Introduction
<Enzymes>
Enzymes are biological, usually protein, catalysts that speed the rate of chemical reactions (by factors of up to 1017). They achieve this by lowering the activation energy required for the reaction to proceed.
Enzymes react specifically with one or more substrates (S) to produce one or more products (P).

Co-factors or Co-enzymes?
Certain enzymes may require the presence of either co-factors (e.g. Mg2+ or Fe2+ ions) or co-enzymes (e.g. NAD+/NADH or NADP+/NADPH) to assist the reaction.
It is noteworthy that many enzyme-catalysed reactions followed by the use of spectrophotometry rely on the differing absorbance characteristics of the oxidised and reduced forms of NAD+/NADH or NADP+/NADPH.
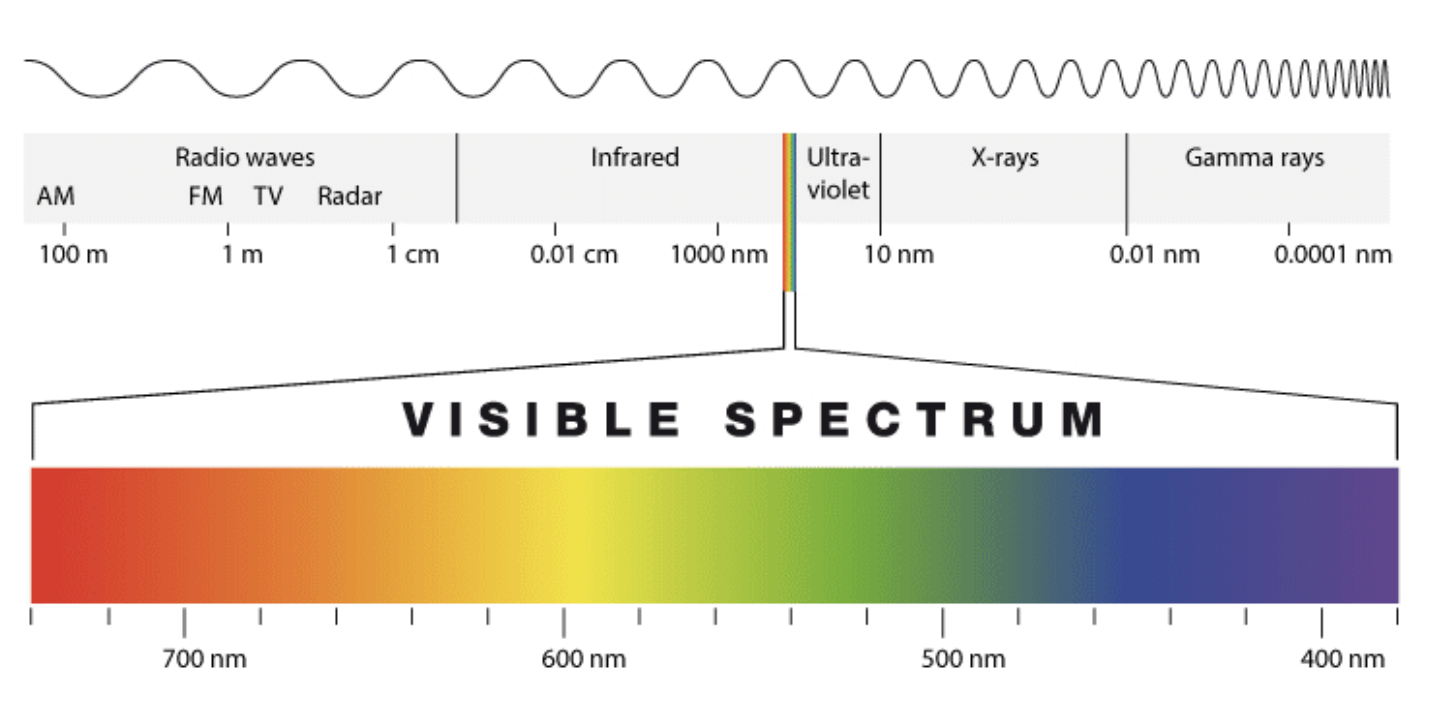
Specifically, NADPH absorbs strongly at a wavelength of 340 nm whilst NADP+ does not. Thus, reactions involving either the appearance or disappearance of NADPH can be monitored spectrophotometrically.

It is noteworthy that many enzyme-catalysed reactions followed by the use of spectrophotometry rely on the differing absorbance characteristics of the oxidised and reduced forms of NAD+/NADH or NADP+/NADPH.
Specifically, NADPH absorbs strongly at a wavelength of 340 nm whilst NADP+ does not. Thus, reactions involving either the appearance or disappearance of NADPH can be monitored spectrophotometrically.
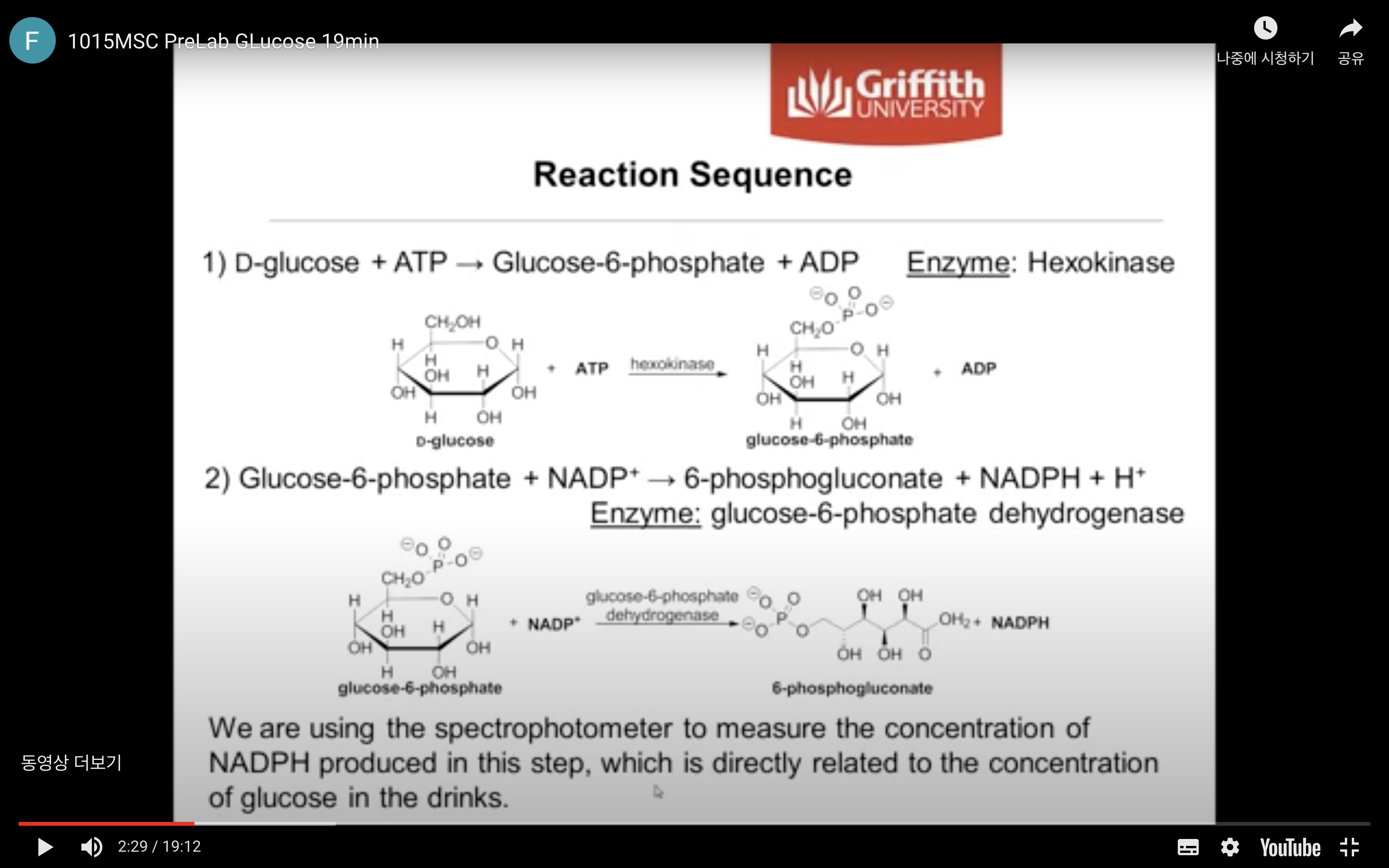
The reaction sequence


Spectrophotometers, Beer's Law & Chromophores!
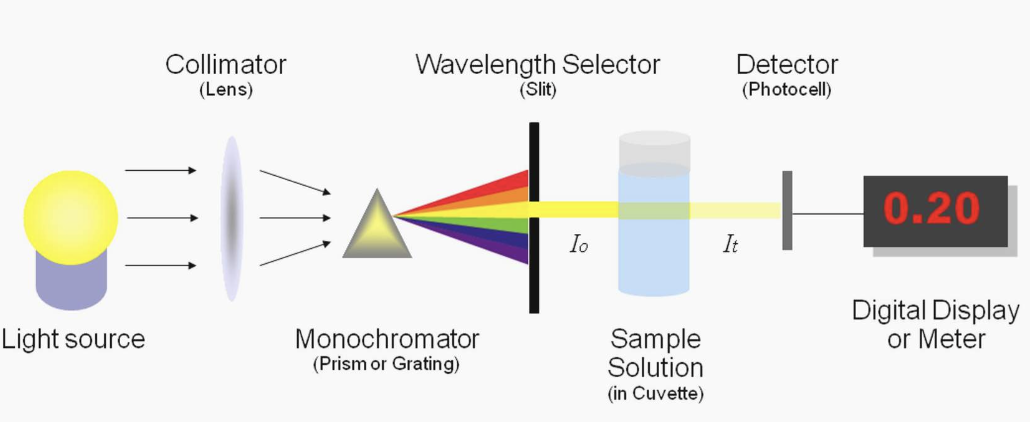
