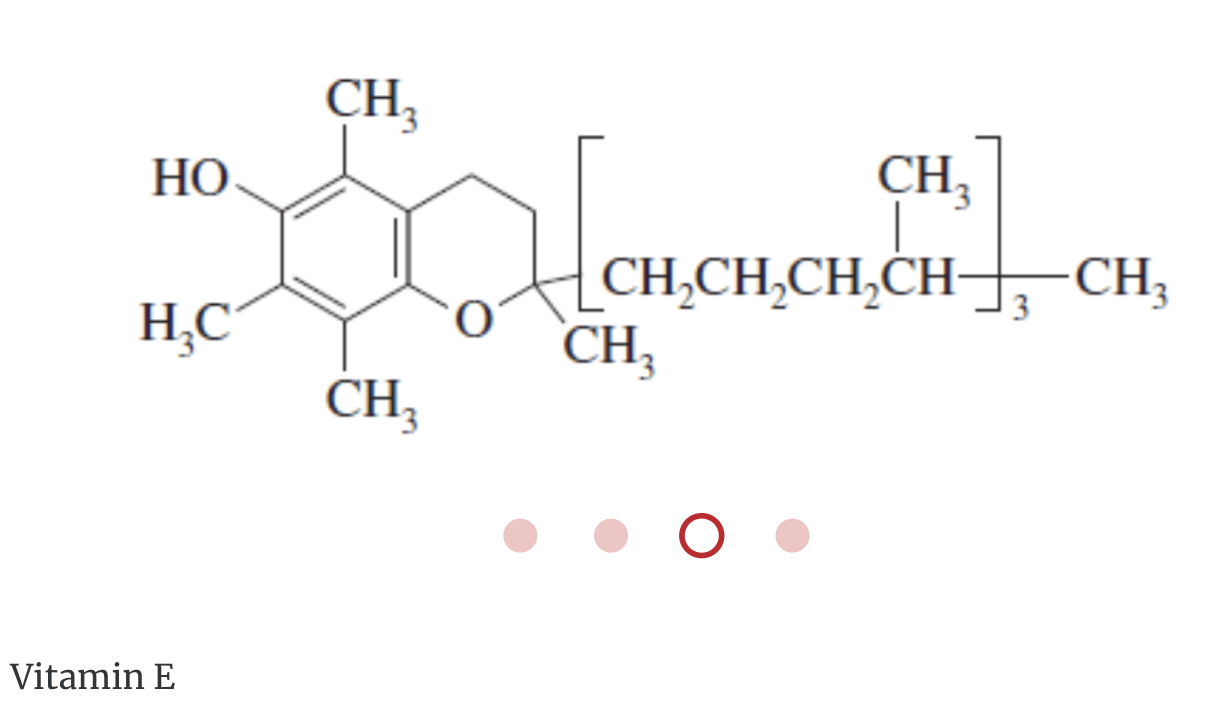

Hydrophobic Lipids and Biology
The hydrophobic nature of lipids has many important biological consequences. The water insolubility of lipids results in:
- lipid aggregation that causes atherosclerosis
- lipid aggregation that forms biological membranes
A lipid is in a hostile environment when it is surrounded by water. Lipid molecules aggregate to minimize their contact with water when in water. The hydrophilic part of lipid molecules is attracted to water and forms an interface with it, but the hydrophobic part distances itself from water molecules.
Complex lipids such as phospholipids and sphingolipids have two hydrophobic alkyl groups. Complex lipids form liposomes in aqueous mixtures. Liposomes are bounded by two layers of lipid. The hydrophobic alkyl chains are covered by hydrophilic groups on both the liposome’s inside and outside. Liposomes have a water core as shown here.

