Mini Lectures


* More than one -OH groups = polyhydroxyl alchols
*One R group attached to hydroxyl carbon -> primary
*Two R groups attached to hydroxyl carbon -> secondary
*Three R groups attached to hydroxyl carbon -> tertiary alchol
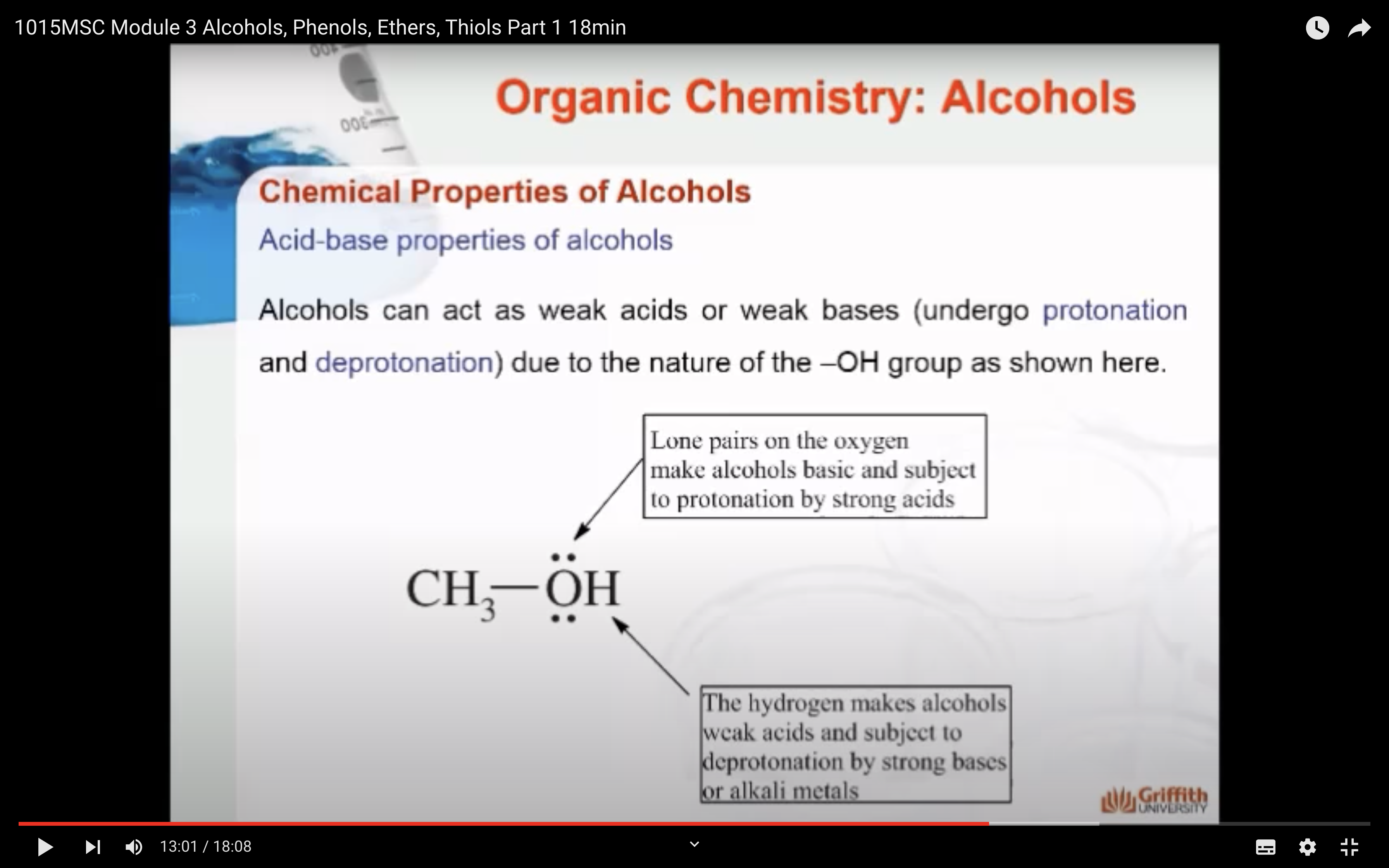
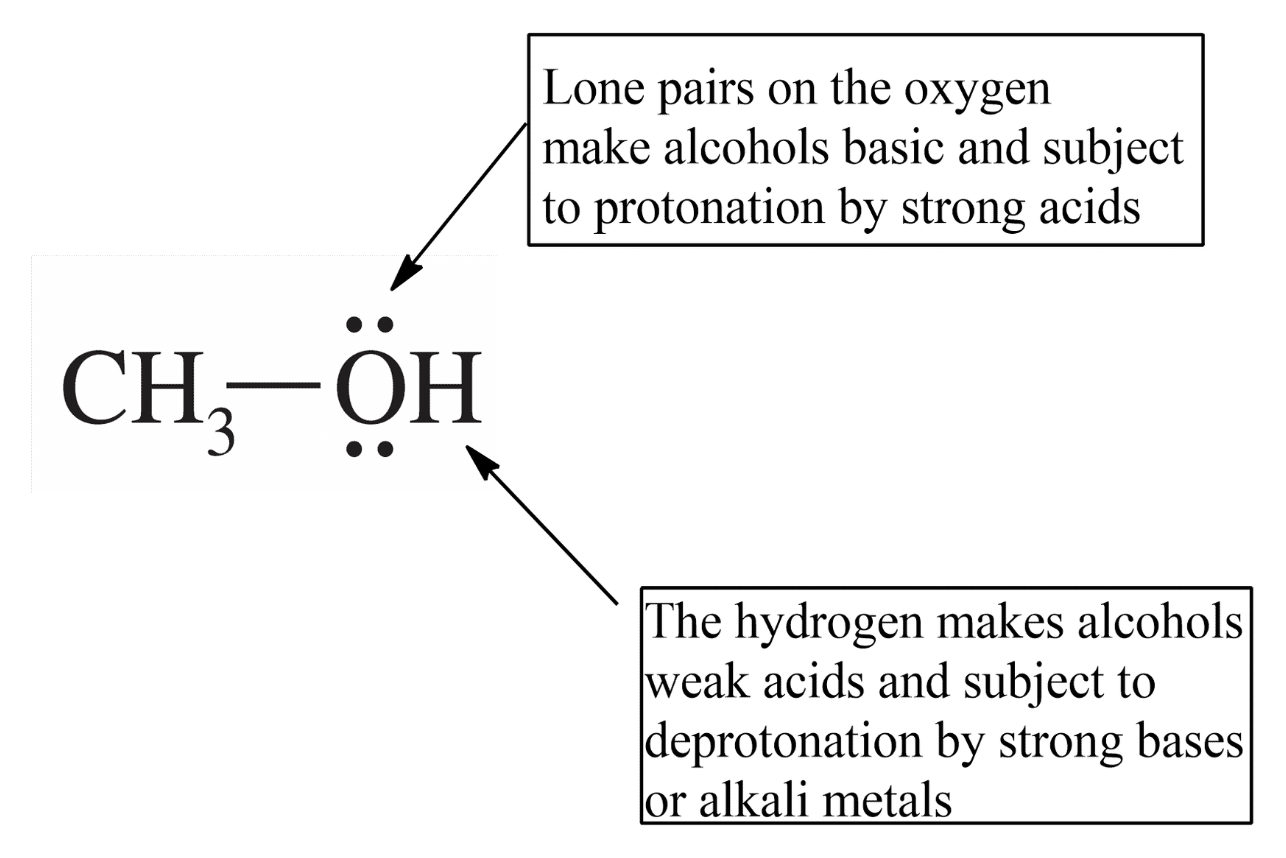
*Because Alchol has hydrogen bonding
-> water soluble
-> increases boiling points
REACTION with Alcohols
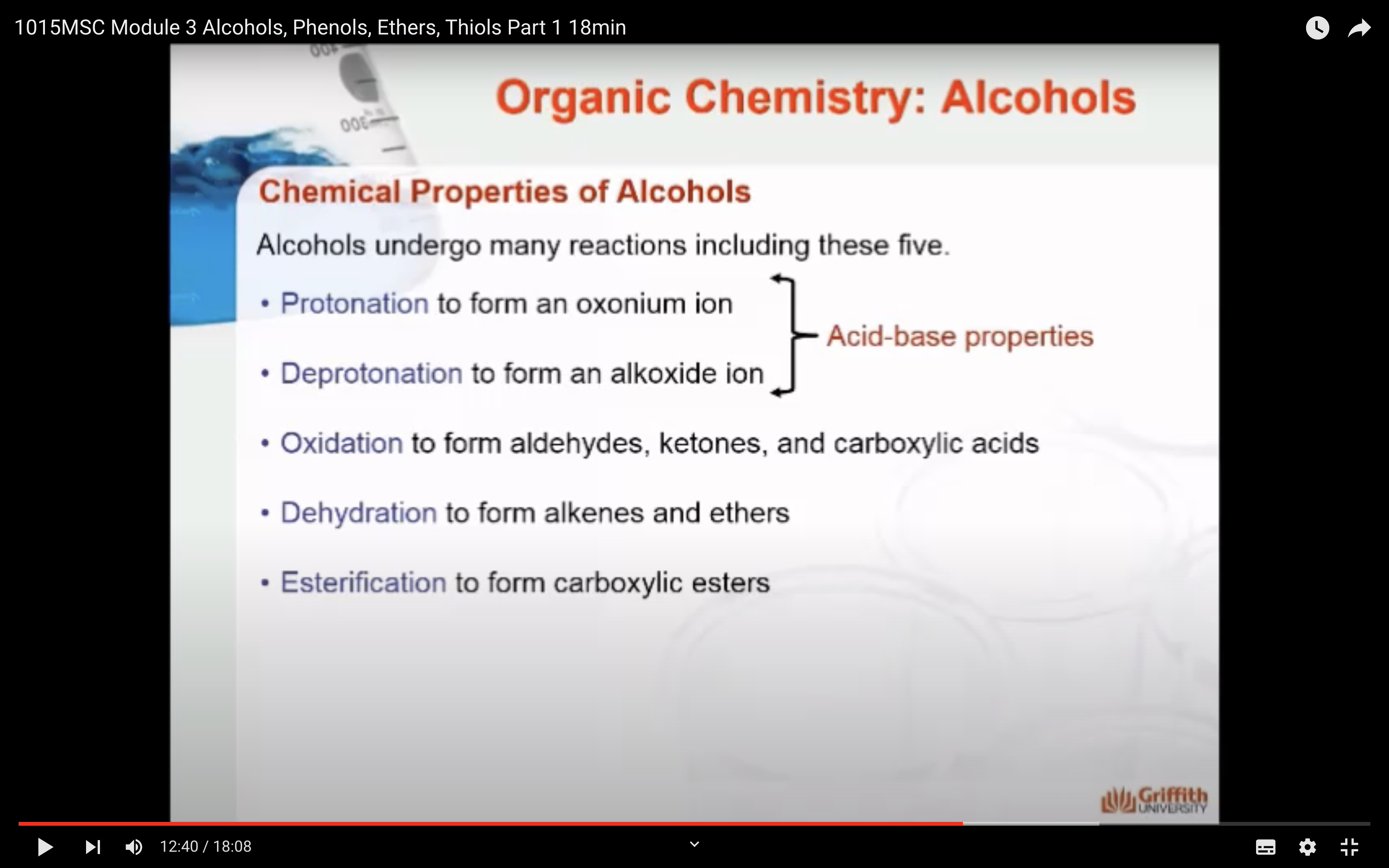
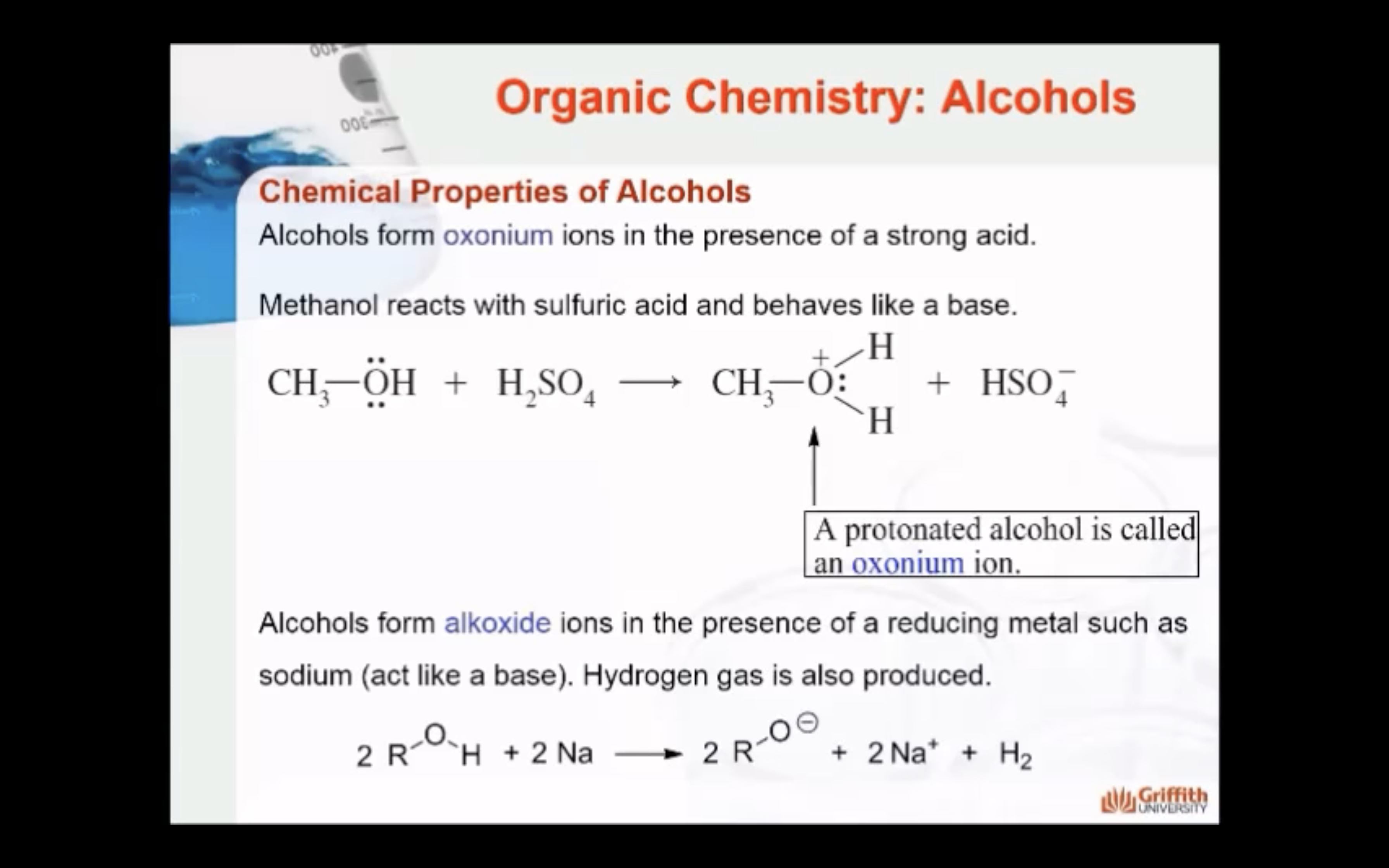
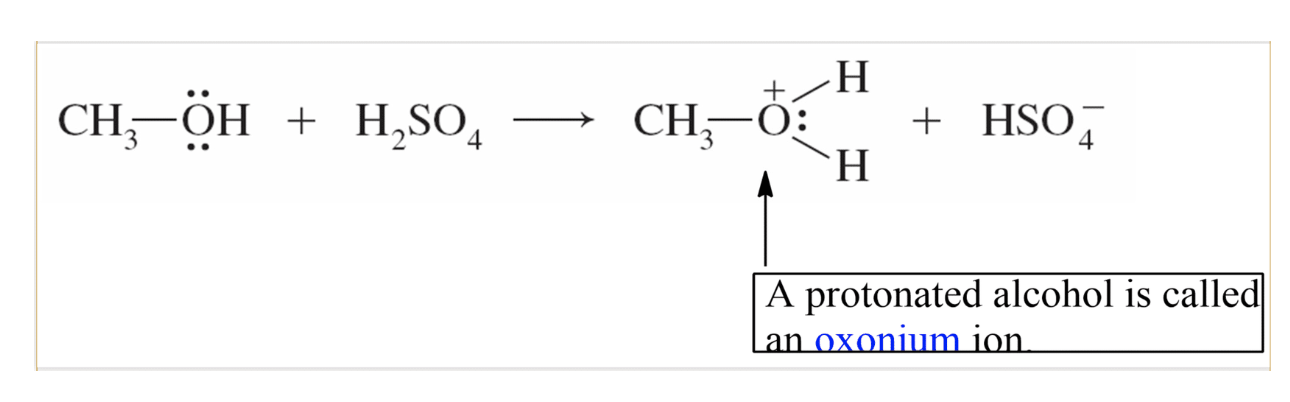
1)Protonation -> oxonium ion is formed
2)Deprotonation -> alkoxide ion is formed
=> Acid base properties
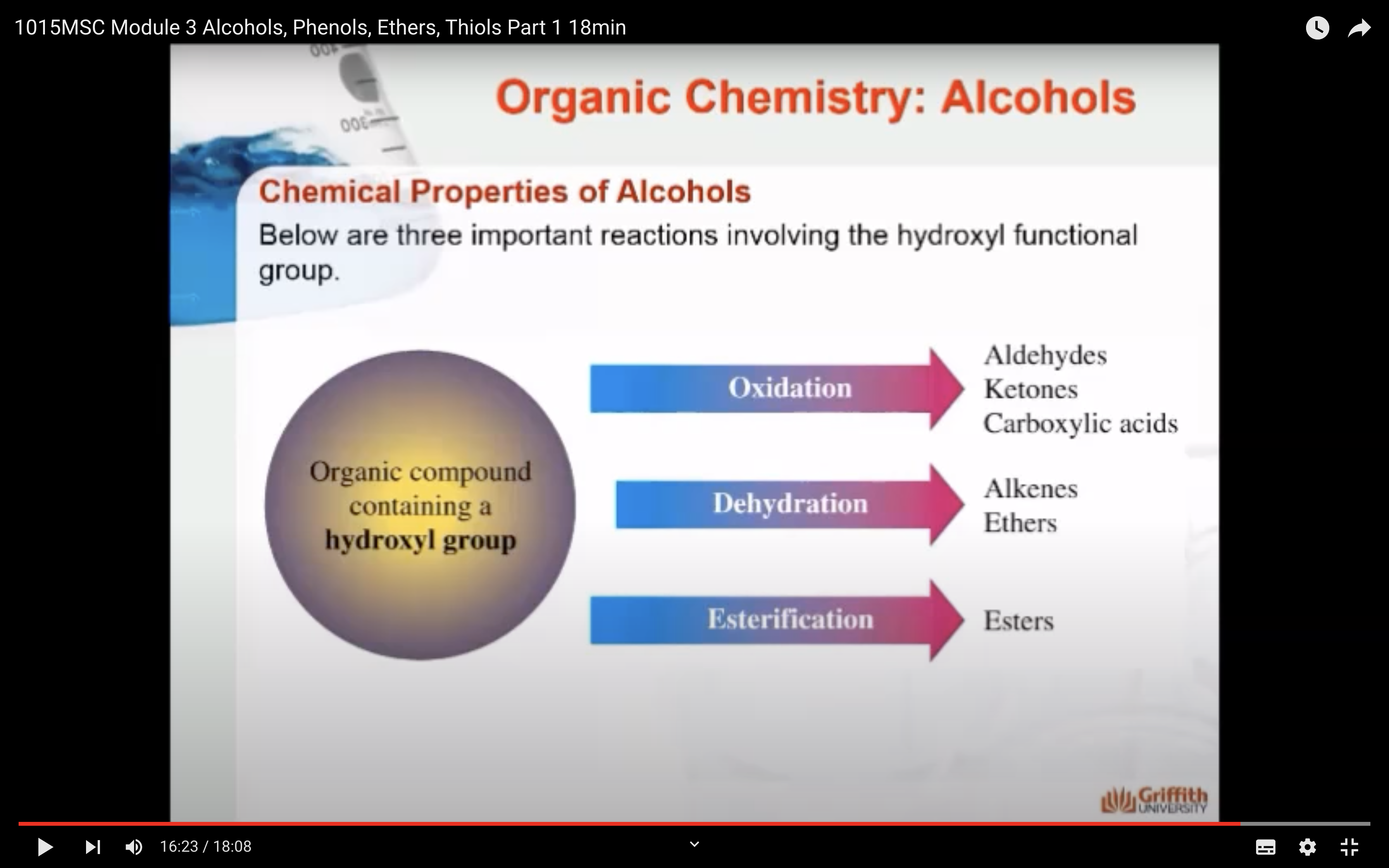
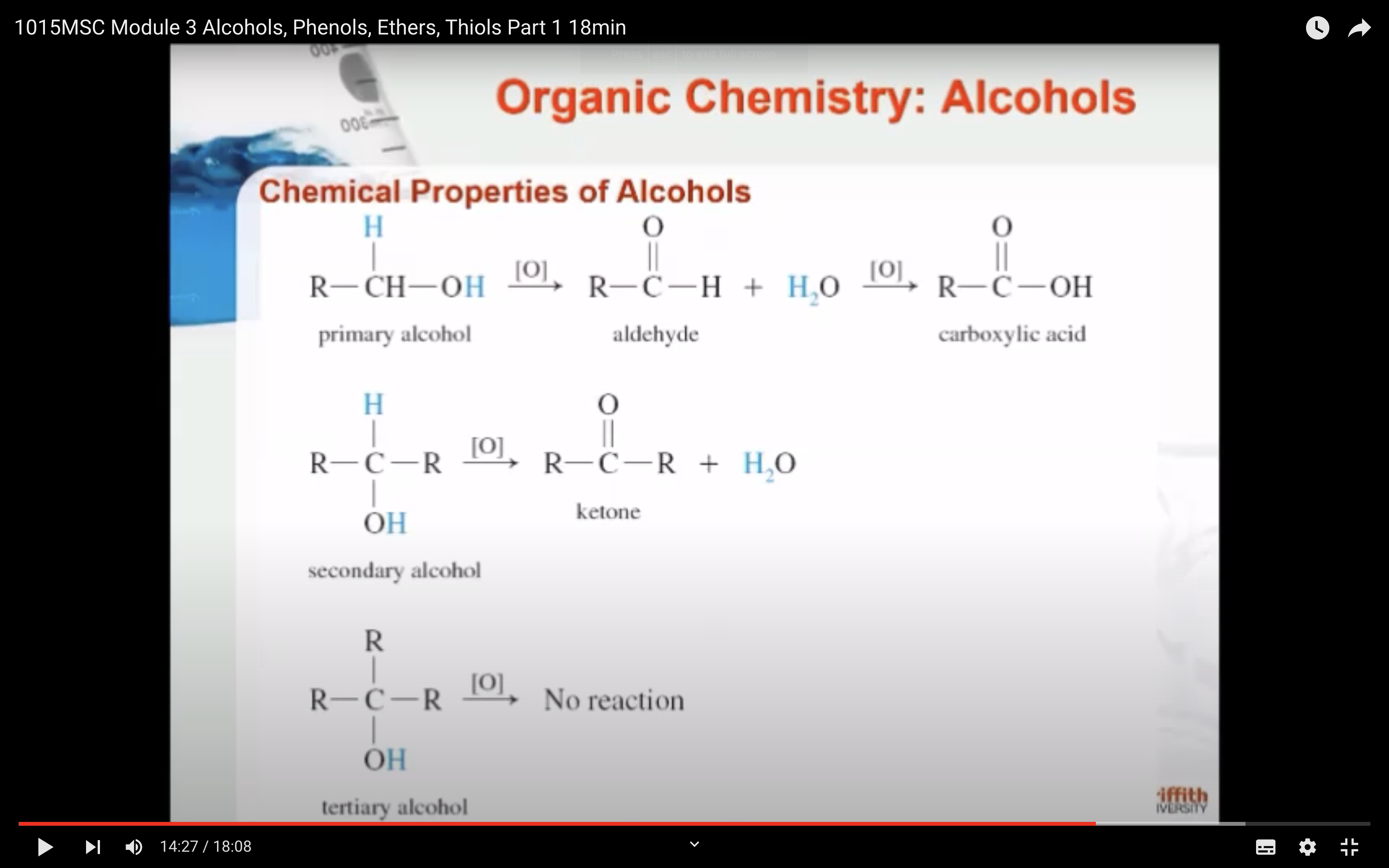
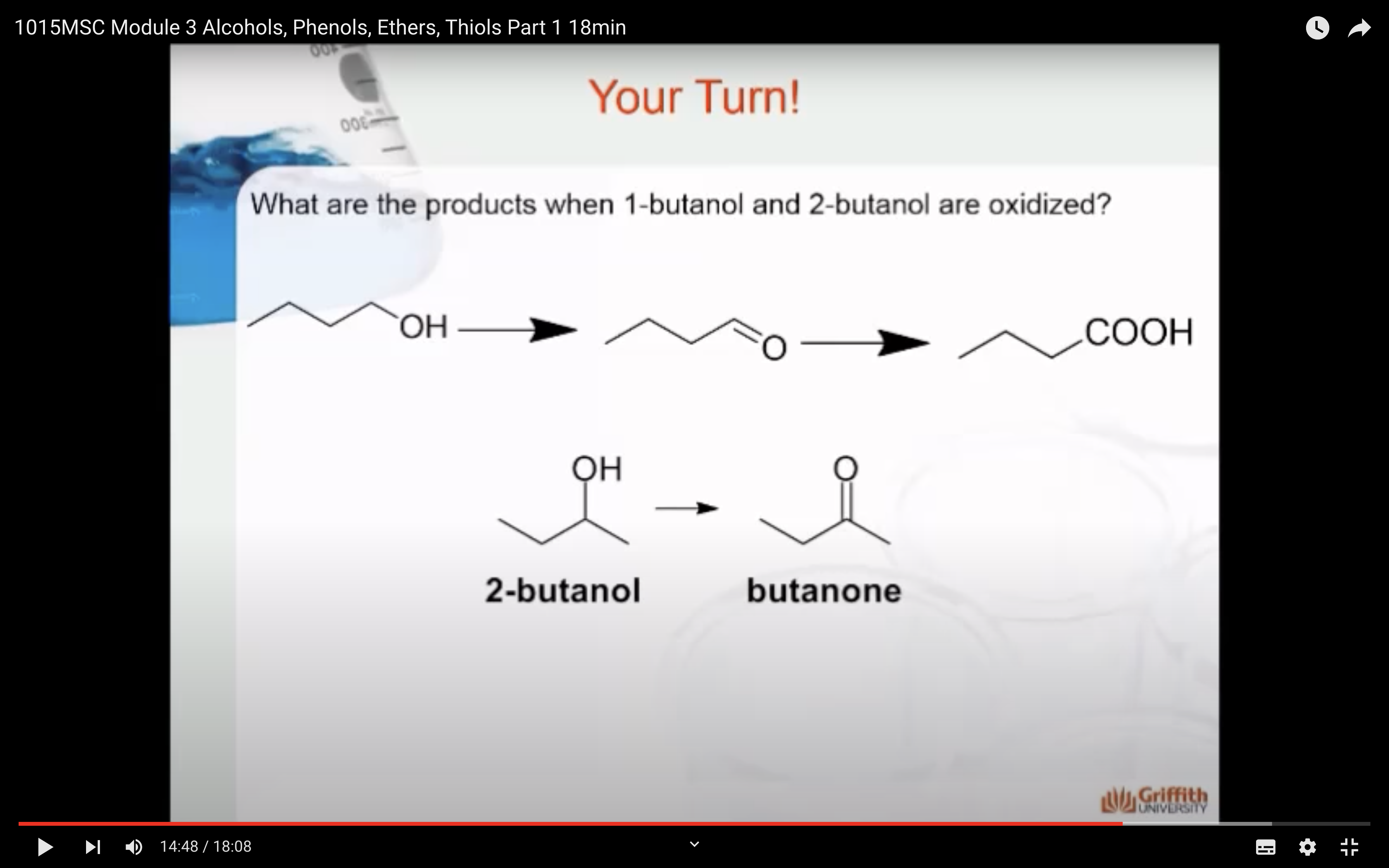
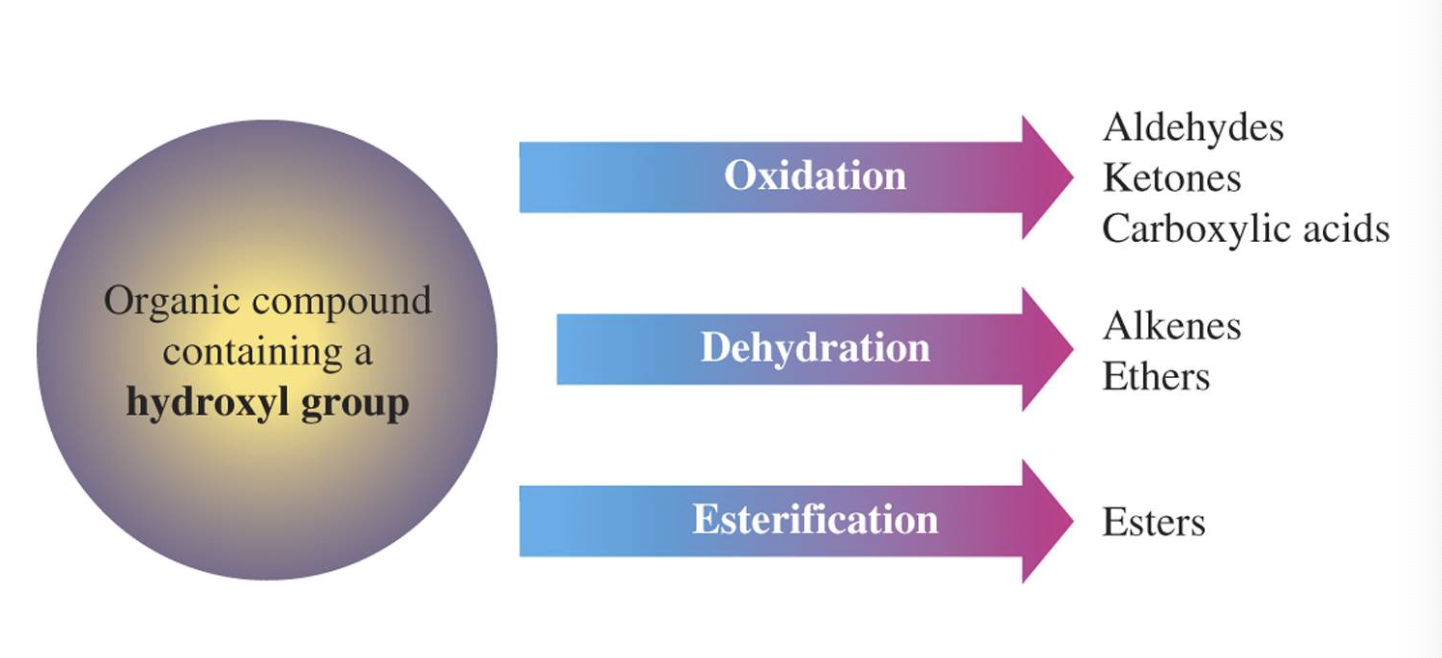
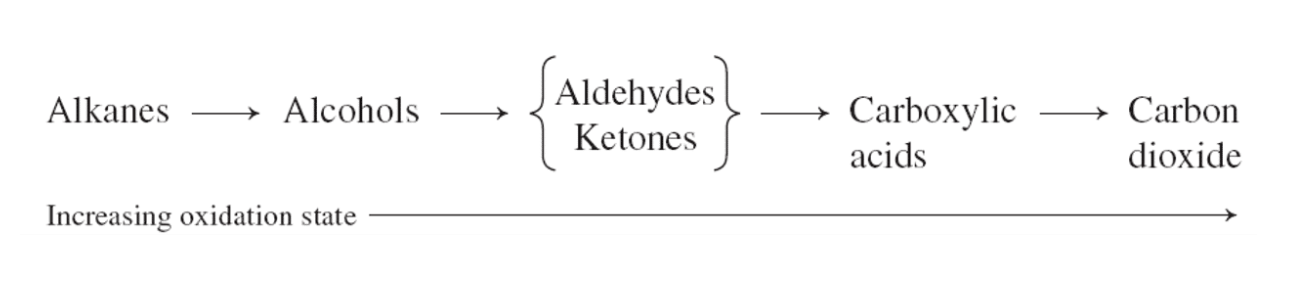
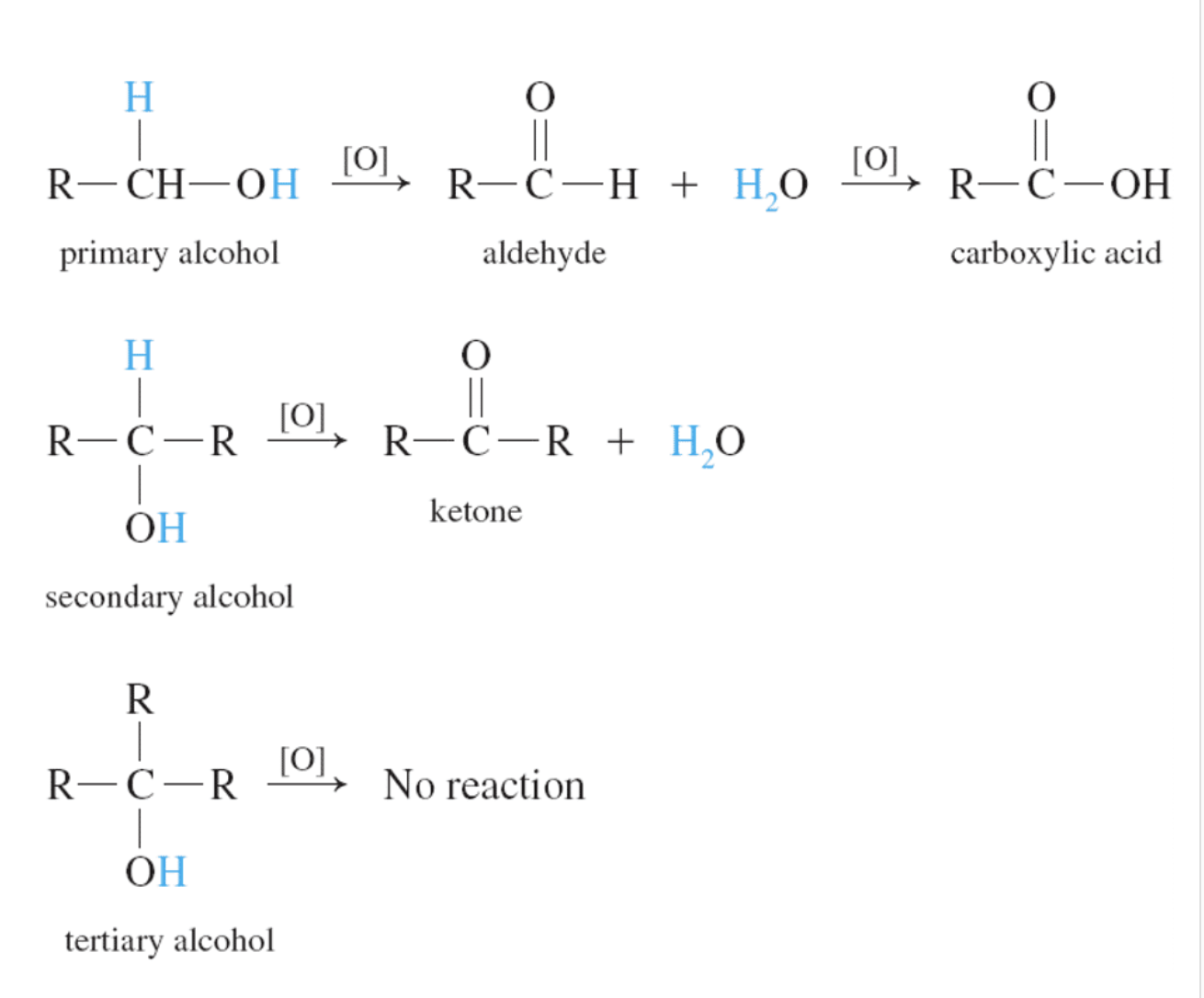
3)Oxidation (산소를 더하는 반응을 해서 수소를 잃어서 반응물로는 물이 생성) -> aldehydes, ketones ad carboxylic acids