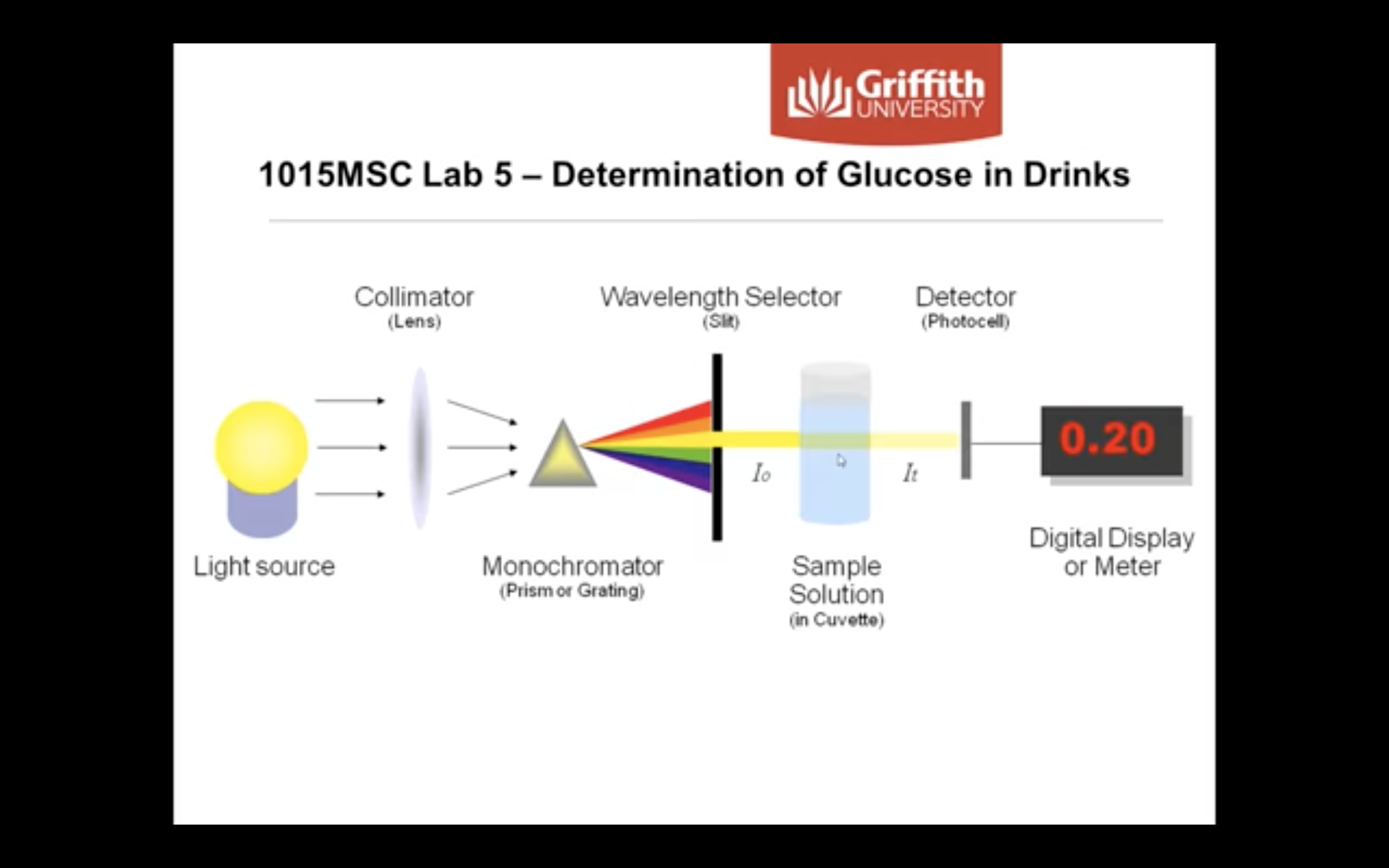
<How does a spectrophotometer work?>
Transmittance : the amount of light that passes completely through the sample and strikes the detector
Absorbance : measurement of light that is absorbed by the sample
-The detector senses the light being transmitted through the sample and converts this information into a digital display

<Beer-Lambert's Law>
-kinetics : study of reaction rates
-measure the mass of a precipitate as it is forming, or measure the volume or even the pressure of a gas that is produced over time
-Spectroscopy : the study of how light interacts with matter
-All molecules absorb and emit light differently, this depends on the types of bonds
-If the products and reactants interact with light different enough that there is a color change as the reaction occurs, we can monitor this process with an instrument called a spectrophotometer
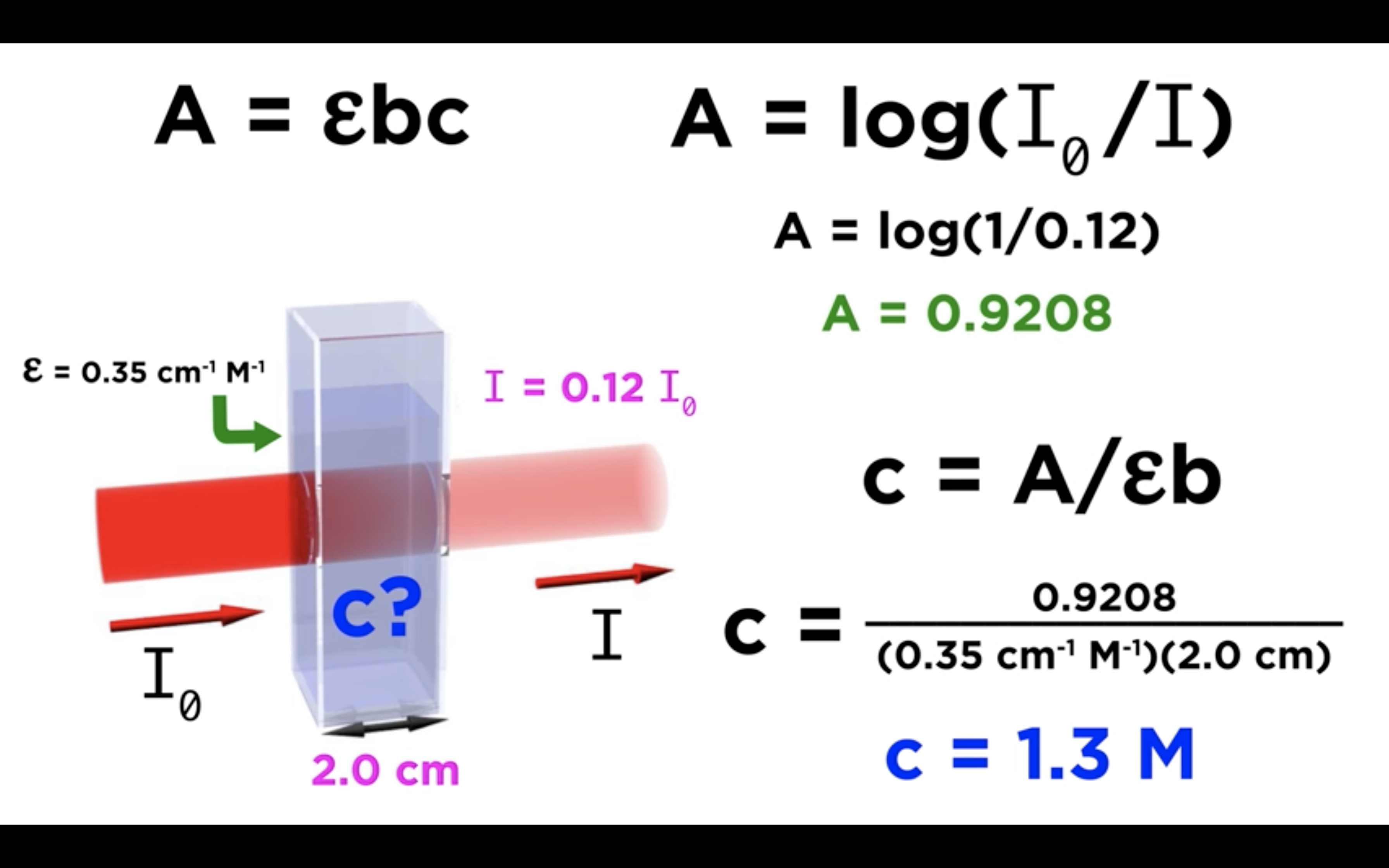
*Equation that relates the amount of material in a sample to the absorption of light passing through it, and this is called Beer's law
-Absorbance is measured by comparing the intensity of the beam of light as it enters the sample, and the intensity as it leaves the sample and strikes the detector, which are I0 and I respectively

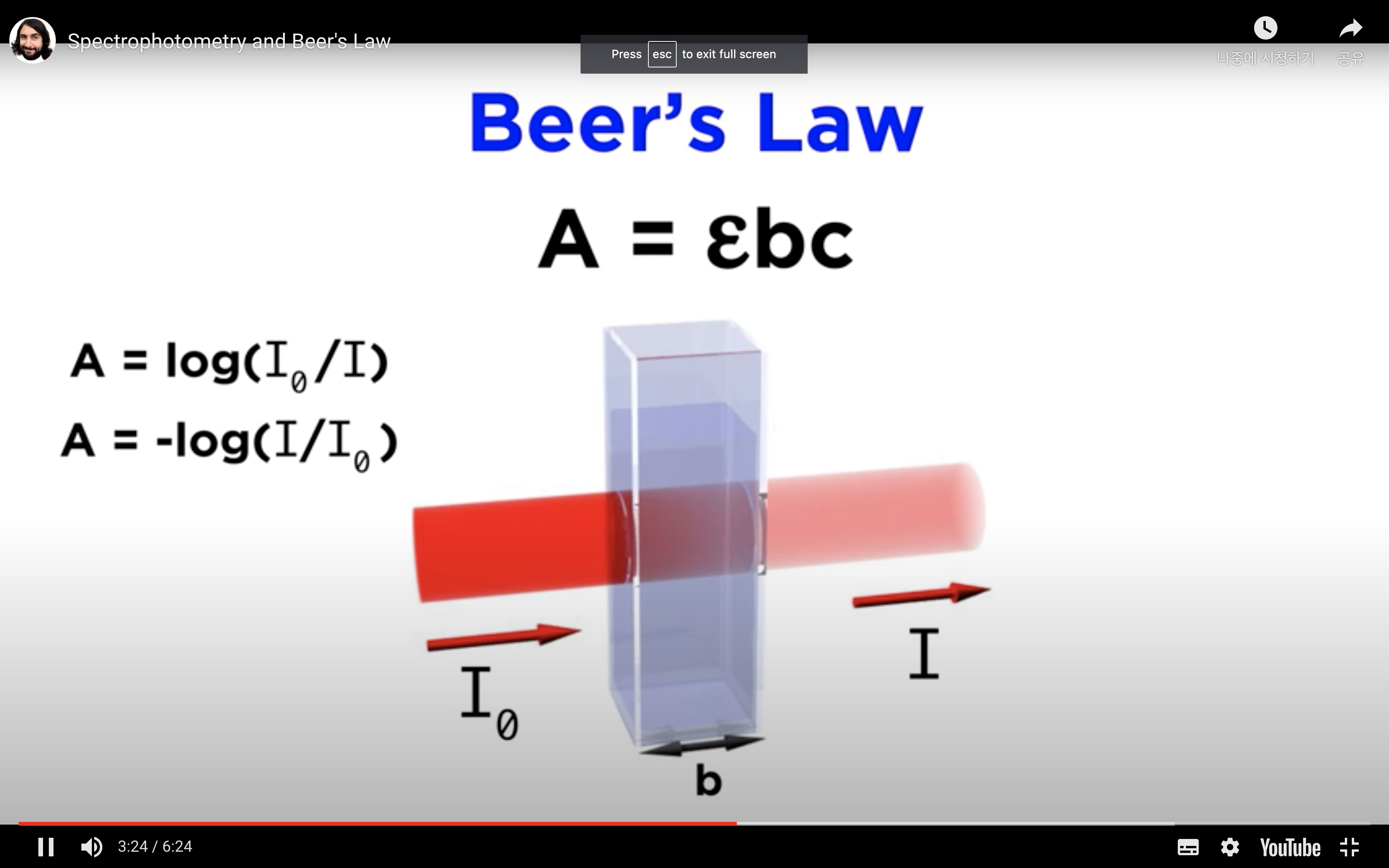
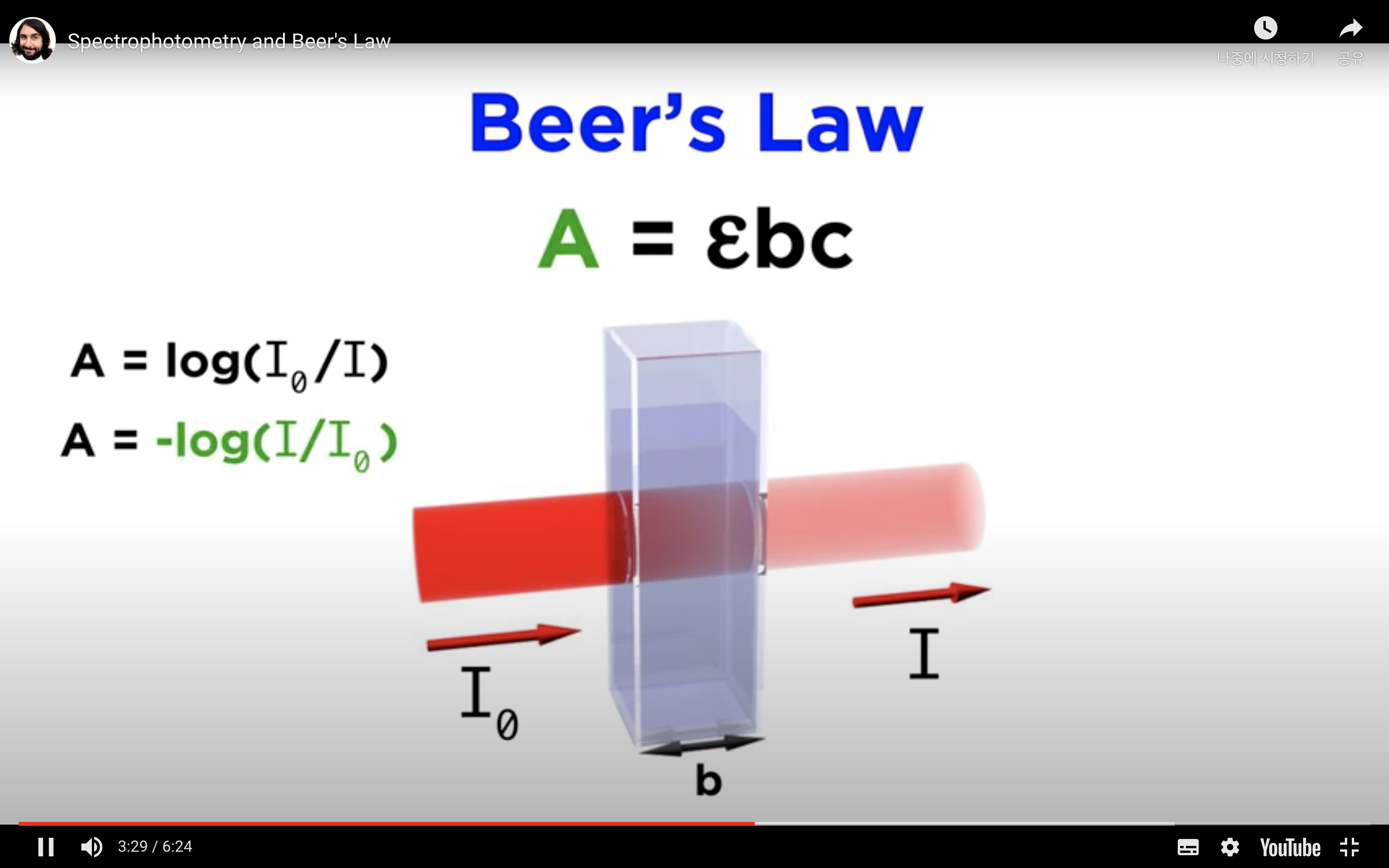
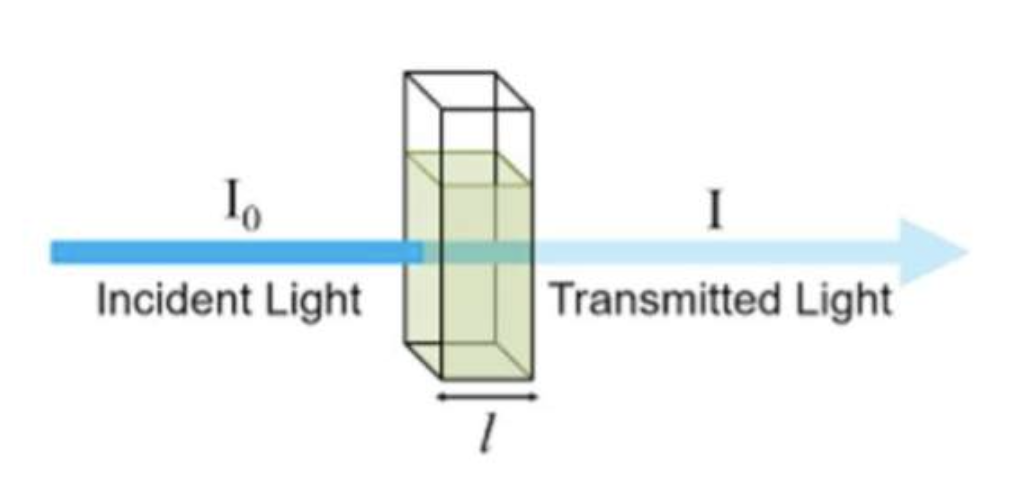
When monochromatic light of intensity I0 is incident upon a solution (typically contained in a 1cm cuvette), a fraction, I, emerges from the sample.
