ORIGINS OF DRUGS



Plants and plant derivatives are the major sources of drugs. NOTE: (Infographics shown below as examples and for your interest only - not examinable)
Plant-based drugs account for approximately 30% of all pharmaceuticals.
A number of medicines (including tablets, injections, capsules, creams, mixtures and vaccines) contain animal products or are animal derived.


Microbial products in the cosmetic industry
Microbes have made a phenomenal contribution to the health and well-being of people throughout the world. In addition to producing many primary metabolites, such as amino acids, vitamins and nucleotides, they are capable of making secondary metabolites, which constitute half of the pharmaceuticals on the market today.
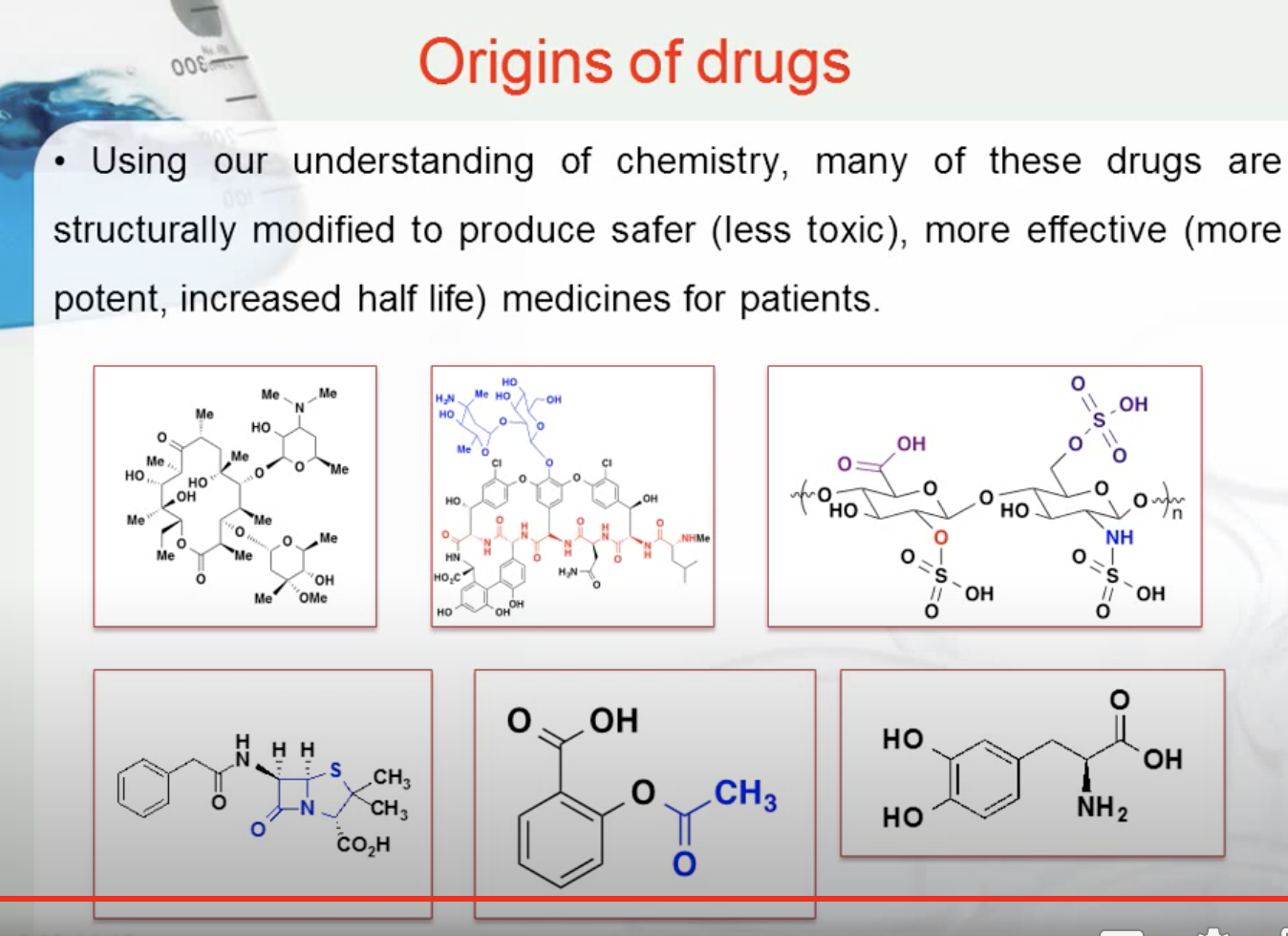
Using our understanding of chemistry, many of these drugs are structurally modified to produce safer (less toxic), more effective (more potent, increased half-life) medicines for patients.
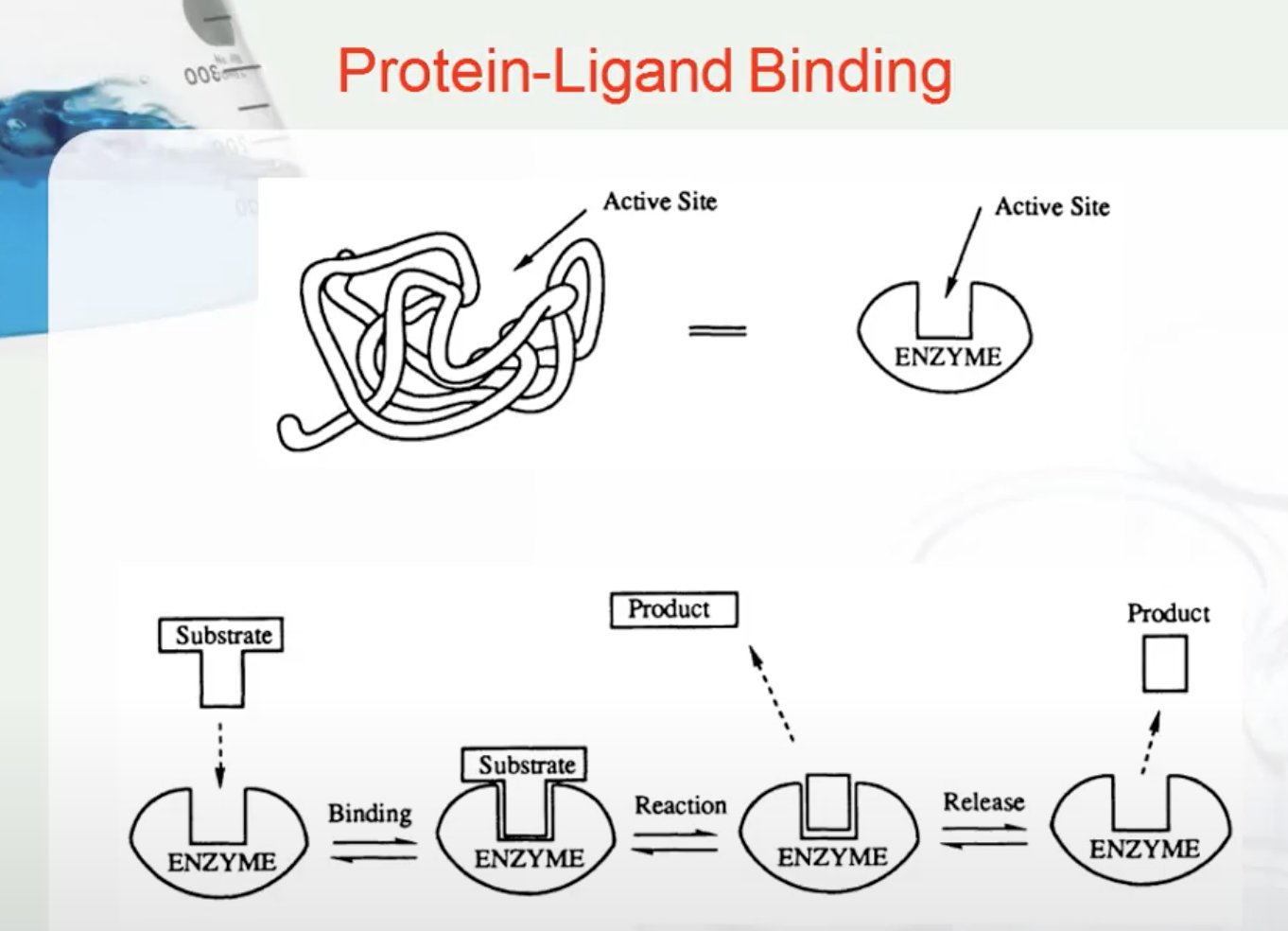
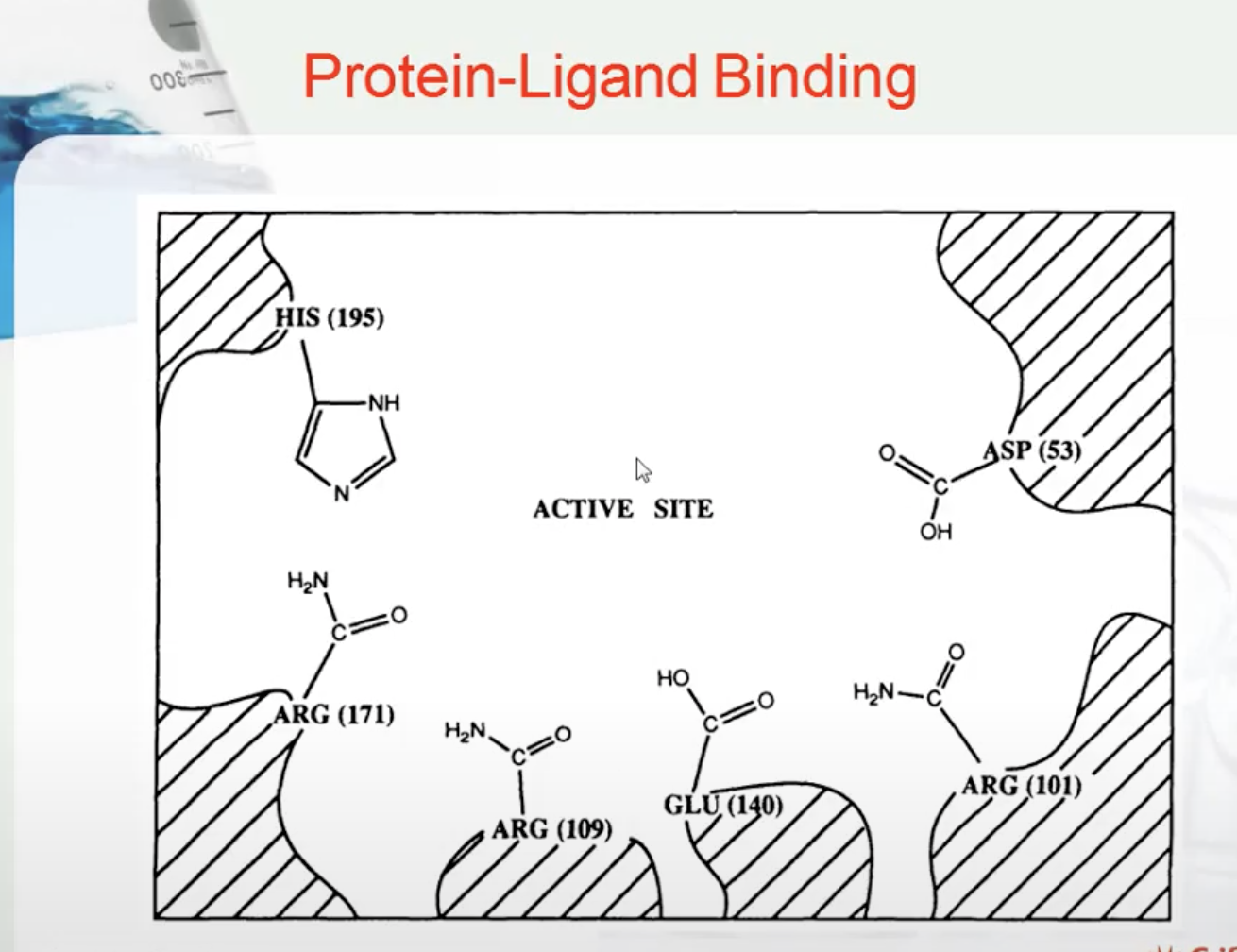
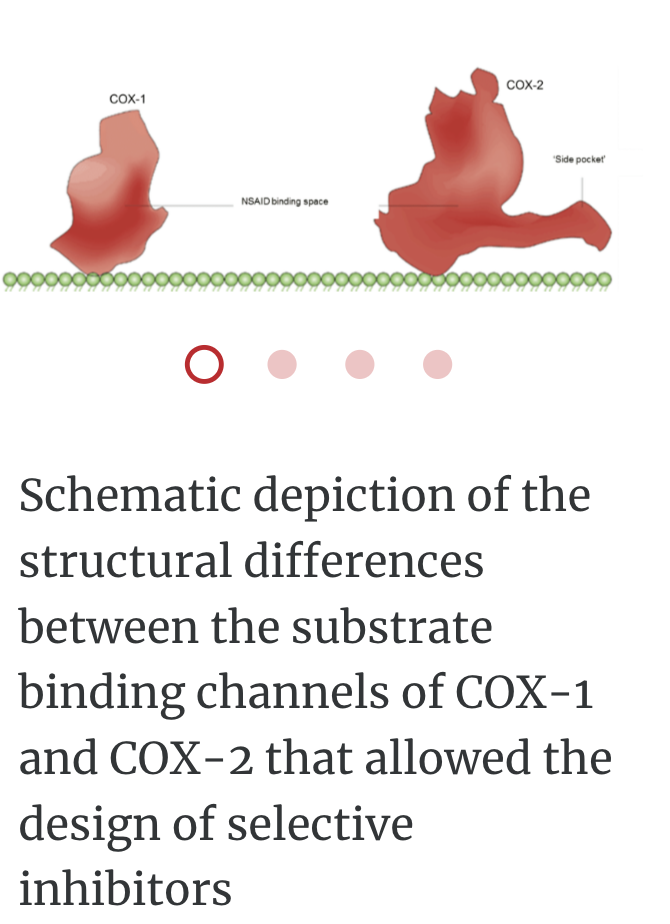
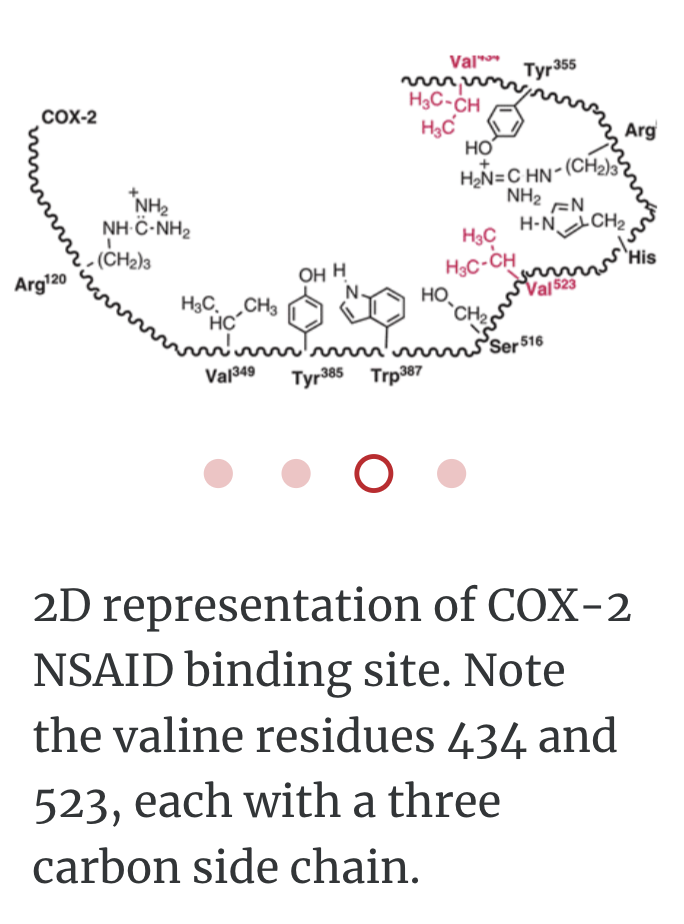
PROTEIN LIGAND BINDING

