Chemical Equilibrium
Part 1: Chemical equilibrium: Reversible reactions, Rates of reaction, Intro to chemical equilibrium
Learning Outcomes 1-2
Key Concepts
- Reversible reactions
- Rates of reactions
- Chemical equilibrium
What Are Reversible Reactions?




Effective and ineffective Collision


What is Dynamic Equilibrium?



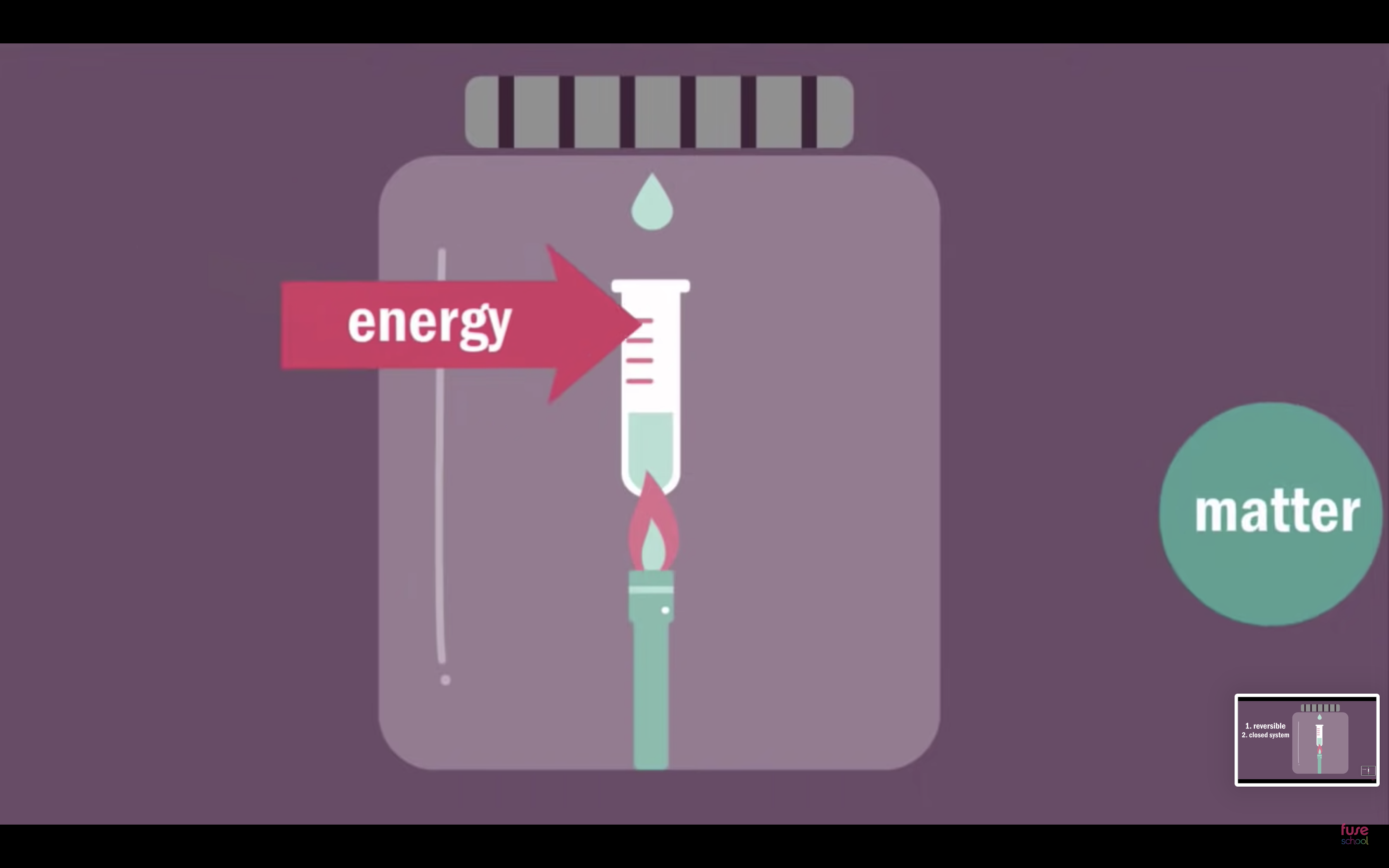
- 조건 : 1. reversible 2. closed system ( only energy can come in )
Part 2: Chemical equilibrium - Le Châtelier’s Principle: Effect of: Concentration, temperature, volume, & catalyst
Learning Outcomes: 3-6
Key Concept
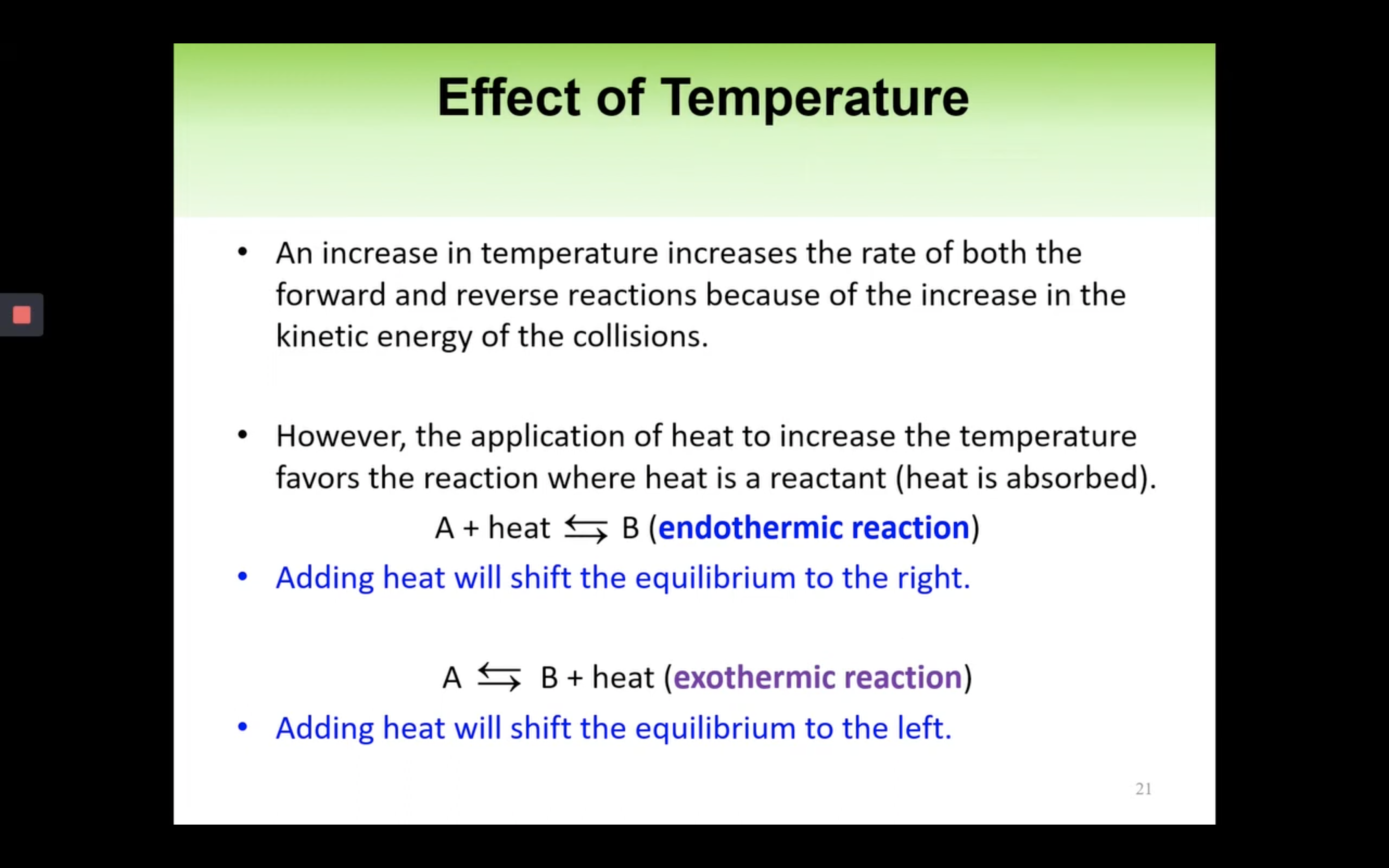
- Le Châtelier’s Principle
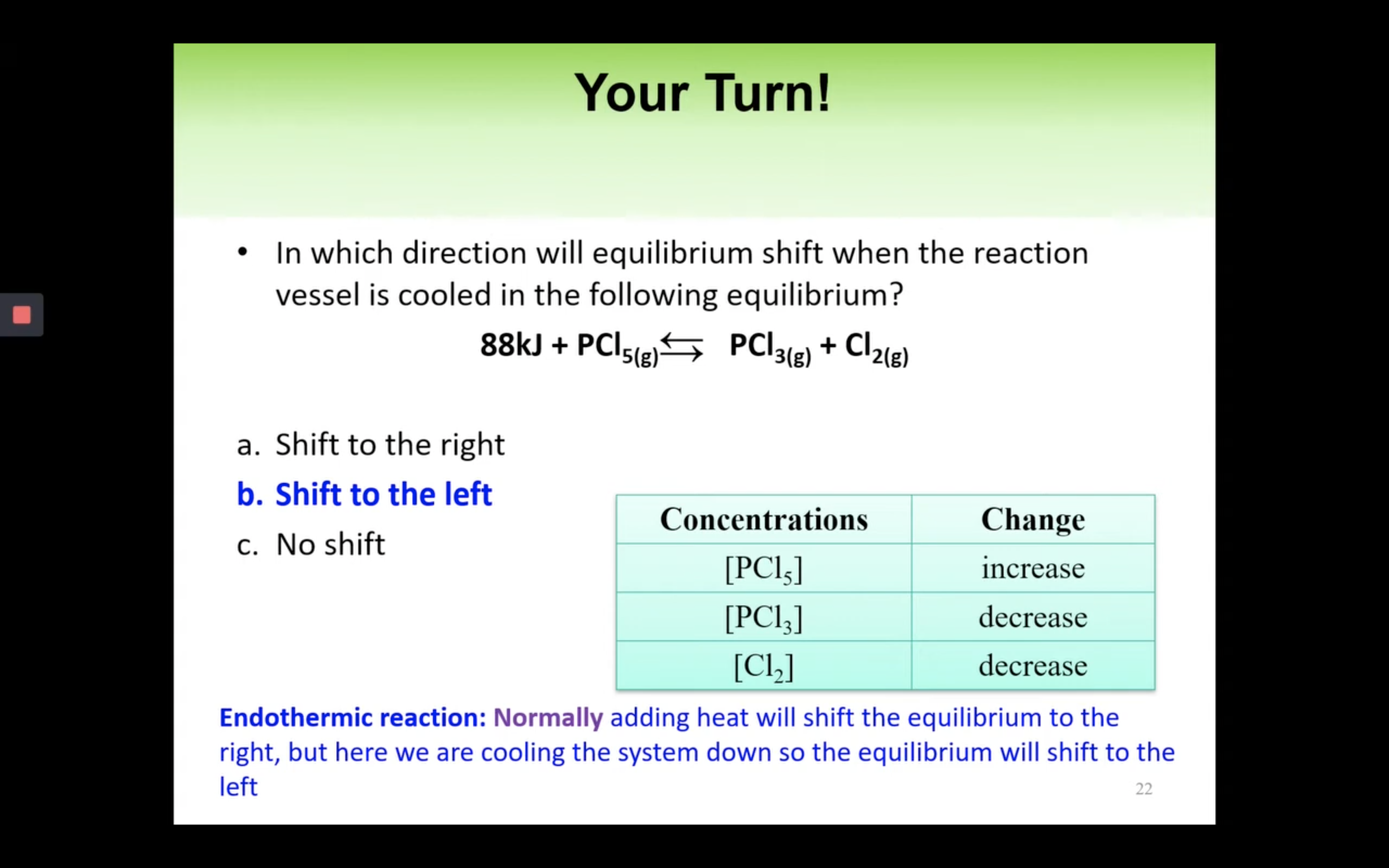
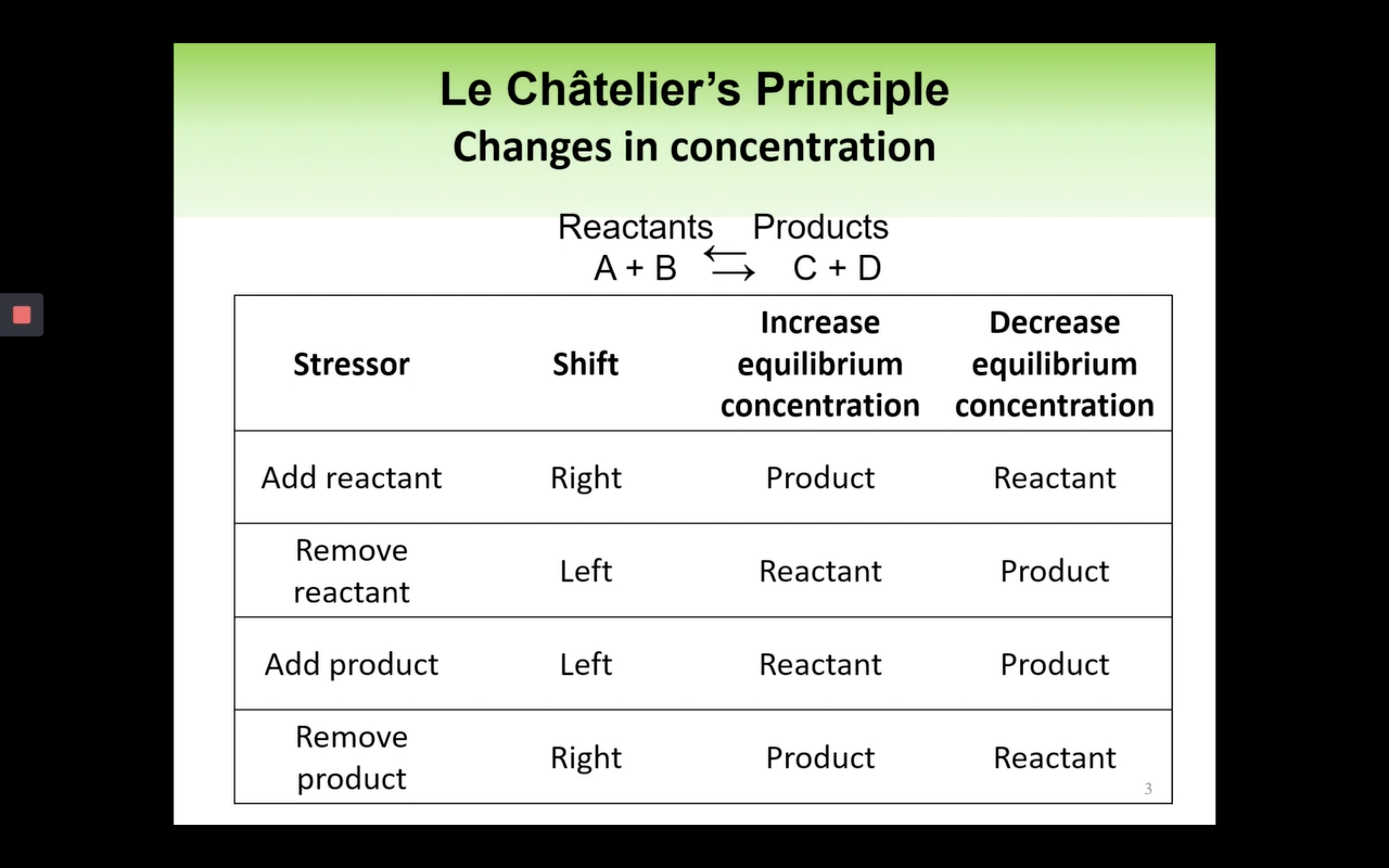
Le Châtelier’s Principle-effect of [Concentration], Volume (g) and temperature
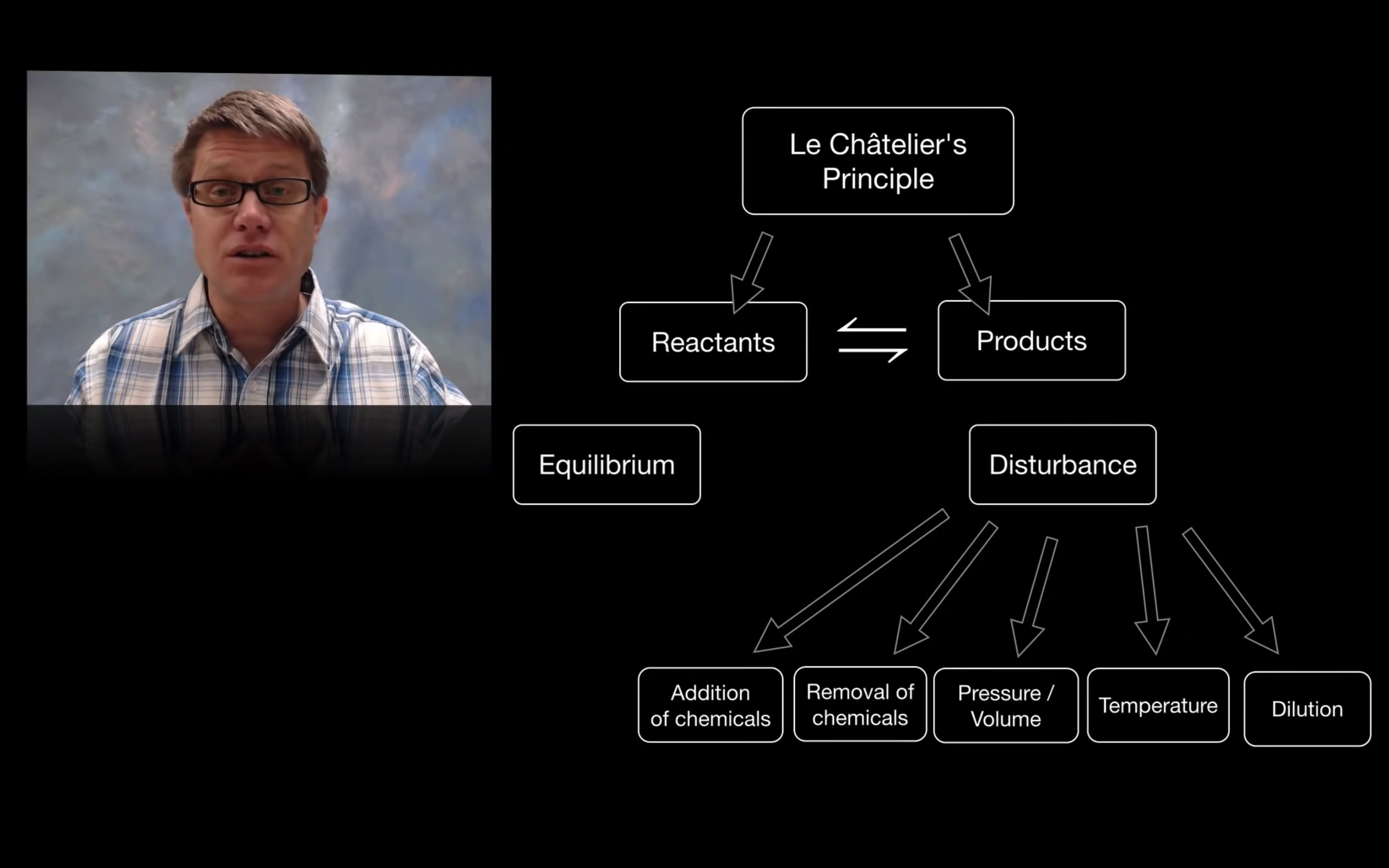
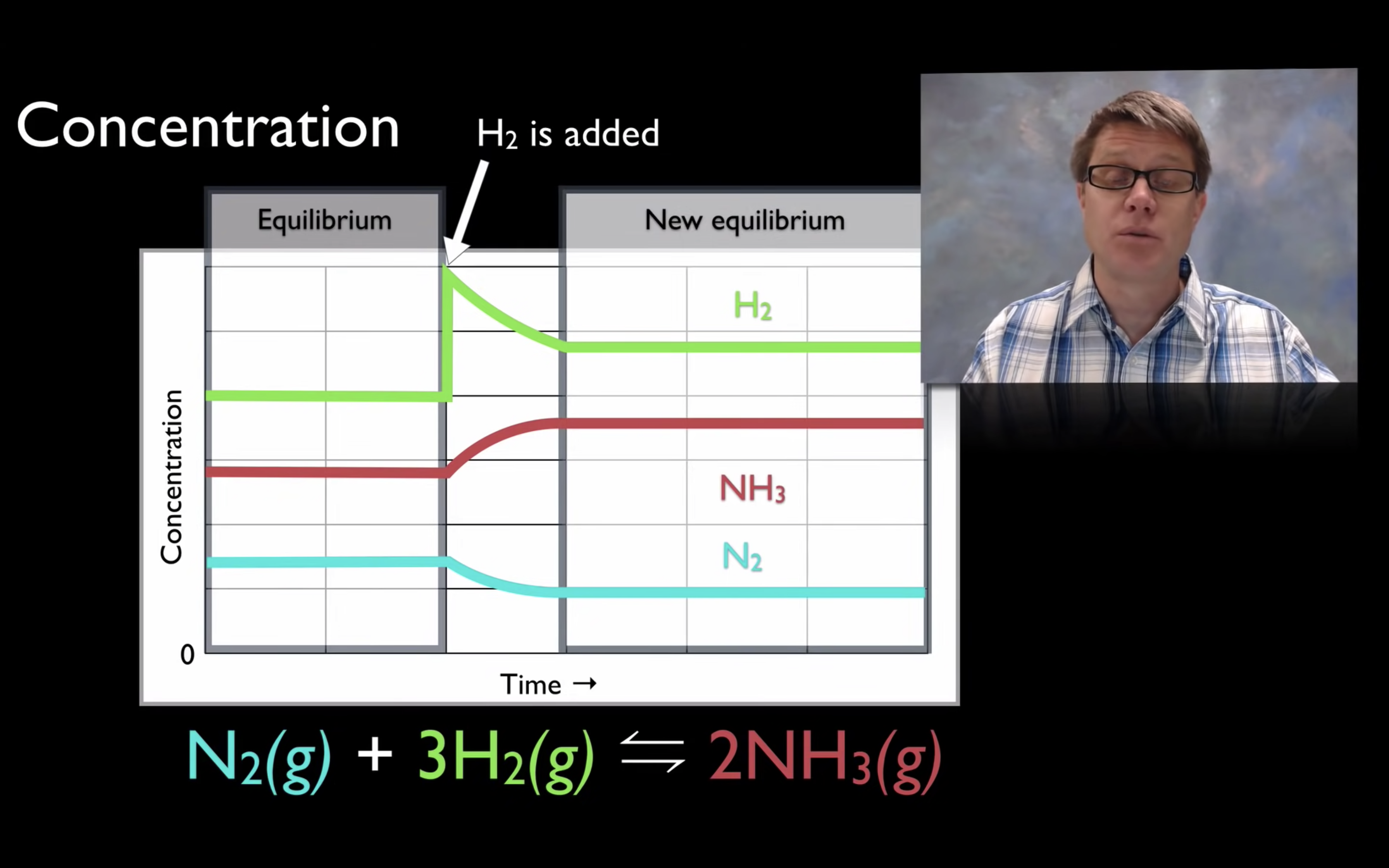
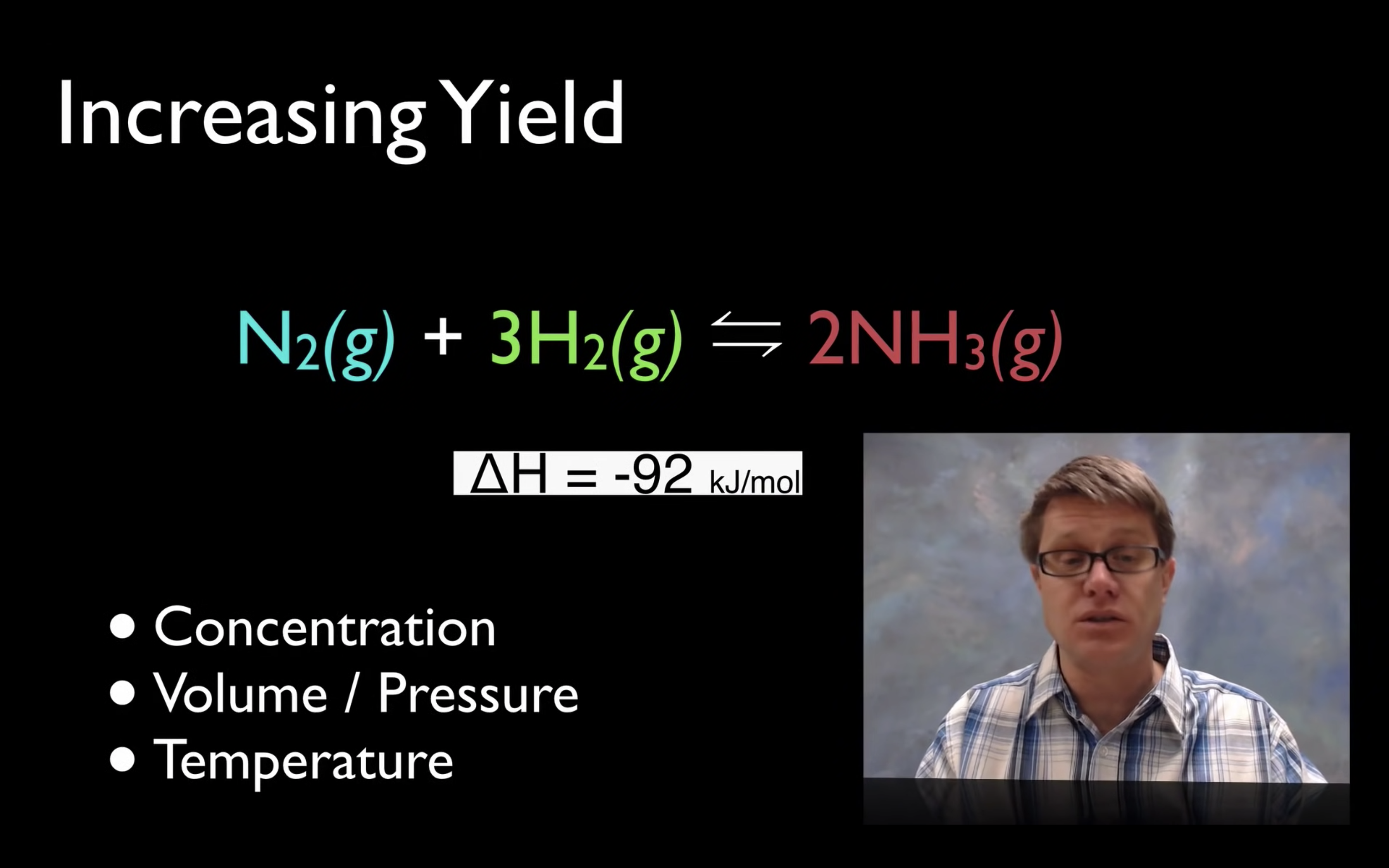

By changing the concentration (adding more reactants), volume and pressure, and temperature,
yield can increase so the reaction can shift forward or backward.
Worked out Question- Temperature & Catalyst
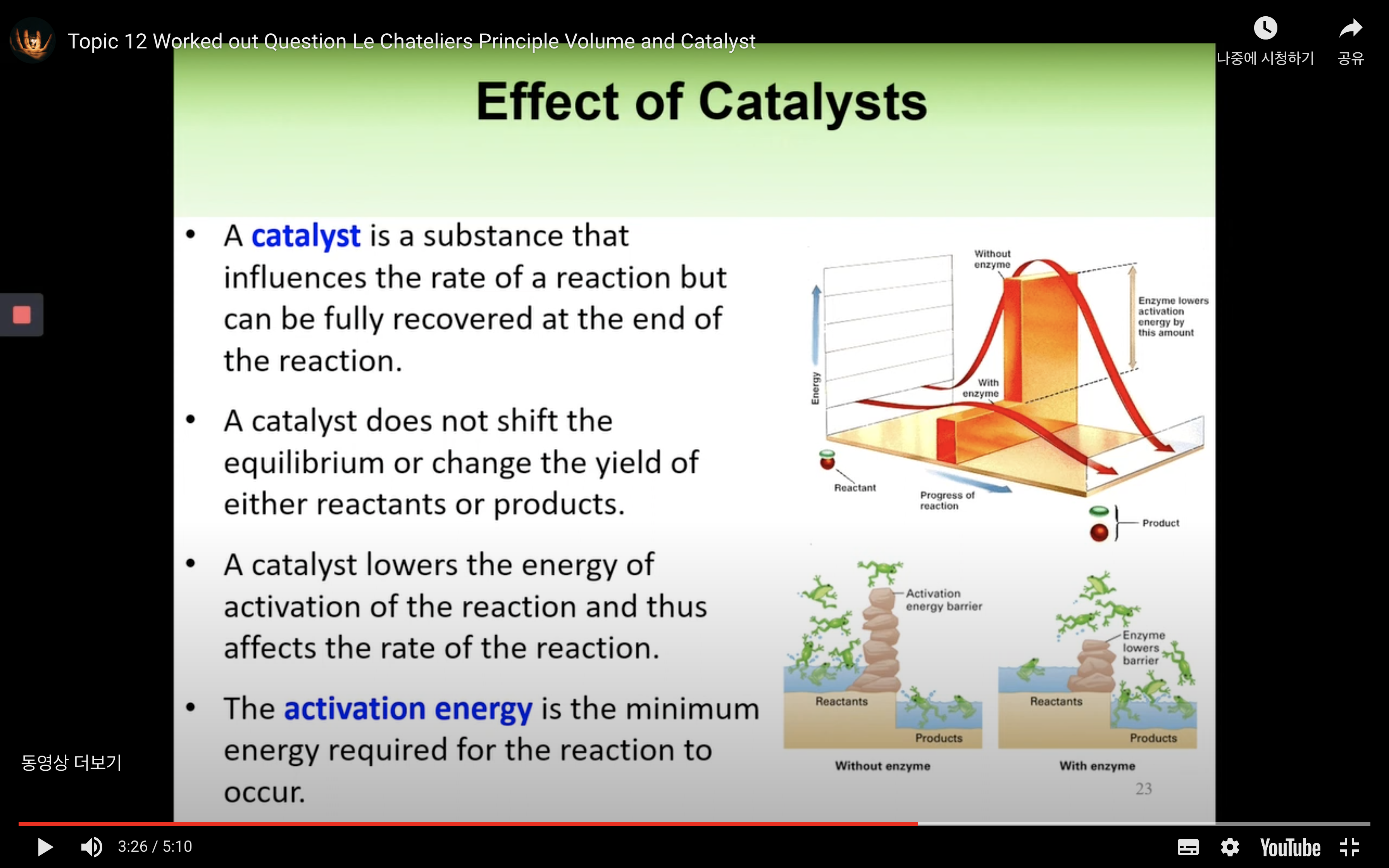

-catalyst is a substance that influences the rate of reaction but can be fully recovered at the end of the reaction.
A catalyst does not shift the equilibrium or change the yield of either reactants or products.
A catalyst lower the energy of activation of the reaction and thus affects the rate of the reaction.
Activation energy is the minimum energy required for the reaction to occur.