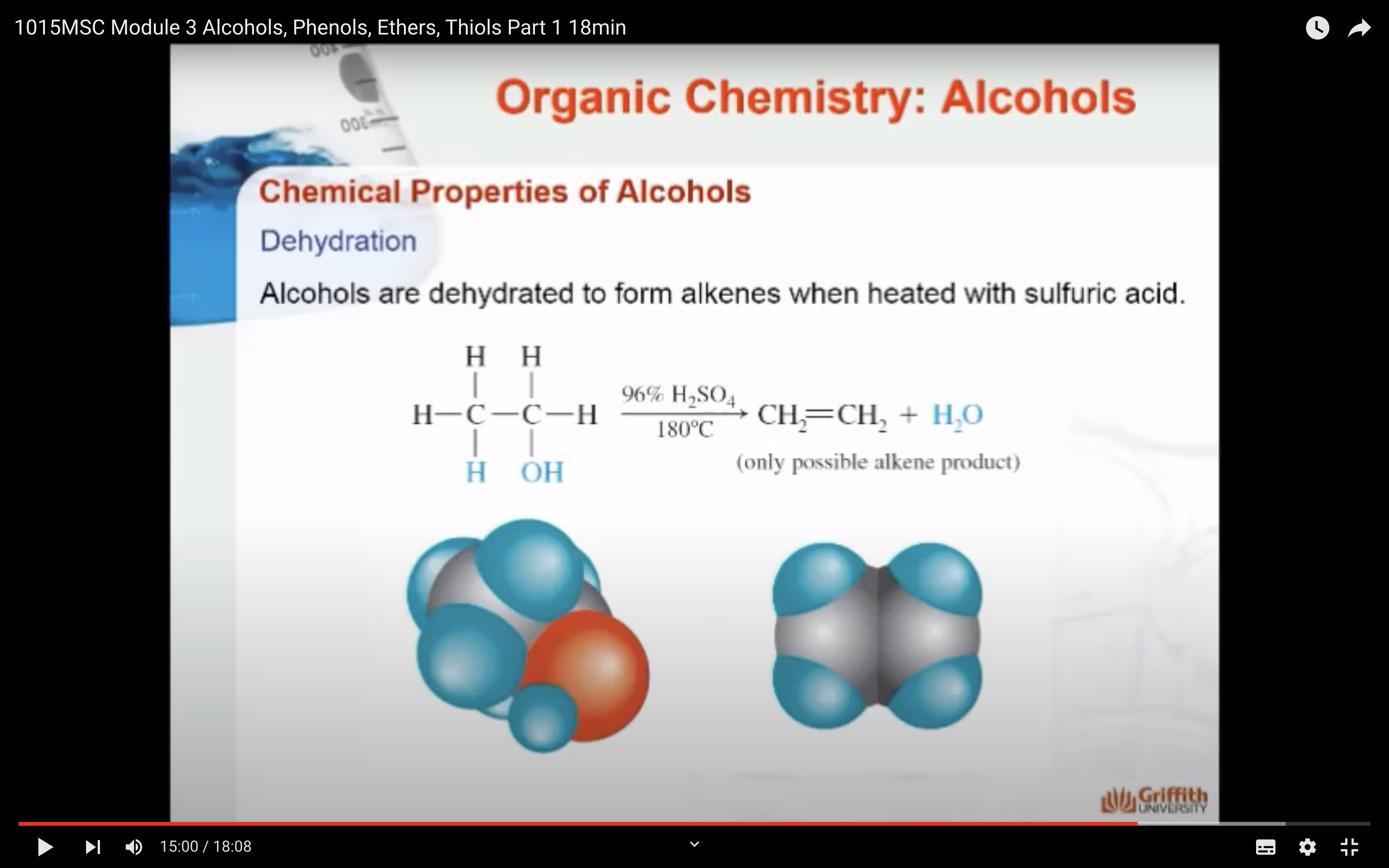
4)Dehydration (OH, H 가 떨어져 나가서 반응물로 물이 생성) -> form alkenes and ethers
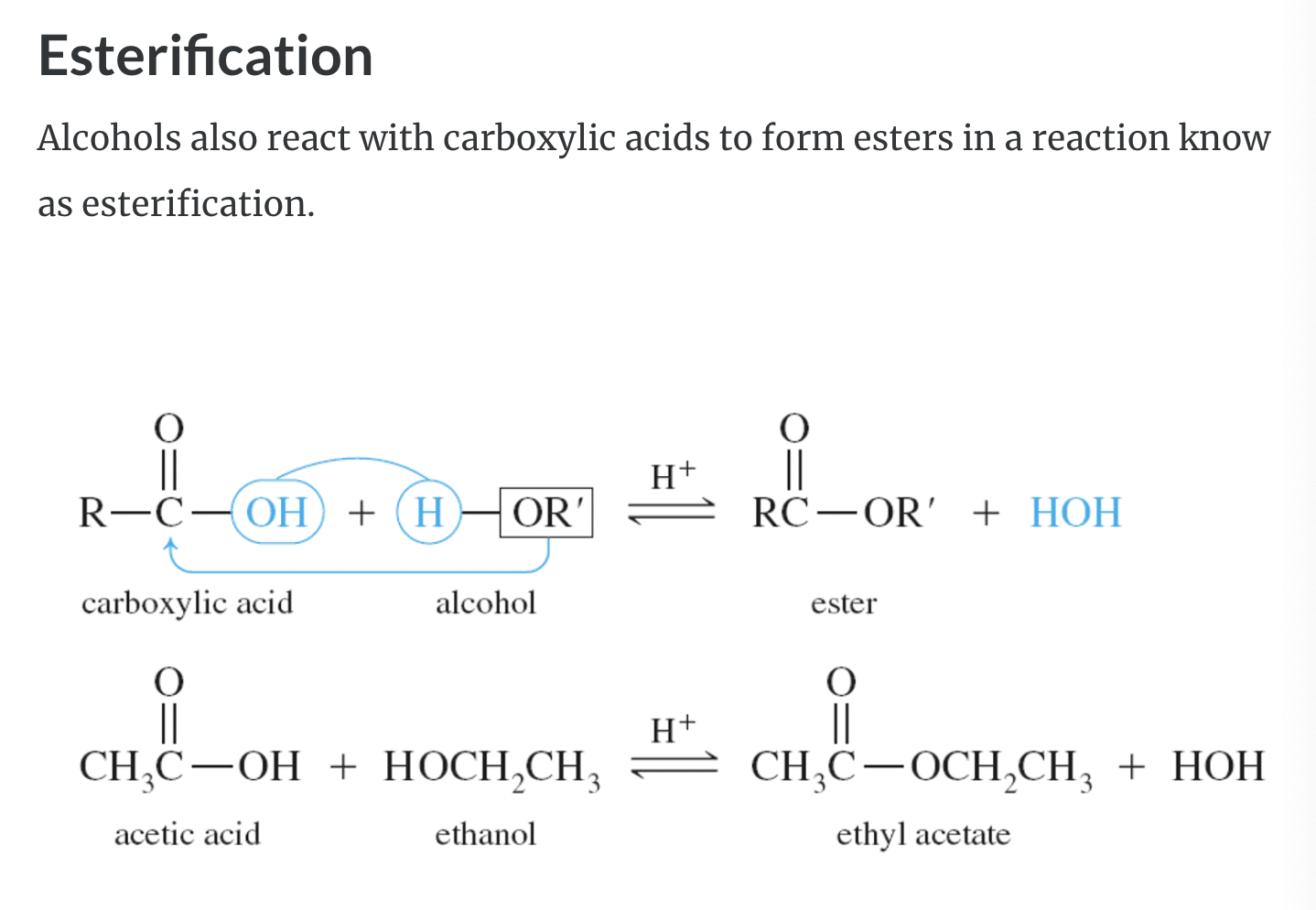
5)Esterification -> form carboxylic esters

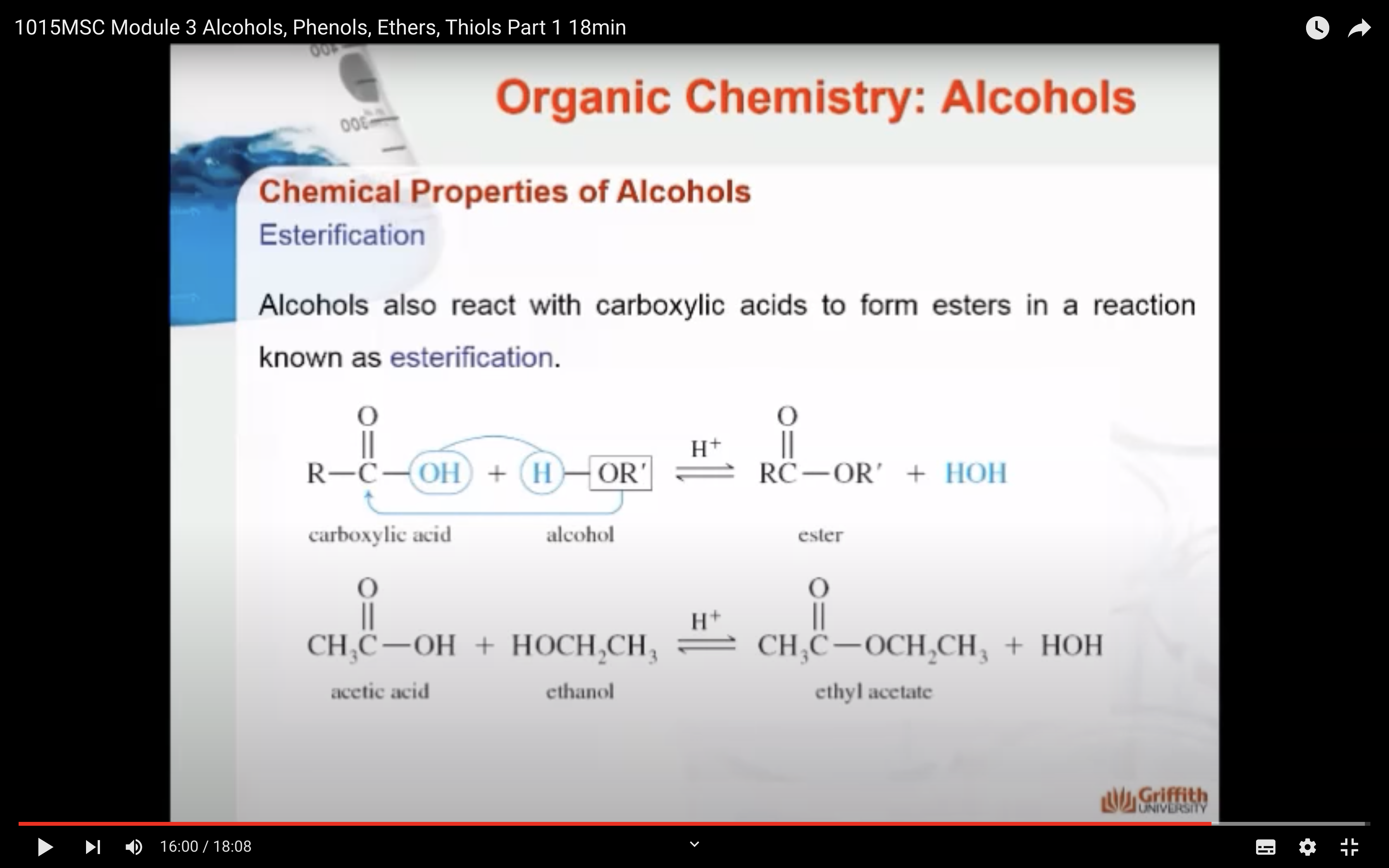
-Esterification : carboxylic acid 의 COOH 중 OH가 alcohol(ROH) 의 H 와 만나서 물을 만들고 carboxylic acid 그룹의 카르보닐기가 alcohol 에 남은 OR 가 만나서 ester (COOR) 를 만든다
SYNTEHSIS of Alcohol
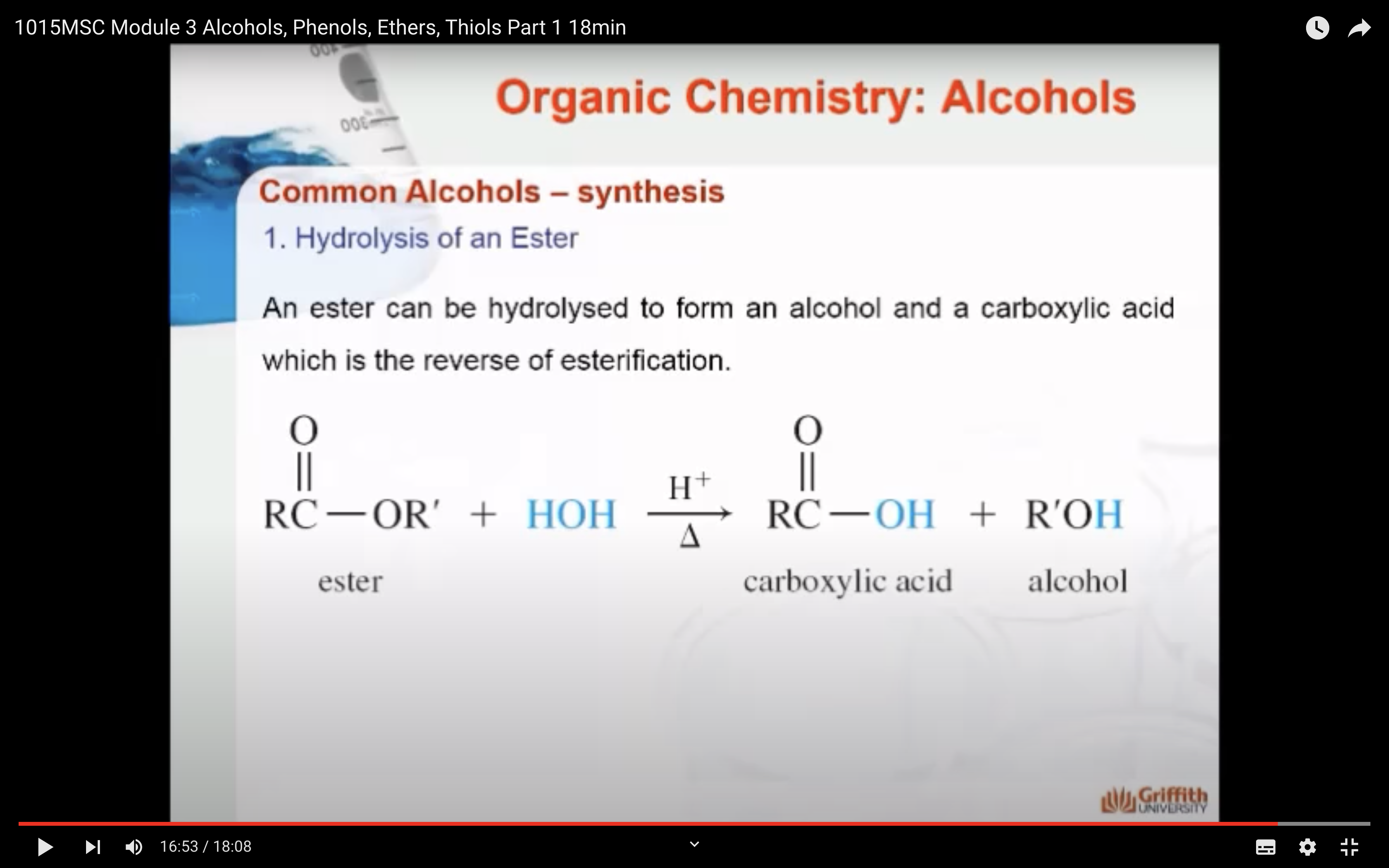
1)Hydrolysis of an ester (ester + H2O -> Carboxylic acid + ROH,alcohol)
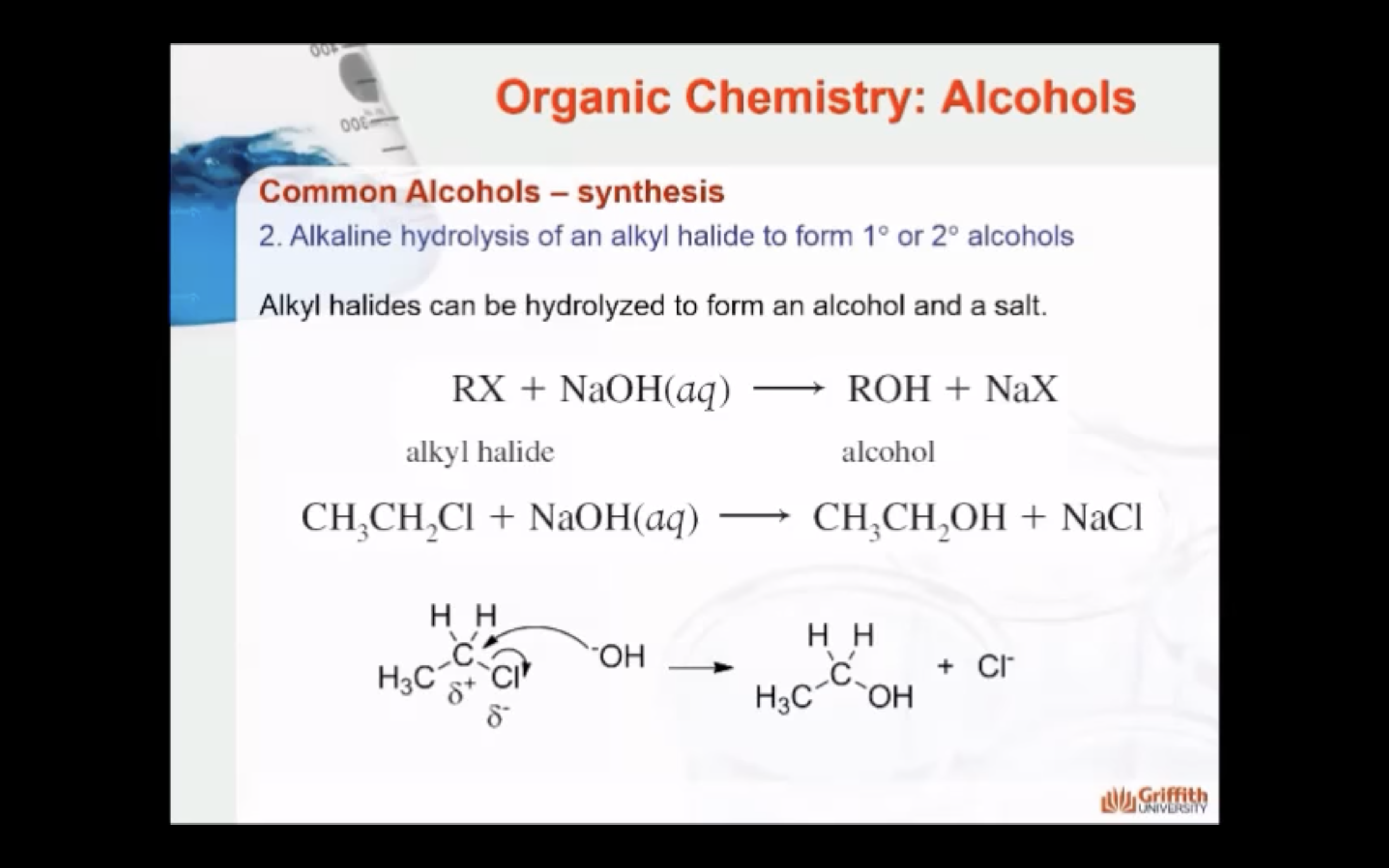
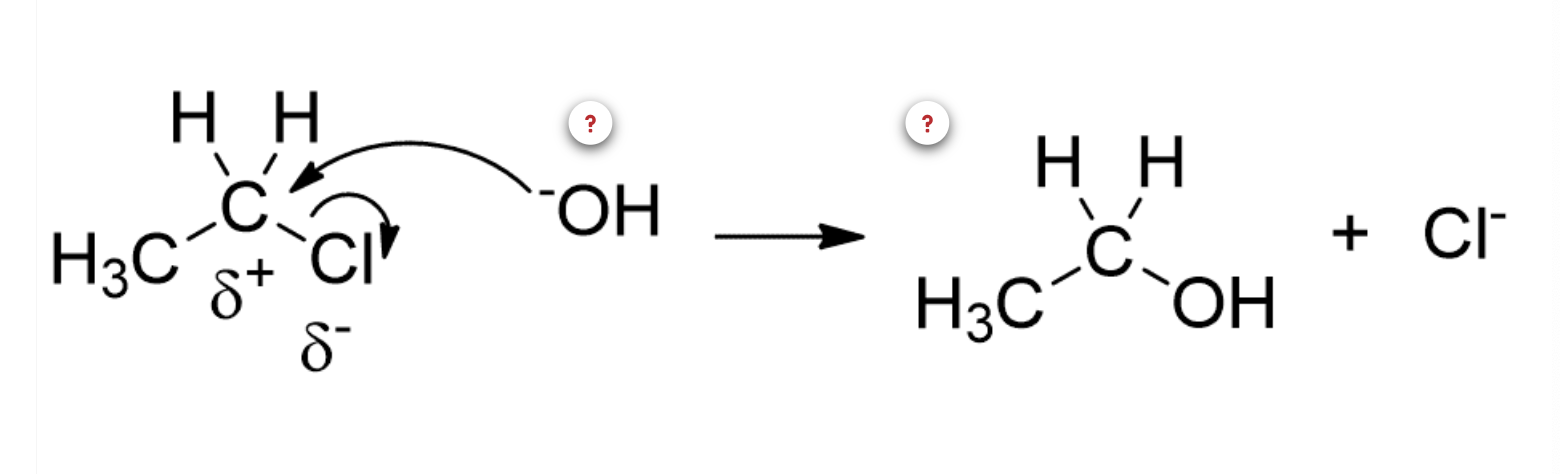
2)Alkaline hydrolysis of an alkyl halide (RX, alkyl halide + NaOH -> ROH, alcohol + NaX, salt)
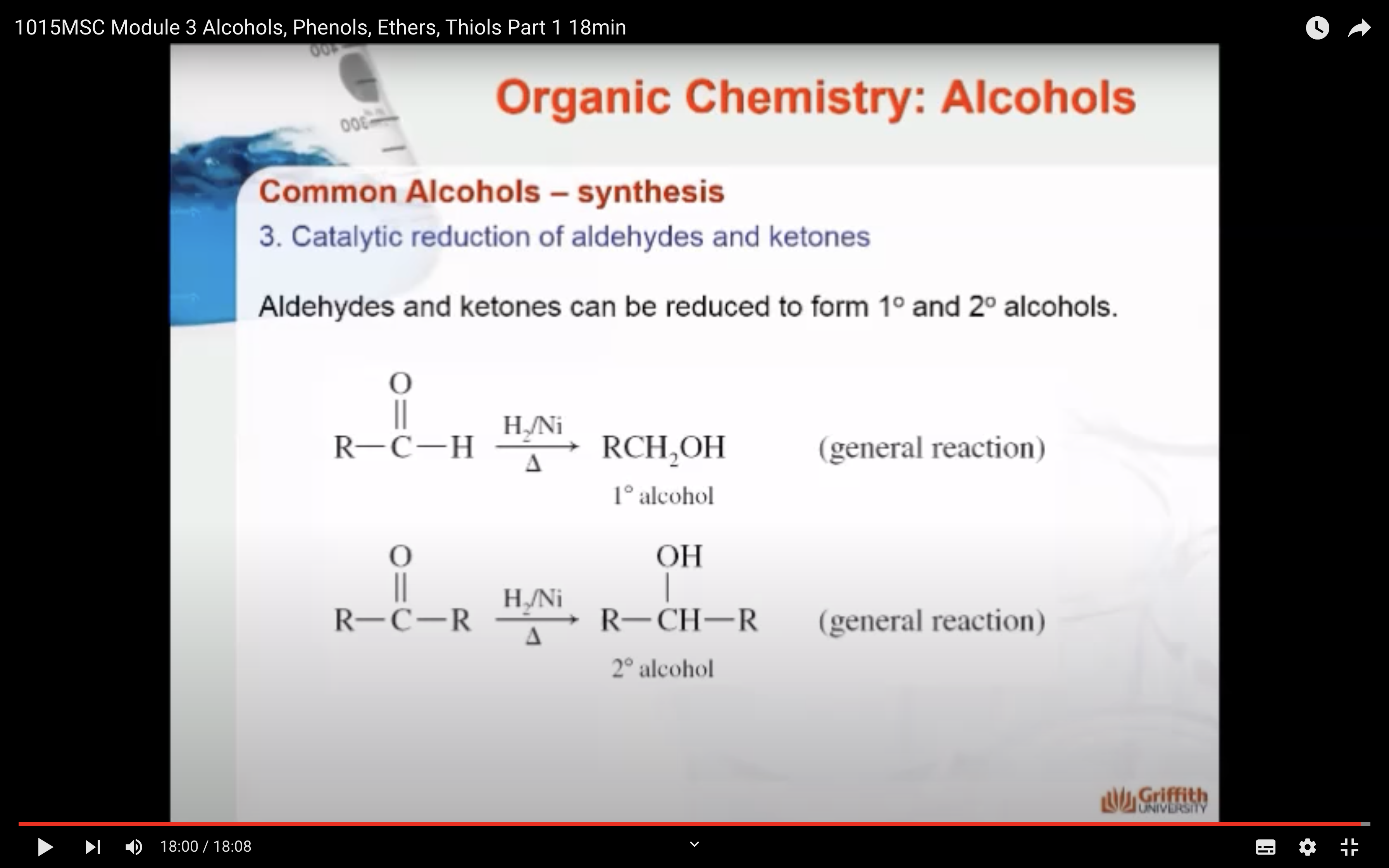
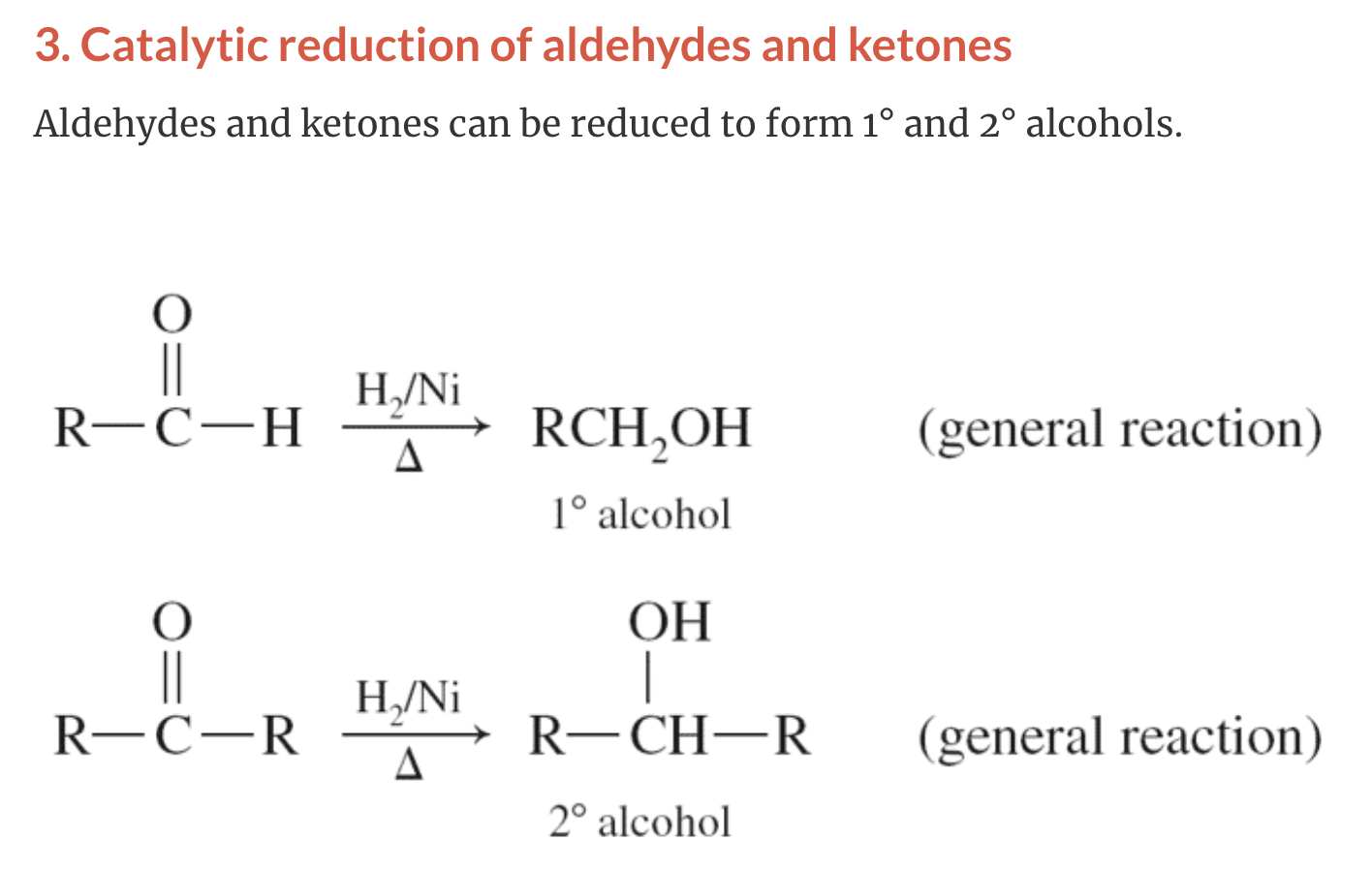
3)Catalytic reduction of aldehydes and ketones (aldehyde +H2 -> primary alcohol, ketone + H2 -> secondary alcohol)

PHENOLS
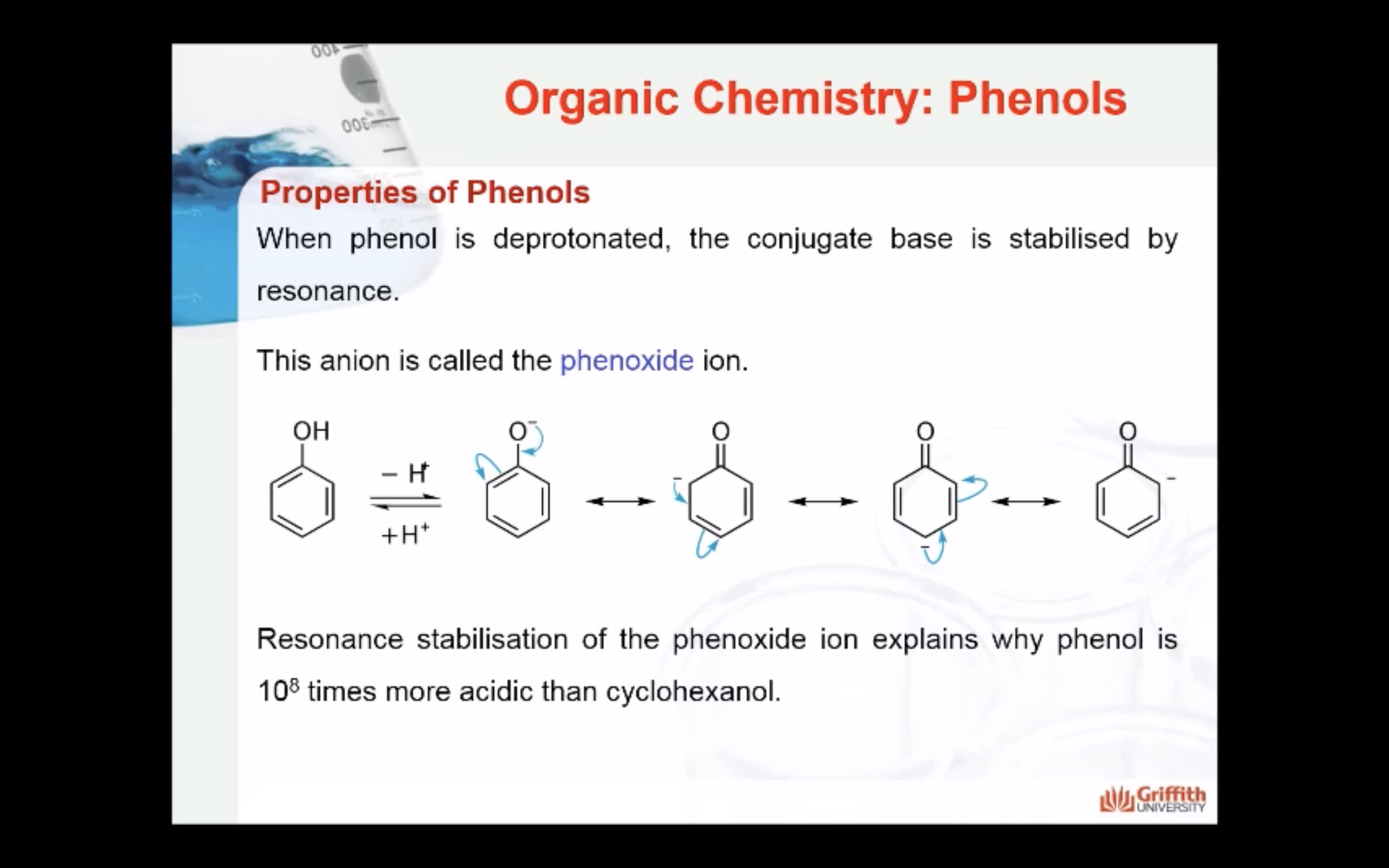