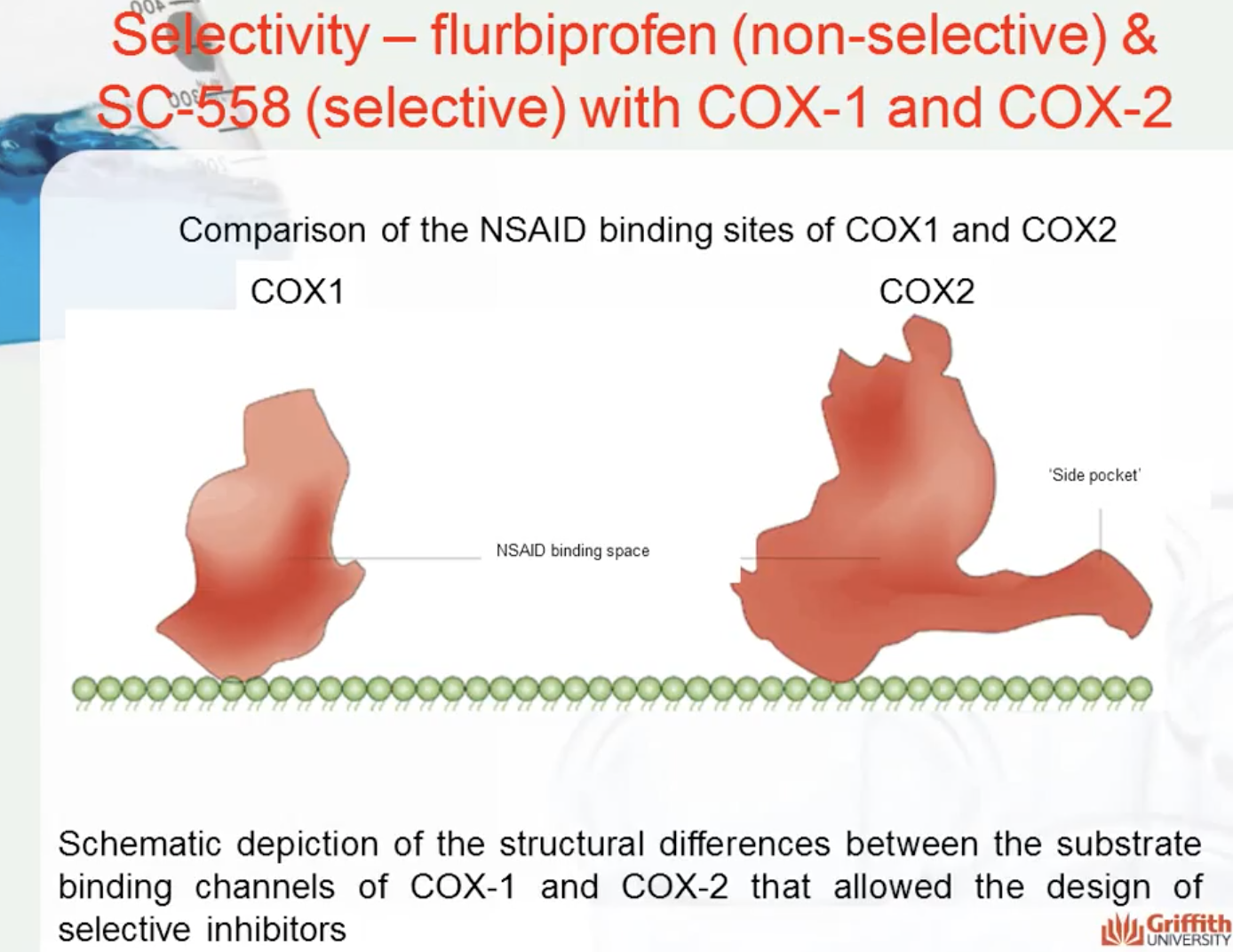
COX1- gastrointestinal health
COX2- causes inflammation
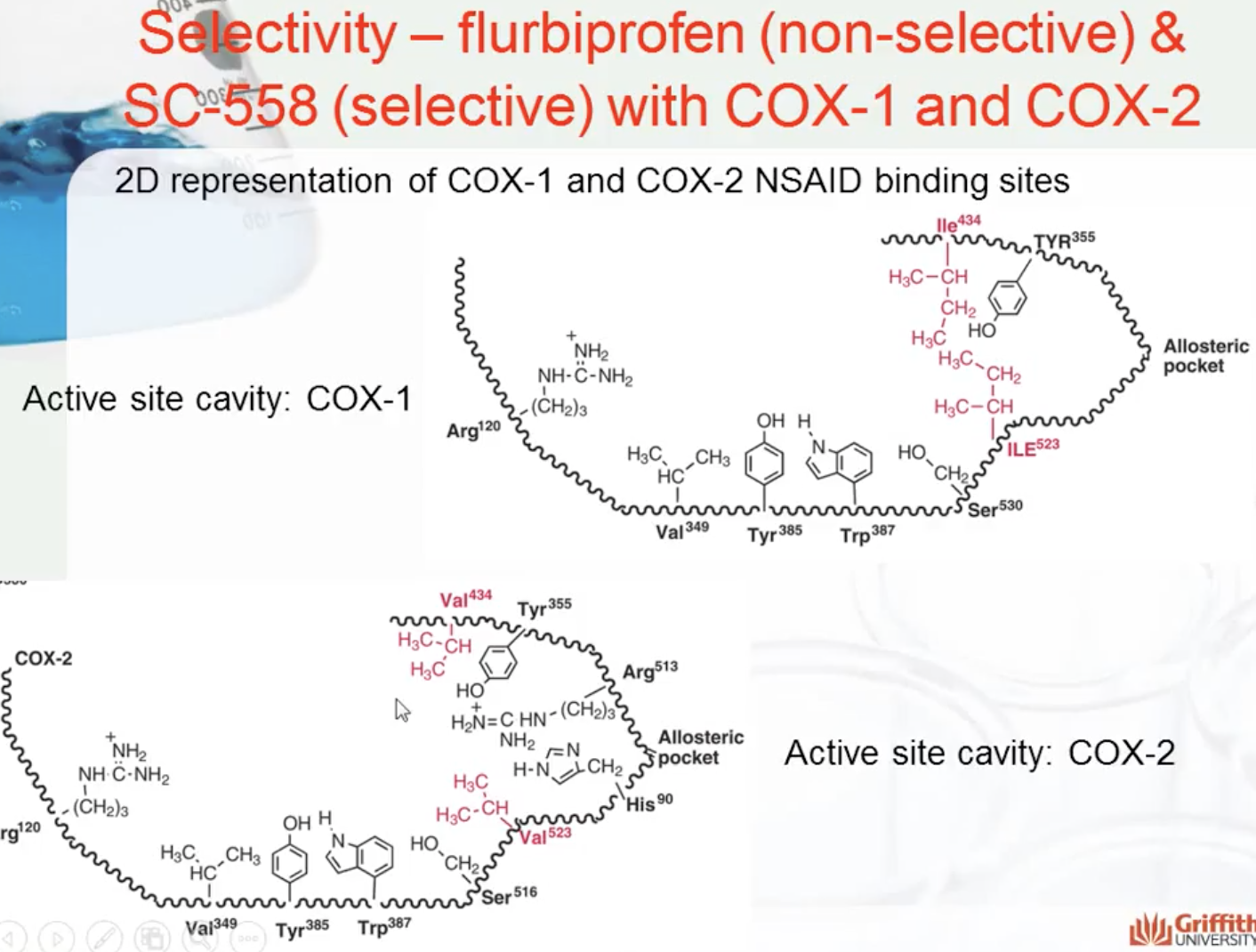
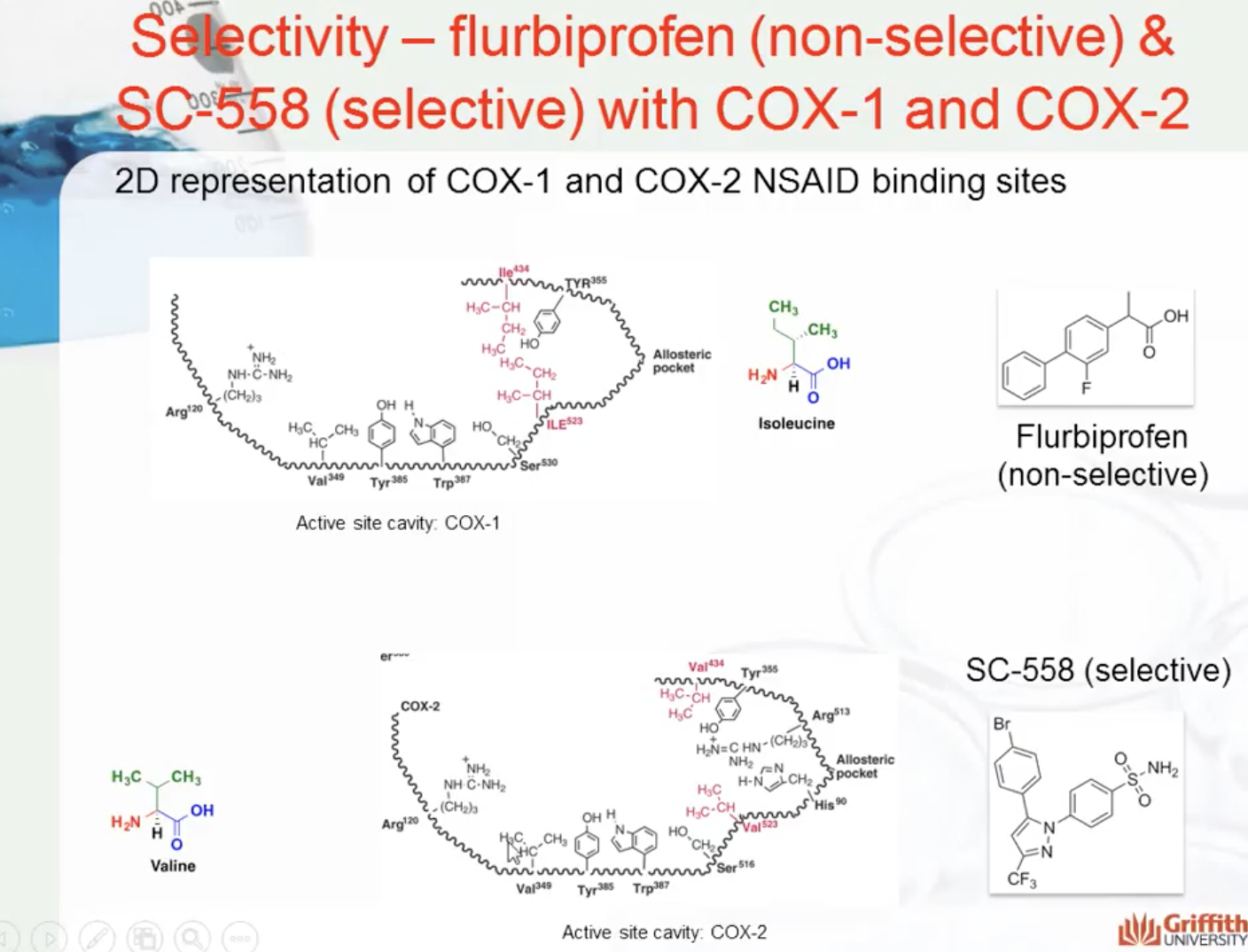
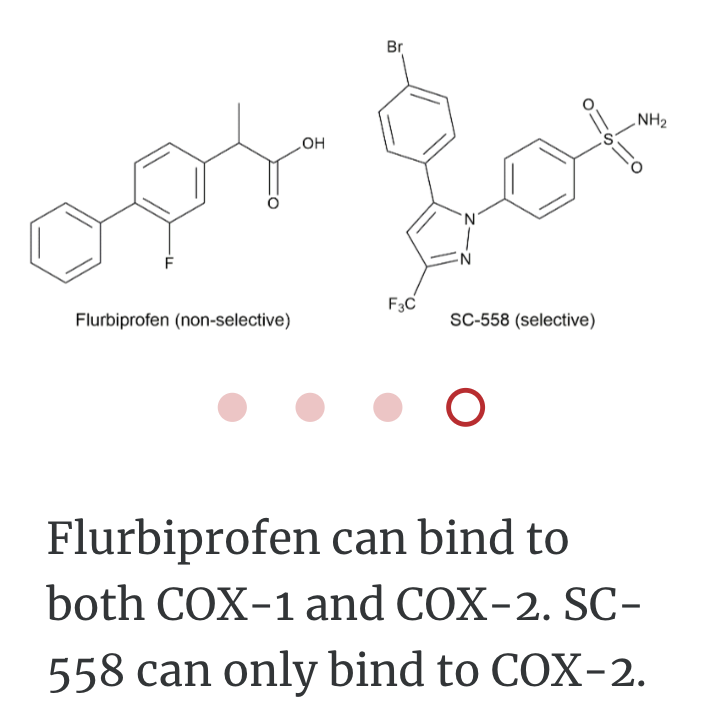
SC-558 only binds to COX2
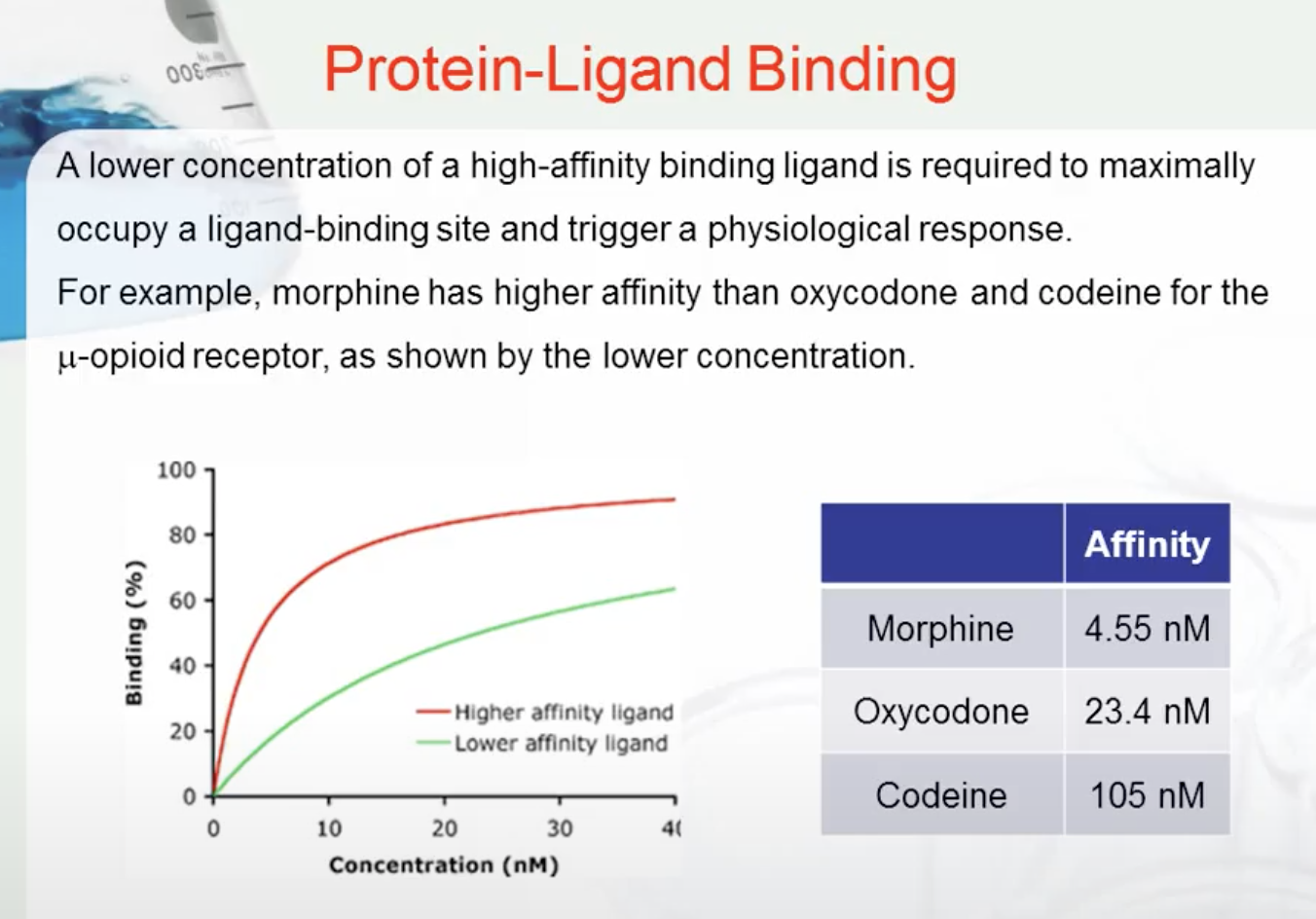
A ligand is a molecule which produces a signal by binding to a site on a target protein. Binding occurs by intermolecular forces such as ionic bonds, hydrogen bonds and Van der Waals forces. The interaction of a ligand with a protein binding site can be measured as a binding affinity. The greater the intermolecular forces between the ligand and the receptor, the greater the binding affinity.
