Mini lecture
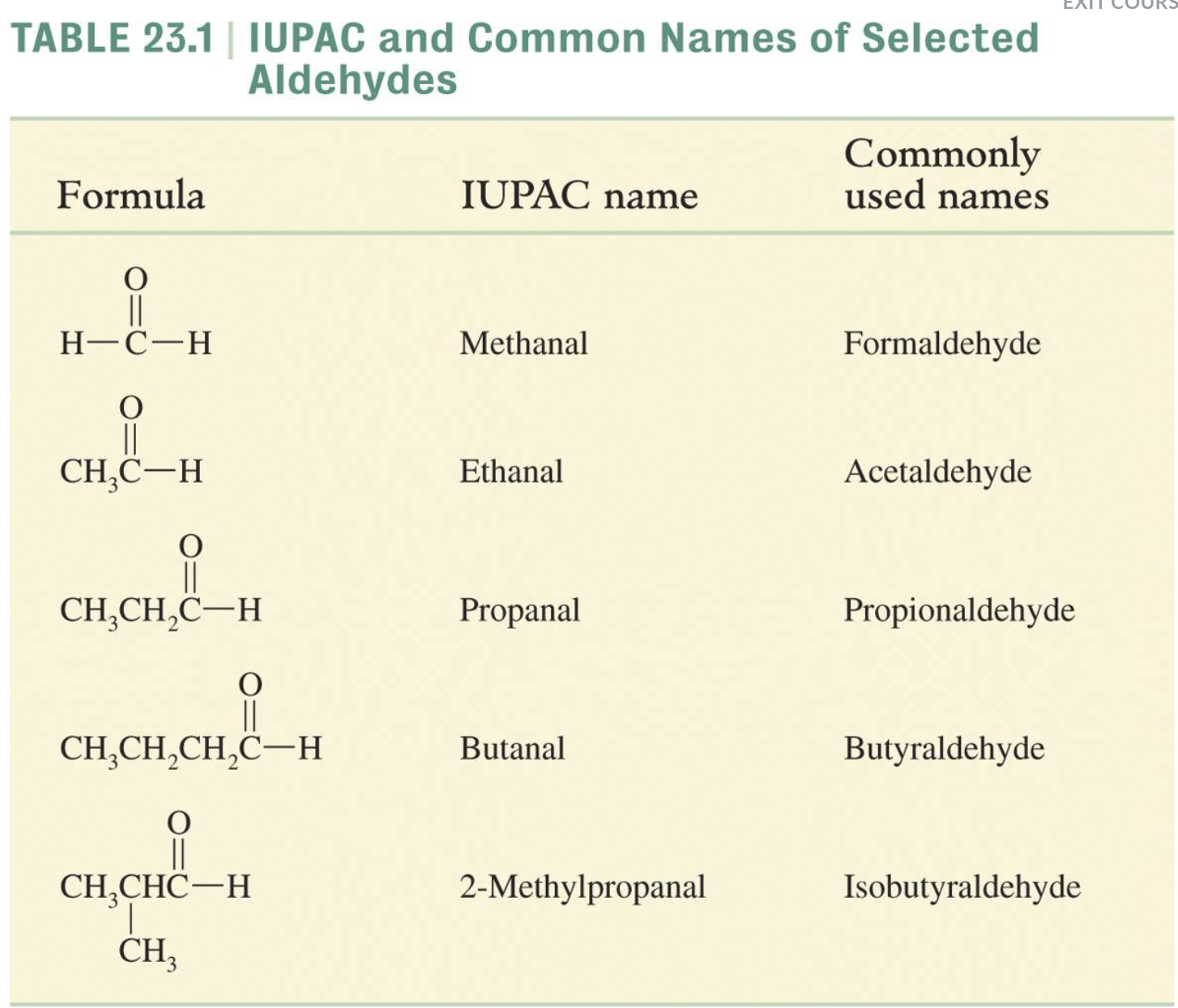
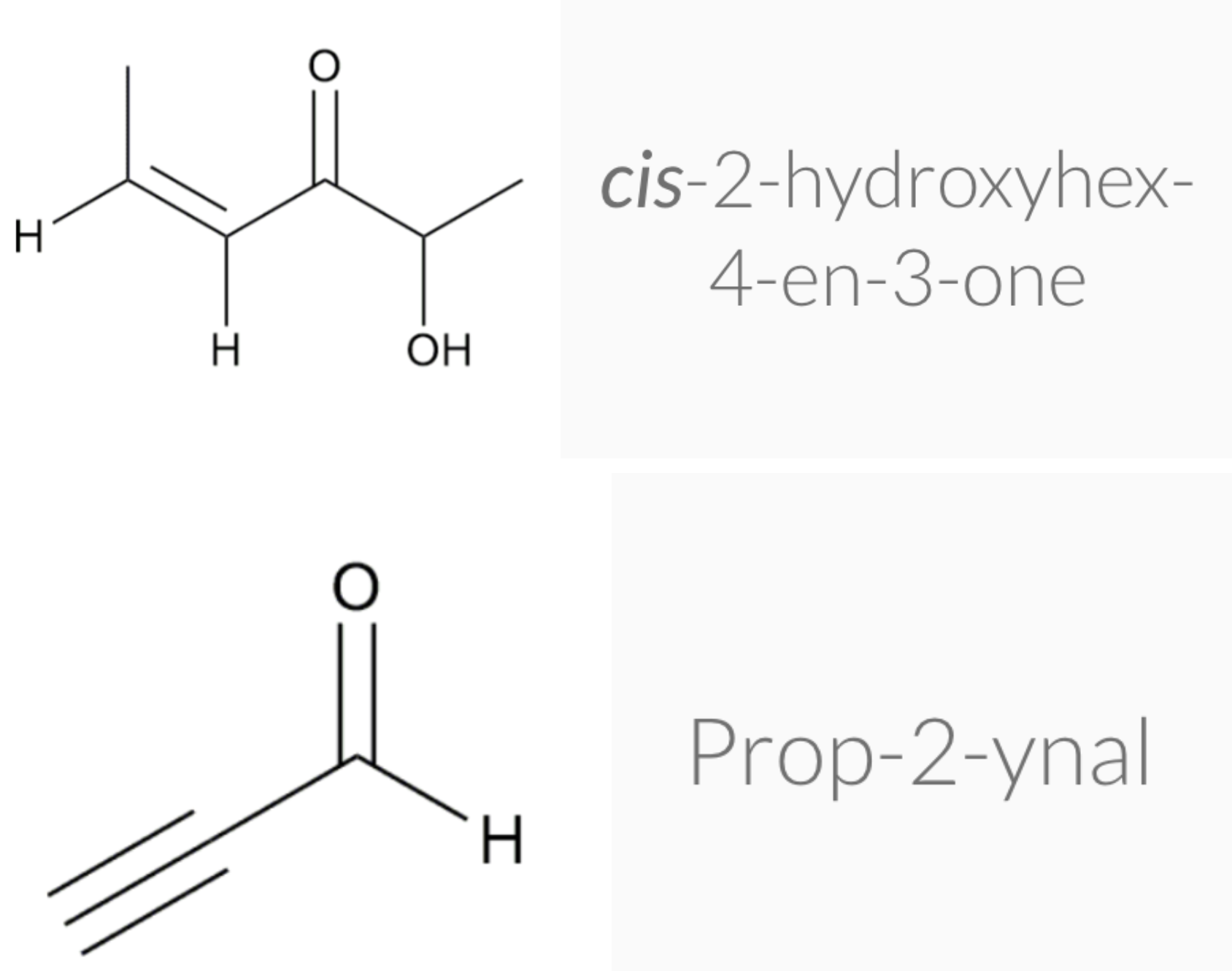
-Aldehydes -> nal
-Ketone -> one
-Aldehydes are more highly oxidised than ketones



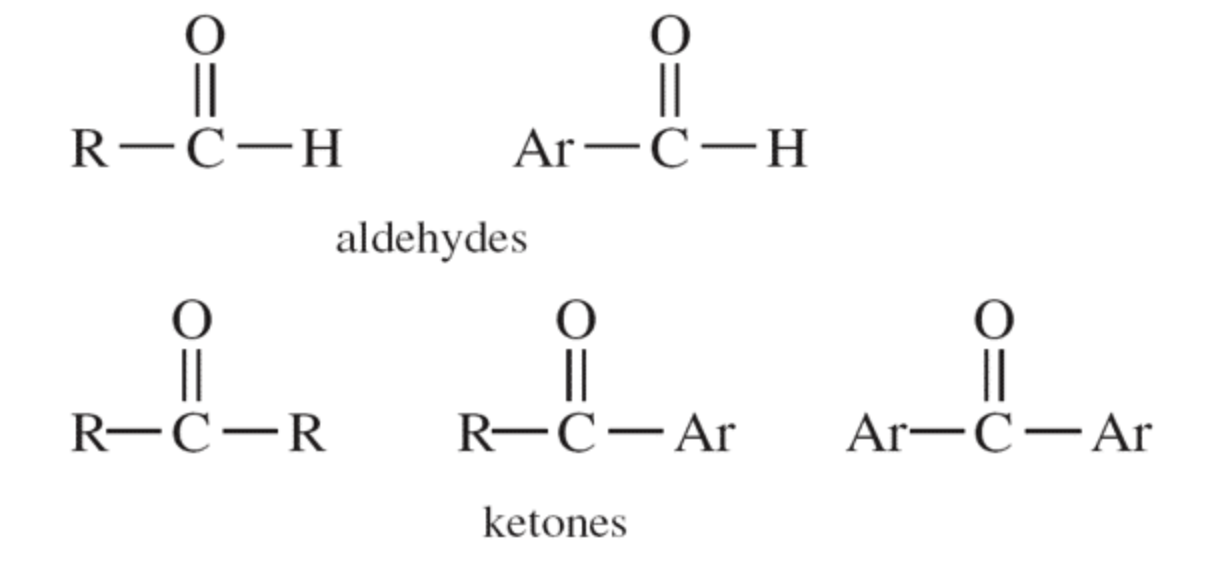
Structures of Aldehydes and Ketones
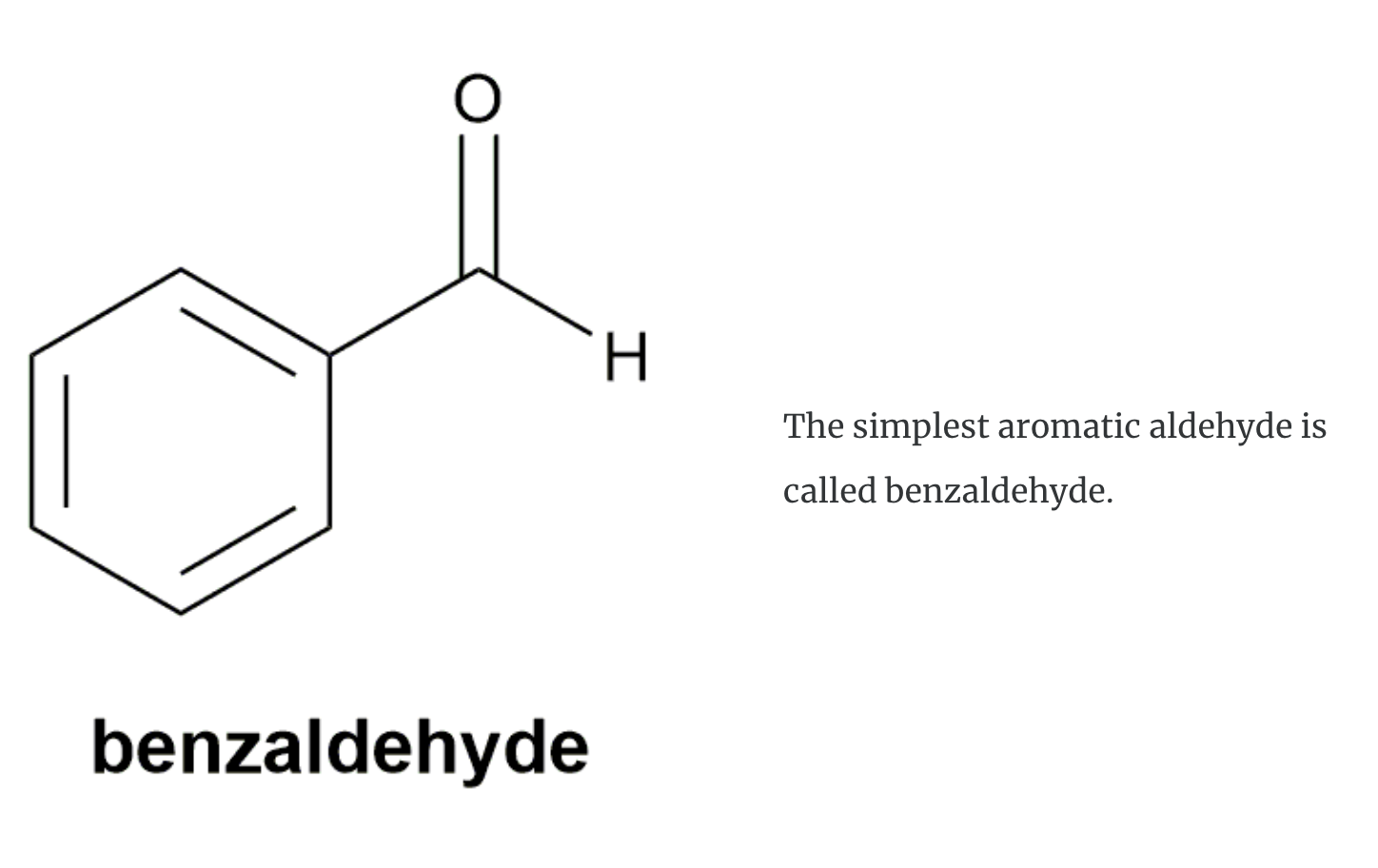
Aldehydes (i.e. RCHO) and ketones (i.e. R2CO) are compounds with a chemical structure that contains the carbonyl (C=O) functional group as shown in these structures (R– is an alkyl group and Ar– is an aromatic group).
