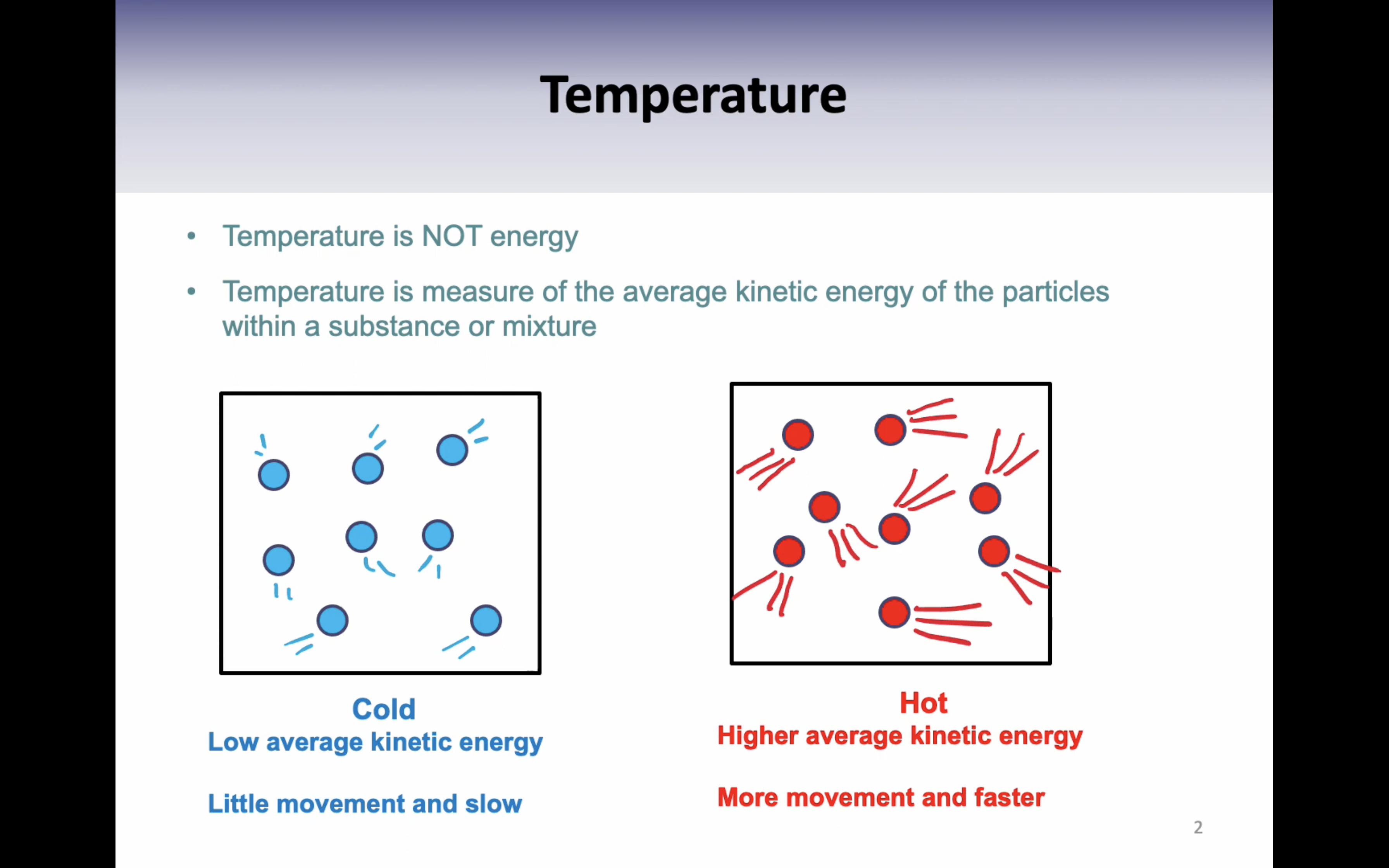
VIDEO 1: GENERAL PROPERTIES OF LIQUIDS
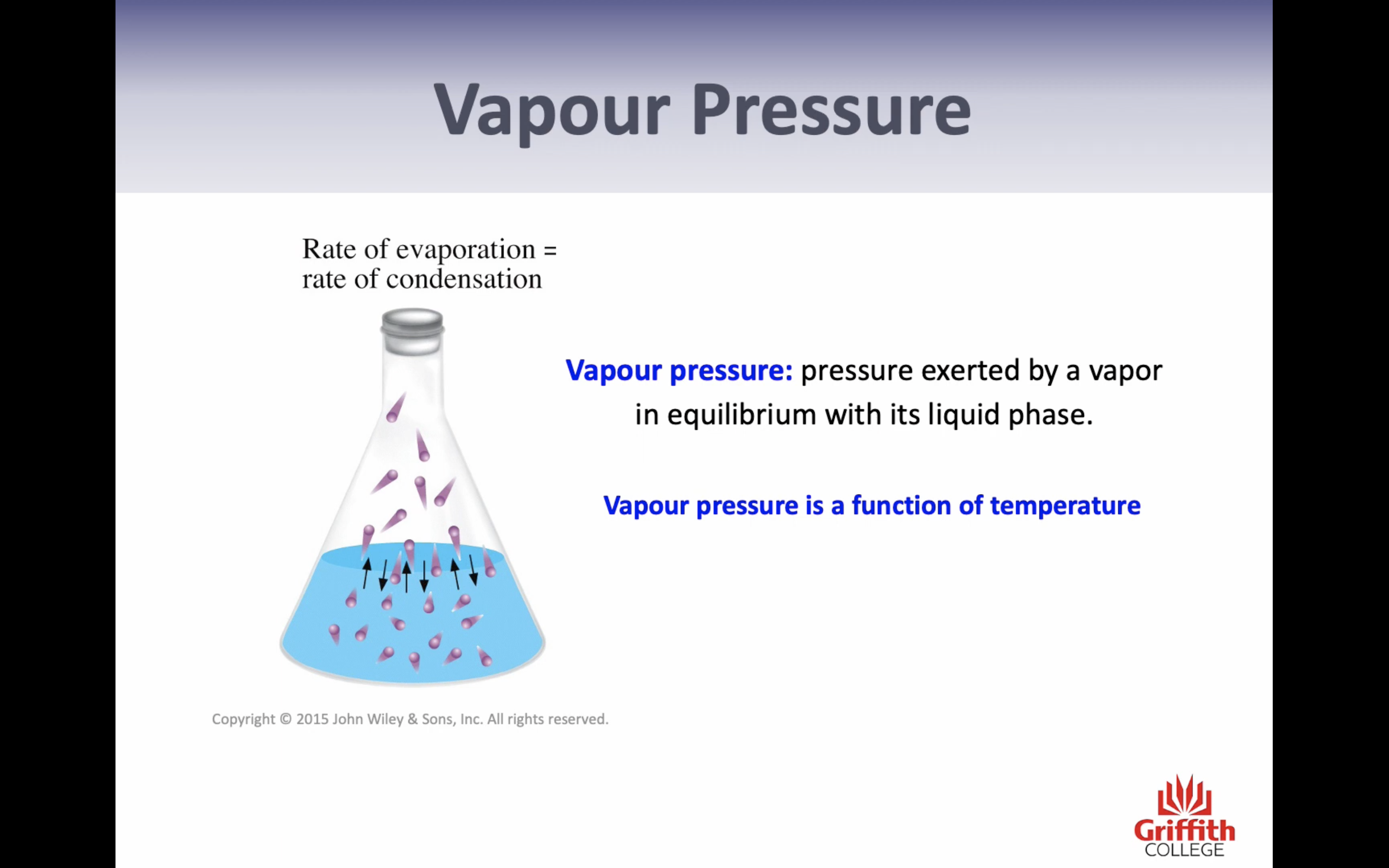
*Vapor pressure = pressure exerted by a vapor in equilibrium with its liquid phase
*(If tempertature increases, vapor pressure increases)
*(= 온도가 올라가서 액체상태의 분자들이 활발하게 움직이게 되고 분자들이 증발하려고 할때,
액체상태에 머무르게 하도록 누루는 vapor pressure도 증가하게 된다)


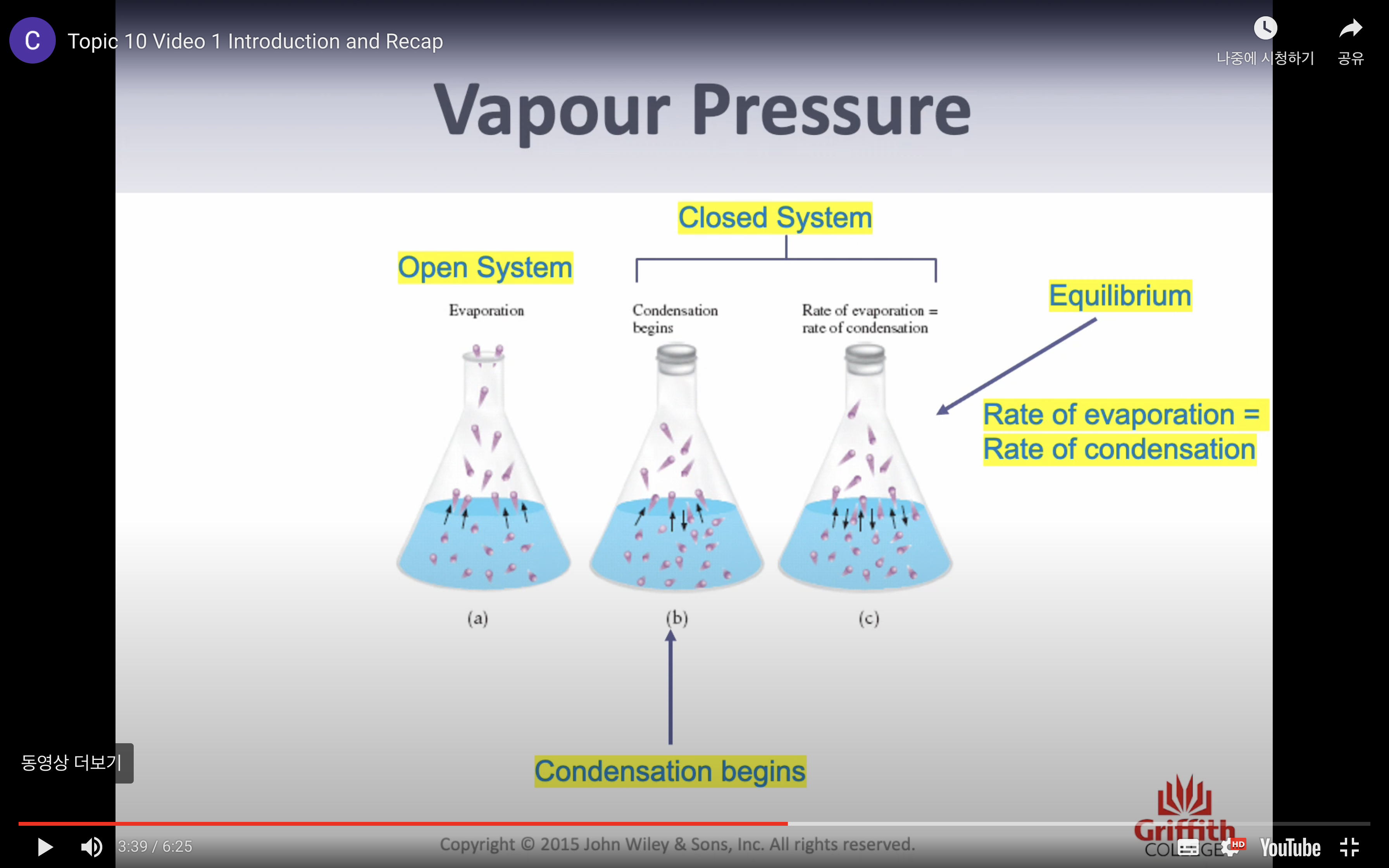
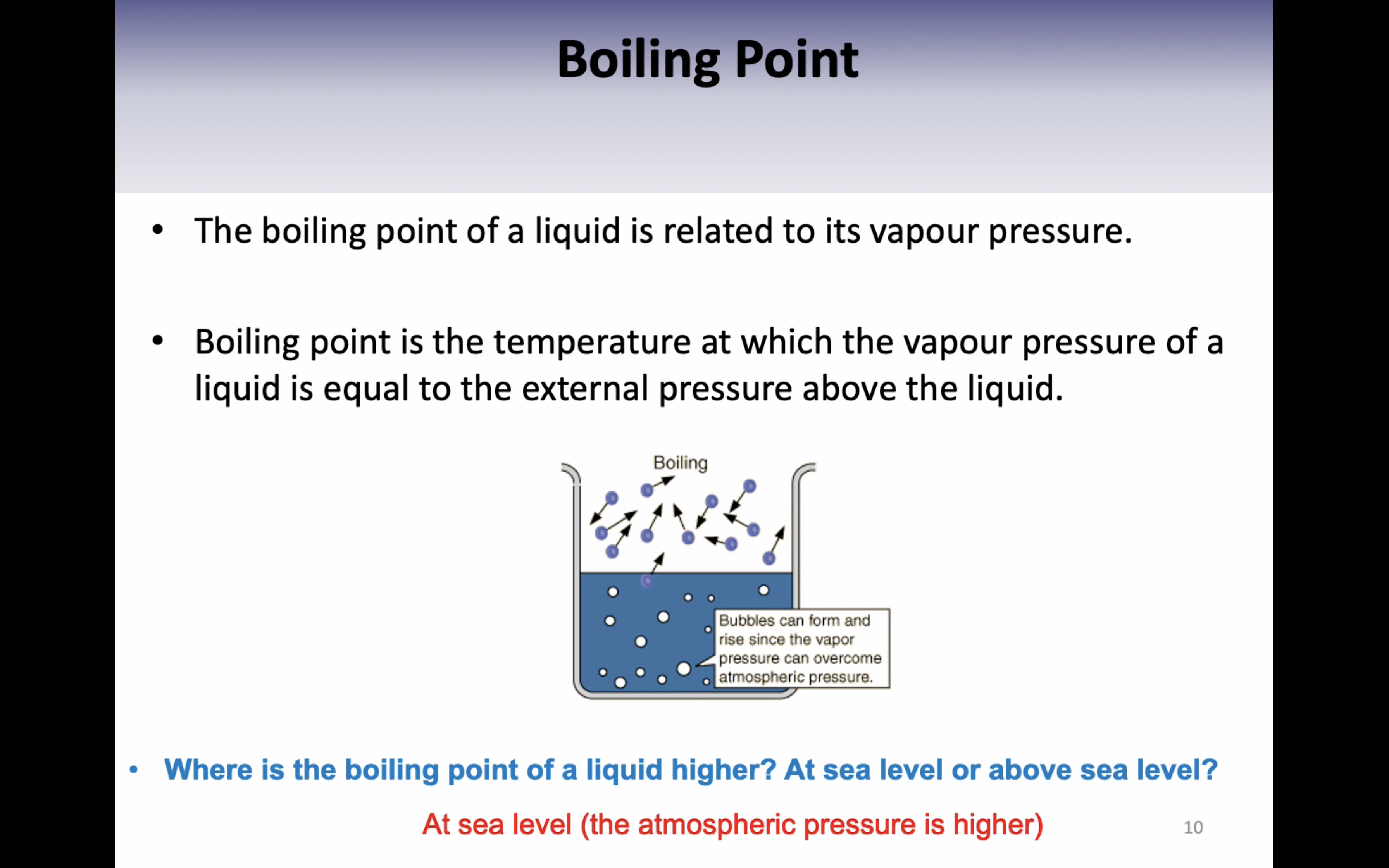