A lower concentration of a high-affinity binding ligand is required to maximally occupy a ligand-binding site and trigger a physiological response. For example, morphine has higher affinity than oxycodone and codeine for the μ-opioid receptor, as shown by the lower concentration.
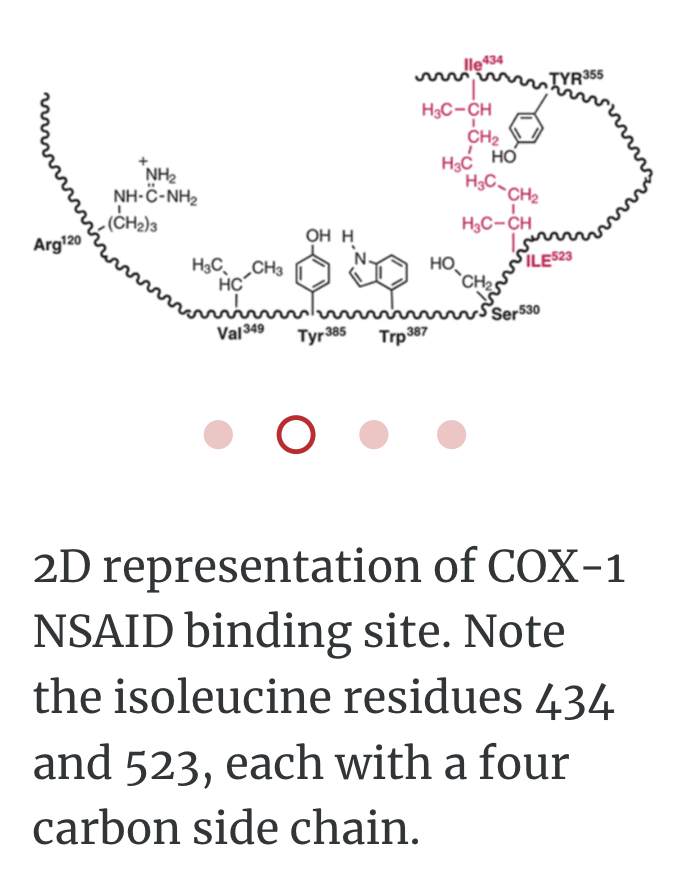
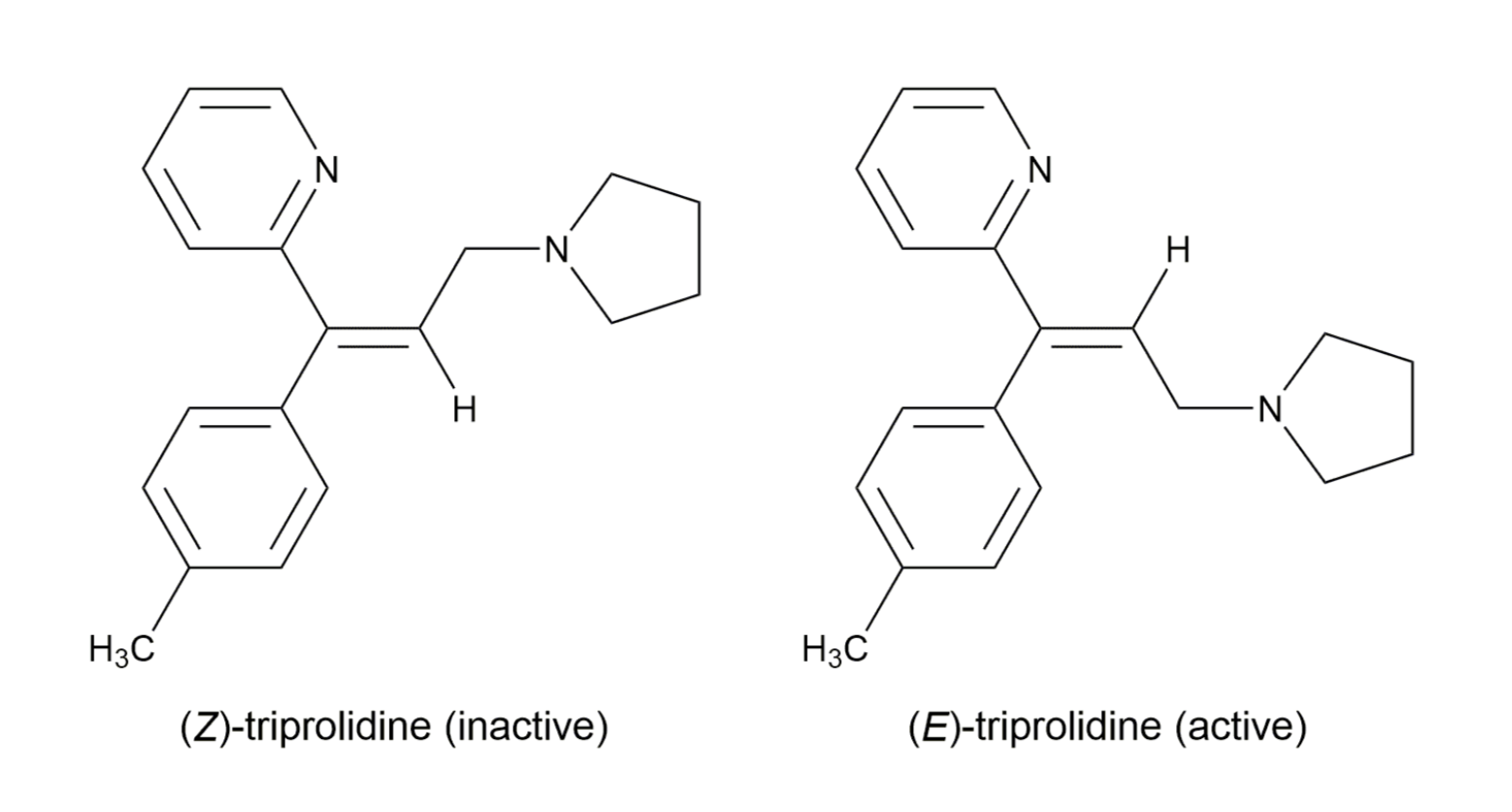
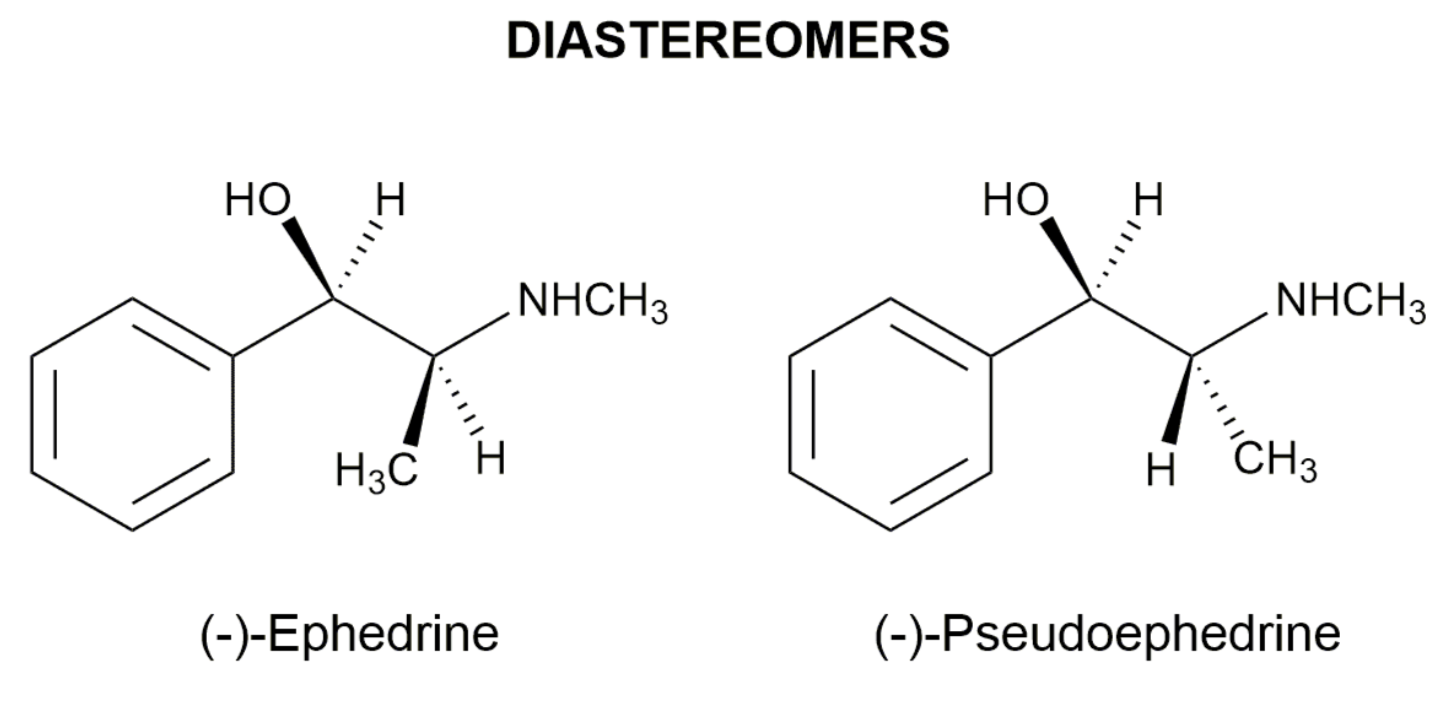
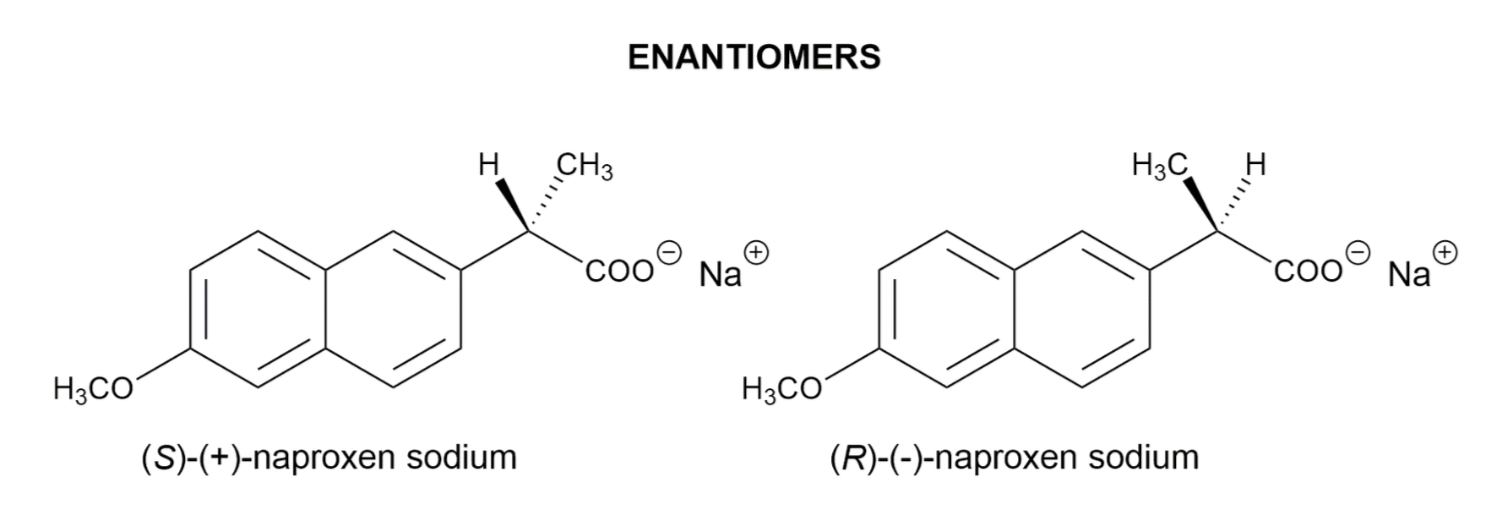
In order to bind right enzyme, it needs to have right SHAPE (stereo), and right attraction (functional group)
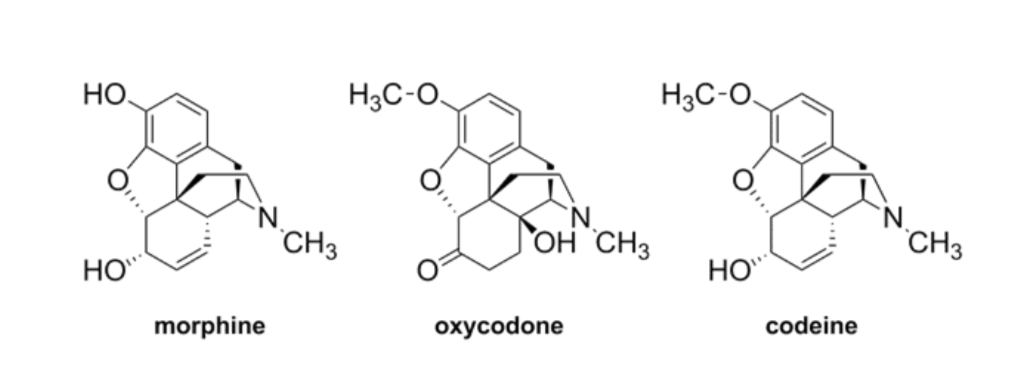
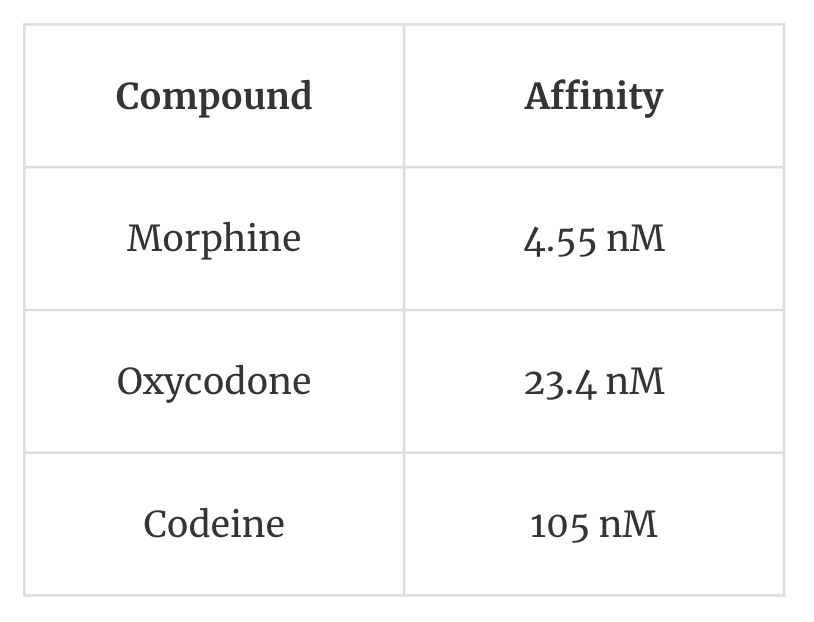
Morphine has high affinity than codeine
