LEARNING CONTENT
Topic 2 Unsaturated Hydrocarbons
- Nomenclature of Alkenes and alkynes
- Geometric isomerism of Alkenes
- Cycloalkenes
Learning Outcomes
- Be able to name alkenes systematically and also be able to to draw a structural formula of an alkene based on the IUPAC systematic name
- Be able to predict alkene structural isomers
- Be able to name and interpret the names of cycloalkenes
- Be familiar with the concept of geometric isomers of alkenes
- Be able to name and interpret the names of simple alkenes using the cis/trans nomenclature
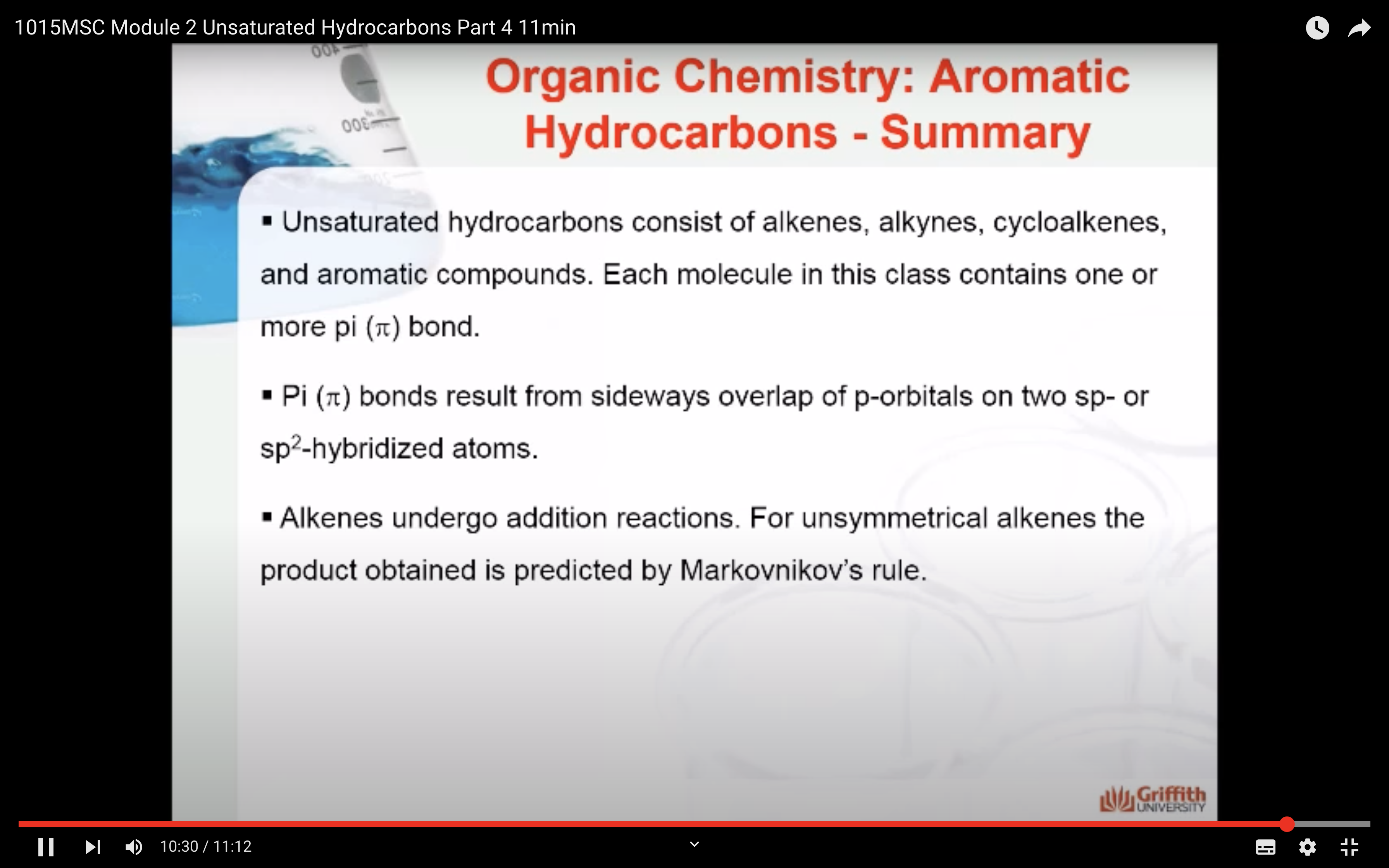
- Reactions of alkenes and alkynes (addition reactions, Markovnikov's rule)
Part 1

Part2

Part 3

Part 4
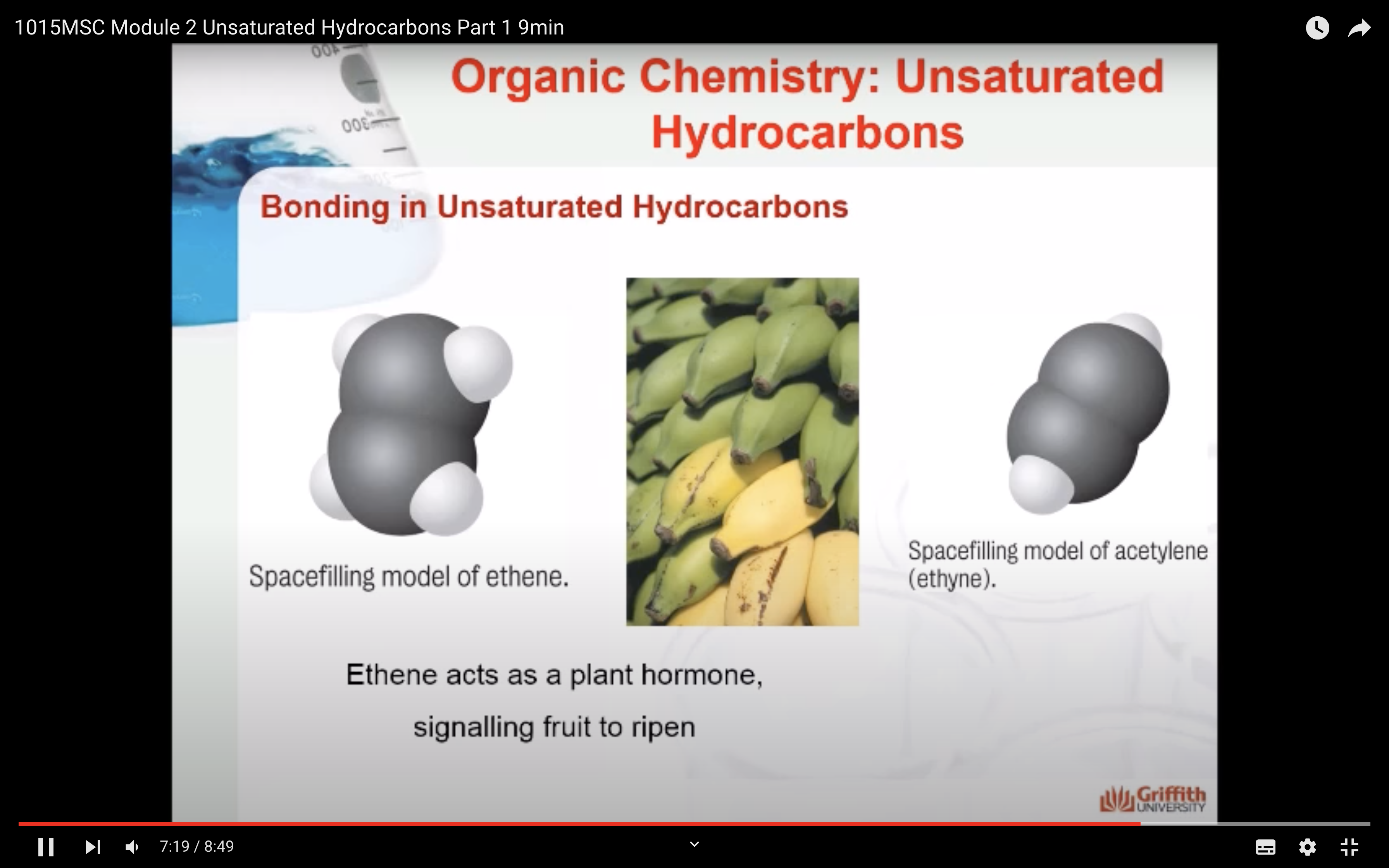
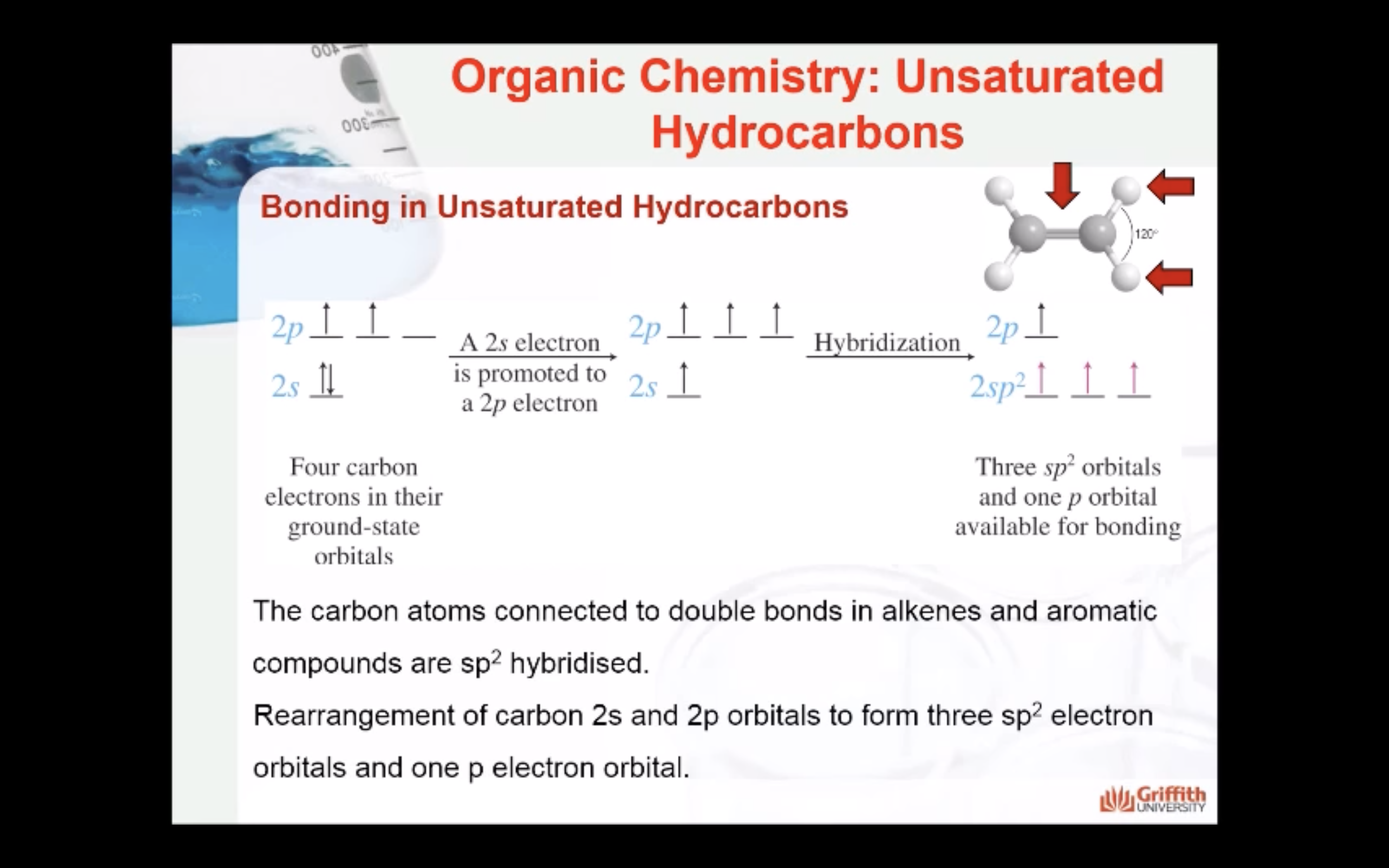
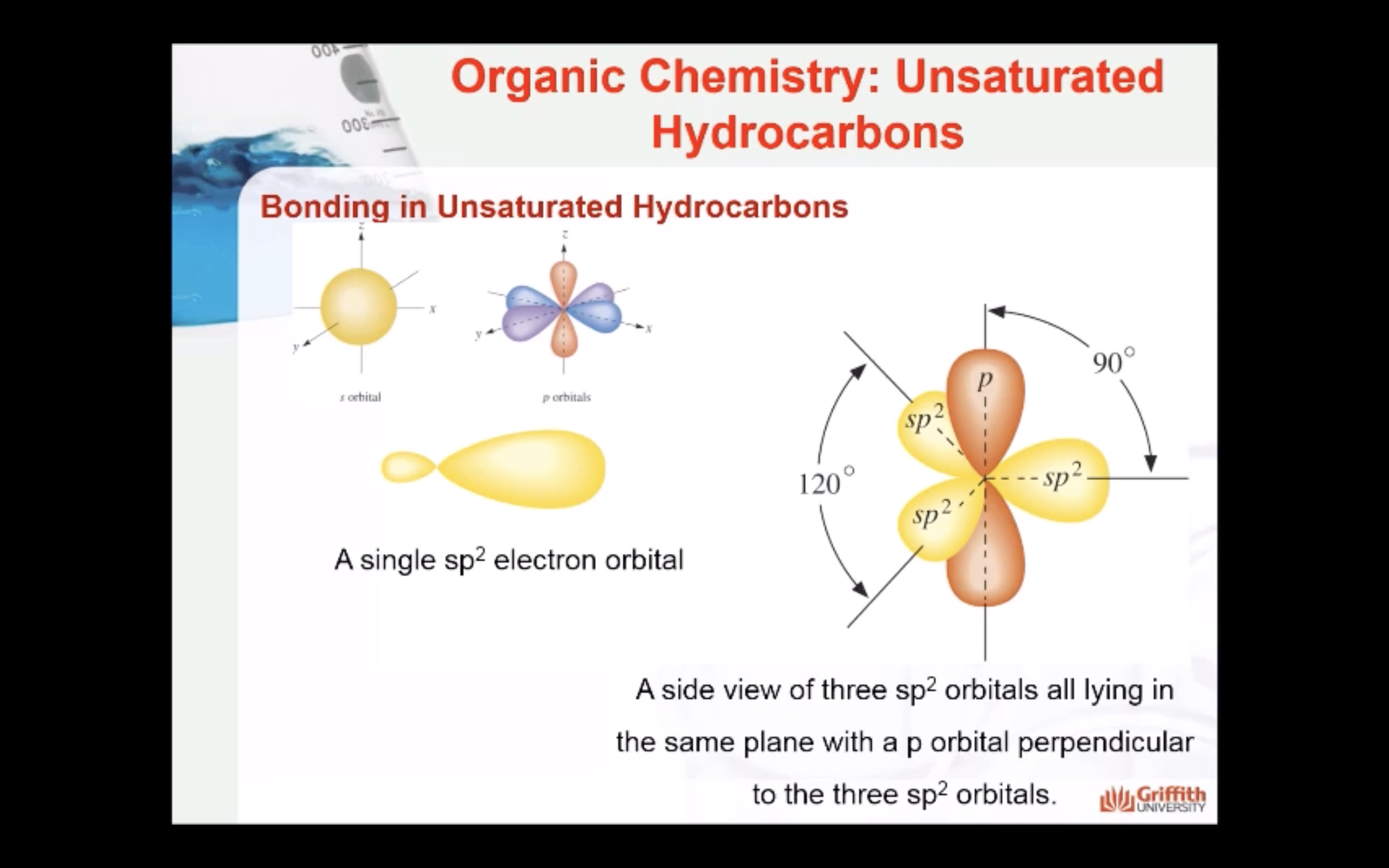
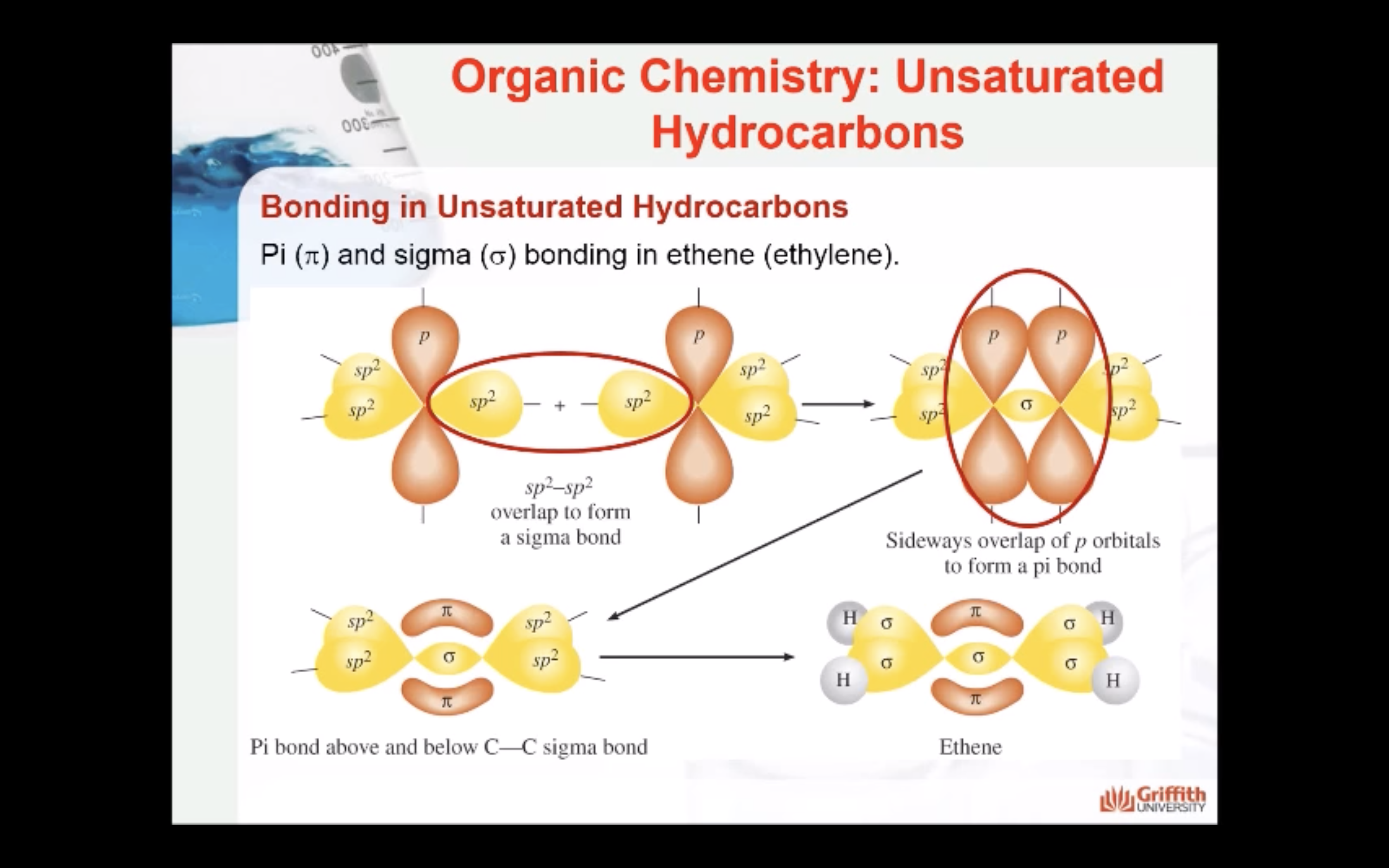
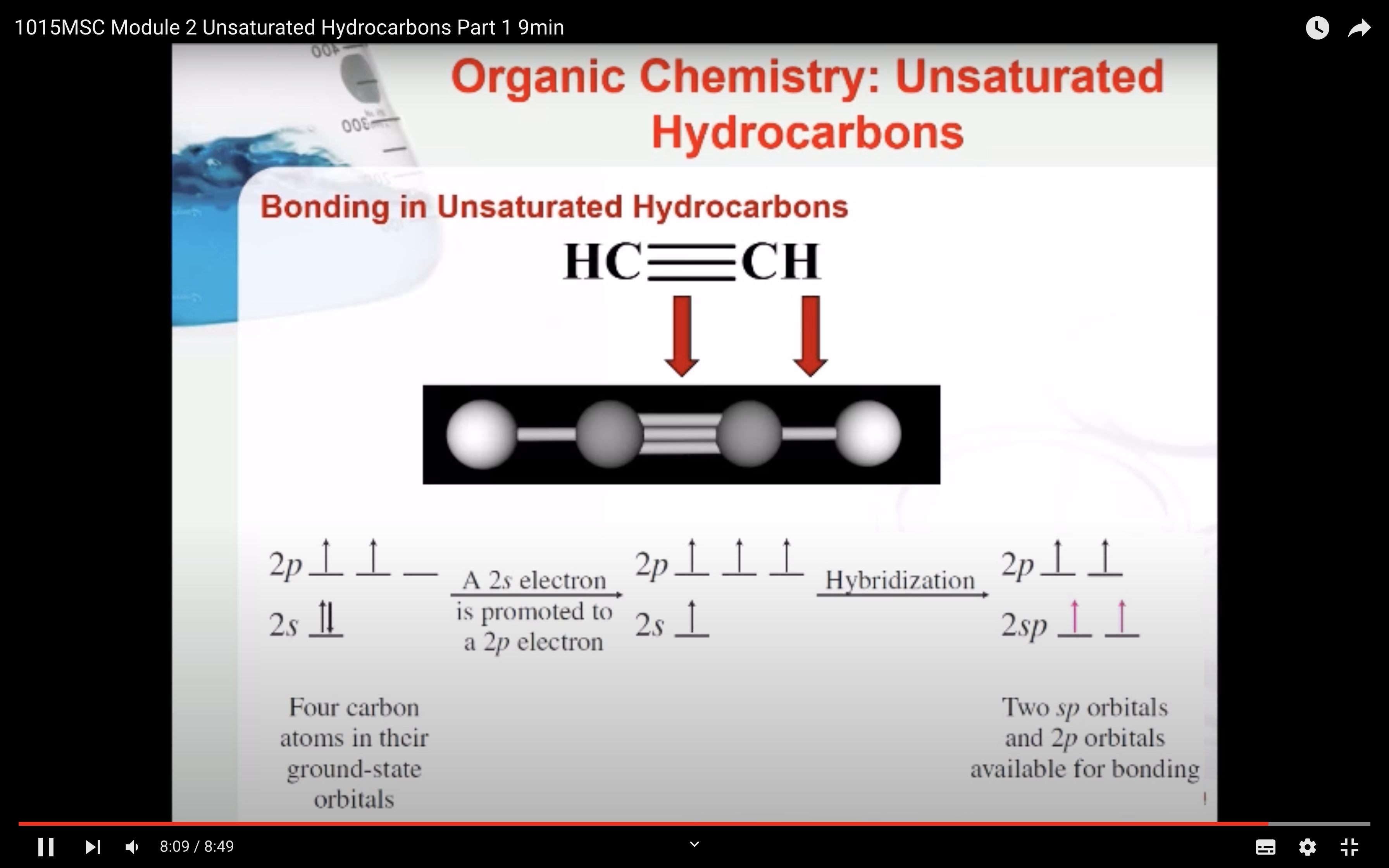
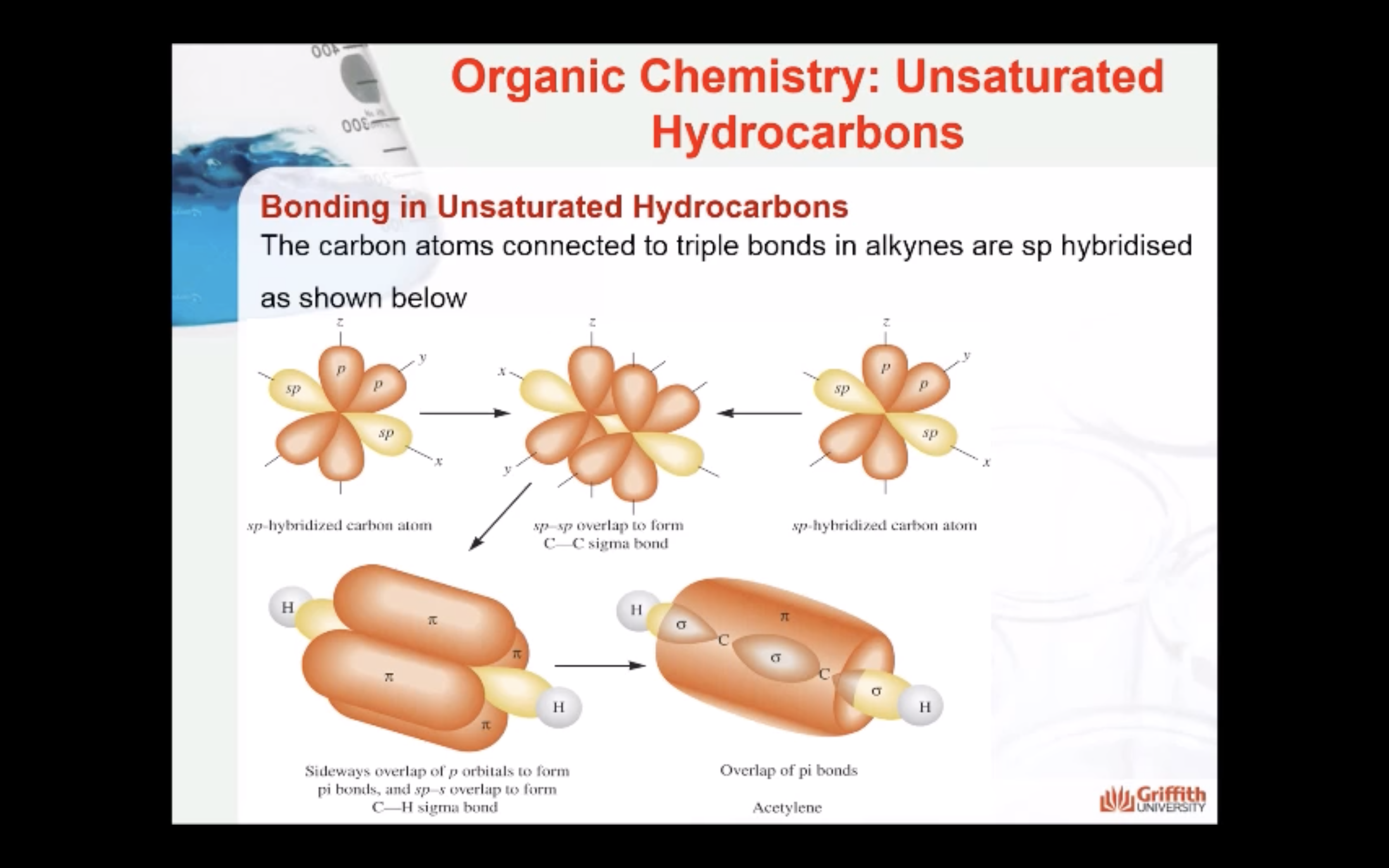
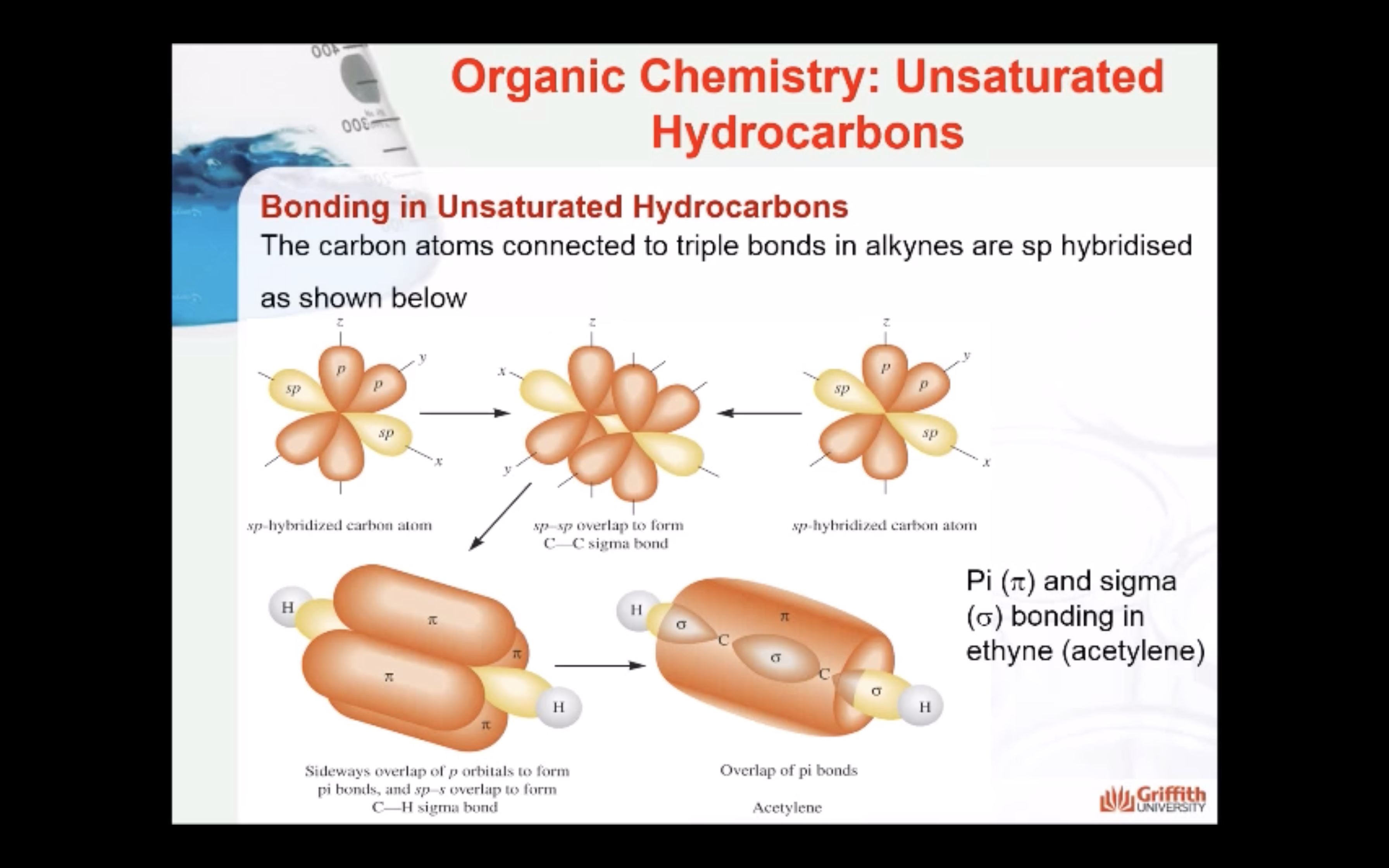
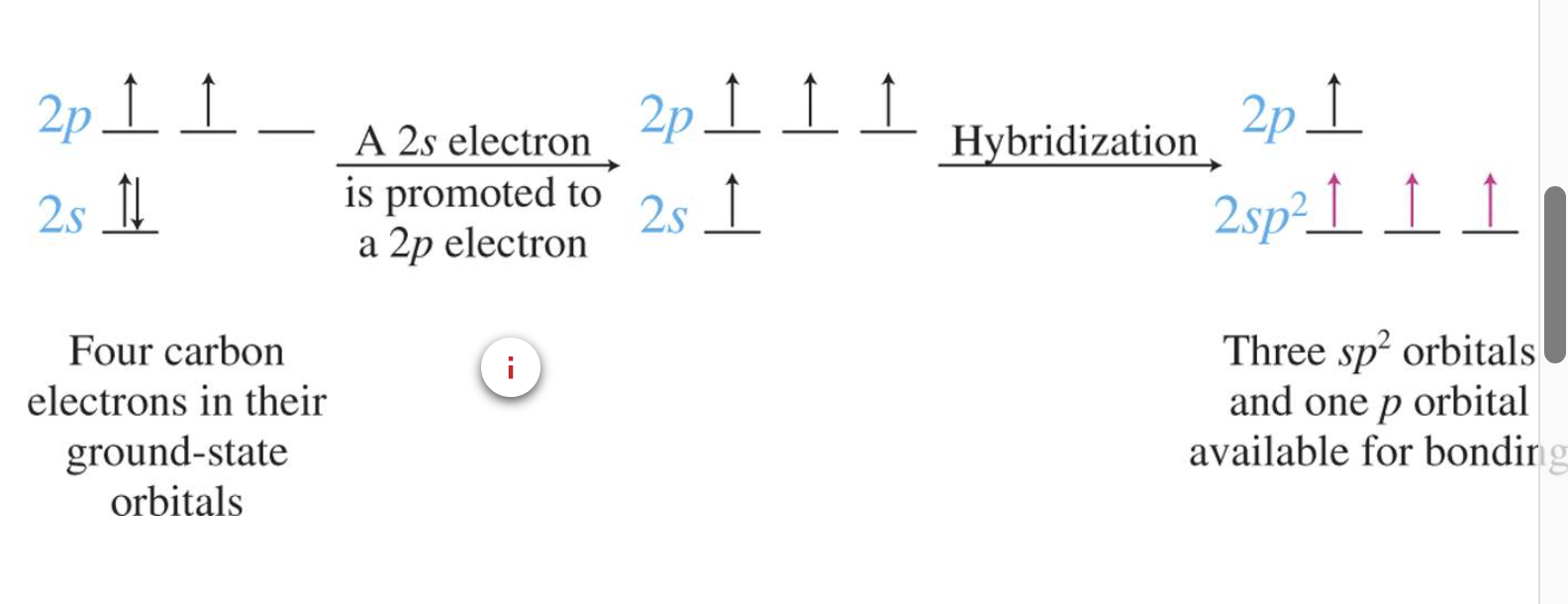
Bonding in Unsaturated Hydrocarbons

Hybridisation in Alkenes
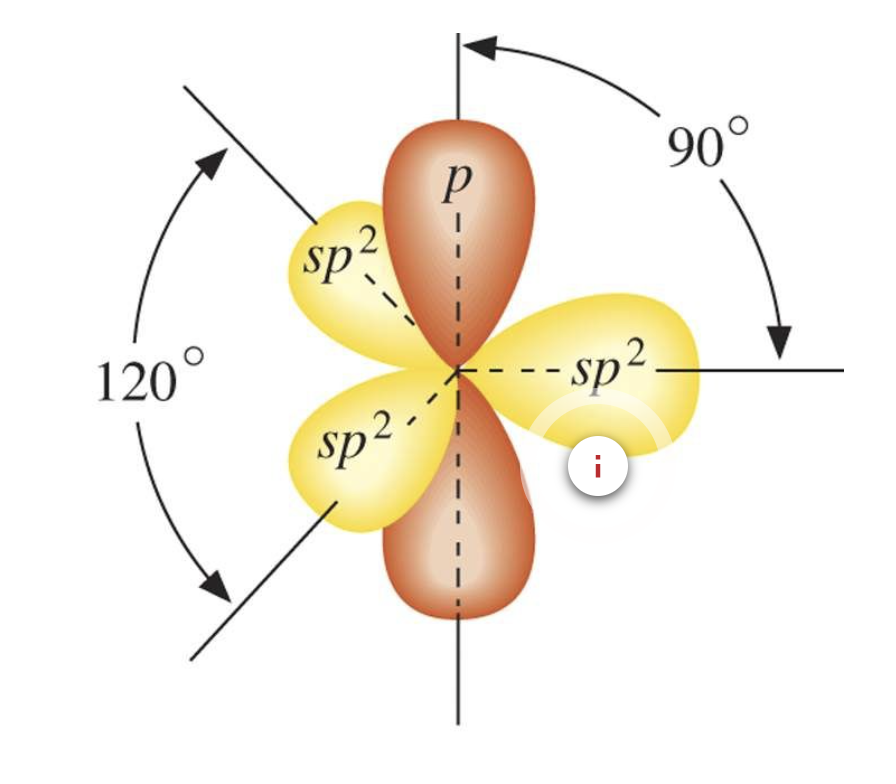
