Learning Content
Part 1: Organic Chemistry: History and Scope.
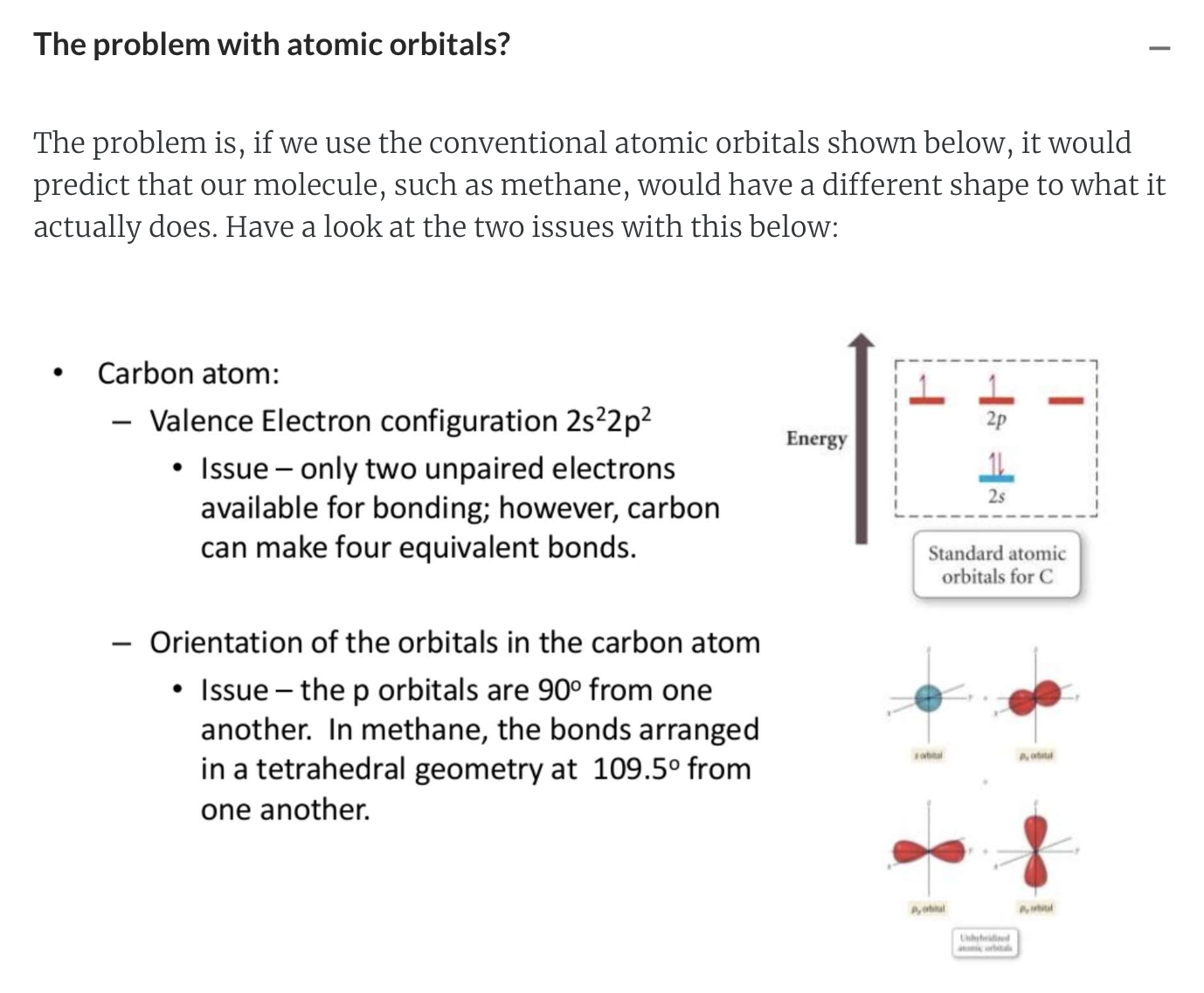
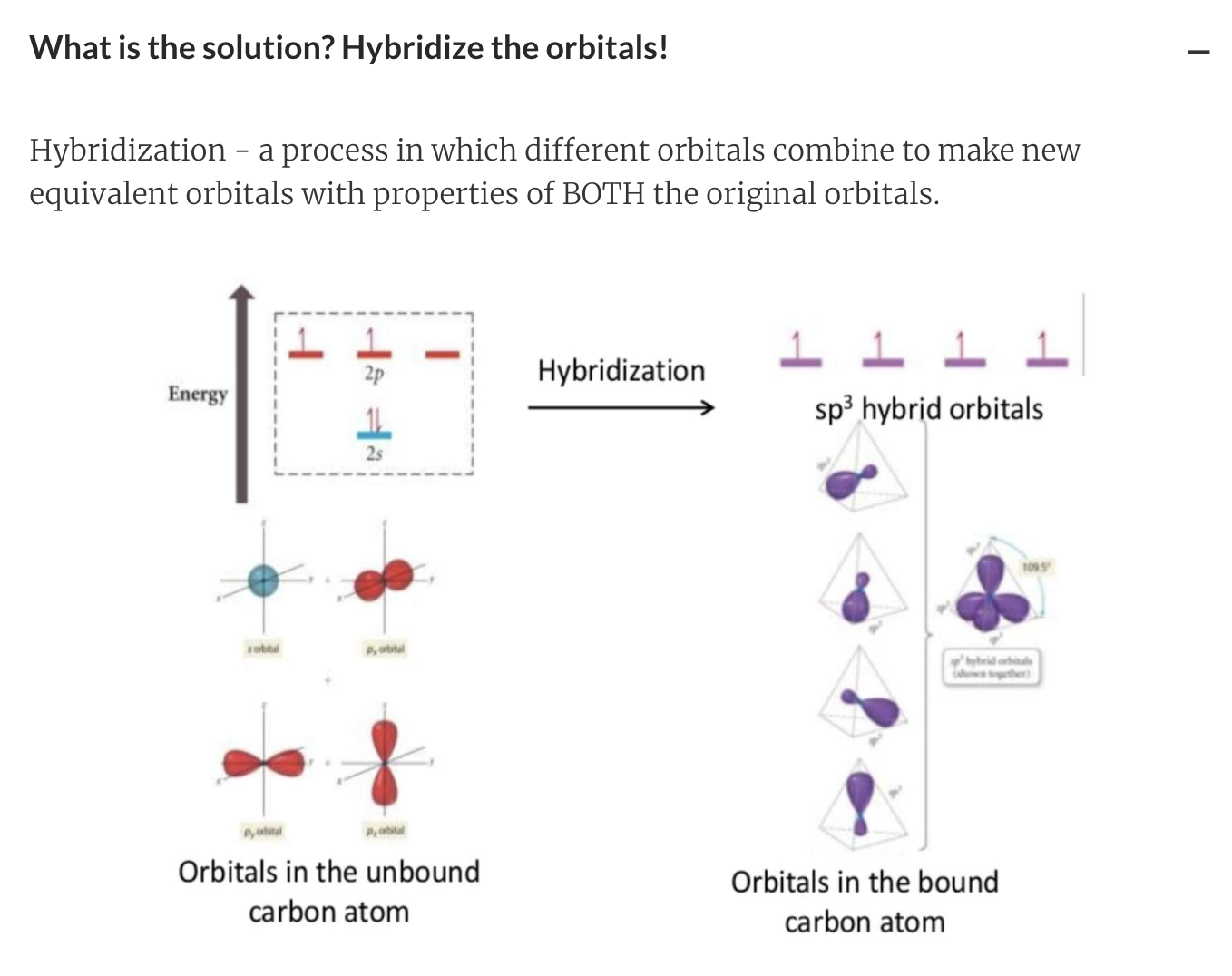
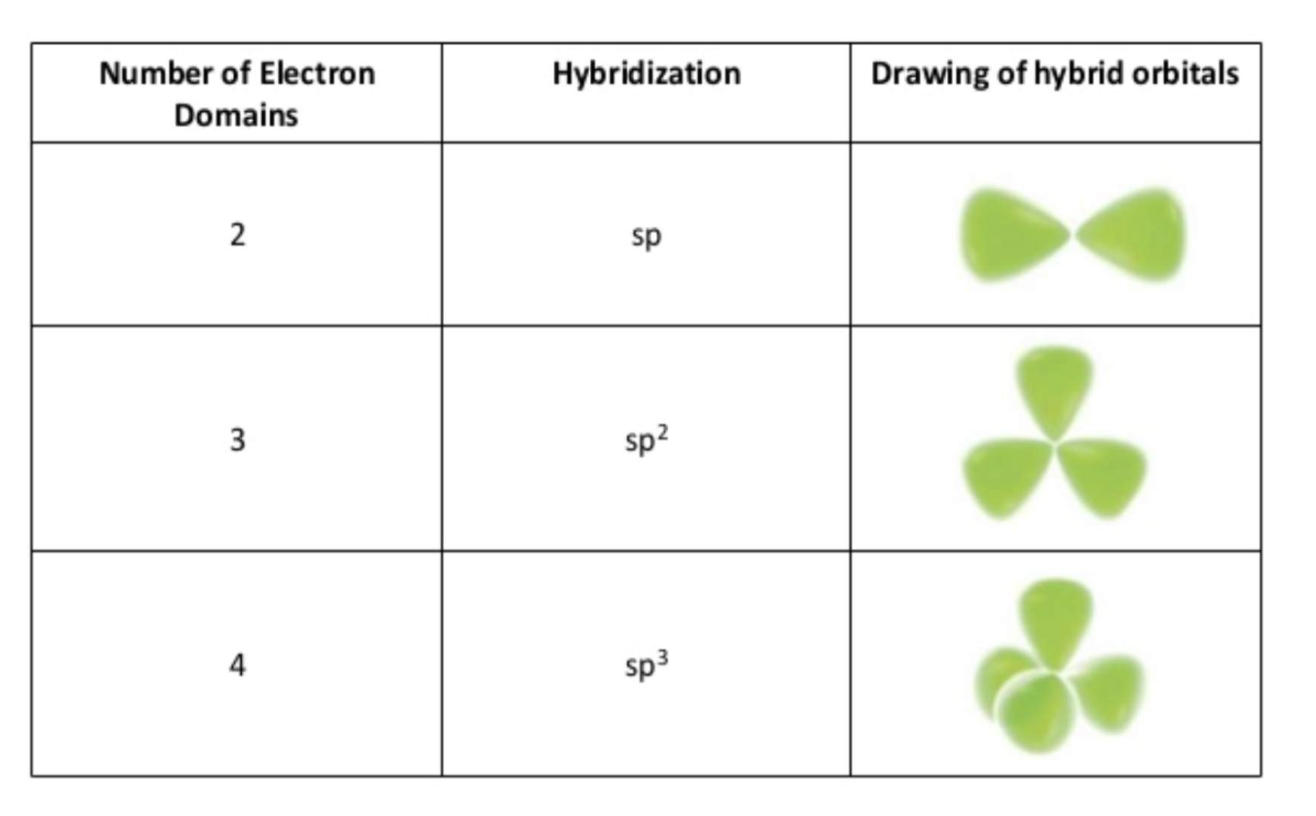
The Carbon Atom: Bonding and Shape
Learning outcomes
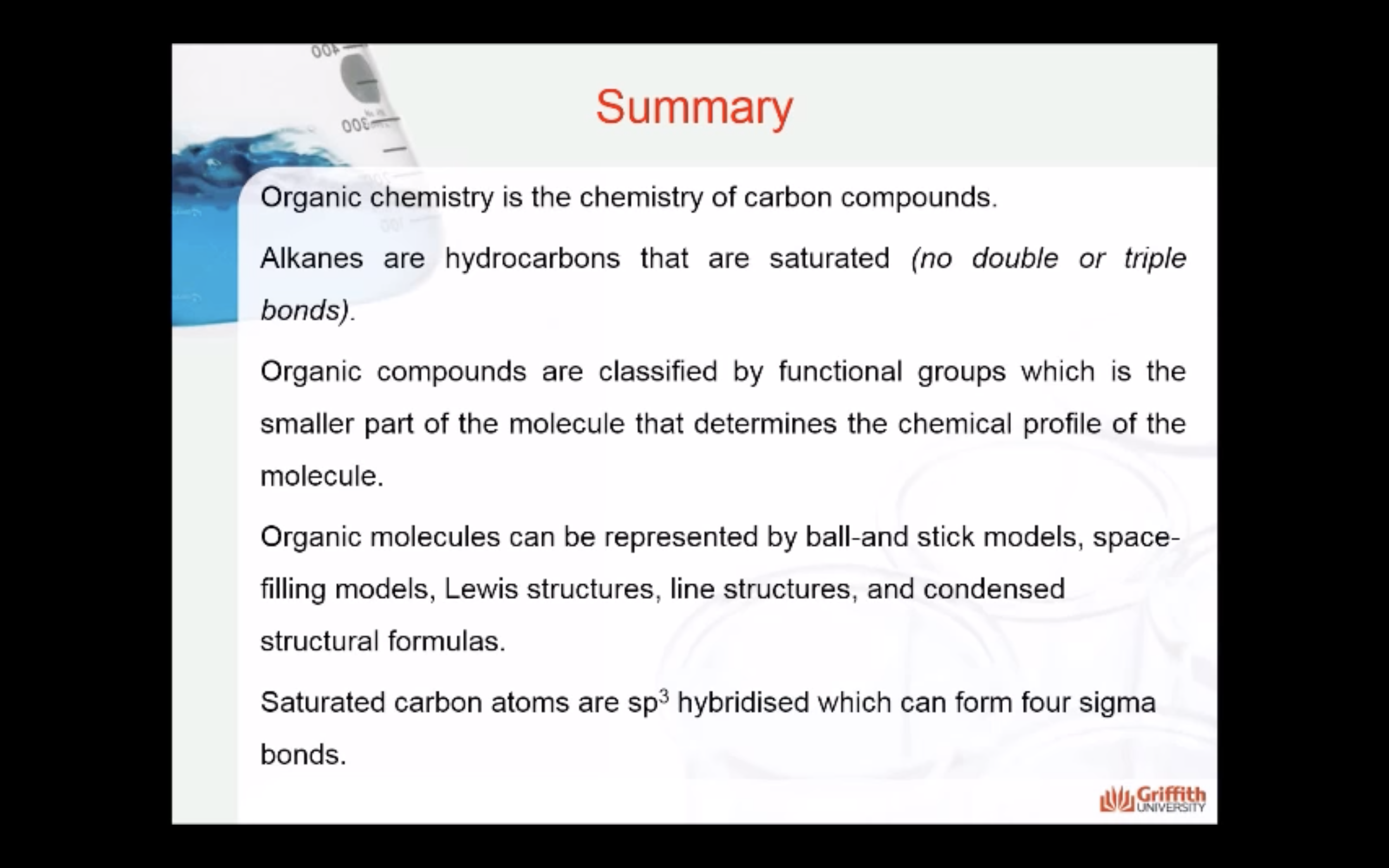
- Know that organic chemistry is the chemistry of carbon compounds and that the bonding in organic compounds is mostly covalent
- Have a basic understanding of typical properties of organic, compared to inorganic compounds
- Be aware of the concept of functional groups in organic chemistry
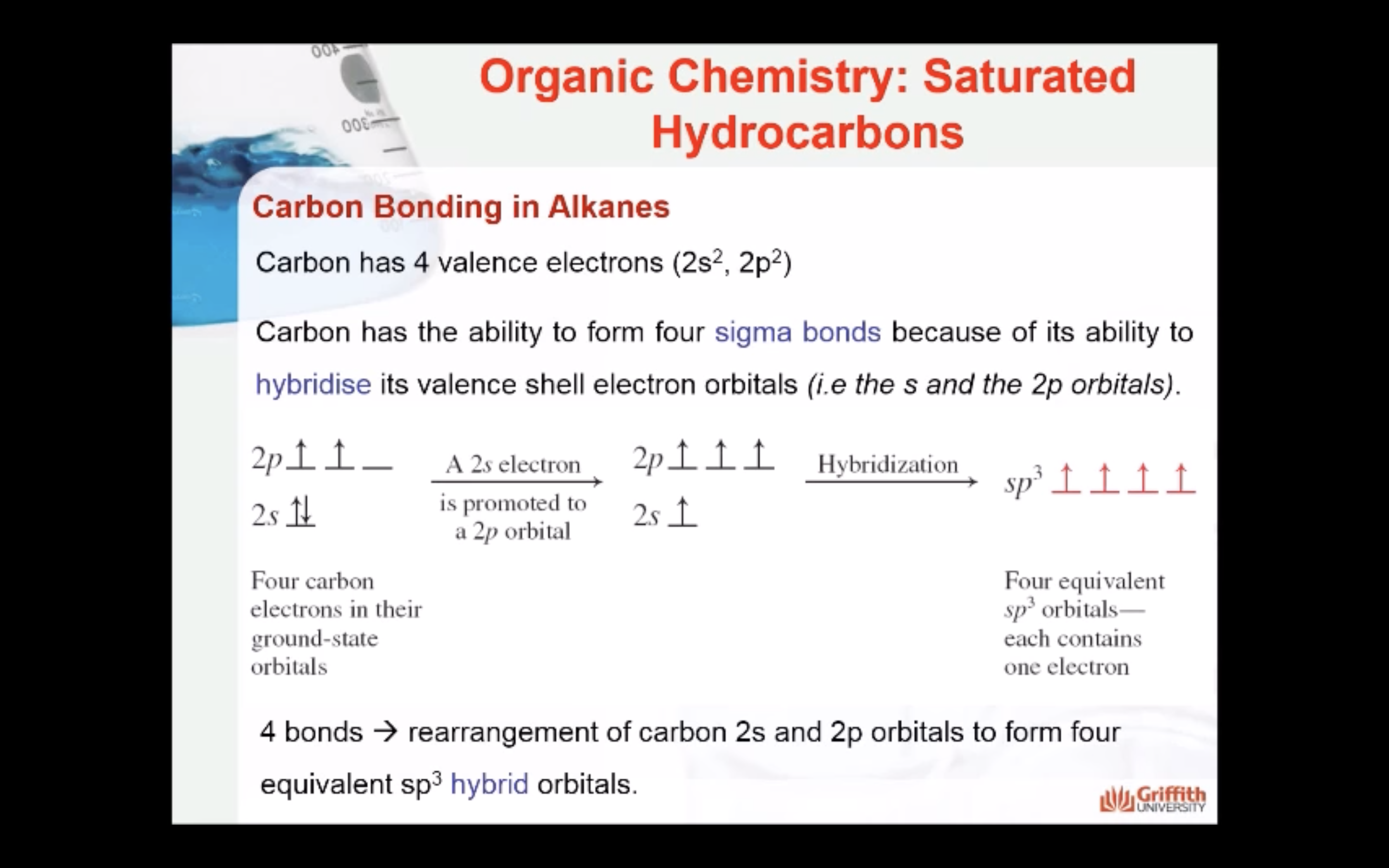
- Understand in general terms why carbon bonding is covalent
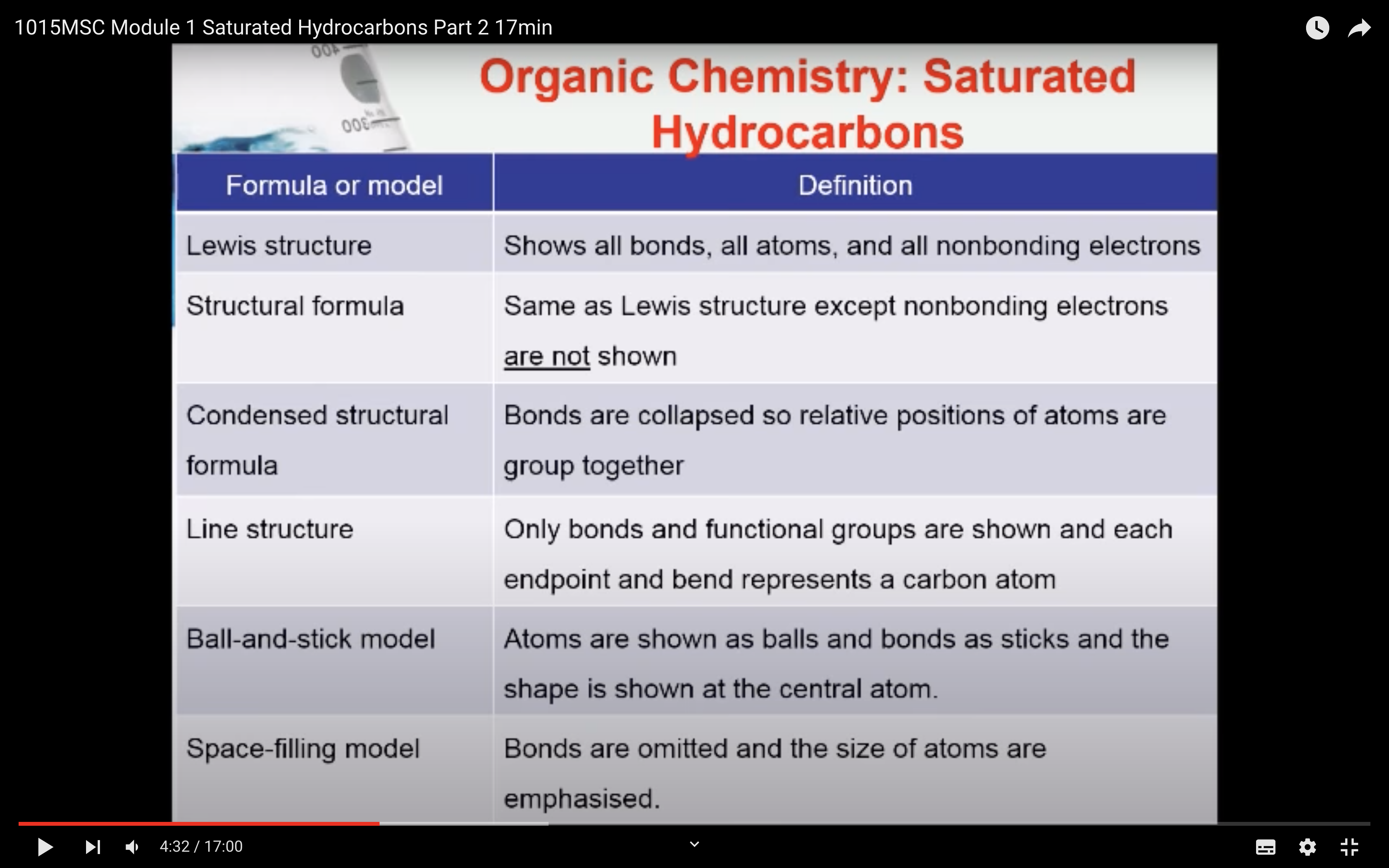
- Have an understanding of the distinctions between empirical, molecular and structural formulae of organic compounds
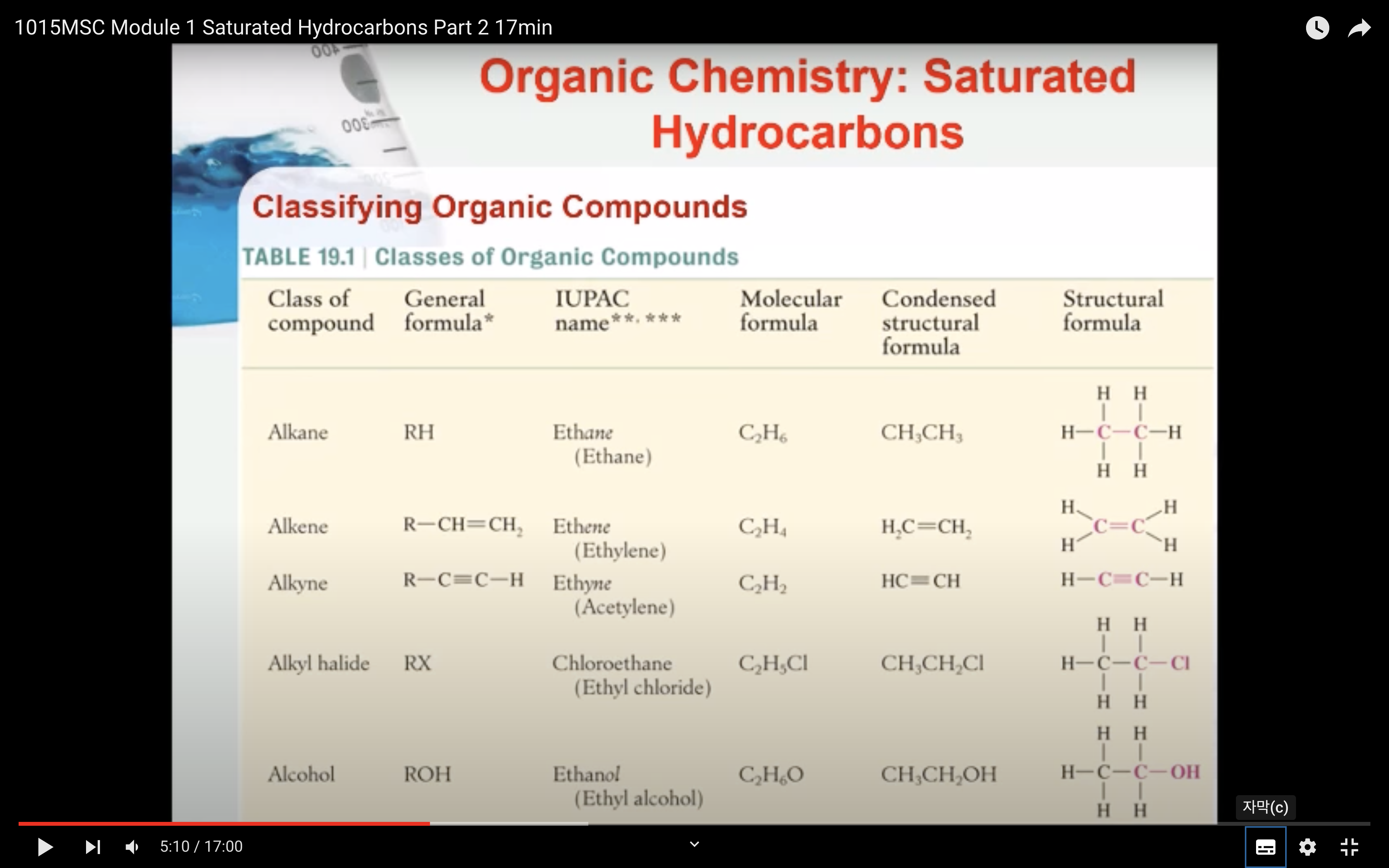
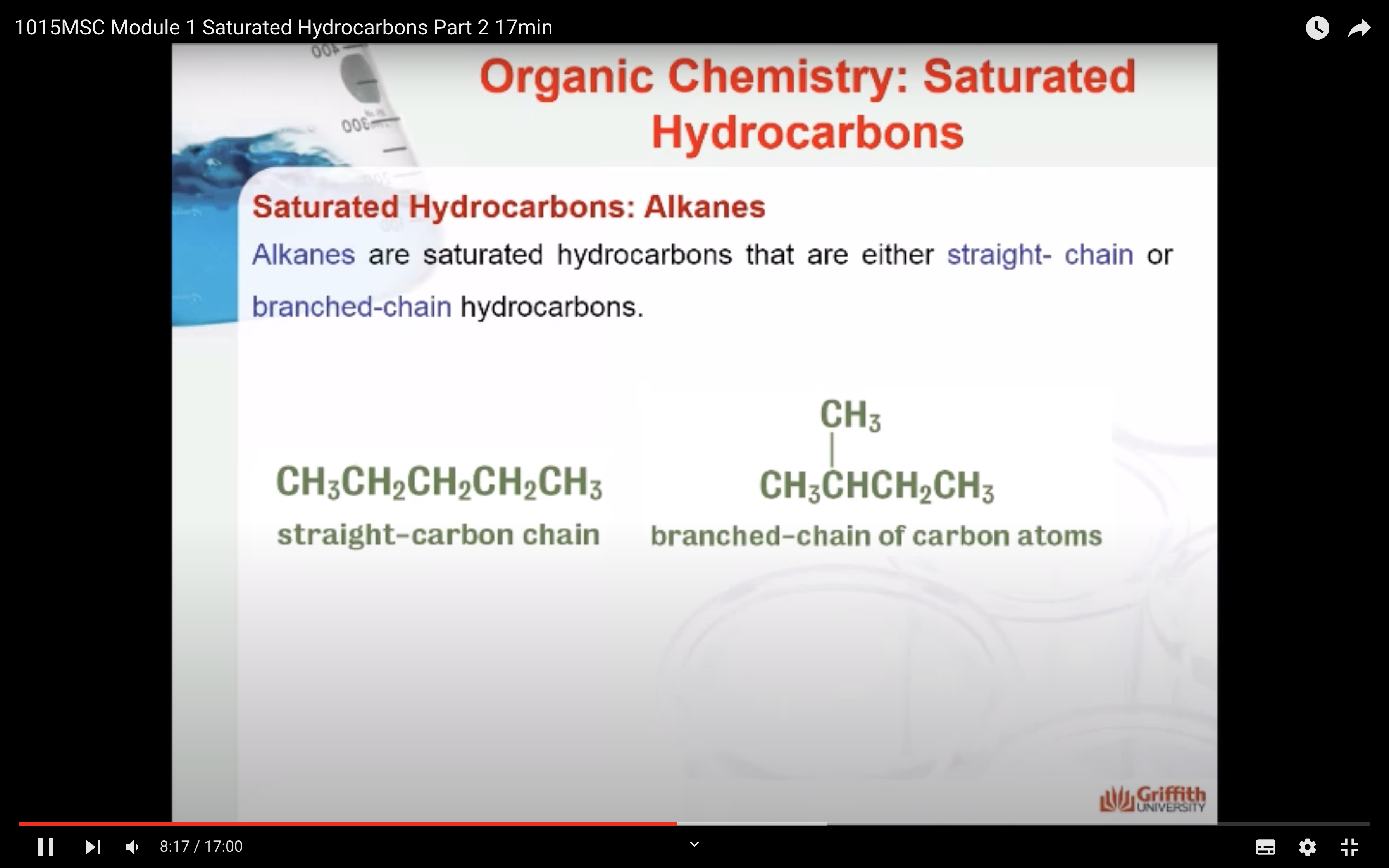
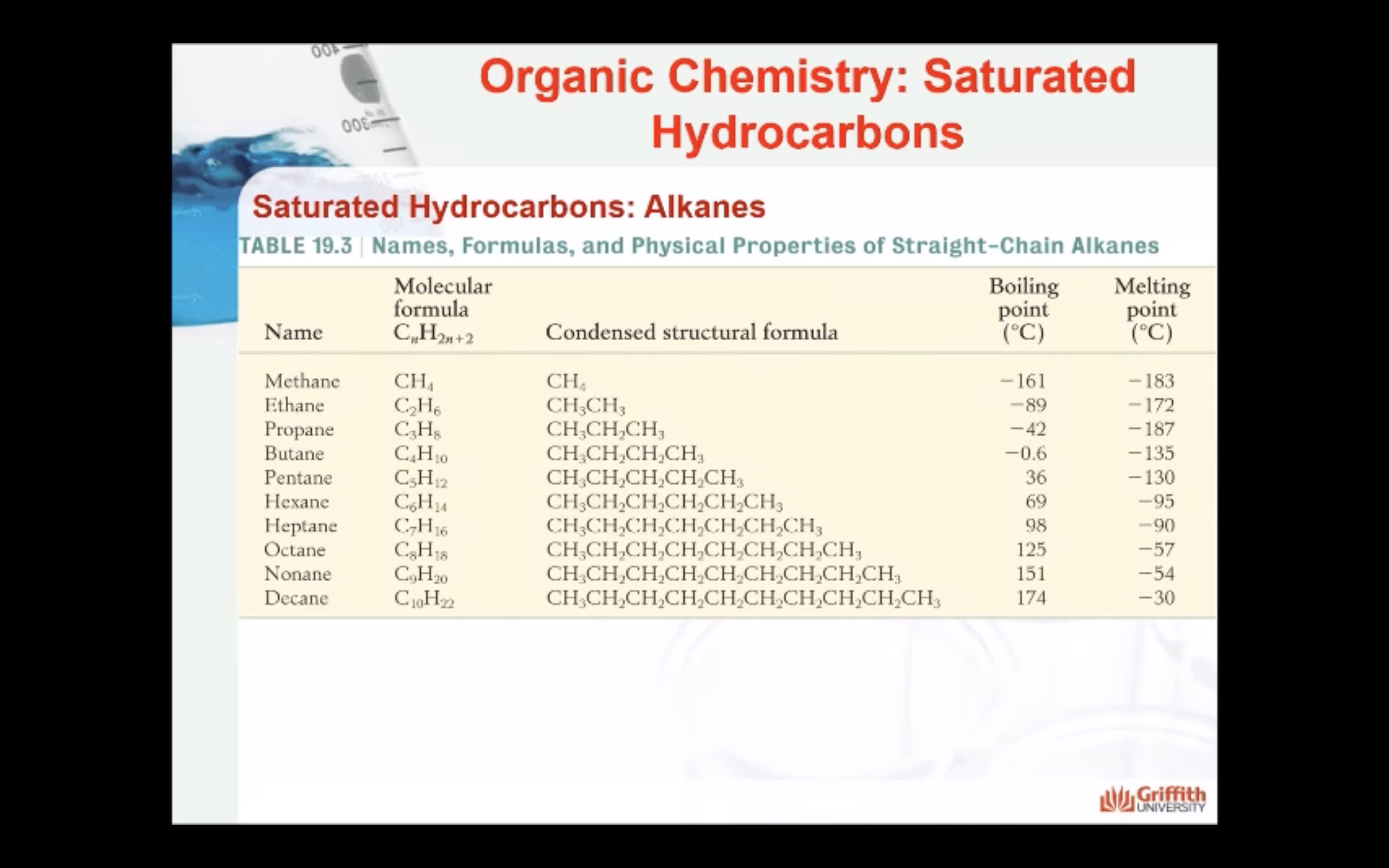
- Know the different sub-groups of hydrocarbon compounds
- understand the general formula of the alkane homologous series
Revision Module

Energy levels of Electrons

The Covalent Bond : Sharing Electrons

Electronegativity, Bond Polarity and Dipole
When two different kinds of atoms share a pair of electrons, a bond forms in which electrons are shared unequally.
This results in one atom having a partial positive charge and the other negative with respect to each other.
This difference occurs because the two atoms exert unequal attraction for the pair of shared electrons.
The attractive force that an atom of an element has for shared electrons is known as its electronegativity.
A partial positive charge is represented by δ+ and a partial negative charge by δ–.




You can predict whether bonding in binary compounds will be ionic or covalent based on:
- The elements involved. Metal/nonmetal will generally be ionic; two nonmetals will generally be covalent.
- Difference in electronegativity. Greater than 1.7-1.9 will generally be ionic, less than that will generally be covalent.
Predict whether bonding in the following compounds will be ionic or covalent. Drag the card at the top to the correct pile below.
Lewis Structures of Compounds
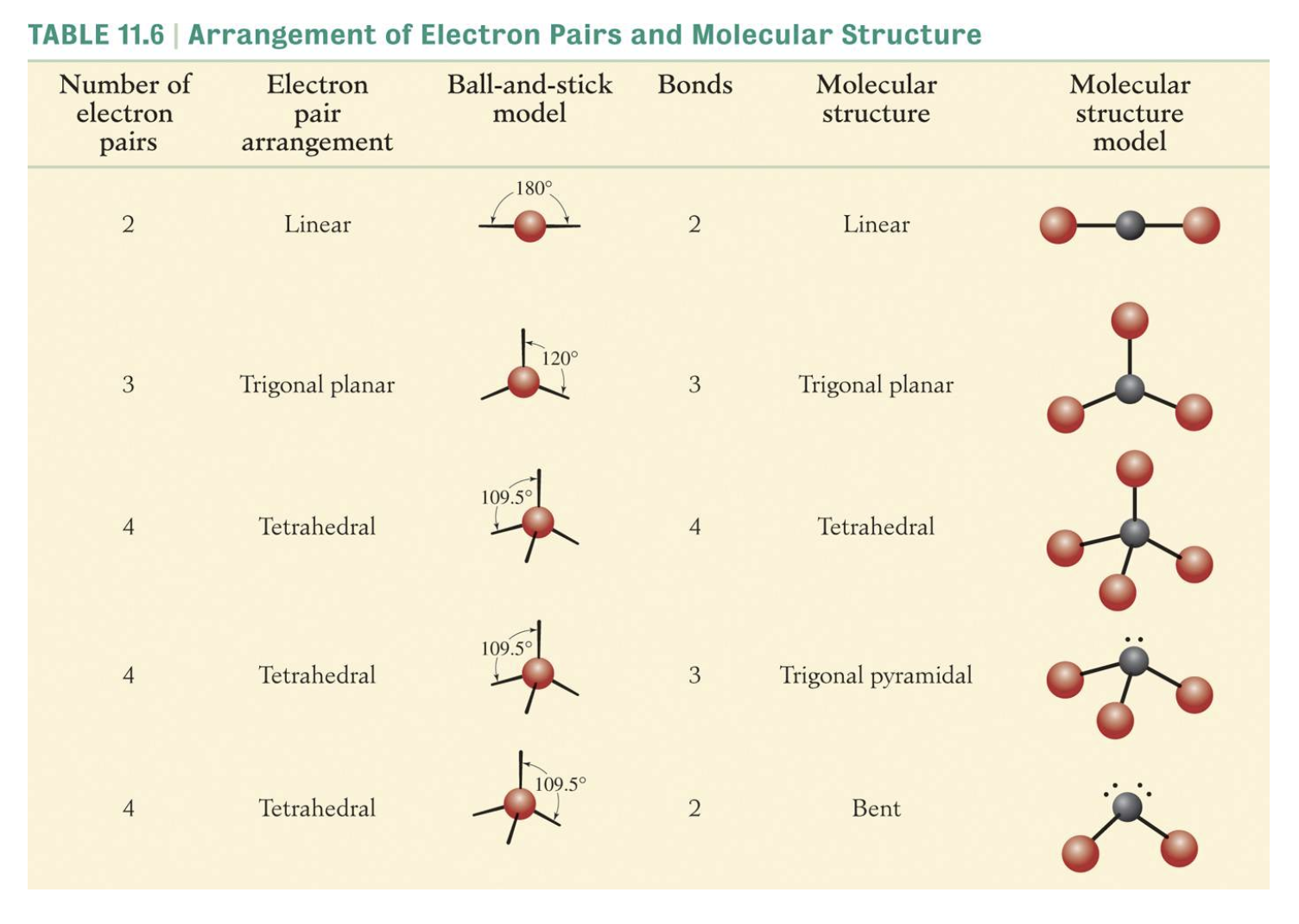
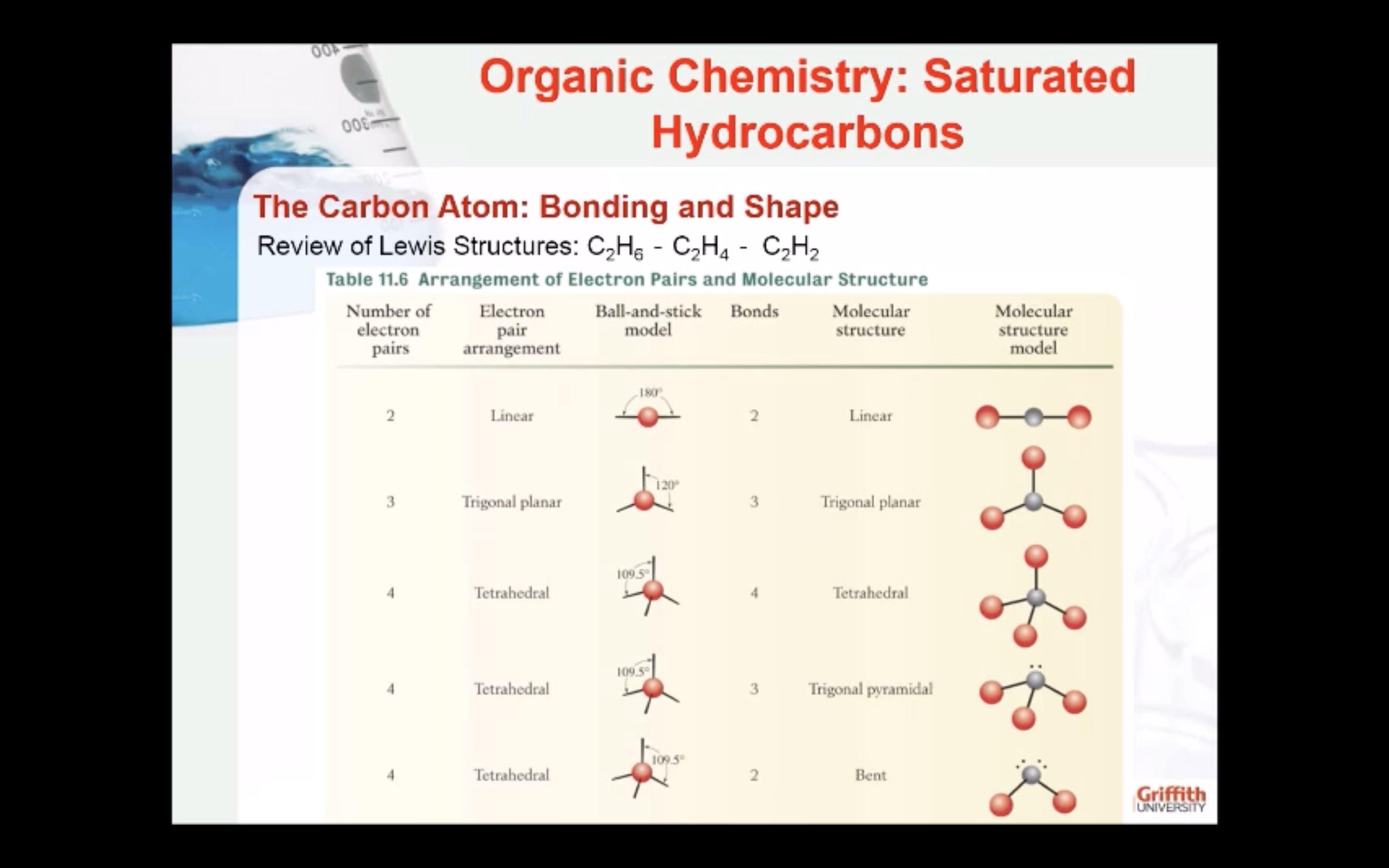
Molecular shape and VSEPR model
The Valence Shell Electron Pair Repulsion (VSEPR) Model is based on the idea that an electron pair will repel each other electrically and will seek to minimise this repulsion.
The electron pairs will be arranged around a central atom as far apart as possible.