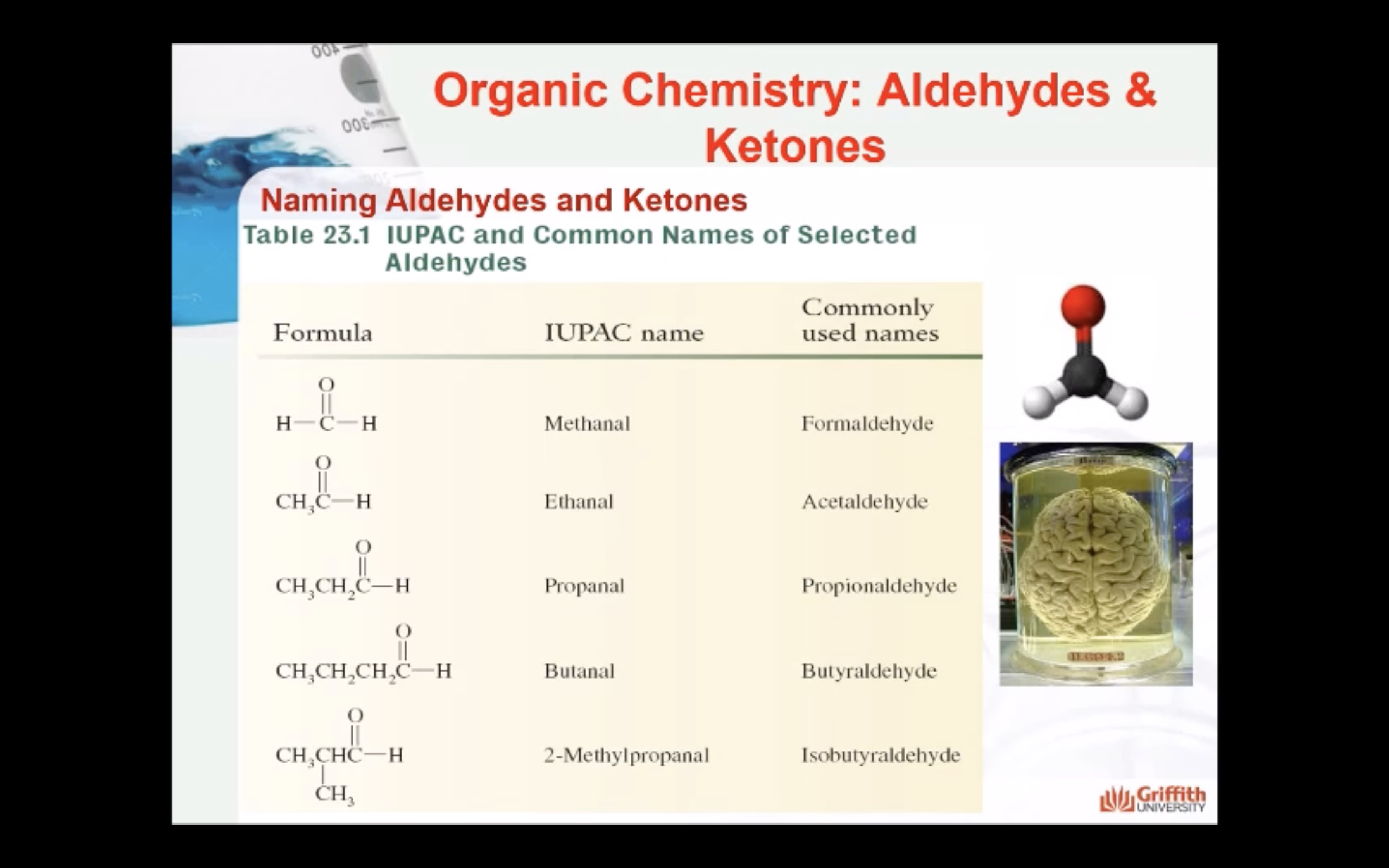
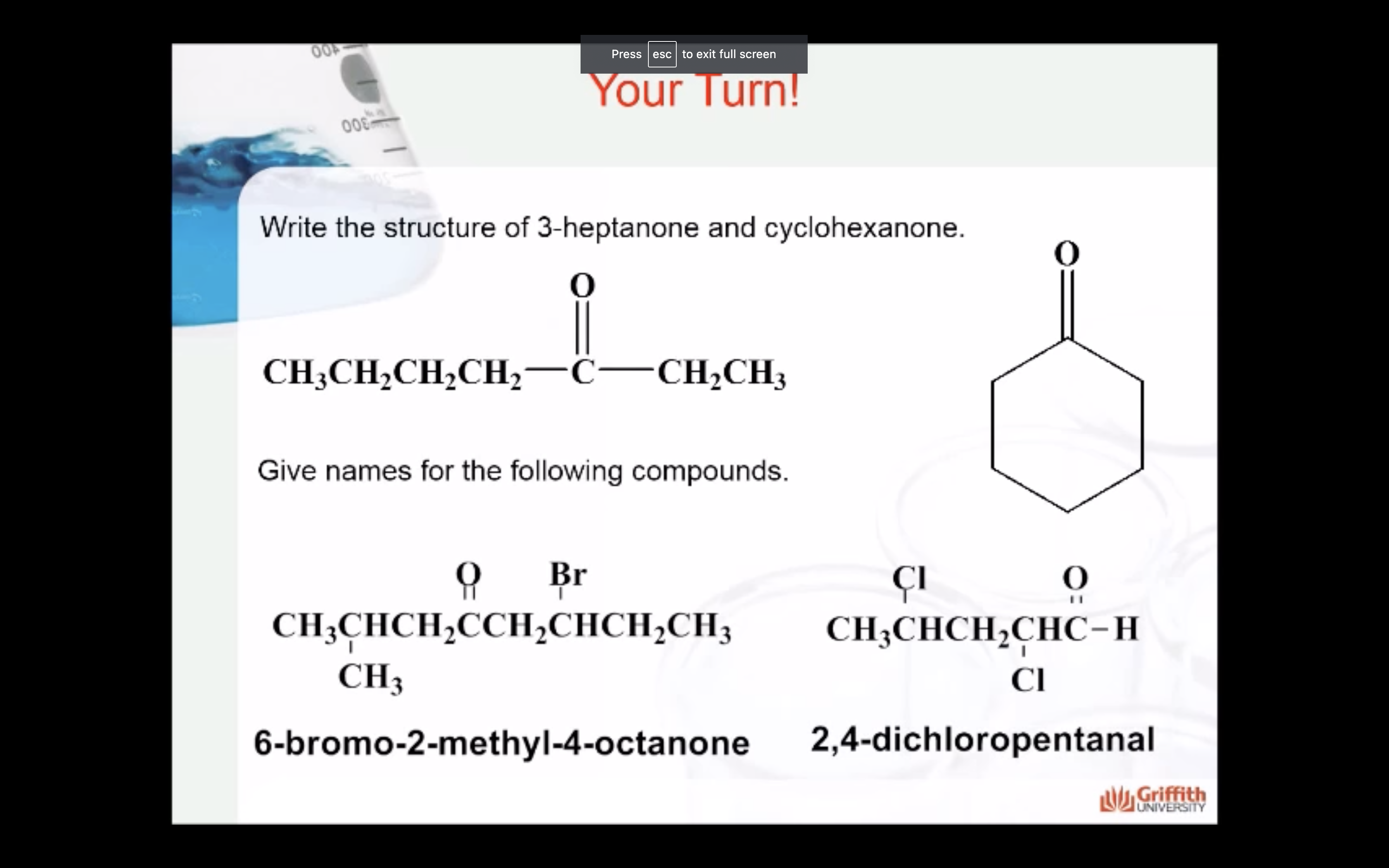
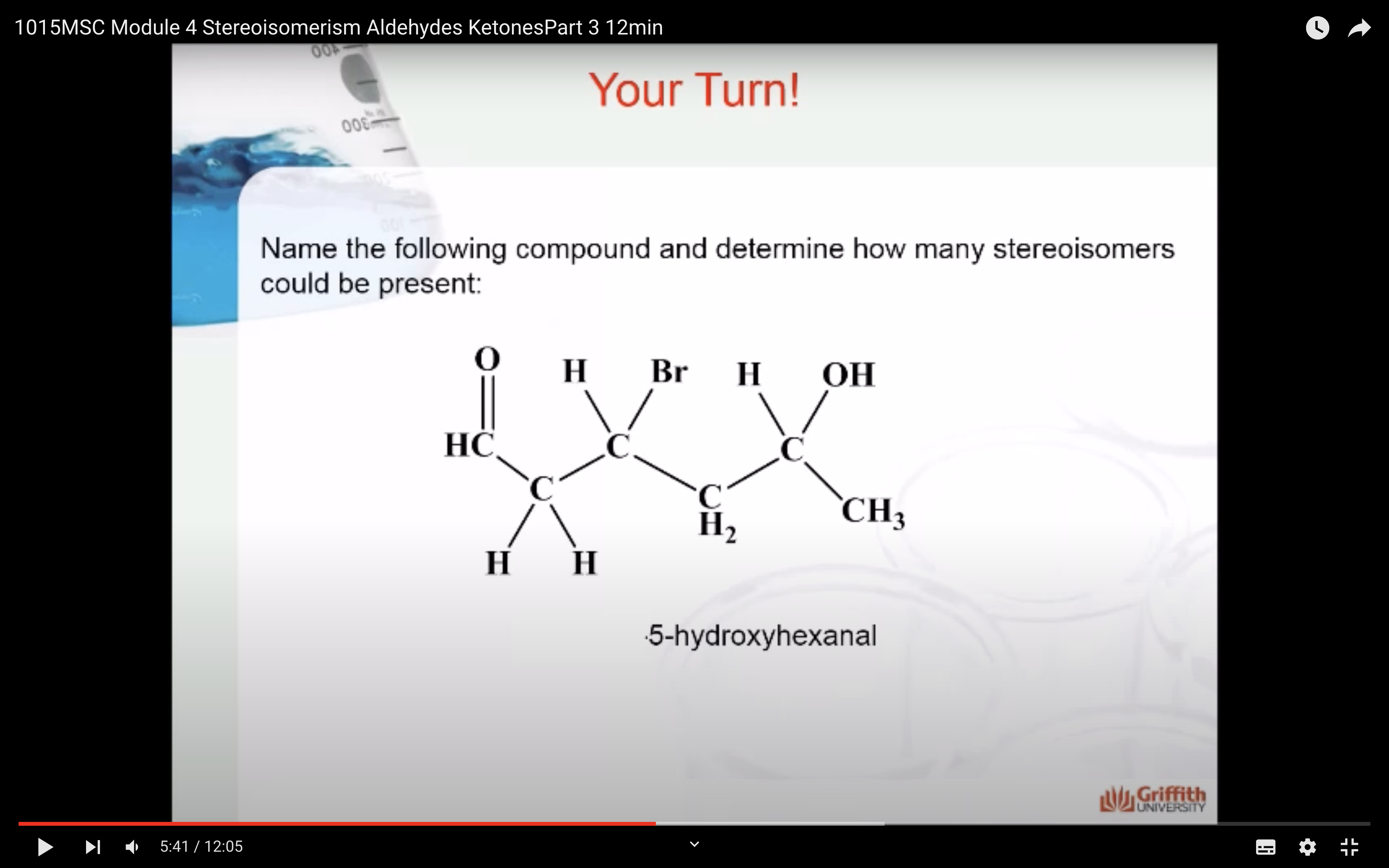
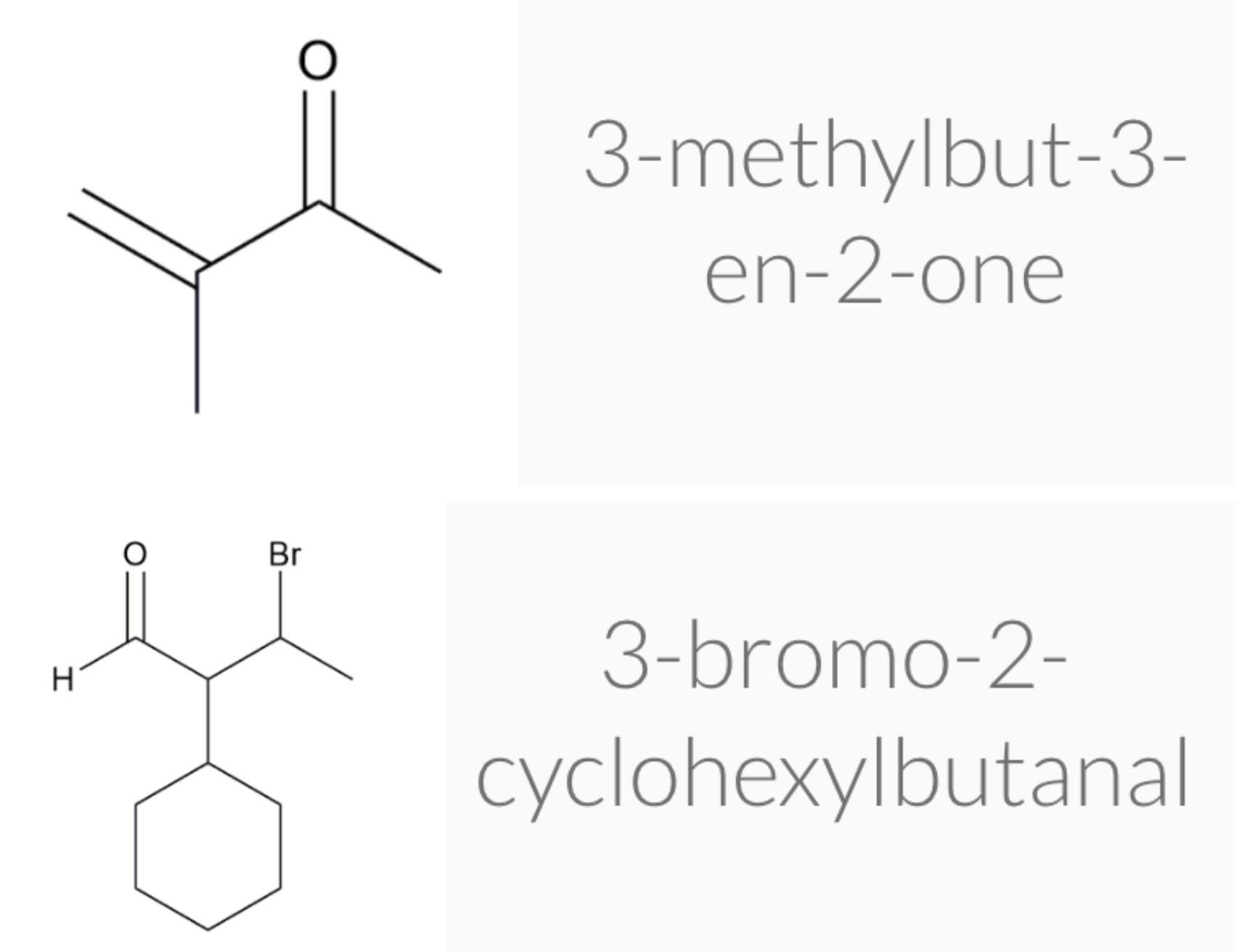
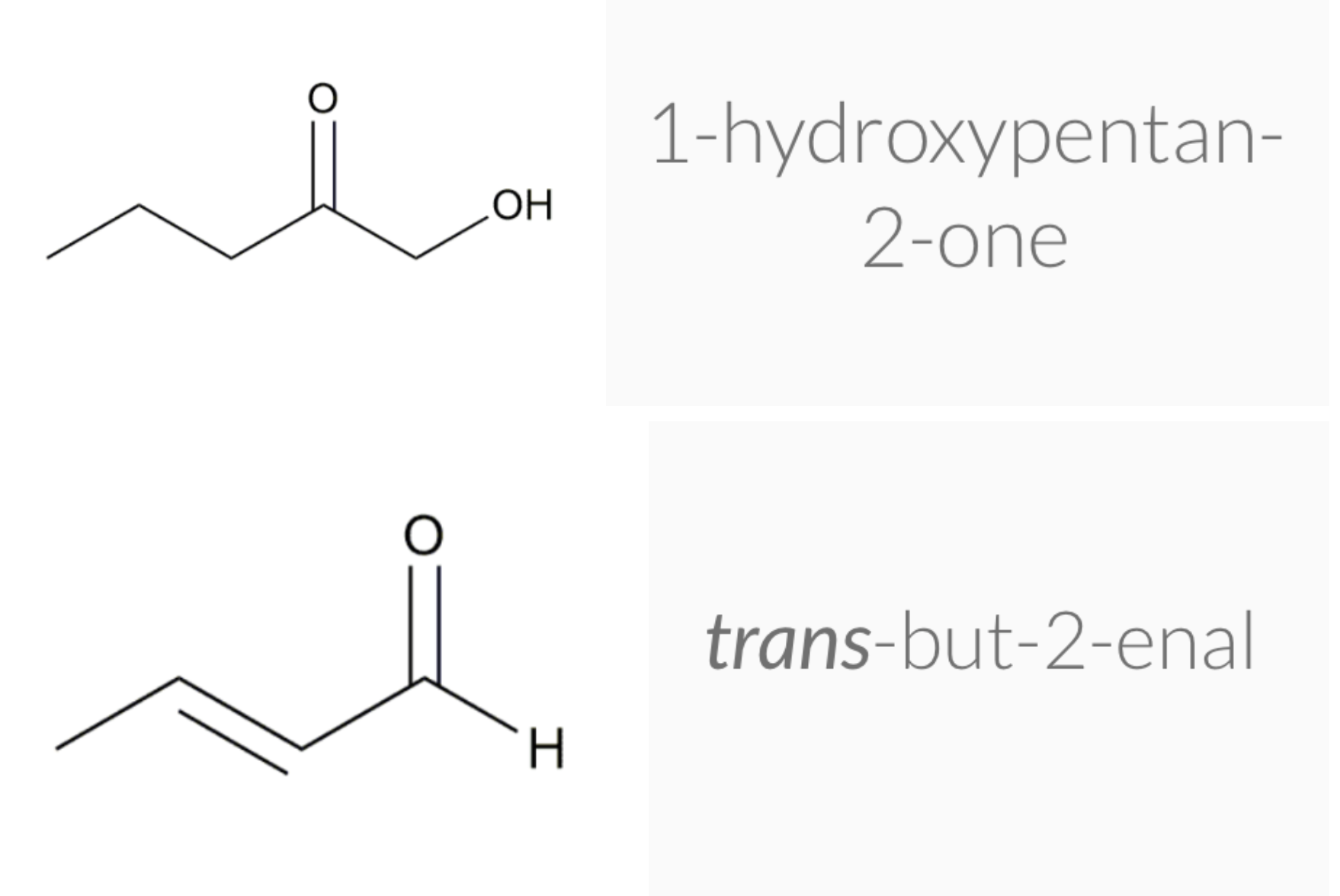
ALDEHYDE NAMING
- Name the longest continuous carbon chain containing the CHO group.
- The CHO carbon is the #1 carbon atom. Notice that the aldehyde group is not given a number in the name.
