













Molecules of heredity
Within the cell, the nucleus contains chromosomes made of protein and a single molecule of the nucleic acid, deoxyribonucleic acid (DNA)..
Passed from parents to offspring, DNA contains the specific instructions that make each type of living creature unique.
The unique structure of chromosomes keeps DNA tightly wrapped around spool-like proteins, called histones. Without such packaging, DNA molecules would be too long to fit inside cells. For example, if all of the DNA molecules in a single human cell were unwound from their histones and placed end-to-end, they would stretch 6 feet.
The nucleic acids and their building blocks & primary structure
Molecules of heredity
Within the cell, the nucleus contains chromosomes made of protein and a single molecule of the nucleic acid, deoxyribonucleic acid (DNA)..
Passed from parents to offspring, DNA contains the specific instructions that make each type of living creature unique.
The unique structure of chromosomes keeps DNA tightly wrapped around spool-like proteins, called histones. Without such packaging, DNA molecules would be too long to fit inside cells. For example, if all of the DNA molecules in a single human cell were unwound from their histones and placed end-to-end, they would stretch 6 feet.



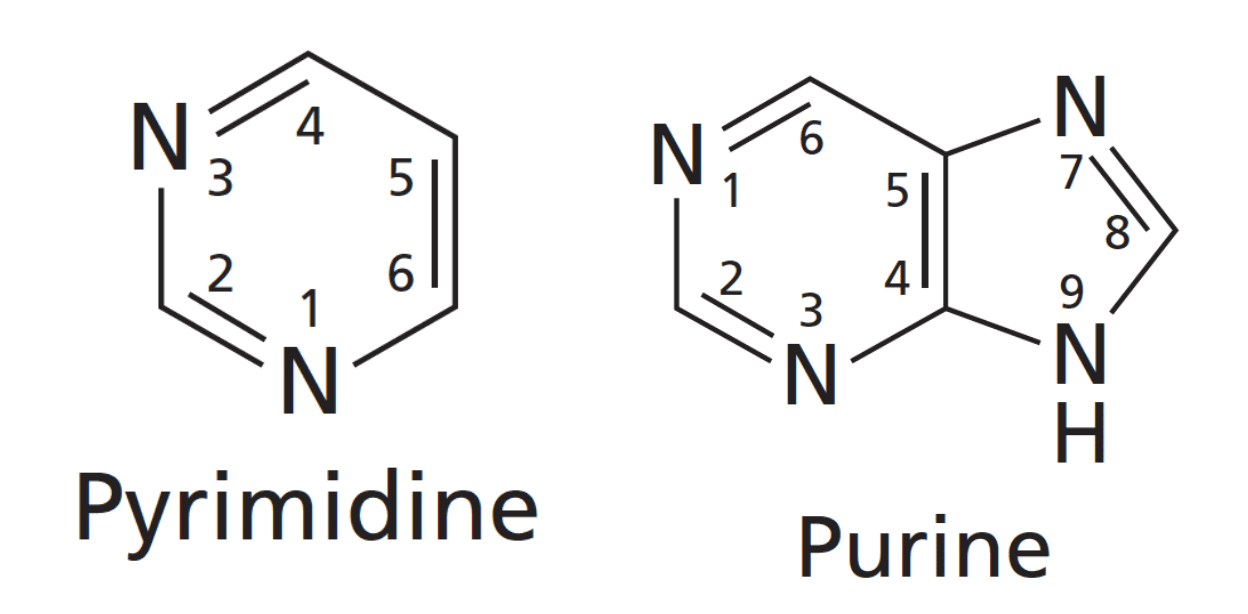
What is a nucleoside?
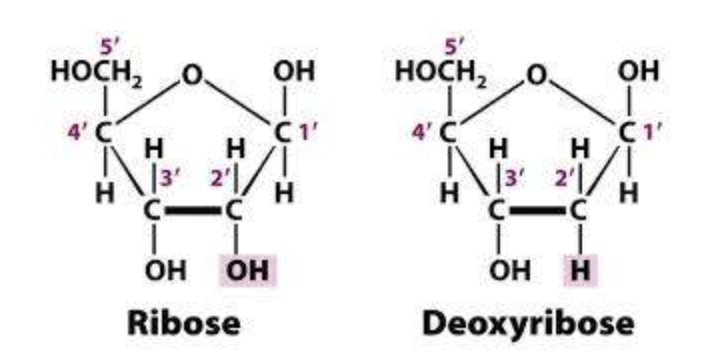
Nucleosides, are structural subunits of DNA and RNA. They are made up of the nucleobases like those we have just studied, plus a sugar which is either ribose or deoxyribose
The link between the nucleobase and the sugar is called a beta-N-glycosidic bond because the bond from the sugar goes from the anomeric carbon C1 to the Nitrogen at N1 for the pyrimidines and N9 for the purines. Why beta then?......because the nucleobase is on the same side as the 5'-CH2OH and so the anomeric configuration of the ribose or deoxy is beta! This bond is highlighted in red for Adenosine below.

Naming of nucleosides...
Notice in the above table that adenine when bonded to a sugar becomes either adenosine if it's bonded to ribose or deoxyadenosine if it's bonded to deoxyribose - and the same is true of the other purine nucleoside guanine becomes guanosine or deoxyguanosine.
For the purine nucleosides the rule is: -nine becomes -osine (ie guanine - guanosine)
What about the pyrimidine nucleosides? - well for cytosine and thymine it's pretty simple, the rule is: -ine becomes -dine:
- cytosine - cytidine
- thymine - thymidine
But because there is ALWAYS an exception to the rule: uracil becomes uridine.