(In order of stronger bonding)
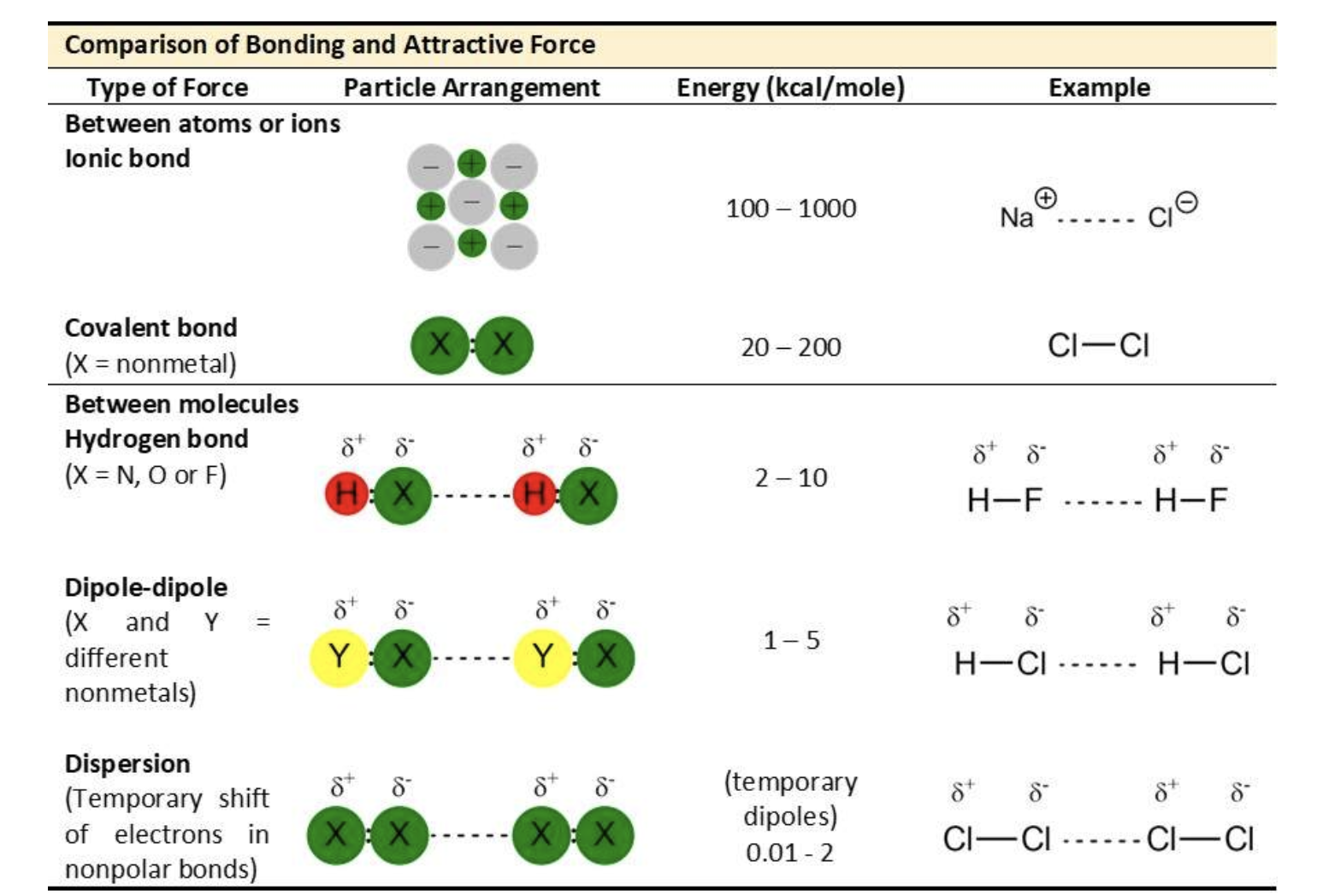
Ion-Ion
Ion-Dipole
Dipole-Dipole
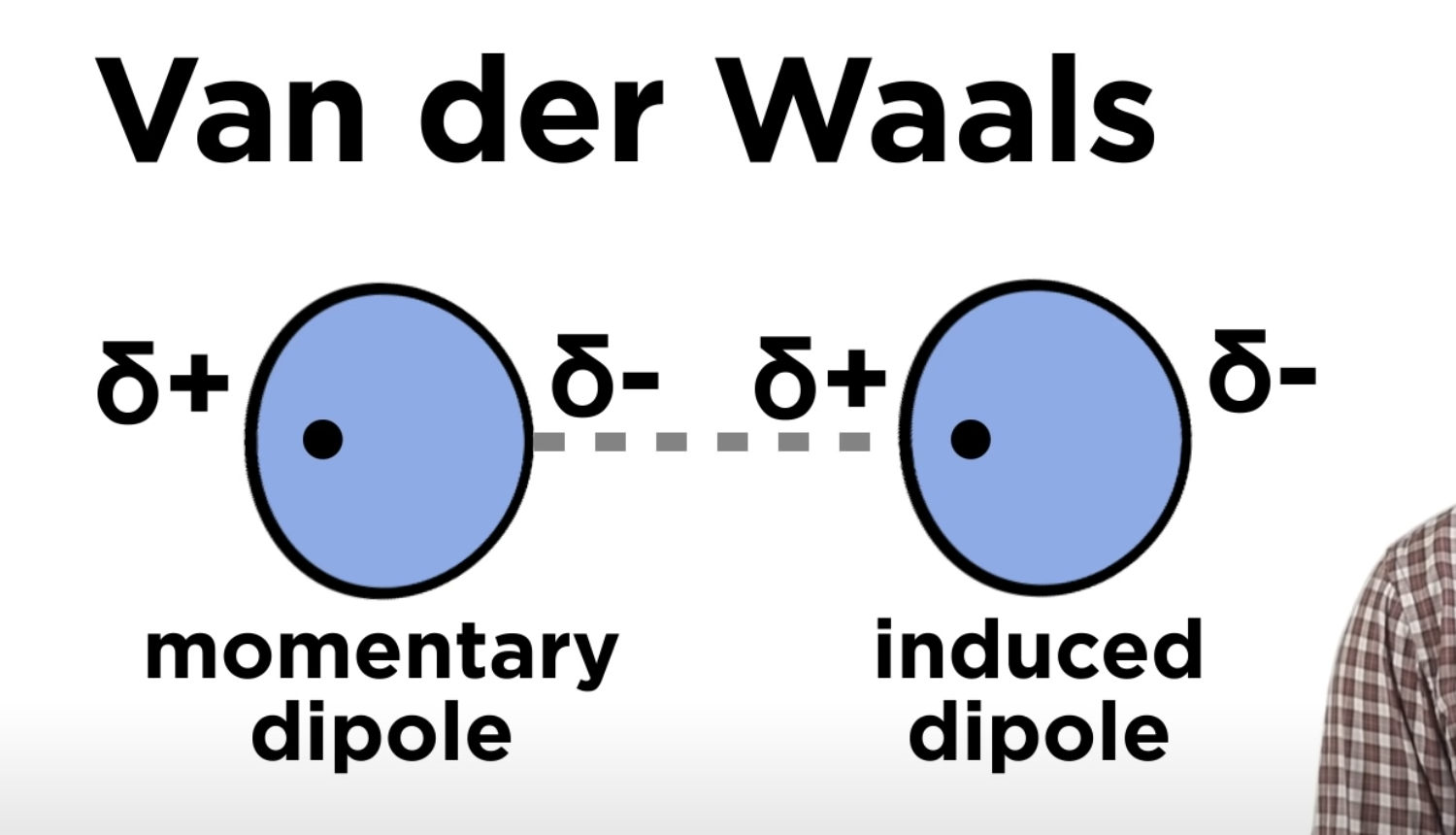
Van der Waal's (London dispersion)
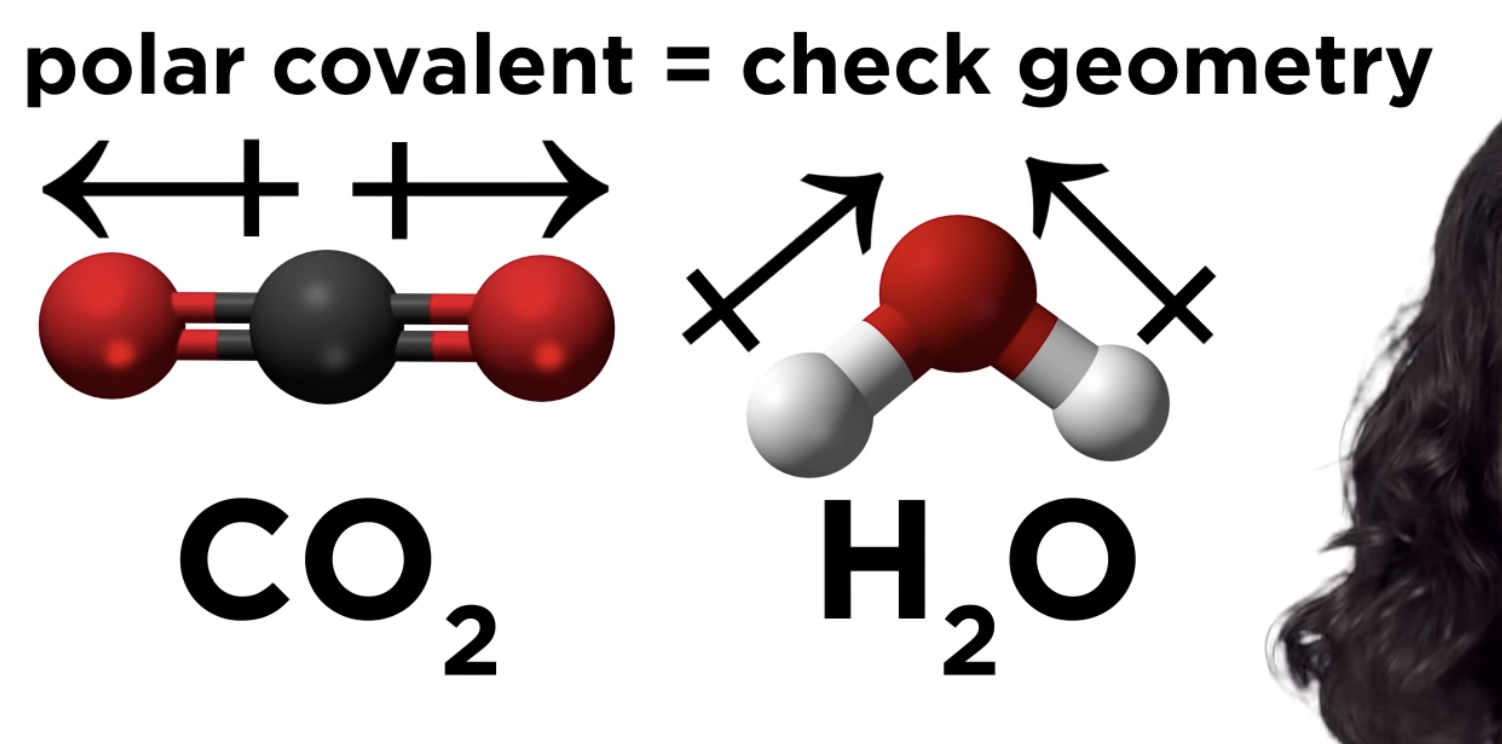
Polar Covalent pond -> check geometry
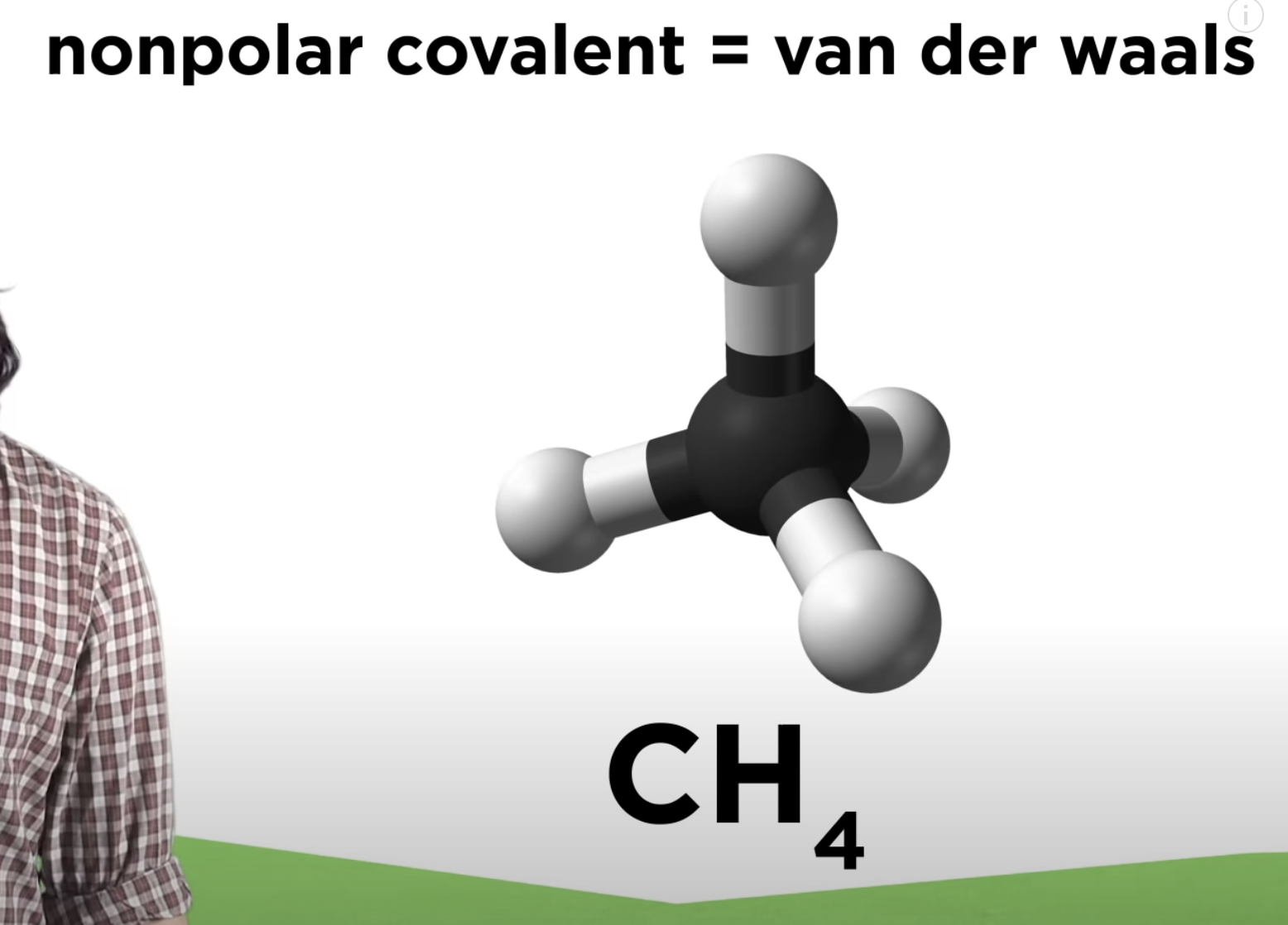
-If that polar covalent is non polar -> only van der waals
-If that polar covalent is polar -> dipole-dipole

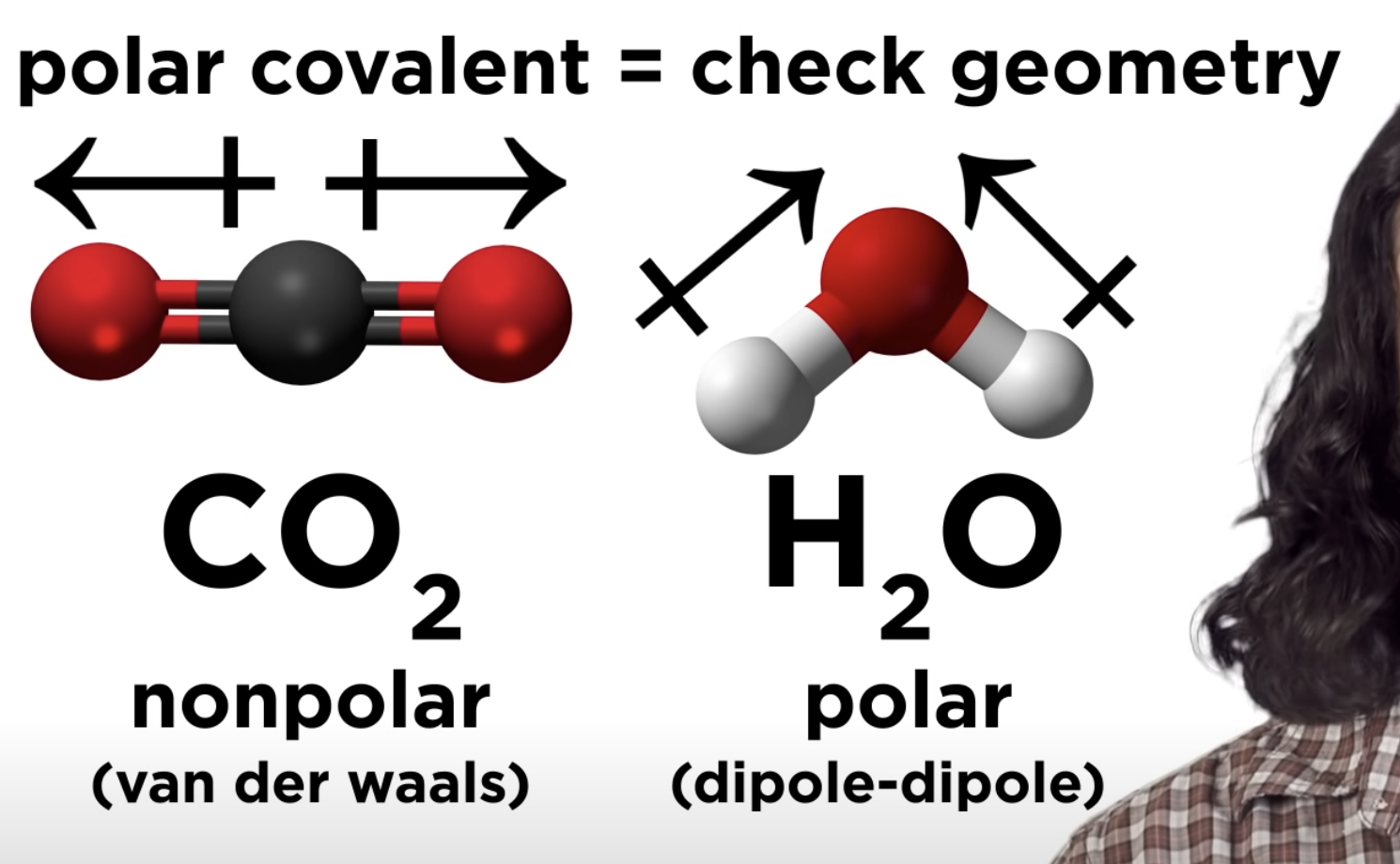
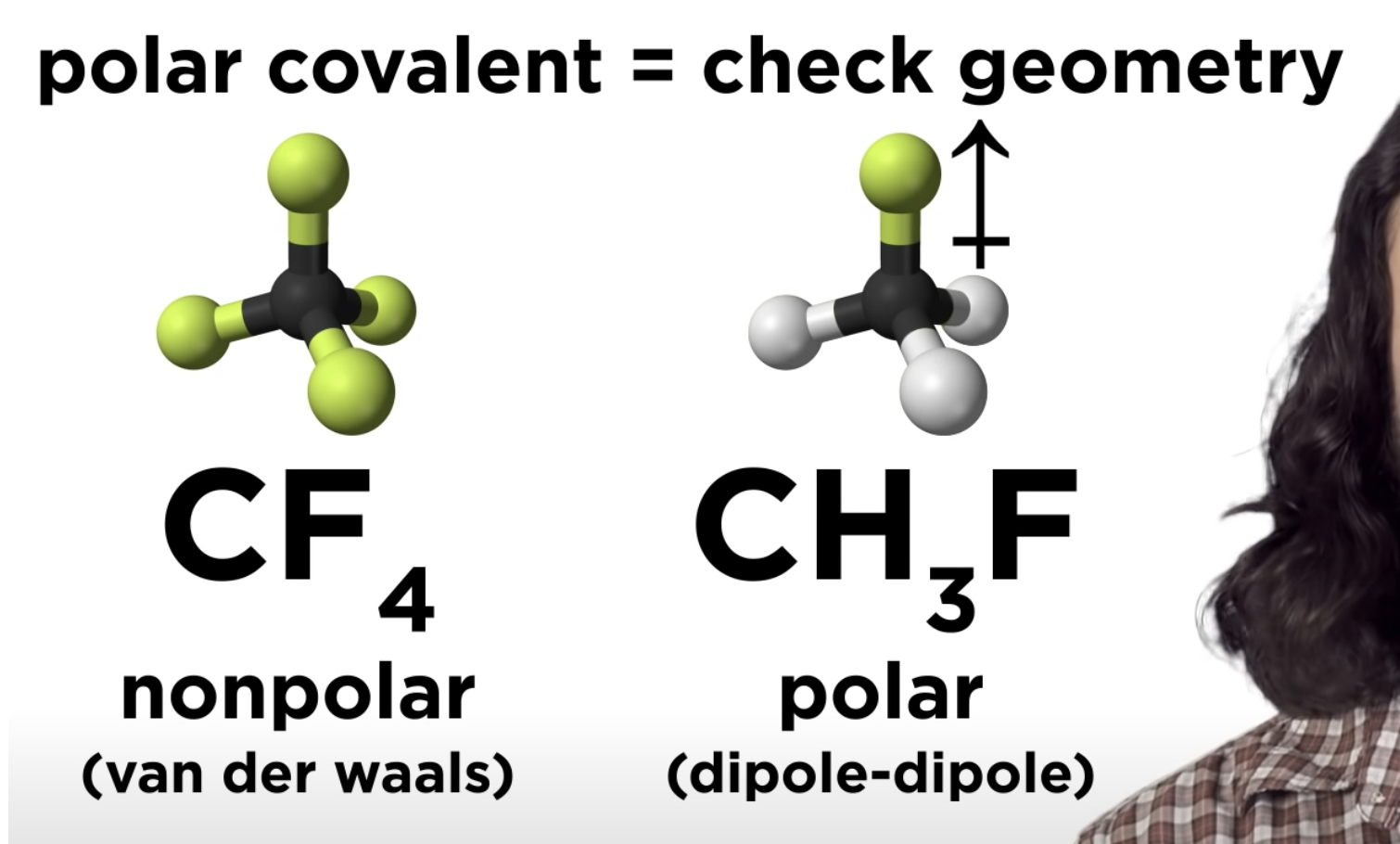
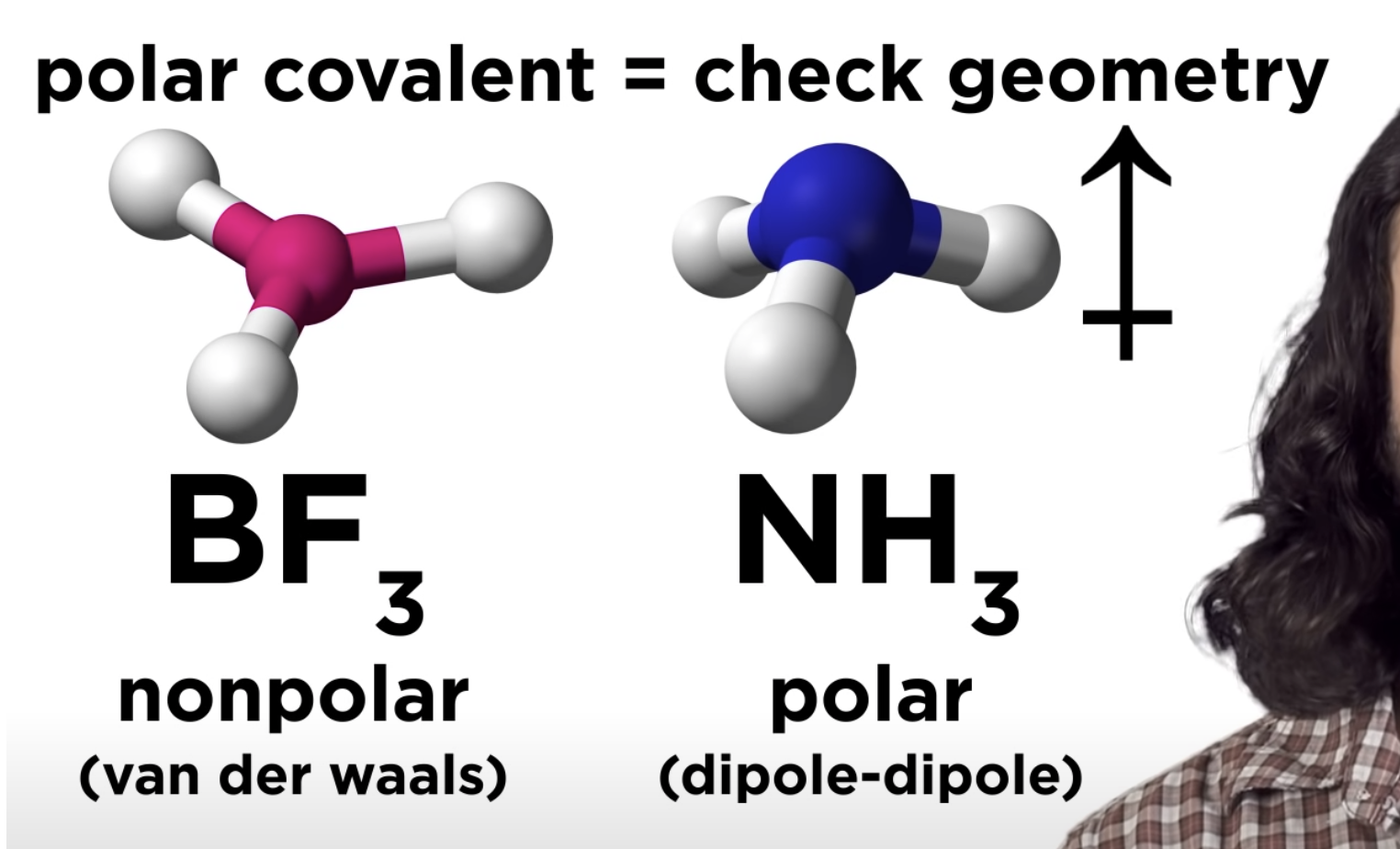
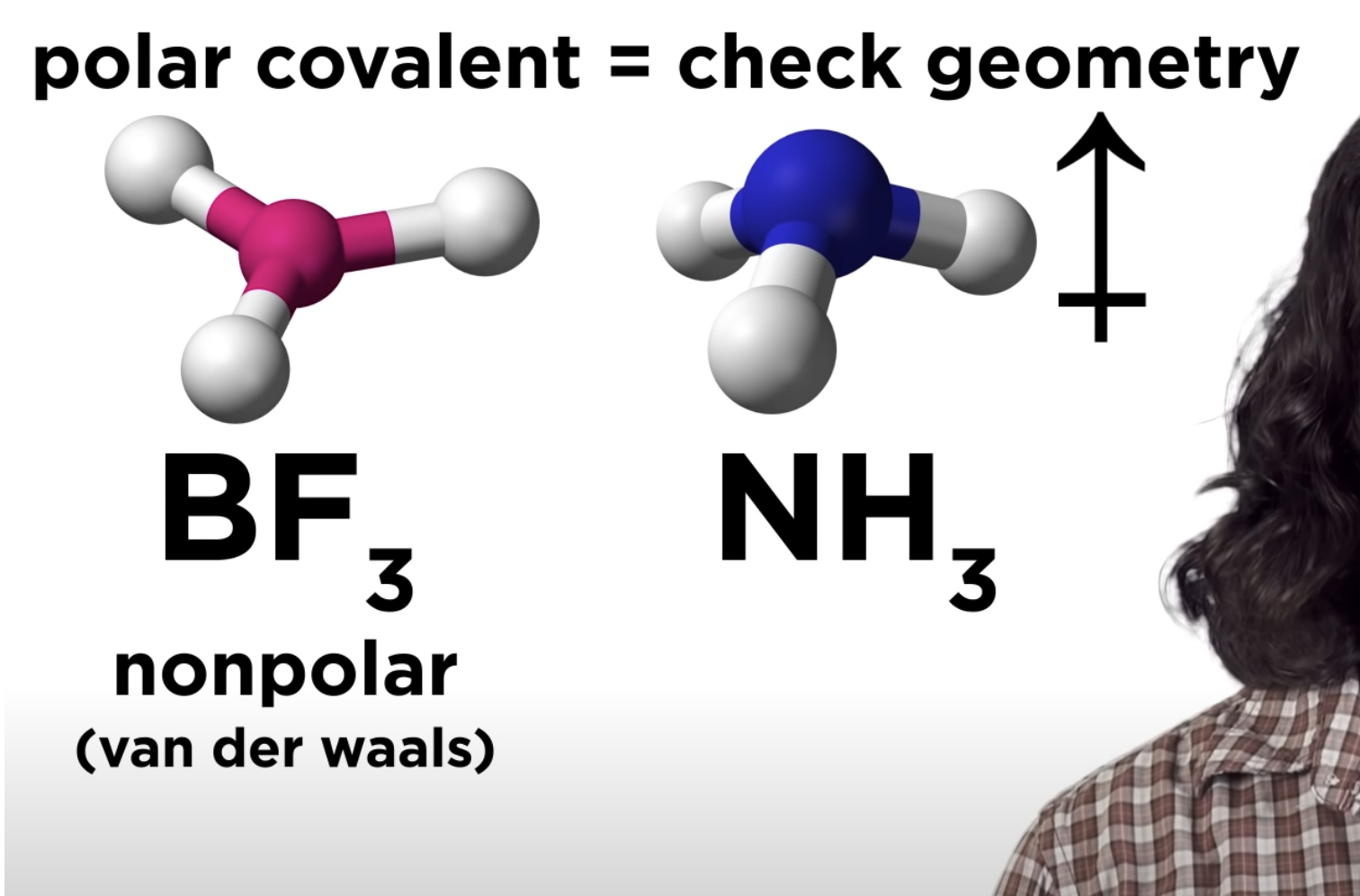
Intermolecular Forces

Perhaps the most important noncovalent interaction in biological molecules is the hydrogen bond. A hydrogen bond is in attractive interaction between a hydrogen atom bonded to an electronegative atom (fluorine, oxygen or nitrogen) and an unshared electron pair on another electronegative atom. This attraction creates a noncovalent "bridge" between them. It is, in essence, a very strong dipole-dipole interaction.
These hydrogen bonds are more like intermolecular attractions than true bonds. Hydrogen bonds are weak compared to ionic and covalent bonds. They do not bind atoms into molecules, but rather they establish important links between molecules such as H2O, or between different parts of a large molecule, such as a protein or nucleic acid.
Even though single hydrogen bonds are weak, large molecules such as proteins may contain hundreds of these bonds. Collectively, these hydrogen bonds provide considerable strength that maintains the three-dimensional shape of large molecules. As we will see, the structure of a molecule determines its function.

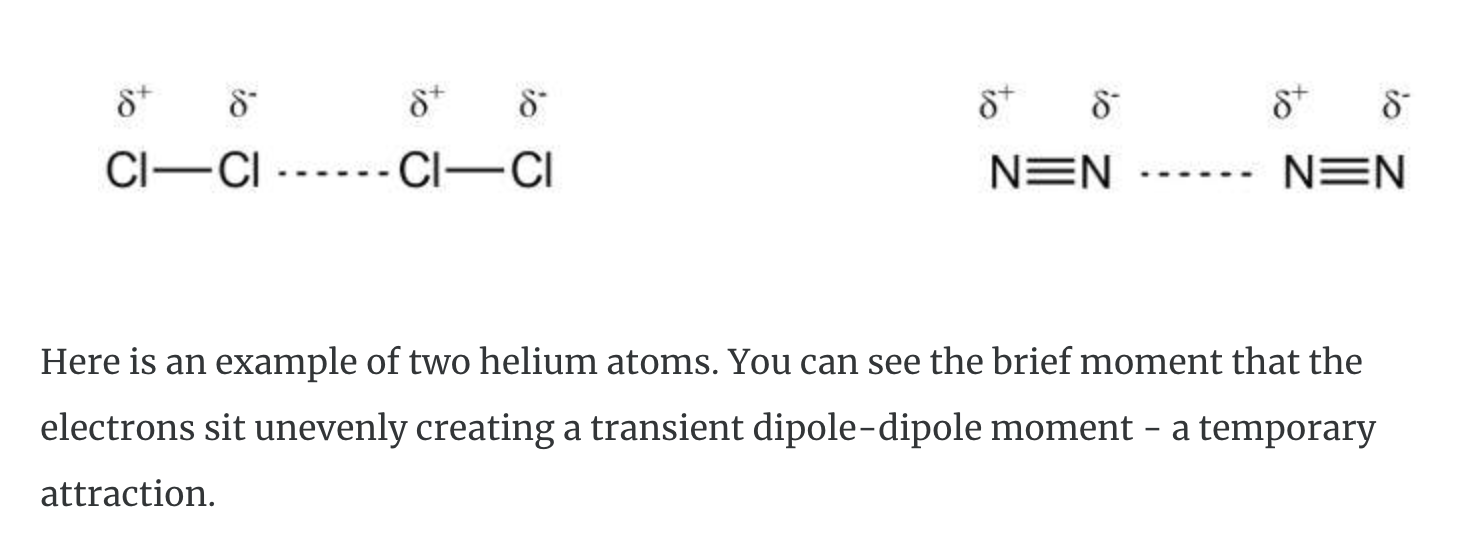
We have learned that some covalent molecules have dipoles. That means this kind of covalent bond has a separation of positive and negative charges. The attractive forces between polar molecules, called dipole-dipole attractions hold them together. For example, in a sample of HCl, the delta positive hydrogen atom of one dipole attracts the delta negative chlorine atom in another molecule.

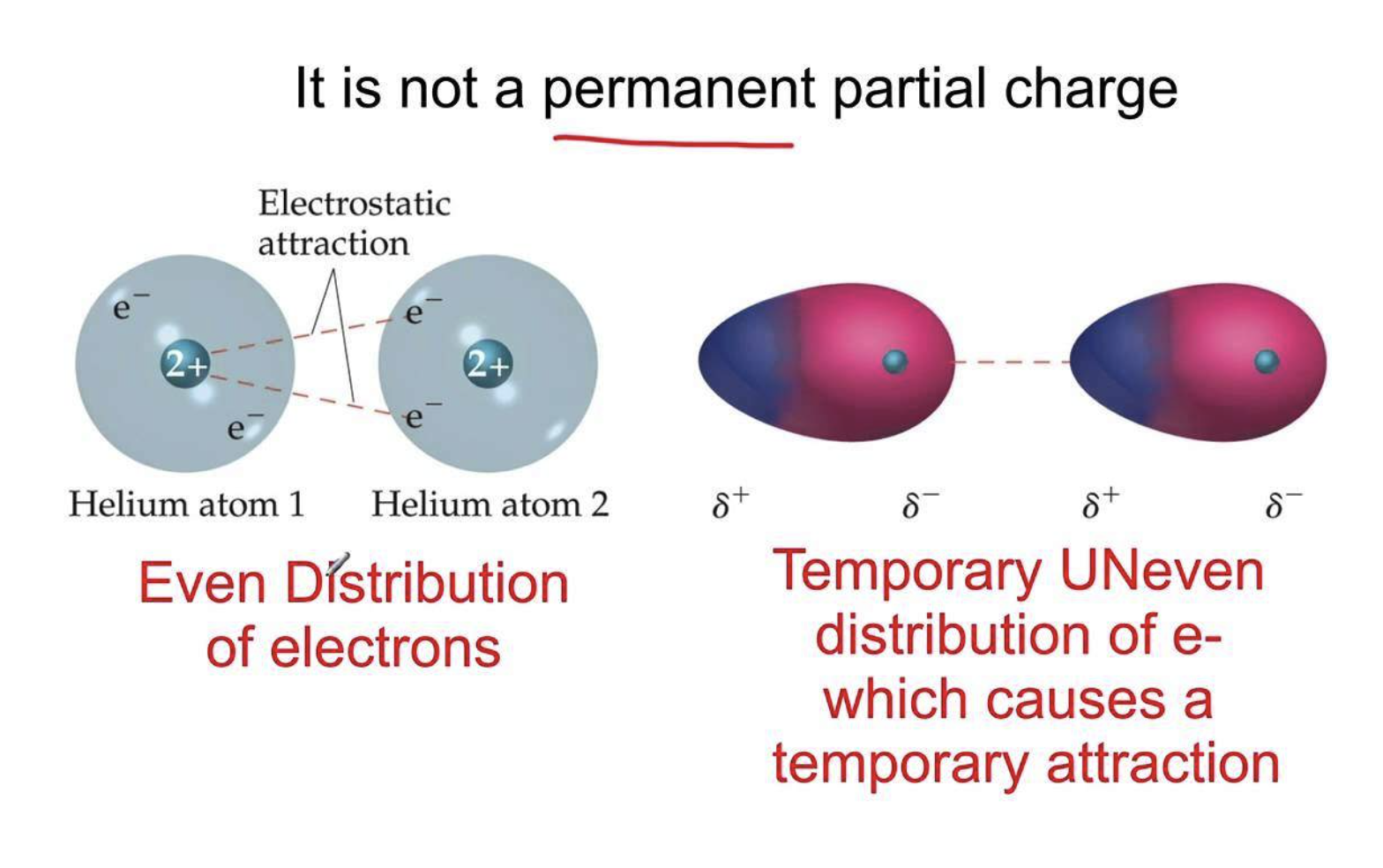
Very weak attractions called dispersion forces (or London Forces) occur between non-polar compounds when more electrons are momentarily present at one end of the molecule to form a temporary dipole. Although these dispersion forces are the weakest of the dipole-dipole interactions, they make it possible for non-polar molecules to form liquids and solids at low temperature.

