Gas Laws
1: The kinetic-molecular theory
2: Measurement of pressure of gases
3: Empirical laws of gases
4: Ideal gas law
K = °C + 273
1 atm = 760 torr = 760 mm Hg
STP: standard Temperature (273 K) and pressure (1 atm).
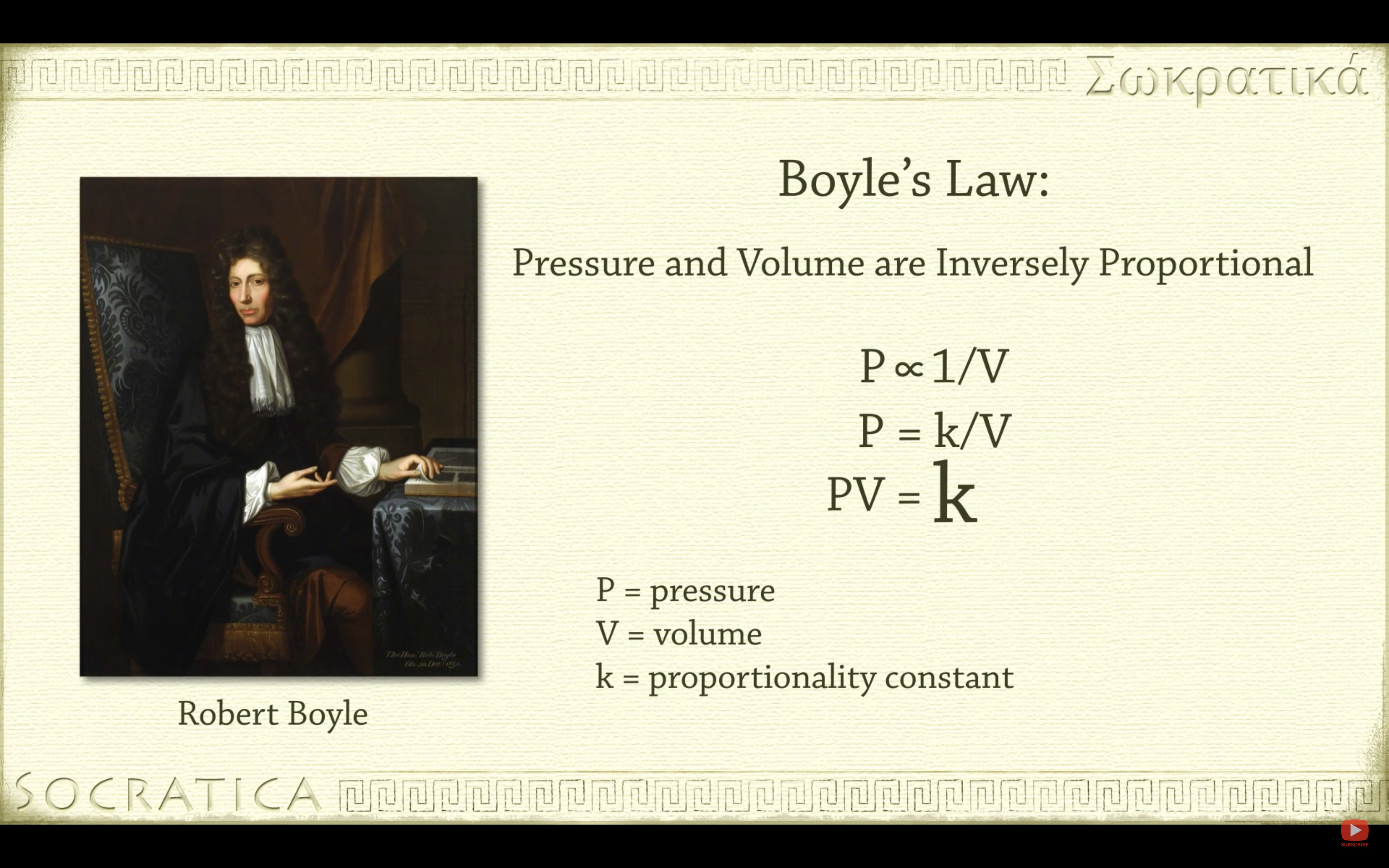
Boyle's Law



Charles's Law



Gay-Lussac's Law


Use the Combined gas law to deduce the equation you need based on what is provided in the question. e.g. if the question gives you P and V and T is constant, cover up the T's and you are left with P1V1 = P2V2






How to Use the Ideal Gas Law in Two Easy Steps

Ideal gas Law & Gas Stoichiometry Worked examples




반응형
'Griffith college Tri3 2022 > 1001GRC (Chem)' 카테고리의 다른 글
| WEEK12 - Topic 14 (0) | 2023.01.07 |
|---|---|
| WEEK10 - Topic 12 (0) | 2022.12.29 |
| WEEK9 - Topic 11 (0) | 2022.12.17 |
| WEEK8 - Topic 10 (1) | 2022.12.10 |
| WEEK7 - Topic 9&10 (0) | 2022.12.03 |