It needs less to have similar affect as codeine
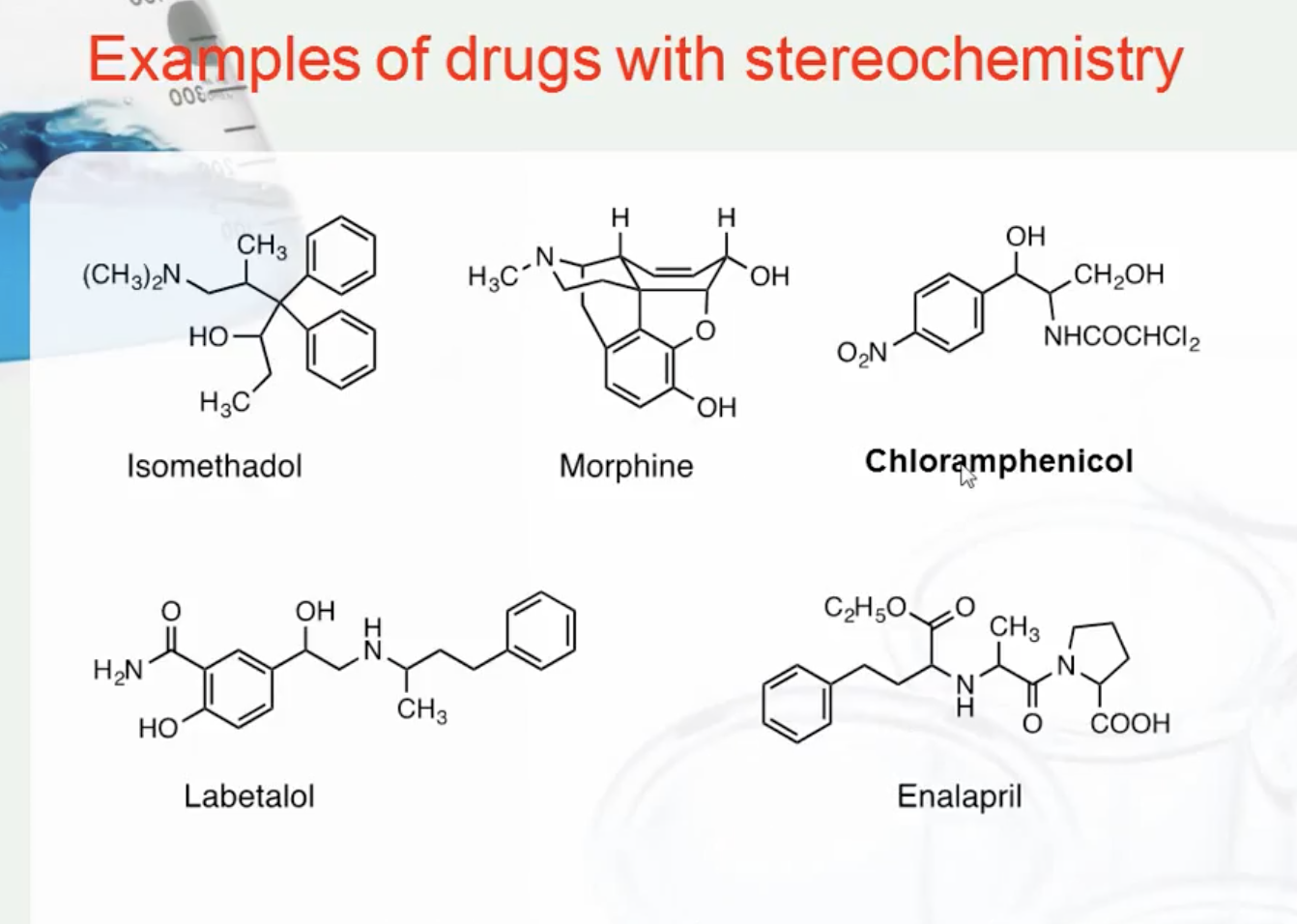
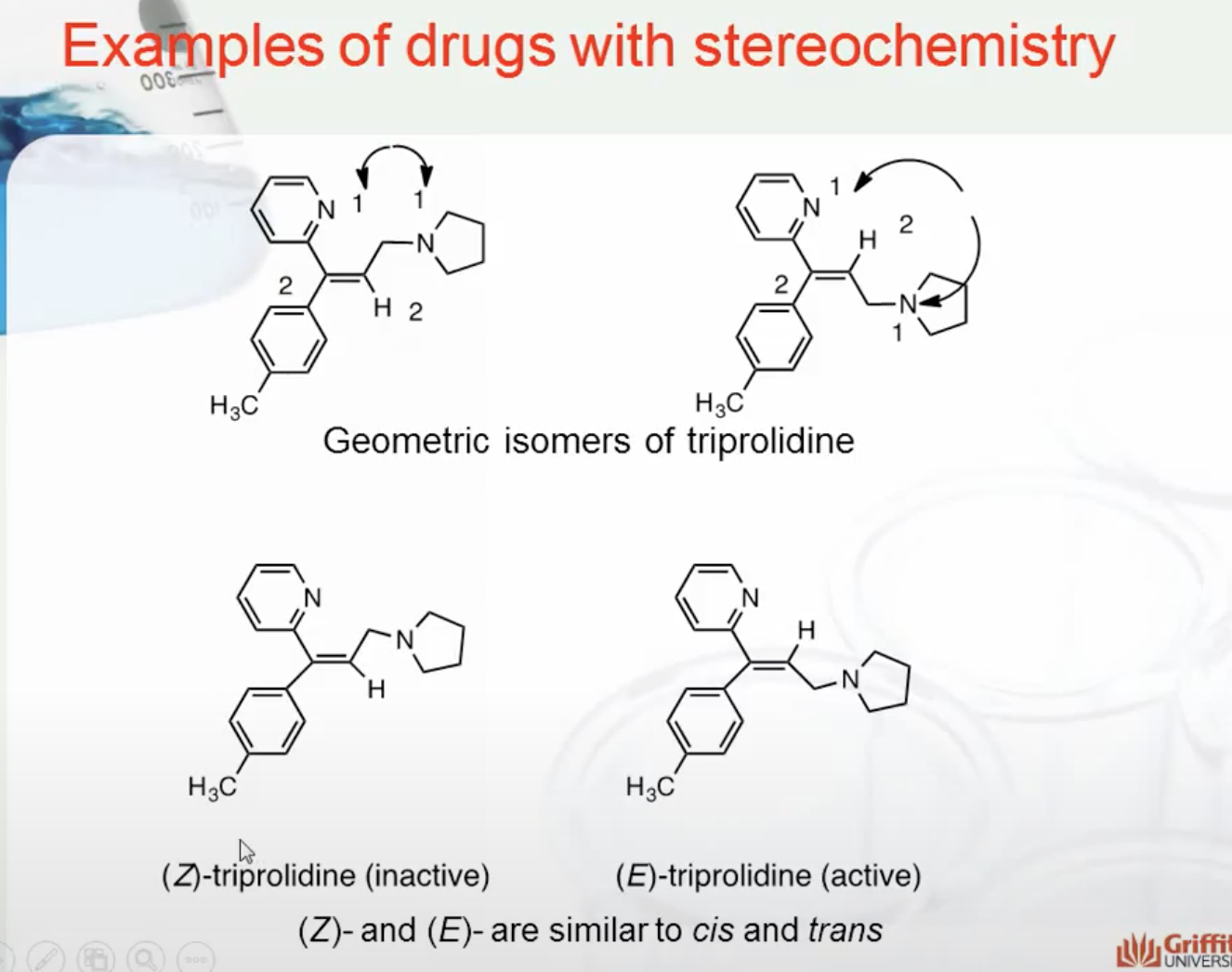
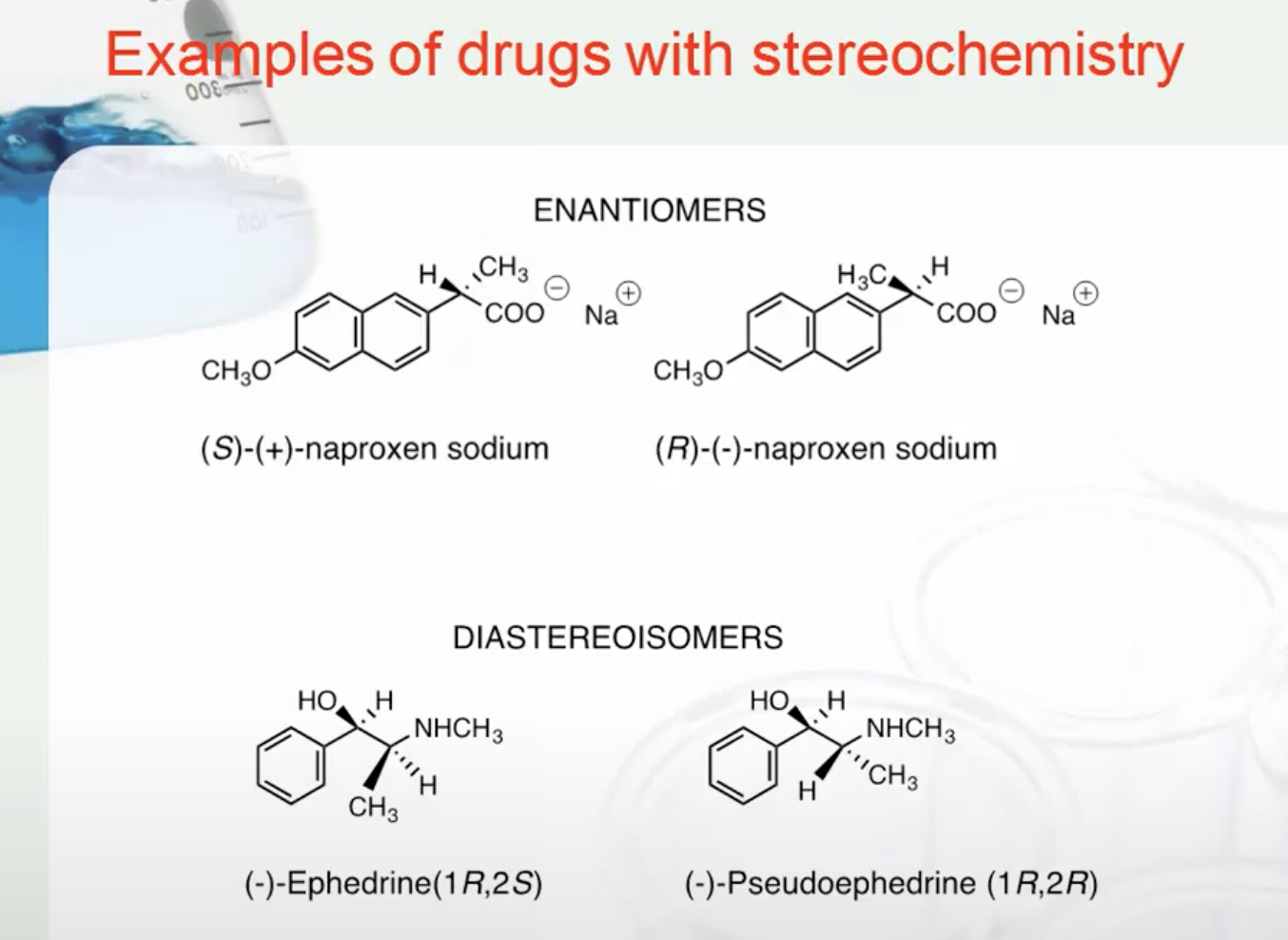
EXAMPLES OF DRUGS WITH STEREOCHEMISTRY
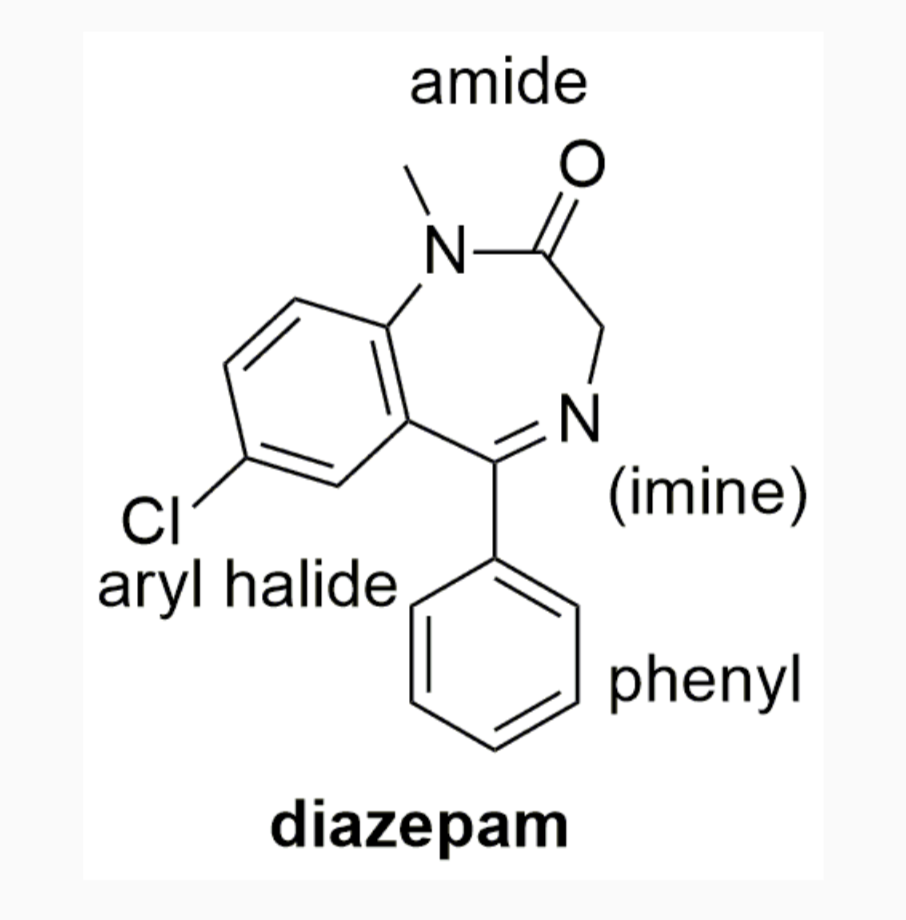
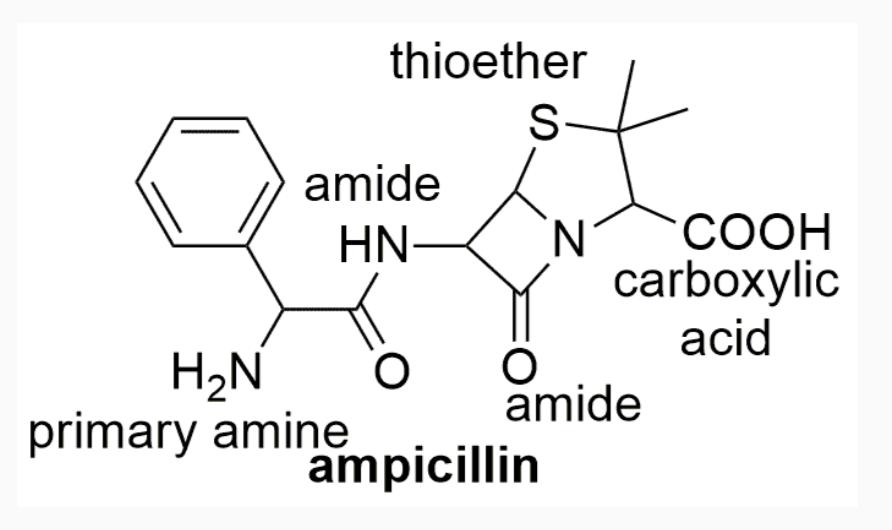
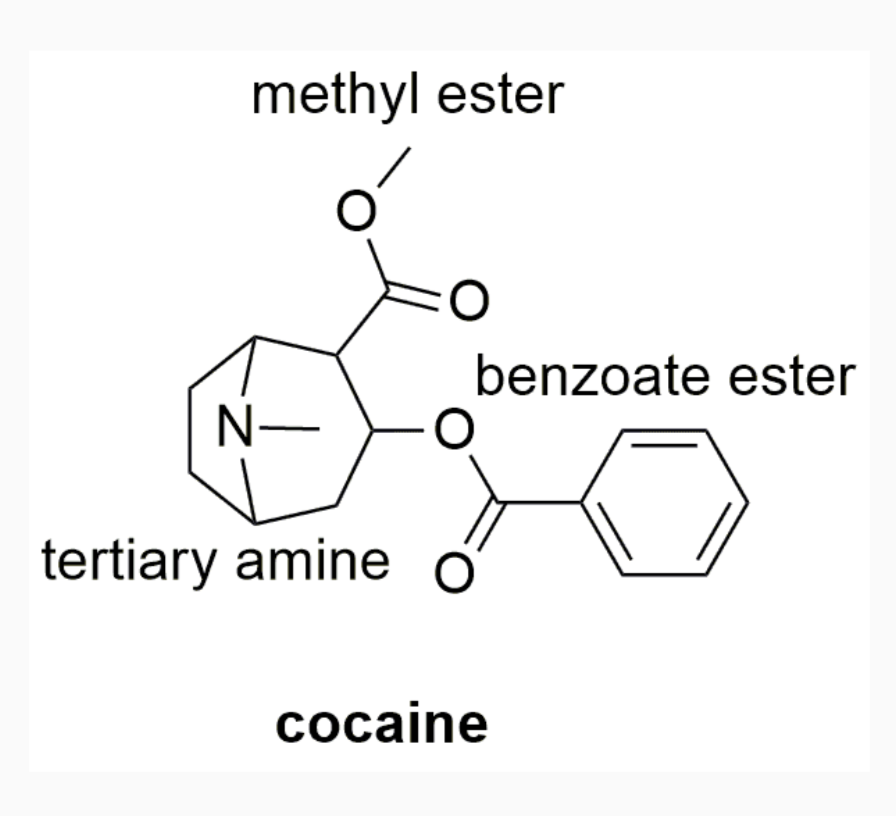
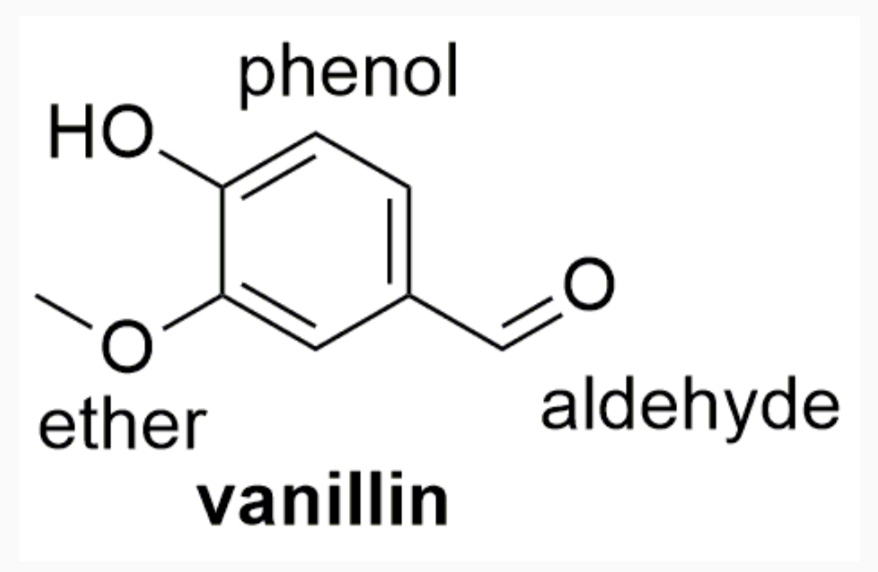
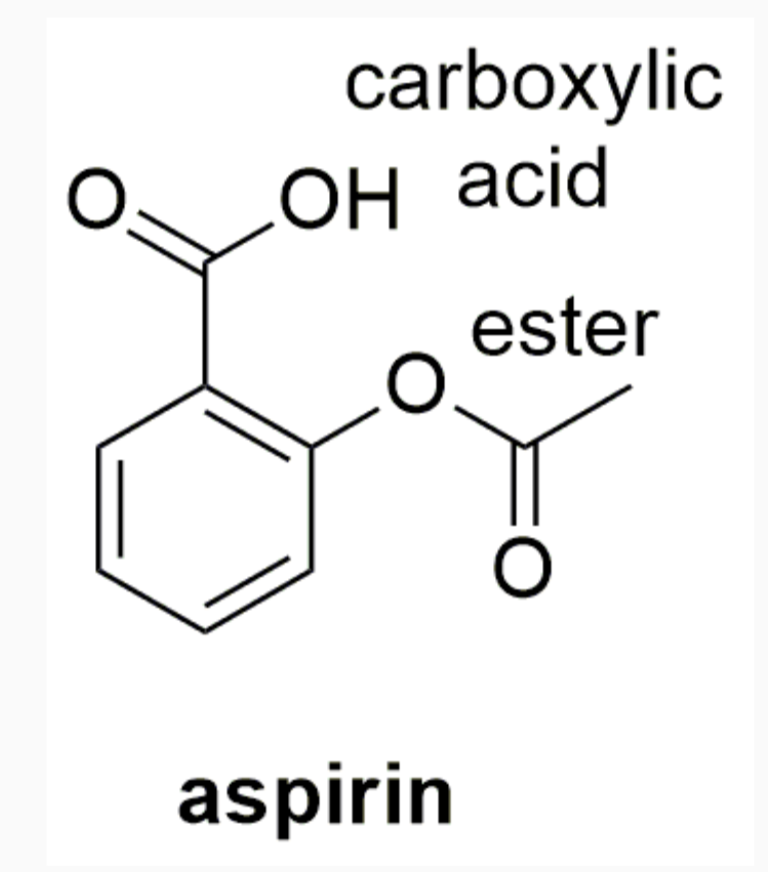
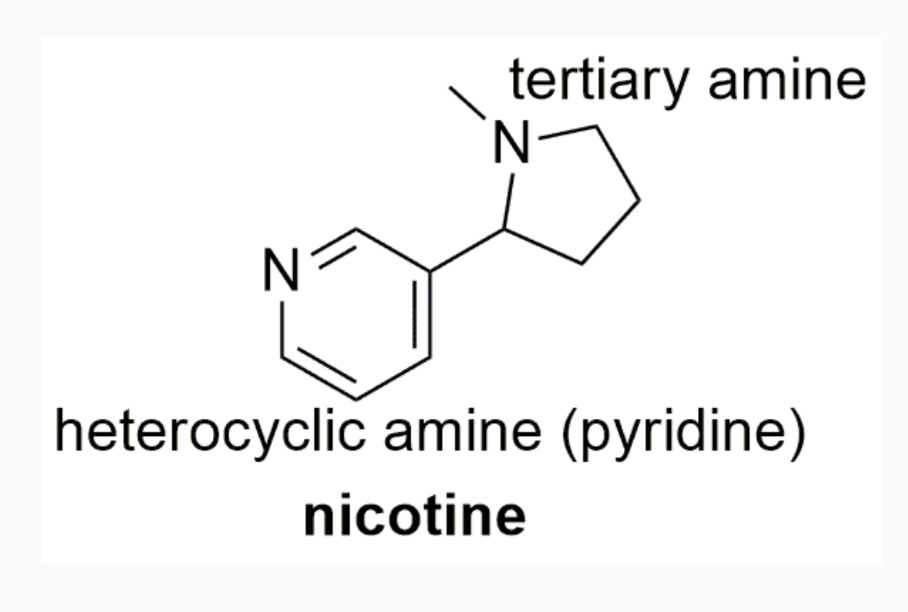
INTERMOLECULAR FORCES
1. Dispersion forces
- weakest intermolecular force (0.5-1.0 kcal/mole)
- electrostatic
- occurs between nonpolar groups (e.g. hydrocarbons)
- highly distance and temperature dependent