Reactions of Acids
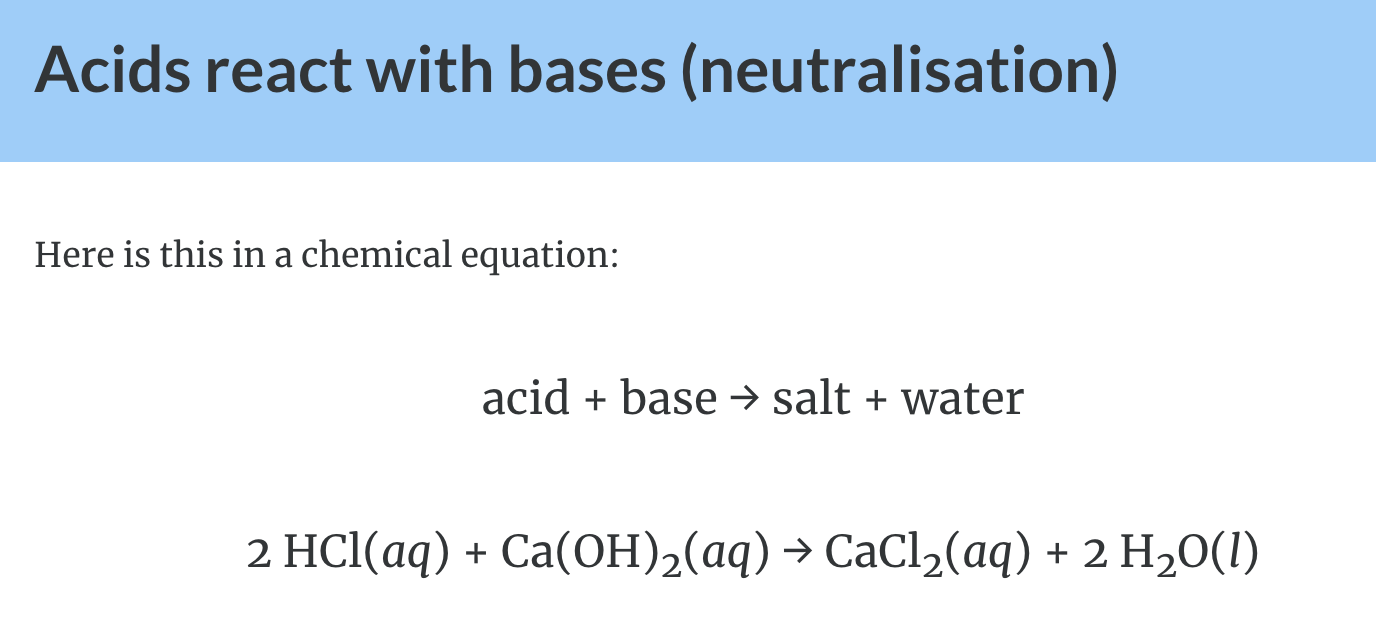
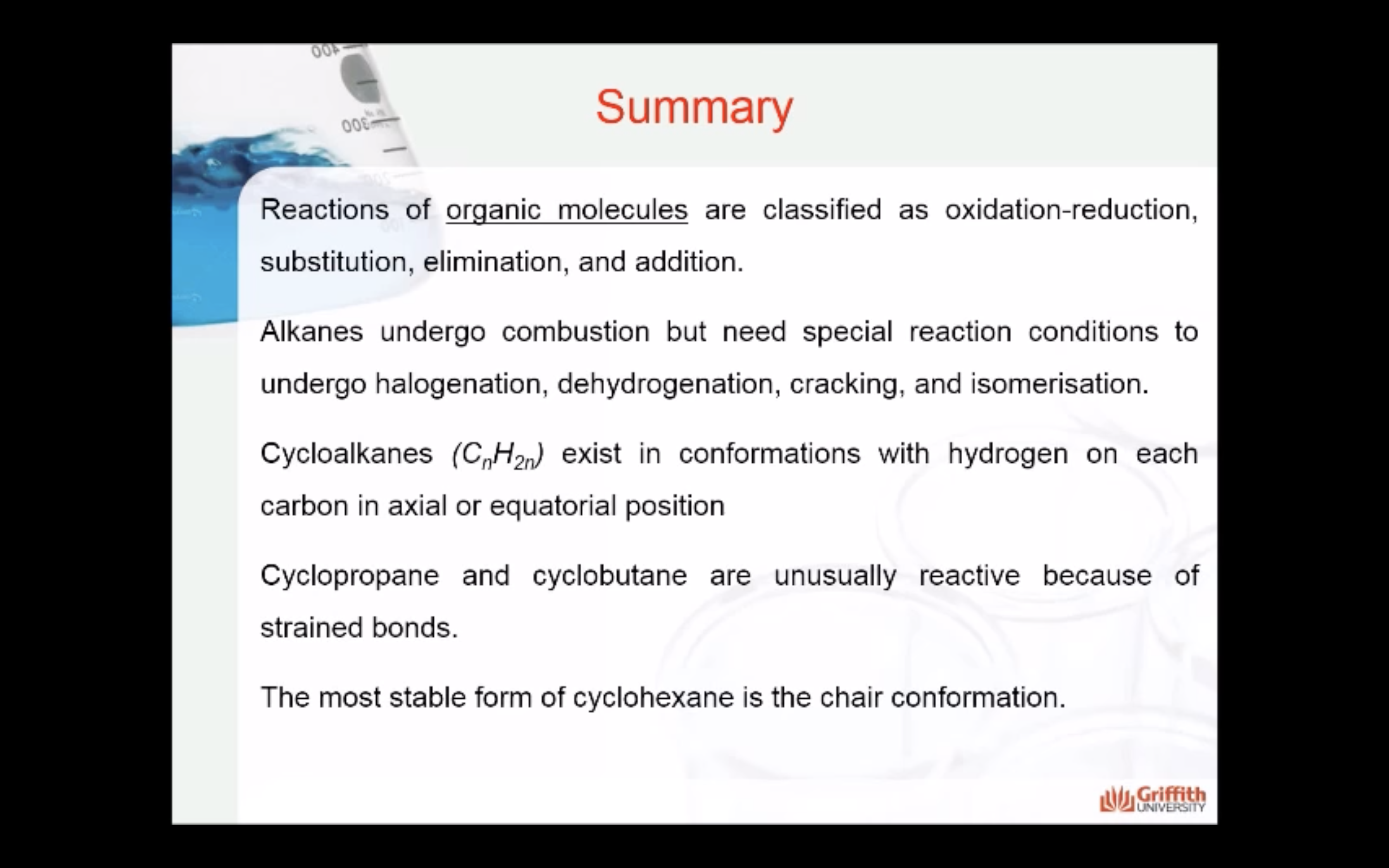
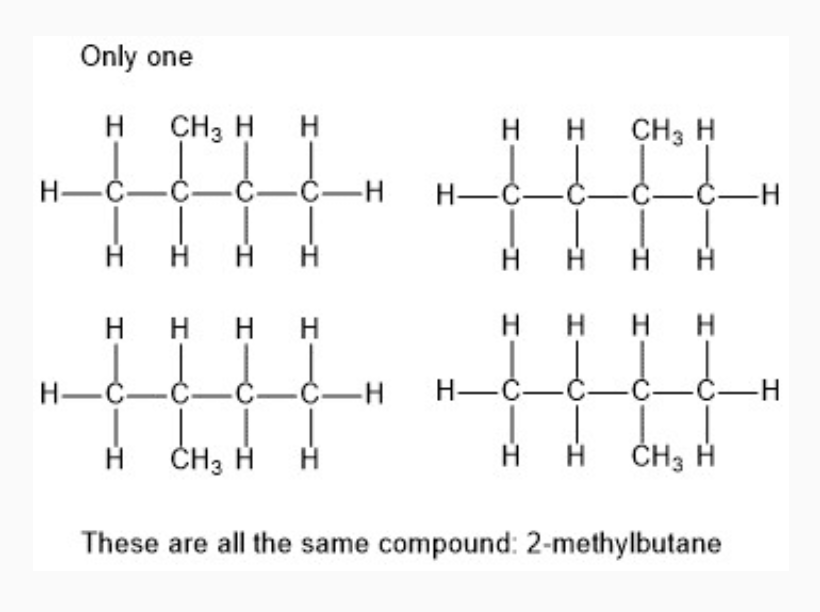
Topic 1 Saturated Hydrocarbons
Mini lecture


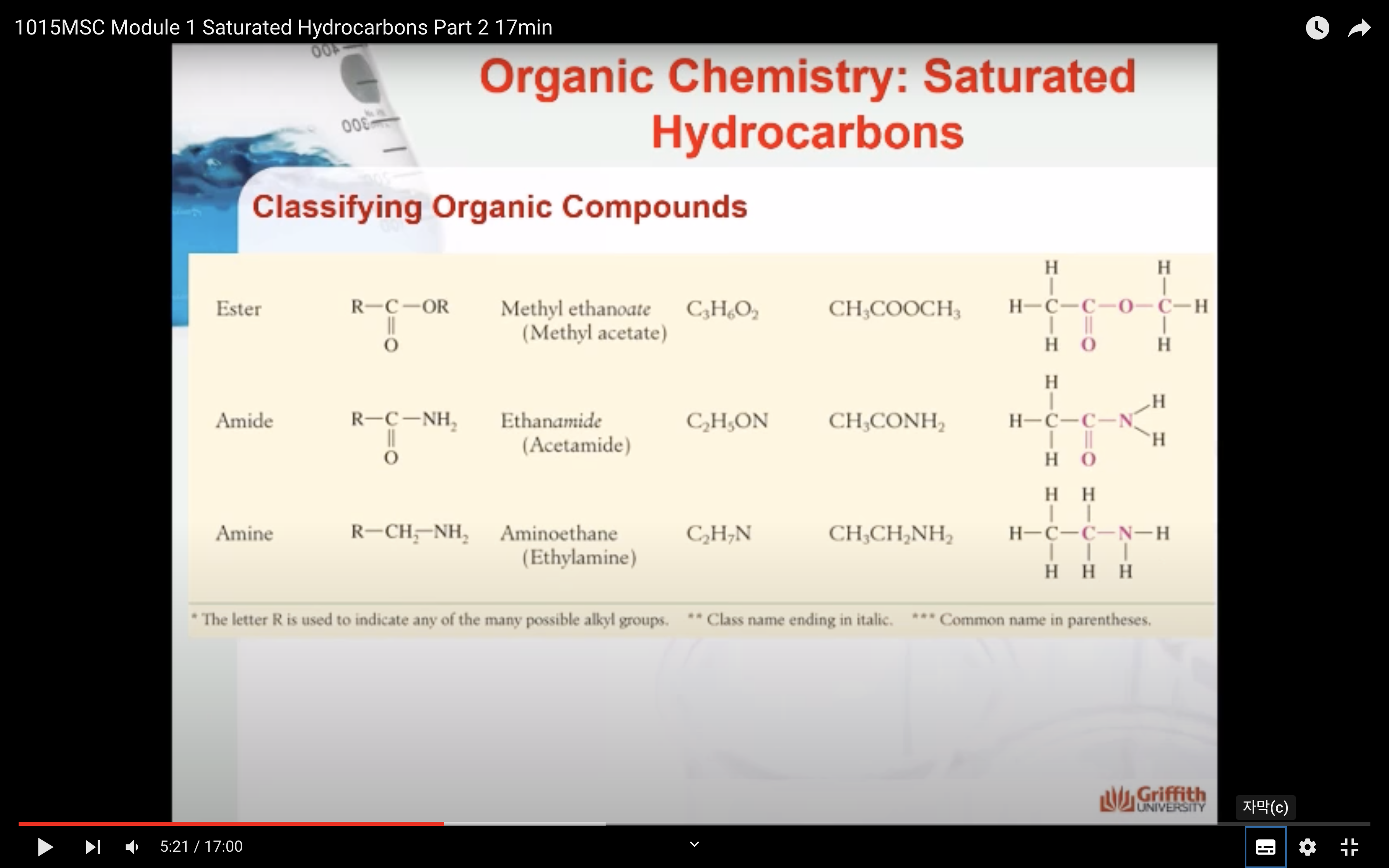
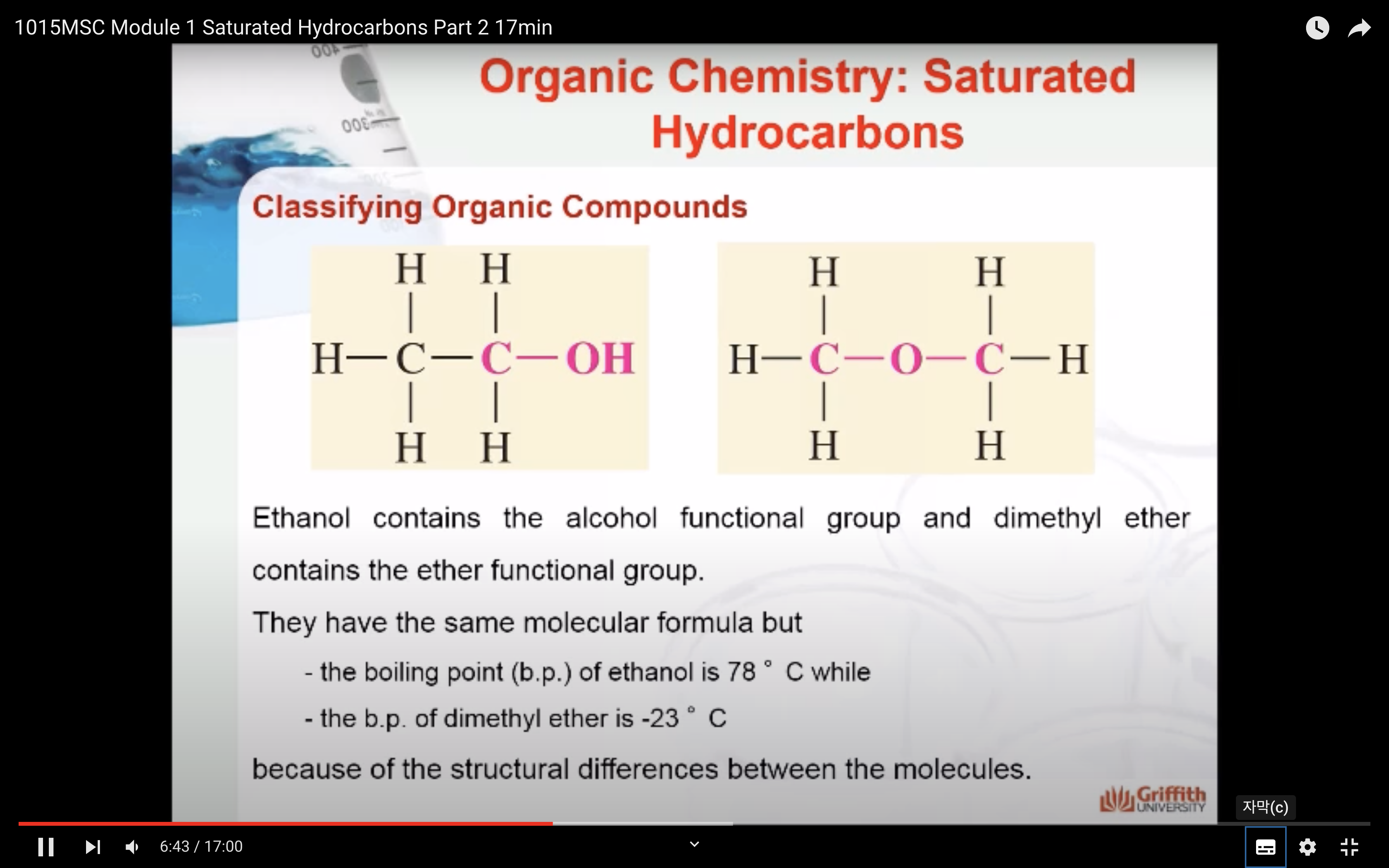
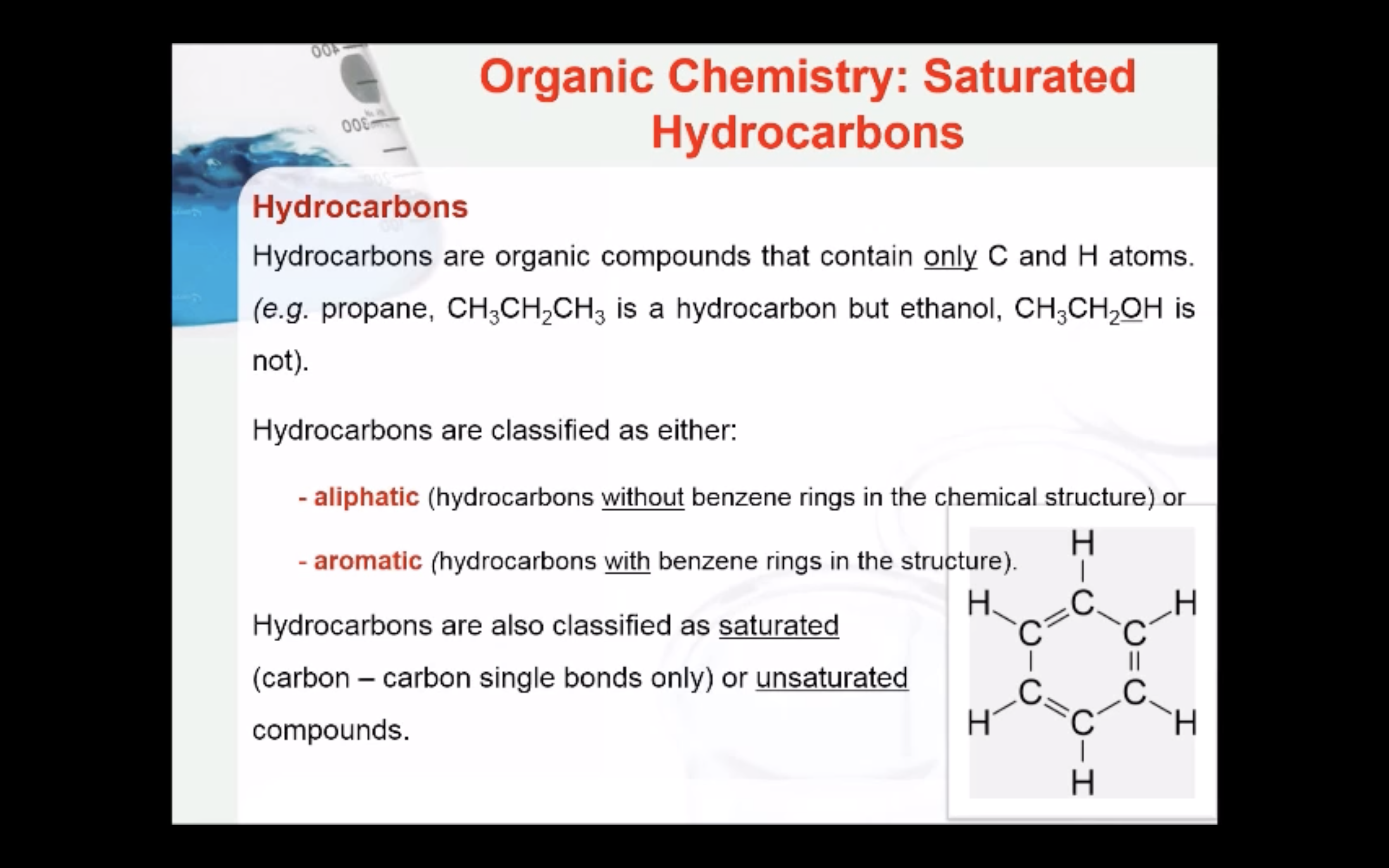
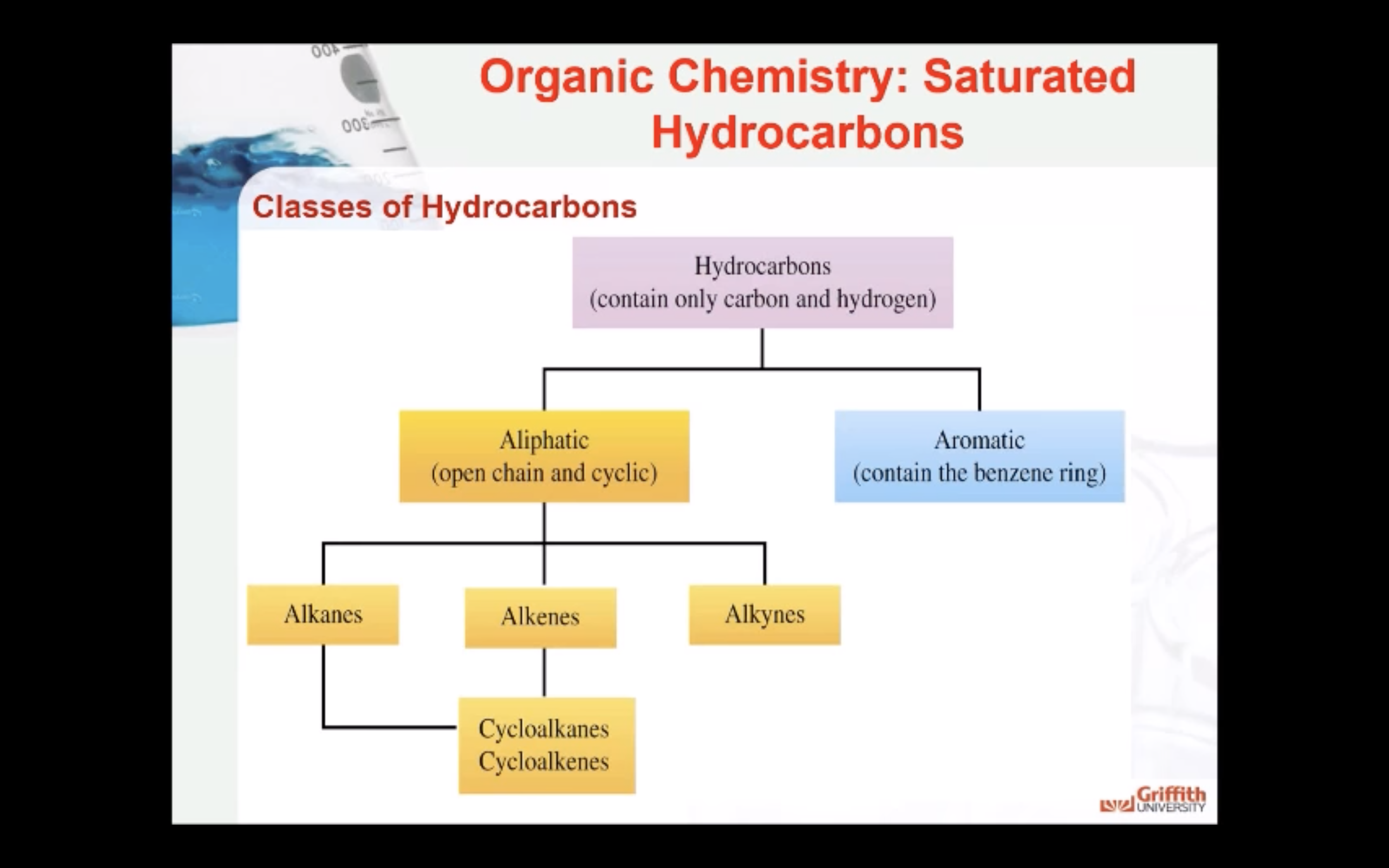
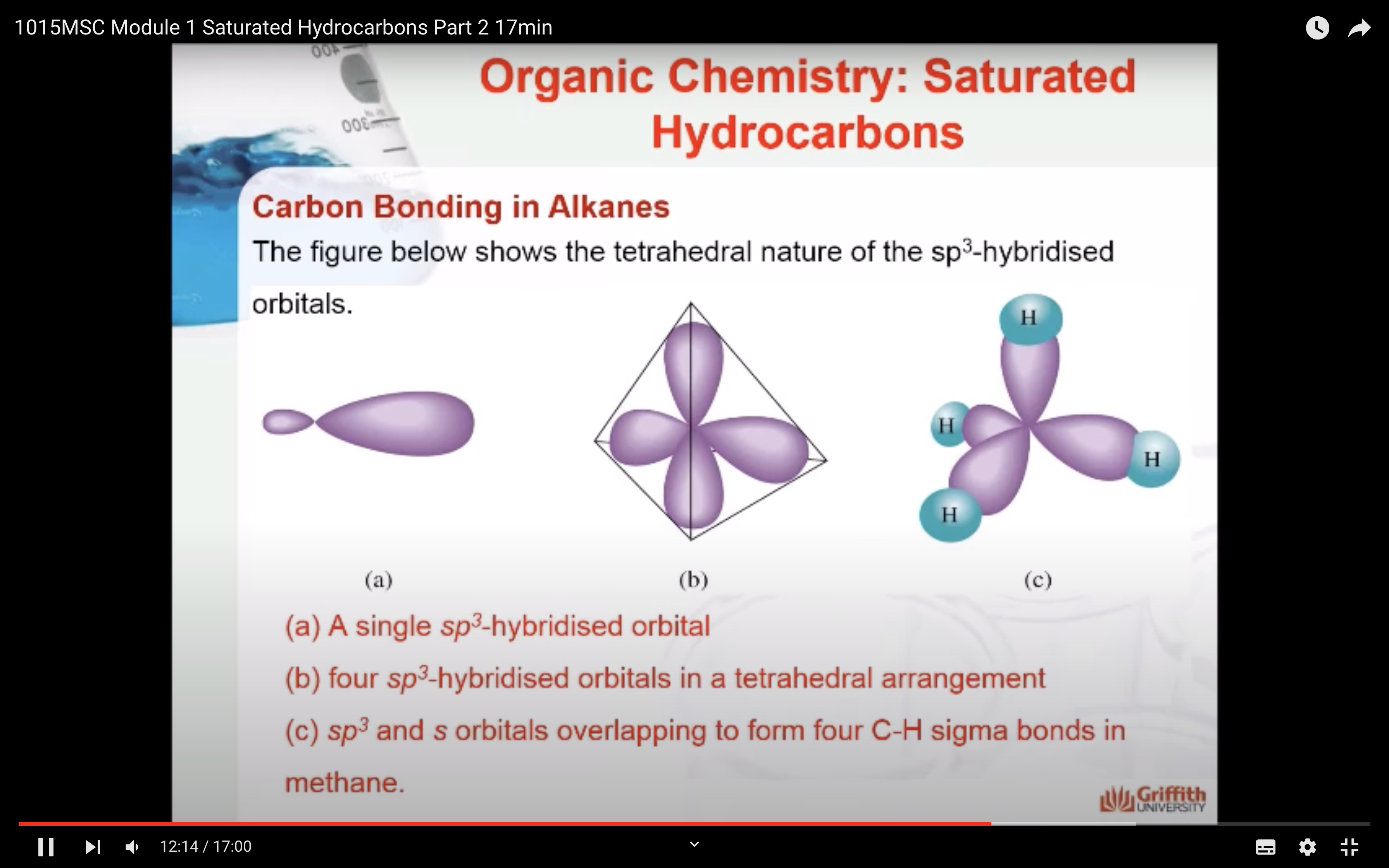
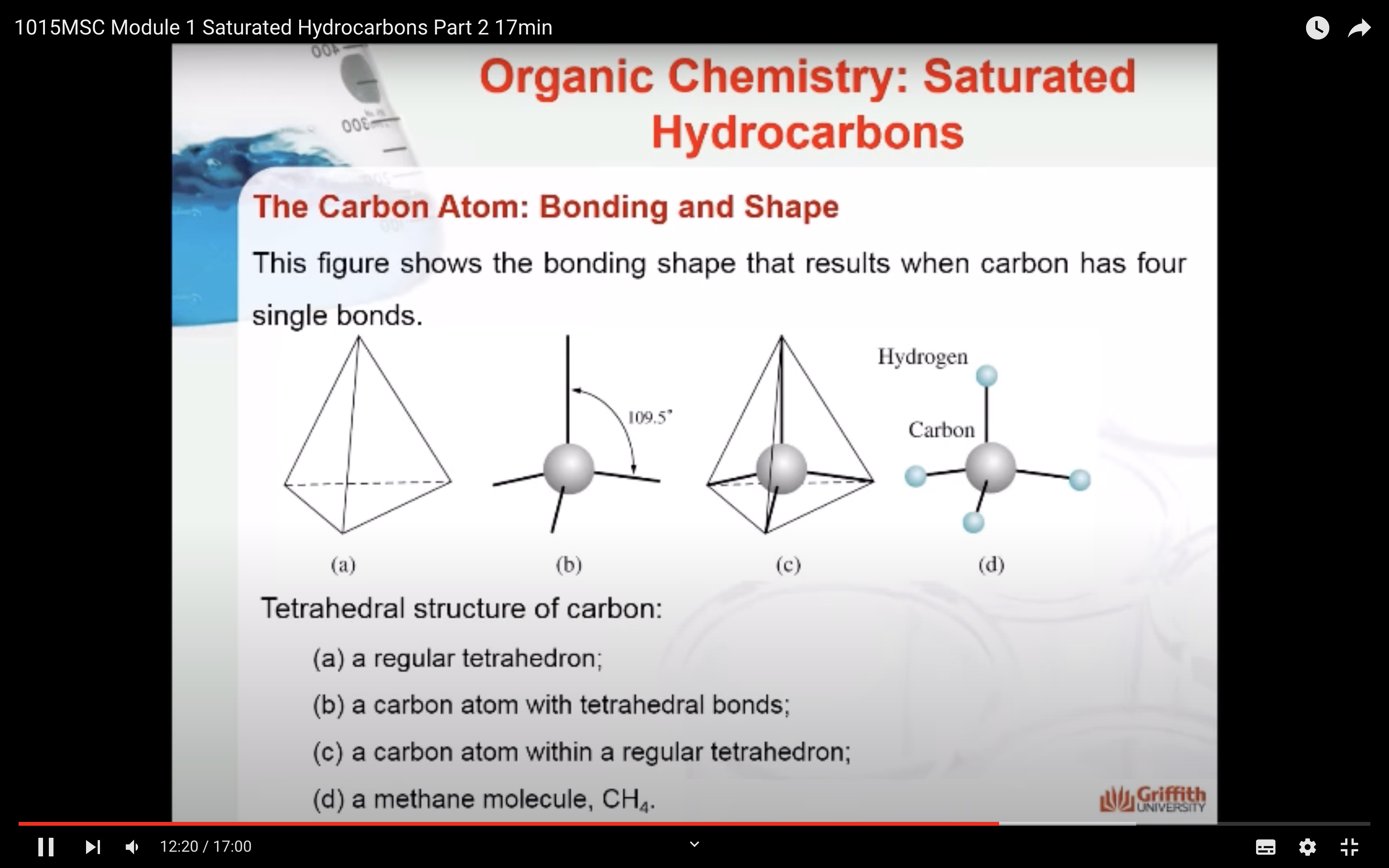
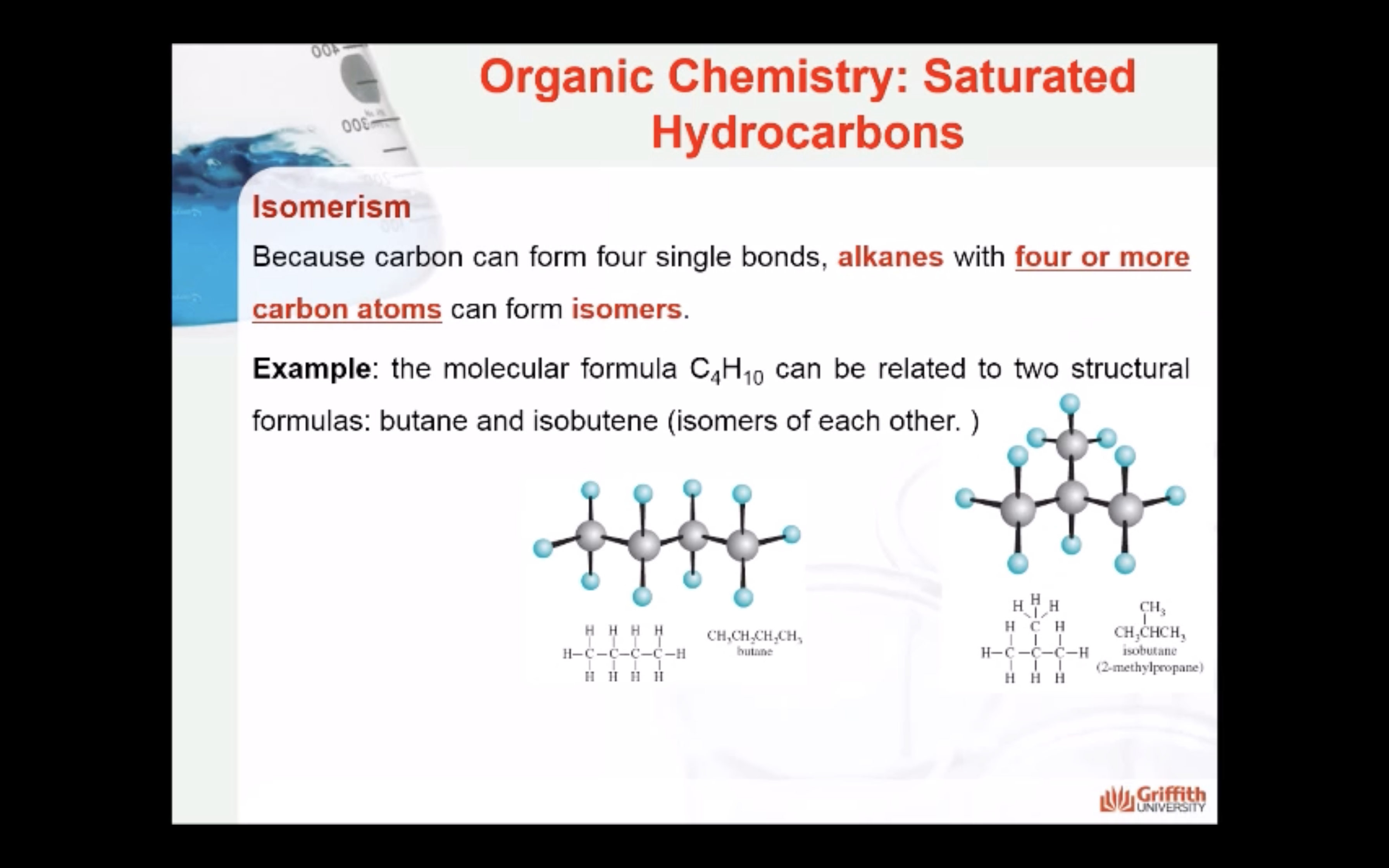
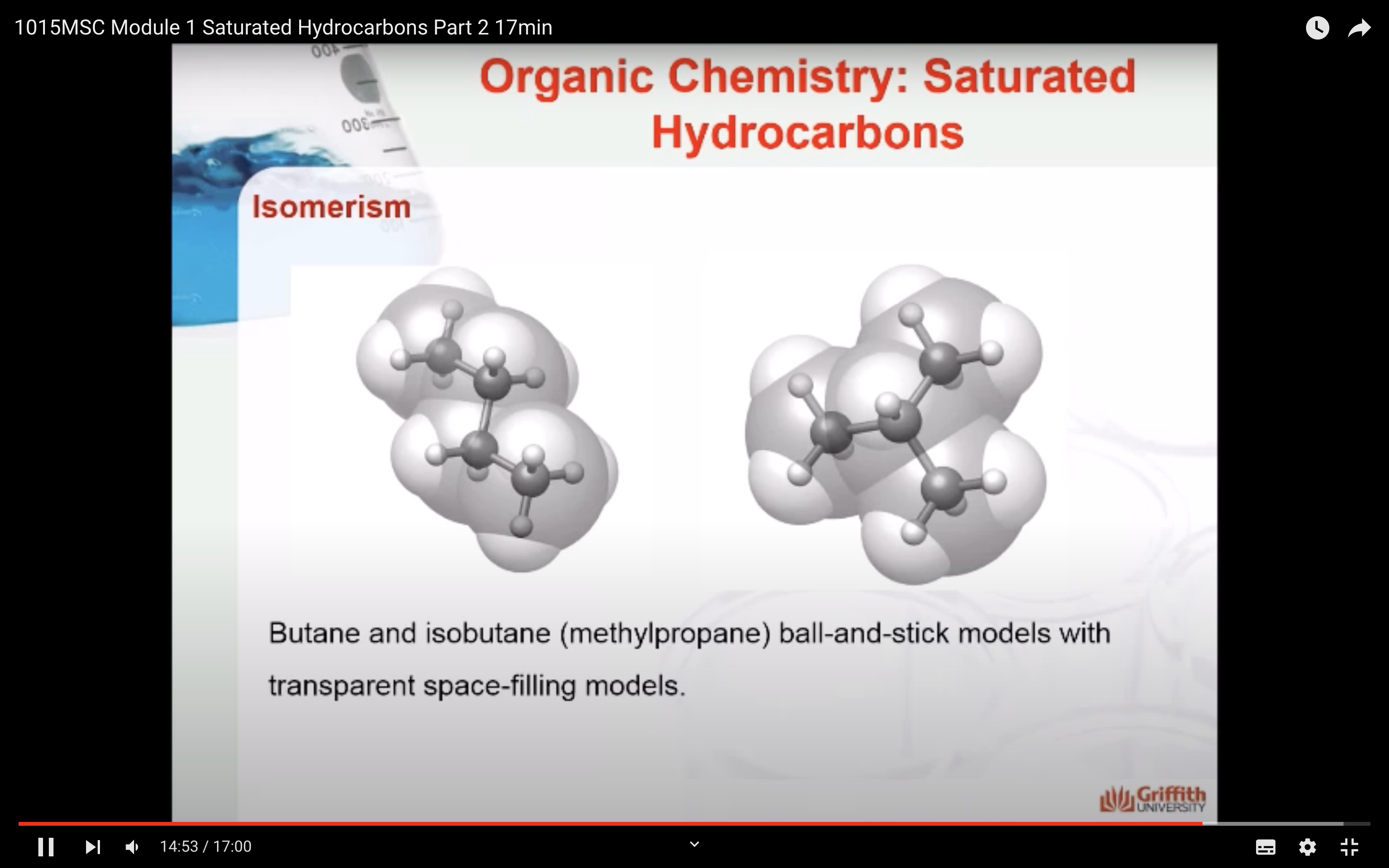
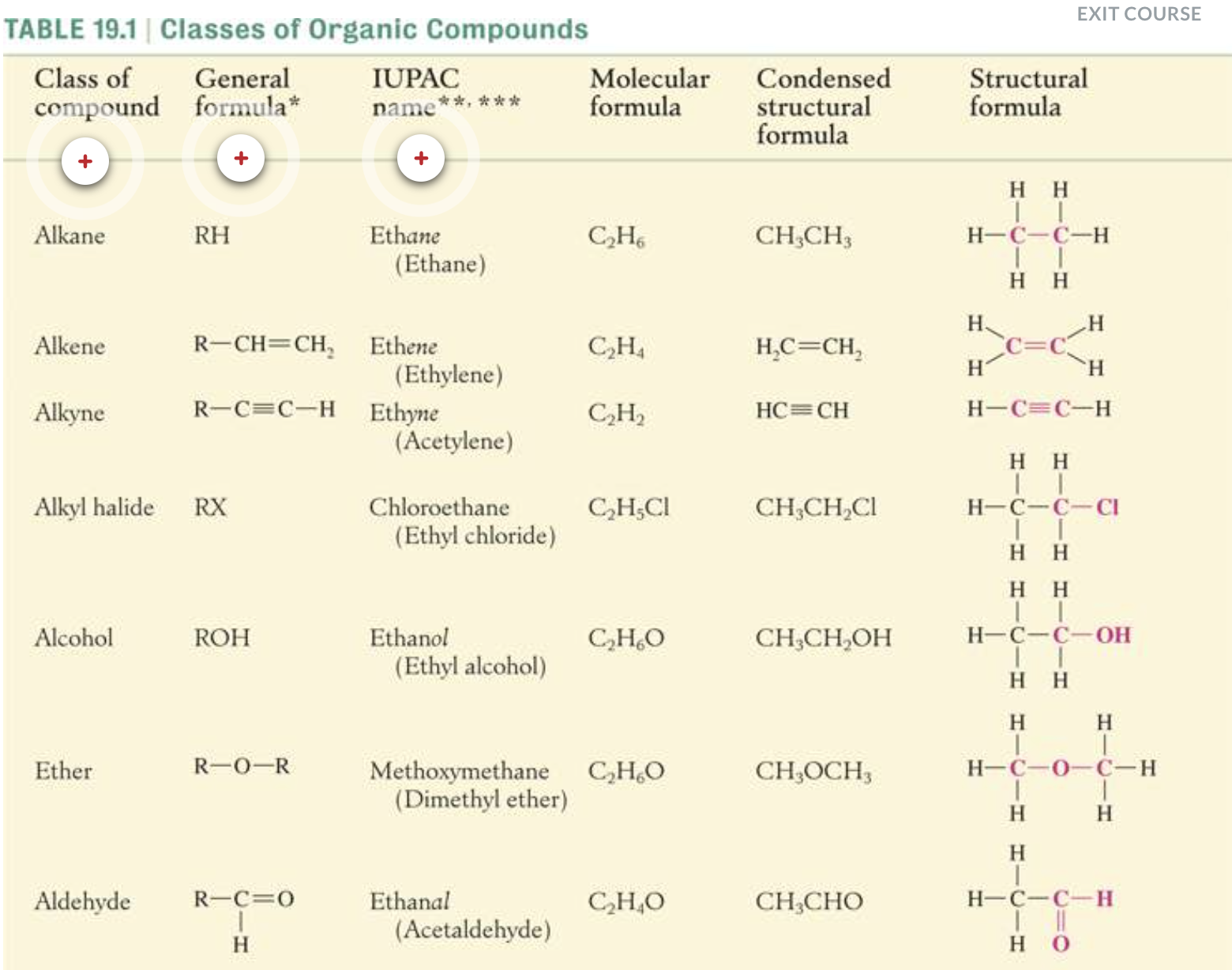
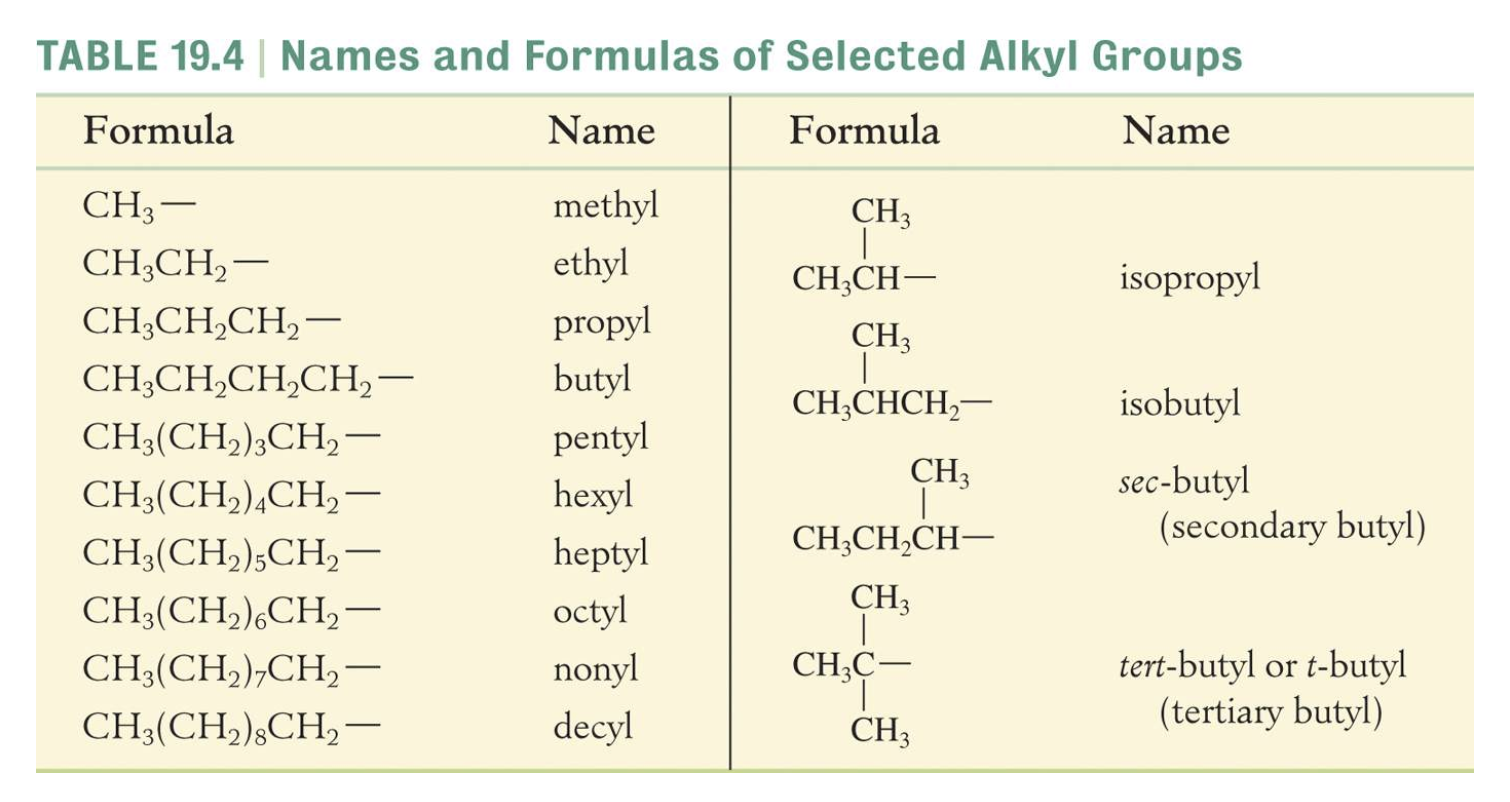
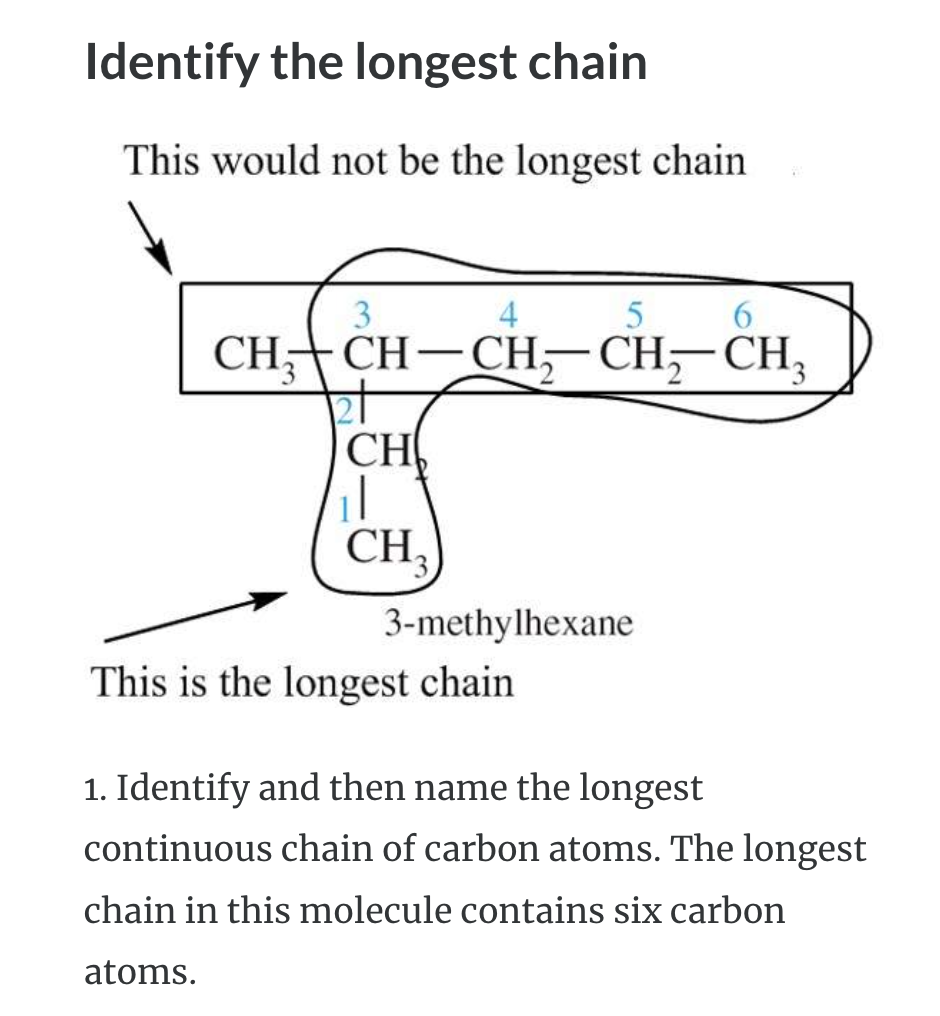
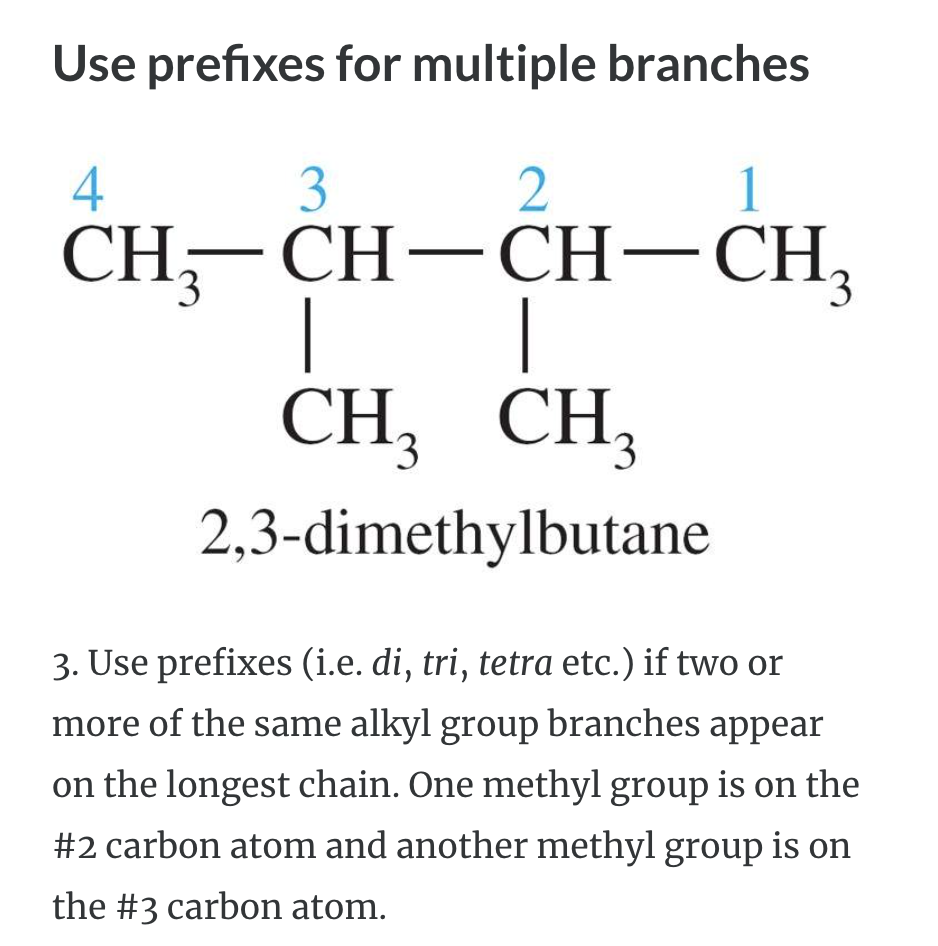
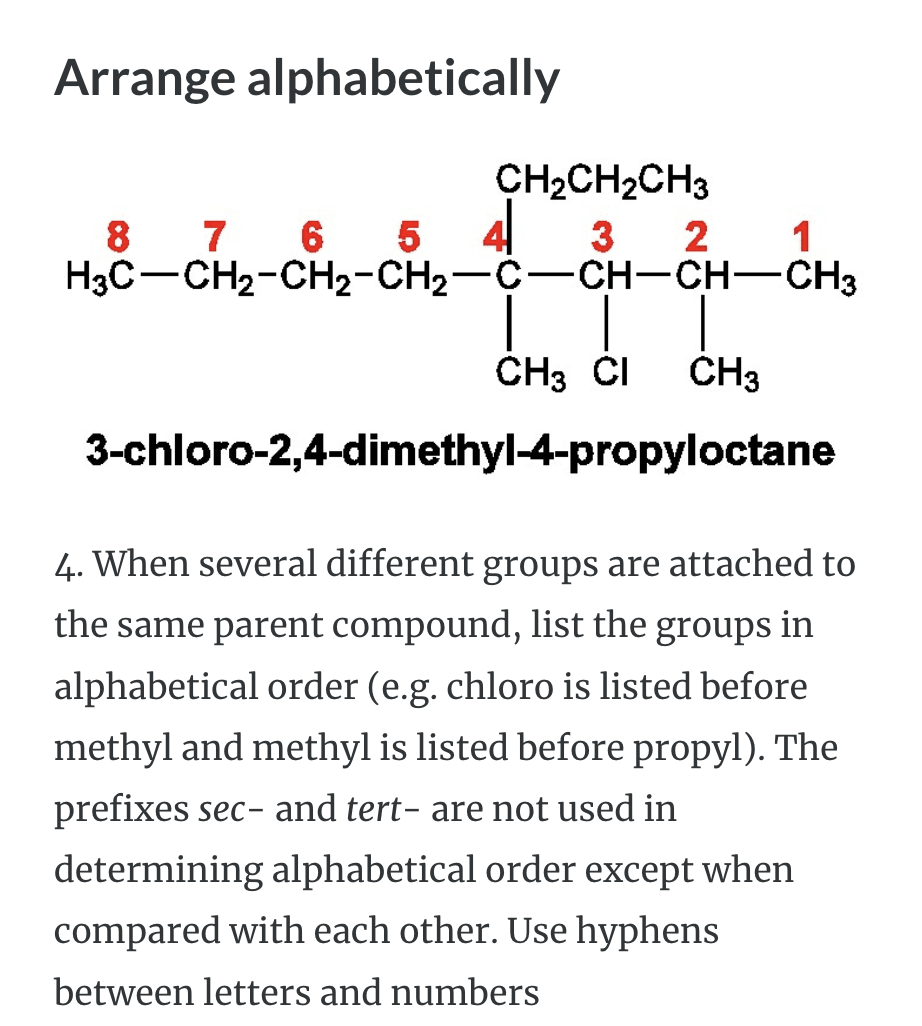
NAMING ORGANIC COMPOUNDS