So the name gives you hint as to the structure of the nucleoside

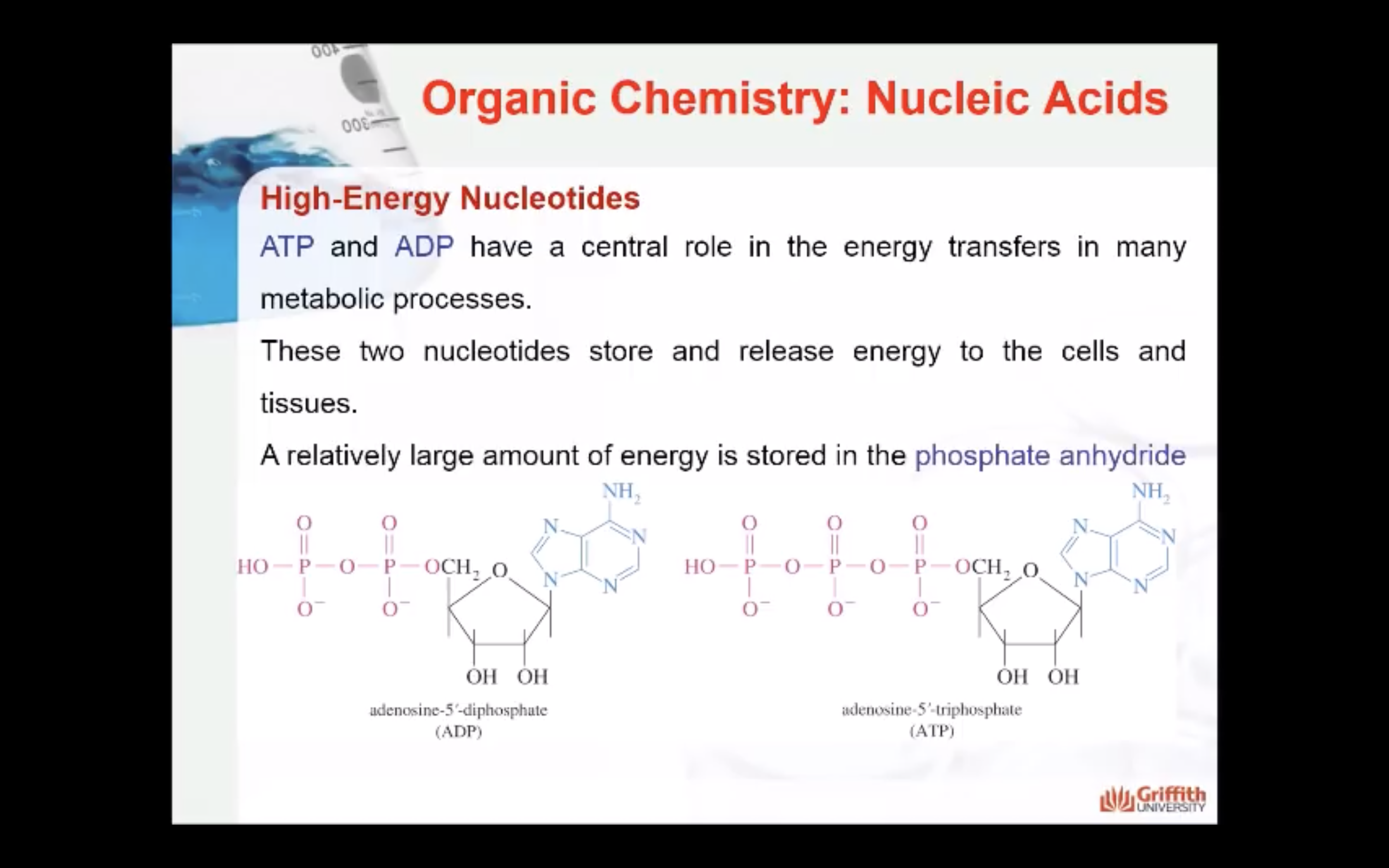
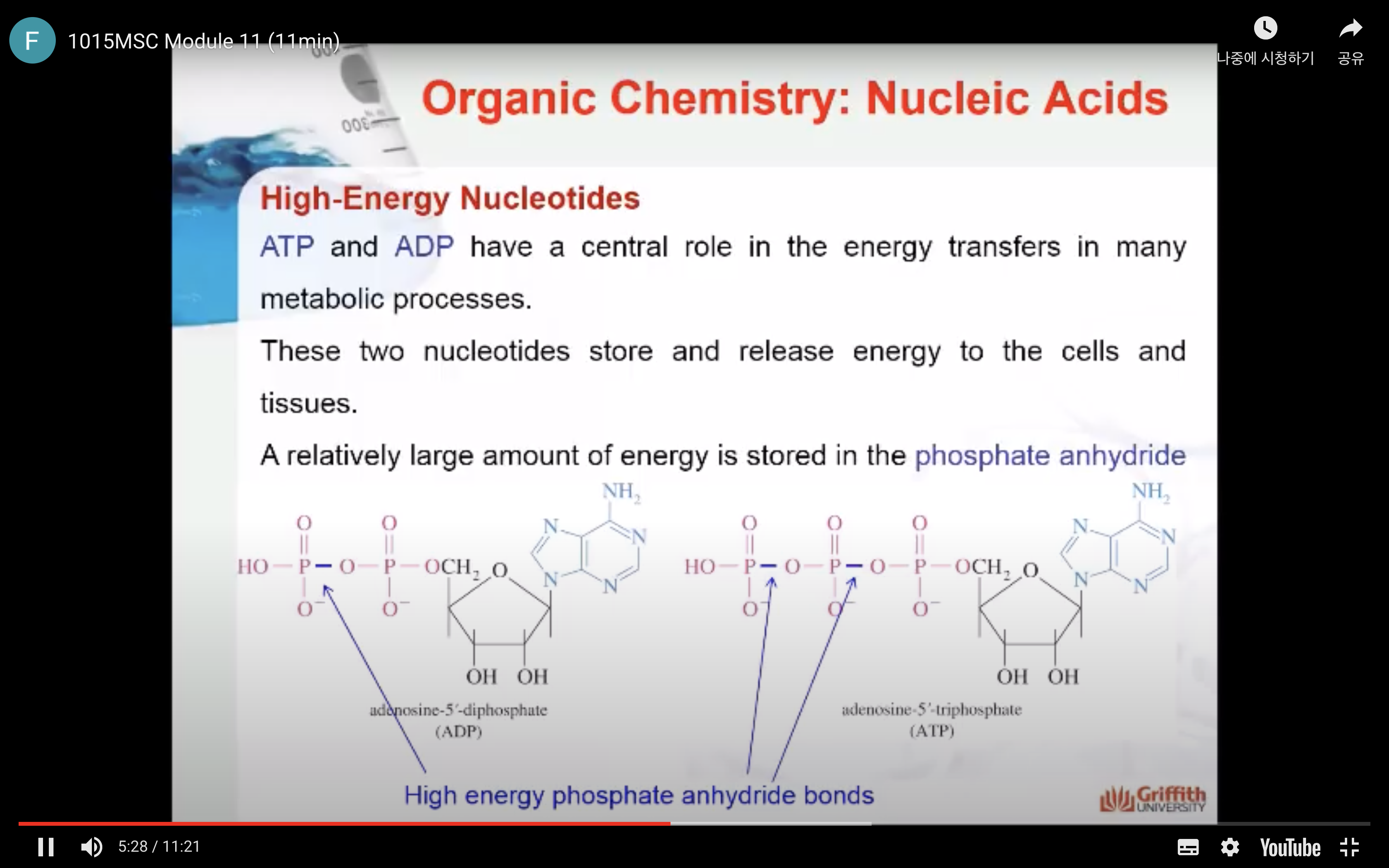
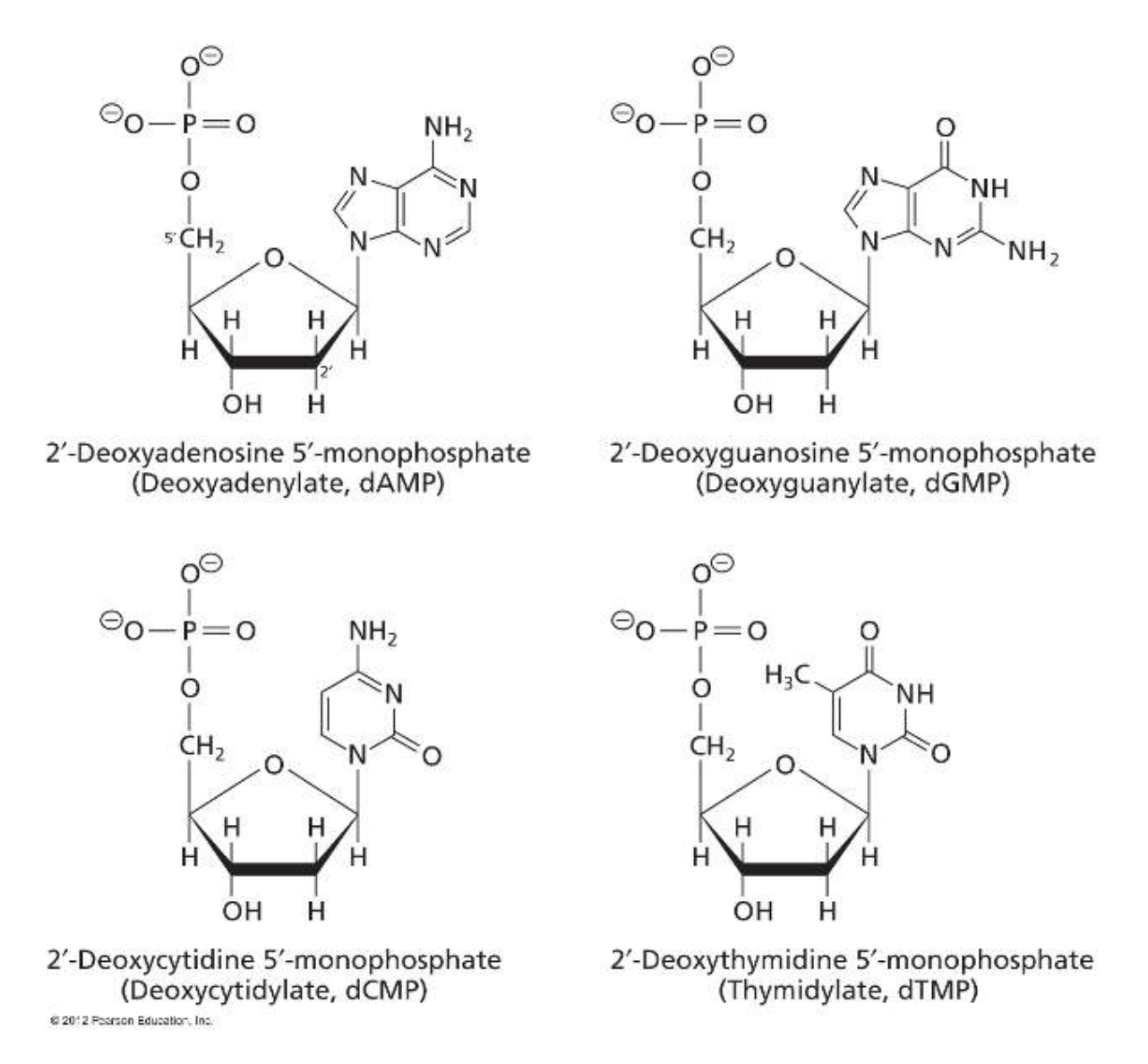
Nucleotides are the building blocks of nucleic acids
Nucleic acids (DNA and RNA) are one of the major classes of macromolecules, that like proteins and polysaccharides, contain similar monomeric units joined by covalent bonds to produce large polymers. The monomer units of nucleic acids are called nucleotides that are derived from the nucleosides.
Nucleotides are comprised of 3 components: a 5 carbon sugar, a nucleobase and one or more phosphate groups.