In this case the %transmittance (T) is given by:
T = (I/Io) × 100%
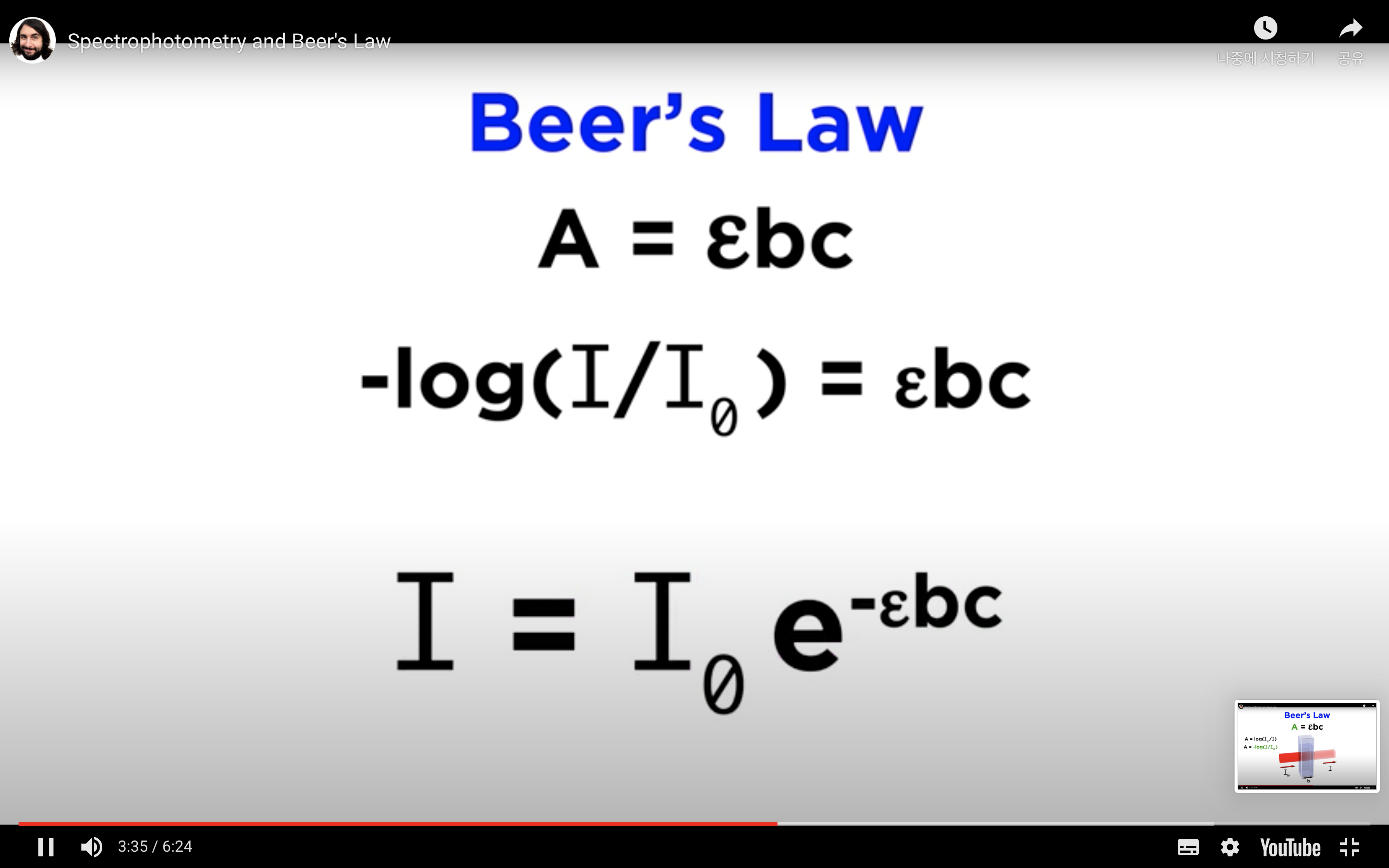
The absorbance (A) is:
A = -log10T = - log10(I/Io) or log10(Io/I)
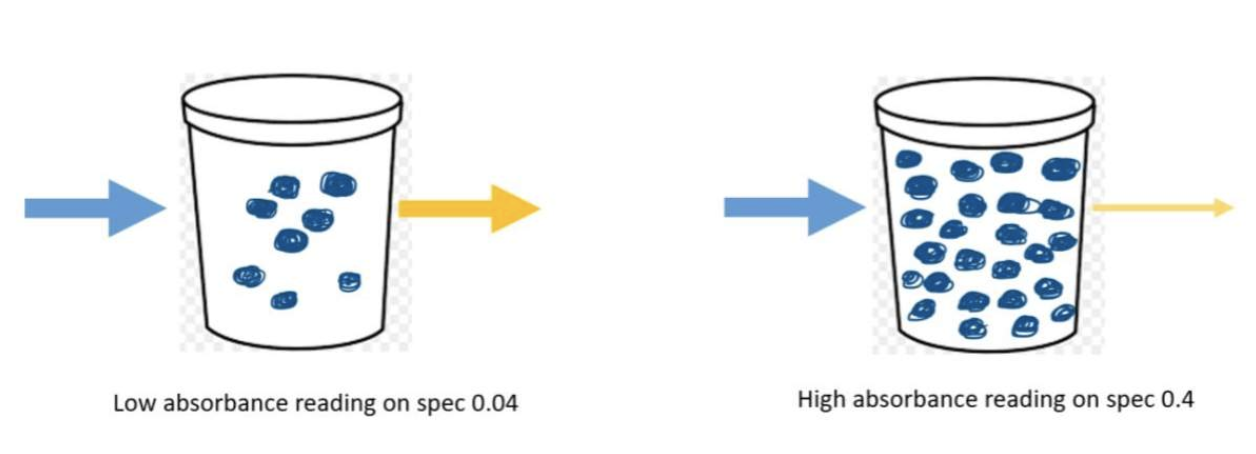
The more molecules in the cuvette, the less light is transmitted through to the other side. The less molecules in the cuvette, the more light makes it to the detector on the other side.
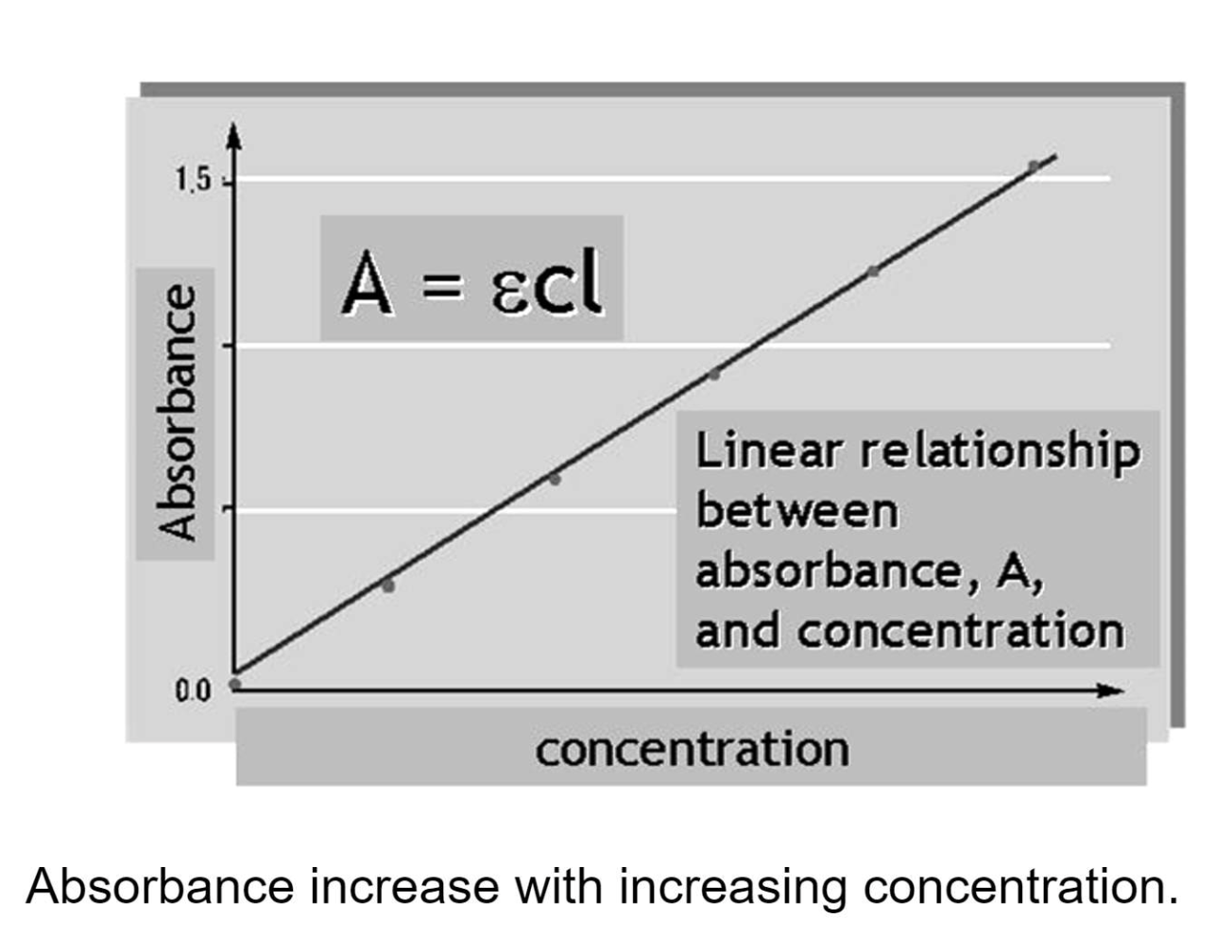
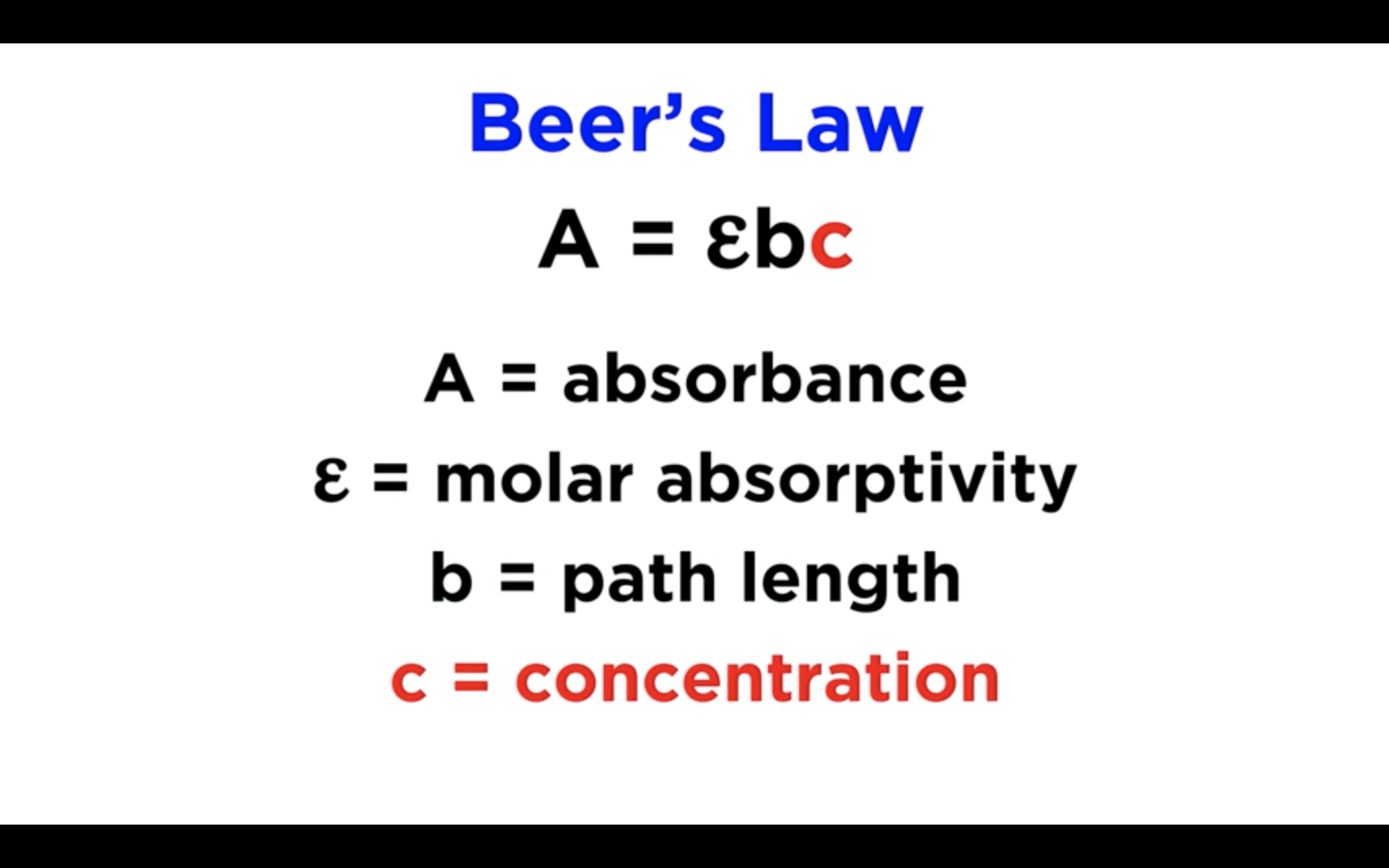
The Beer-Lambert Law relates the absorbance (A) to:
e = Molar extinction coefficient (M-1 cm-1)
c = concentration of solution (M)
l = pathlength of cuvette (cm)
Overall, A = e × c × l

<Chromophore, auxochrome>
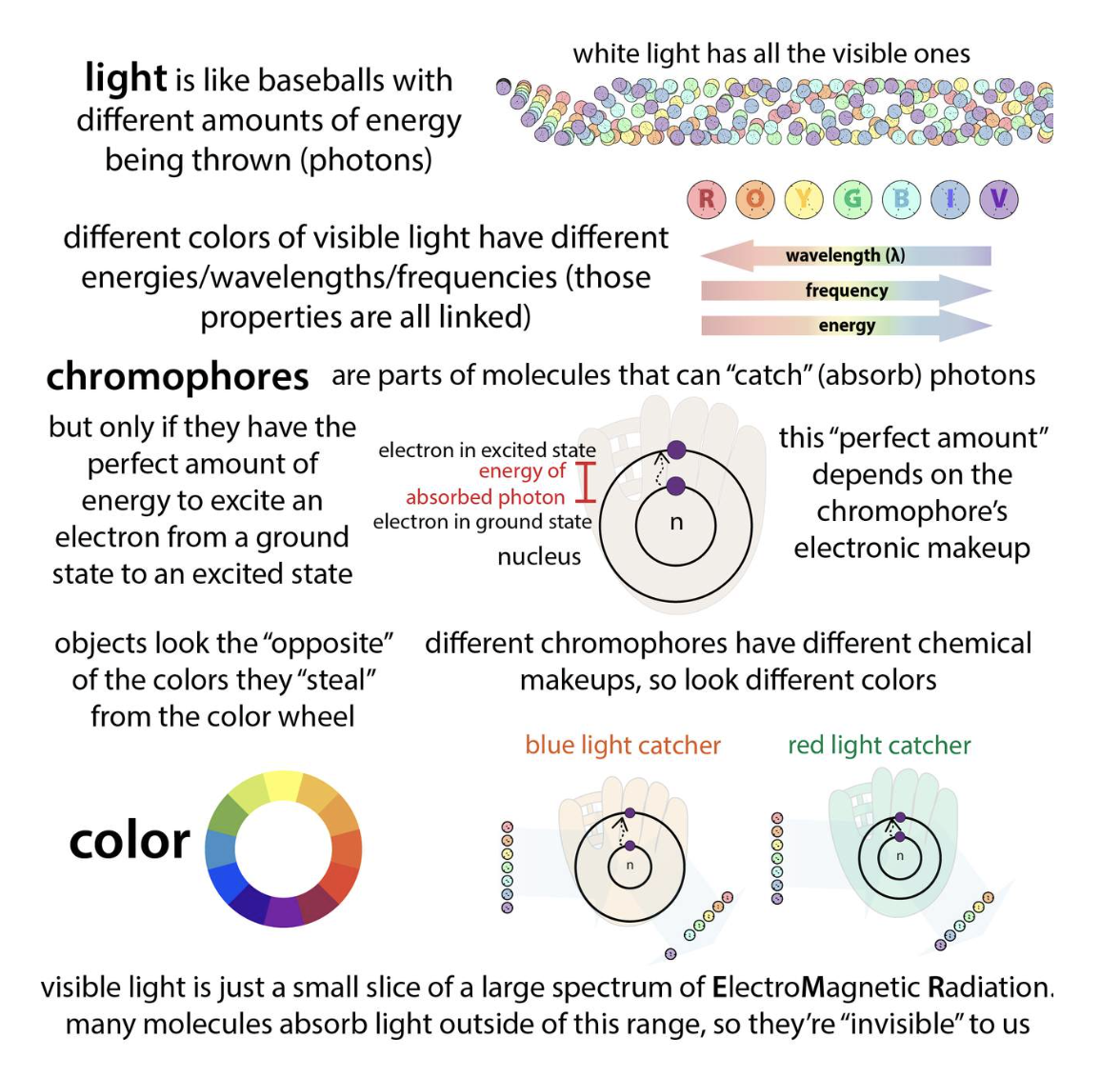
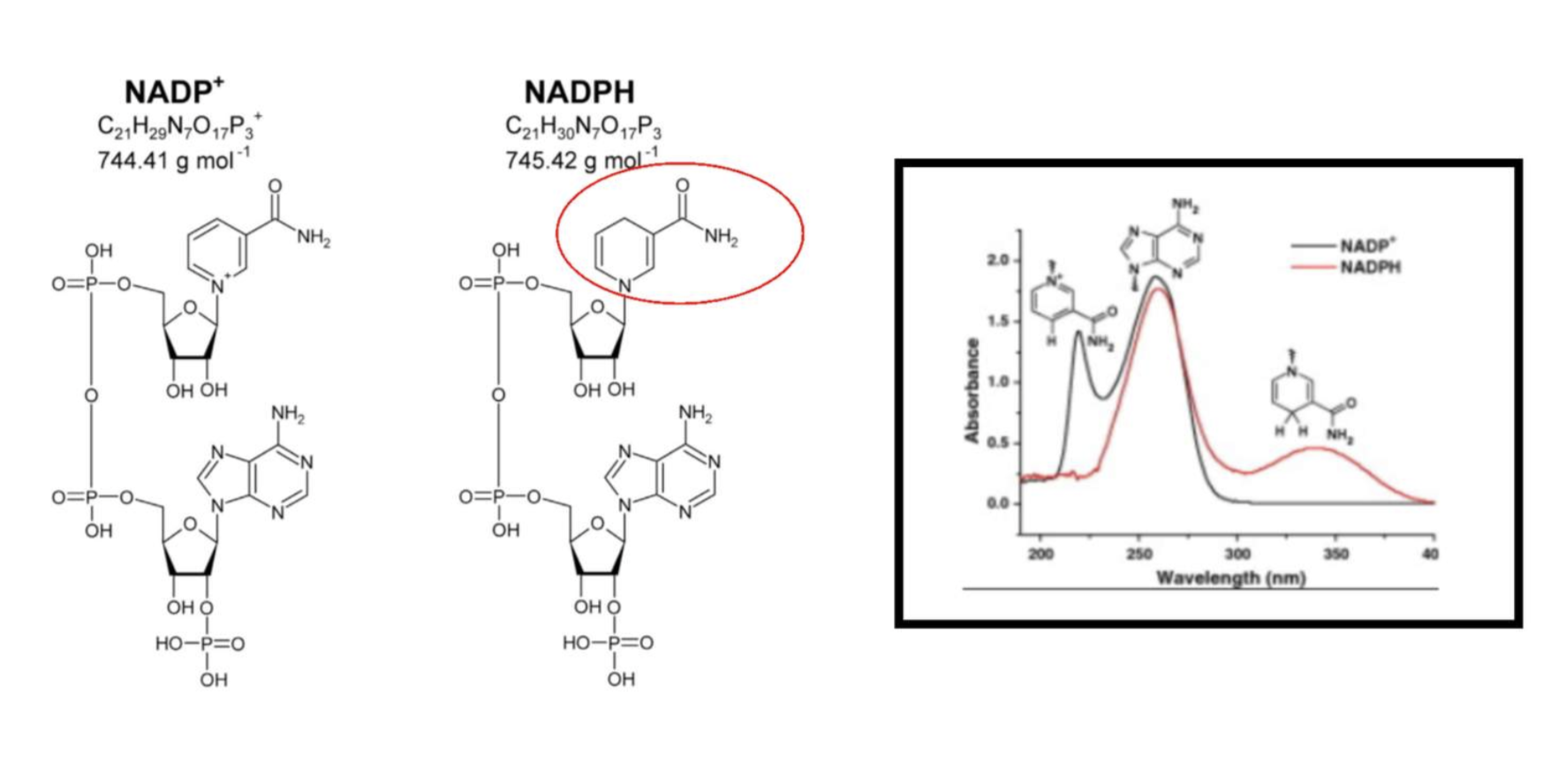
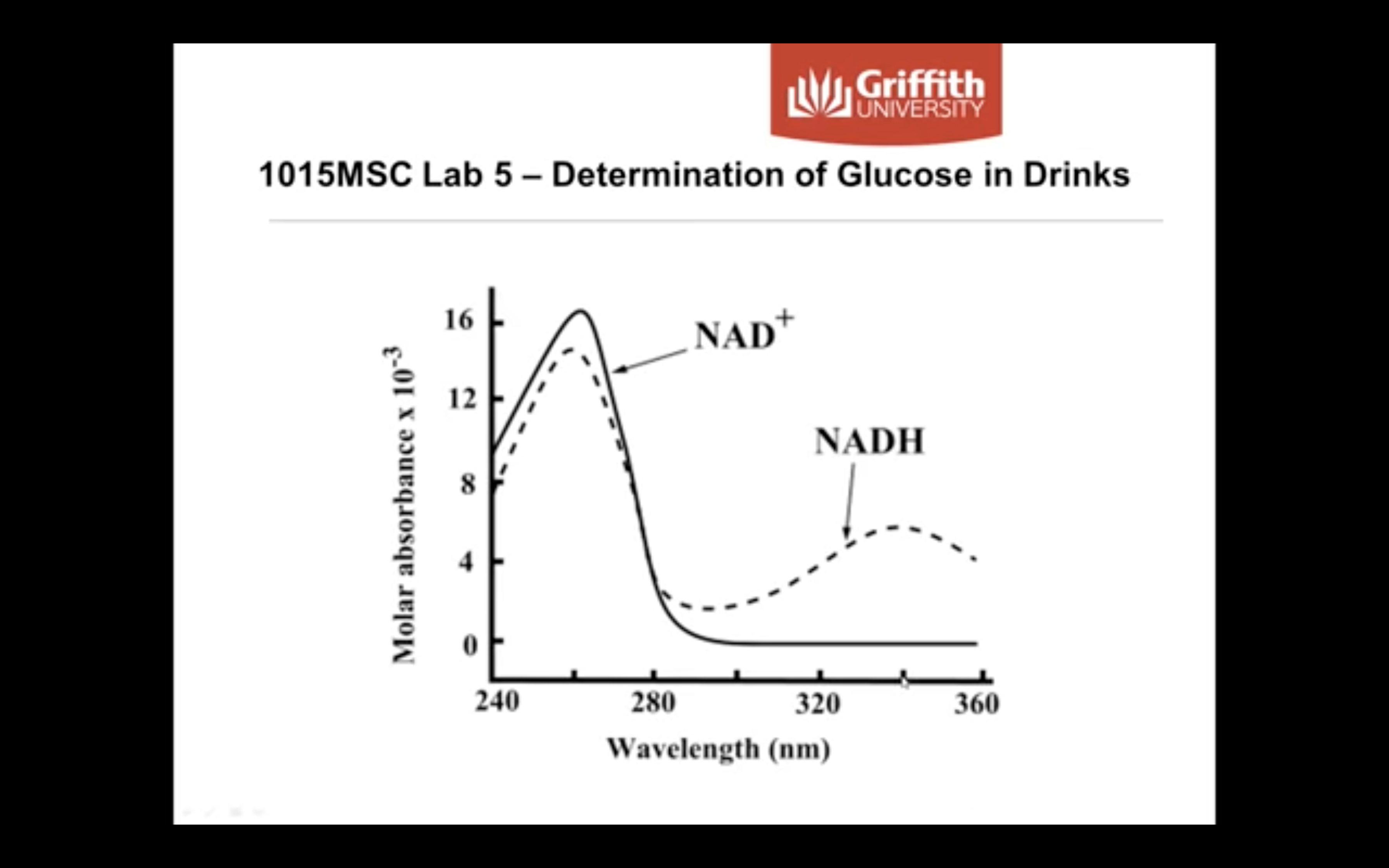
Below you can see the light absorbance difference that results from NADP+ and NADPH. After learning about Chromophores above, you should be able to determine which part of these molecules are responsible for the absorbance of light at different wavelengths.