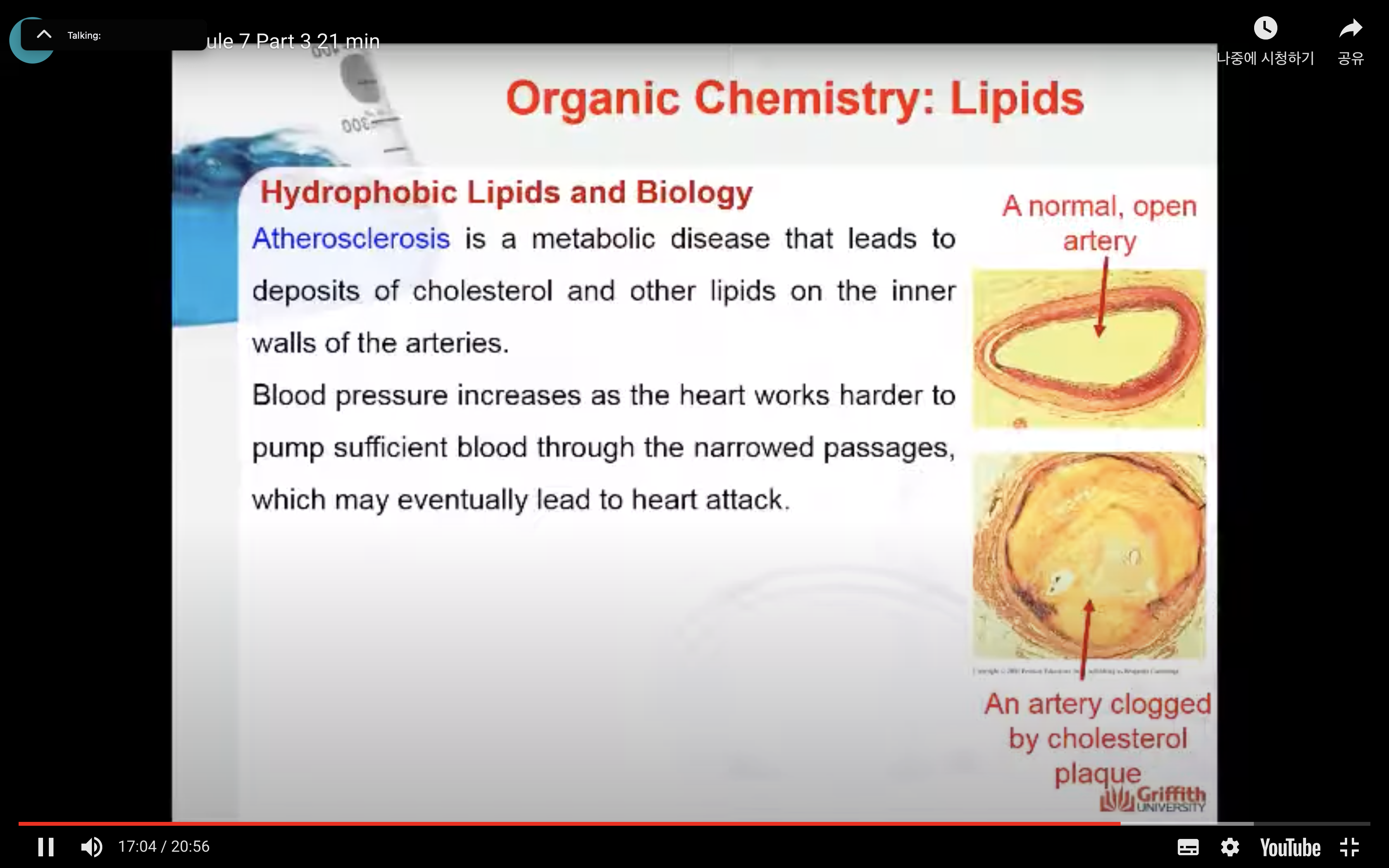
Atherosclerosis
Atherosclerosis is a metabolic disease that leads to deposits of cholesterol and other lipids on the inner walls of the arteries. Blood pressure increases as the heart works harder to pump sufficient blood through the narrowed passages, which may eventually lead to heart attack. Plaque formation begins because of a lipid’s natural tendency to aggregate. Improper transport of cholesterol through the blood contributes to atherosclerosis. Cholesterol (and other lipids) must be packaged for transport because lipids aggregate in the aqueous bloodstream.