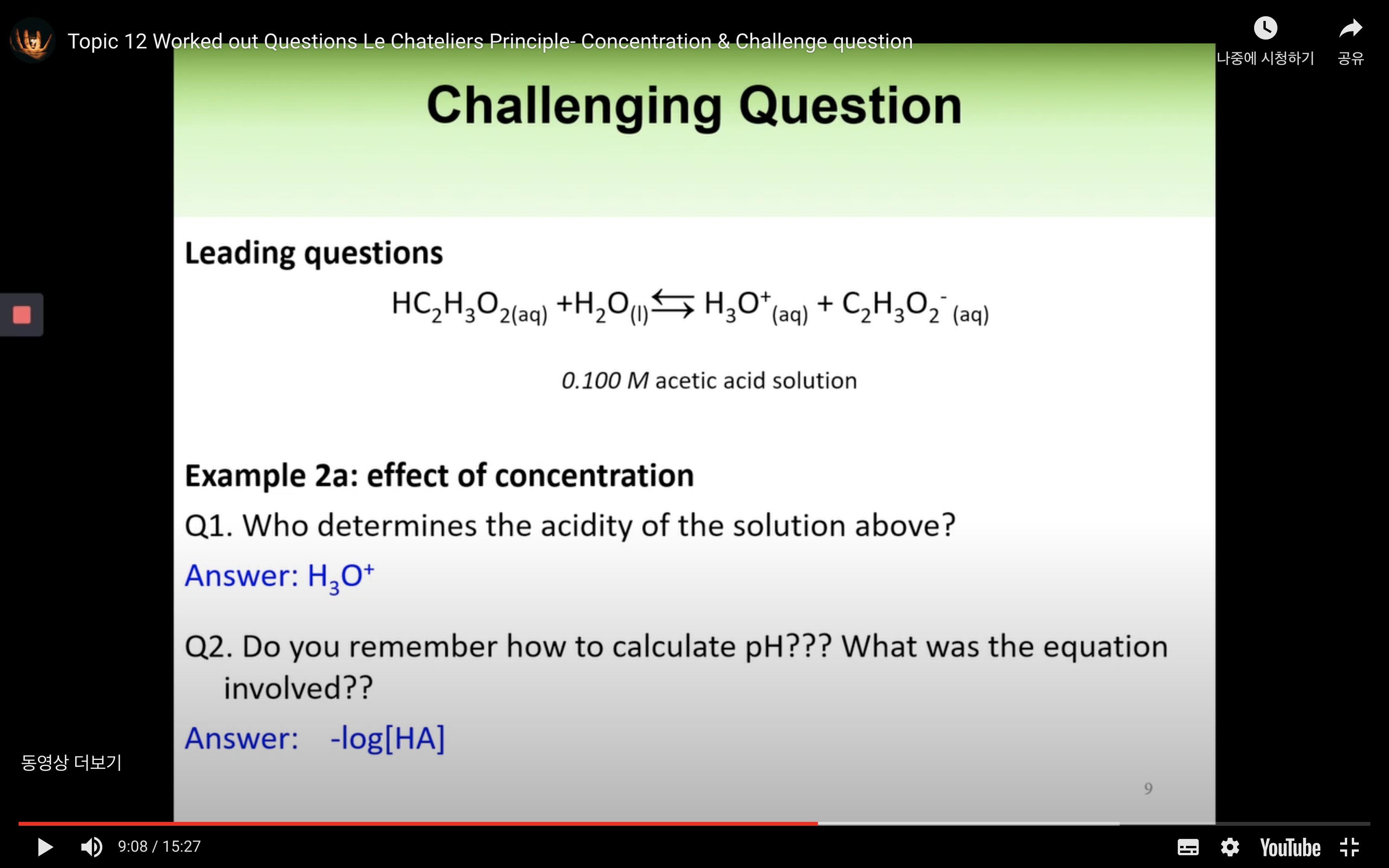
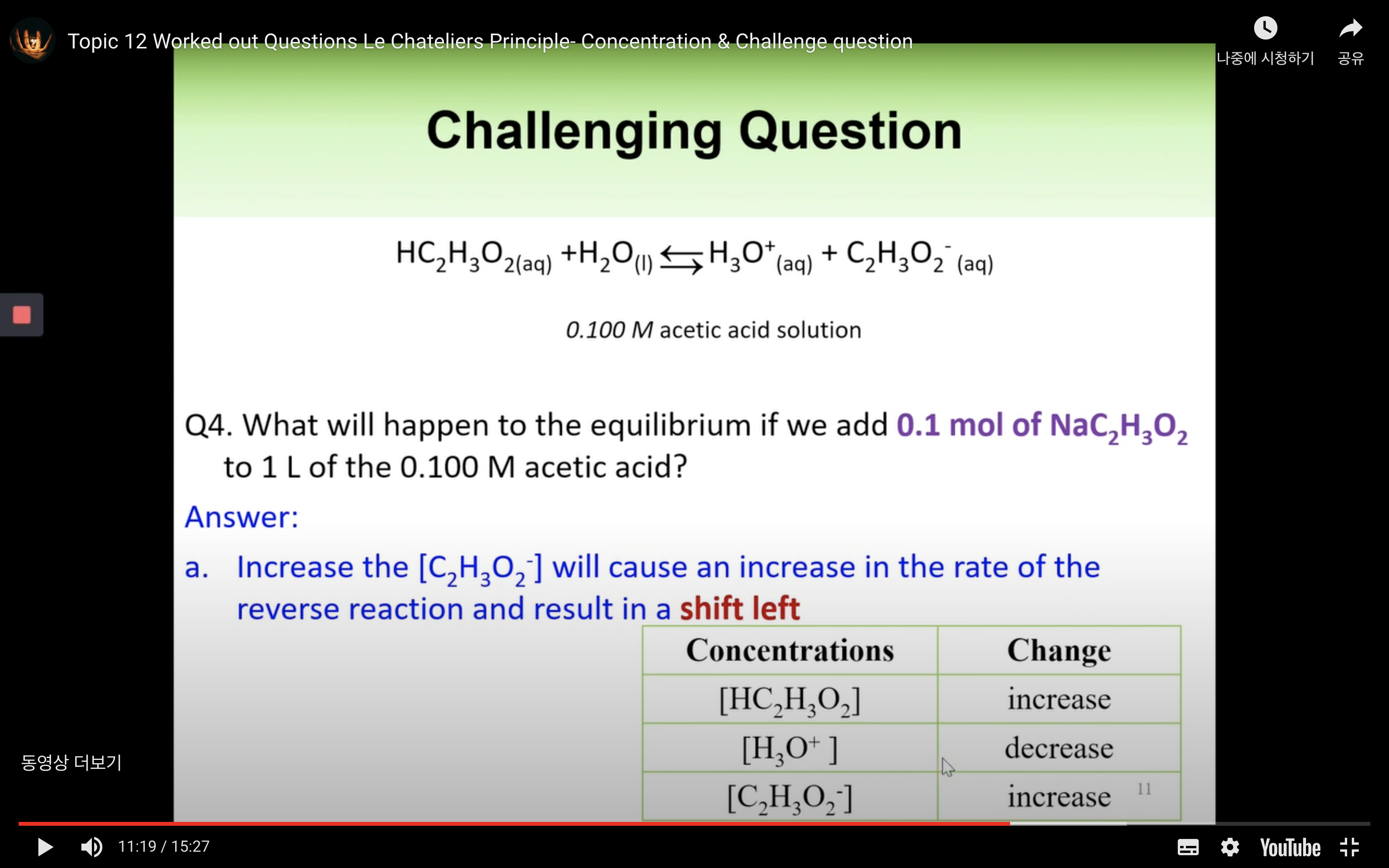
Worked out Questions- Concentration & Challenge Question





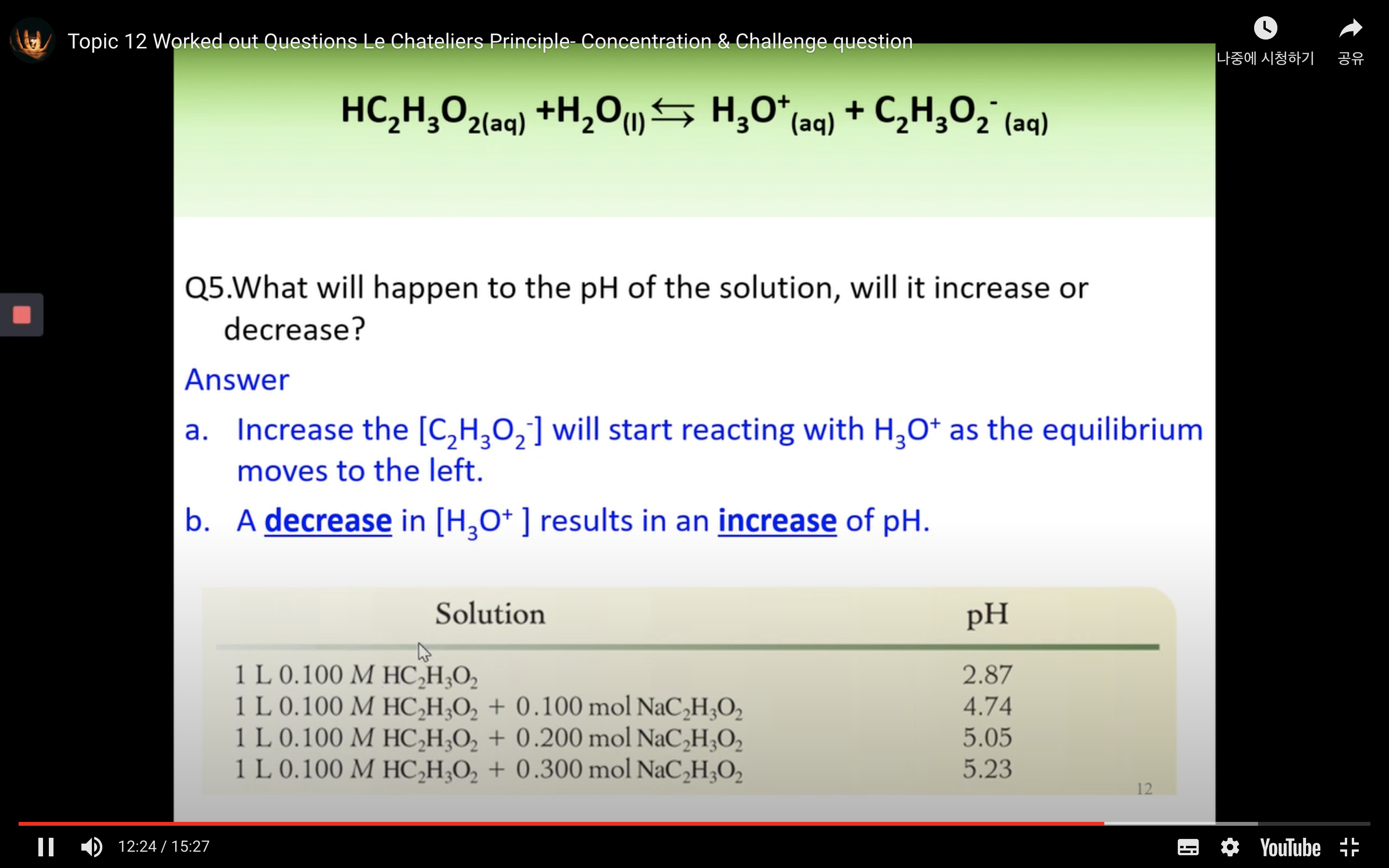

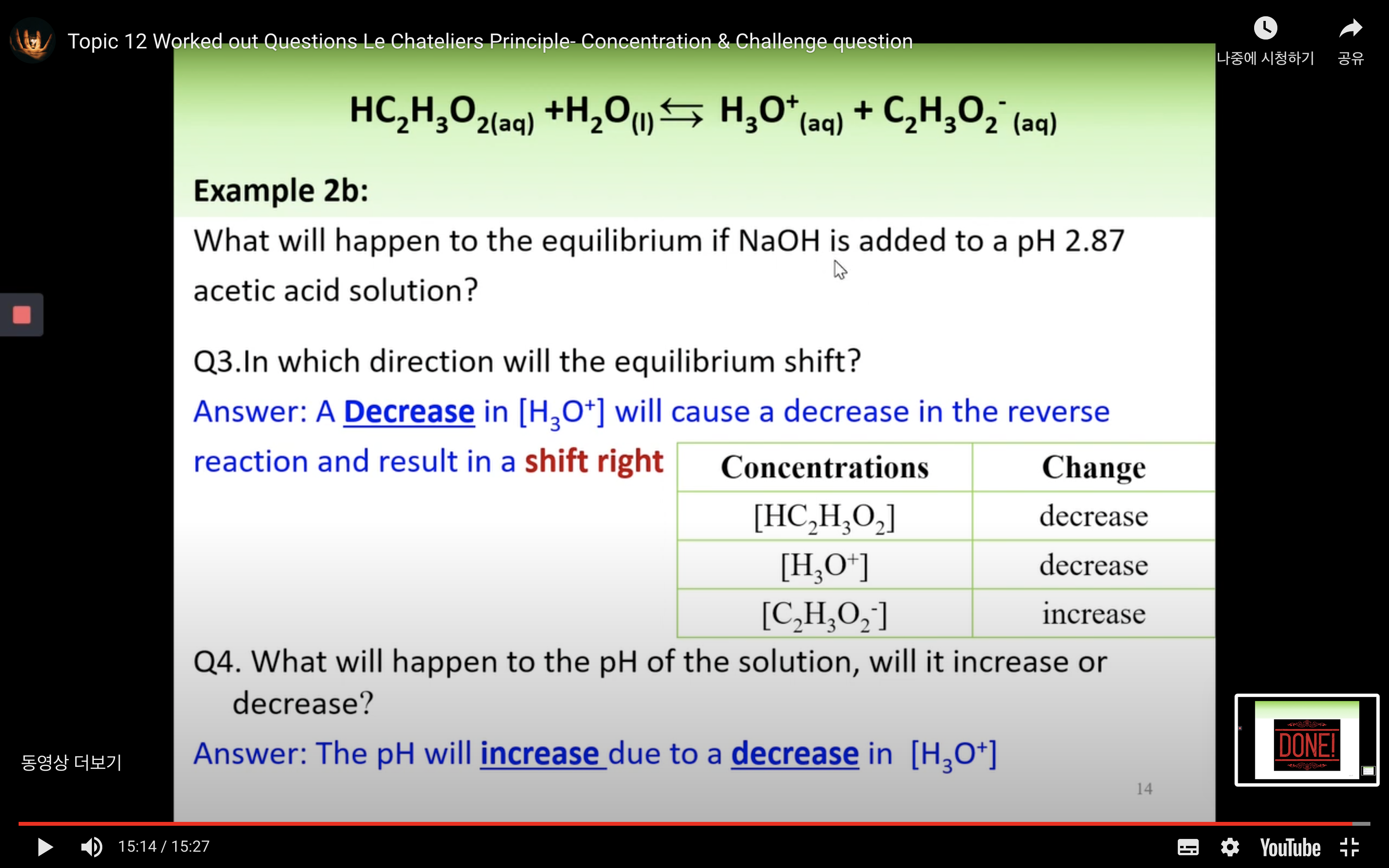
Part 3: Chemical equilibrium - Equilibrium constants: Keq, Kw, Ka
Learning Outcomes: 7-8
Key Concepts
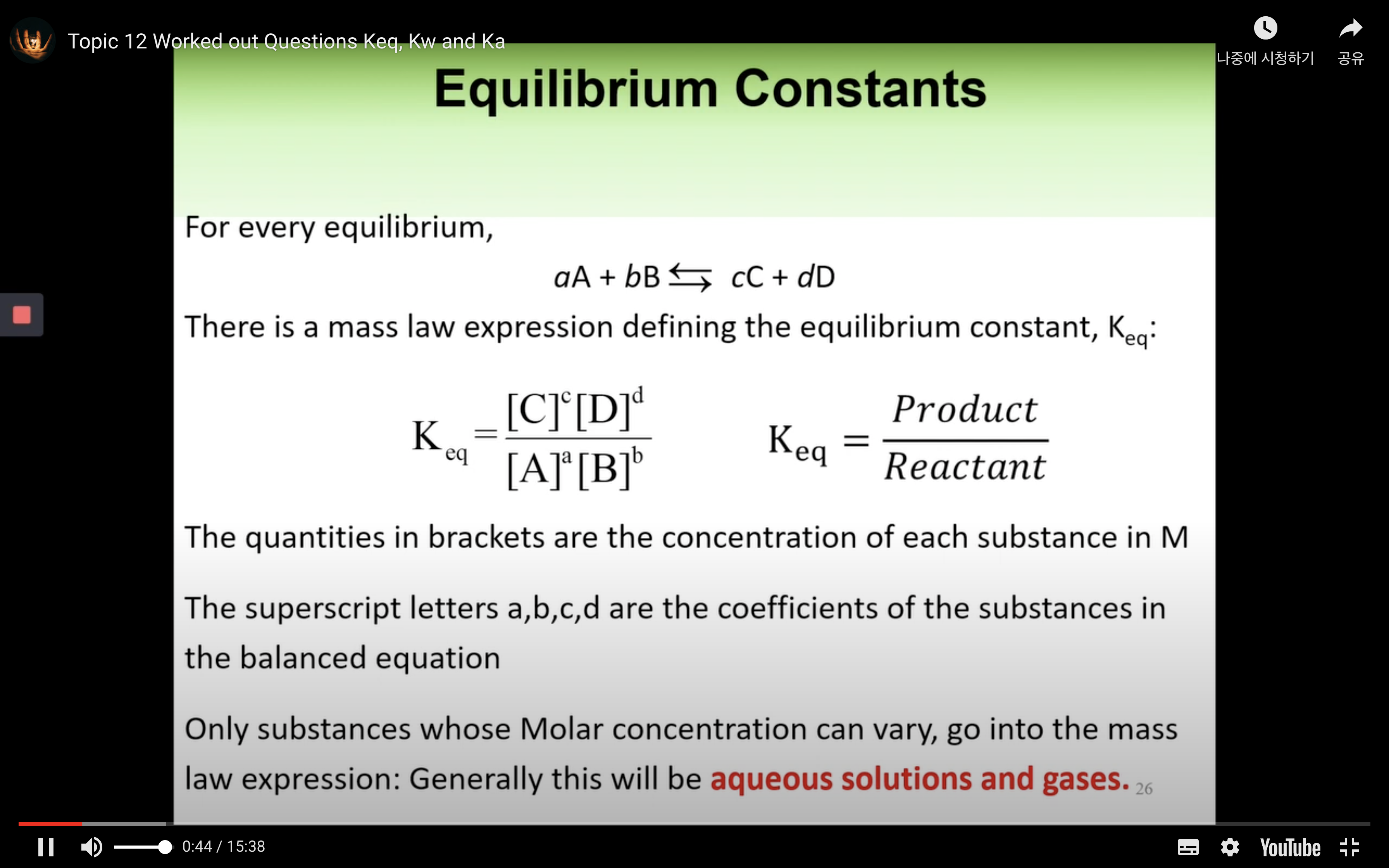
- Chemical equilibrium constants
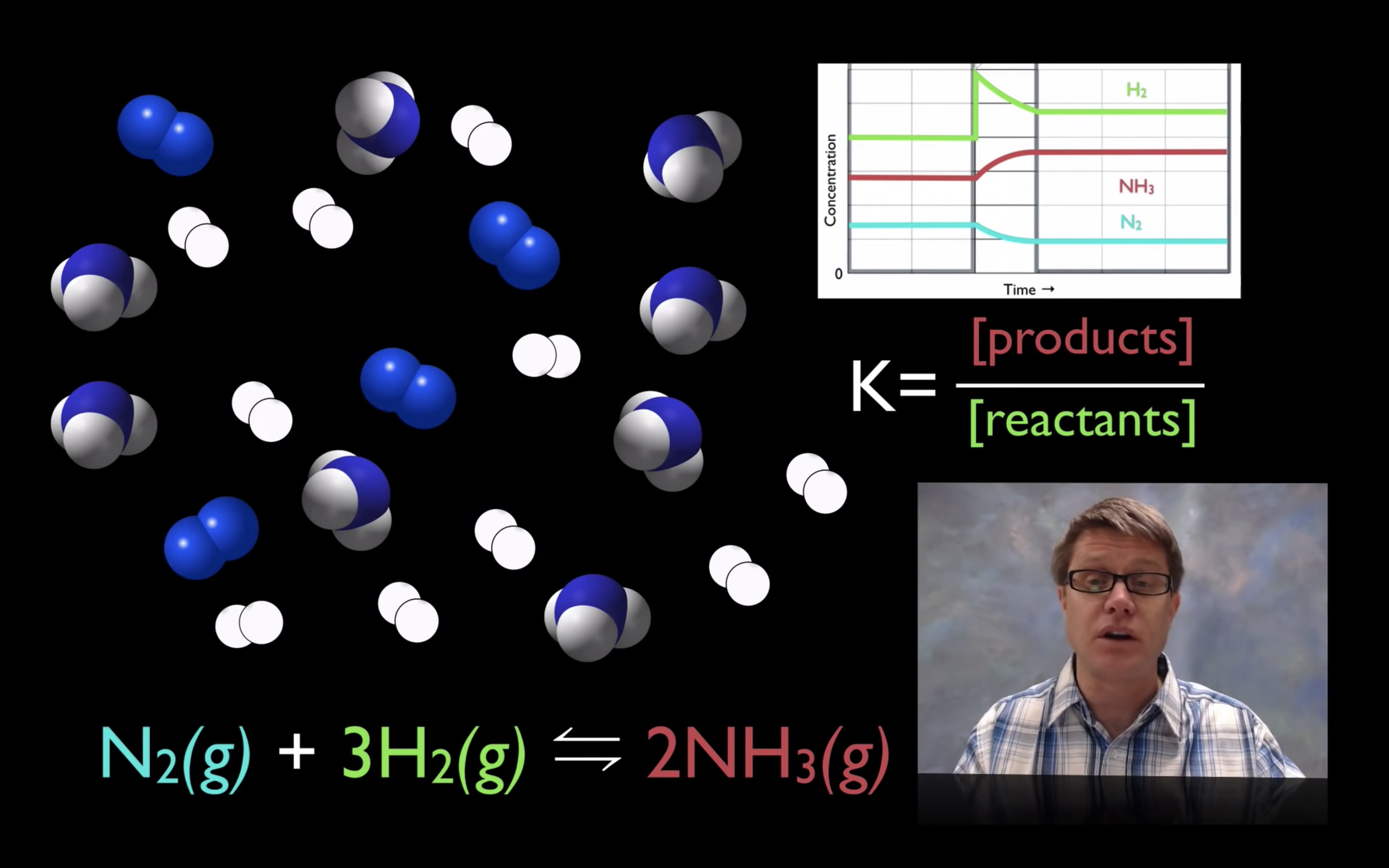
Kc or Keq
Please note that in this video they use Kc and we use both Kc and Keq. They are equivalent!

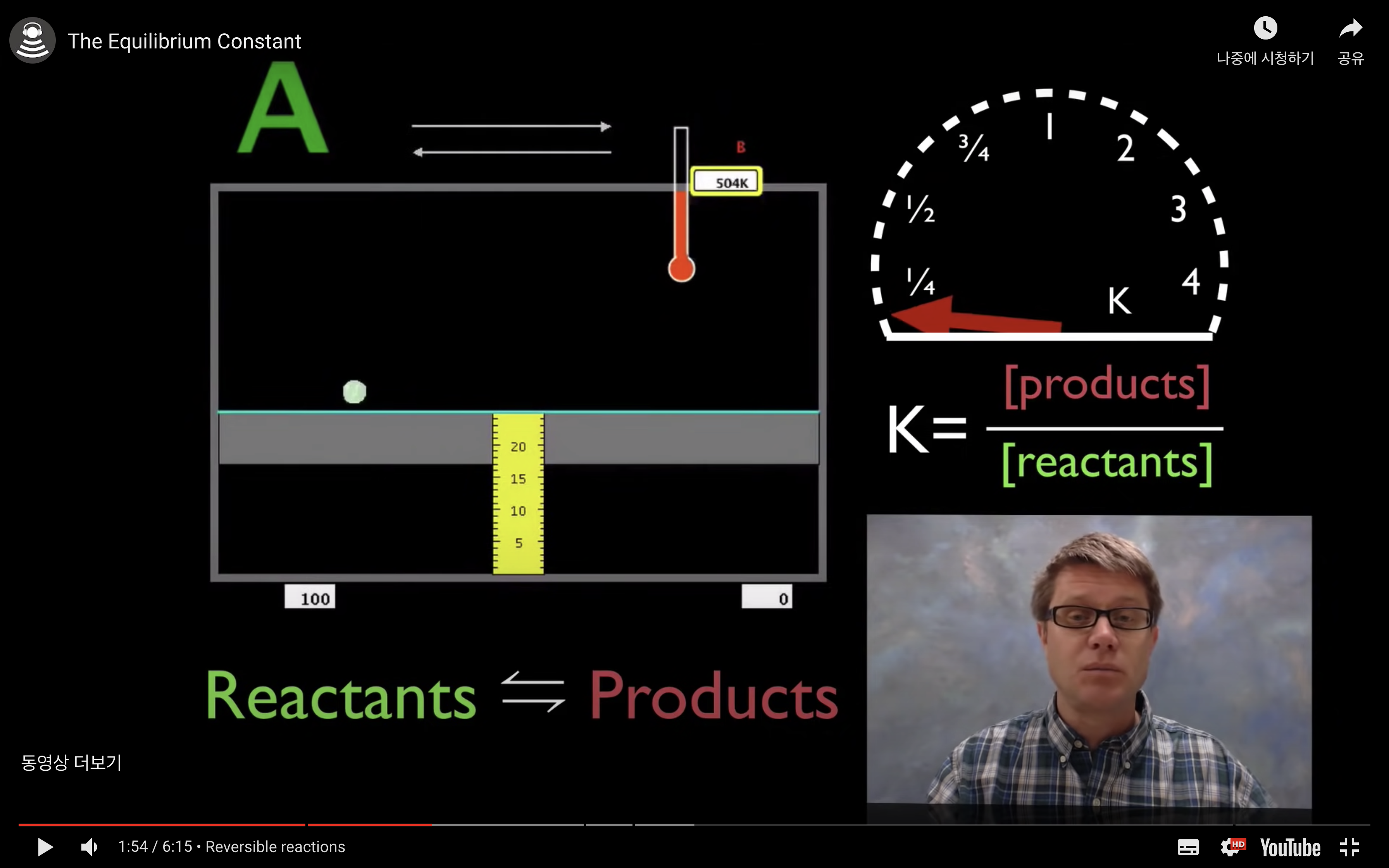
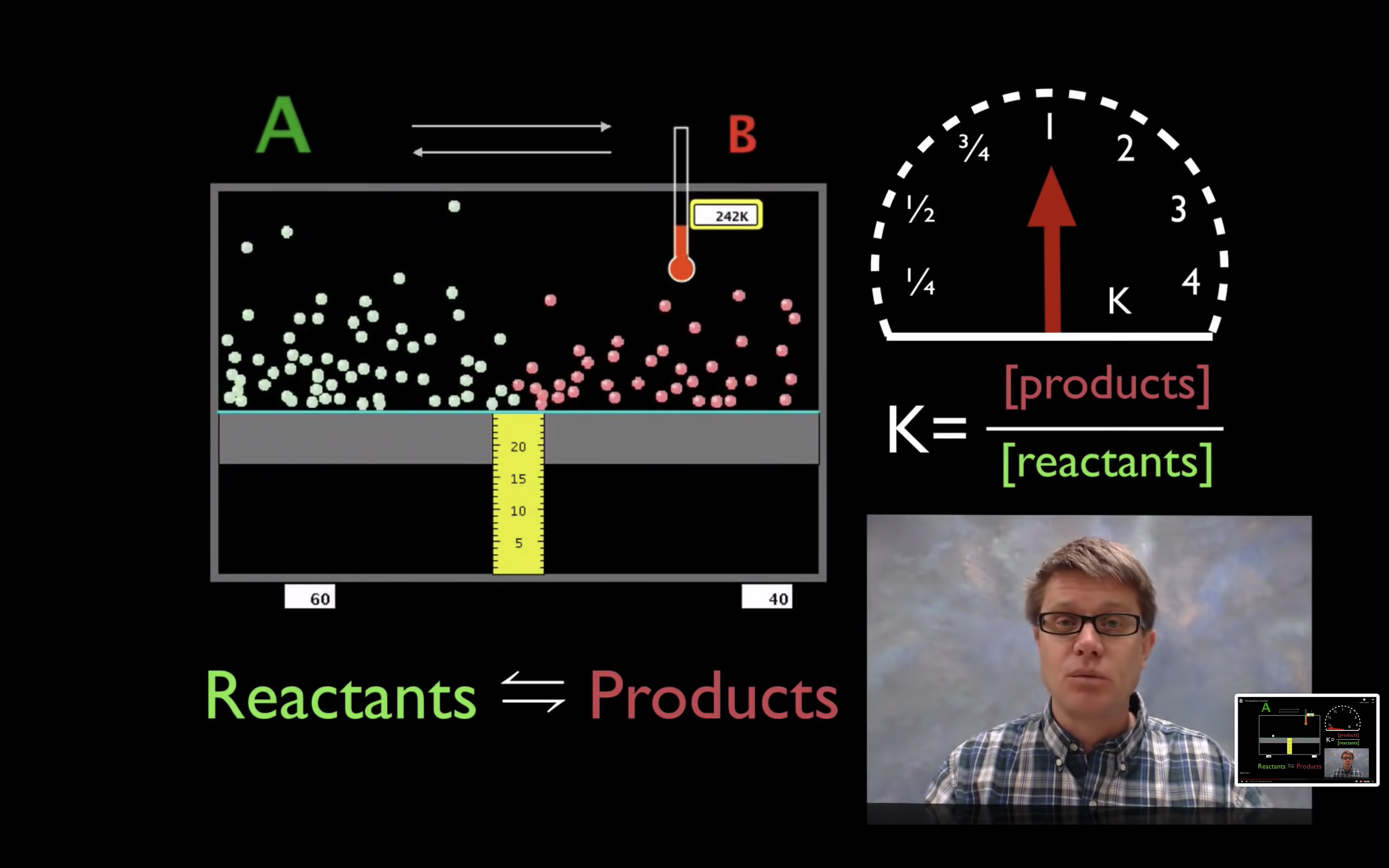
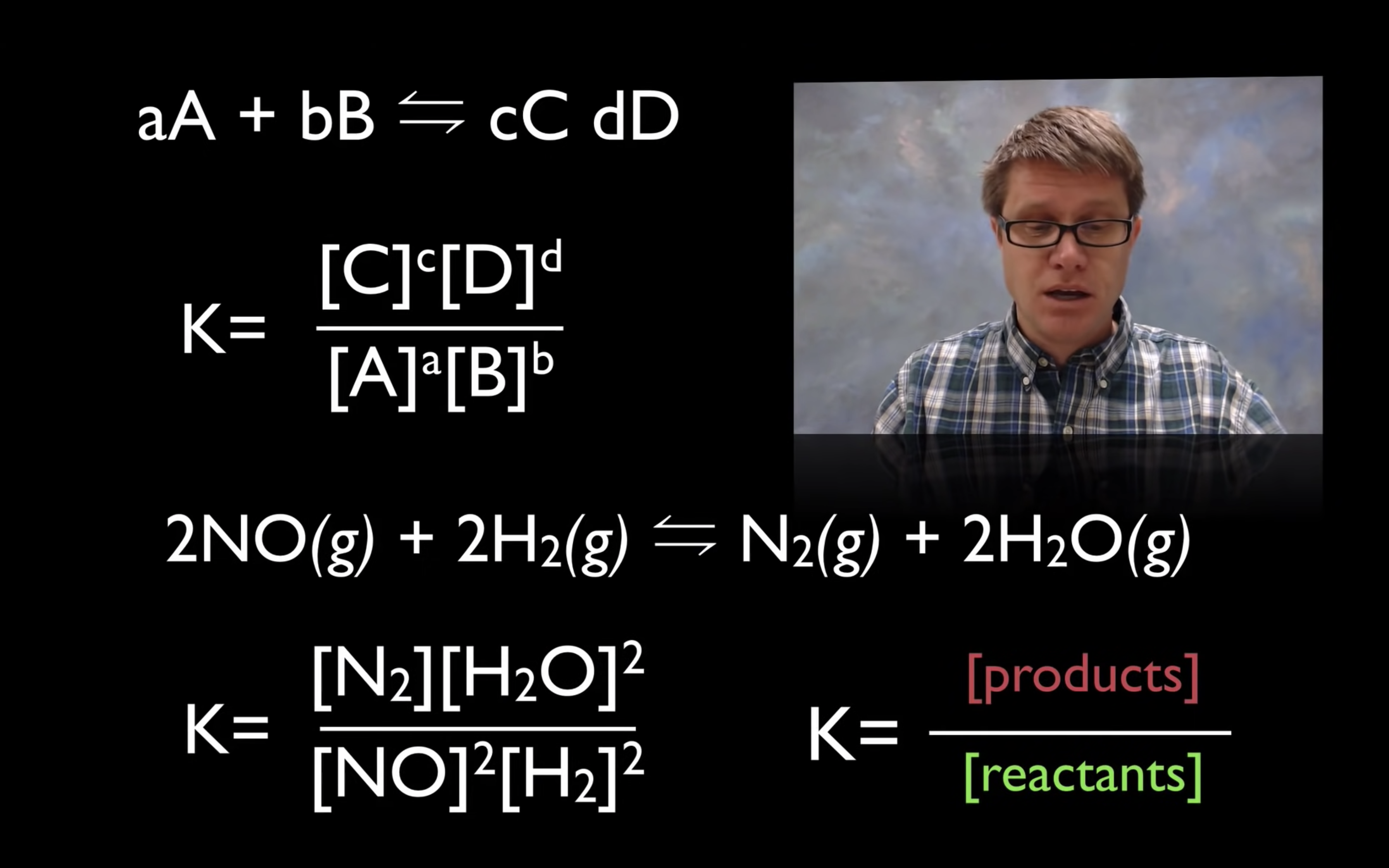
