Learning Outcomes
1: Identify moles and molar mass and carry out calculations involving them
2: Calculate the percent composition
3: Determine empirical and molecular formulas
Key Concepts
1: The mole and molar mass of compounds
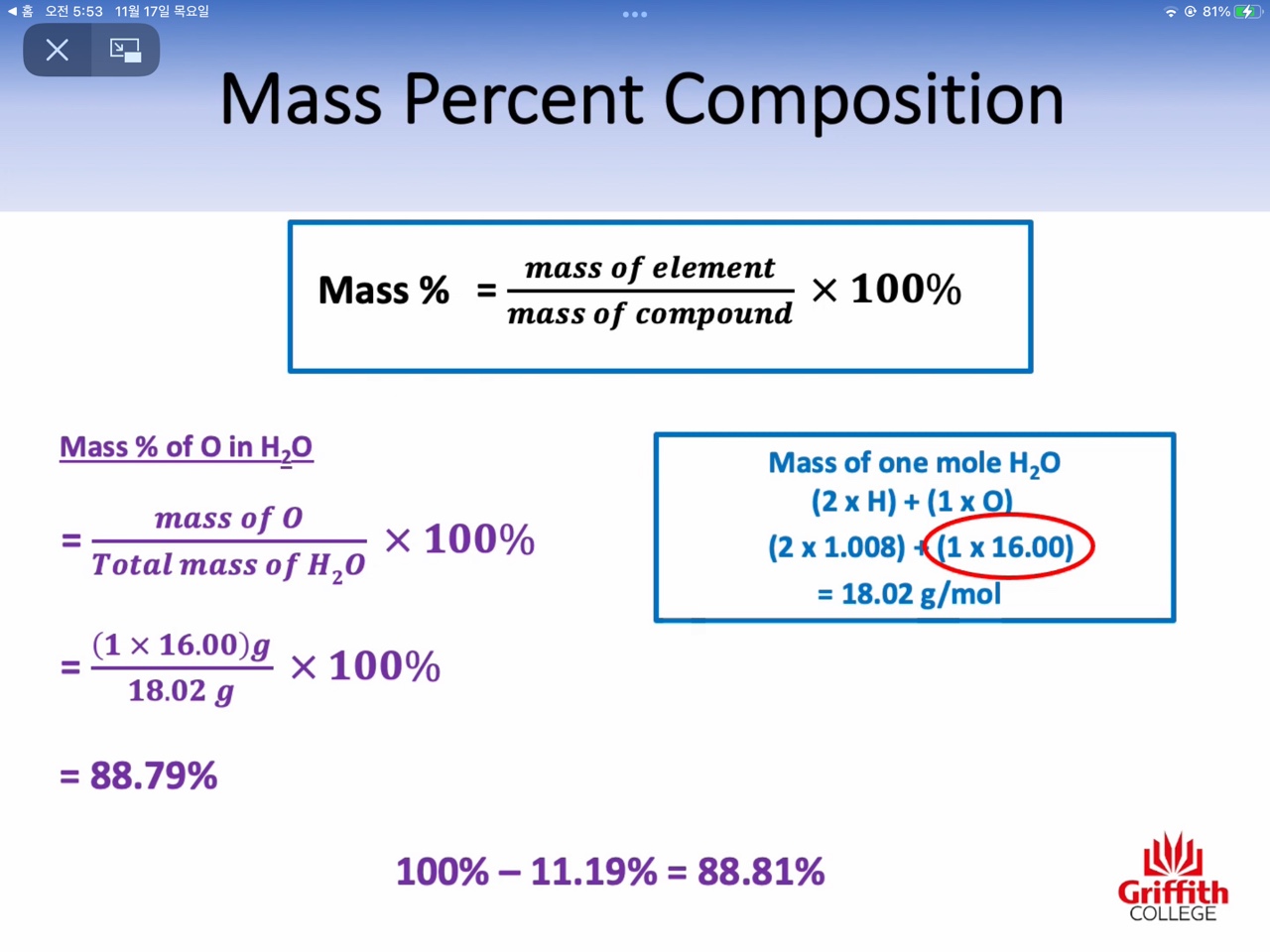