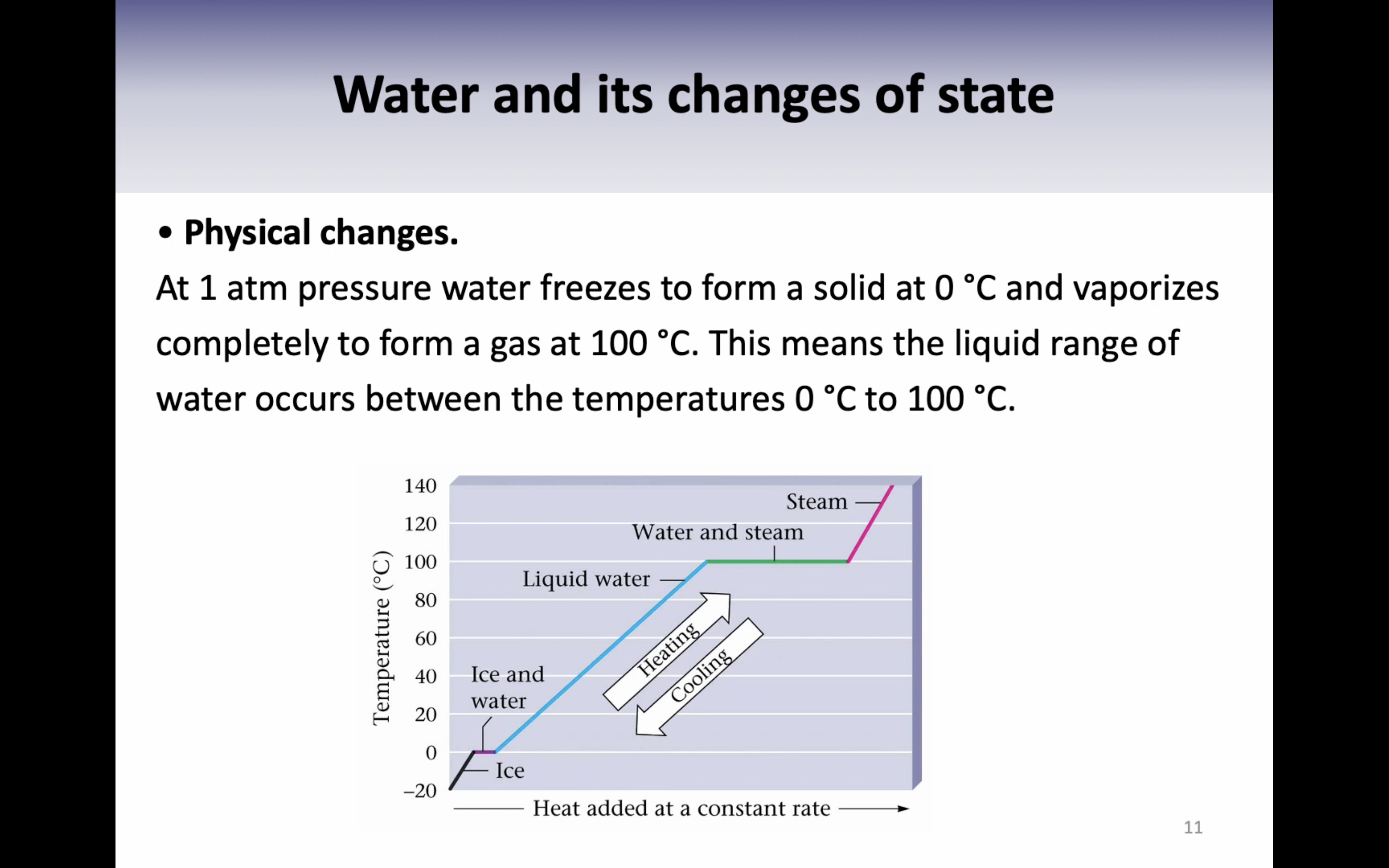
*Boiling point is the temperature where the vapour pressure of a liquid is equal to the external pressure above the liquid.
*(vapor pressure 가 낮아지면 boiling point도 낮아진다, 두개가 같은 개념이라고 보면 되는데 vapor pressure 는 압력에 관한거고 boiling point 는 온도에 관한 것)
*(vapor pressure 가 액체상태에 가해지는 압력이라고 했는데 그 압력이 사라지고 외부 압력과 같아지게 되면 액체상태의 분자들이 증발할 수 있게 된다)
VIDEO 2: INTERMOLECULAR FORCES AND HYDROGEN BONDING
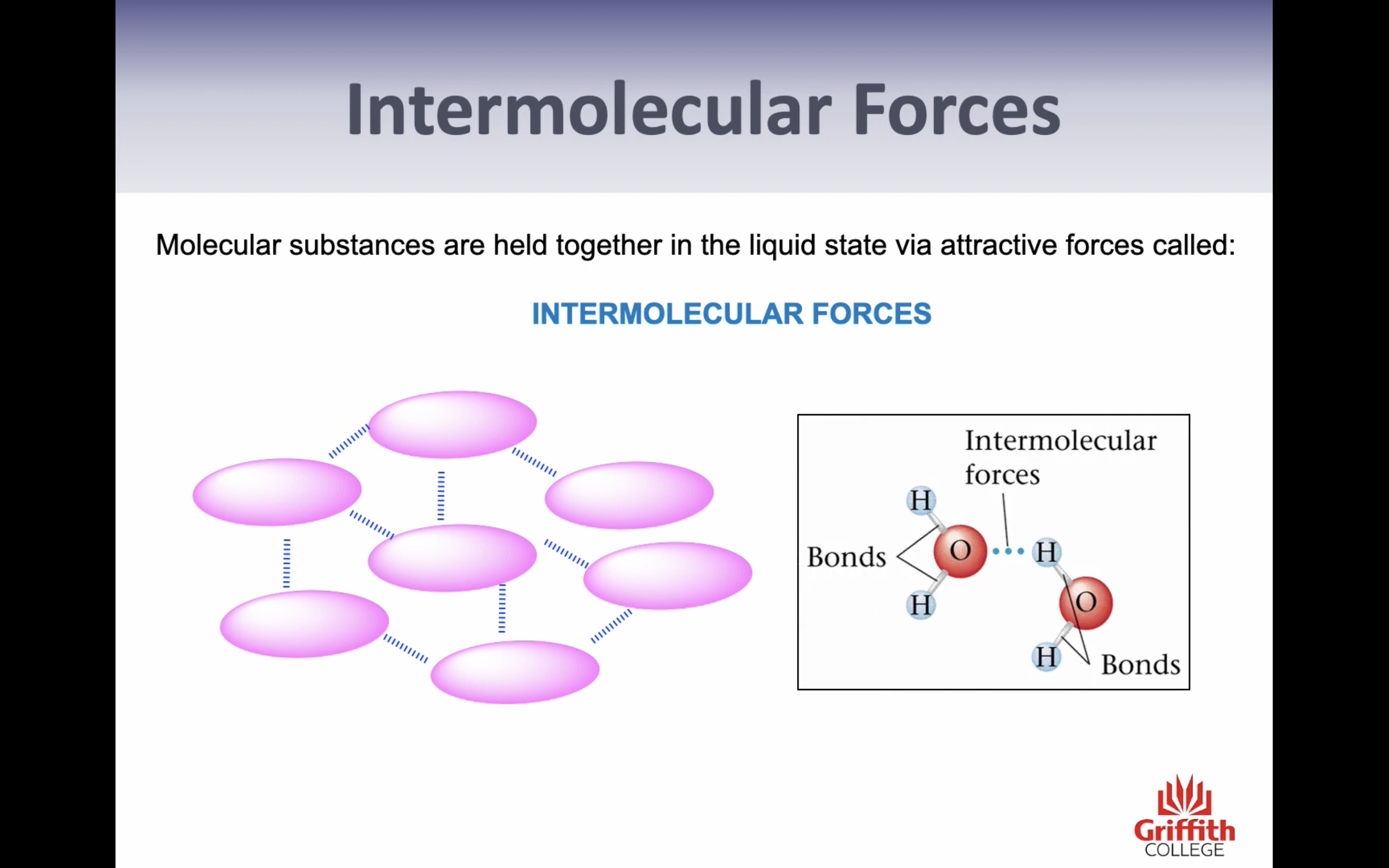