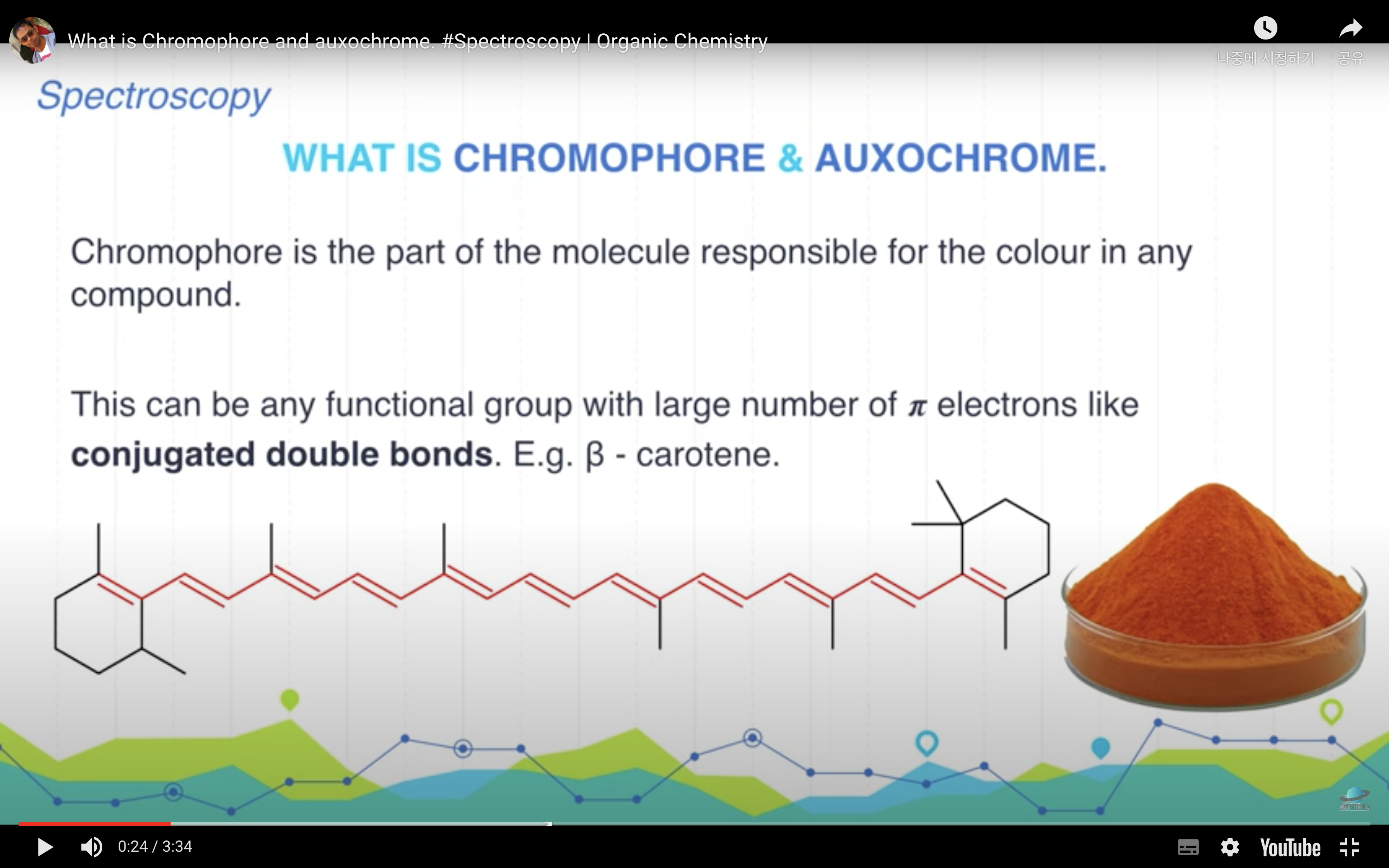

*Chromophore : part of the molecule responsible for the color in any compound
-> it can be any functional group with large number of pi electrons like conjugateddouble bonds
*Auxochrome : part of the molecule responsible for the intensity of color in any compound. These are functional groups with lone pairs of electrons or non bonding electrons.