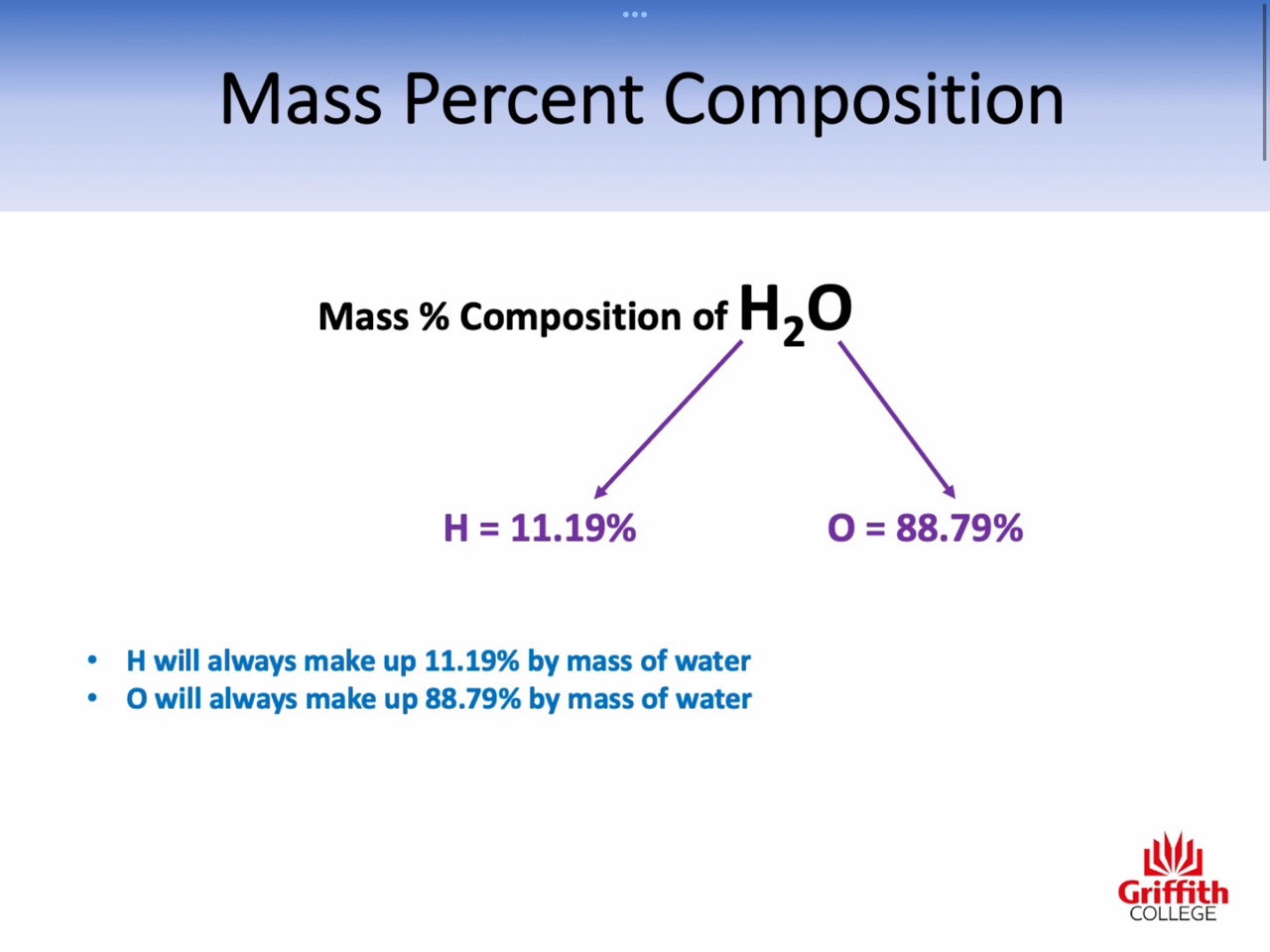
2: Percent composition of compounds
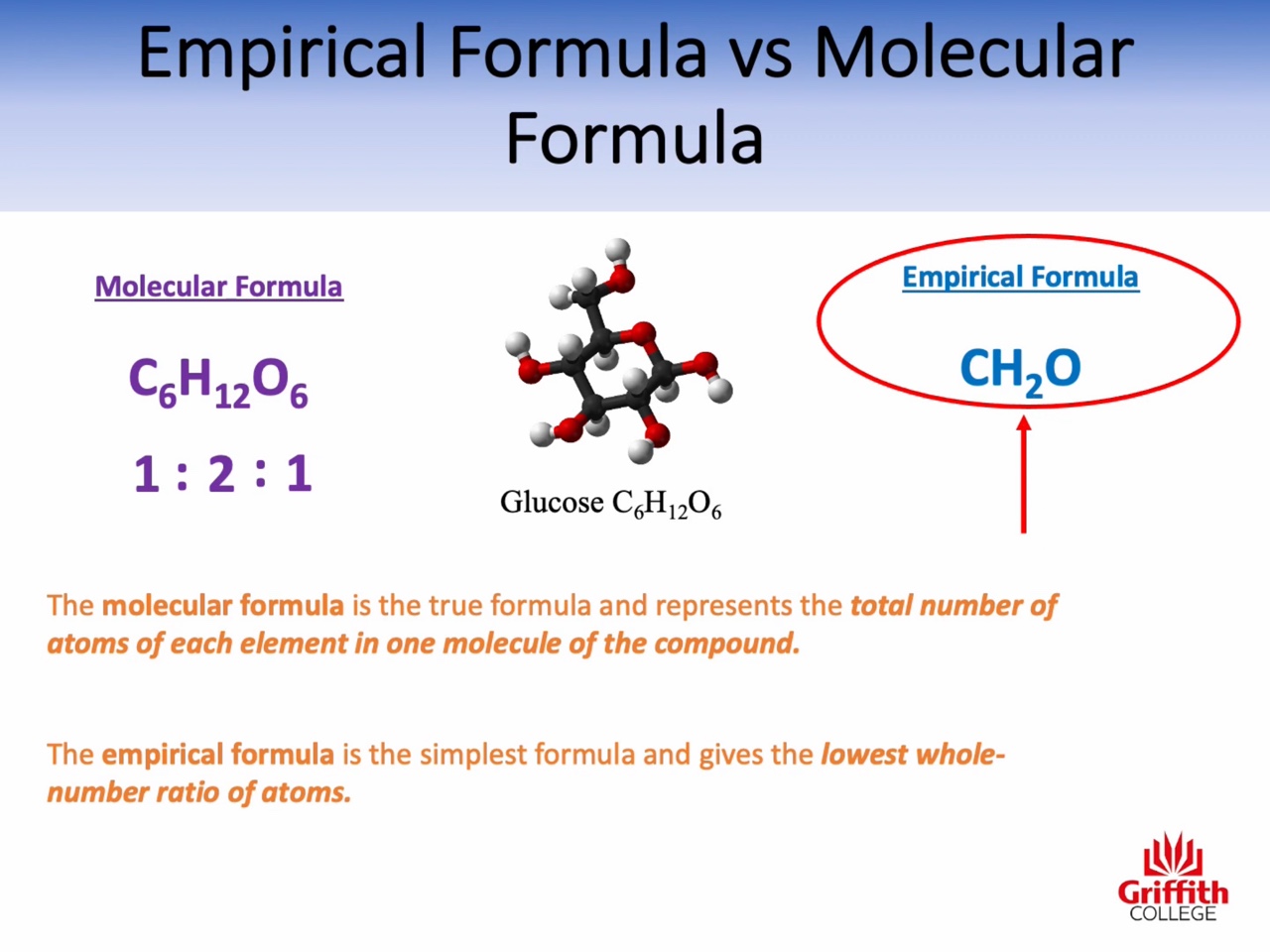