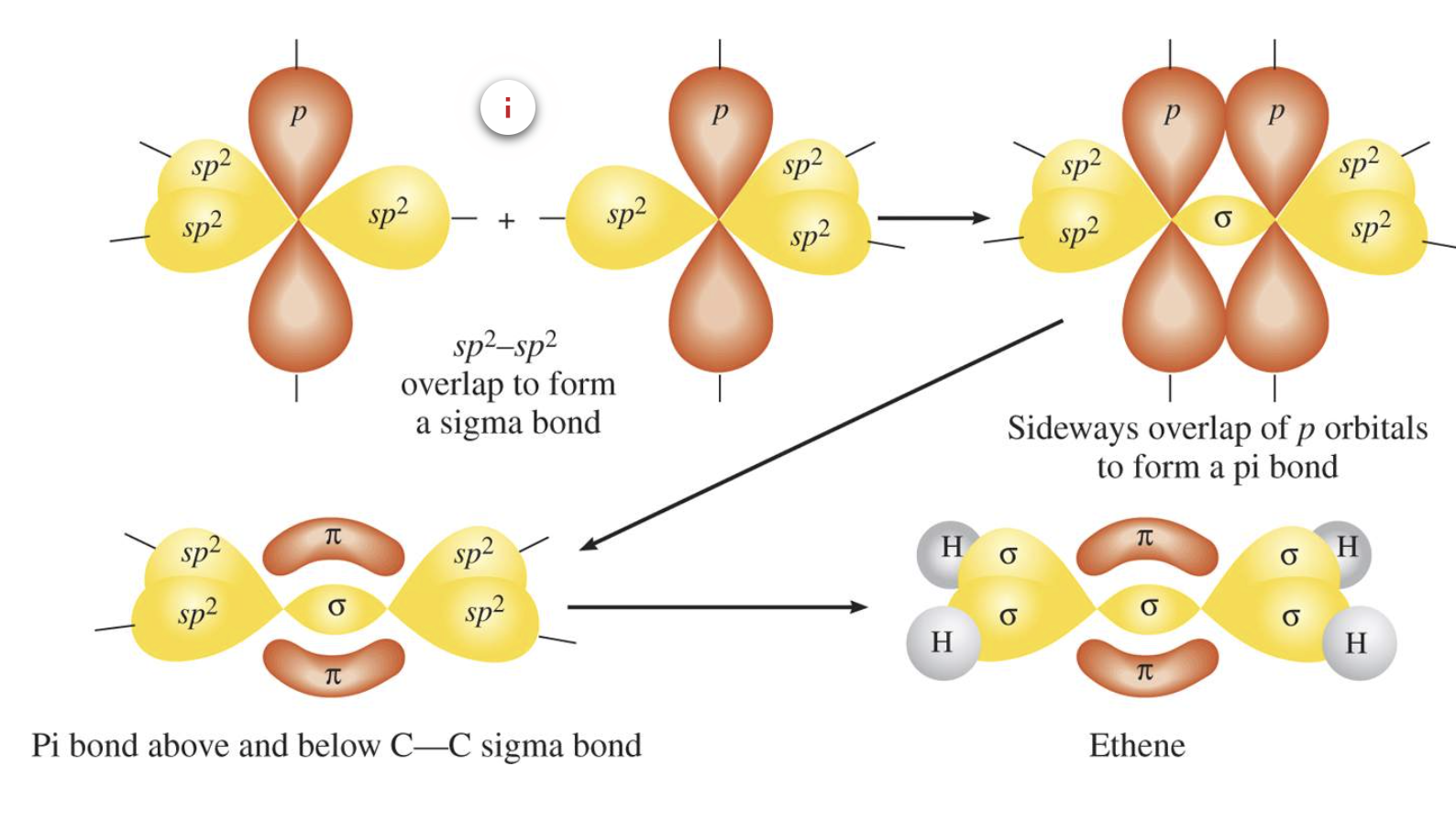
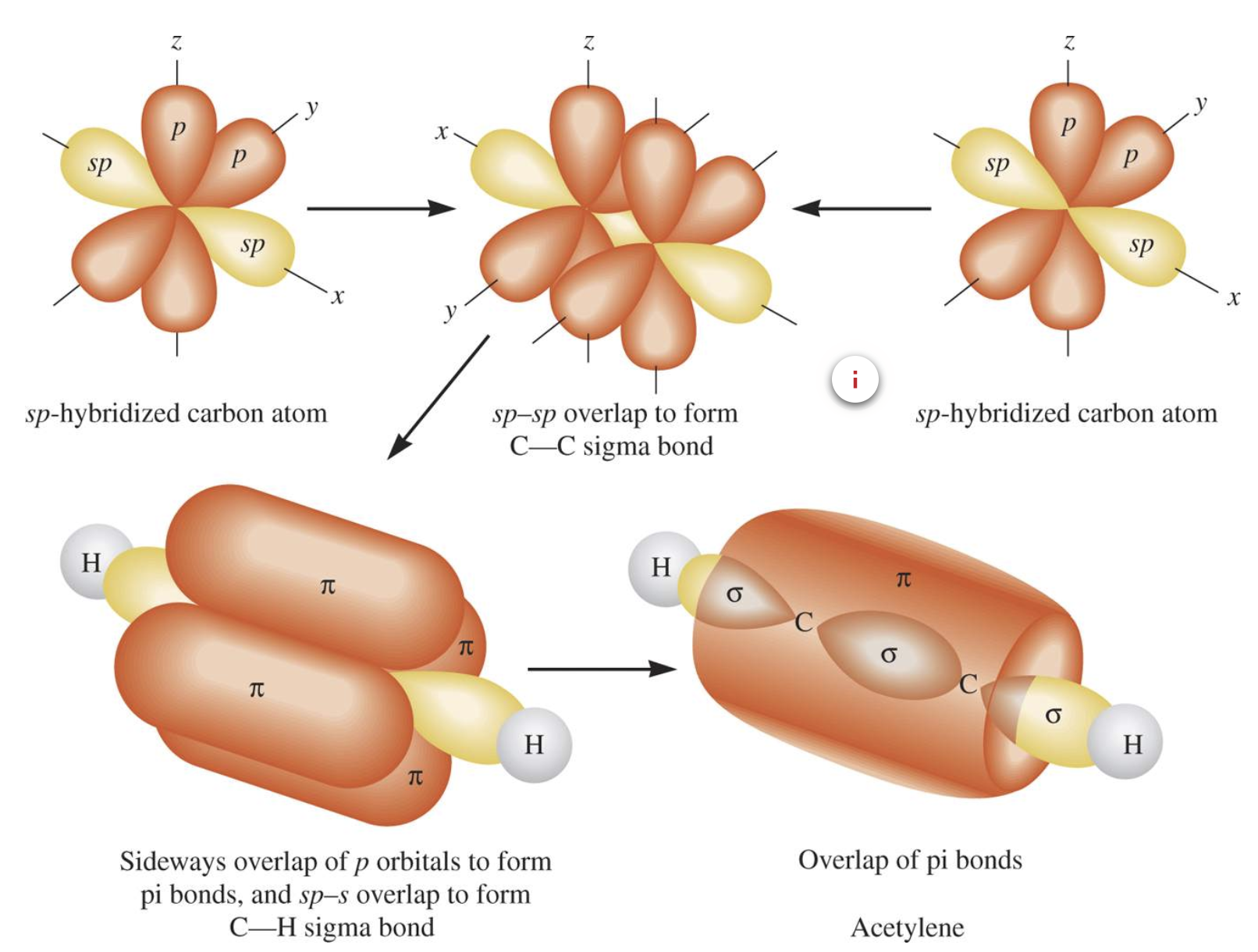
Hybridisation in Alkynes
https://www.youtube.com/watch?v=vHXViZTxLXo
https://www.youtube.com/watch?v=mTHW9W-2-N8
https://www.youtube.com/watch?v=YcSPPKESpwc
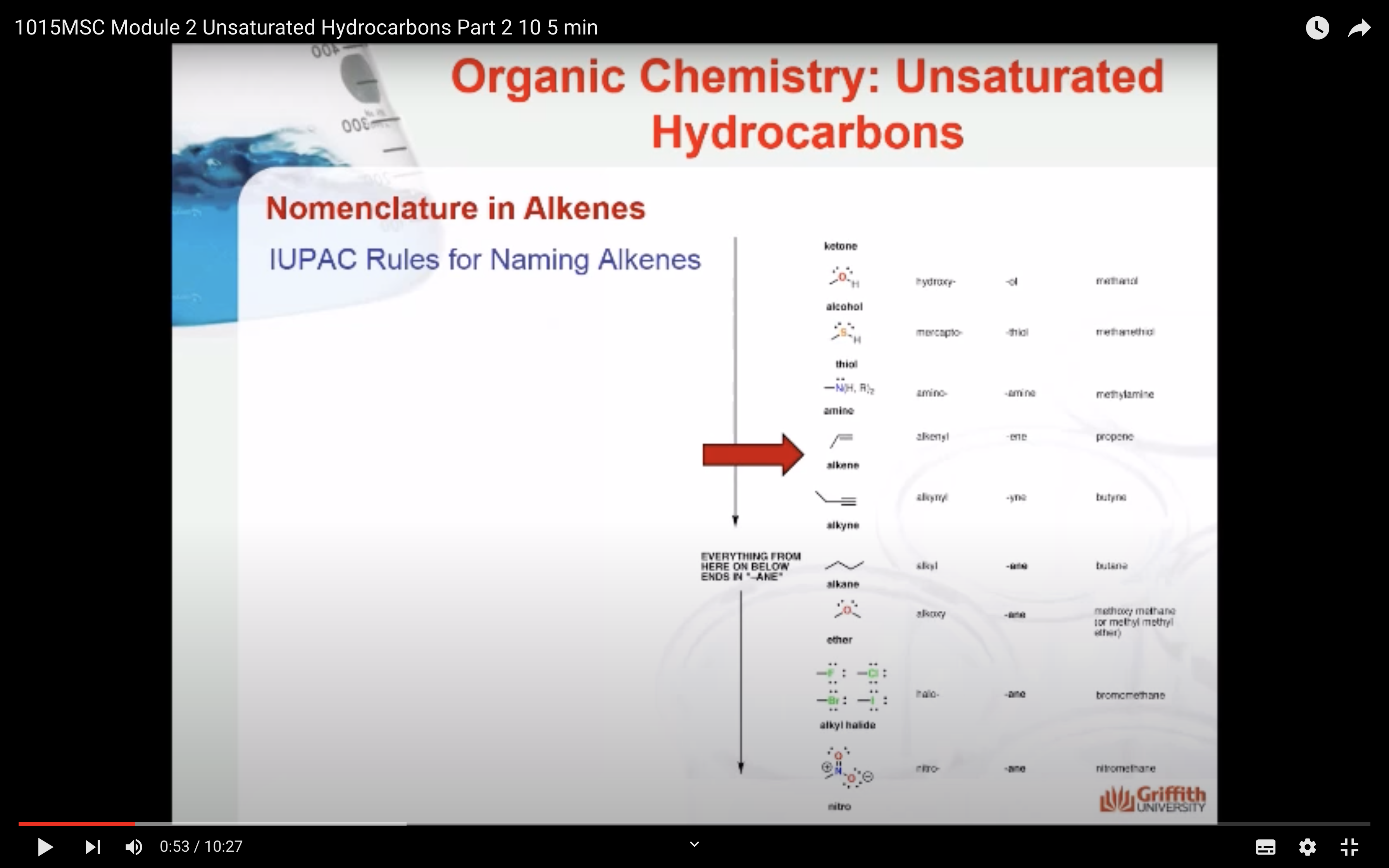
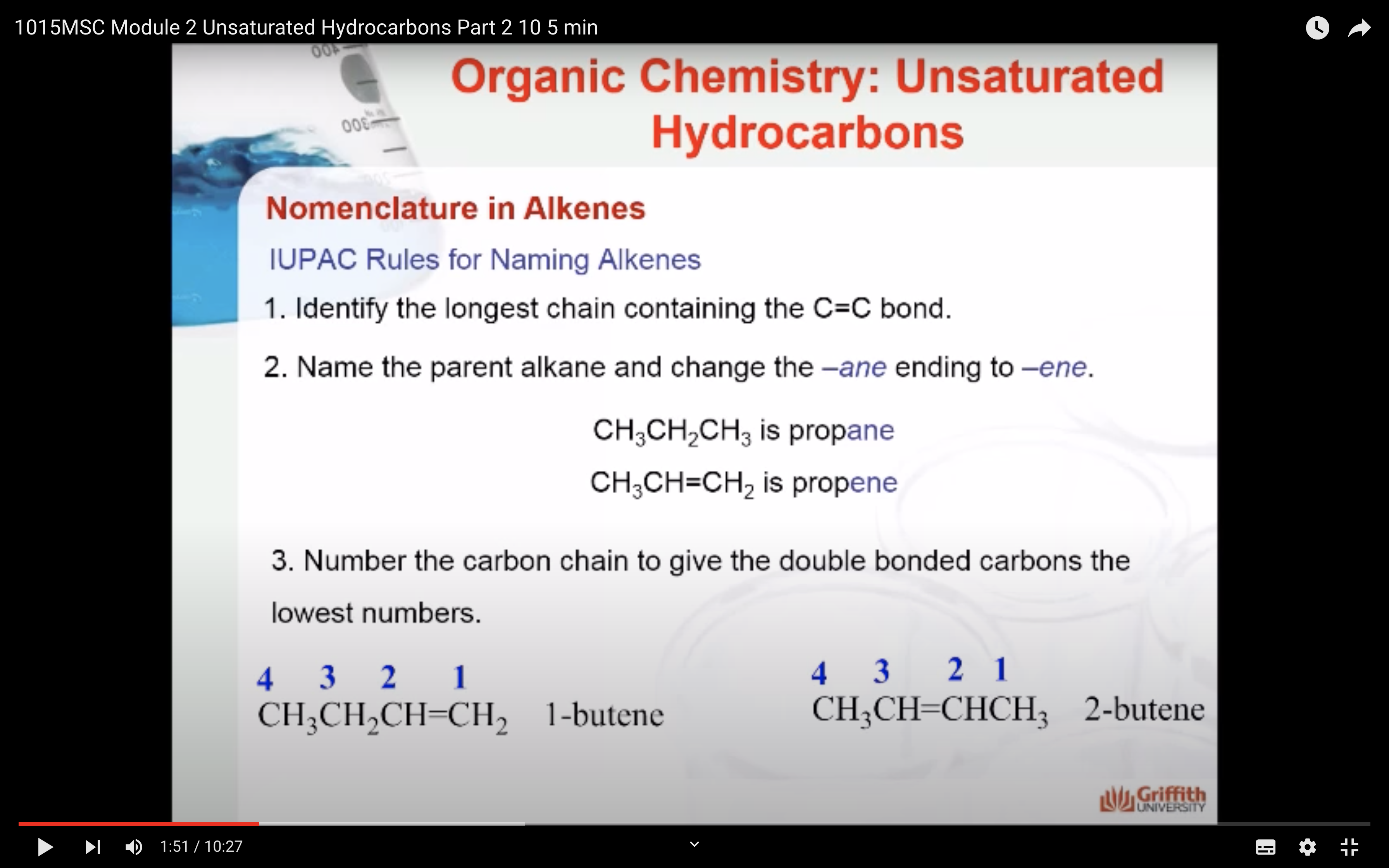
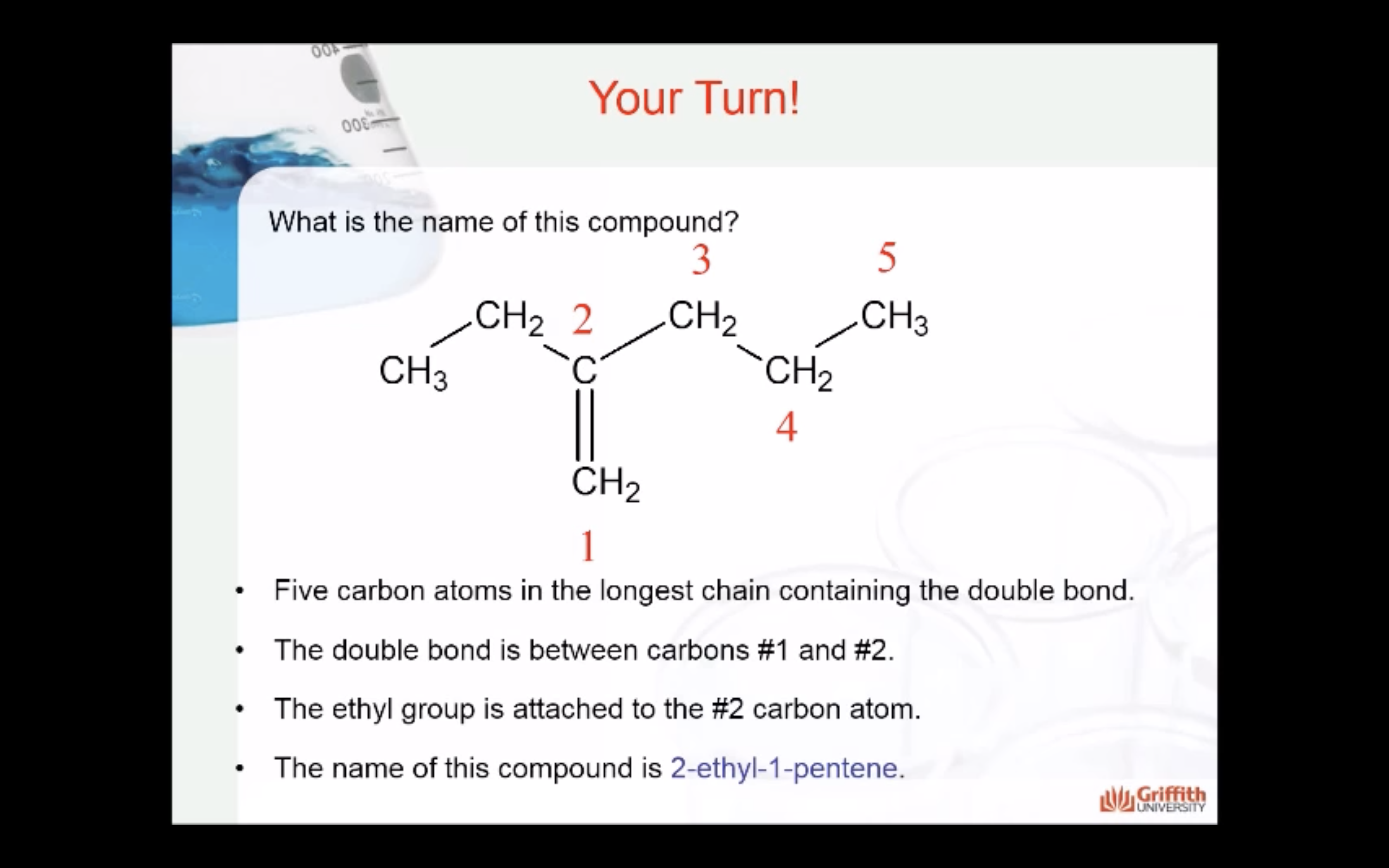
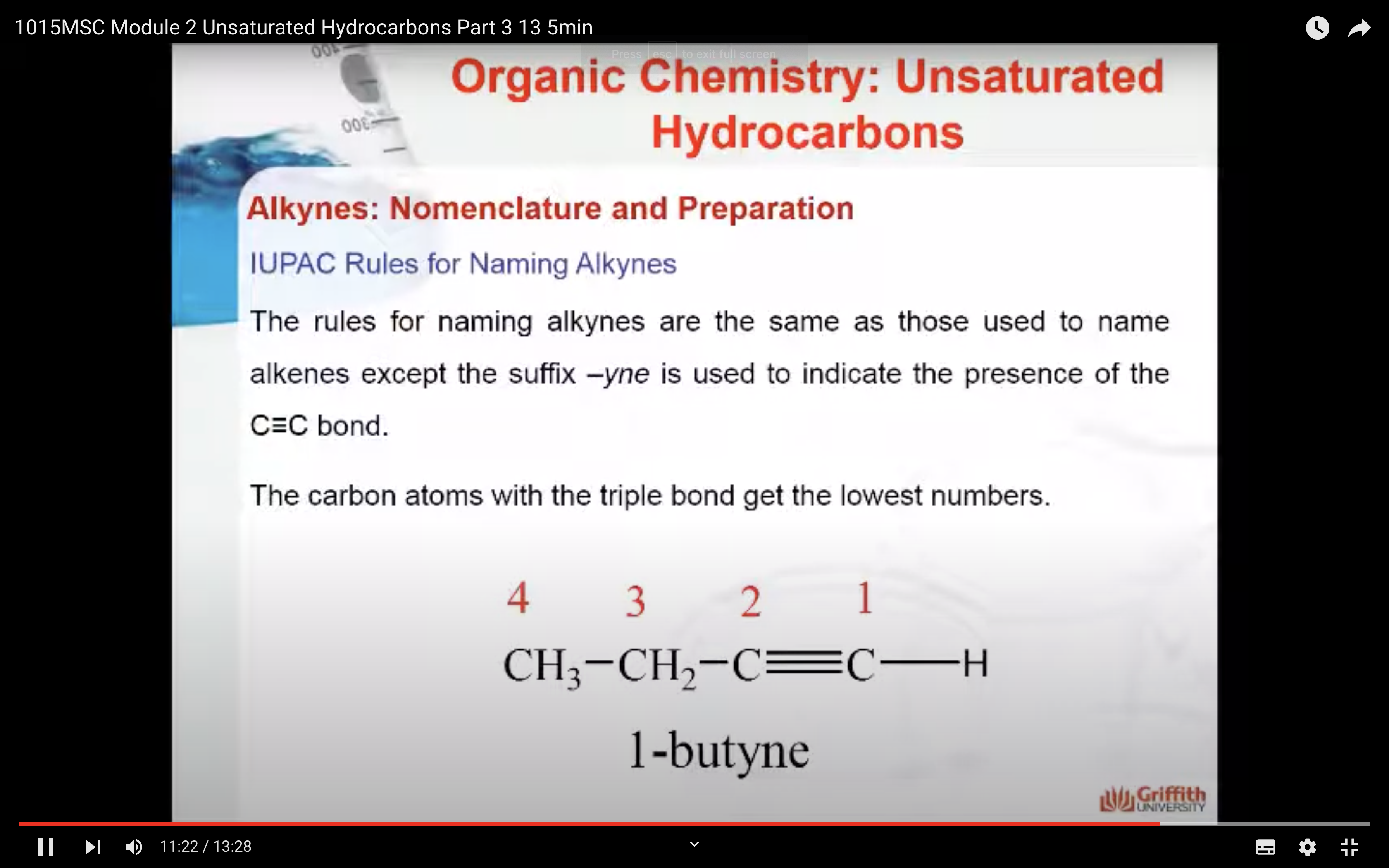
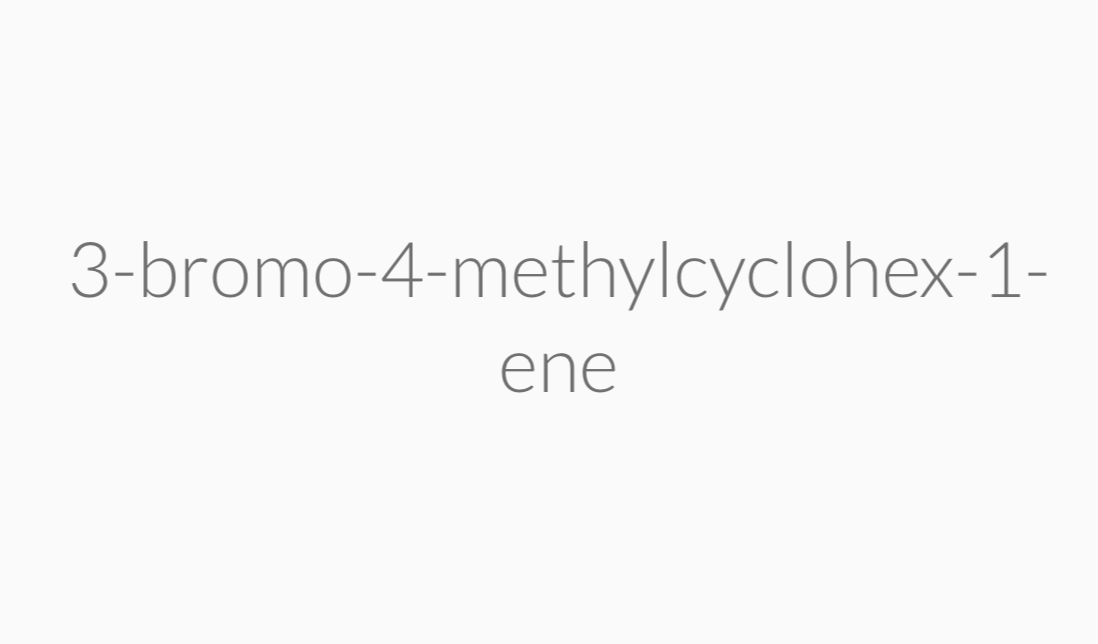
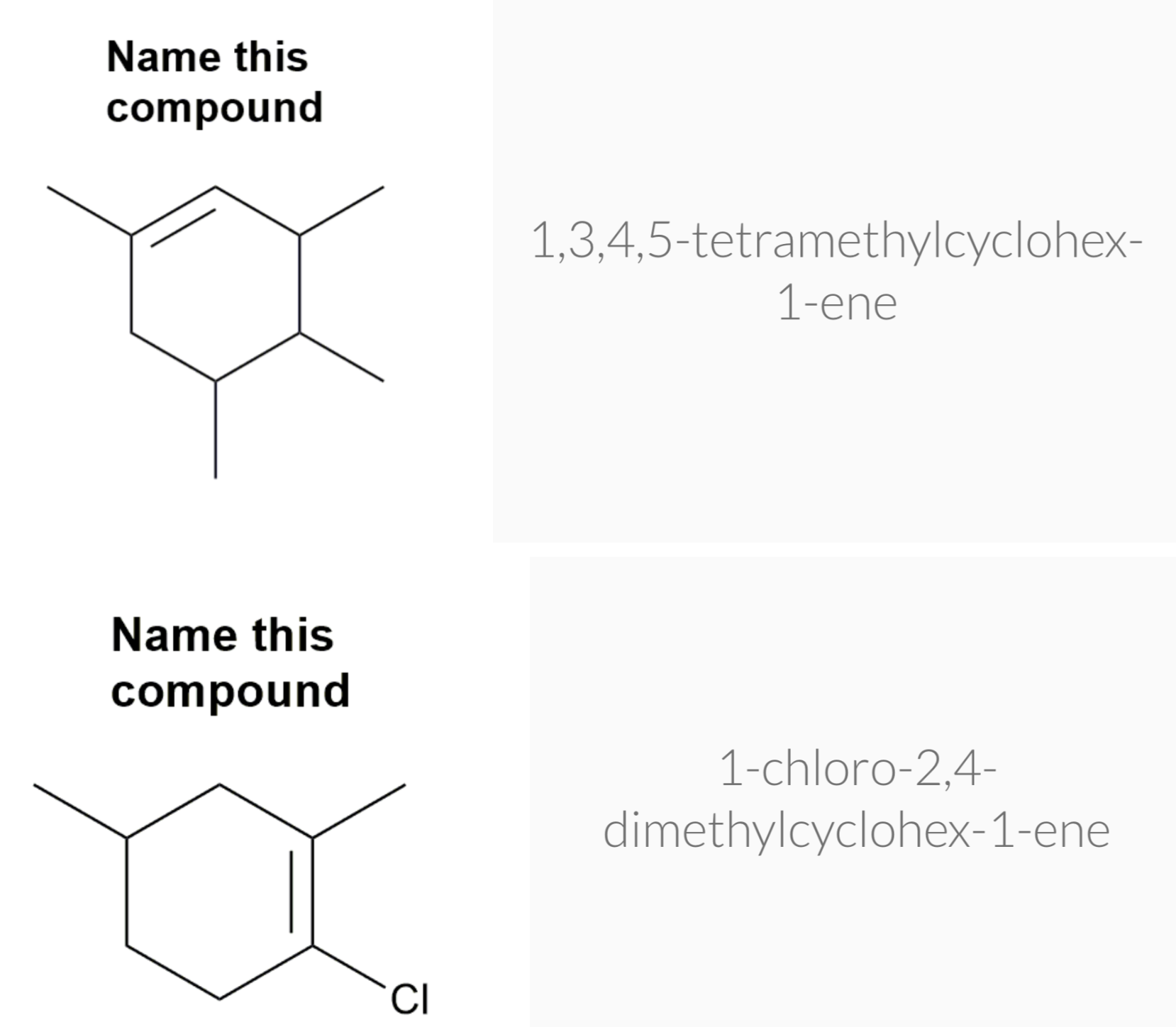
IUPAC Rules for Naming Alkenes
https://www.degruyter.com/document/doi/10.1515/pac-2019-0104/html
https://www.youtube.com/watch?v=nWI4Itkg7PU
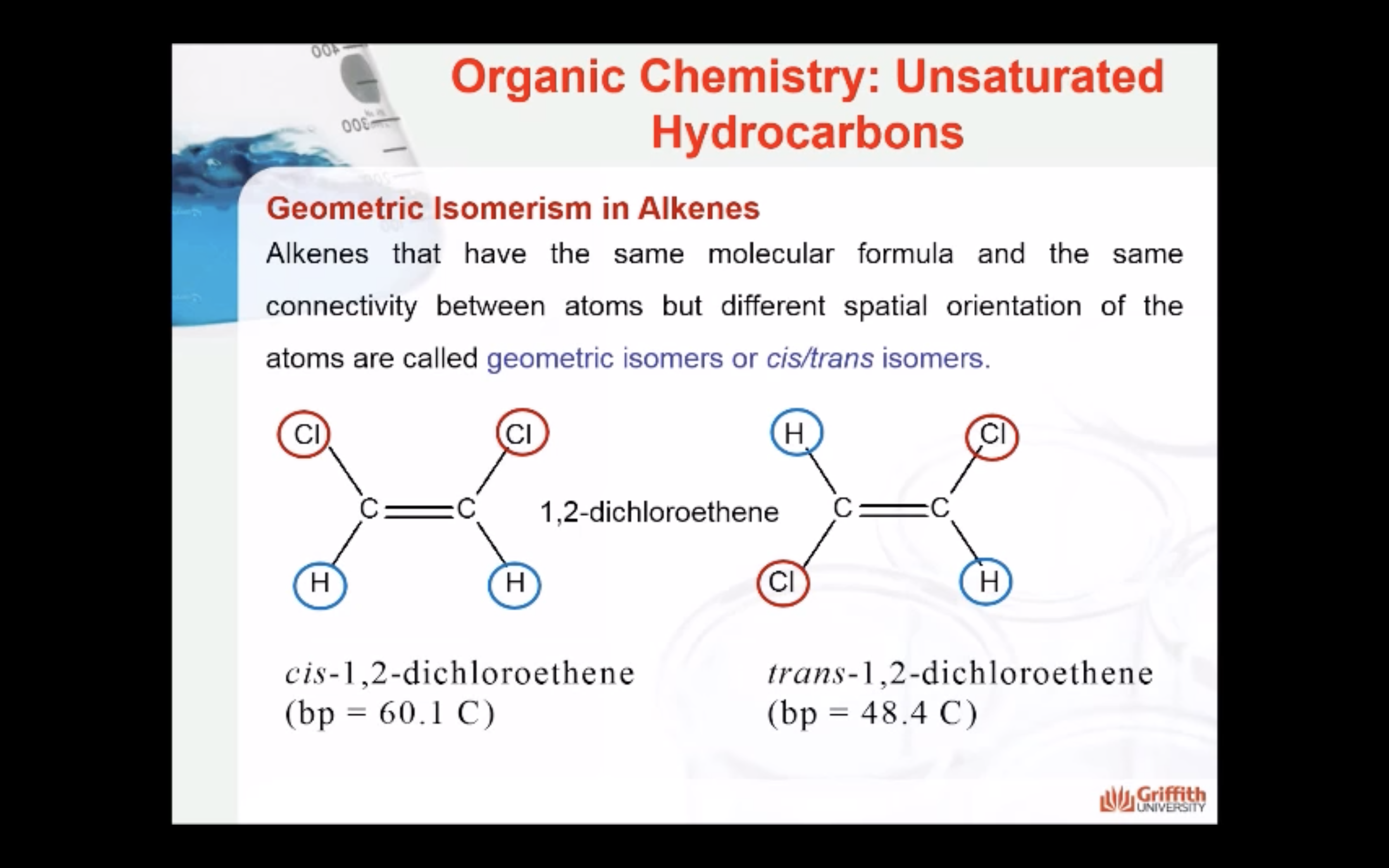
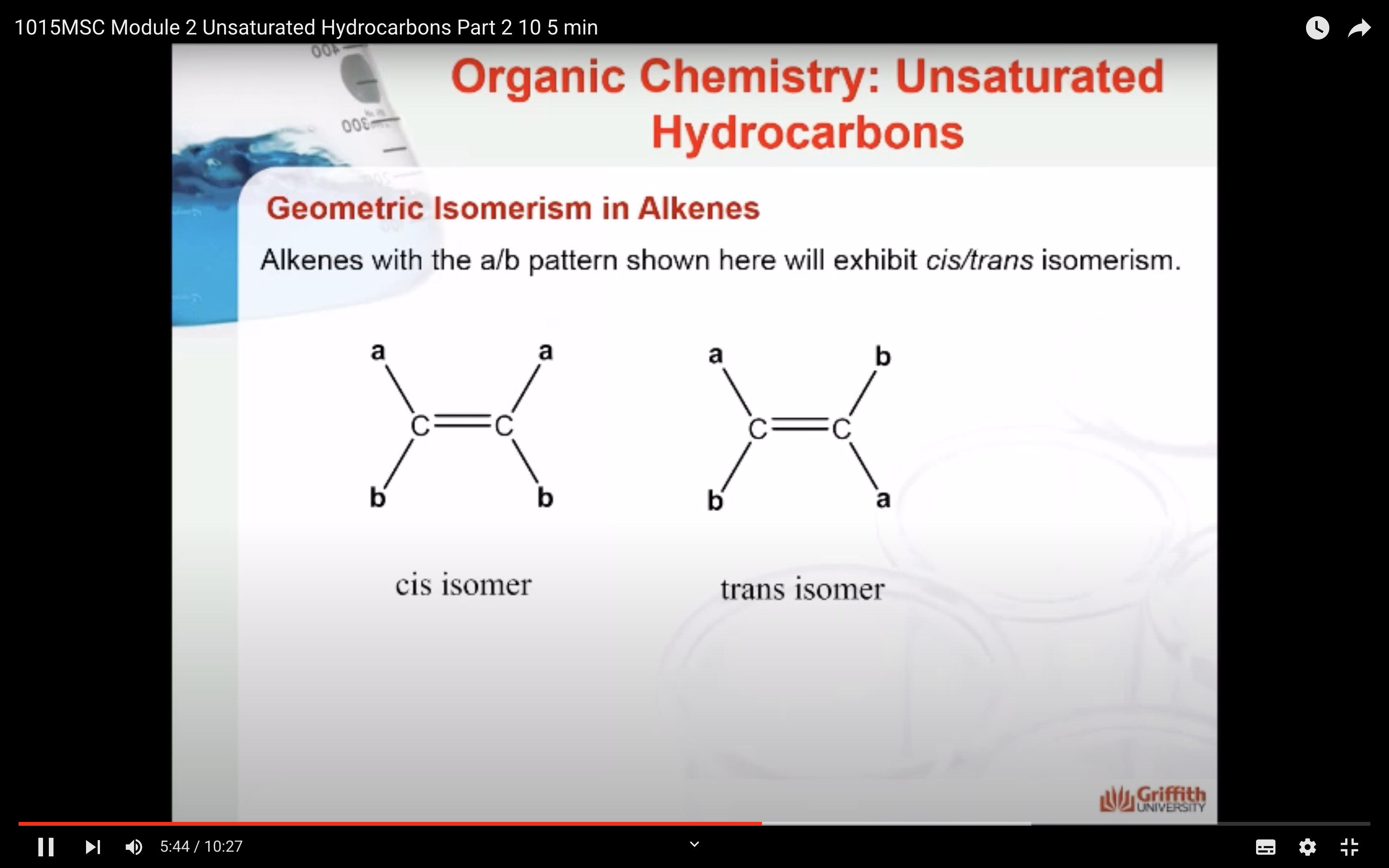
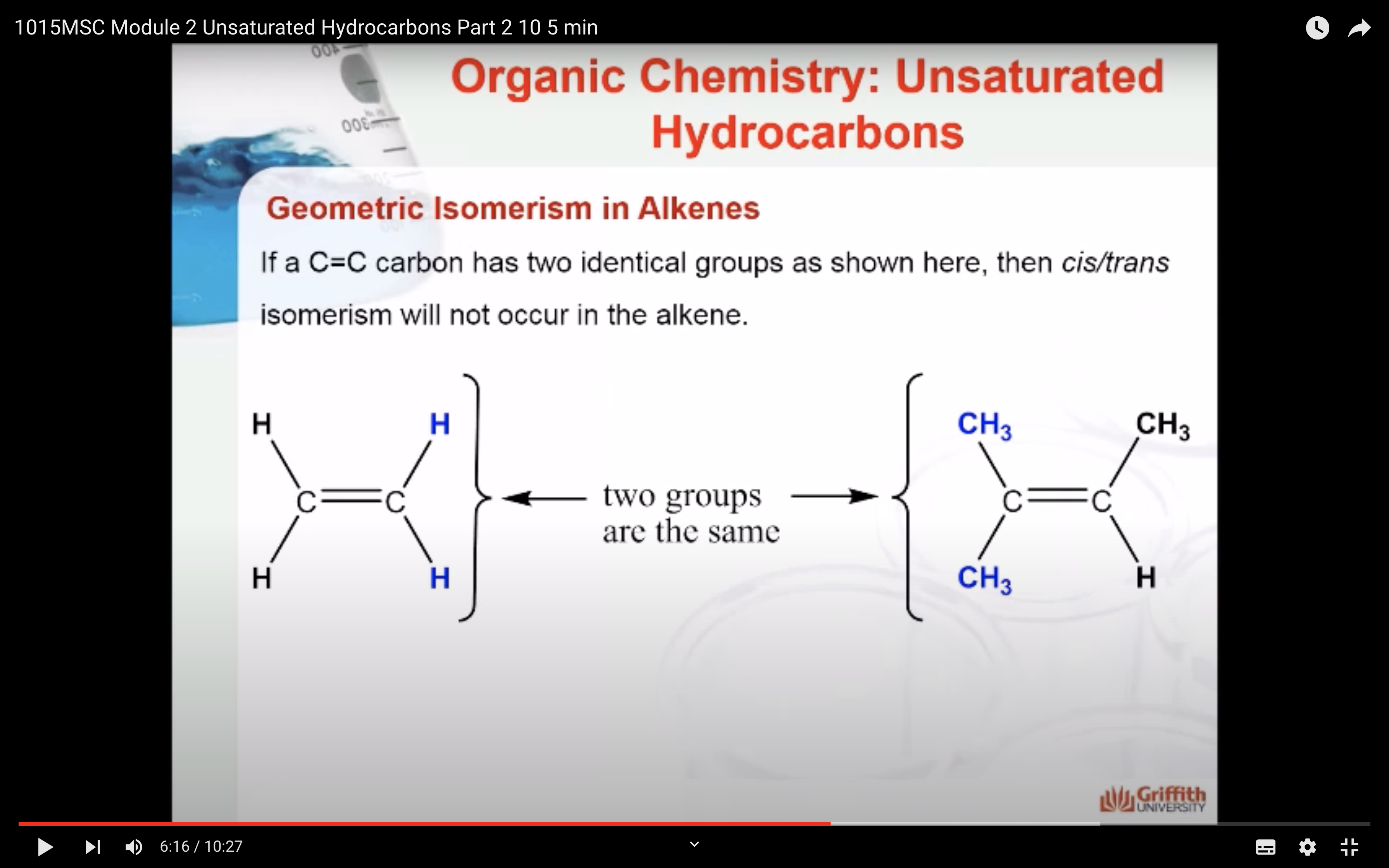
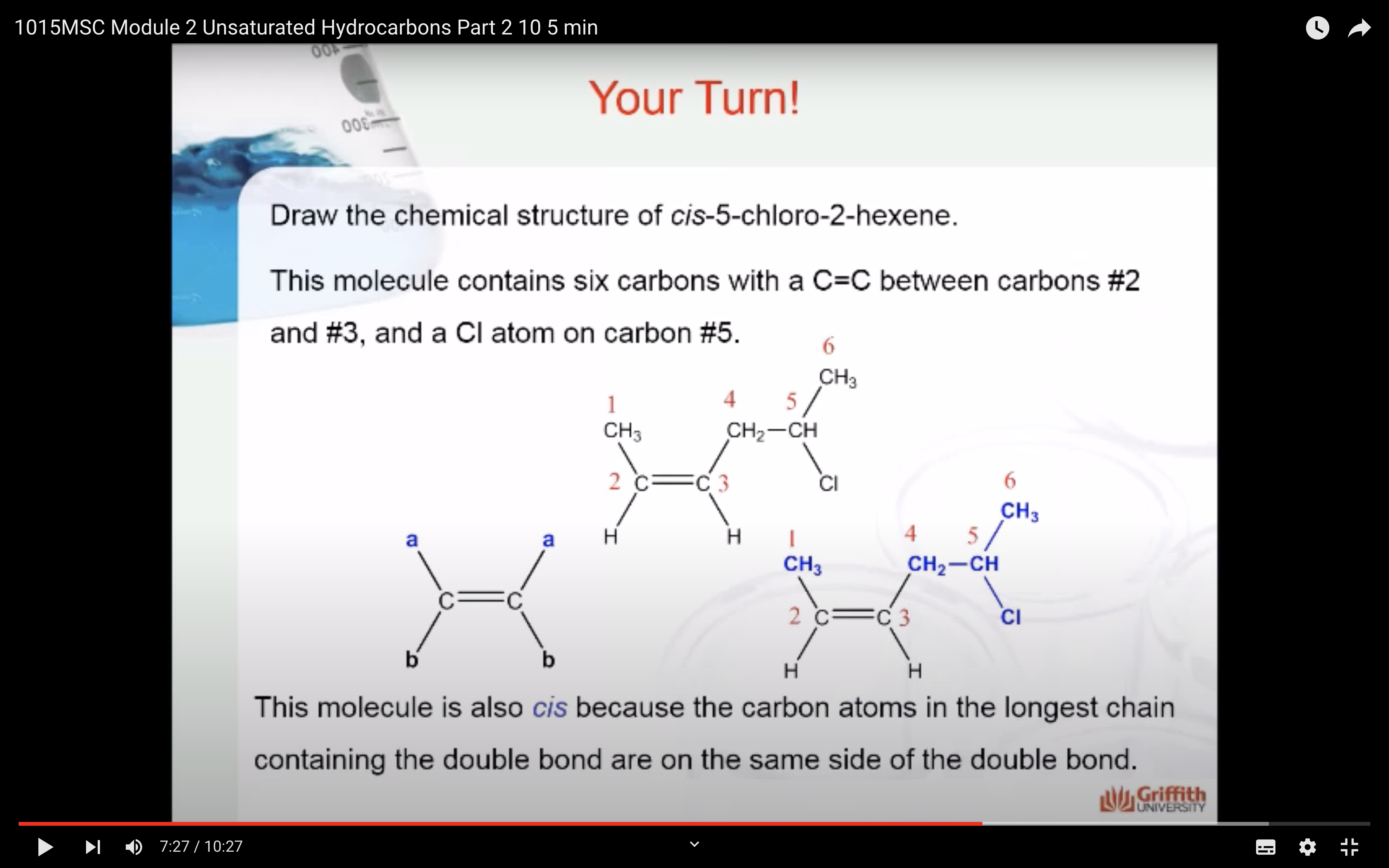
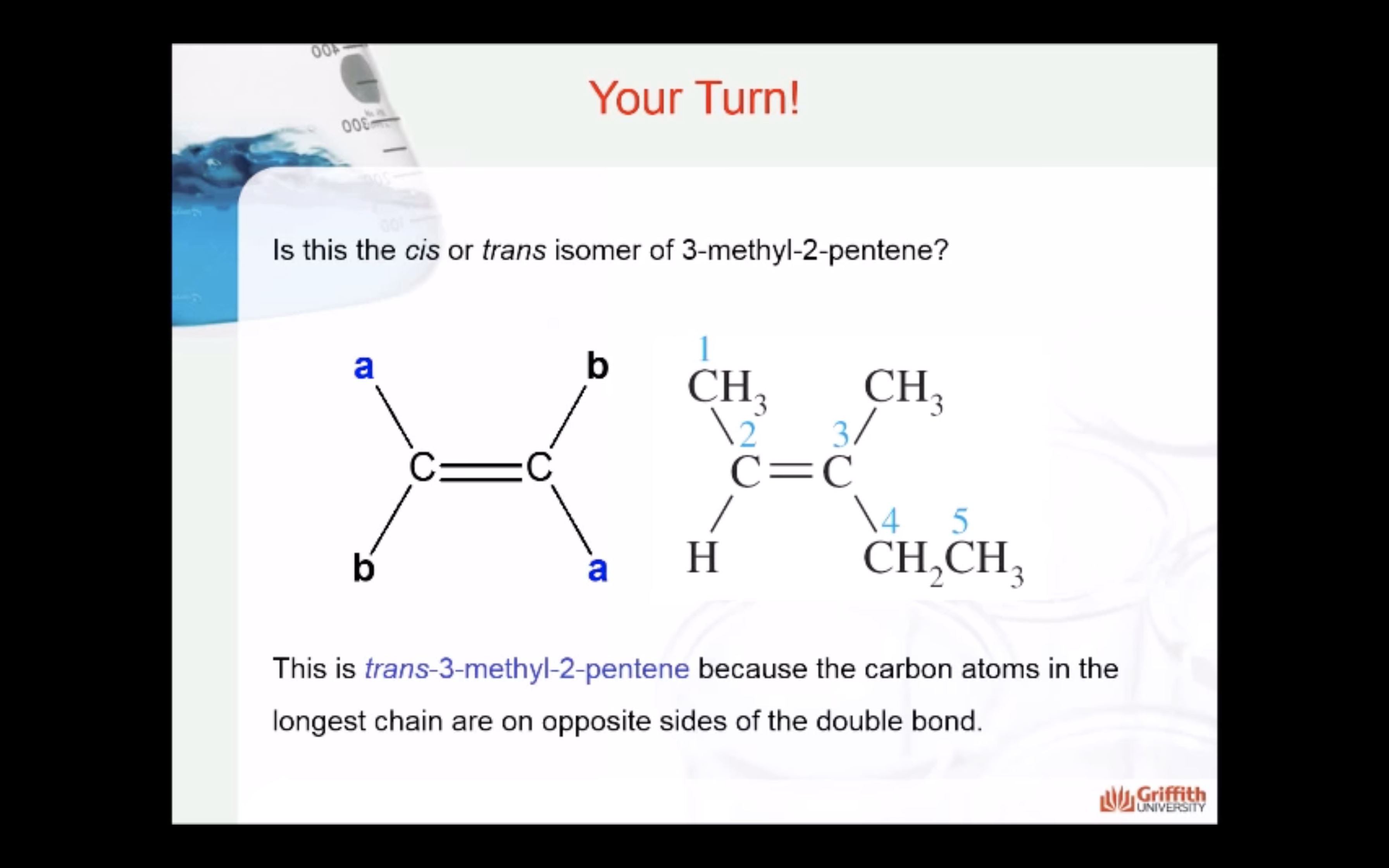
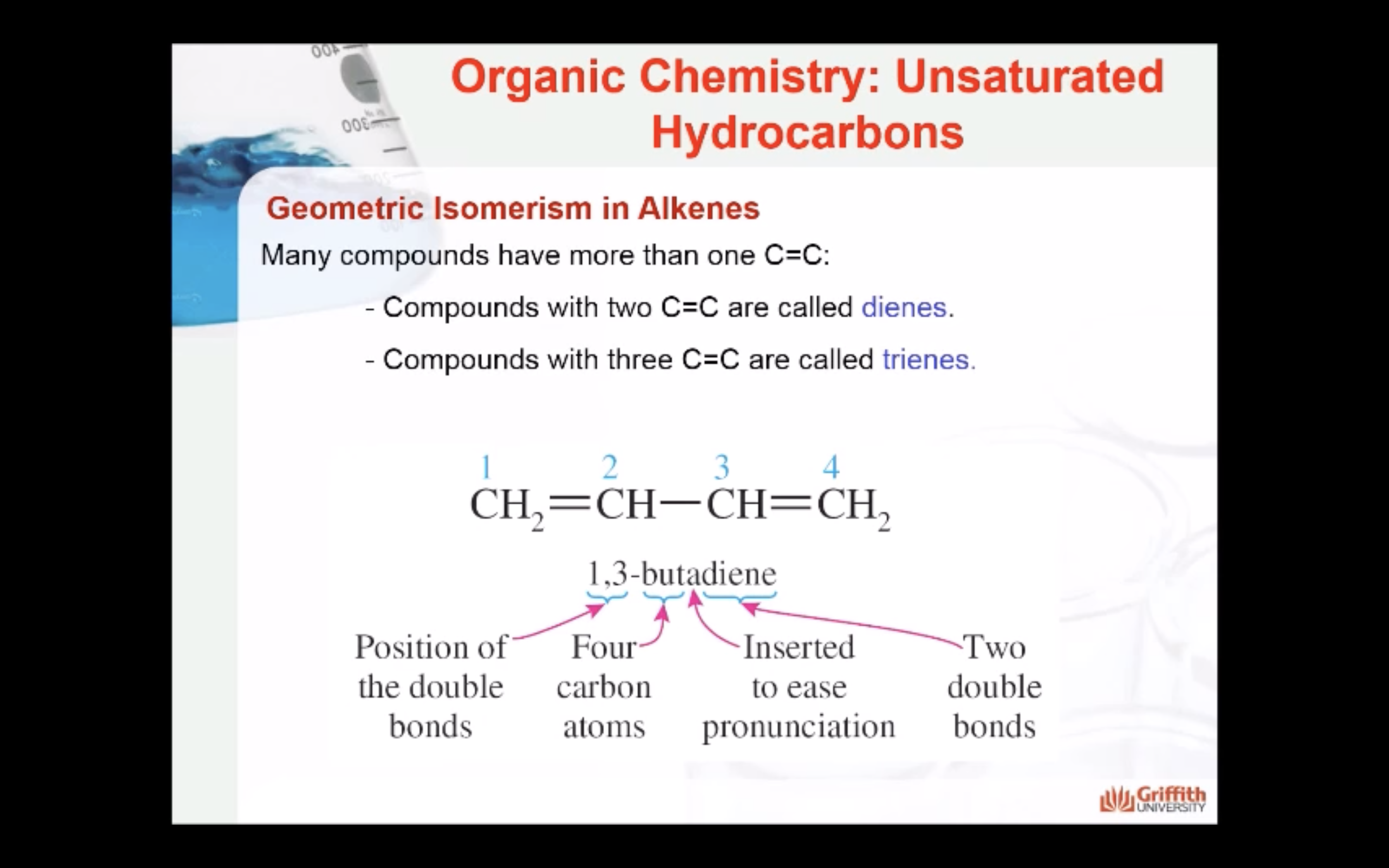
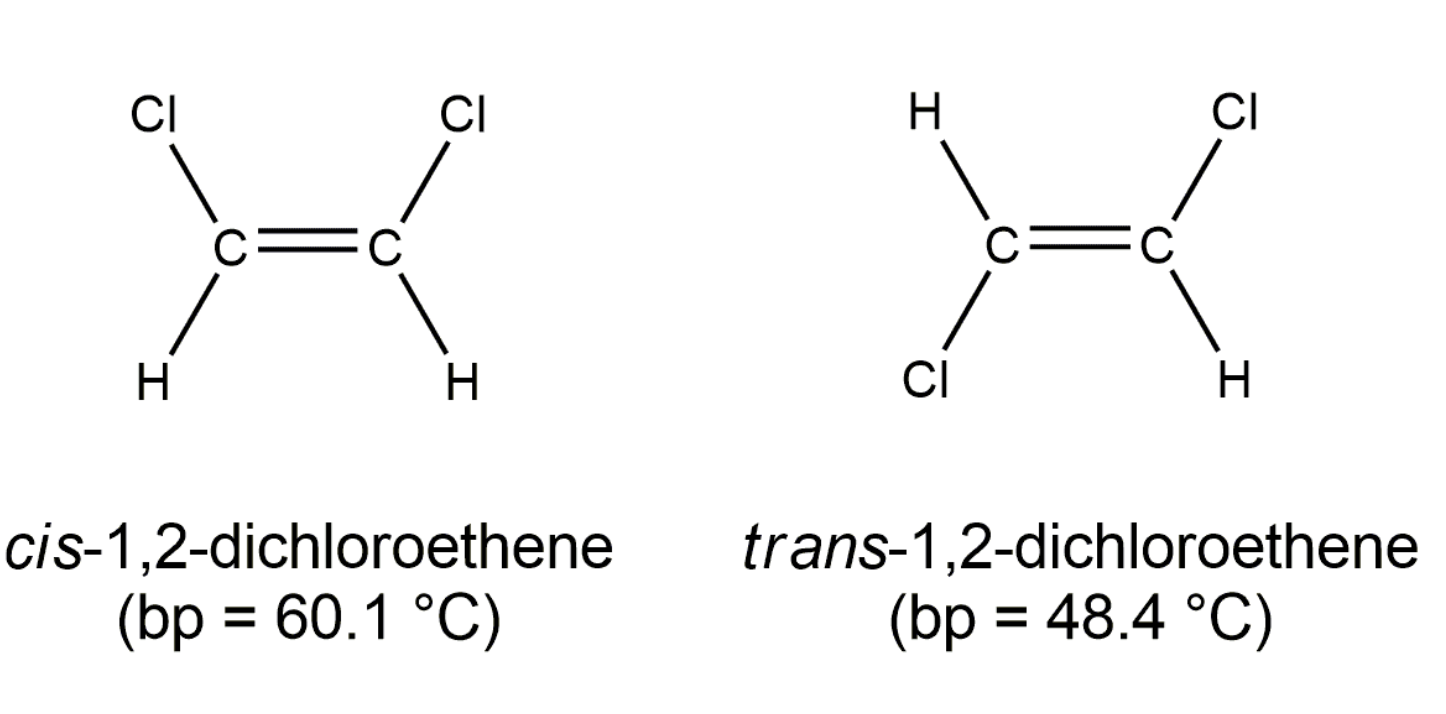
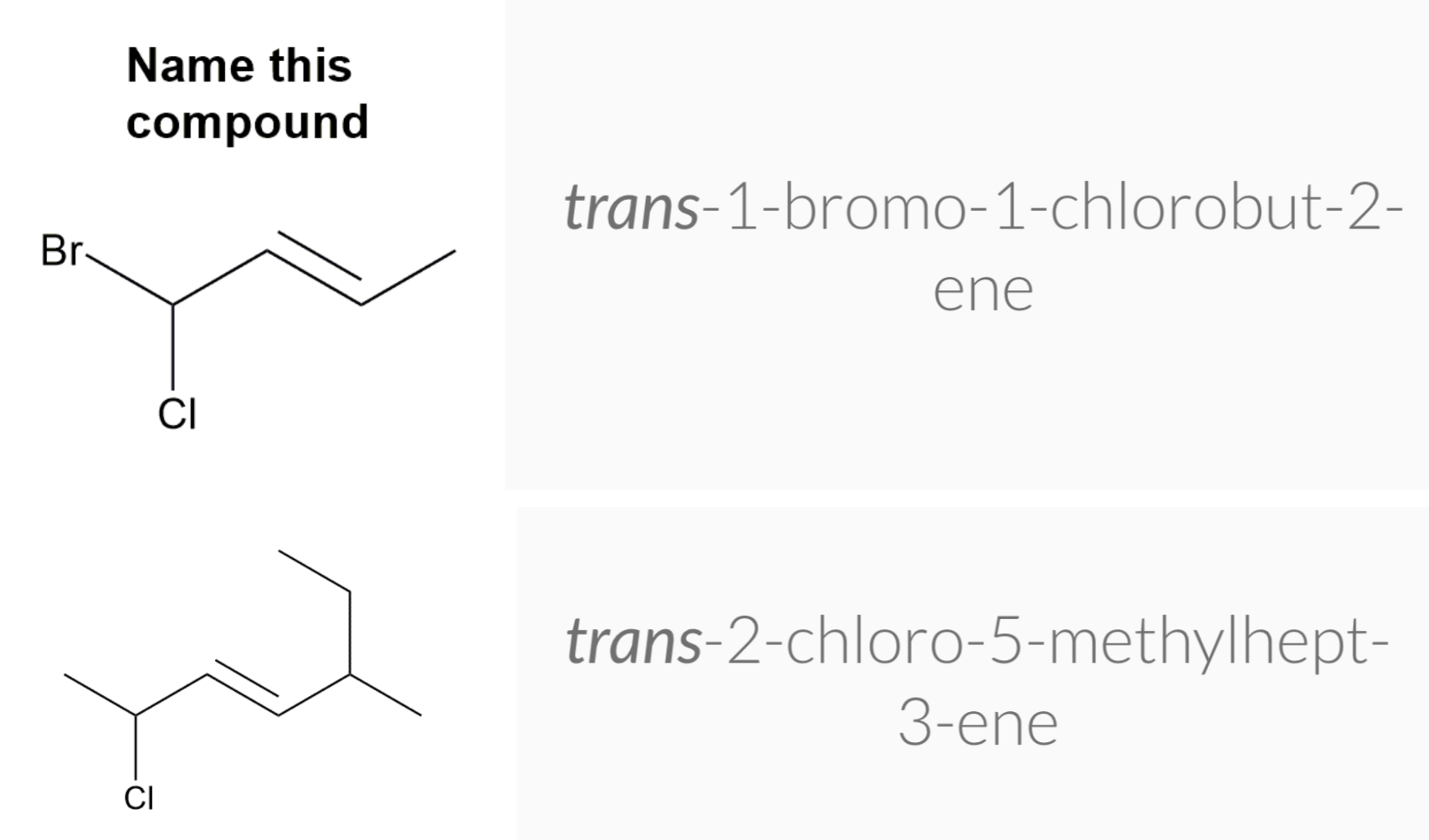
Geometric Isomerism in Alkenes
Alkenes that have the same molecular formula and the same connectivity between atoms but different spatial orientation of the atoms are called geometric isomers or cis/trans isomers.