(Auxochrome themselves can not impart color to any compound but they can only increase the intensity of color)
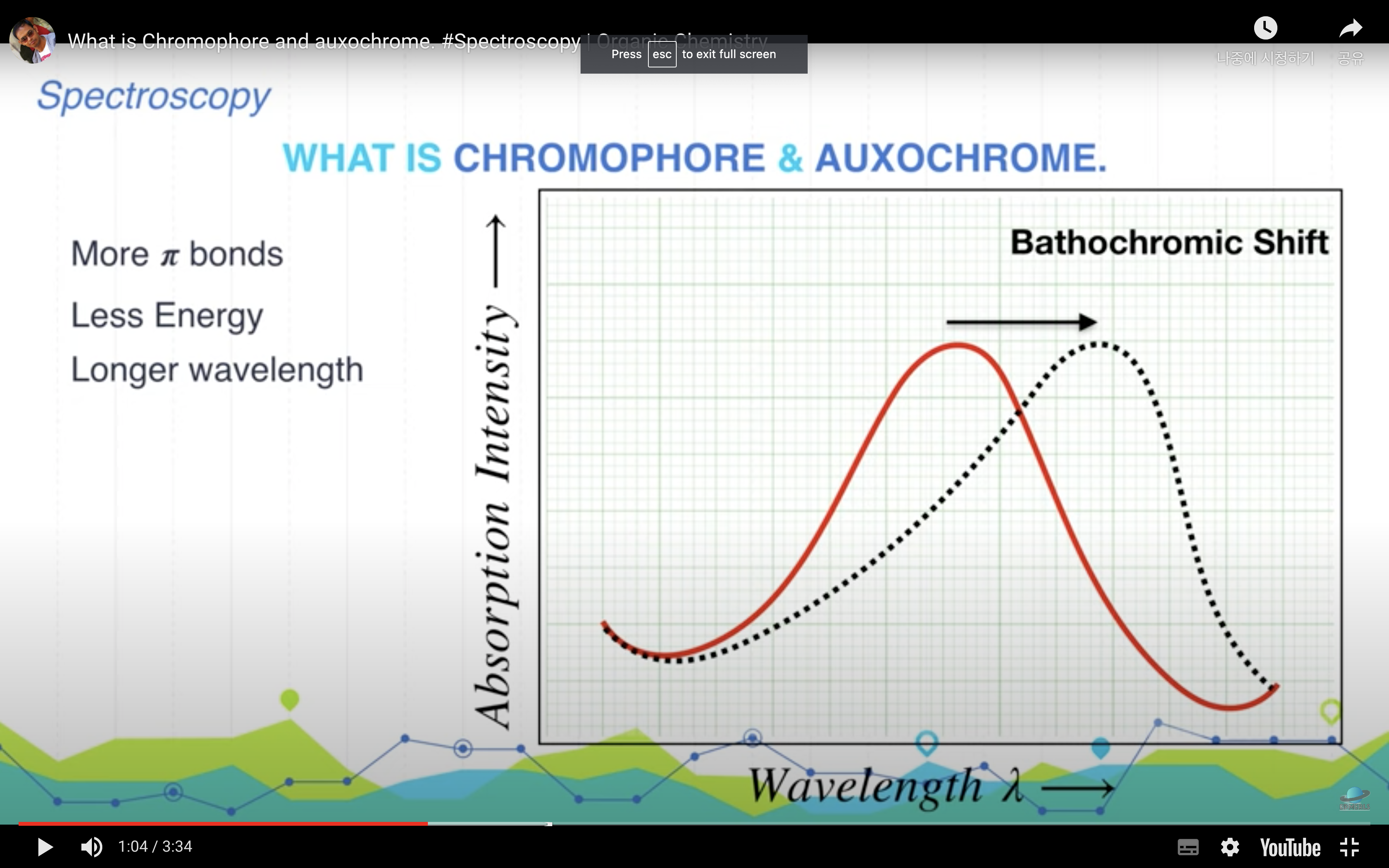
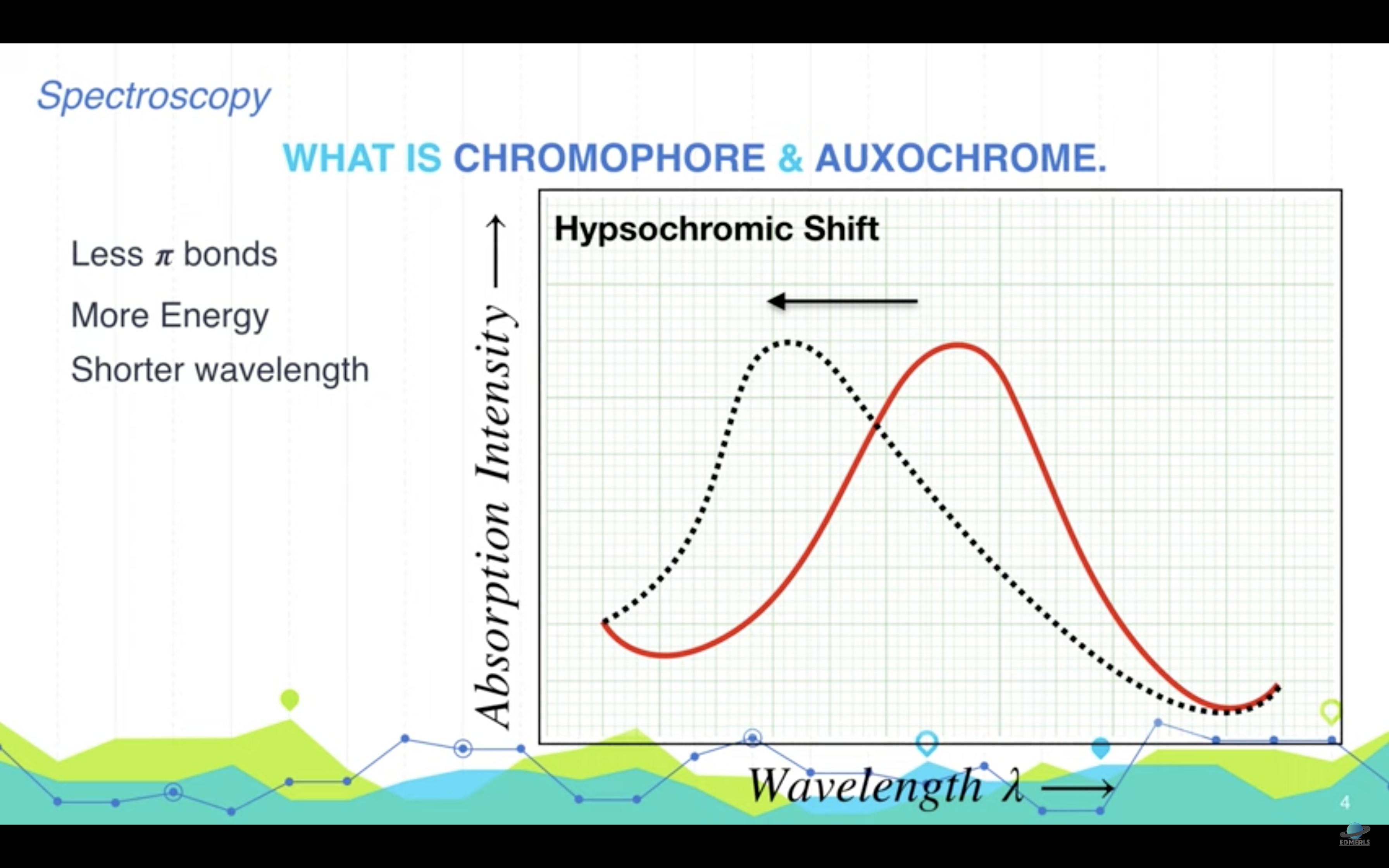
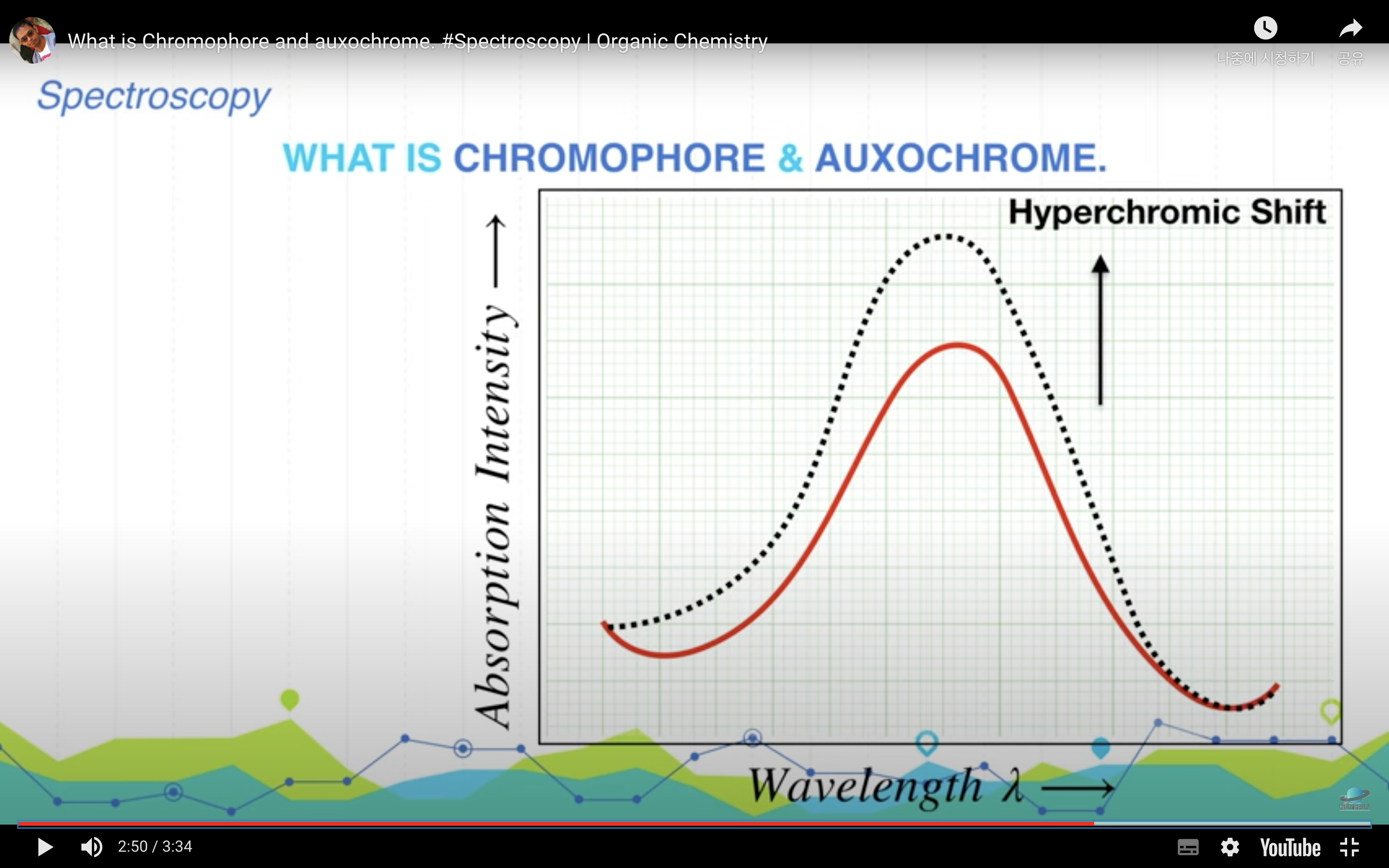
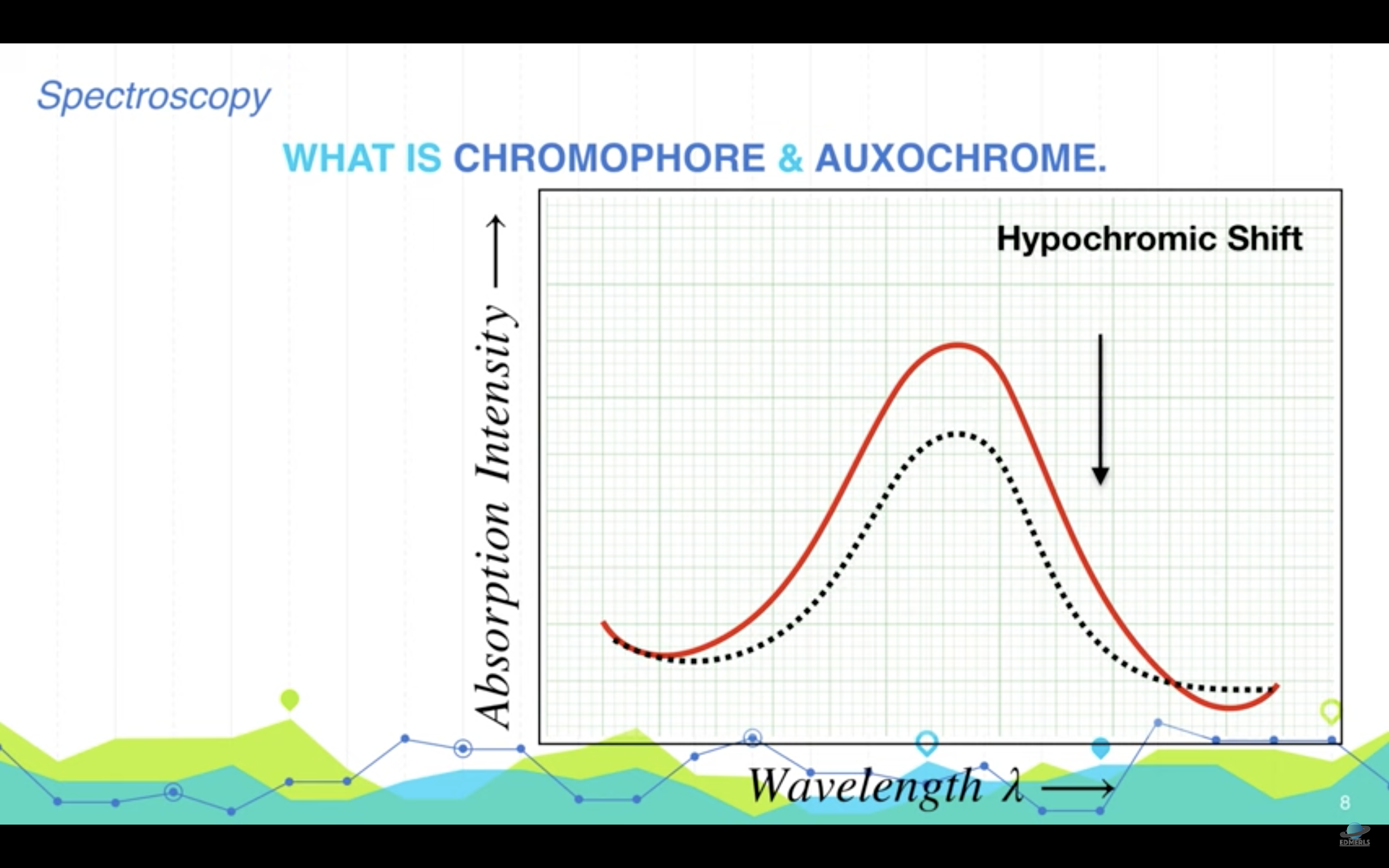
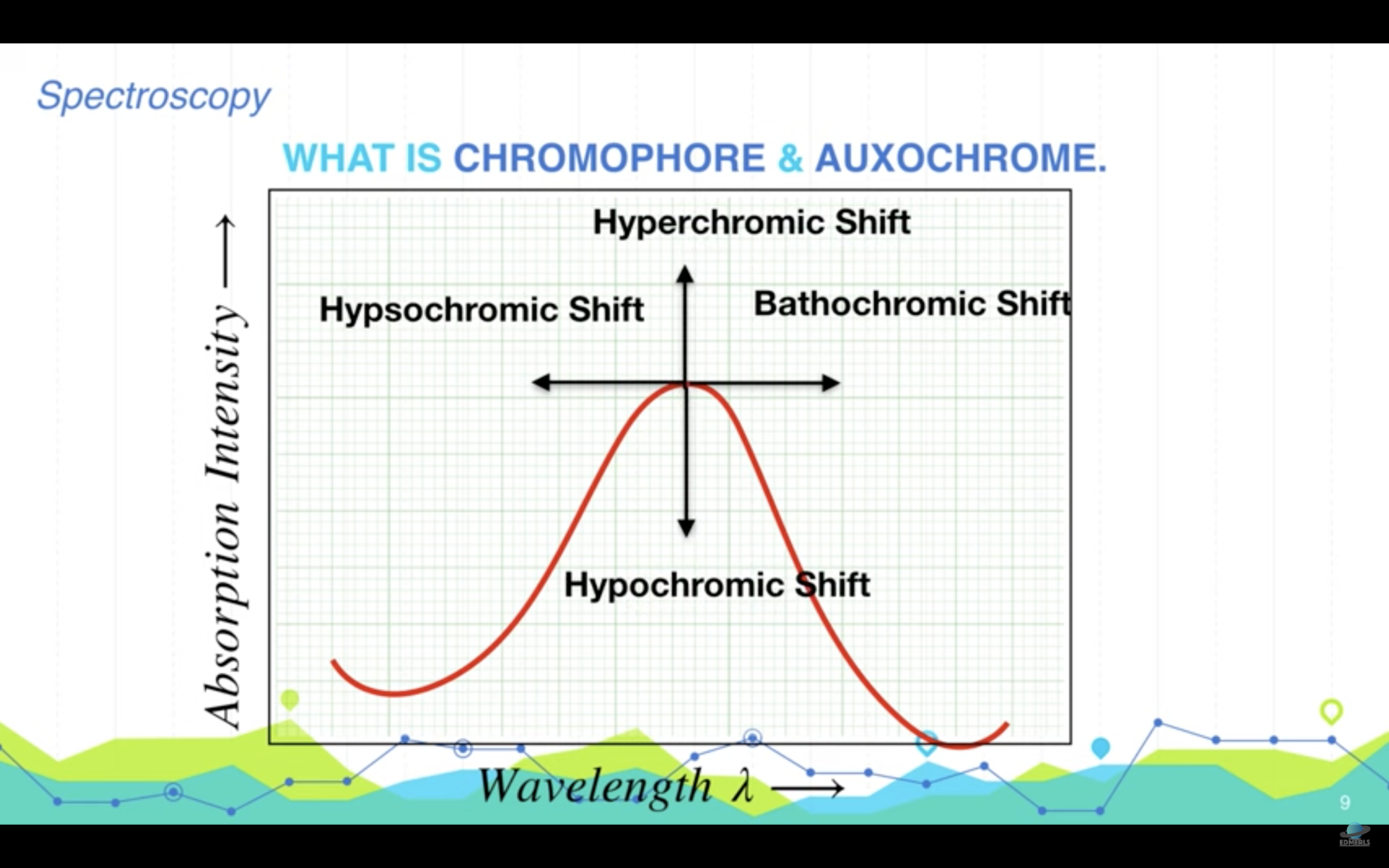
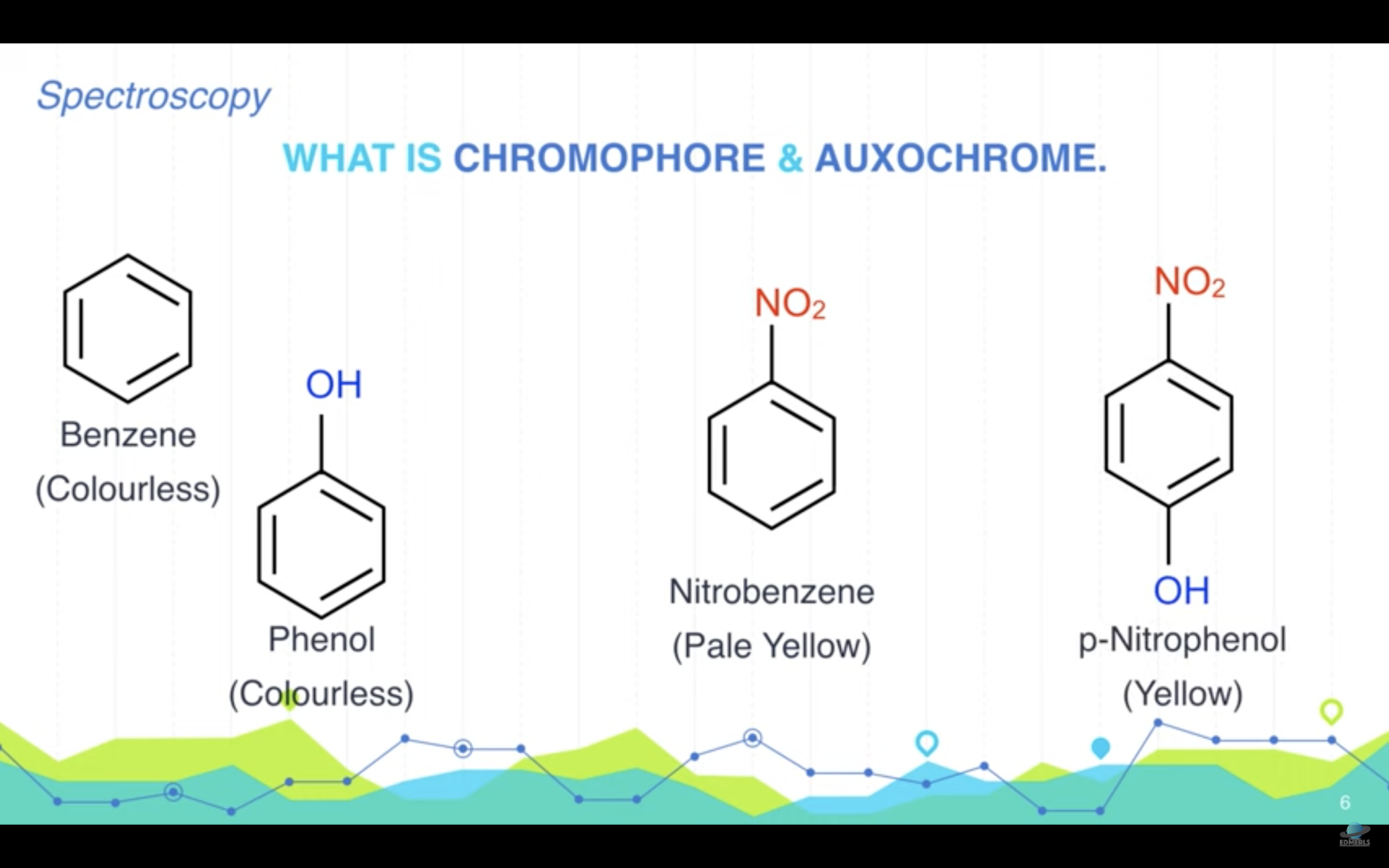