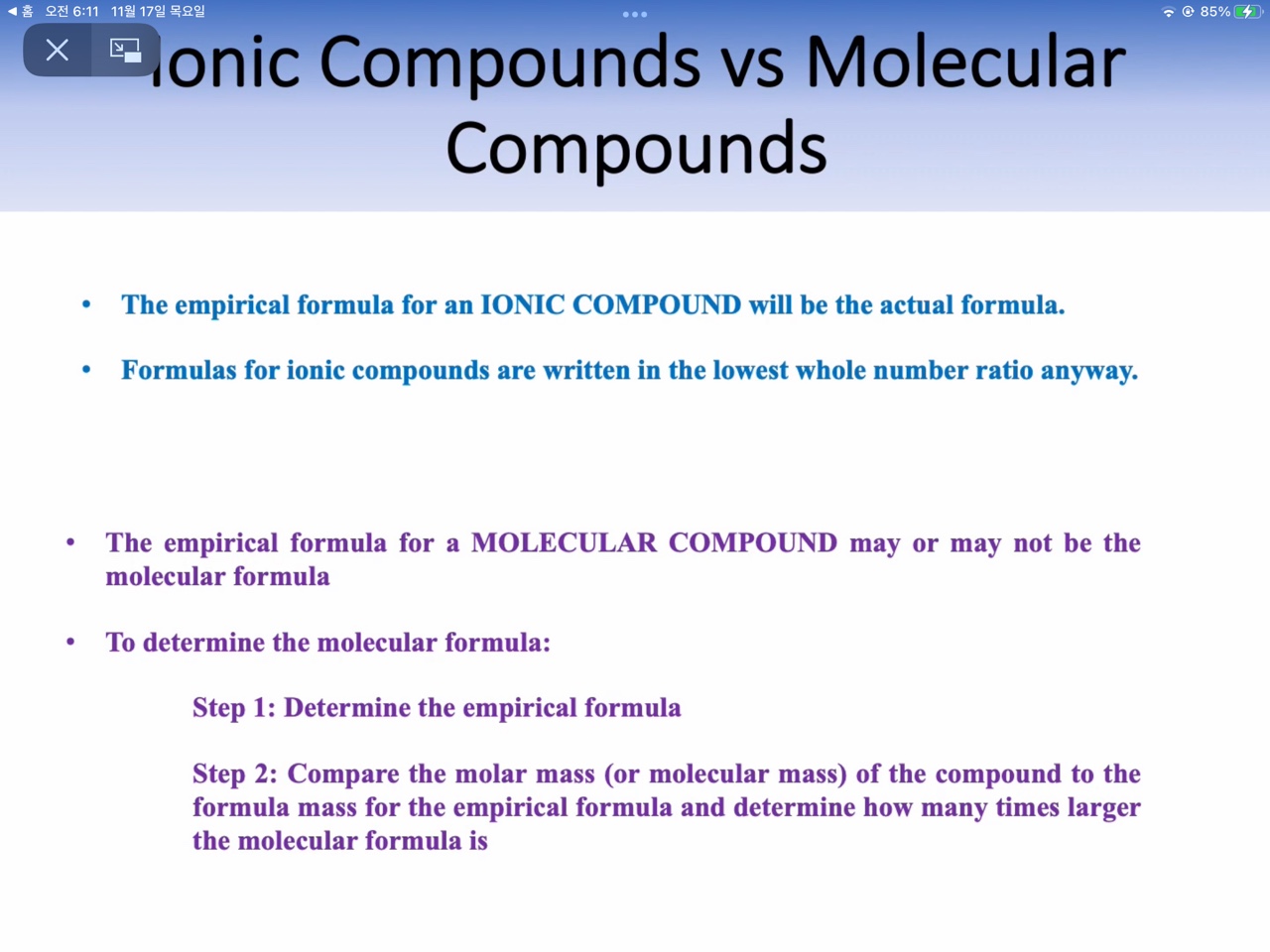
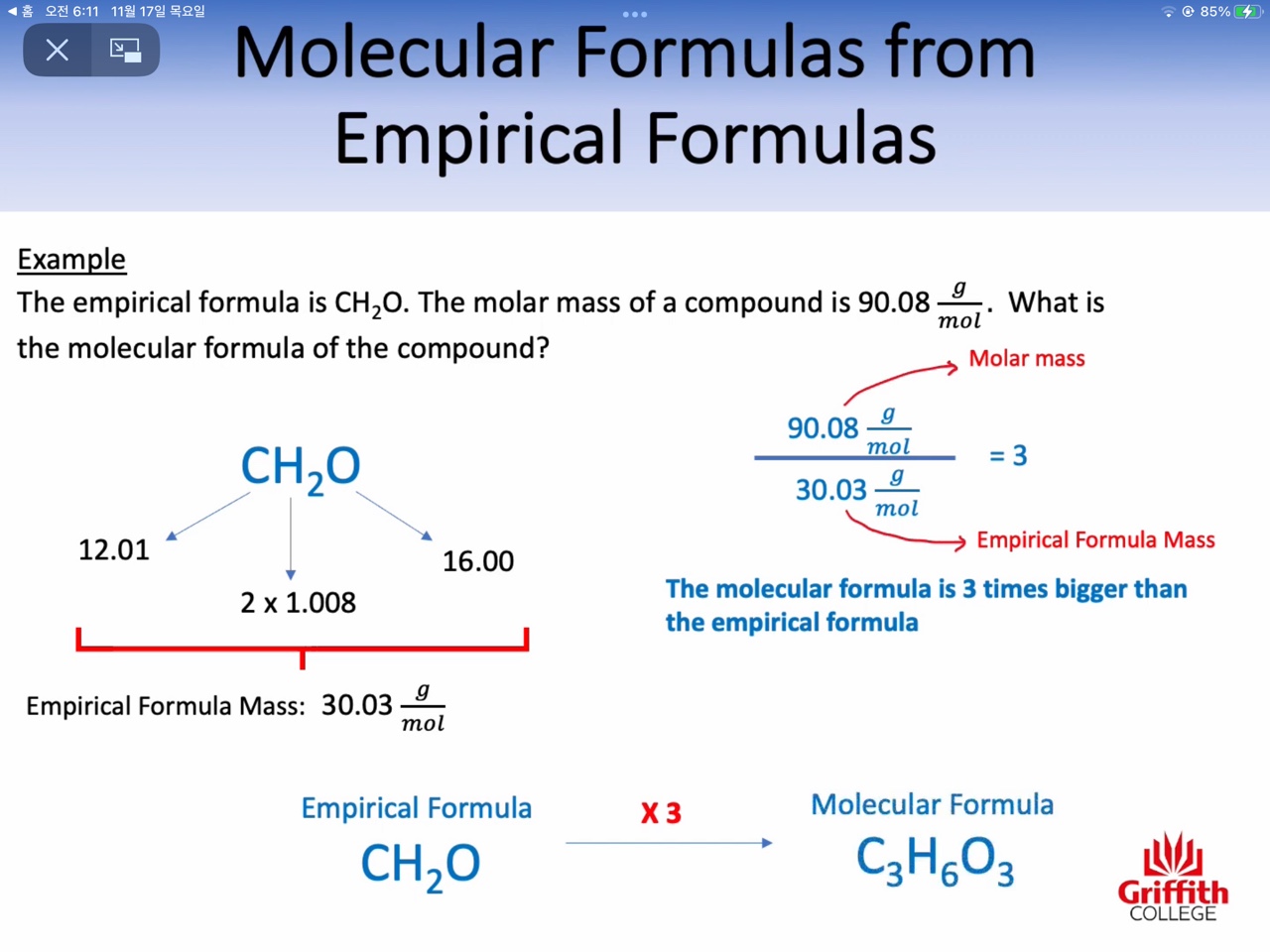
3: Empirical formula versus molecular formula
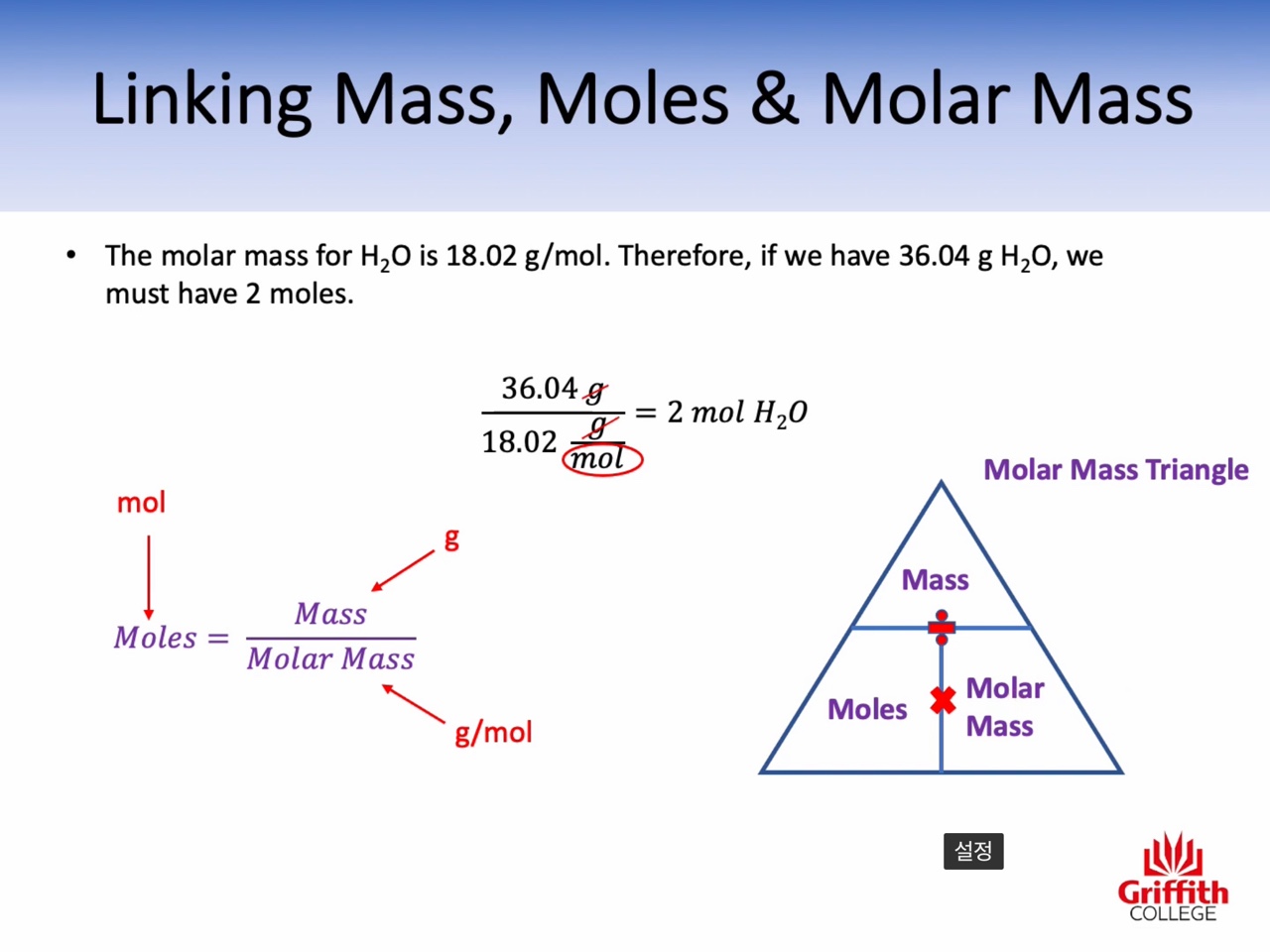