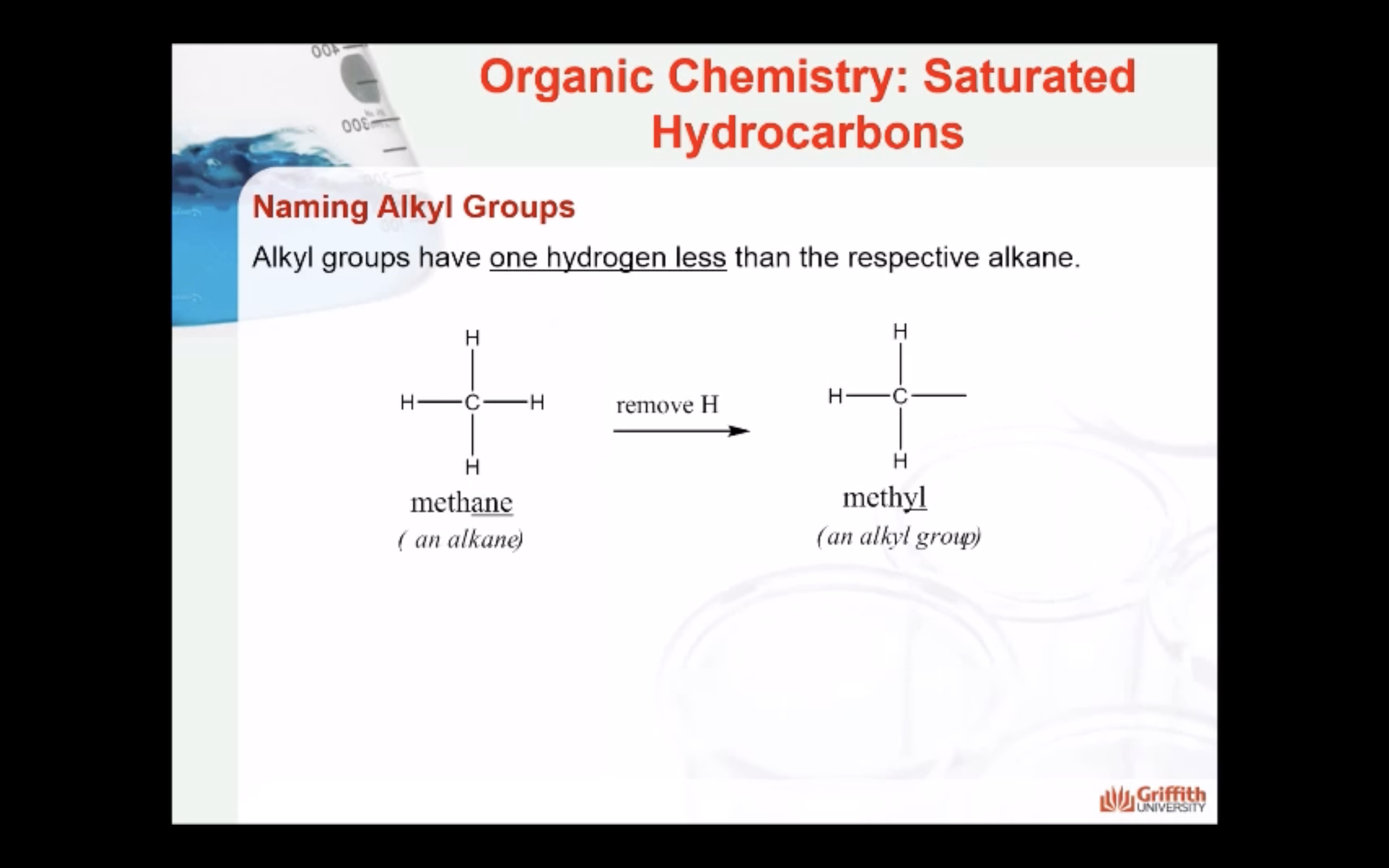
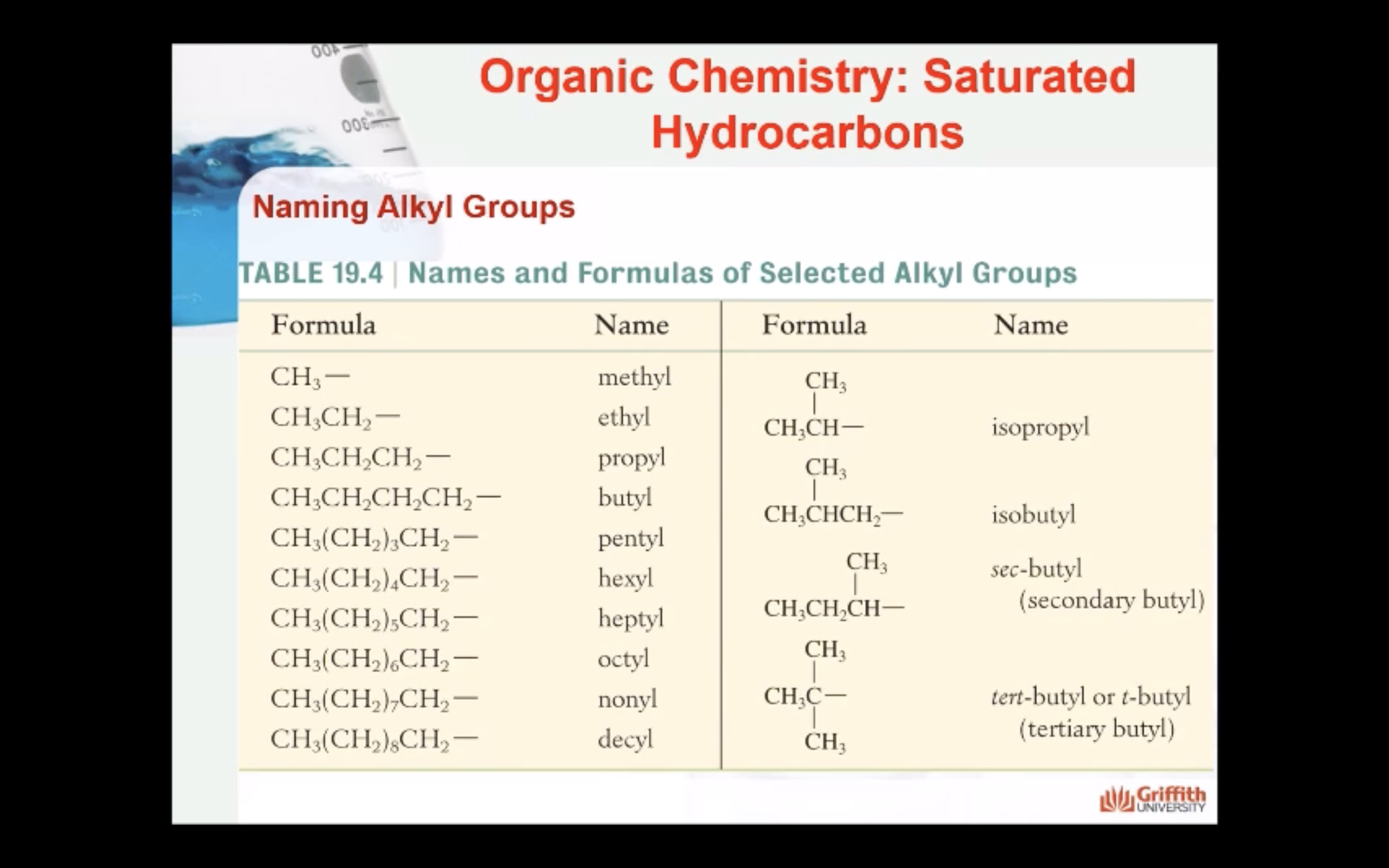
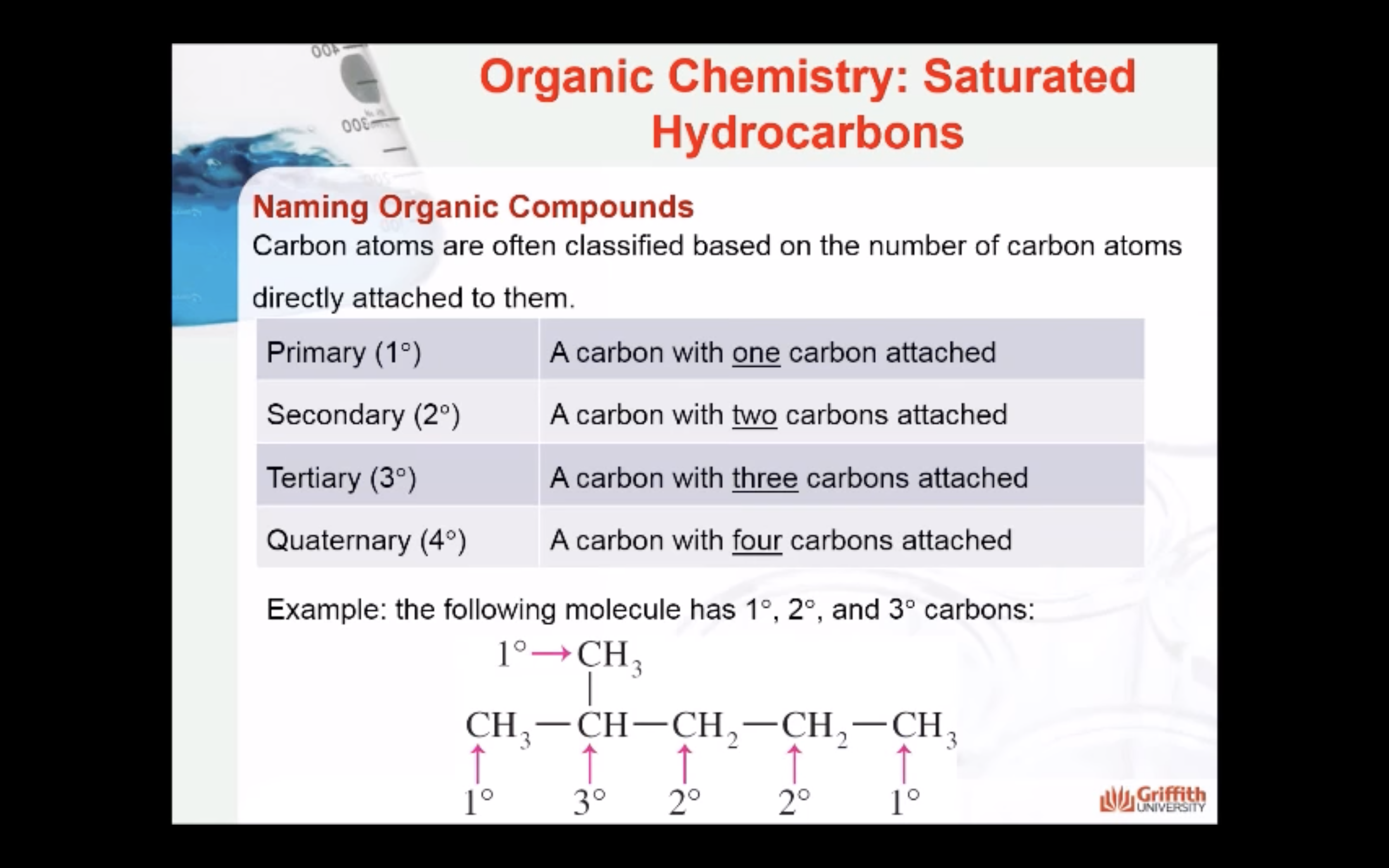
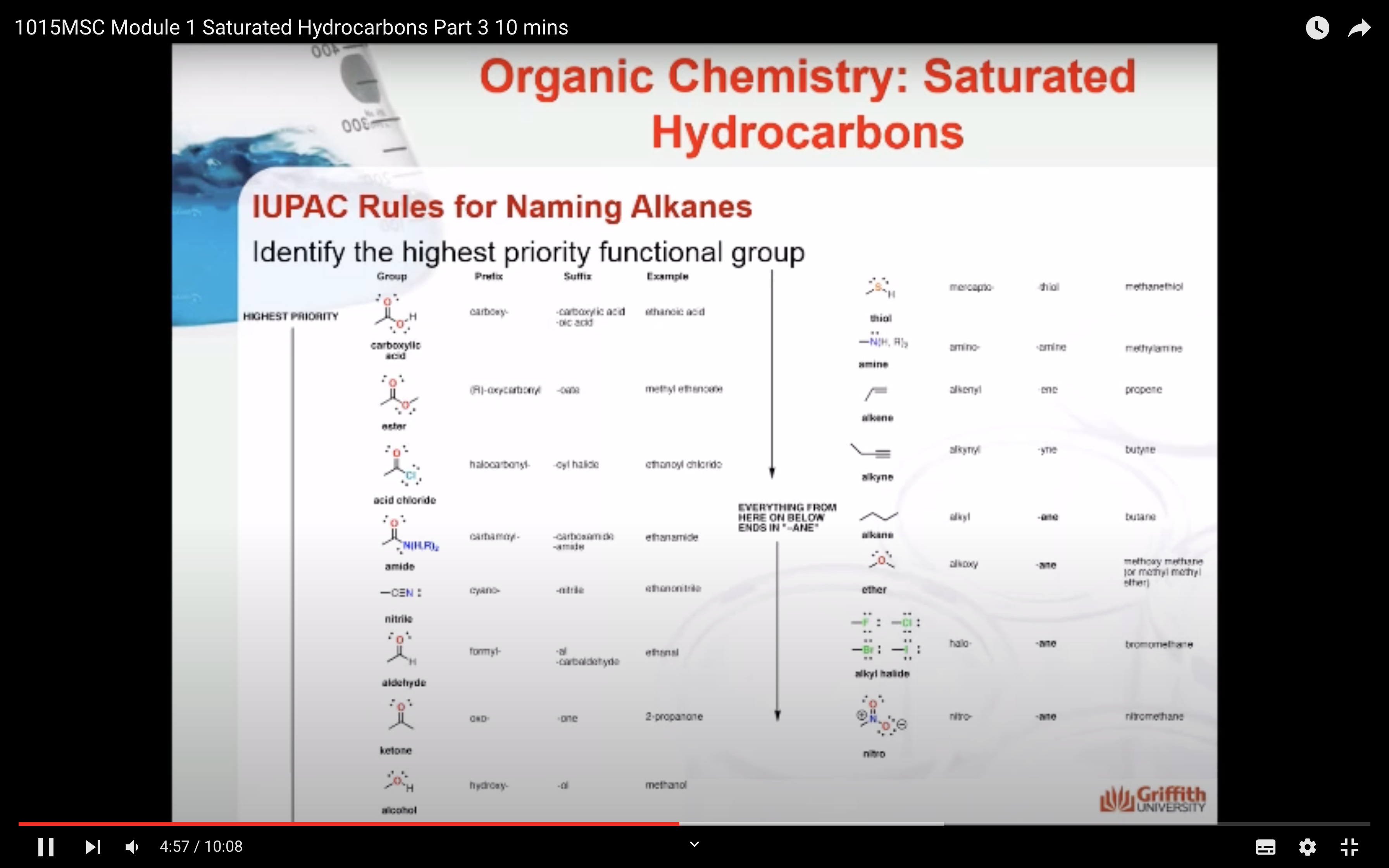
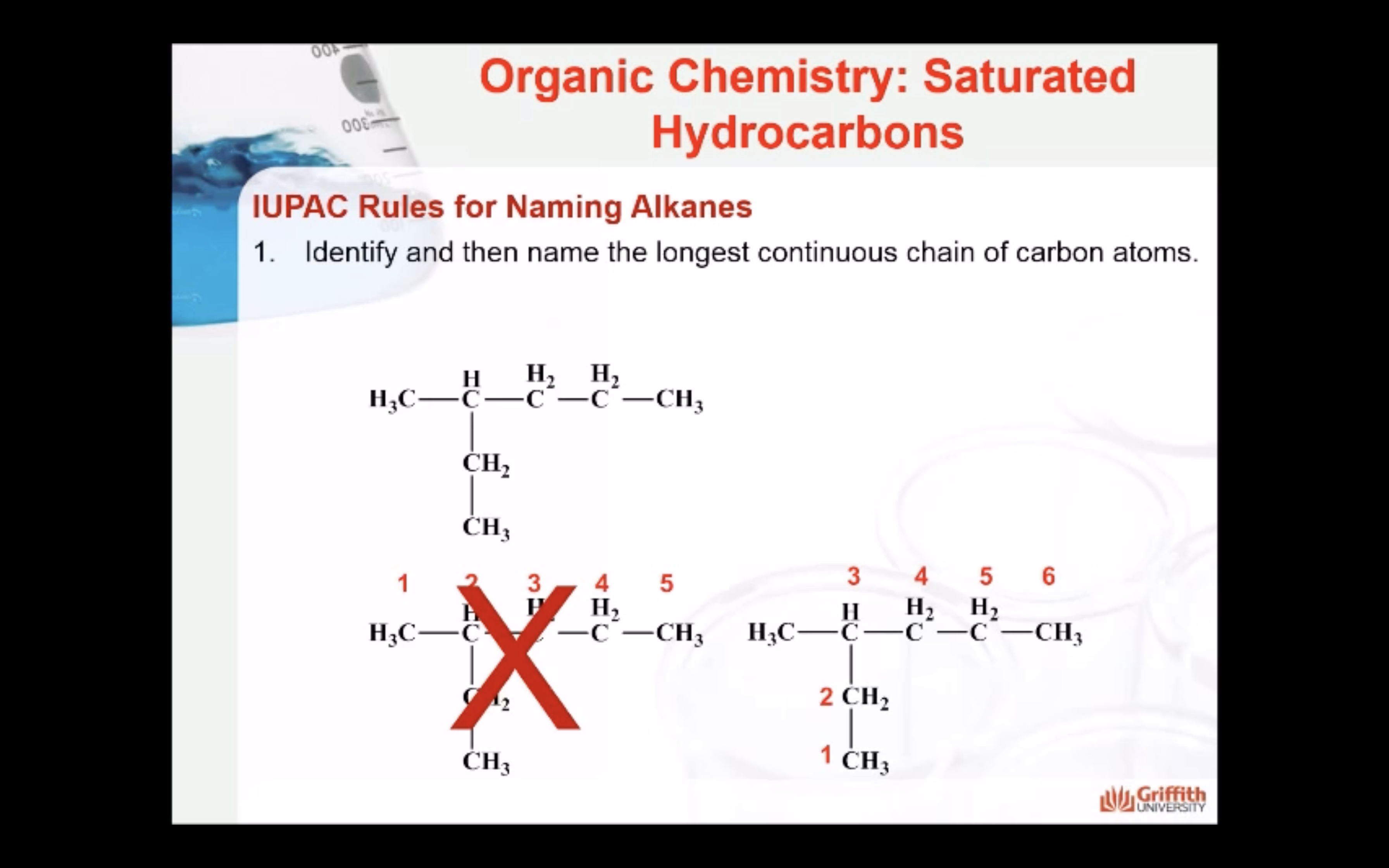
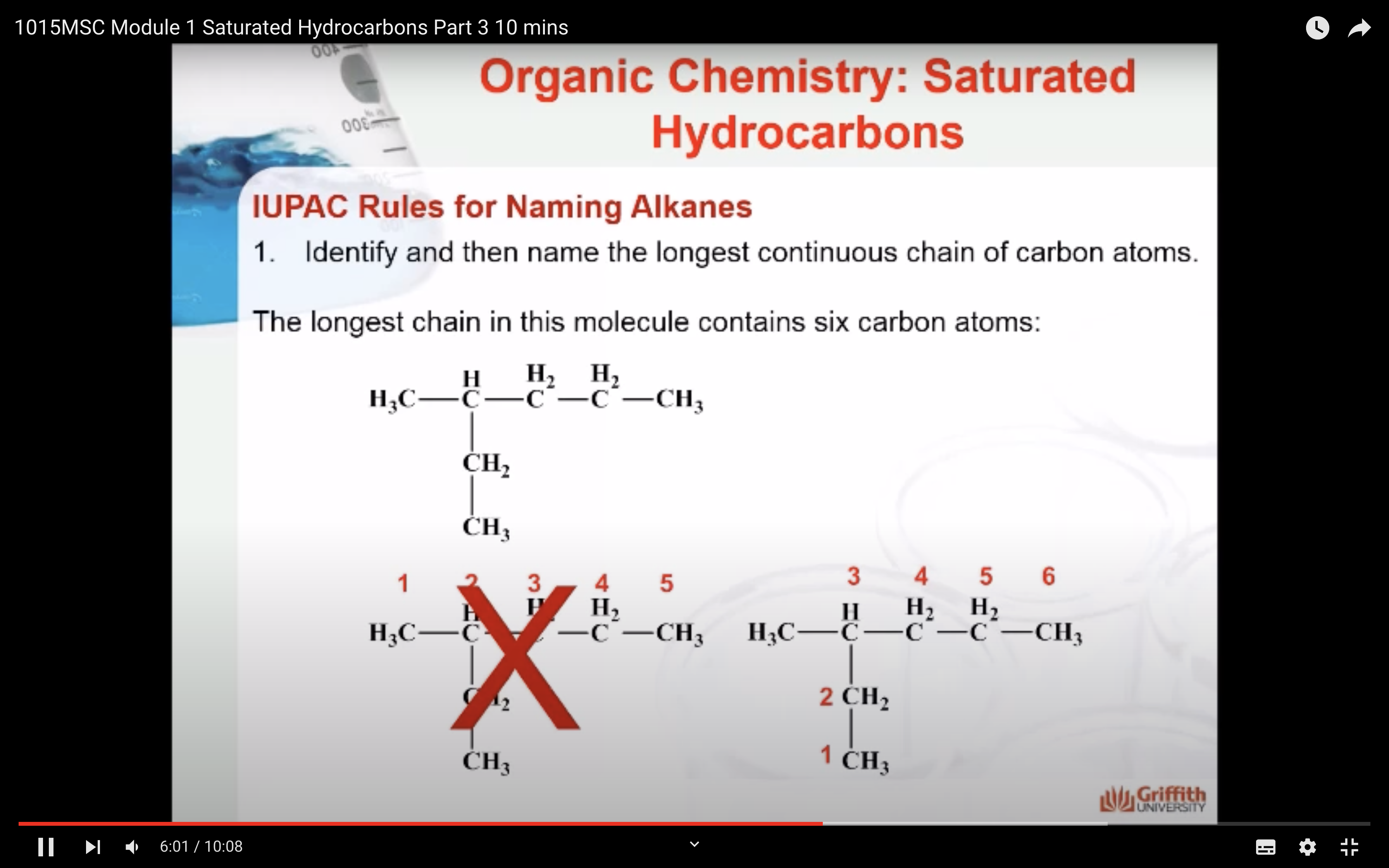
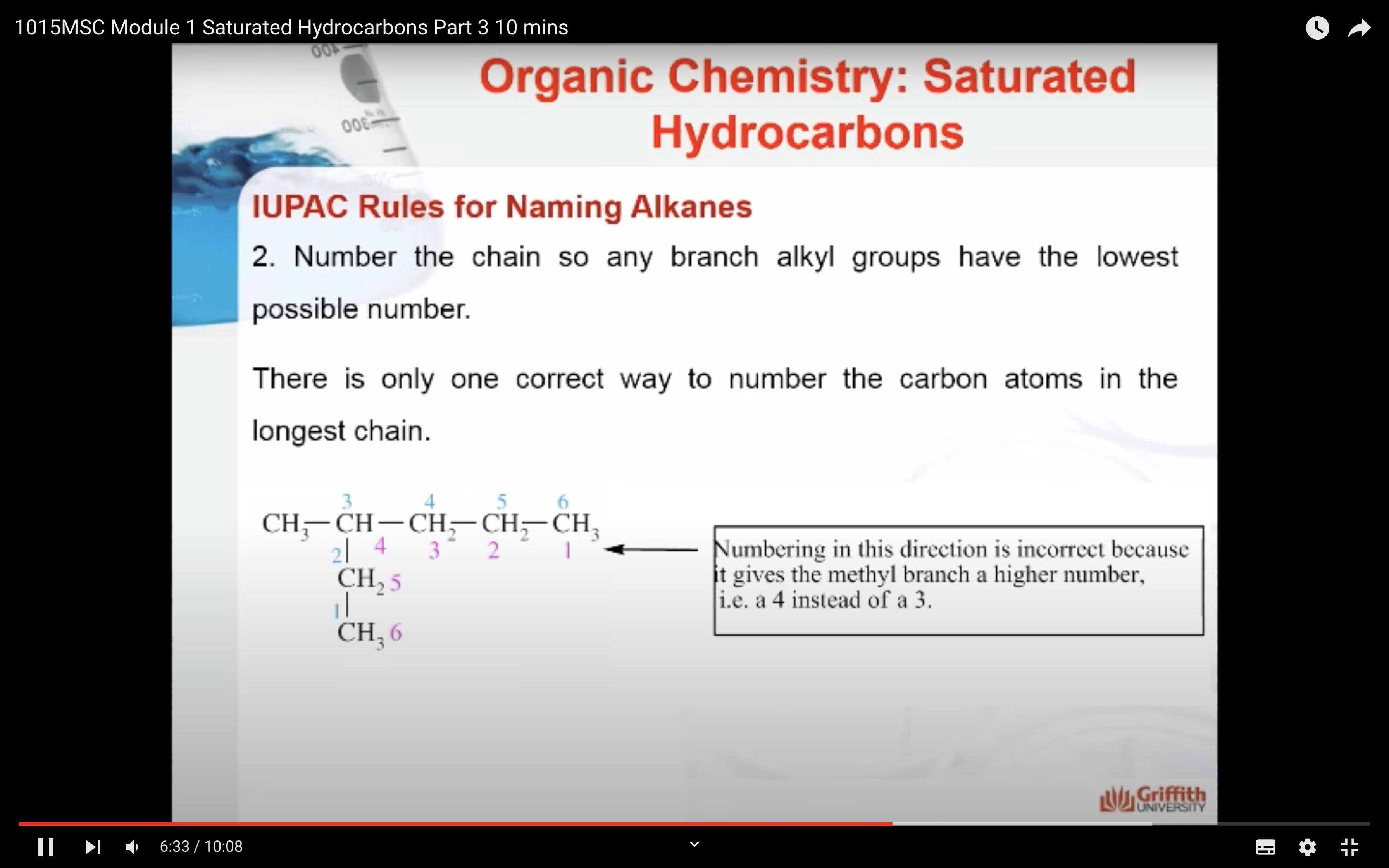
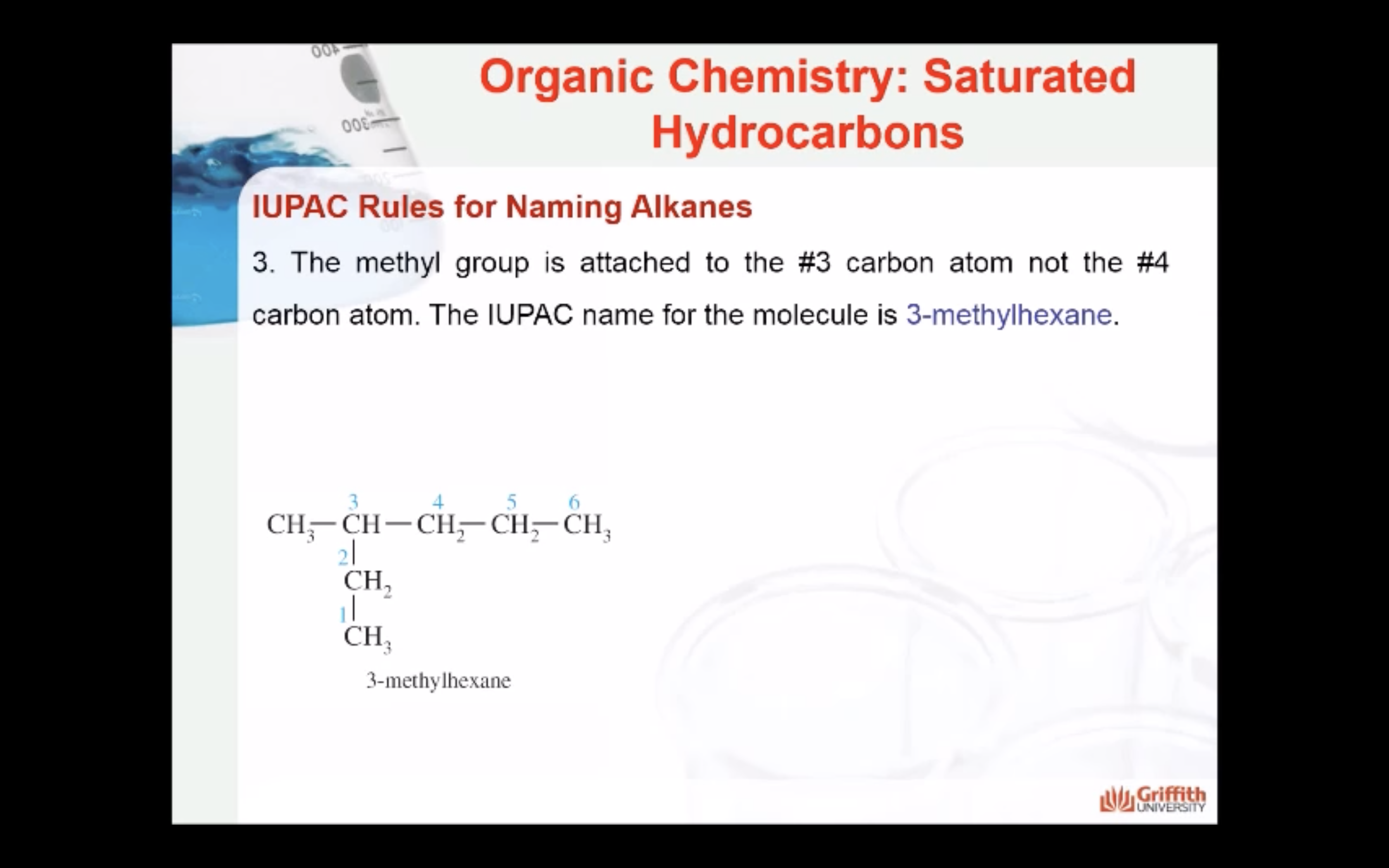
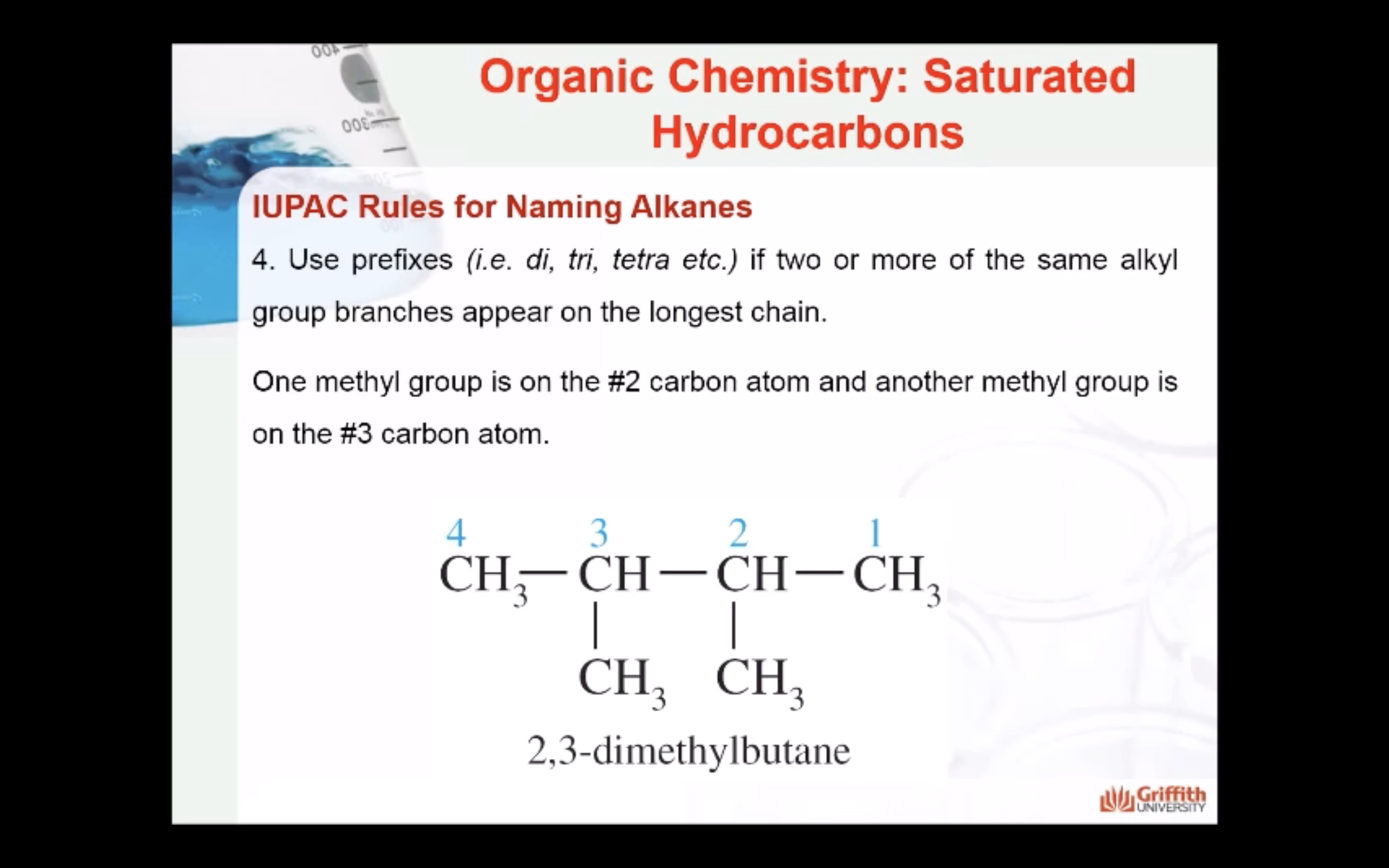
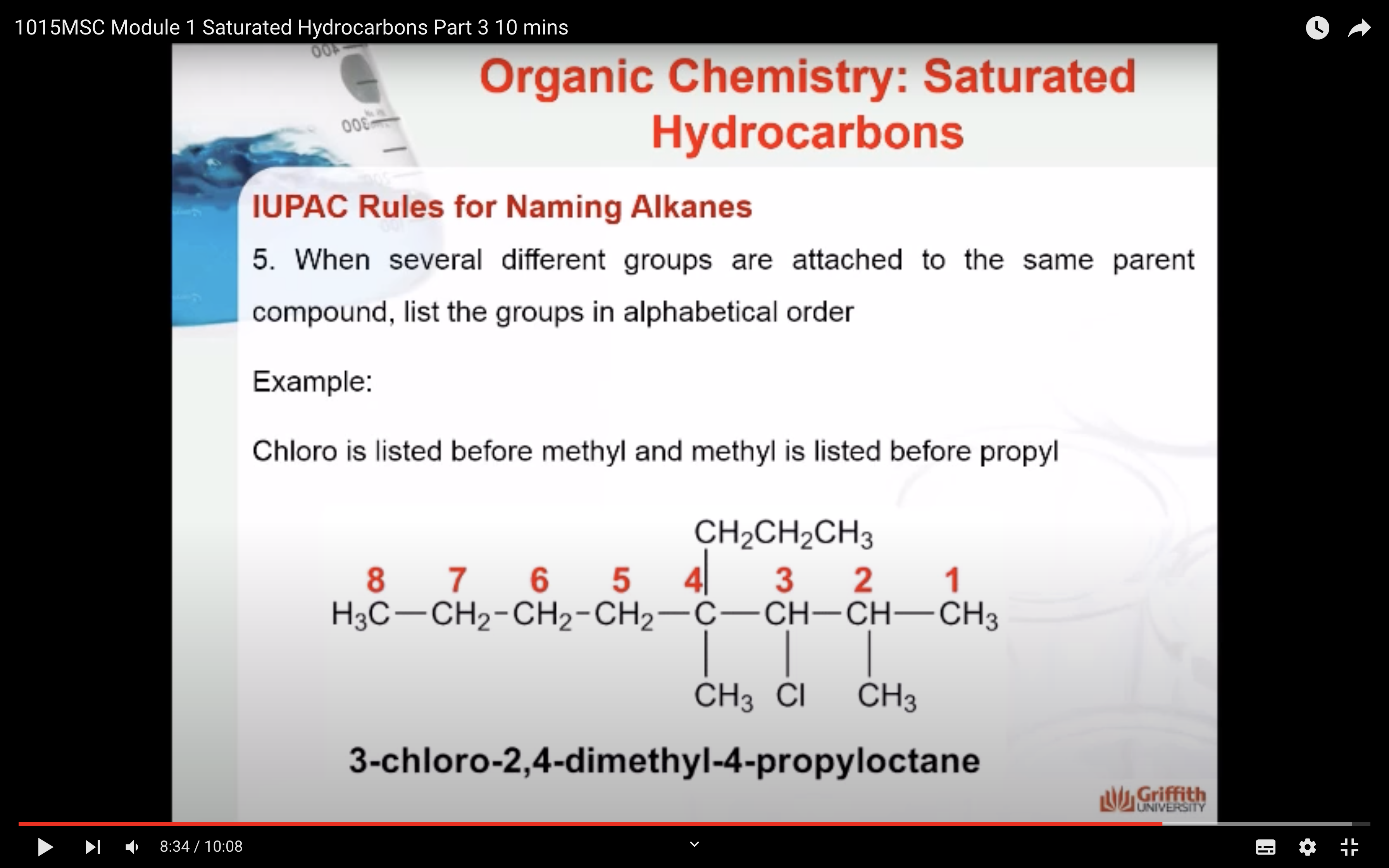
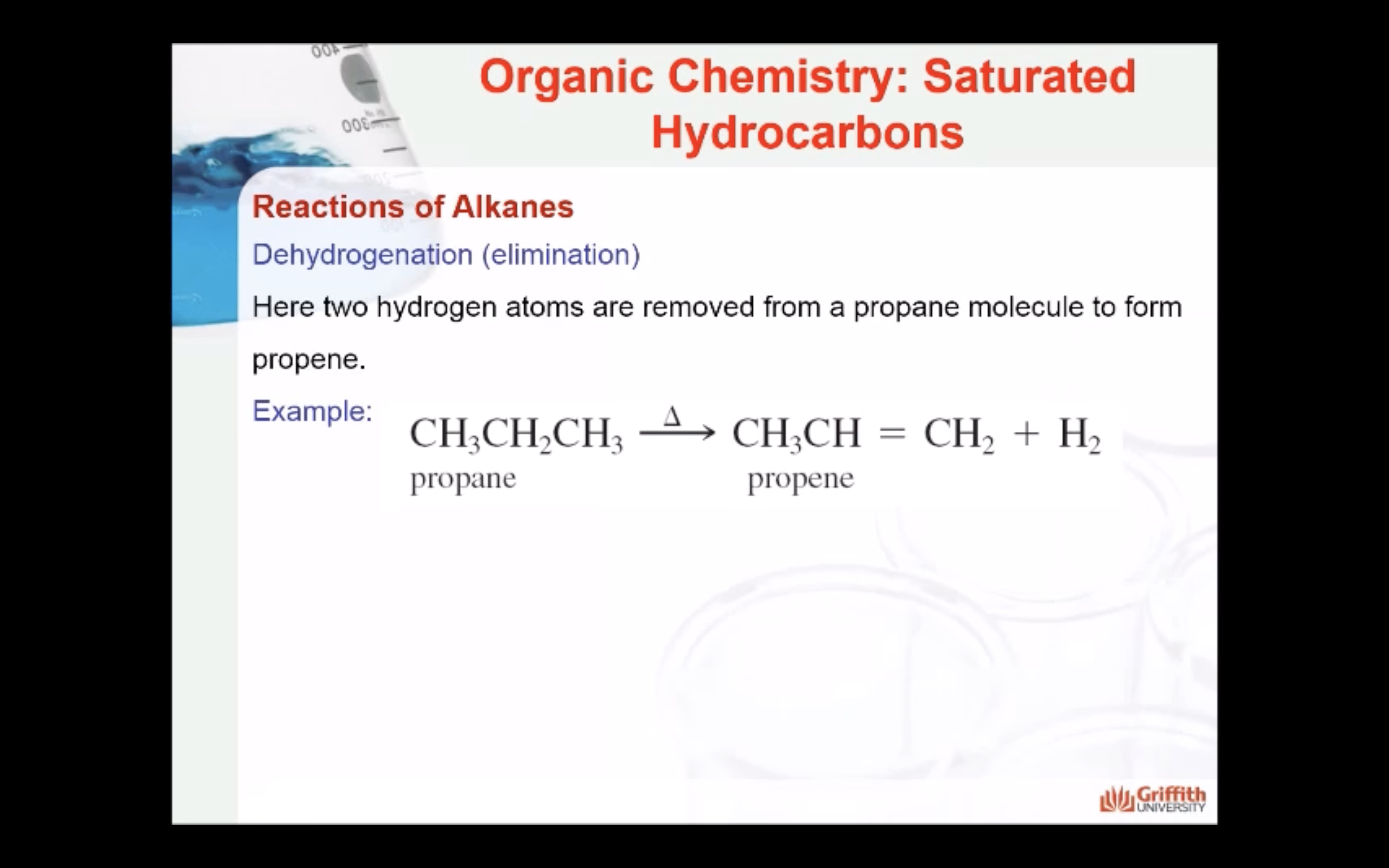
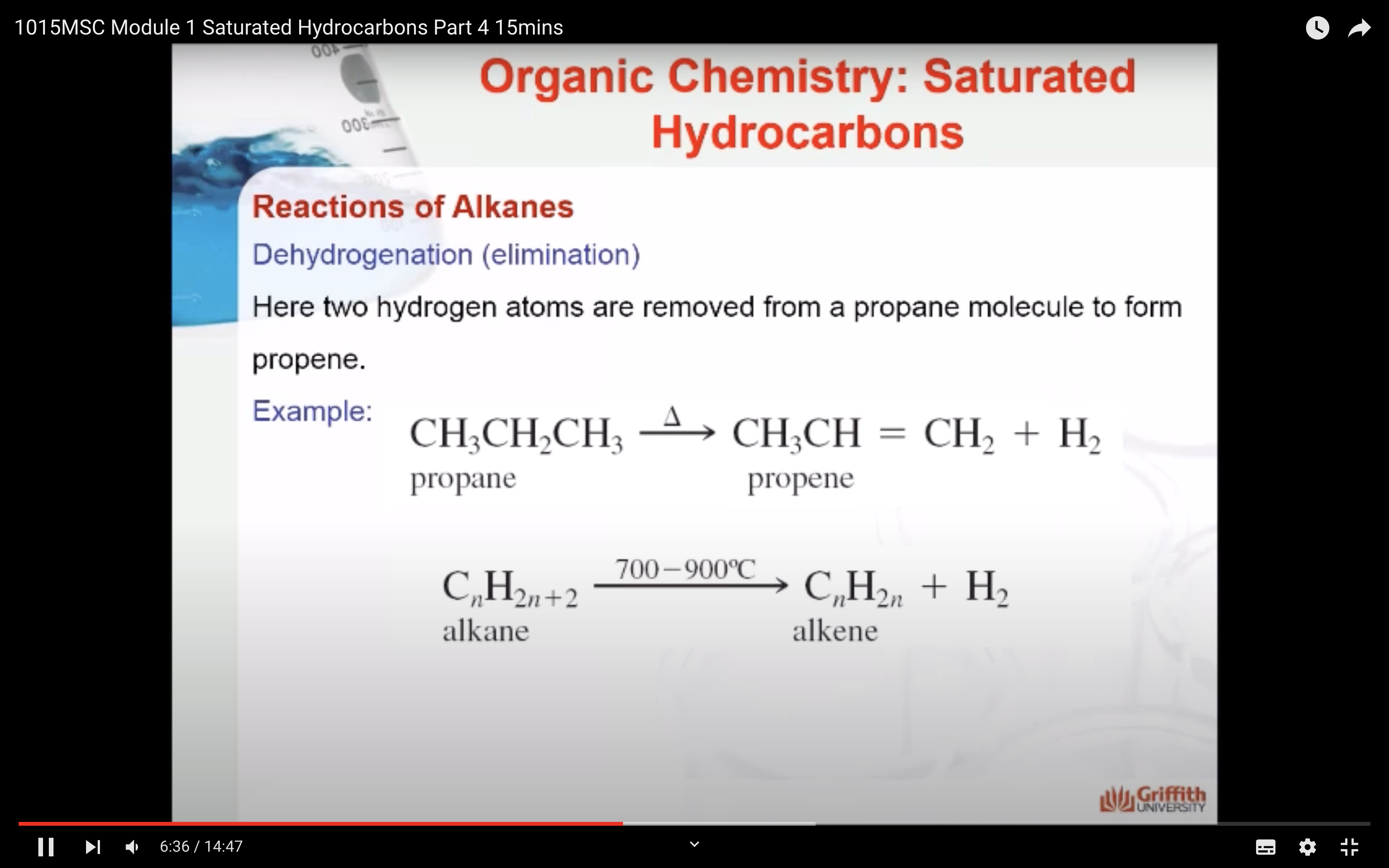
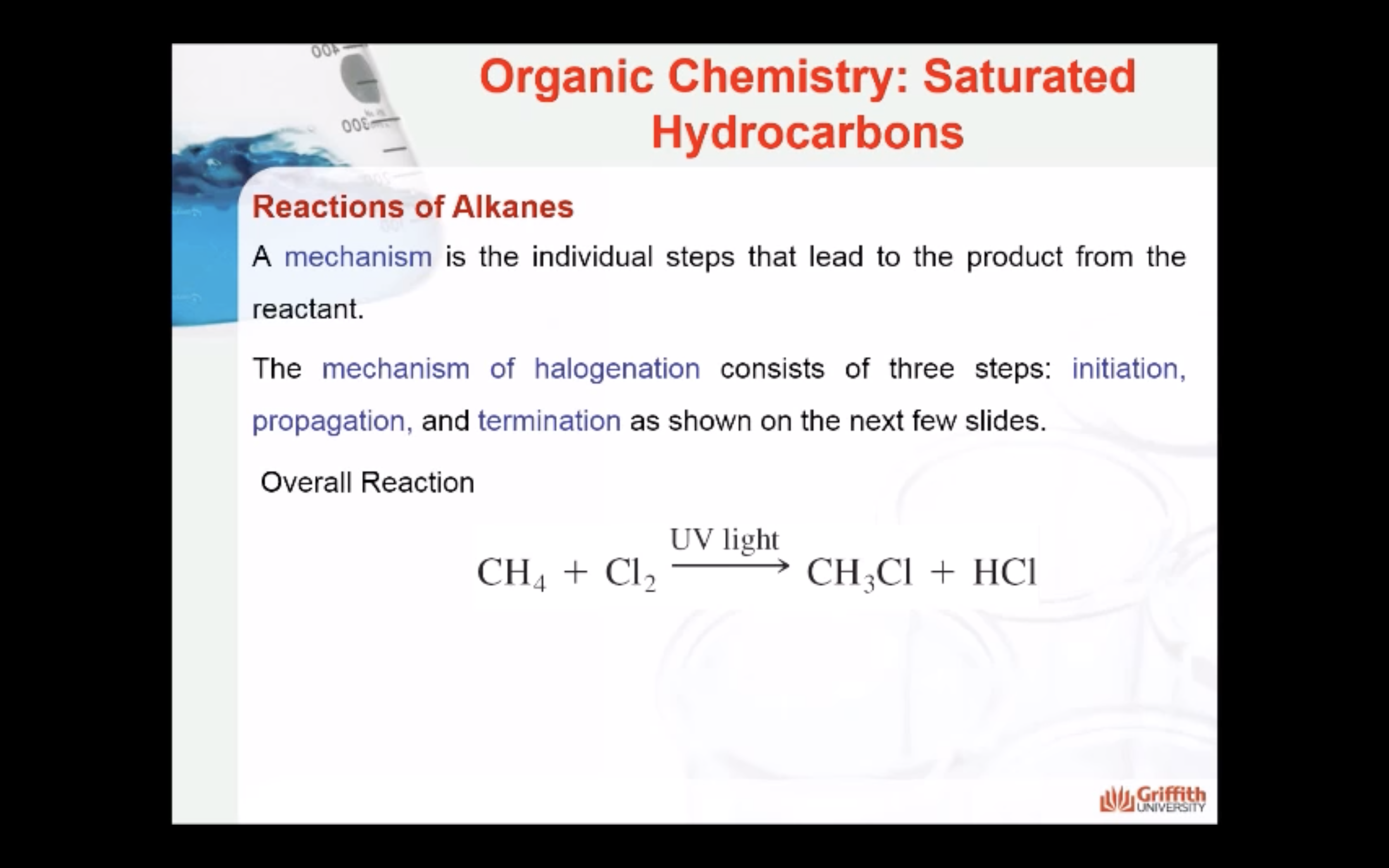
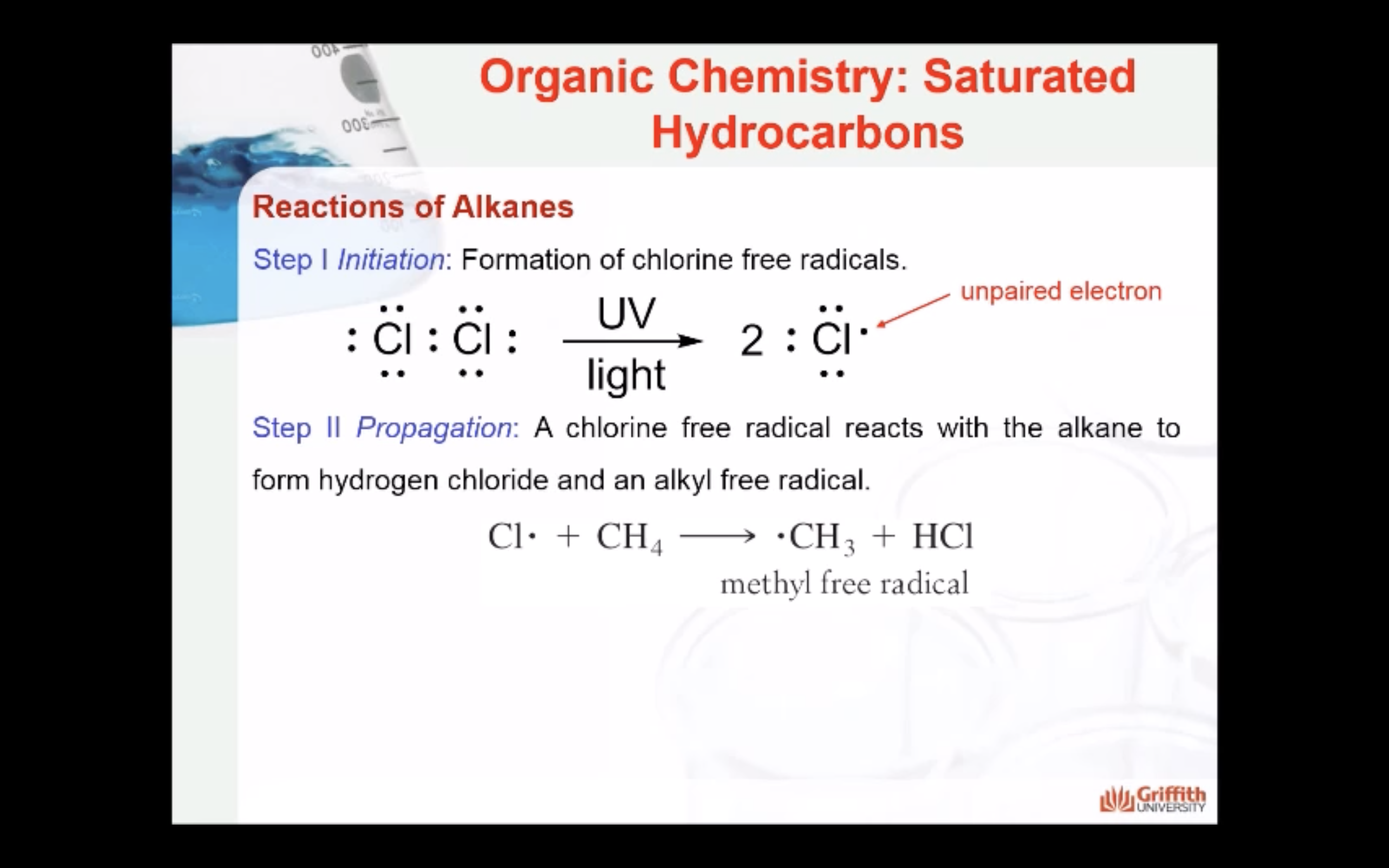
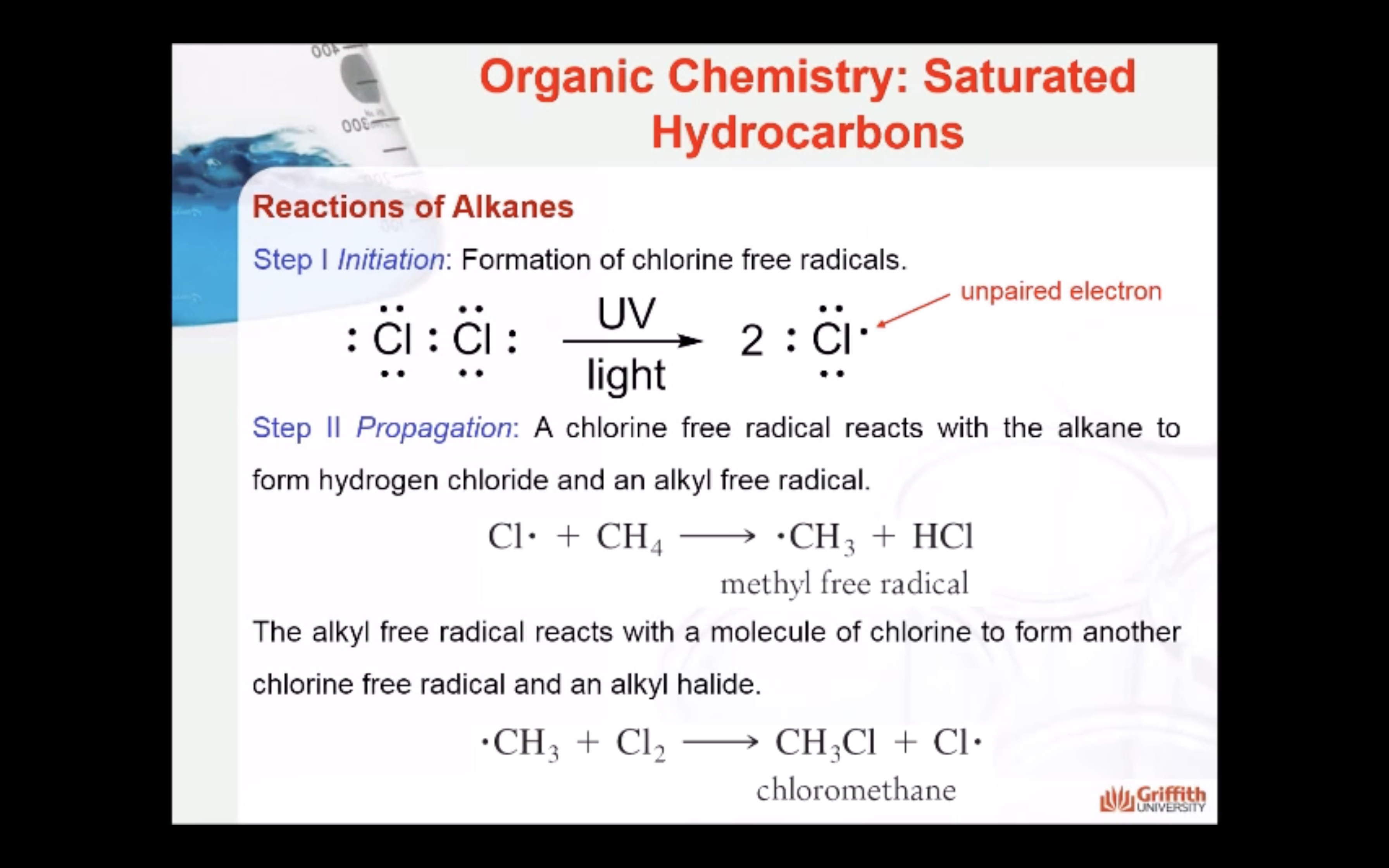
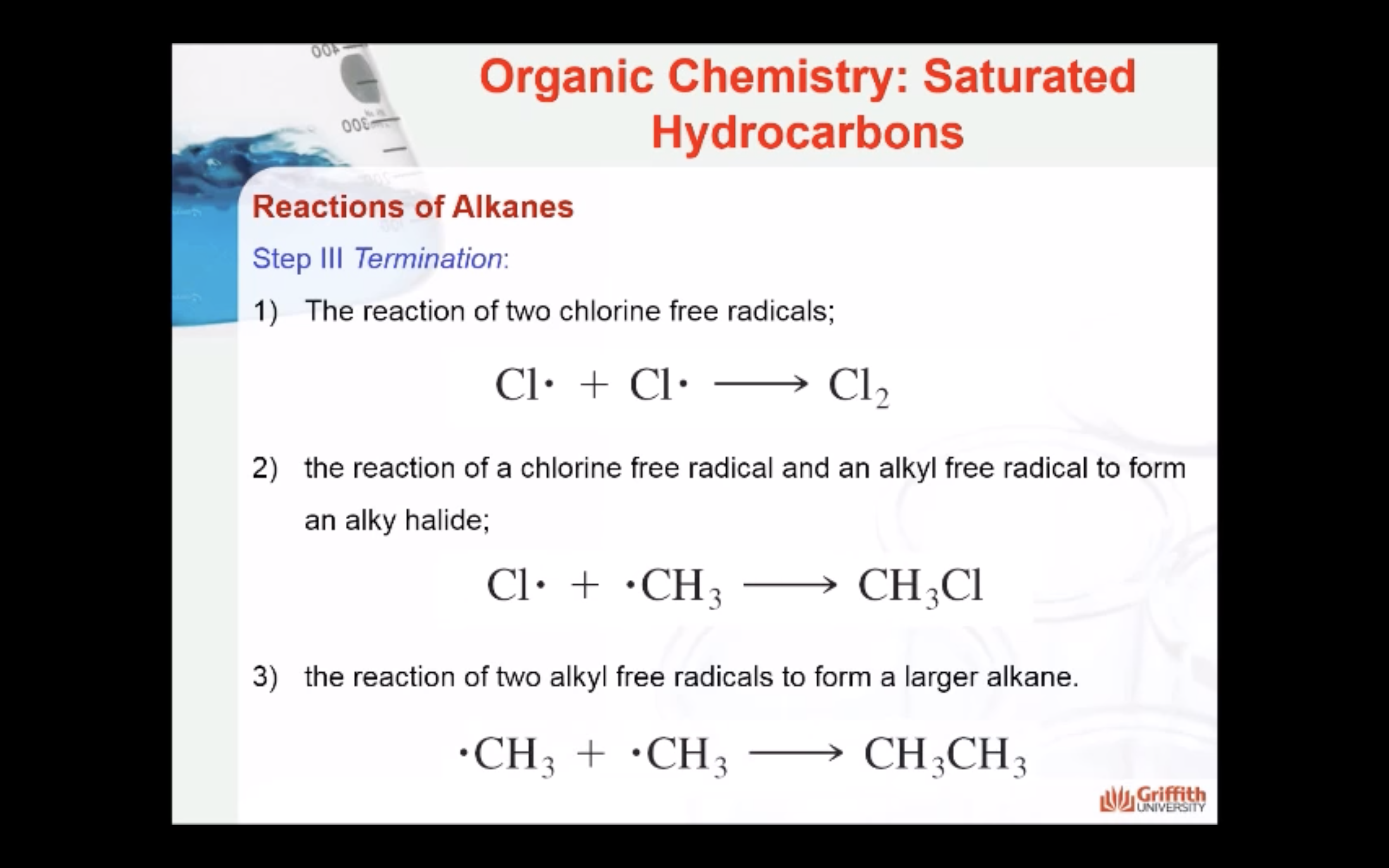
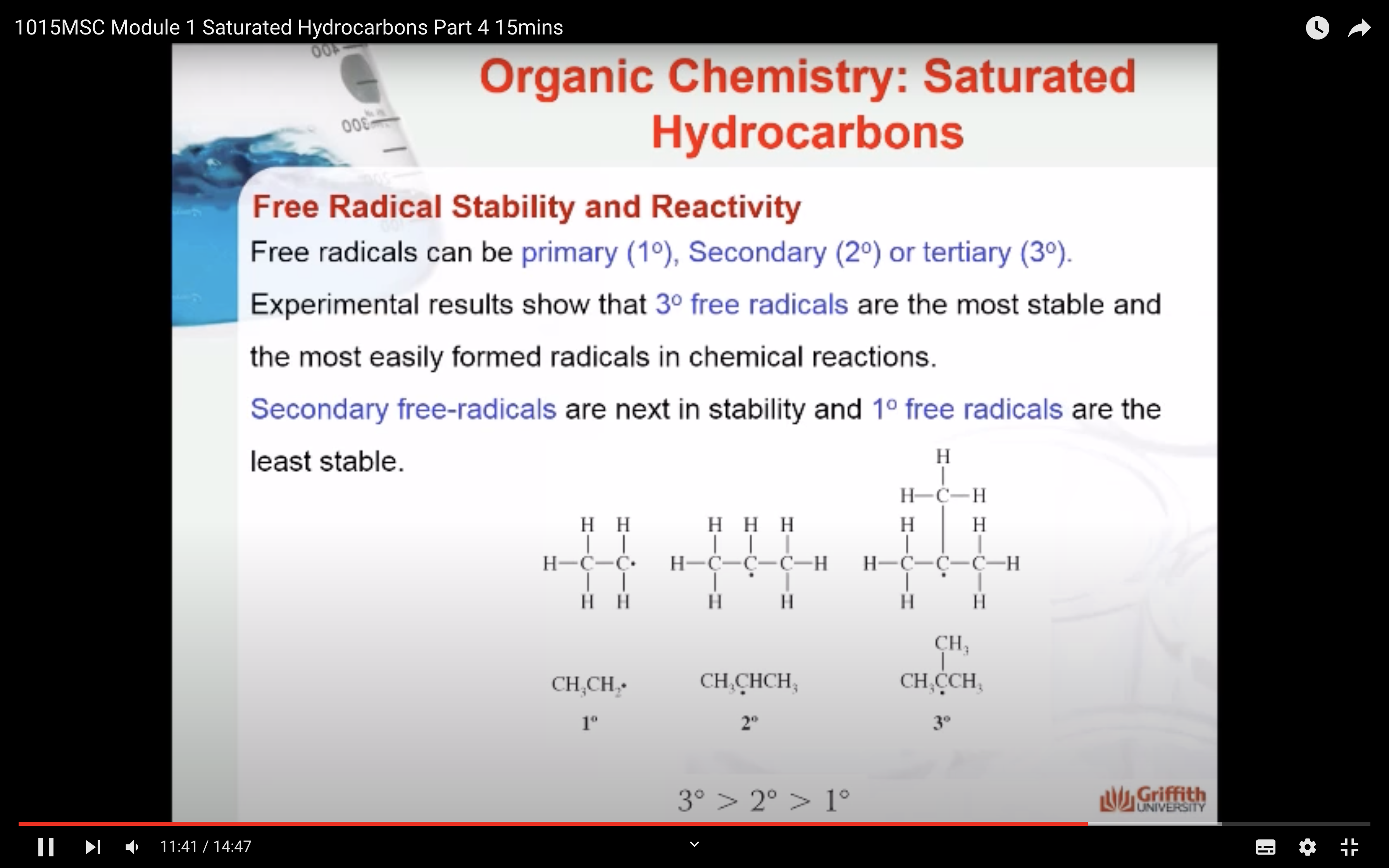
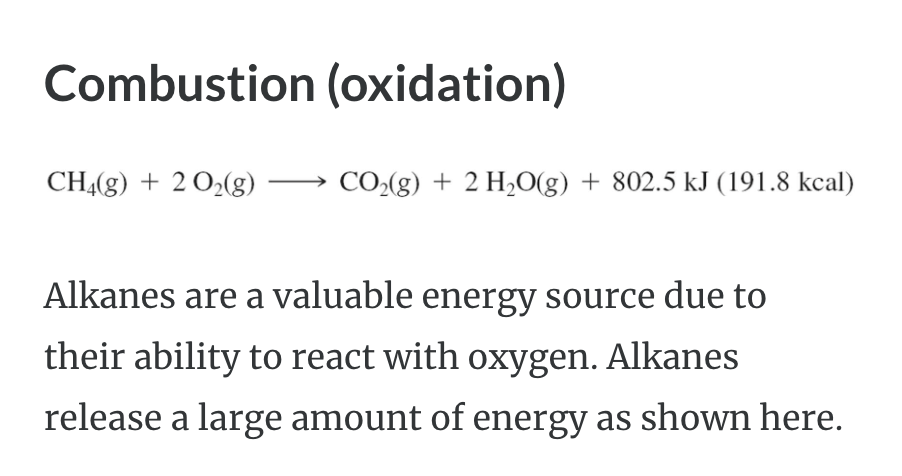
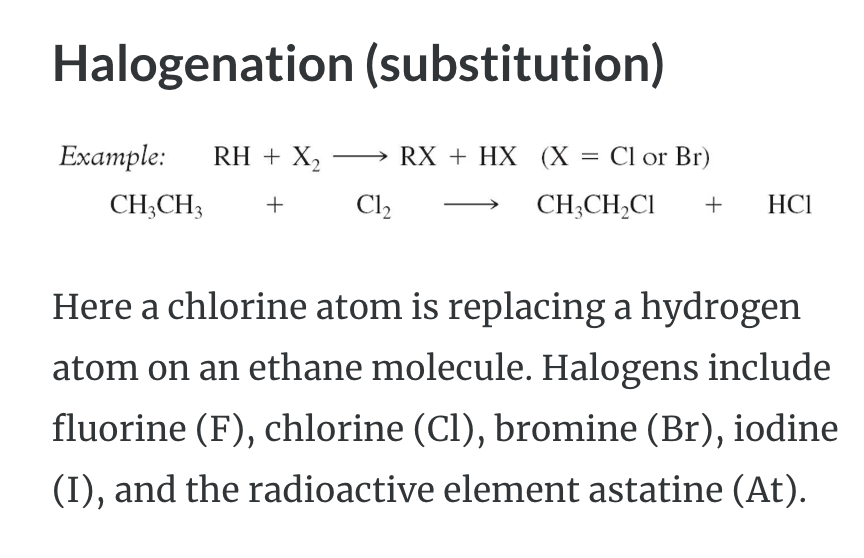