-hydroxyl group attached to an aromatic ring
-phenol is deprotonated -> it is called Phenoxide
-Resonance stabilisation of phenoxide ion is so stable that it explains why phenol is more acidic than cyclohexanol
ETHERS
-aryl / alkyl + O : alkoxyl group
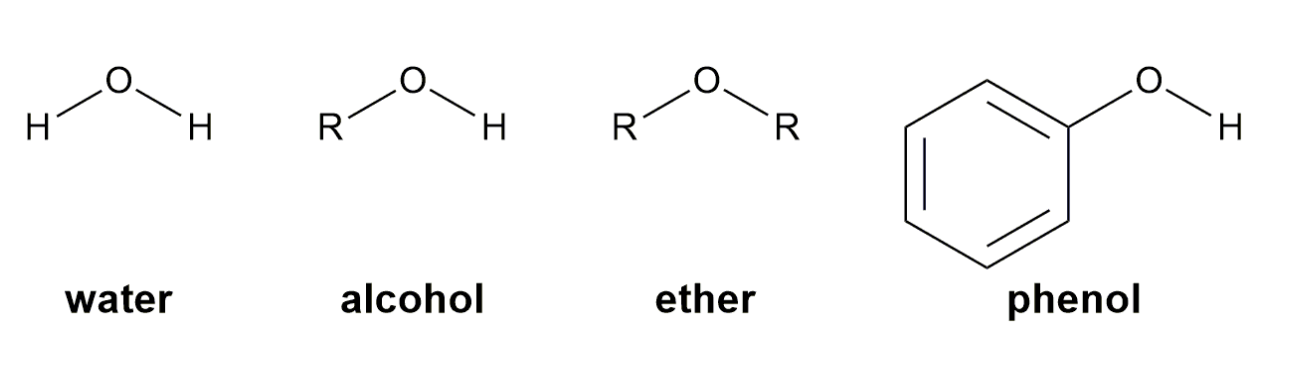
-HOH : water / ROH : alcohol / ROR : ether
-ether is polar -> water soluble
-However depending on the length of the rest of the chain, it can be non-polar enough to dissolve in organic comounds
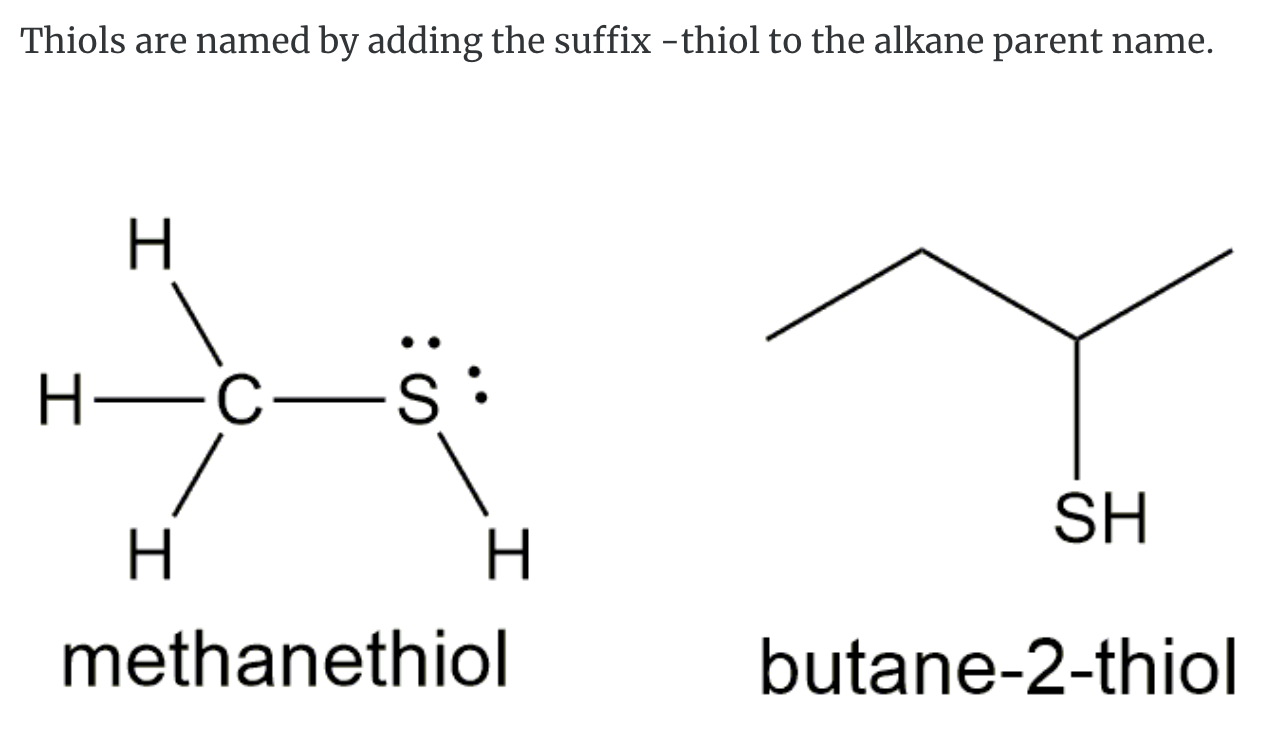
THIOLS
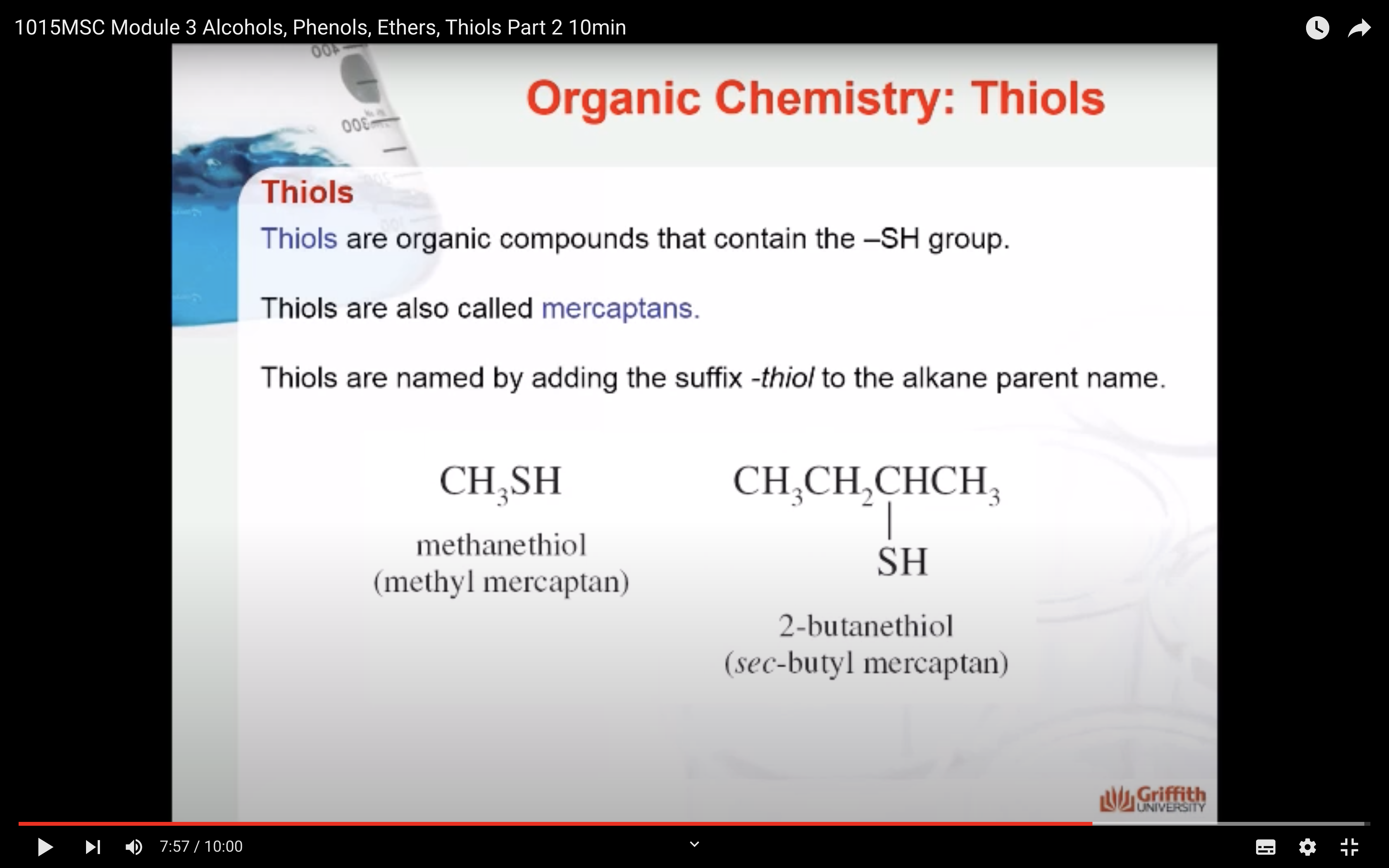
-SH group, mercaptans = thiol
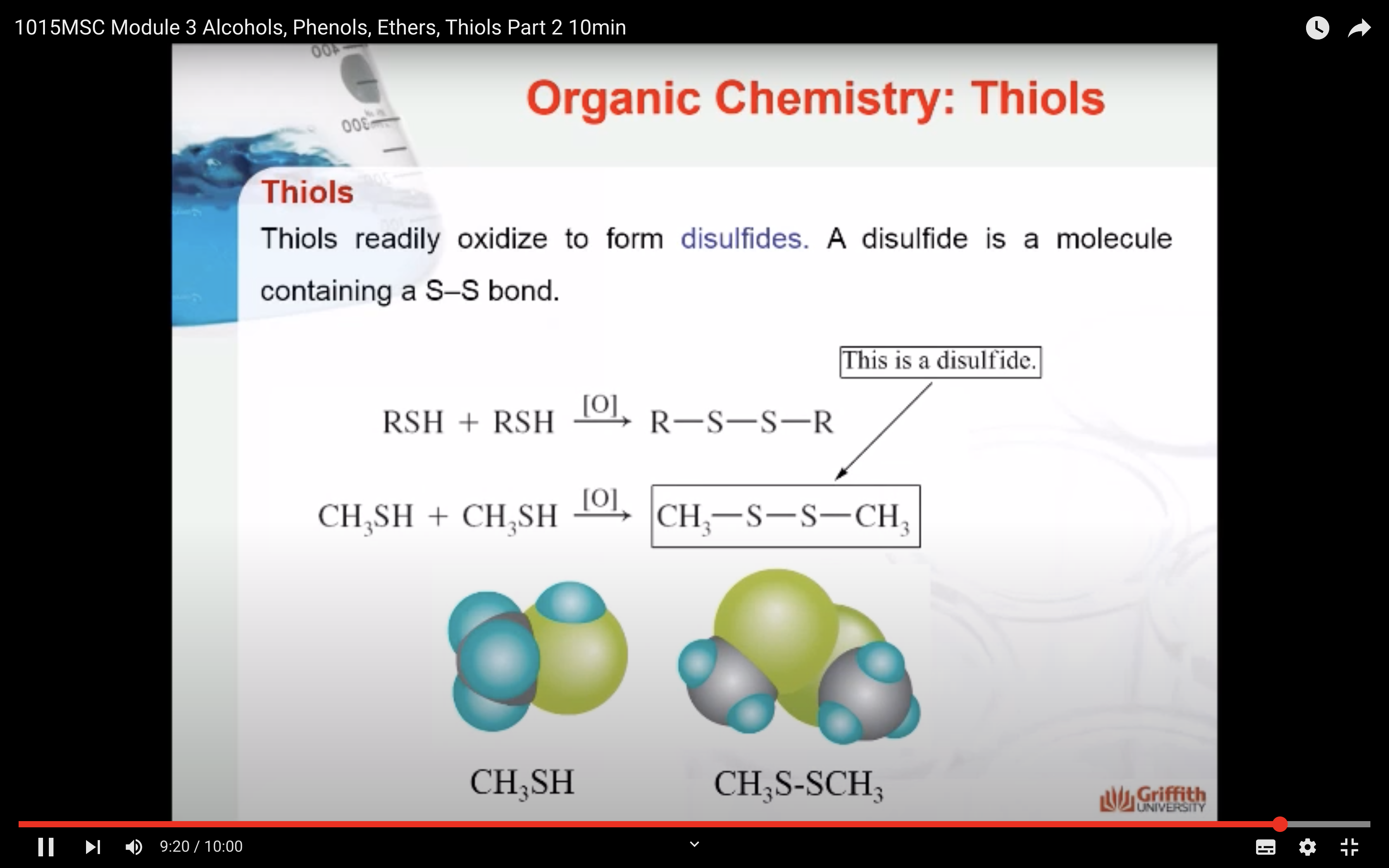
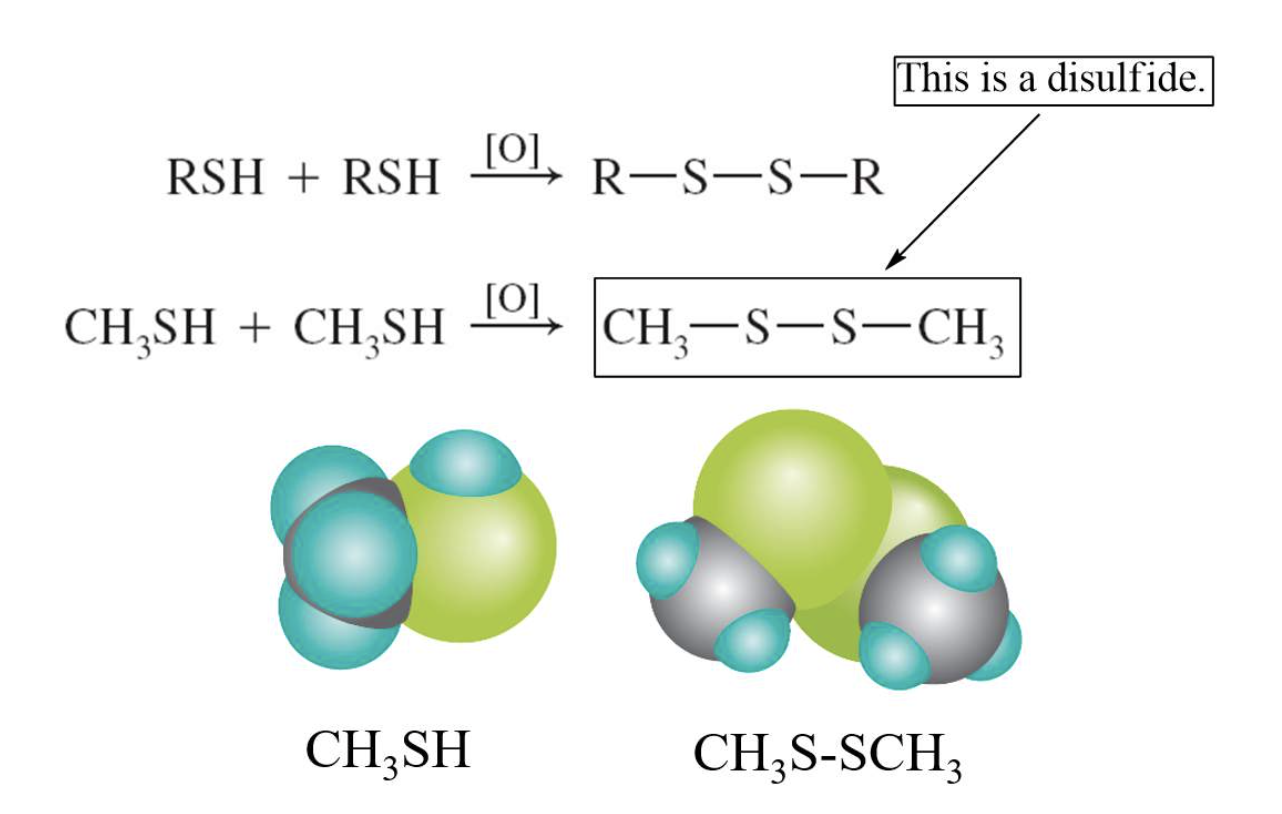
-oxidised from disulfides (RSSR)

Alcohols
-Functional groups
Heteroatom: In organic chemistry, it is an atom other than carbon or hydrogen. Acetamide (shown here) has two heteroatoms: one oxygen atom and one nitrogen atom. The carbon atoms and the hydrogen atoms are not heteroatoms.