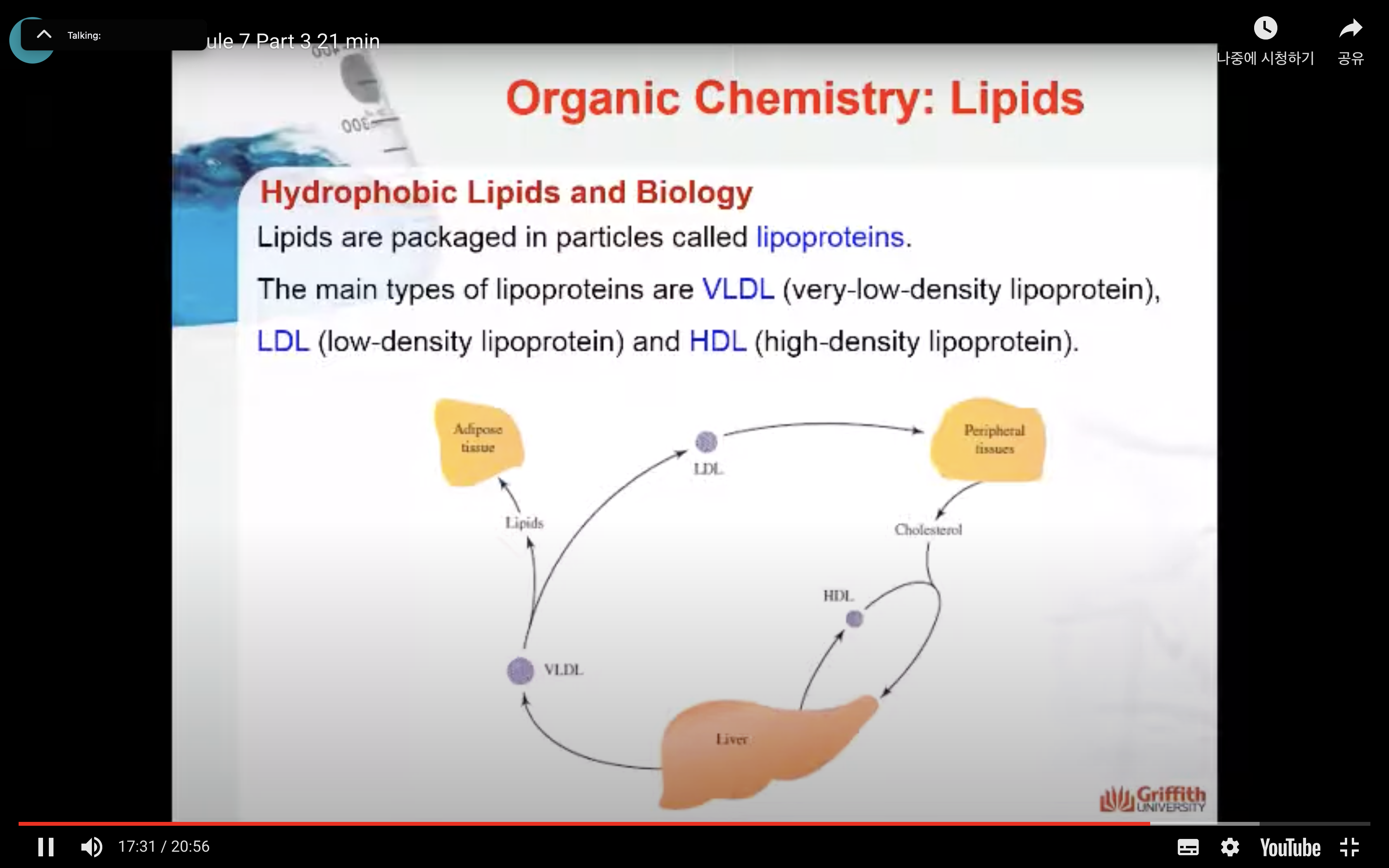
Lipids are packaged in particles called lipoproteins. The main types of lipoproteins are VLDL (very–low–density lipoprotein), LDL (low–density lipoprotein) and HDL (high–density lipoprotein). The lipid distribution system through the bloodstream using these lipoproteins is shown here.