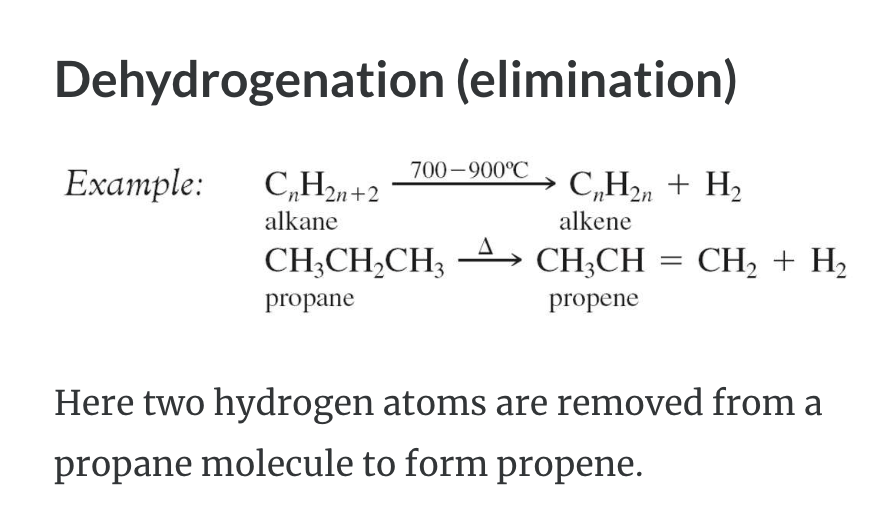
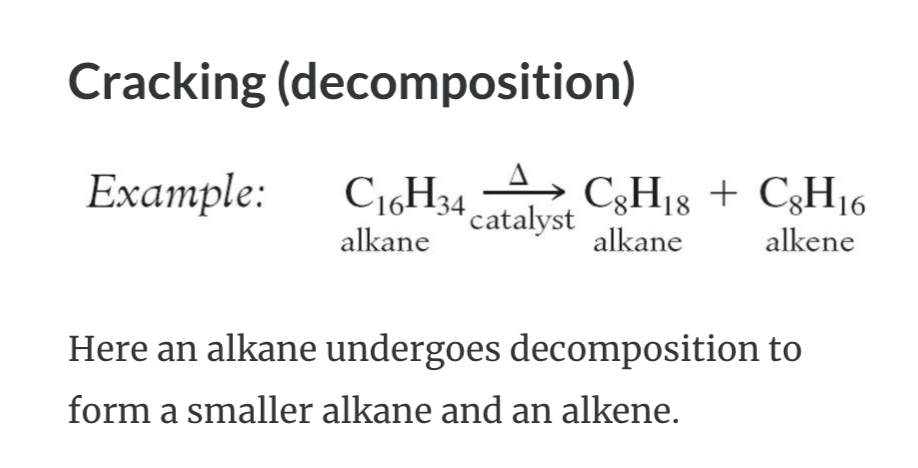
REACTIONS OF ALKANES
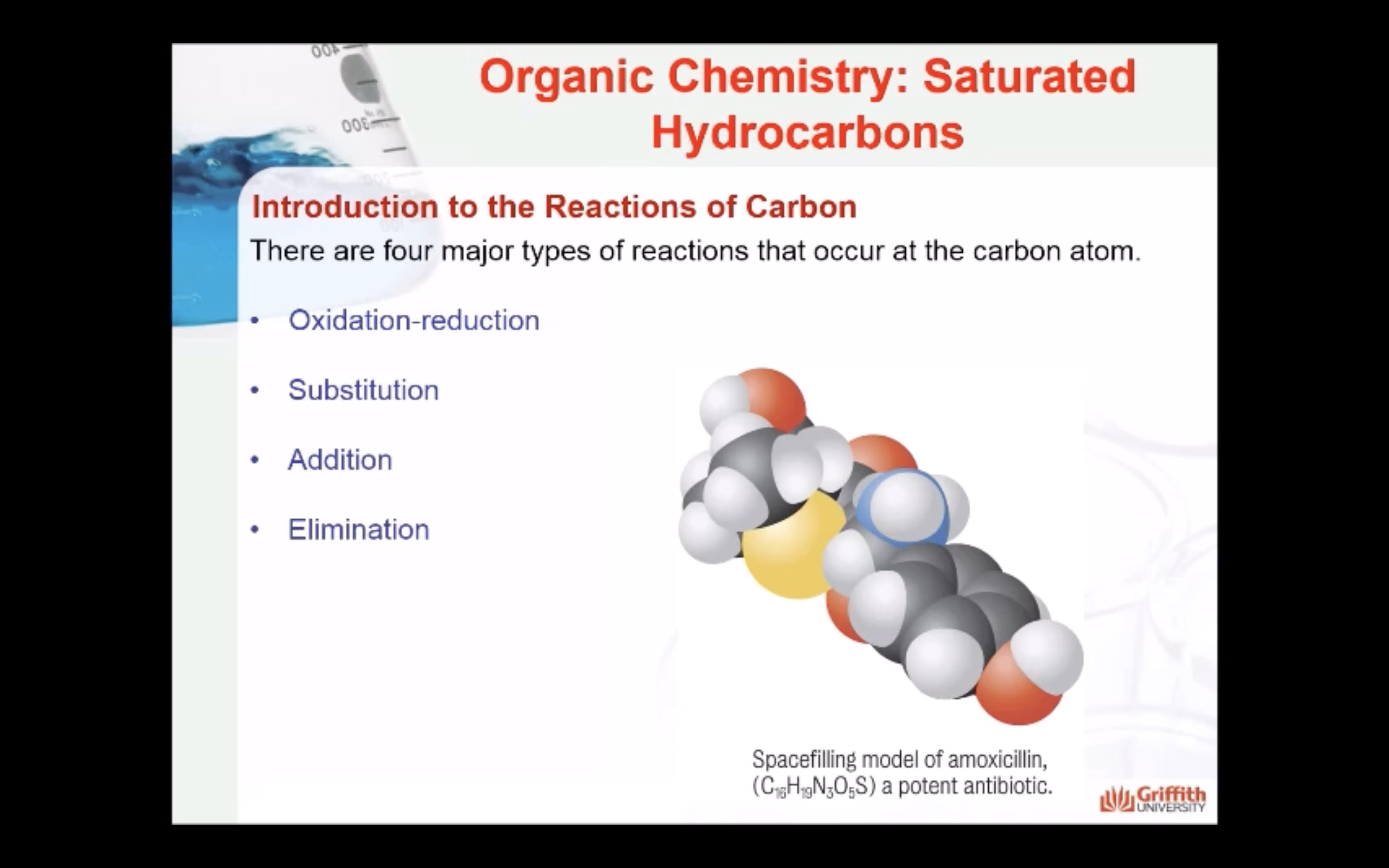
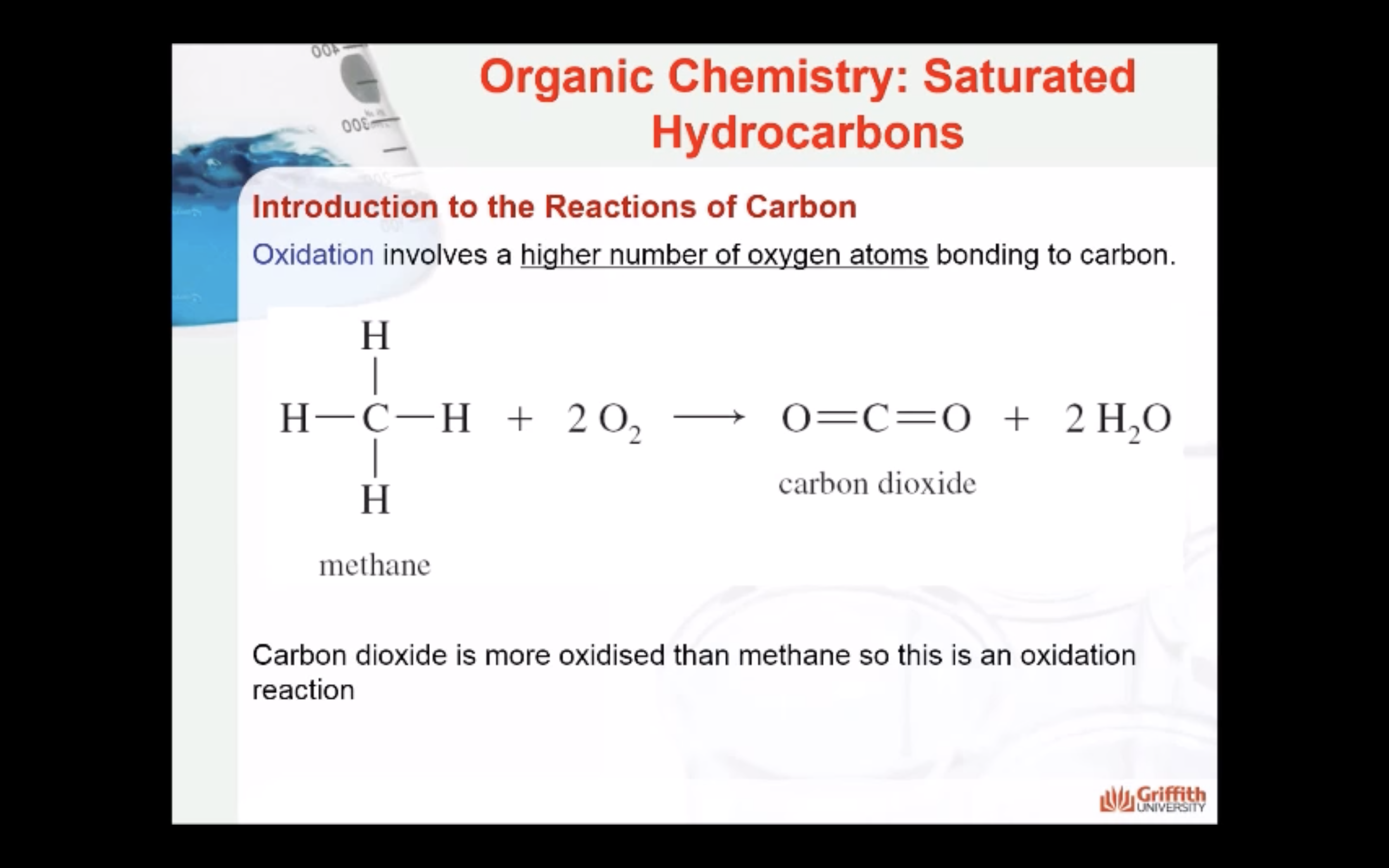
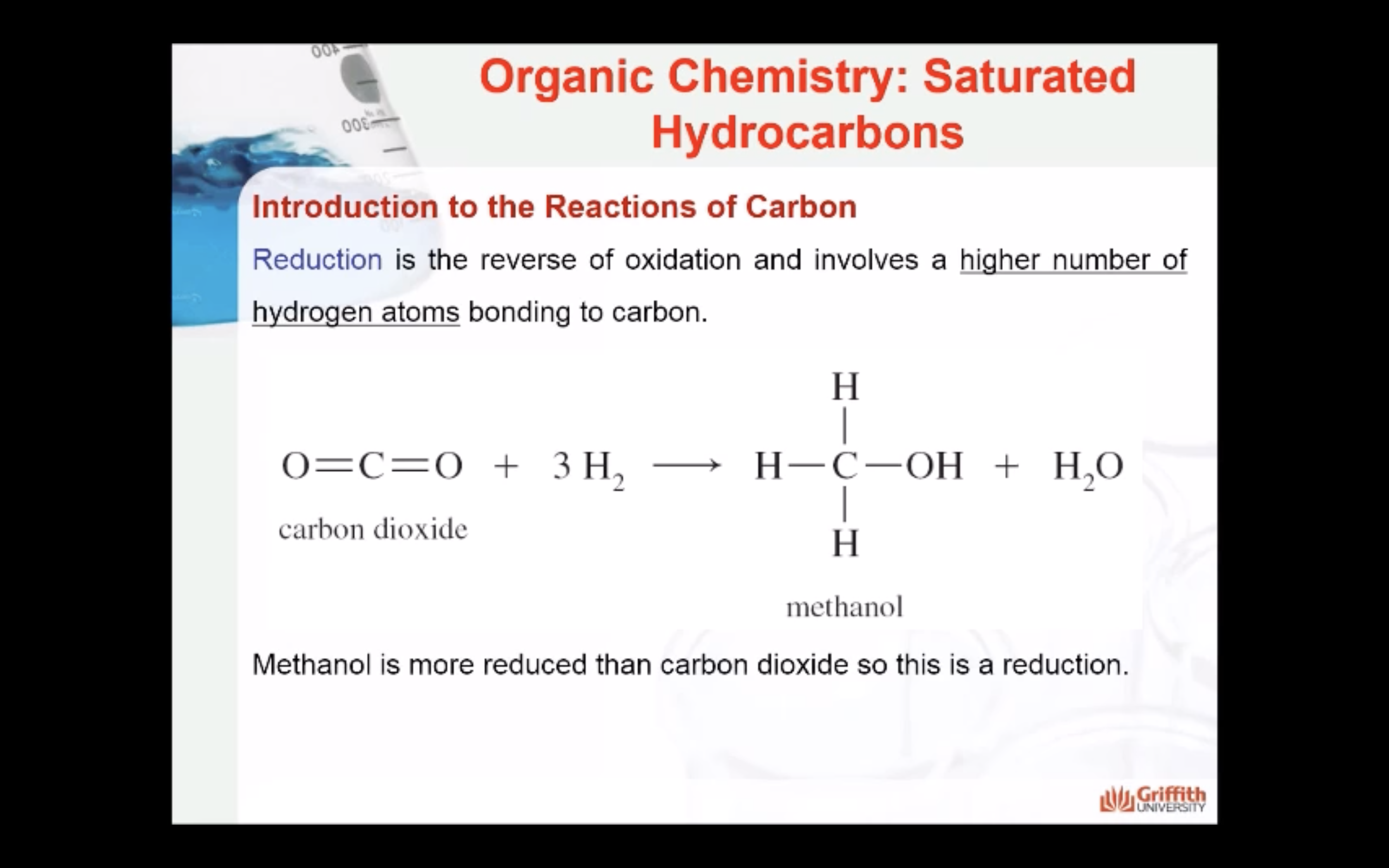
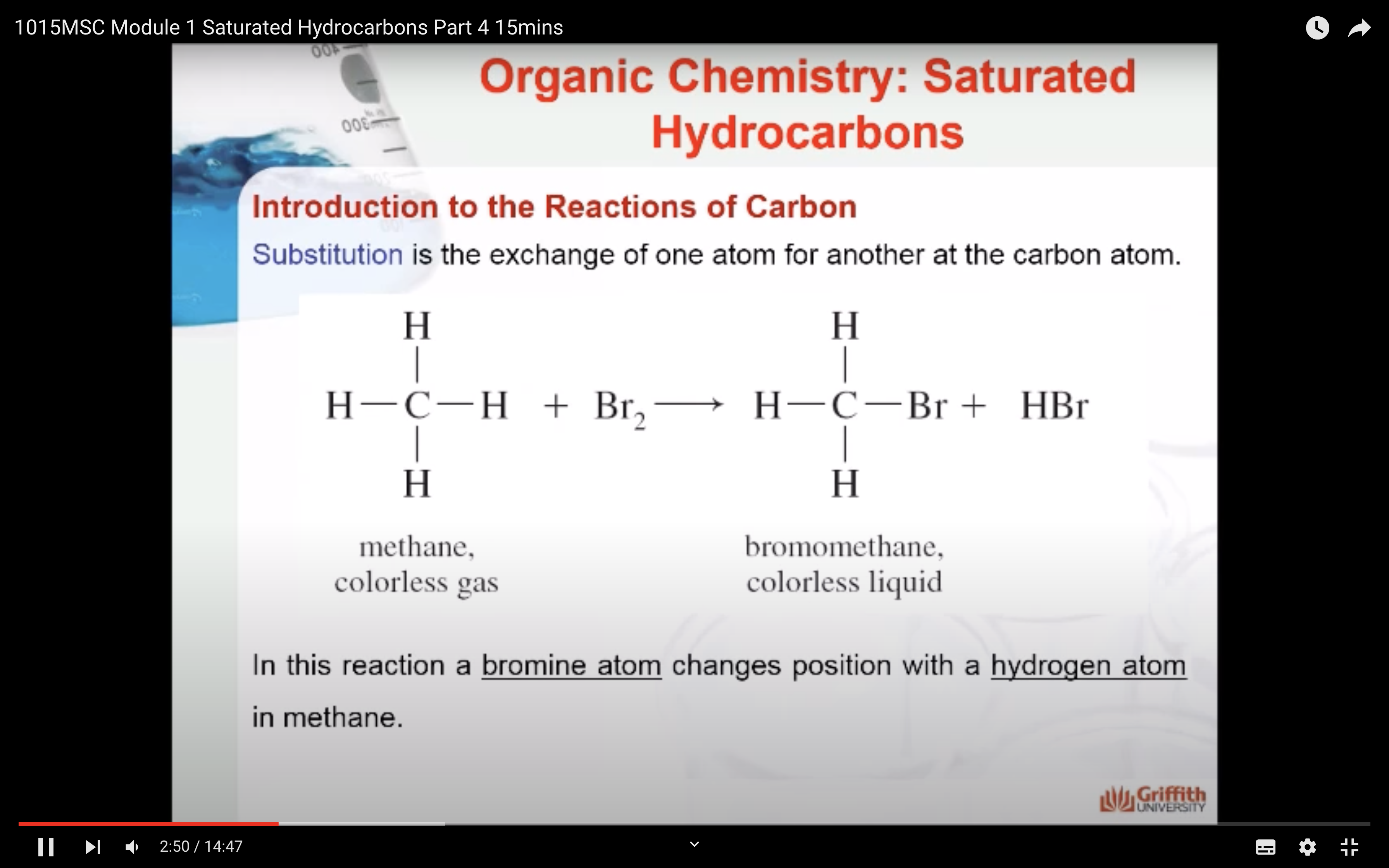
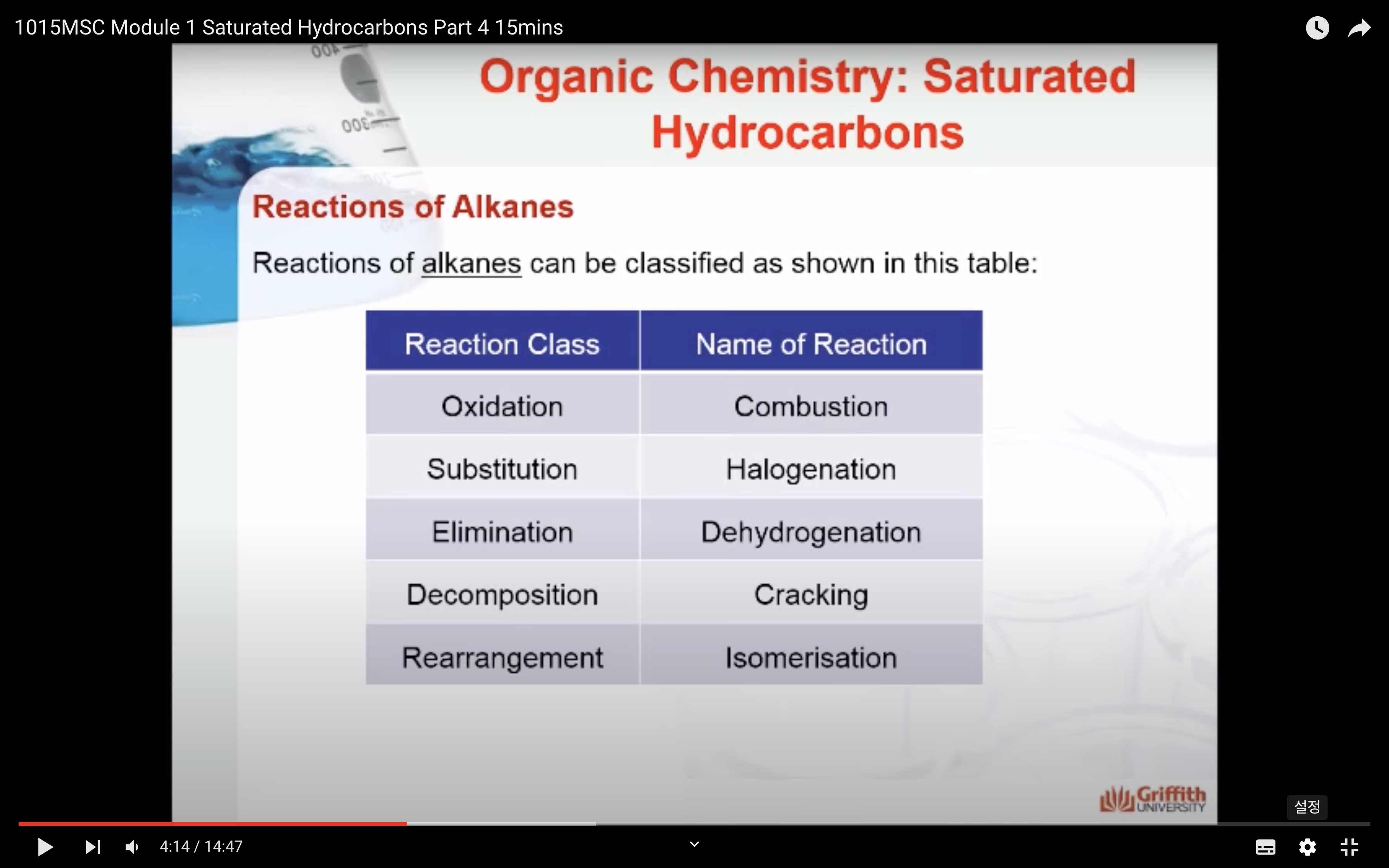
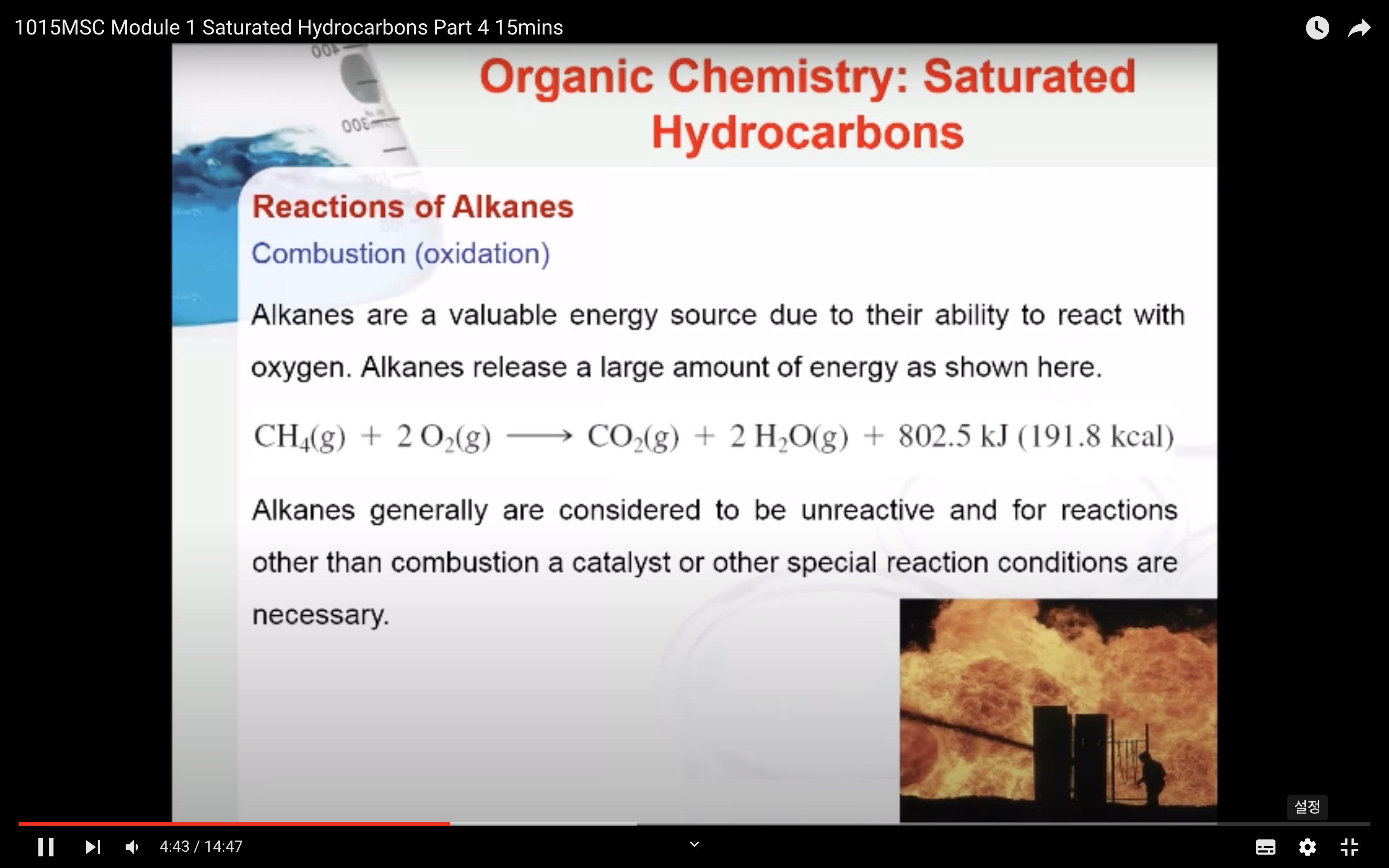
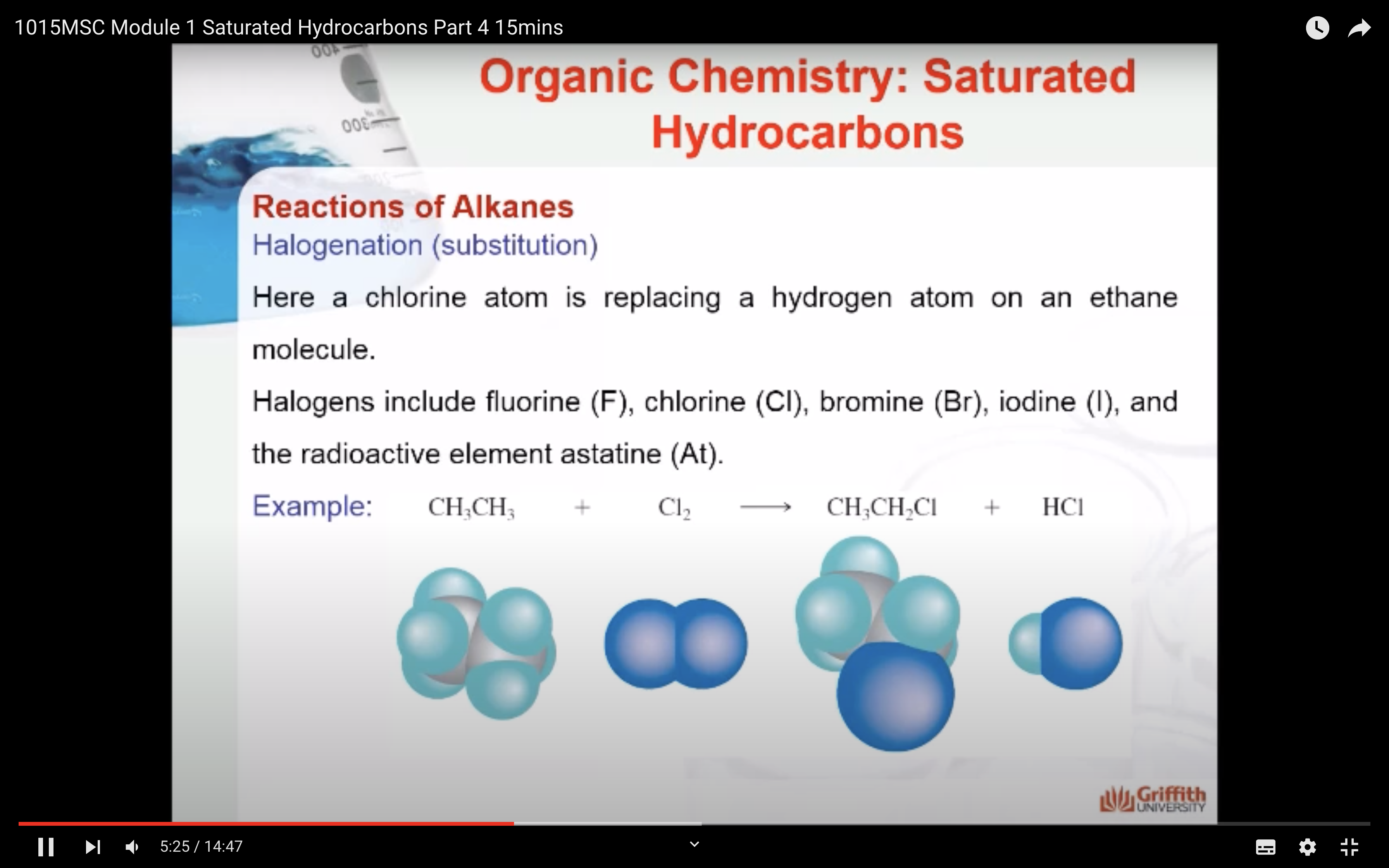
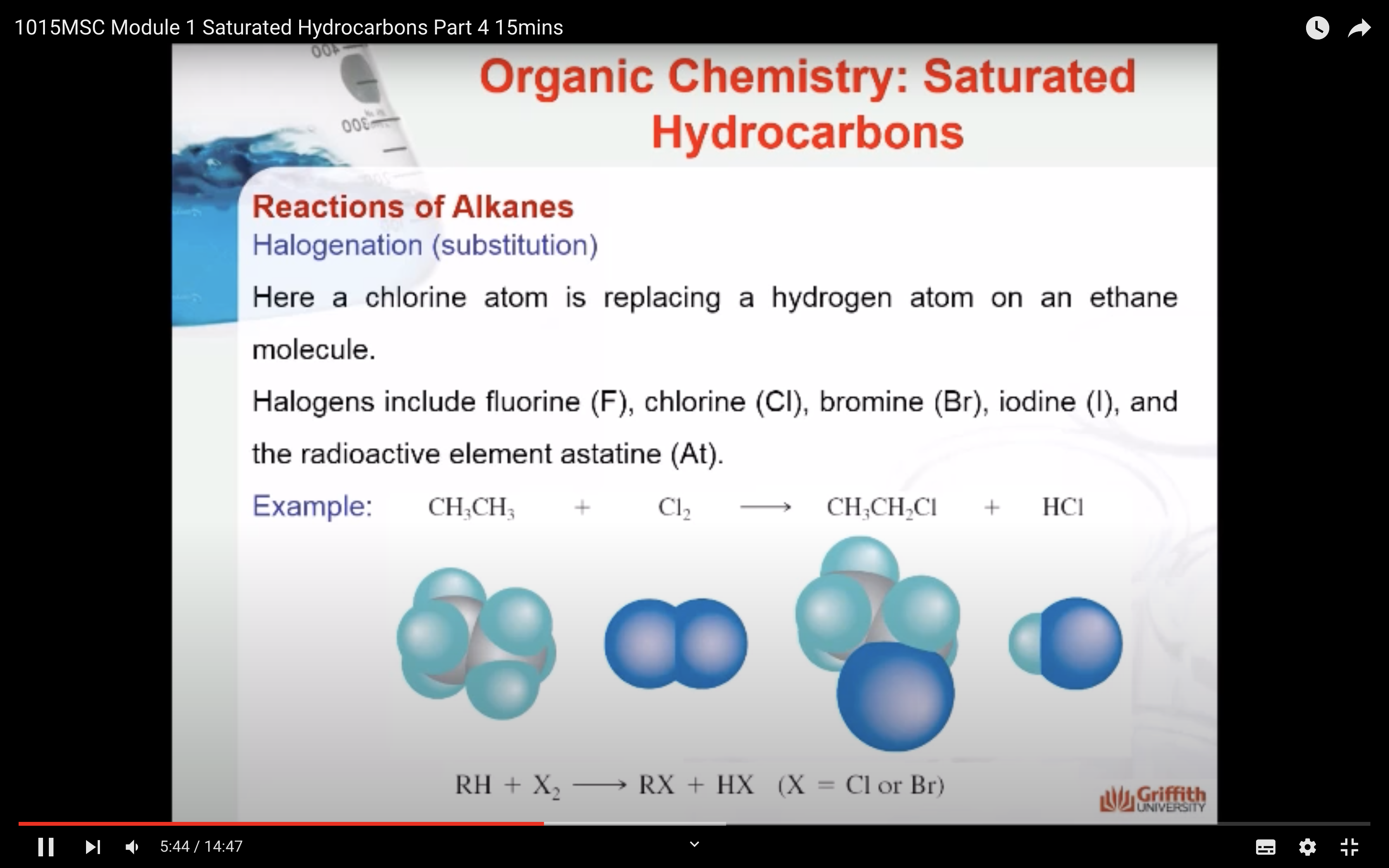
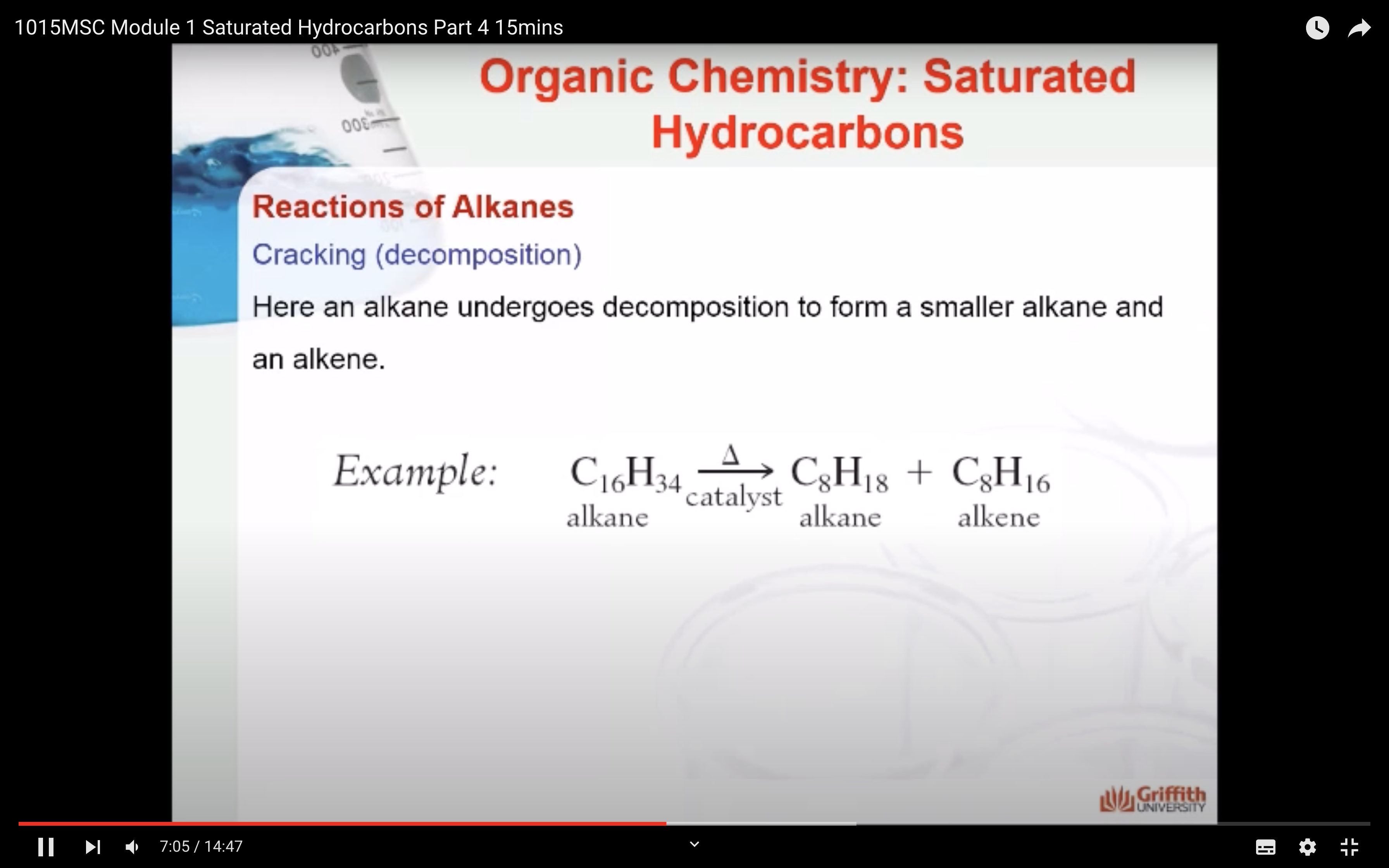
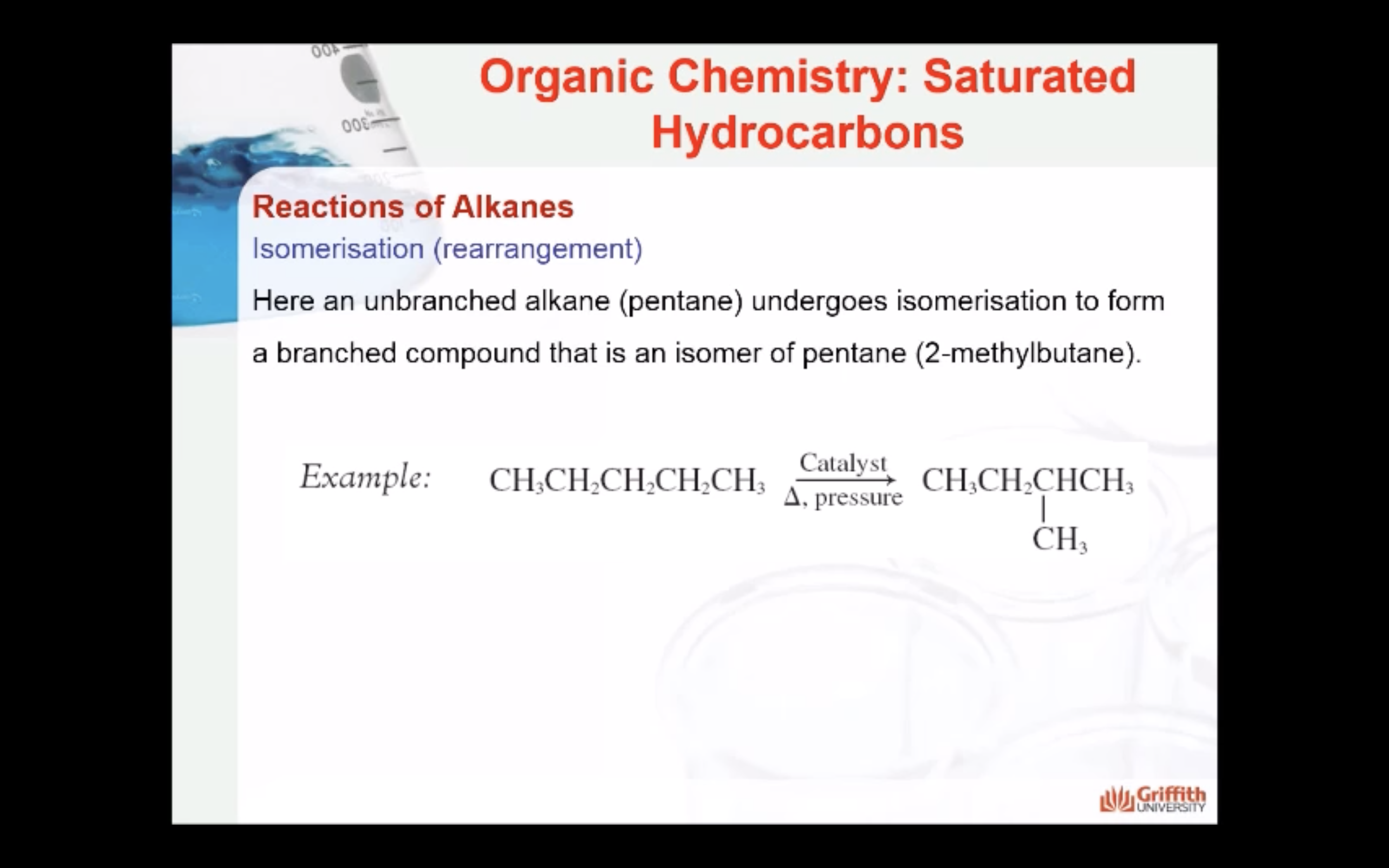
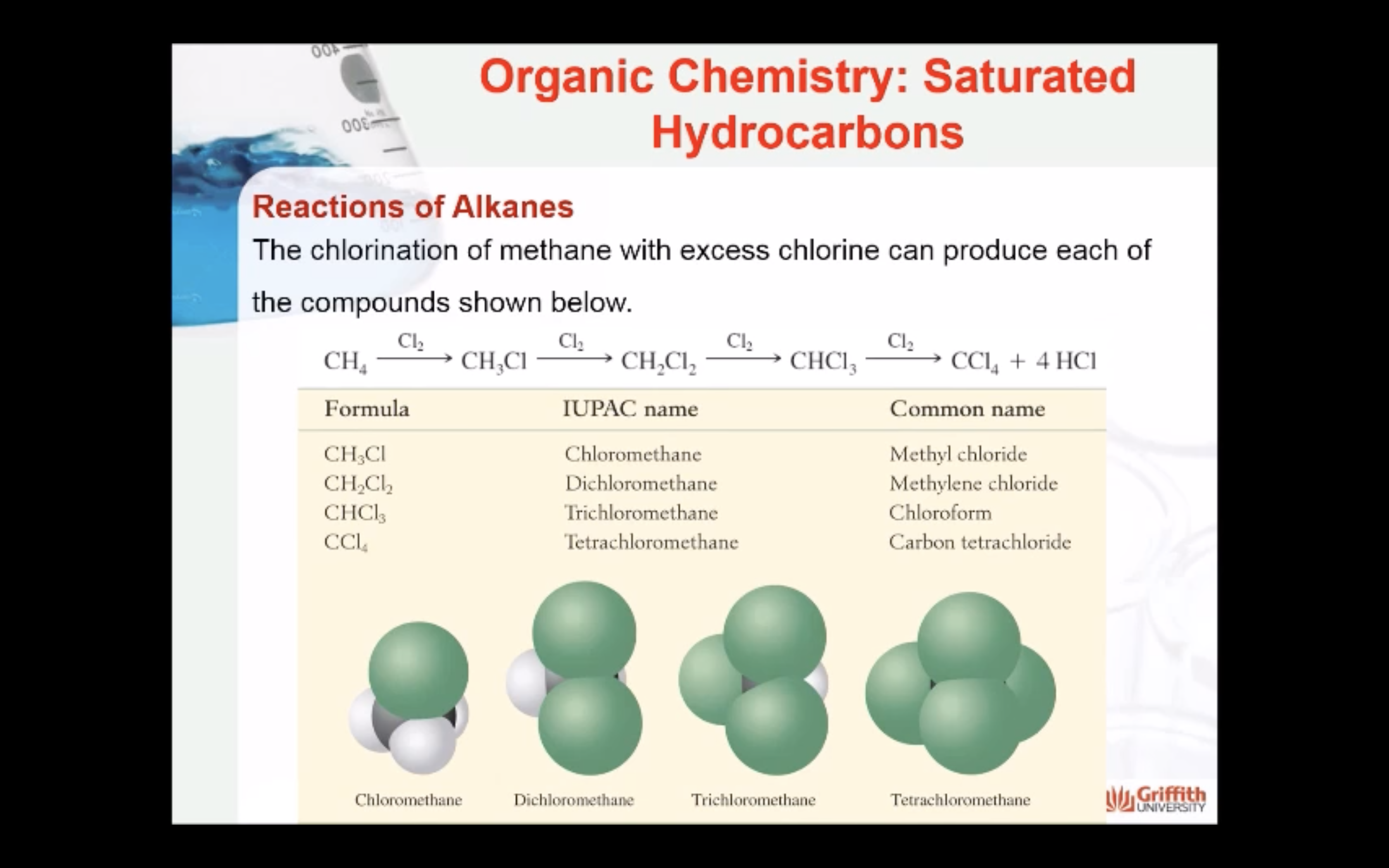
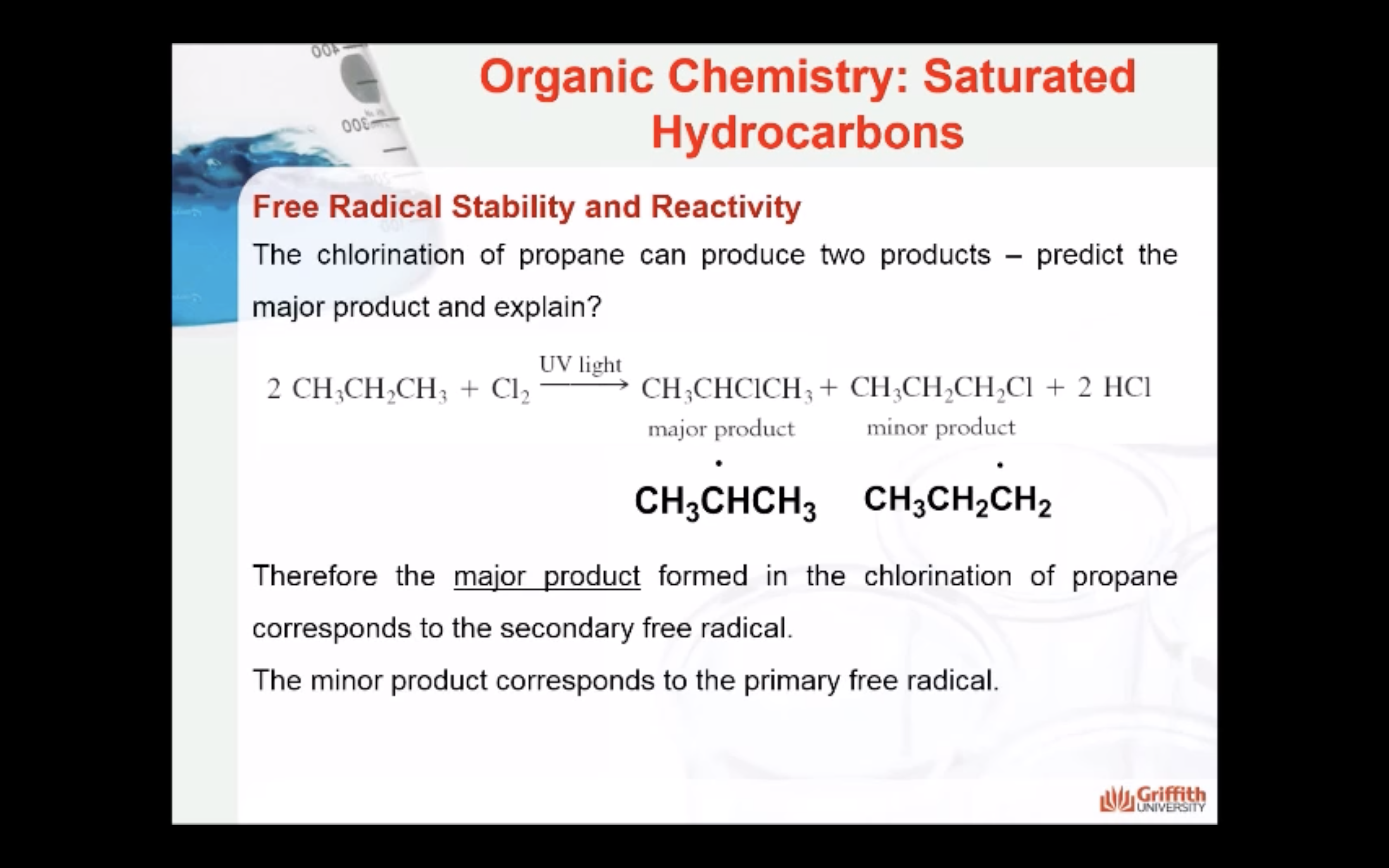
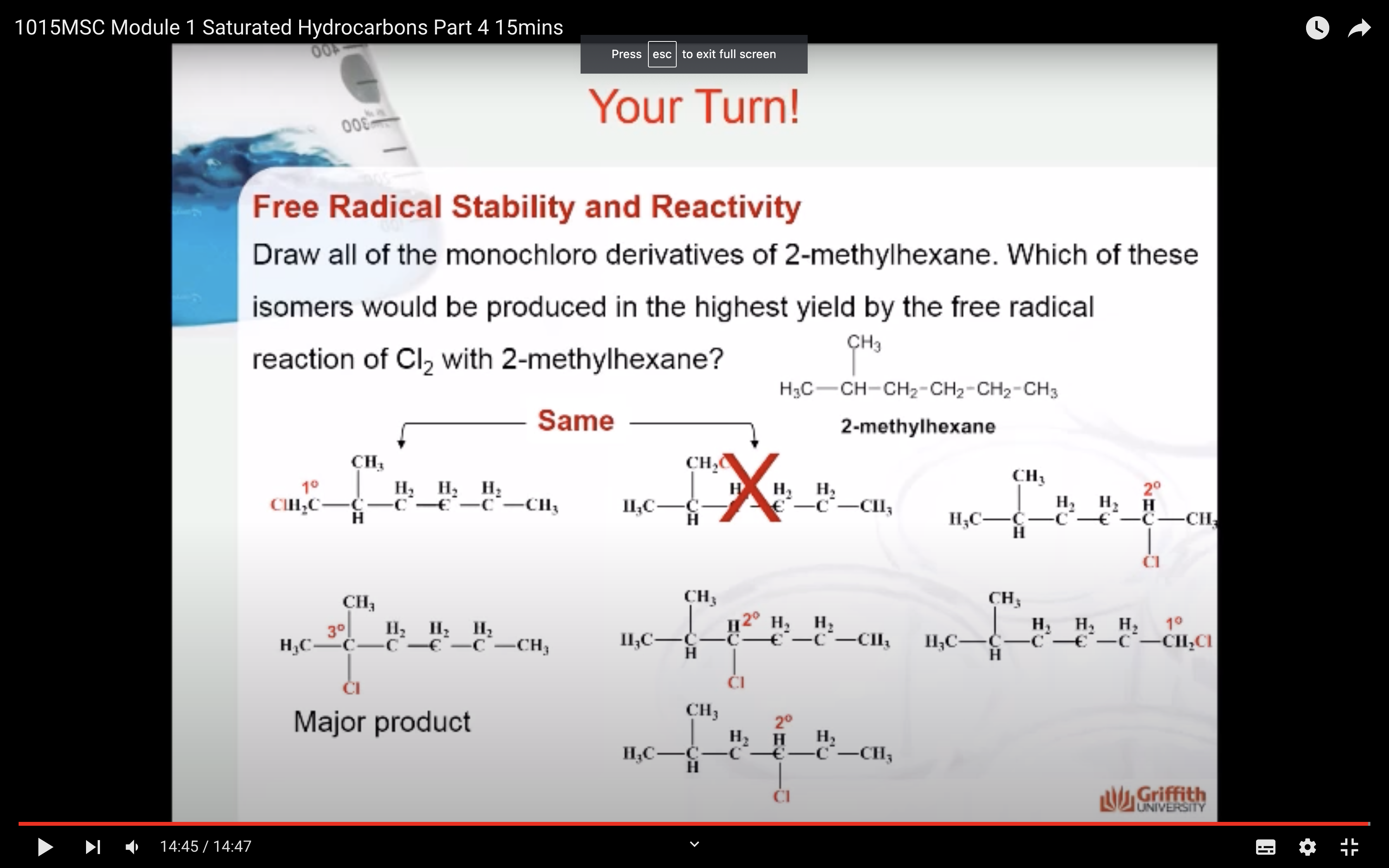
MECAHNISM OF HALOGENATION
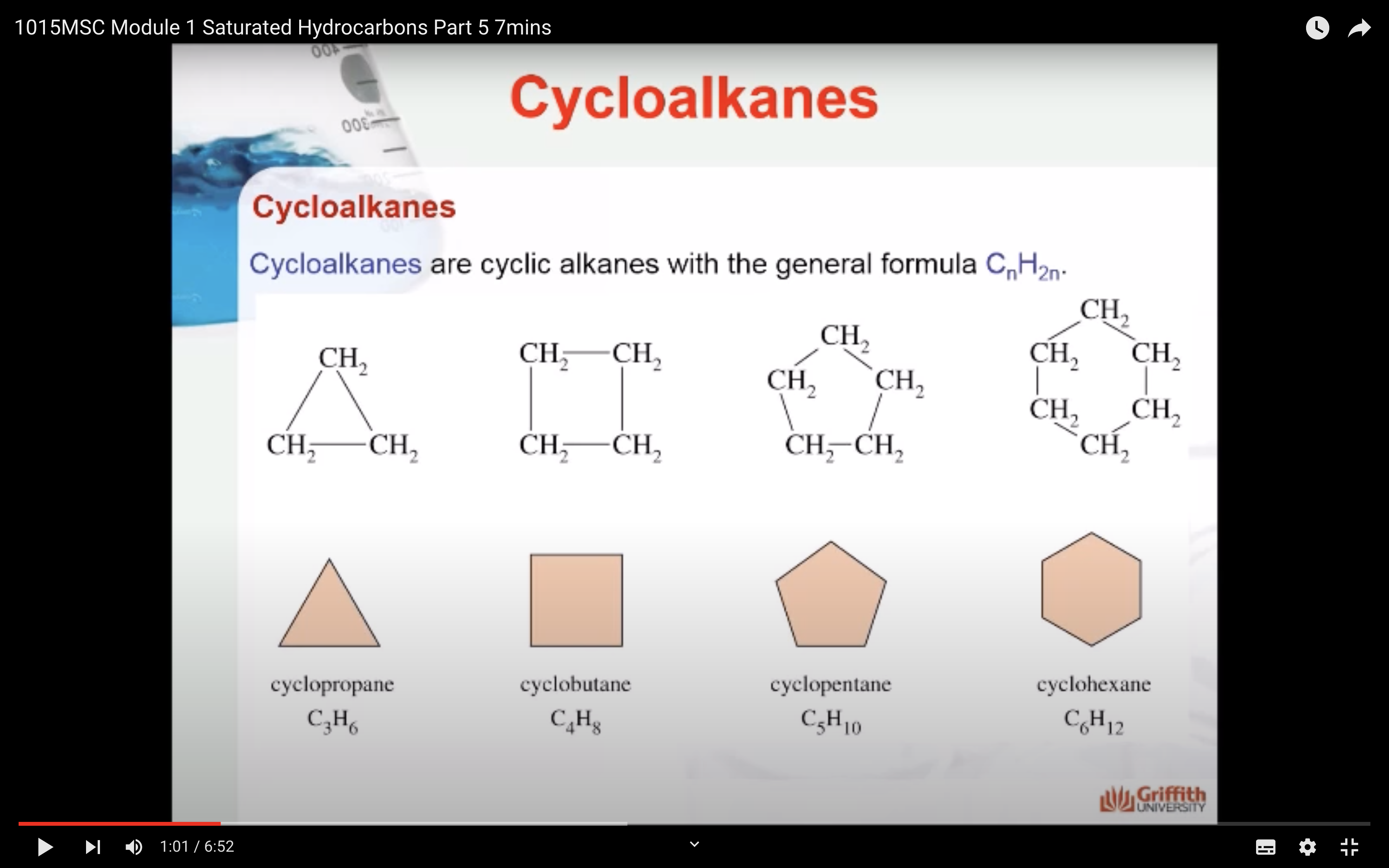
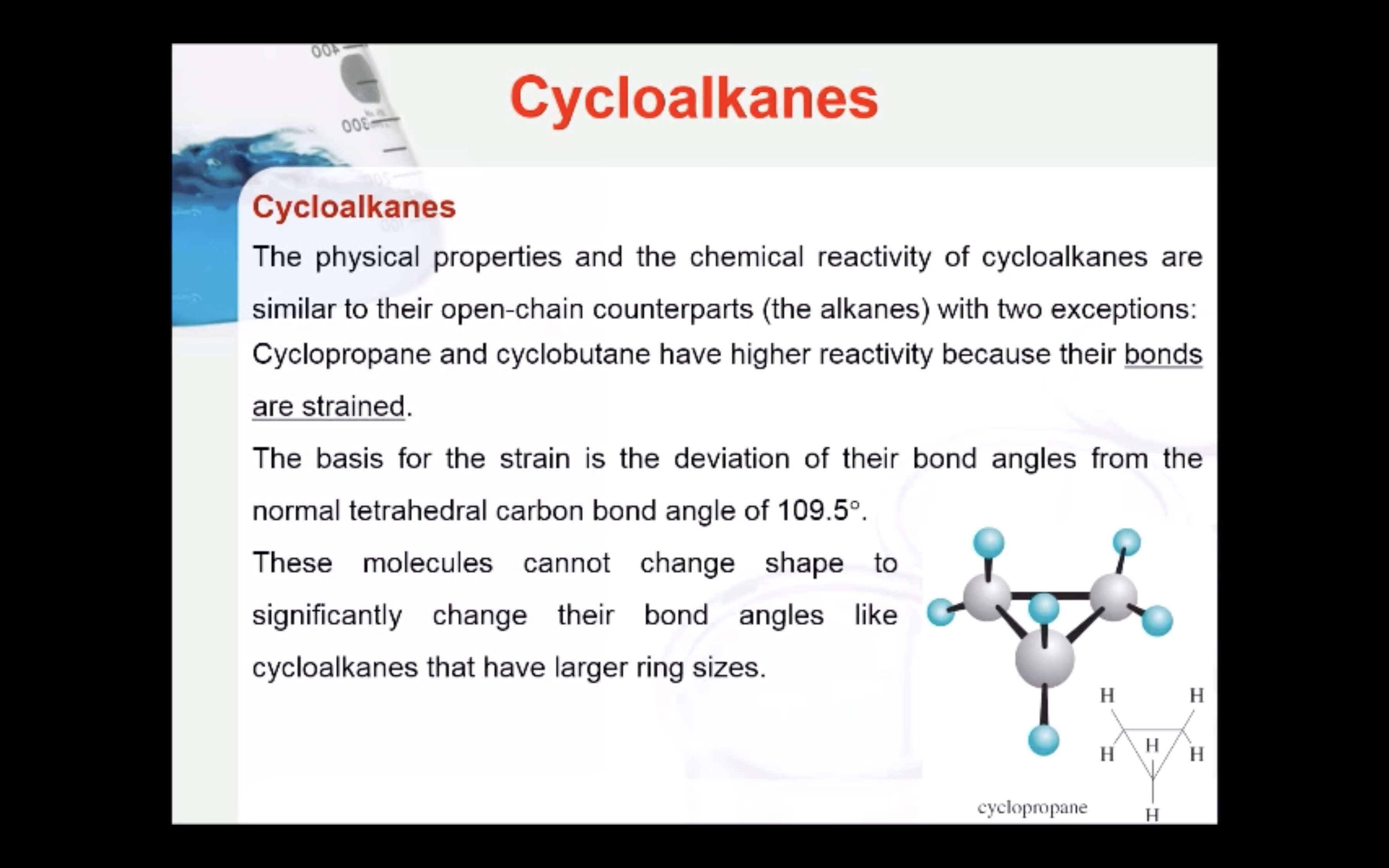