- Drop an –e from the corresponding alkane parent name and add the suffix –al.
- Number and name groups attached to the longest carbon chain.
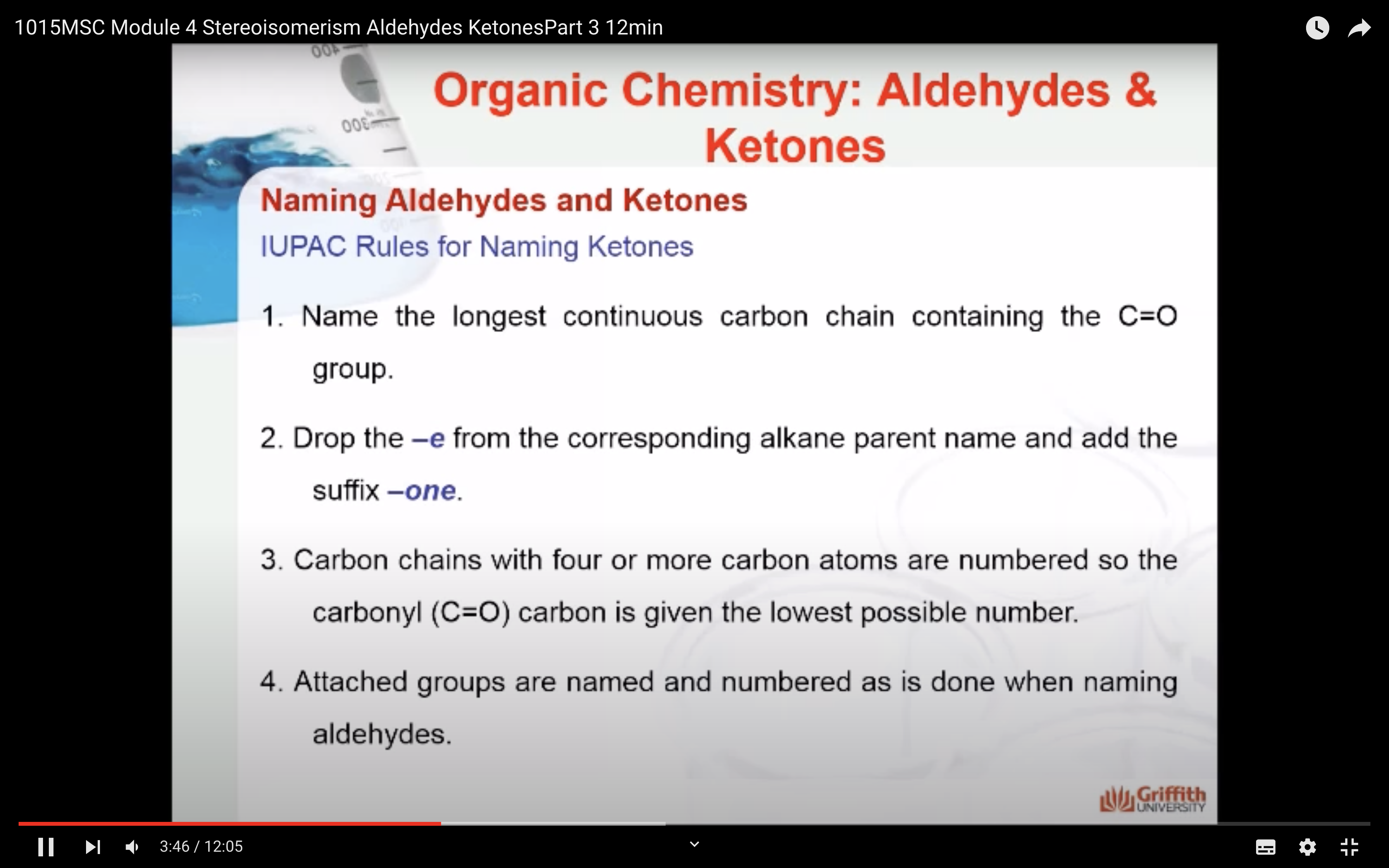
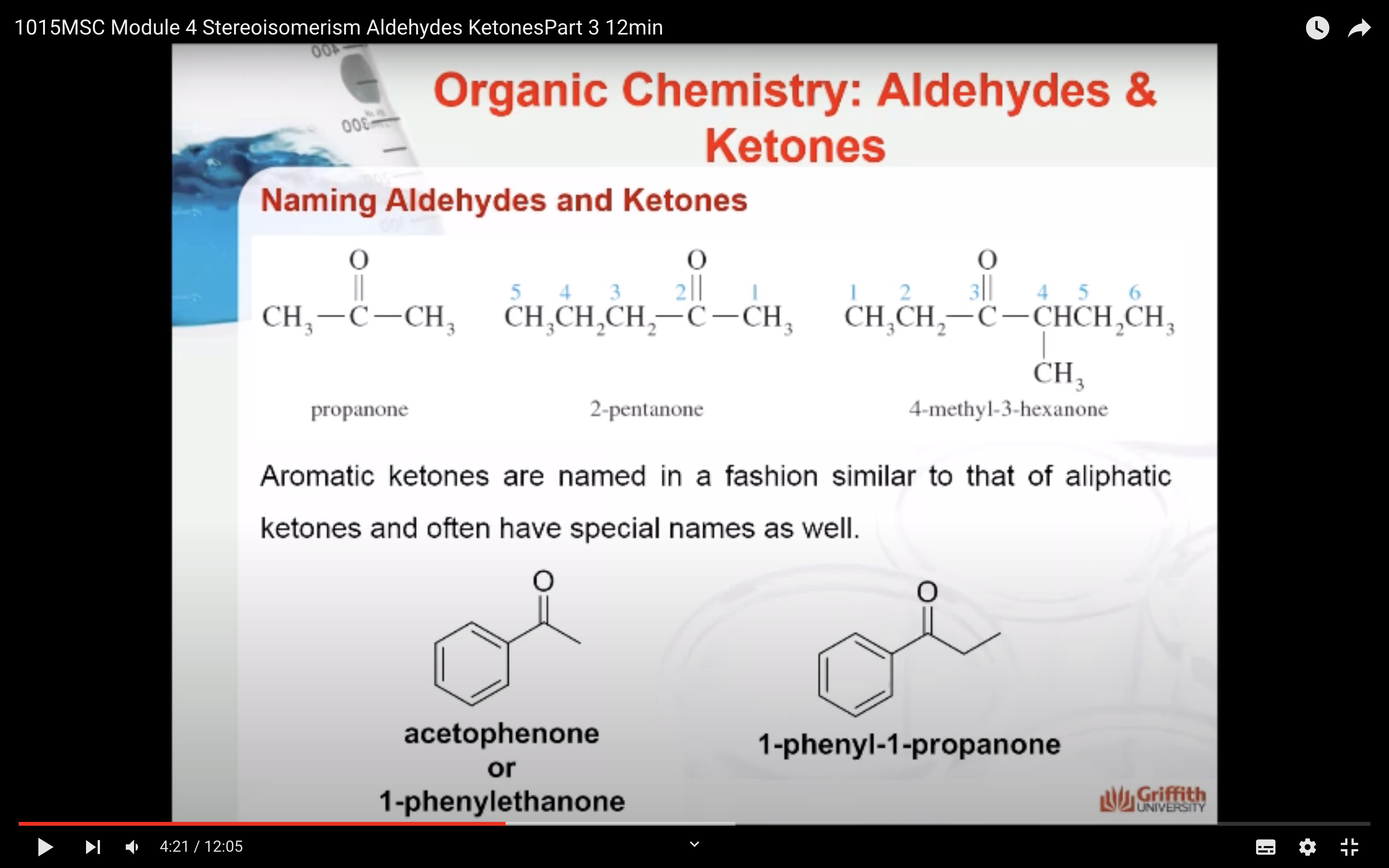
KETONE NAMING
- Name the longest continuous carbon chain containing the C=O group.