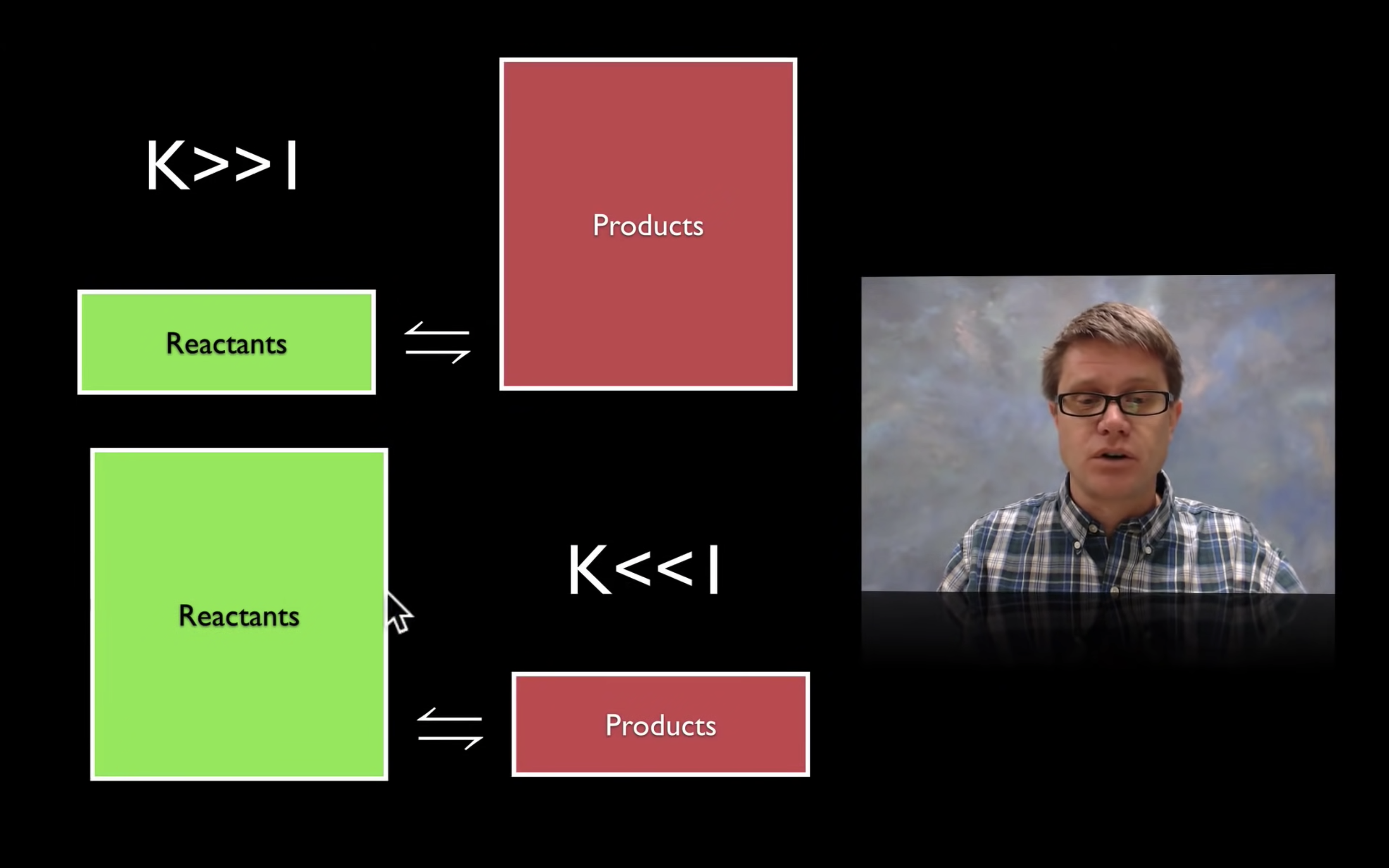
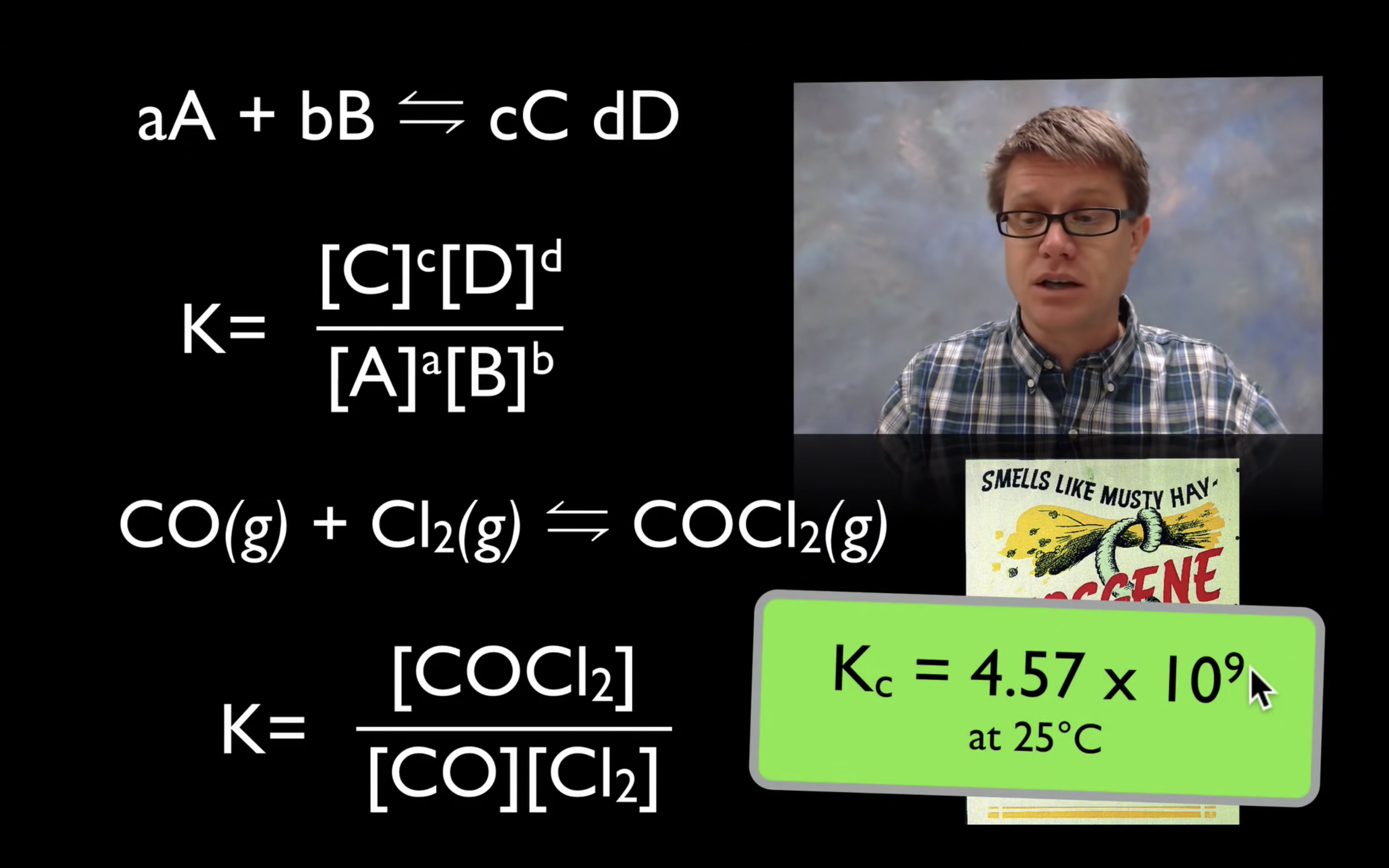
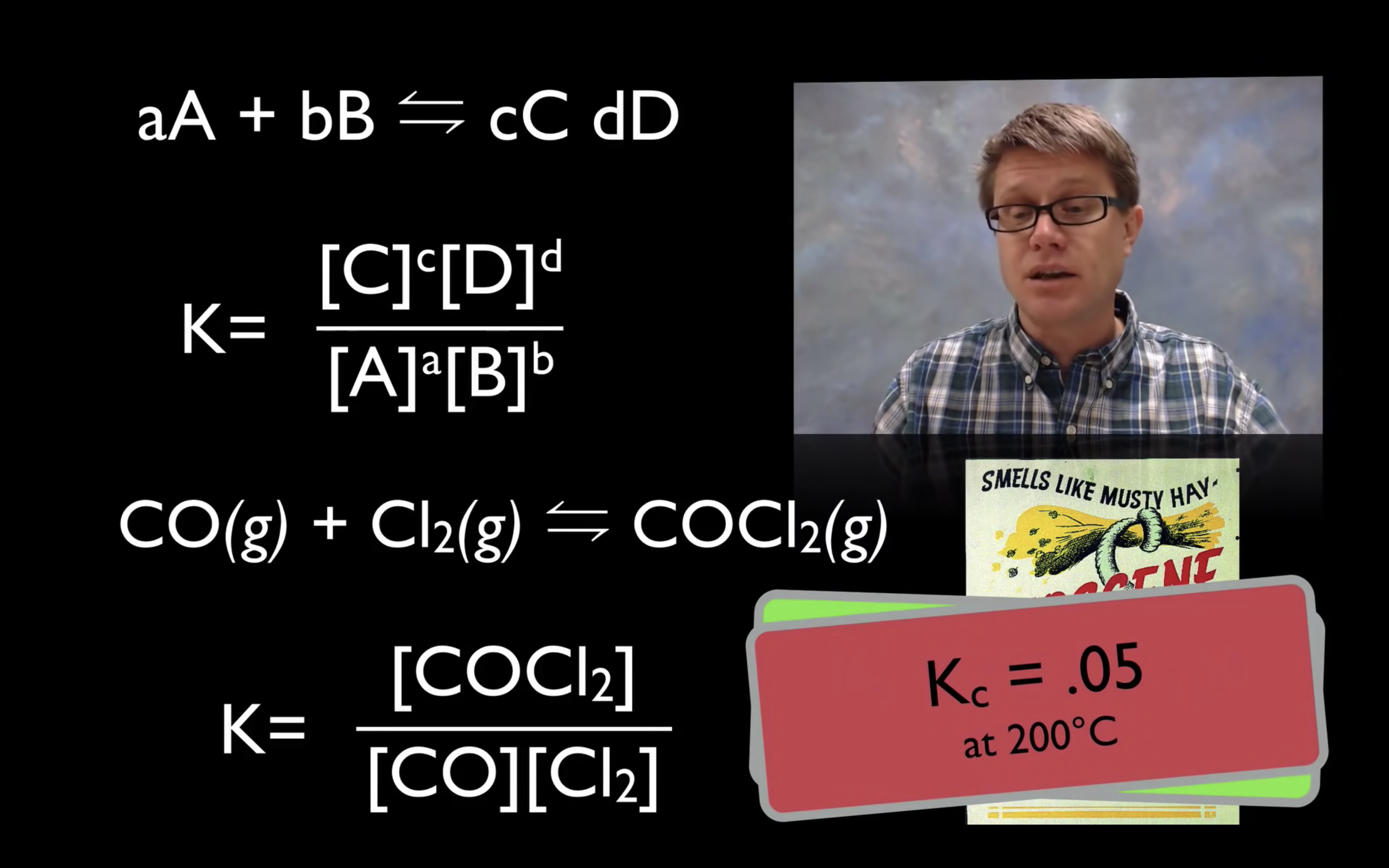
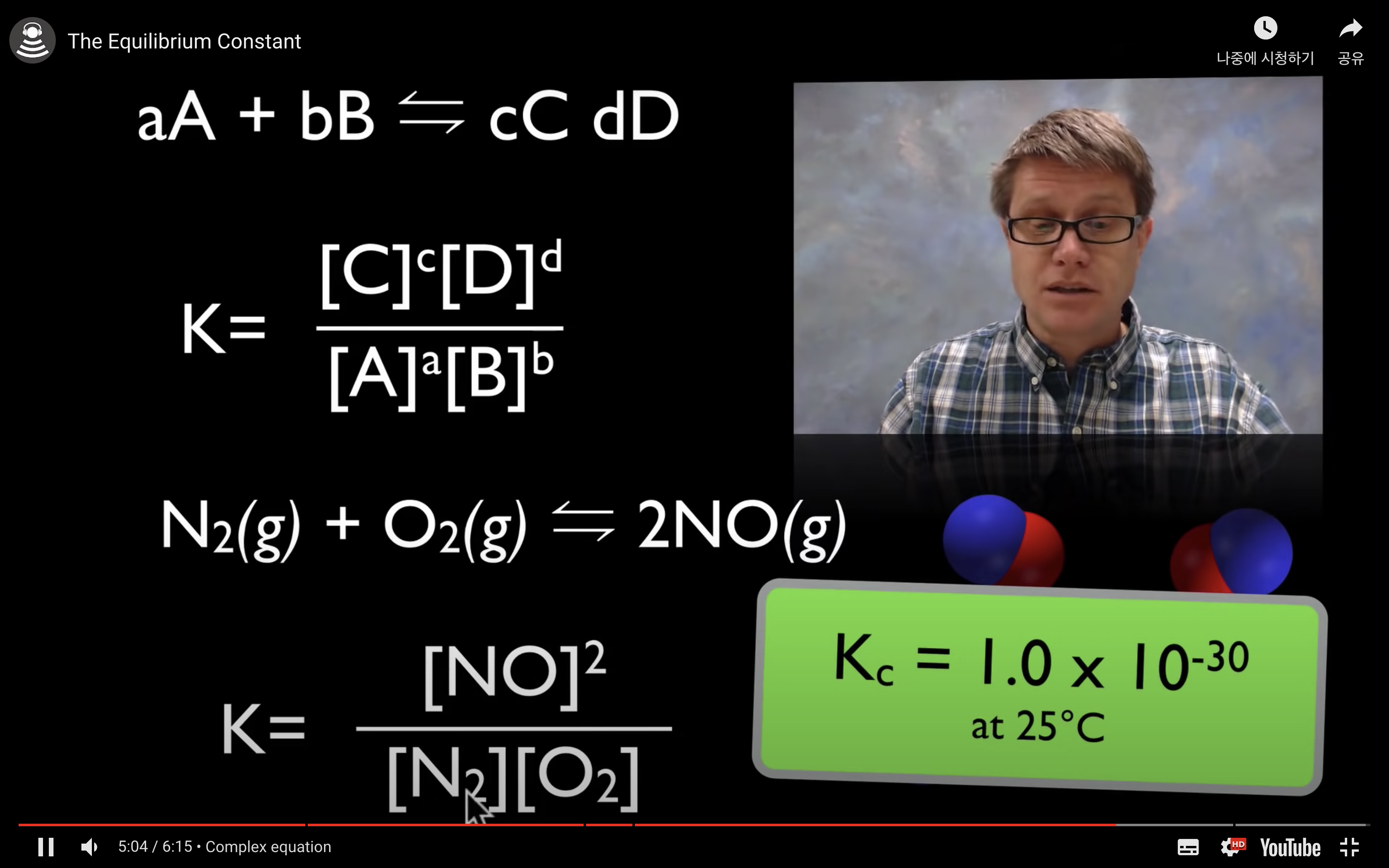
kc,keq are the equilibrium constant.
kc = [product]/[reactant]
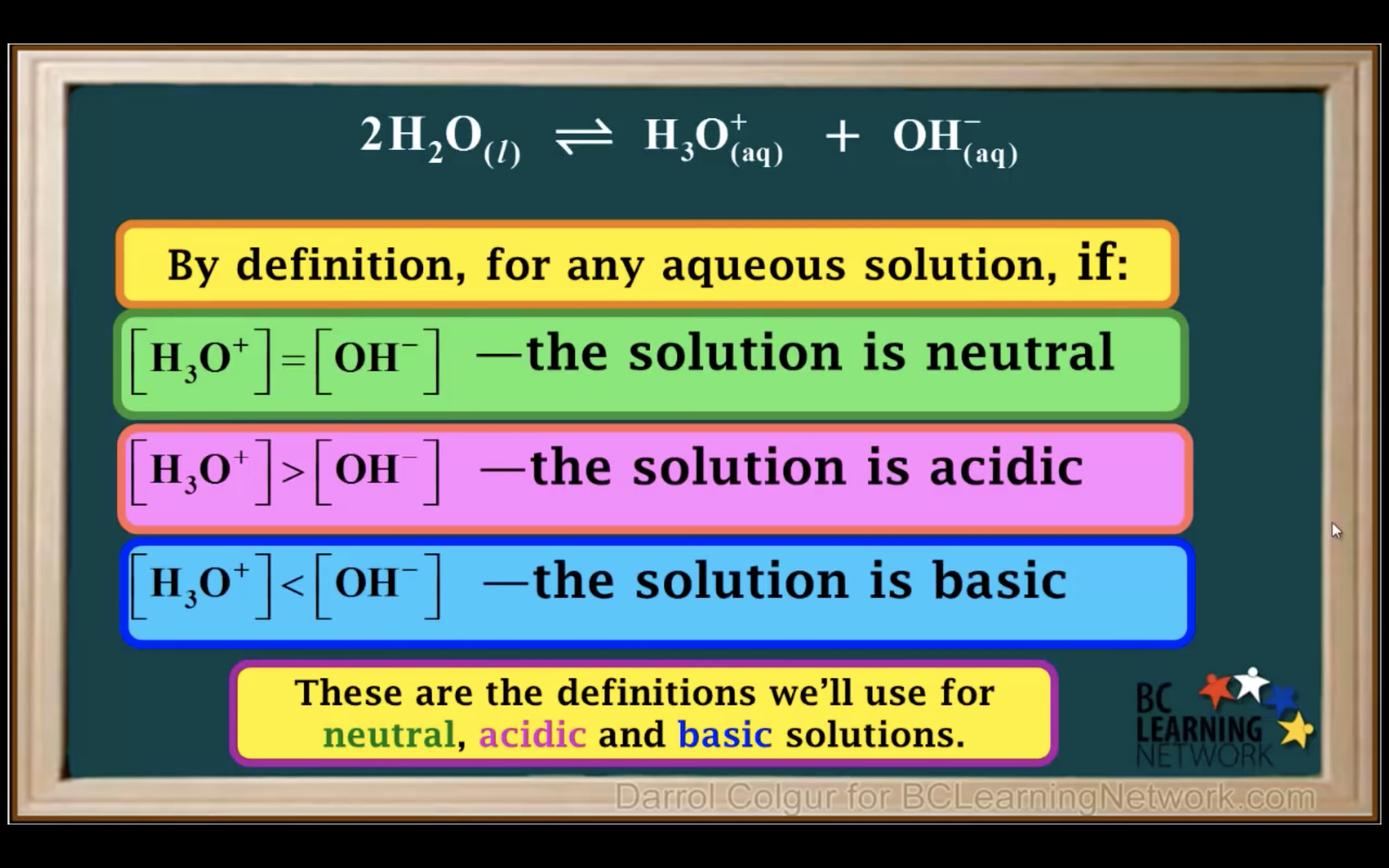
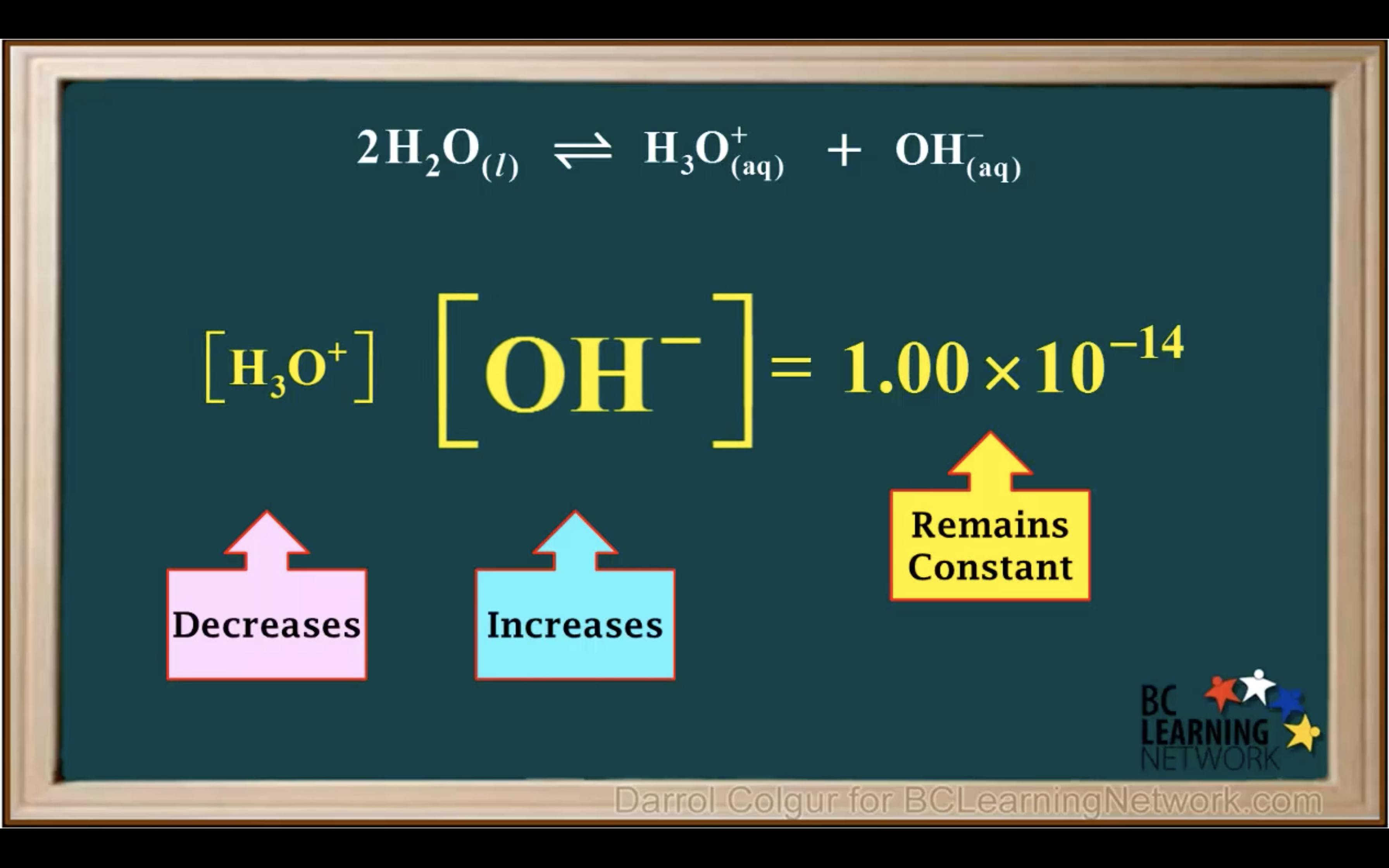
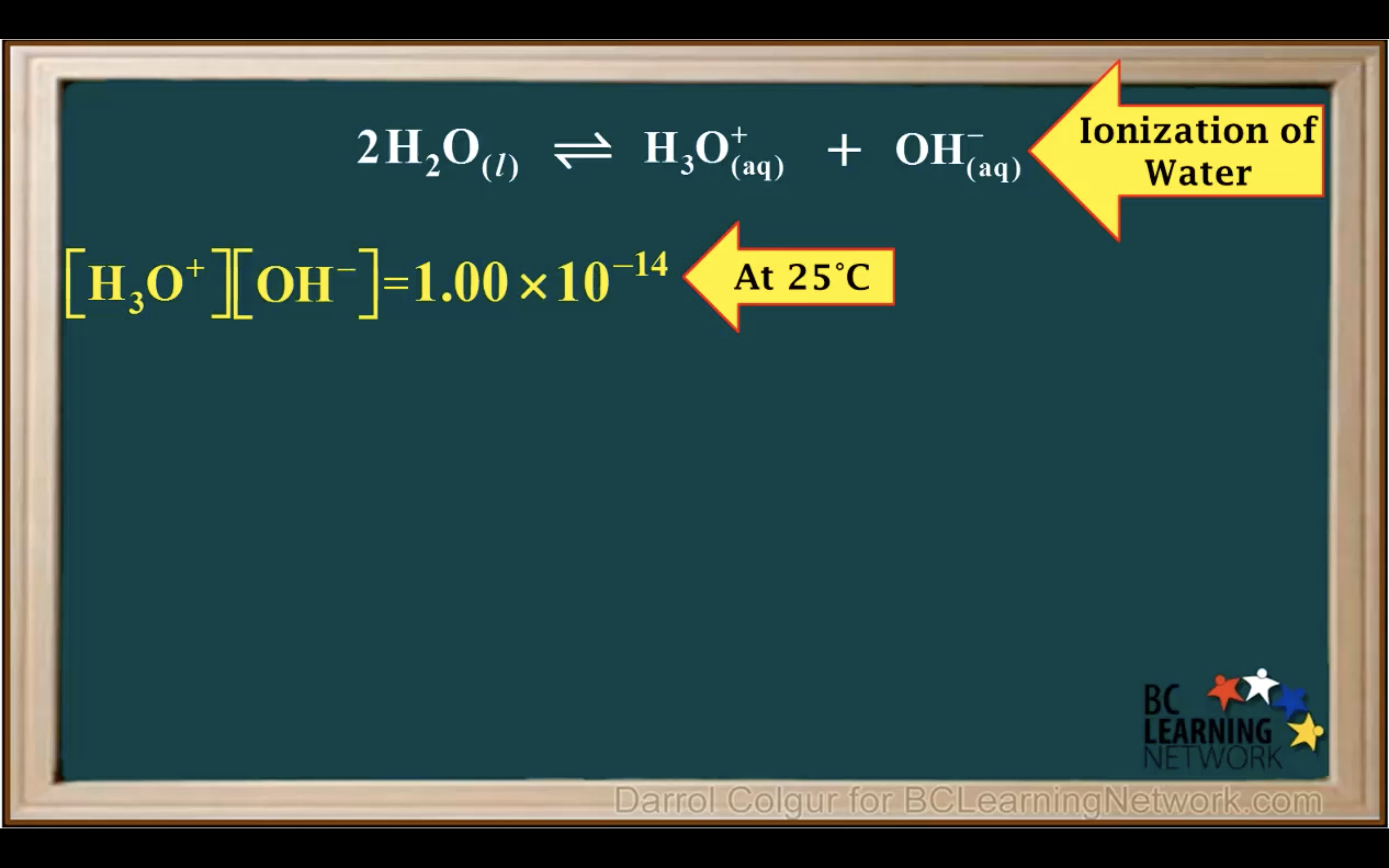
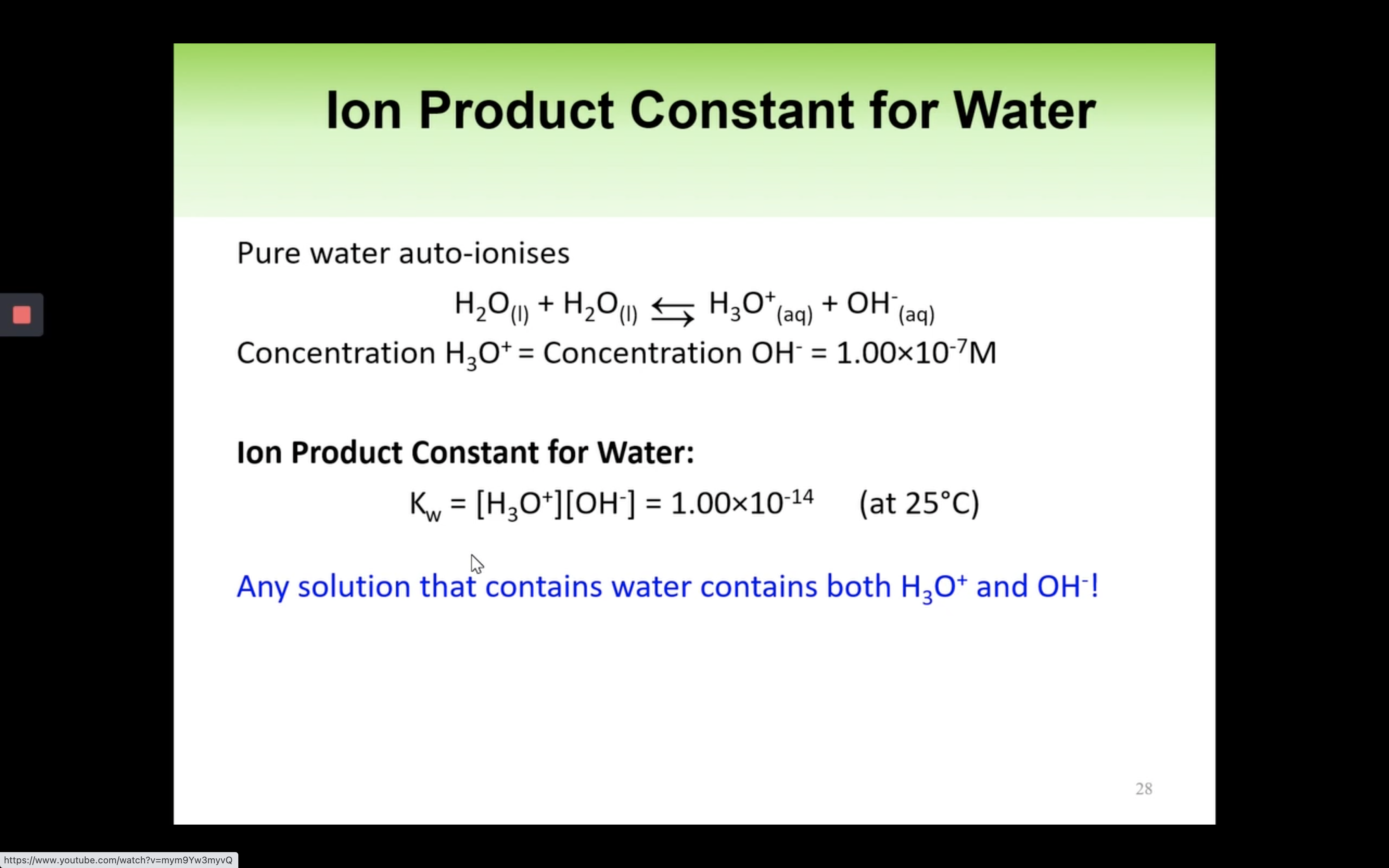
Kw Introduction - The water ionisation constant
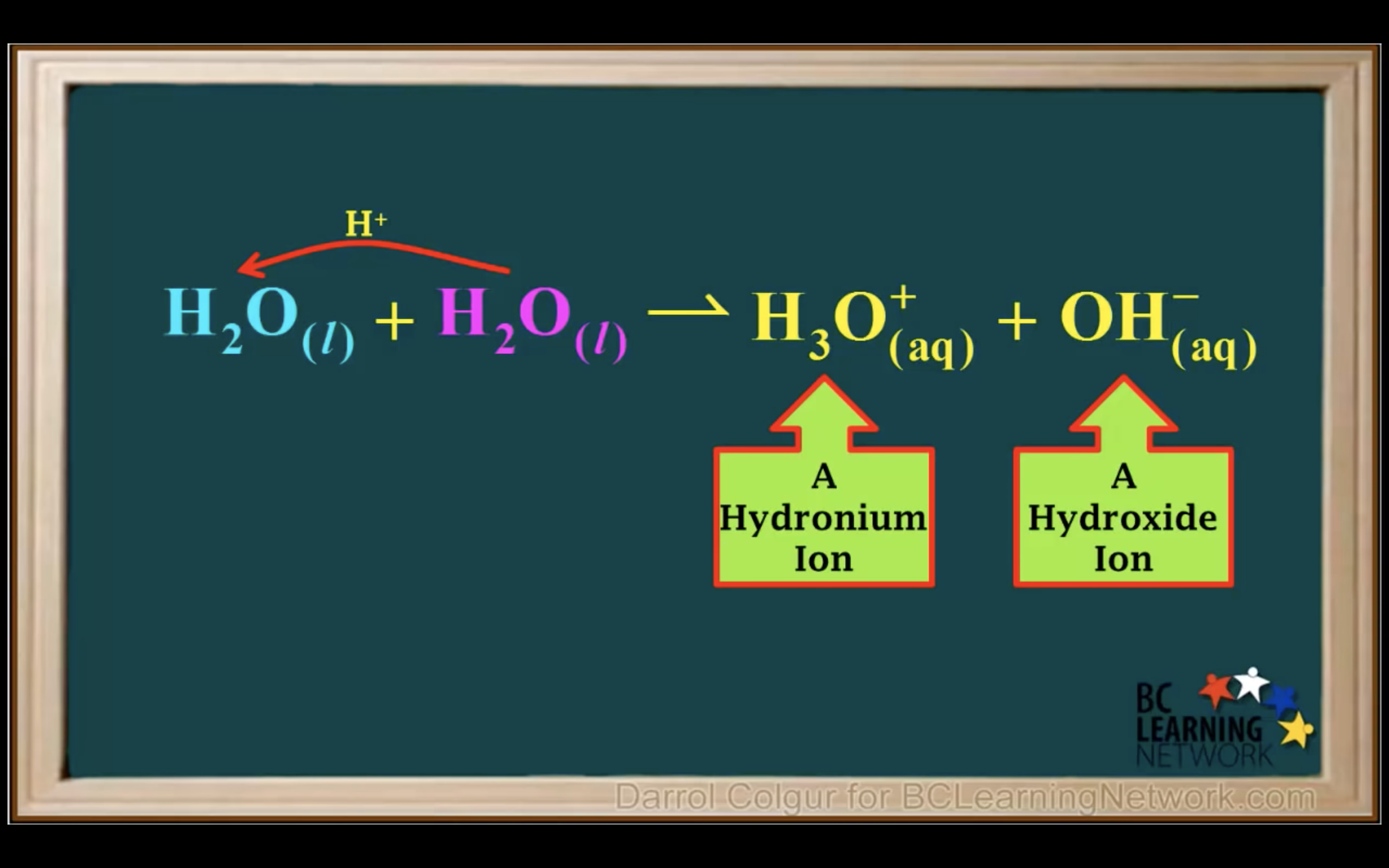
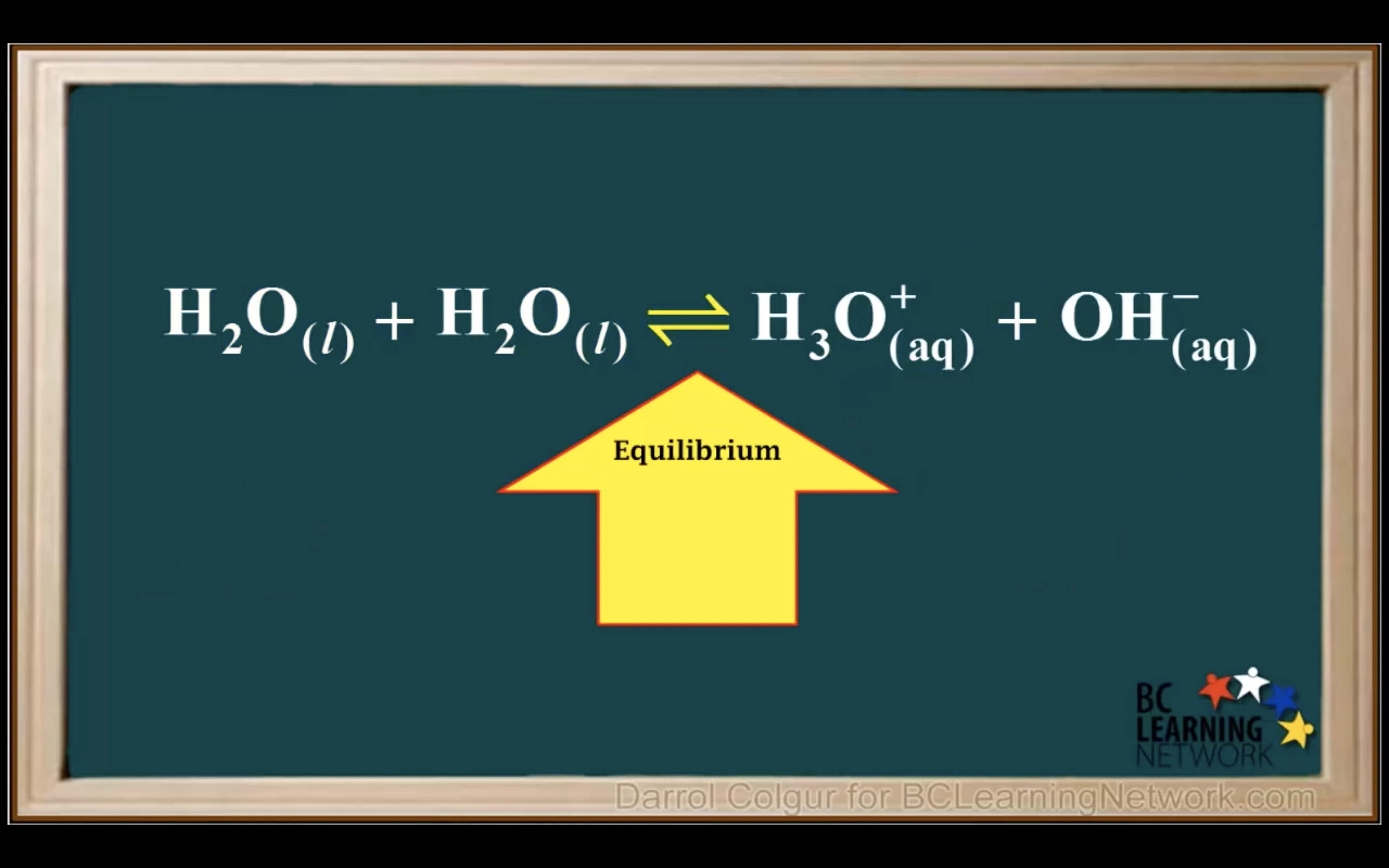
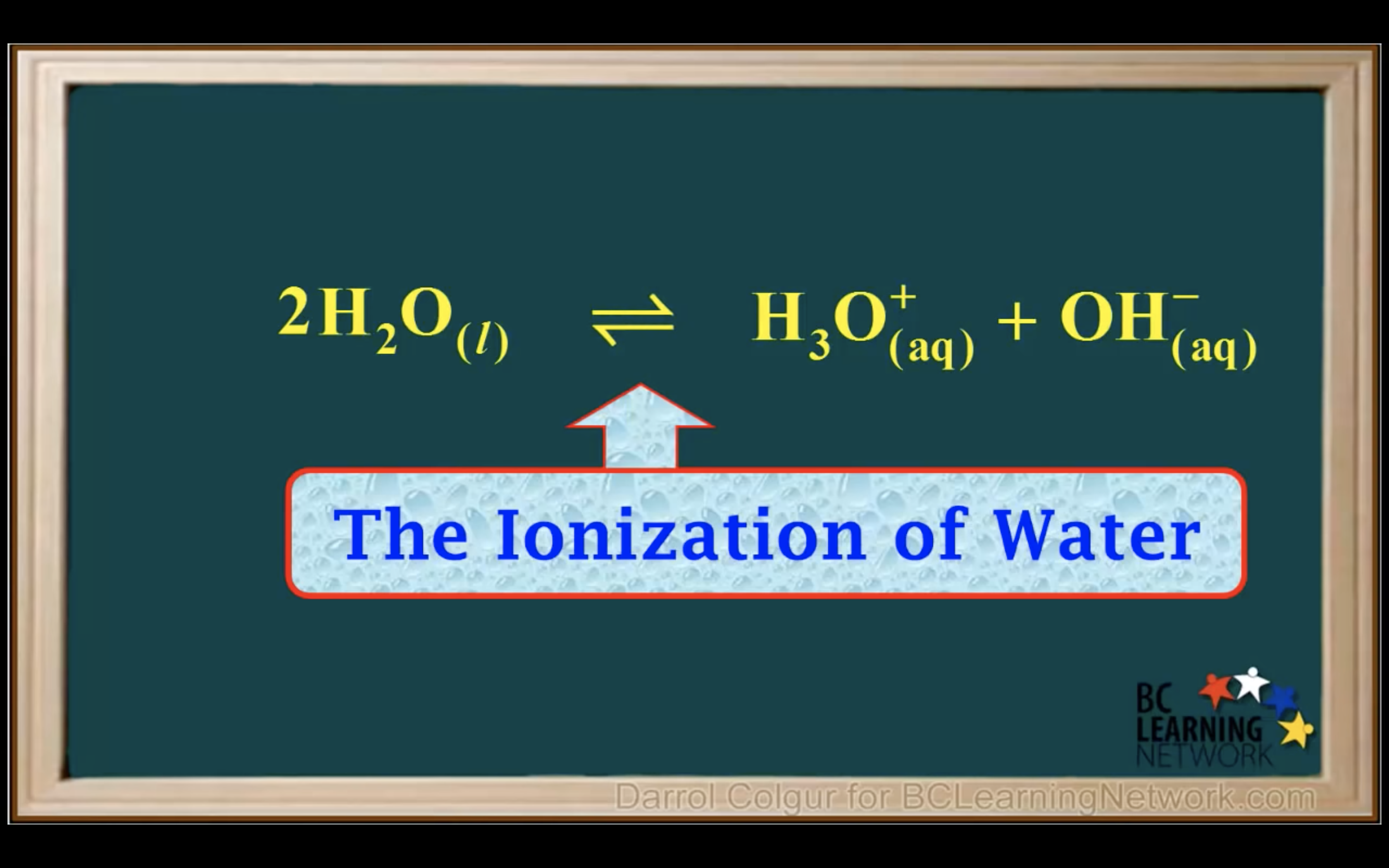
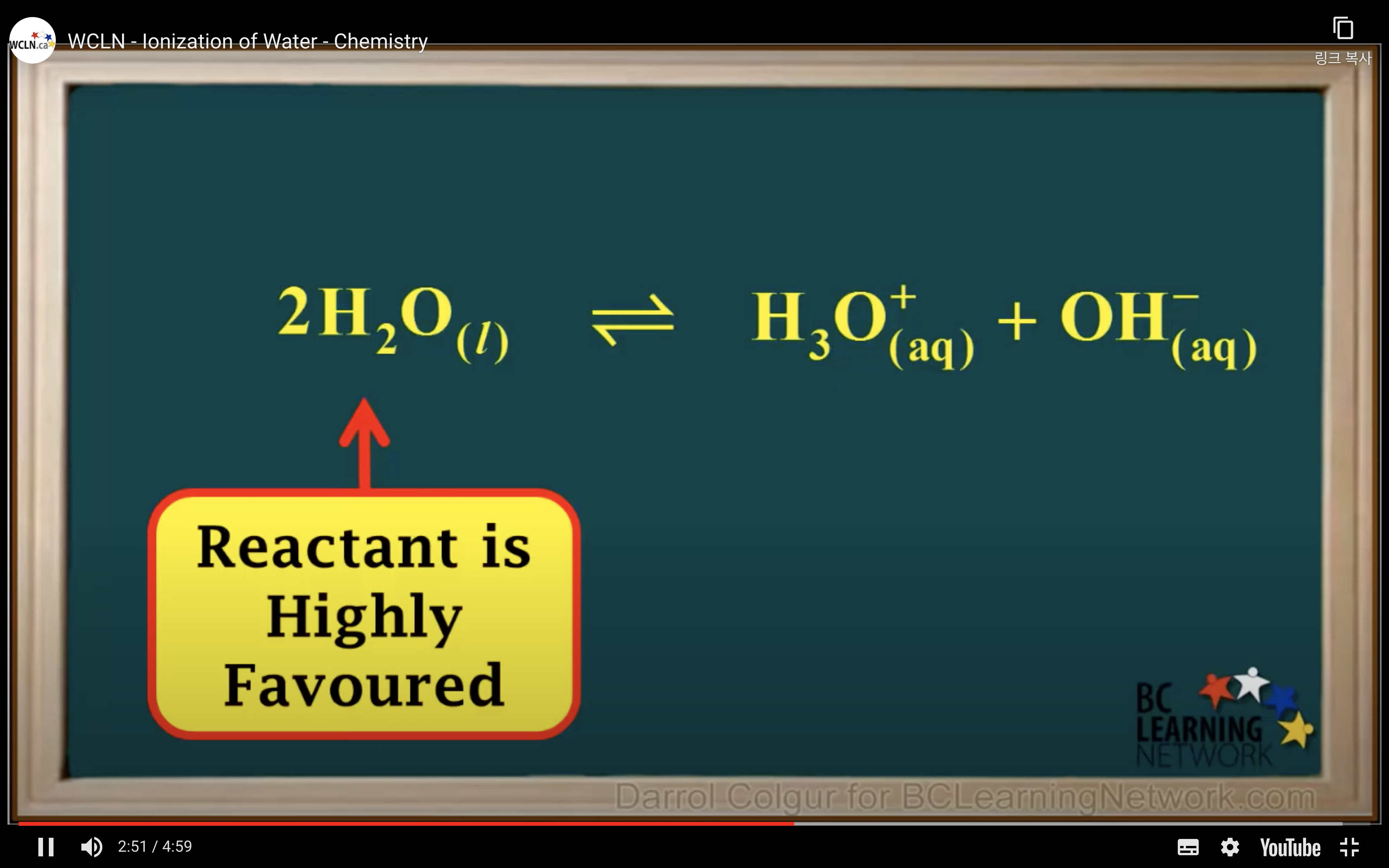
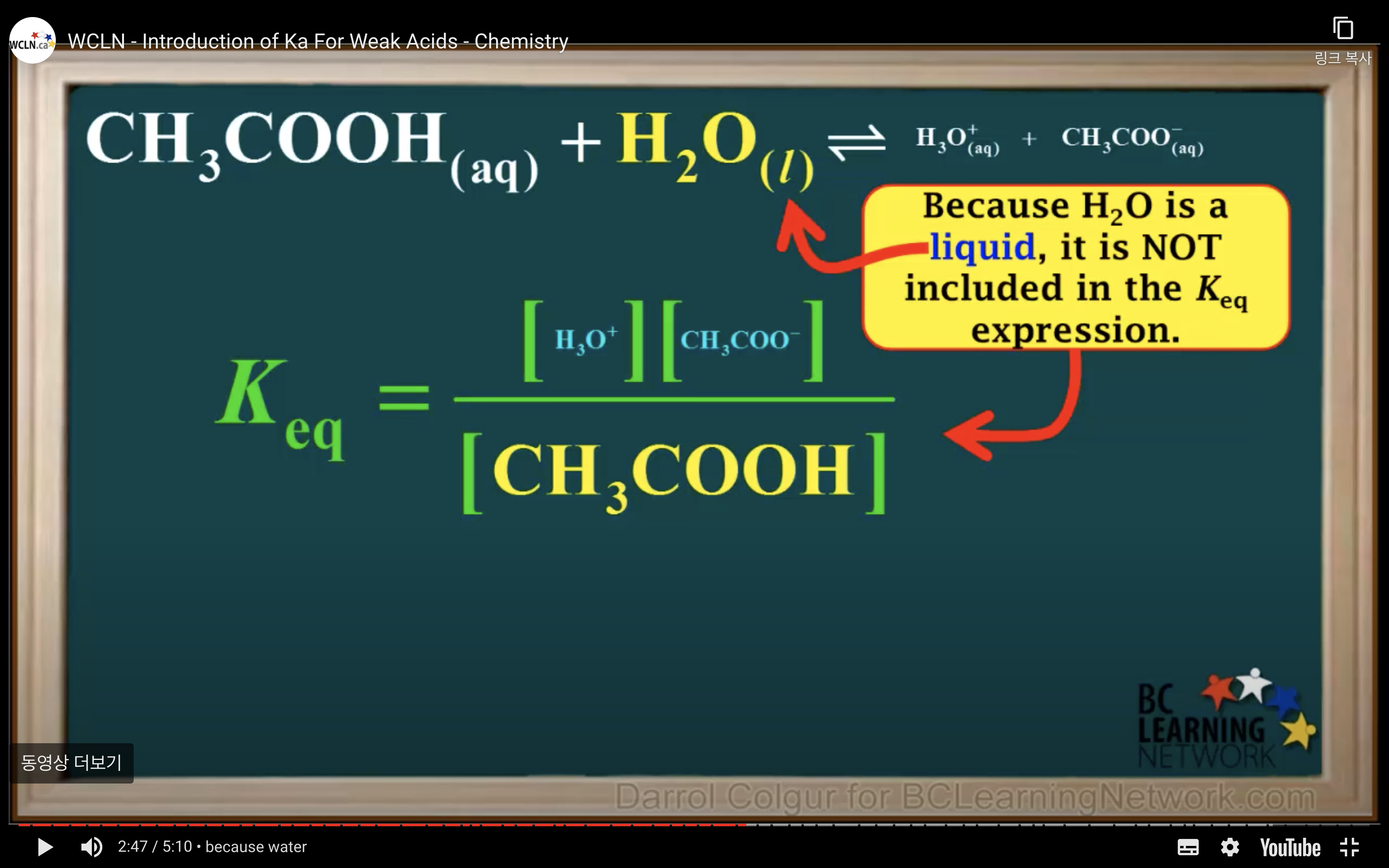