CYCLOALKANES
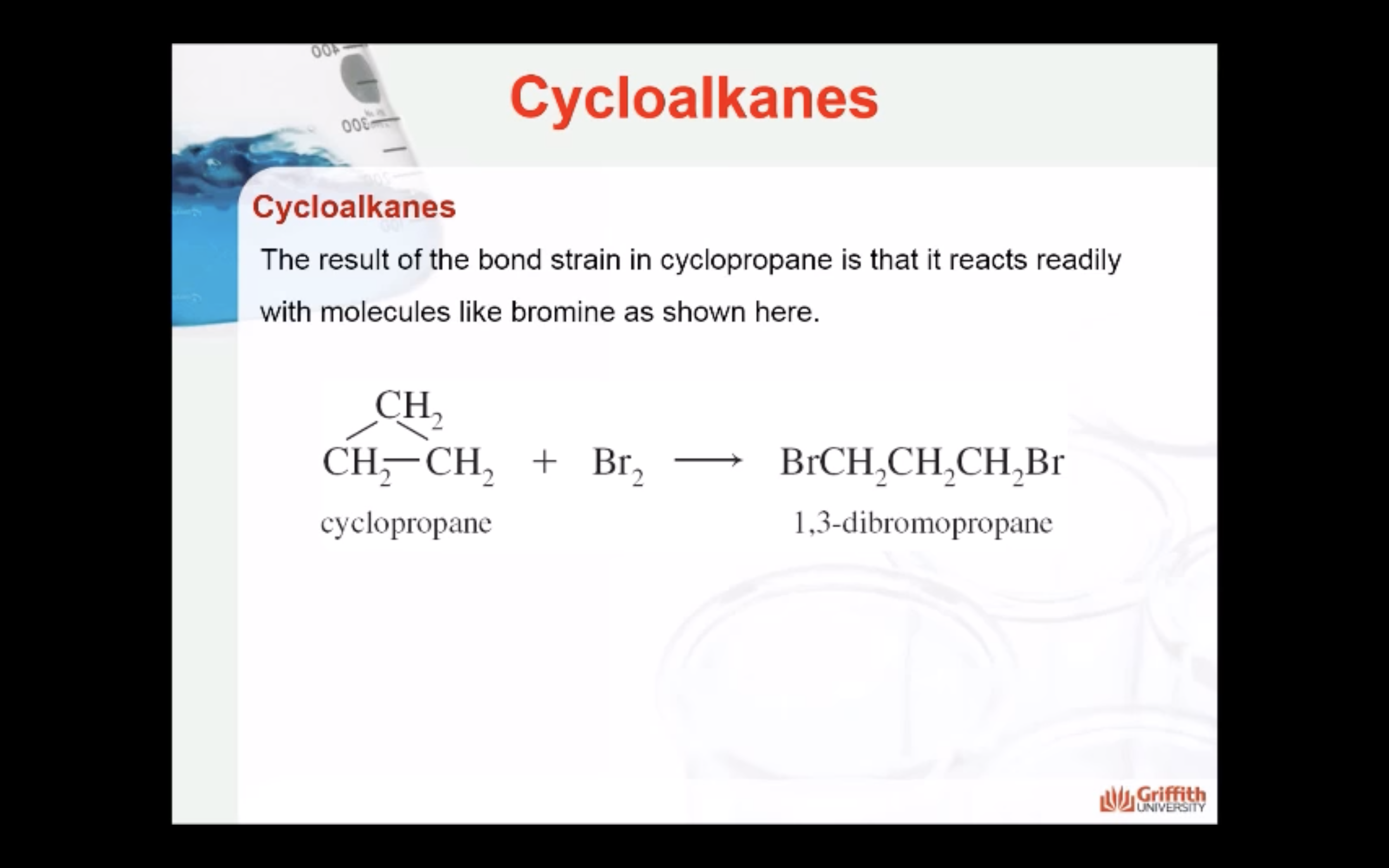
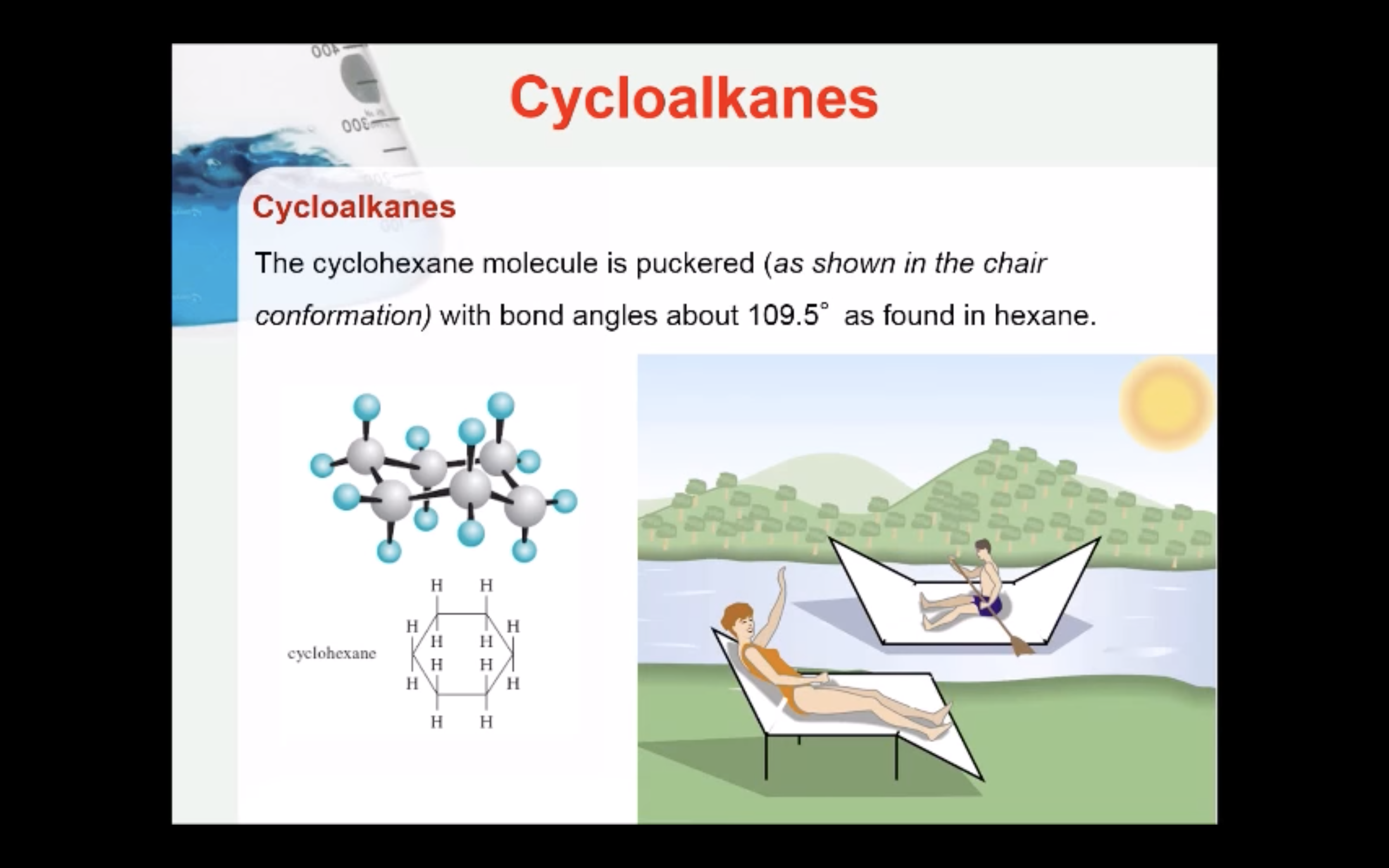
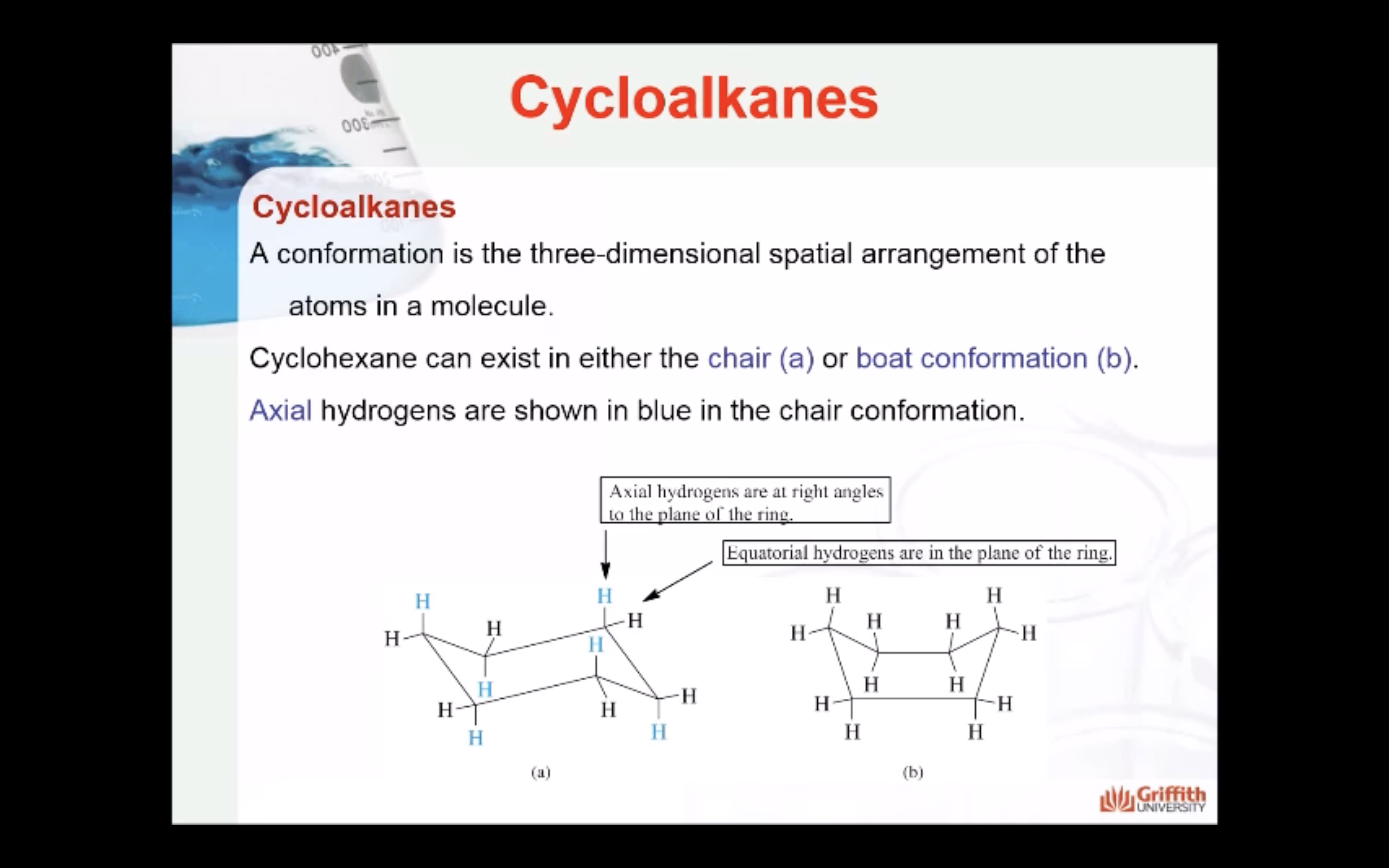
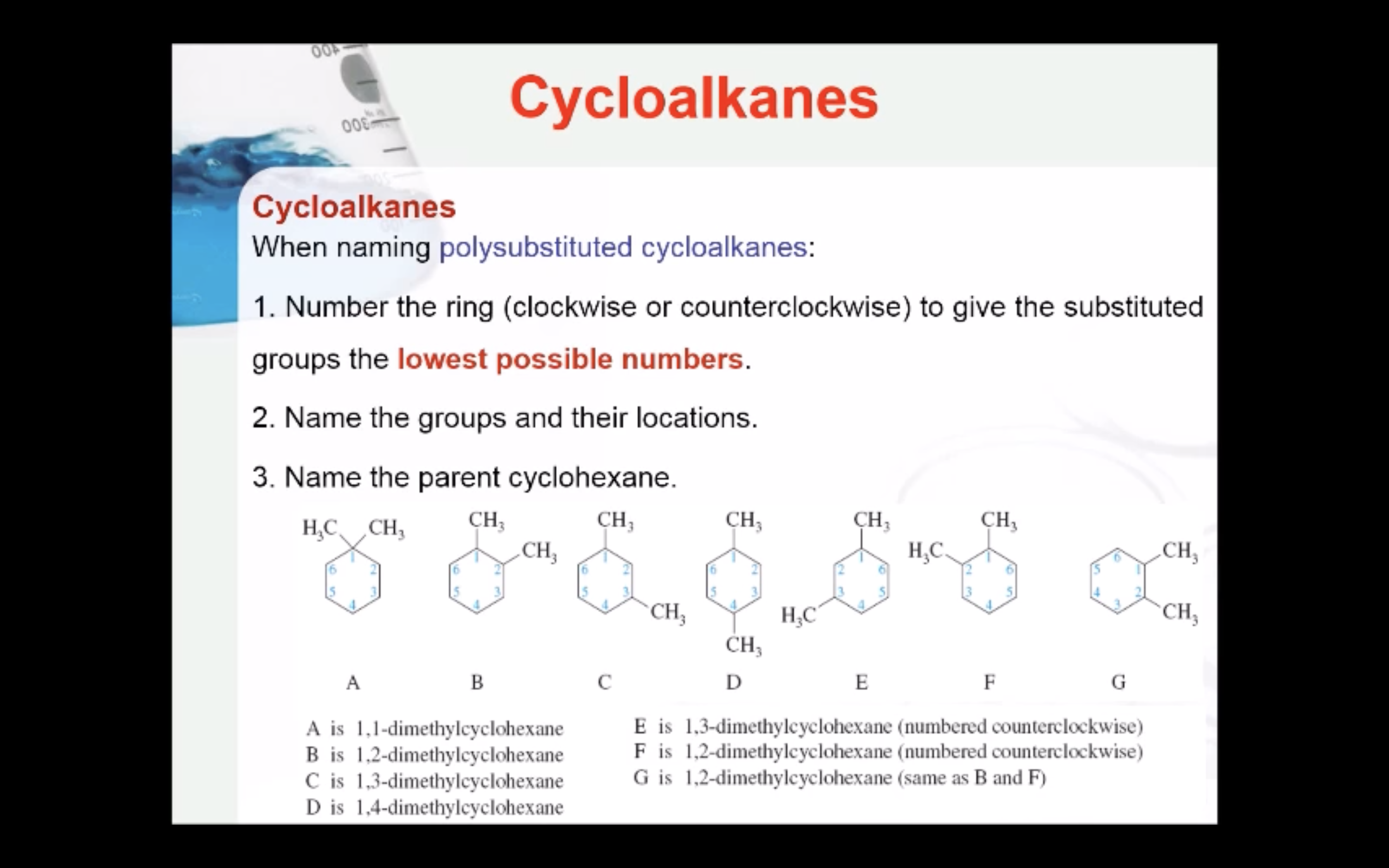
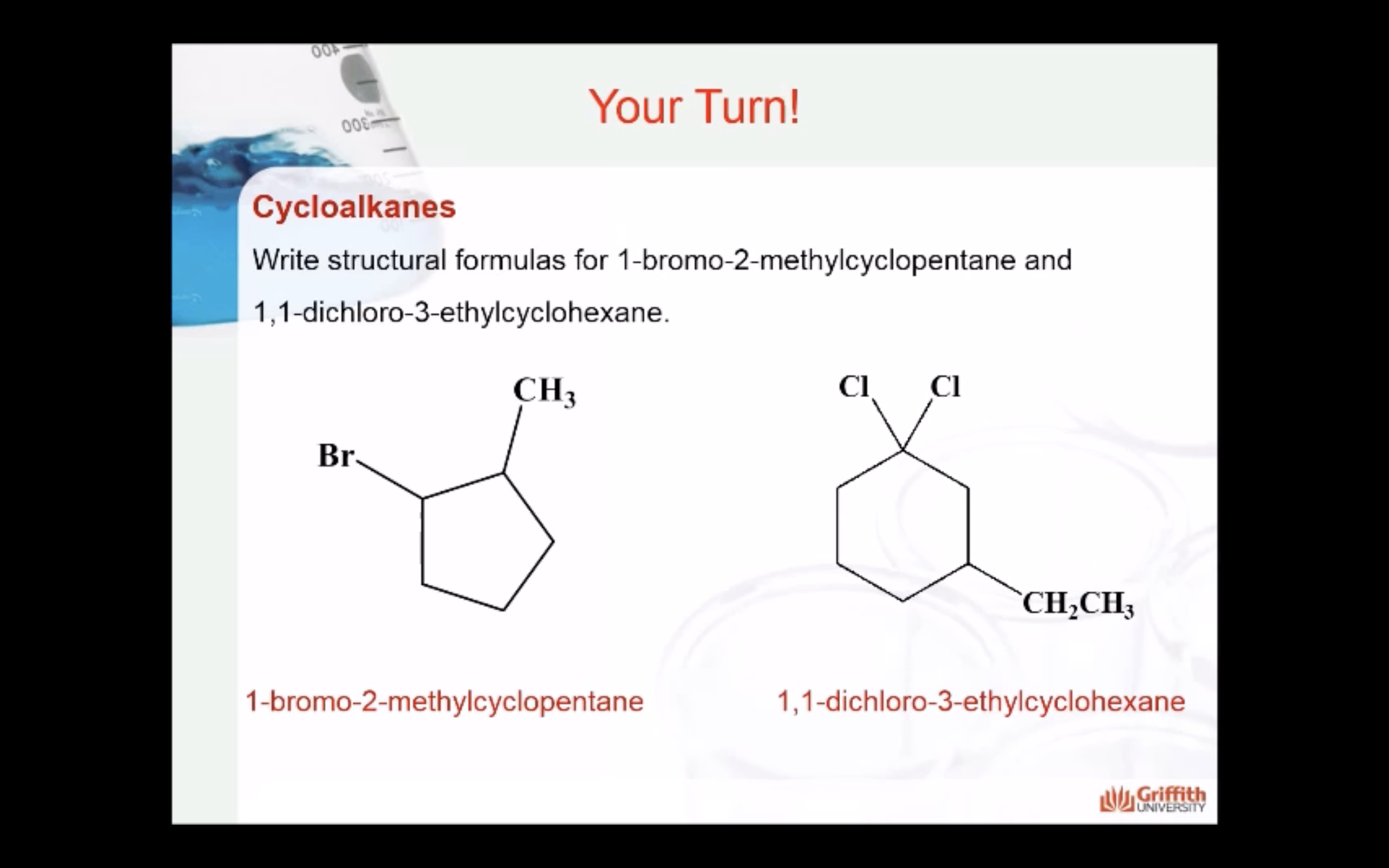
IMAGES
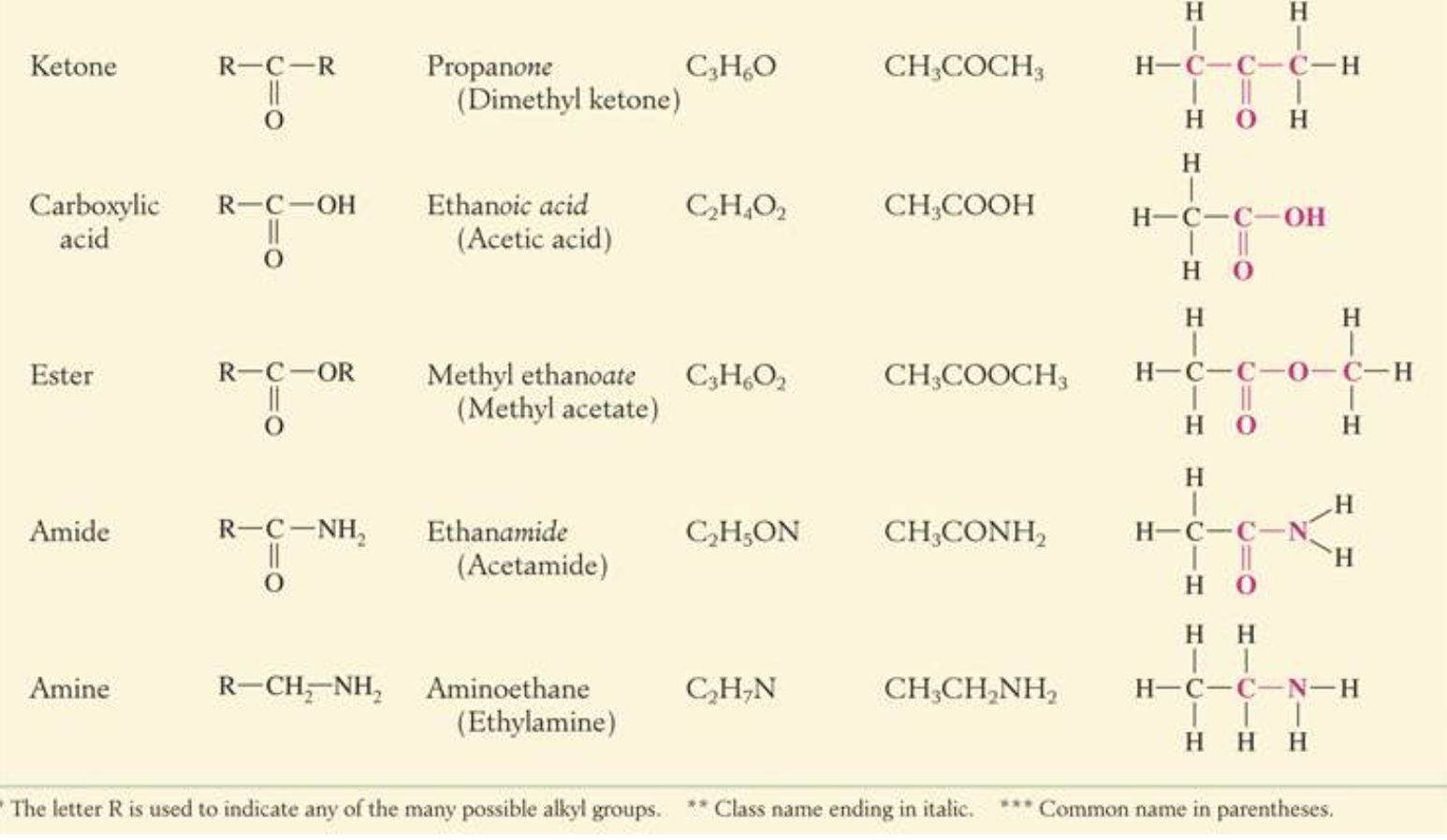
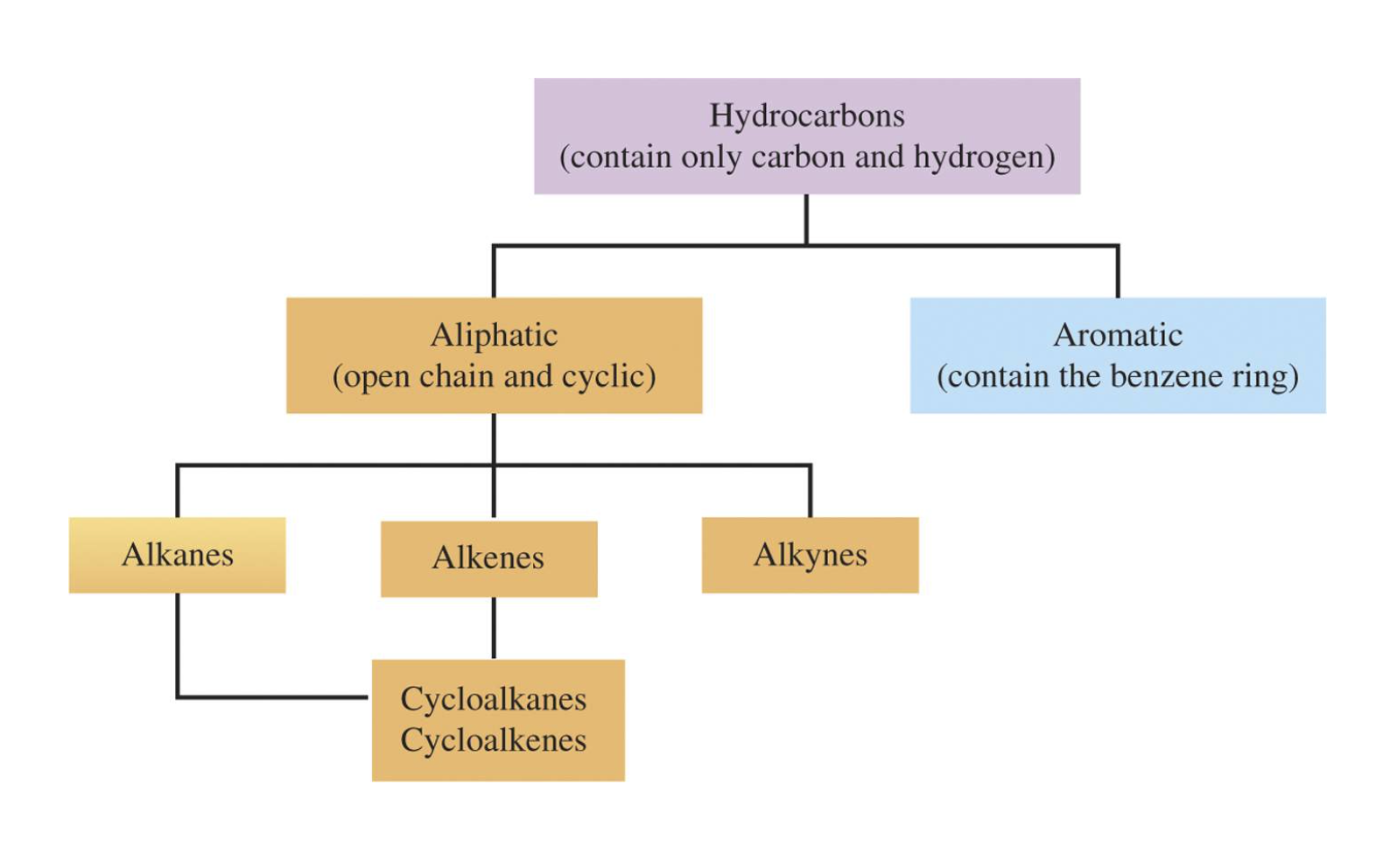
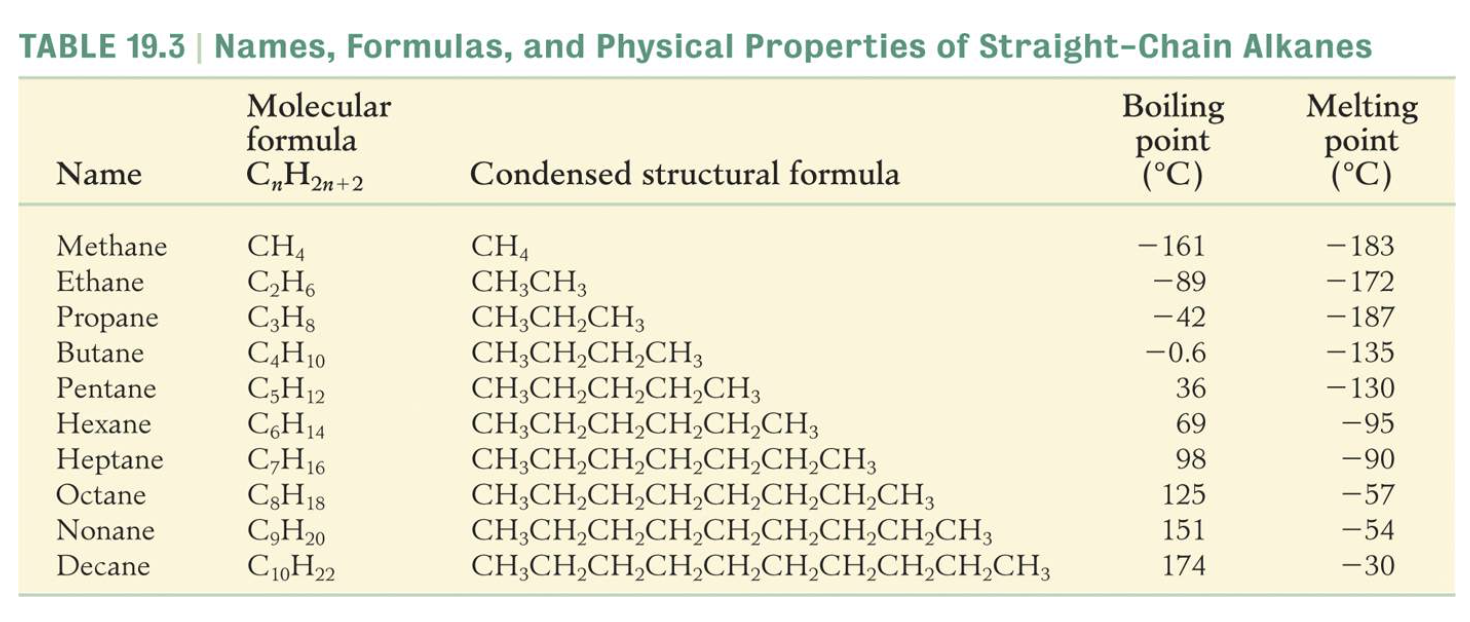
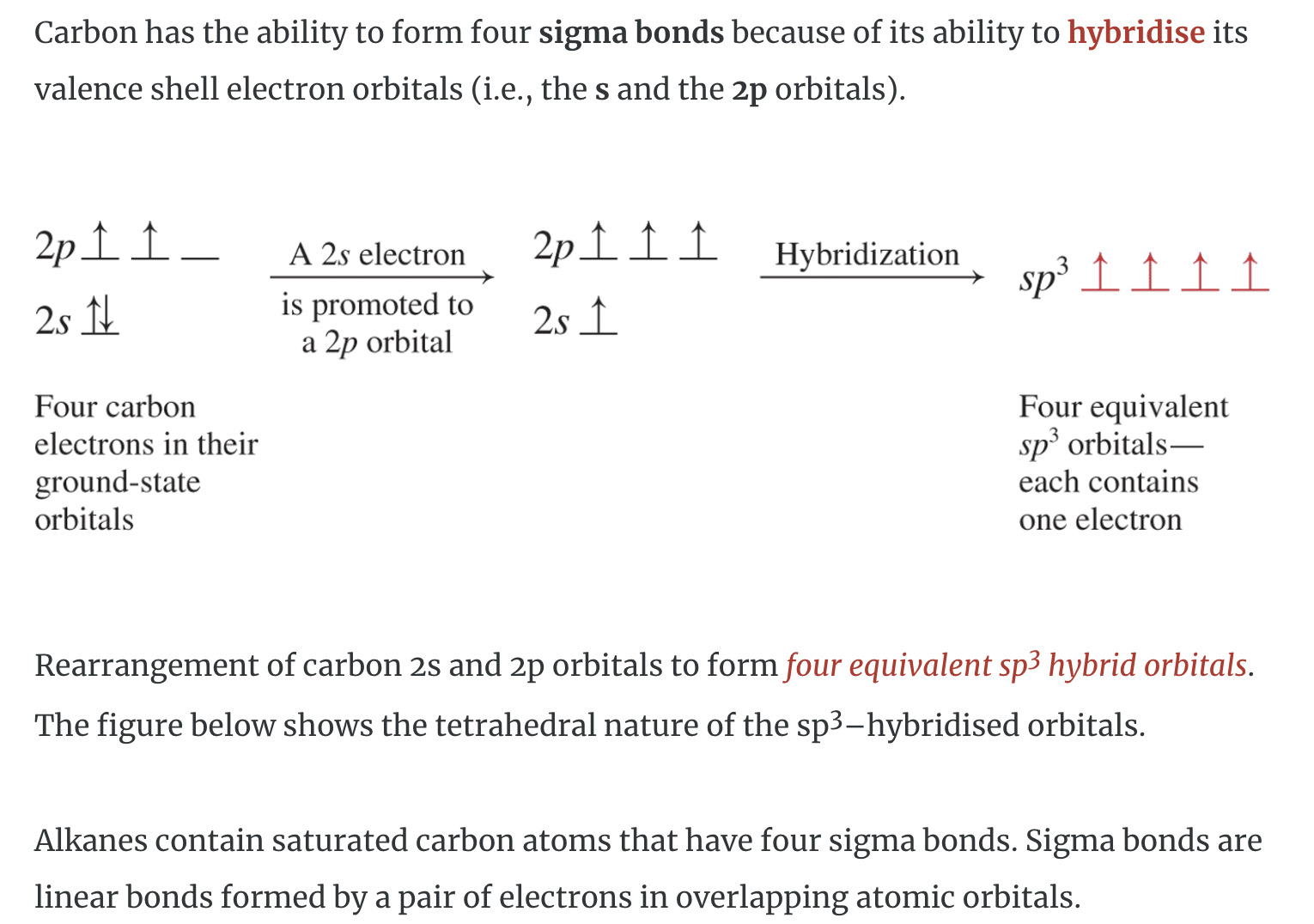



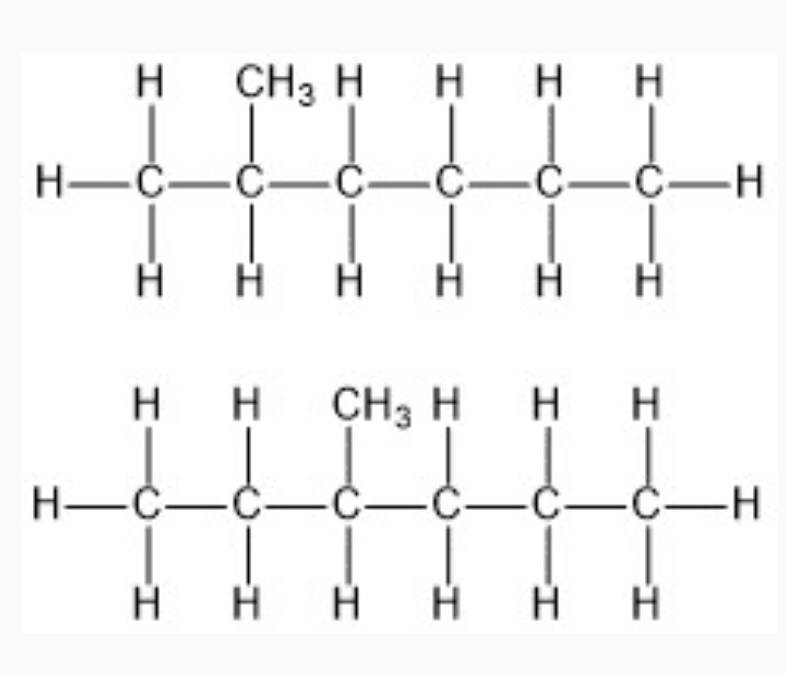

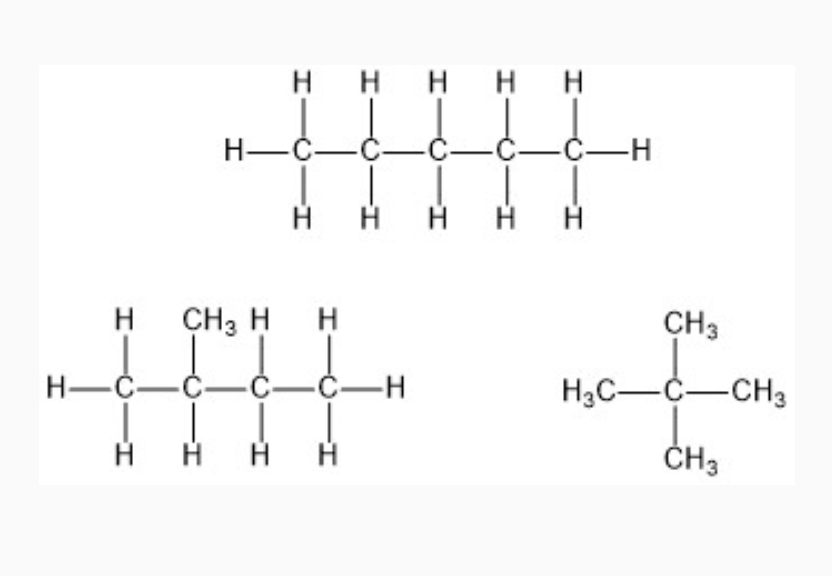
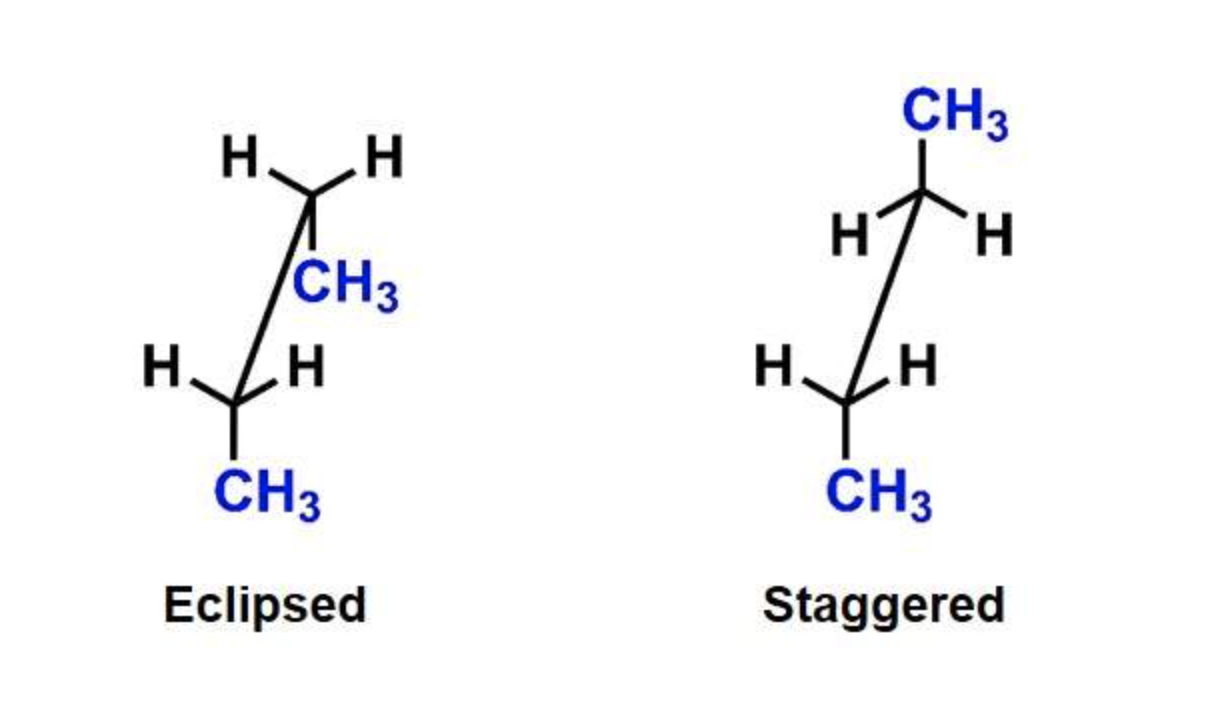
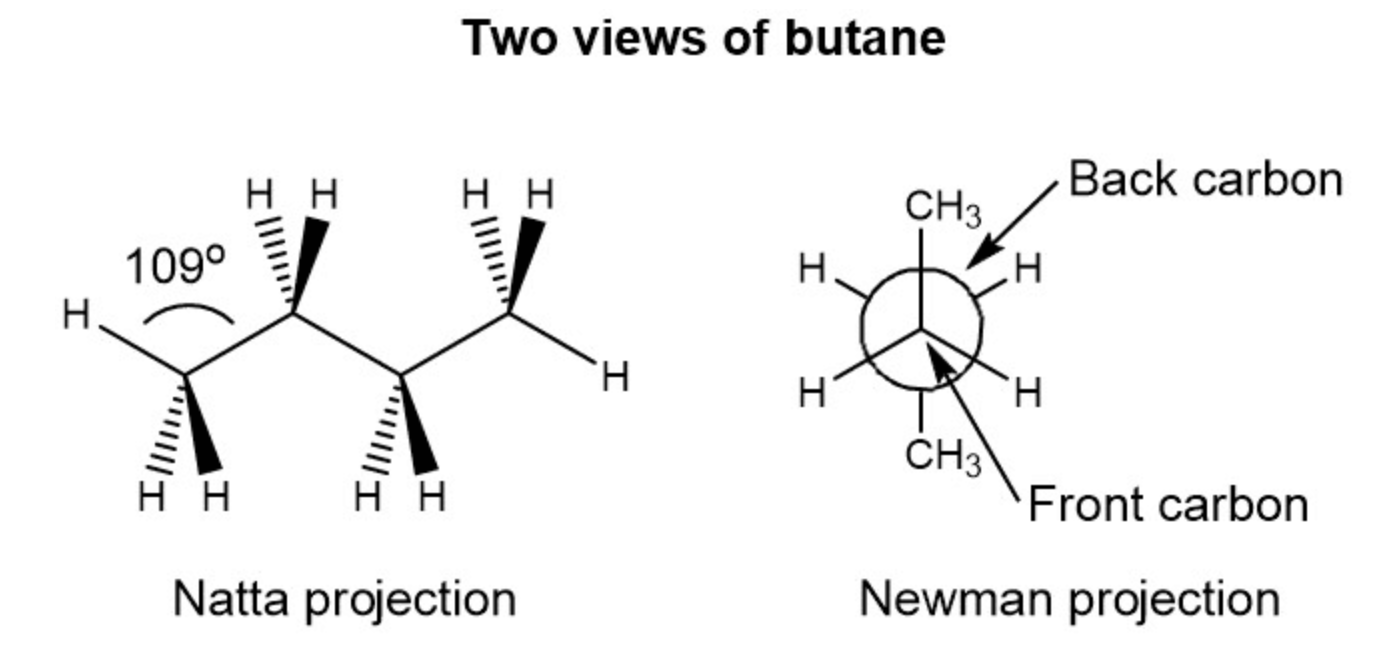















YOUTUBE VIDEOS
https://www.youtube.com/watch?v=D5NZERv_EDQ
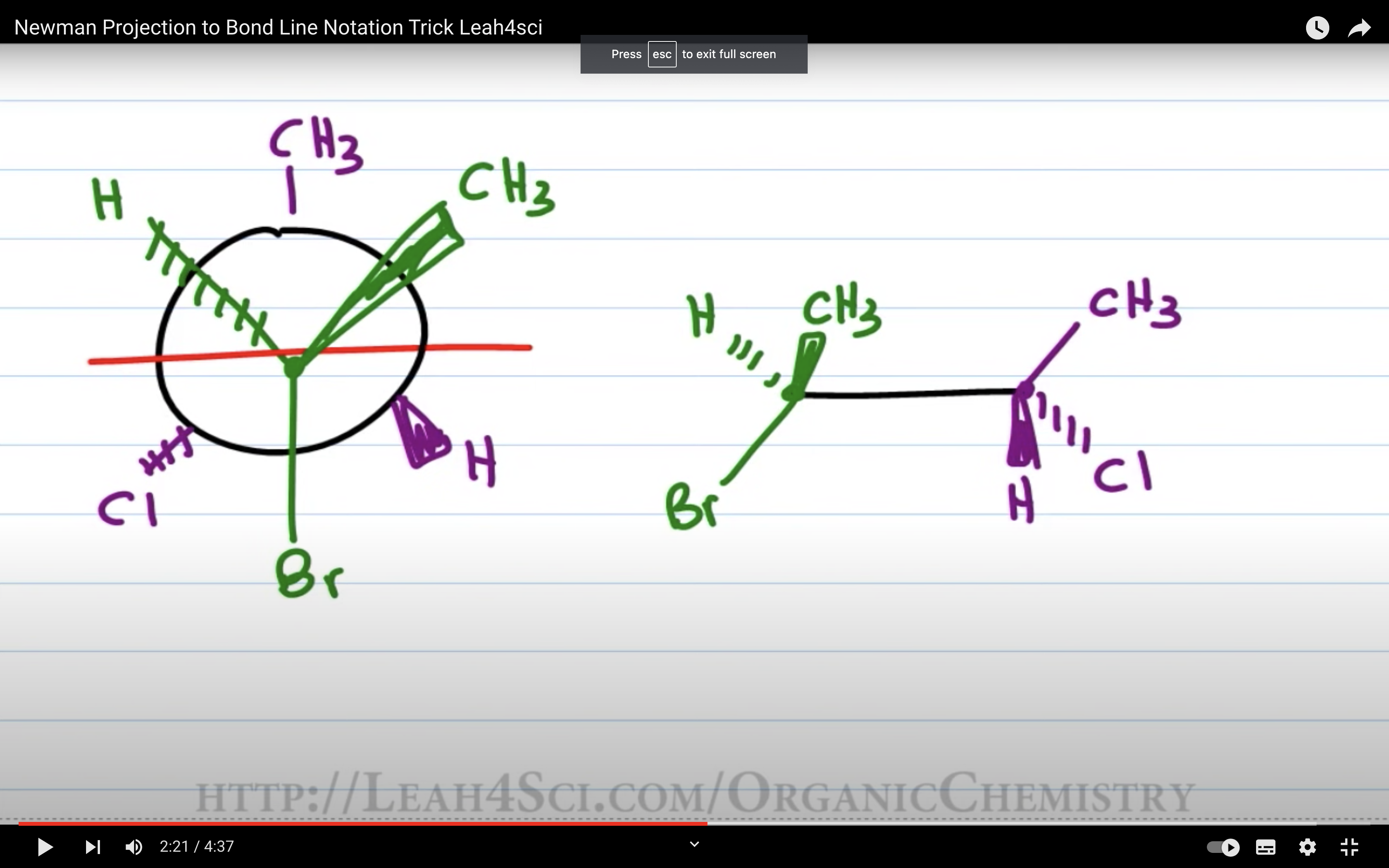
초록이를 왼쪽에 둘 경우 newman projection에서 오른쪽에 있는 애들을 튀어나온 애들로 치고
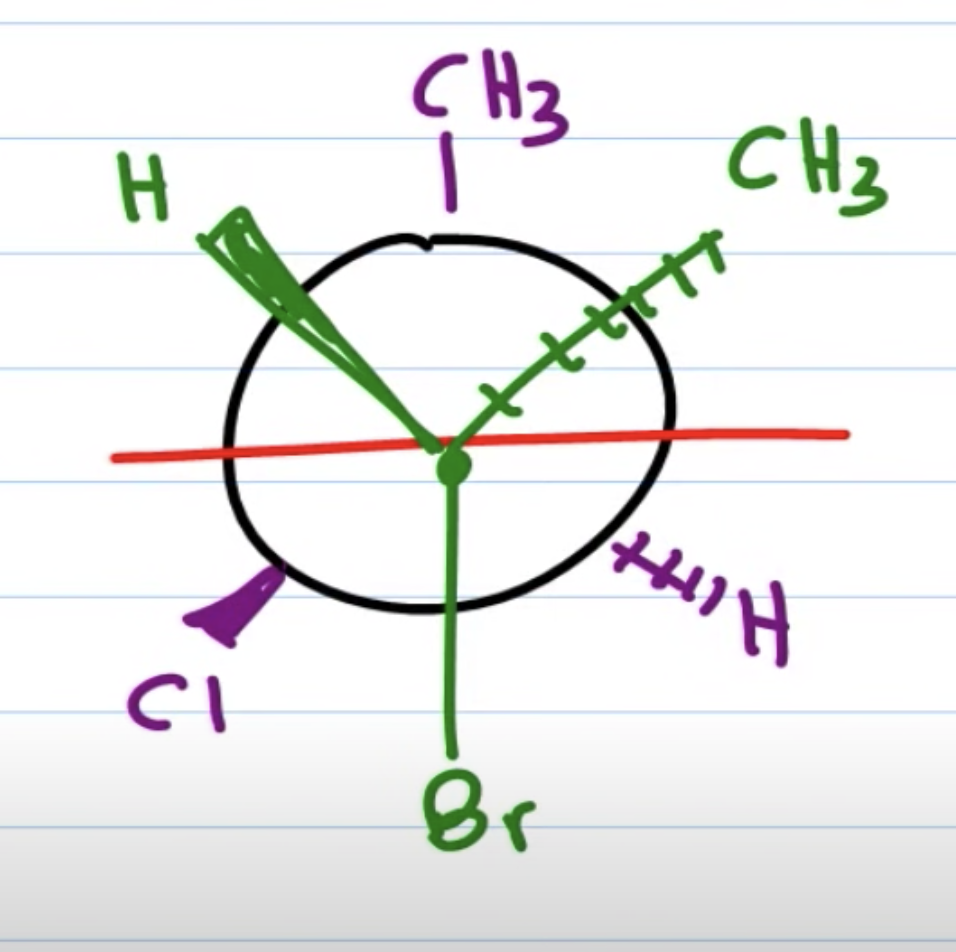