Mole, Molar Mass, Mass and Moles
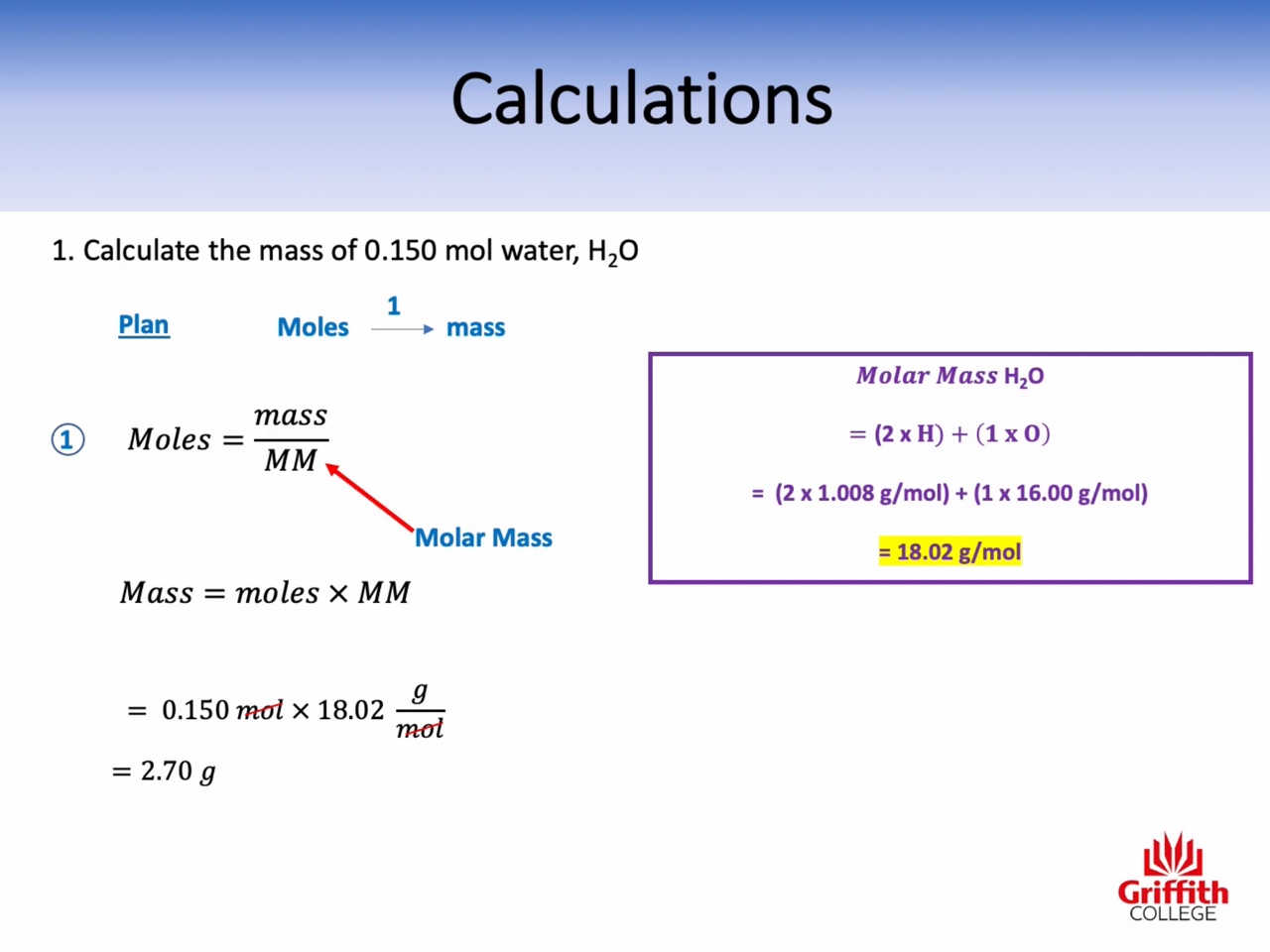
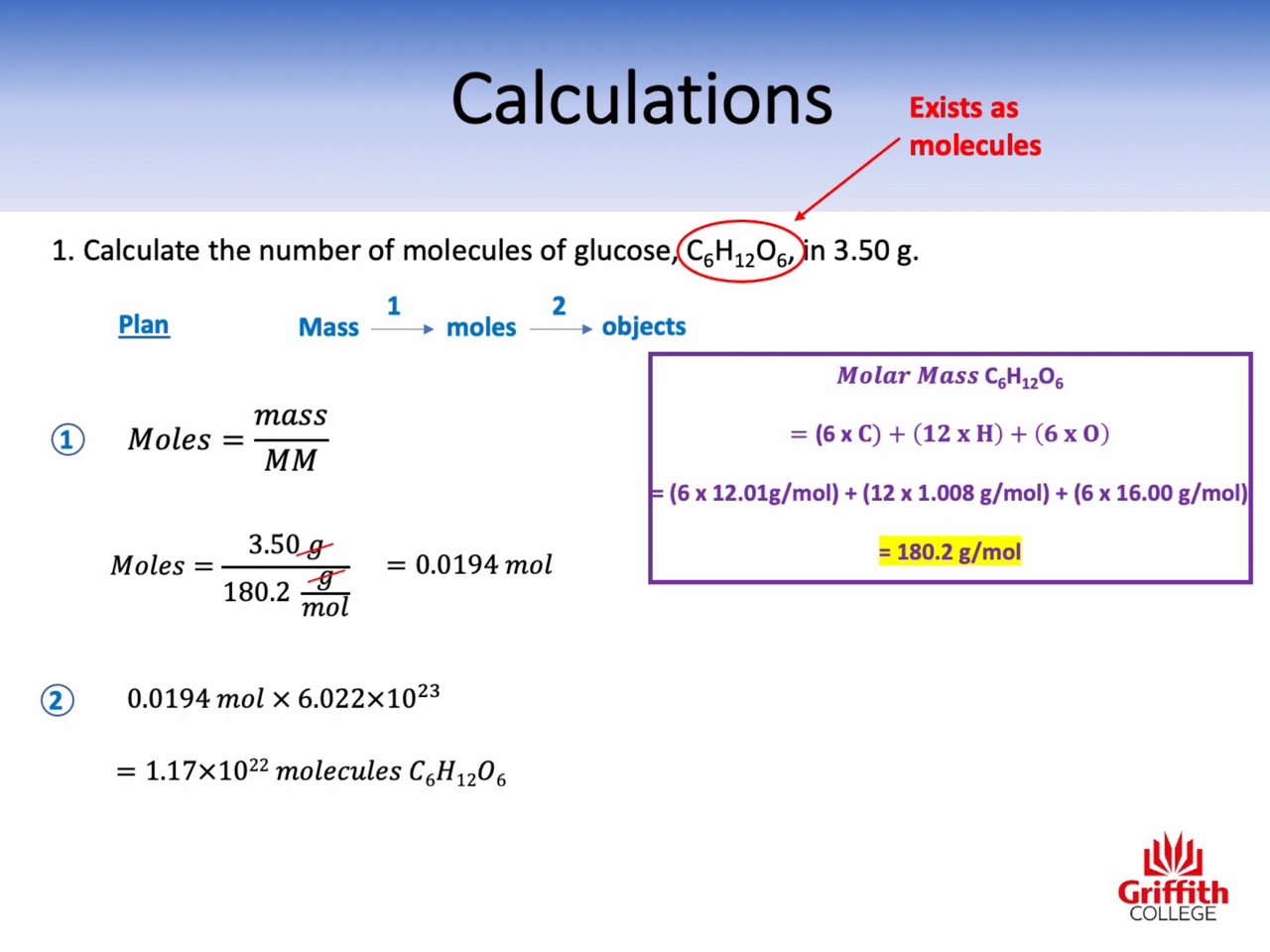