2. Dipole-Dipole Bonding
- stronger (1.0 to 10 kcal/mole)
- occurs electrostatically between electron deficient and electron rich atoms (dipoles)
- hydrogen bonding is a specific example of this bonding and serves as a prime contributor to hydrophilicity
- Dipole results from an unequal sharing of the pair of electrons in a covalent bond.
- Found when the two covalently bonded atoms differ greatly in electronegativity

3. Hydrogen Bonding
- Stronger bond than van der Waal
- Less affected by temperature or distance
- Give hydrophilic character to chemical
- Accounts for water-solubilizing properties of organic compounds

4. Ionic Bonding
- electrostatic attraction between cations and anions
- common in inorganic compounds and salts of organic molecules
- relatively strong (5 kcal/mole)
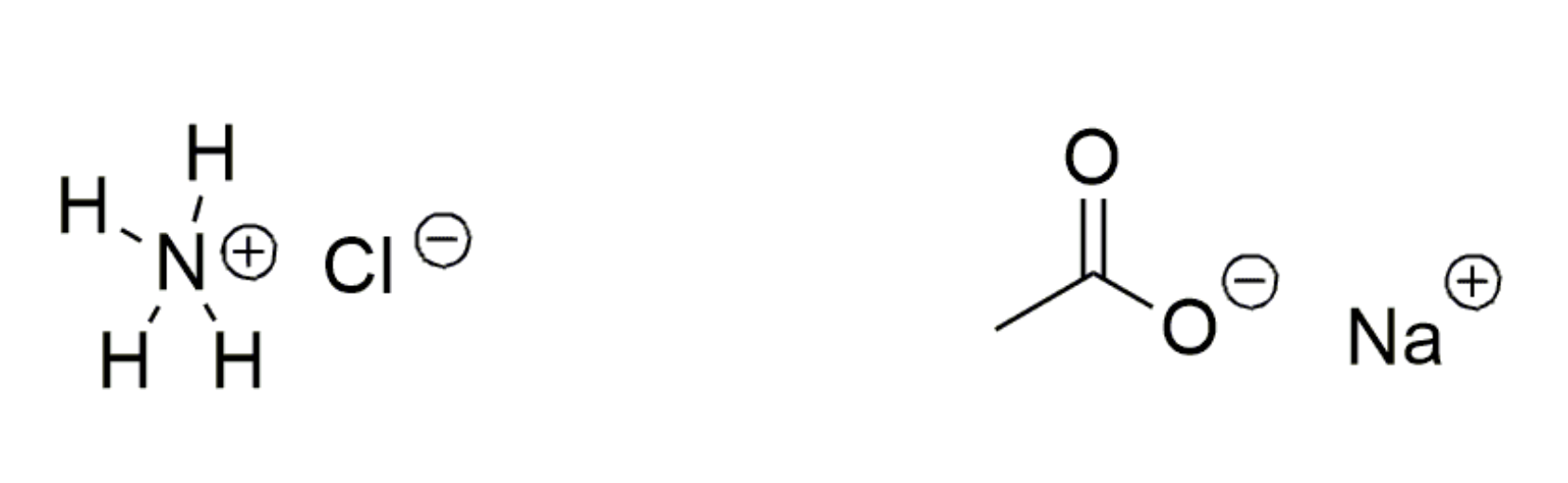
5. Ion-Dipole Bonding
- electrostatic between a cation/anion and a dipole
- relatively strong (1-5 kcal/mole)
- low temperature and distance dependence
- important attraction between organic compounds and H2O
- Electrostatic attraction that occurs between a formally charged ion and a dipole.
- If the salt can dissociate in water (i.e., separate), water solubility can occur.
- Can play a significant role in dissolving organic compounds in water