- Drop the –e from the corresponding alkane parent name and add the suffix –one.
- Carbon chains with four or more carbon atoms are numbered so the carbonyl (C=O) carbon is given the lowest possible number.
- Attached groups are named and numbered as is done when naming aldehydes.

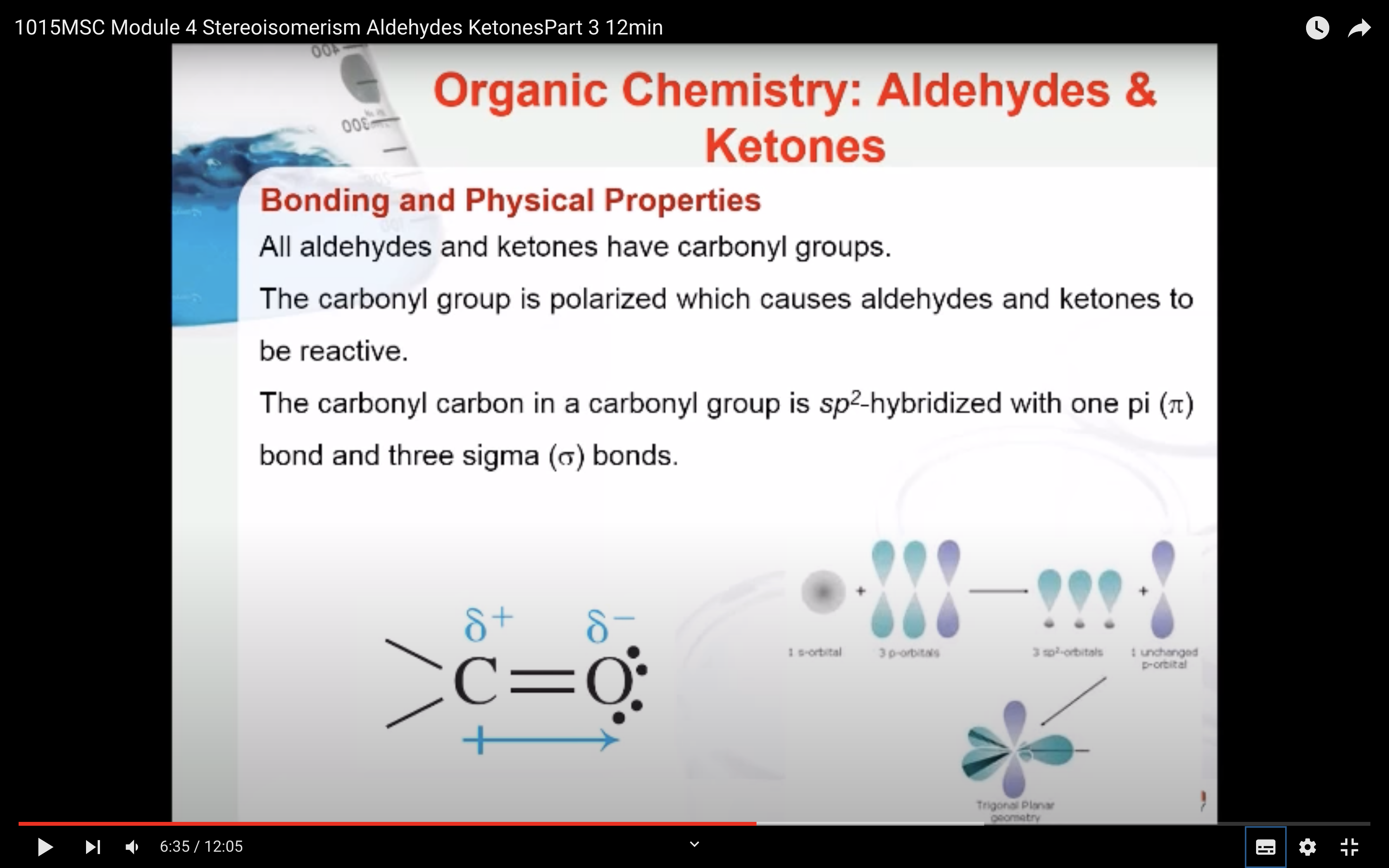
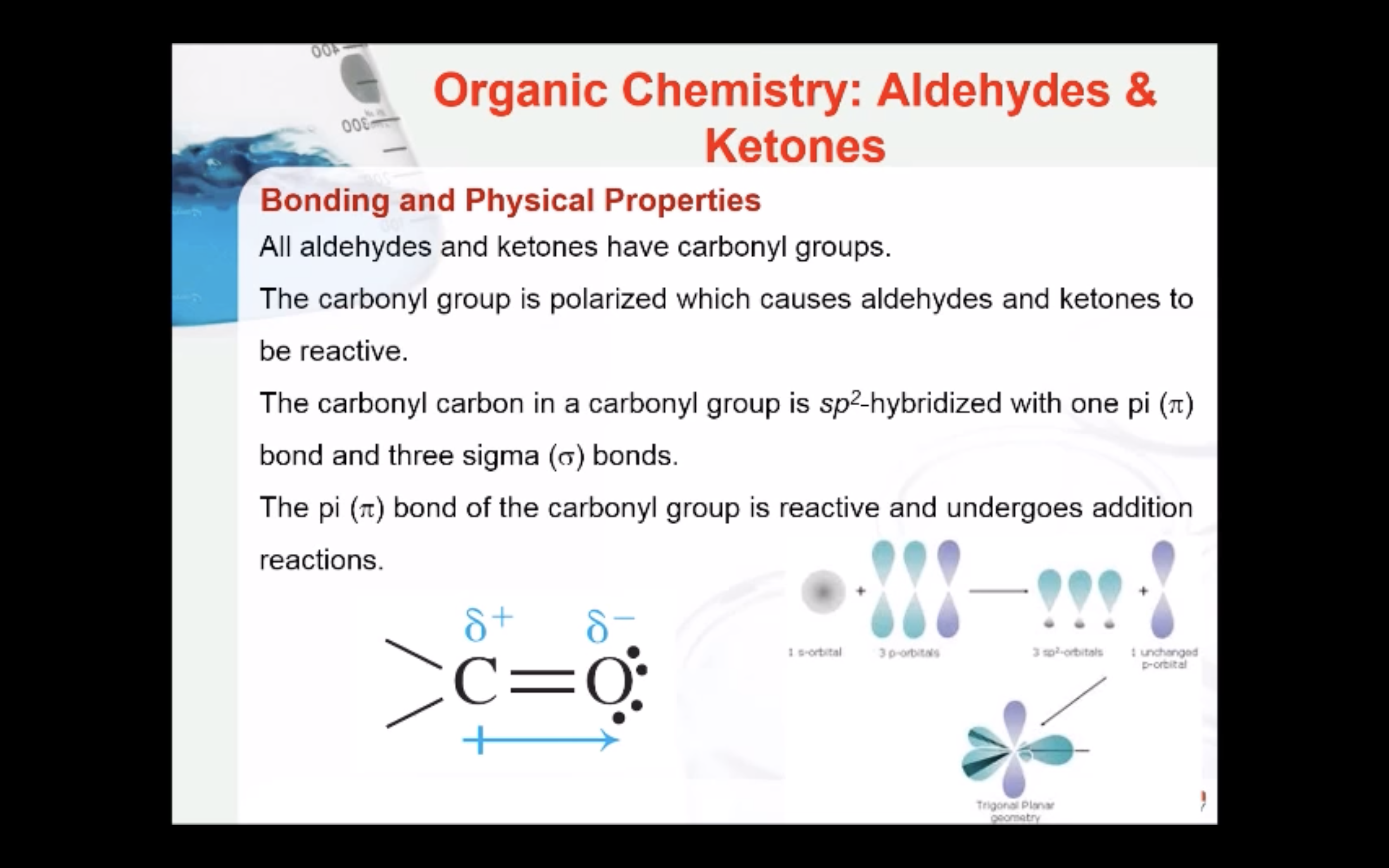
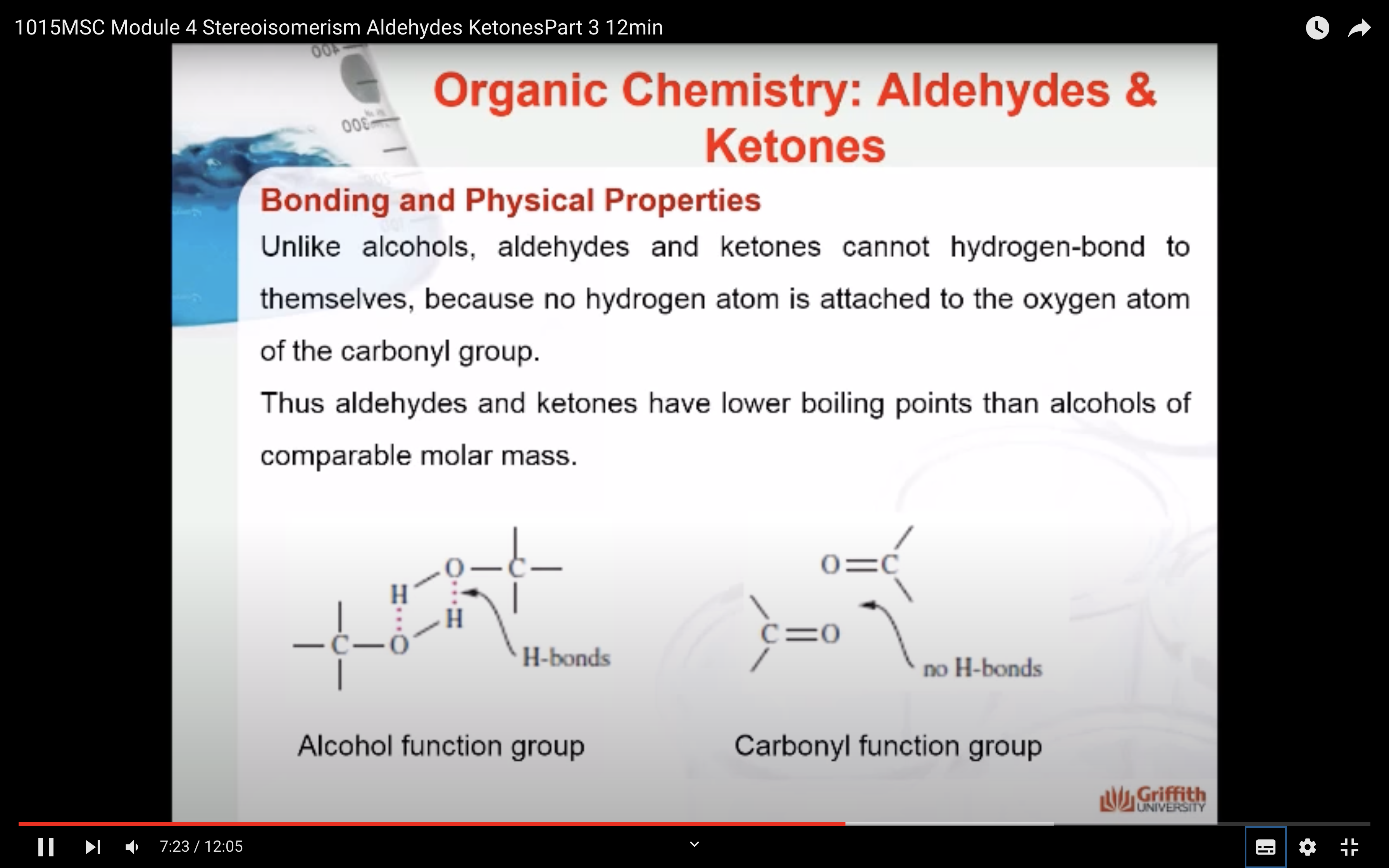
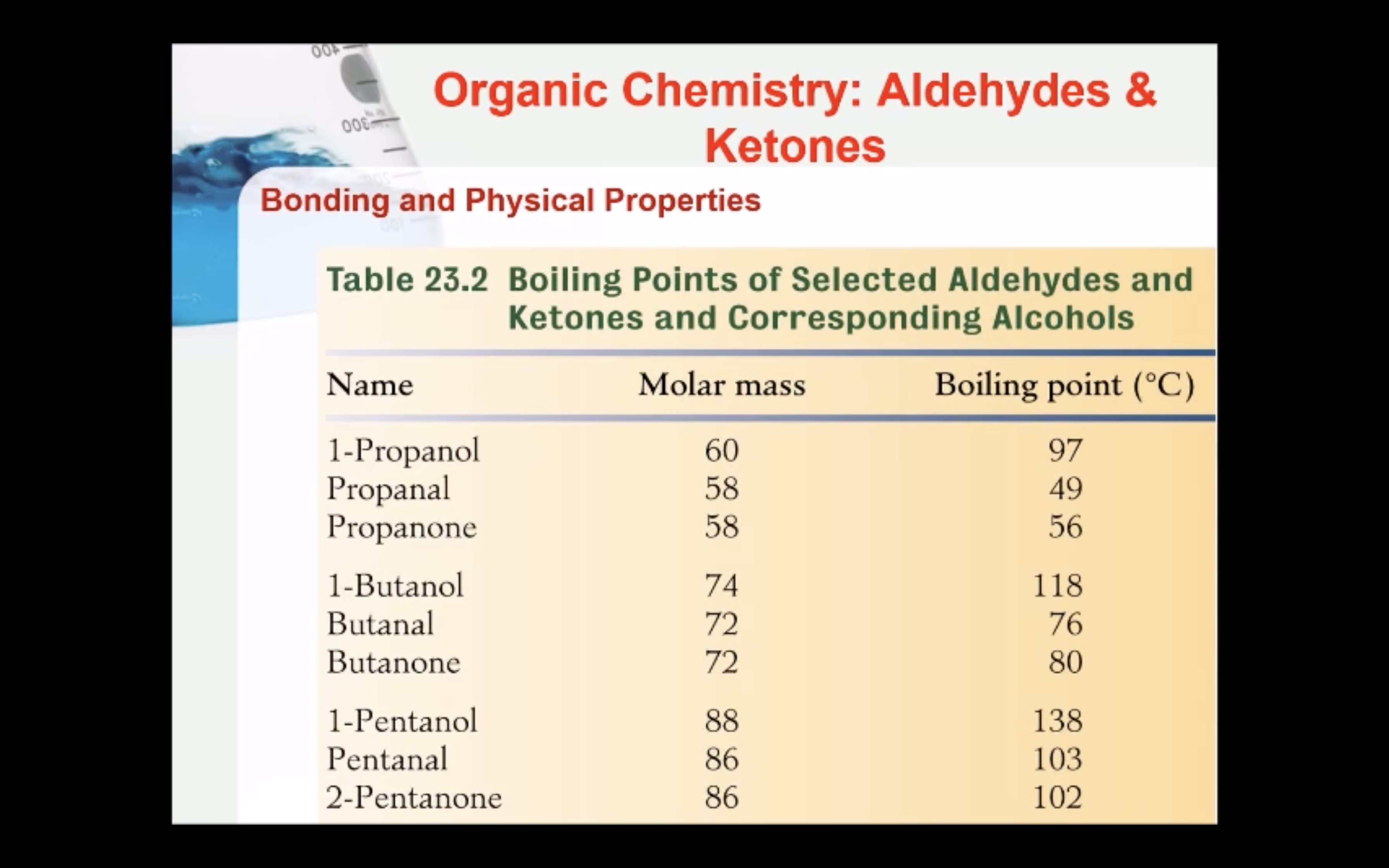
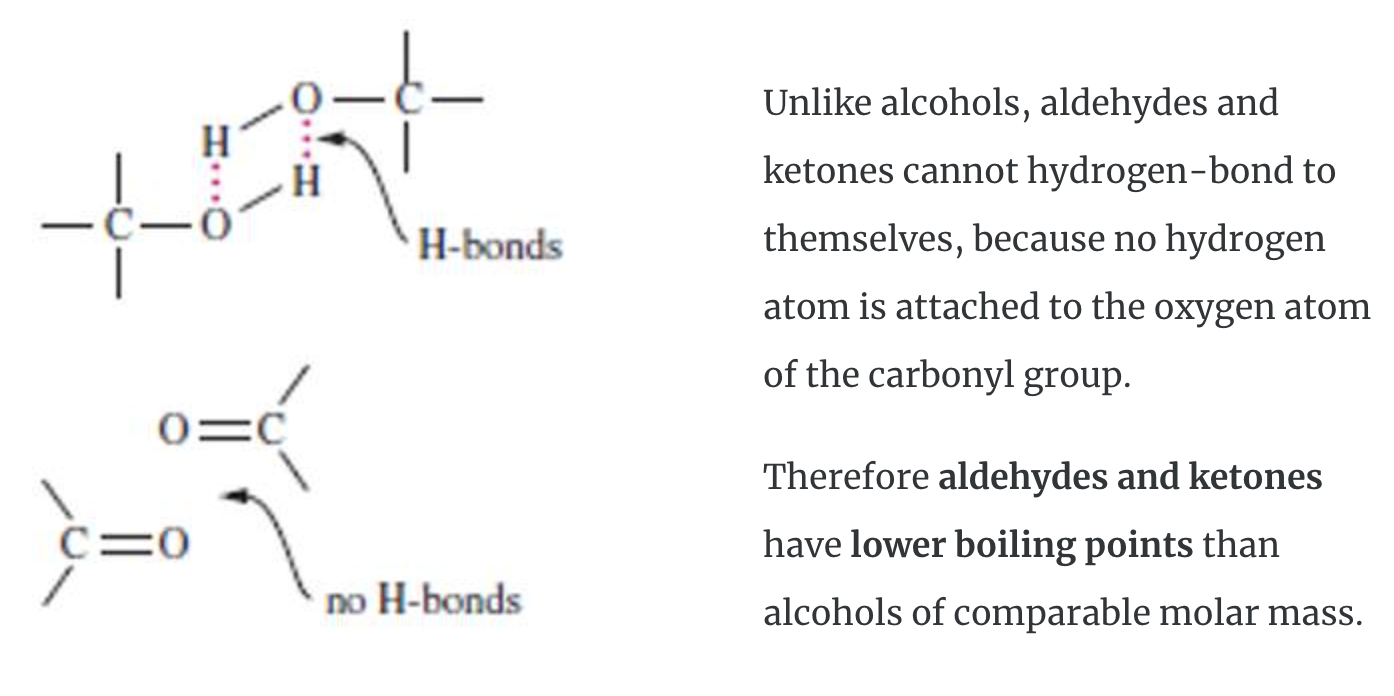
Bonding and Physical Properties