H2O is not calculated in the equilibrium K because it is liquid.
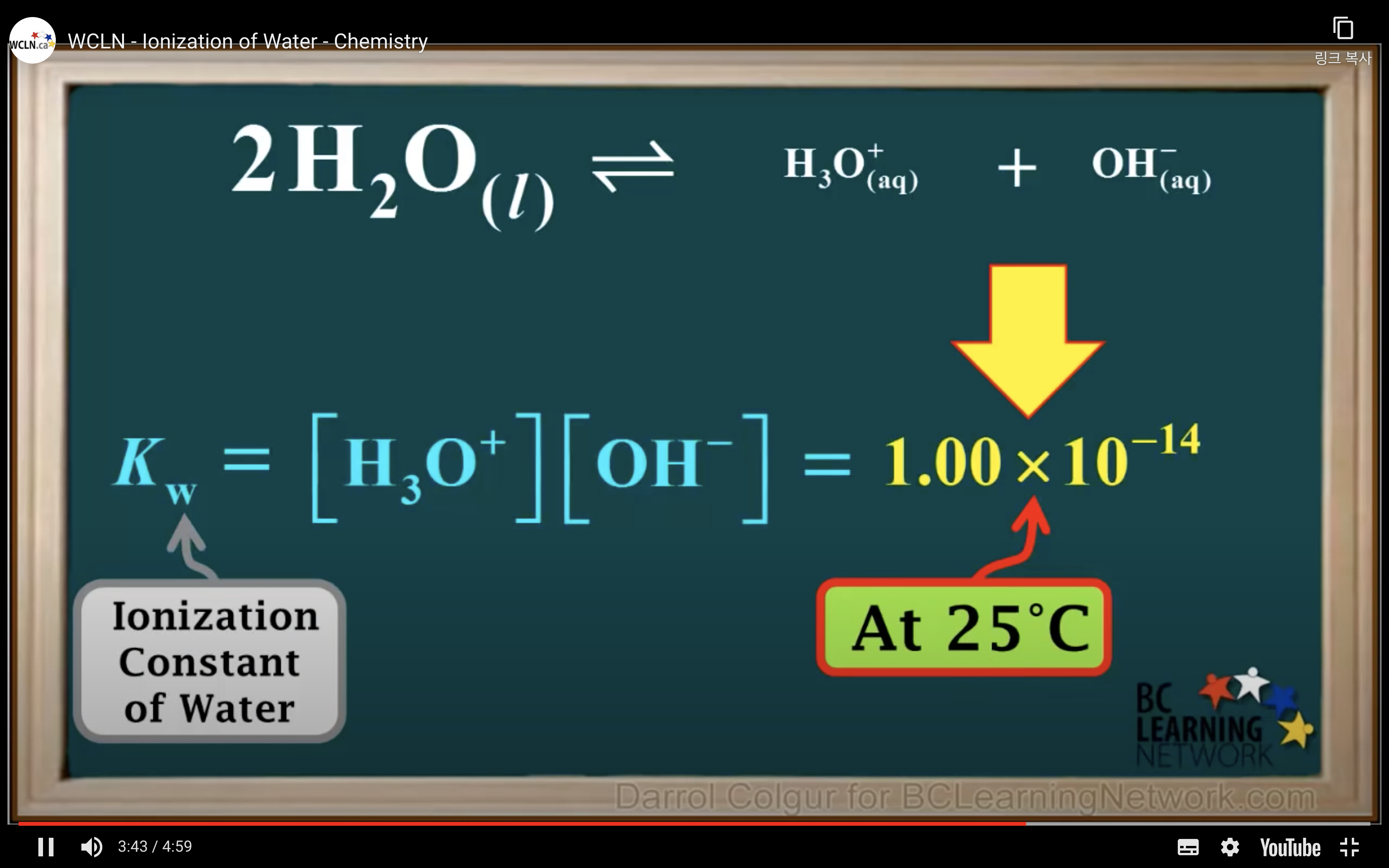
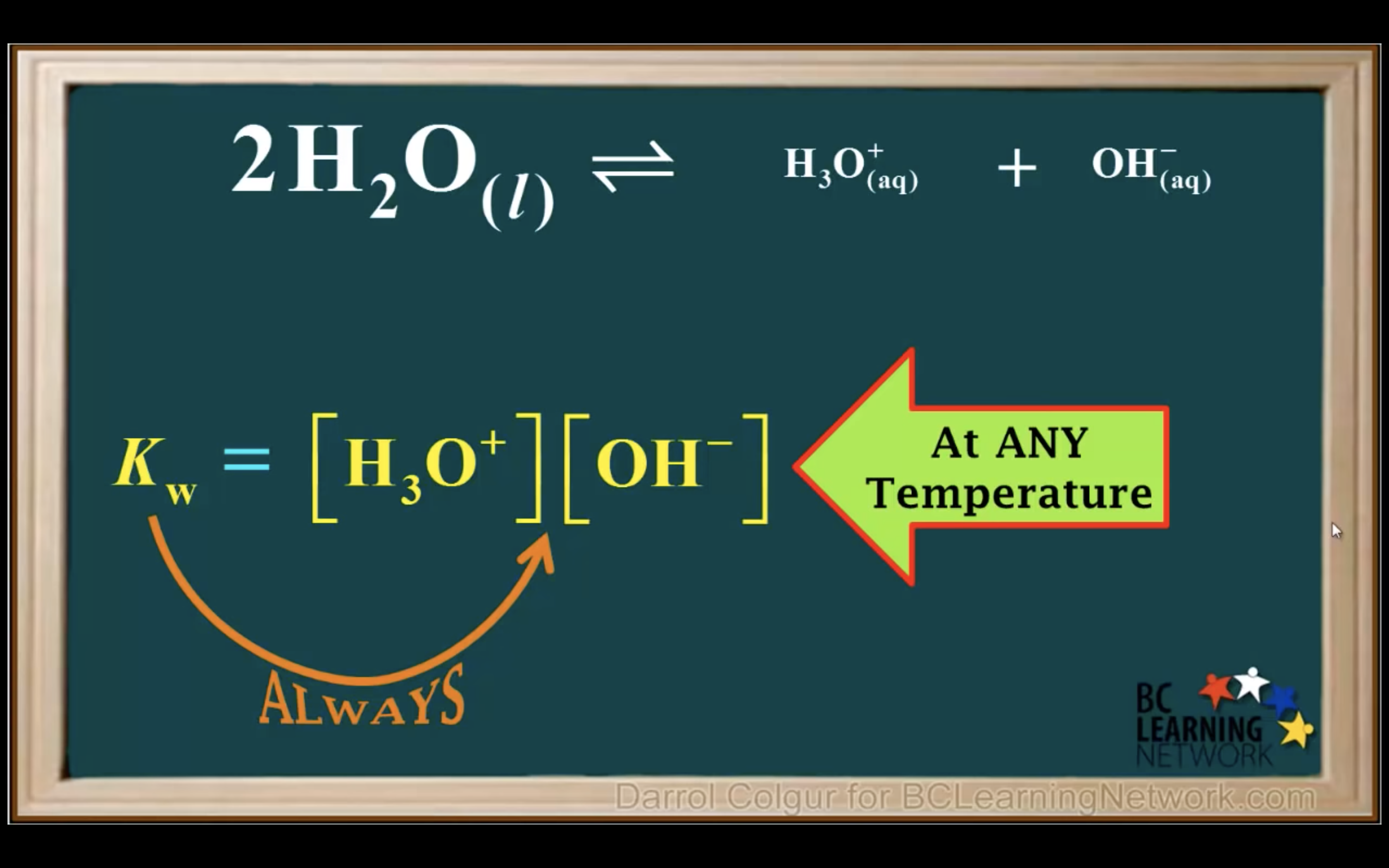
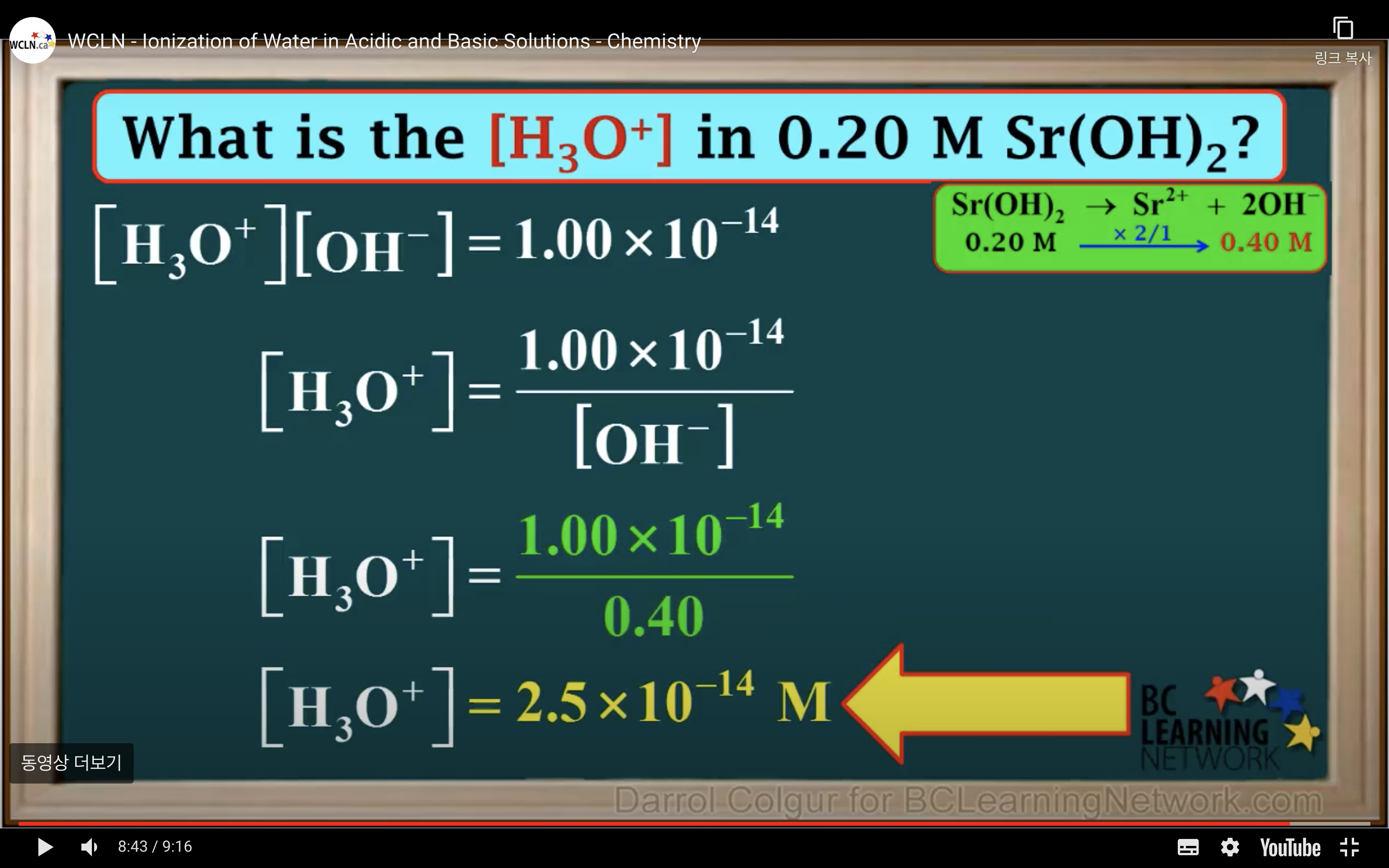
Keq = Kc = equilibrium constant = > for water : kw : ionization constant of water
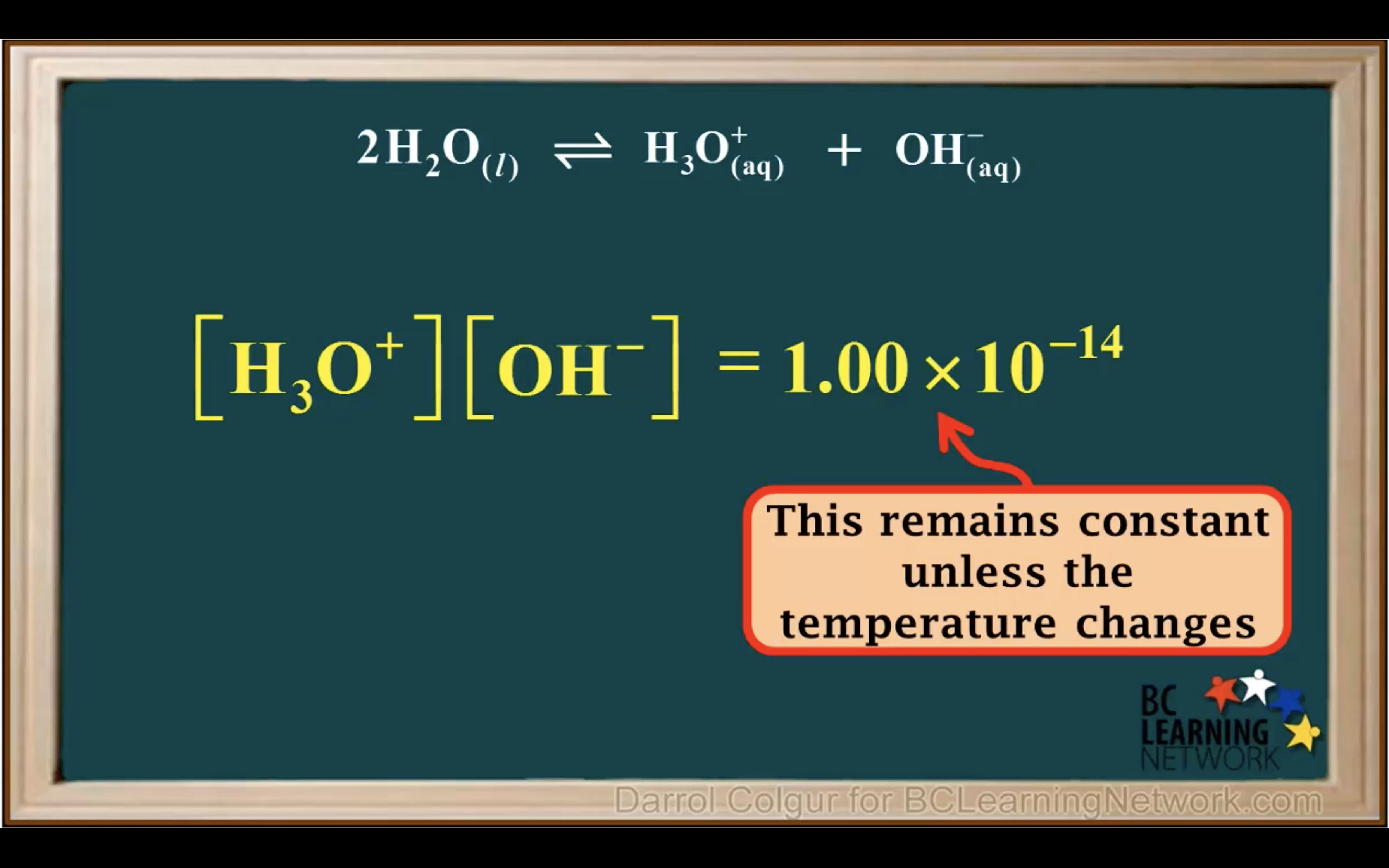
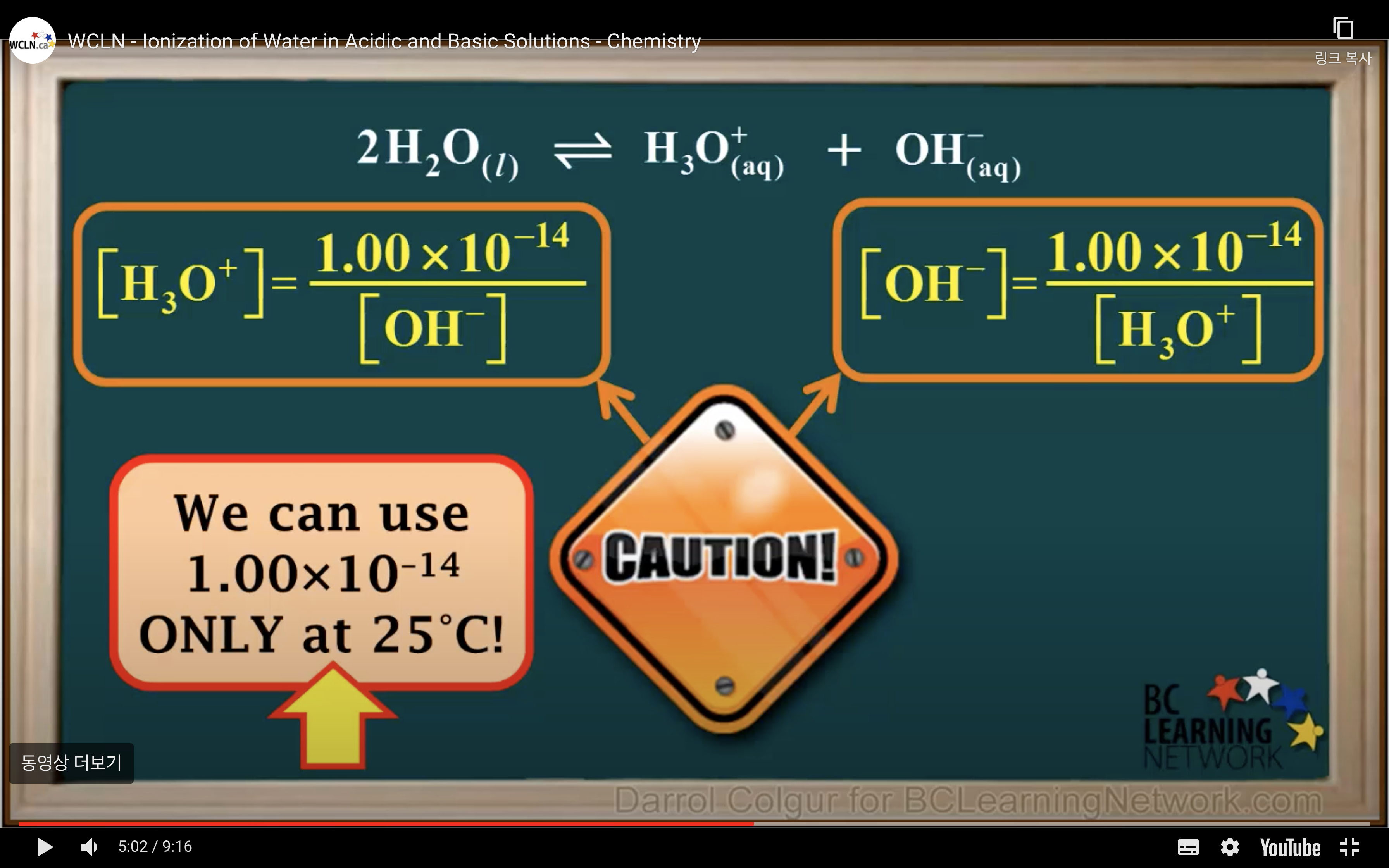
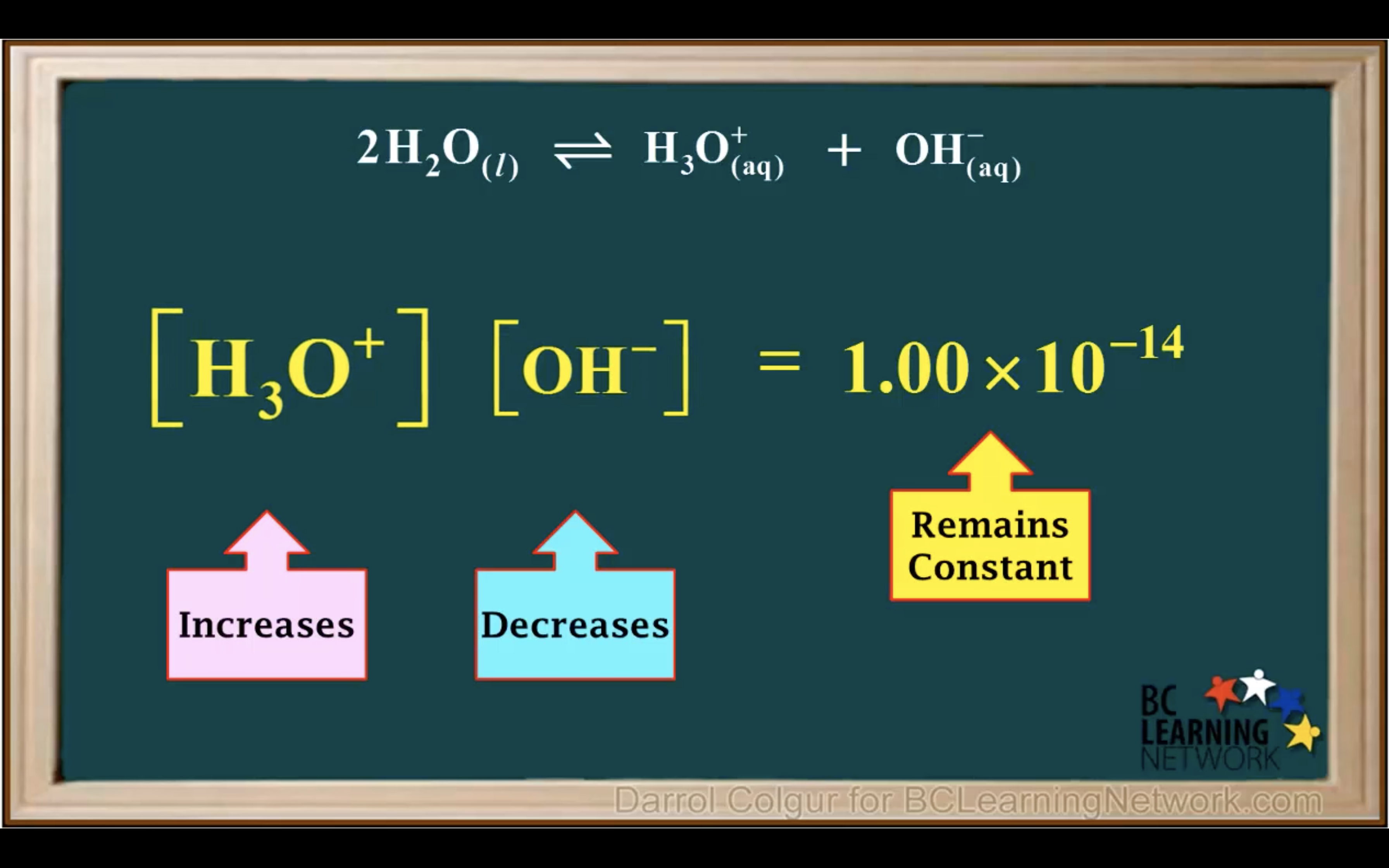
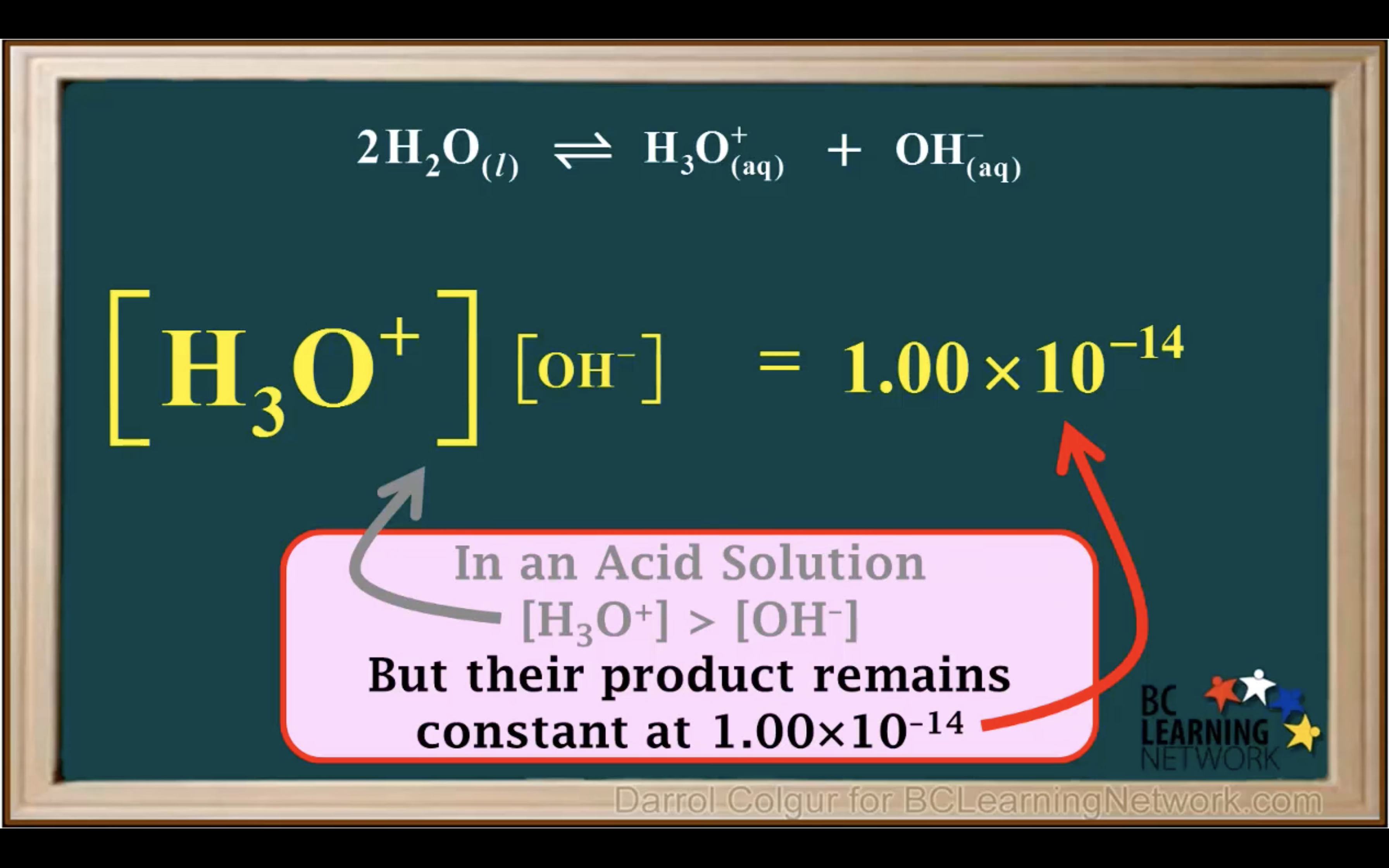
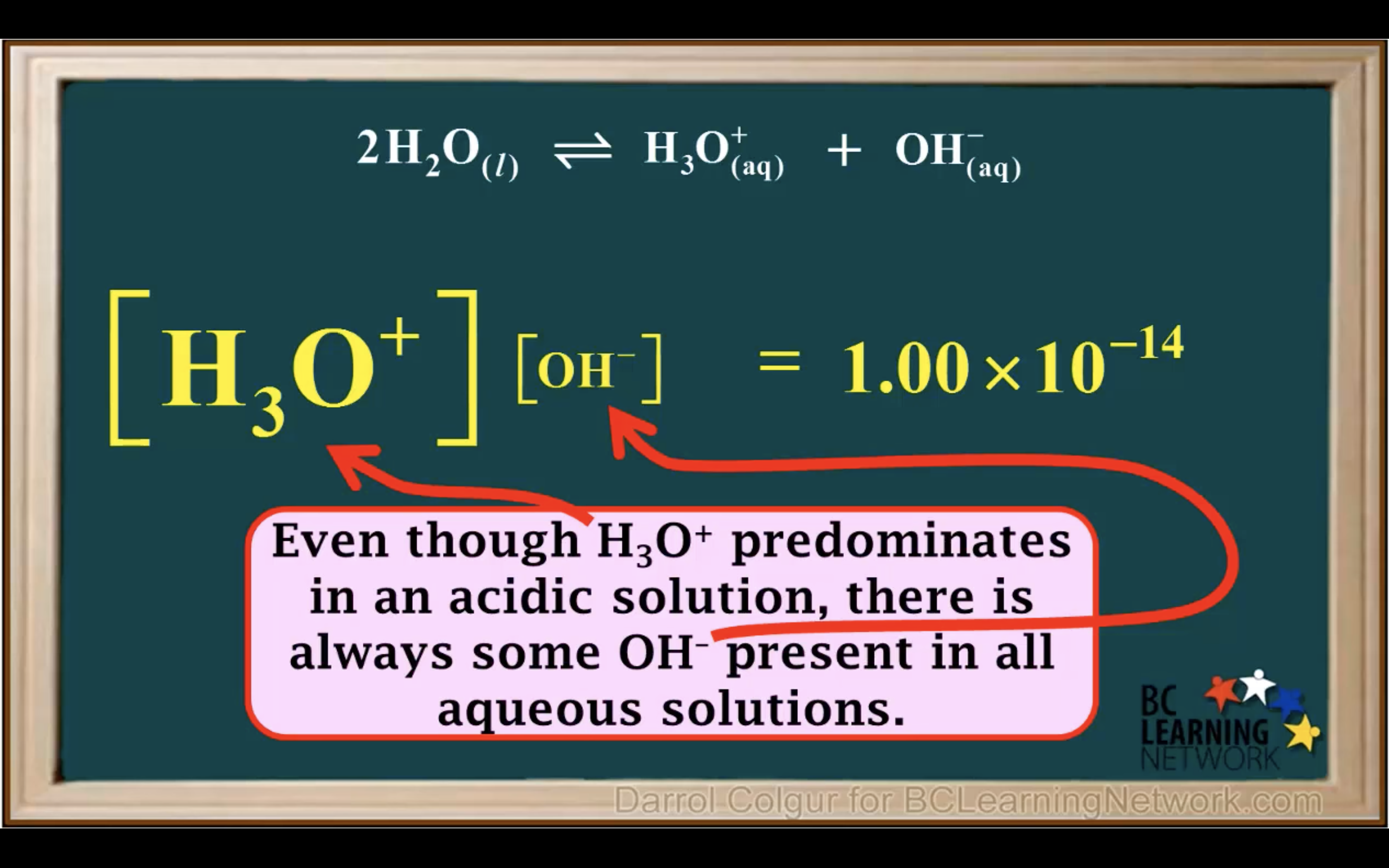
kw = [H3O+][OH-] = 1.00 x 10-14 (It remains constant unless the temp changes)
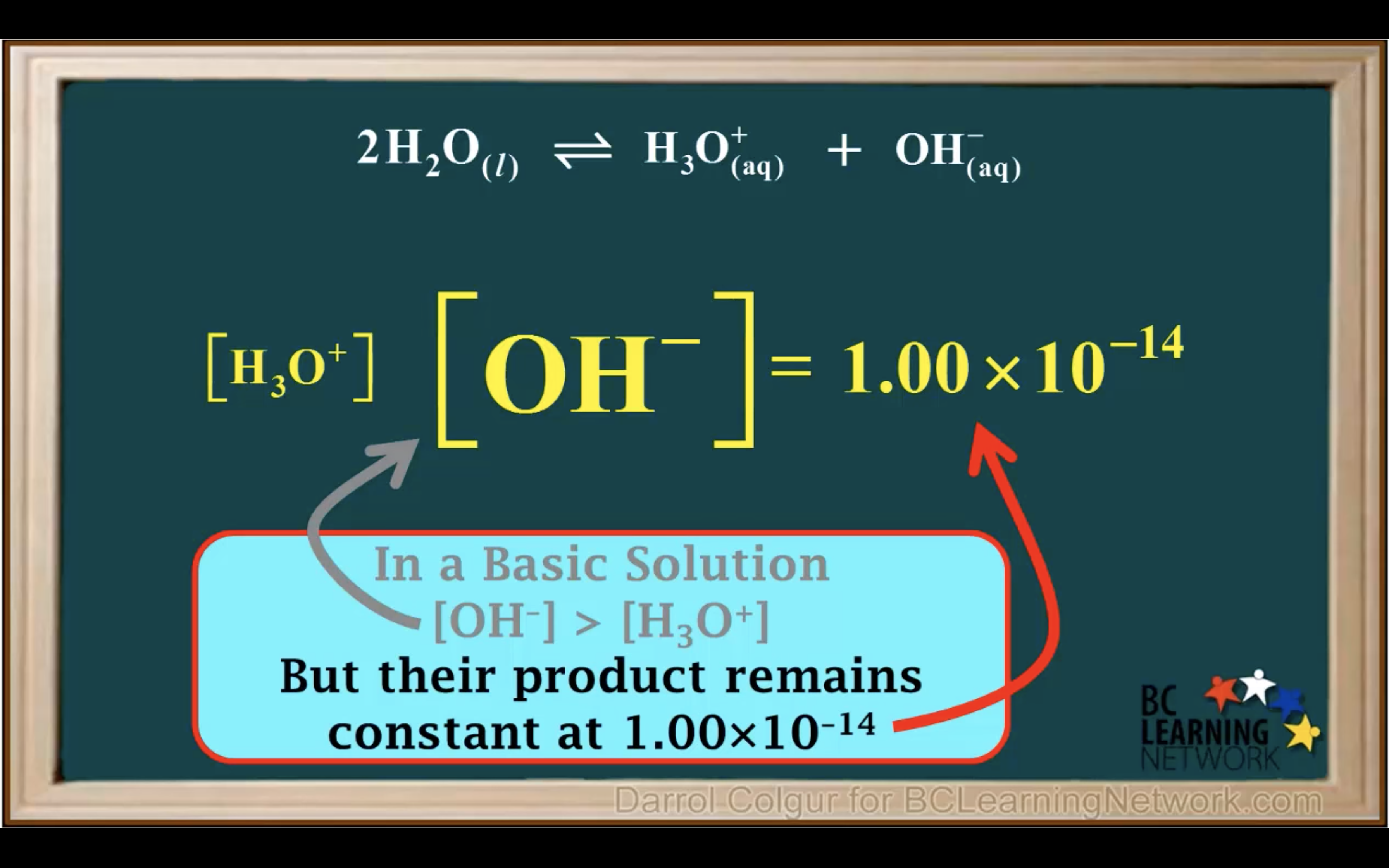
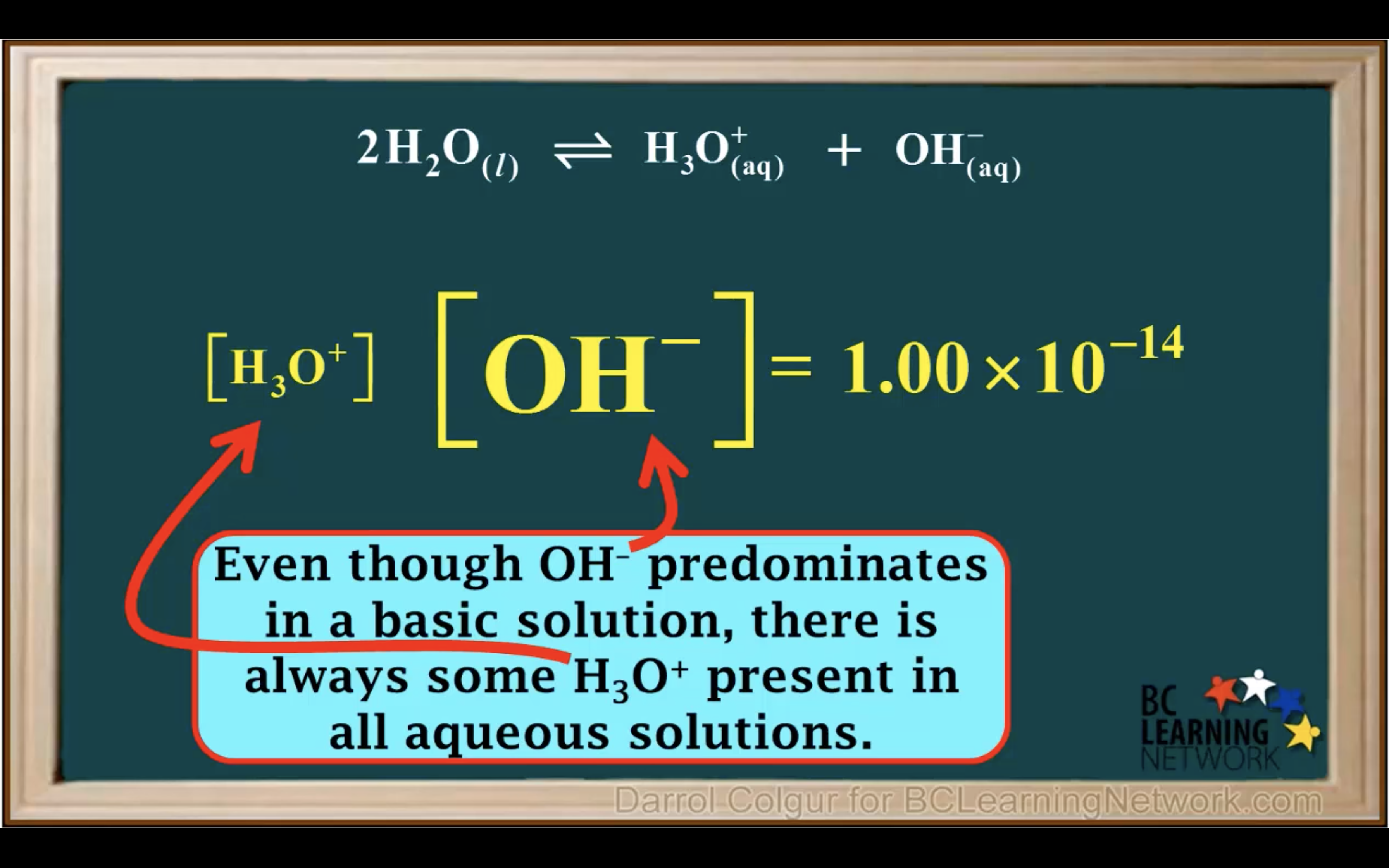
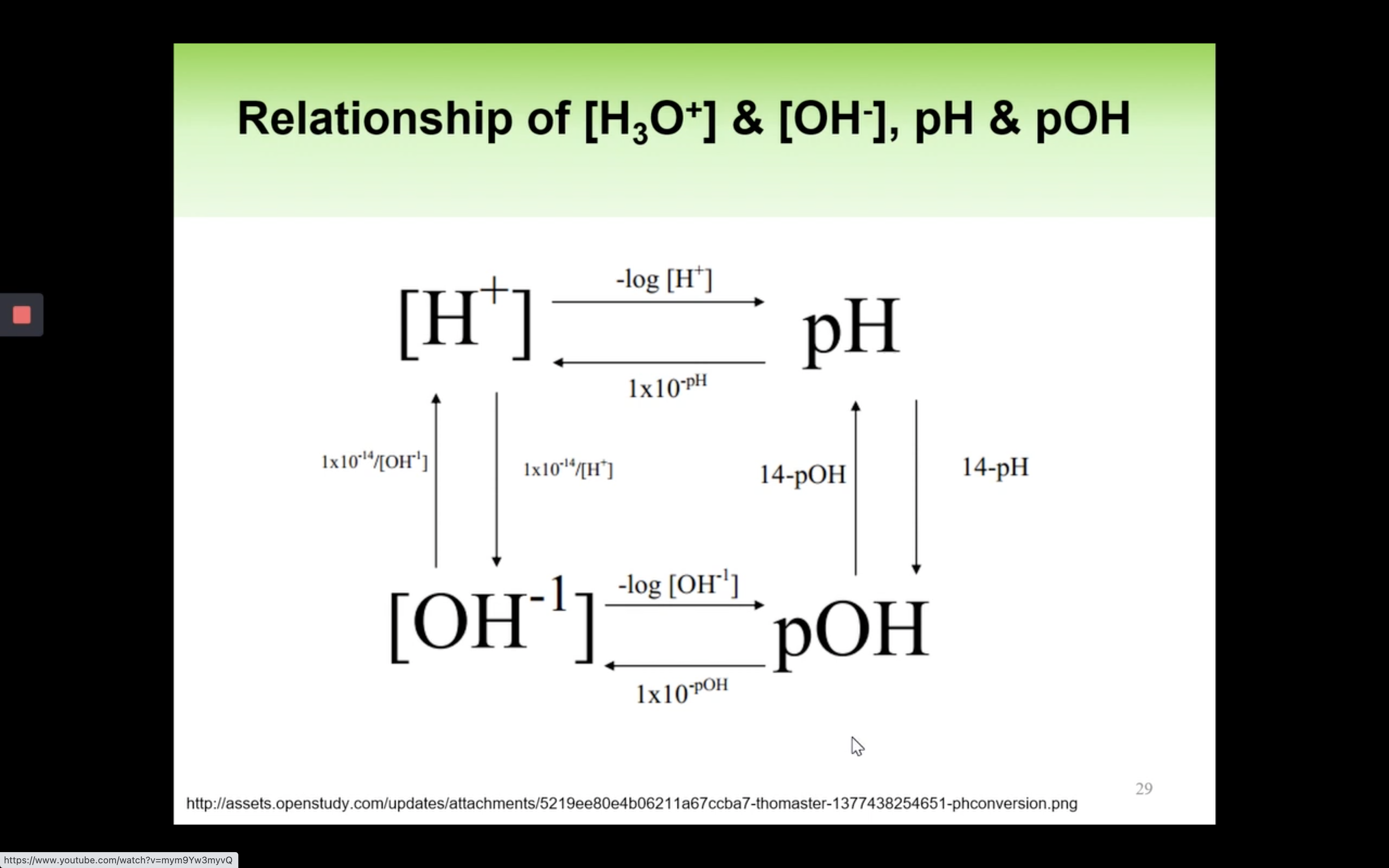