Naming of nucleosides and nucleotides
- nucleoside + 1 phosphate group = nucleotide monophosphate
- nucleoside + 2 phosphate groups = nucleotide diphosphate
- nucleoside + 3 phosphate groups = nucleotide triphosphate


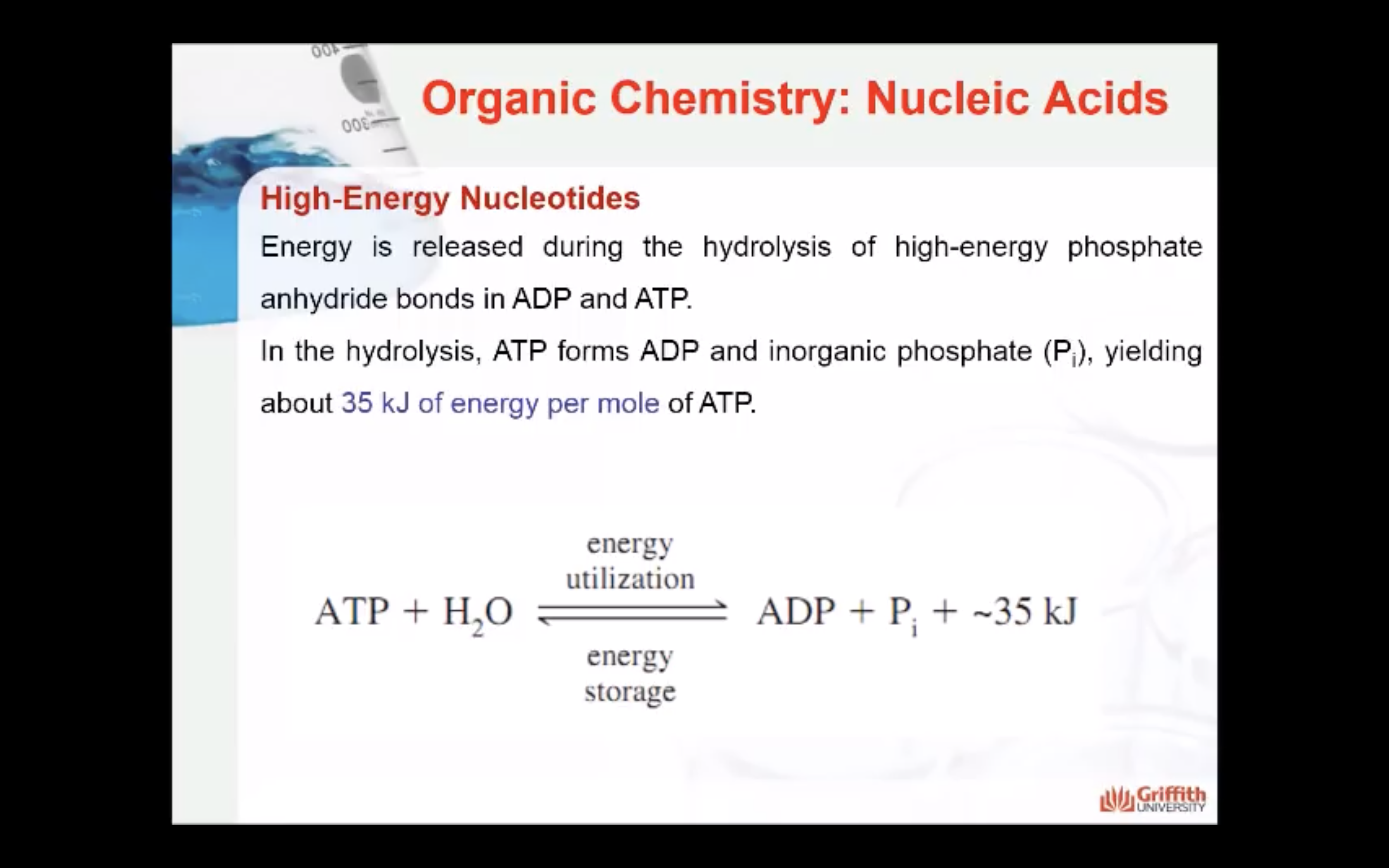
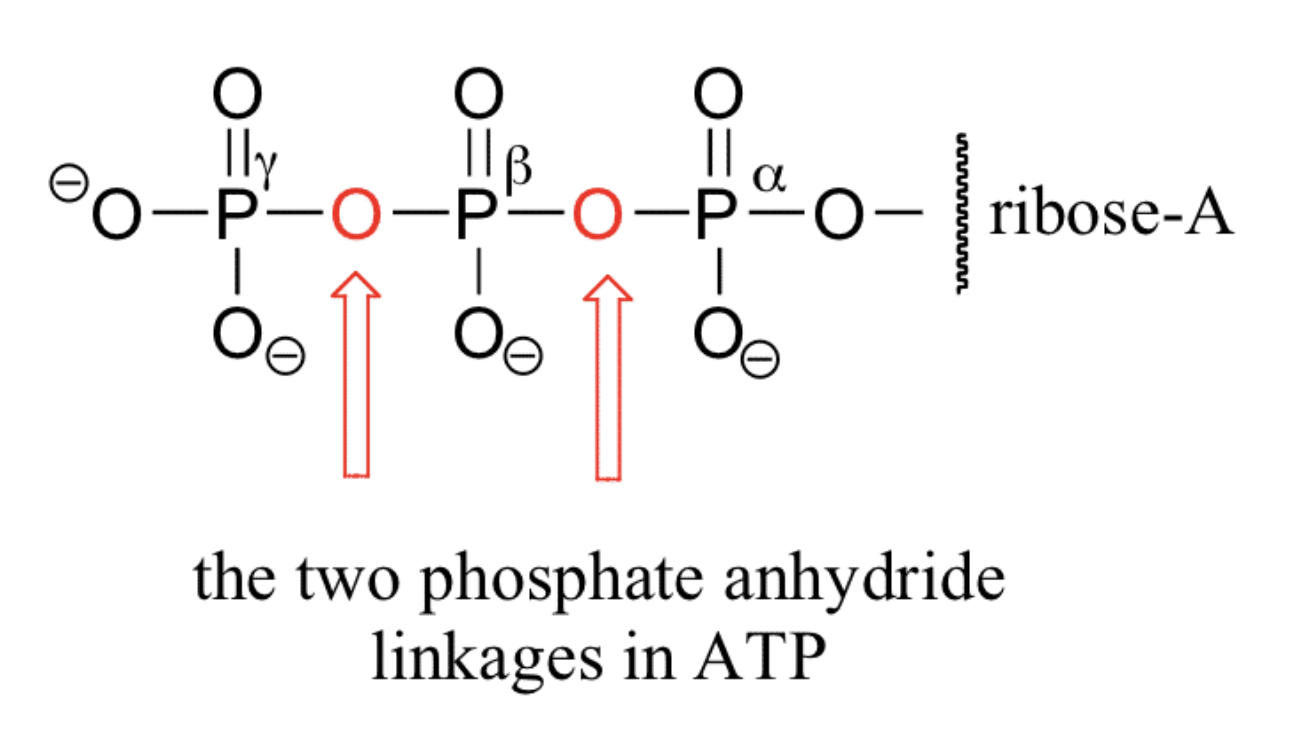
As we described above, nucleotides can have more than one phosphate attached. The bond formed between phosphate groups is called the phosphoester bond. The phosphate ester may be a monophosphate, a diphosphate, or a triphosphate A high-energy phosphate anhydride bond (P-O-P) is formed when two or more phosphates are linked together