The carbonyl carbon in a carbonyl group is sp2-hybridized with one pi (π) bond and three sigma (σ) bonds. The pi (π) bond of the carbonyl group is reactive and undergoes addition reactions. The carbonyl group is polarized which causes aldehydes and ketones to be reactive.
(NOTE: There is an optional link at the end of this lesson if you need to refresh pi and sigma bonds).
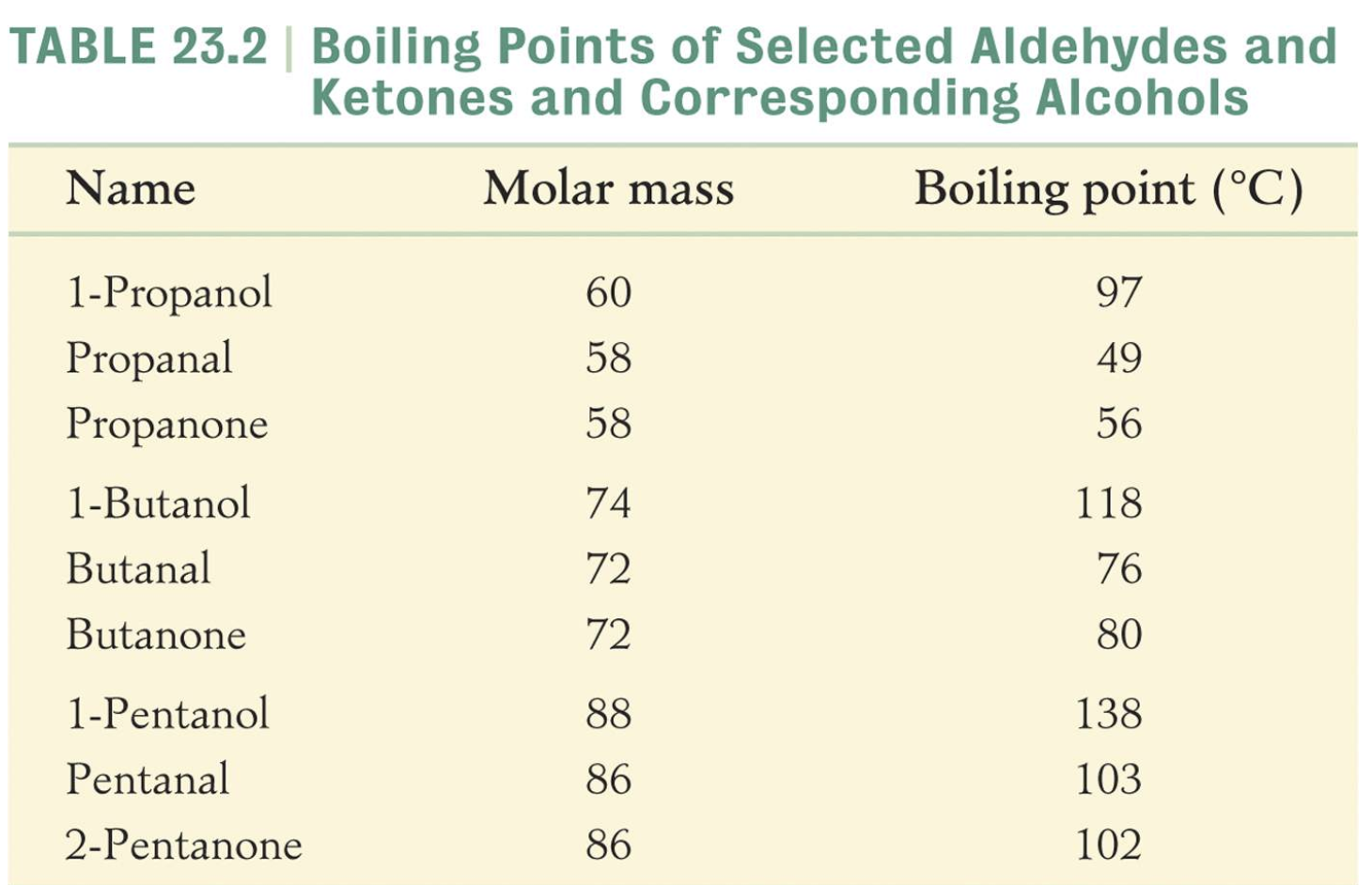

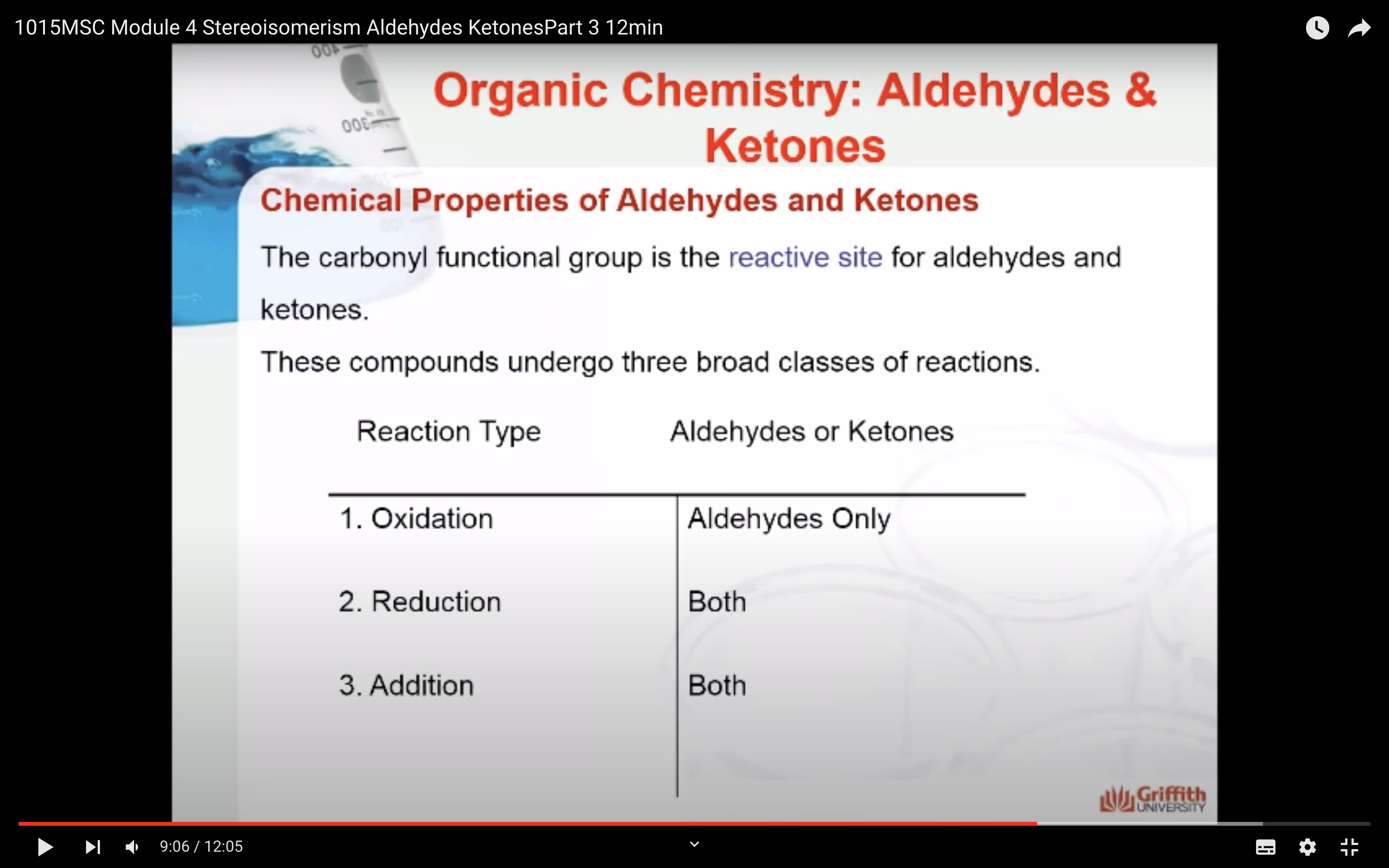
Chemical Properties of Aldehydes and Ketones
The carbonyl functional group is the reactive site for aldehydes and ketones. These compounds undergo three broad classes of reactions.