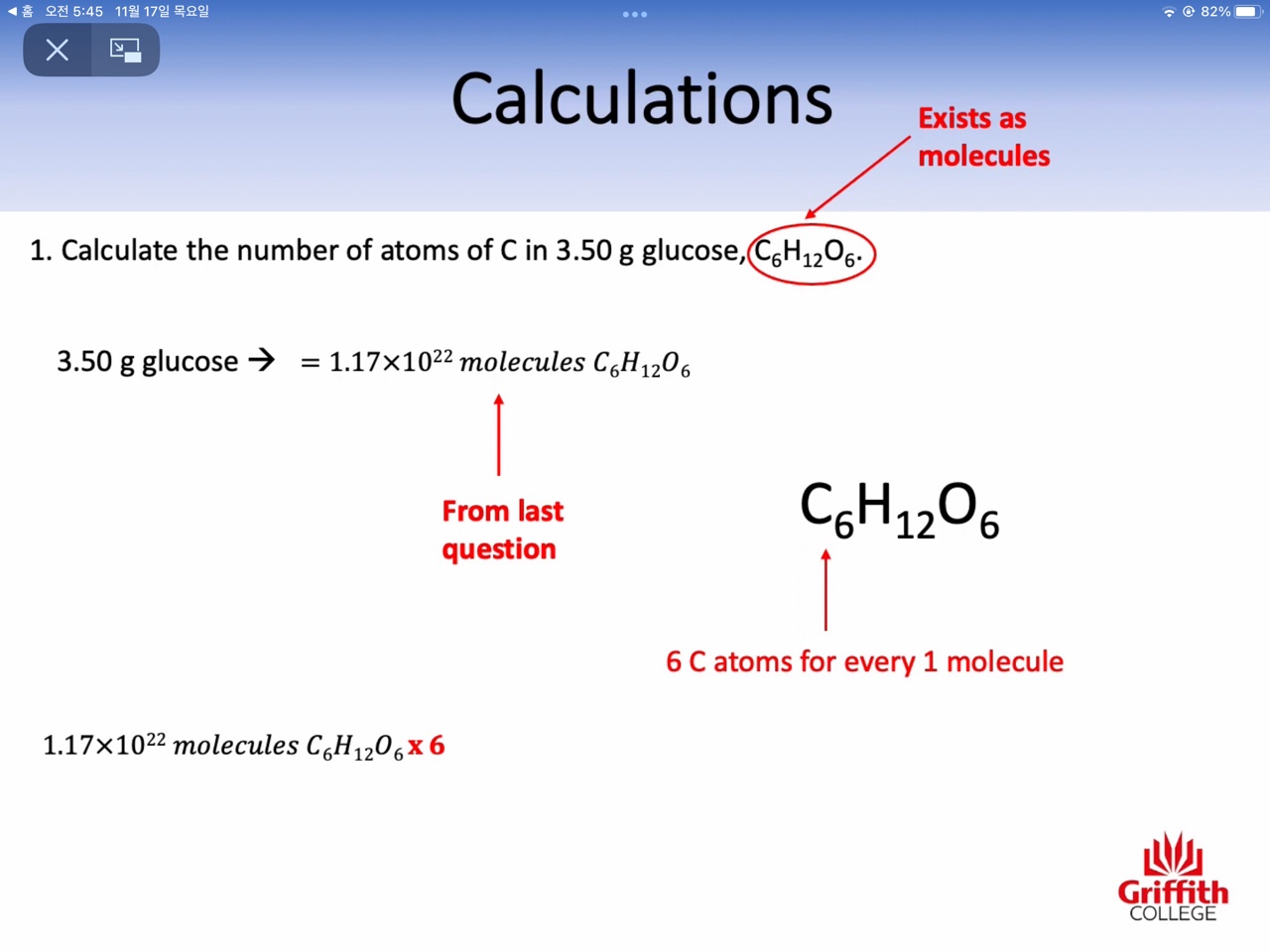
Calculations involving Mass, Moles and Molar Mass
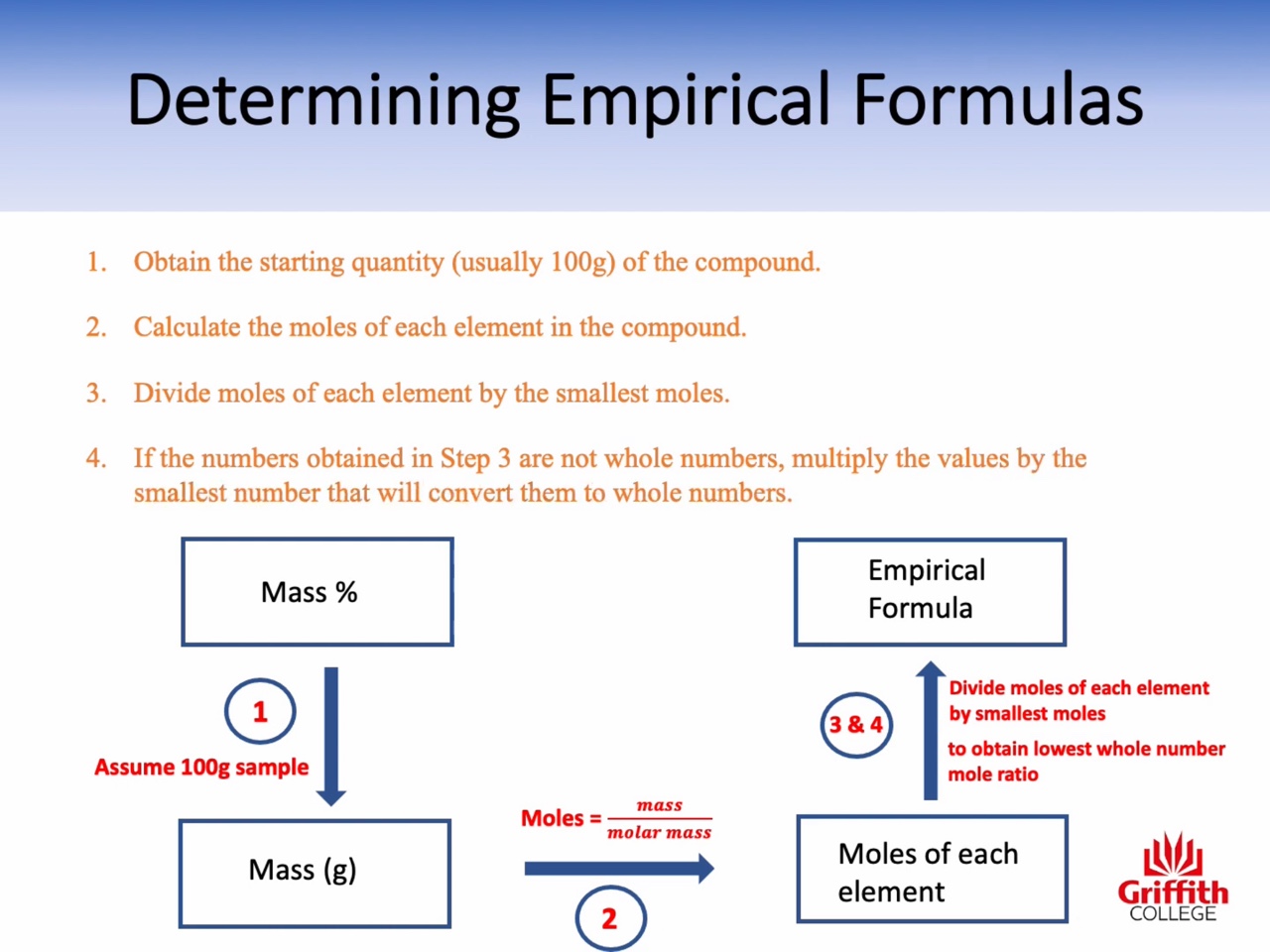