Kw- Ionisation of water in Acidic and Basic solution
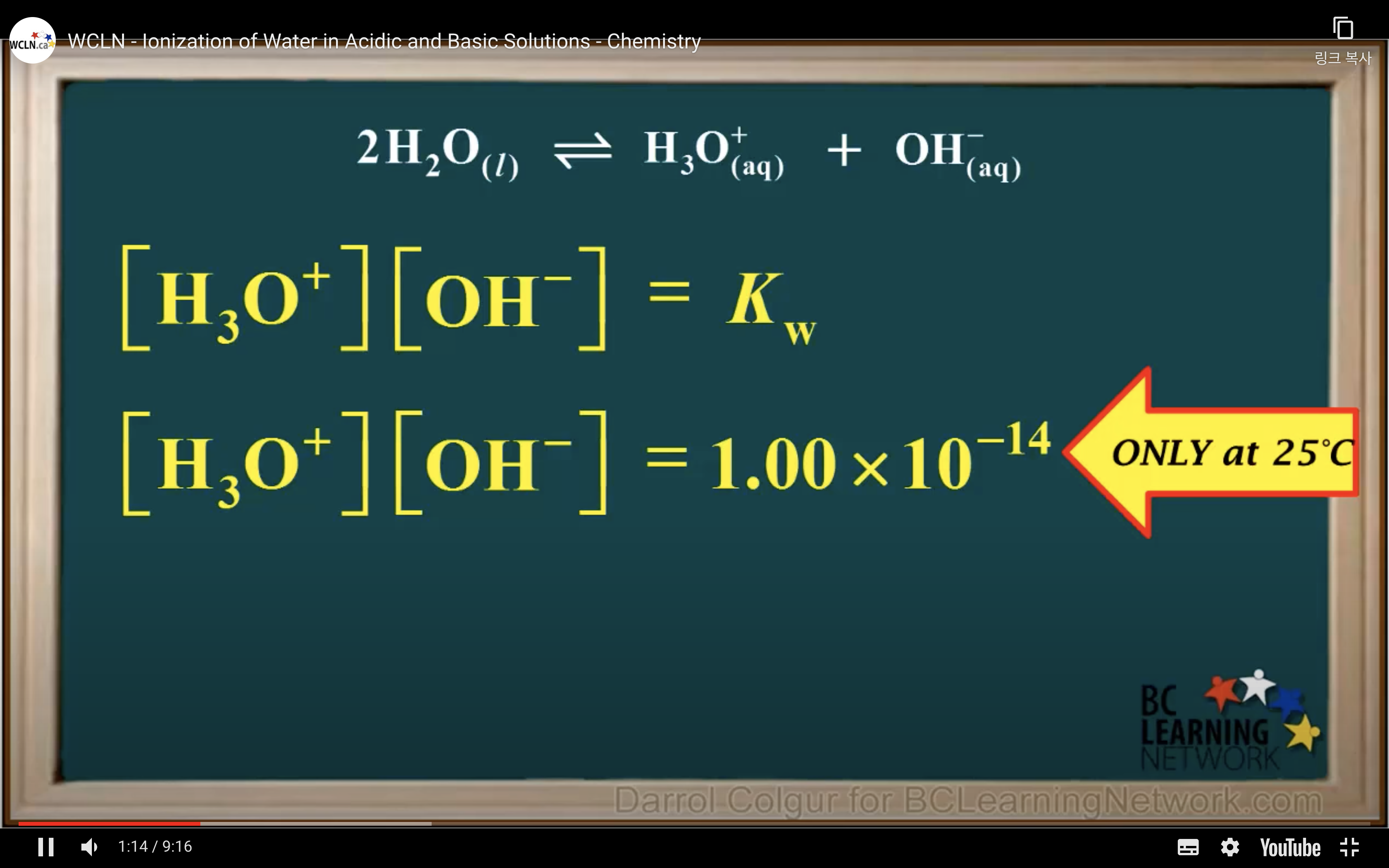
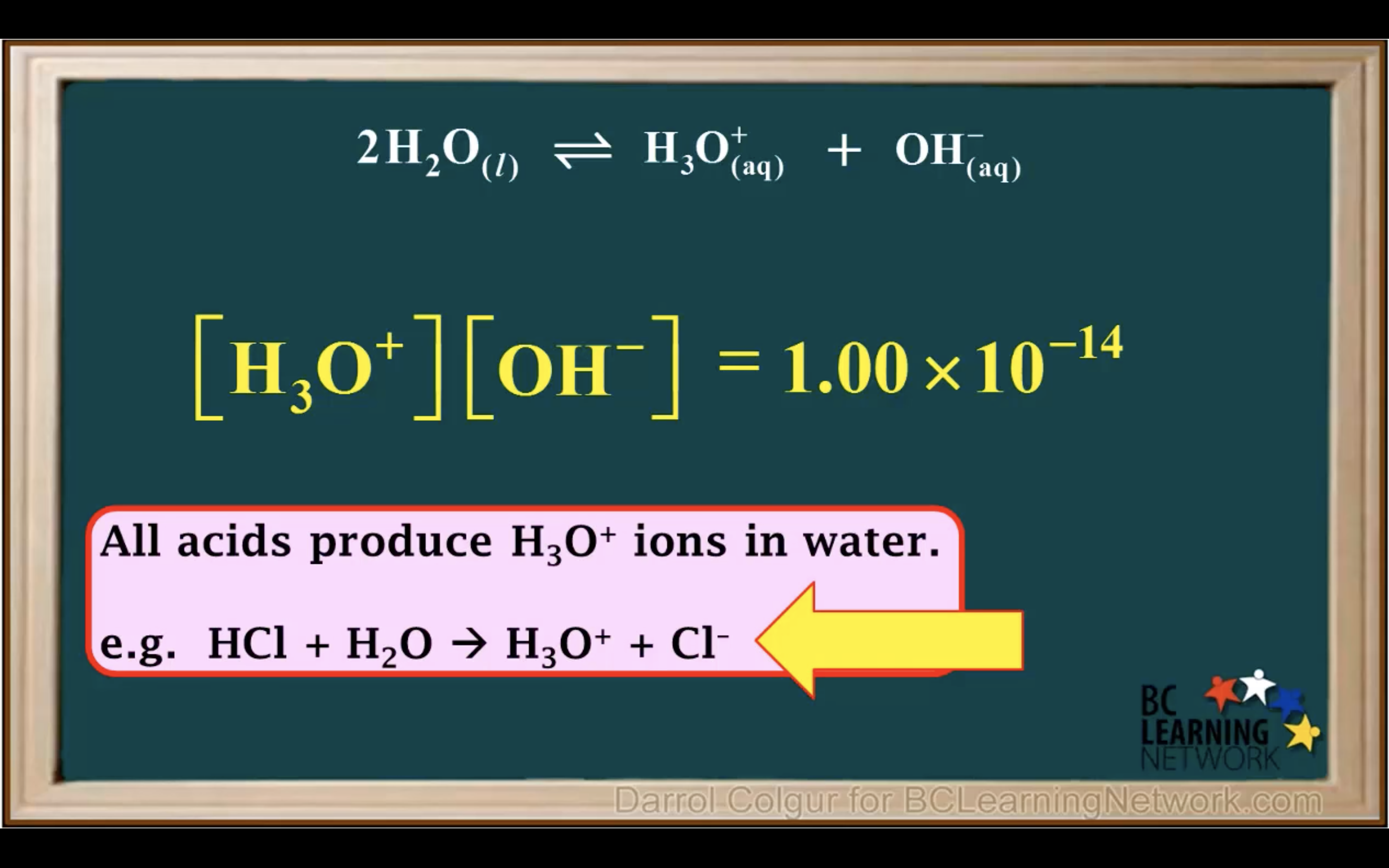
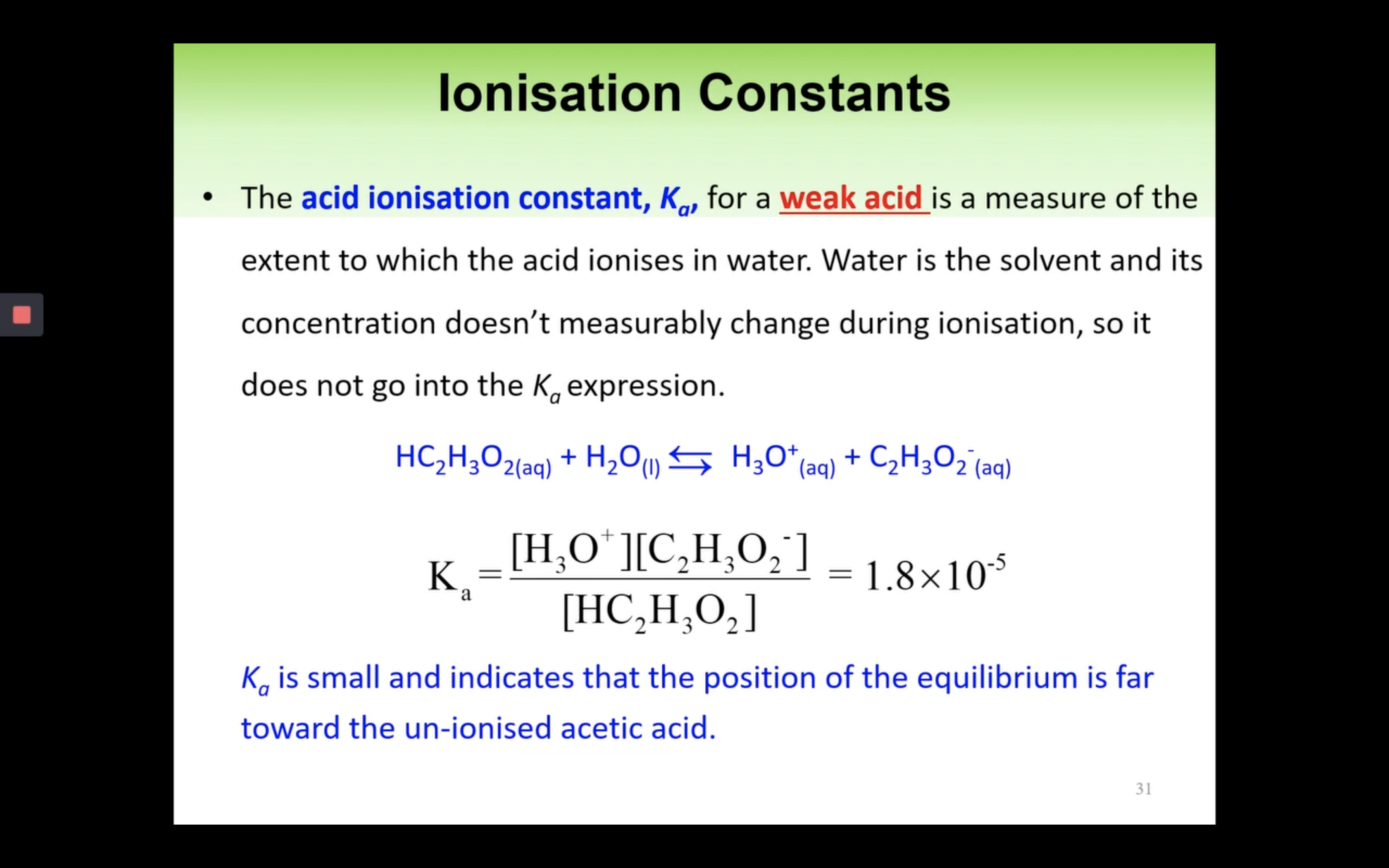
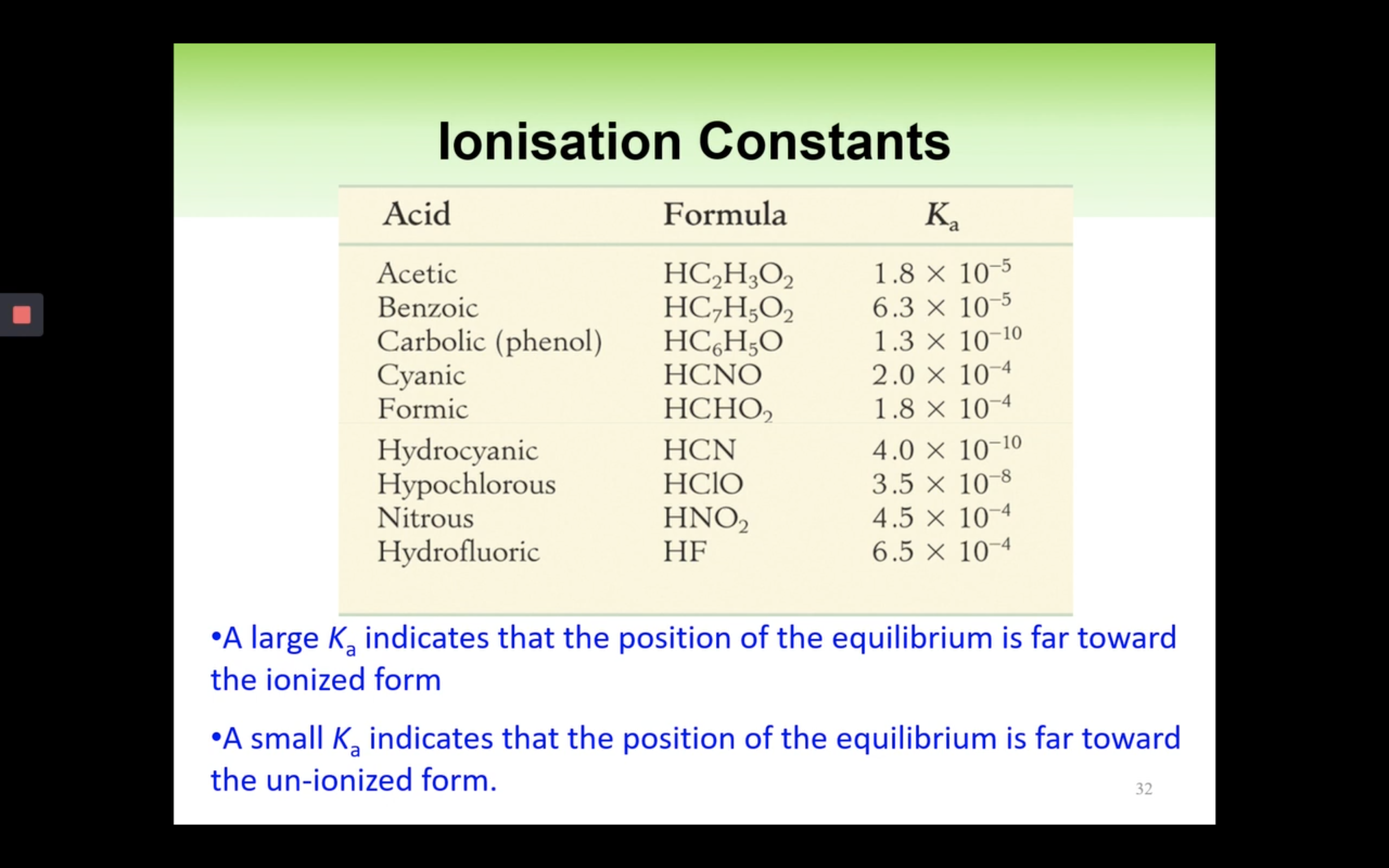
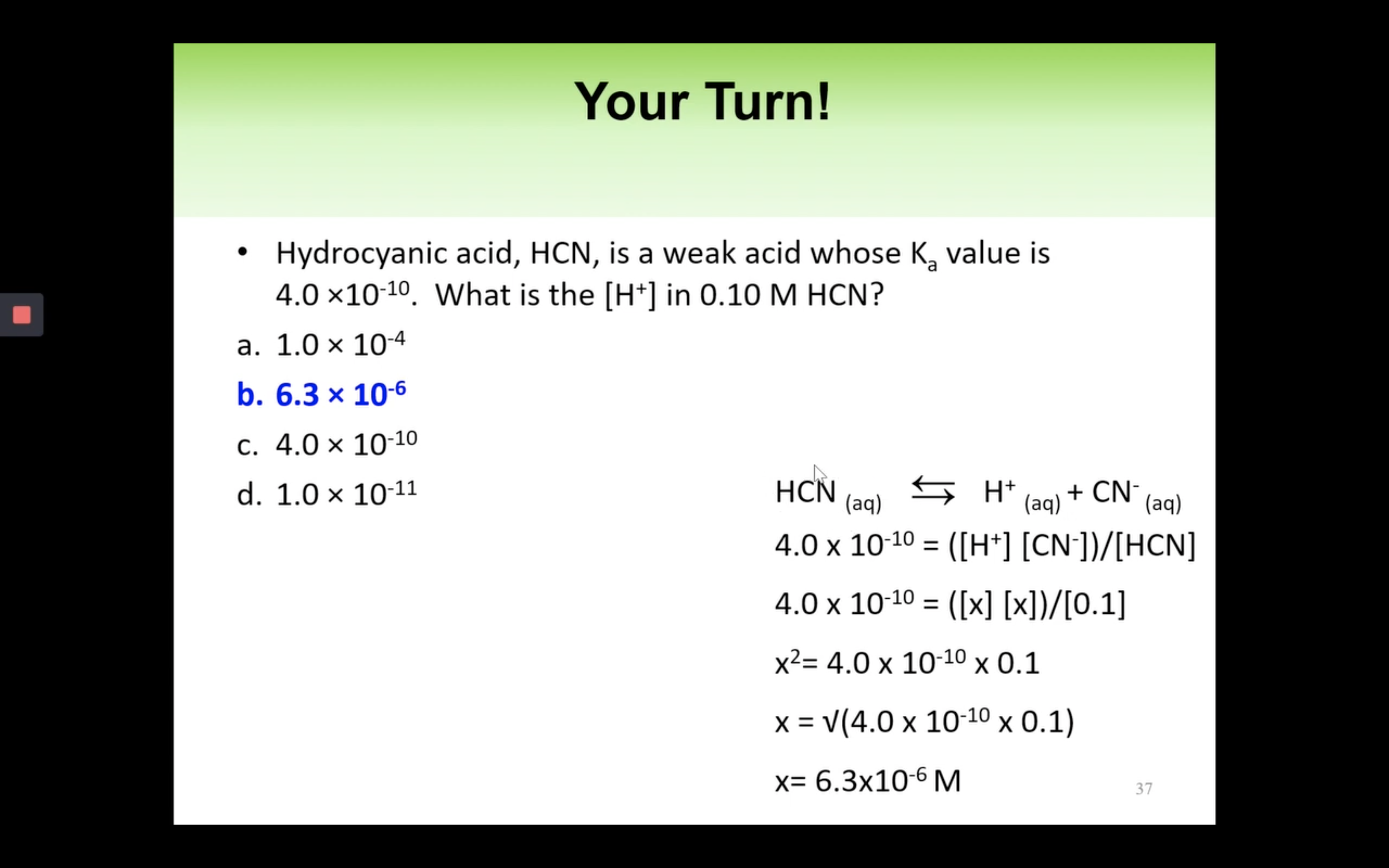
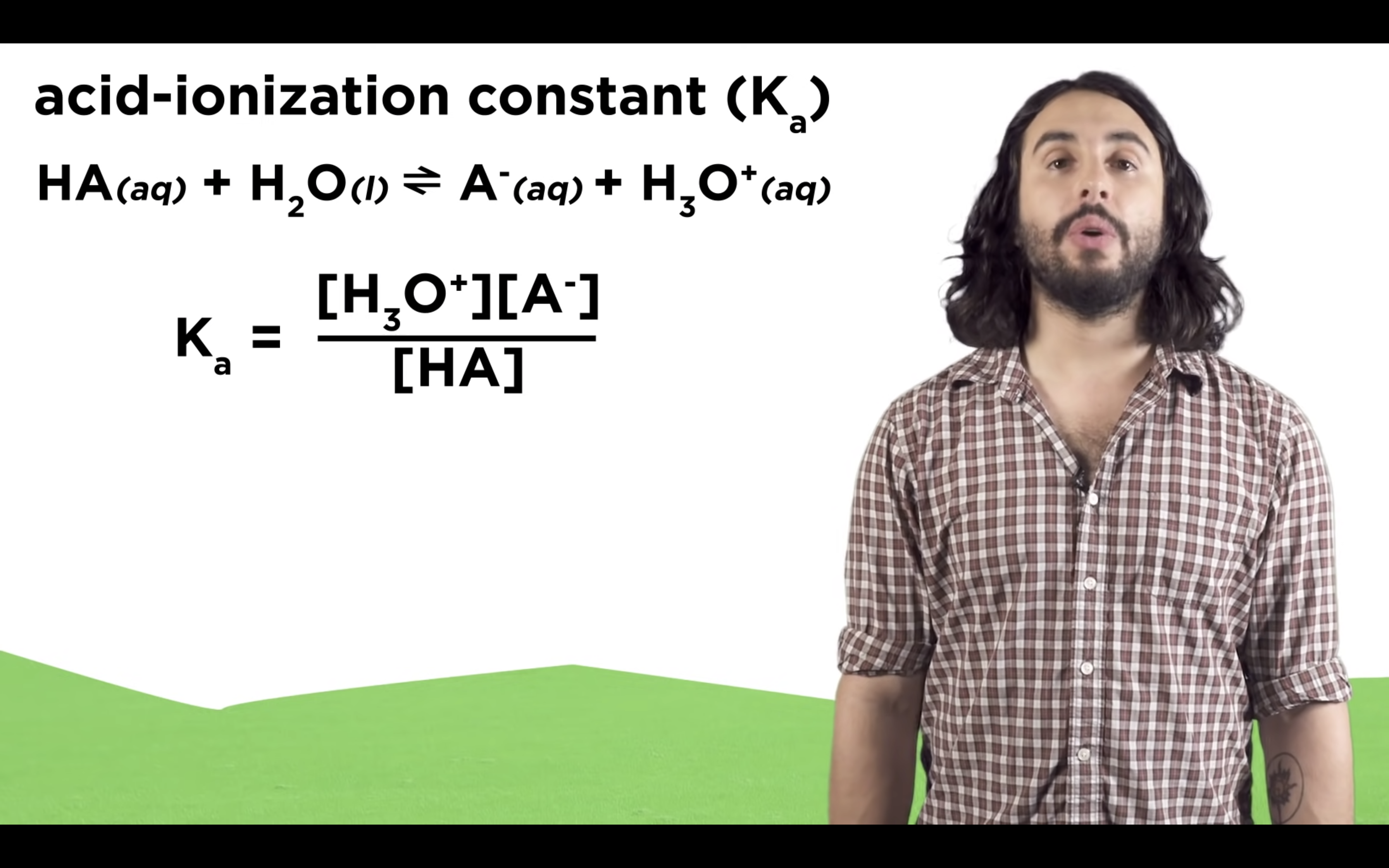
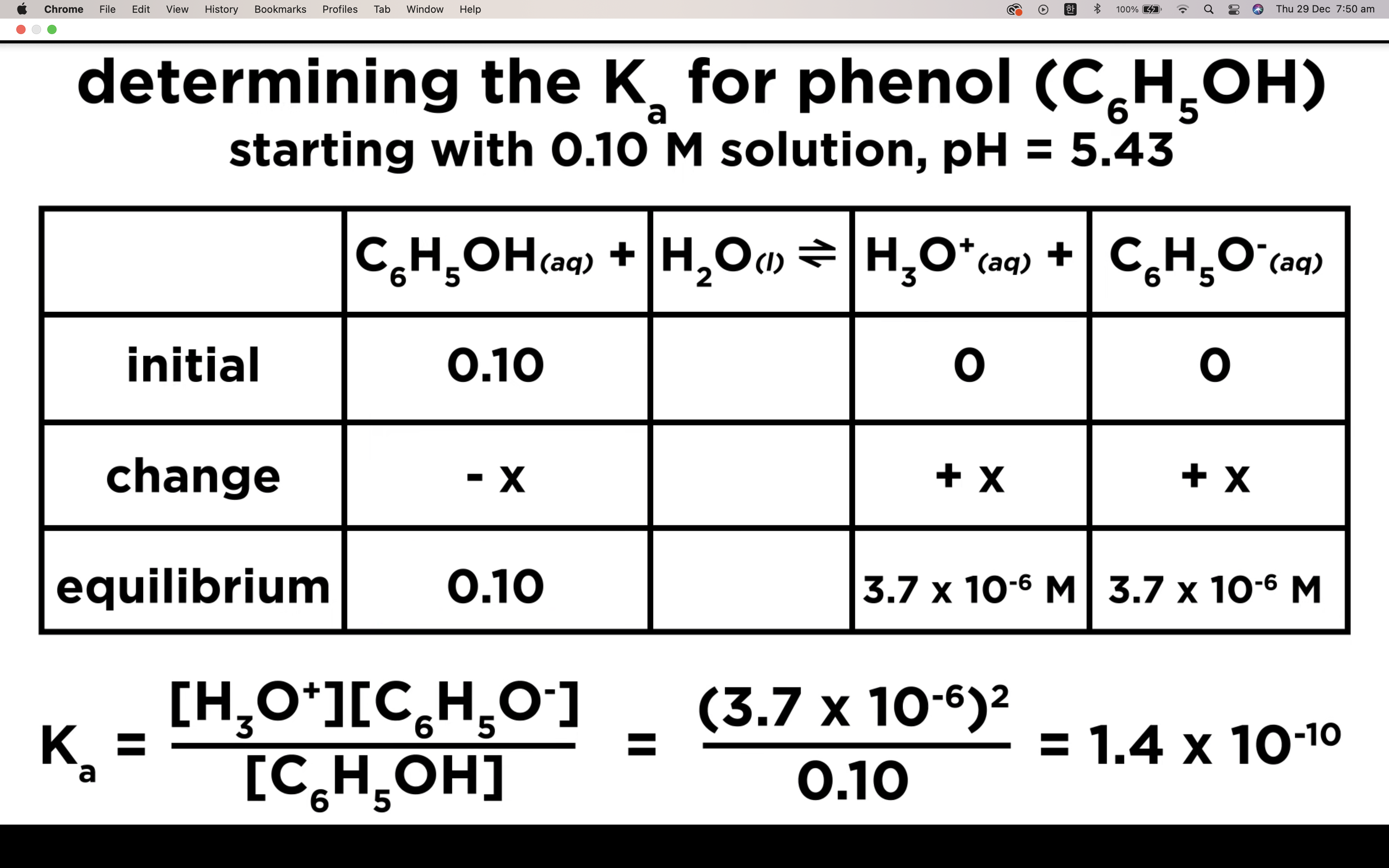
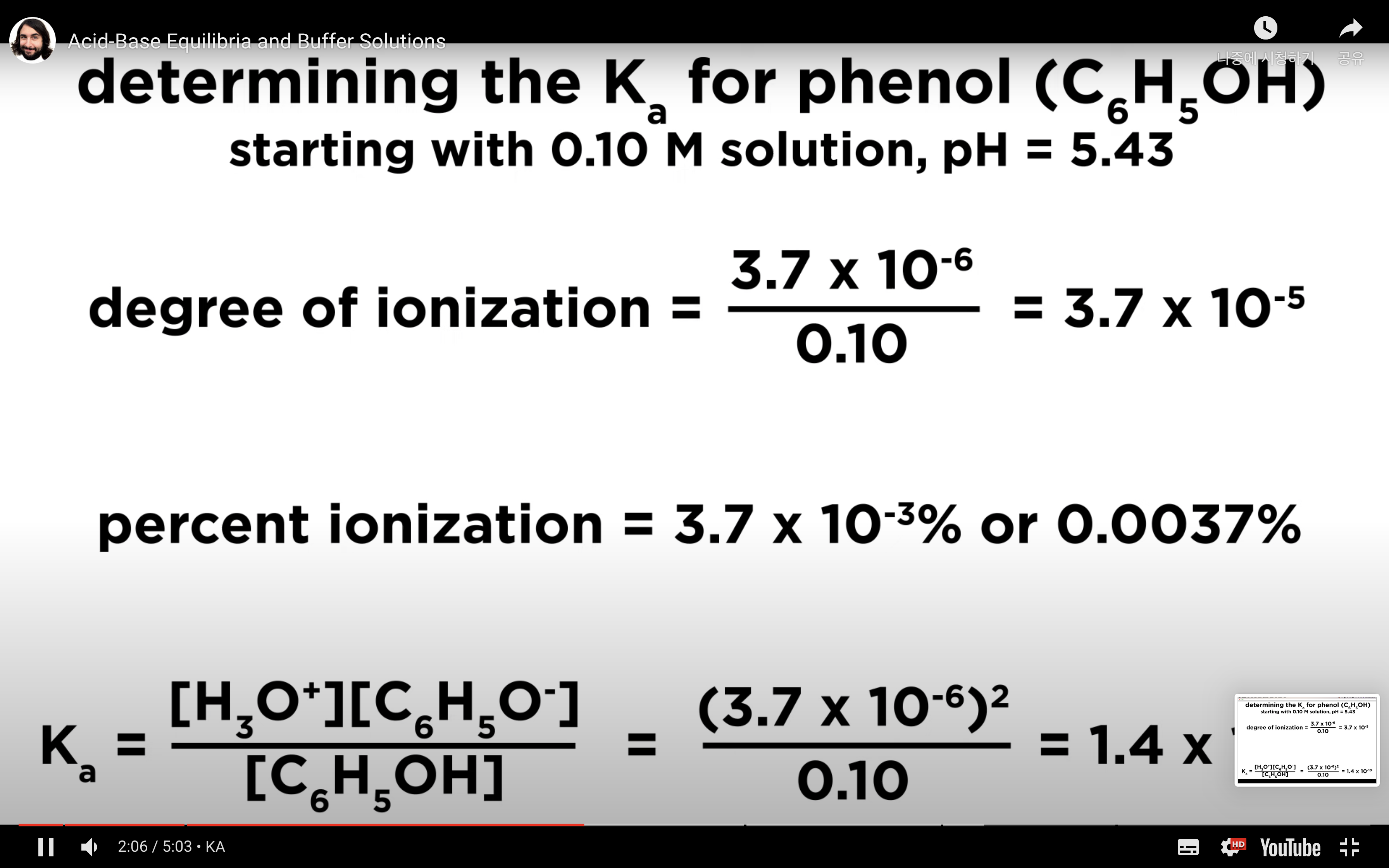
Introduction to Ka for weak acids
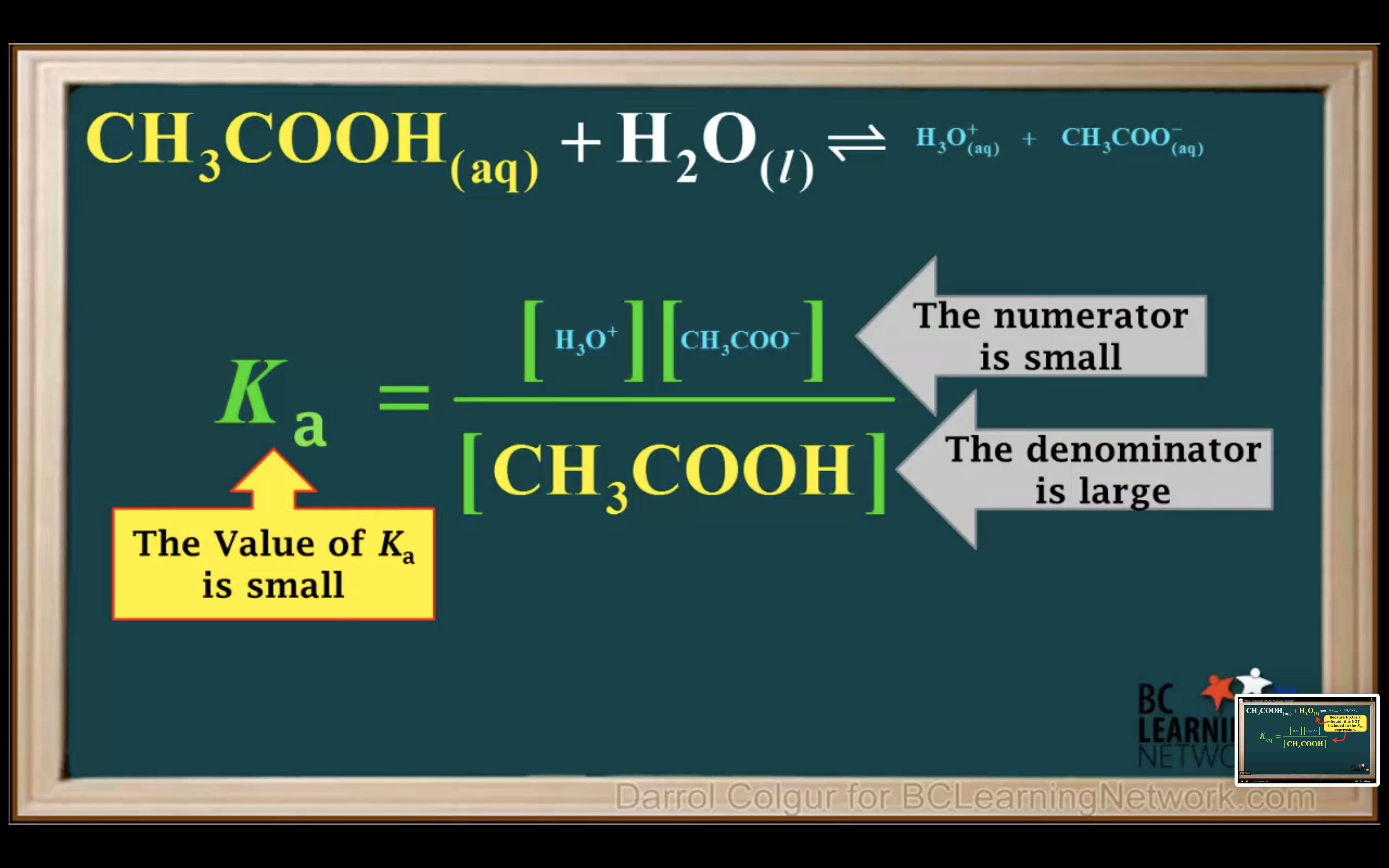
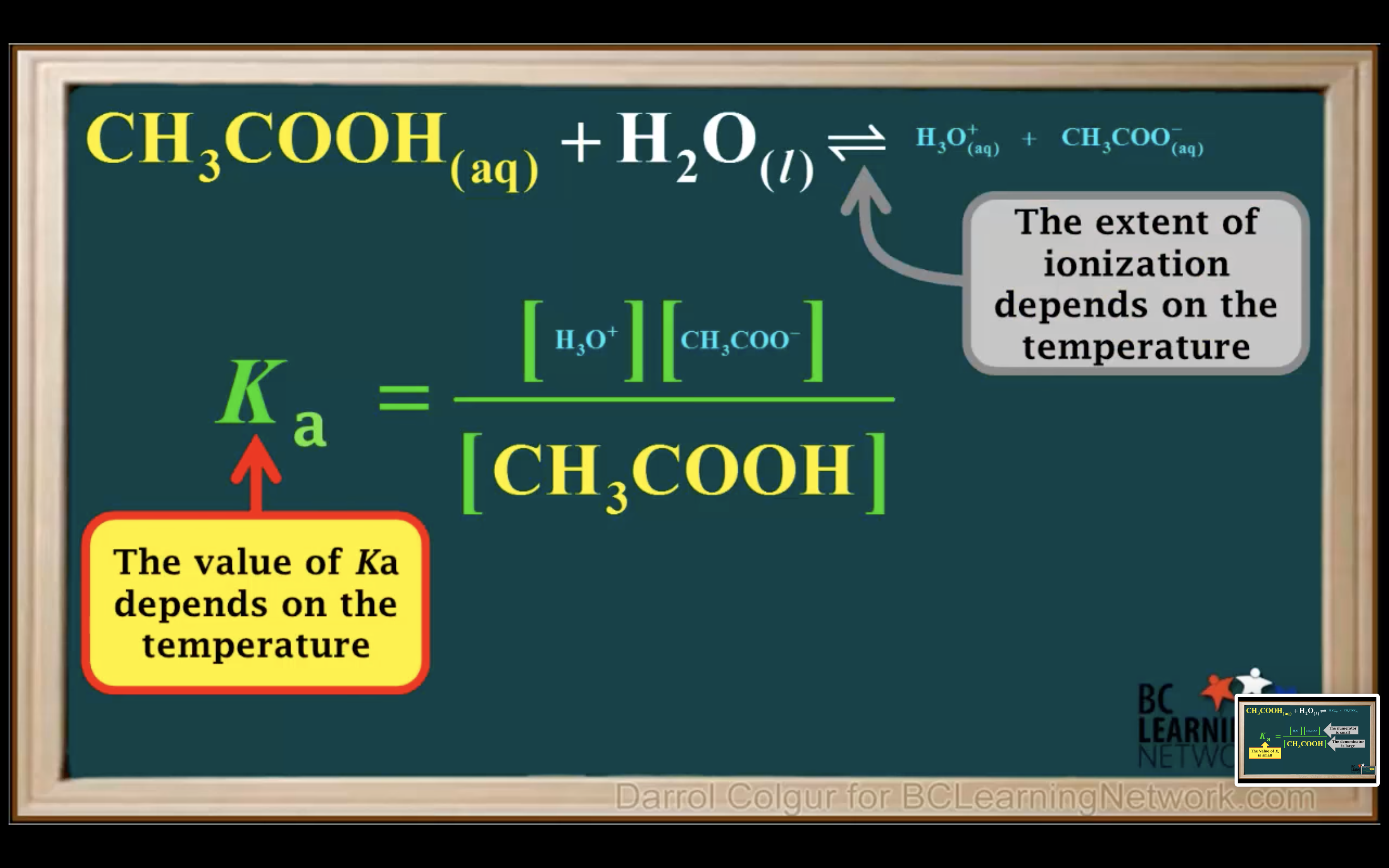
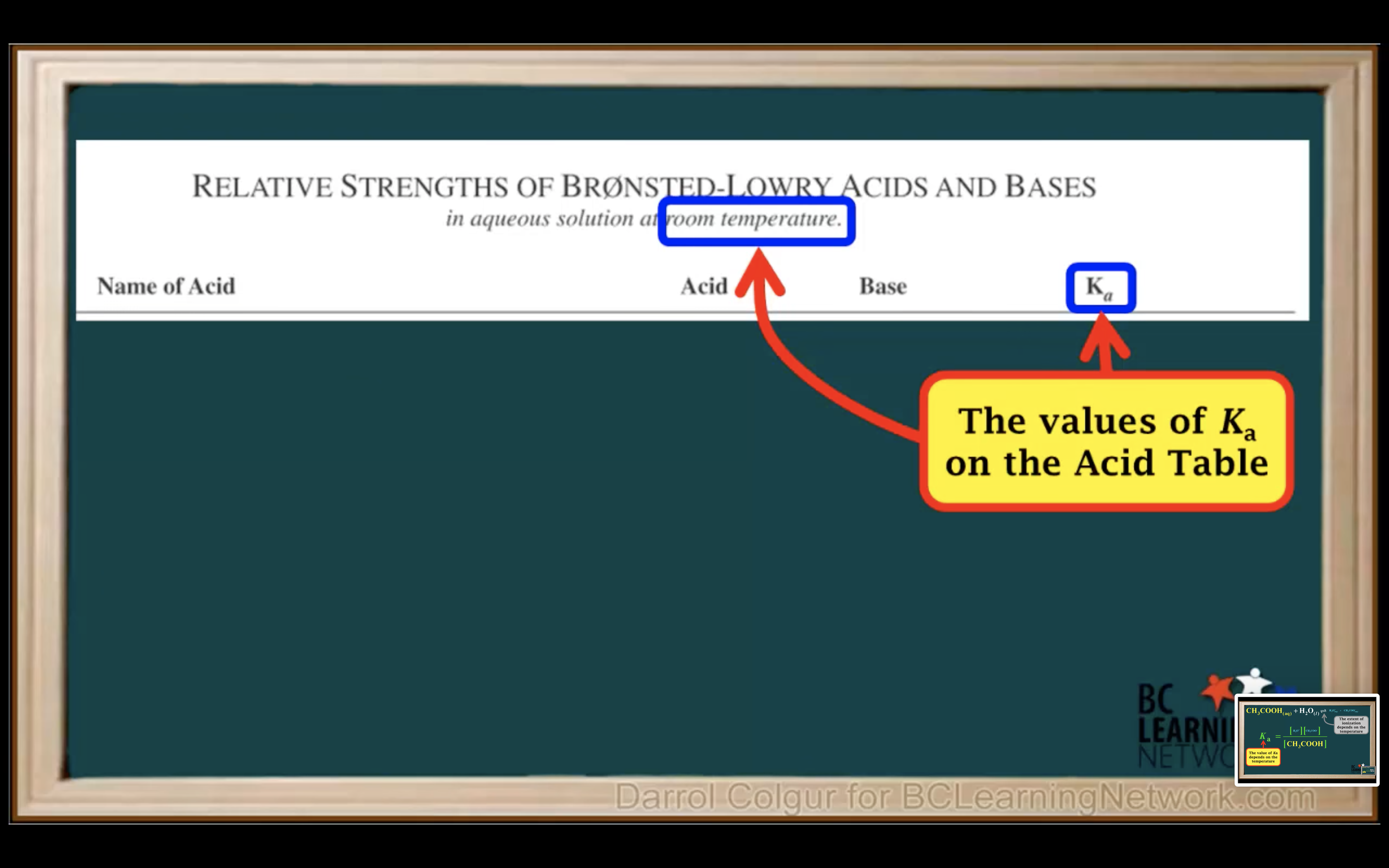
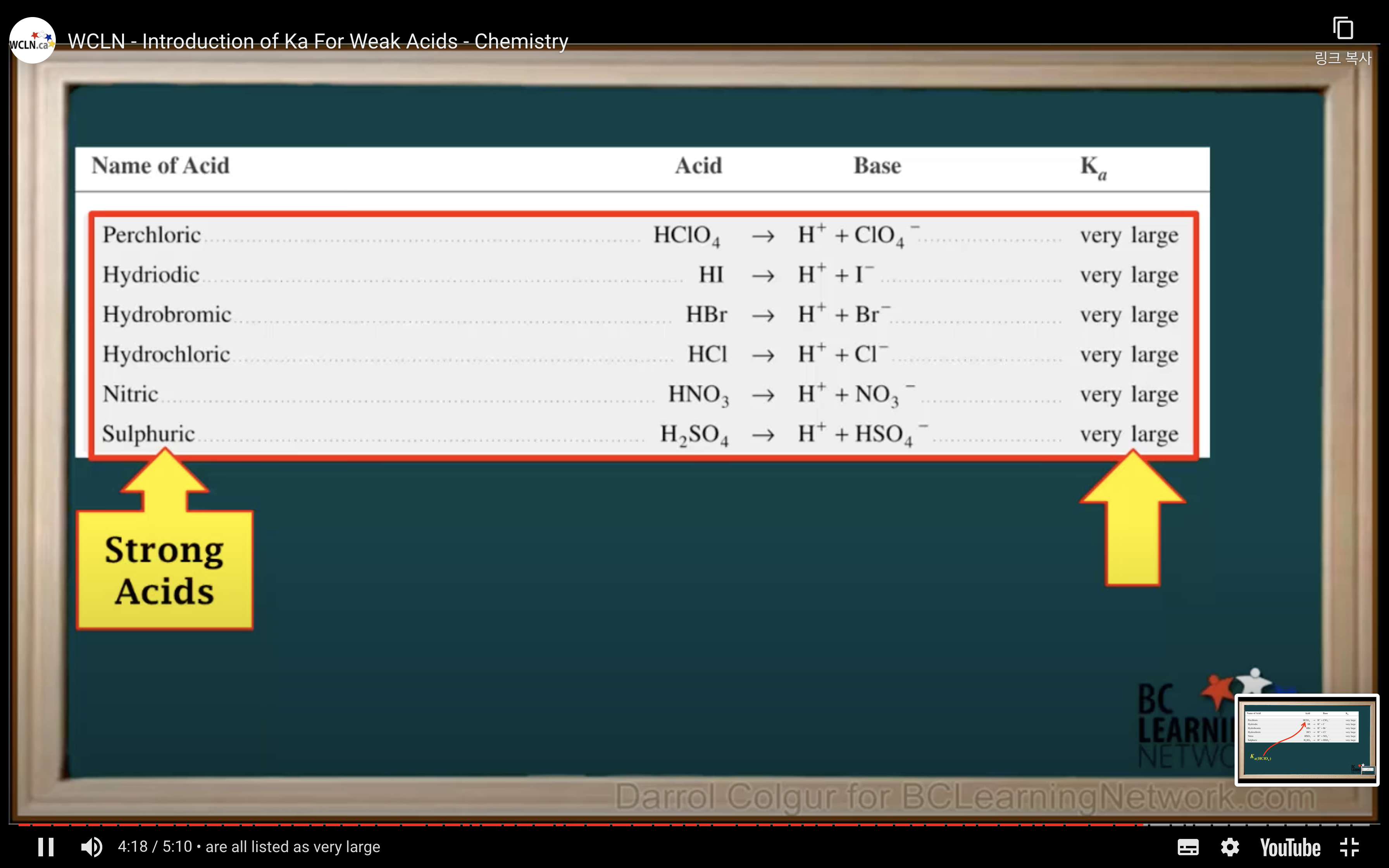
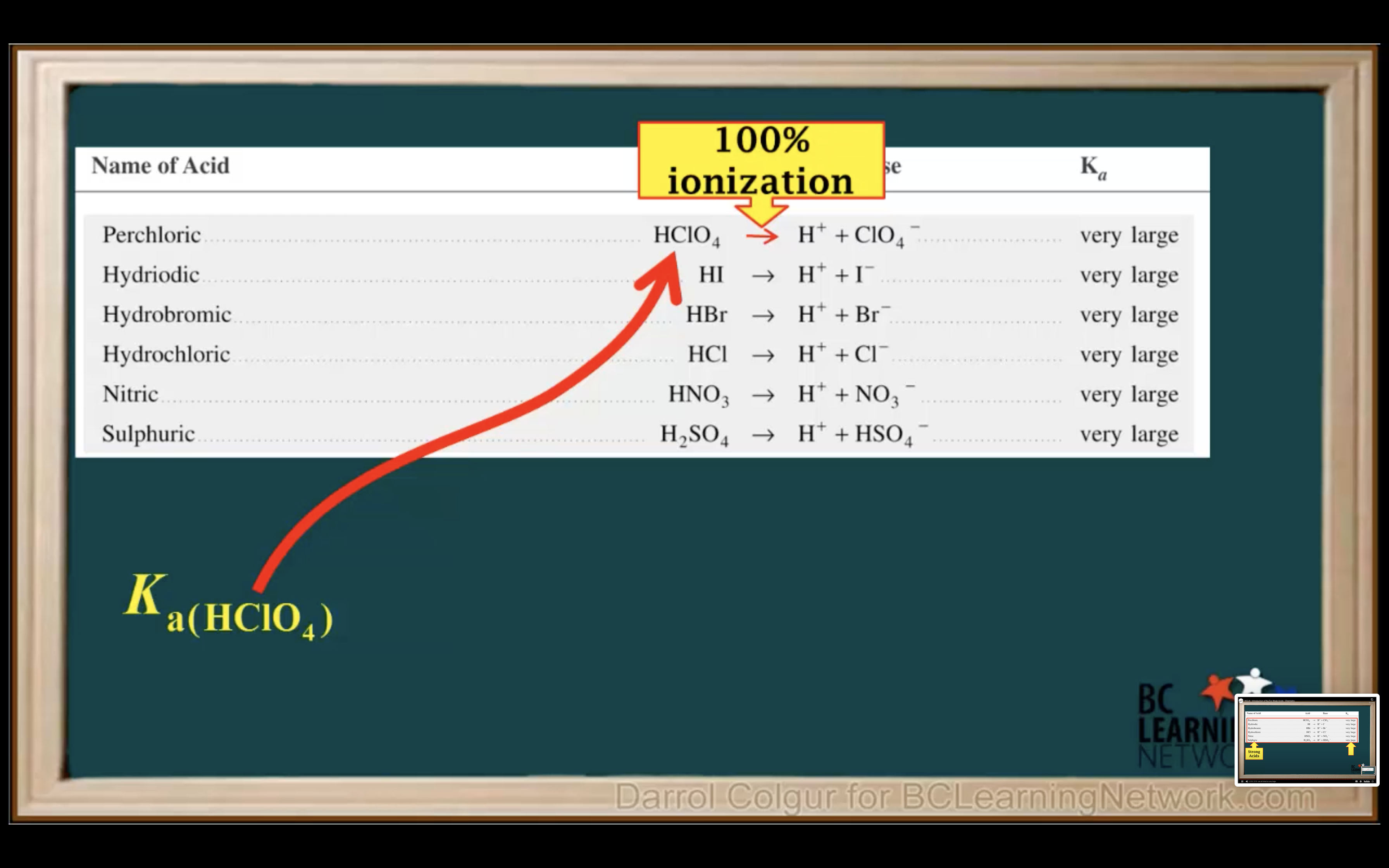
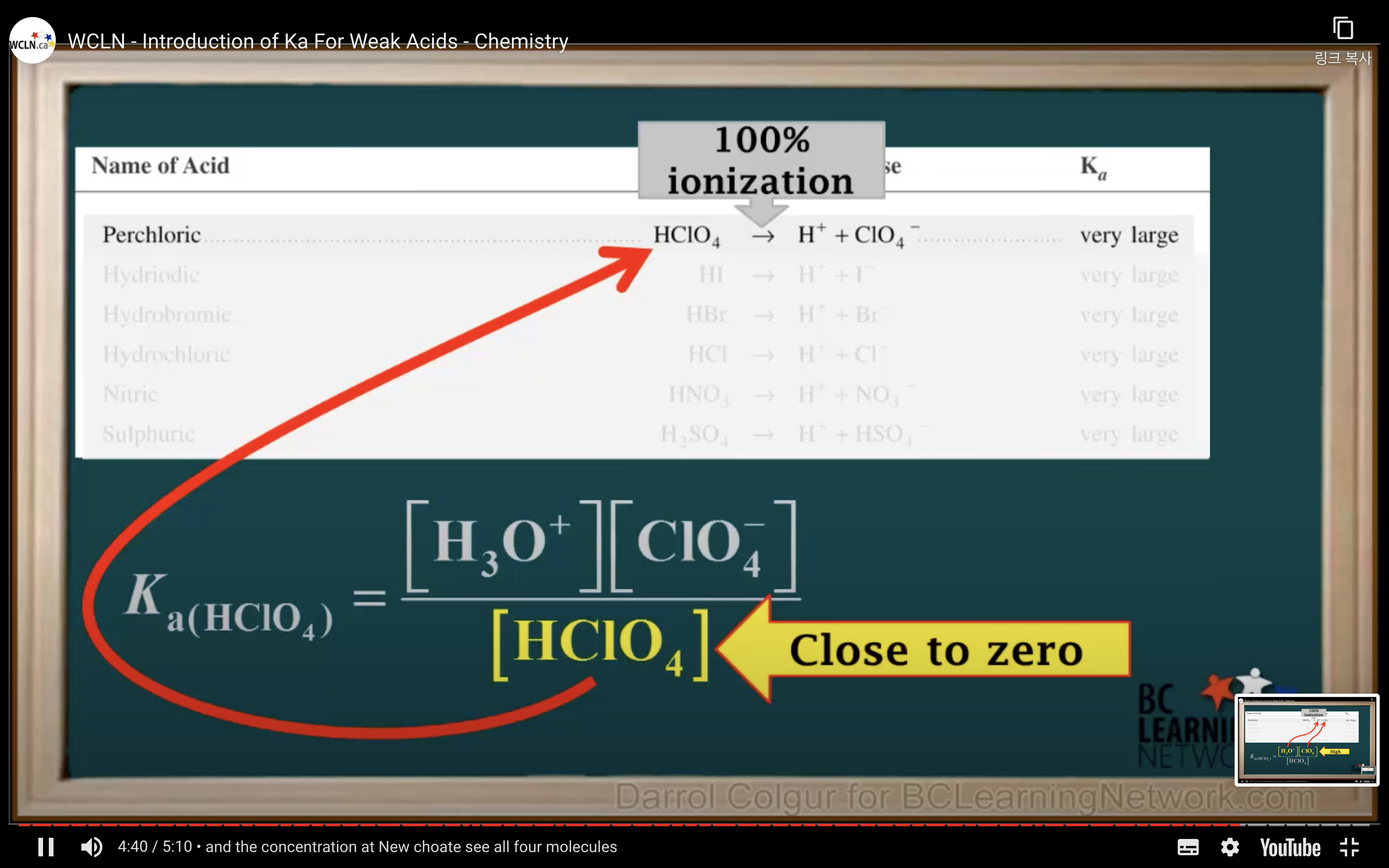