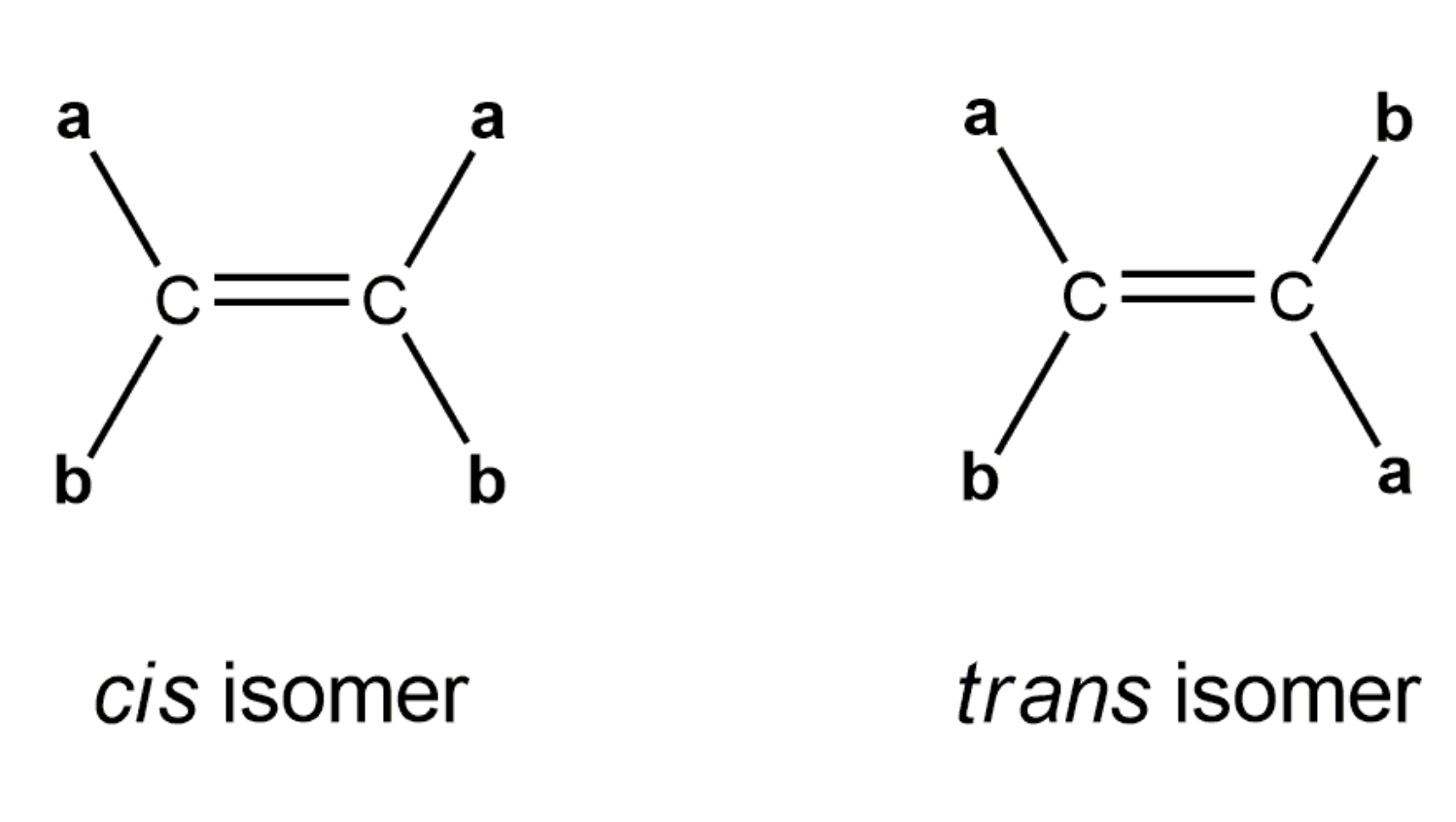
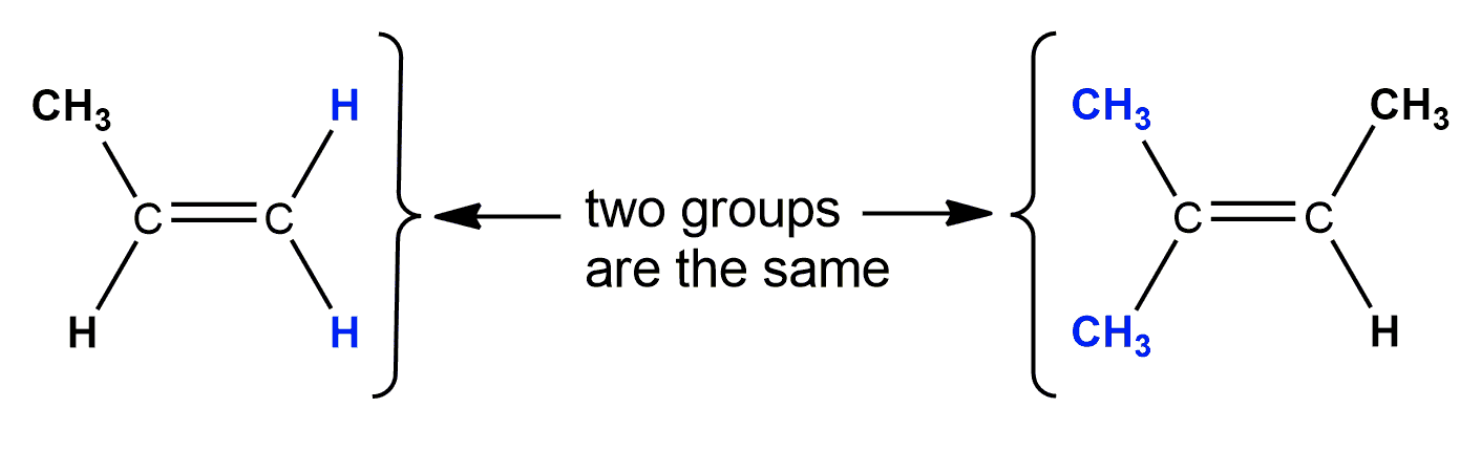
https://www.youtube.com/watch?v=zIUcMVe1yPs
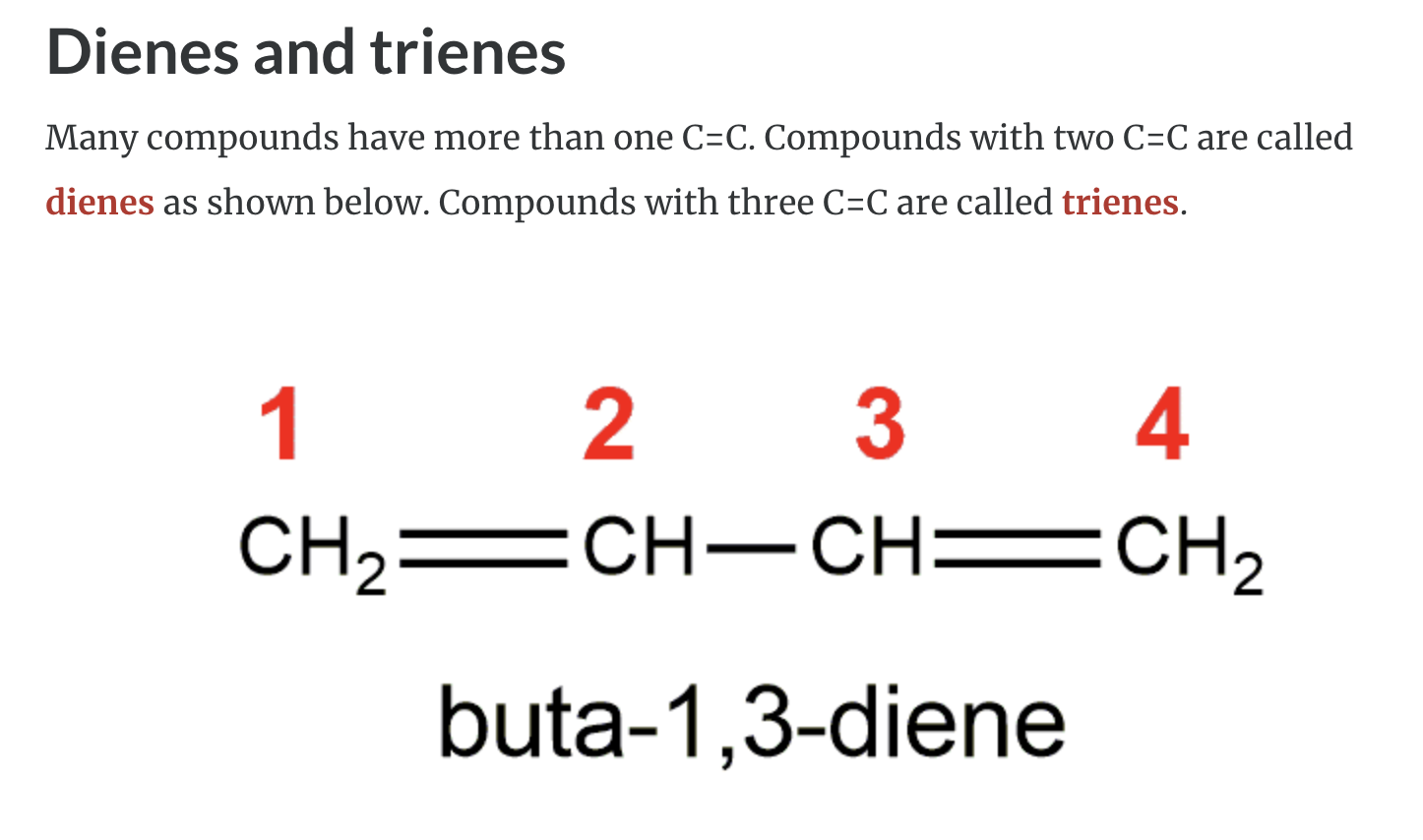
https://www.youtube.com/watch?v=iwriWtfBRXE
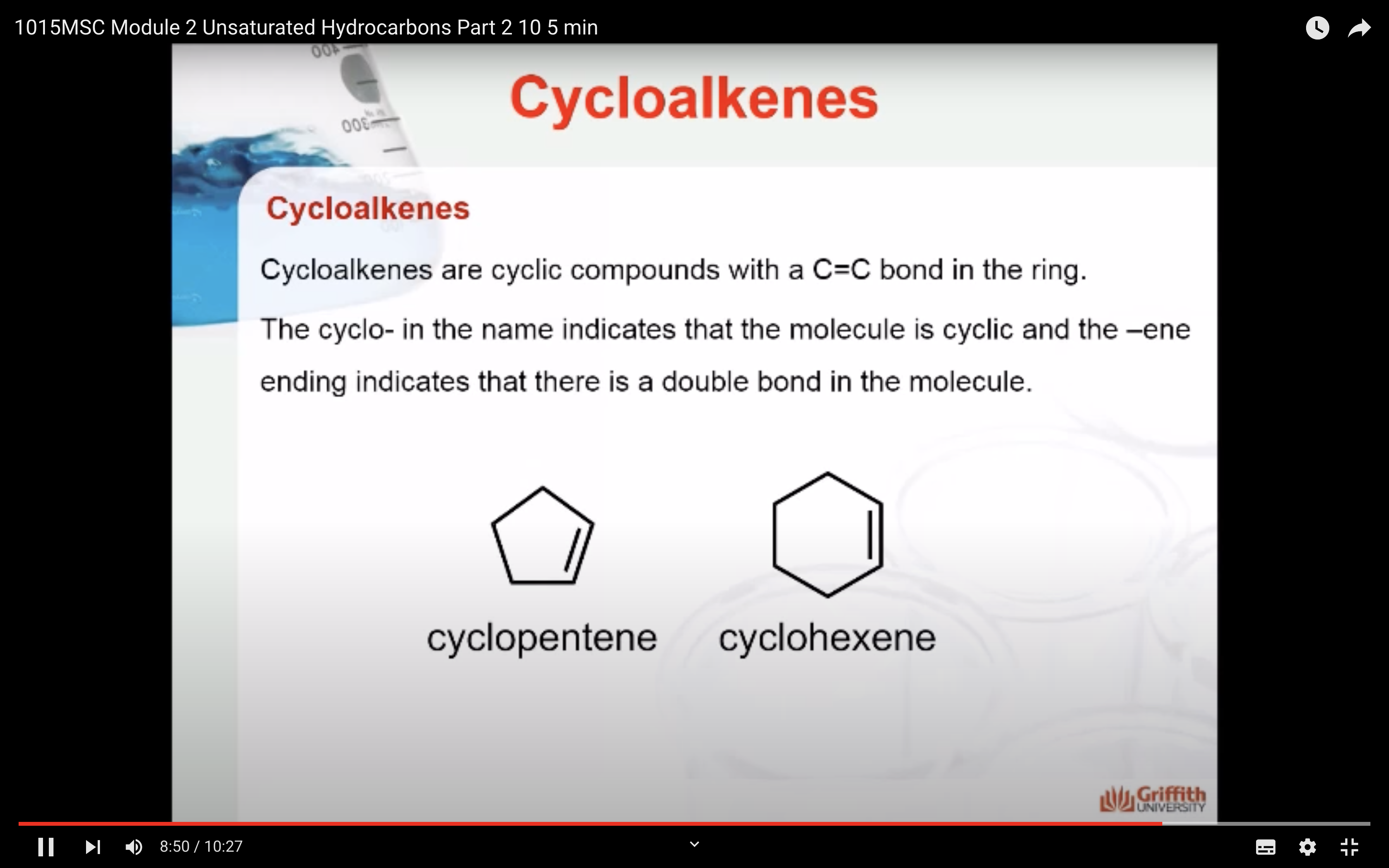
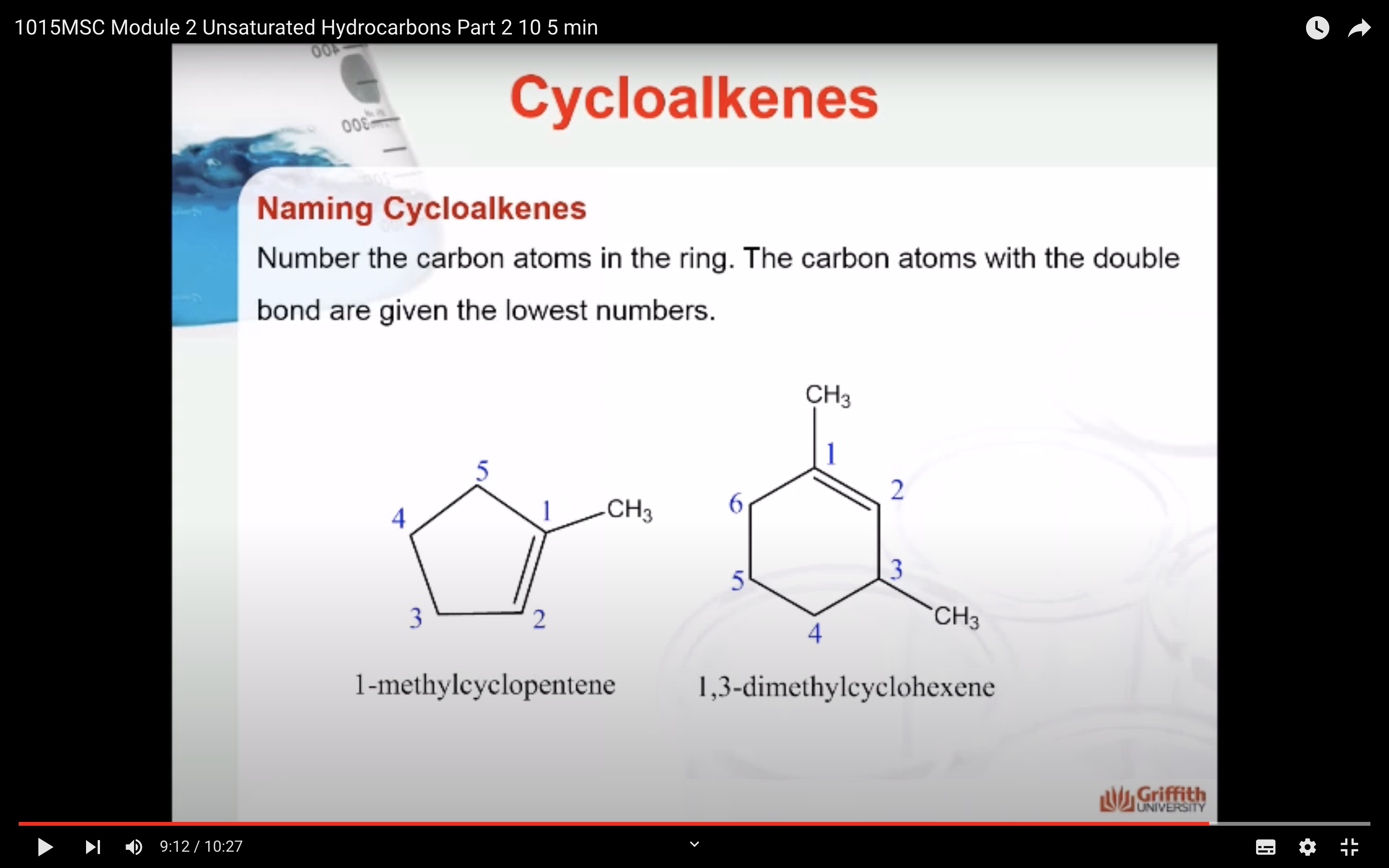
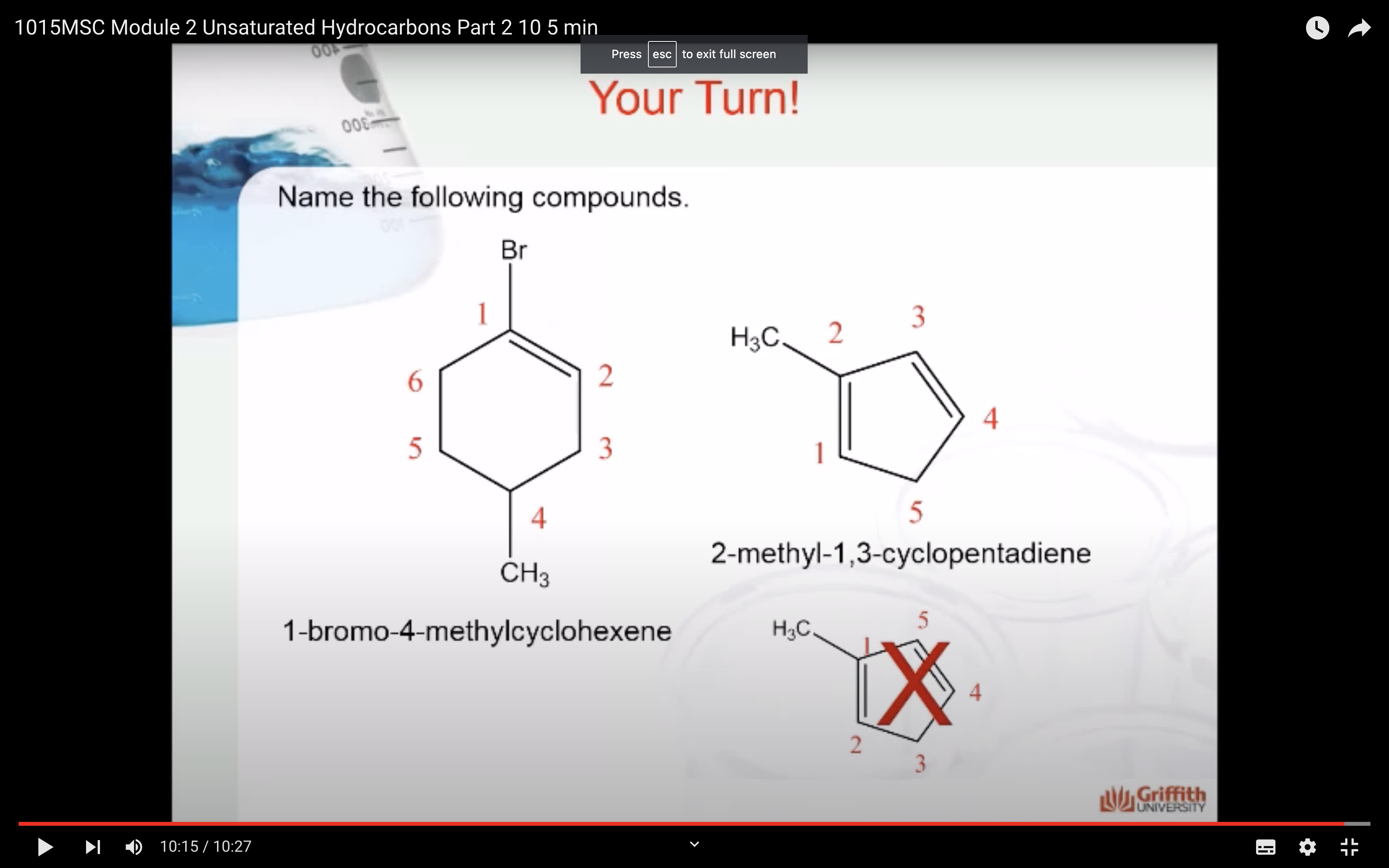
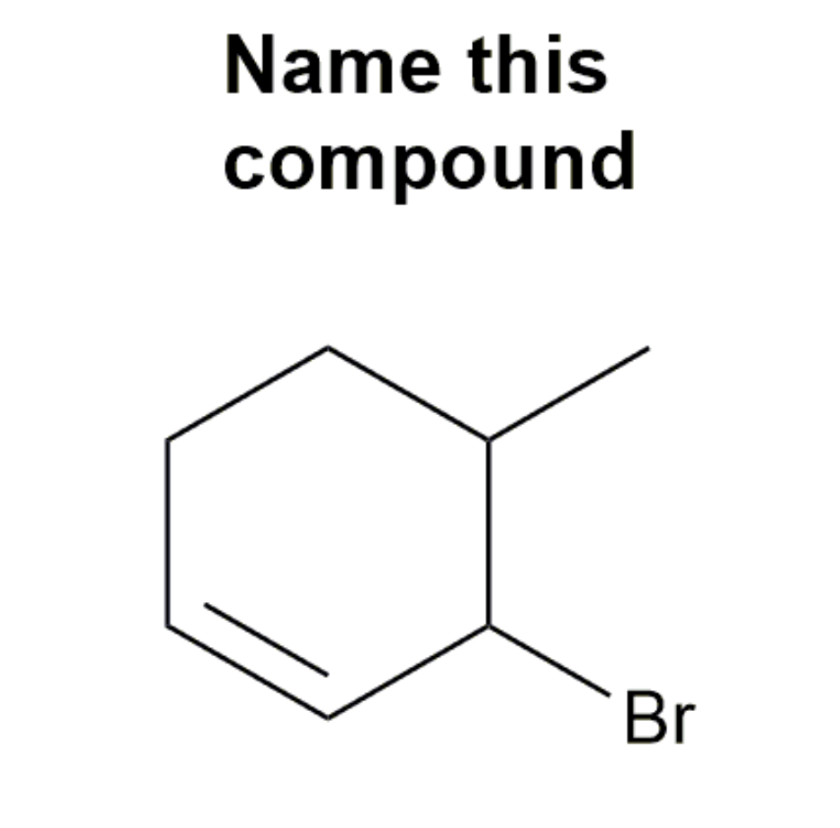
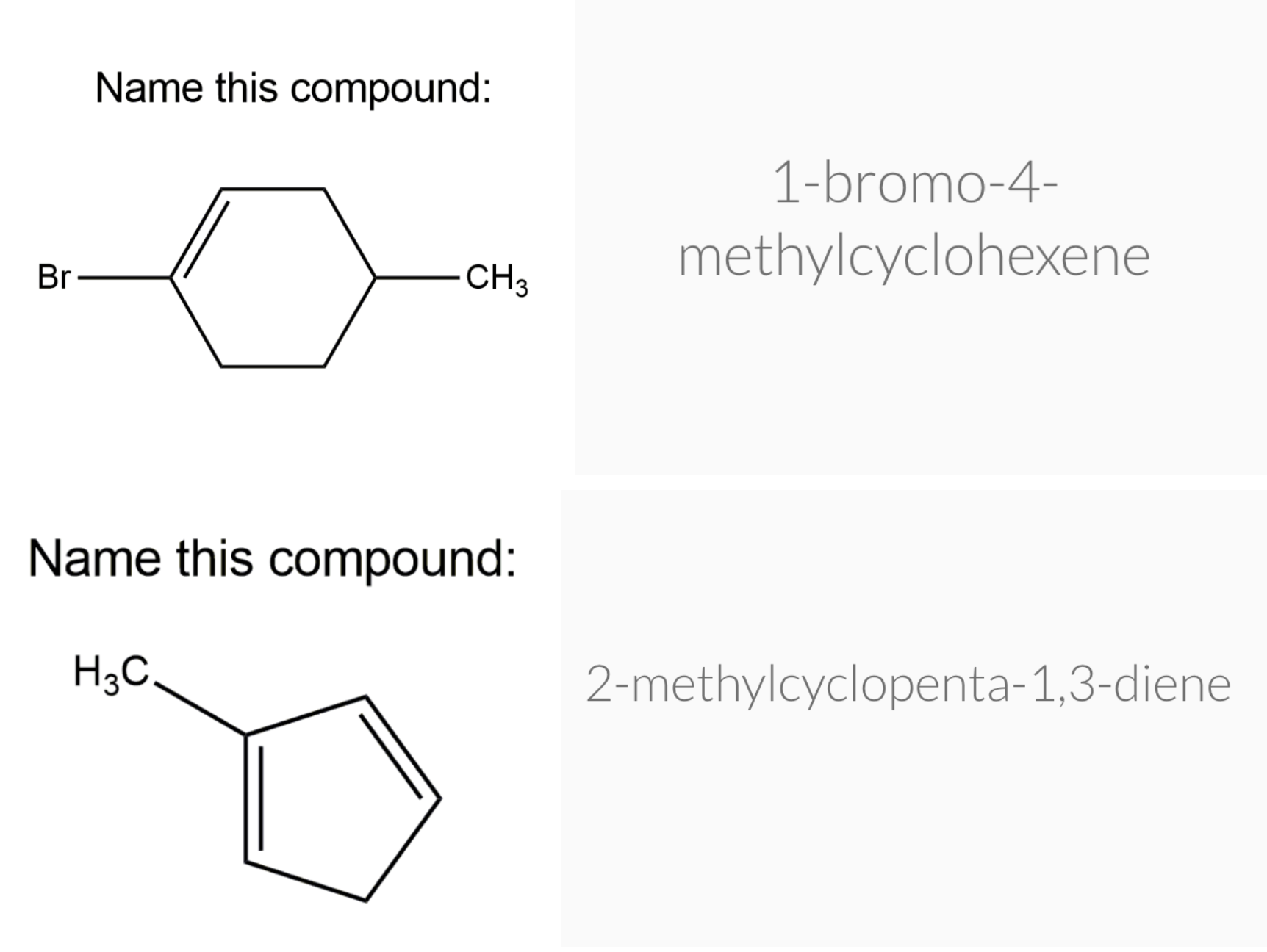
Cycloalkenes
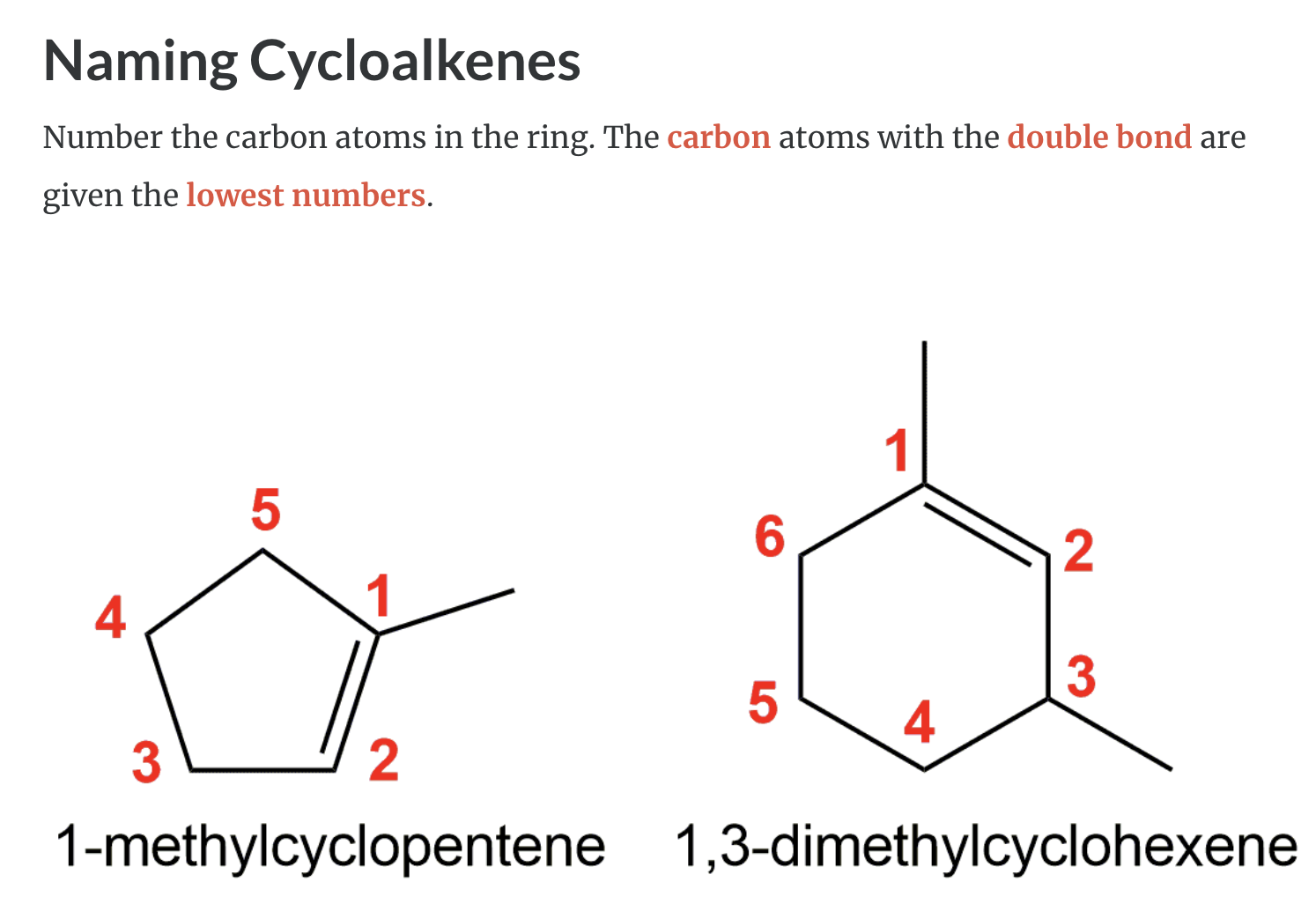
Cycloalkenes are cyclic compounds with a C=C bond in the ring. The cyclo– in the name indicates that the molecule is cyclic and the –ene ending indicates that there is a double bond in the molecule.
https://www.youtube.com/watch?v=B02E4REukXE
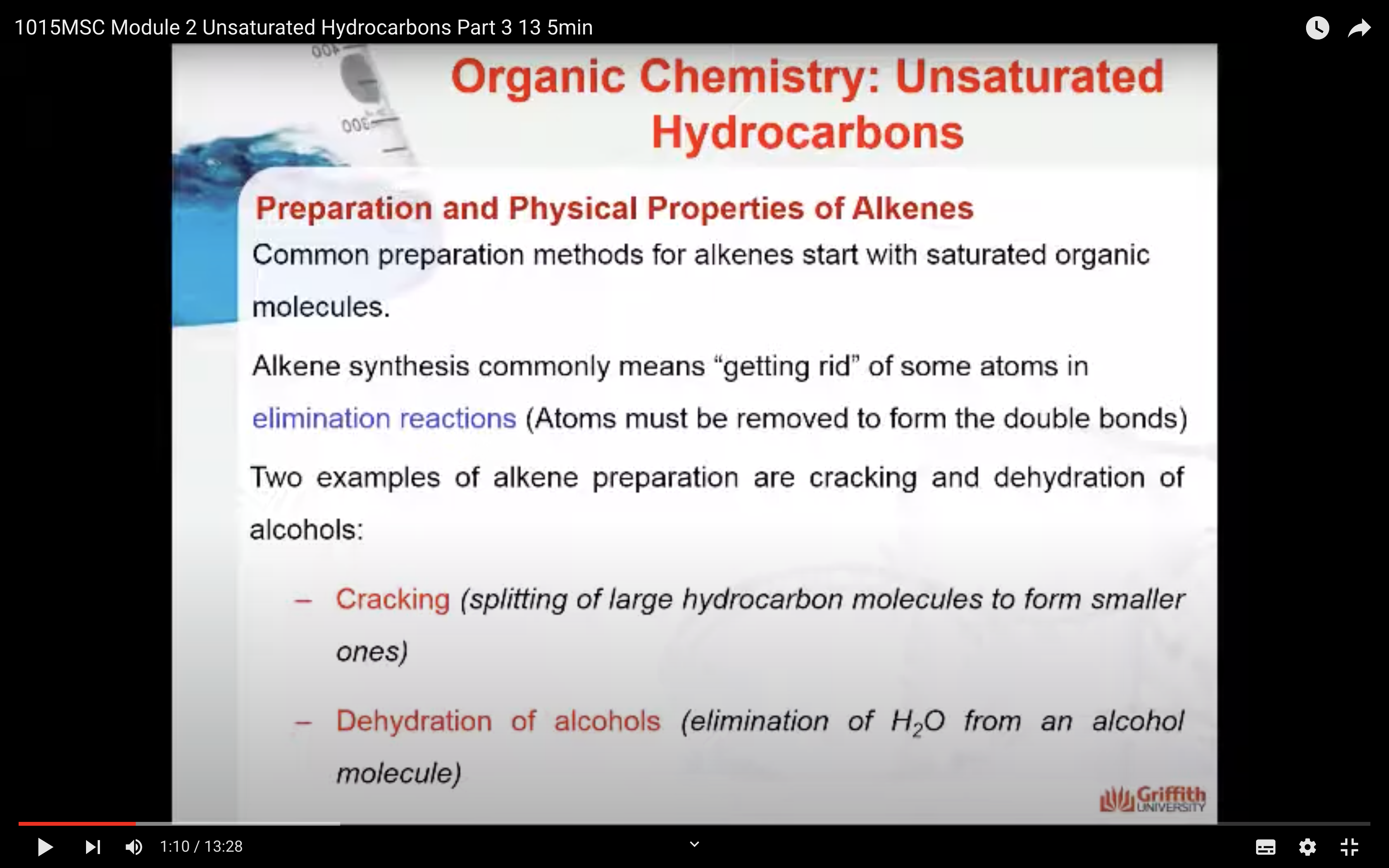
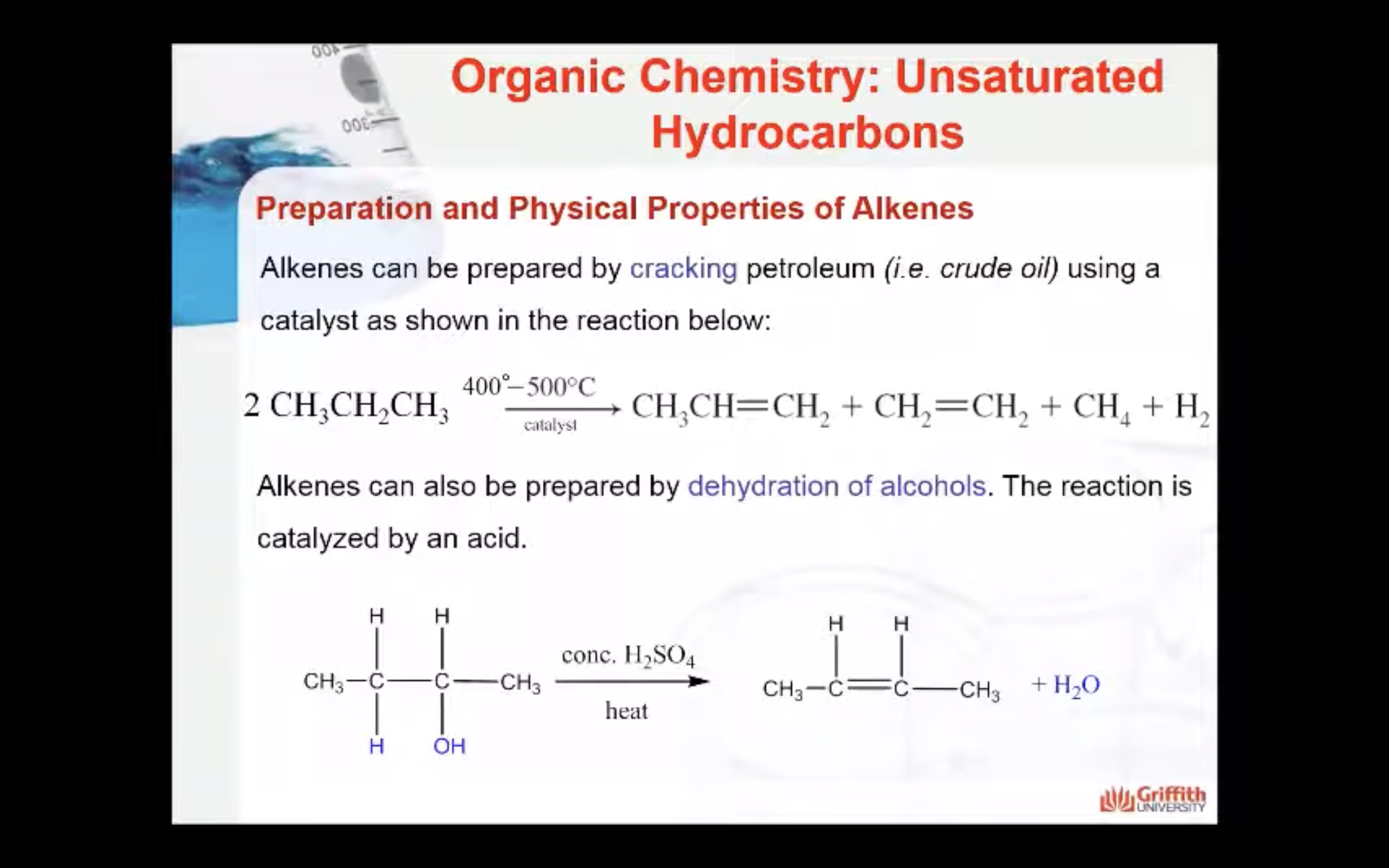
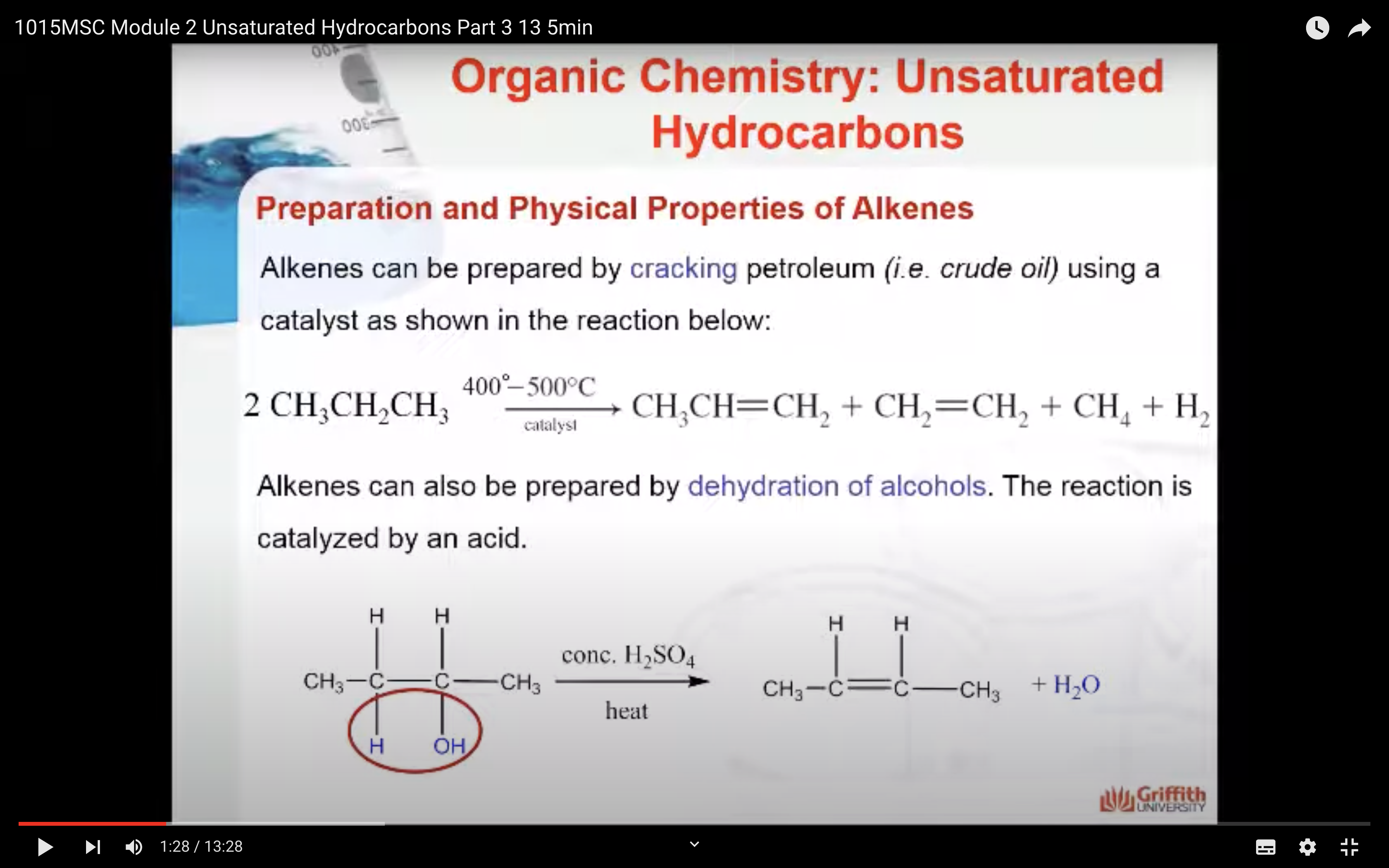
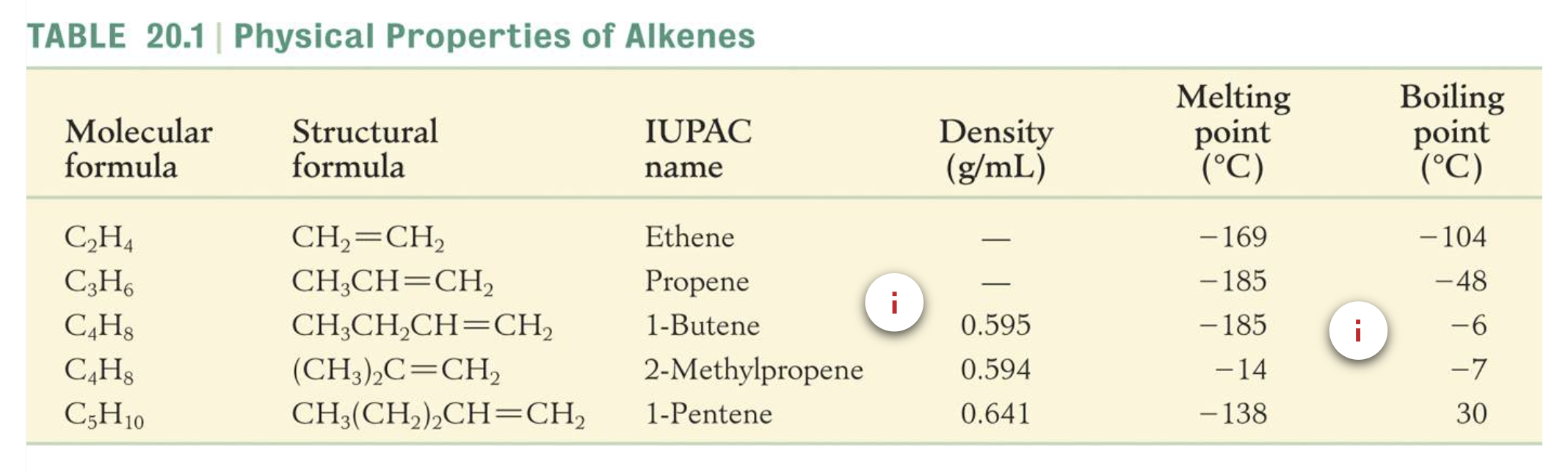
Physical Properties of Alkenes


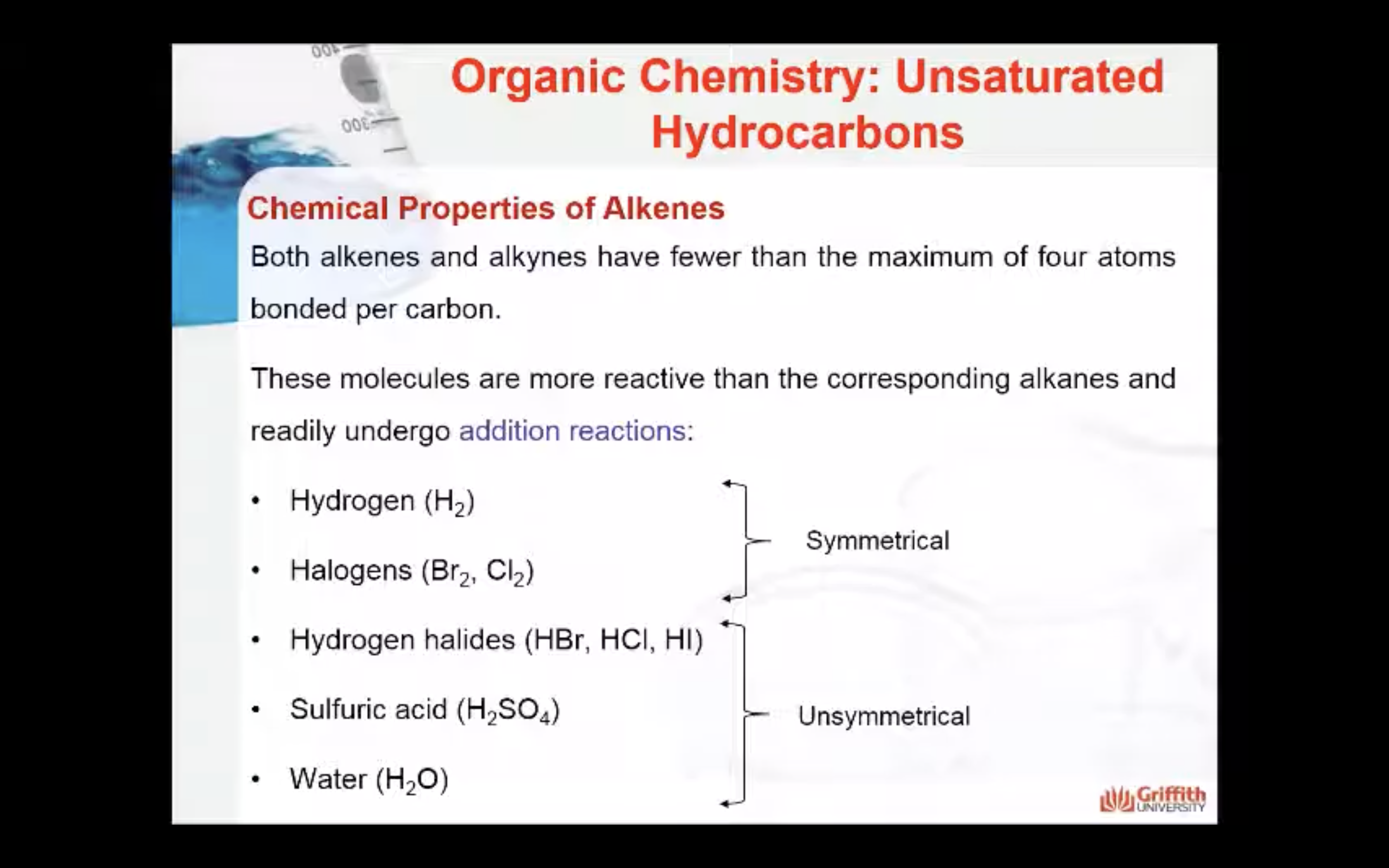