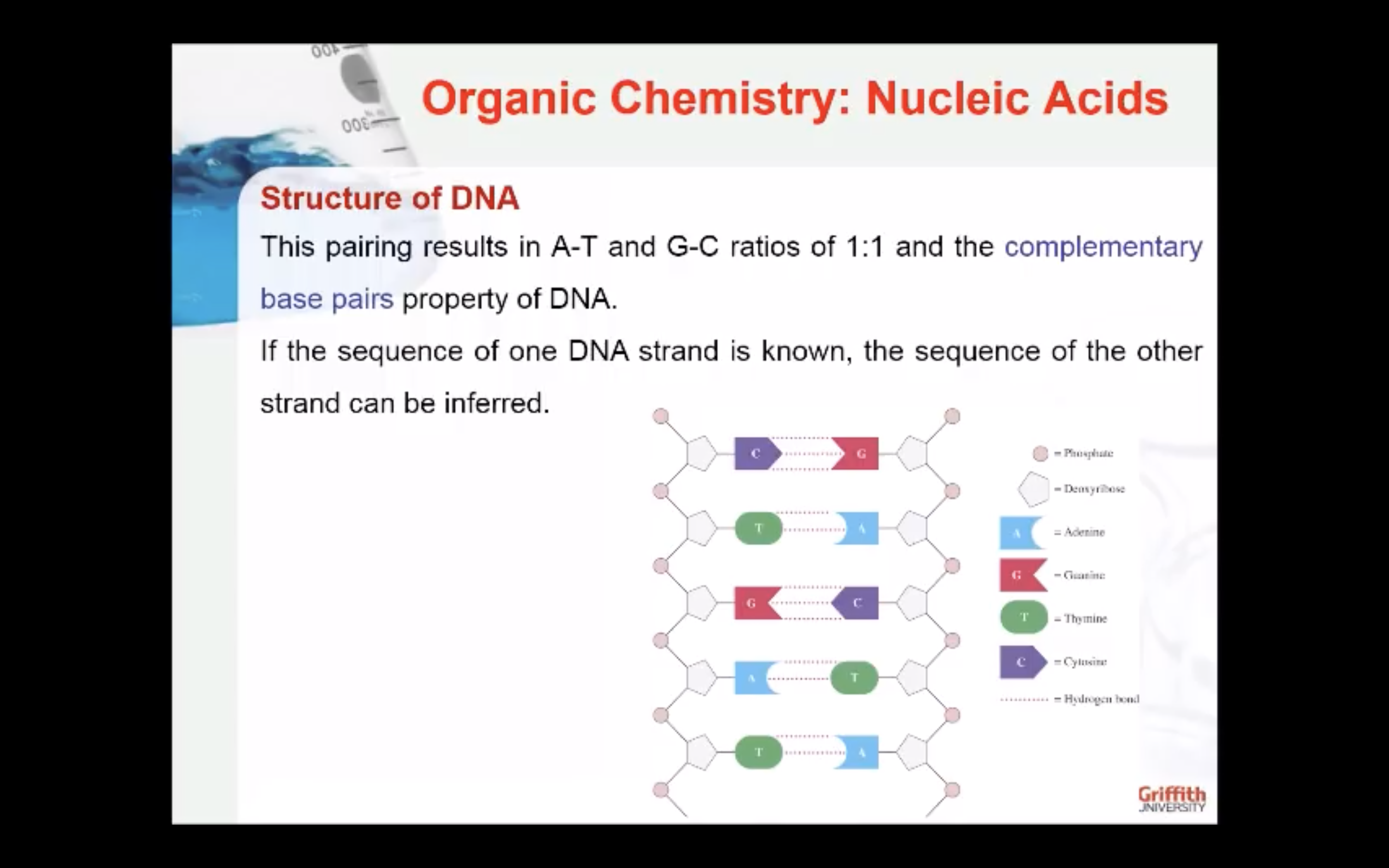
Primary sequence of the nucleic acids.....
Every naturally occurring polynucleotide has a unique structure: defined nucleotide sequence = primary structure
- Sequence of nucleic acid is written from 5' to 3' (= sense or directionality)
- 5' end carries a phosphate group
- 3' end carries a free -OH group
Polarity is important in DNA replication, transcription and translation. Genetic information is stored and encoded in the sequence of bases
Structure of the nucleic acids
The nucleic acids - RNA and DNA are polymeric structures that are formed by the nucleotides (= monomers) linking together in a specific way.