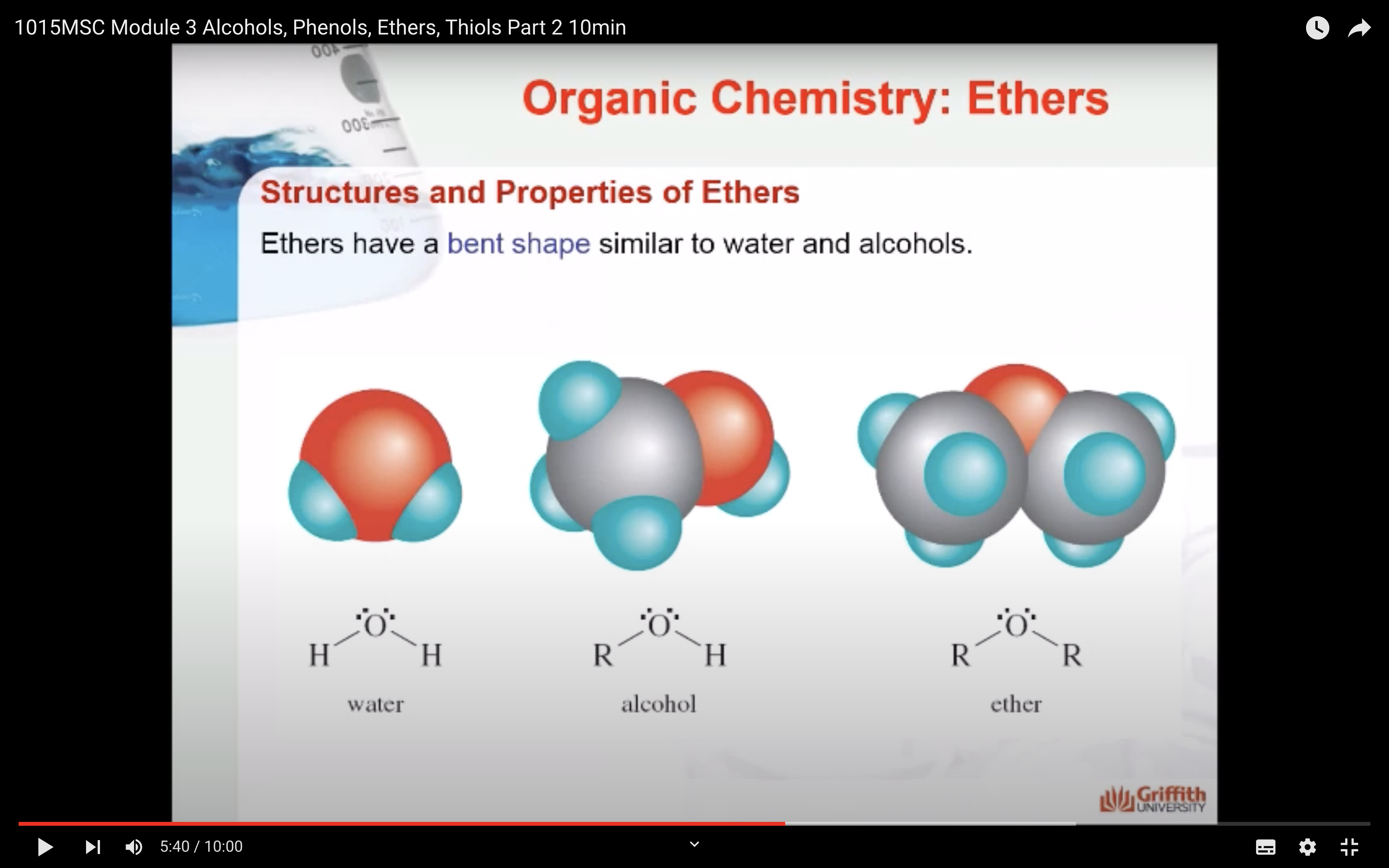
Alcohols, ethers, and phenols are organic compounds that are structural derivatives of water formed by replacing a hydrogen atom with an alkyl group or aromatic ring.
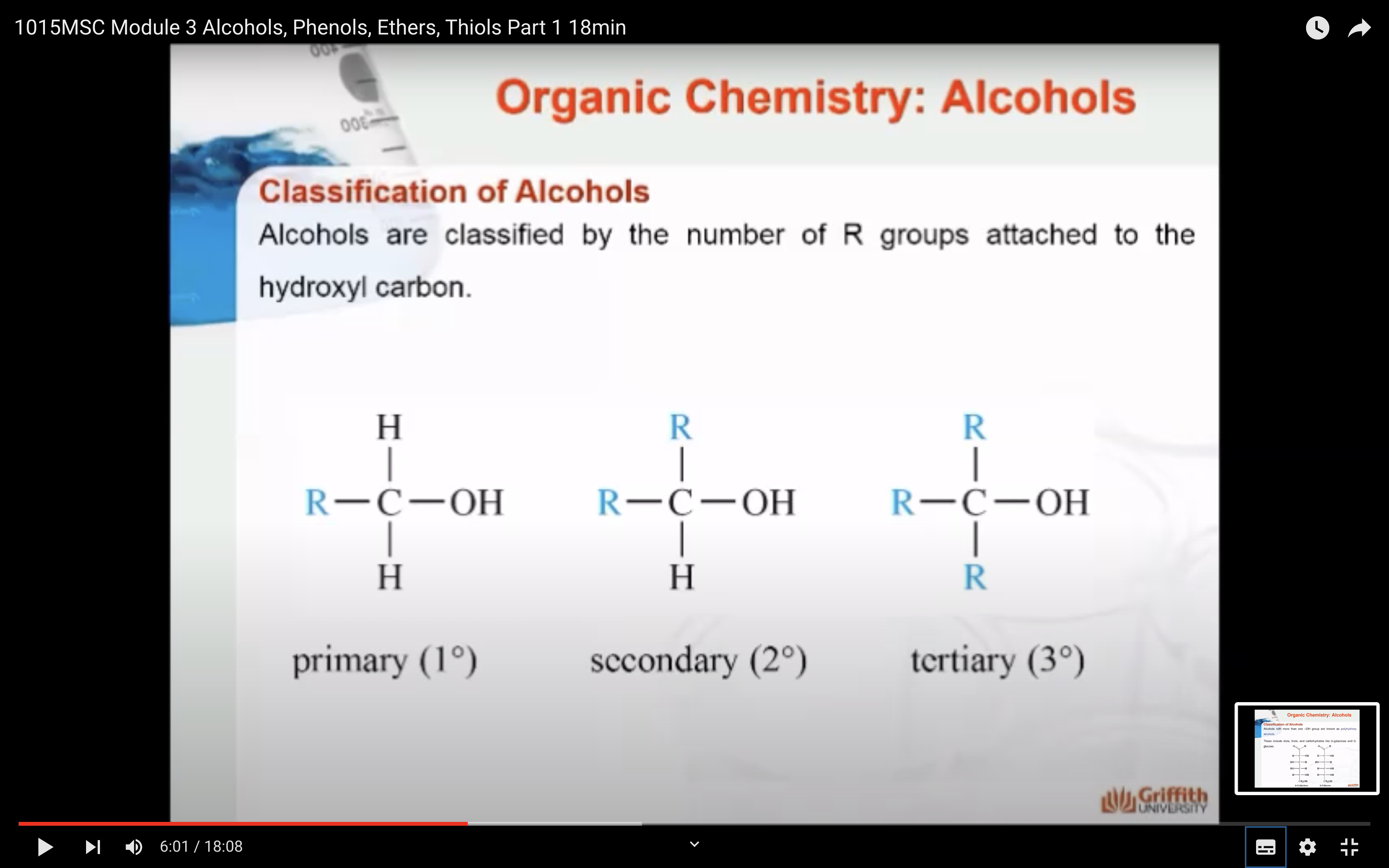
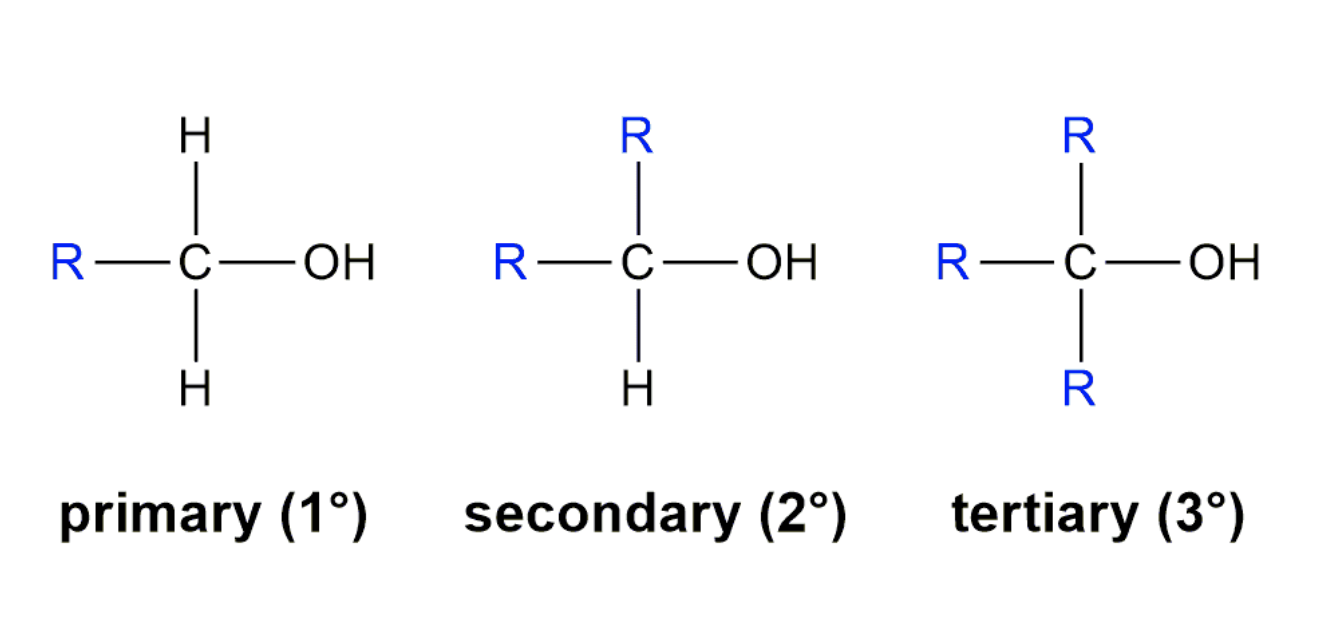
Alcohols are classified by the number of R groups attached to the hydroxyl carbon as shown here.
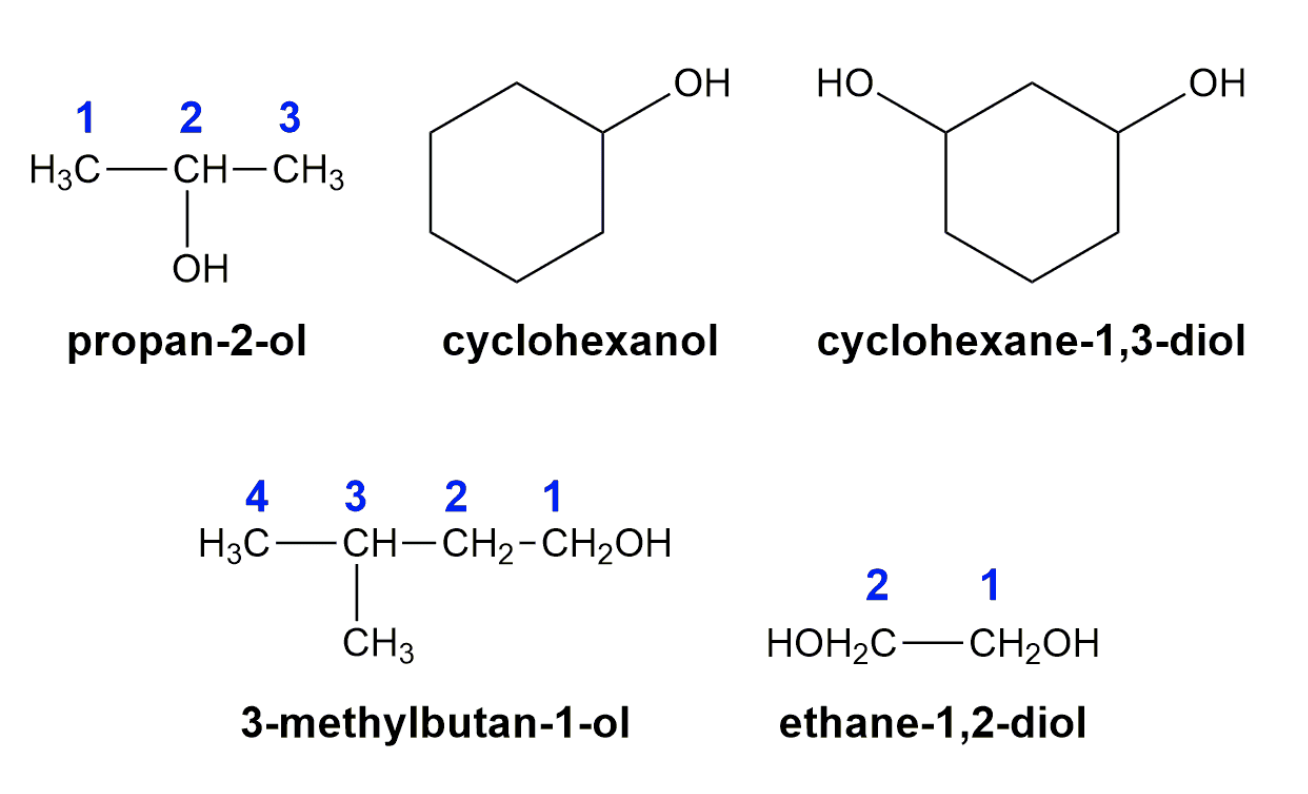
- Primary alcohols have one R group attached to the carbon with the -OH. Examples are ethanol and propan-1-ol.
- Secondary alcohols have two R groups attached to the carbon with the -OH. Examples are propan-2-ol and cyclohexanol.
- Tertiary alcohols have three R groups attached to the carbon with the -OH. Examples are 2-methylpropan-2-ol and 1-methylcyclohexanol.
As the chain length increses, boiling point increases
Note that diols and triols have higher boiling points than similar alcohols with only one -OH group.