SOLUBILITY




OXIDATION, DEMETHYLATION, GLUCURONIDATION increase polarity and water solubility
INCREASE THE WATER SOLUBILITY TO INCREASE THE EXCRETION OF THE COMPOUND
=> This happens in the liver to convert higly lipid soluble to water soluble compound to excrete it from body


A form of a type of sugar called glucose that helps remove harmful substances from the body. Glucuronic acid and the harmful substance combine in the liver and then are passed in the urine.

Attaching glucoronic acid = glucoronide

The relative solubility of an organic compound is a function of the presence of both lipophilic and hydrophilic features within its structure, which serve to determine the extent of interaction of the organic compound with lipid and/or aqueous phases.
Look at the difference below between the solubility of heparin in water and the solubility of aspirin in water.
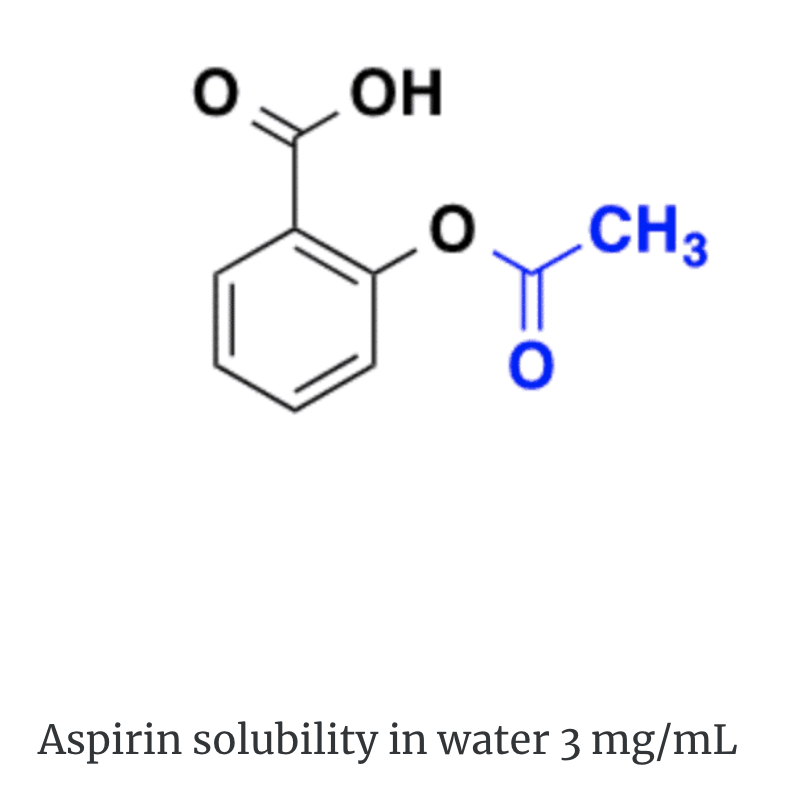