There are two main uses made of spectrophotometers:
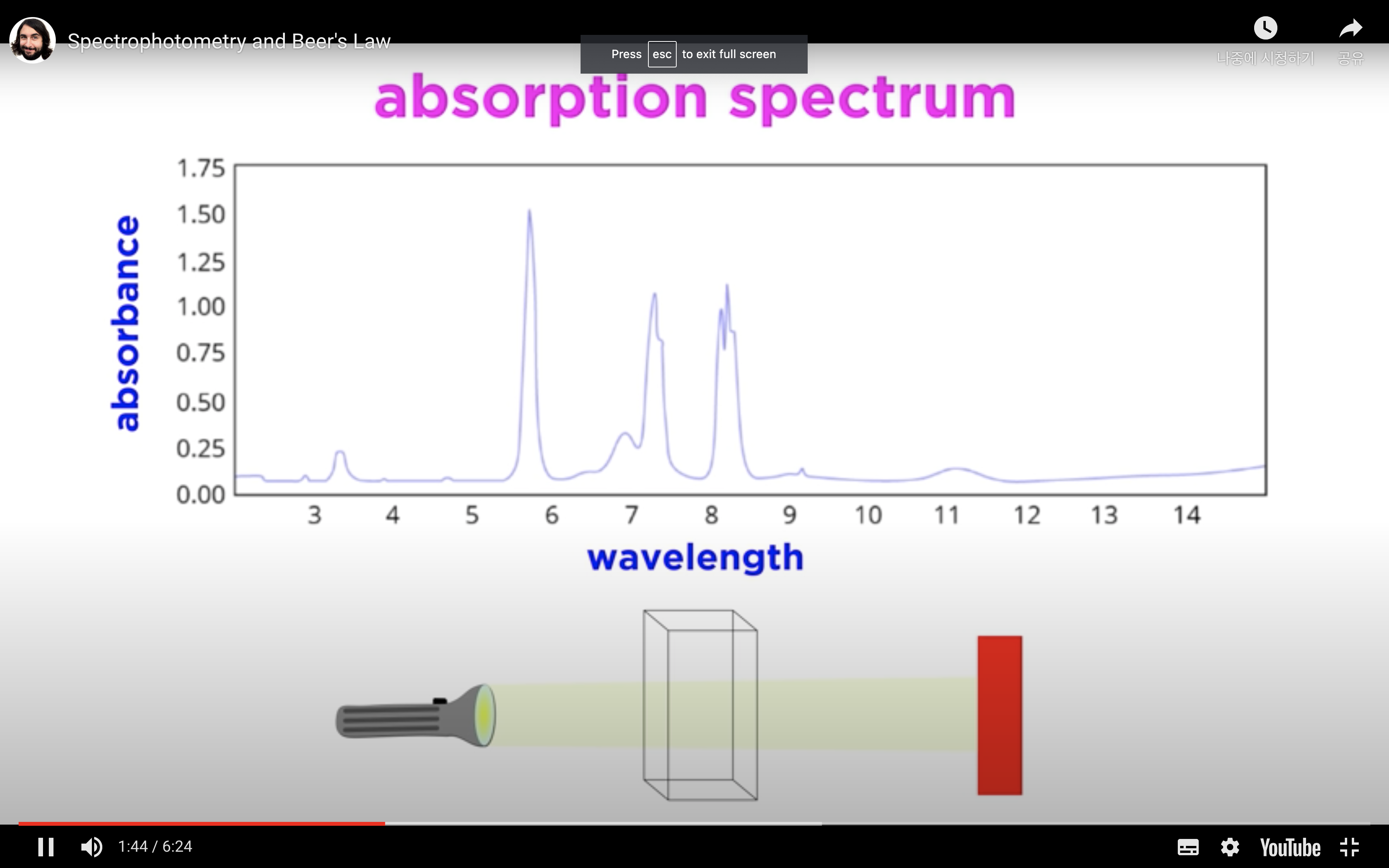
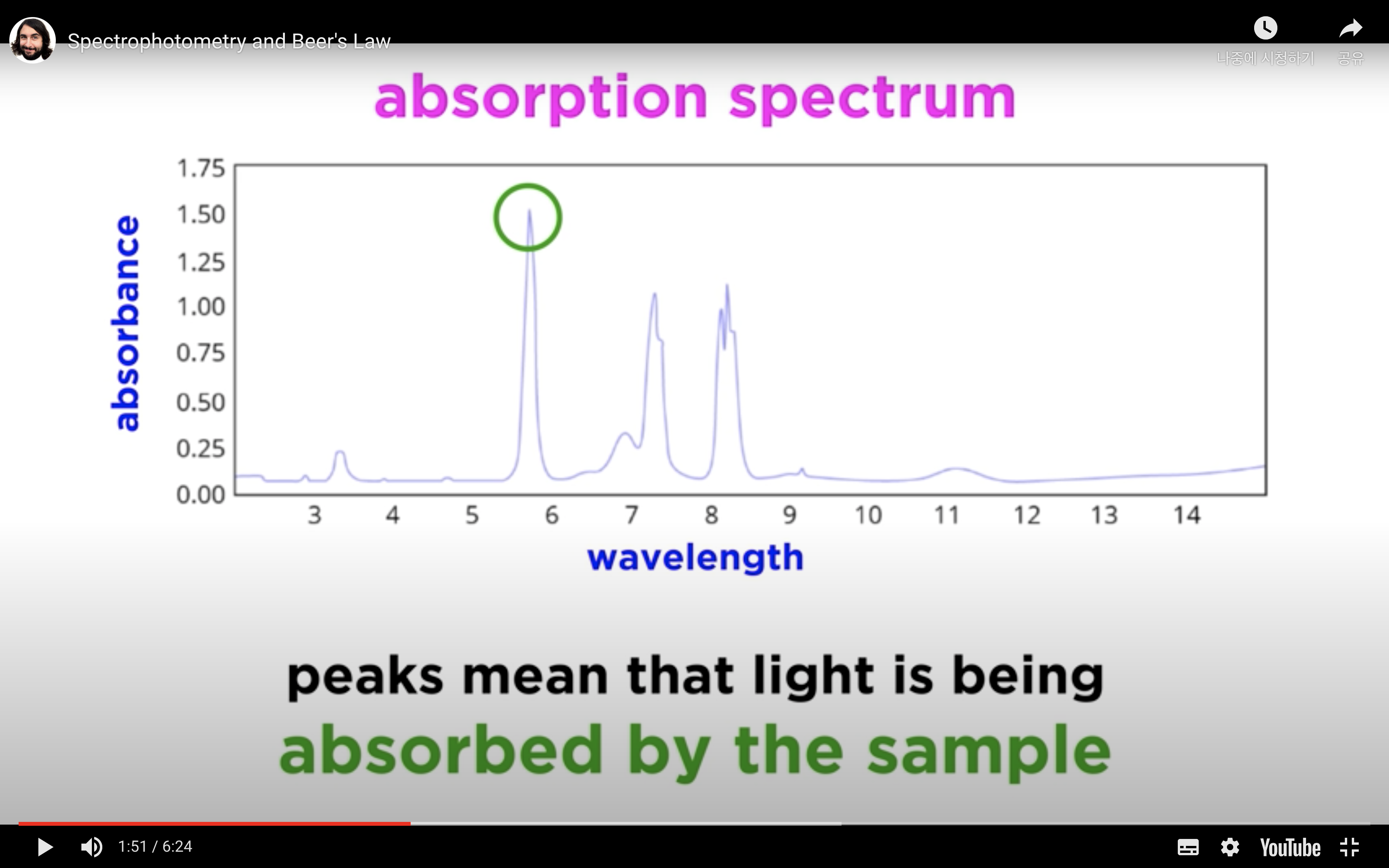
- Absorption spectrum: Identification of a compound by measuring the absorption of light by a solution of the compound at a series of different wavelengths, i.e. to determine the absorption spectrum of a compound.