Alcohols with more than one –OH group are known as polyhydroxy alcohols. These include diols, triols, and carbohydrates like D–galactose and D–glucose.
https://www.youtube.com/watch?v=T9jumUrXvN0
-Naming alchols

-Physical properties of alchols
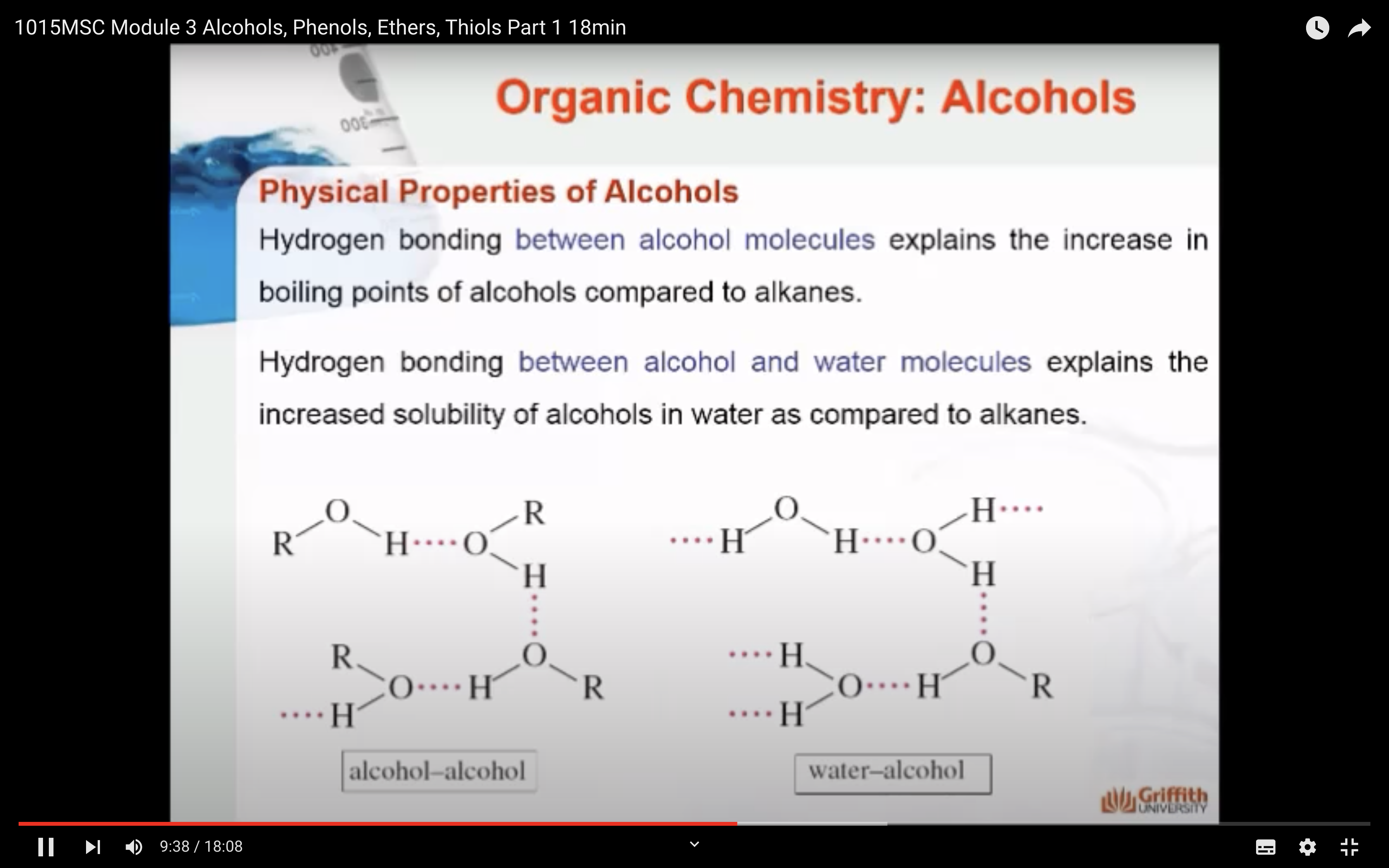
Hydrogen bonding between alcohol molecules explains the increase in boiling points of alcohols compared to alkanes. Hydrogen bonding between alcohol and water molecules explains the increased solubility of alcohols in water as compared to alkanes.
Alcohols have higher boiling points than alkanes with the same number of carbons.
As carbon chain length increases, so does the boiling point.
Diols have higher boiling points than alcohols with the either same number of carbons or similar molar mass. The extra -OH increases the hydrogen bonding between molecules.
Branching (in molecules, not trees) also affects boiling points. A branched–chain alcohol will have a lower boiling point than the corresponding straight–chain alcohol.
-Chemical properties of alchols
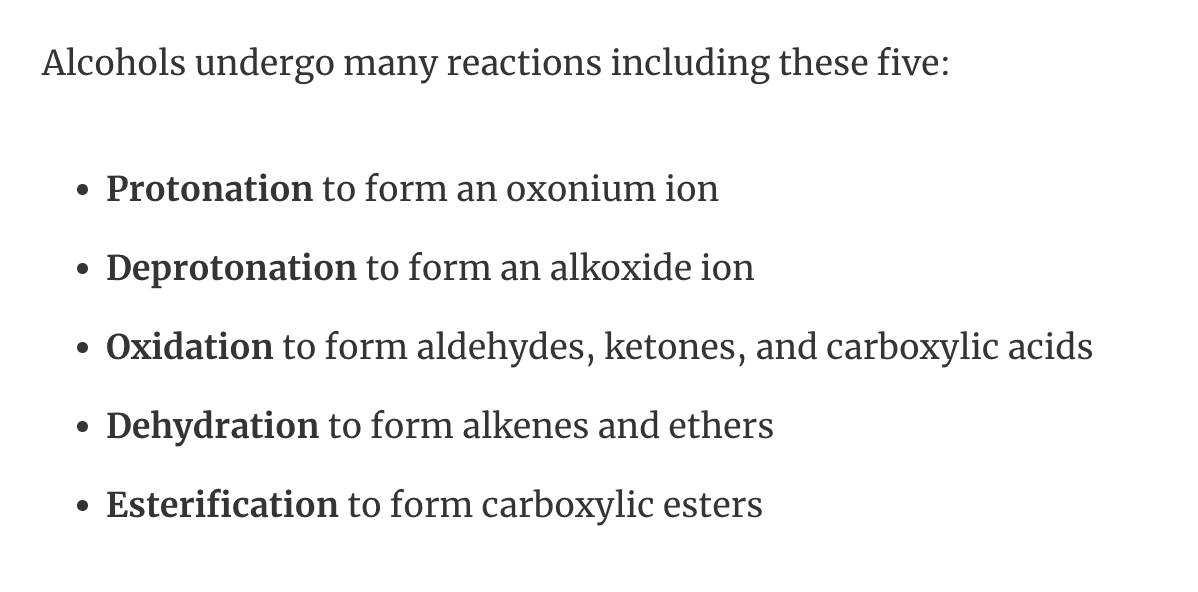
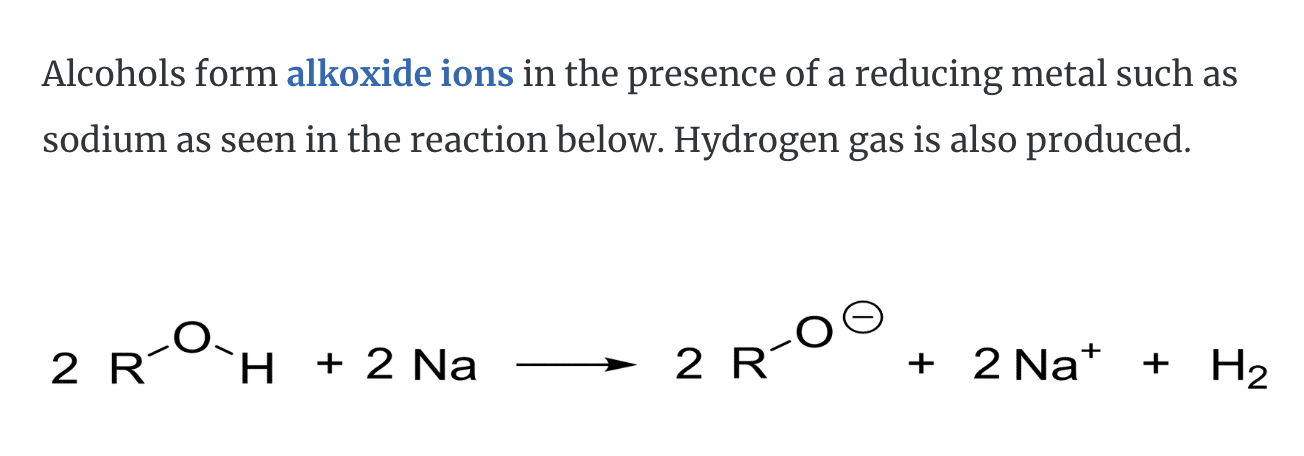
OXIDATION
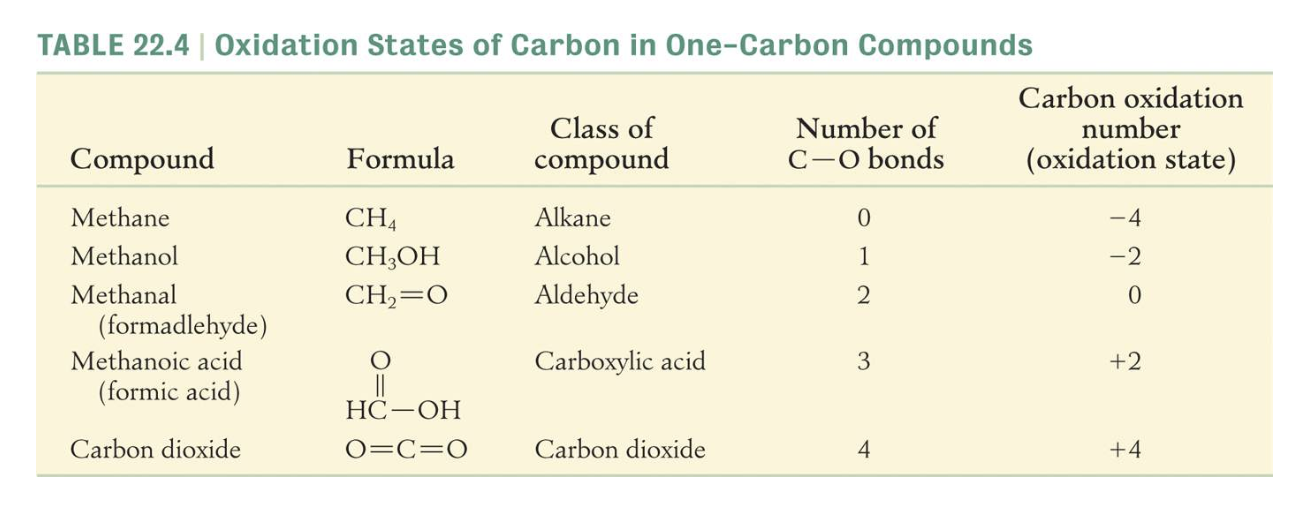
DEHYDRATION
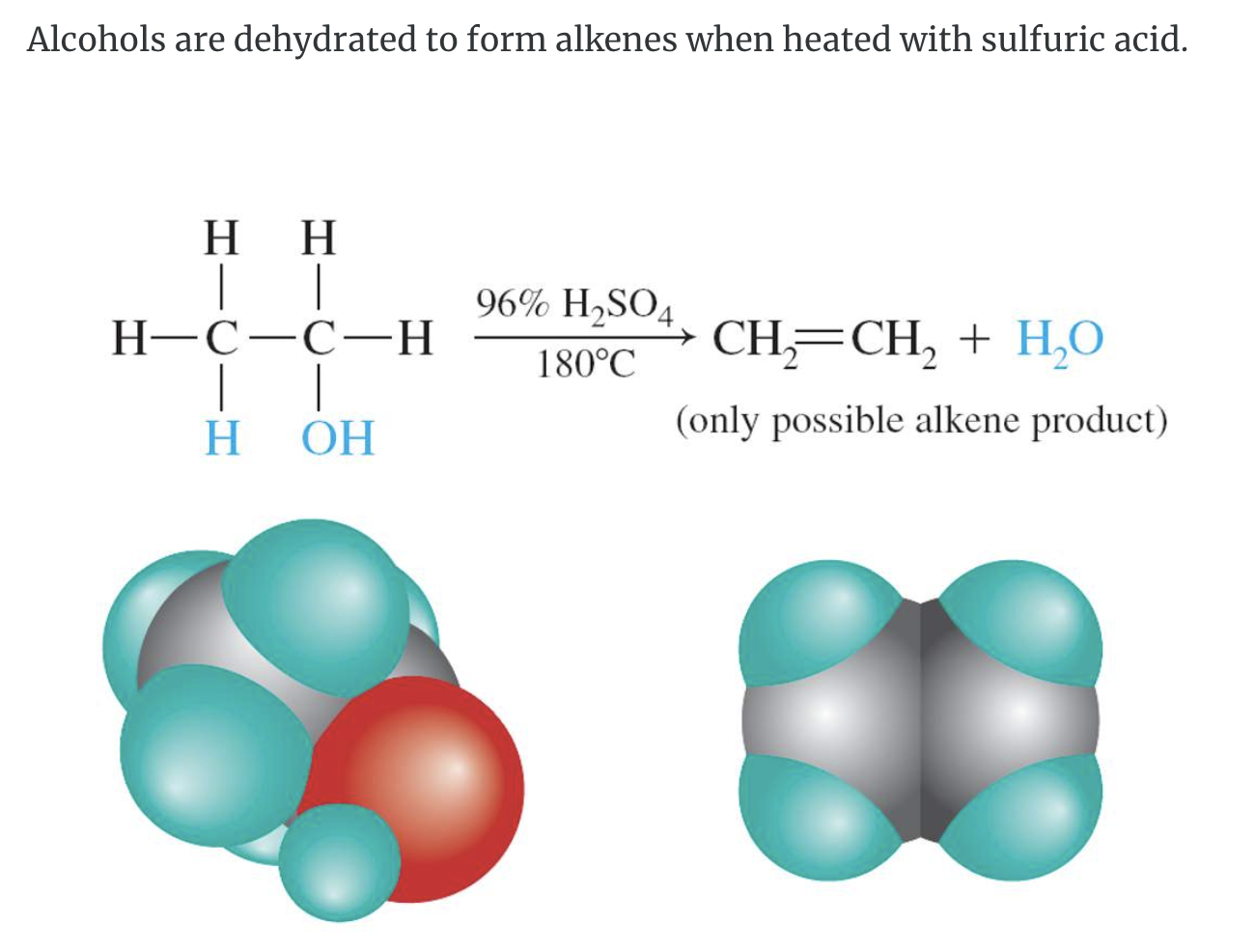
Zaitsev’s rule https://youtu.be/lCHVfpWjnQk