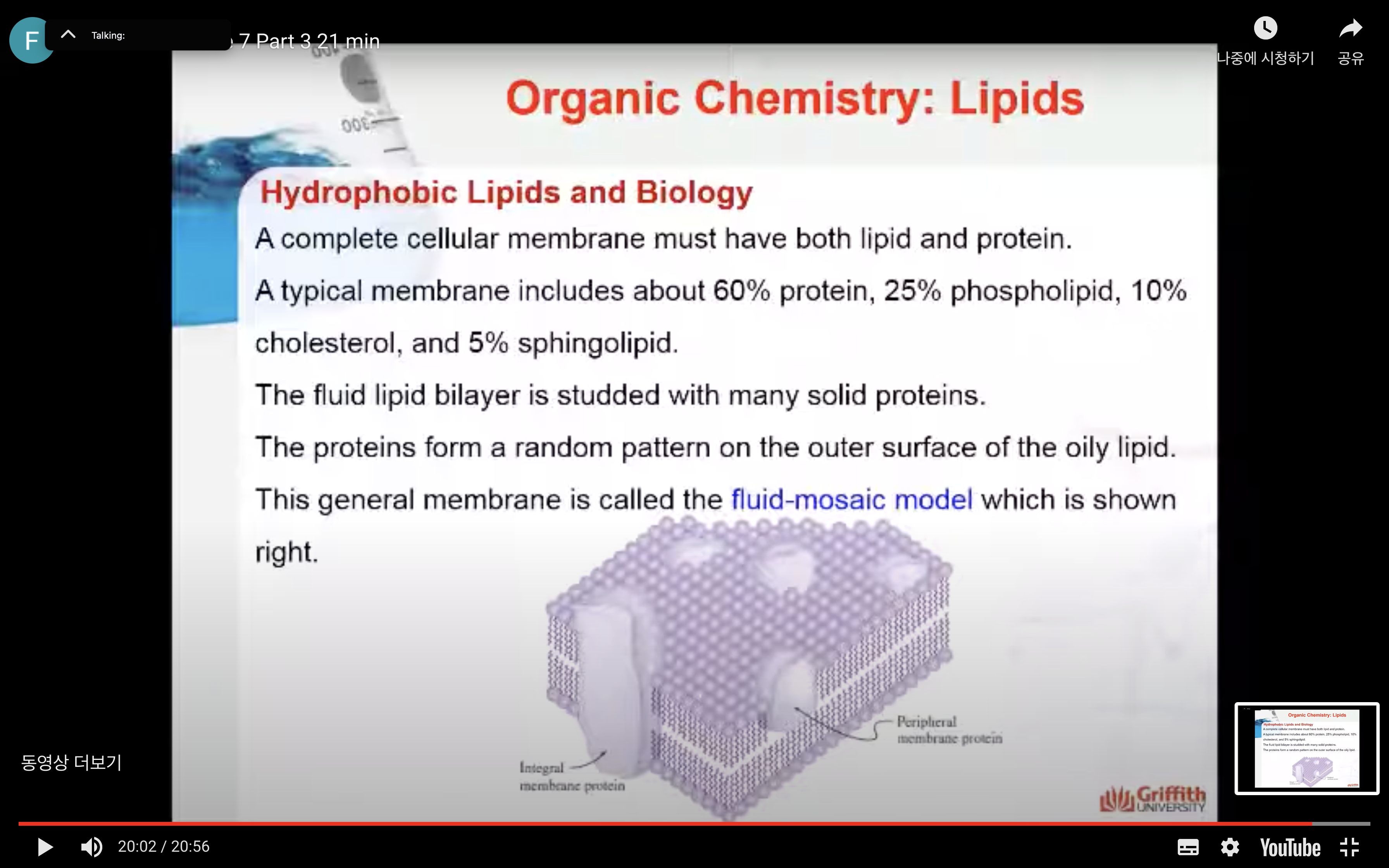
Biological membranes
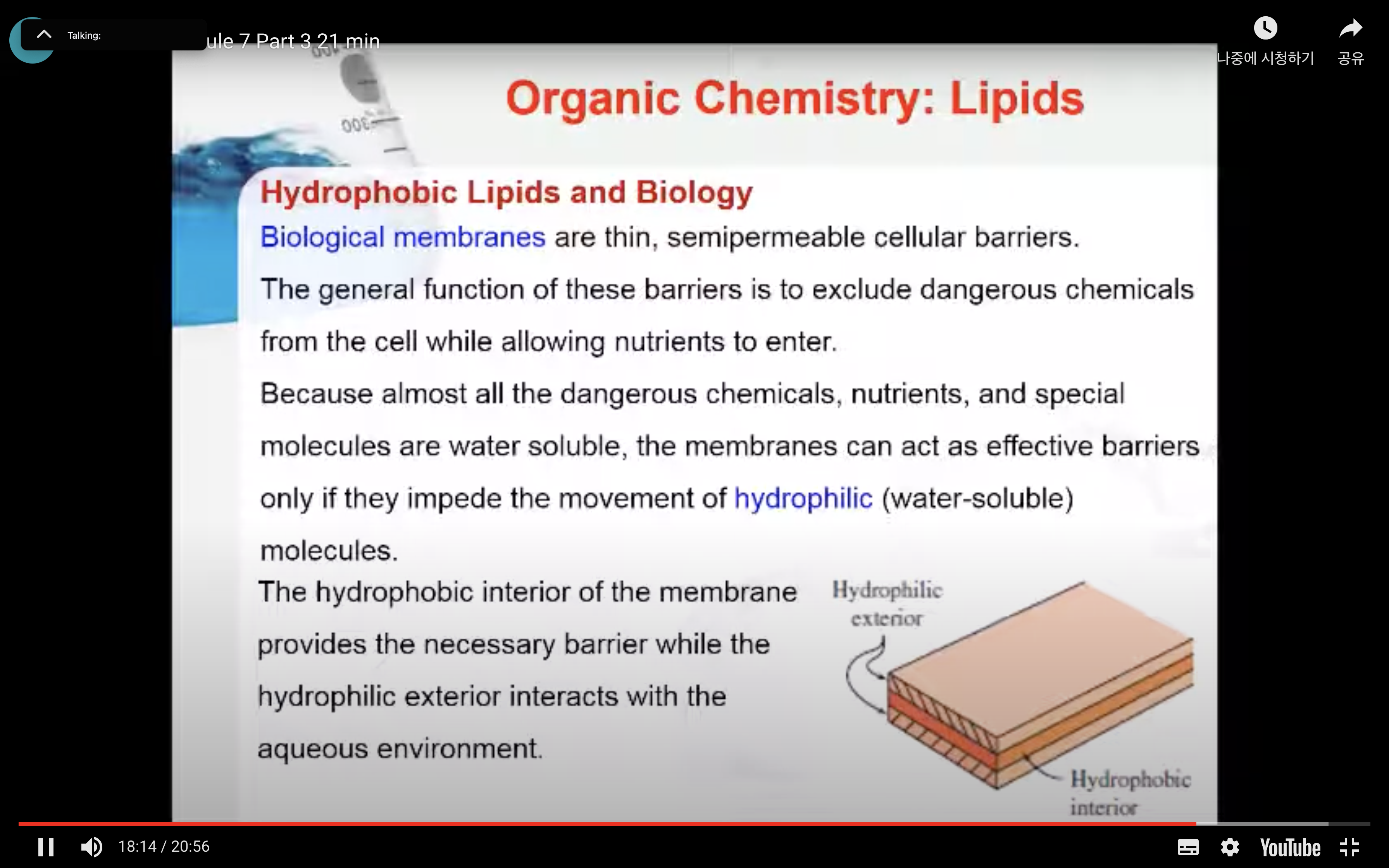
Biological membranes are thin, semipermeable cellular barriers. The general function of these barriers is to exclude dangerous chemicals from the cell while allowing nutrients to enter. Because almost all the dangerous chemicals, nutrients, and special molecules are water soluble, the membranes can act as effective barriers only if they impede the movement of hydrophilic (water-soluble) molecules. The hydrophobic interior of the membrane provides the necessary barrier while the hydrophilic exterior interacts with the aqueous environment.