DNA
Deoxyribonucleic acid (DNA) is a polynucleotide made up of D-2'-deoxyribose, phosphoric acid, and the four bases adenine, guanine, cytosine, and thymine.
RNA
Ribonucleic acid (RNA) is a polynucleotide that, is made up of D-ribose, phosphoric acid, and the four bases adenine, guanine, cytosine, and uracil.
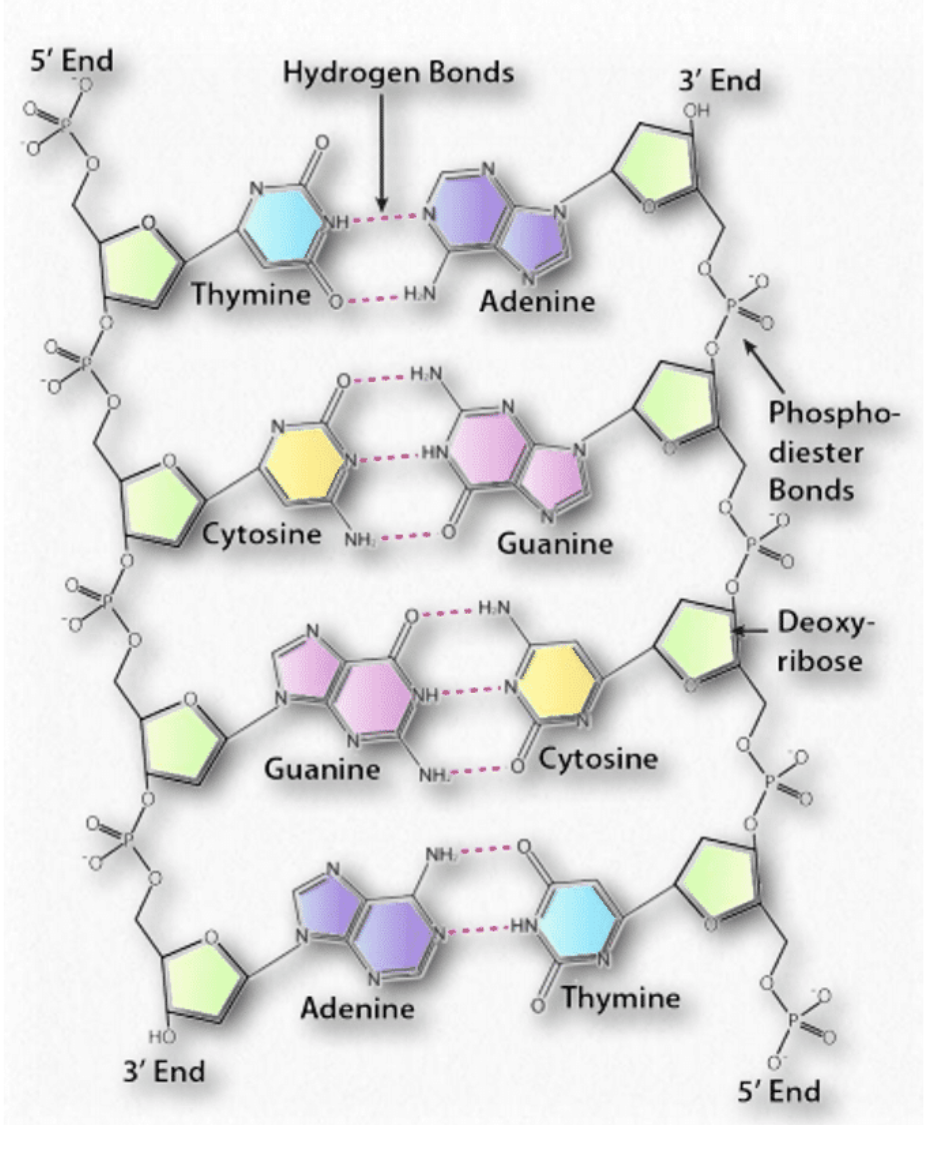
The primary structure of the nucleic acids DNA and RNA is the covalent bonding of the nucleotides from the 5' to 3' end through the sugar phosphodiester bonds.
The primary structure of the nucleic acids DNA and RNA is the covalent bonding of the nucleotides from the 5' to 3' end through the sugar phosphodiester bonds.