- Determination of Concentration: Measurement of the amount of light absorbed by a solution of known concentration, calculation of the concentration of the unknown solution
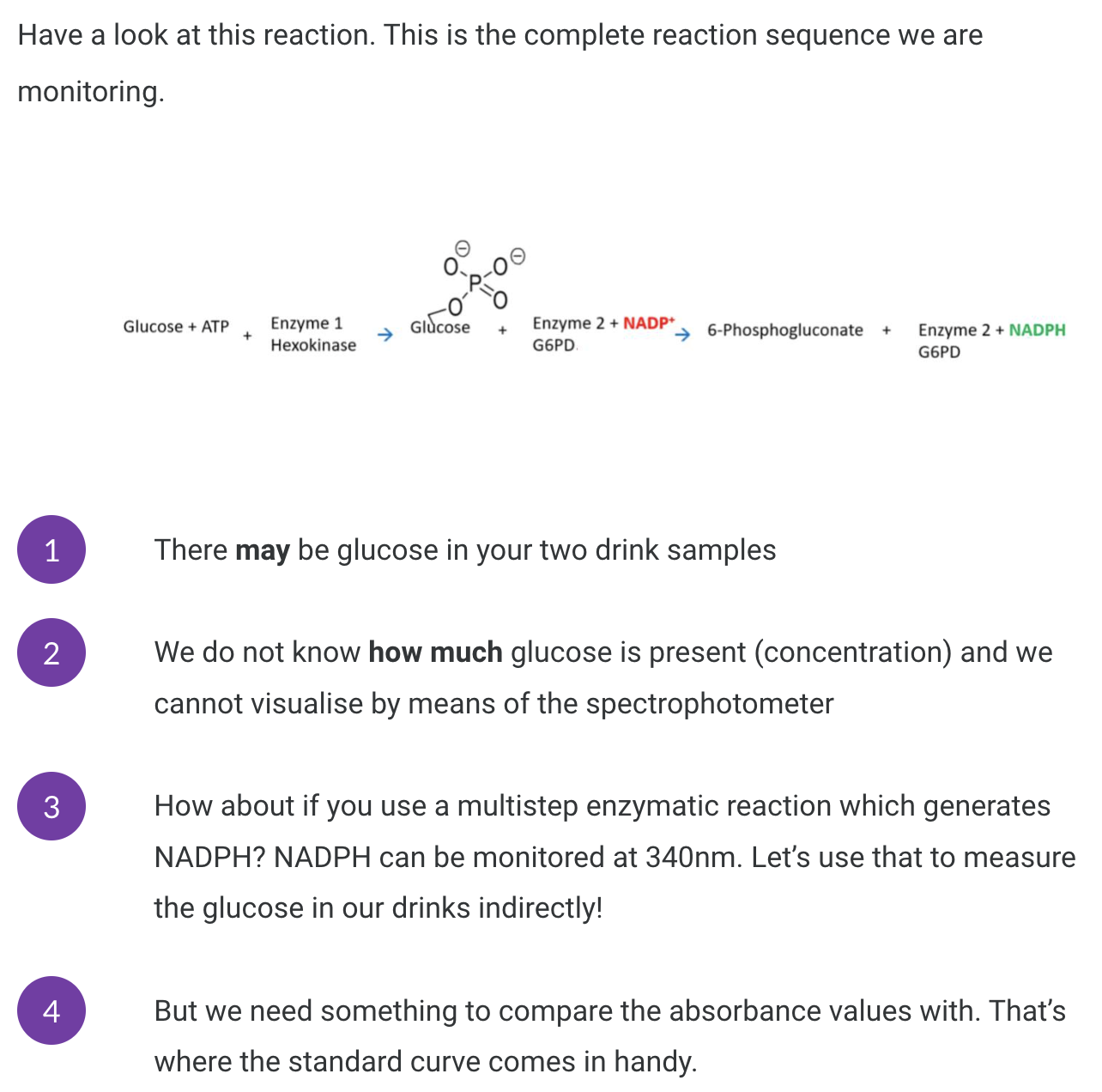
<Our experiment>
<standard curve>
What do I need?
For each pair of students to assay two commercially available drinks & 6 glucose standards, you will be provided with:
- 20 mM glucose standard (5 mL).
- Two (2) samples of drinks (1.5 mL – diluted 1/100);
- TEA buffer pH 7.6 (contains NADP+, ATP, hexokinase, & glucose-6-phosphate dehydrogenase); and
- distilled H2O
What do I do?
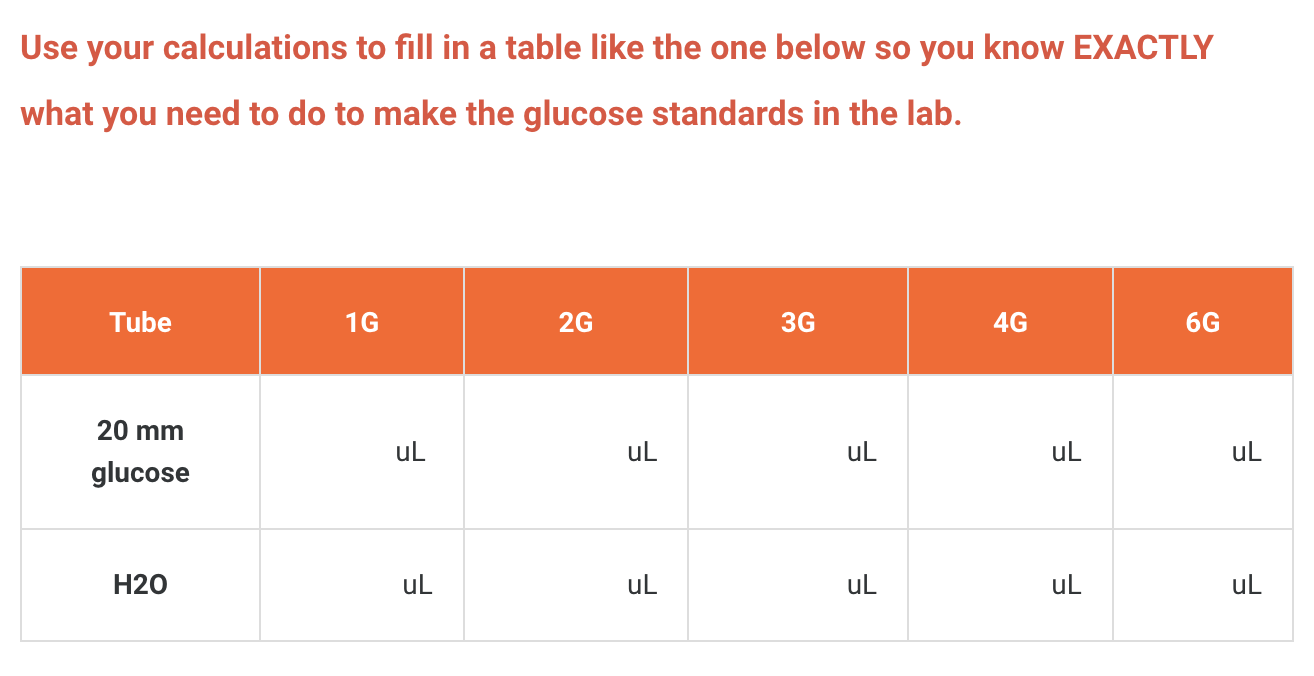
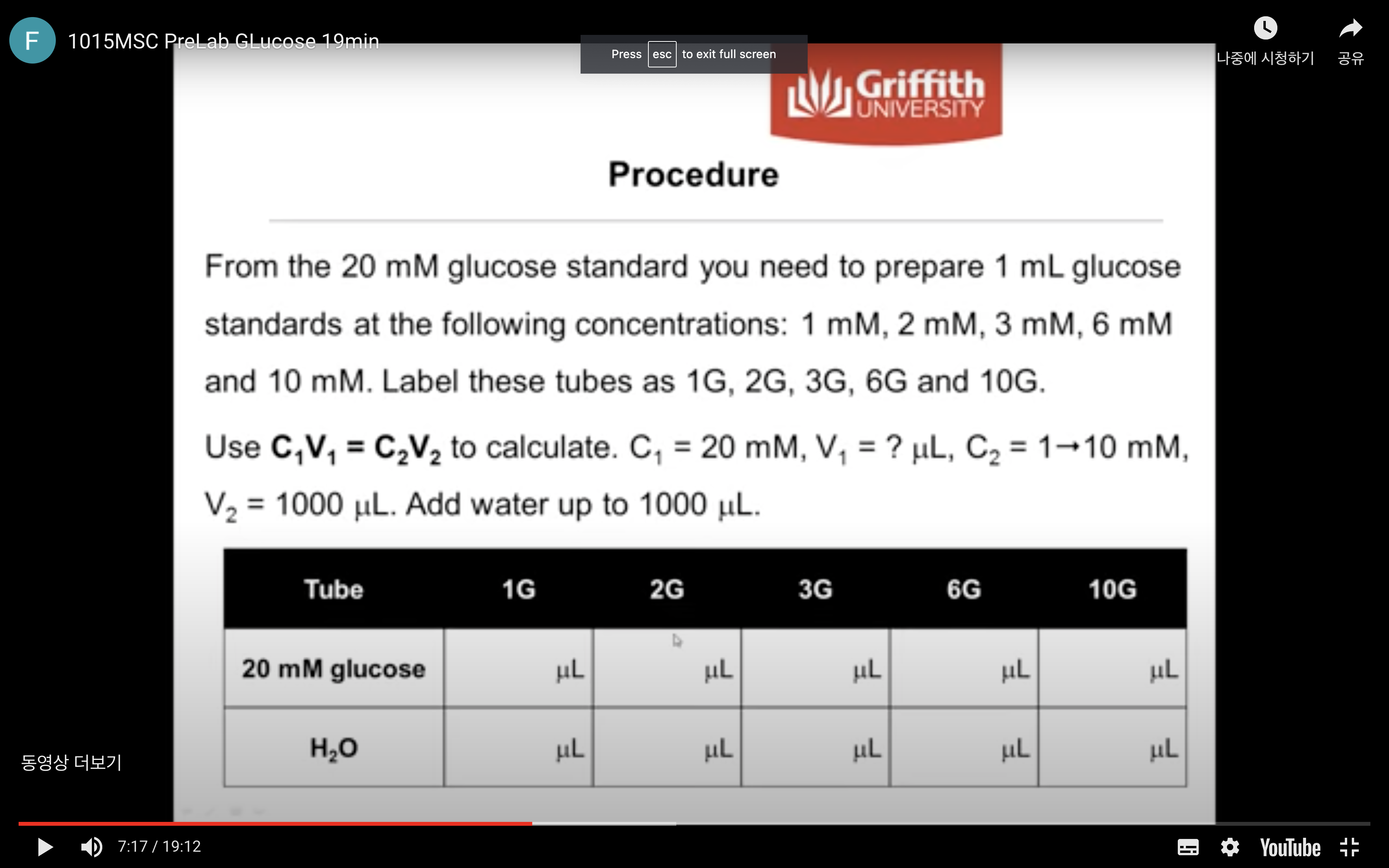
From the 20 mM glucose standard you need to prepare 1 mL glucose standards at the following concentrations:
1 mM, 2 mM, 3 mM, 4 mM and 6 mM. (Label these tubes as 1G, 2G, 3G, 4G and 6G)
Use C1V1 = C2V2 to calculate.
C1 = 20 mM, V1 = ? mL, C2 = 1 → 6 mM, V2 = 1000 uL. Add water up to 1000 uL.
Zero the spectrophotometer
Place distilled water into the cuvette, place the cuvette into the spectrophotometer, and set the spectrophotometer wavelength to 340 nm. Zero the absorbance reading. DO NOT re-zero again throughout the lab experiment.
A1 readings
As you are taking your absorbance readings at 0 minutes, fill them into the first column of the results table just like the one you see to the left.
Then...
Once you have the A1 readings you will add the final reagent in 2 min intervals:
To the first cuvette, add 300 mL of Glucose assay reagent. Immediately start the timer. Pipette the solution a few times to mix the solution.
At t = 2 mins, to the second cuvette, add 300 mL of Glucose assay reagent. Pipette the solution a few times to mix the solution.
Continue for cuvettes 2, 3, 4, 6, D1, & D2 until t = 16 mins.
A2 readings
From t = 18 mins you will take the A2 readings:
At t = 18 mins, to the first cuvette, record the absorbance at 340 nm as A2.
At t = 20 mins, to the second cuvette, record the absorbance at 340 nm as A2.
At t = 22 mins, to the third cuvette, record the absorbance at 340 nm as A2.
Continue for cuvettes 3, 4, 6, D1 & D2 until t = 34 mins.
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK5] Chemistry of Food -Fatty Acids (0) | 2023.03.29 |
|---|---|
| REACTION NOTES (0) | 2023.03.28 |
| [WEEK4] Aldehydes and Ketones (1) | 2023.03.21 |
| [WEEK3] Alcohols, phenols, ethers and thiols (0) | 2023.03.14 |
| [WEEK2] Unsaturated Hydrocarbons (0) | 2023.03.08 |