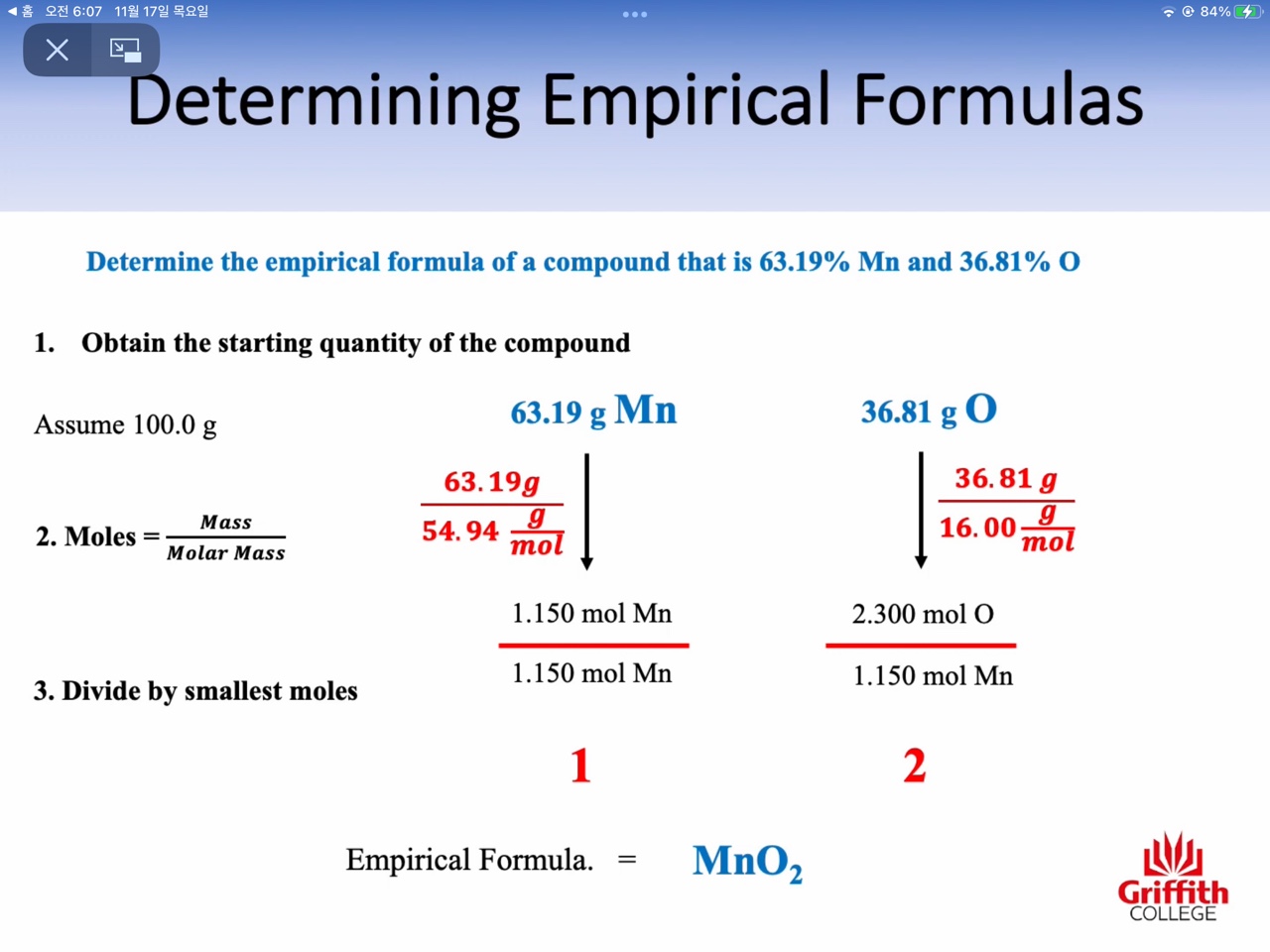
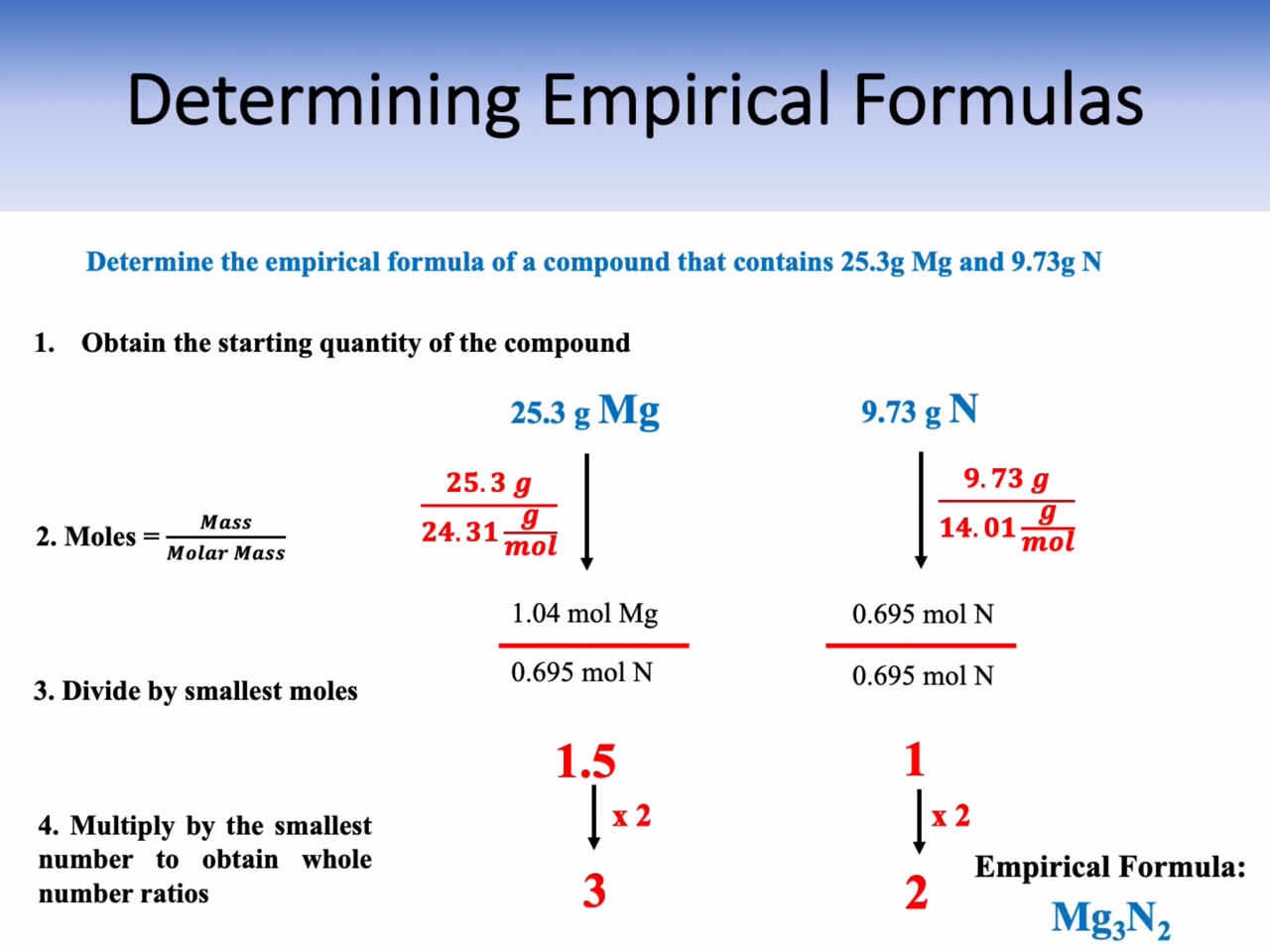
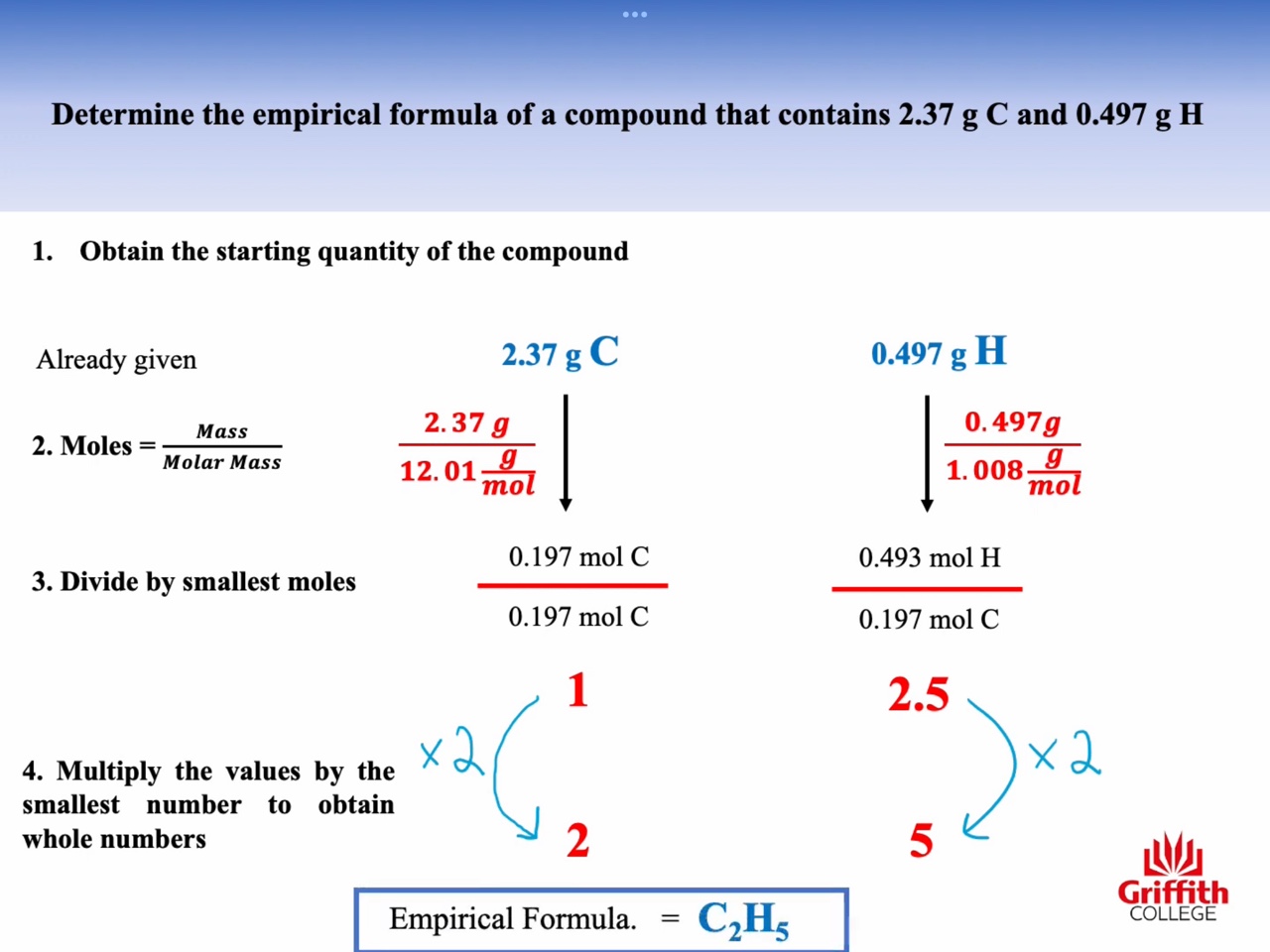
Mass percent, Empirical Formula