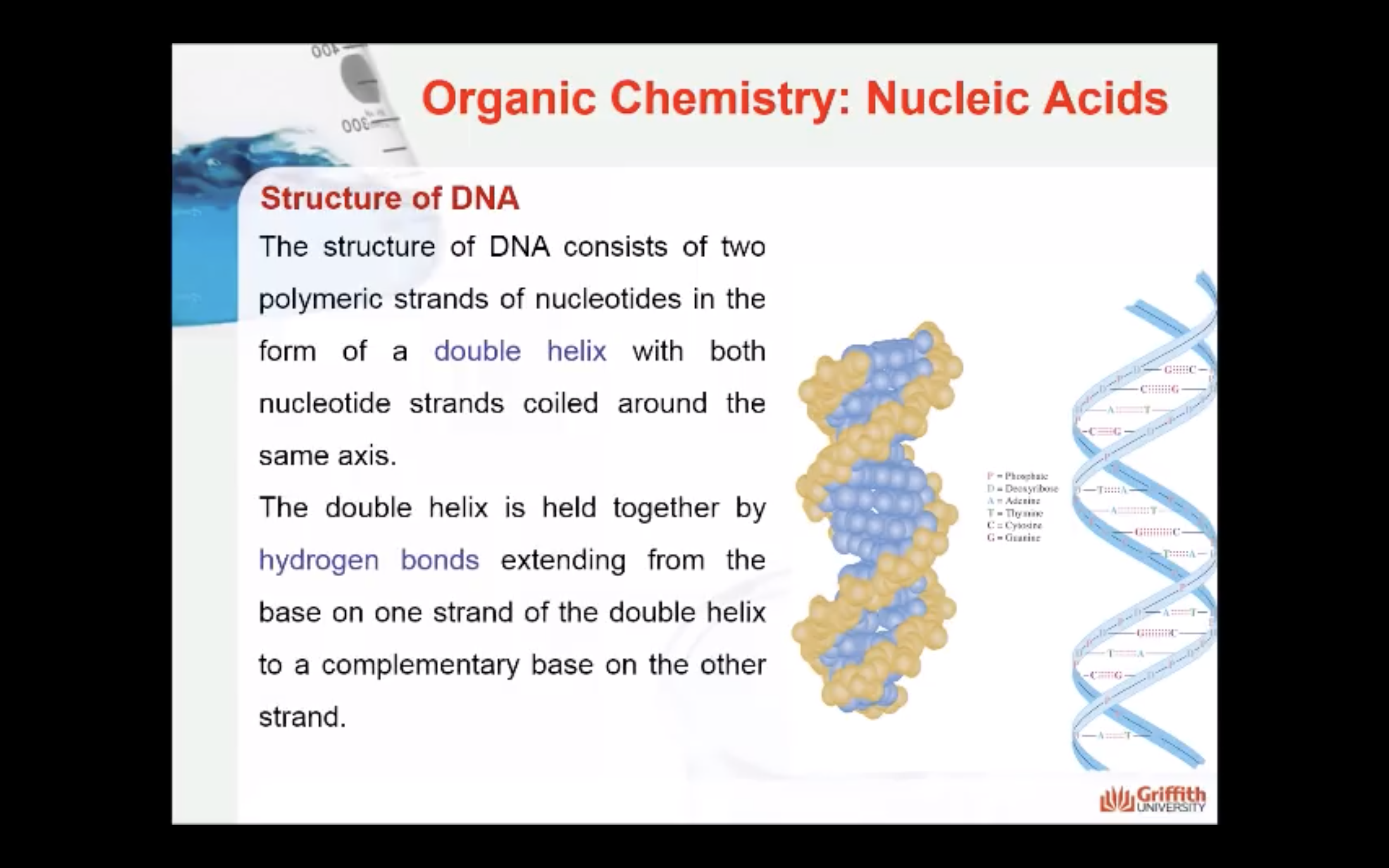
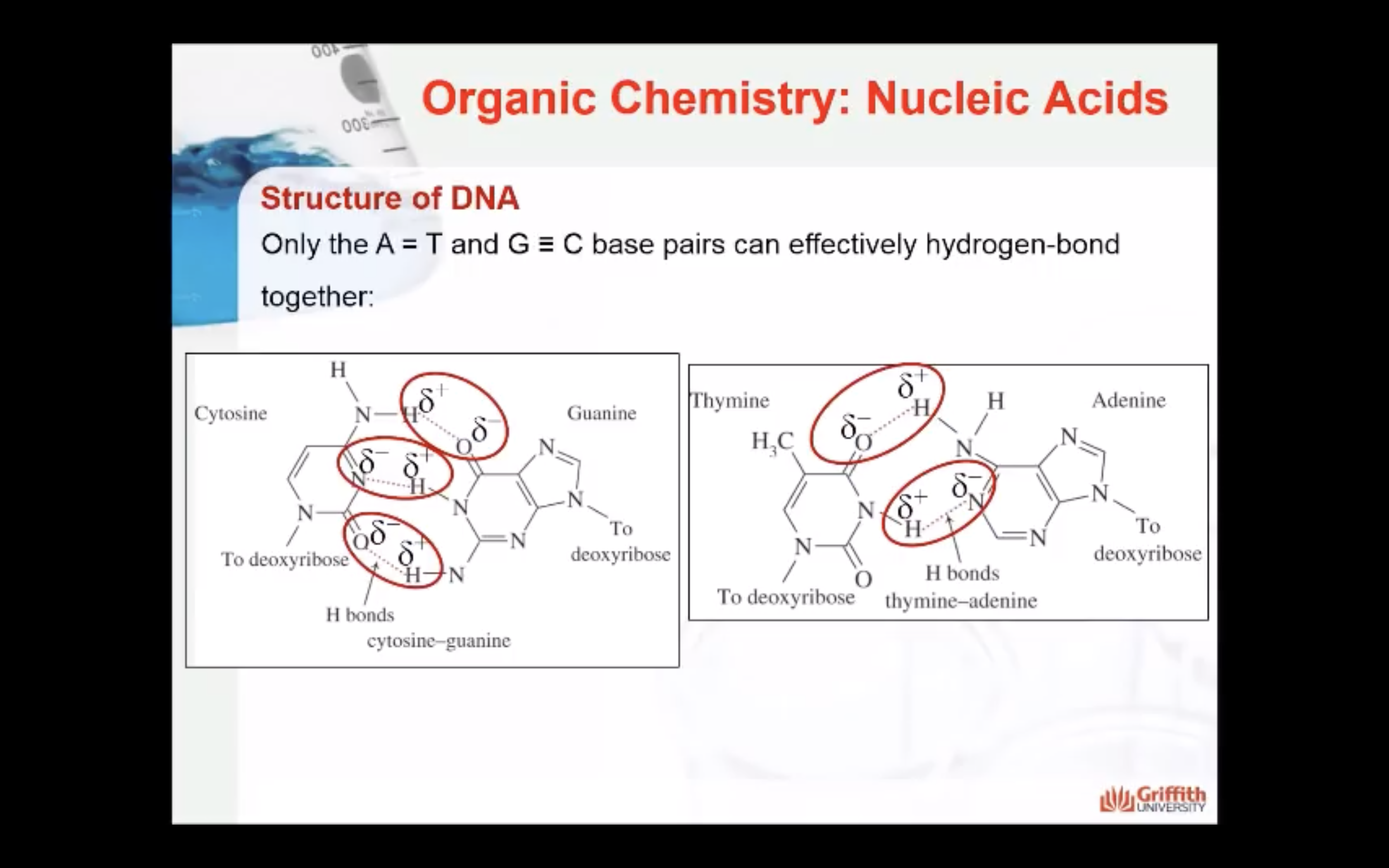
How the bases interact to stabilise the structure of DNA
The two antiparallel strands of DNA are stabilised by hydrogen bonds between the base pairs. Only certain pairing between the bases are found and this maximises the hydrogen bonding and therefore the stability of the double helix structure (more hydrogen bonds = more stability)
Adenine hydrogen bonds with Thymine (A-T) and forms 2 hydrogen bonds
Guanine hydrogen bonds with Cytidine (G-C) and forms 3 hydrogen bonds.