During intramolecular dehydration, if there is a choice of positions for the carbon-carbon double bond, the preferred location is the one that generally gives the more highly substituted alkene–that is, the alkene with the most alkyl groups attached to the double-bond carbons. “The poor get poorer”.
ESTERIFICATION
FAT METABOLISM
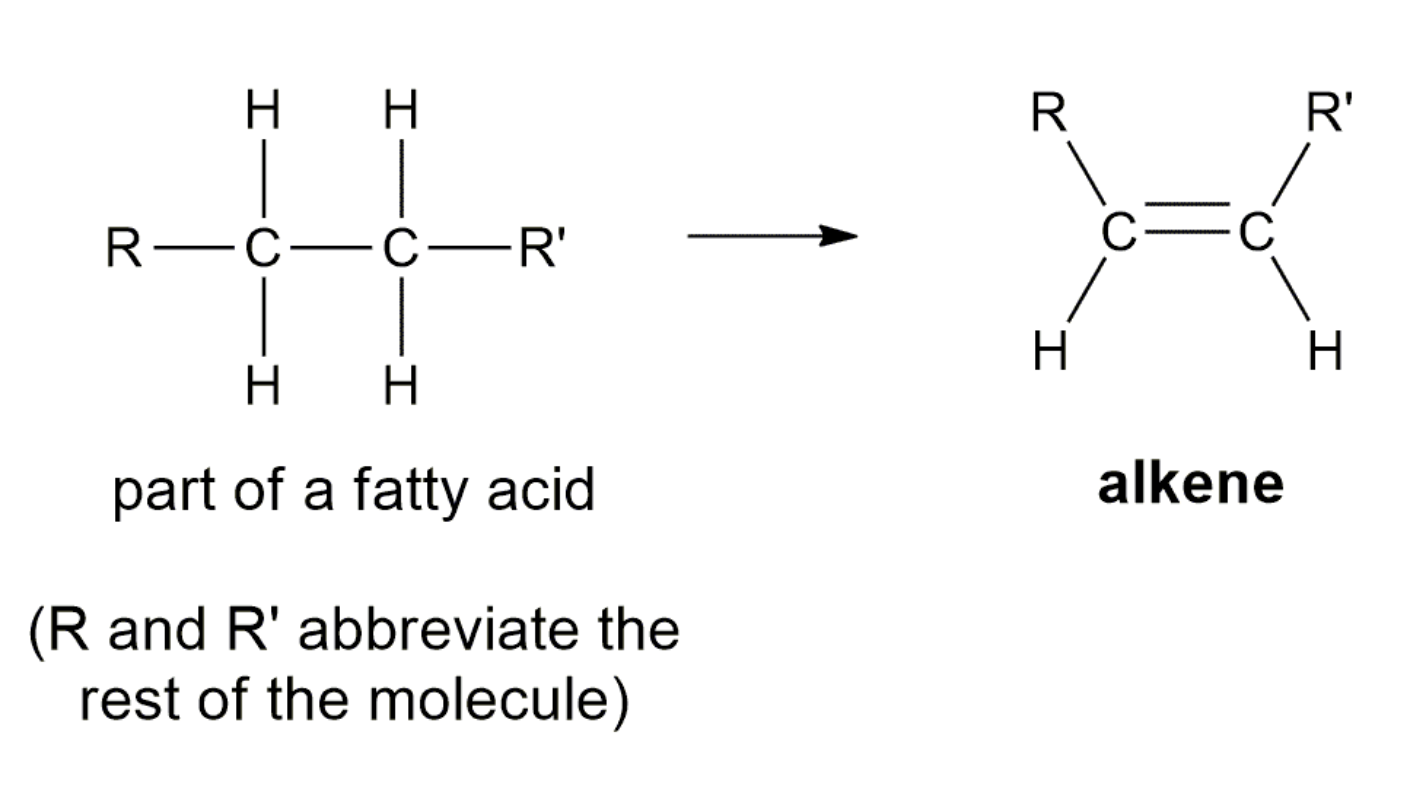
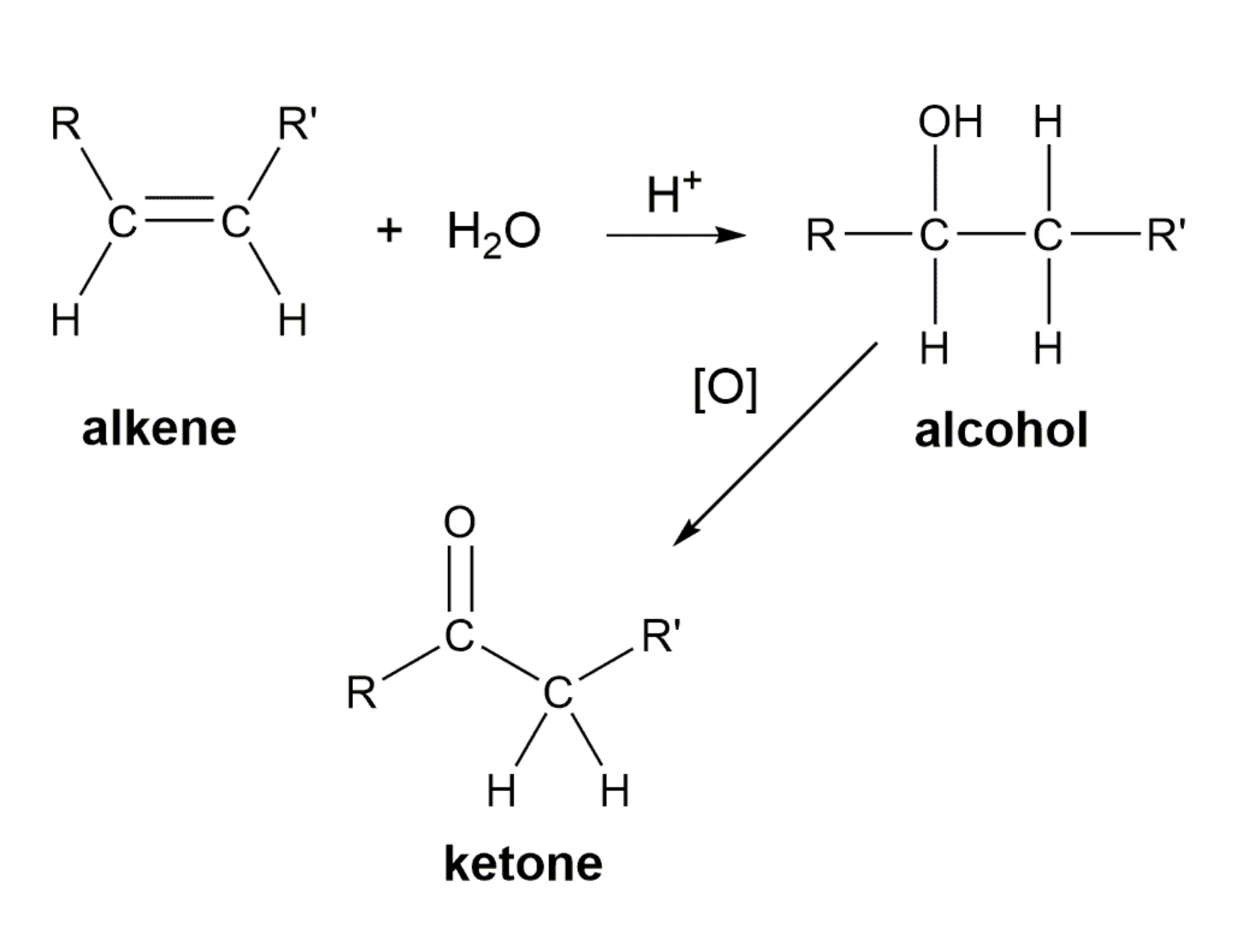
SYNTHESIS OF ALCOHOLS

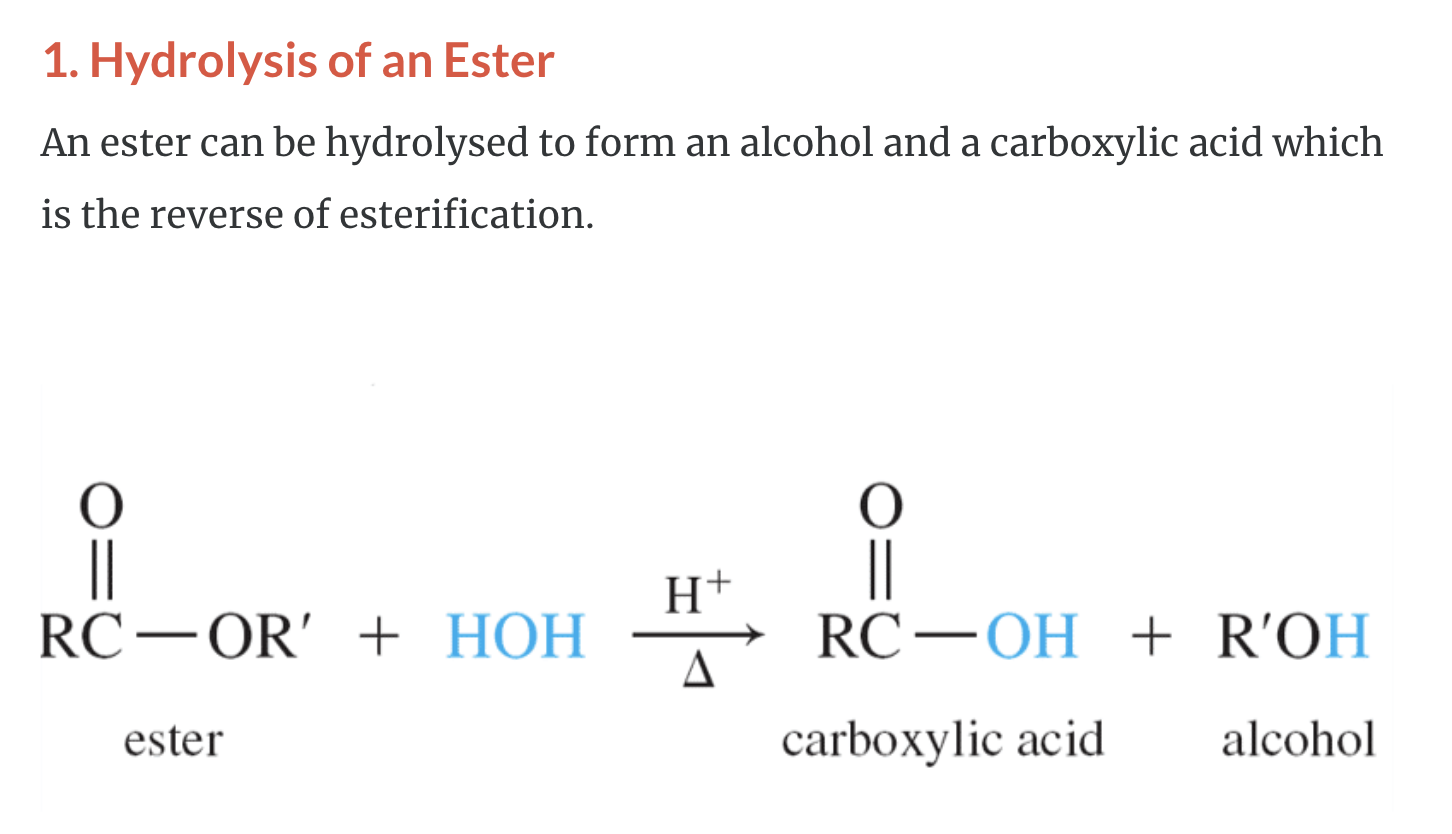
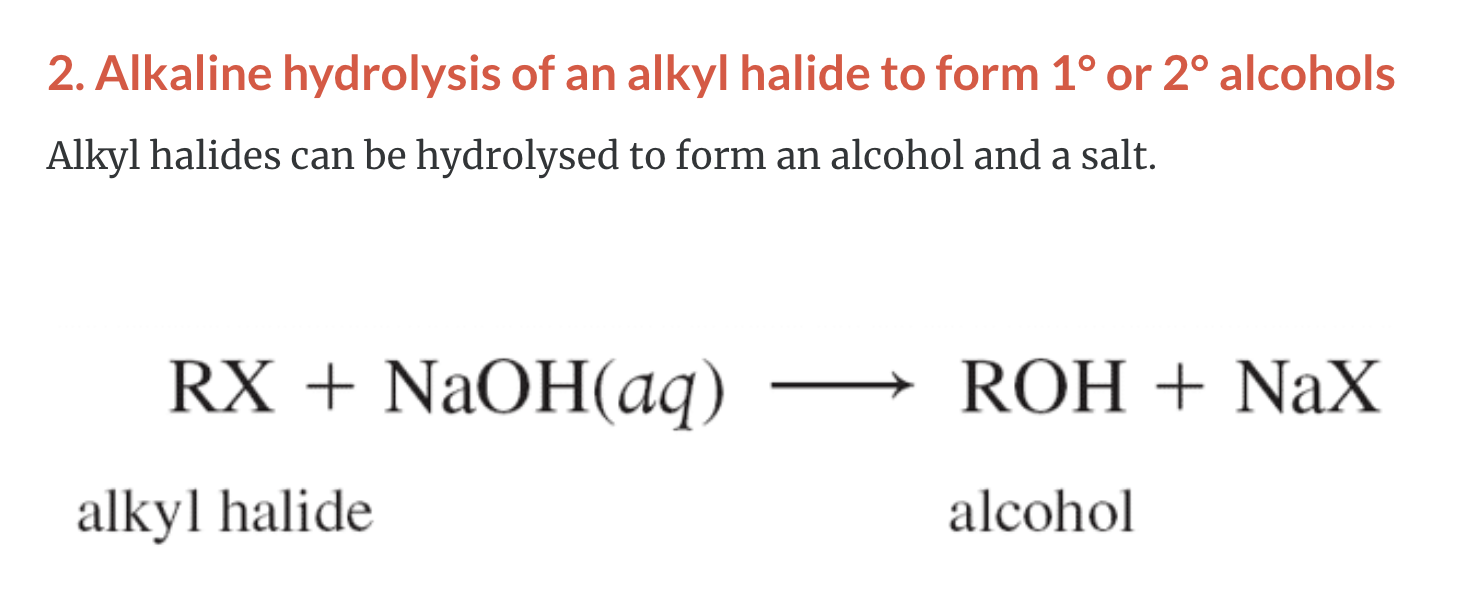
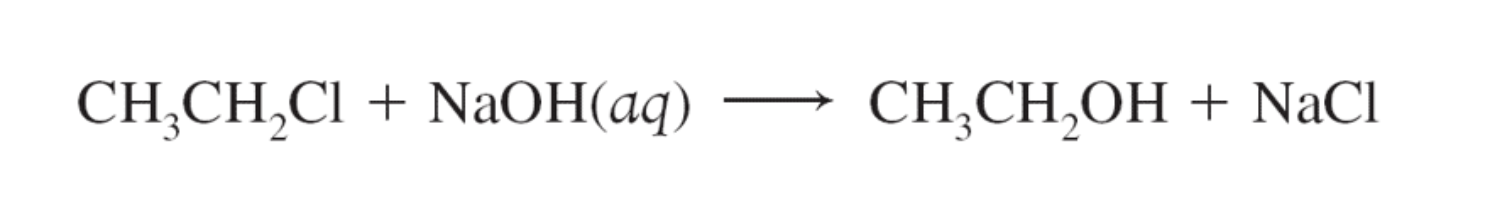
-Common alchols
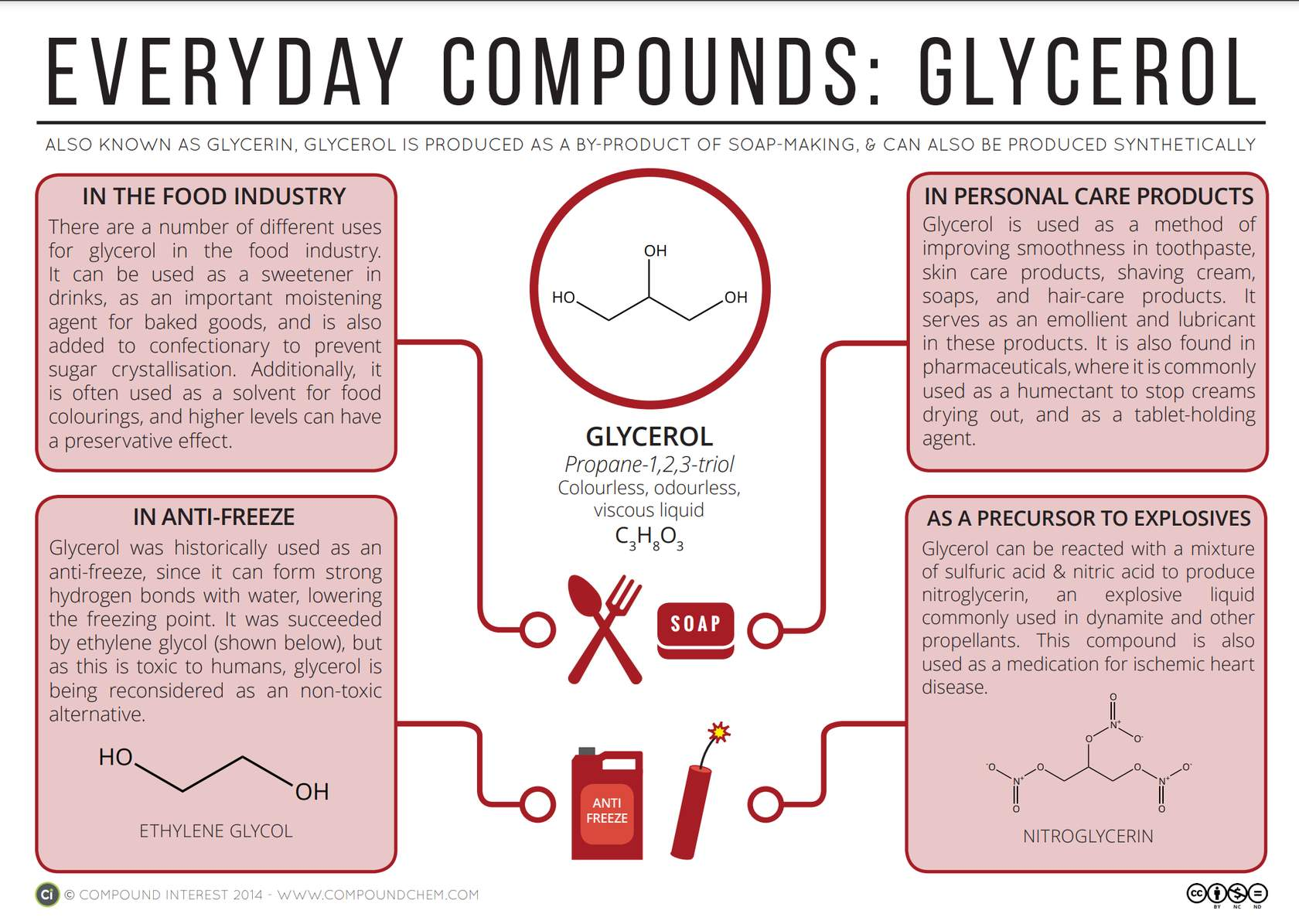
Glycerol (also known as 1,2,3-propanetriol and glycerine)
Glycerol is a polyhydroxy alcohol – it has a high attraction for water, due to the polarity of its hydroxyl groups.
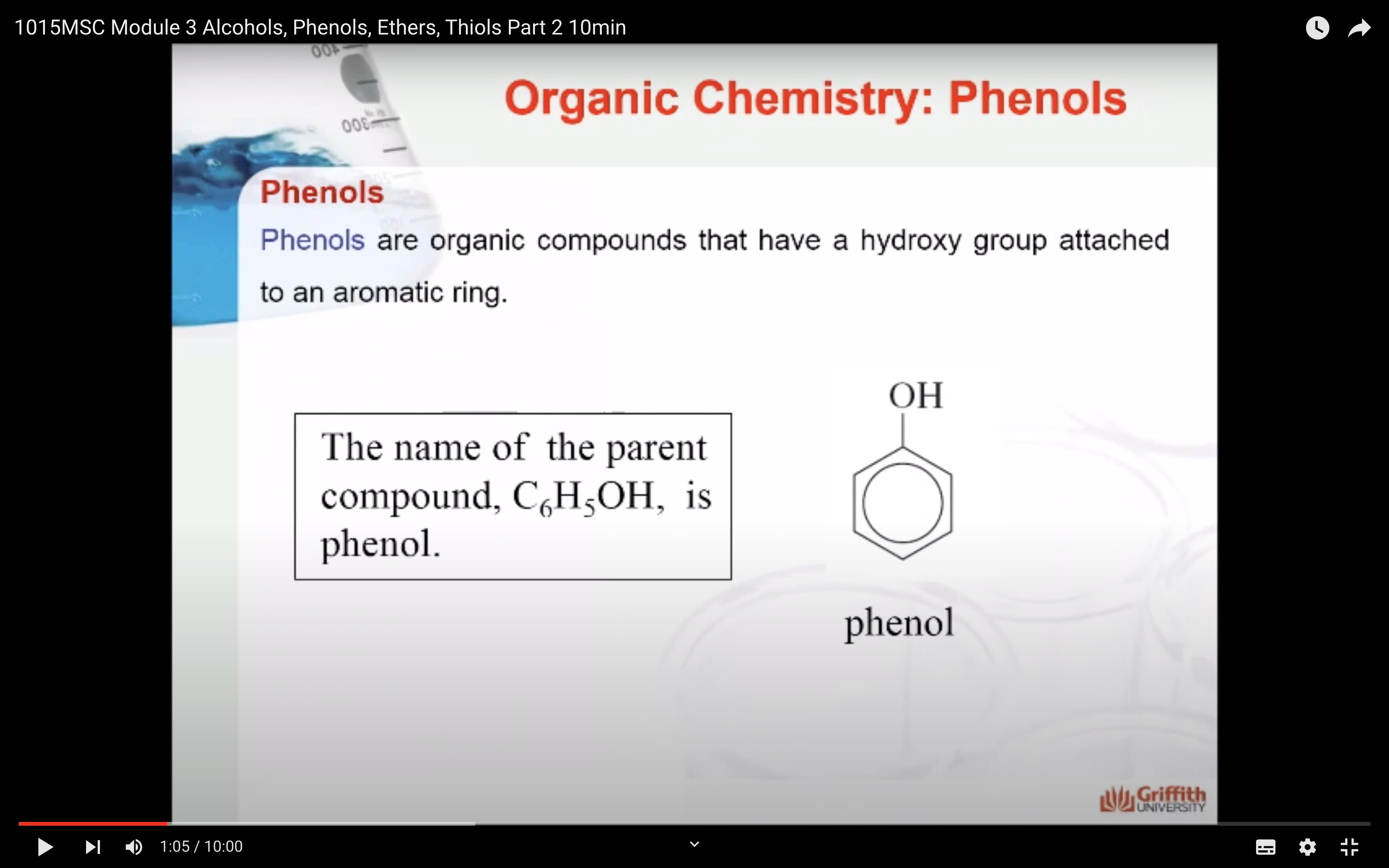
Phenols
-Phenols
Phenols are organic compounds that have a hydroxy group attached to an aromatic ring. They include the compound phenol, as well as substituted phenol compounds.
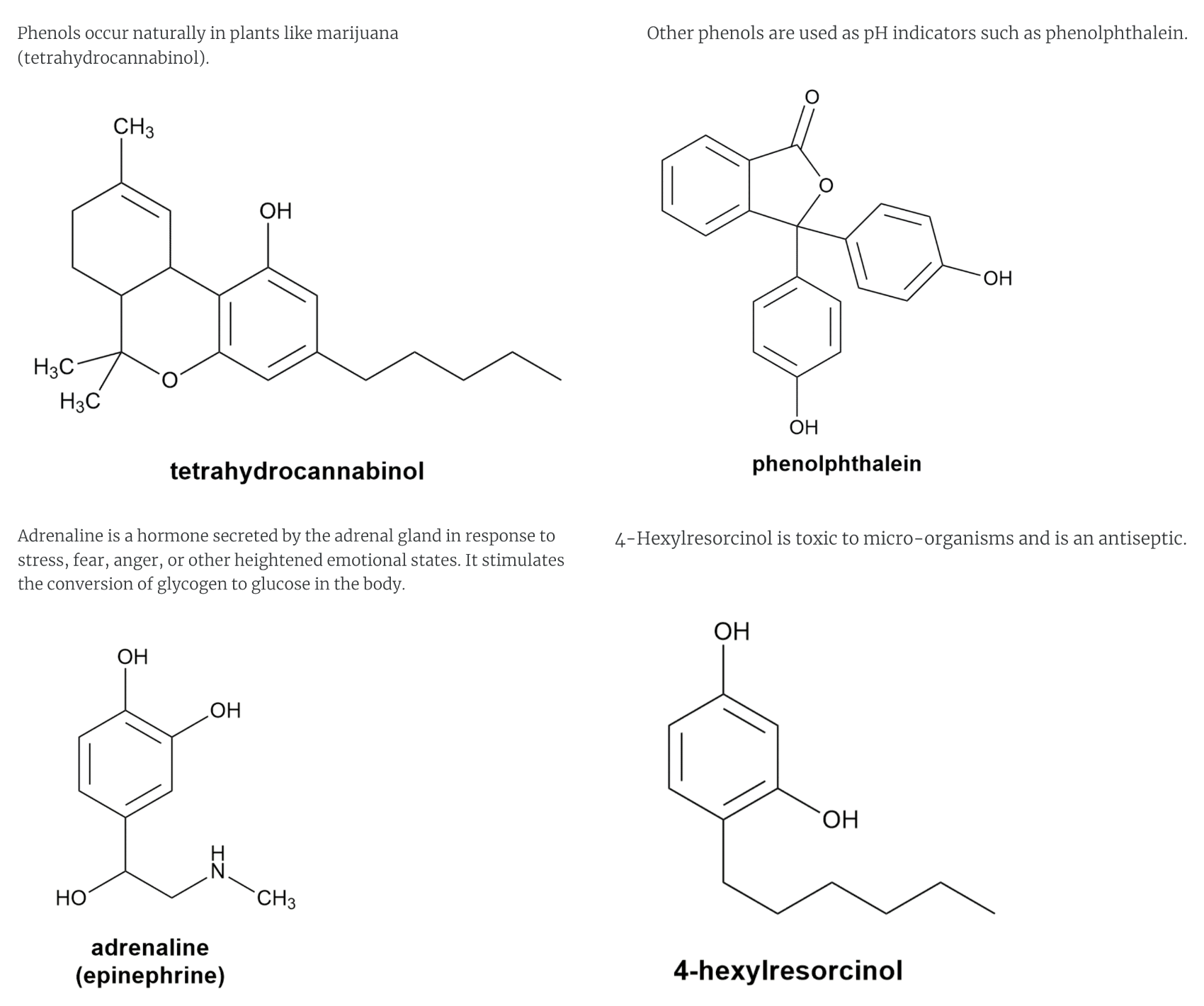
PHENOLS USES
-marijuana, pH indicators, adrenaline,4-hexylresocinol, antiseptics

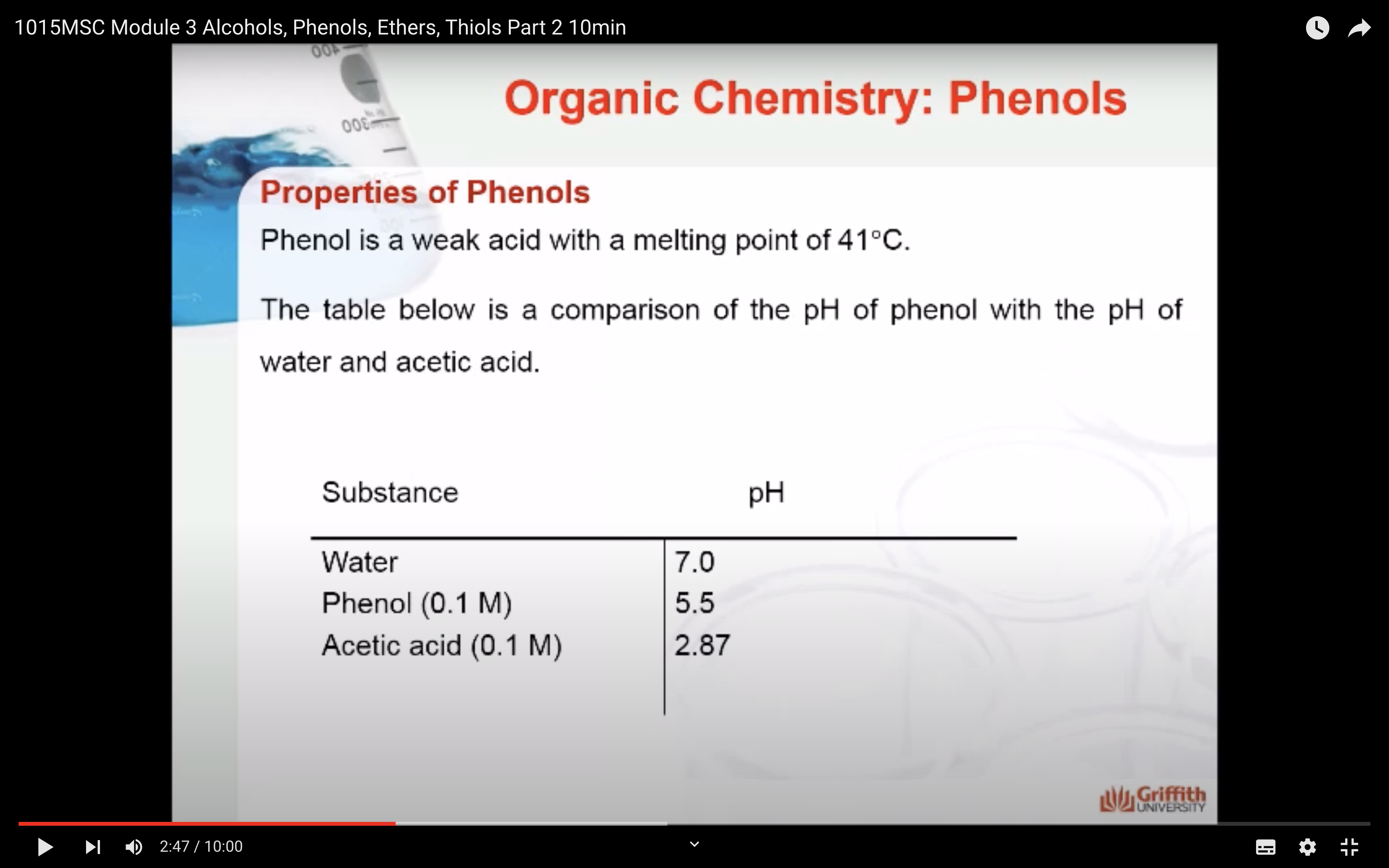
-Properties of phenols
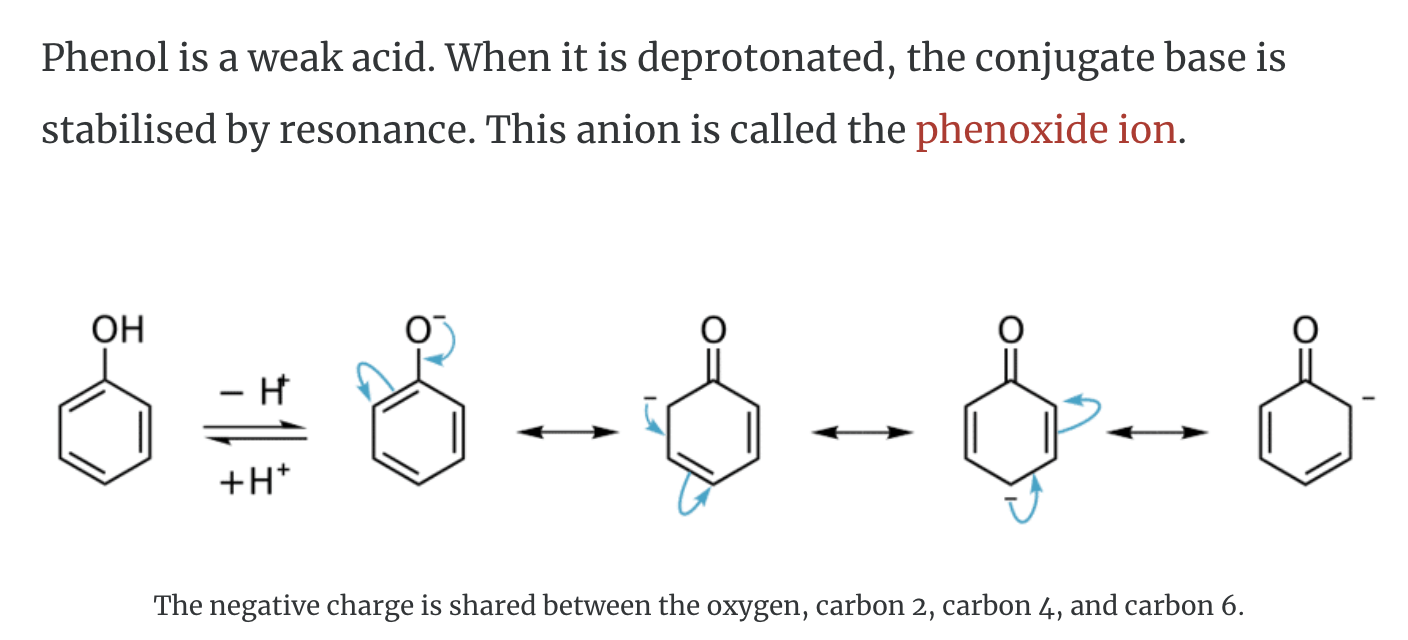
Resonance stabilisation of the phenoxide ion explains why phenol is 108 times more acidic than cyclohexanol. However, it is a weaker acid than carboxylic acids such as acetic acid.