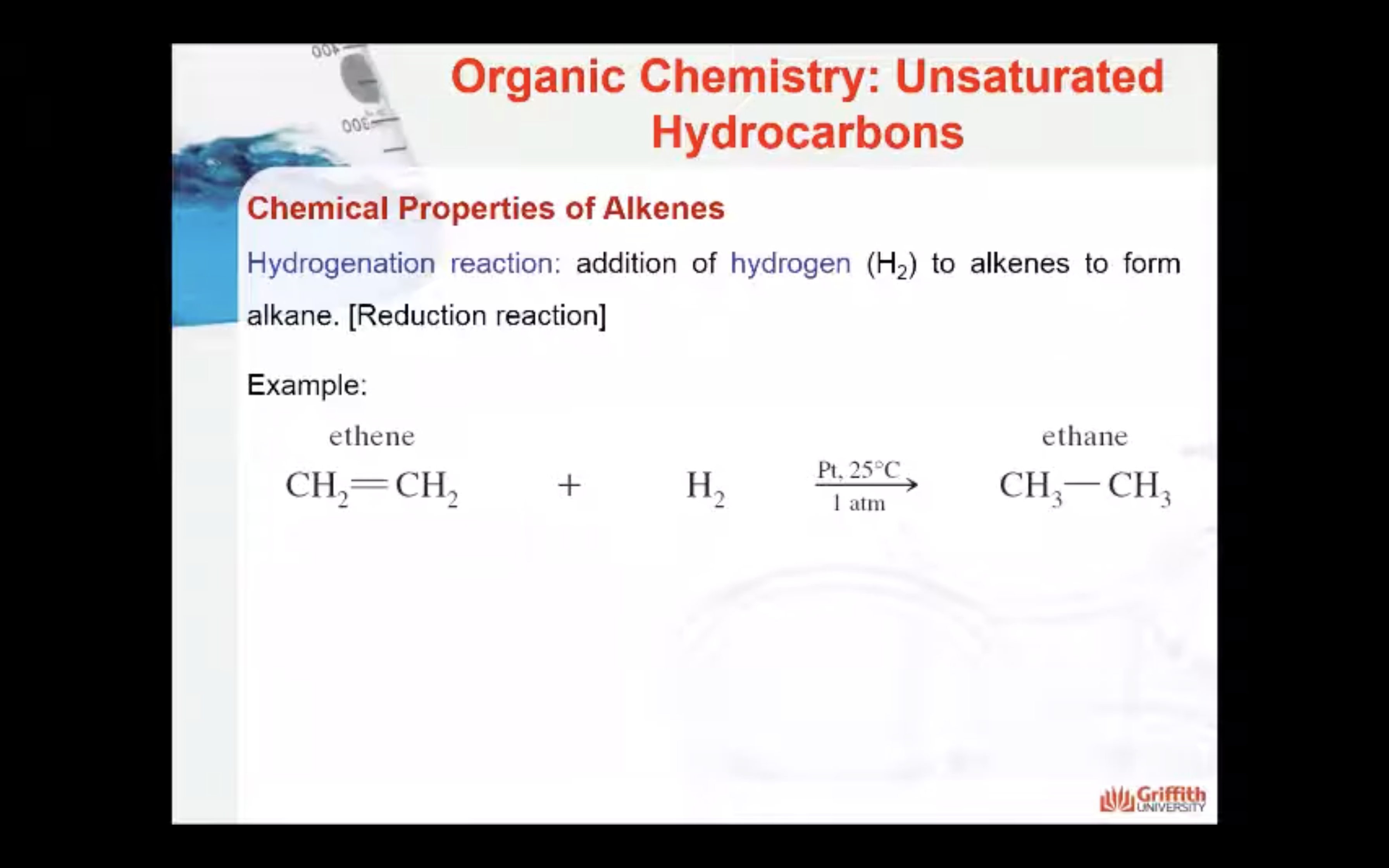
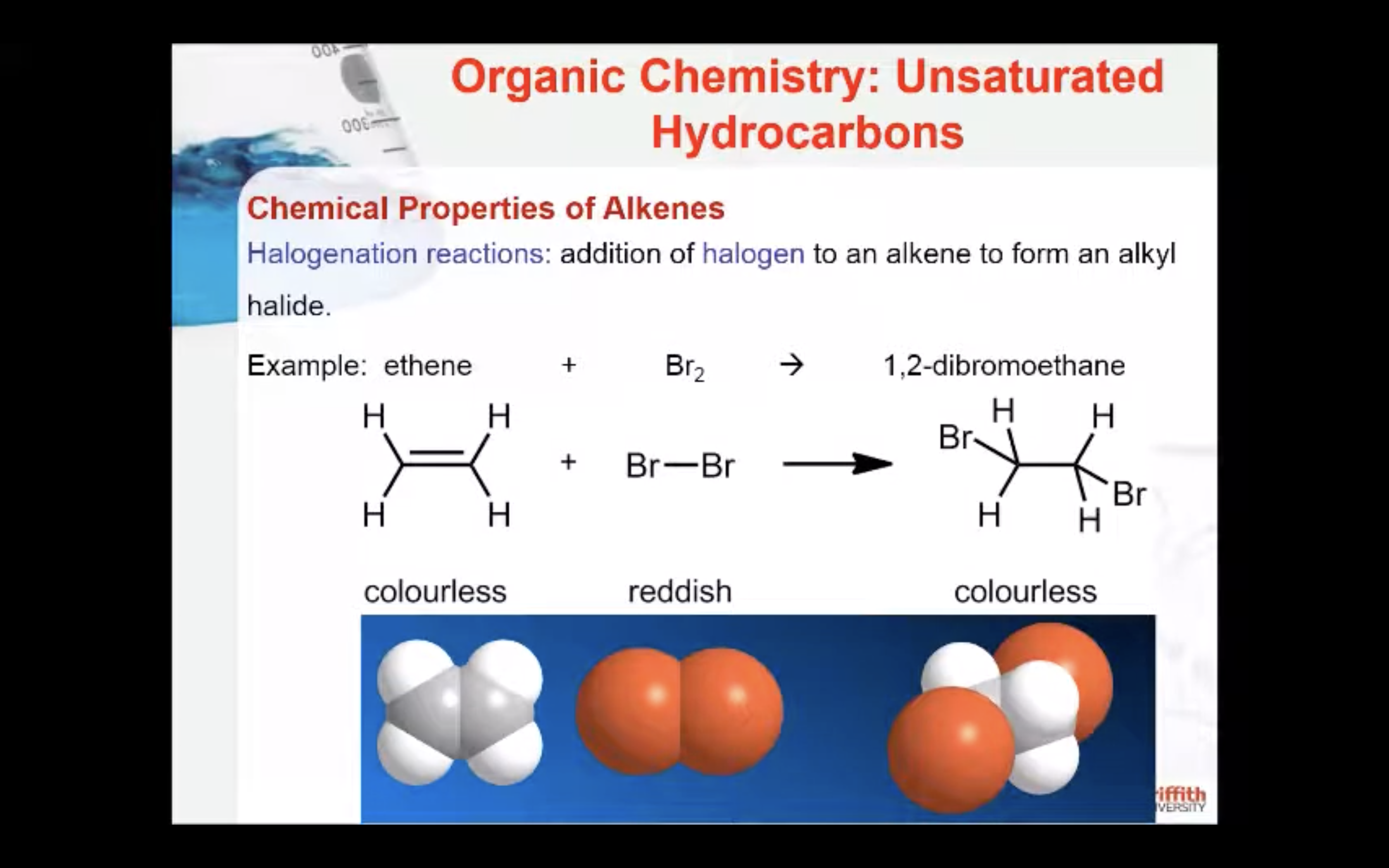
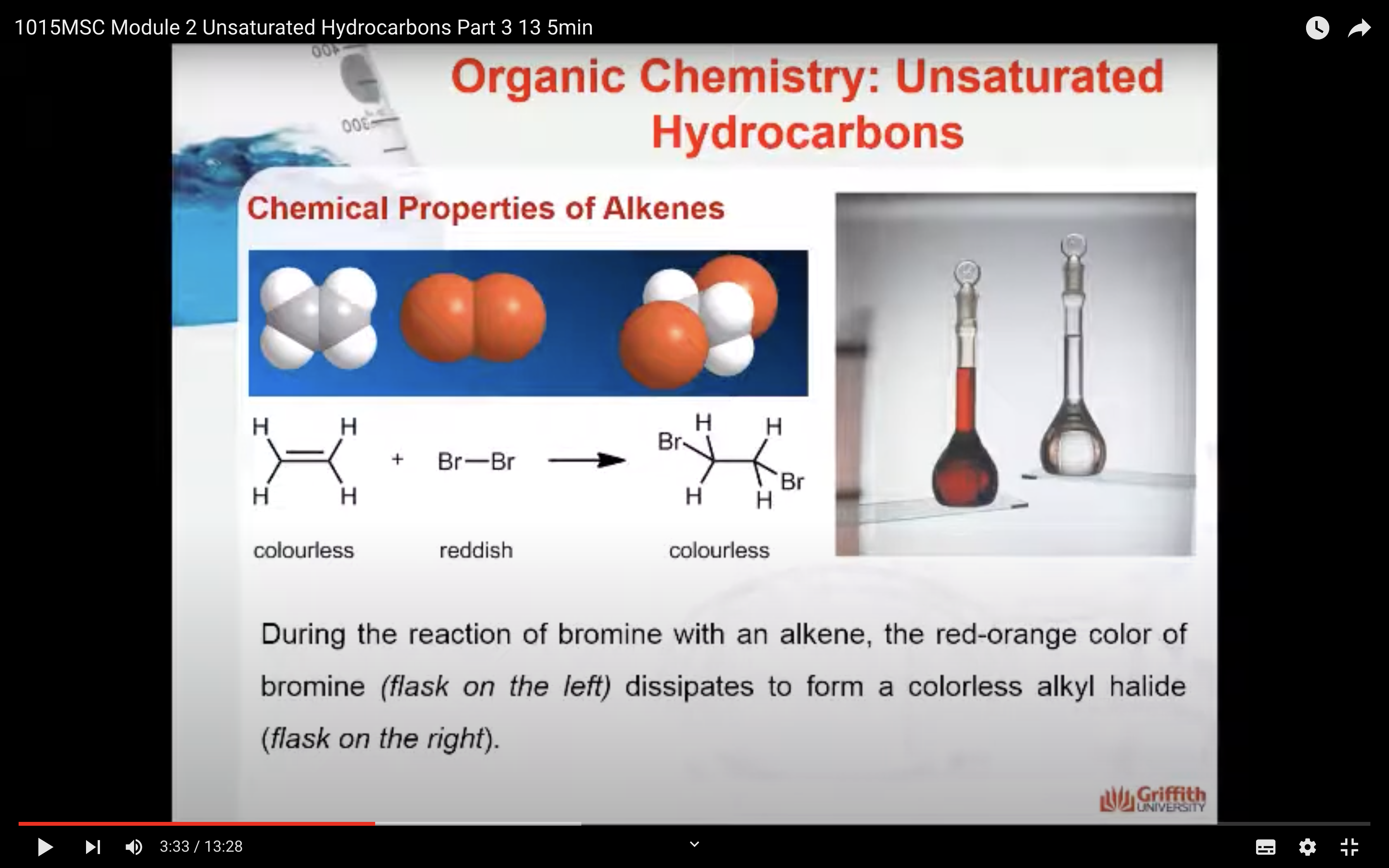
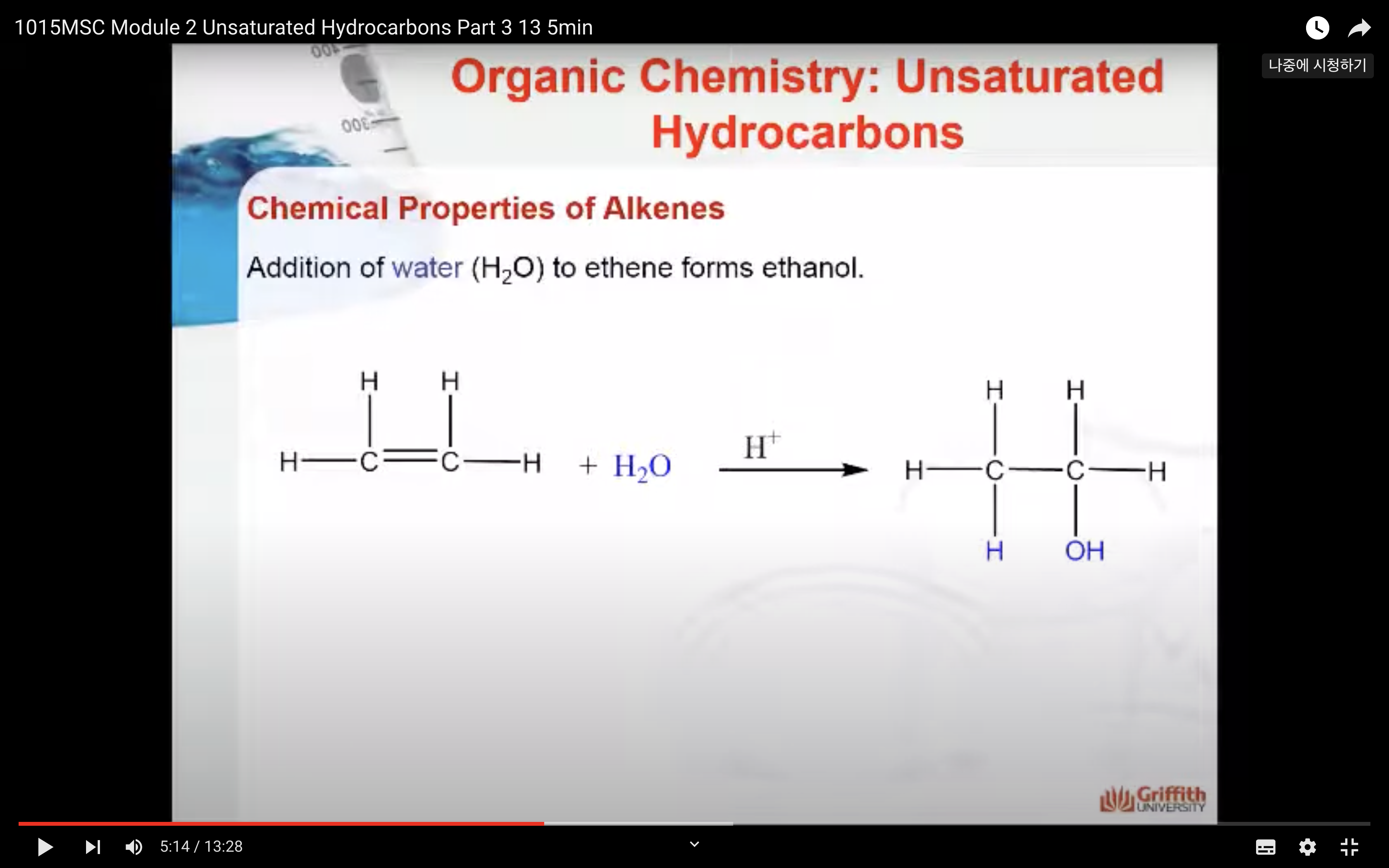
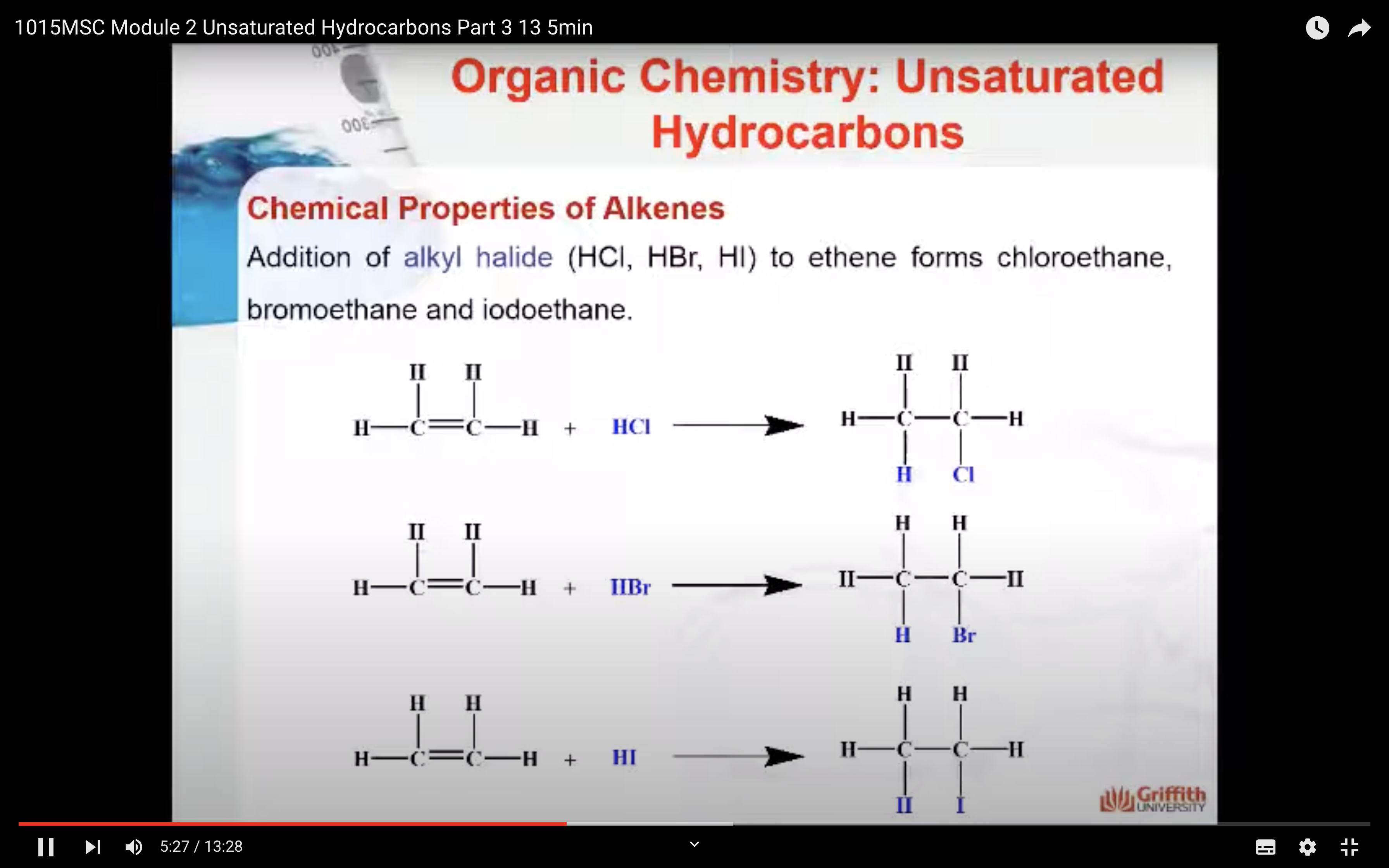
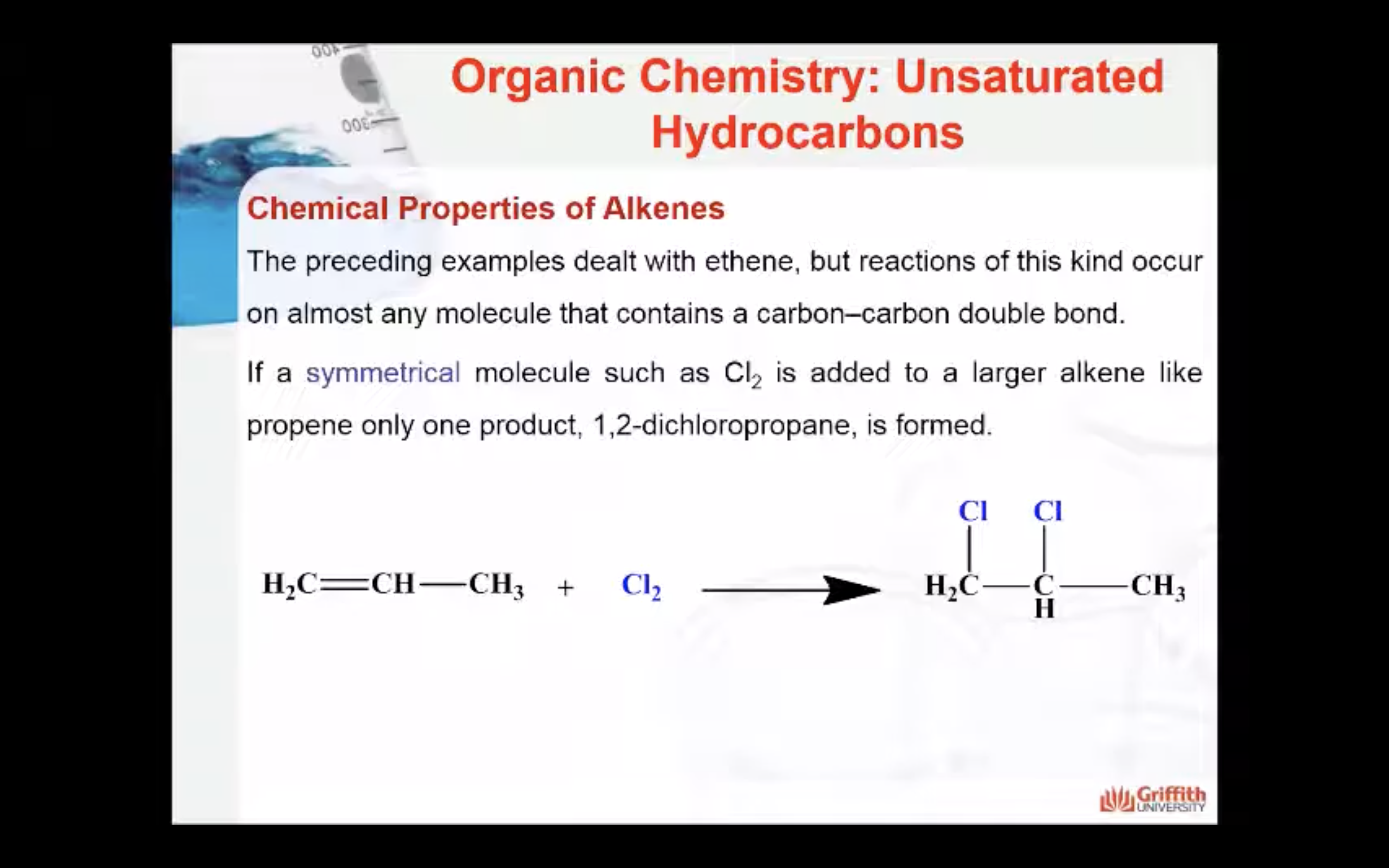
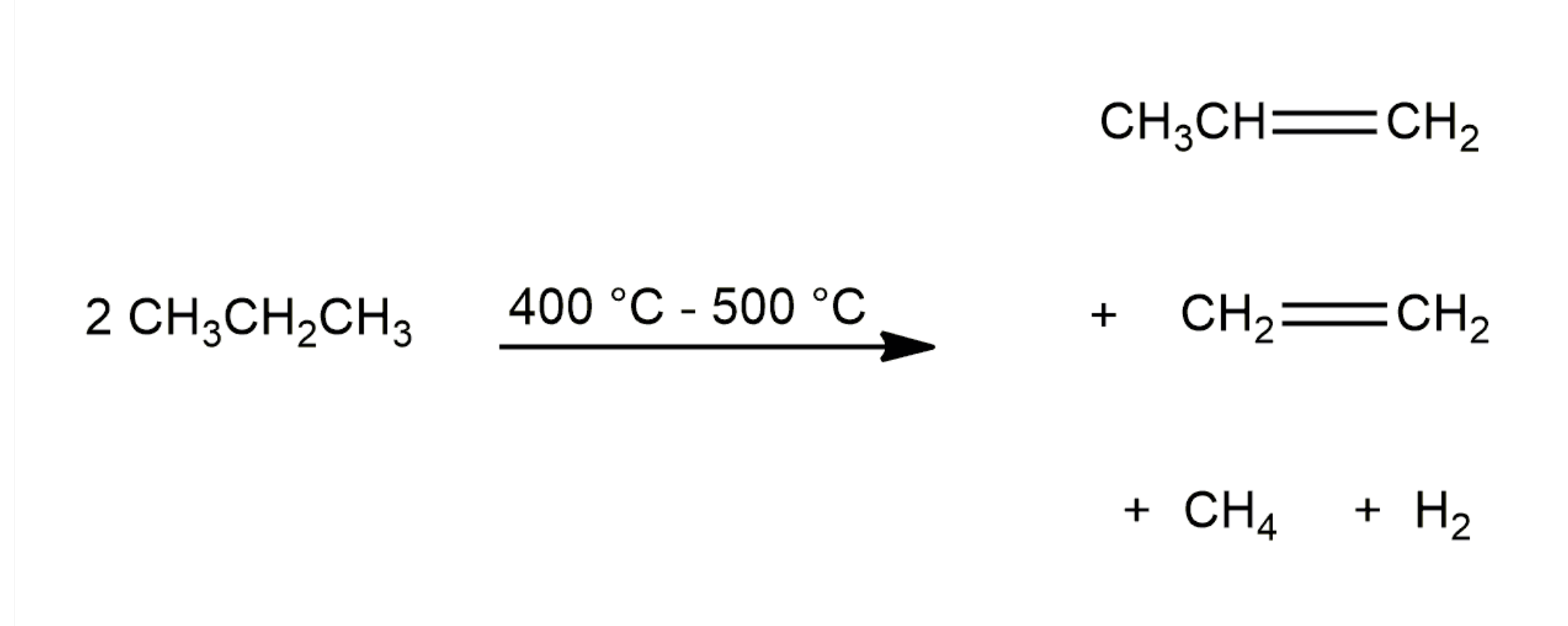
Chemical Properties of Alkenes

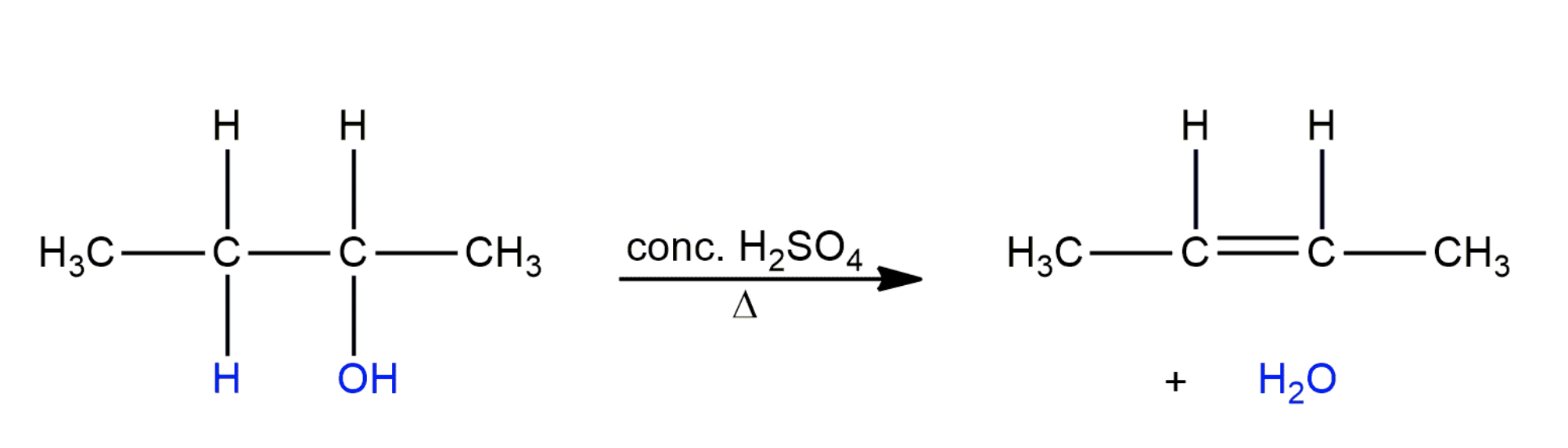
Alkenes can also be prepared by dehydration of alcohols. The reaction is catalysed by an acid.
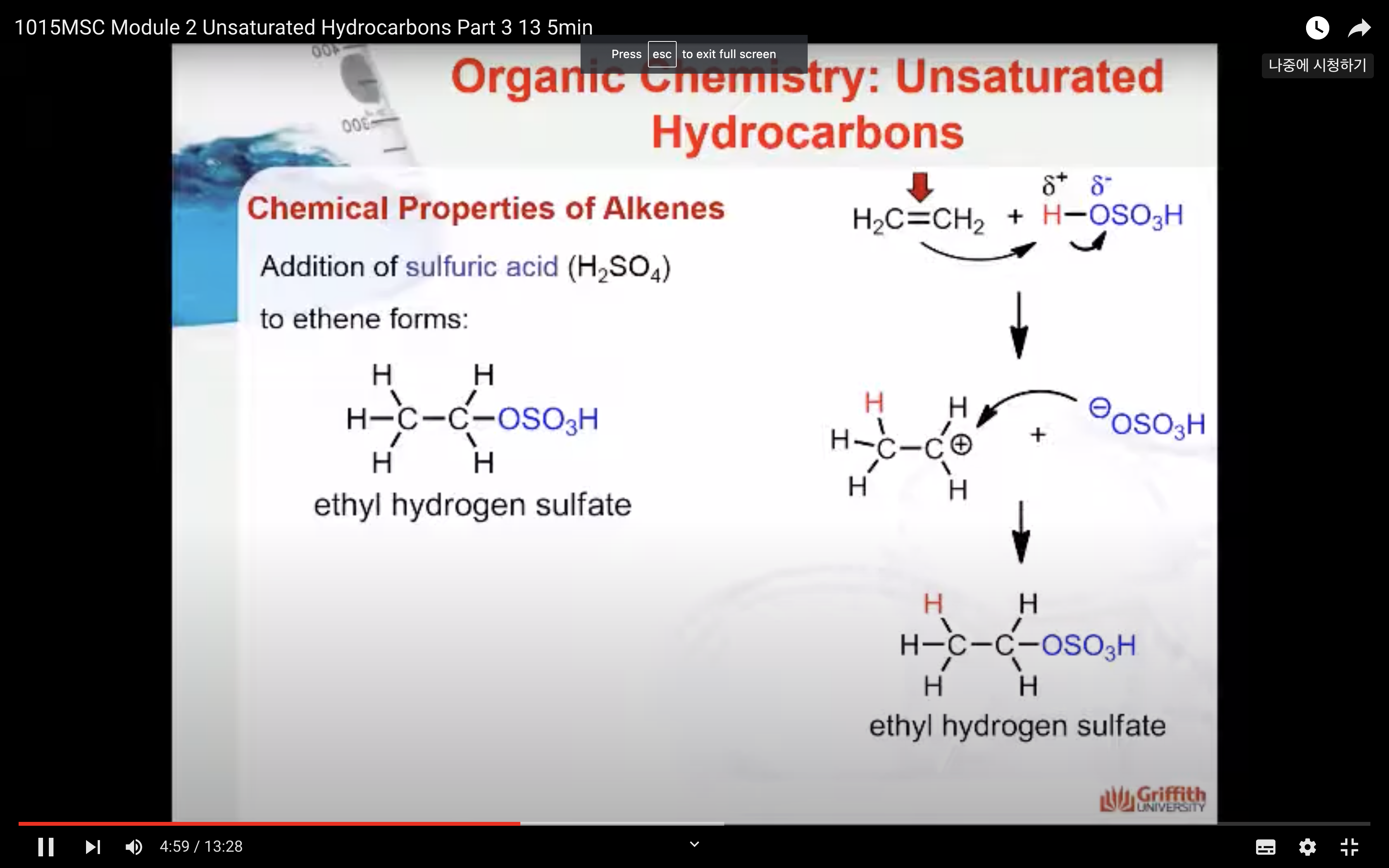
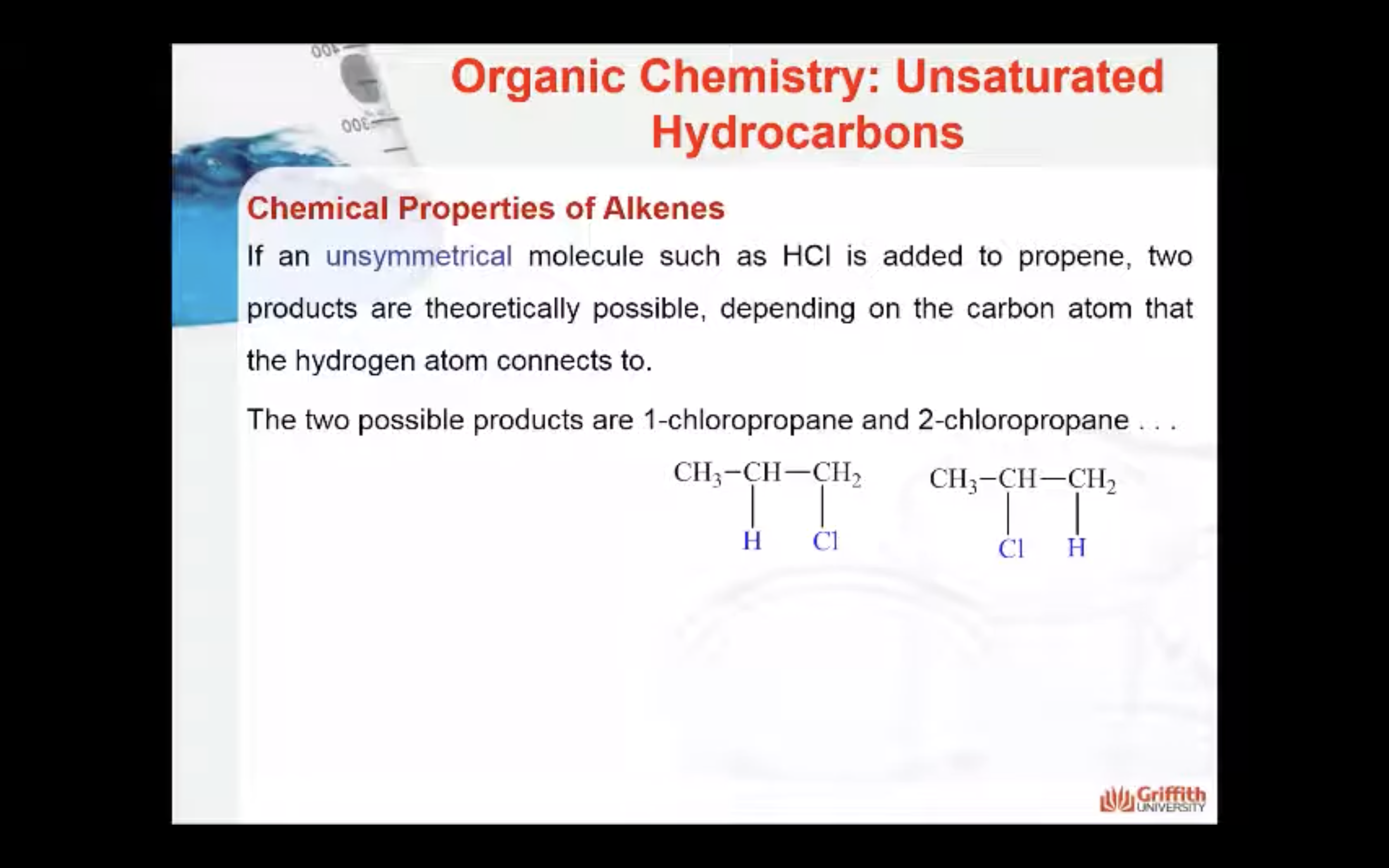
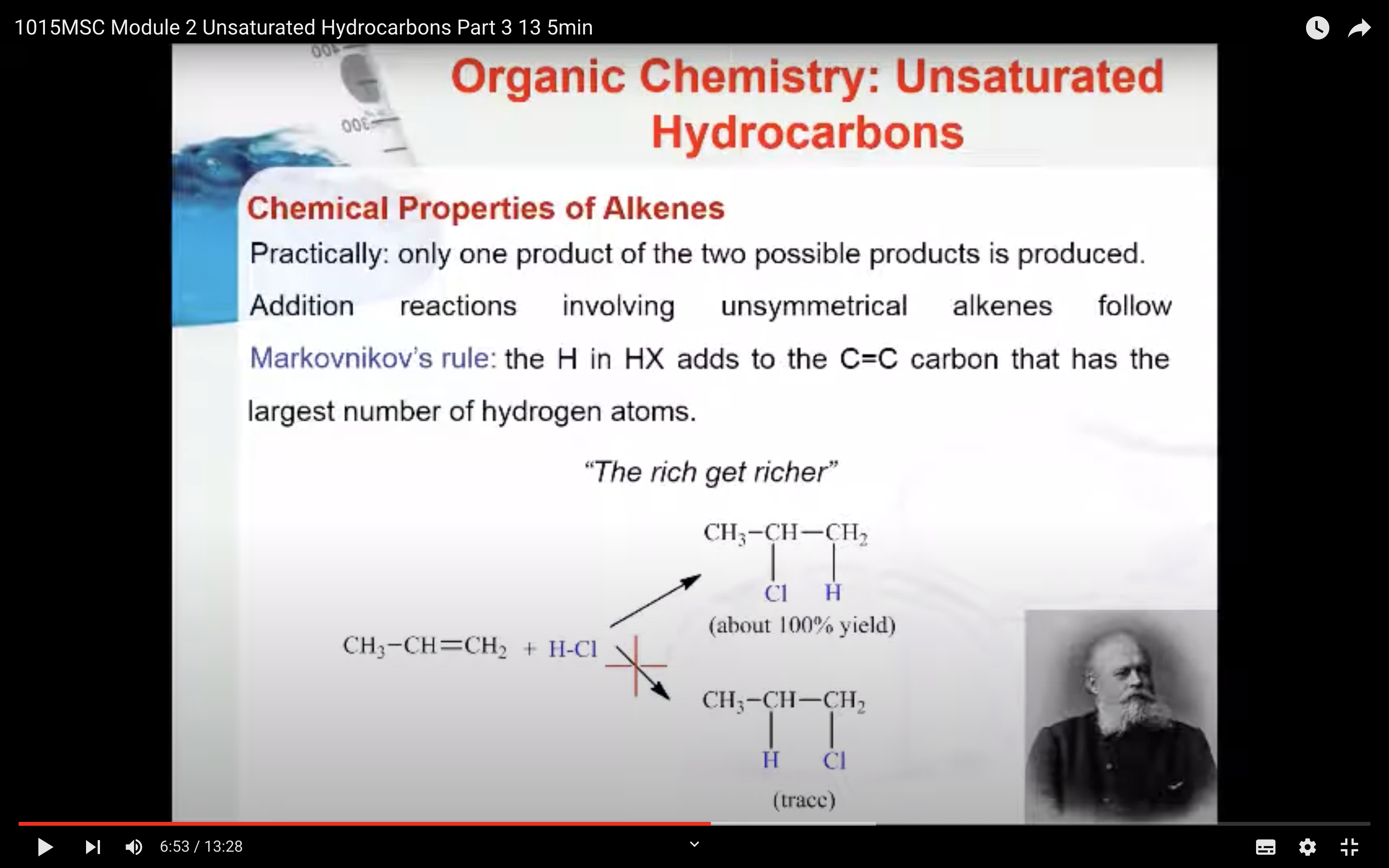
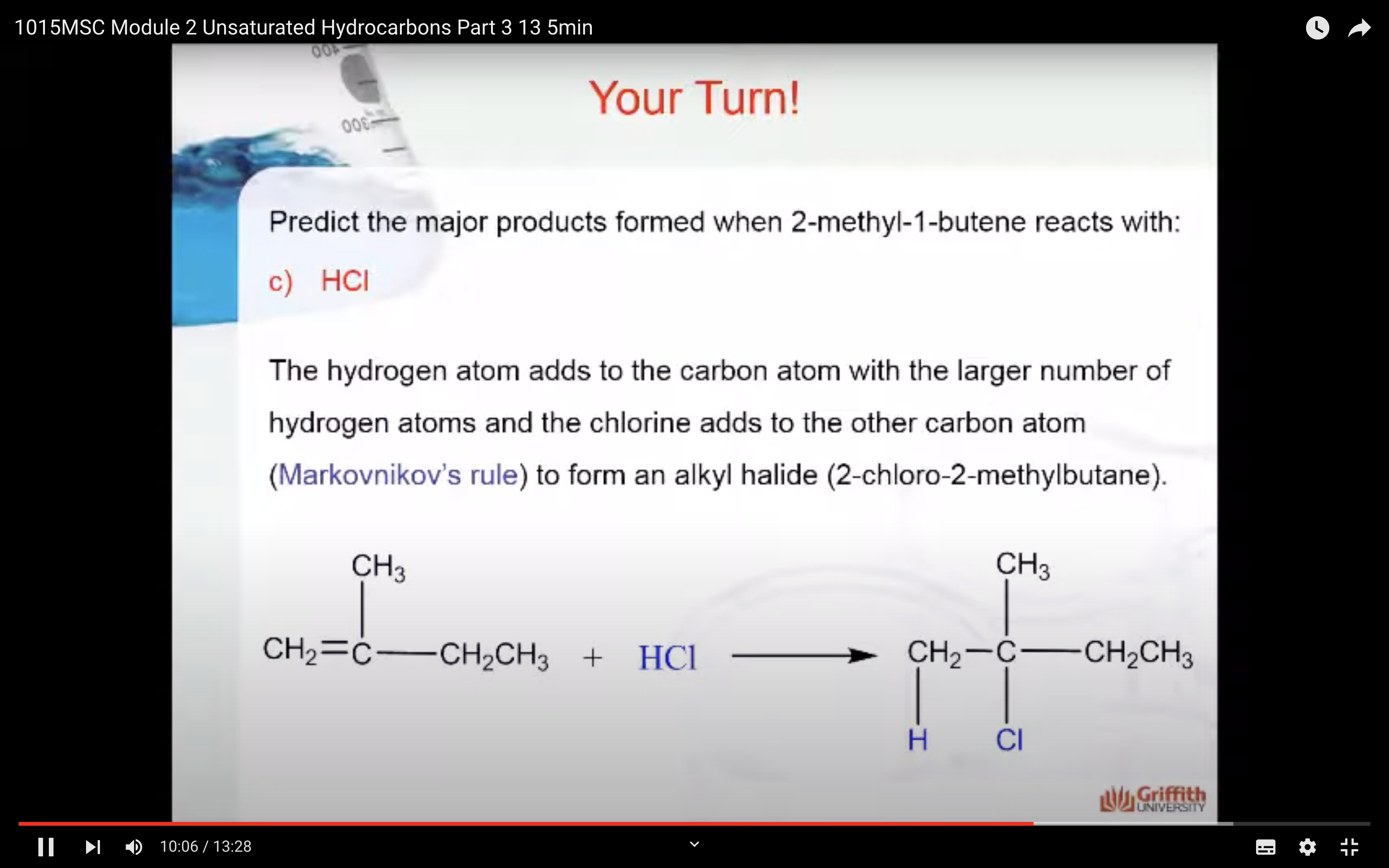
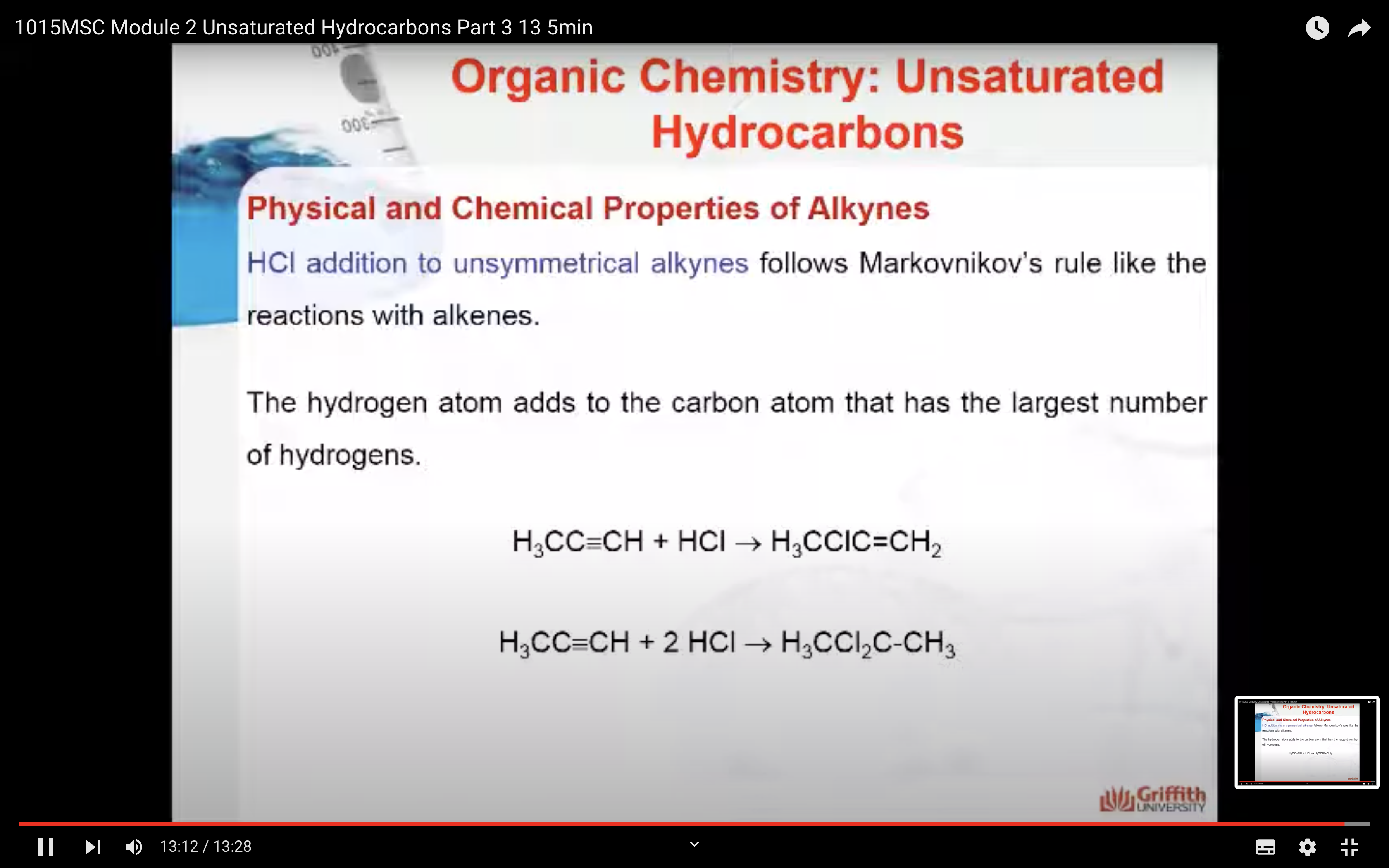
Markovnikov’s Rule
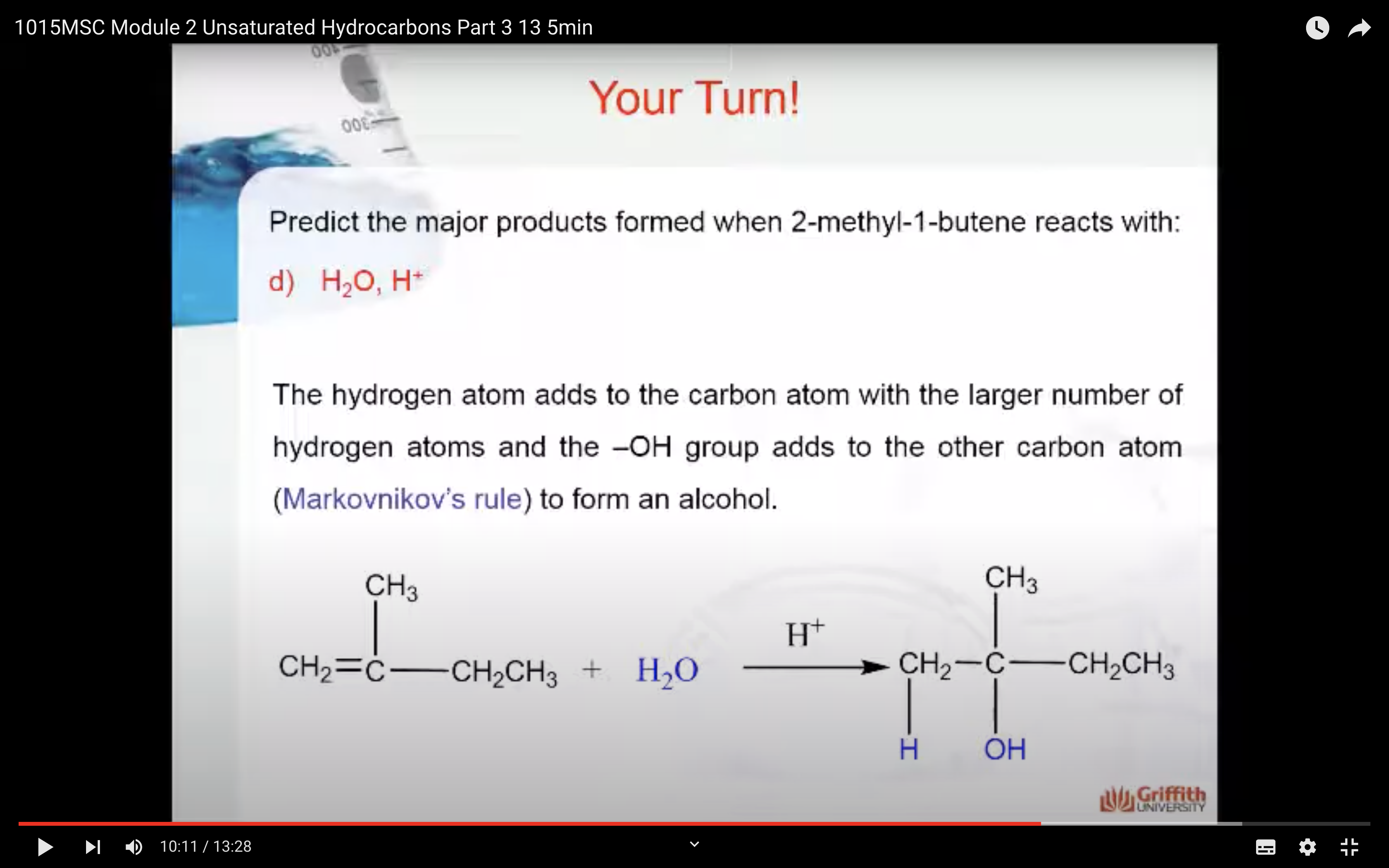
What is Markovnikov’s rule? It is a rule that states the H in HX adds to the C=C carbon that has the largest number of hydrogen atoms. “The rich get richer”. This rule can be explained by a reaction mechanism (i.e. the specific steps from reactants to products).