Details of the double helix structure
Secondary structure of DNA is an alpha helix, it is right handed, or clockwise coiling direction approximately 10 base pairs per 360 degree rotation of helix so each base pair is twisted 36 degrees relative to the adjacent bases.
The base pairs are 0.33 nanometer apart each complete rotation of the molecule which is 10 bases (= 360 ˚) is 3.4 nanometer.
The diameter of the helix is 2.37 nm, and the bases are perpendicular to the long axis of the DNA molecule and interact with each other and further stabilise the helix.
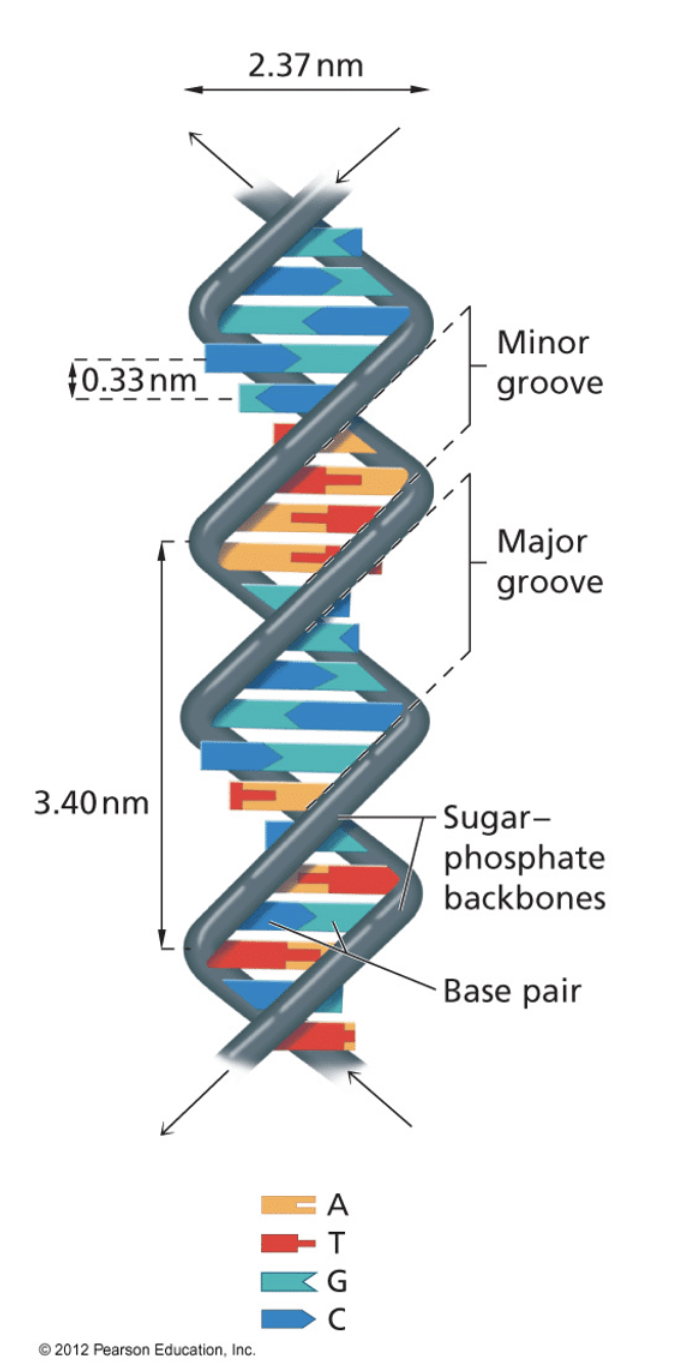
Structural details
- Minor and major groove
- Rise 0.33 nm
- Width 2.37 nm
- Repeats every 10 base units = 3.4 nm
What about the phosphates?
The phosphates are on the outside of the DNA structure along the backbone and are negatively charged.
To balance out these charges DNA phosphates groups form ionic bonds to cations such as Mg2+ or Ca2+ which are called counterions.
DNA counterions reduce the electrostatic repulsion between DNA molecules by screening the negative charges in their backbones.

Bases buried in the interior of the helix away from the aqueous environment increases its stability, whereas the more polar phosphate and sugars ( hydrophilic) are exposed and interact with the aqueous environment
Ionic interactions (ion pair)

Eukaryotic cells normally range between 1– 100µm in diameter. Each human cell contains approximately 2 meters of DNA if stretched end-to-end; yet the nucleus of a human cell, which contains the DNA, is only about 6 μm in diameter??????
DNA packaging
Each chromosome consists of one continuous thread-like molecule of DNA coiled tightly around proteins called histones, and contains a portion of the 6,400,000,000 basepairs (DNA building blocks) that make up your DNA. The way DNA is packaged into chromatin is a factor in how protein production is controlled.
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK12] The Chemistry of Medicine (0) | 2023.05.27 |
|---|---|
| [WEEK12] The Chemistry of Exercise (0) | 2023.05.26 |
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (1) | 2023.05.13 |
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (0) | 2023.05.13 |
| [WEEK6]Chemistry of Food- Carbohydrates & Carbohydrates (0) | 2023.04.02 |