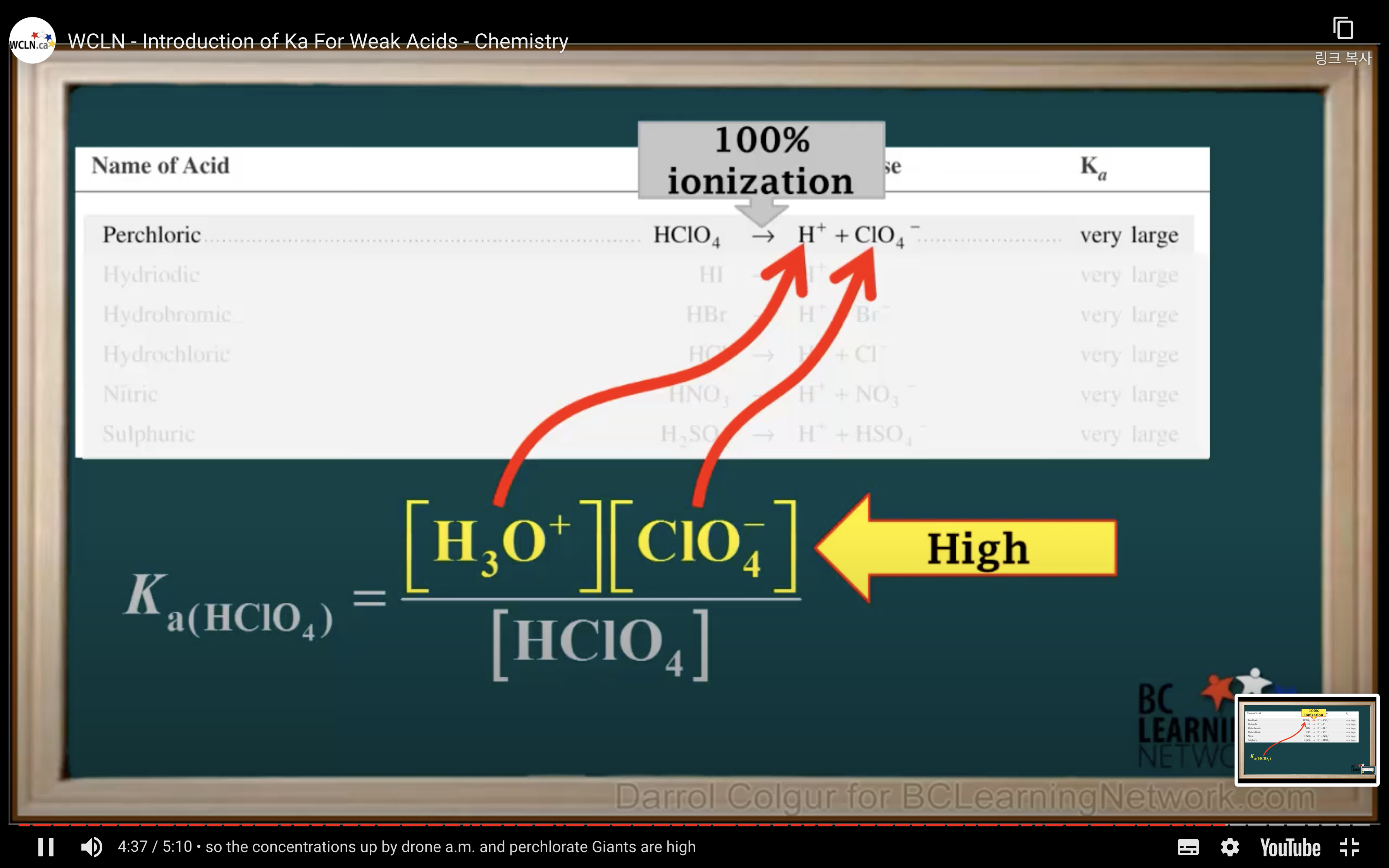
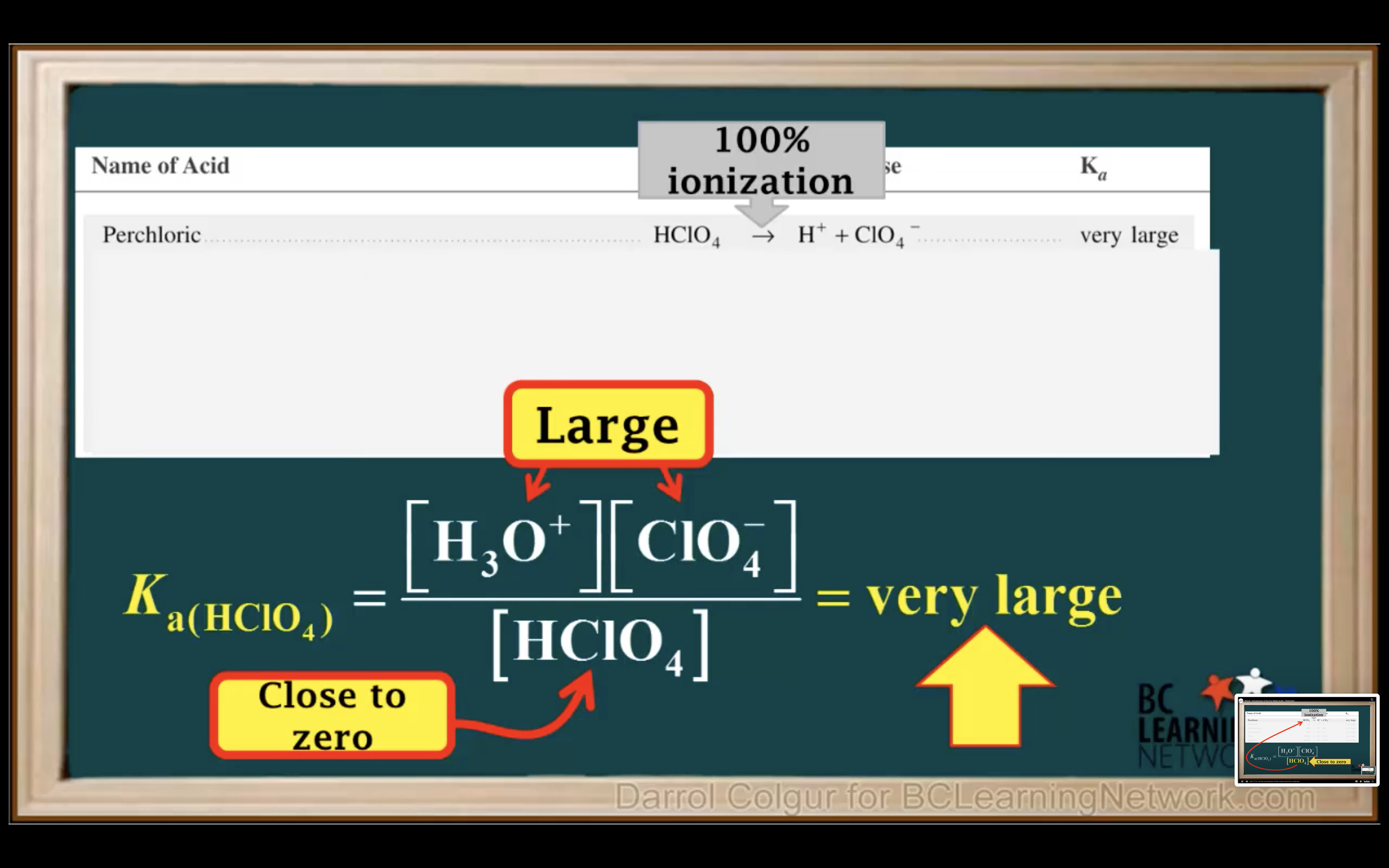
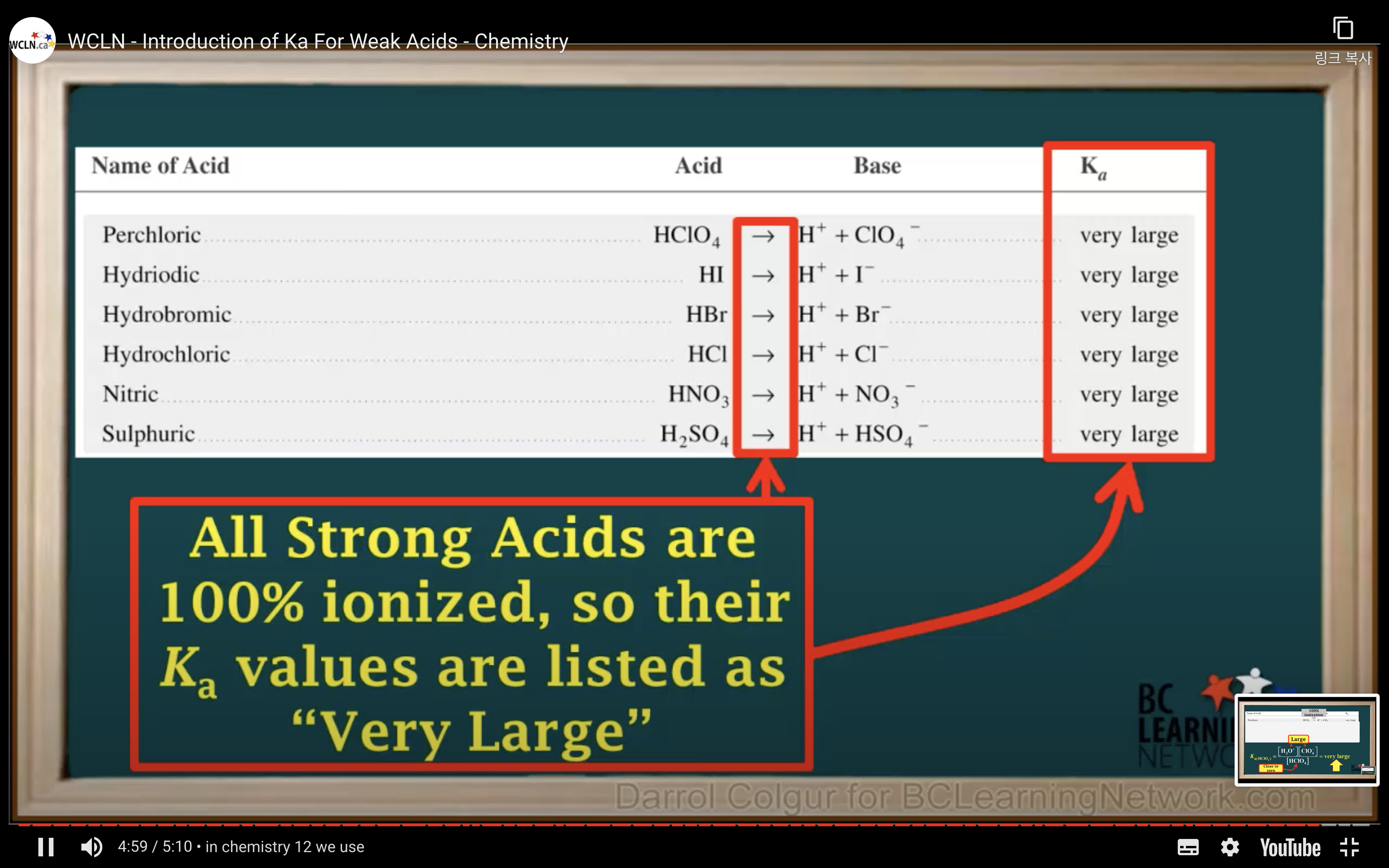
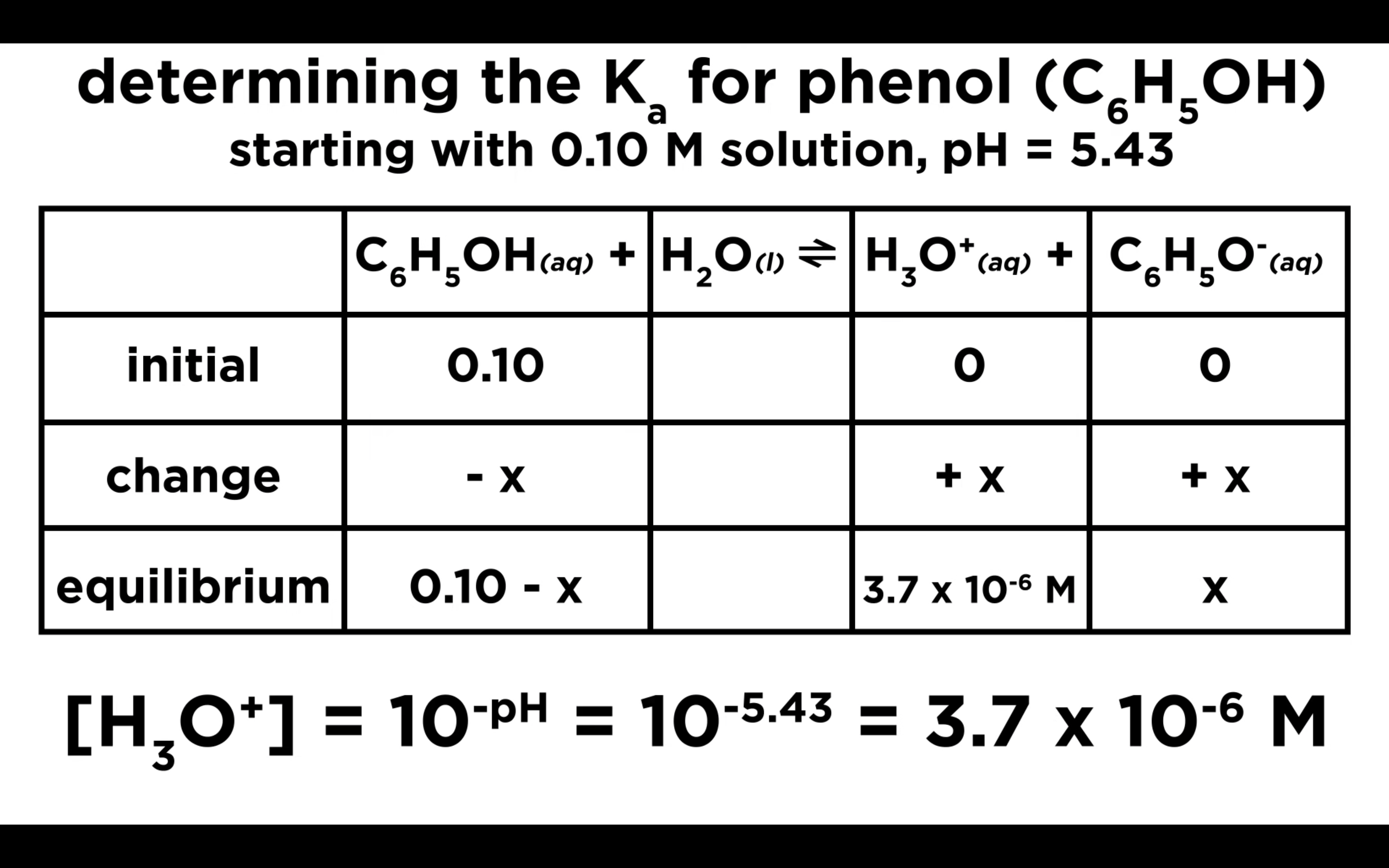
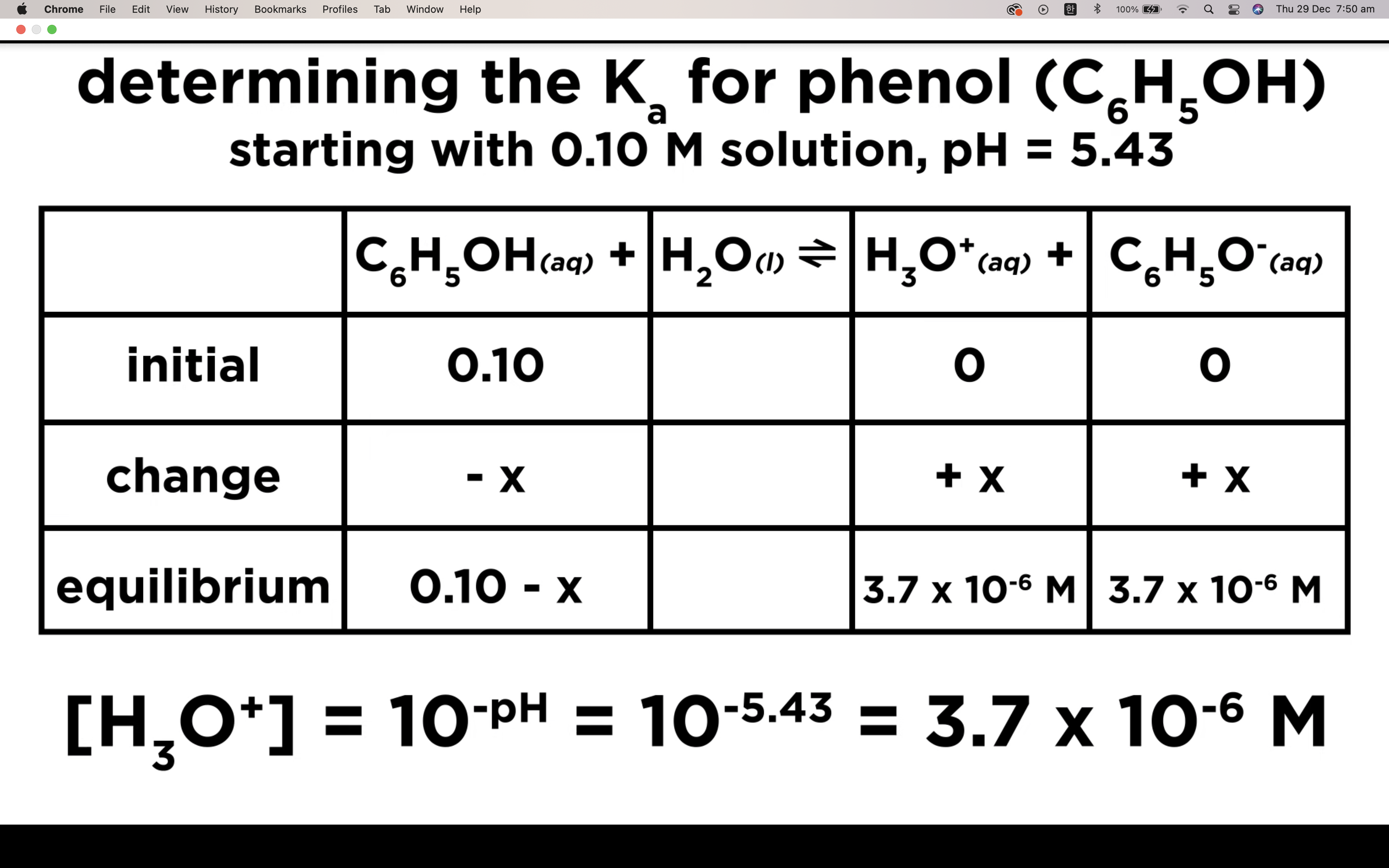
How to calculate Ka for a weak acid

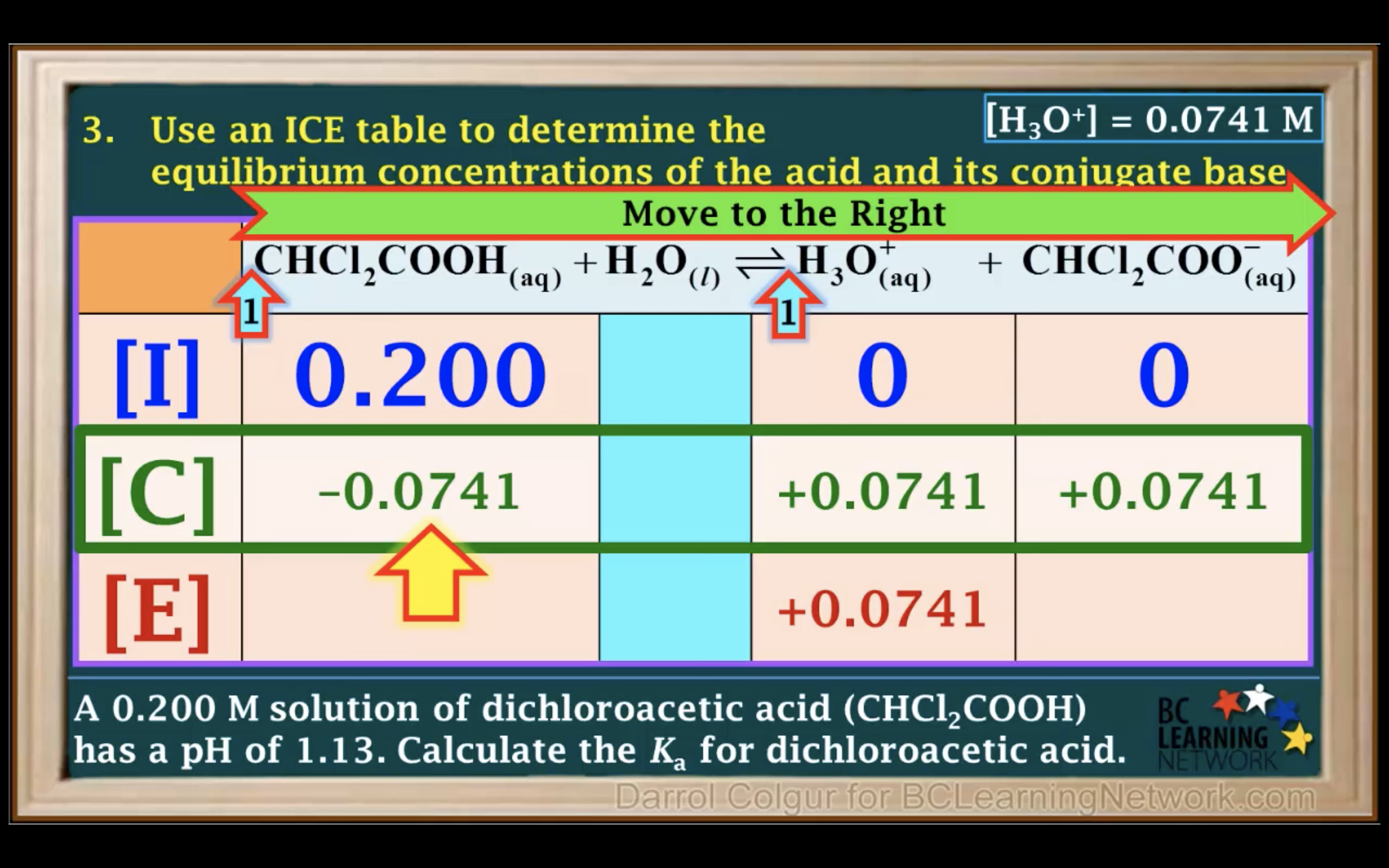
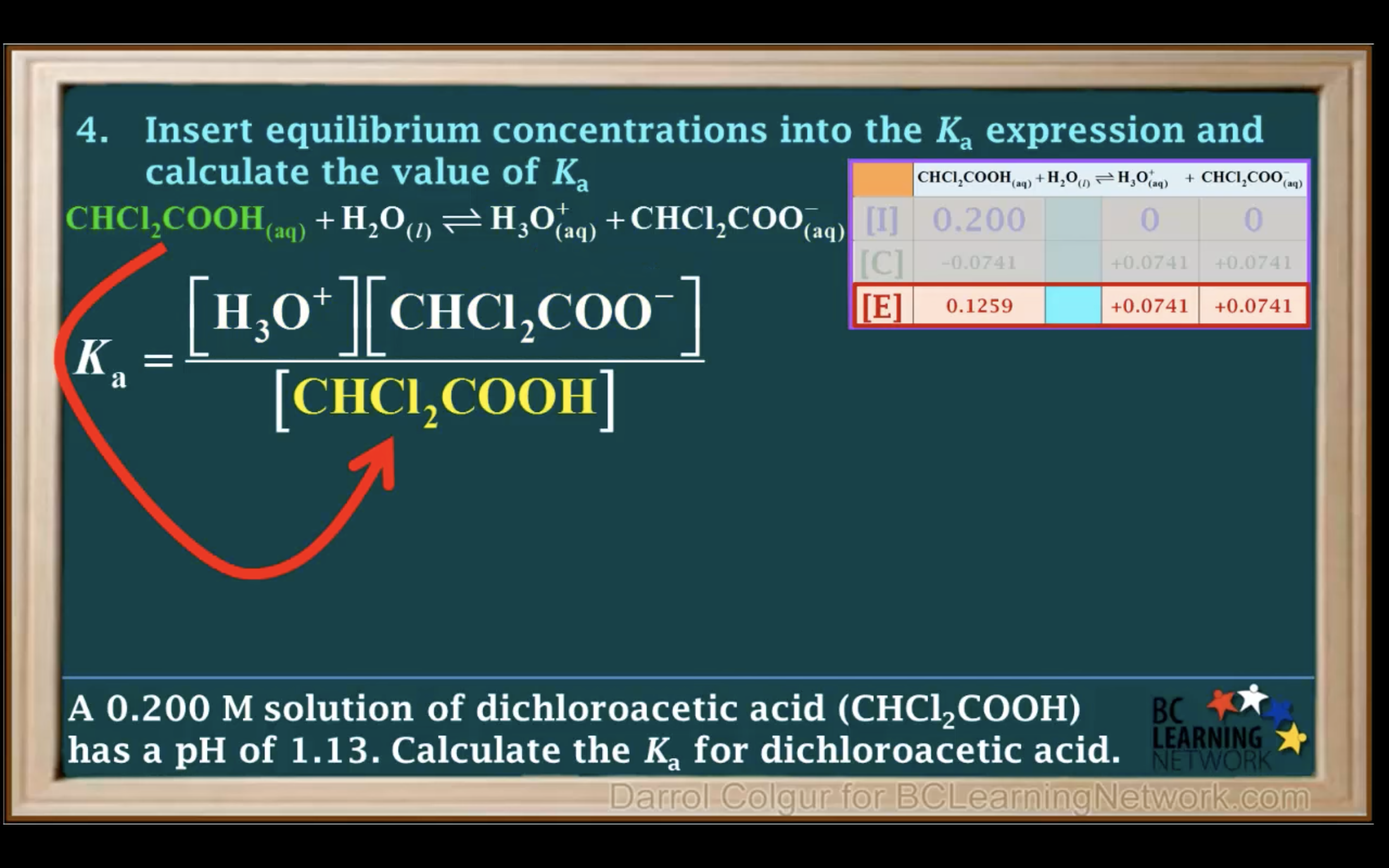
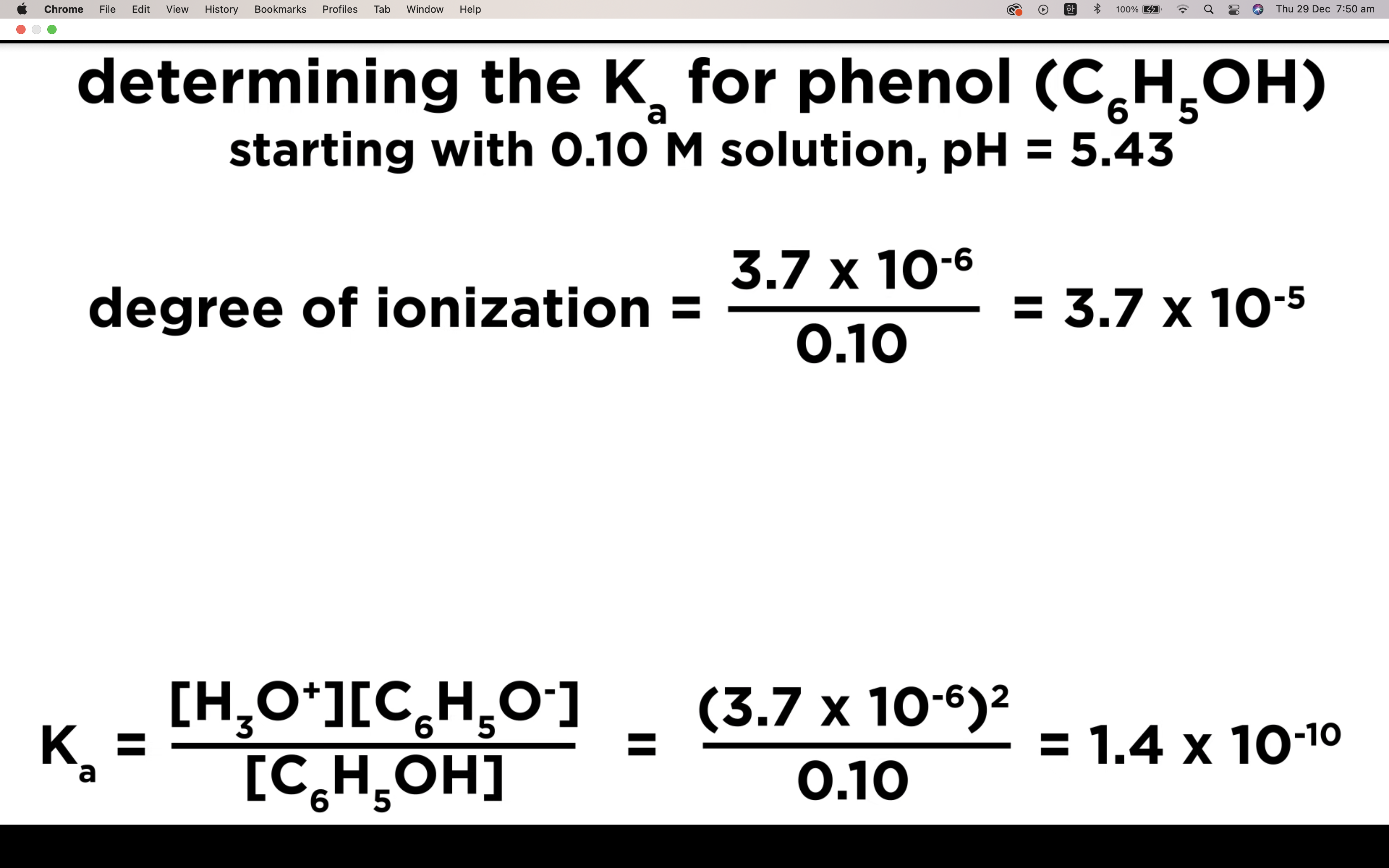
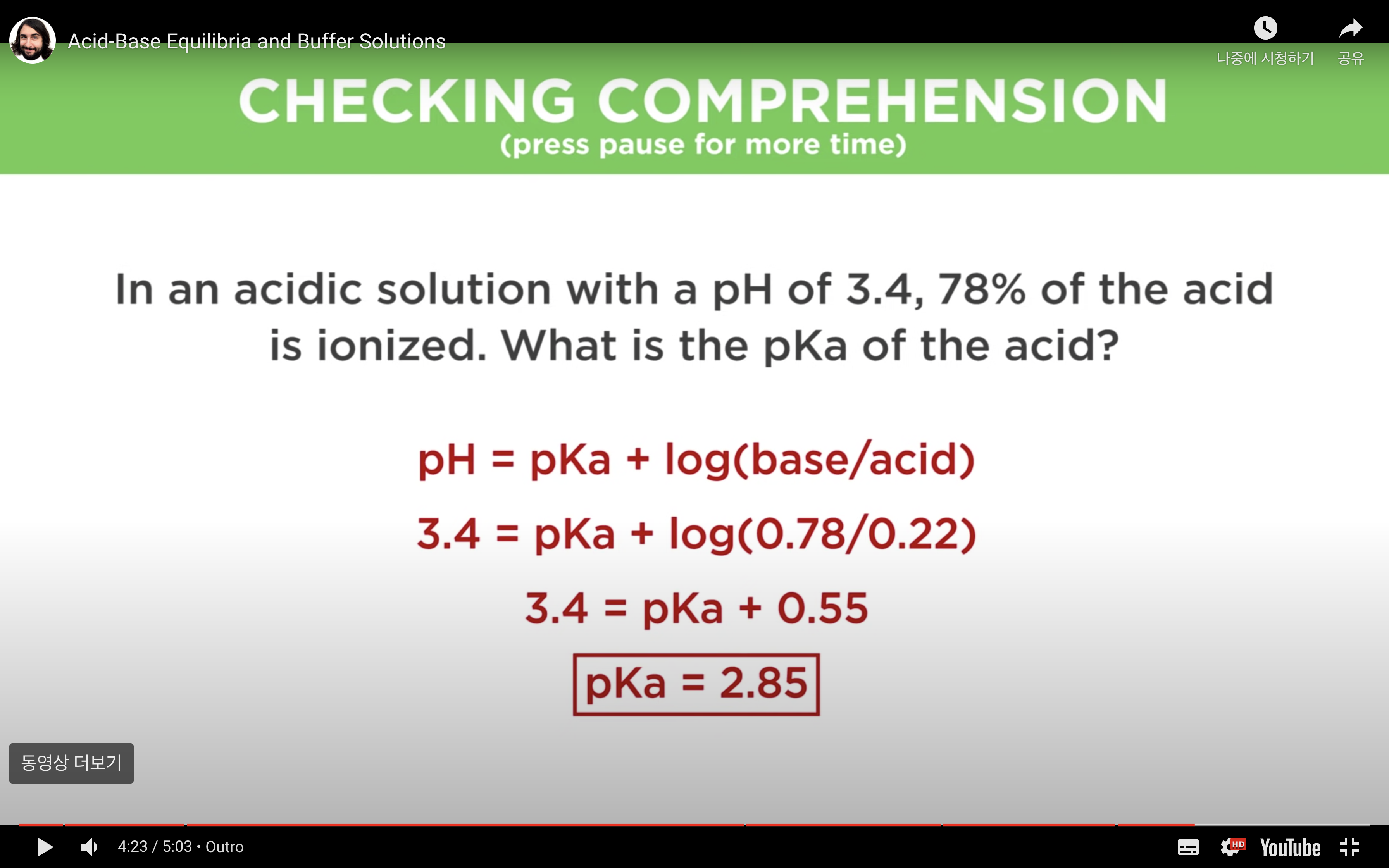
Ka to pH and Percent Ionisation
First step is to see whether it's strong acid or weak acid


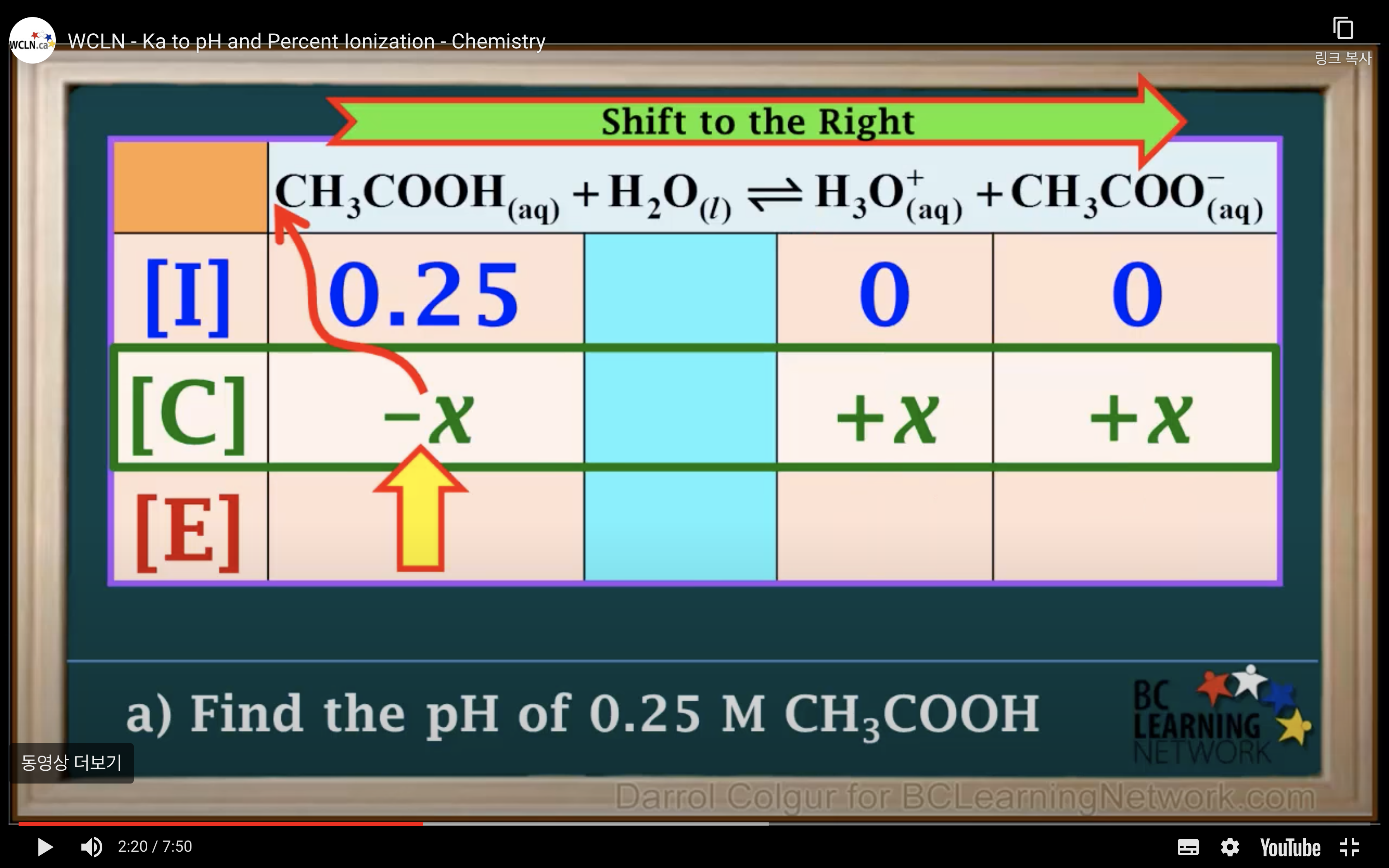
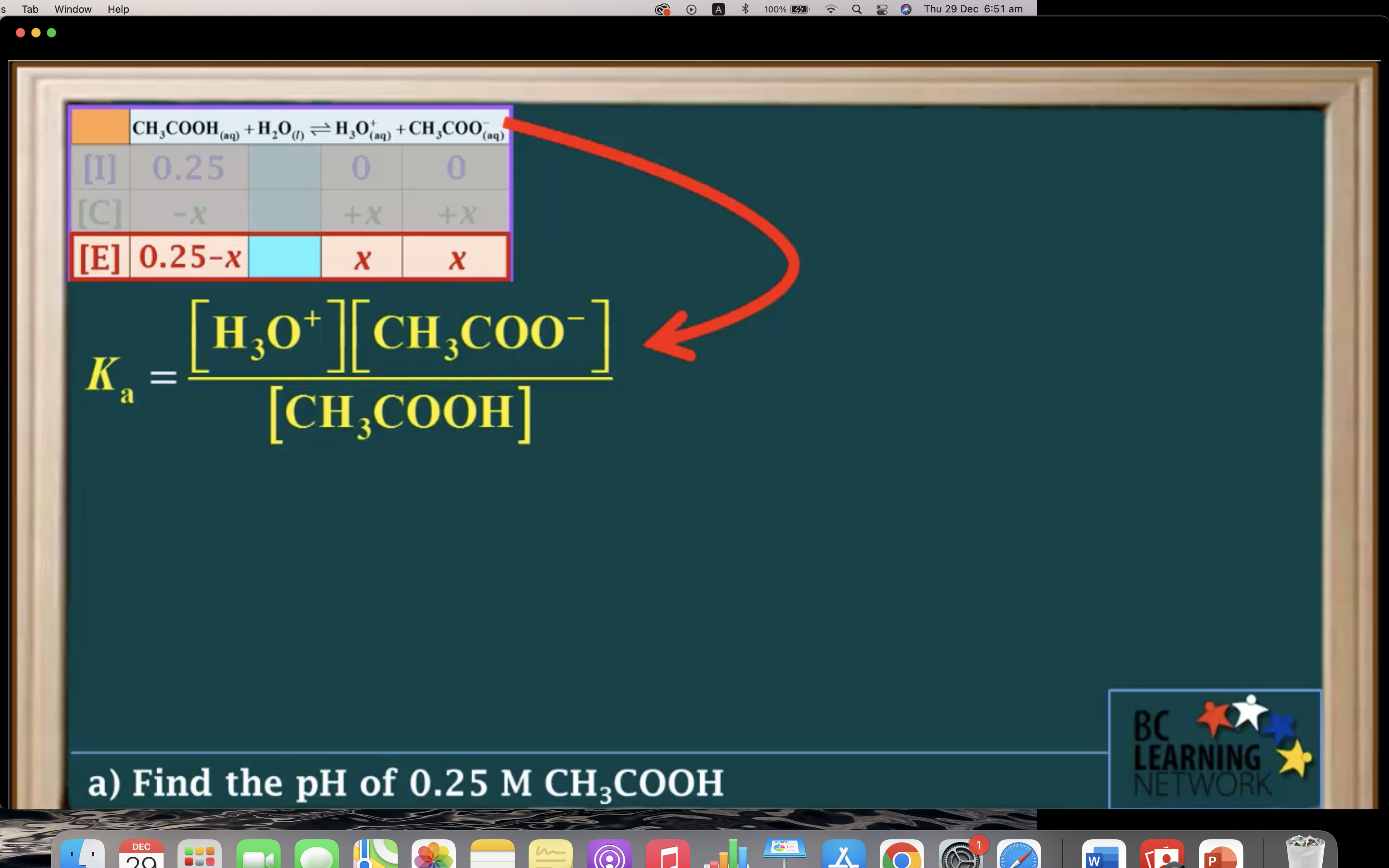
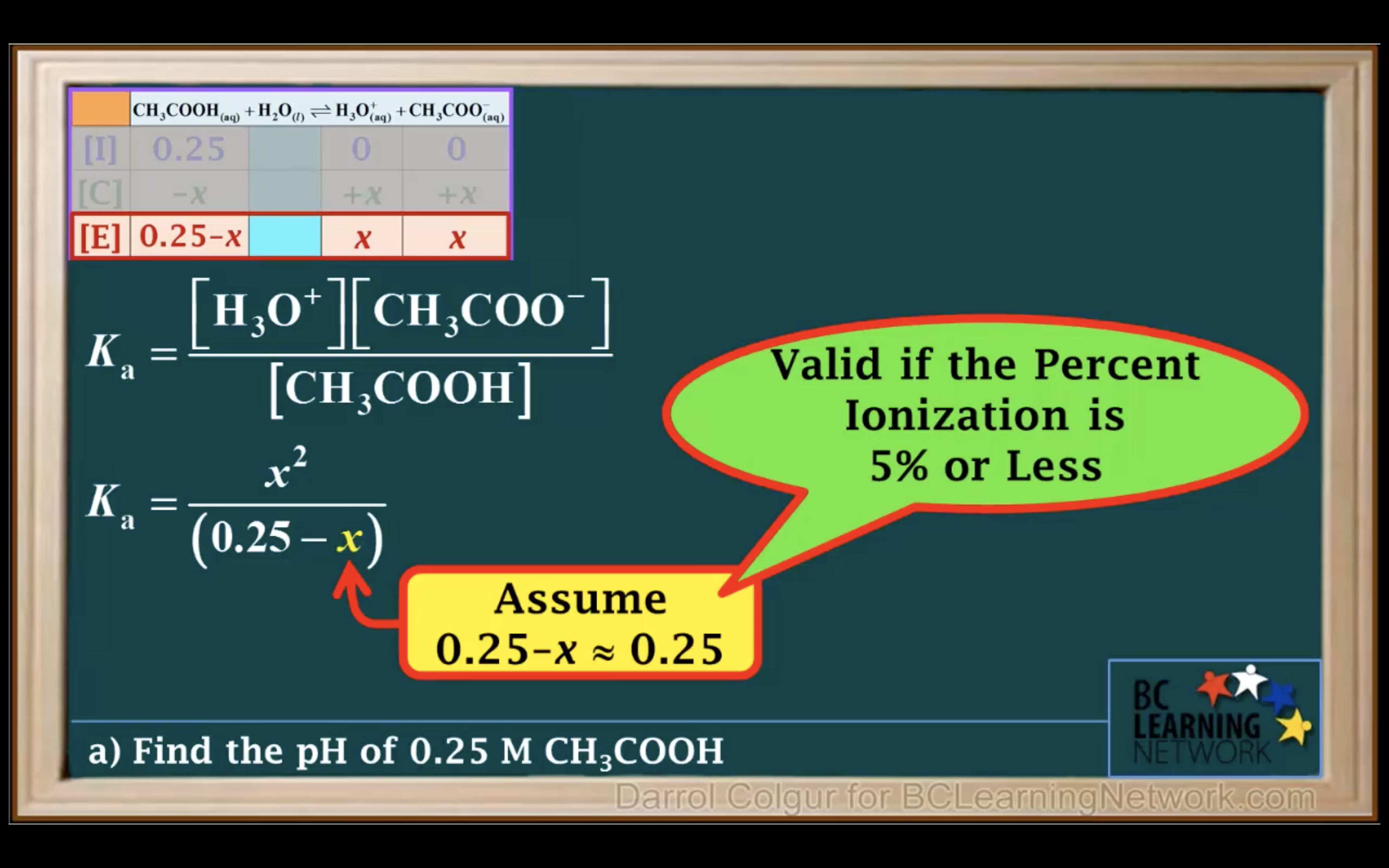