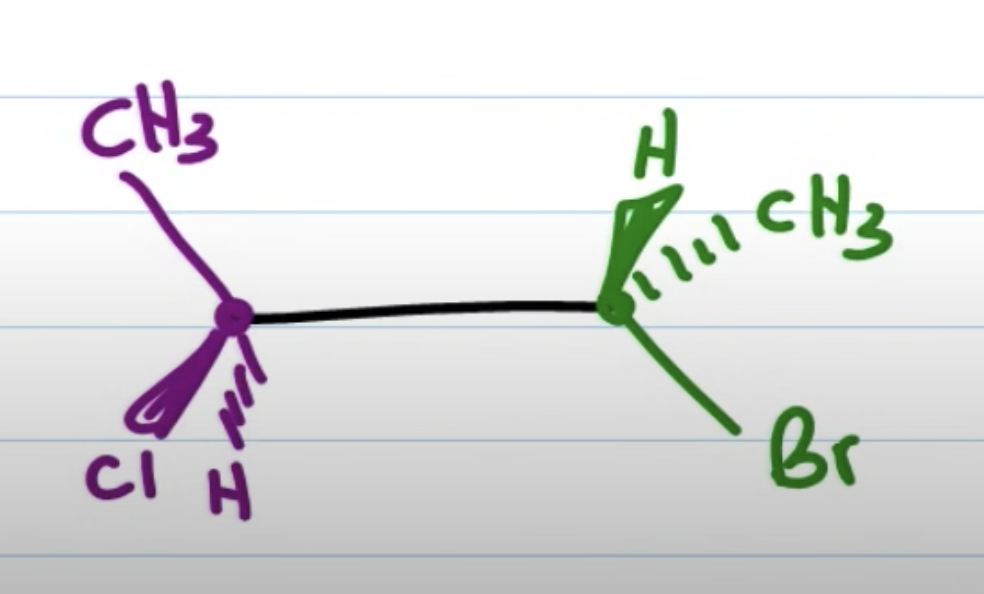
초록이를 오른편에 둘 경우 newman projection 에서 왼쪽에 있는 애들을 튀어나온 애들로 친다
(두 경우 모두 위아래 애들은 위아래에 둔다)
https://www.youtube.com/watch?v=ryHnC0wqTTo&list=PL8dPuuaLjXtONguuhLdVmq0HTKS0jksS4
아직 안 본 영상 자료들
https://www.youtube.com/watch?v=GRVxDqhgOYo&list=PL8dPuuaLjXtONguuhLdVmq0HTKS0jksS4
https://www.youtube.com/watch?v=cpWHgKgKxGk&list=PL8dPuuaLjXtONguuhLdVmq0HTKS0jksS4
https://www.youtube.com/watch?v=HhT2E7wuAgg
sp3 orbitals, pi and sigma bonding CLEARIFYING VIDEO
https://www.youtube.com/watch?v=vHXViZTxLXo
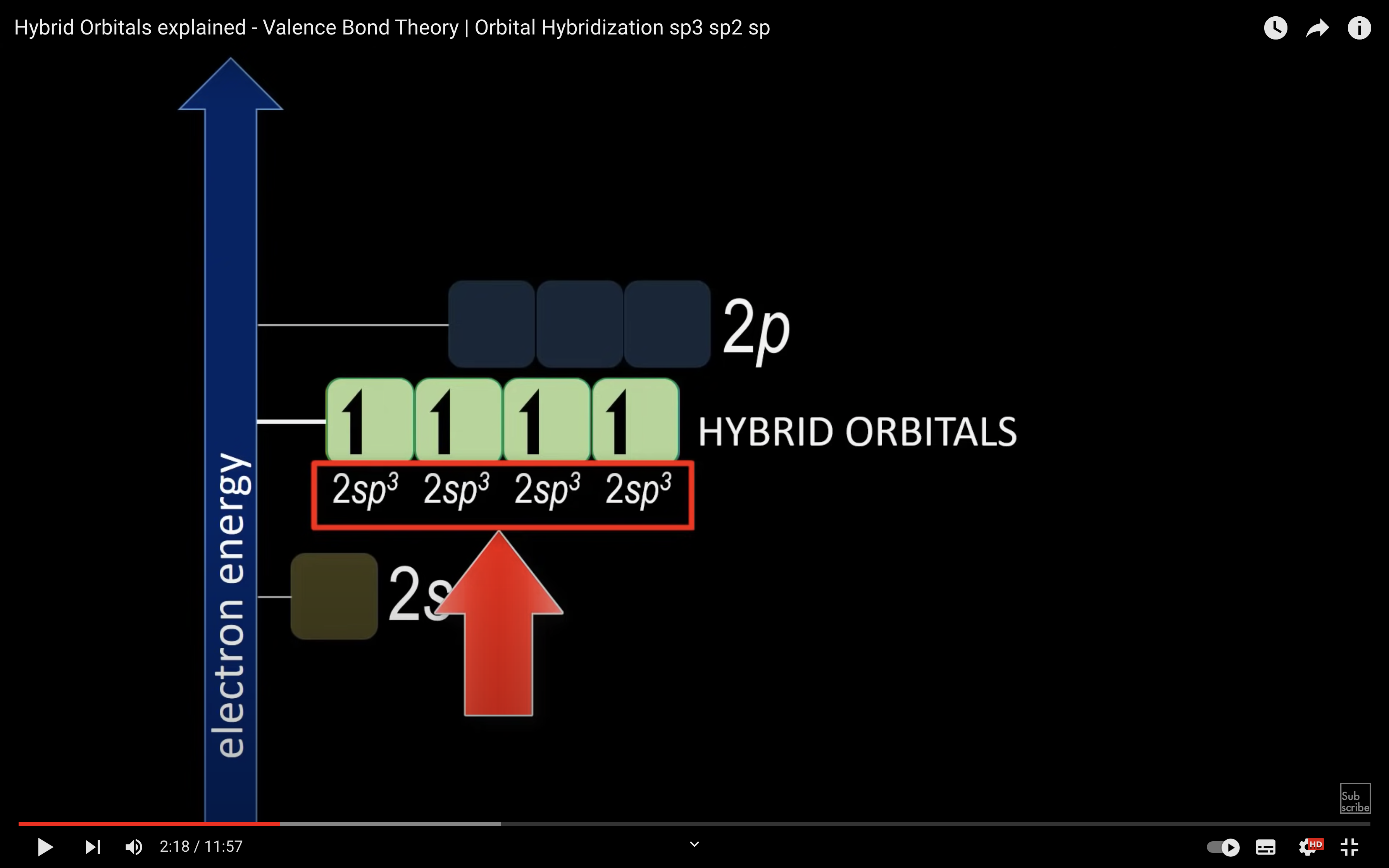
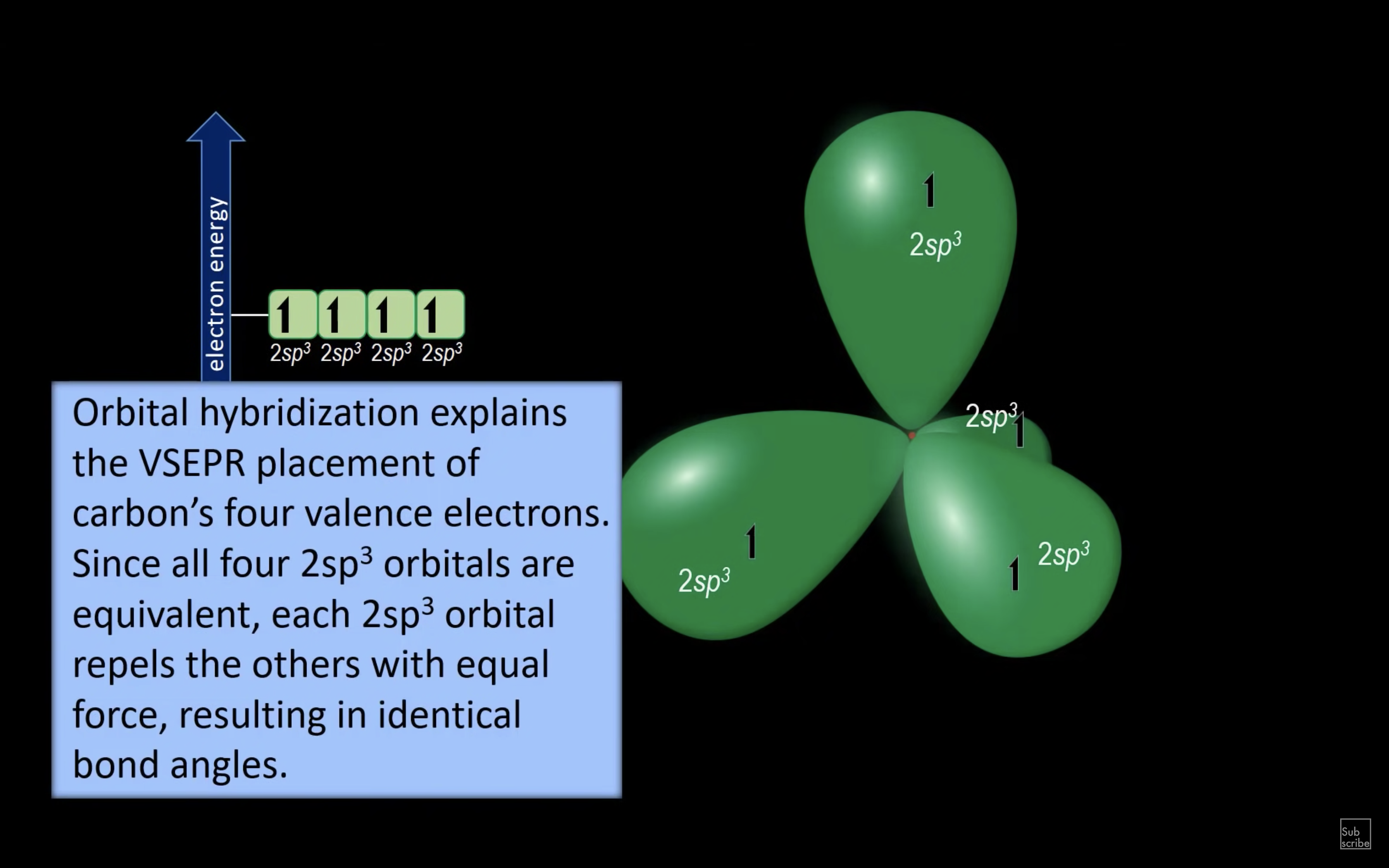
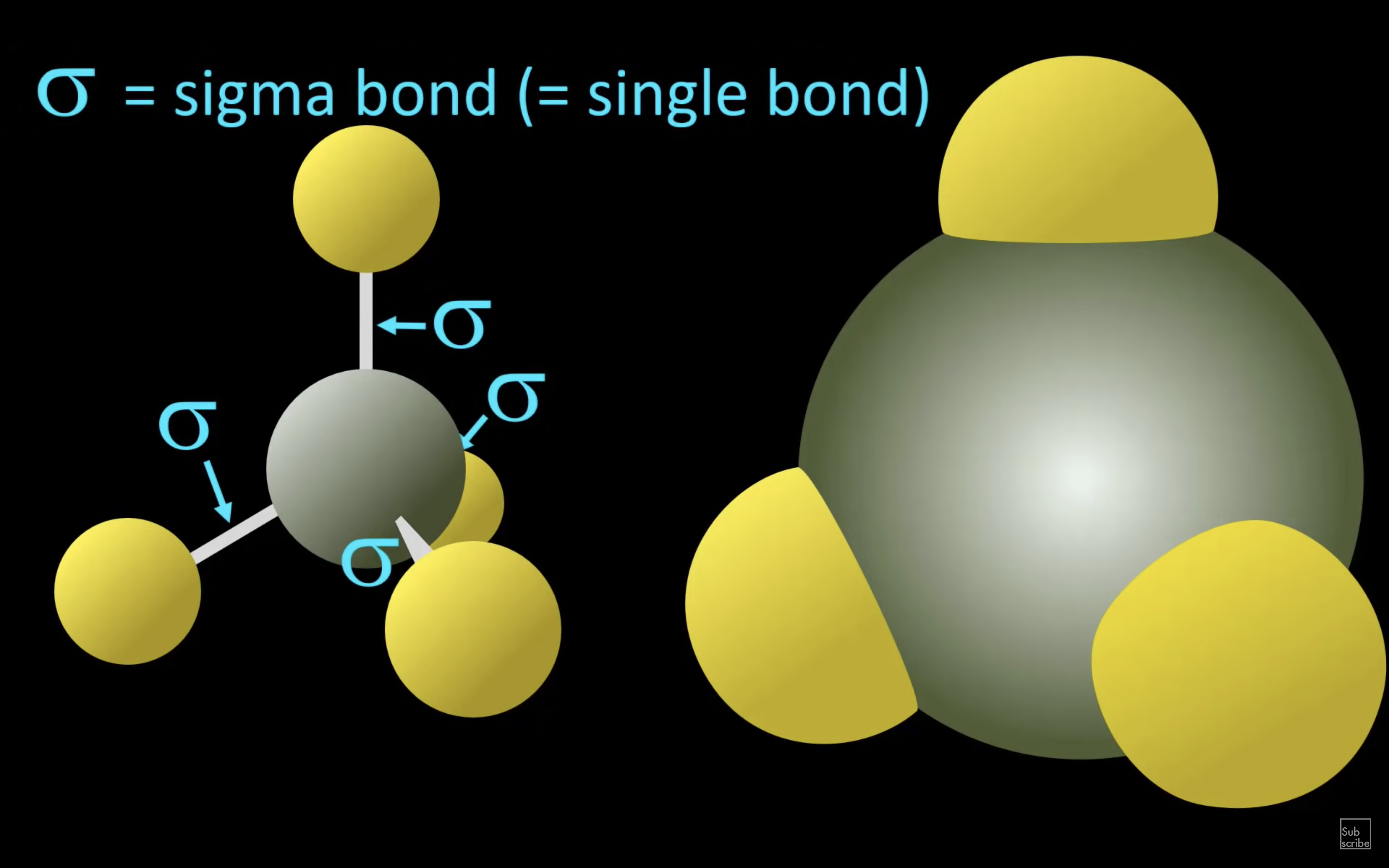
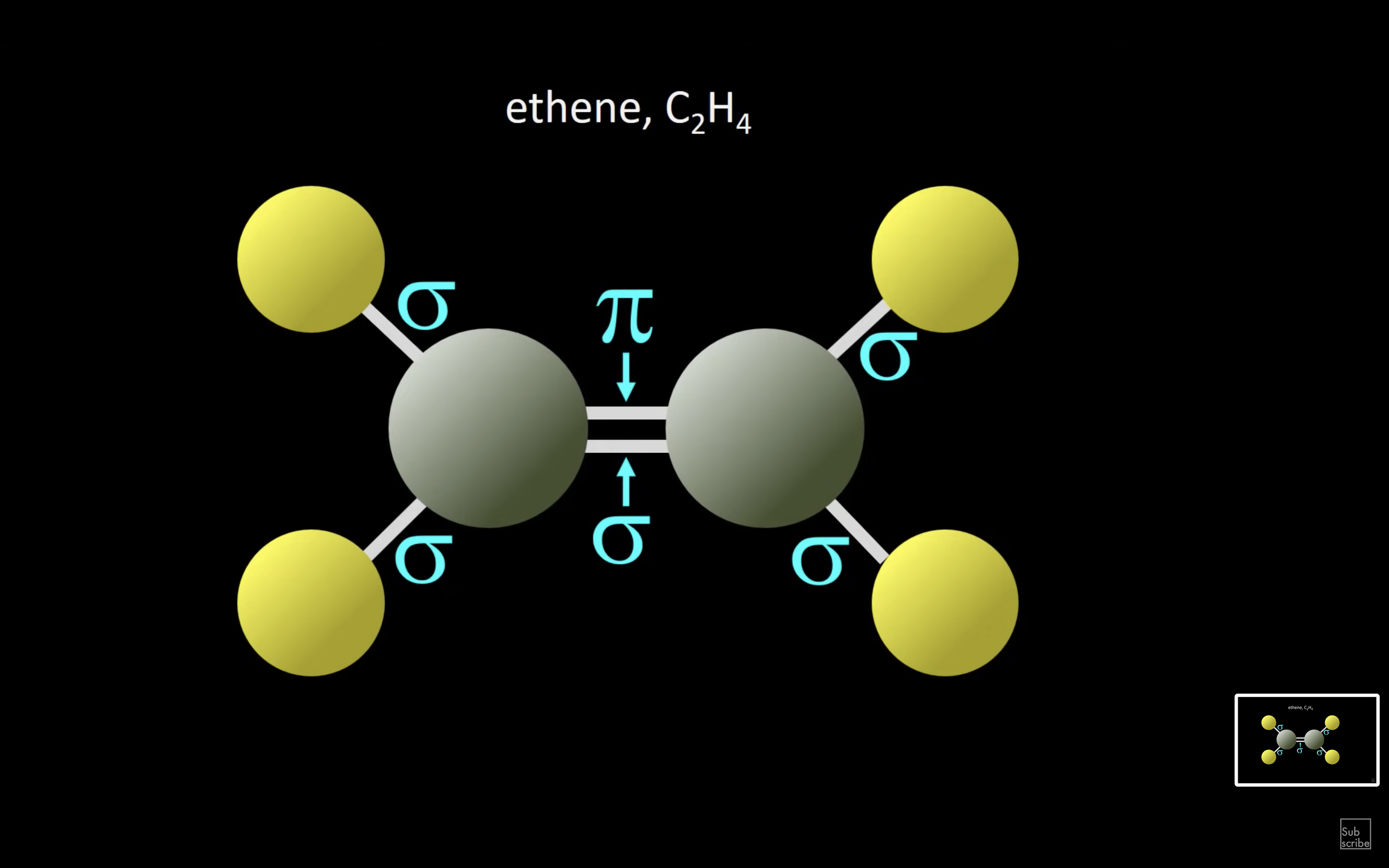
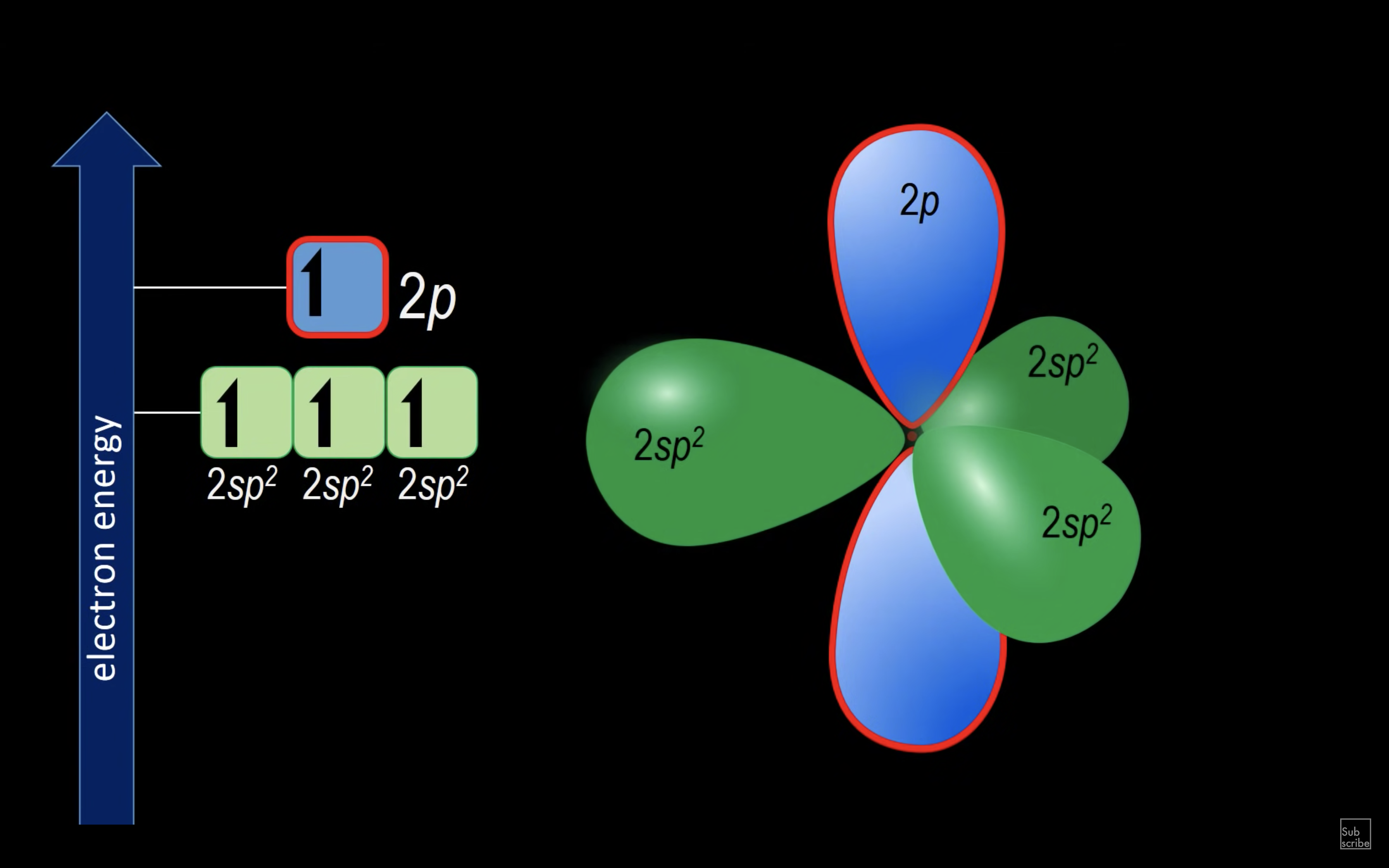