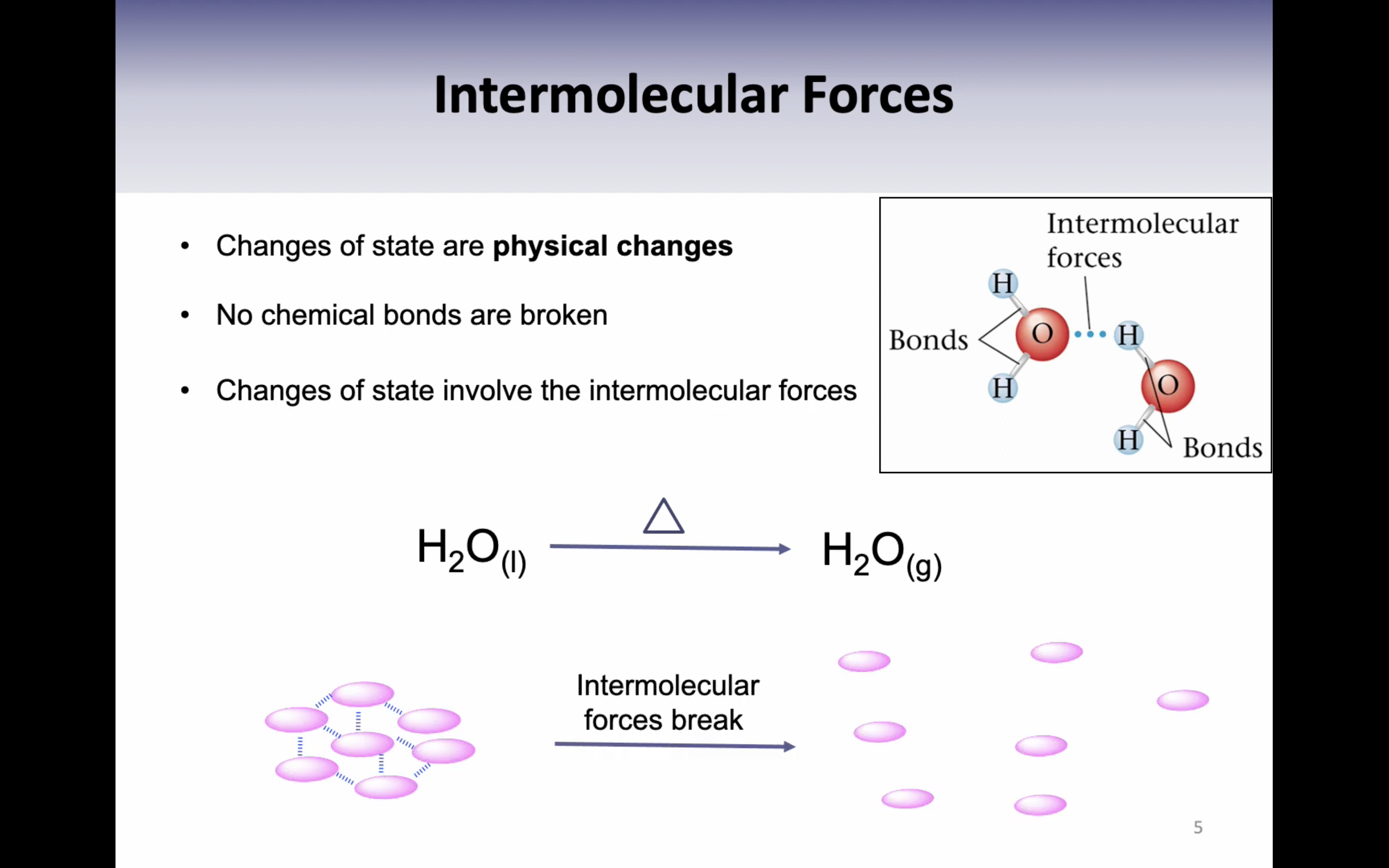
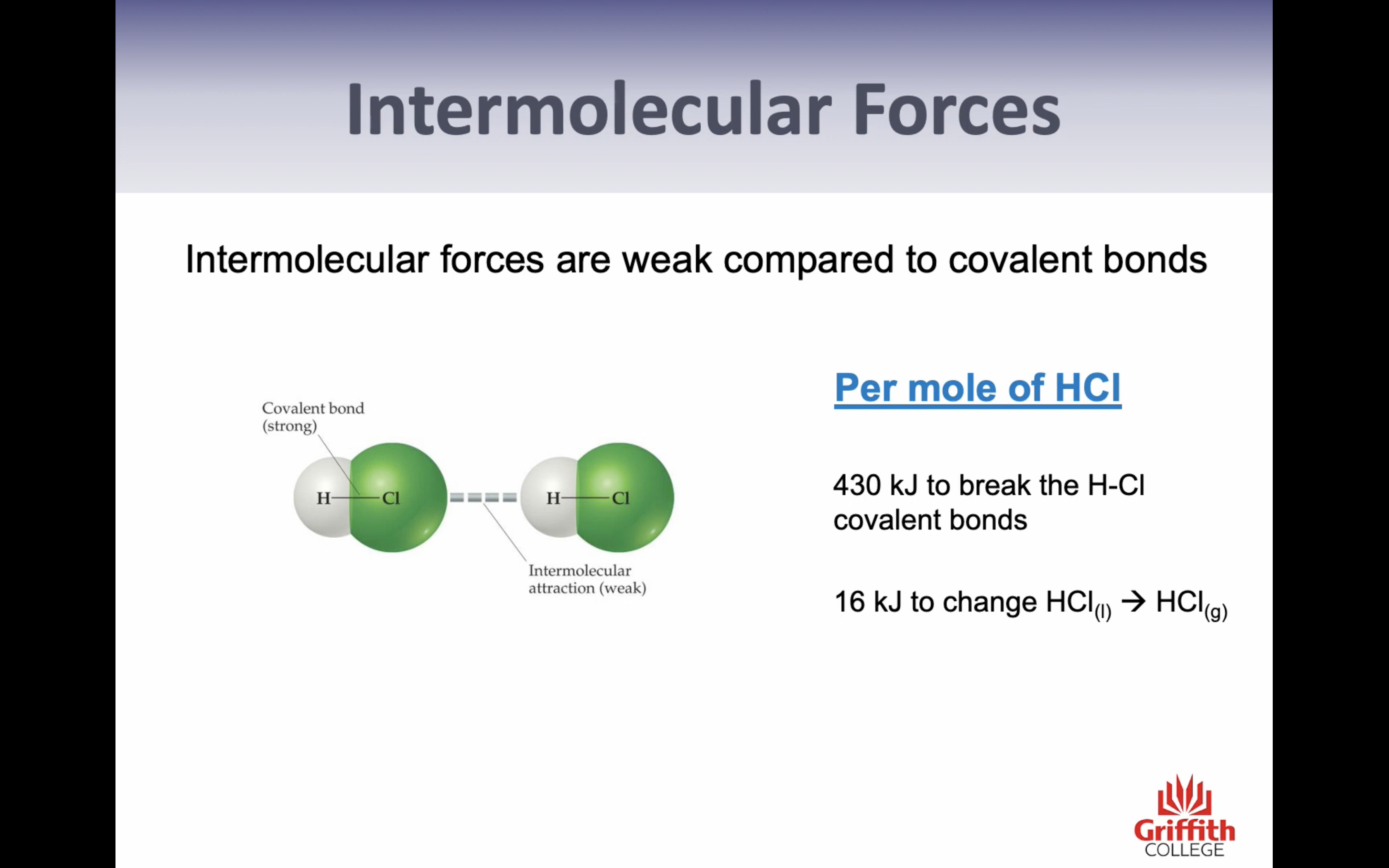
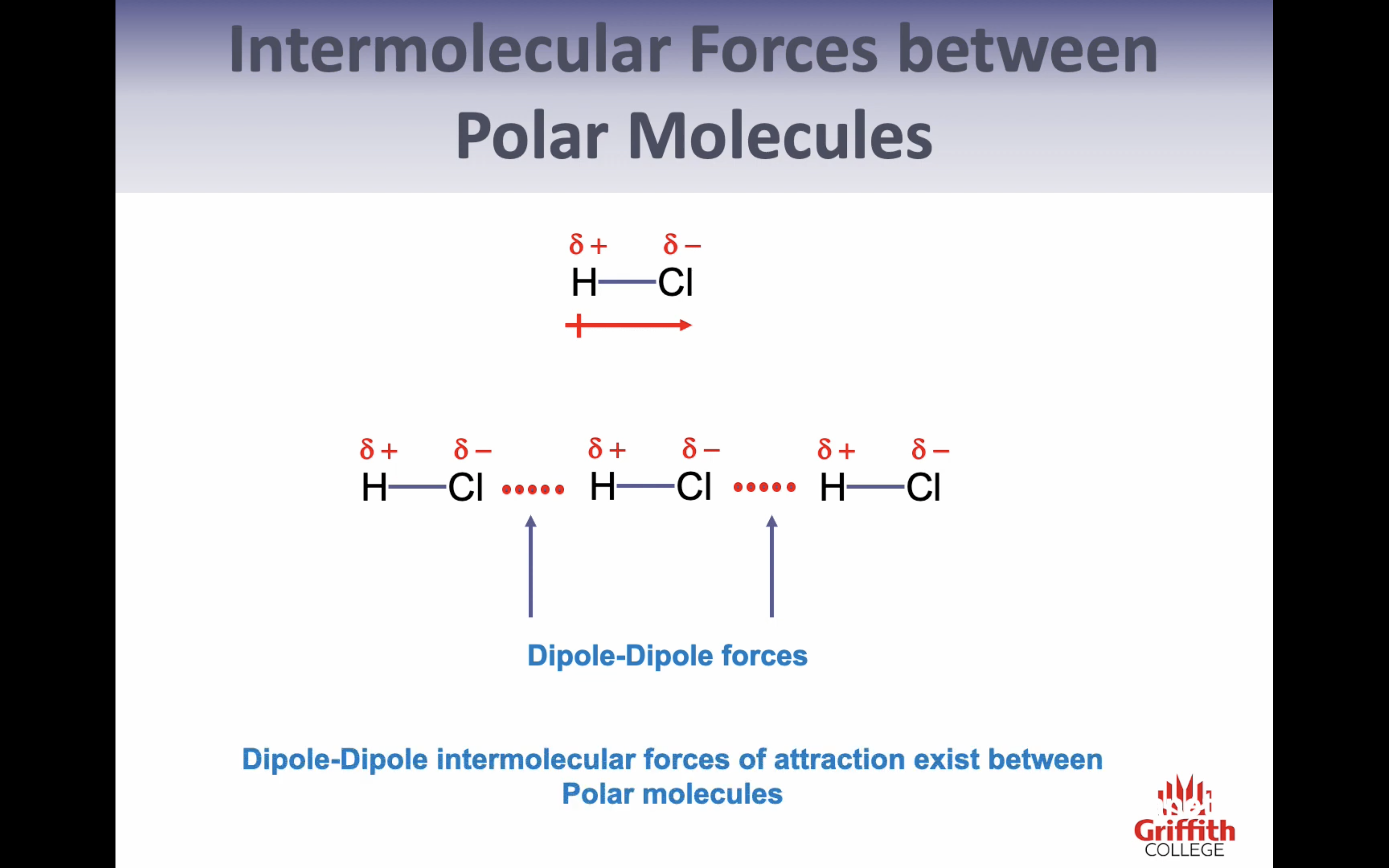
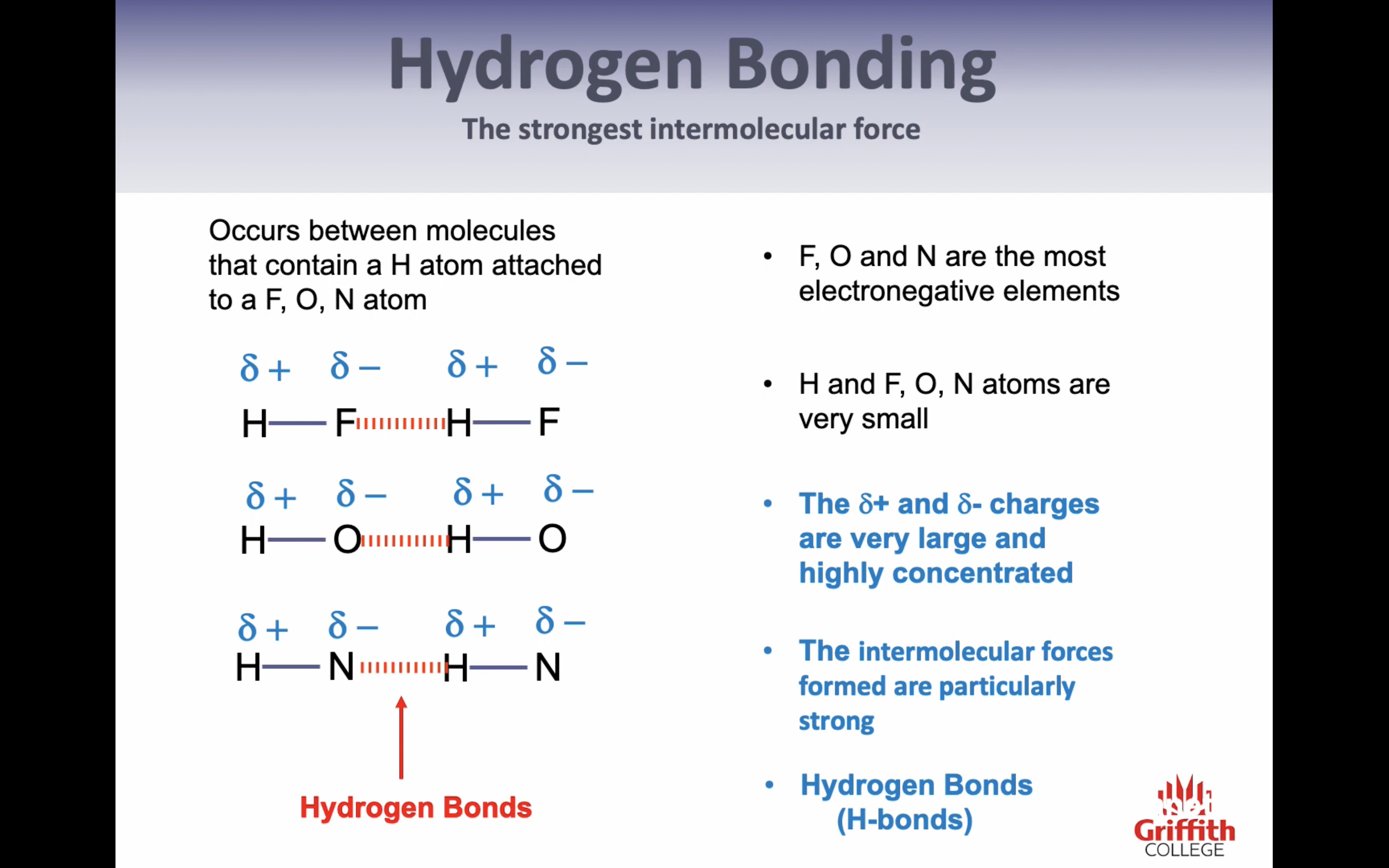
Hydrogen Bonding
-FO and N are the most electronegative elements
-H, FON are very small
-dipoles are very large and highly concentrated
->intermolecular forces formed are particularly strong
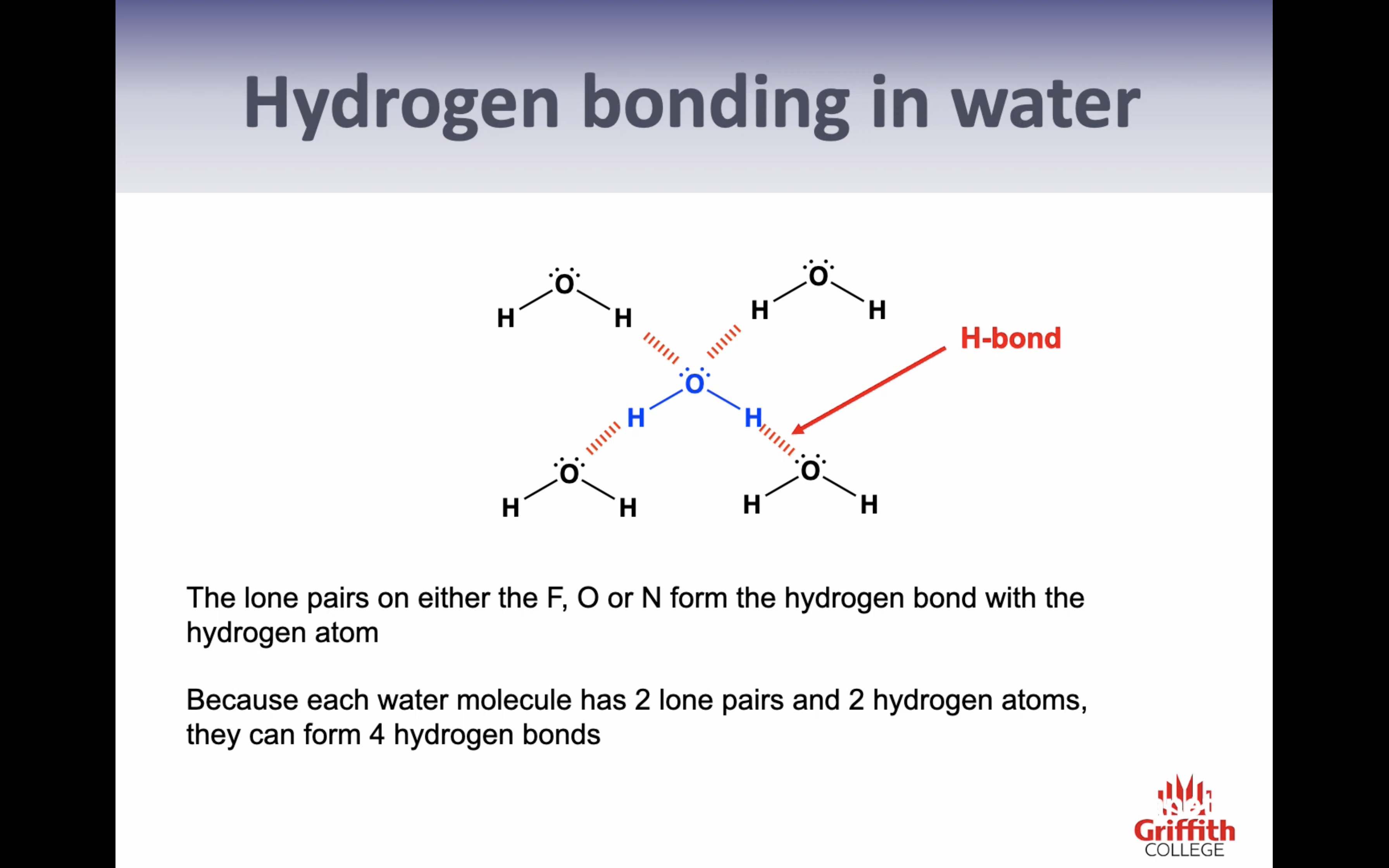

1. FON
2.lone pairs - H (directly)