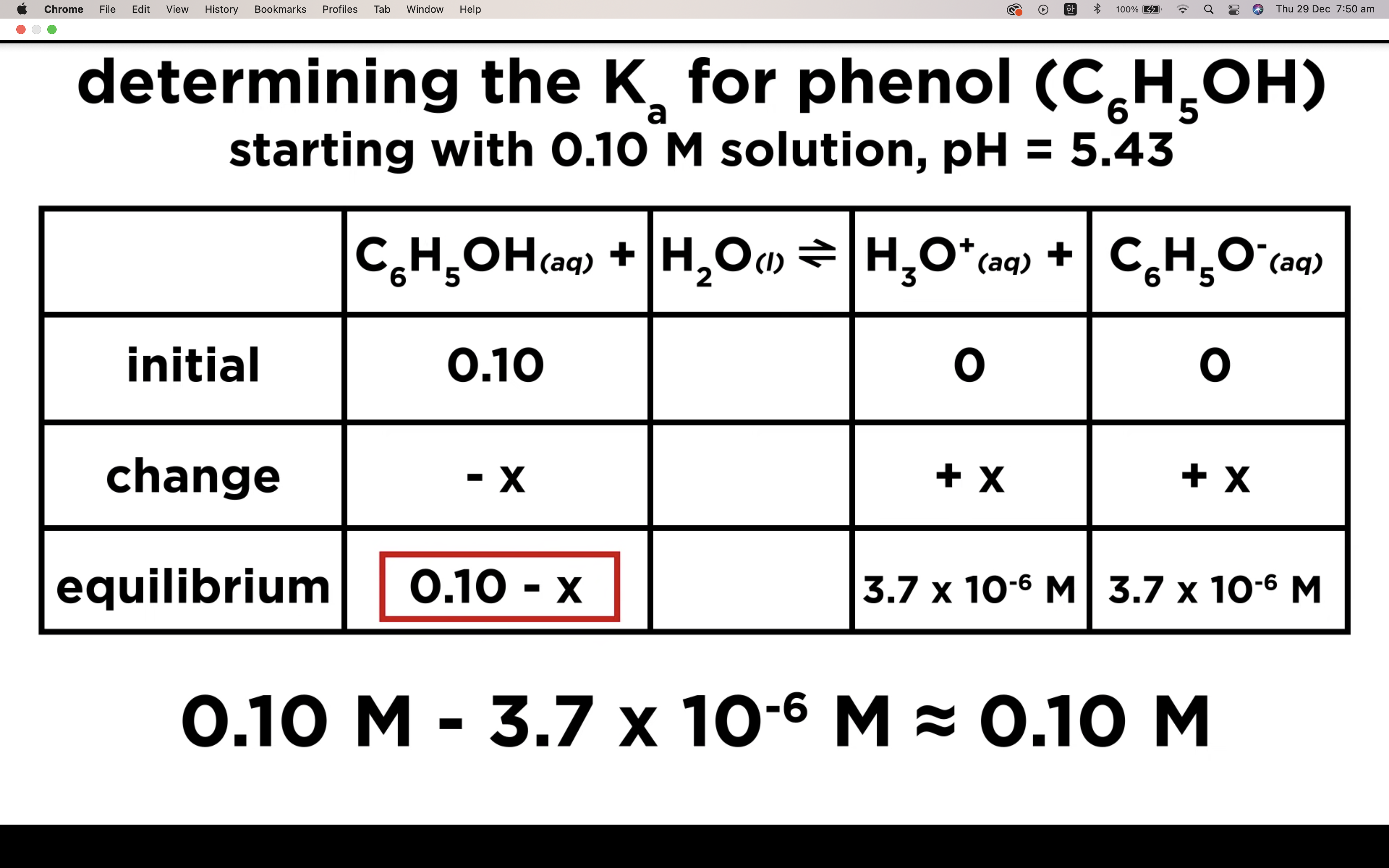
It is a weak acid so we assume it hasn't ionised/ hydrolised that much -> assume 0.25-x ~0.25 !!!!!
(It is only available when the percent ionization is 5% or less!!!)
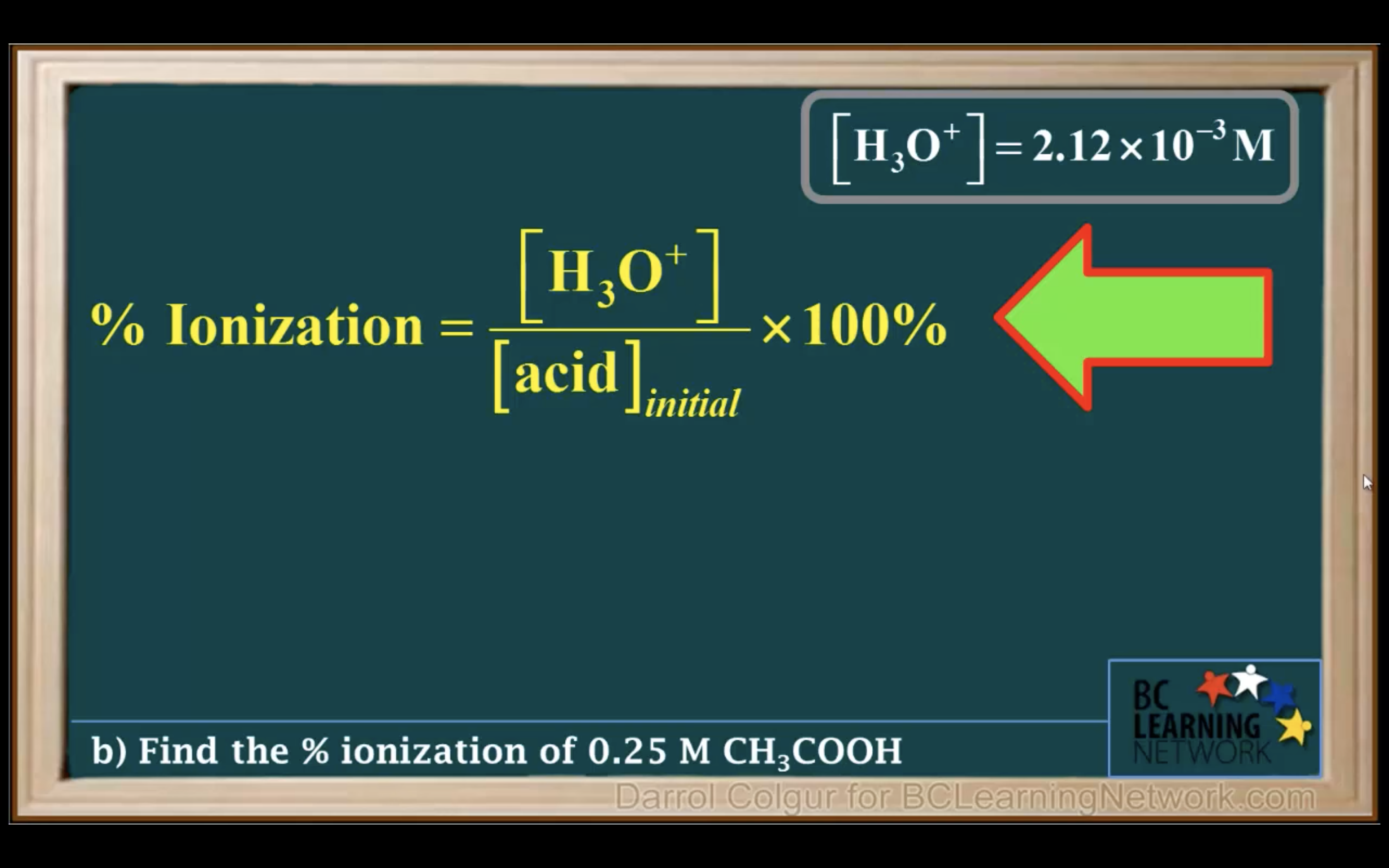
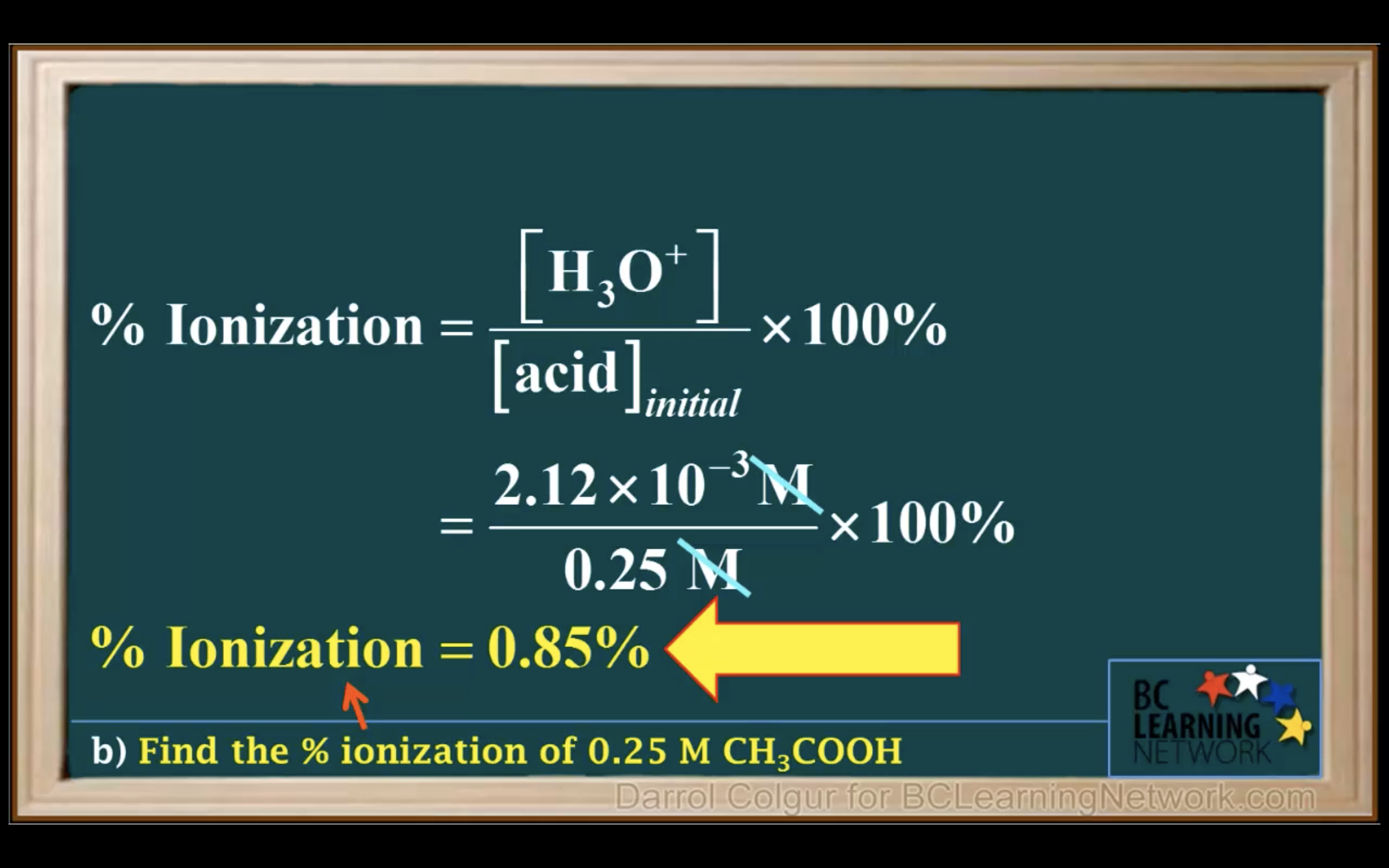
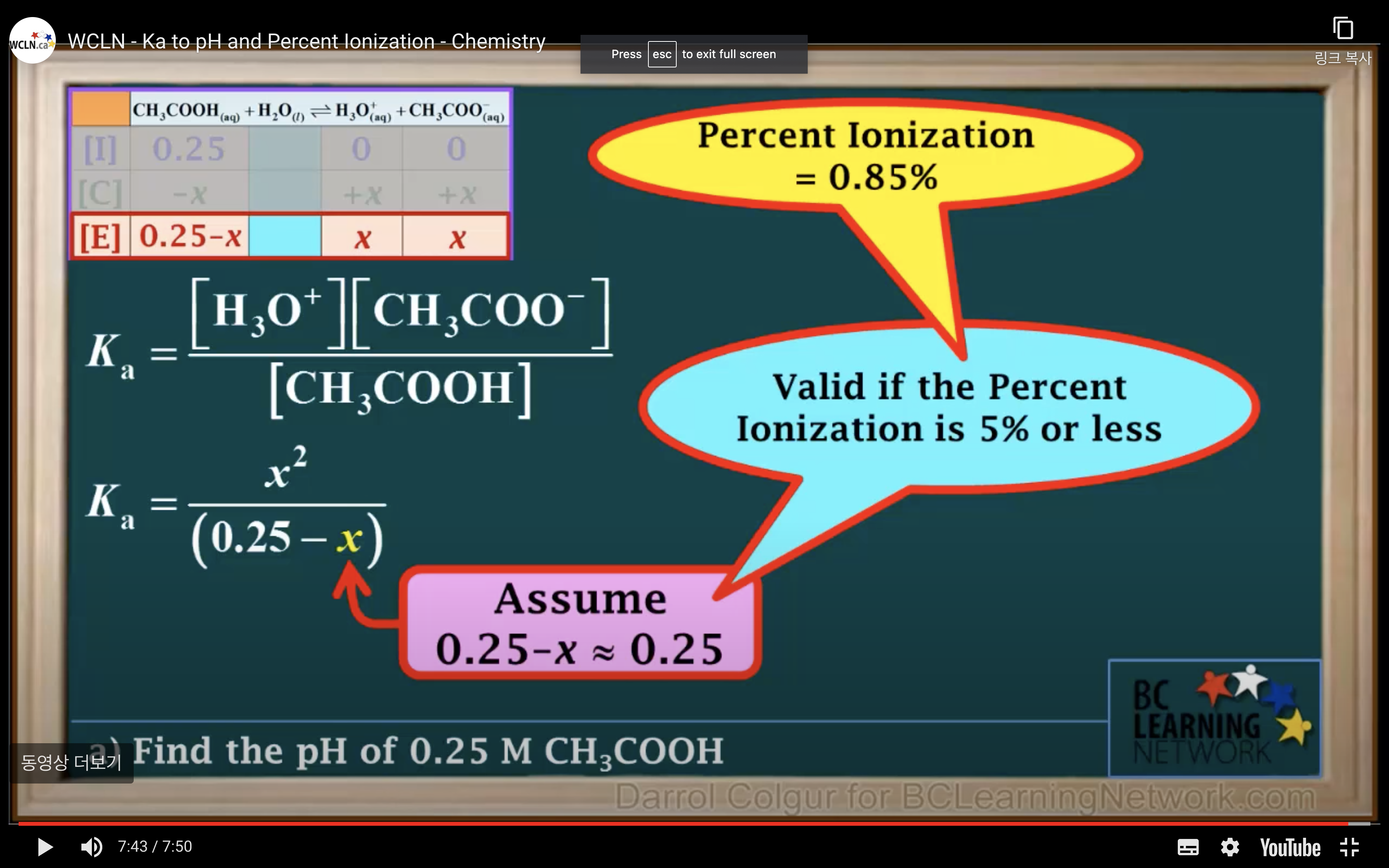
Percentage of ionisation = [H3O+]/[acid]initial x 100%
Worked out questions Keq, Kw & Ka


Part 4: Chemical equilibrium – Buffer solutions: Acid-Base Properties of Salts, pH of buffers
Learning Outcomes: 9-10
Key Concepts
- The acidic & basic properties of salts
- Buffer solutions
Acidic & Basic properties of salts
Introduction to Buffers