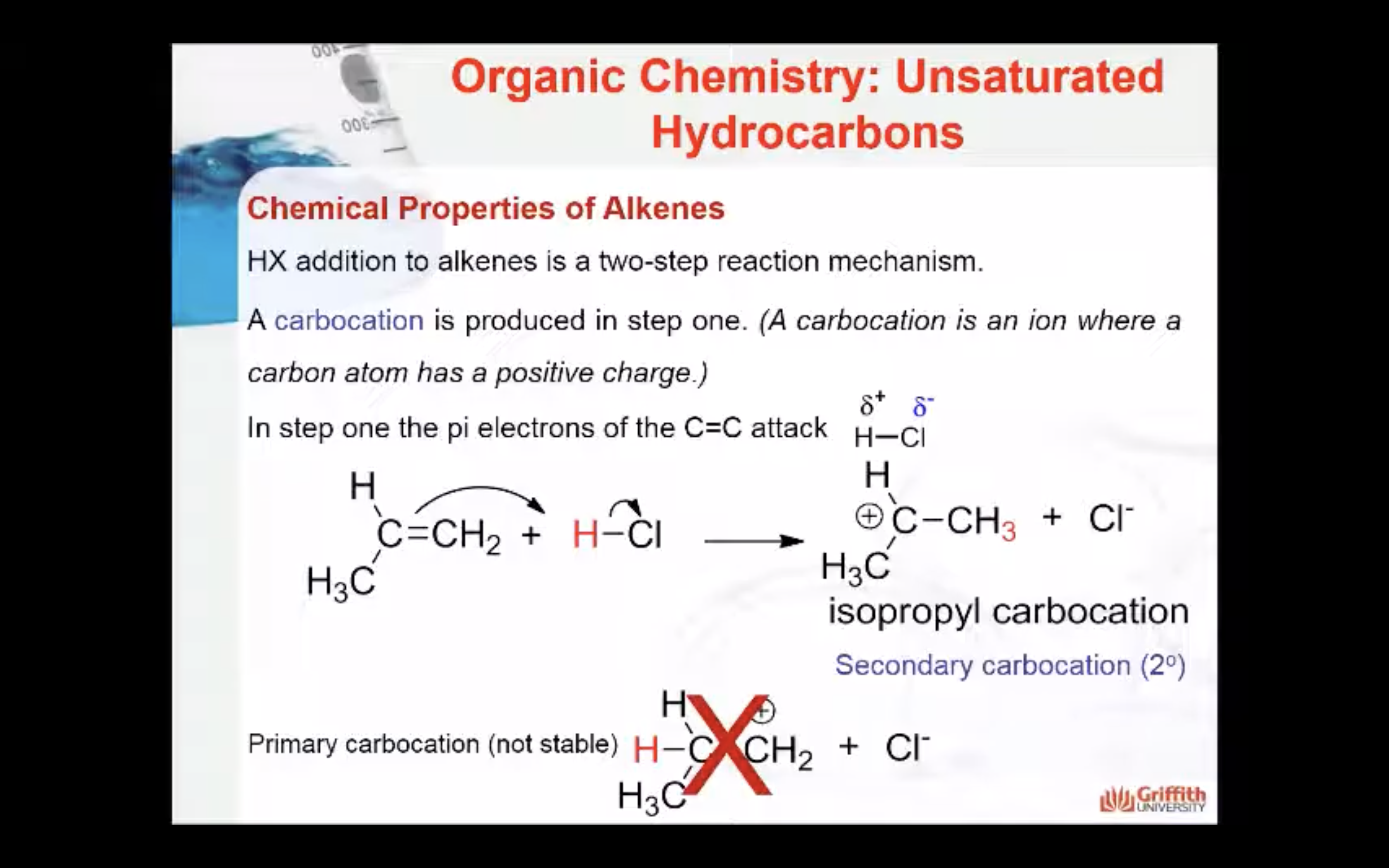
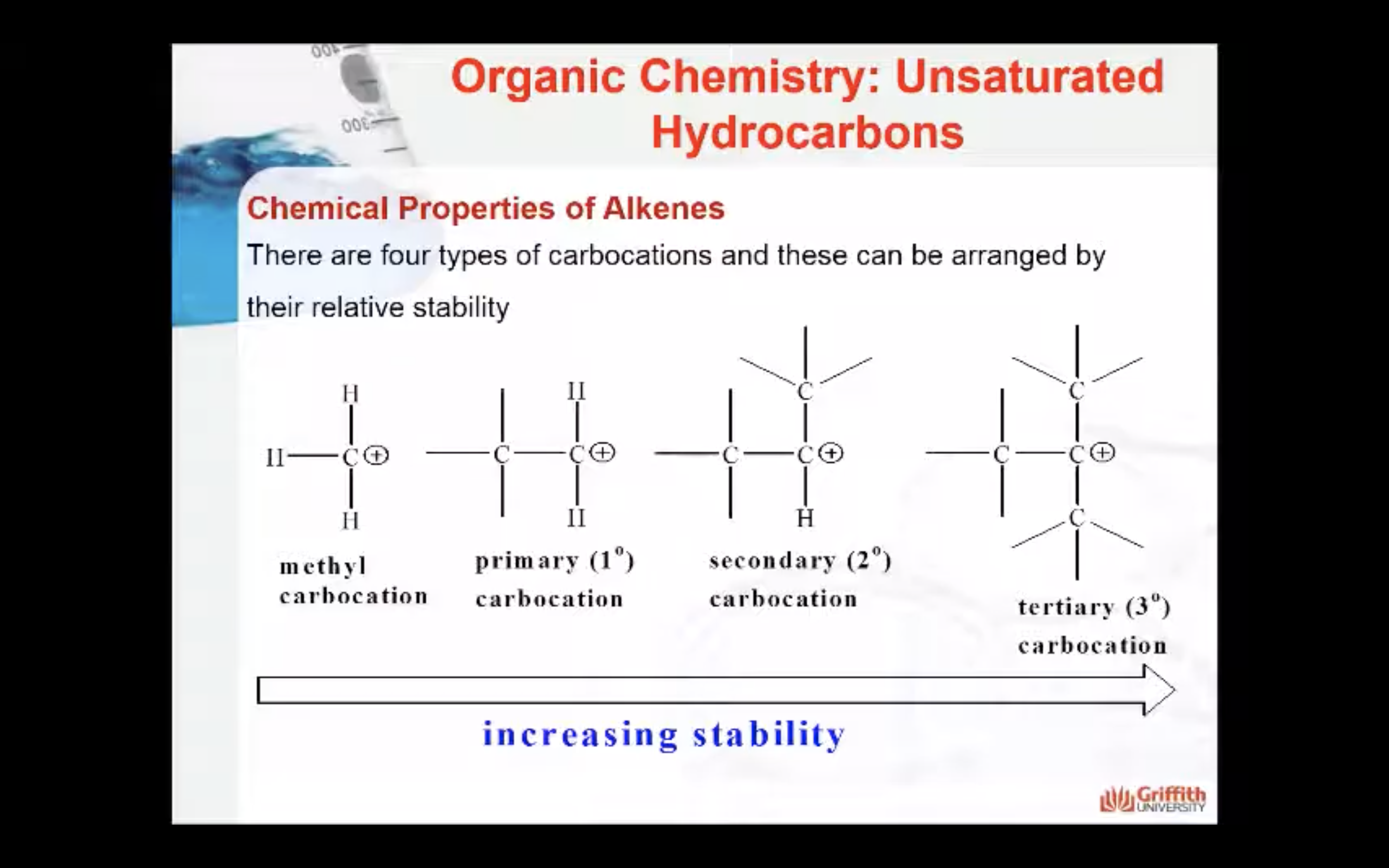
Note: A 2° carbocation is more stable than a 1° carbocation so the 2° carbocation forms preferentially over the 1° carbocation. This difference in carbocation stability is the basis for Markovnikov’s rule. There are four types of carbocations and these can be arranged by their relative stability.
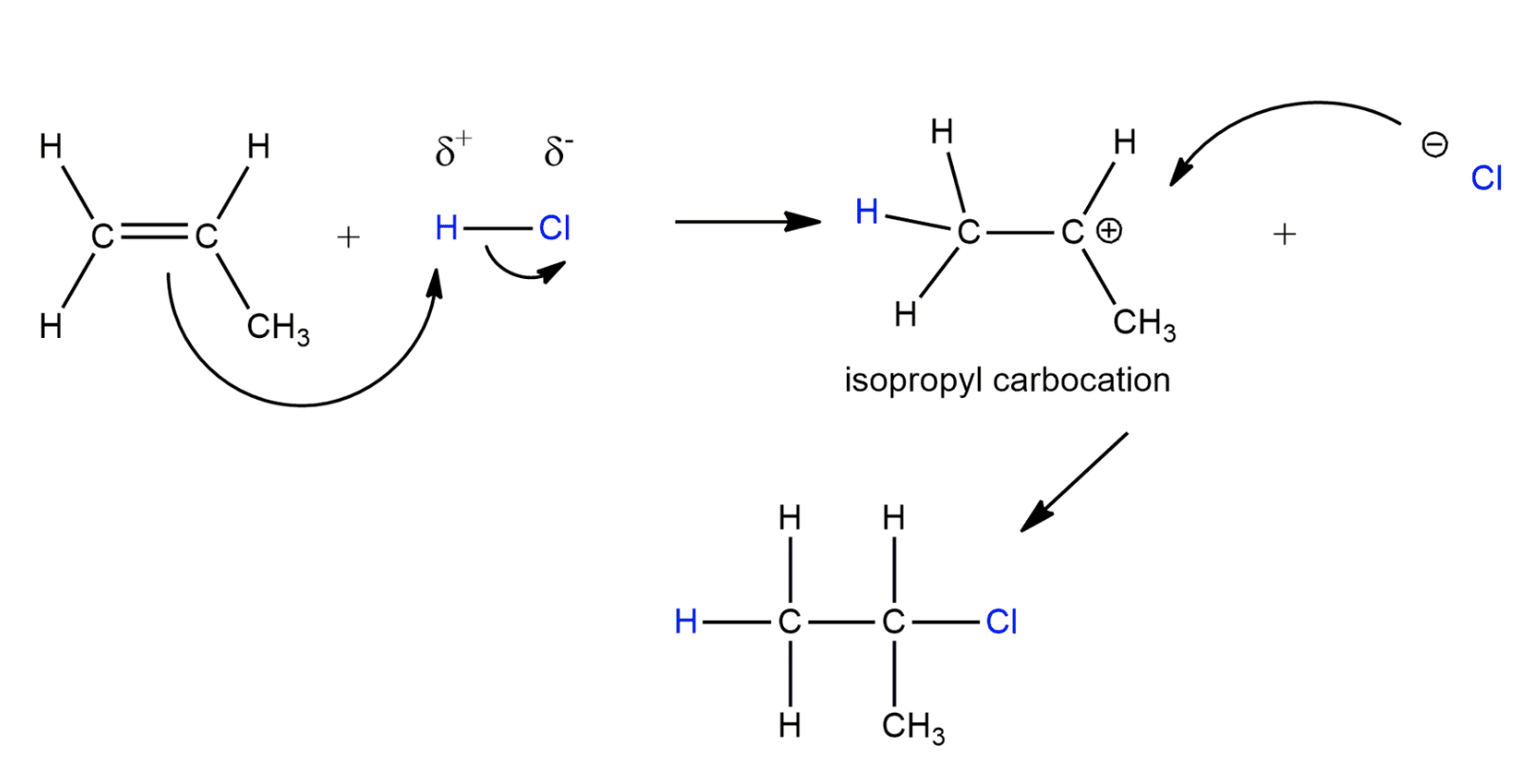
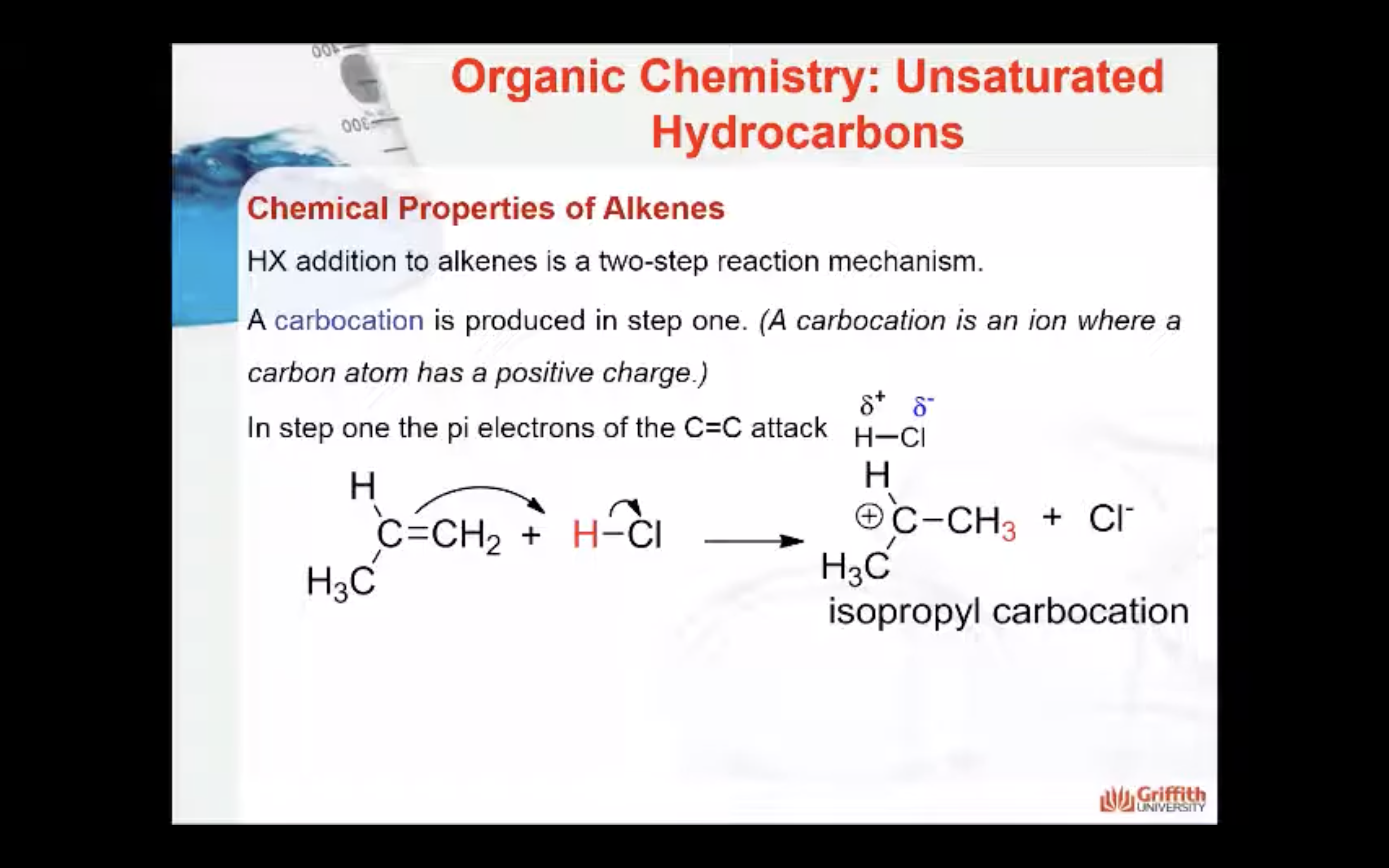
HX addition to alkenes is a two–step reaction mechanism. A carbocation (pronounced carbo-cat-ion) is produced in step one. (A carbocation is an ion where a carbon atom has a positive charge.)
1. In step one a secondary carbocation (2°) is produced when the pi electrons of the C=C attack the δ+ H in HCl.
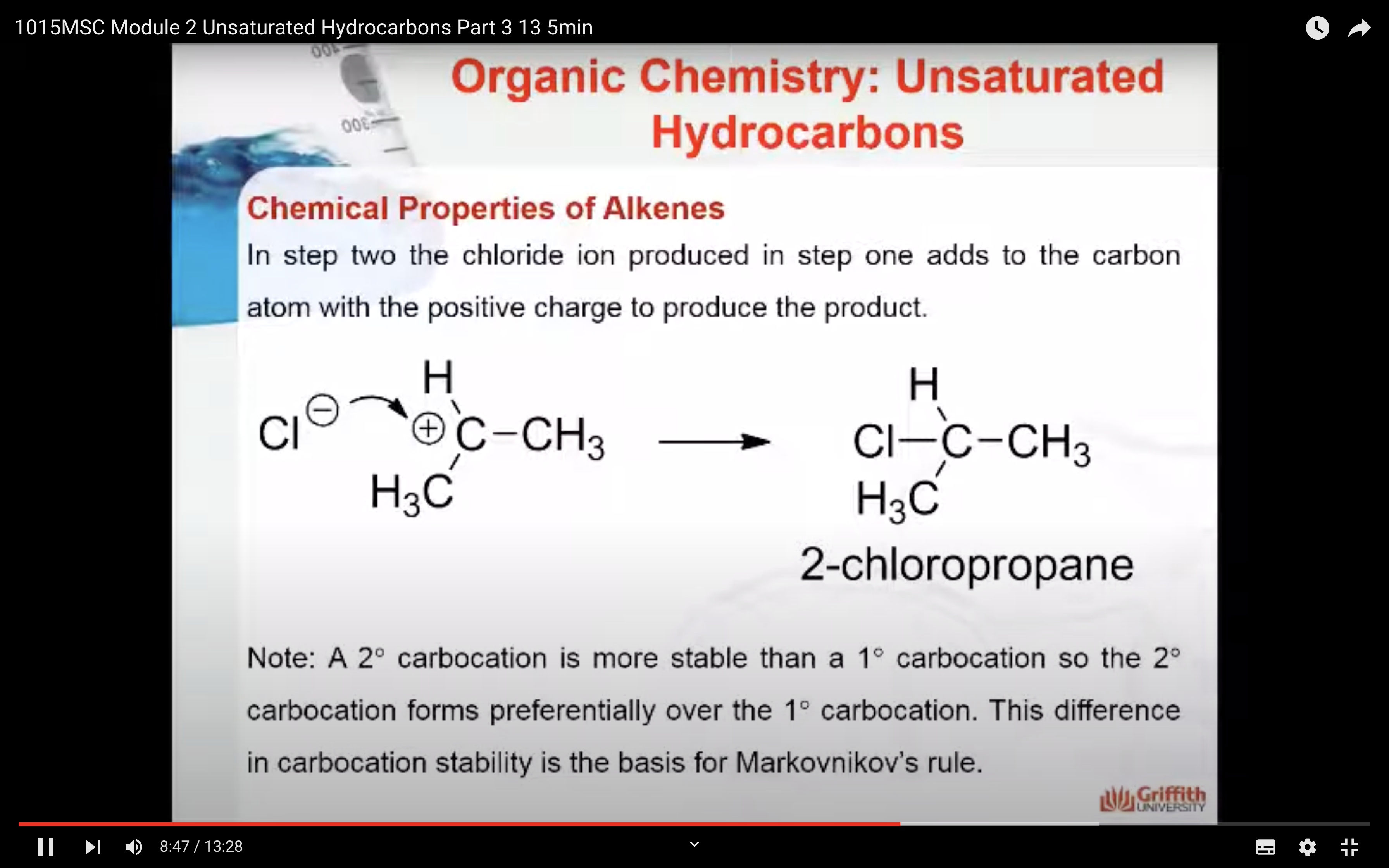
2. In step two the chloride ion produced in step one adds to the carbon atom with the positive charge to form 2-chloropropane.
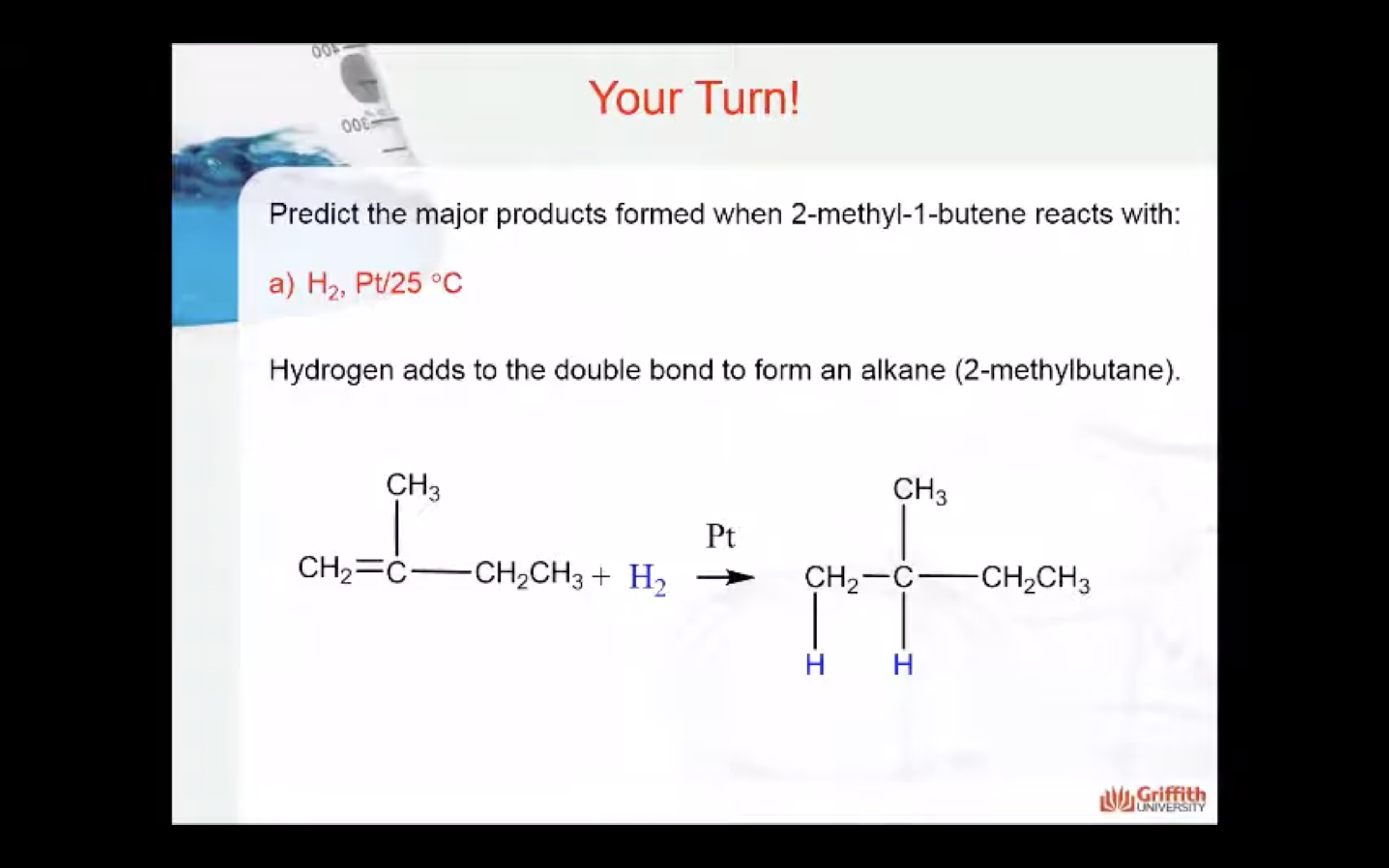
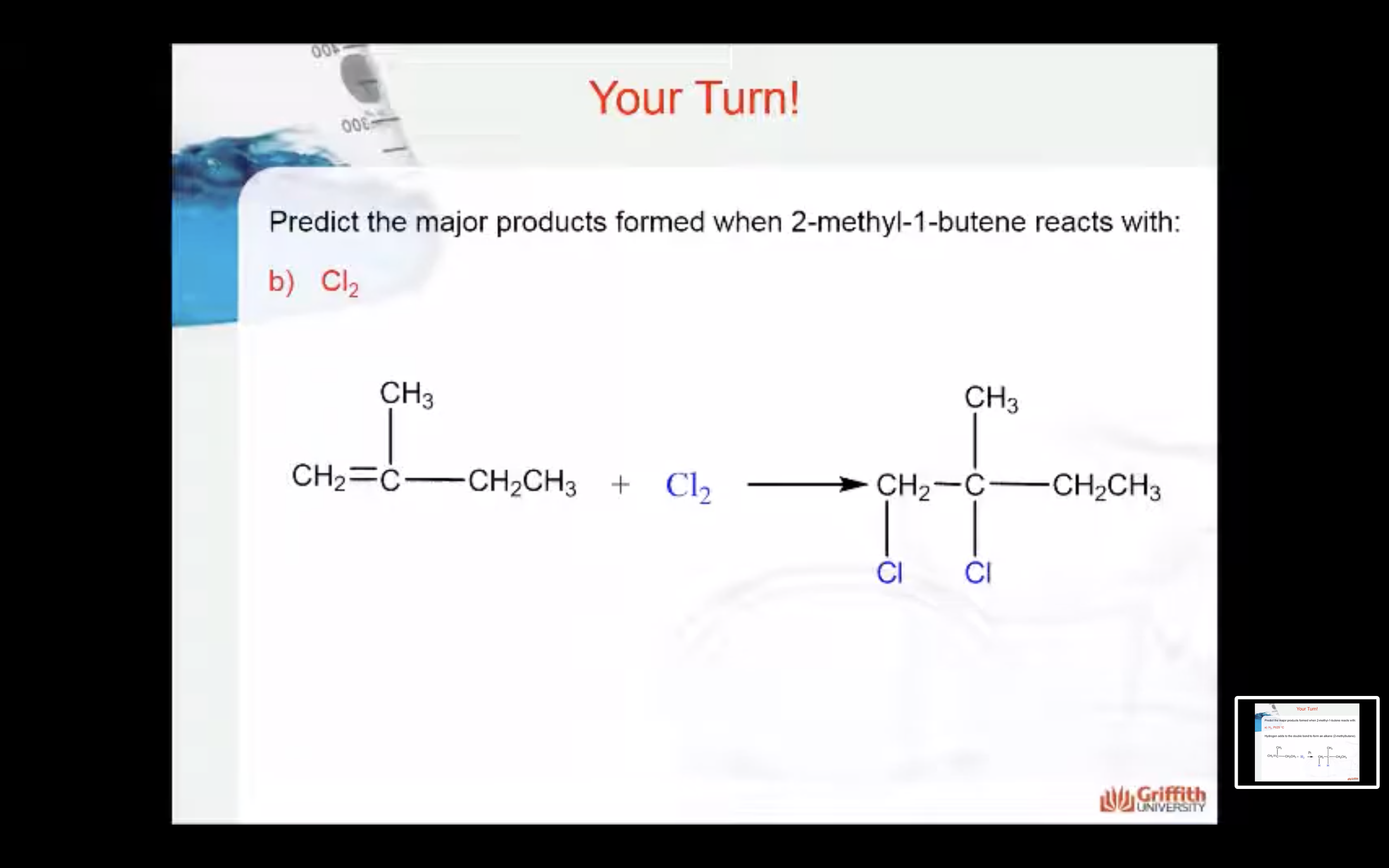
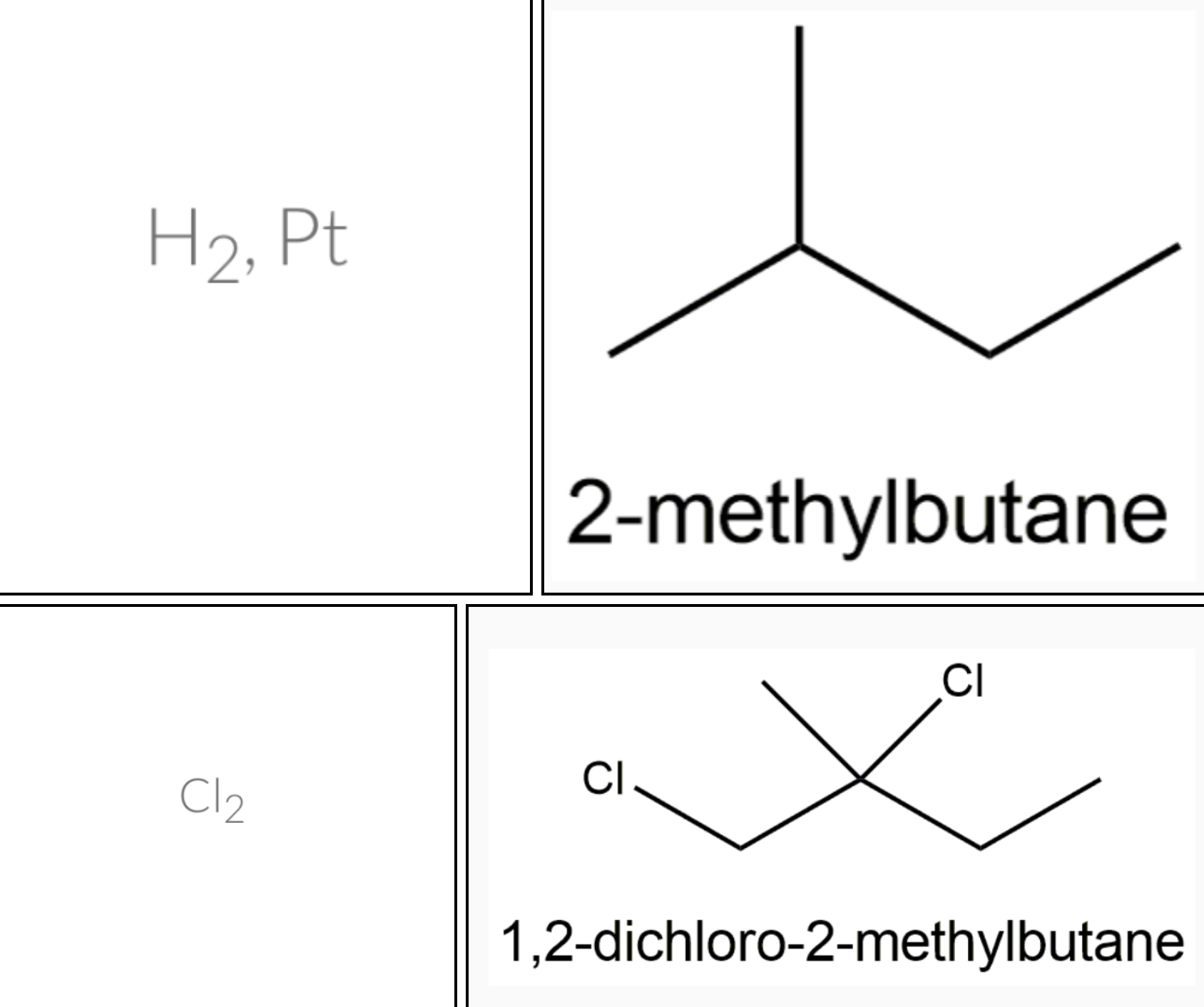
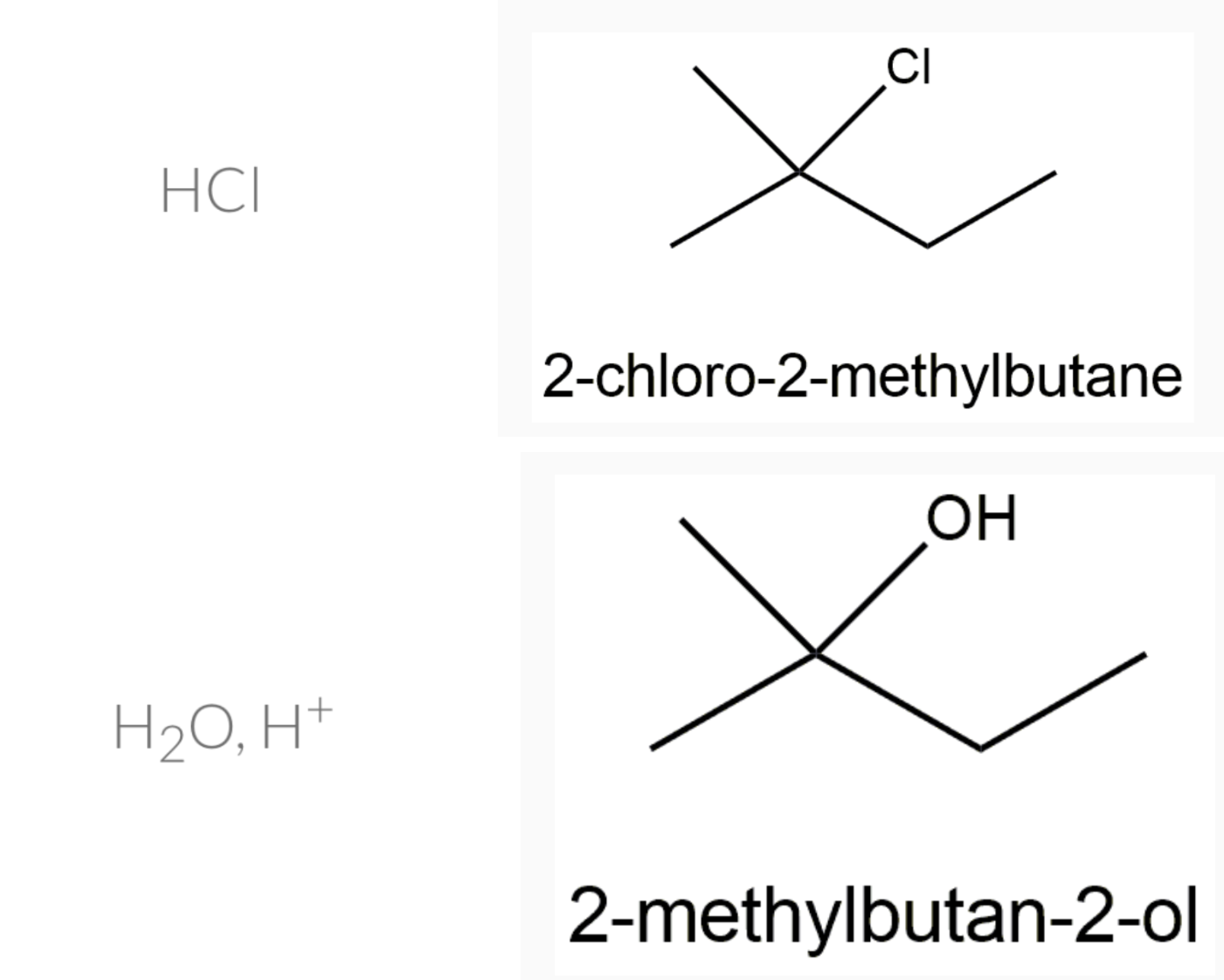
Predict the major product formed when 2–methylbut–1–ene reacts with:
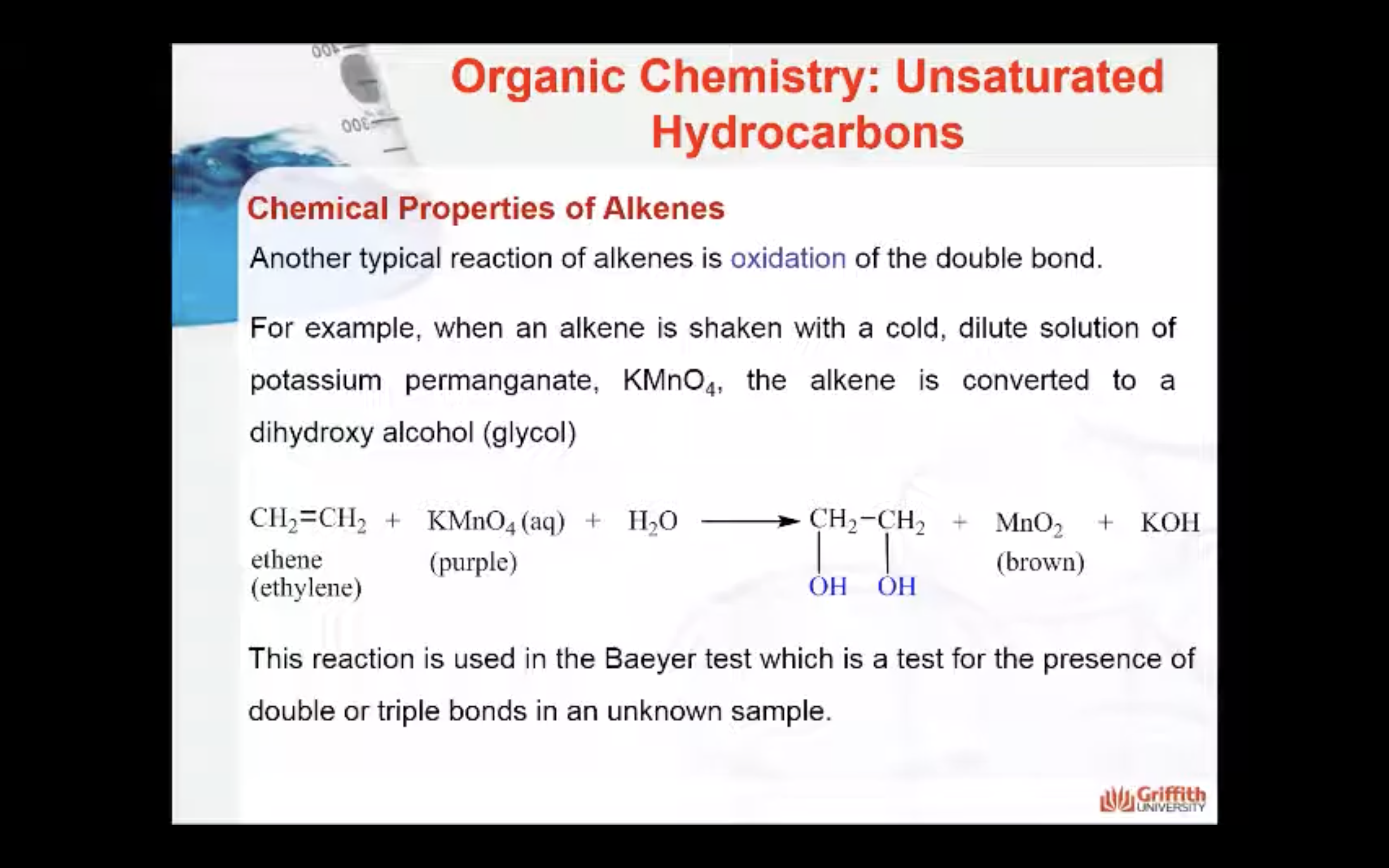
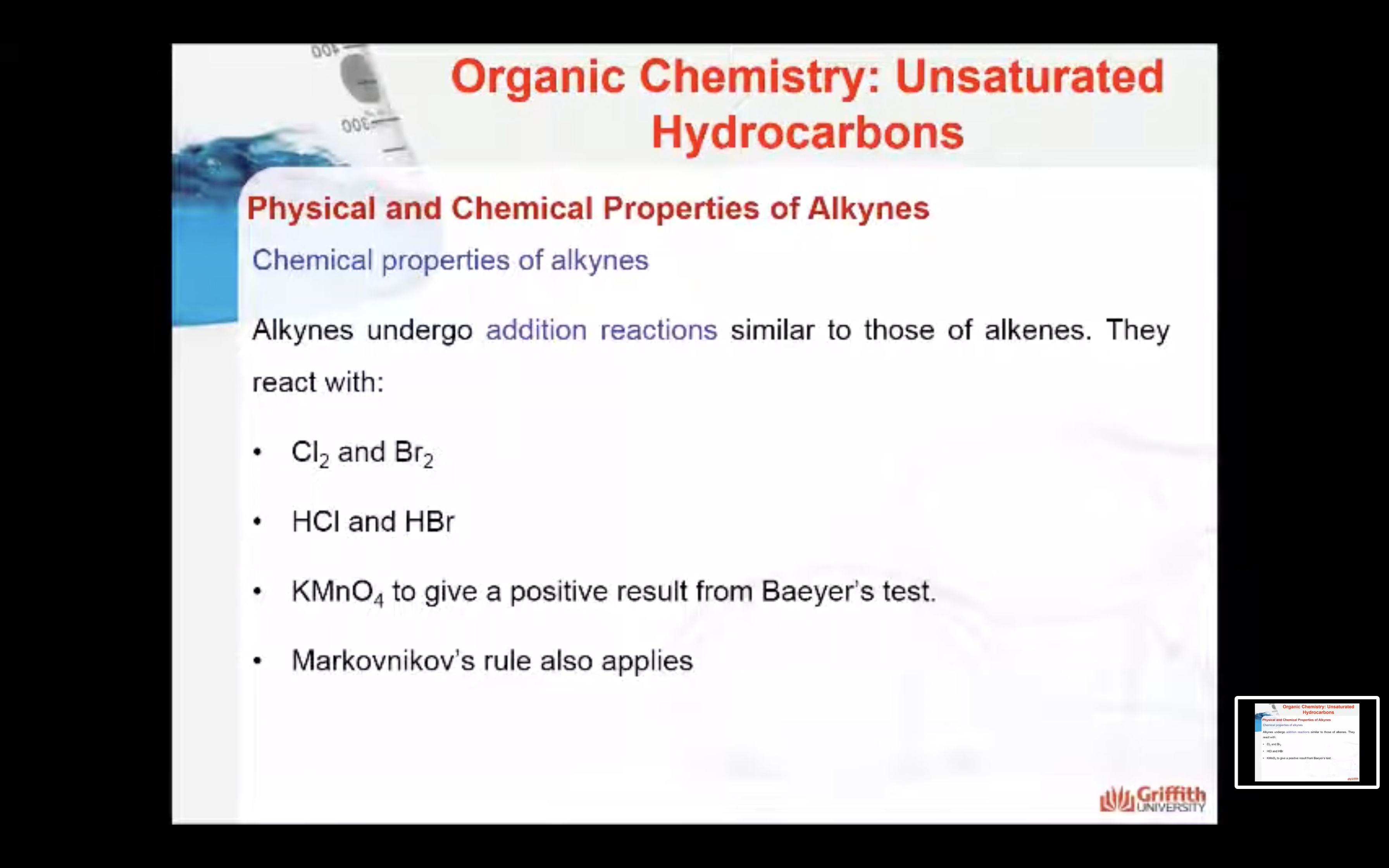
Another typical reaction of alkenes is oxidation of the double bond. For example, when an alkene is shaken with a cold, dilute solution of potassium permanganate, KMnO4, the alkene is converted to a glycol (glycols are dihydroxy alcohols). The reaction below is used in the Baeyer test which is a test for the presence of double or triple bonds in an unknown sample.