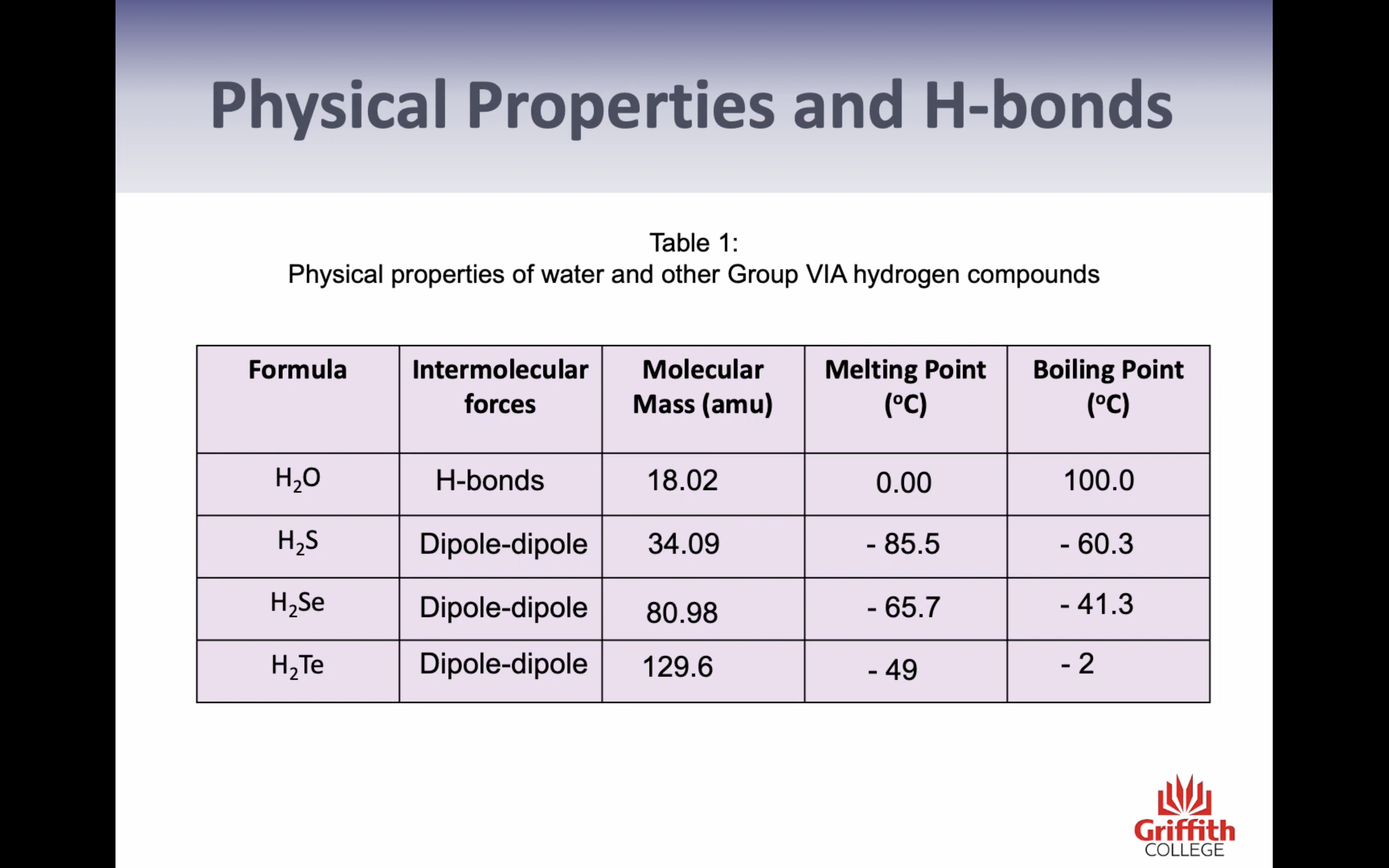
Normally atom size becomes bigger/molarmass increases , melting point increases
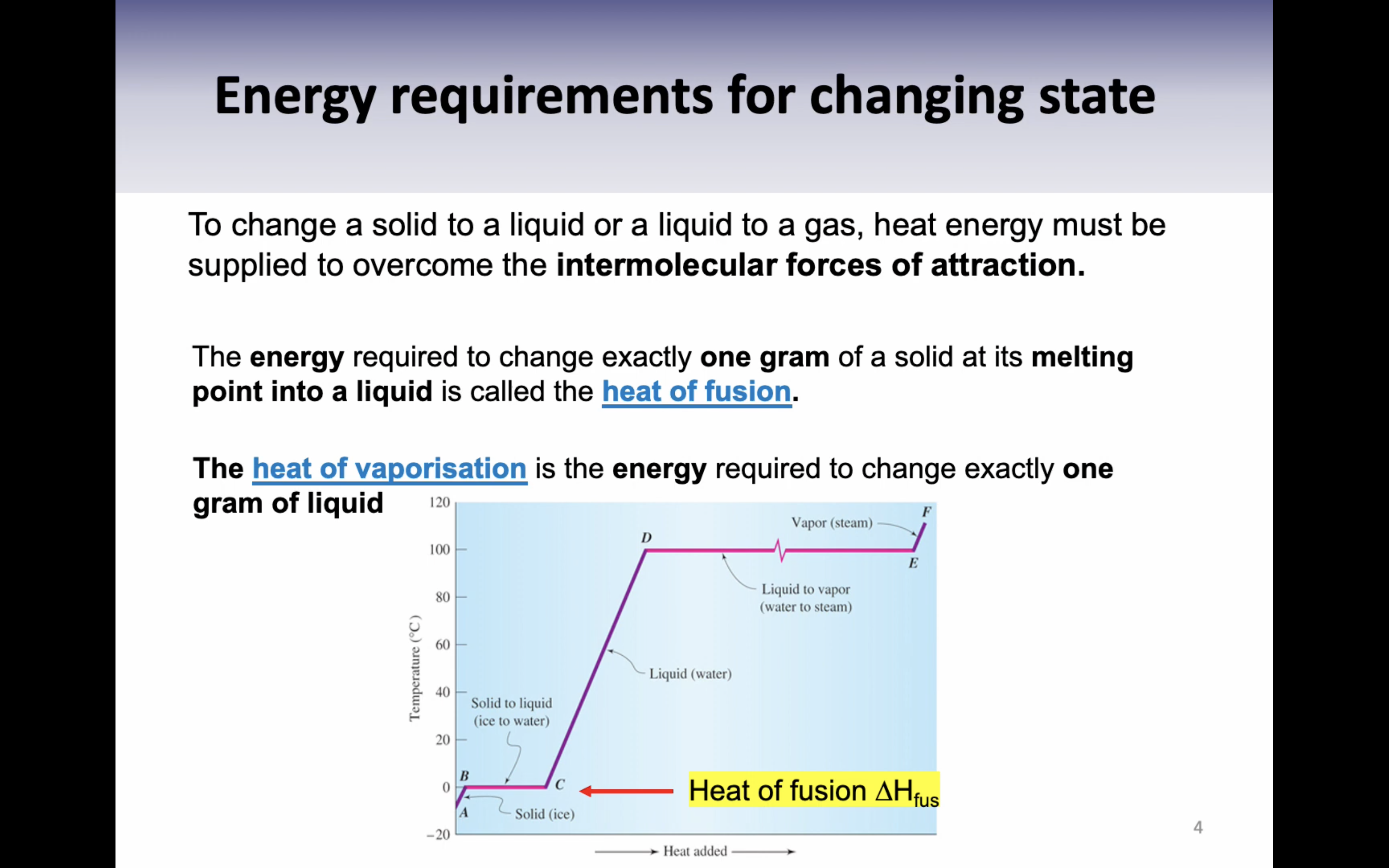
VIDEO 3: ENERGY REQUIREMENTS FOR CHANGING STATE
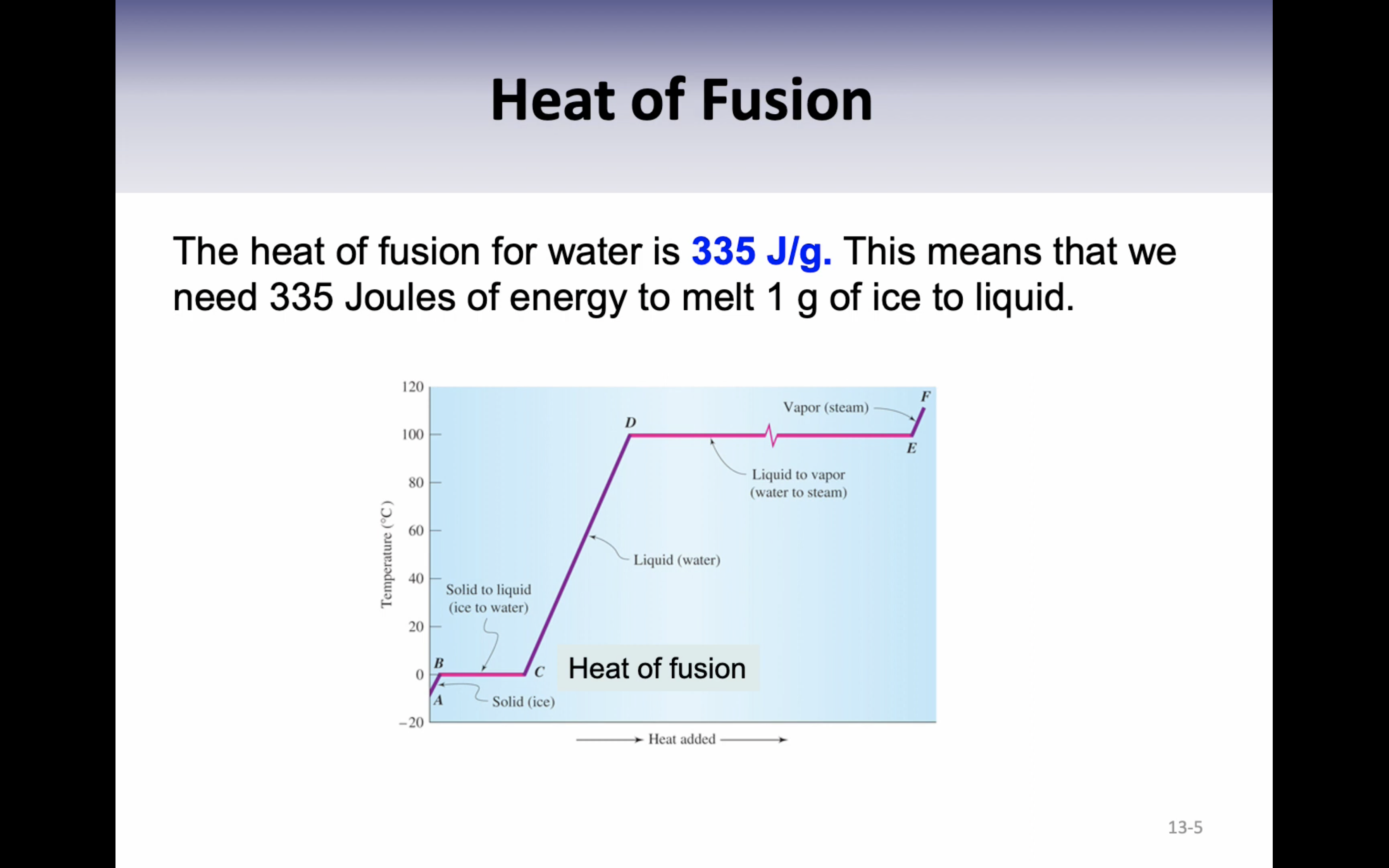
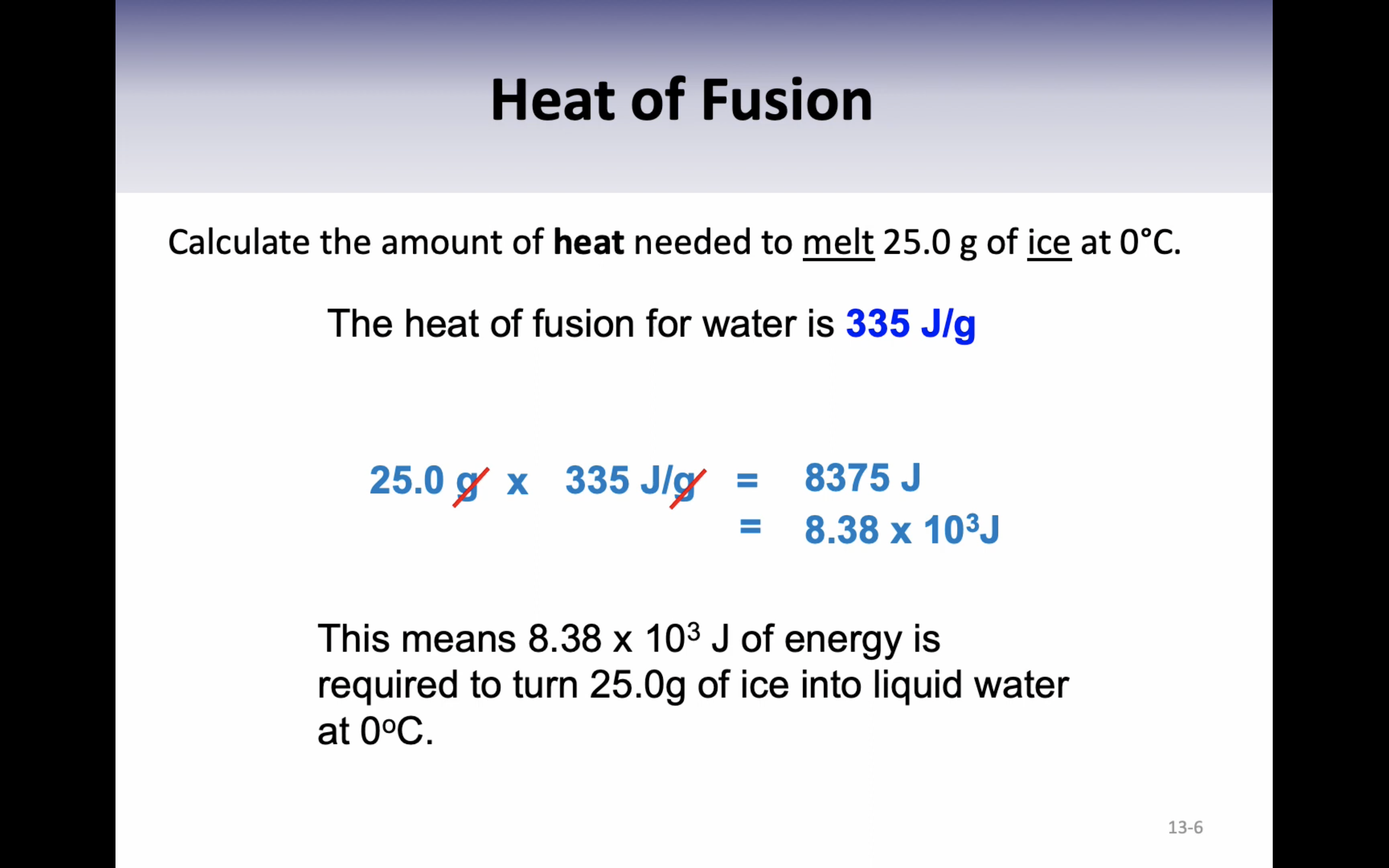
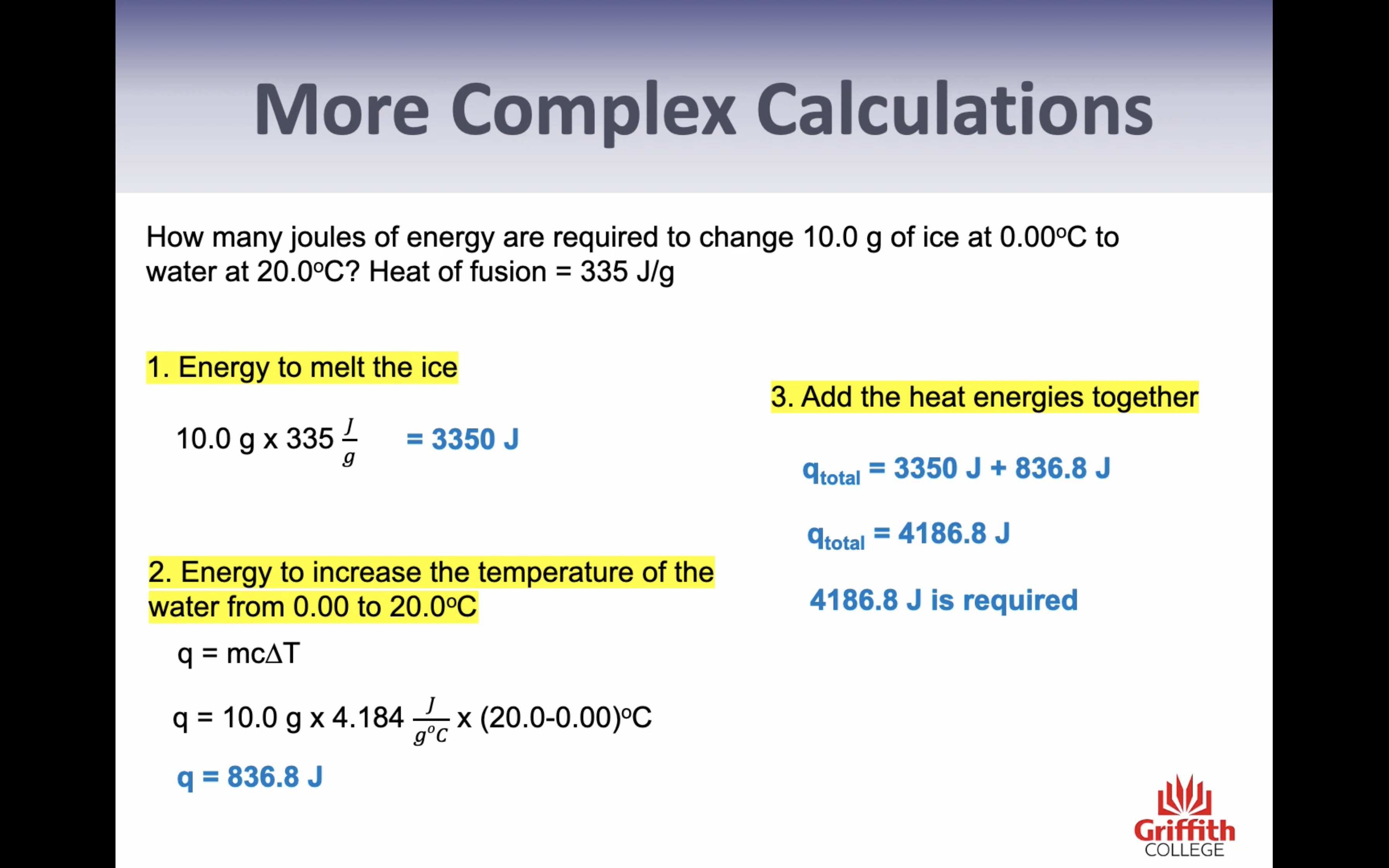
Heat of fusion : the energy required to change exactly one gram of a solid at its melting point into a liquid is called the heat of fusion (336J/g)