Plywood model of simple membrane
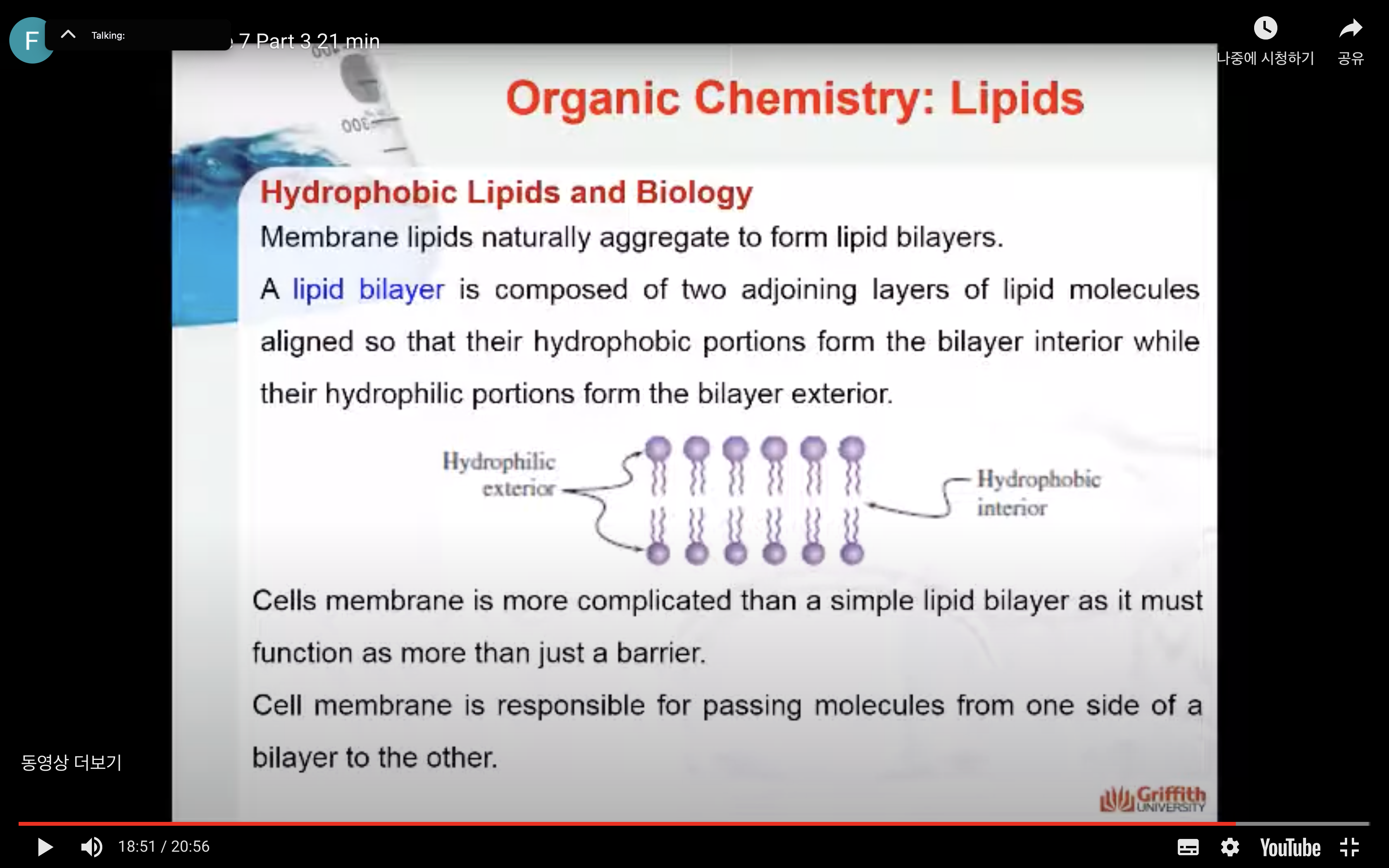
Membrane lipids naturally aggregate to form lipid bilayers. A lipid bilayer is composed of two adjoining layers of lipid molecules aligned so that their hydrophobic portions form the bilayer interior while their hydrophilic portions form the bilayer exterior.