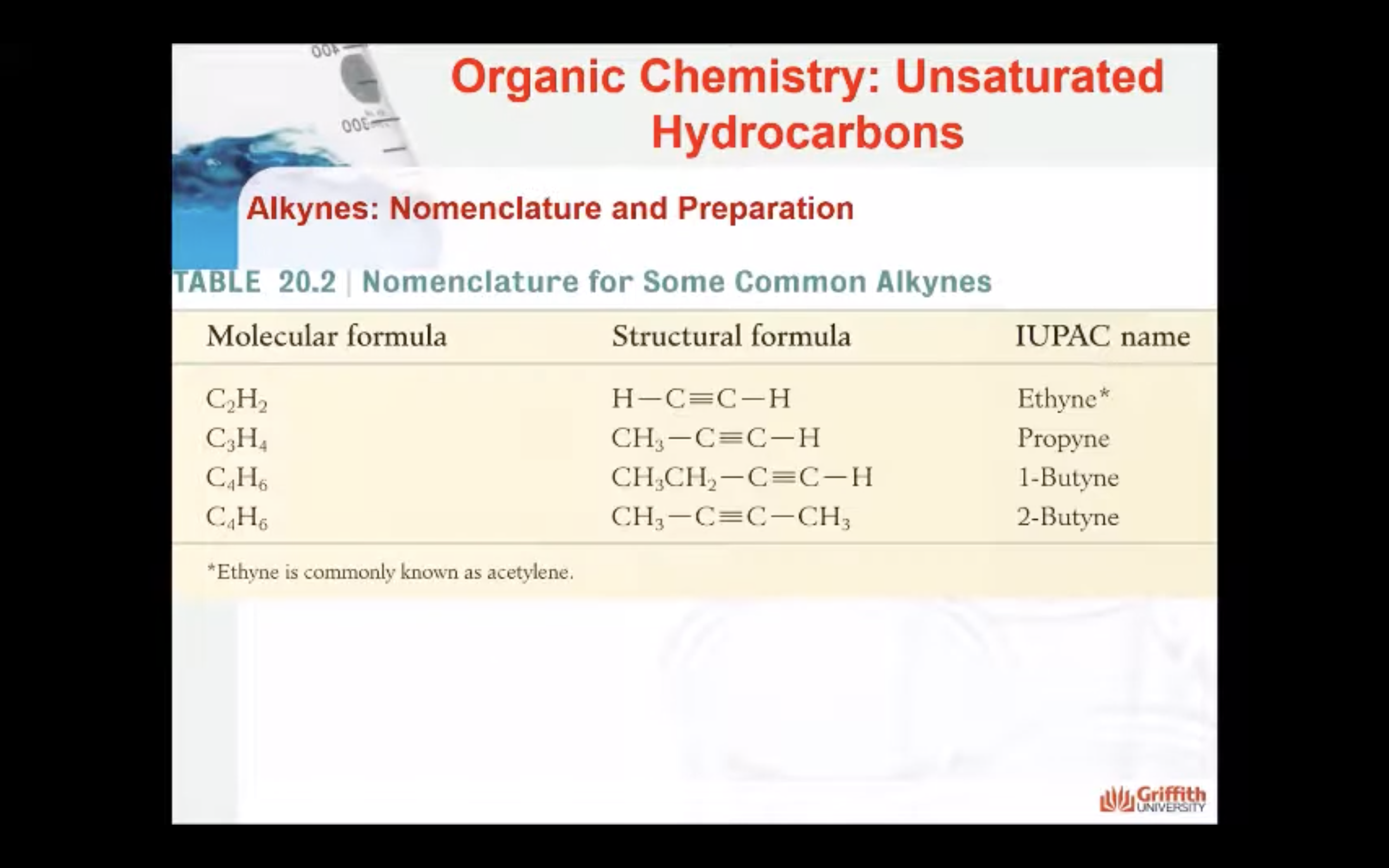
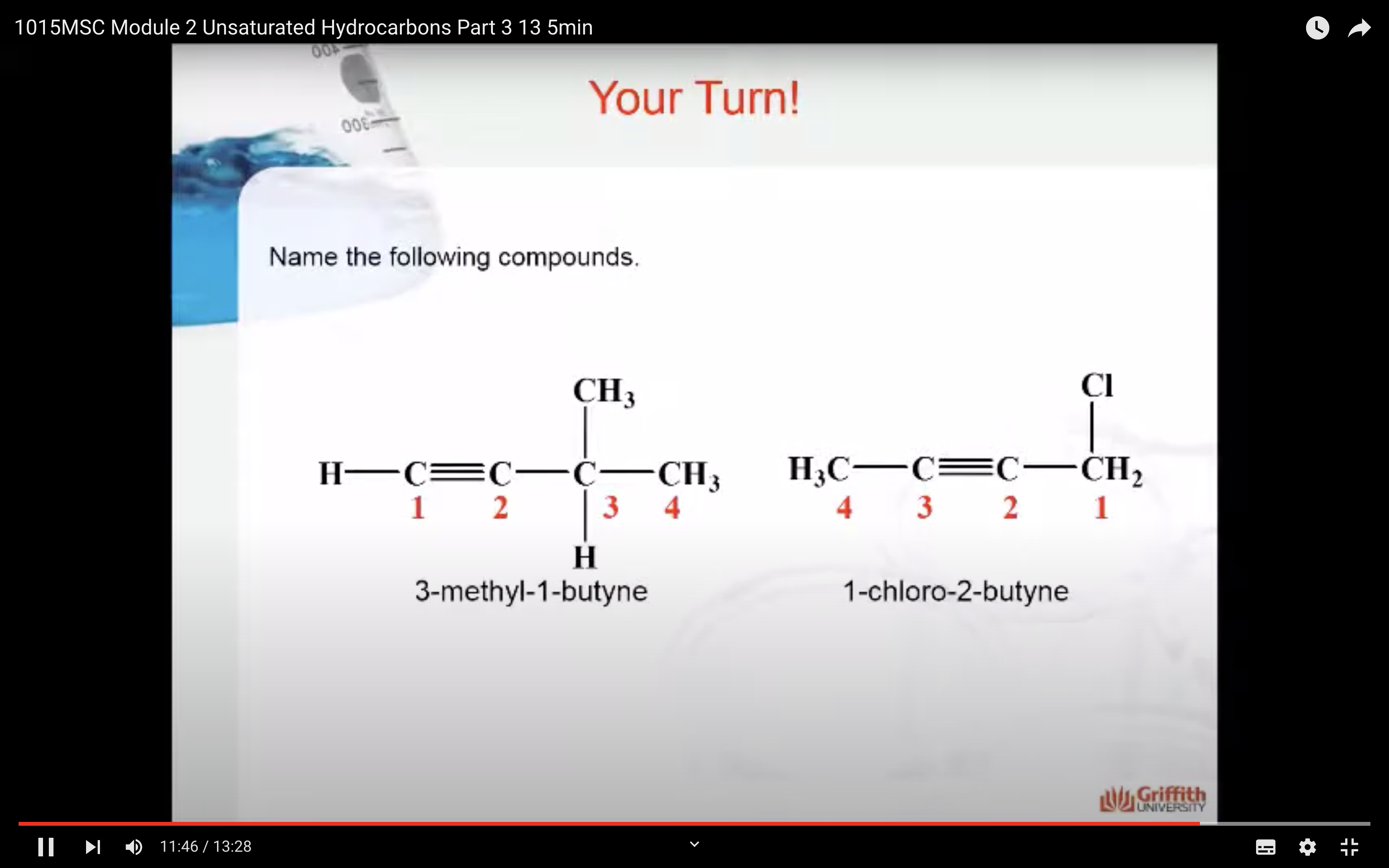
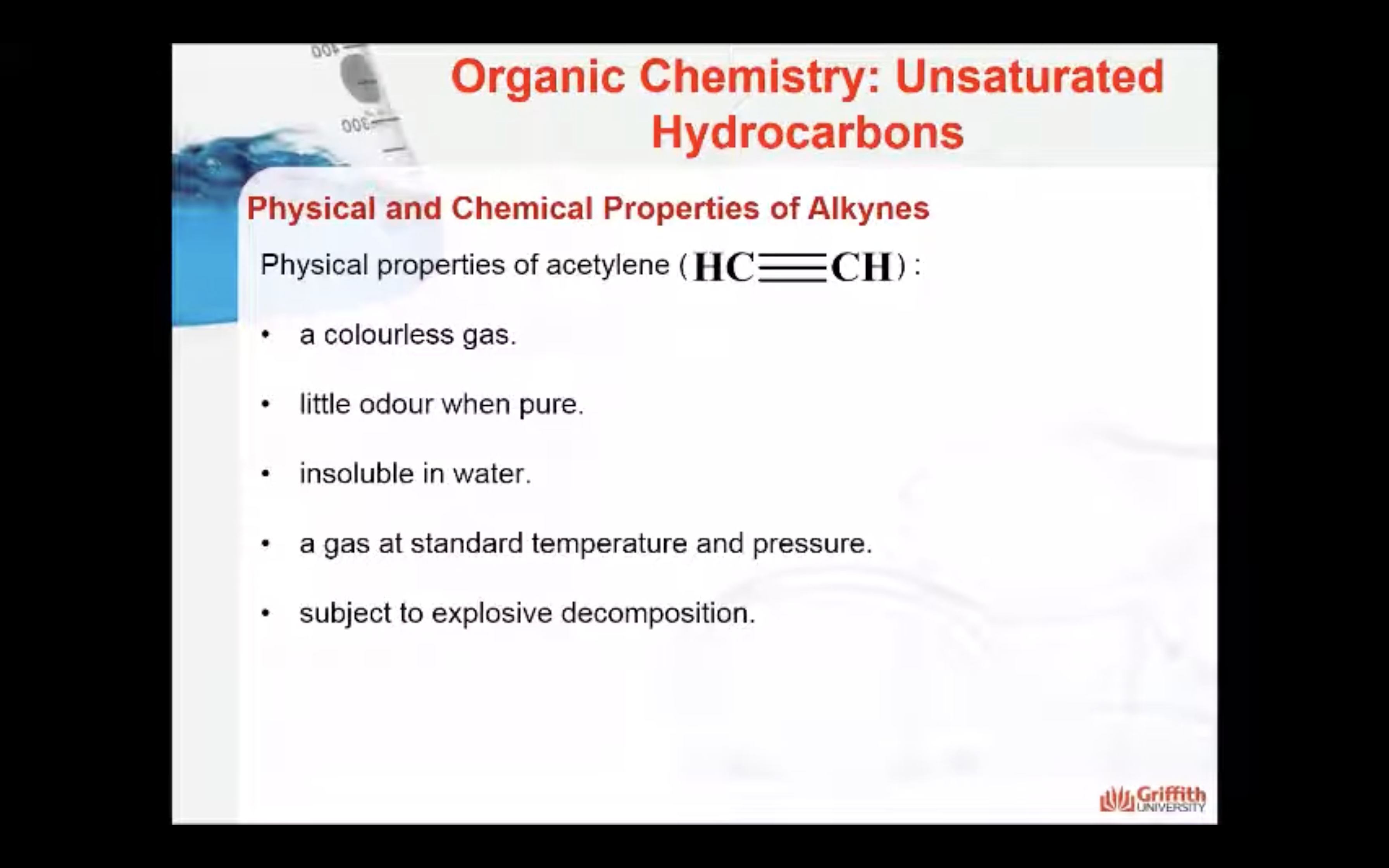
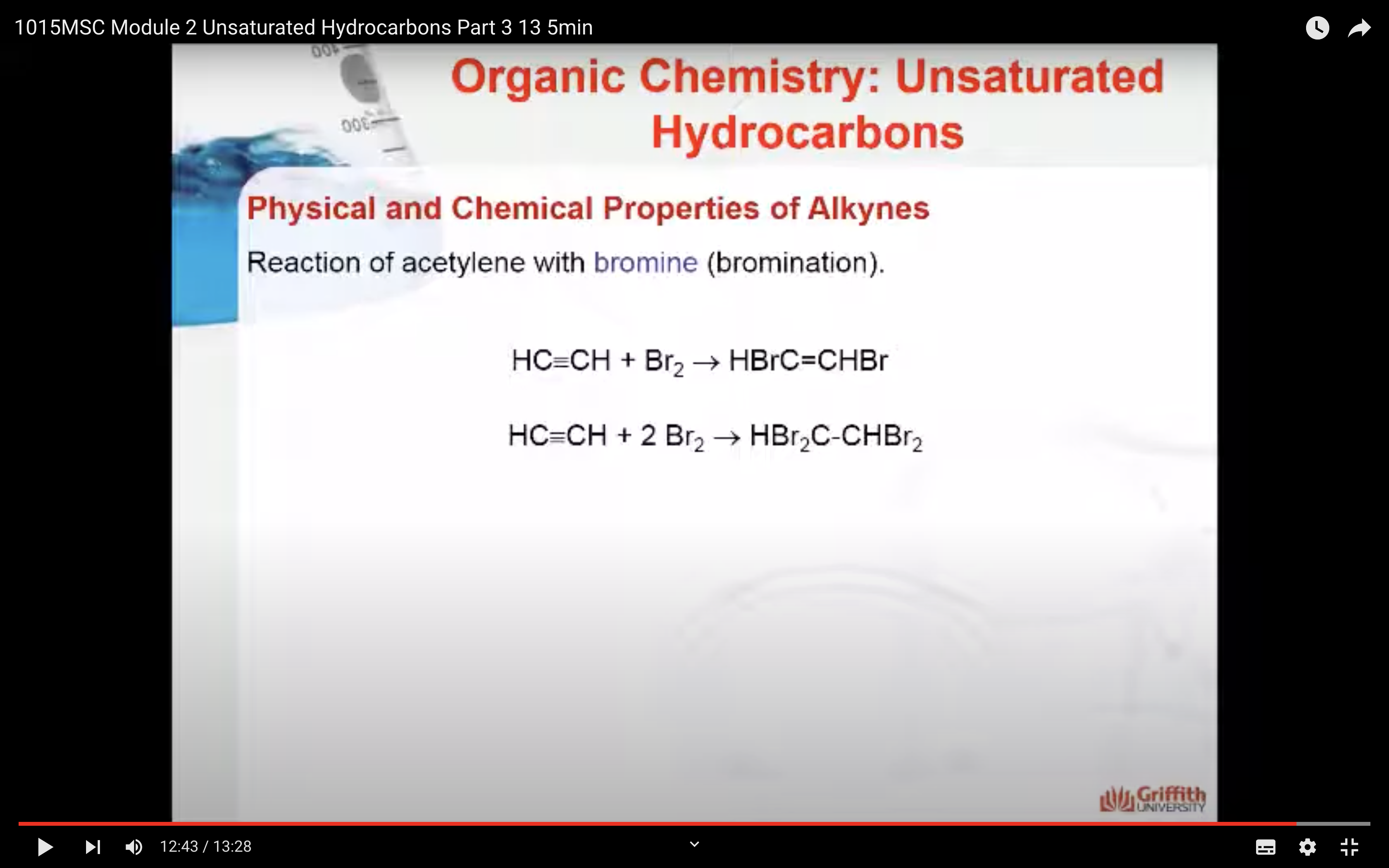
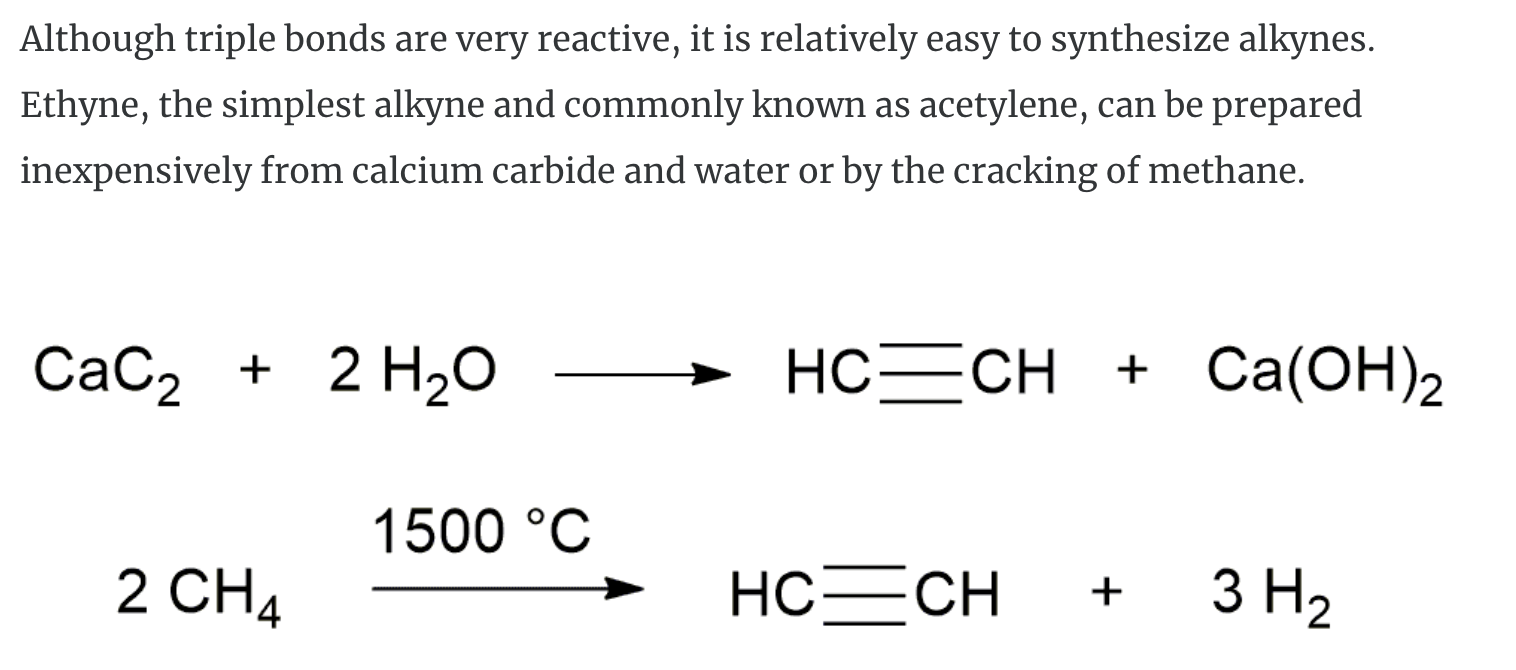
Alkynes


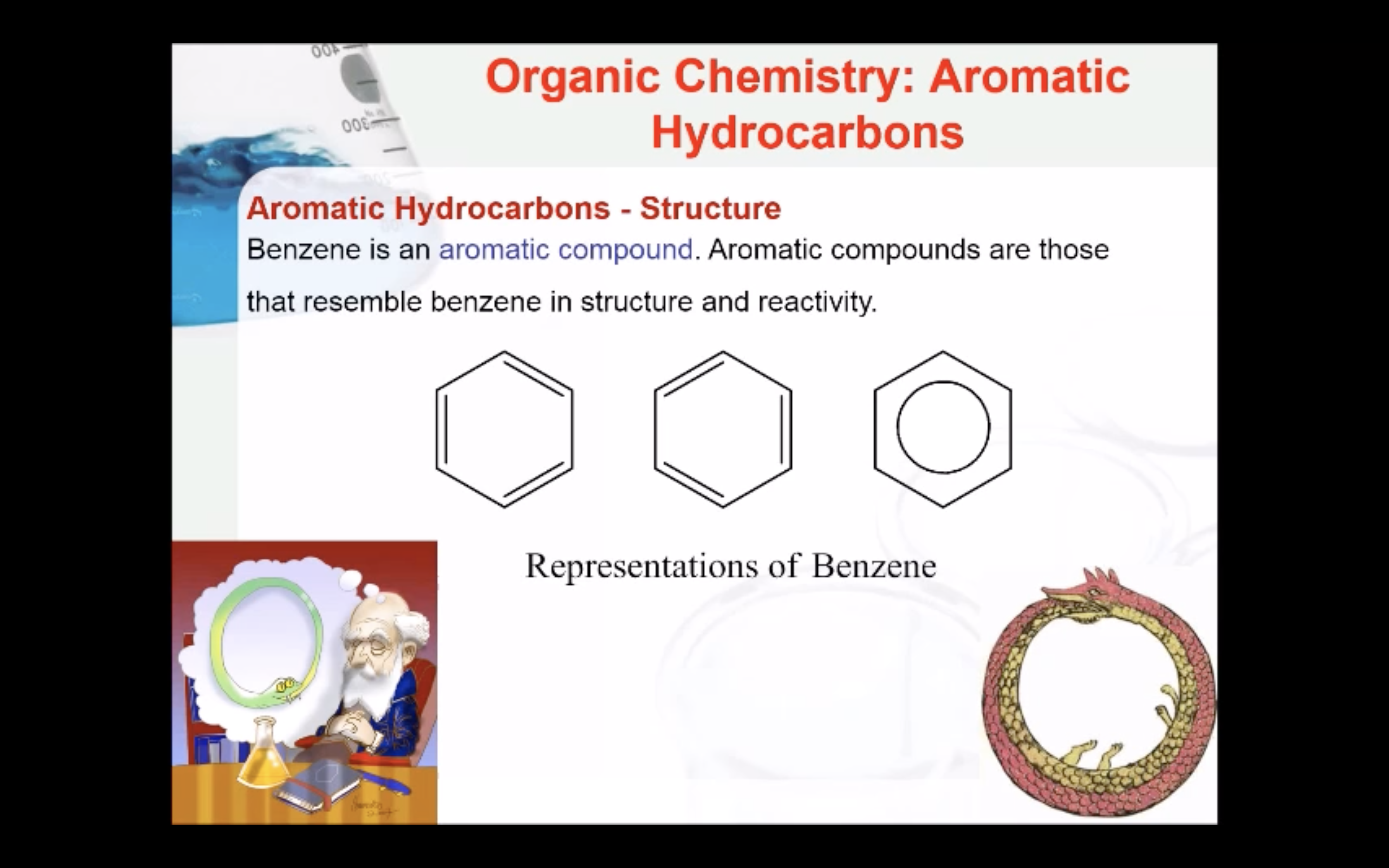
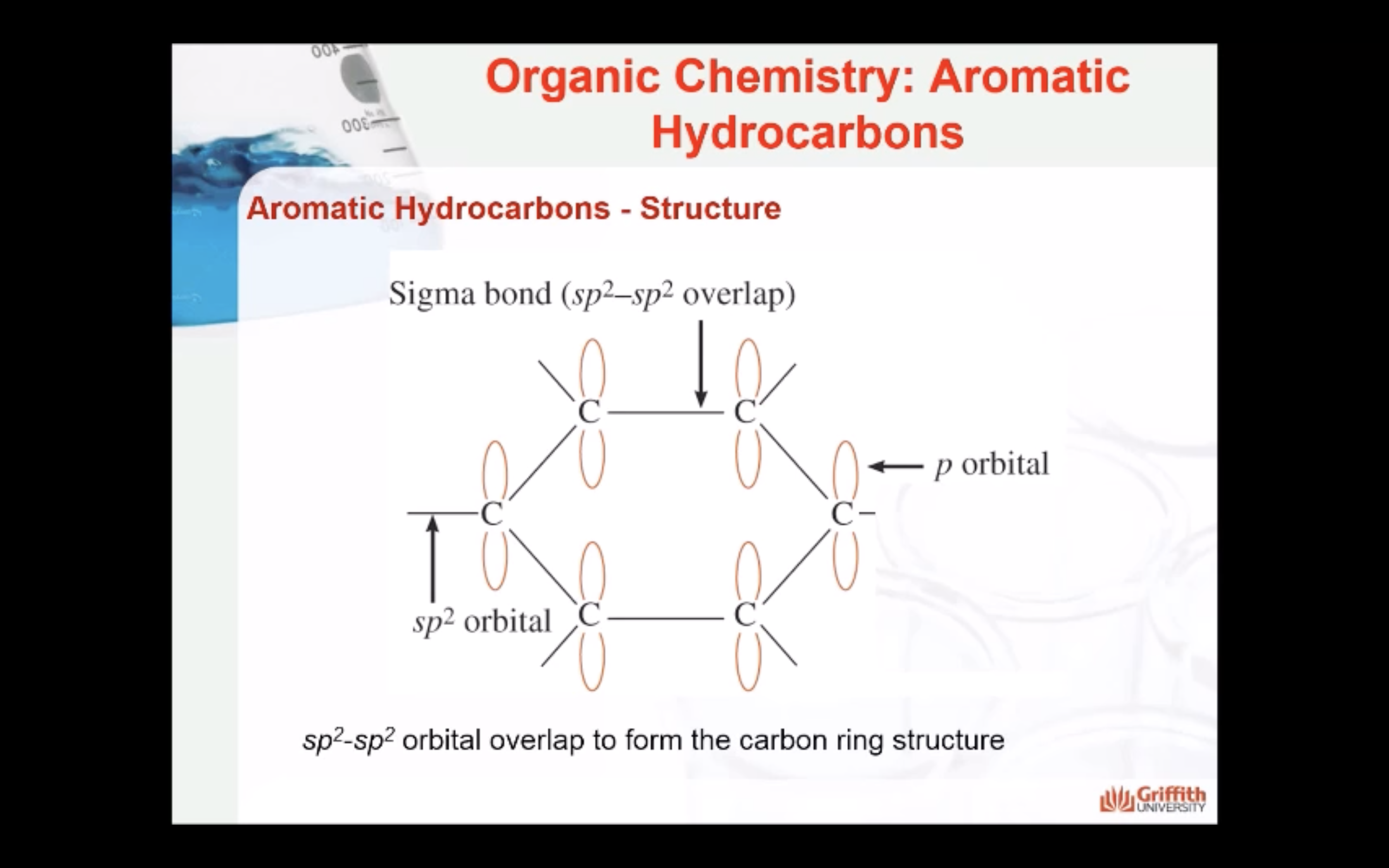
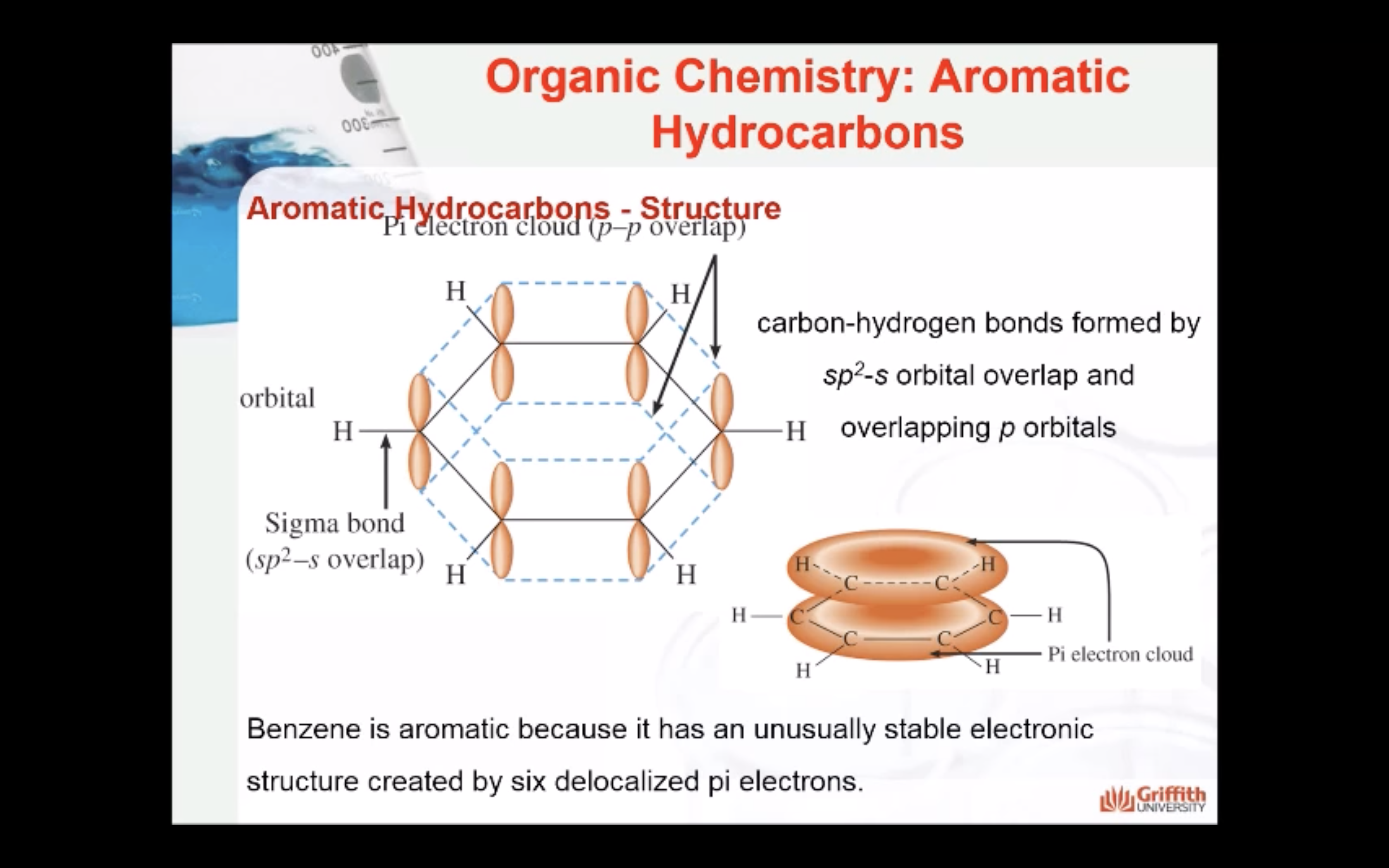
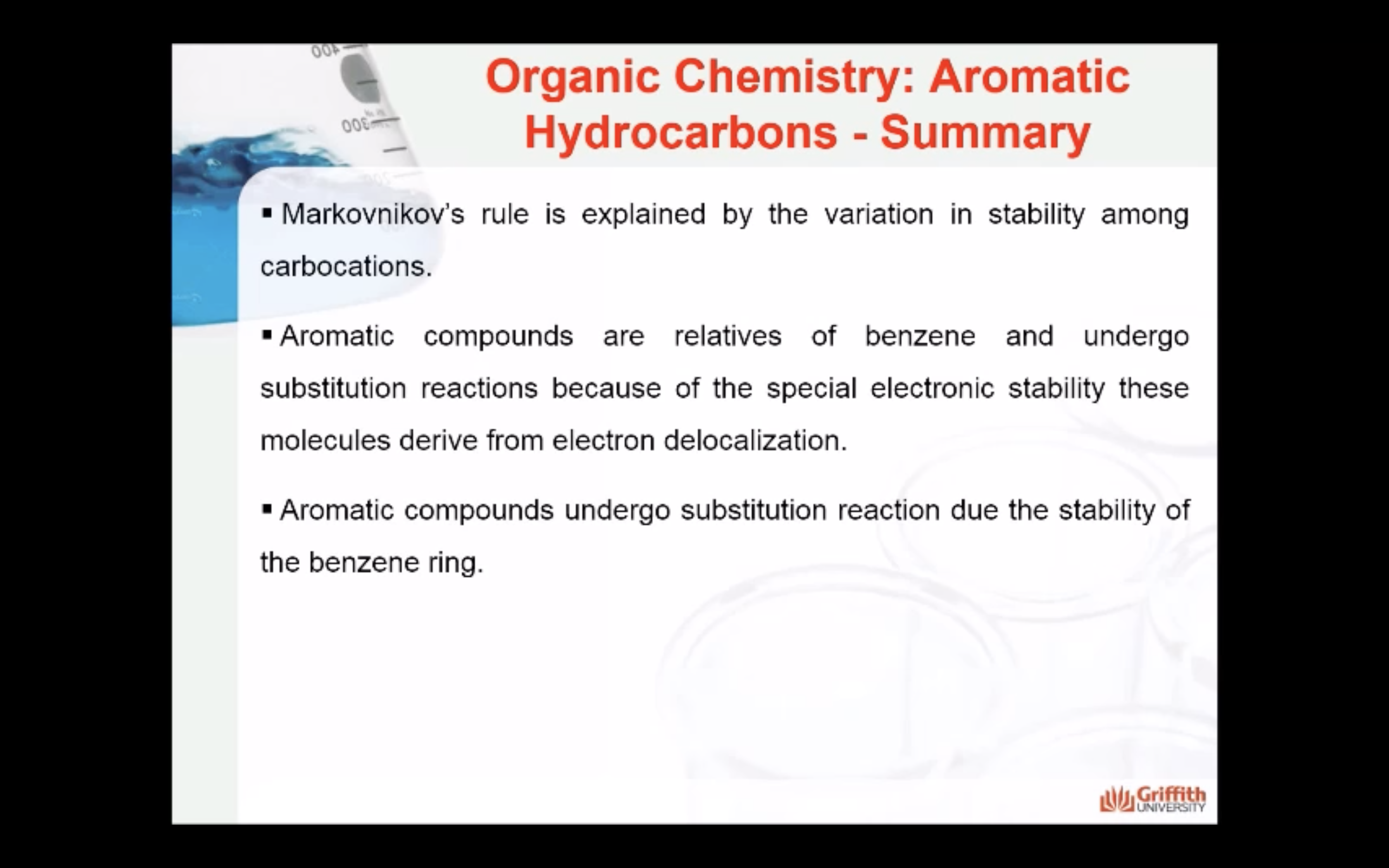
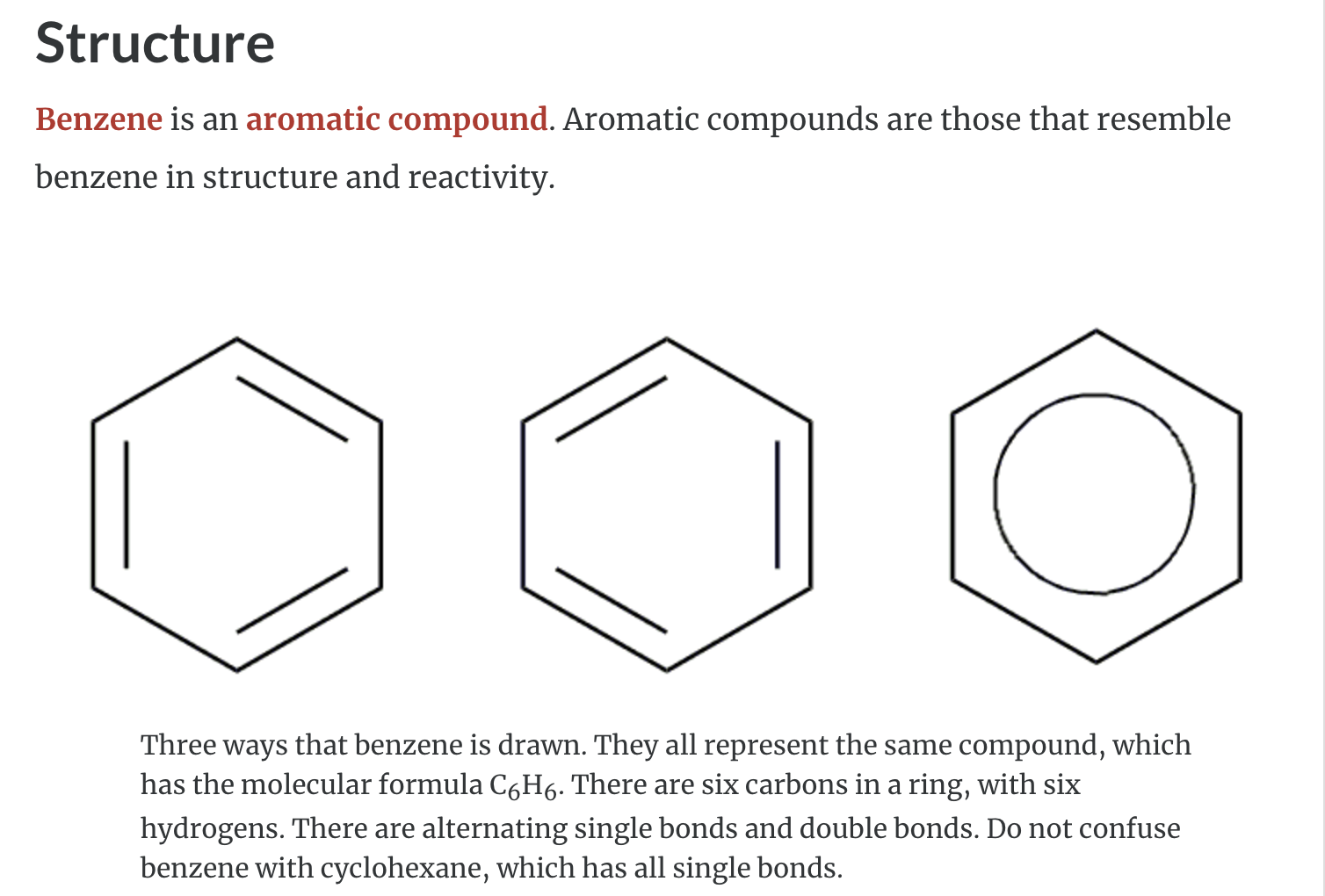
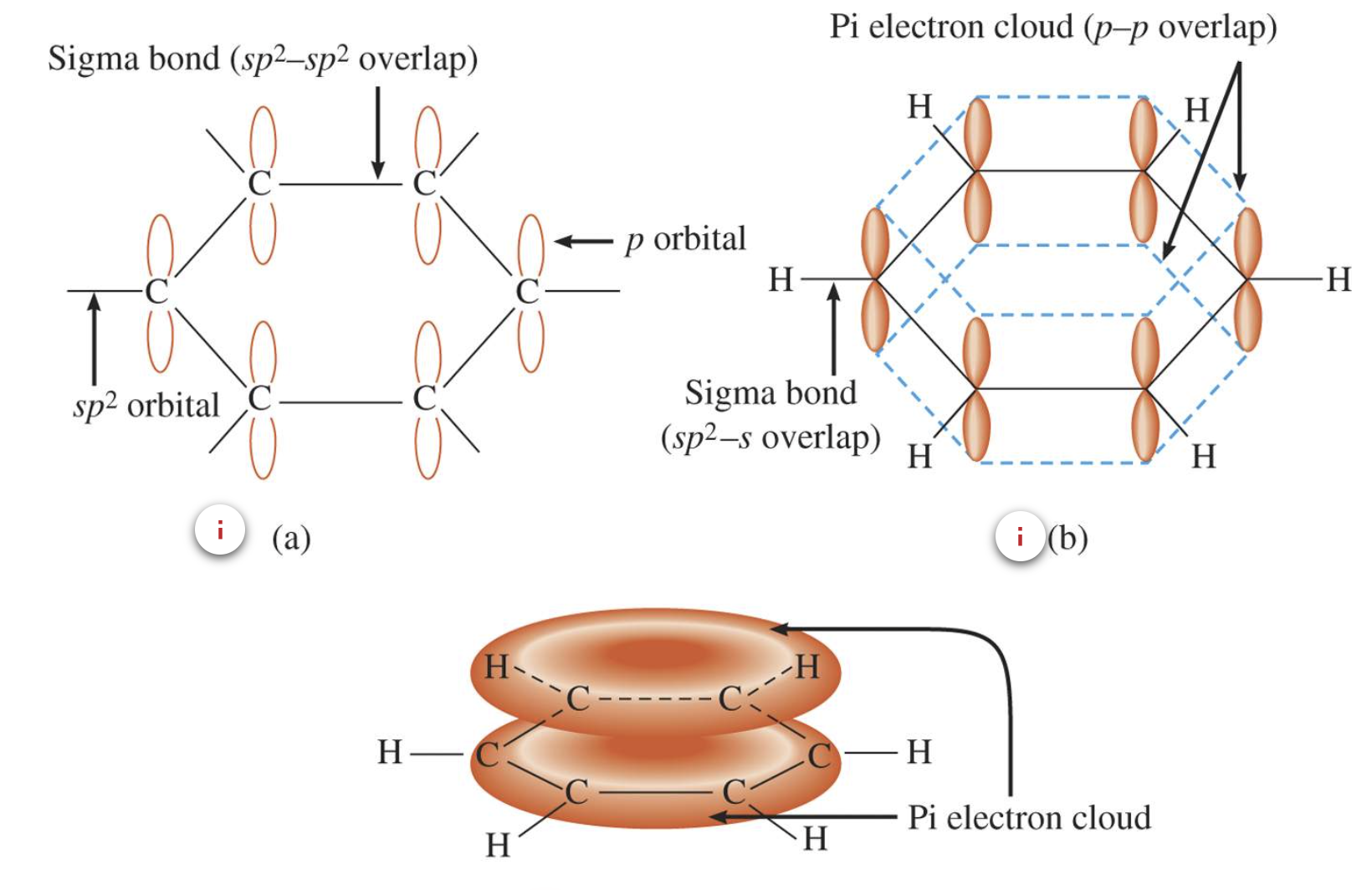
Aromatic Hydrocarbons
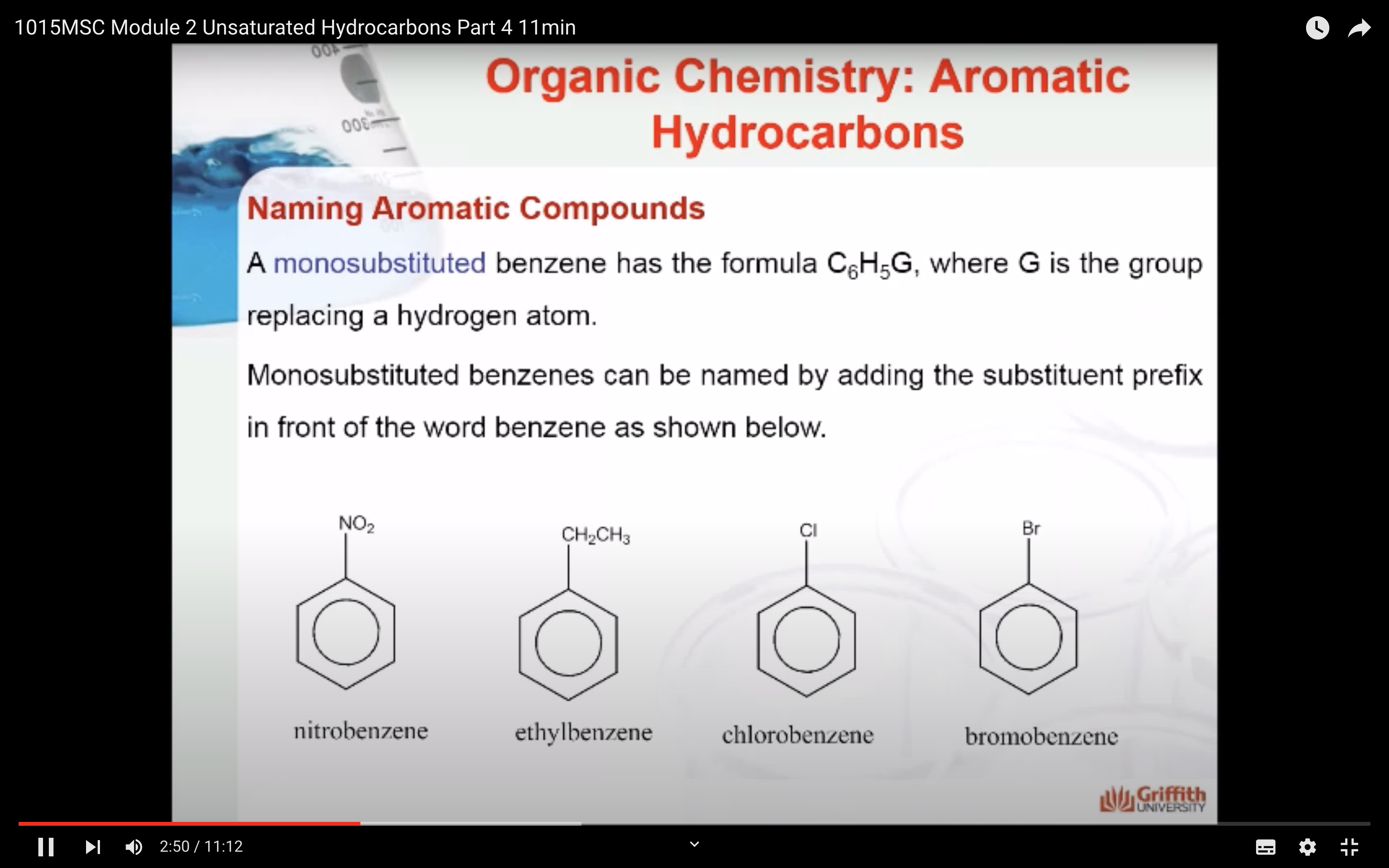
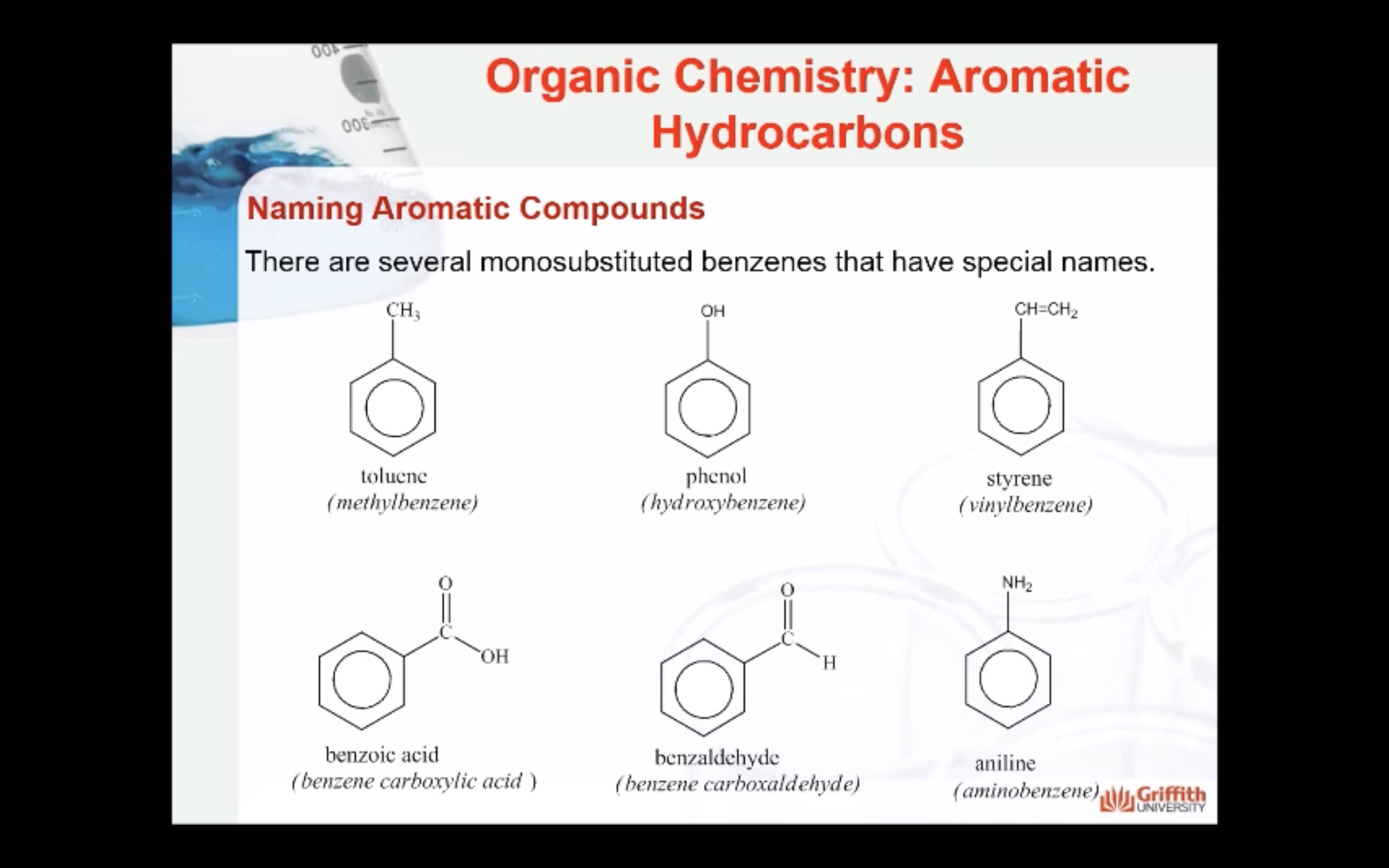
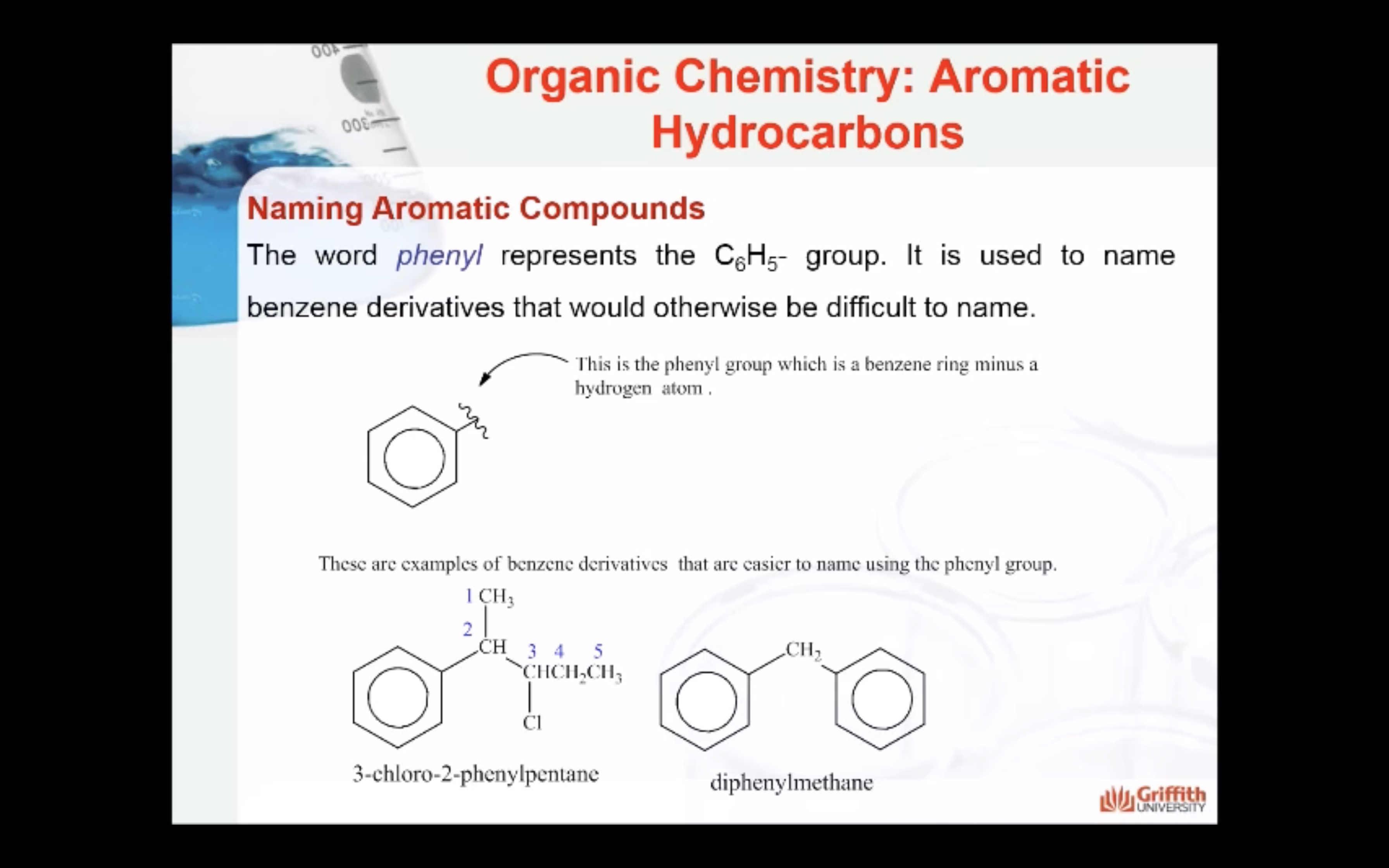