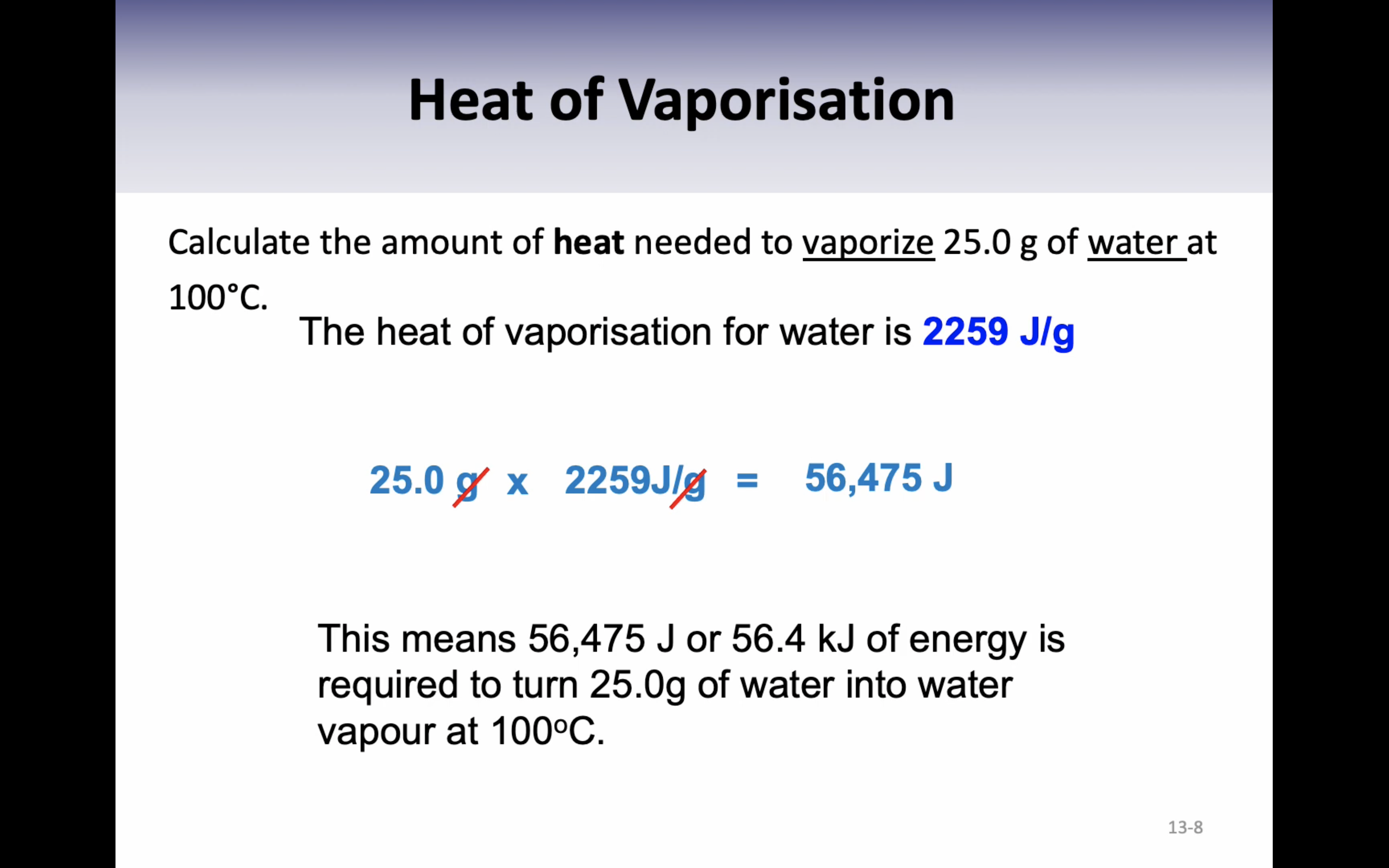
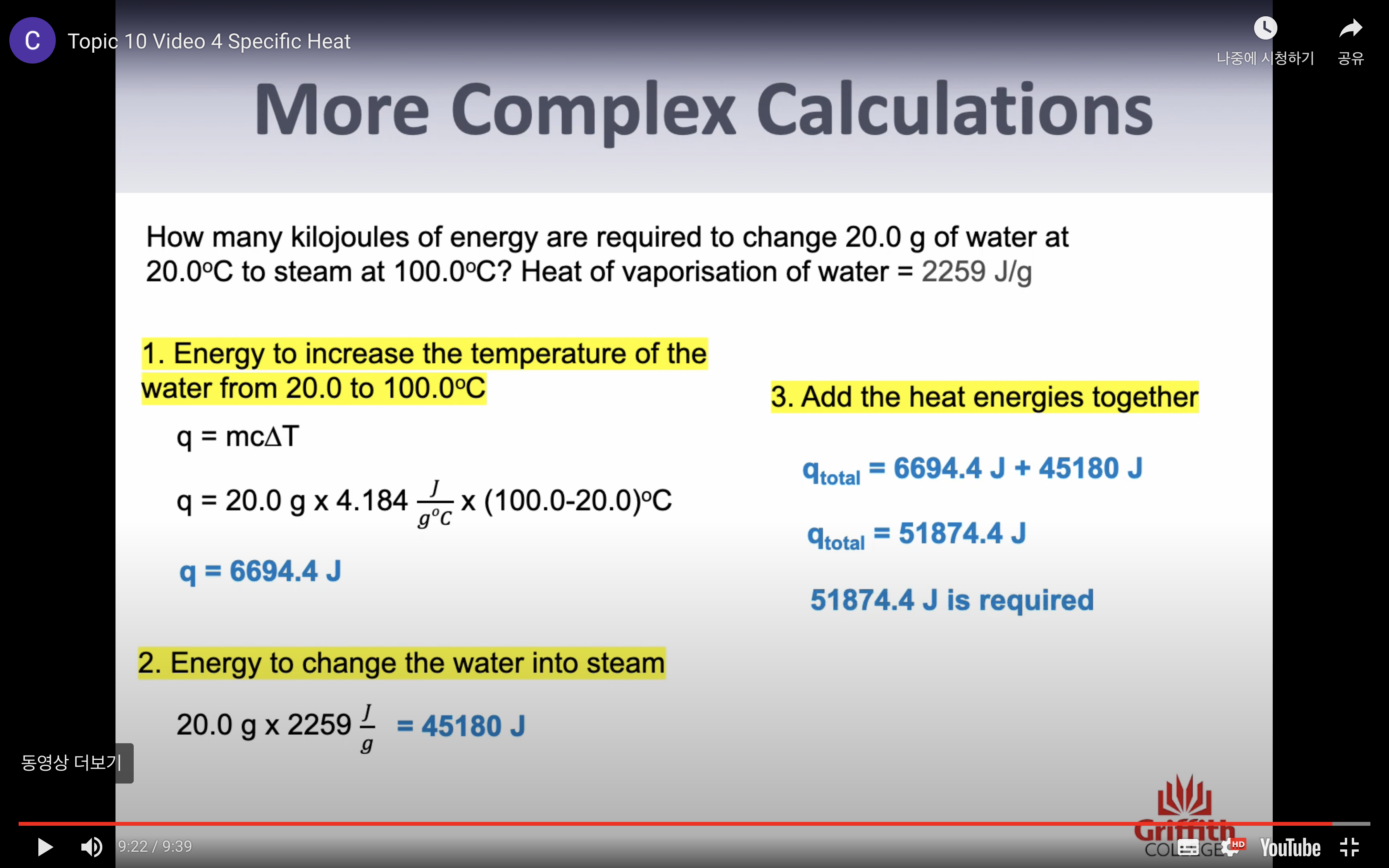
Heat of vaporisation : the energy required to change exactly one gram of liquid (2259J/g)
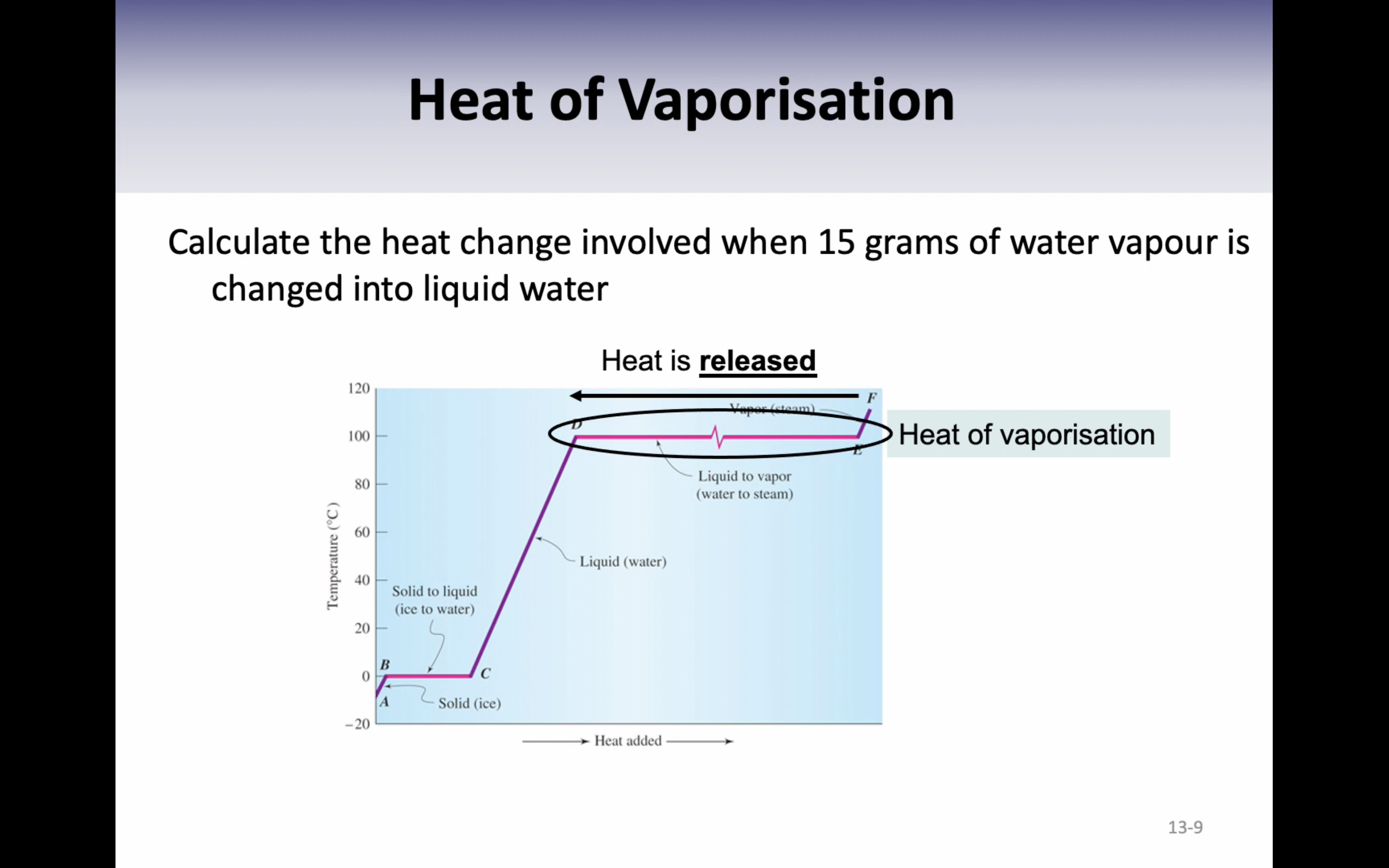

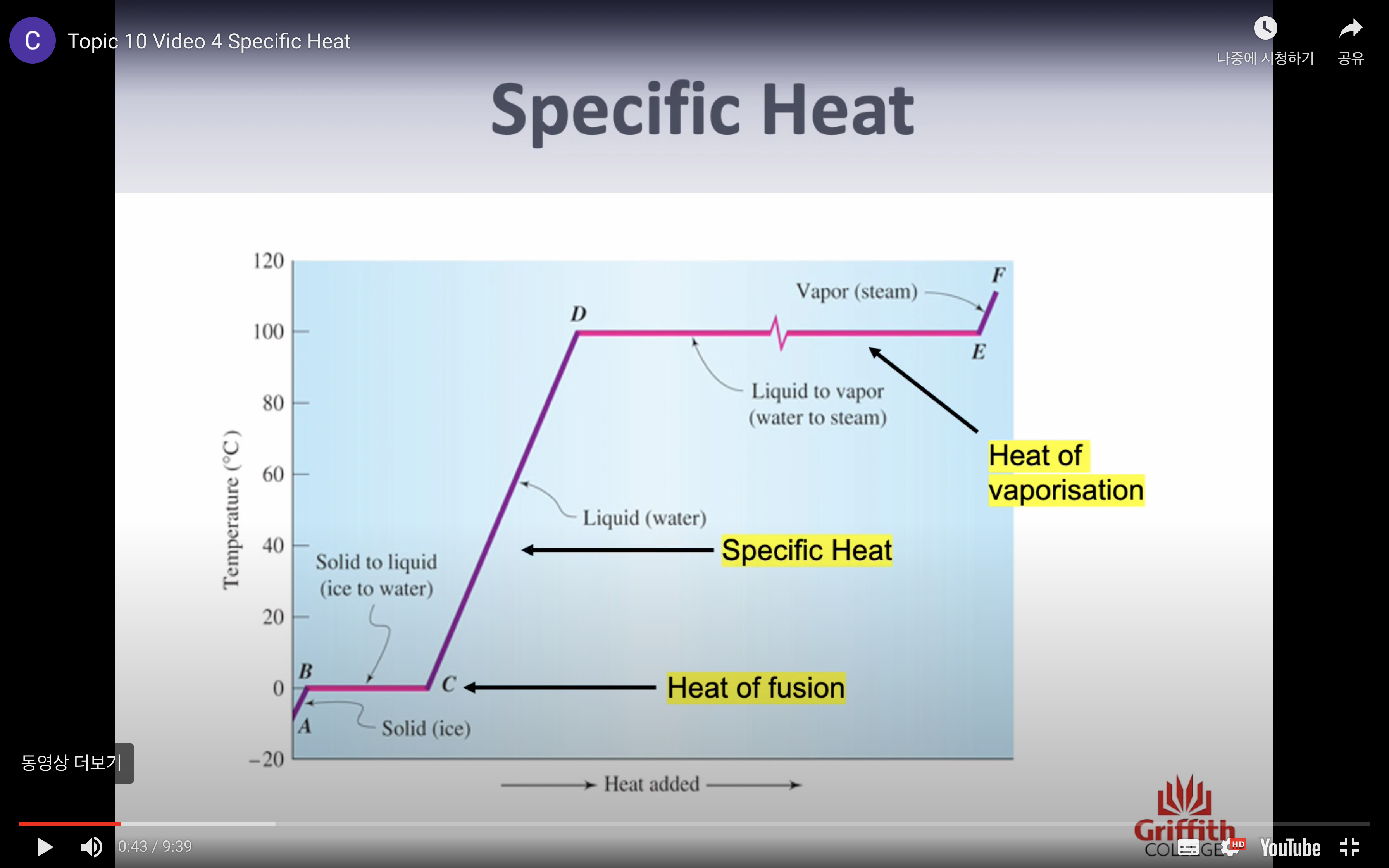
VIDEO 4: SPECIFIC HEAT
-Heat of fusion (change in state= energy needed to change 1g of solid to liquid) (we have specific number)