Ethers
-Ethers
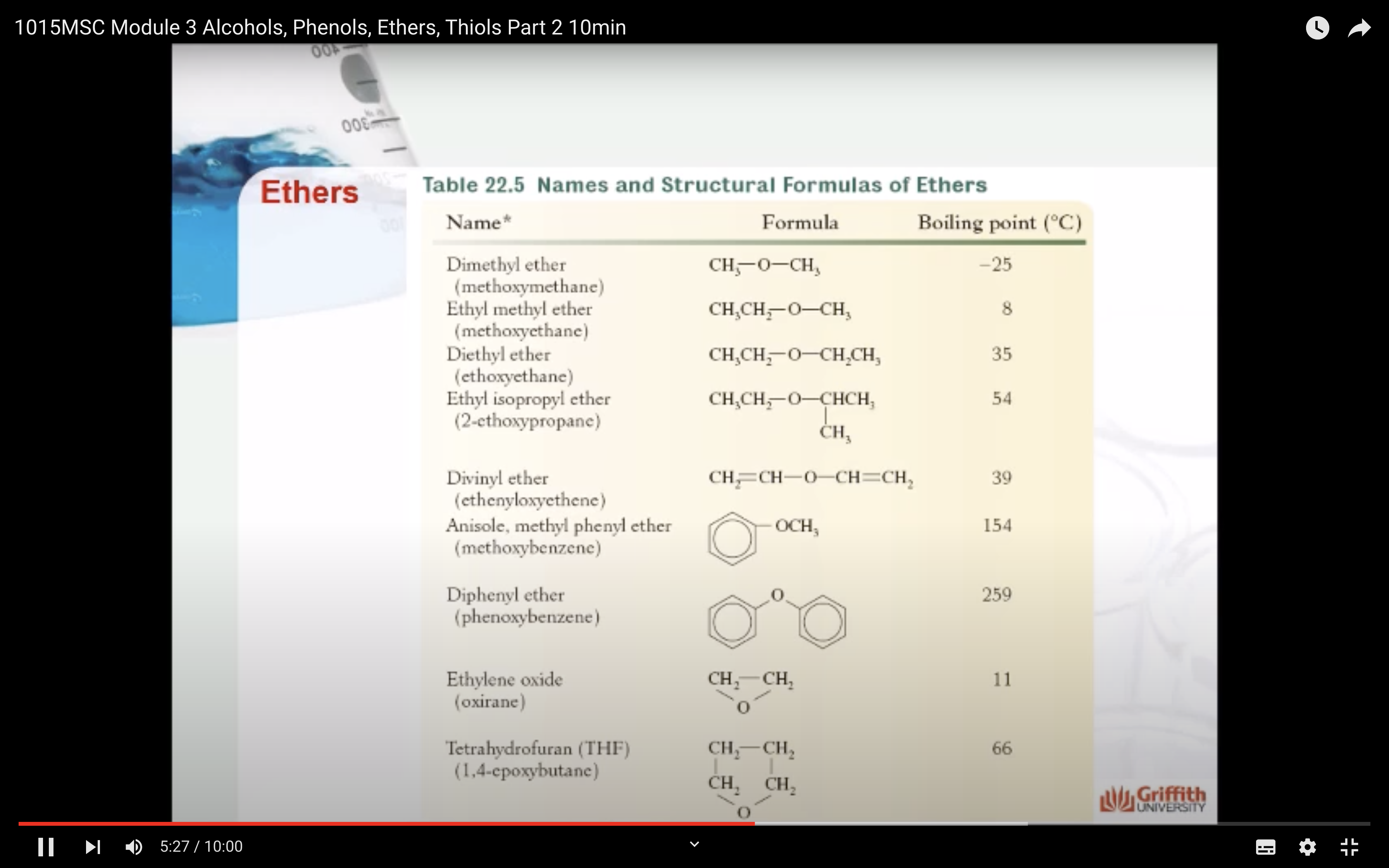
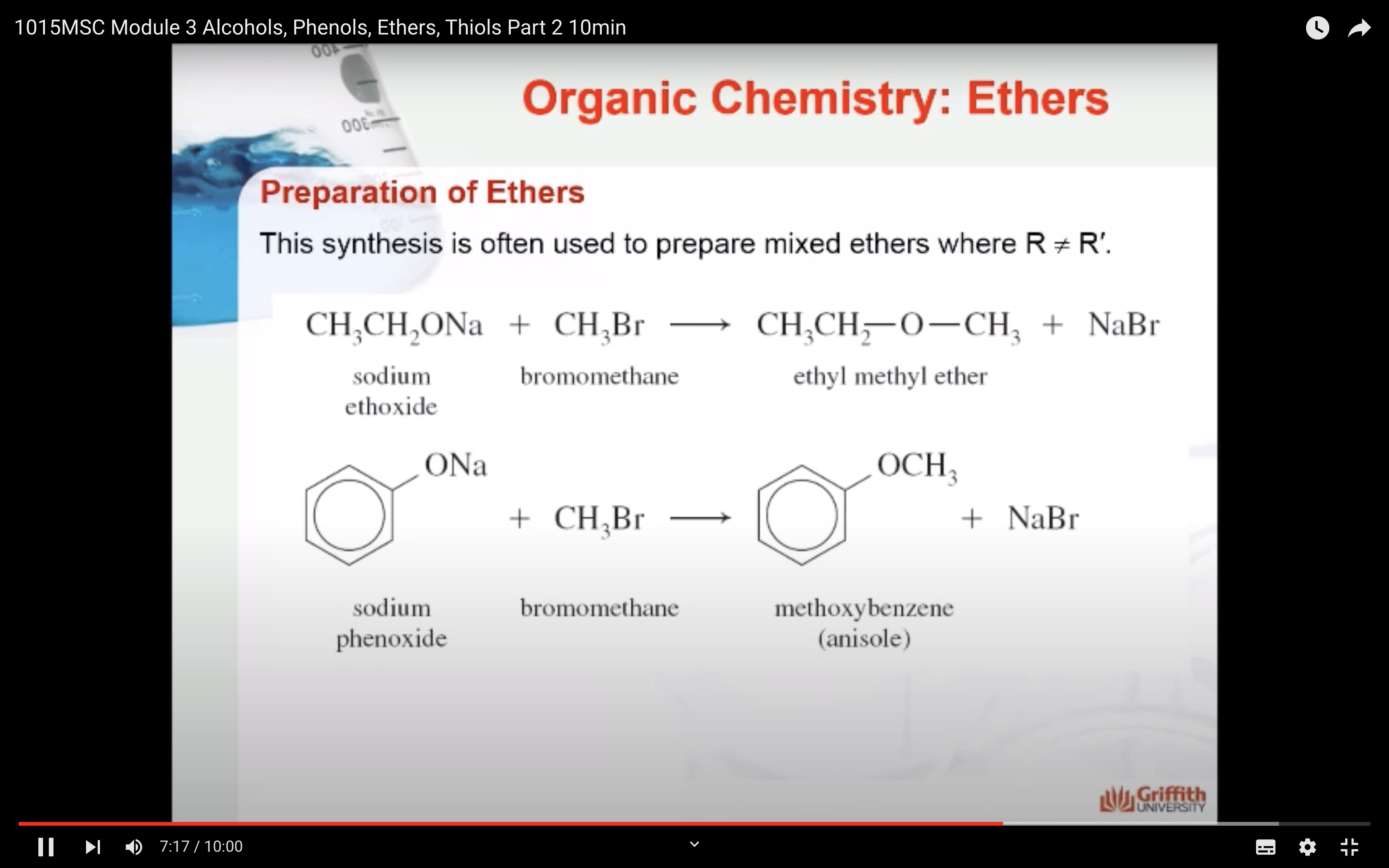
Ethers are organic compounds that have the general formula ROR′ where both R groups (alkyl or aromatic) can be the same or different.
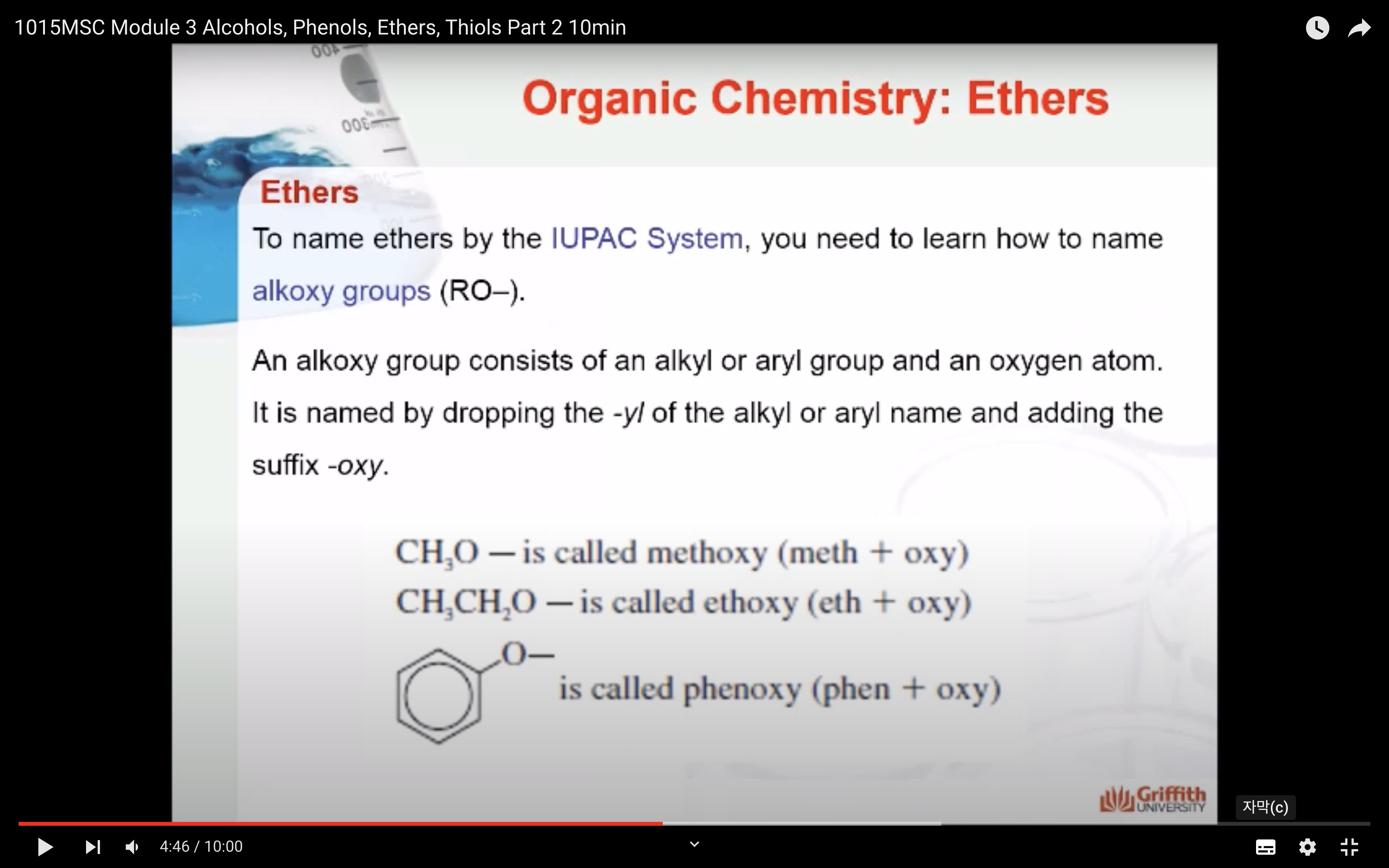
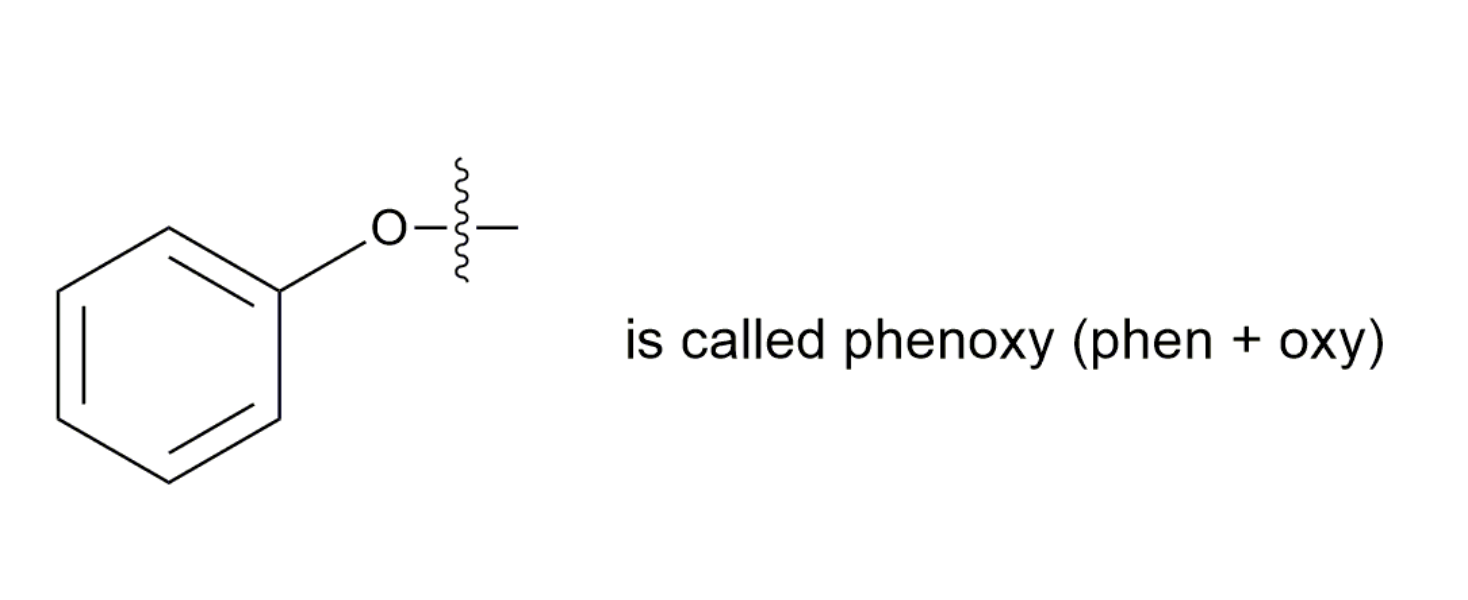
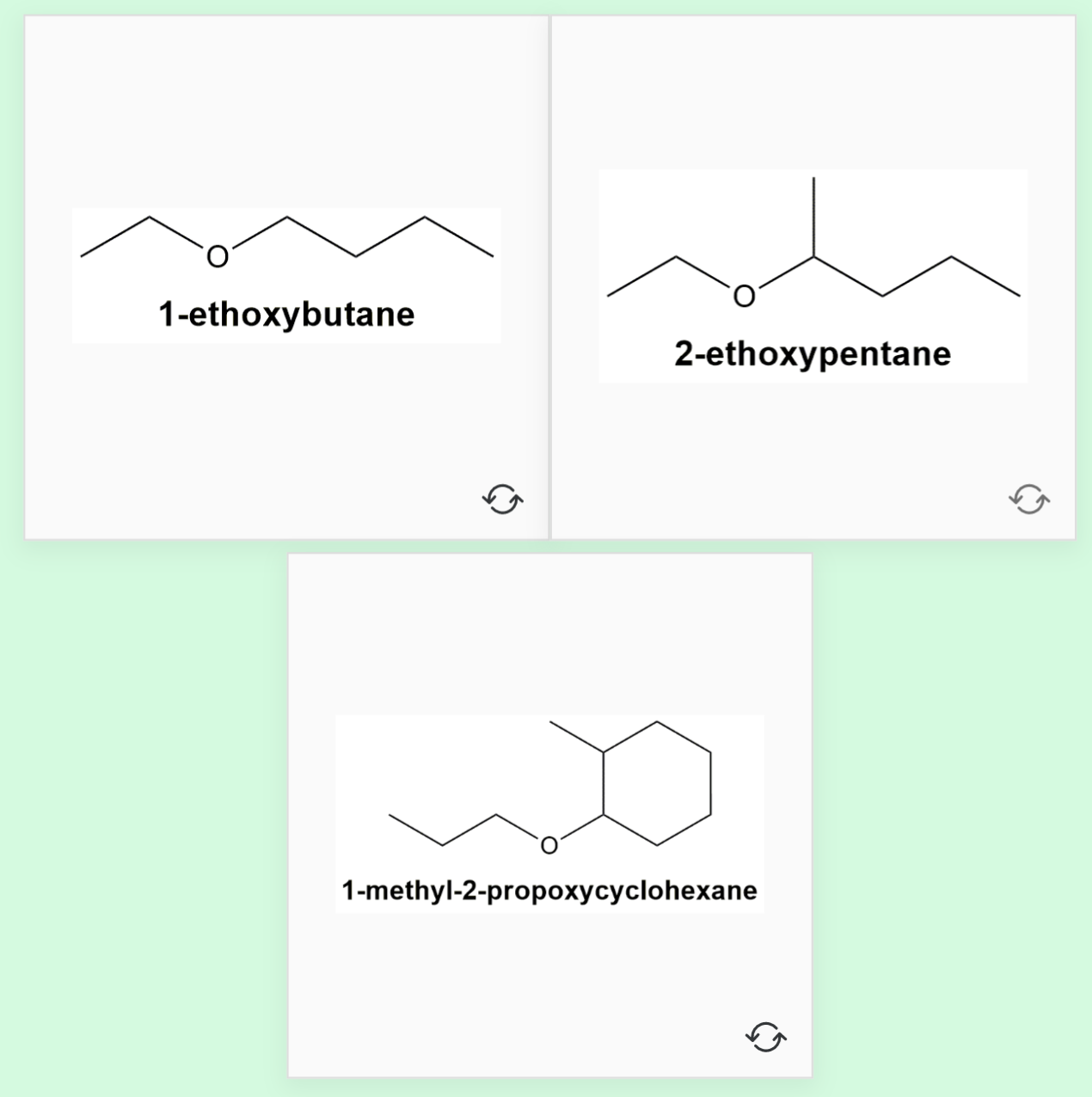
To name ethers by the IUPAC System, you need to learn how to name alkoxy groups (RO–).
An alkoxy group consists of an alkyl or aryl group and an oxygen atom. It is named by dropping the -yl of the alkyl or aryl name and adding the suffix -oxy.
- CH3O- is called methyoxy (meth + oxy)
- CH3CH2O- is called ethyoxy (eth + oxy)
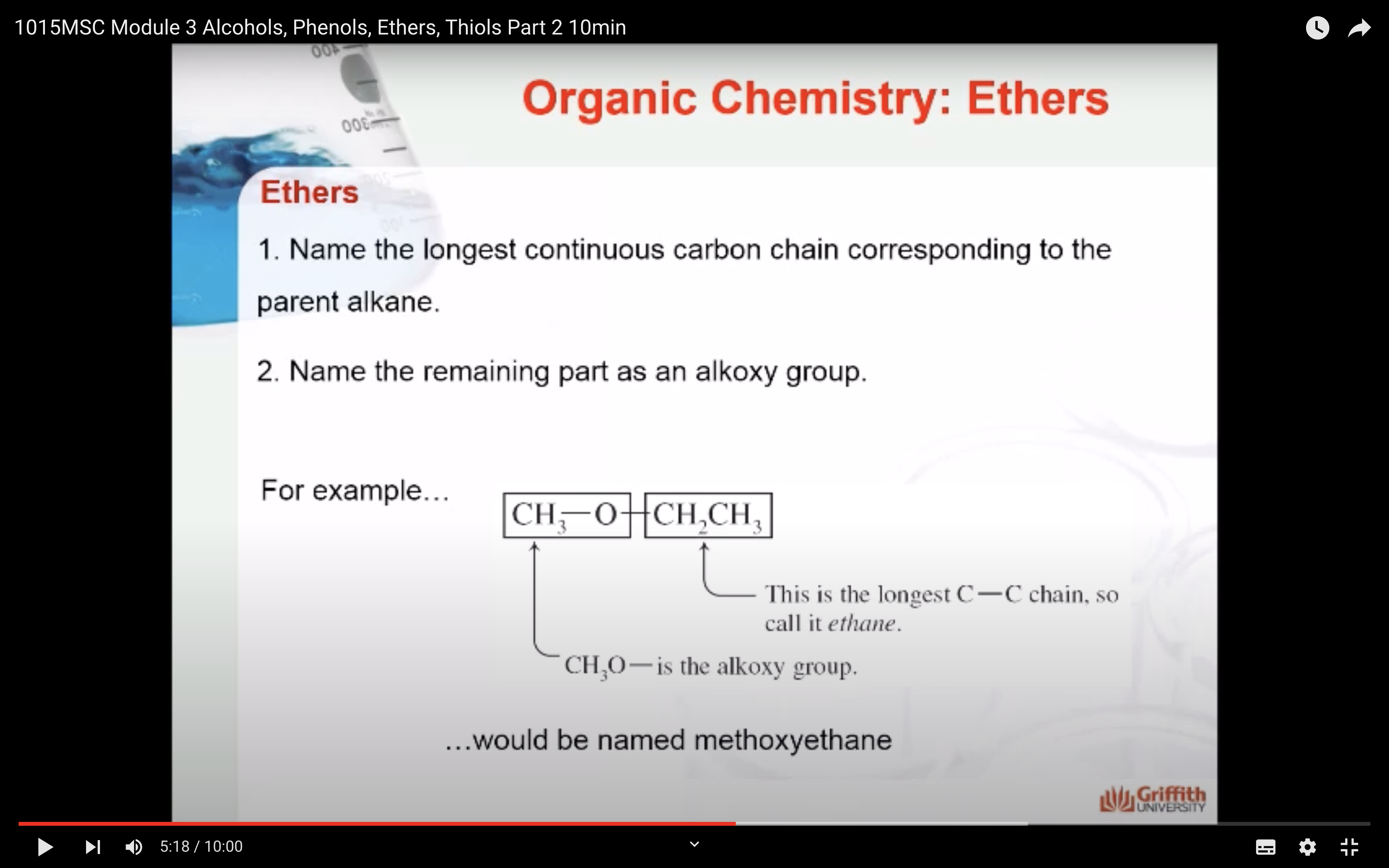
1. Name the longest continuous carbon chain corresponding to the parent alkane.
2. Name the remaining part as an alkoxy group.
For example, CH3-O-CH2-CH3 would be called methoxyethane. The longest carbon chain is two carbons, so it is called ethane. CH3O- is the alkoxy group, in this case it is methoxy.
-Properties of ethers
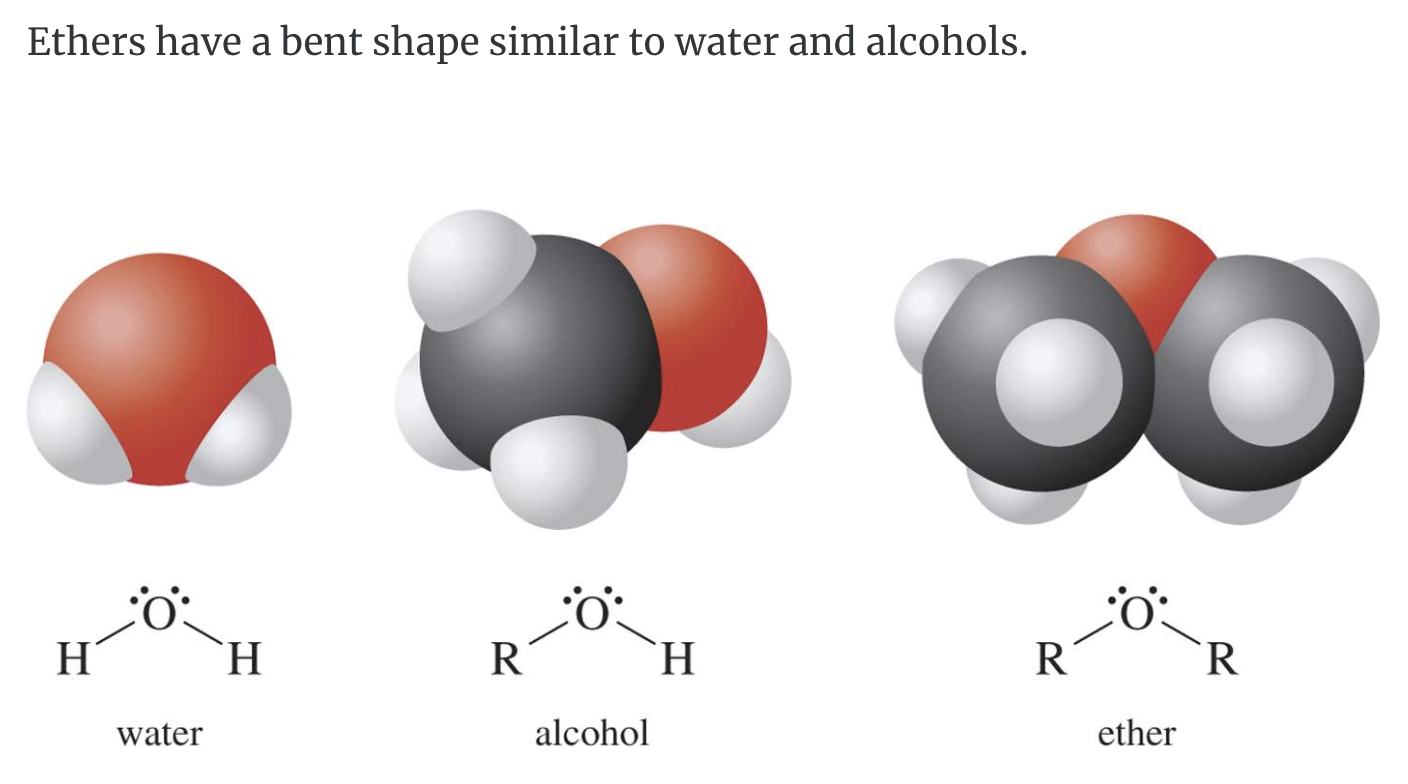
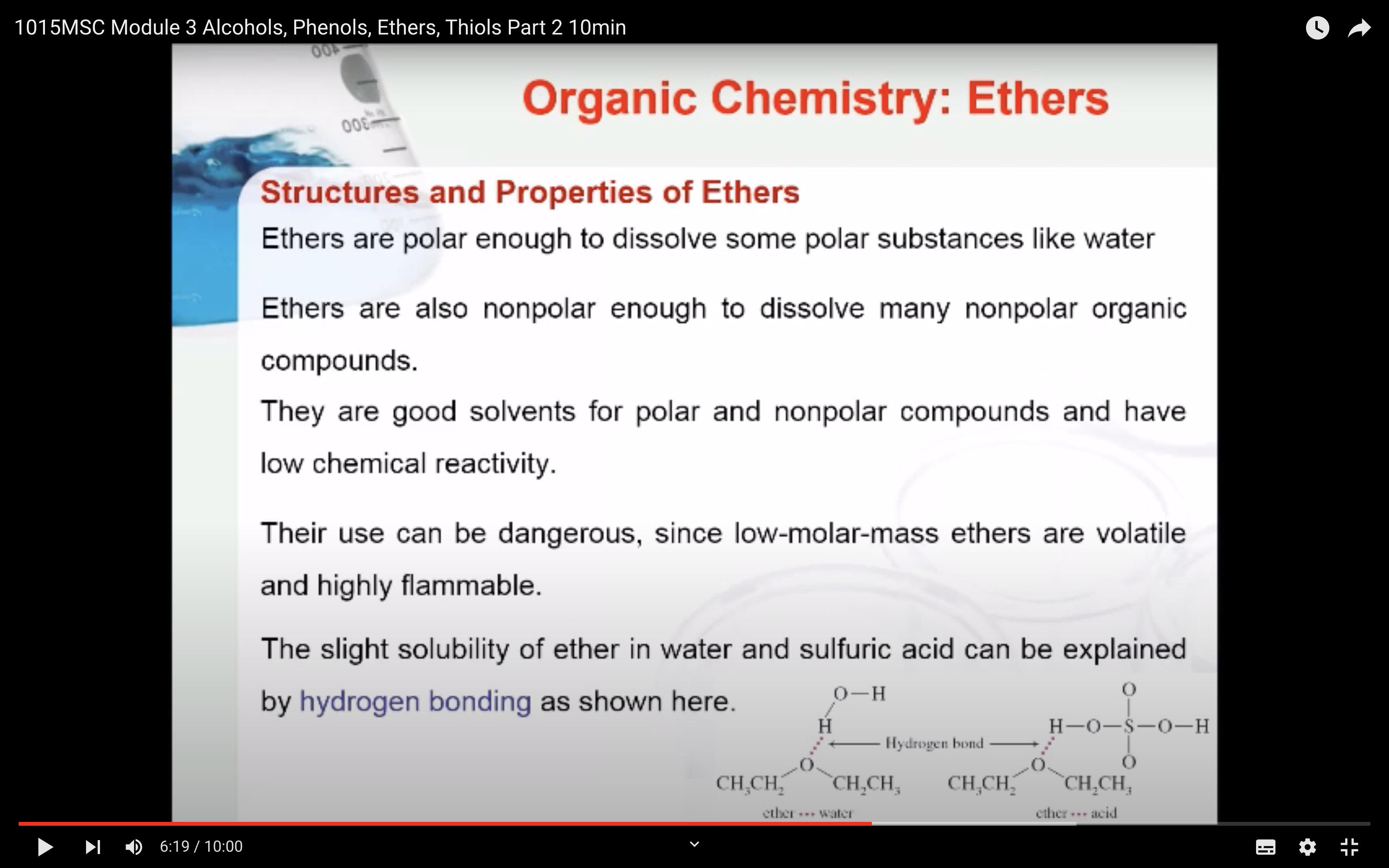
Ethers are polar enough to dissolve some polar substances like water but also nonpolar enough to dissolve many nonpolar organic compounds.