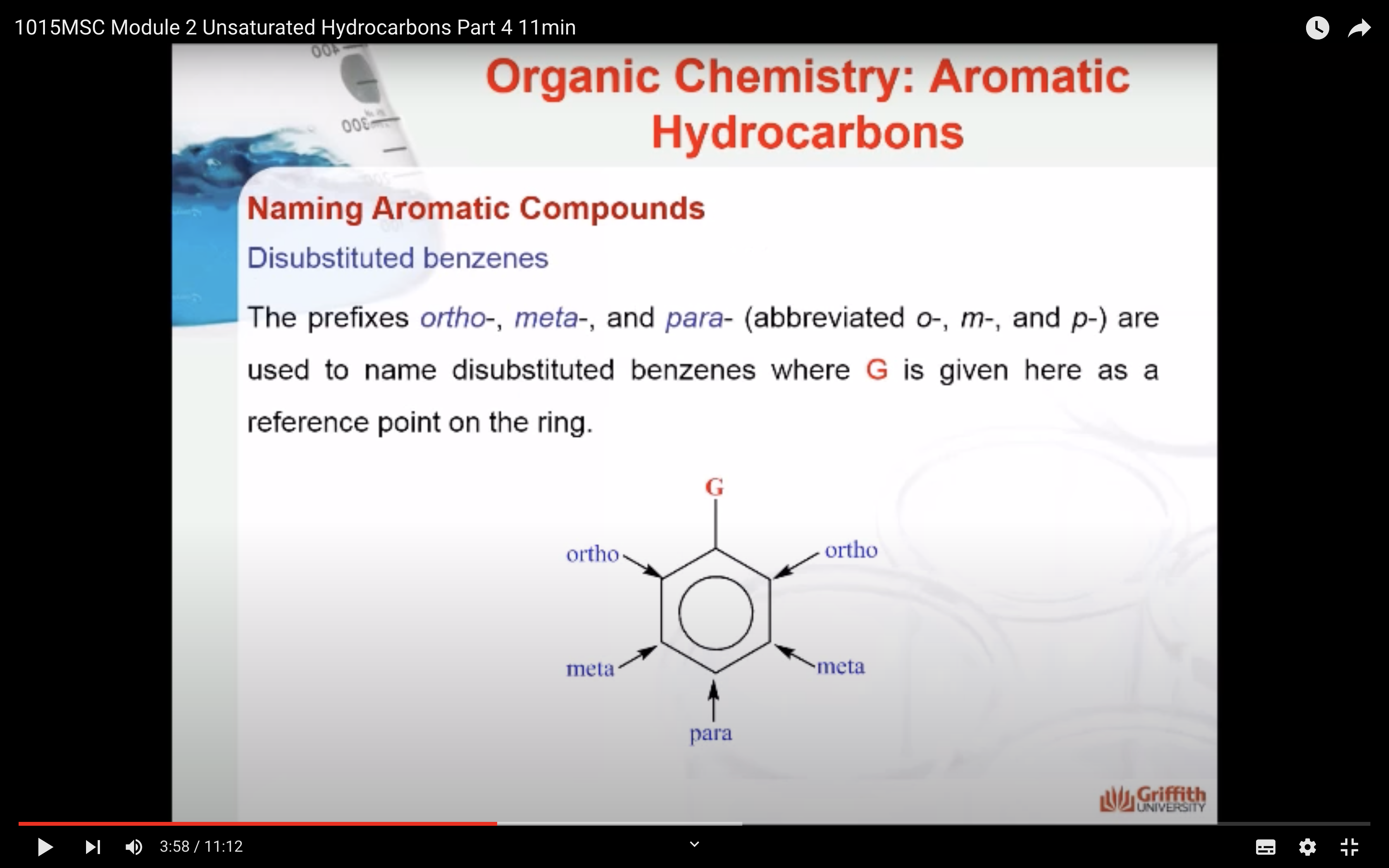
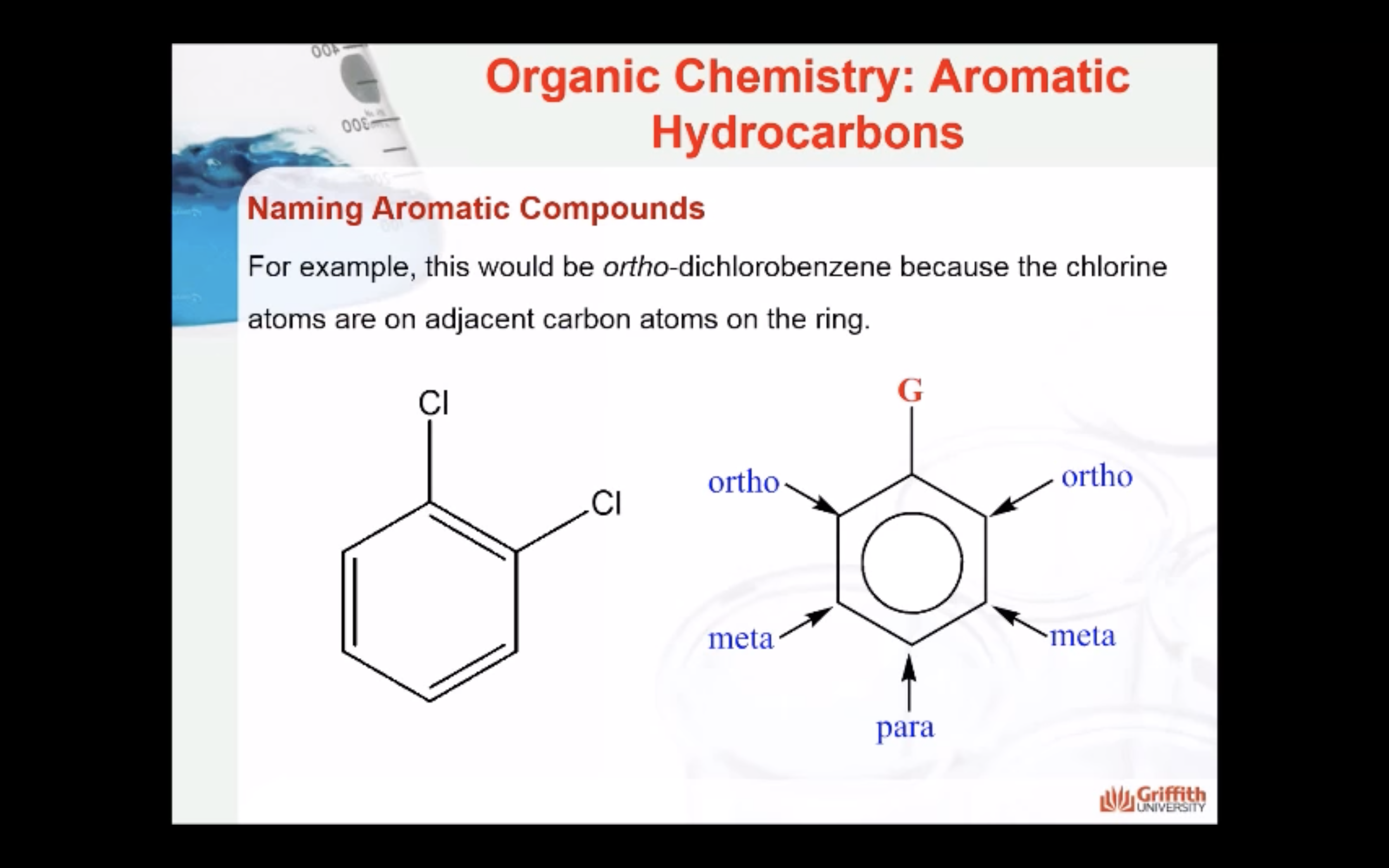
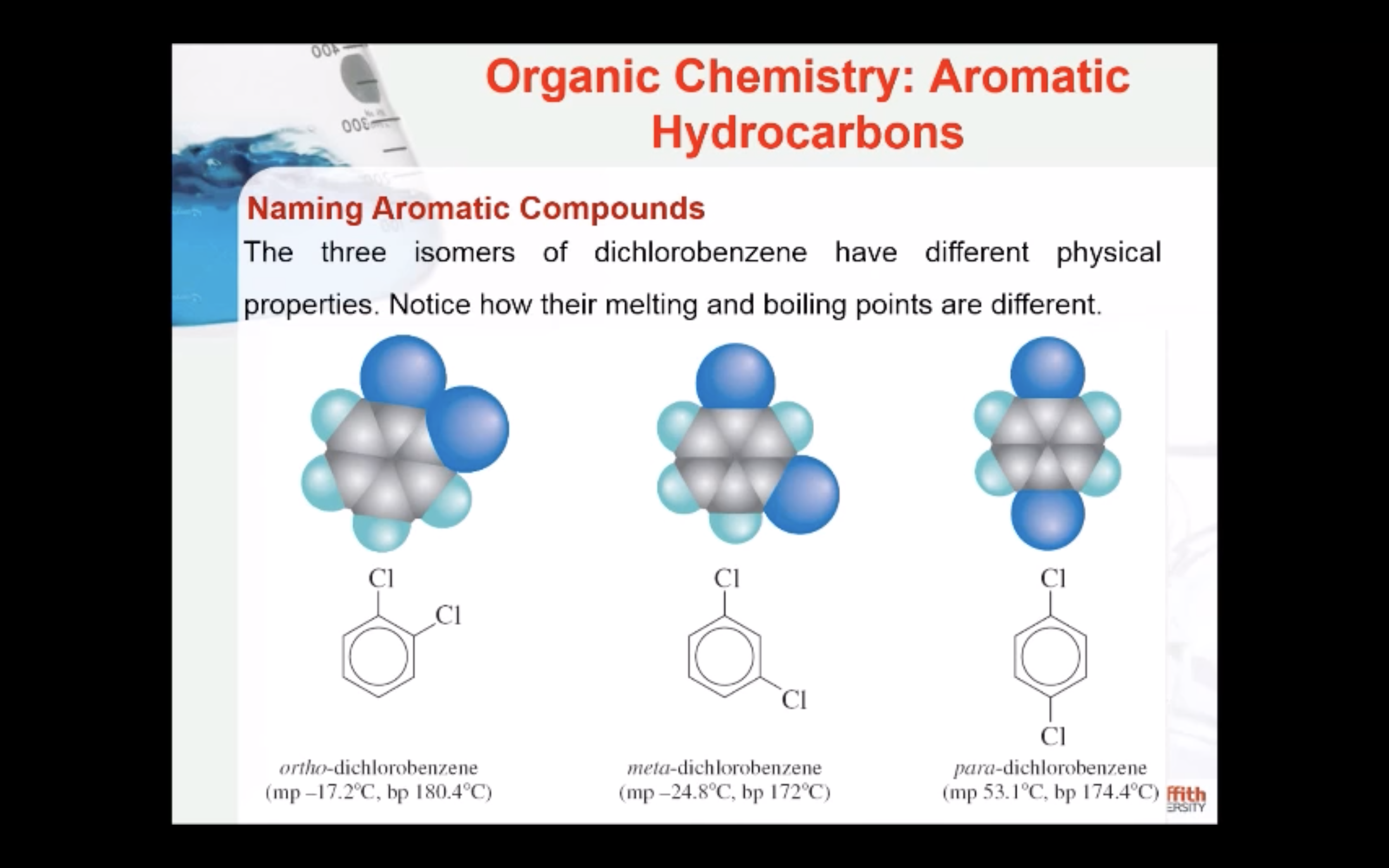
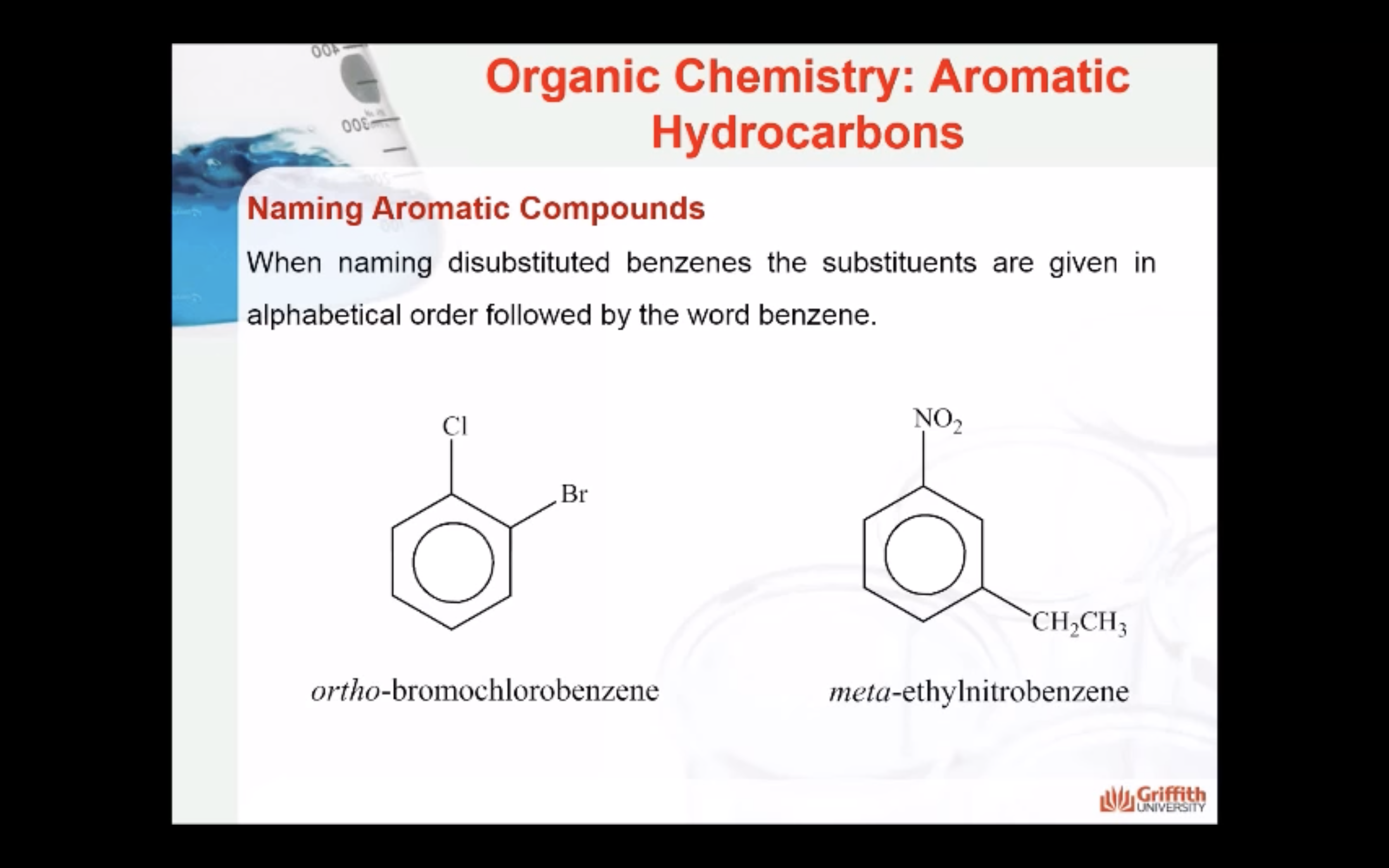
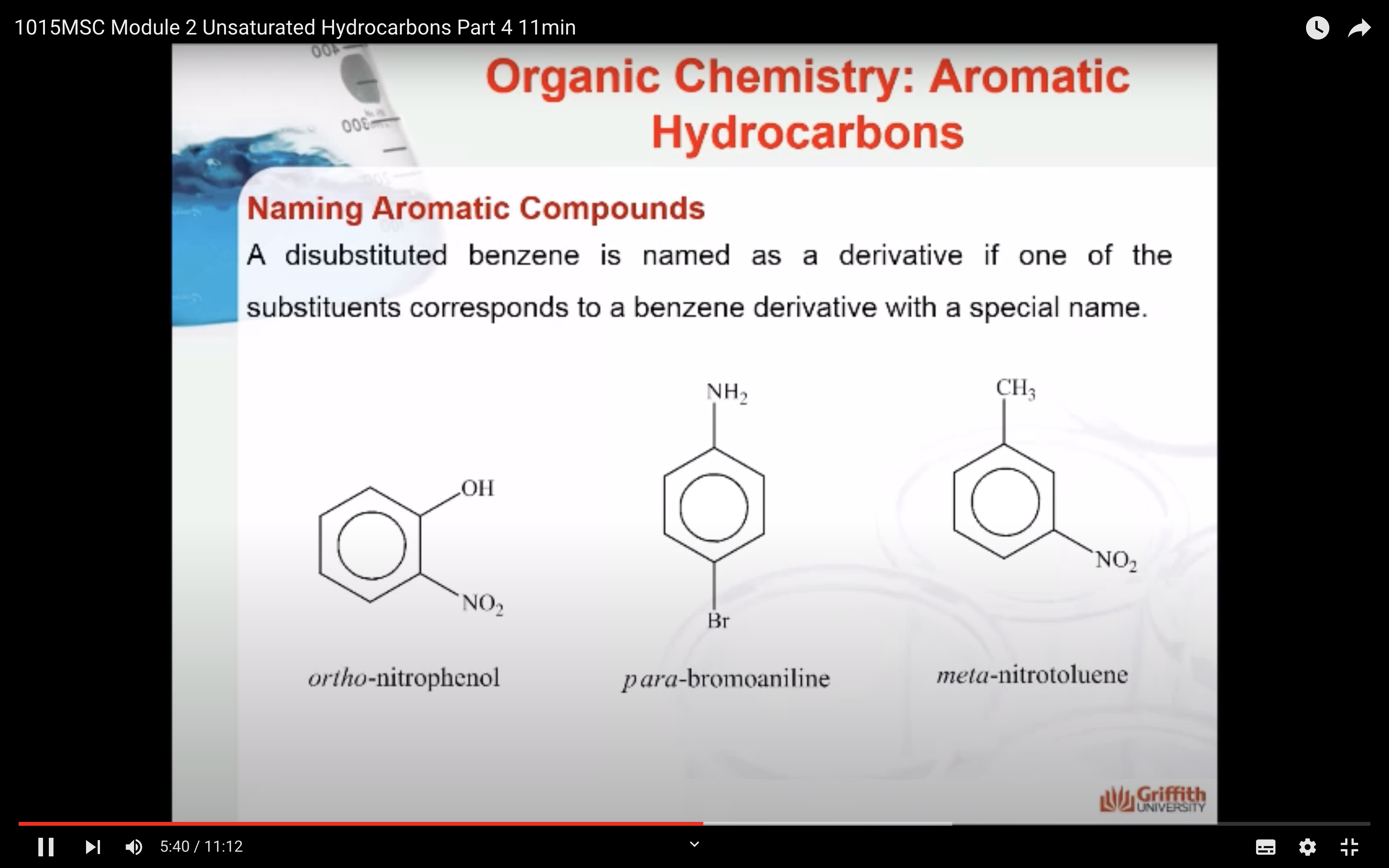
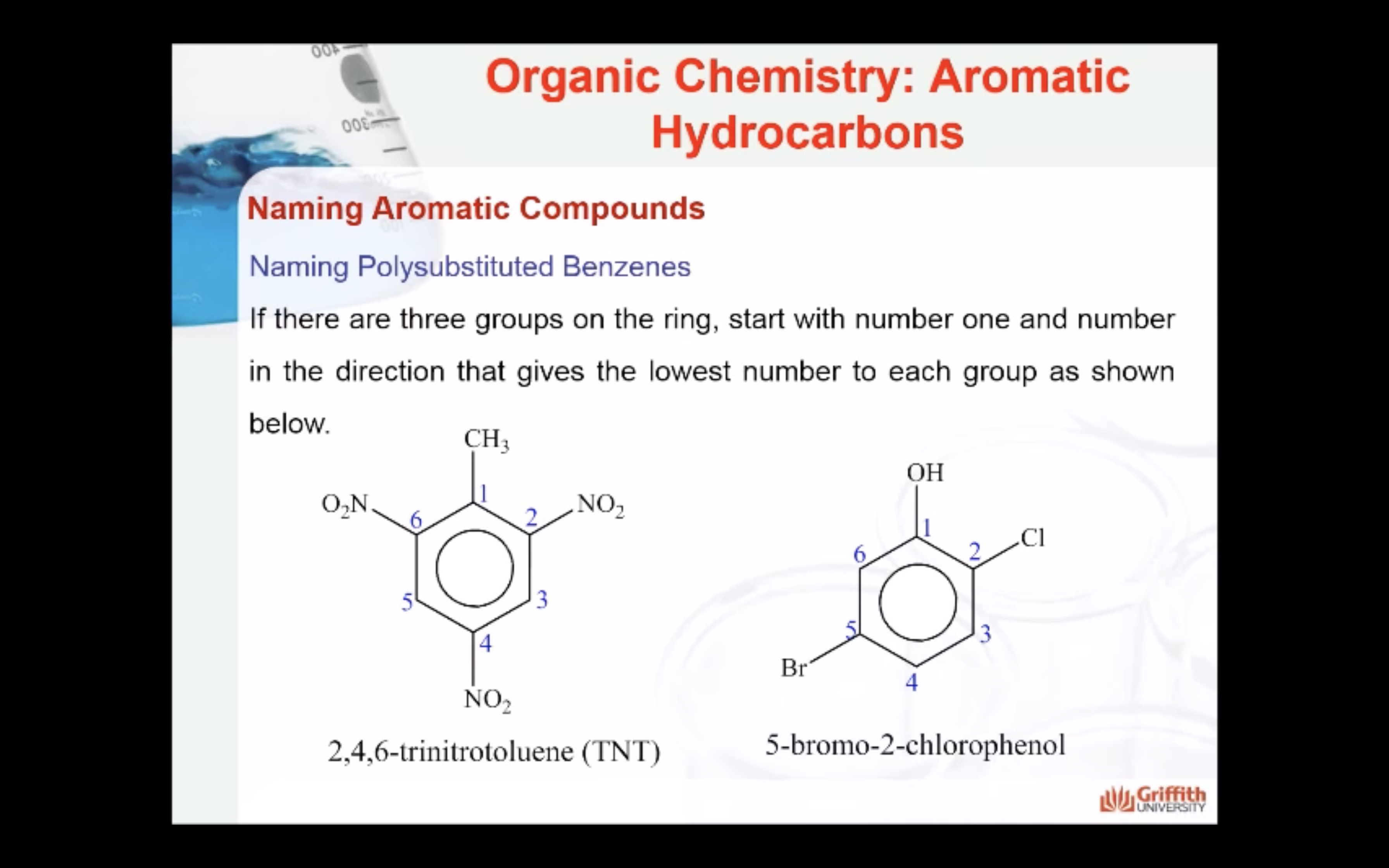
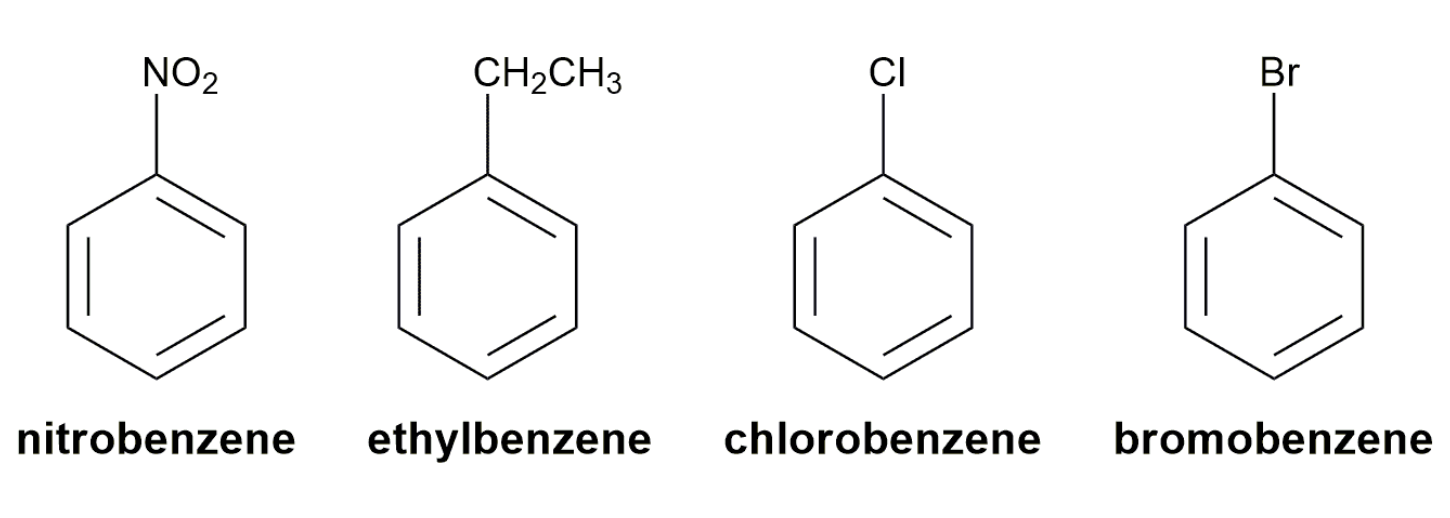
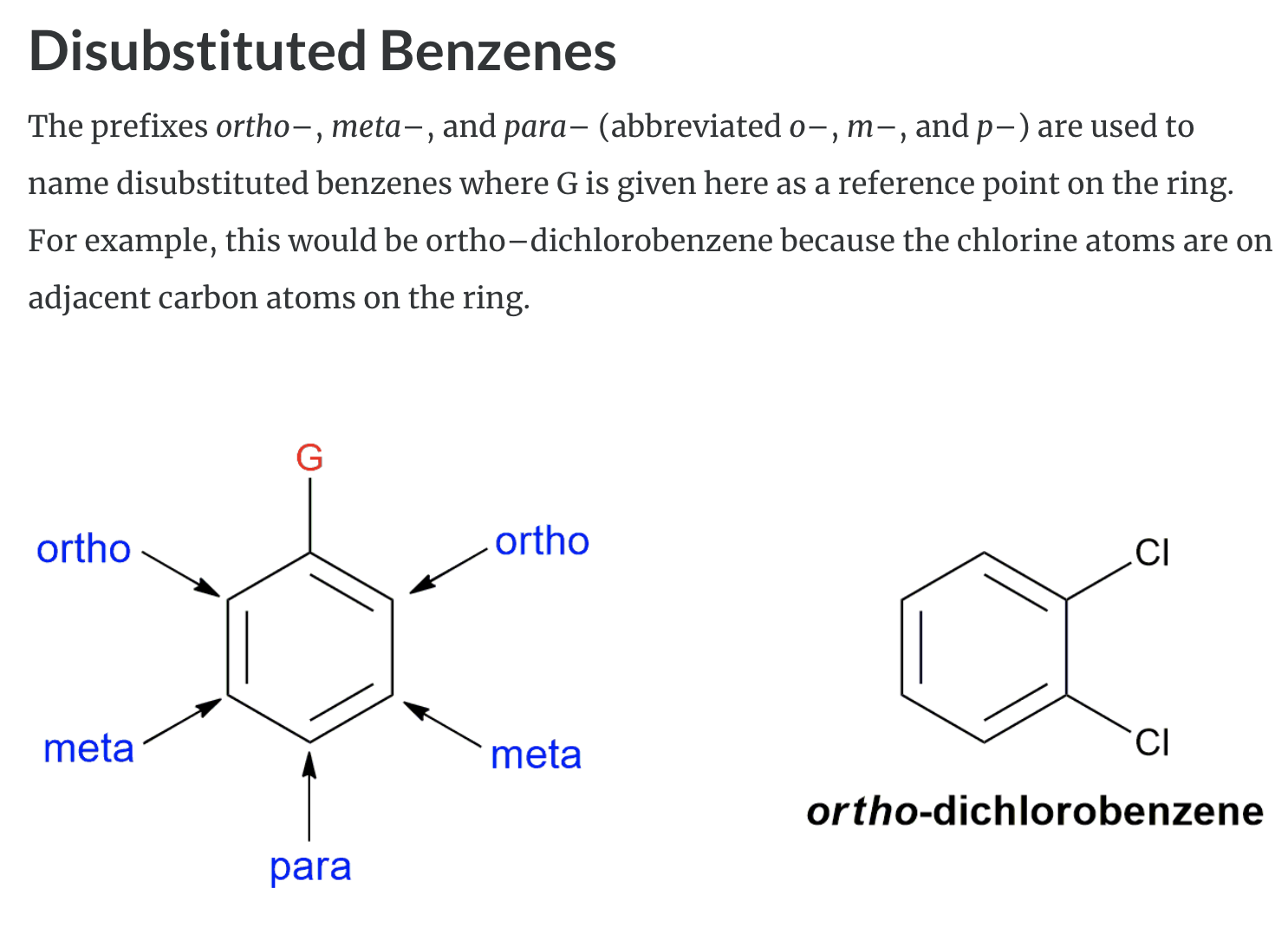
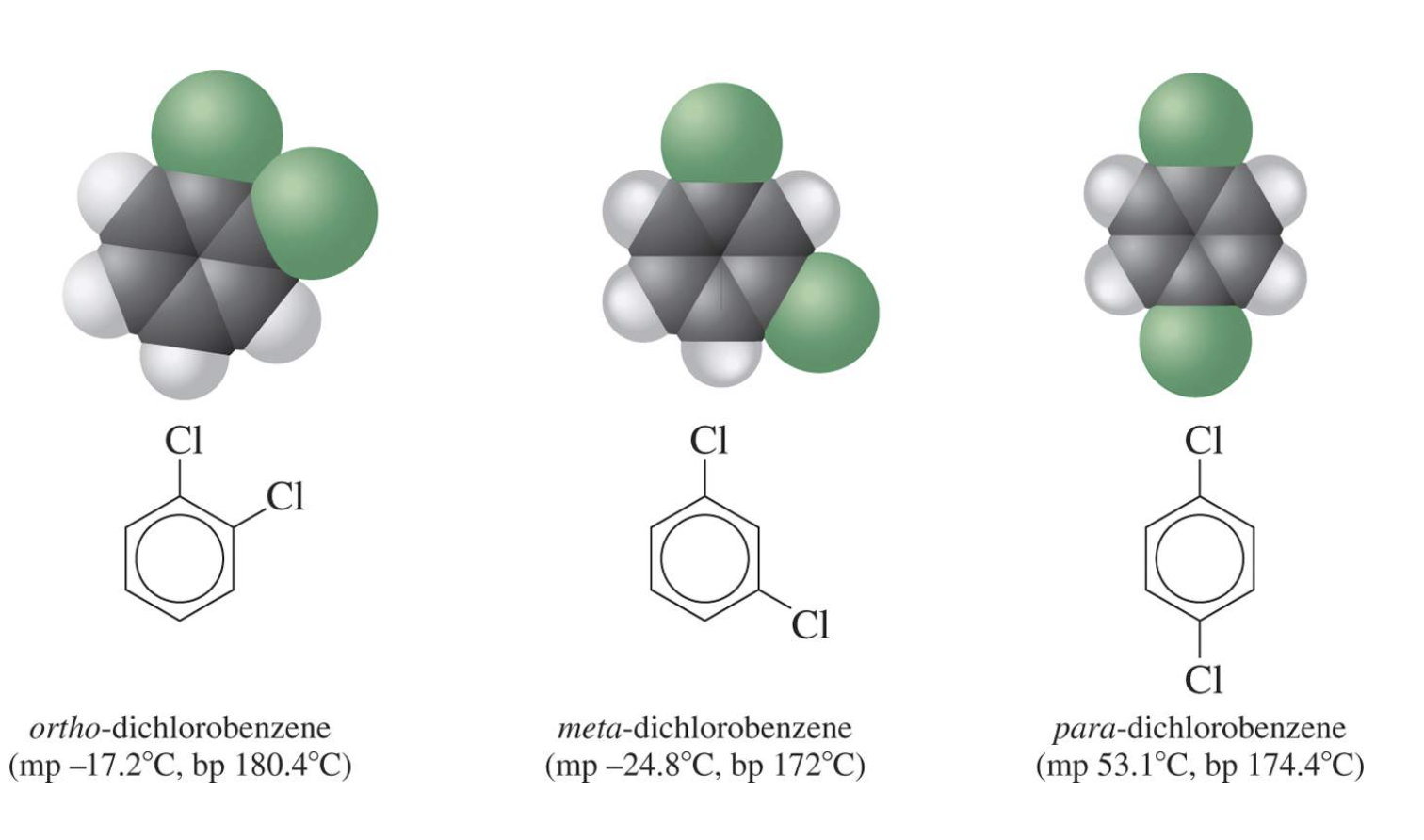
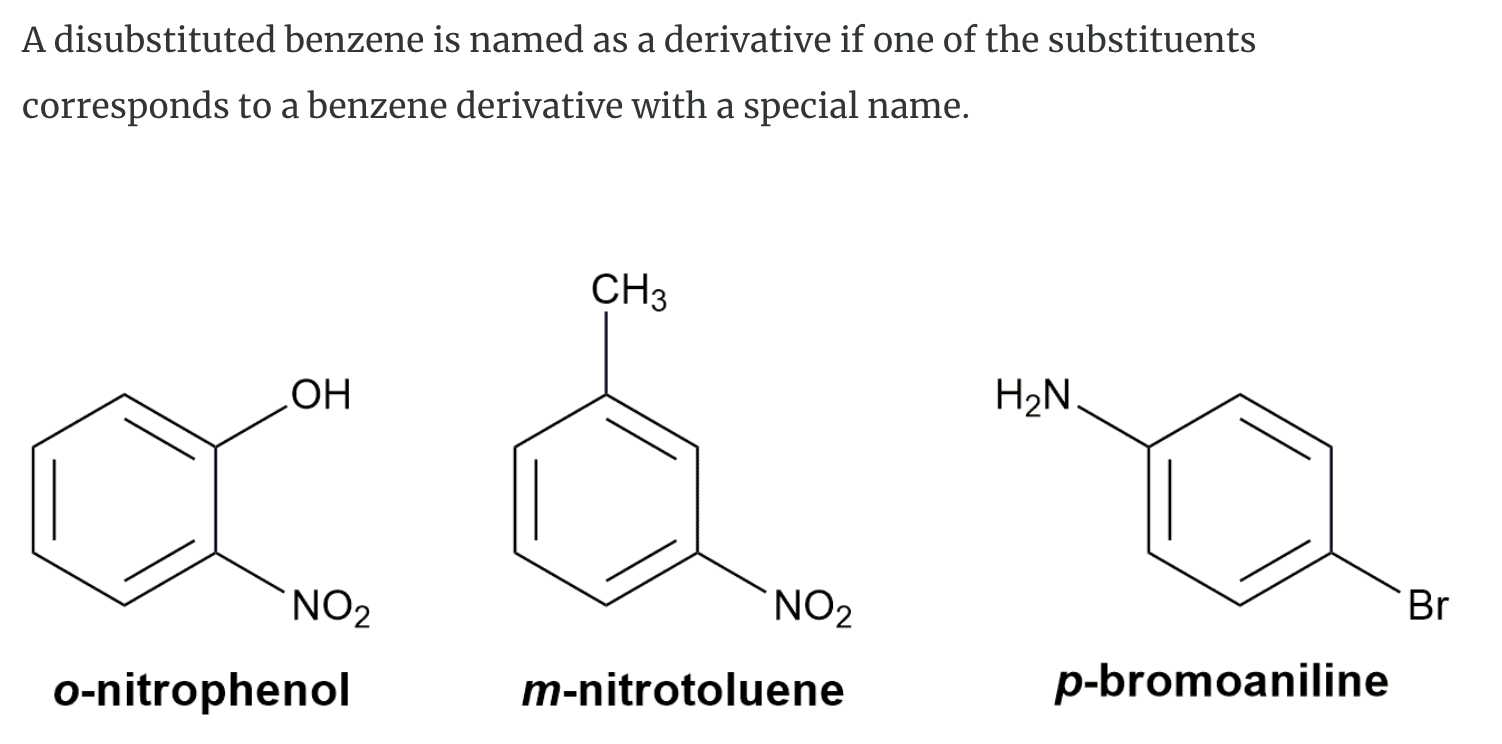
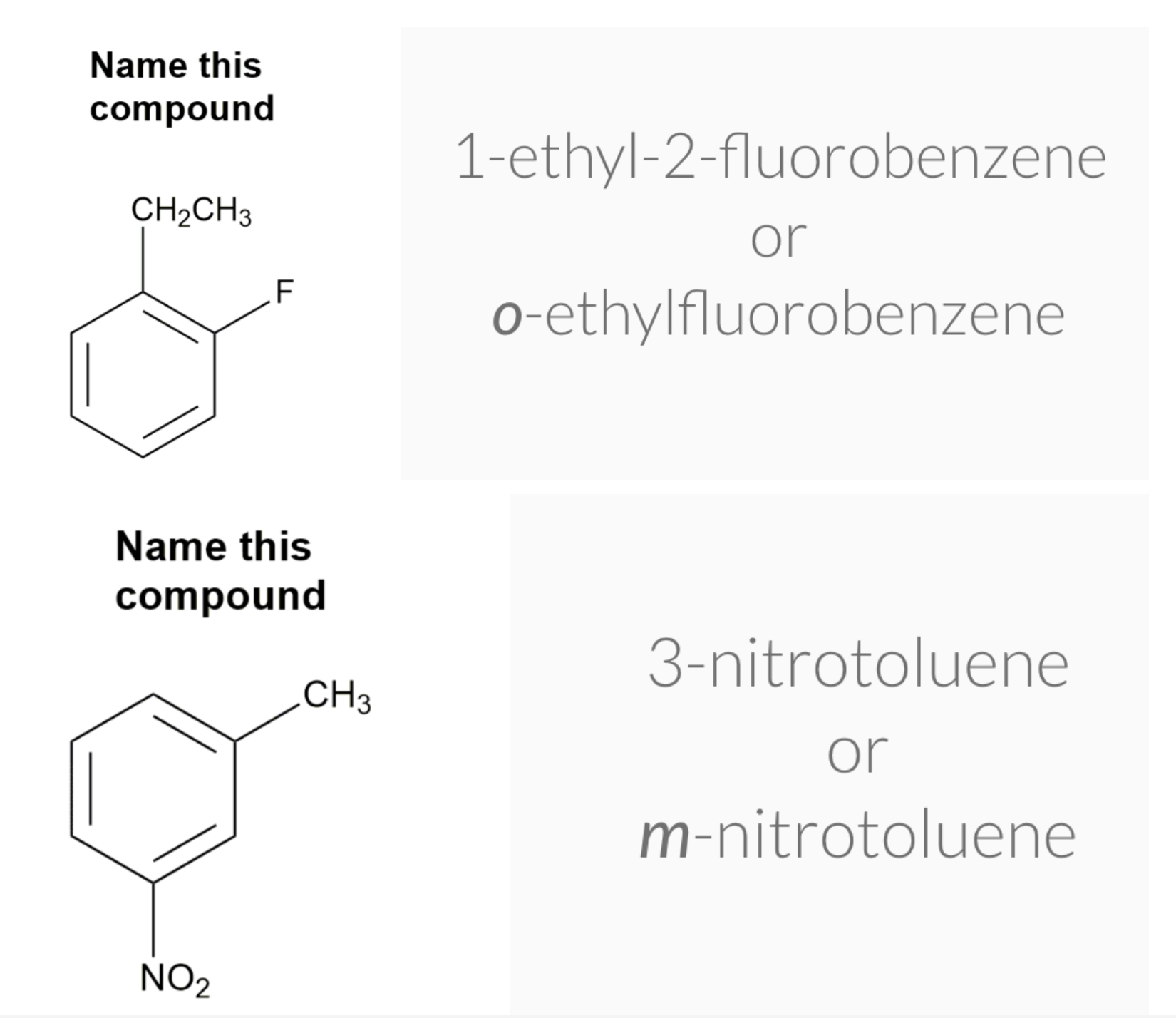
Substituted benzenes are the most common benzene derivatives because substitution is the most common reaction type for benzene.