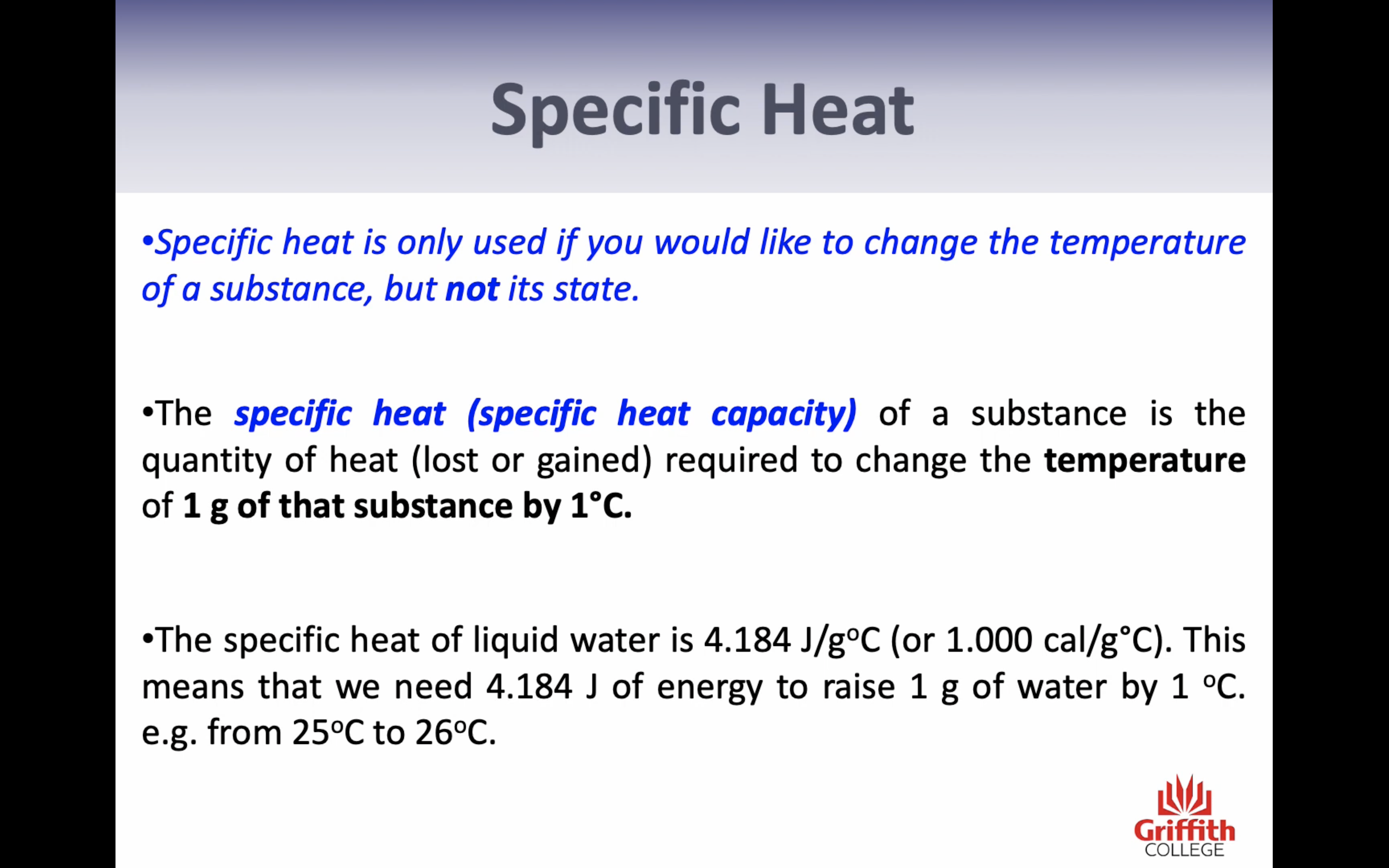
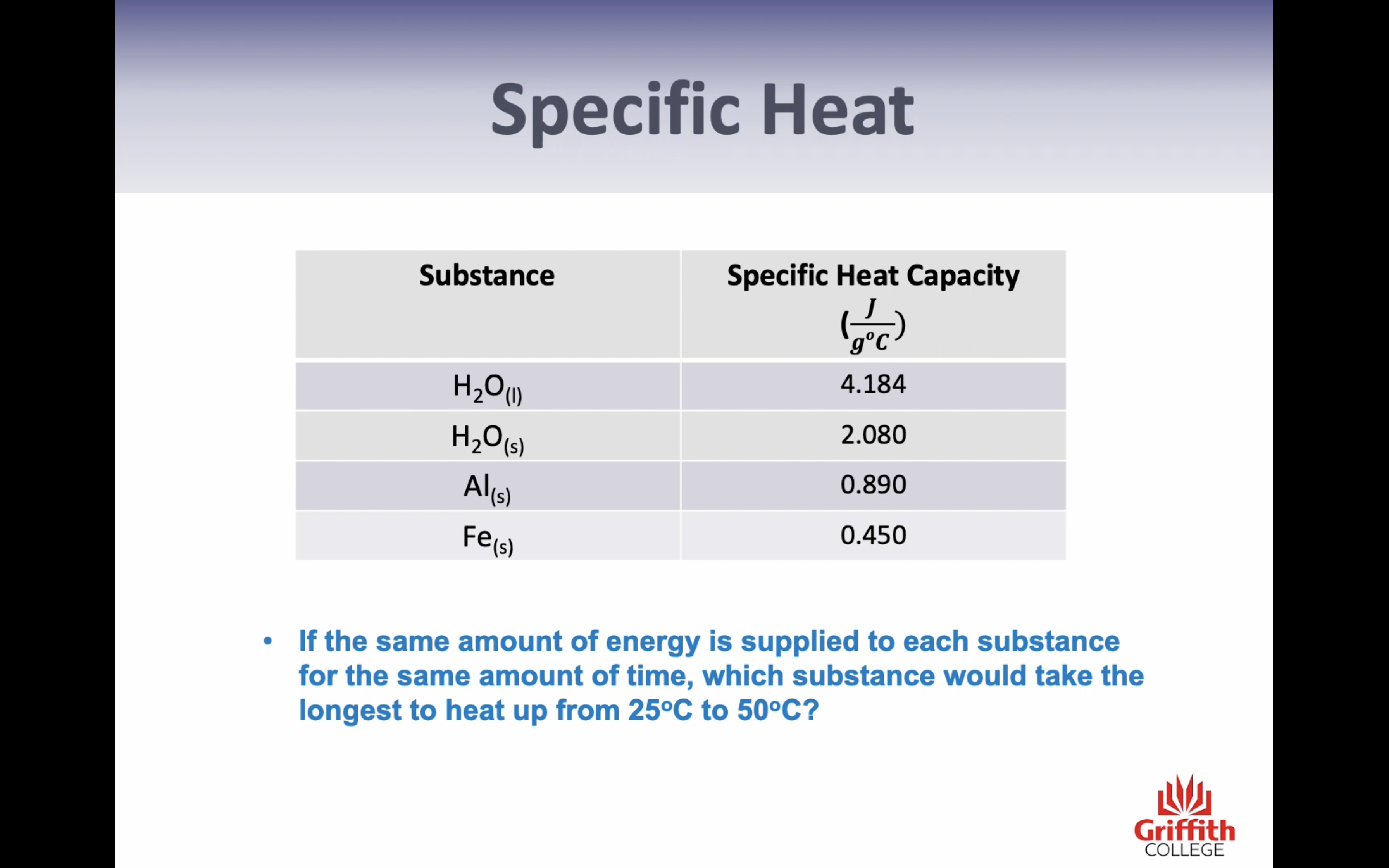
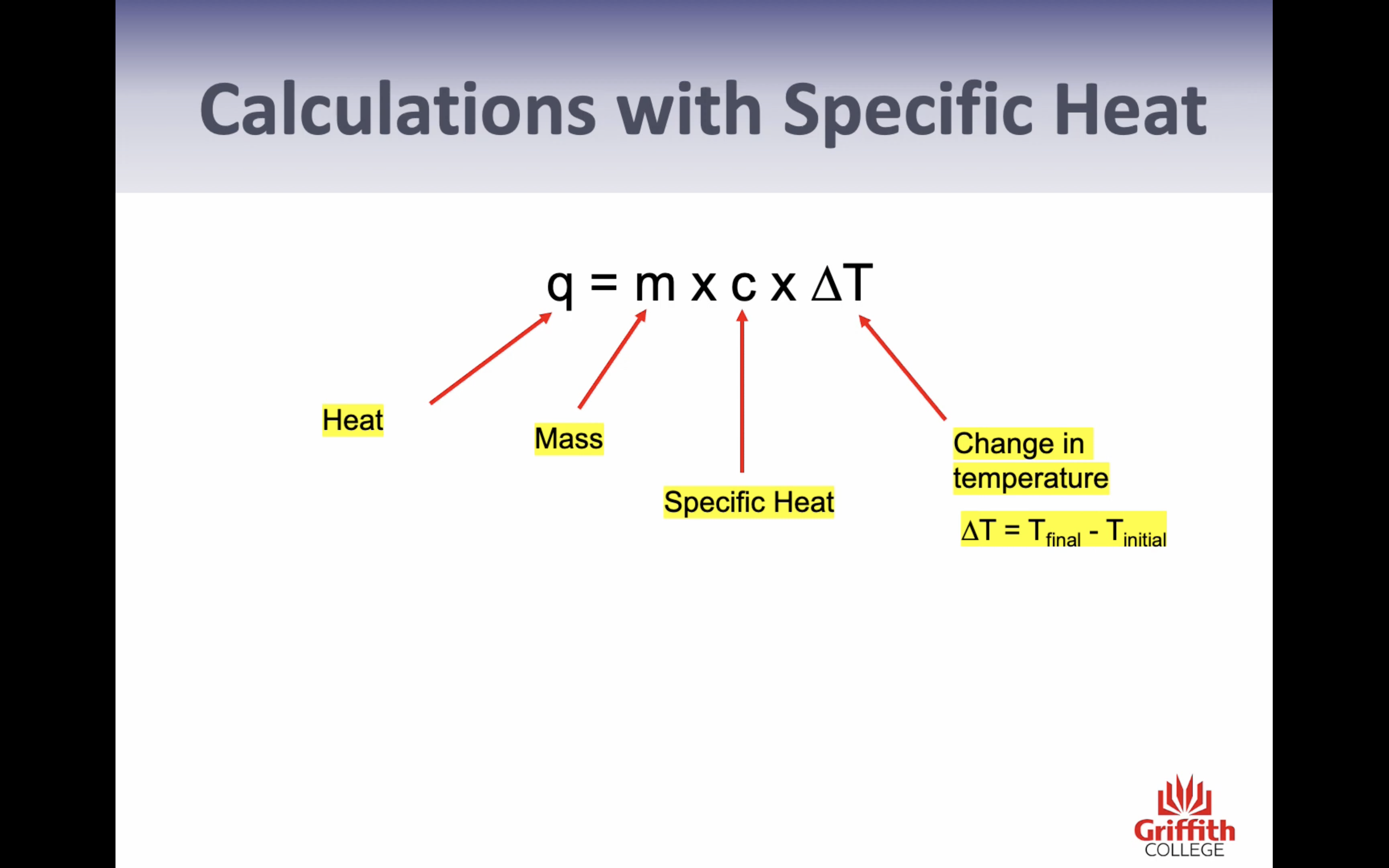
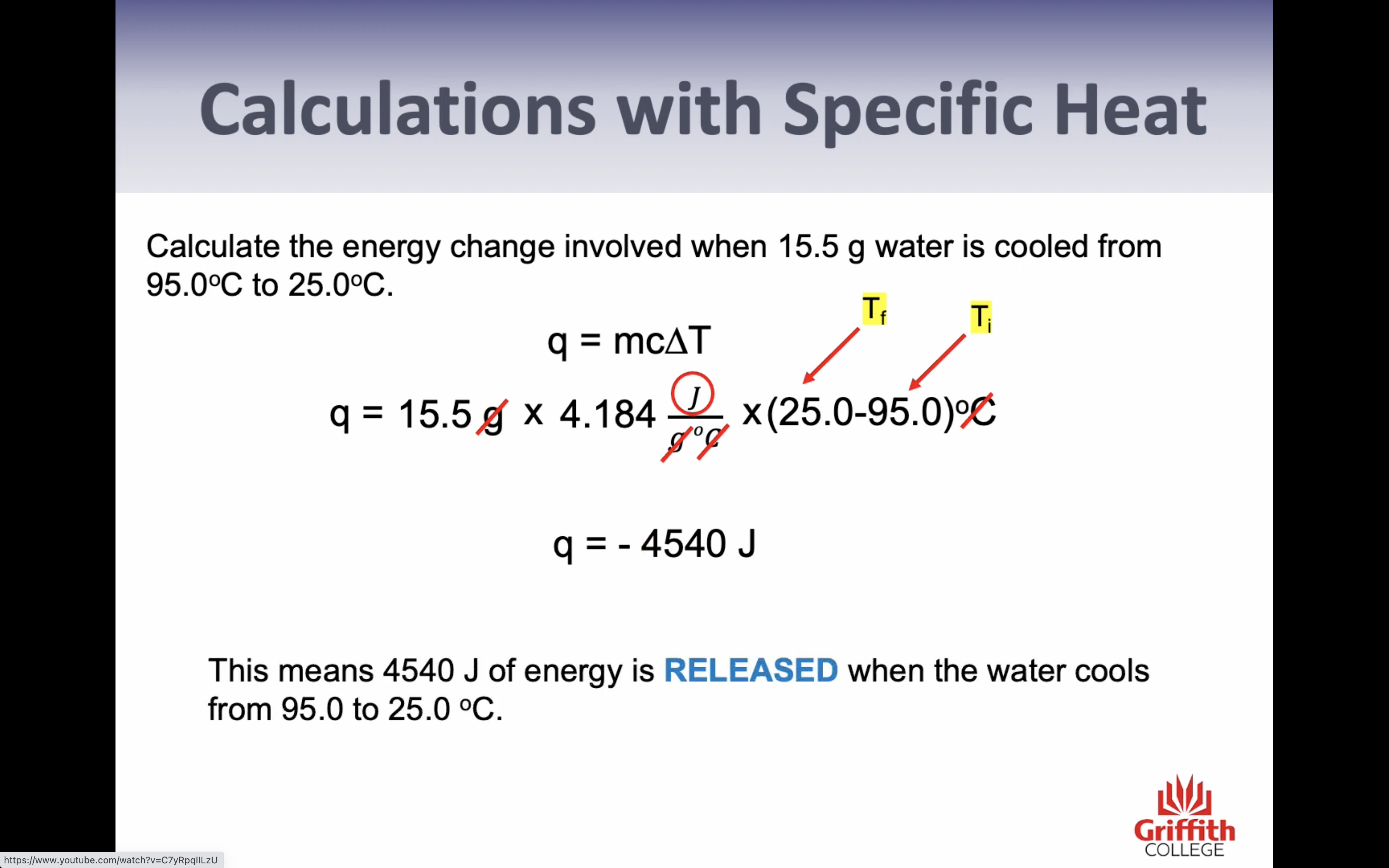
-Specific heat (only change in temp= energy needed to chance 1g of the substance by 1c) (q=mcT)
-Heat of vaporisation (change in state = energy needed to change 1g of liquid to gas) (specific number)
VIDEO 5: CHALLENGING QUESTIONS


VIDEO: TOPIC NOTES SOLUTIONS AND CONCENTRATIONS
전 주랑 동일
VIDEO: How to calculate Percent Concentration
전 주랑 동일
VIDEO: Molarity and Dilutions
전 주랑 동일
Self-Check Quiz Topic 10
- How many grams of (NH4)2SO4 are needed to make a 0.25 L solution at a concentration of 6 M?
- What is the concentration of a solution with a volume of 2.5 litres containing 660 grams of Ca3(PO4)2 ?
- How many grams of CuF2 are needed to make 6.7 litres of a 1.2 M solution?
- What is the molarity of a solution which contains 85.00 g of NaNO3 dissolved in 2.00L of solution?
- If I add water to 100 mL of a 0.15 M NaOH solution until the final volume is 150 mL, what will the molarity of the diluted solution be?
'Griffith college Tri3 2022 > 1001GRC (Chem)' 카테고리의 다른 글
| WEEK10 - Topic 12 (0) | 2022.12.29 |
|---|---|
| WEEK9 - Topic 11 (0) | 2022.12.17 |
| WEEK7 - Topic 9&10 (0) | 2022.12.03 |
| WEEK6- Topic 8&9 (0) | 2022.11.23 |
| WEEK5 - Topic 7 (0) | 2022.11.17 |