The slight solubility of ether in water and sulfuric can be explained by hydrogen bonding as shown here.
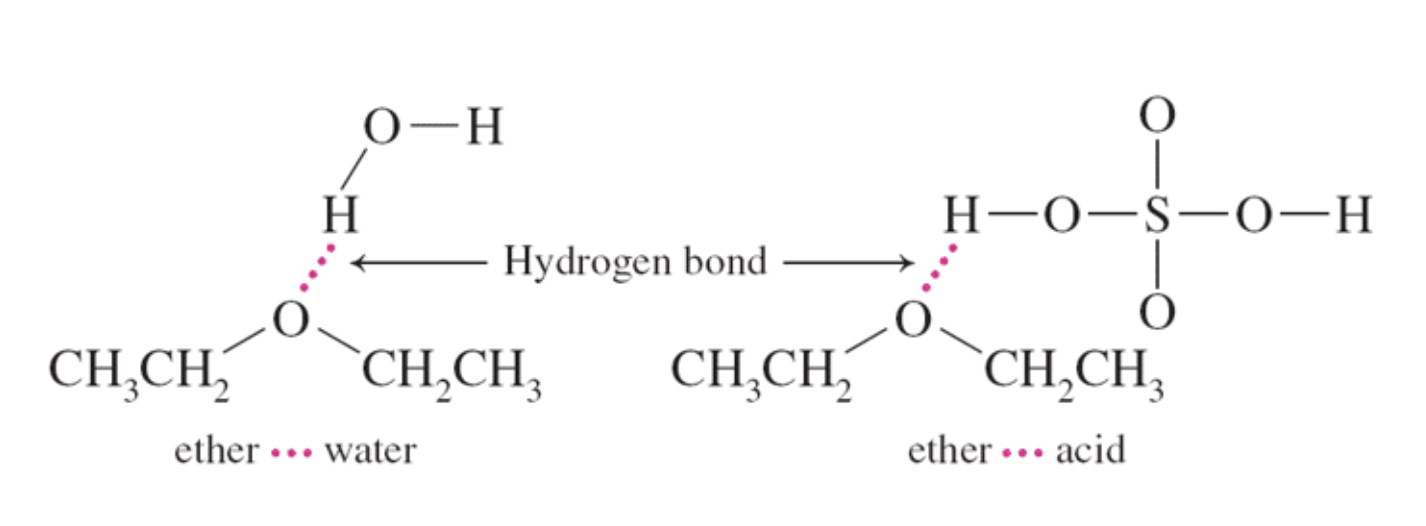
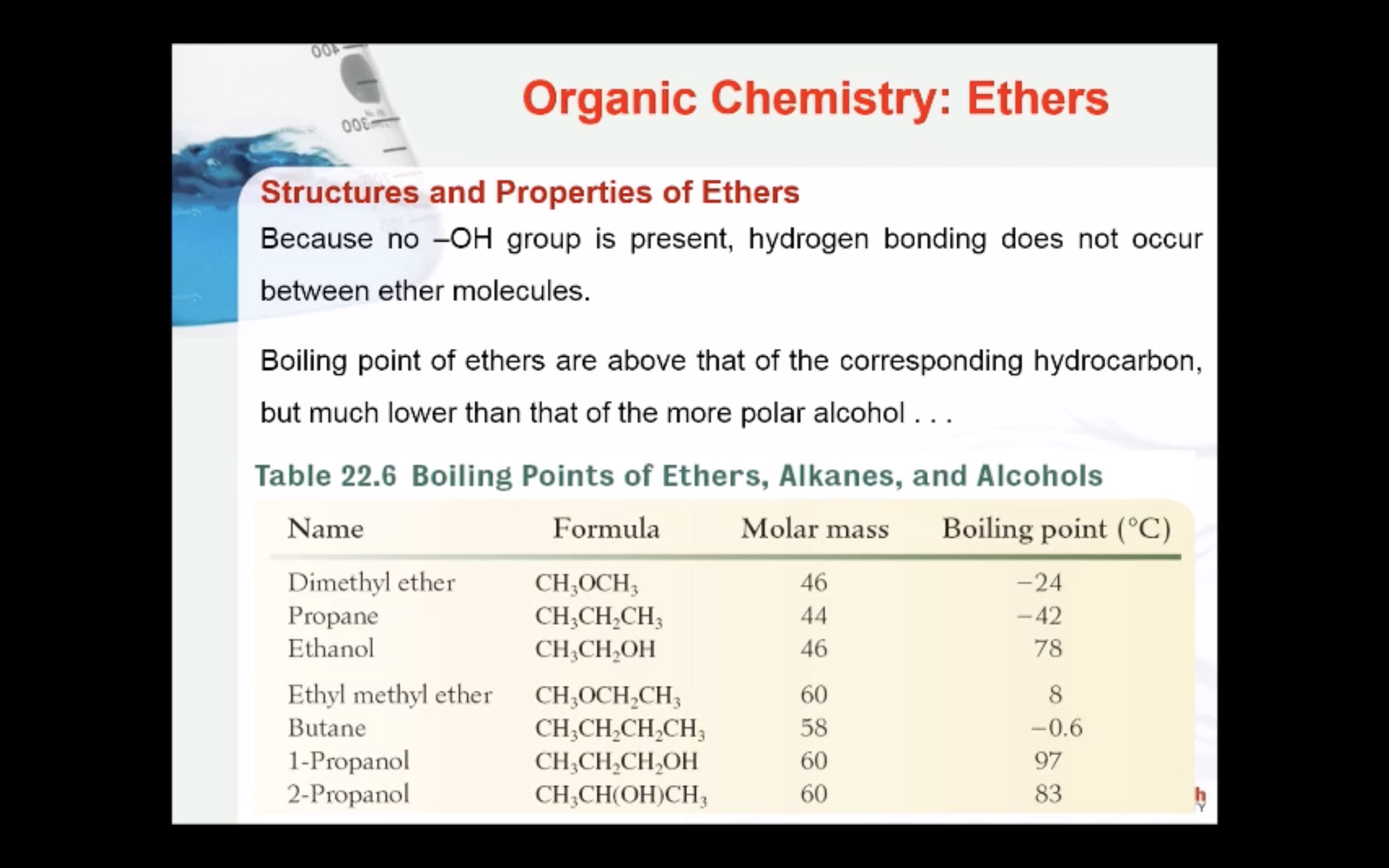
Ethers are good solvents for polar and nonpolar compounds and have low chemical reactivity. However, their use can be dangerous, since low-molar-mass ethers are volatile and highly flammable.
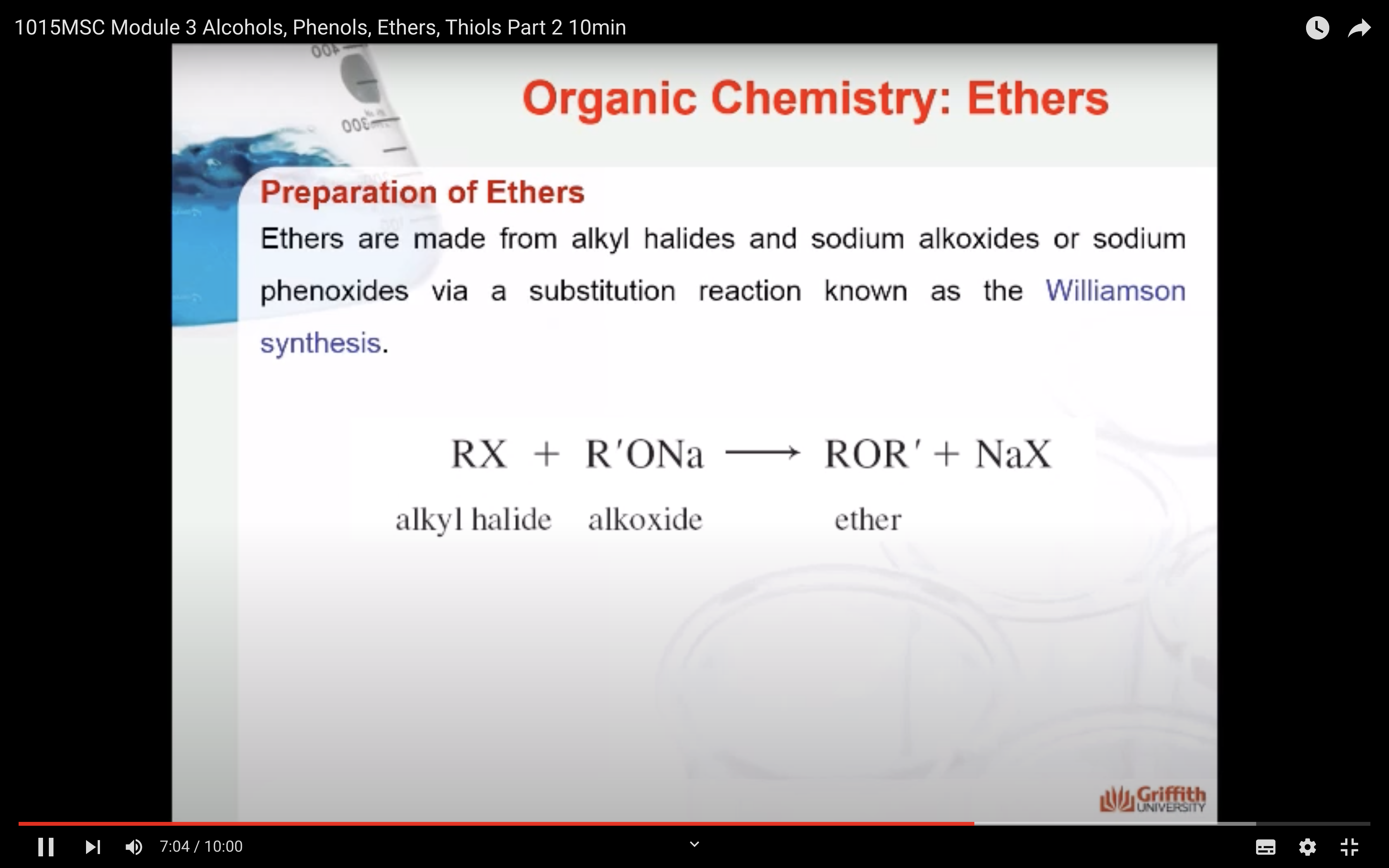
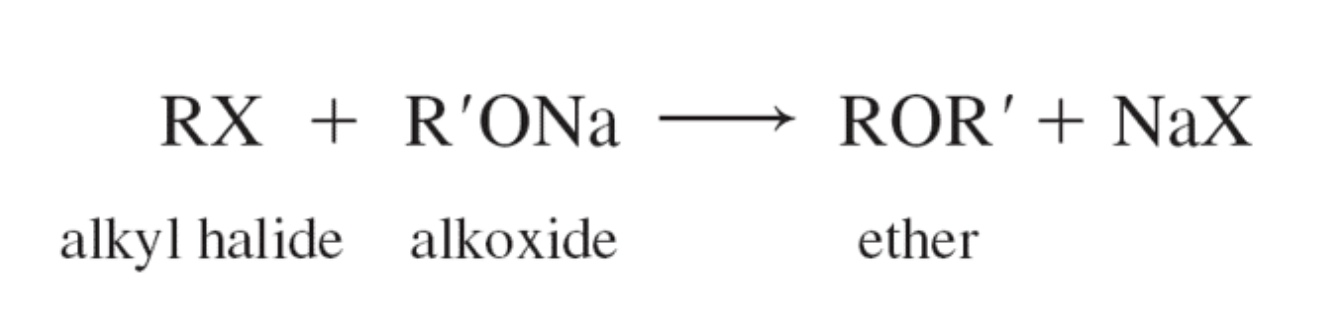
PREPARATION OF ETHERS
-made from alkyl halides, sodium alkoxides, or sodium phenoxides via substitution reaction known as the Williamson synthesis
Thiols
-Thiols
Thiols are organic compounds that contain the –SH group as shown below. Thiols are also called mercaptans.
- Foul odours
- Lower boiling points than alcohols – why?
- (S has low electronegativity than the O so the bonding between tiols are less stronger than alcohols)
- Thiols boil at lower temperatures than alcohols because hydrogen bonds can’t form between thiol molecules but can form between alcohol molecules.
- Readily oxidized to form disulfides
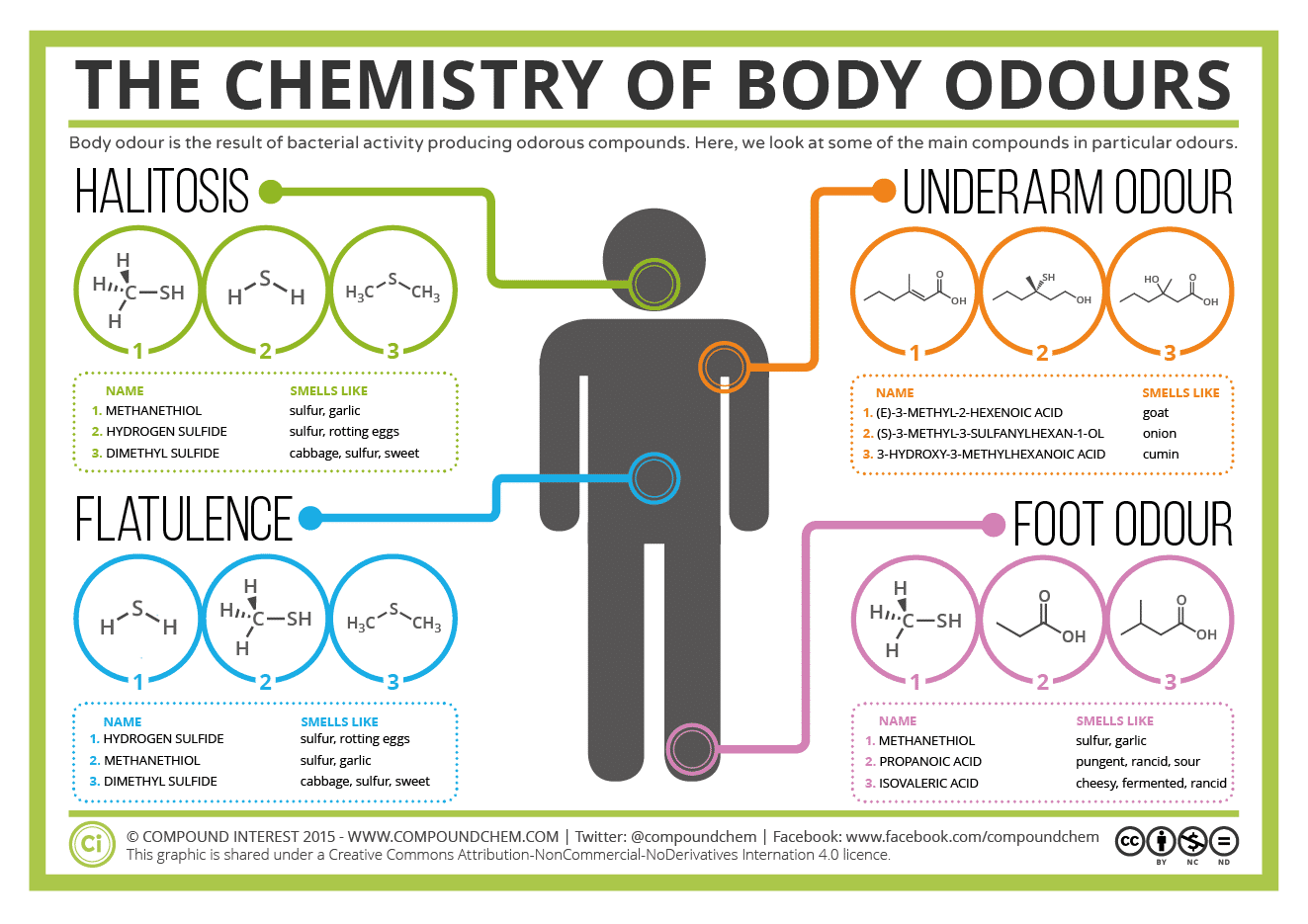
-Smell of skunk is due to thiol componenets including trans-2-butene-1-thiol and 3-methylbutane-1-thiol
-The strong odour associated wiith natural gas is due to the additive methanetiol (CH3SH)
Thiols readily oxidize to form disulfides. A disulfide is a molecule containing a S–S bond. The disulfide structure often binds proteins into biologically useful three-dimensional shapes.
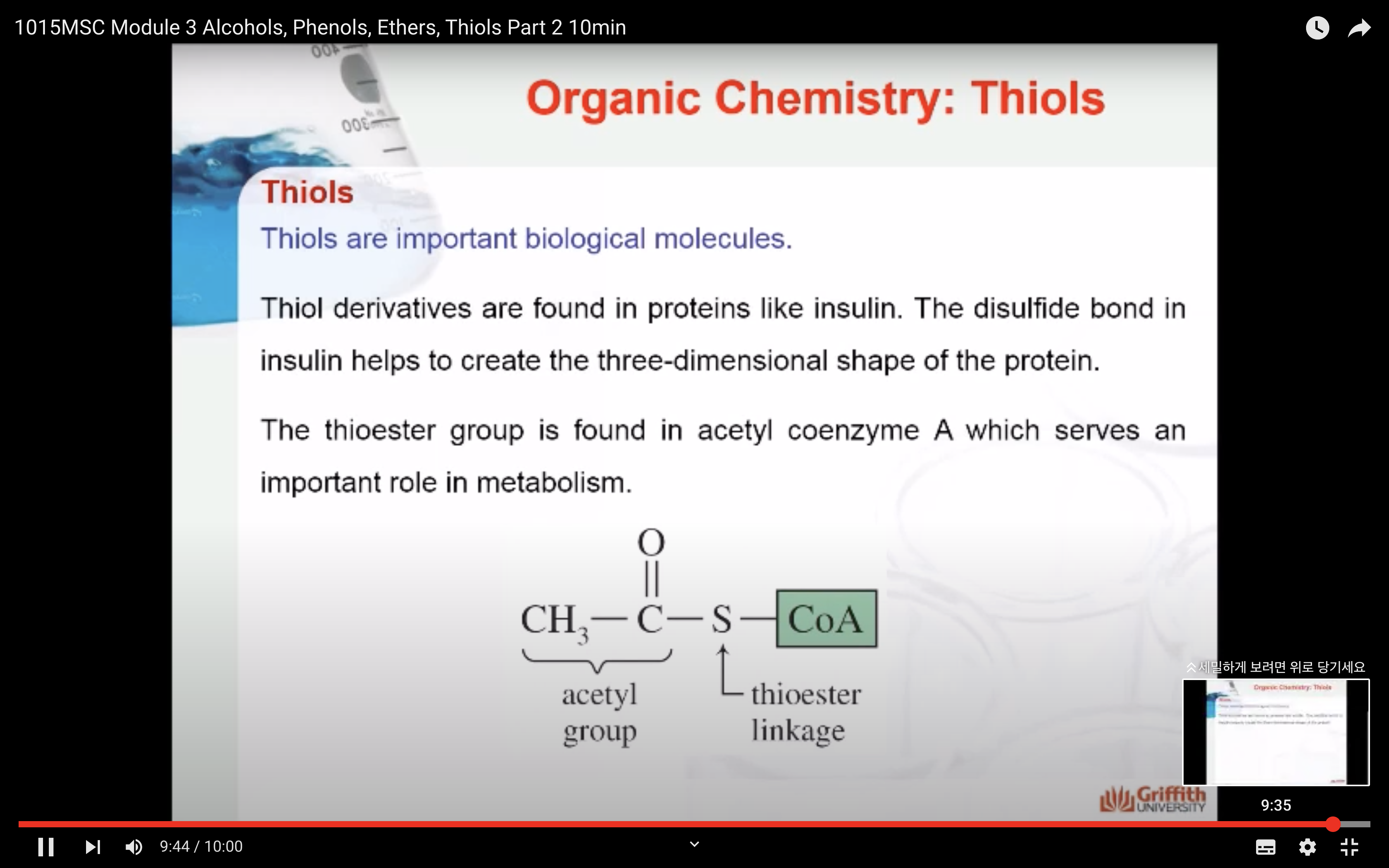
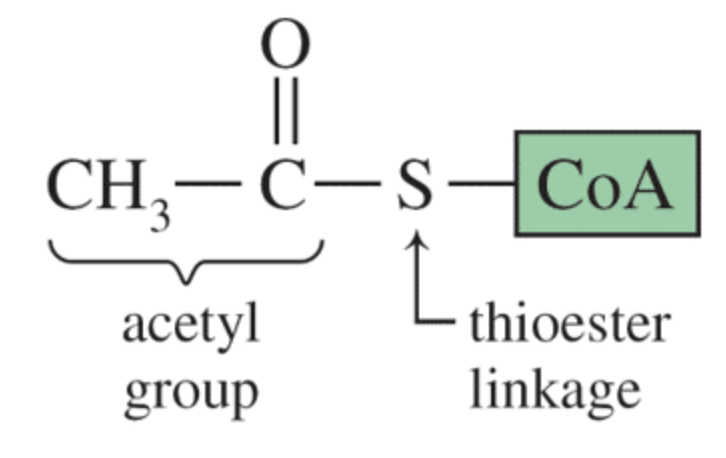
Thiols are important biological molecules.
Thiol derivatives are found in proteins like insulin. The disulfide bond in insulin helps to create the three-dimensional shape of the protein.
The thioester group is found in acetyl coenzyme A which serves an important role in metabolism.
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| REACTION NOTES (0) | 2023.03.28 |
|---|---|
| Lab 3 Glucose Concentration in Drinks (0) | 2023.03.27 |
| [WEEK4] Aldehydes and Ketones (1) | 2023.03.21 |
| [WEEK2] Unsaturated Hydrocarbons (0) | 2023.03.08 |
| [WEEK1] Saturated Hydrocarbons (0) | 2023.03.01 |