Polarity. The electrons of the hydrogen atoms are strongly attracted to the oxygen atom, and are actually closer to its nucleus than to those of the hydrogens. Therefore polarity plays a pivotal role in solubility. A polar solute will dissolve in a polar solvent whereas a non-polar solvent will dissolve in a non-polar solvent.
Non-polar compounds like aspirin will dissolve in lipid media. Polar and ionic compounds like heparin will dissolve in aqueous media.
LIKE DISSOLVES LIKE
Lipid media
Examples of lipid media include water-immiscible solvents such as hexane, di-ethyl ether, ethyl acetate. Octanol is used as a simple model for the phospholipid bilayer. Other examples include cell membranes (shown below), blood vessels and GI track membranes, and the blood-brain barrier.
The predominant intermolecular bonds in these cases are van der Waal interactions. They are found in the aliphatic and aromatic portions of organic molecules (present to some extent in ALL organic compounds).
Aqueous media
Examples of aqueous media include water, blood, and cellular cytosol.
In this case, dipole-dipole bonding (hydrogen bonding) and ion-dipole bonding is important. They are also an important characteristic of many of the organic functional groups.
Examination of the structure of chloramphenicol (as seen below) indicates the presence of both lipophilic (nonpolar) and hydrophilic (polar) groups. The presence of oxygen and nitrogen containing functional groups usually enhances water solubility. While lipid solubility is enhanced by nonionizable hydrocarbon chains and ring systems.
Carboxylate









DRUG METABOLISM









CHOLESTEROL BIOSYNTHESIS

(Lipid transport pathway)
- Exogenous (originating from outside an organism)
- Endogenous (originating from inside an organism)


Exogenous pathway
Dietary lipids are absorbed from food in the form of cholesterol & fatty acids. Fatty acids are then re-esterified &, along with cholesterol, incorporated into chylomicrons. During circulation, the chylomicrons are degraded by lipases. The liberated free acids are then available for storage or energy generation
Endogenous pathway
Begins in the liver with the formation of VLDL. Metabolism of VLDL is similar to the chylomicrons, in that the lipoprotein lipase reduces the triglyceride content of VLDL & increases the availability of free fatty acids. The formed IDL is then metabolised to LDL & transported to the liver. LDL is responsible for almost 2/3rds of total plasma cholesterol.
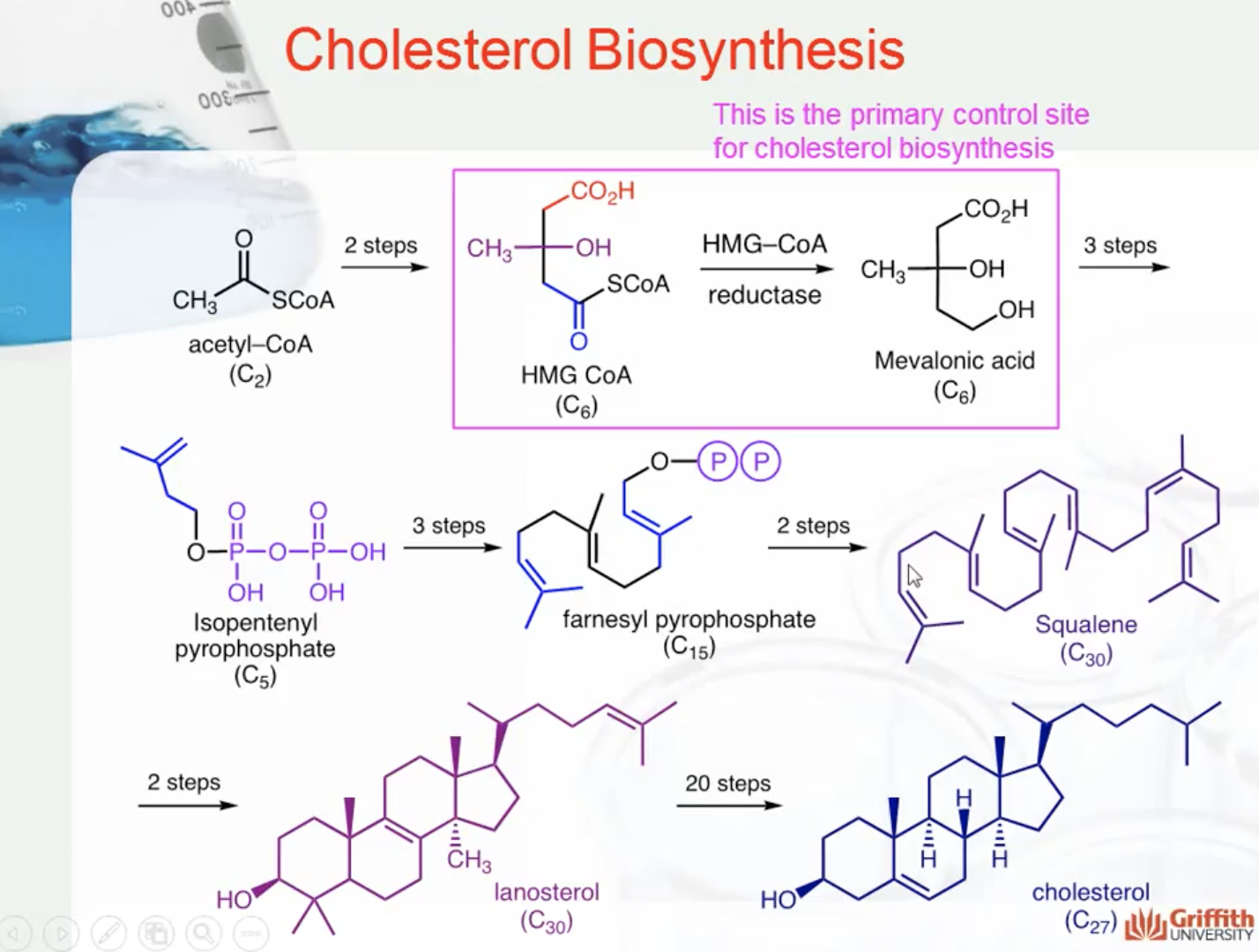




HMG CoA Reductase Inhibitors
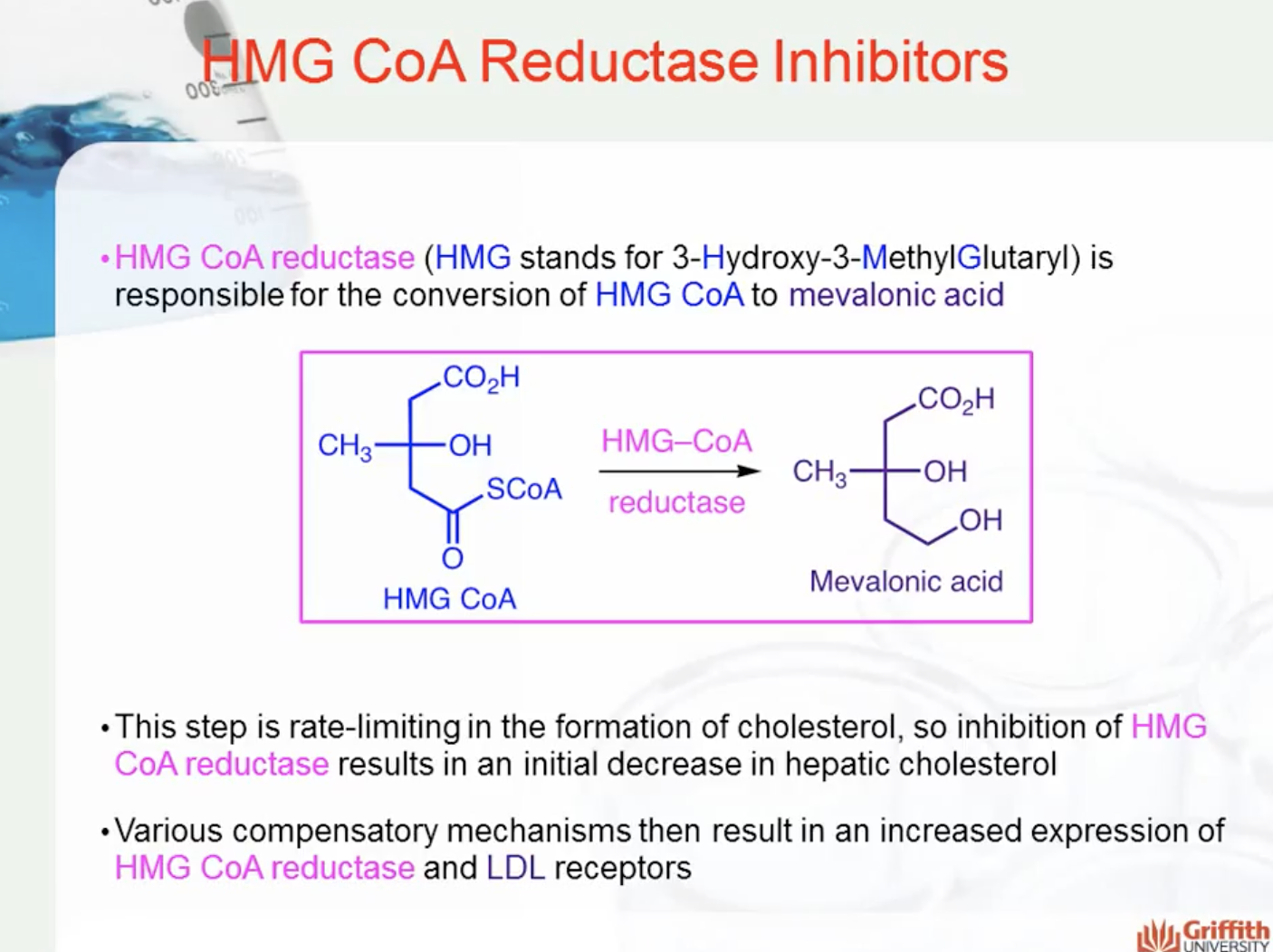
HMG CoA reductase (HMG stands for 3-Hydroxy-3-MethylGlutaryl) is responsible for the conversion of HMG CoA to mevalonic acid (seen in the diagram below). This step is rate-limiting in the formation of cholesterol, so inhibition of HMG CoA reductase results in an initial decrease in hepatic cholesterol. Various compensatory mechanisms then result in an increased expression of HMG CoA reductase and LDL receptors.
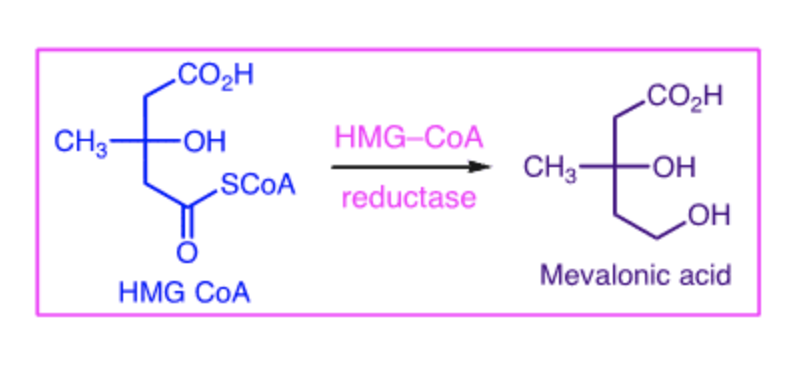
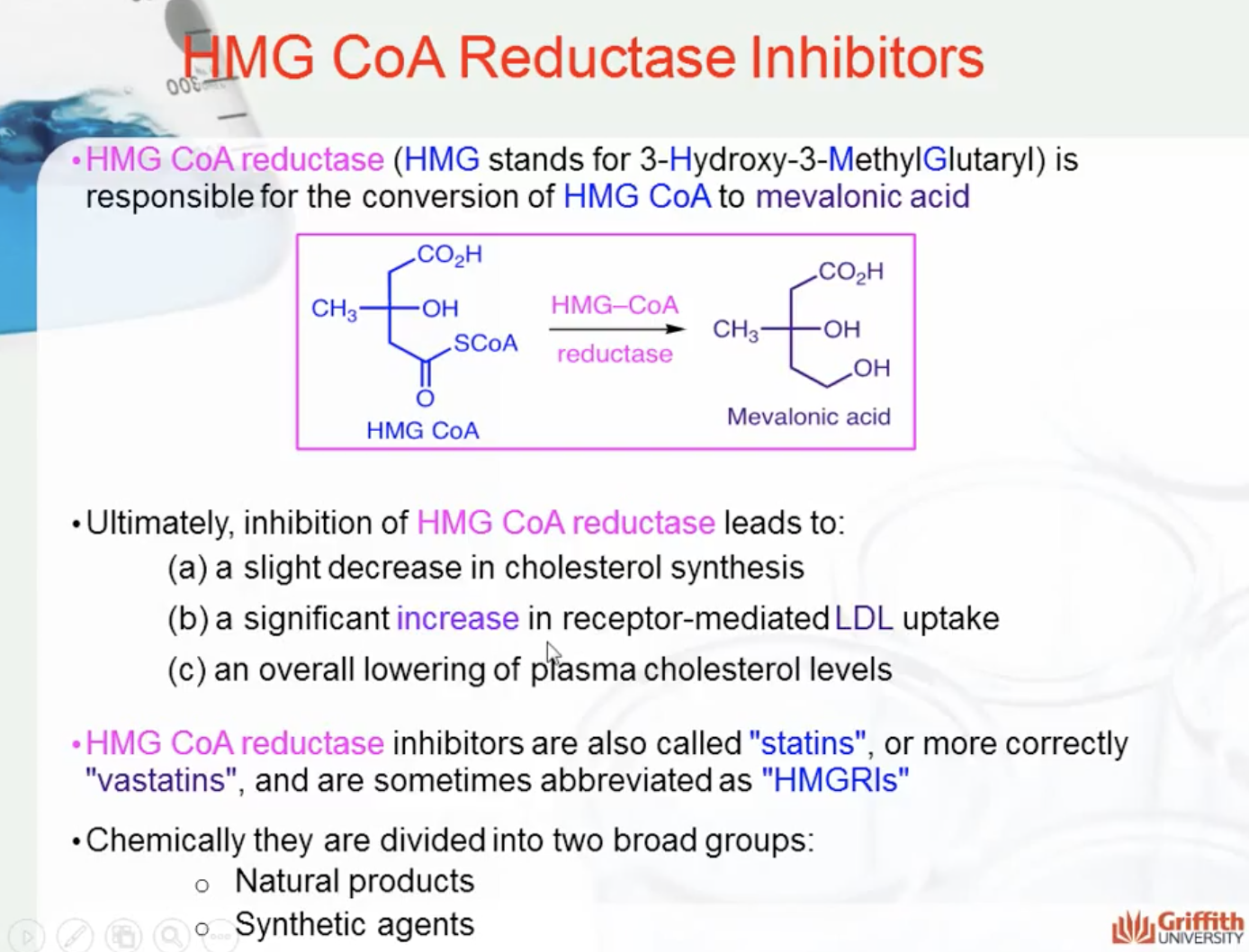
Ultimately, inhibition of HMG CoA reductase leads to:
- a slight decrease in cholesterol synthesis
- a significant increase in receptor-mediated LDL uptake
- an overall lowering of plasma cholesterol levels
HMG CoA reductase inhibitors are also called "statins", or more correctly "vastatins", and are sometimes abbreviated as "HMGRIs". Chemically they are divided into two broad groups:
- Natural products
- Synthetic agents
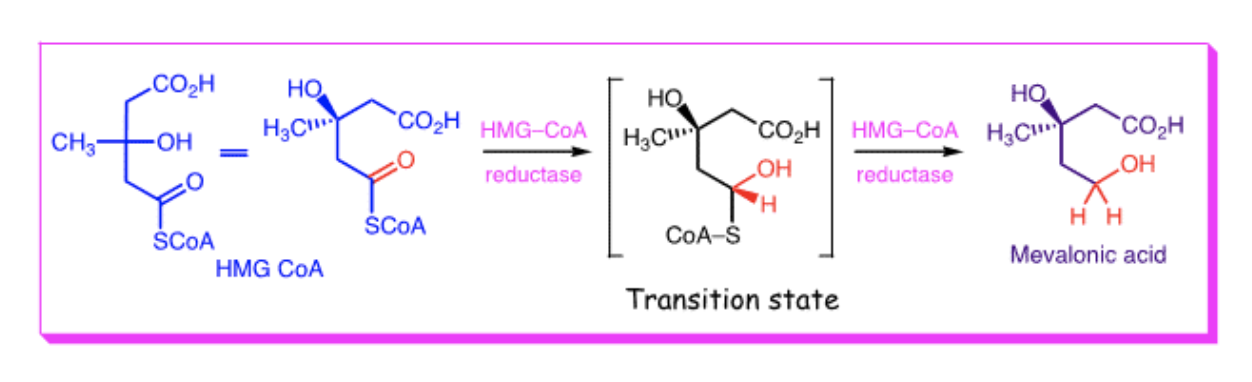
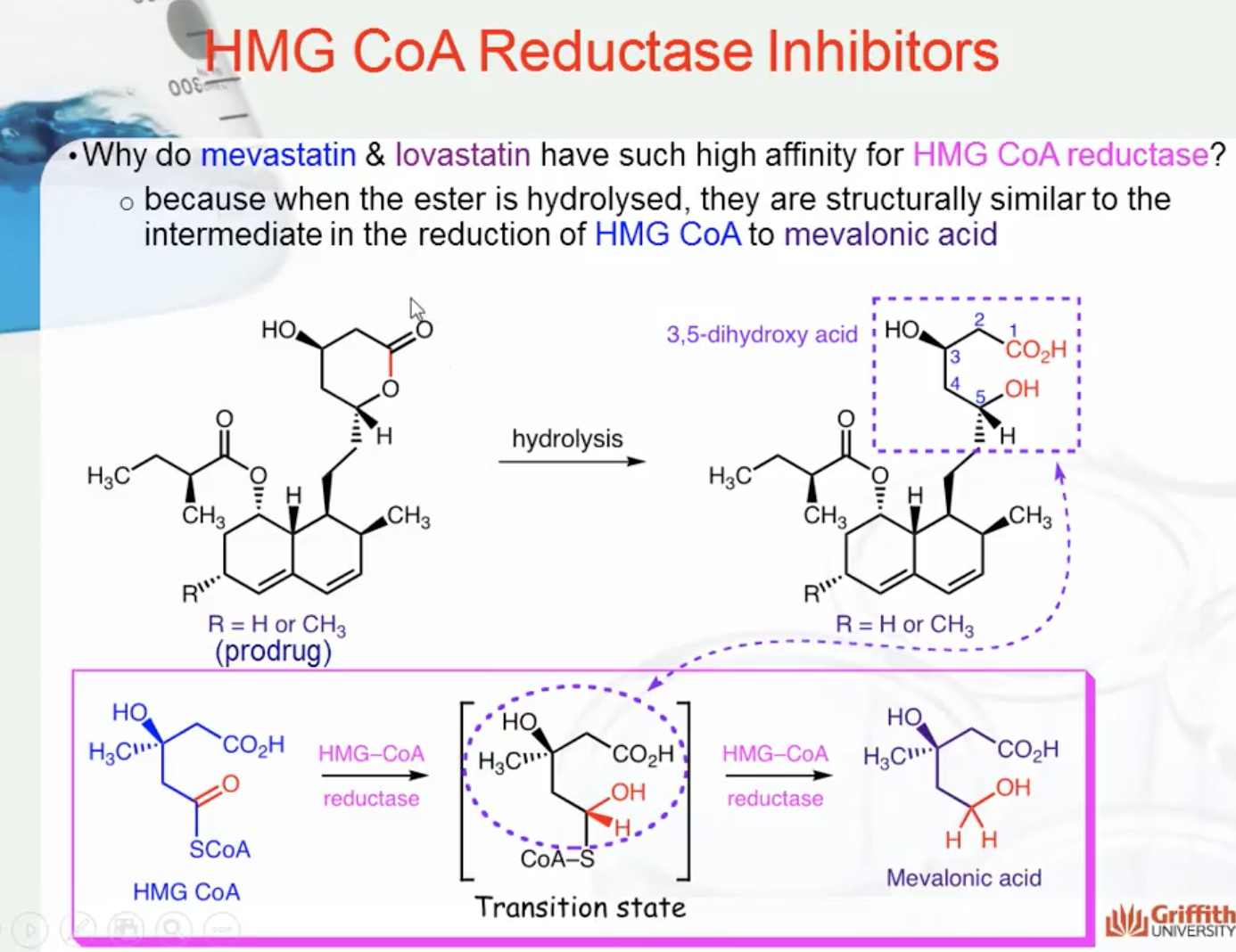
In 1976 a natural product (from a penicillium species) was discovered to have affinity for HMG CoA reductase 10,000 times greater than HMG CoA itself. This molecule was mevastatin. Later, a related structure (lovastatin) was found to be more than twice as potent as mevastatin (i.e. double the affinity for binding to HMG CoA reductase).