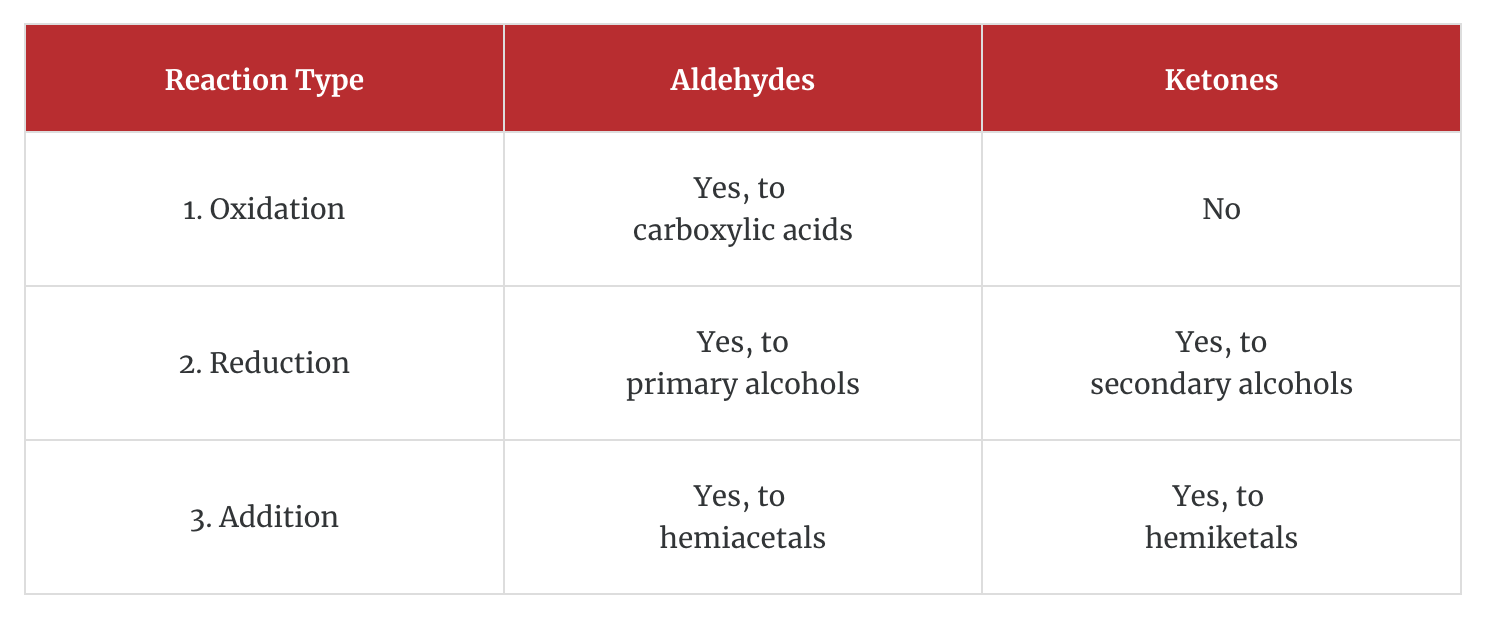
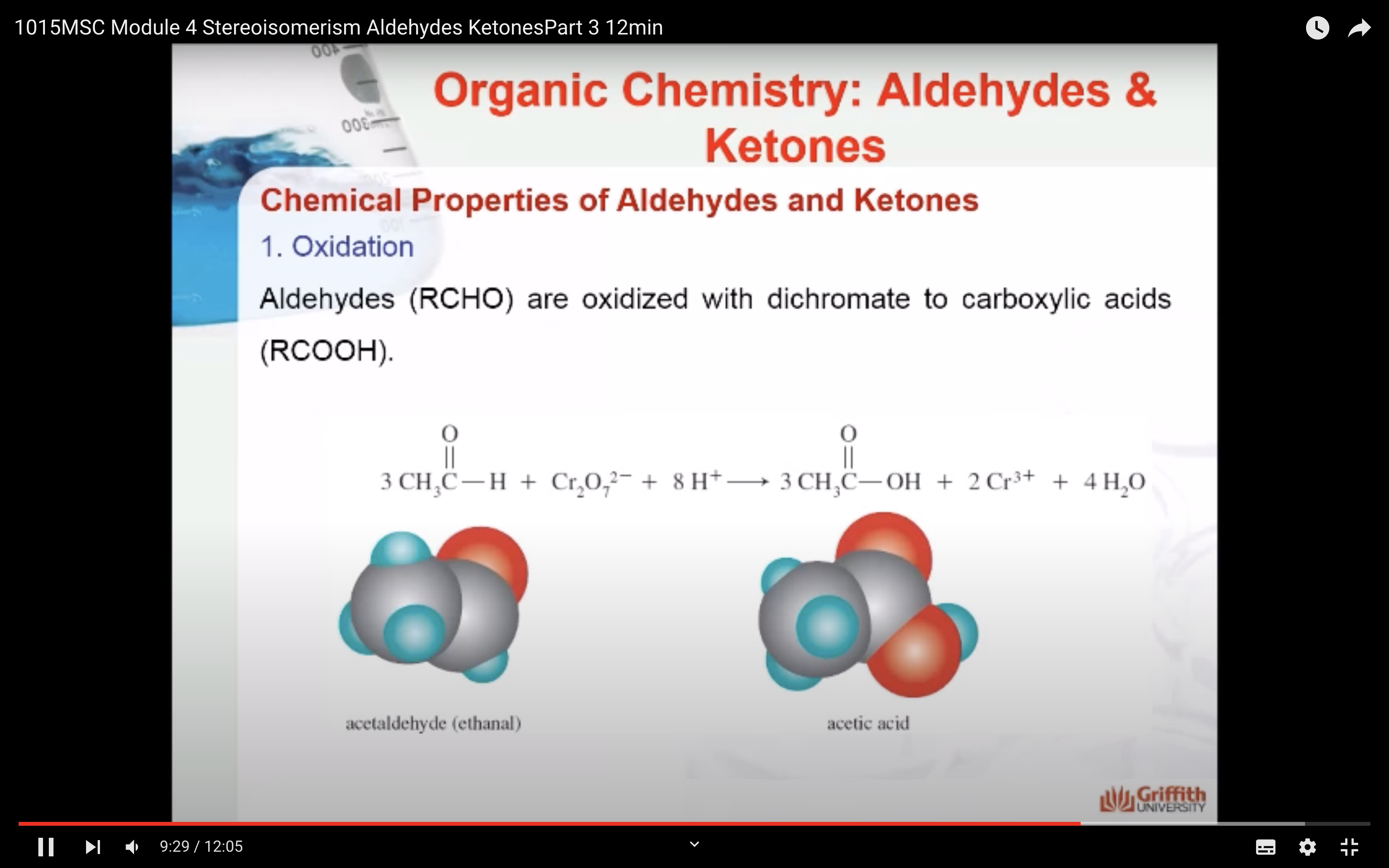
1. Oxidation
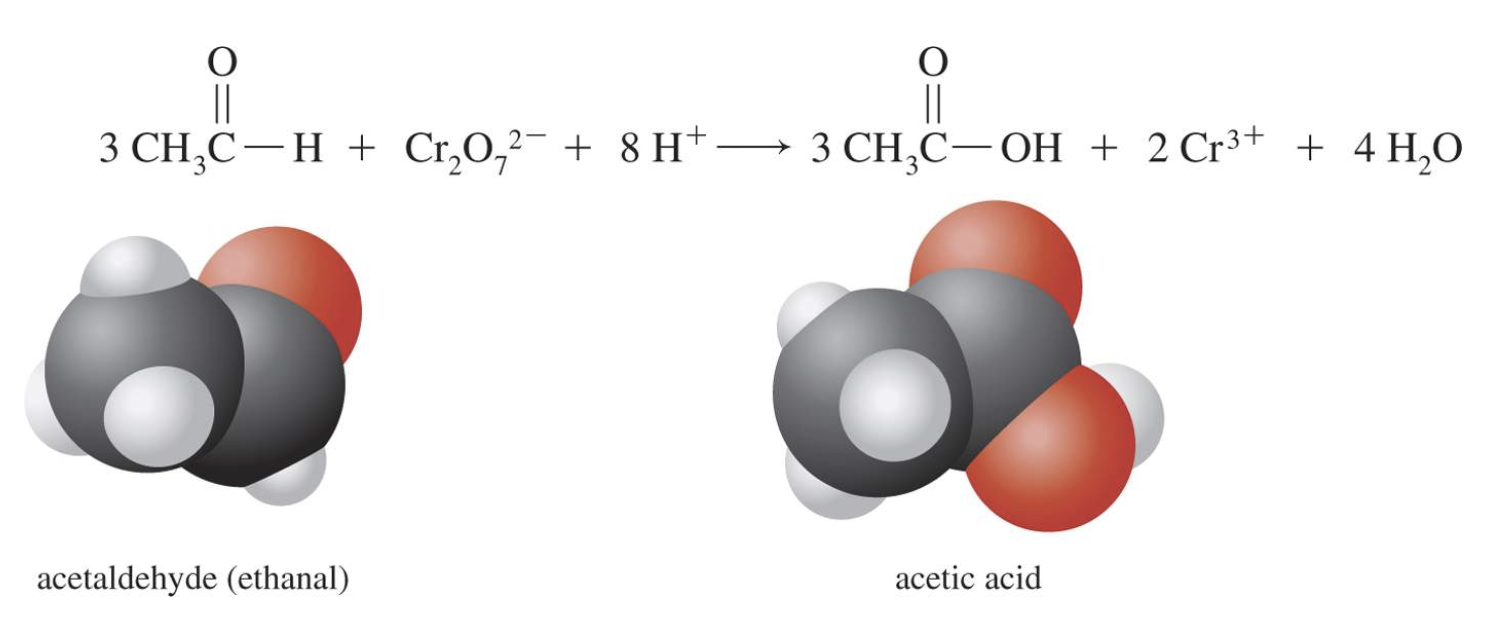
Aldehydes (RCHO) are oxidized with dichromate to carboxylic acids (RCOOH). Here ethanal is oxidized to acetic acid.
In this instance, the oxidizing agent is dichromate, and the reaction is performed under acidic conditions. There are many different oxidizing agents that could be used to convert an aldehyde to a carboxylic acid. You don't need to balance this using redox half reactions. The important aspect is what happens to the organic compound.