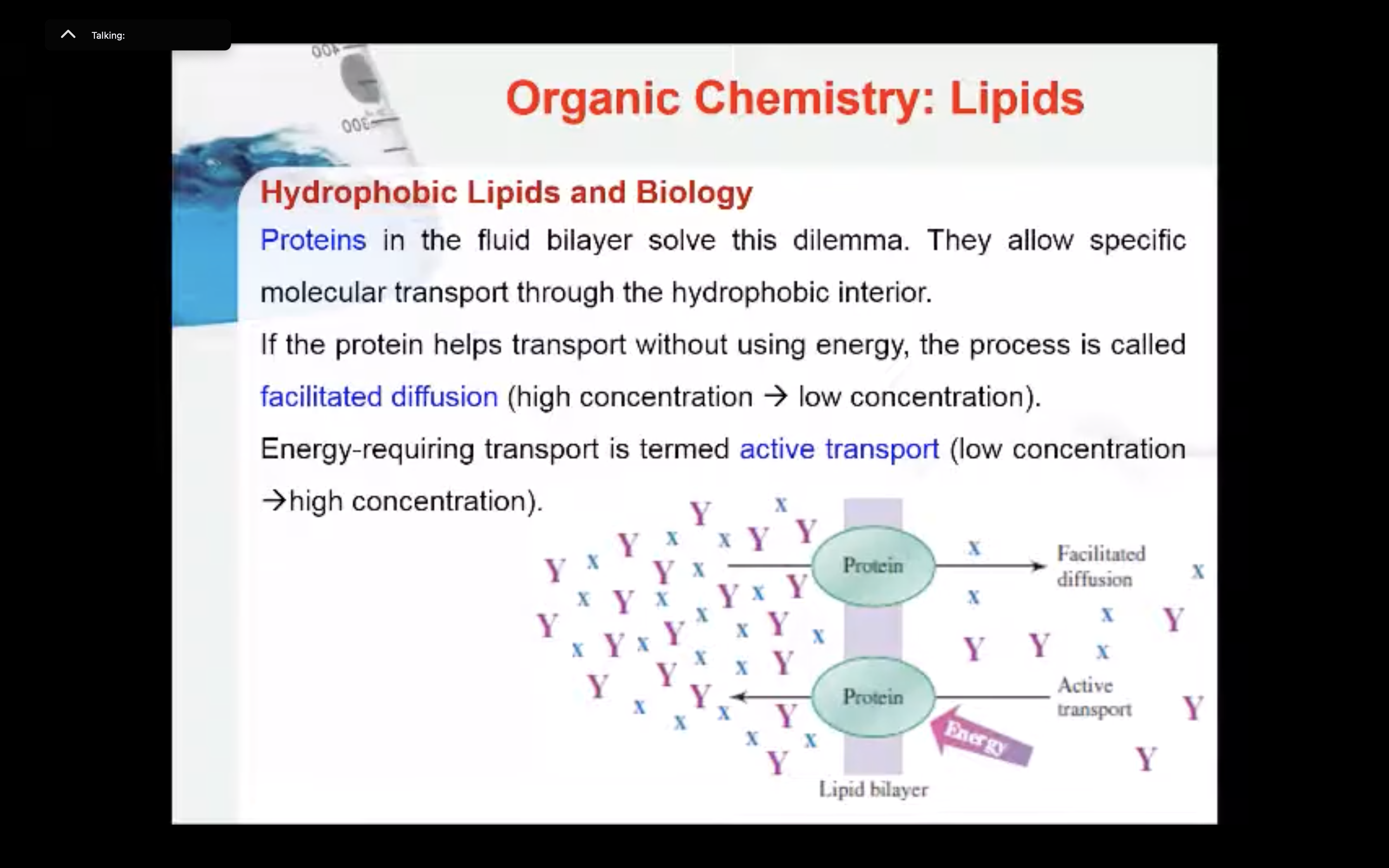
All known cells in today’s world need a membrane that is more complicated than a simple lipid bilayer. A membrane must function as more than just a barrier. Tasks such as passing molecules from one side of a bilayer to the other are an essential part of life. Proteins in the fluid bilayer solve this dilemma. They allow specific molecular transport through the hydrophobic interior. If the protein helps transport without using energy, the process is called facilitated diffusion. Energy–requiring transport is termed active transport.



-Phospholipids yield a phosphate and a nitrogen-containing base as well as glycerol and fatty acids upon hydrolysis
-Sphingolipids yield an unsaturated amino alcohol (sphingosine) and a carbohydrate or phosphate and nitrogen base in addition to a fatty acid upon hydrolysis
-Glycolipids yield sphingosine and a carbohydrate as well as a fatty acid upon hydrolysis
-Steroids possess the steroid nucleus containing 17 carbon atoms
-Miscellanesous lipids include lipoproteins



'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK6] Stereoisomerism (0) | 2023.03.31 |
|---|---|
| [WEEK5] Carboxylic Acids, Esters (0) | 2023.03.30 |
| [WEEK5] Chemistry of Food -Fatty Acids (0) | 2023.03.29 |
| REACTION NOTES (0) | 2023.03.28 |
| Lab 3 Glucose Concentration in Drinks (0) | 2023.03.27 |