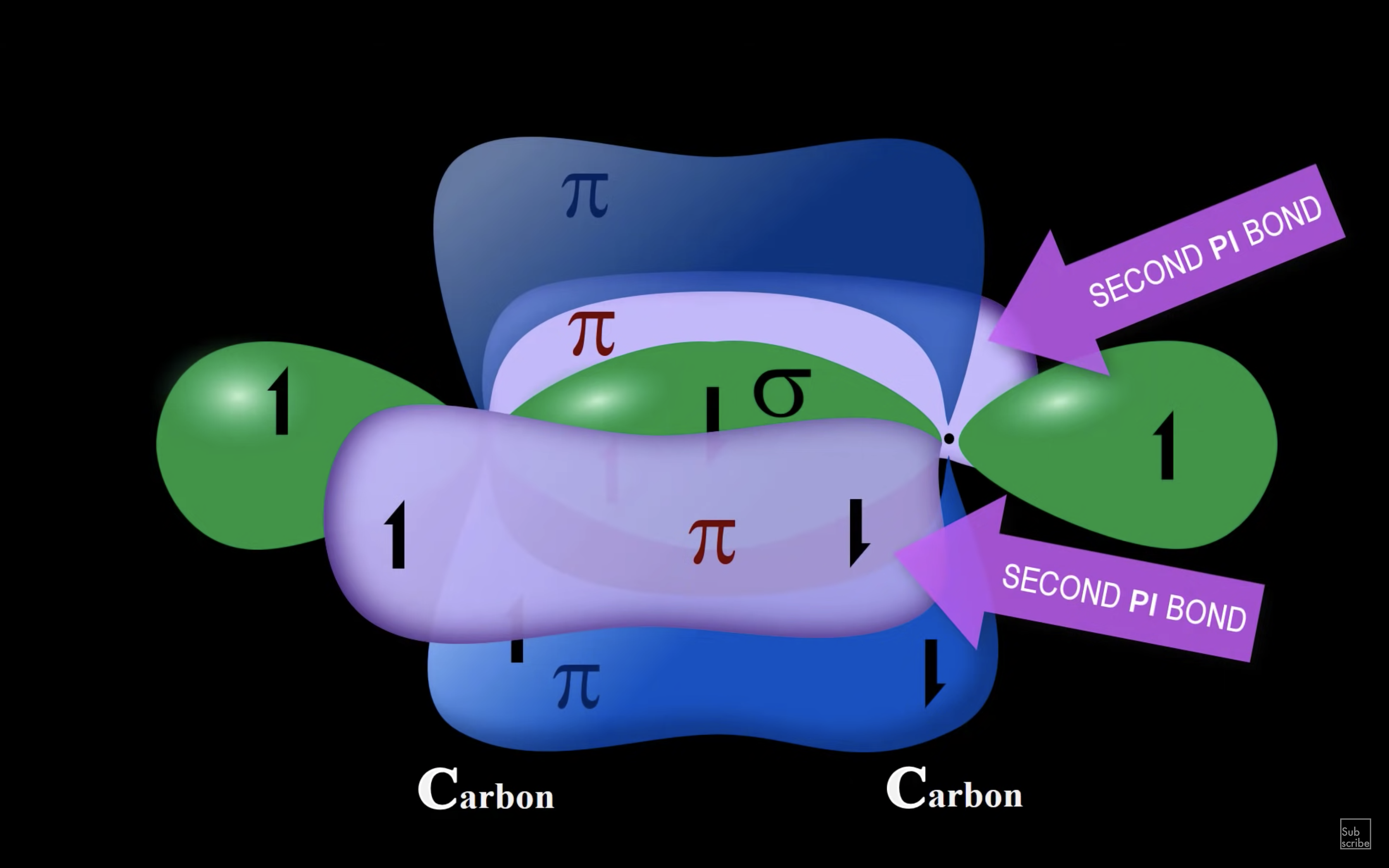
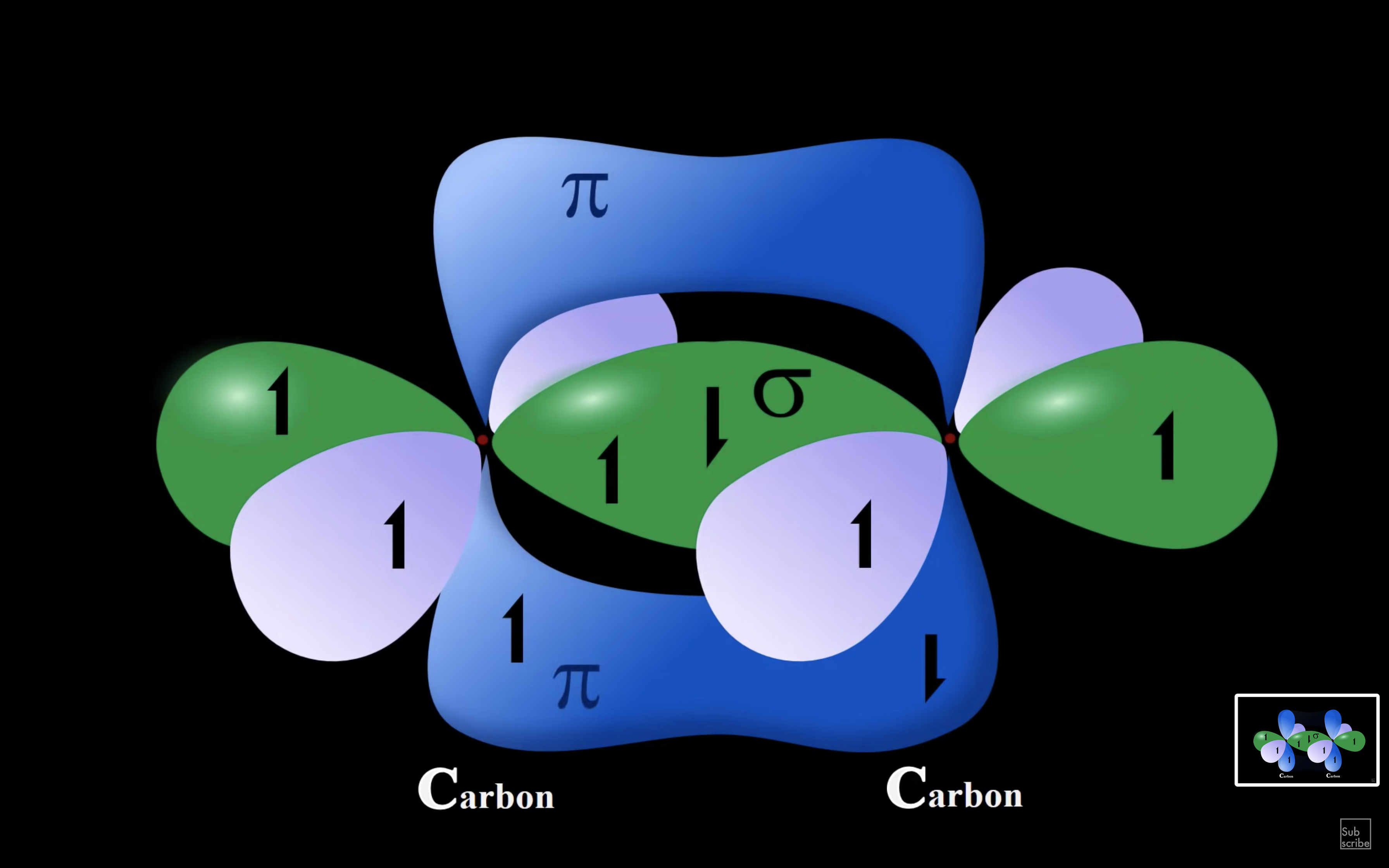
ETHENE
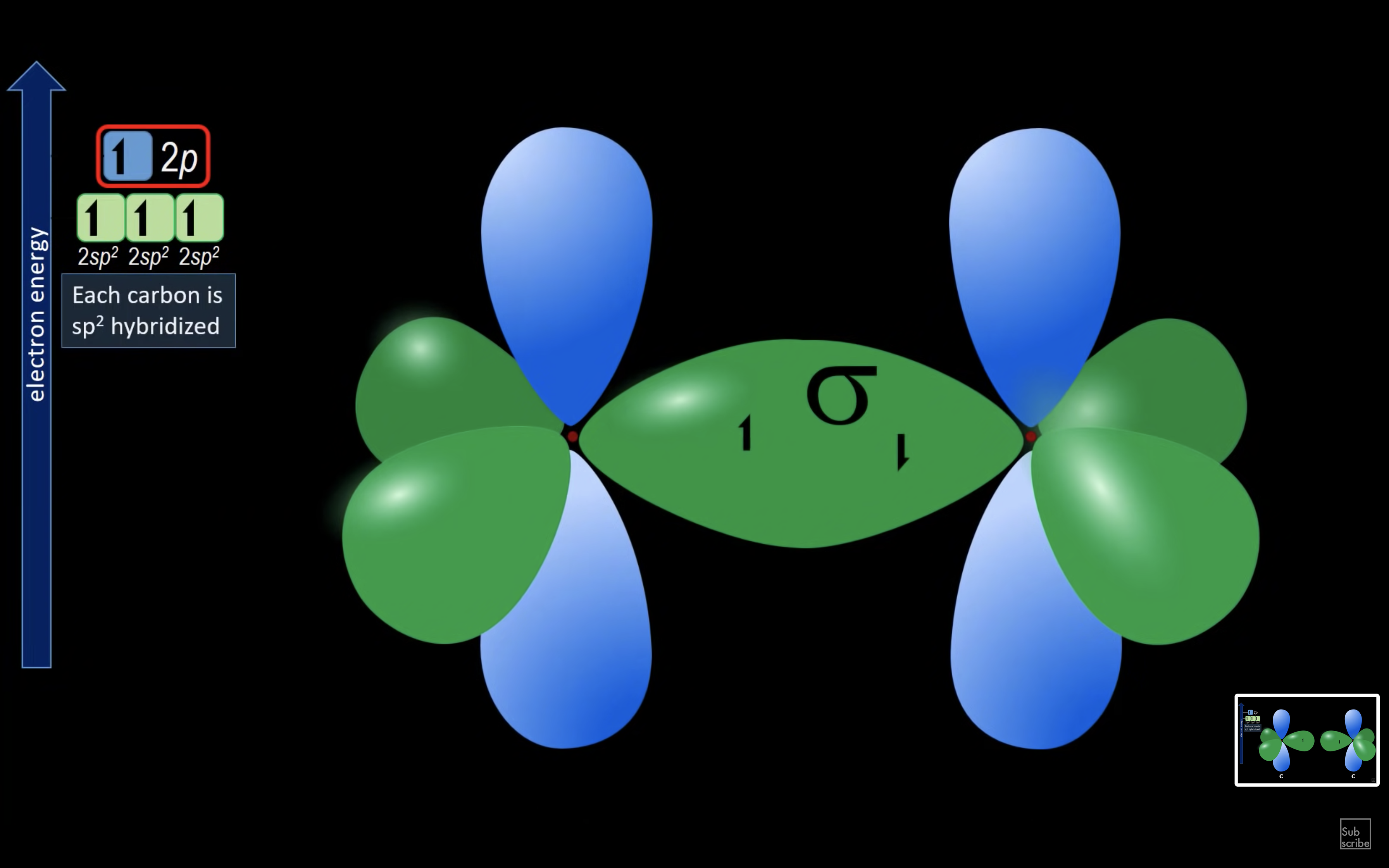
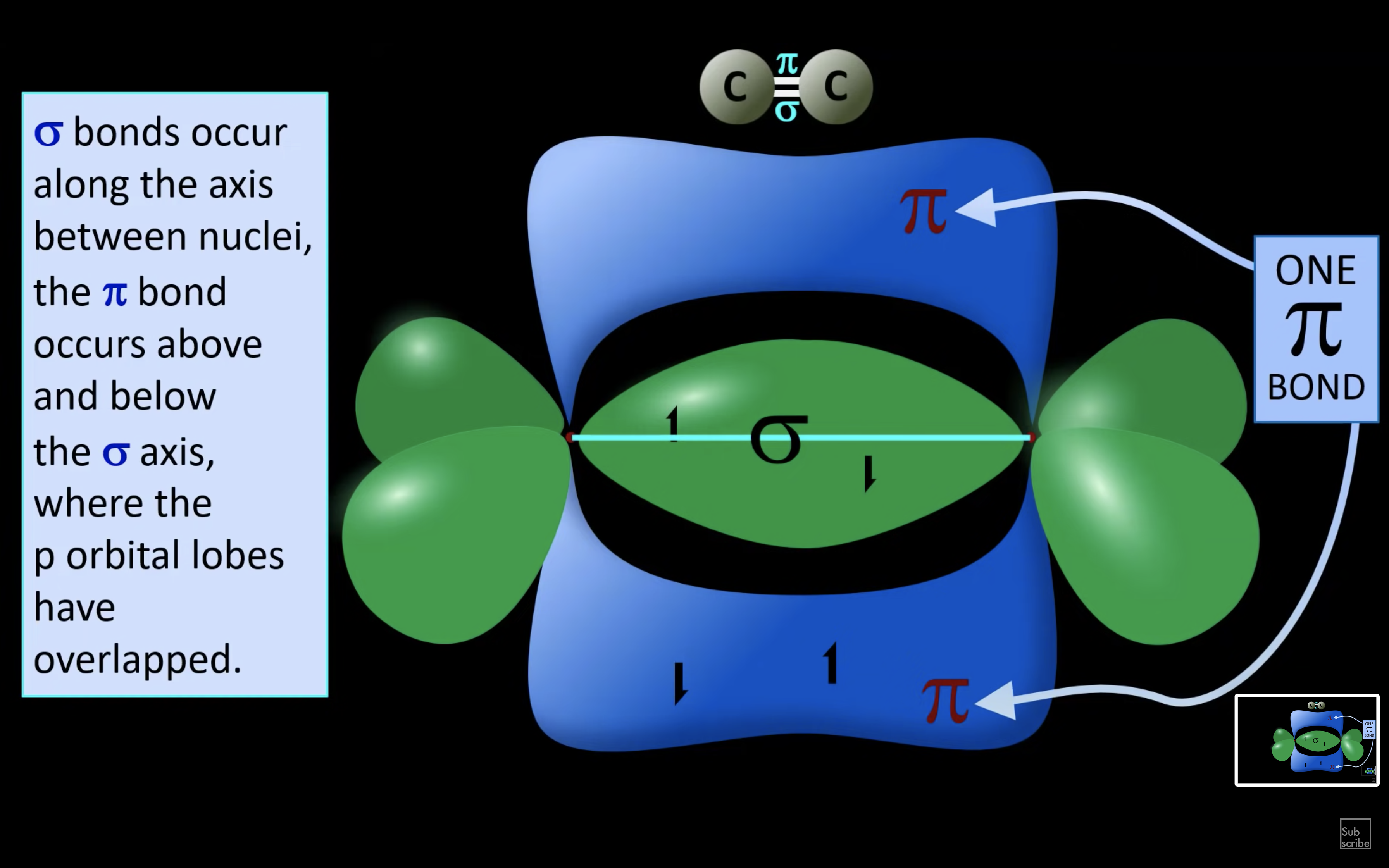
ETHYNE
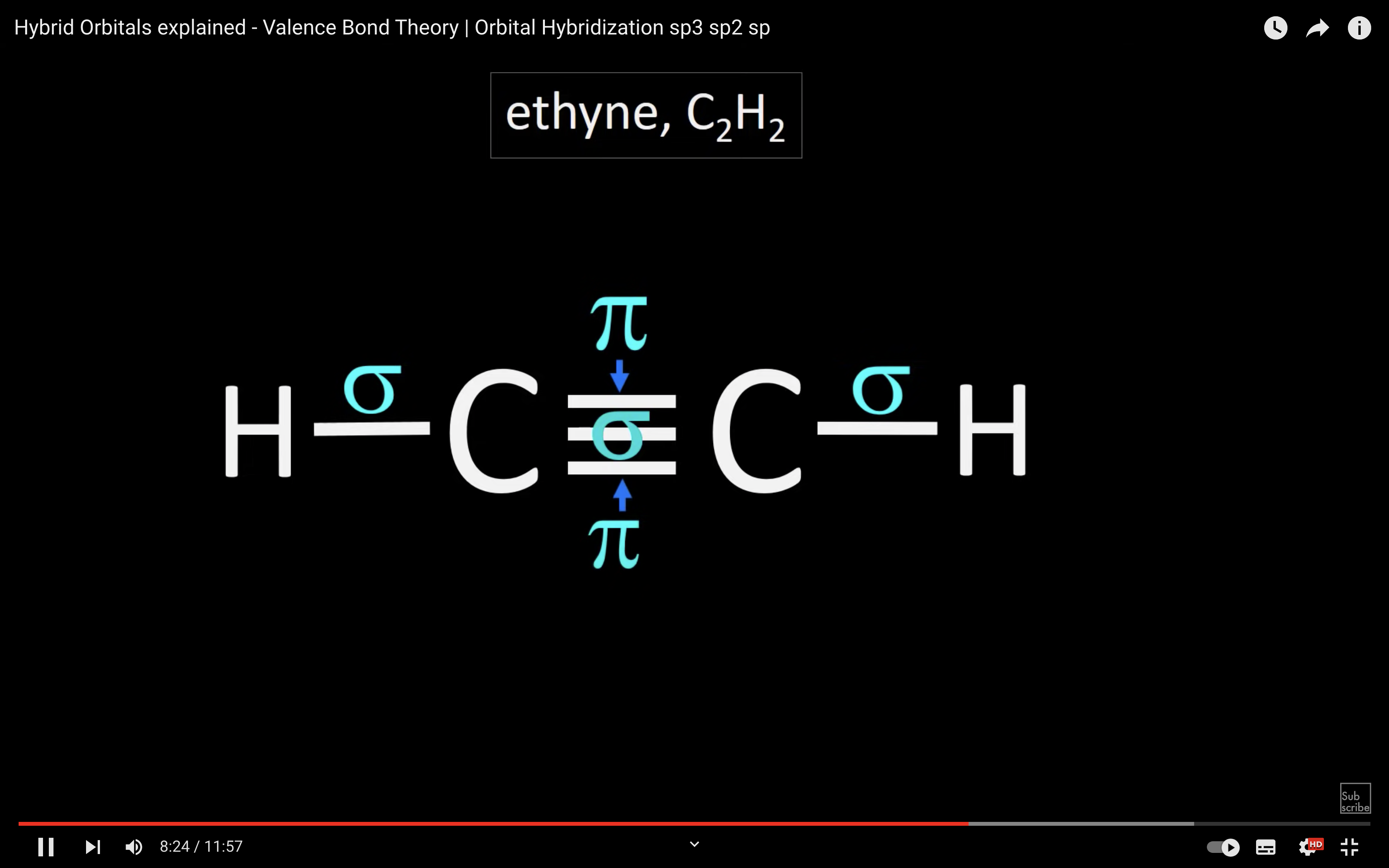
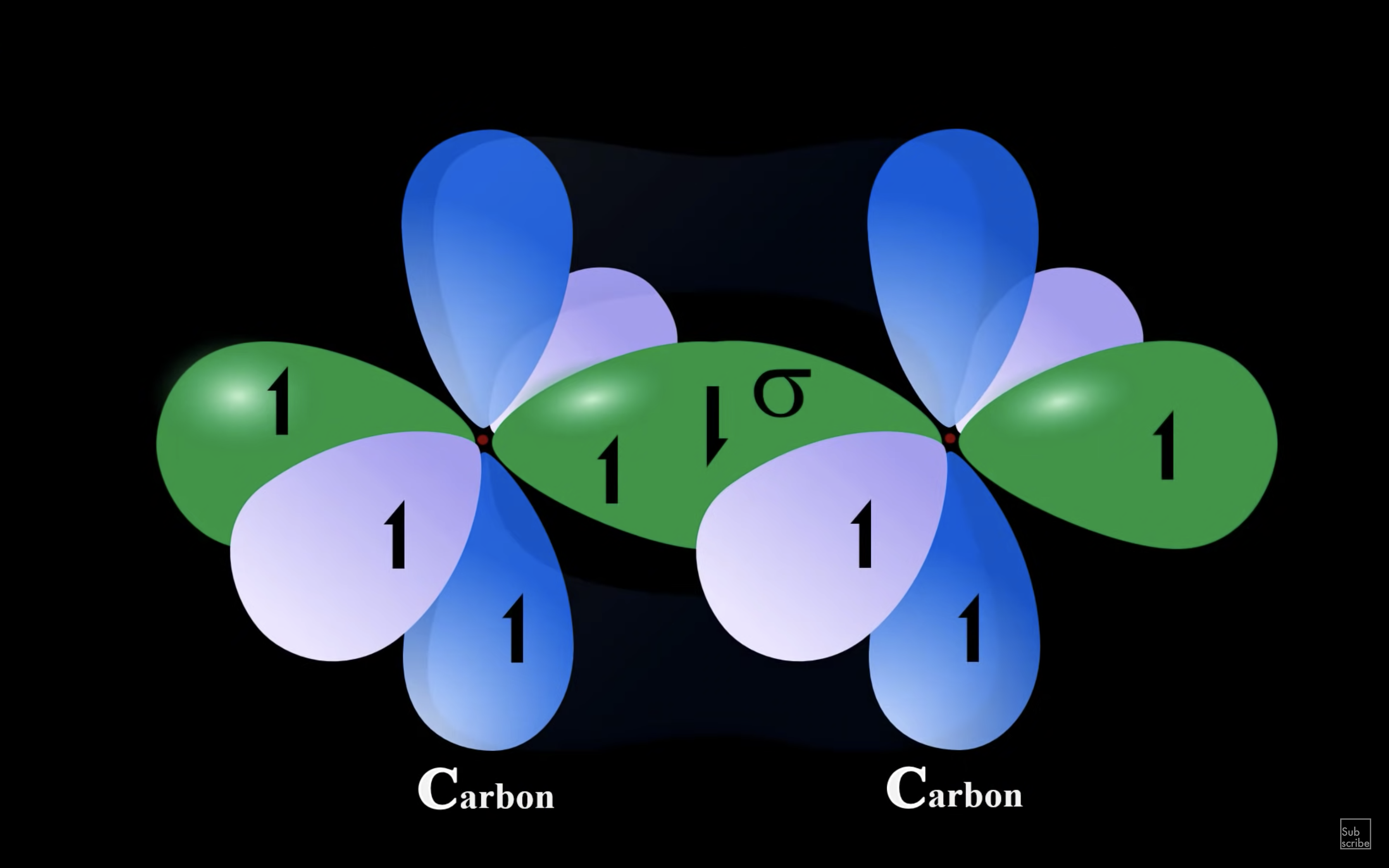
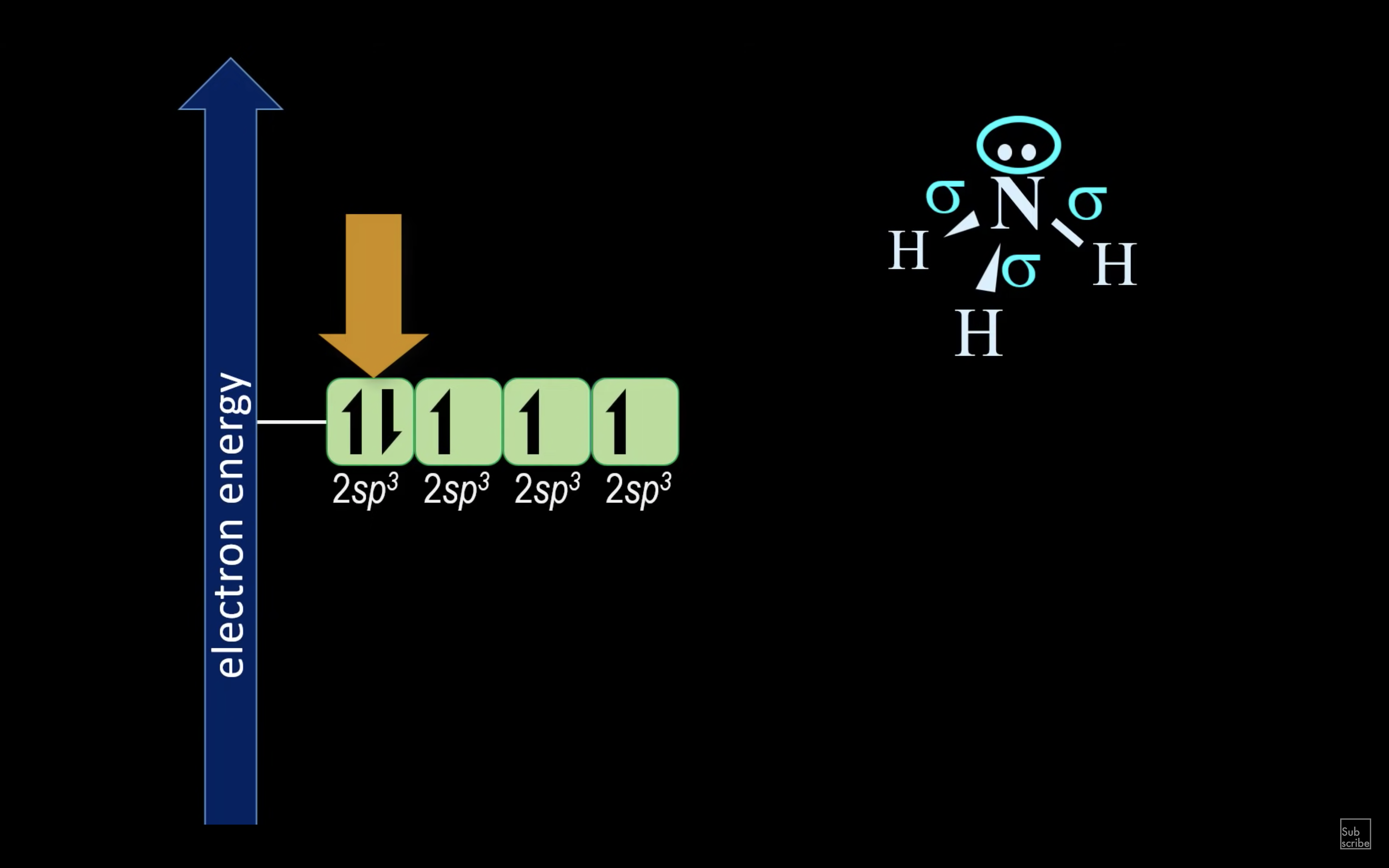
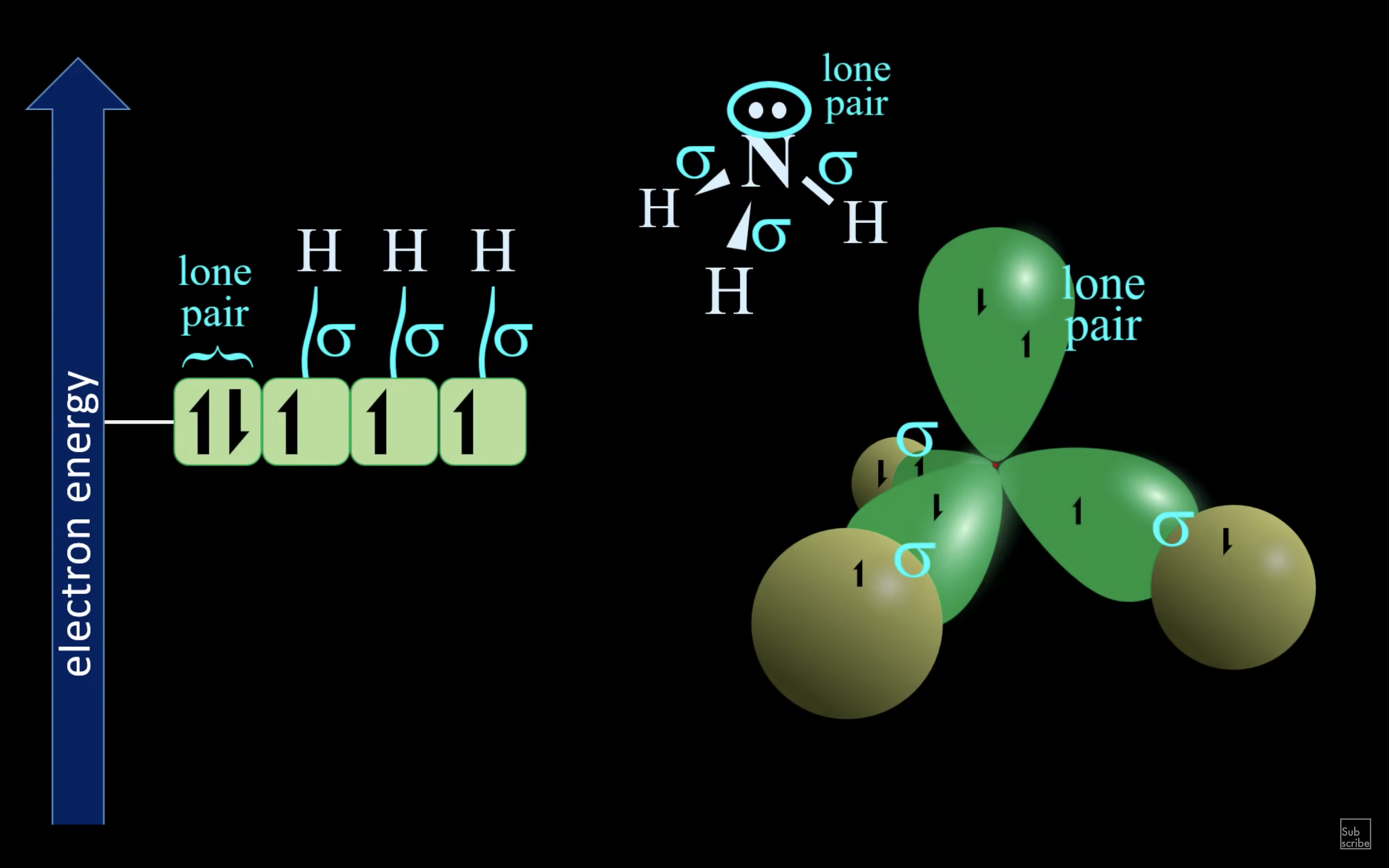
NH3
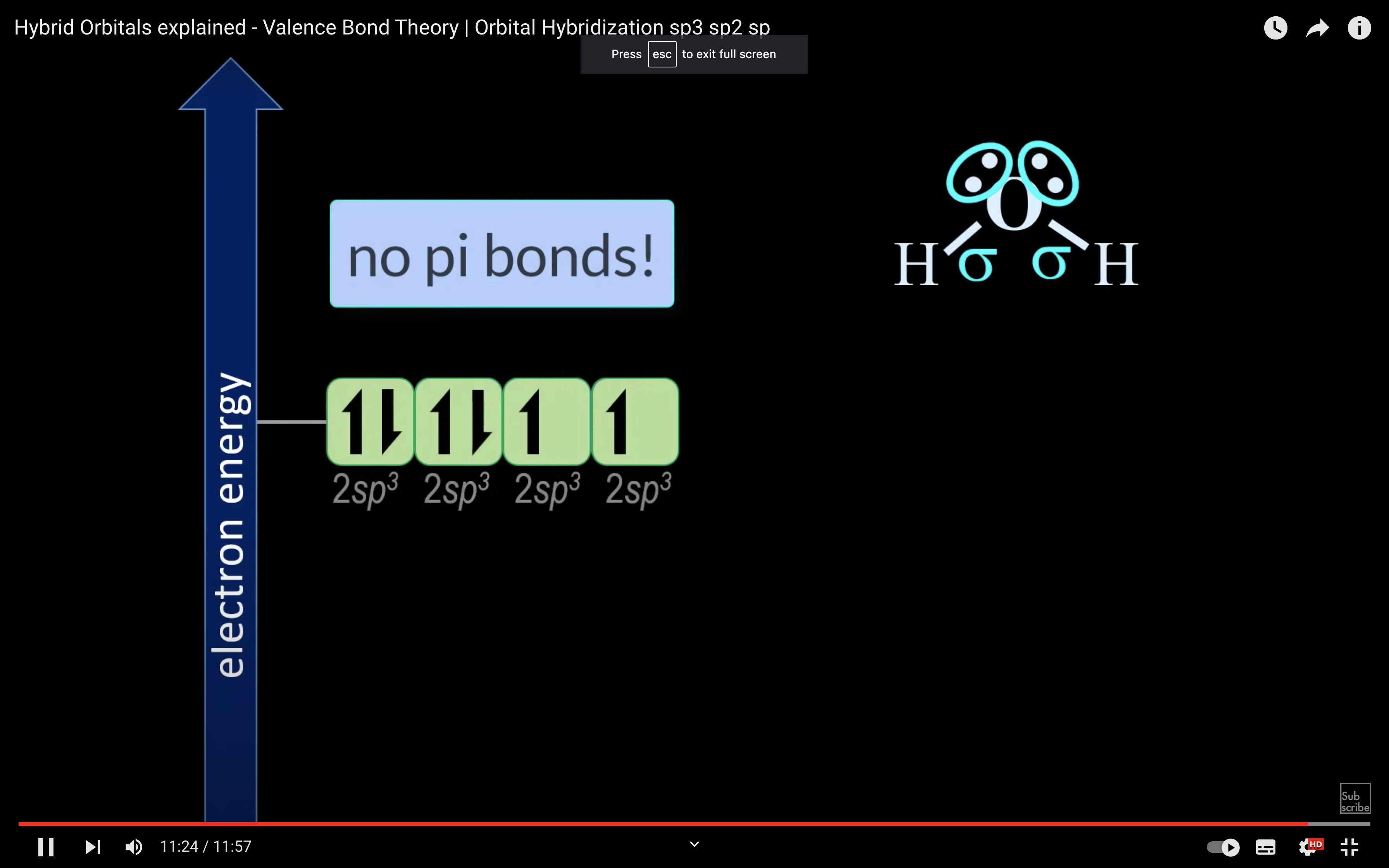
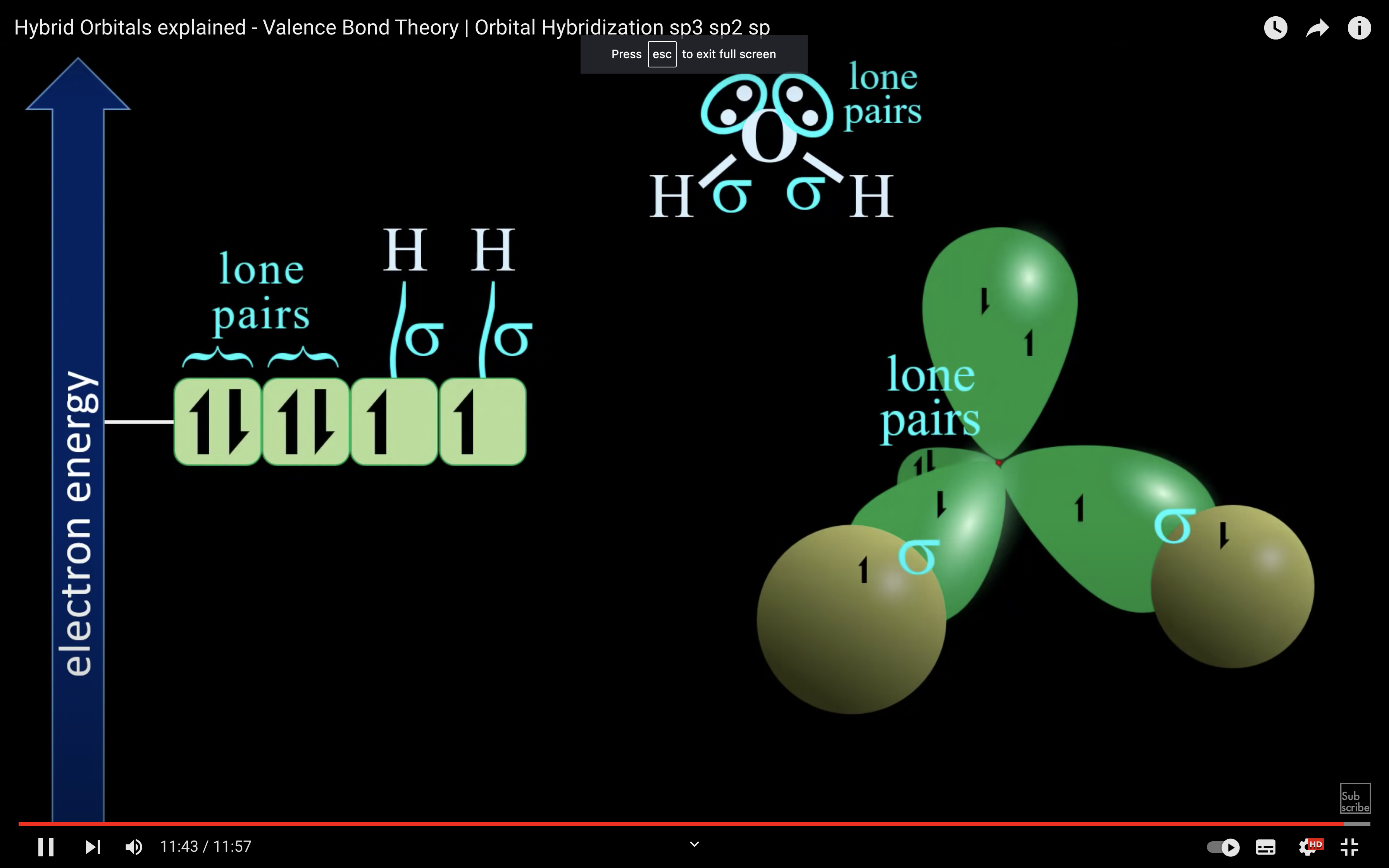
H2O
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| REACTION NOTES (0) | 2023.03.28 |
|---|---|
| Lab 3 Glucose Concentration in Drinks (0) | 2023.03.27 |
| [WEEK4] Aldehydes and Ketones (1) | 2023.03.21 |
| [WEEK3] Alcohols, phenols, ethers and thiols (0) | 2023.03.14 |
| [WEEK2] Unsaturated Hydrocarbons (0) | 2023.03.08 |