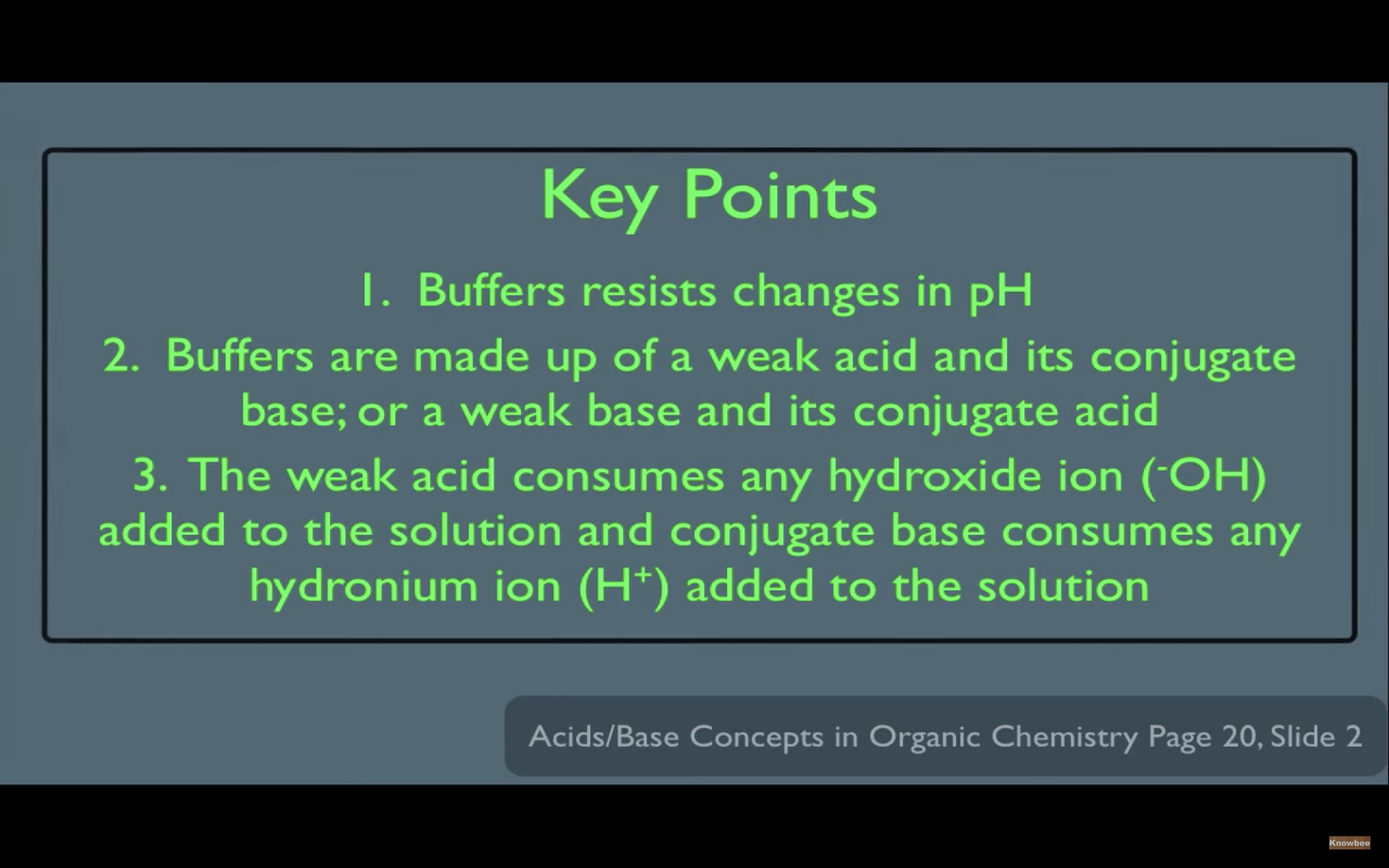
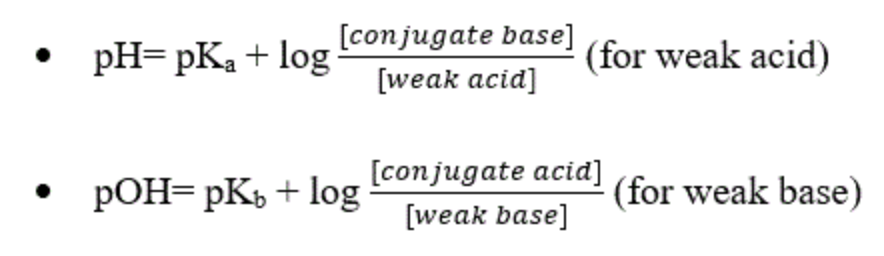
Acid-base Equilibria & Buffer Solutions



https://general.chemistrysteps.com/the-henderson-hasselbalch-equation/
The Henderson–Hasselbalch Equation - Chemistry Steps
The Henderson–Hasselbalch equation relates the pH of a buffer solution to the initial concentration of its components.
general.chemistrysteps.com
'Griffith college Tri3 2022 > 1001GRC (Chem)' 카테고리의 다른 글
| WEEK12 - Topic 14 (0) | 2023.01.07 |
|---|---|
| WEEK11 - Topic 13 (0) | 2023.01.07 |
| WEEK9 - Topic 11 (0) | 2022.12.17 |
| WEEK8 - Topic 10 (1) | 2022.12.10 |
| WEEK7 - Topic 9&10 (0) | 2022.12.03 |