Why do mevastatin & lovastatin have such high affinity for HMG CoA reductase? When the ester is hydrolysed, they are structurally similar to the intermediate in the reduction of HMG CoA to mevalonic acid. You can see that similarity in the diagram below.

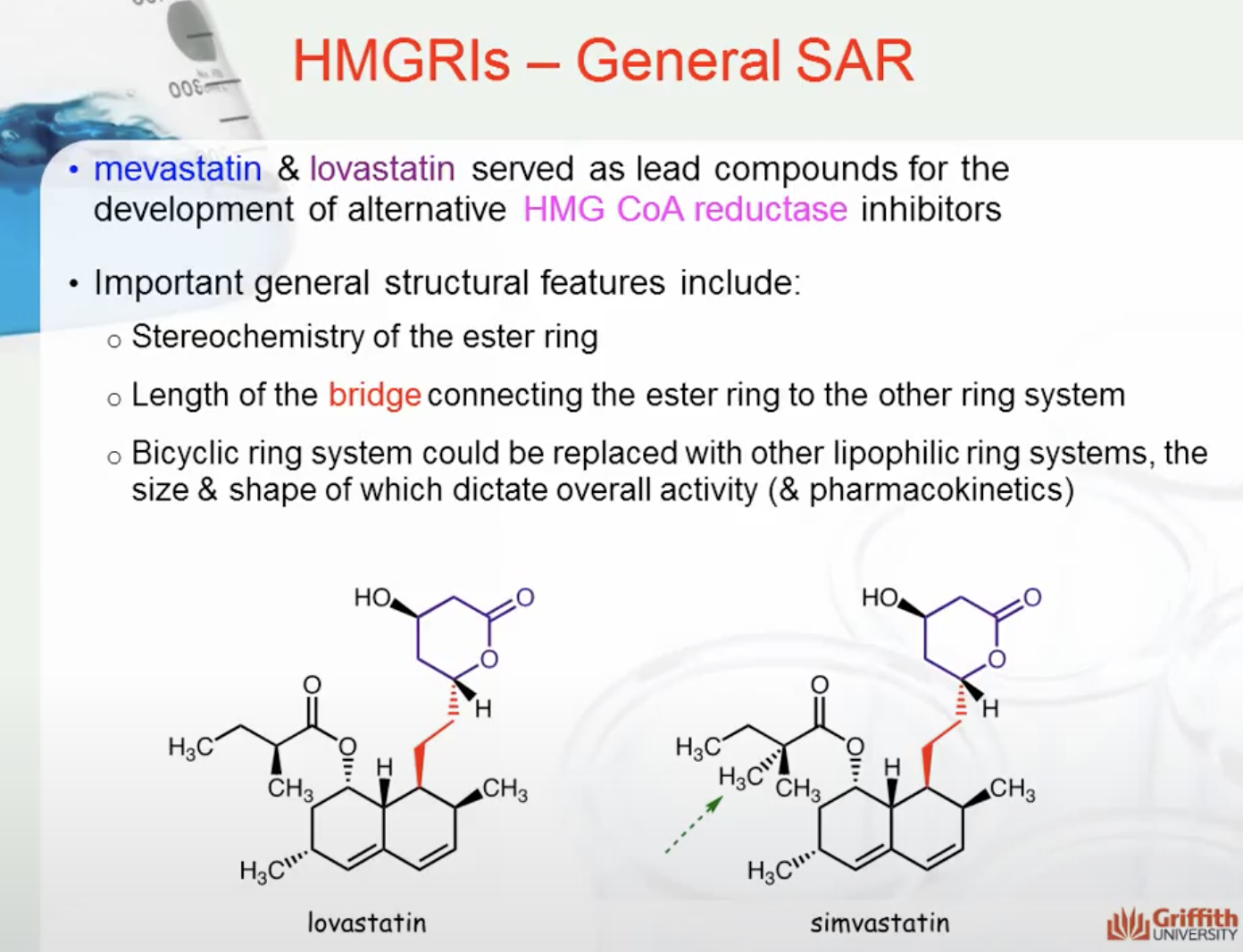
Mevastatin & lovastatin served as lead compounds for the development of alternative HMG CoA reductase inhibitors. Important general structural features include:
- Stereochemistry of the ester ring
- Length of the bridge connecting the ester ring to the other ring system
- Bicyclic ring system could be replaced with other lipophilic ring systems, the size & shape of which dictate overall activity (& pharmacokinetics)

A significant breakthrough in the development of the statins was the discovery of the 3,5-dihydroxy acid analogues. These are "hydrolysed ester" analogues, & are more hydrophilic than the corresponding ester compounds. Note: acid + hydroxyl functional groups versus an ester, as well as the additional hydroxyl in the bicyclic lipophilic portion. It is suggested that the increased hydrophilicity of these compounds leads to better selectivity (for hepatic tissue) & therefore a reduction in side effects.
Following the realisation that 3,5-dihydroxy acid analogues were better agents, structural variation in the "bicyclic ring" portion (= lipophilic) was investigated. Initially, these modifications were to simplify the complexity of the bicyclic ring system of mevastatin & lovastatin. Compounds with significant HMG CoA reductase inhibition were developed.

From 1996 to 2012 under the trade name Lipitor, atorvastatin was the world's best-selling drug, with more than US $125 billion in sales. It treats cardiovascular disease by inhibiting HMG CoA reductase, resulting in lower cholesterol levels.
'Griffith college Tri1 2023 > 1015 MSC (Chem2)' 카테고리의 다른 글
| [WEEK12] The Chemistry of Exercise (0) | 2023.05.26 |
|---|---|
| [WEEK11] Nucleic Acids (0) | 2023.05.16 |
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (1) | 2023.05.13 |
| [WEEK10] Enzymes, Metals in Biological System & Chemistry of Medicine (0) | 2023.05.13 |
| [WEEK6]Chemistry of Food- Carbohydrates & Carbohydrates (0) | 2023.04.02 |