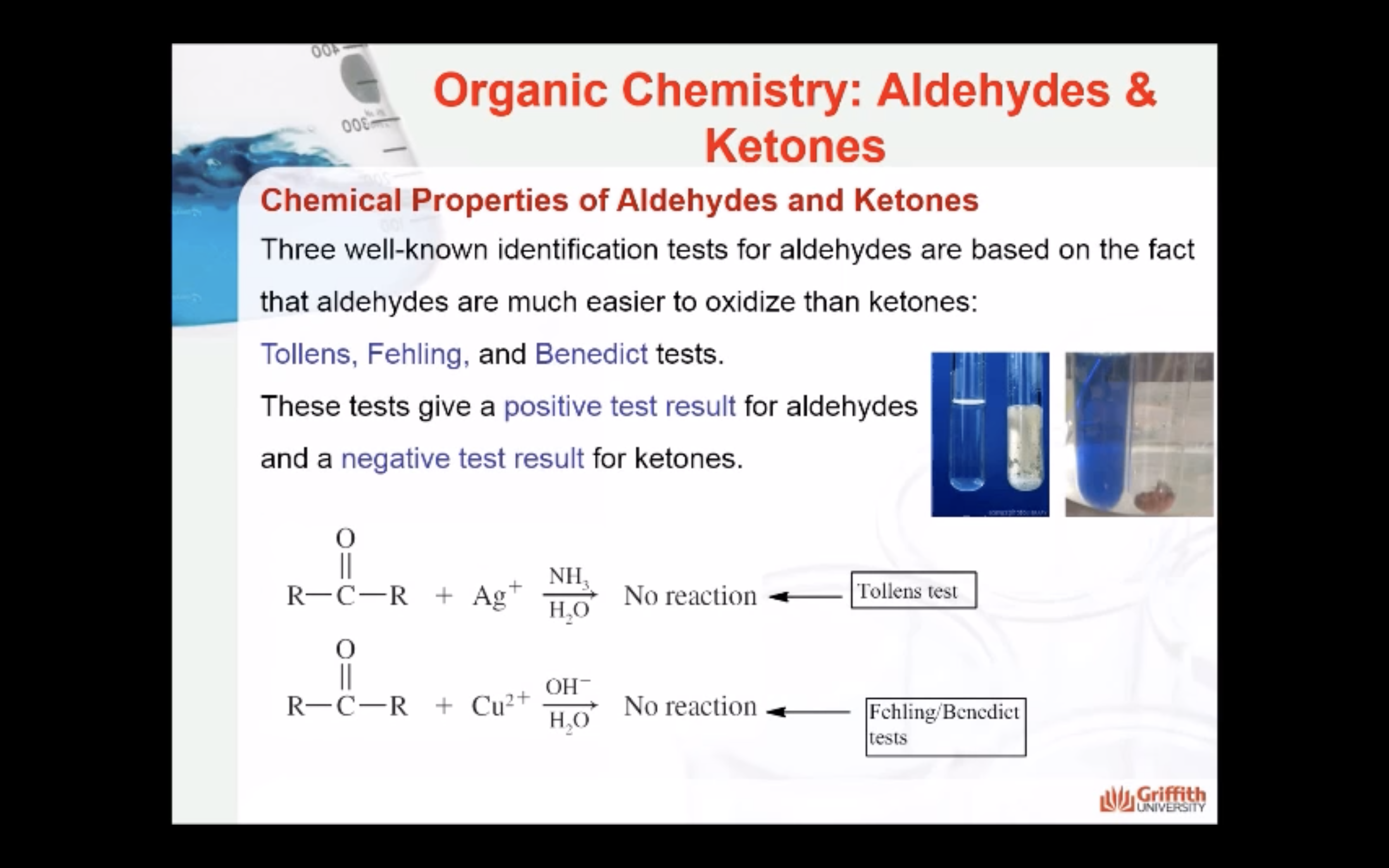
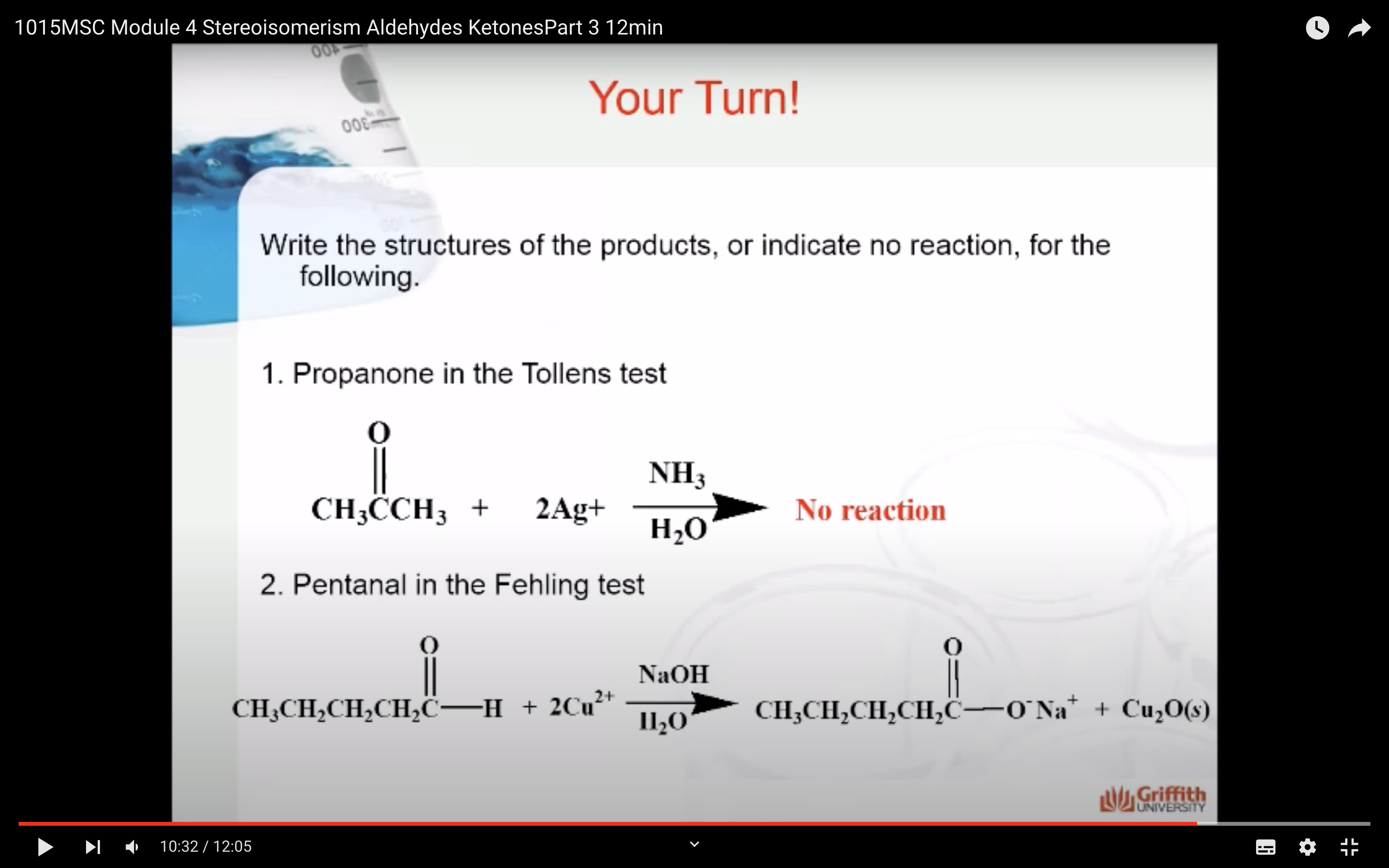
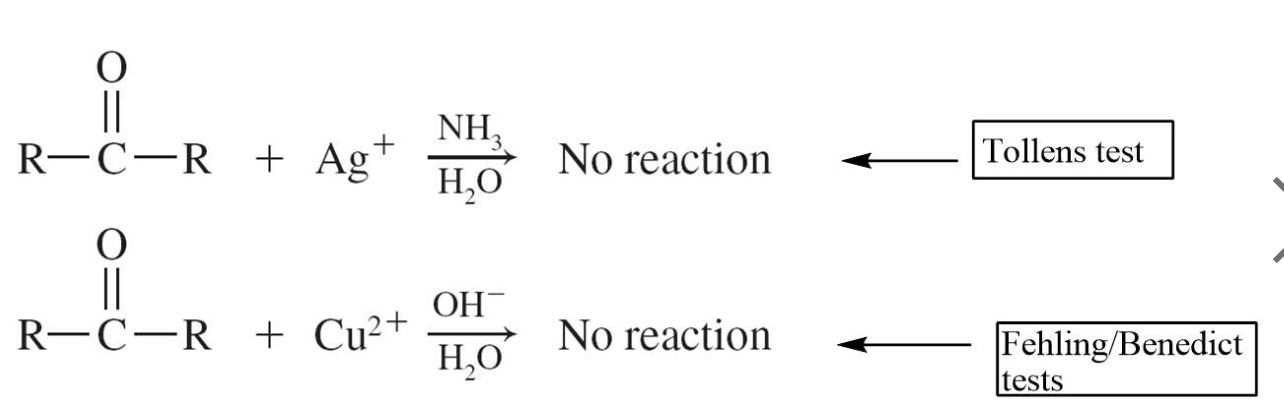
Three well-known identification tests for aldehydes are based on the fact that aldehydes are much easier to oxidize than ketones. These are the Tollens, Fehling, and Benedict tests.
These tests give a positive test result for aldehydes and a negative test result for ketones.