A substituted benzene is derived by replacing one or more hydrogen atoms of benzene by another atom or group of atoms. A monosubstituted benzene has the formula C6H5G, where G is the group replacing a hydrogen atom. Monosubstituted benzenes can be named by adding the substituent prefix in front of the word benzene as shown below.
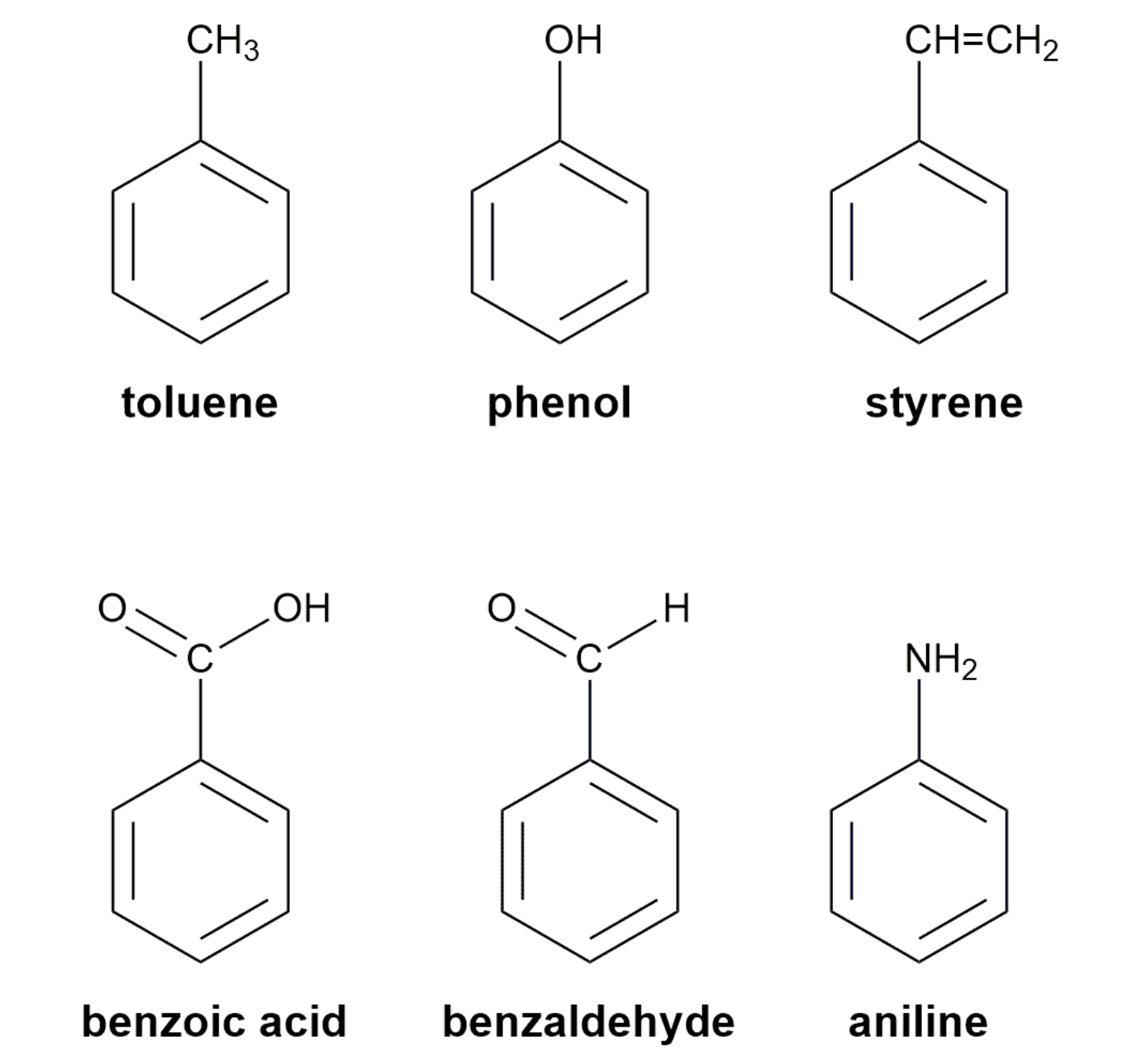
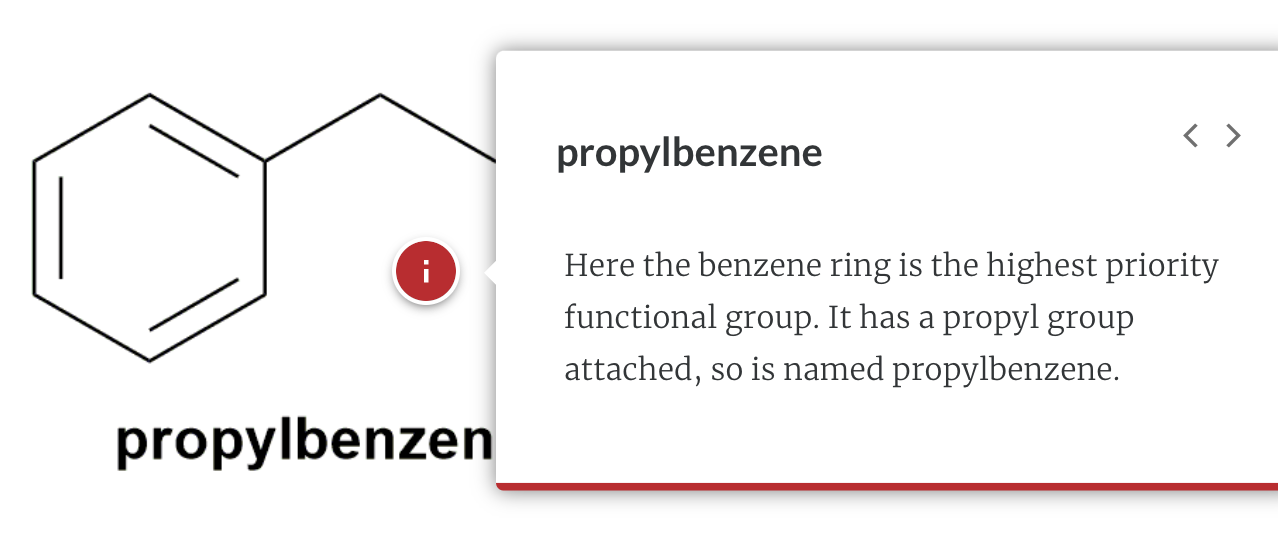
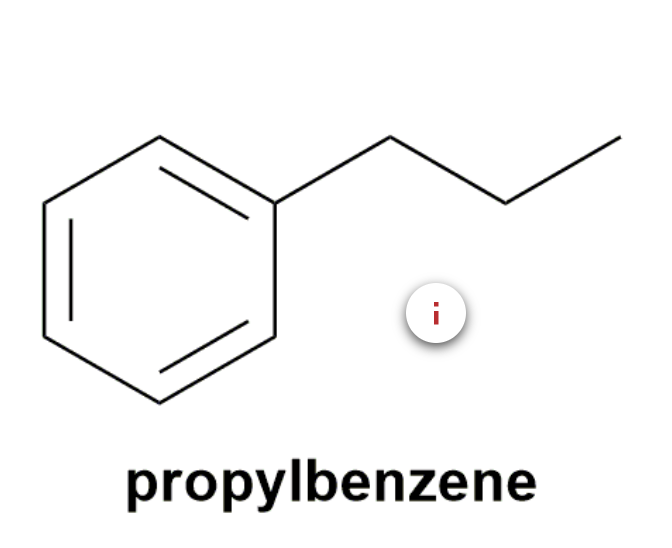
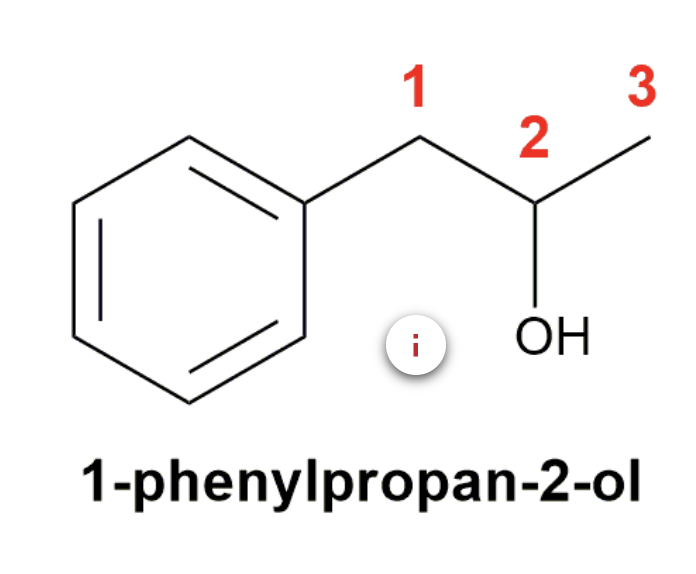

The dimethylbenzenes have the special name xylene.
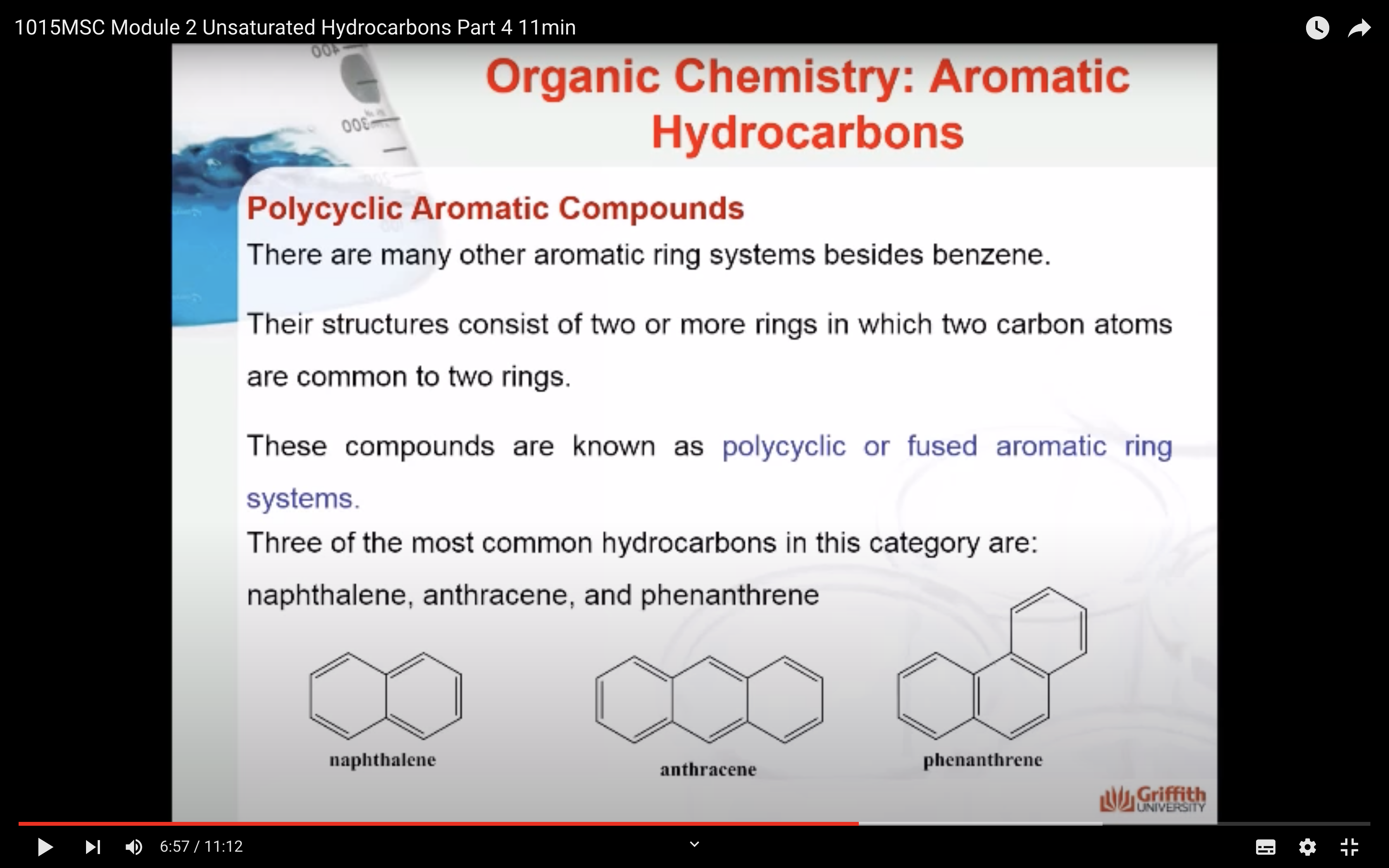
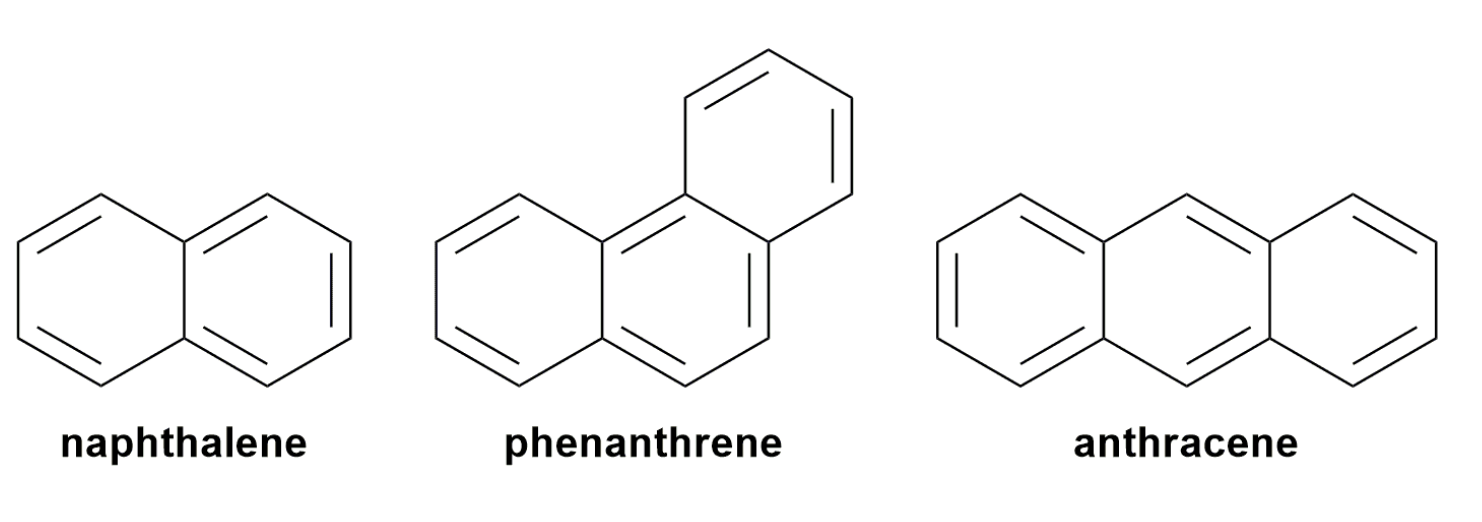
Polycyclic Aromatic Compounds
There are many other aromatic ring systems besides benzene. Their structures consist of two or more rings in which two carbon atoms are common to two rings. These compounds are known as polycyclic or fused aromatic ring systems. Three of the most common hydrocarbons in this category are naphthalene, anthracene, and phenanthrene.

Sources and Physical Properties of Aromatic Hydrocarbons
Aromatic hydrocarbons (to include benzene, toluene, napthalene etc.) are produced from petroleum. A limited amount can be prepared from coal tar. Coal tar is only a by–product of coke. Consequently, this method is no longer the principal source of aromatic hydrocarbons.
Aromatic hydrocarbons have properties common to all hydrocarbons. They are nonpolar substances, they are insoluble in water but soluble in most organic solvents, and have densities less than the density of water. They also burn readily, usually with smoky yellow flames.
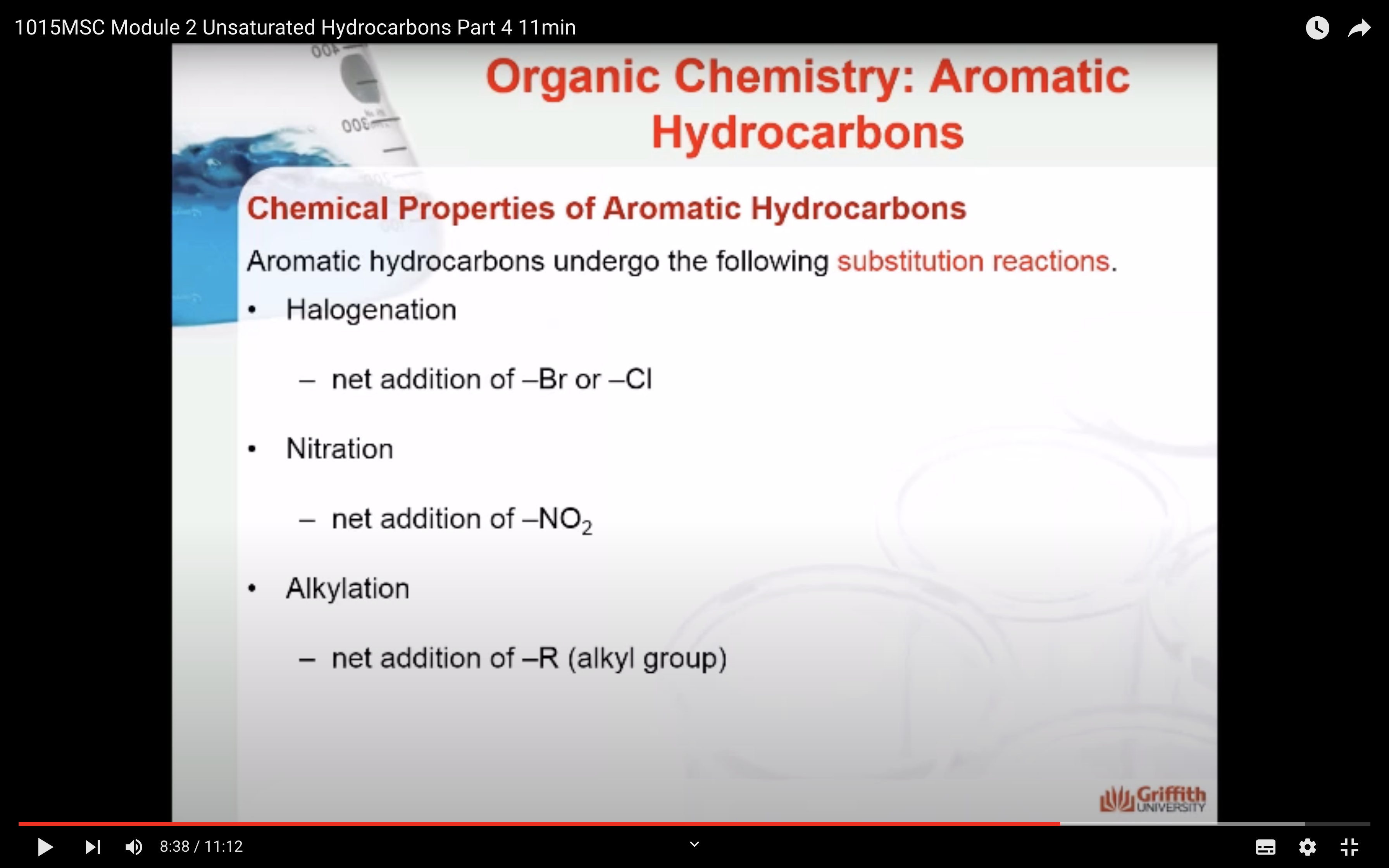
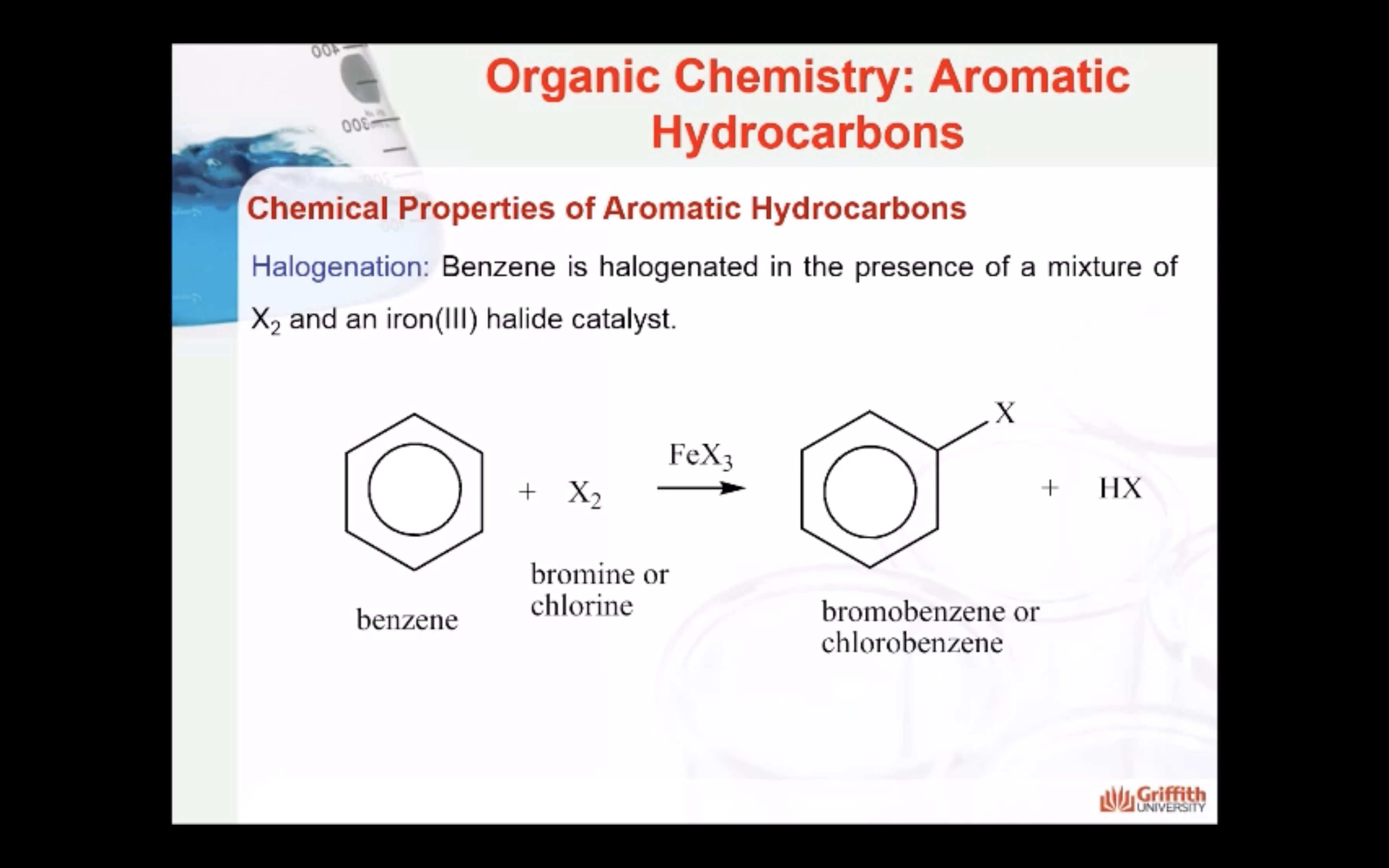
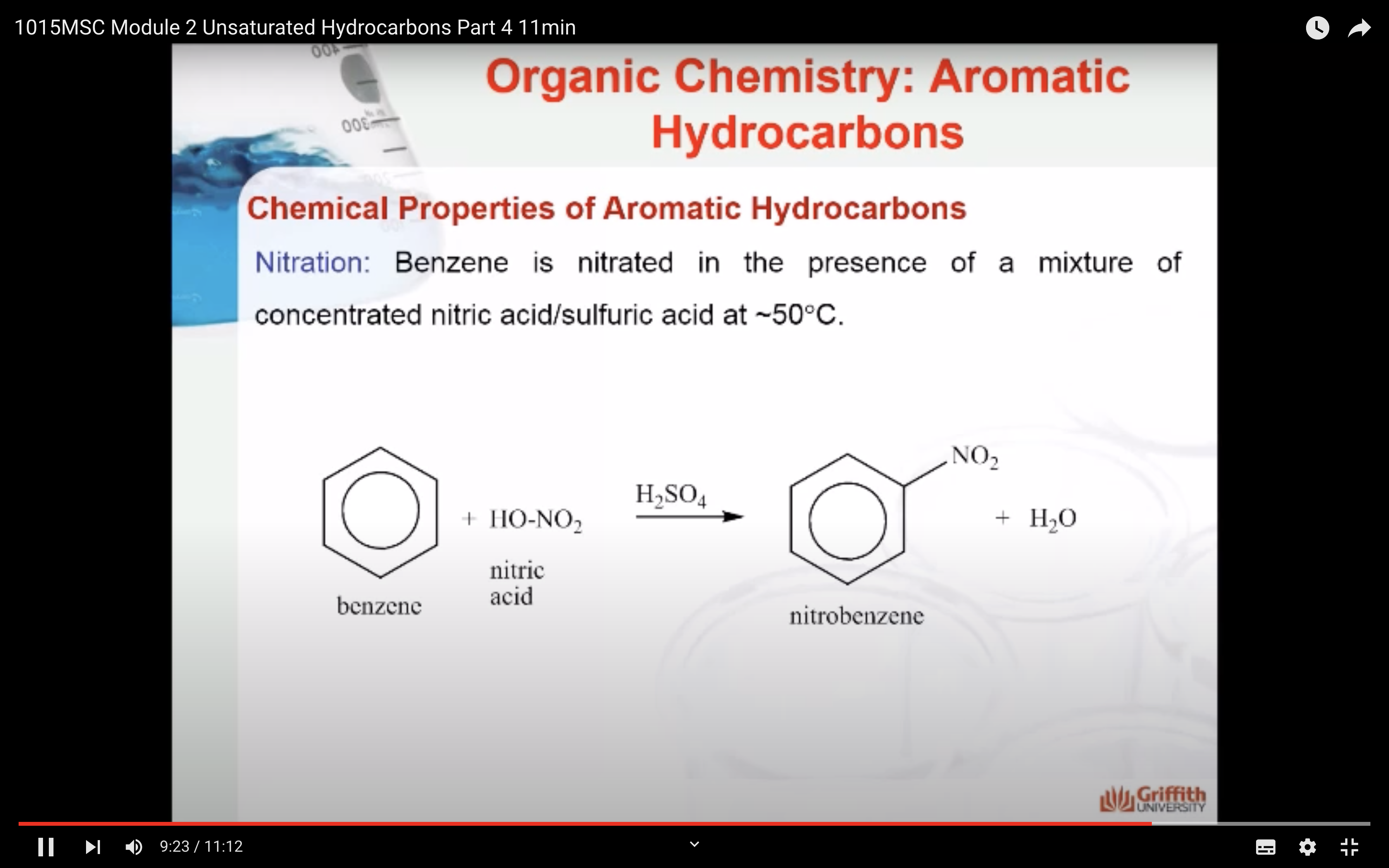
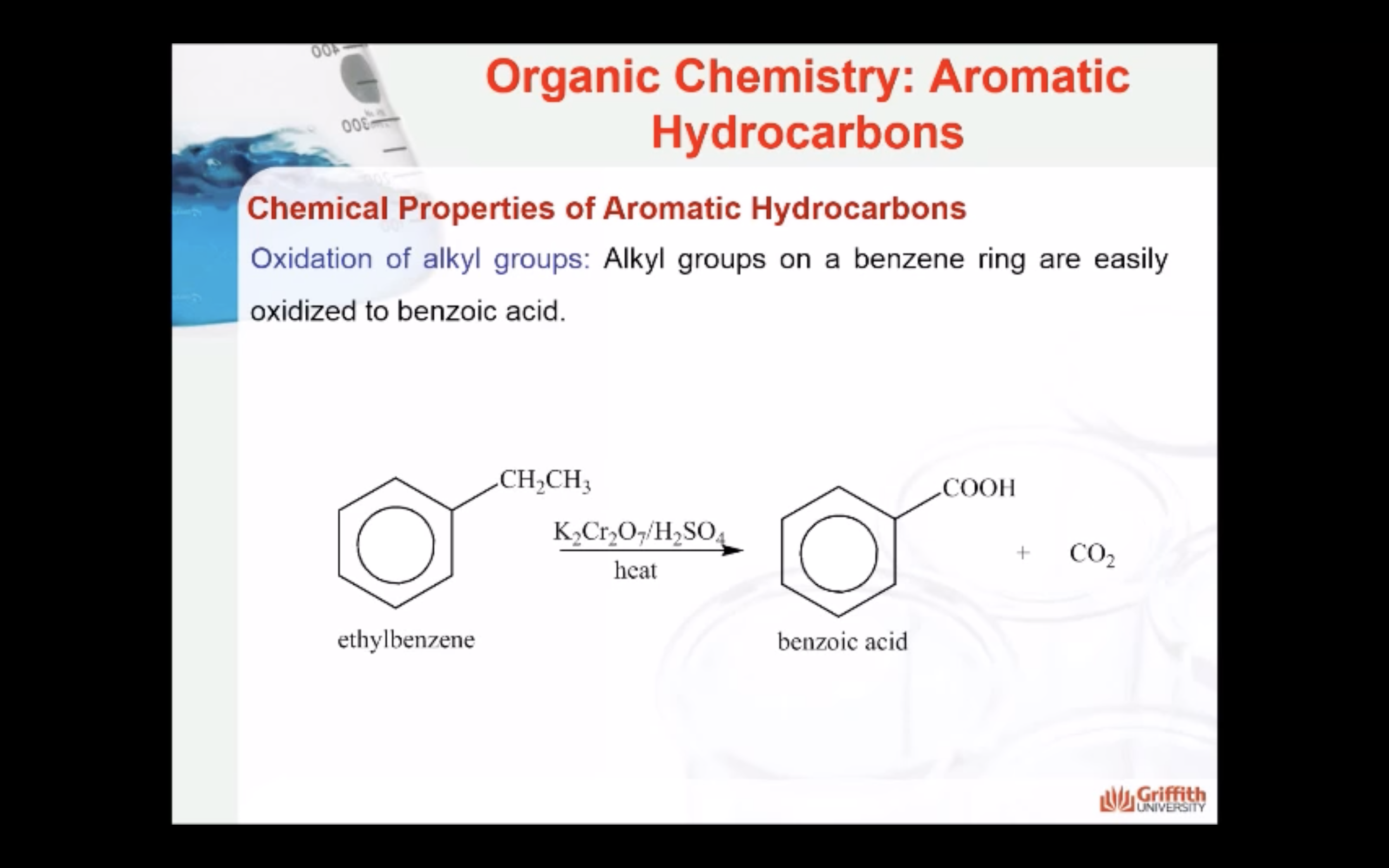
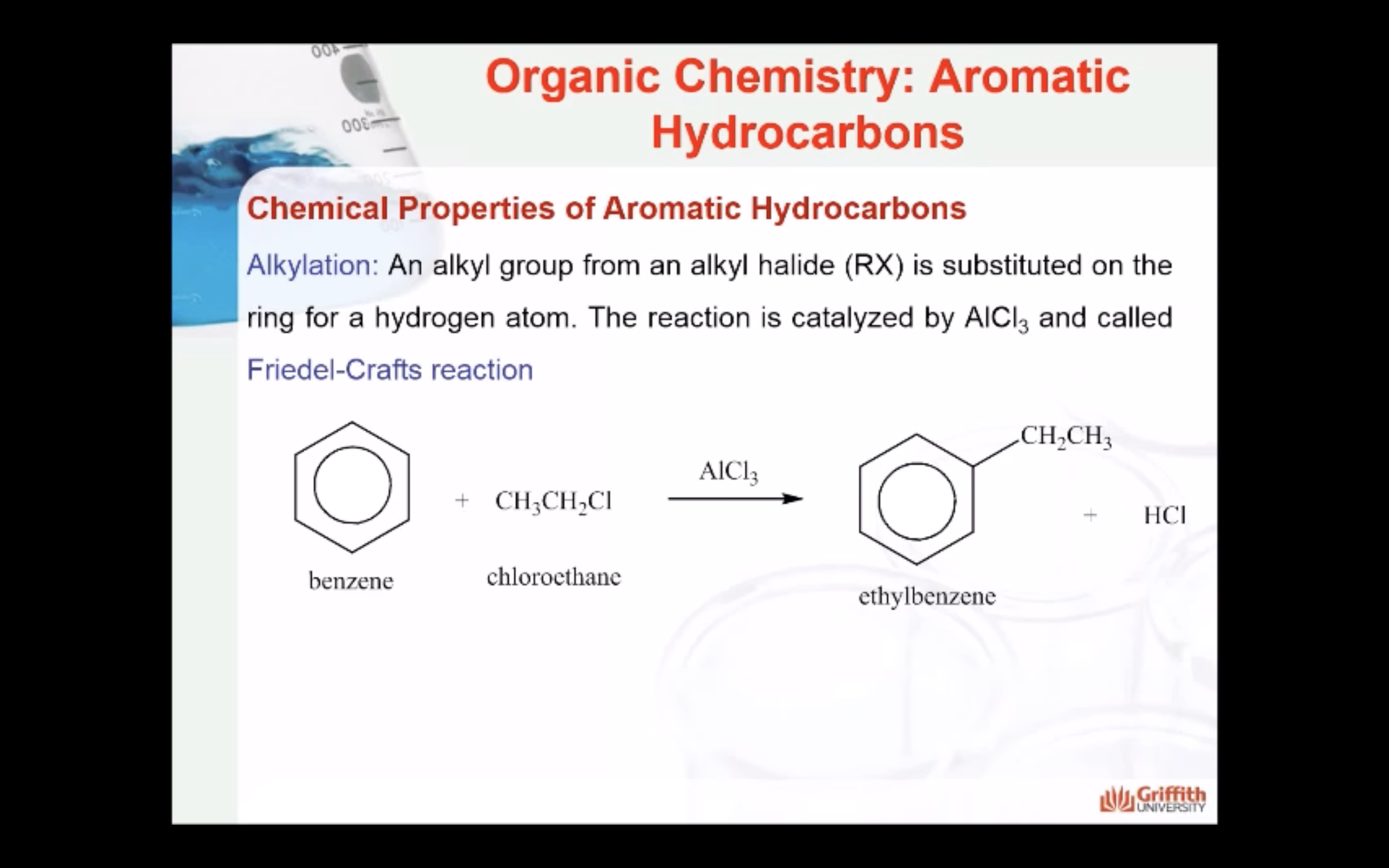
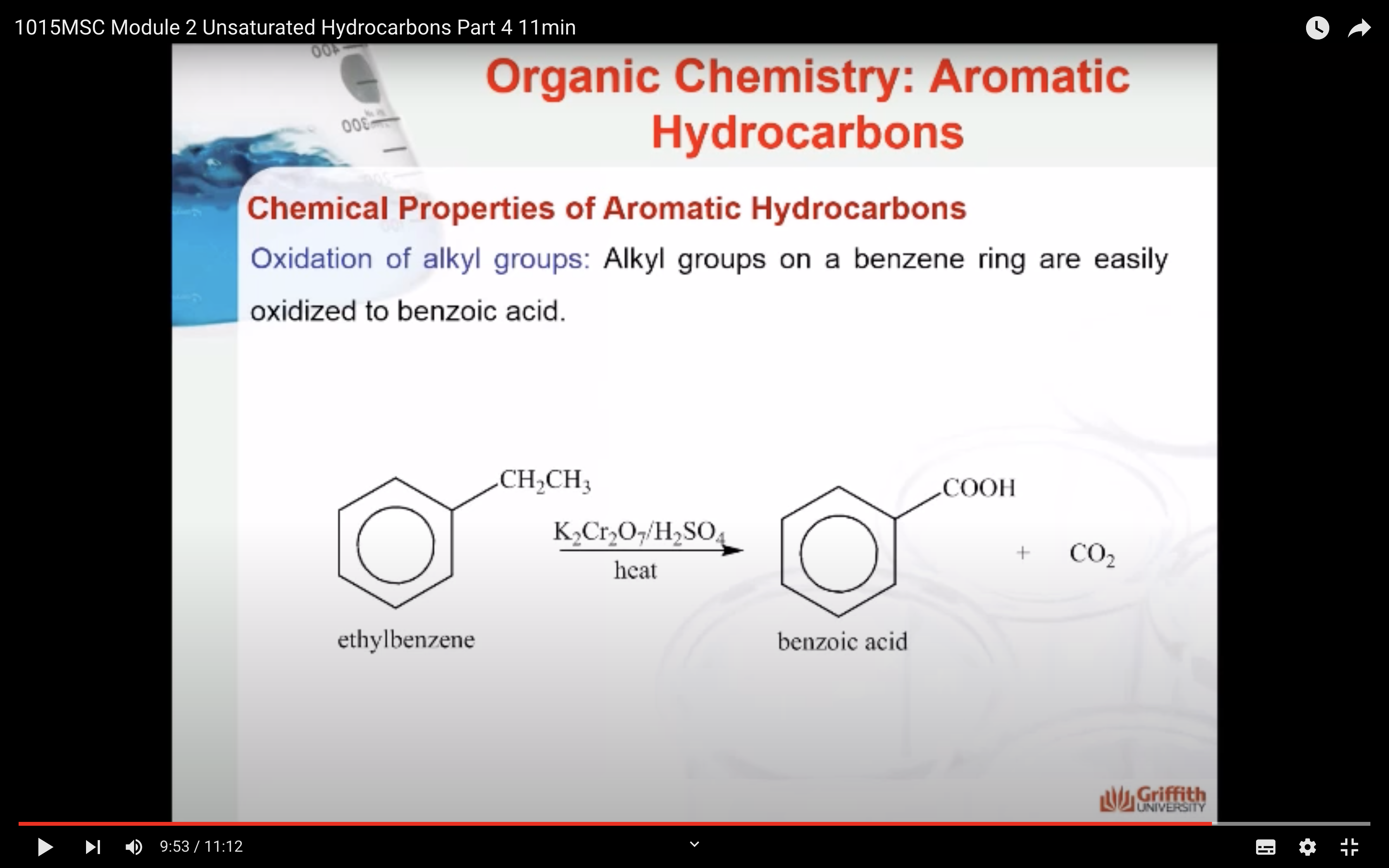
Chemical Properties of Aromatic Hydrocarbons
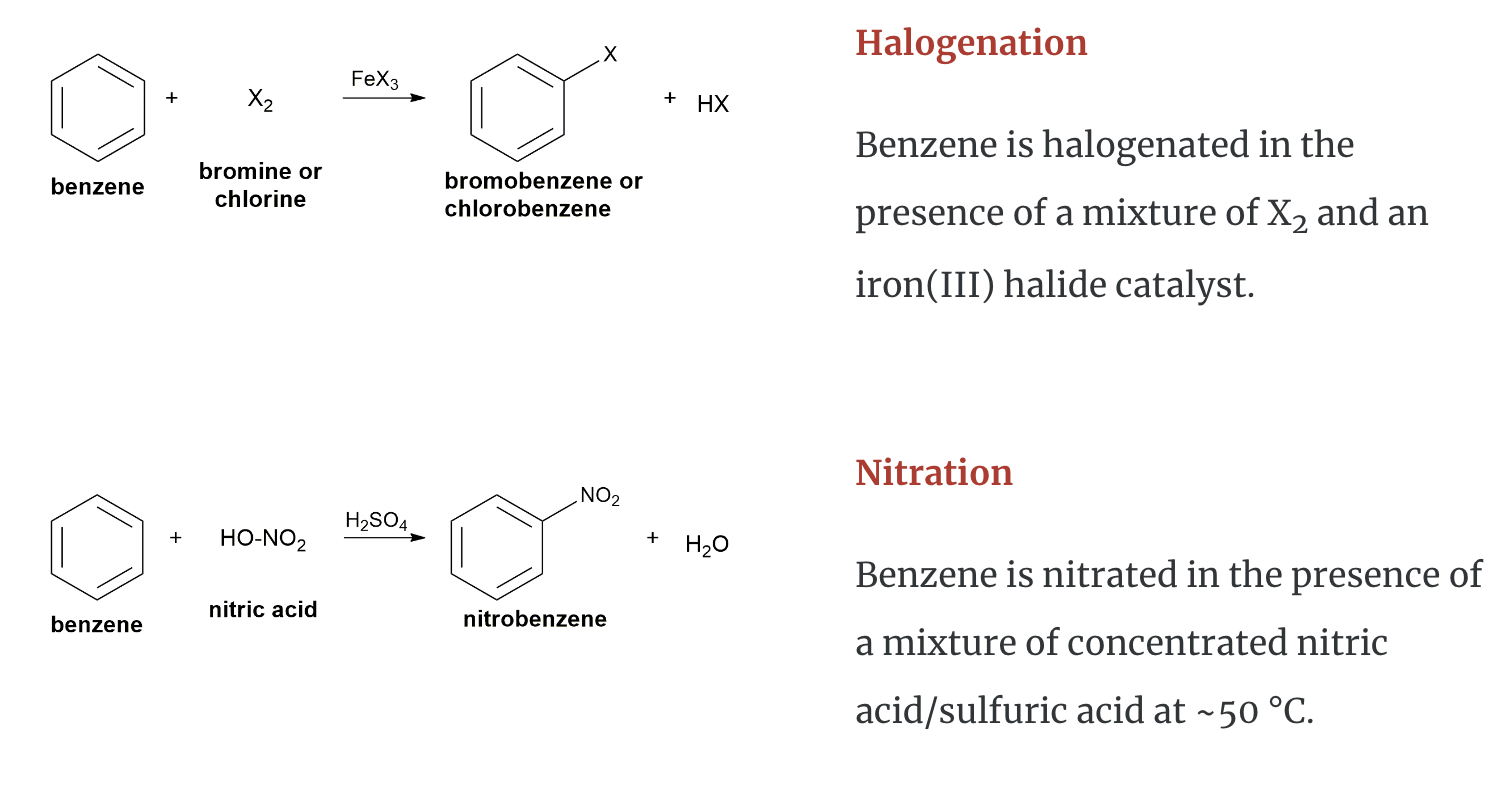
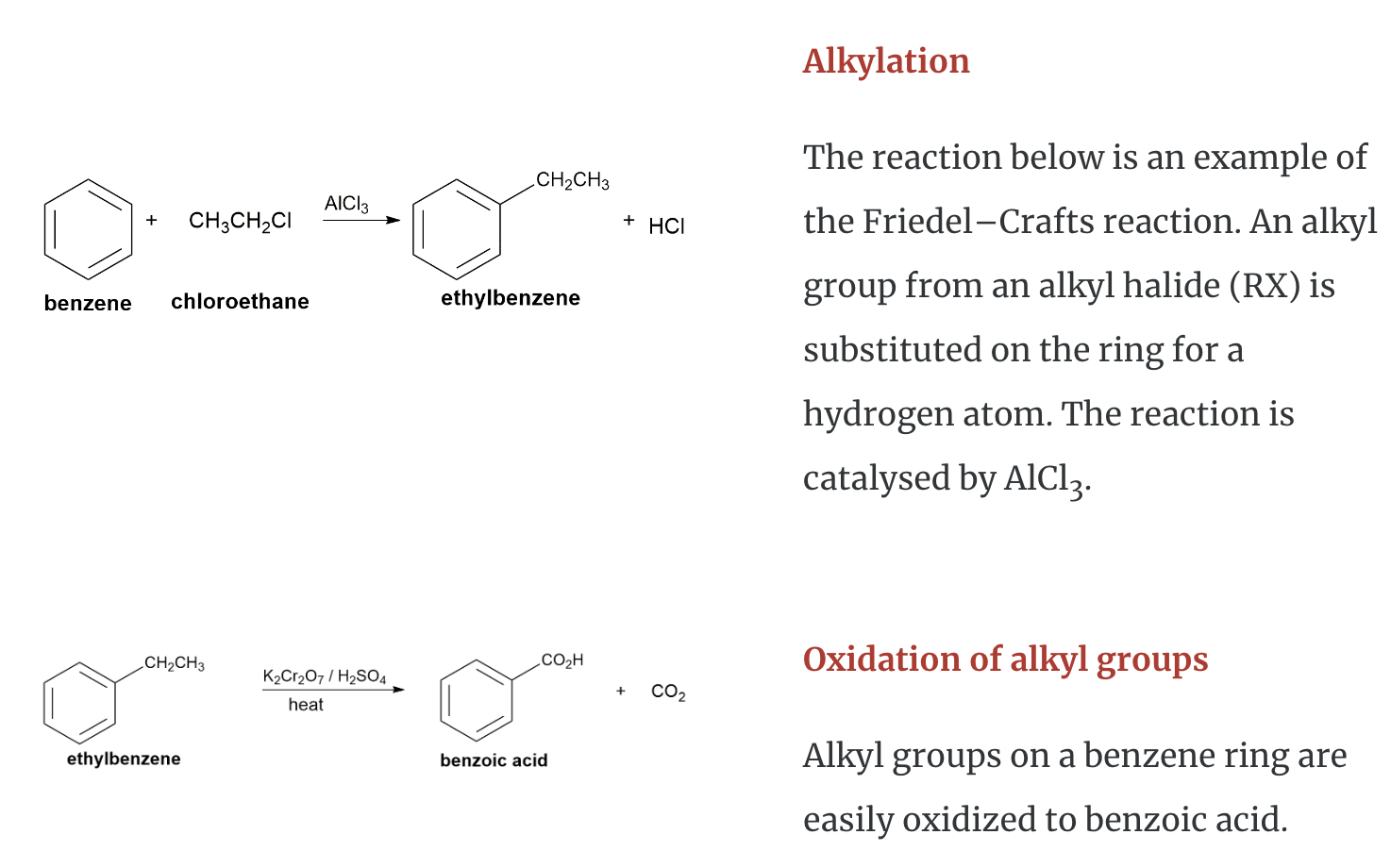
Aromatic hydrocarbons (to include benzene, toluene, napthalene etc.) are produced from petroleum. A limited amount can be prepared from coal tar. Coal tar is only a by–product of coke. Consequently, this method is no longer the principal source of aromatic hydrocarbons.
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| REACTION NOTES (0) | 2023.03.28 |
|---|---|
| Lab 3 Glucose Concentration in Drinks (0) | 2023.03.27 |
| [WEEK4] Aldehydes and Ketones (1) | 2023.03.21 |
| [WEEK3] Alcohols, phenols, ethers and thiols (0) | 2023.03.14 |
| [WEEK1] Saturated Hydrocarbons (0) | 2023.03.01 |