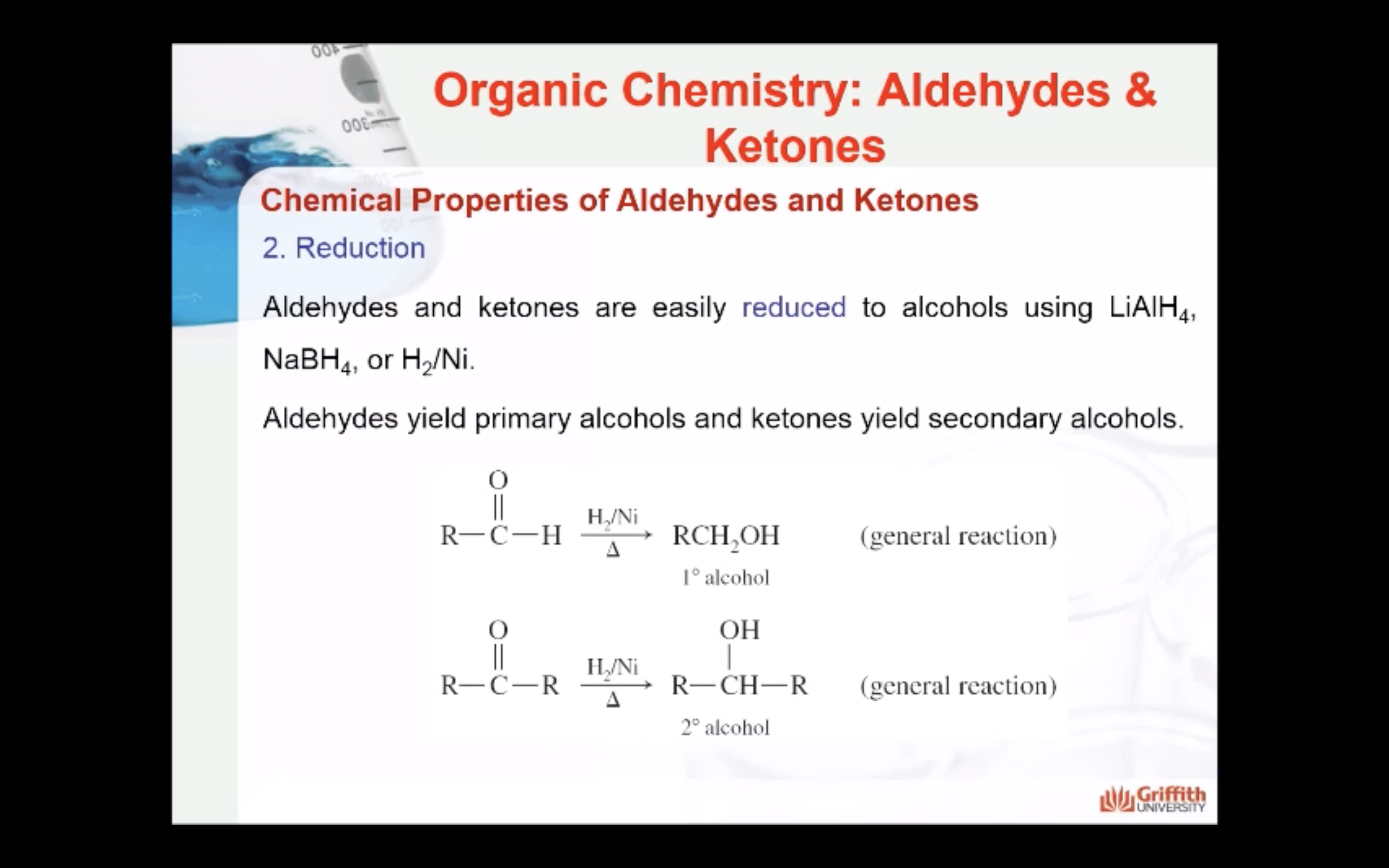
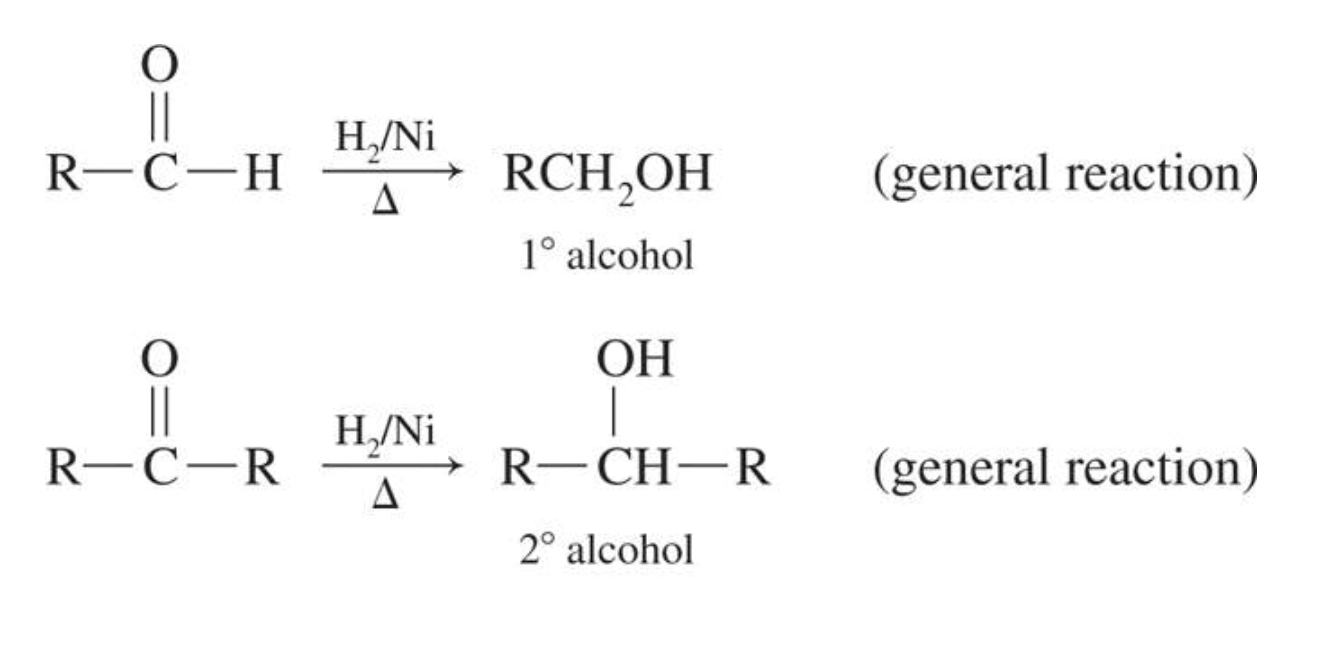
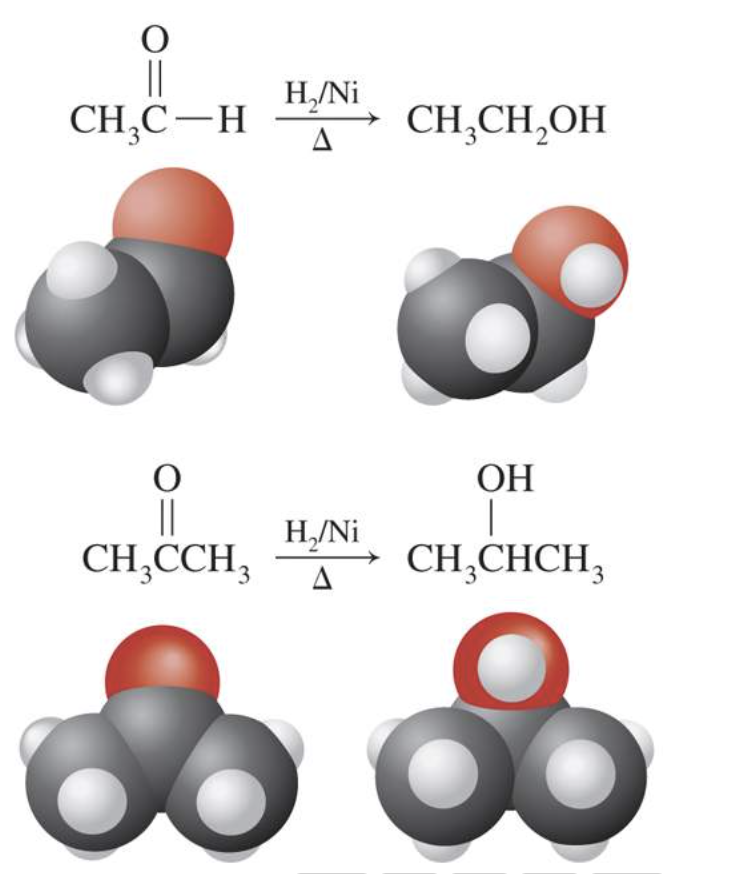
2. Reduction
Aldehydes and ketones are easily reduced to alcohols using reducing agents such as LiAlH4, NaBH4, or H2/Ni. Aldehydes yield primary alcohols and ketones yield secondary alcohols.
Have a look through these reactions below to see the difference.
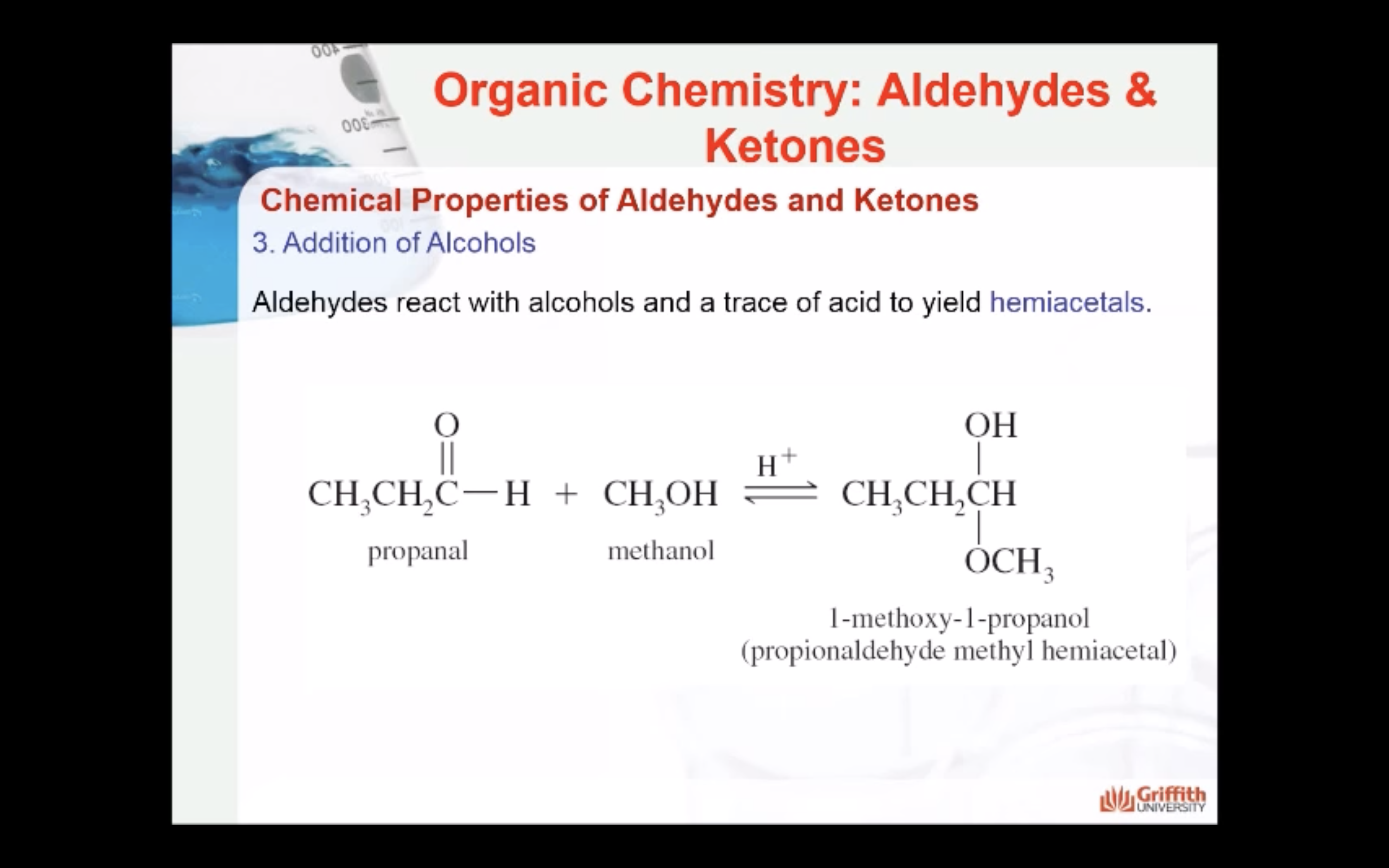
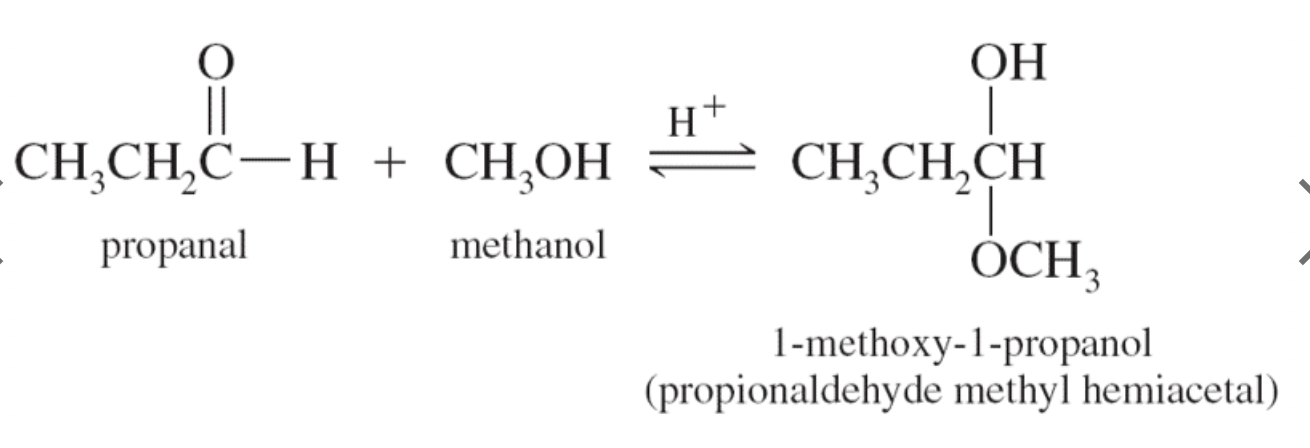
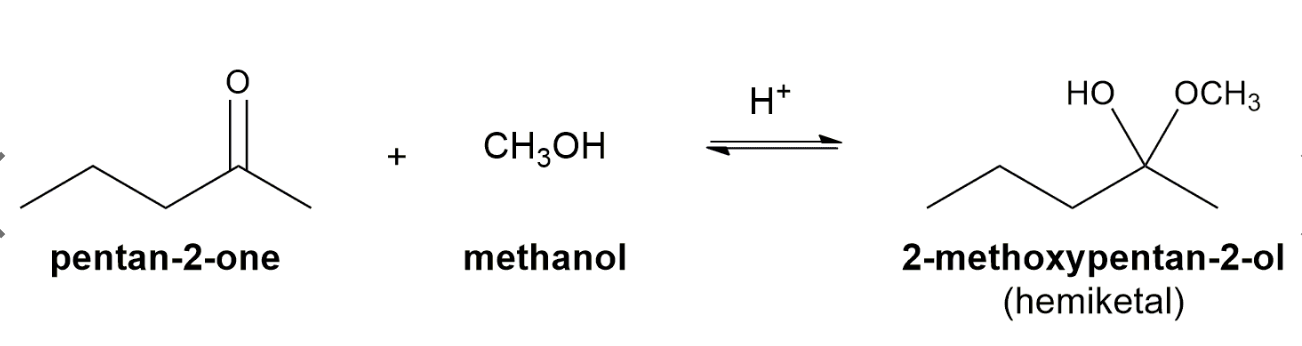
3. Addition of Alcohols
Aldehydes react with alcohols and a trace of acid to yield hemiacetals.
Ketones react with alcohols and a trace of acid to yield hemiketals.
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| REACTION NOTES (0) | 2023.03.28 |
|---|---|
| Lab 3 Glucose Concentration in Drinks (0) | 2023.03.27 |
| [WEEK3] Alcohols, phenols, ethers and thiols (0) | 2023.03.14 |
| [WEEK2] Unsaturated Hydrocarbons (0) | 2023.03.08 |
| [WEEK1] Saturated Hydrocarbons (0) | 2023.03.01 |