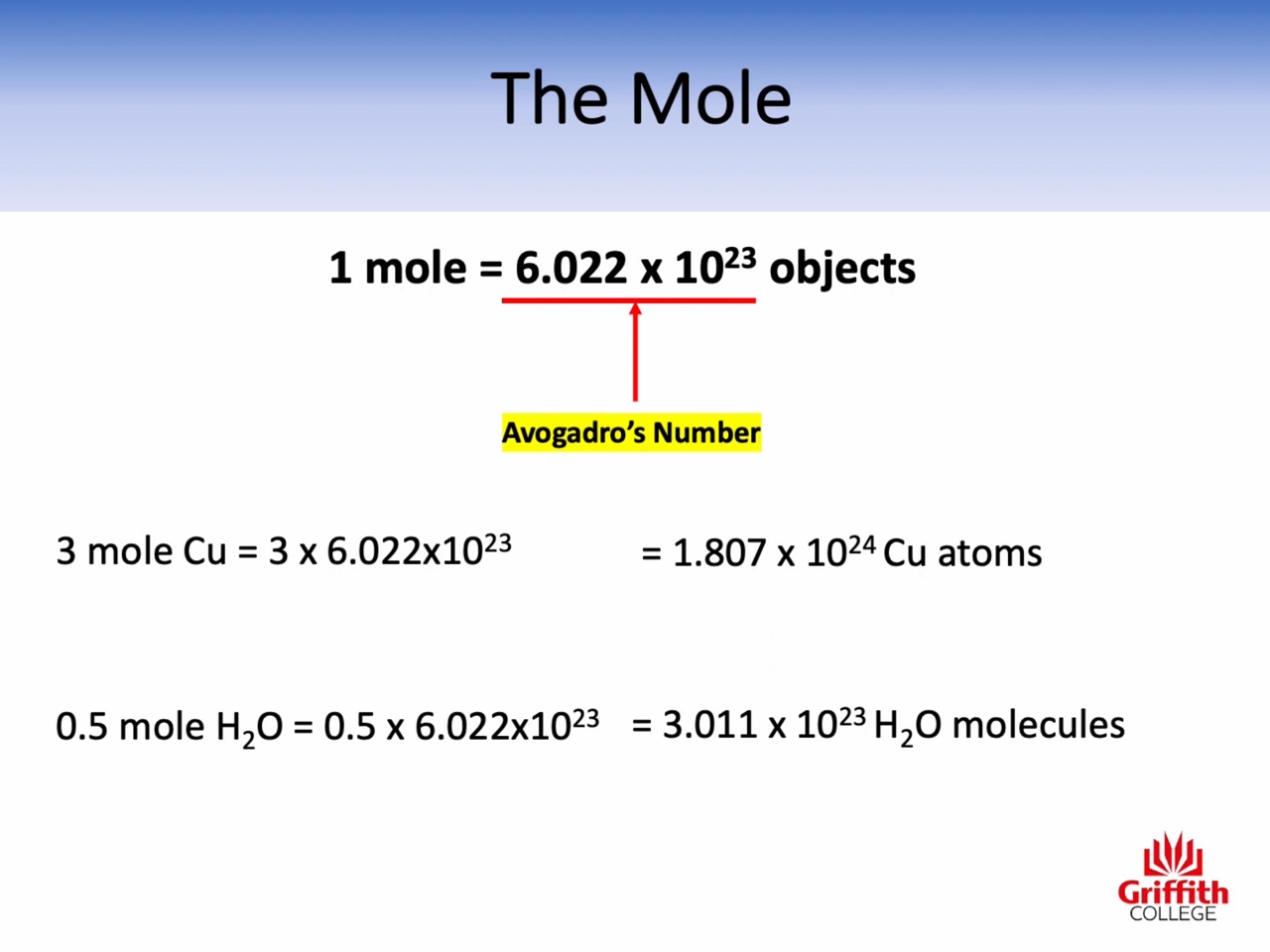
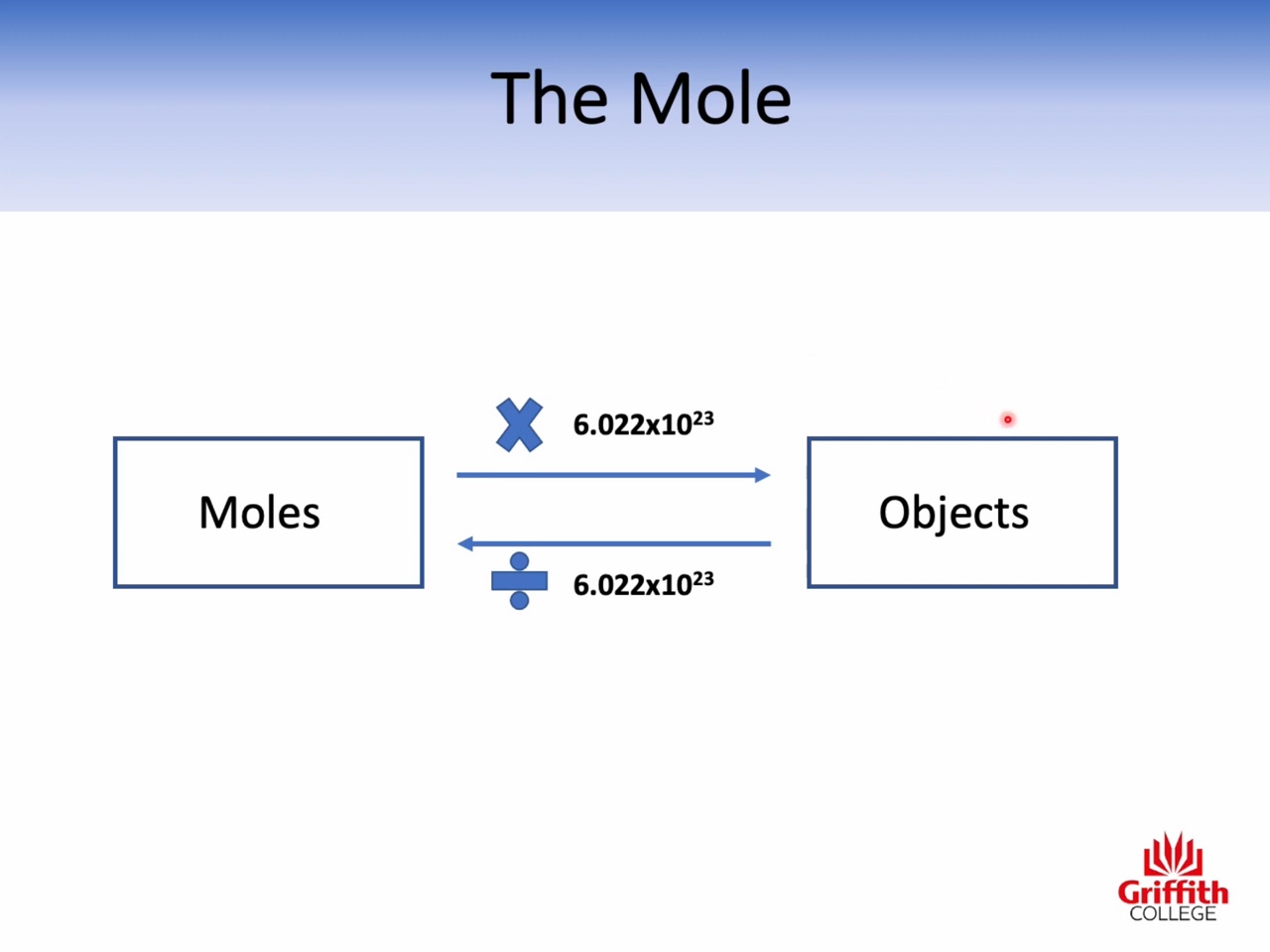
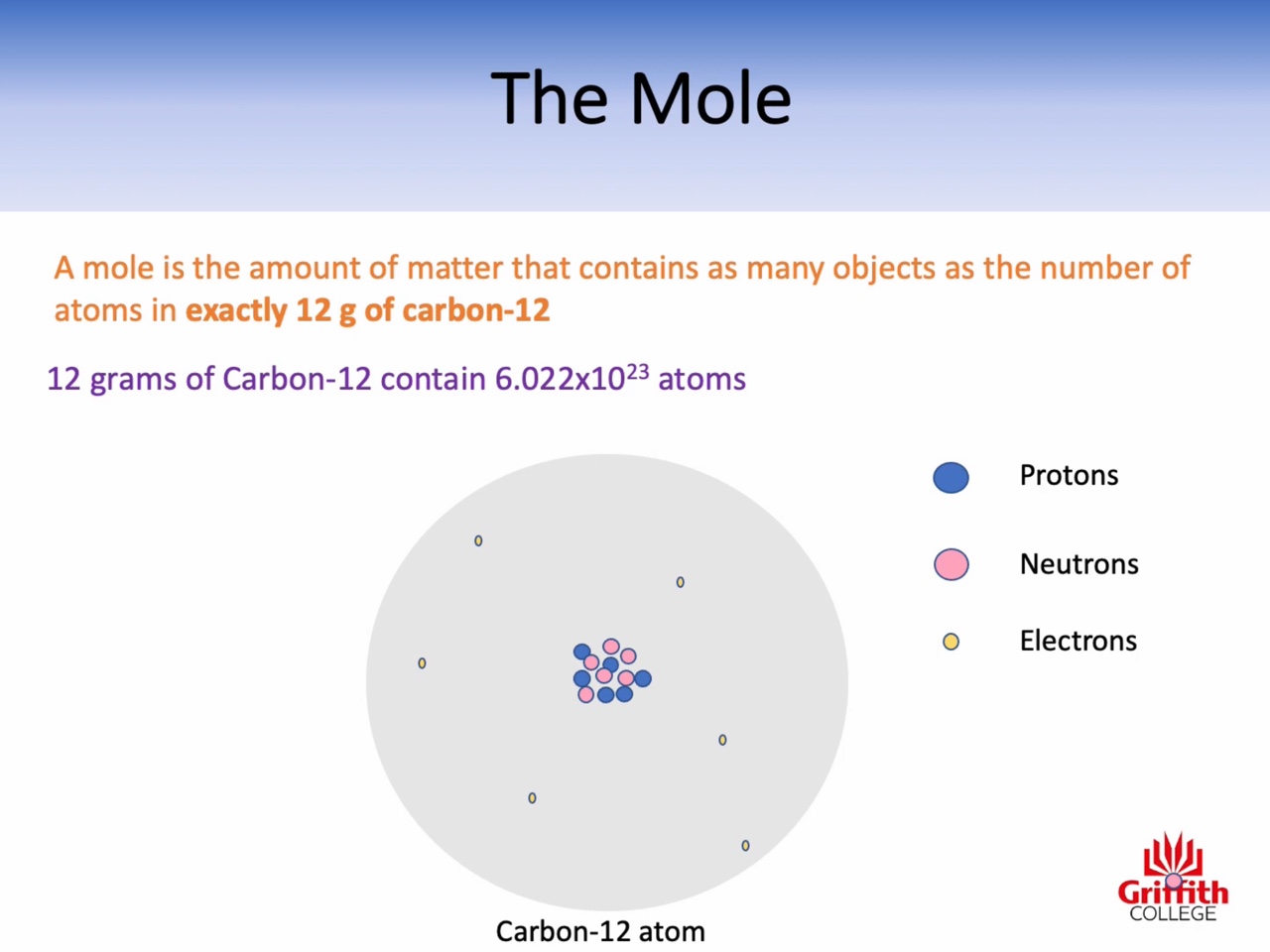
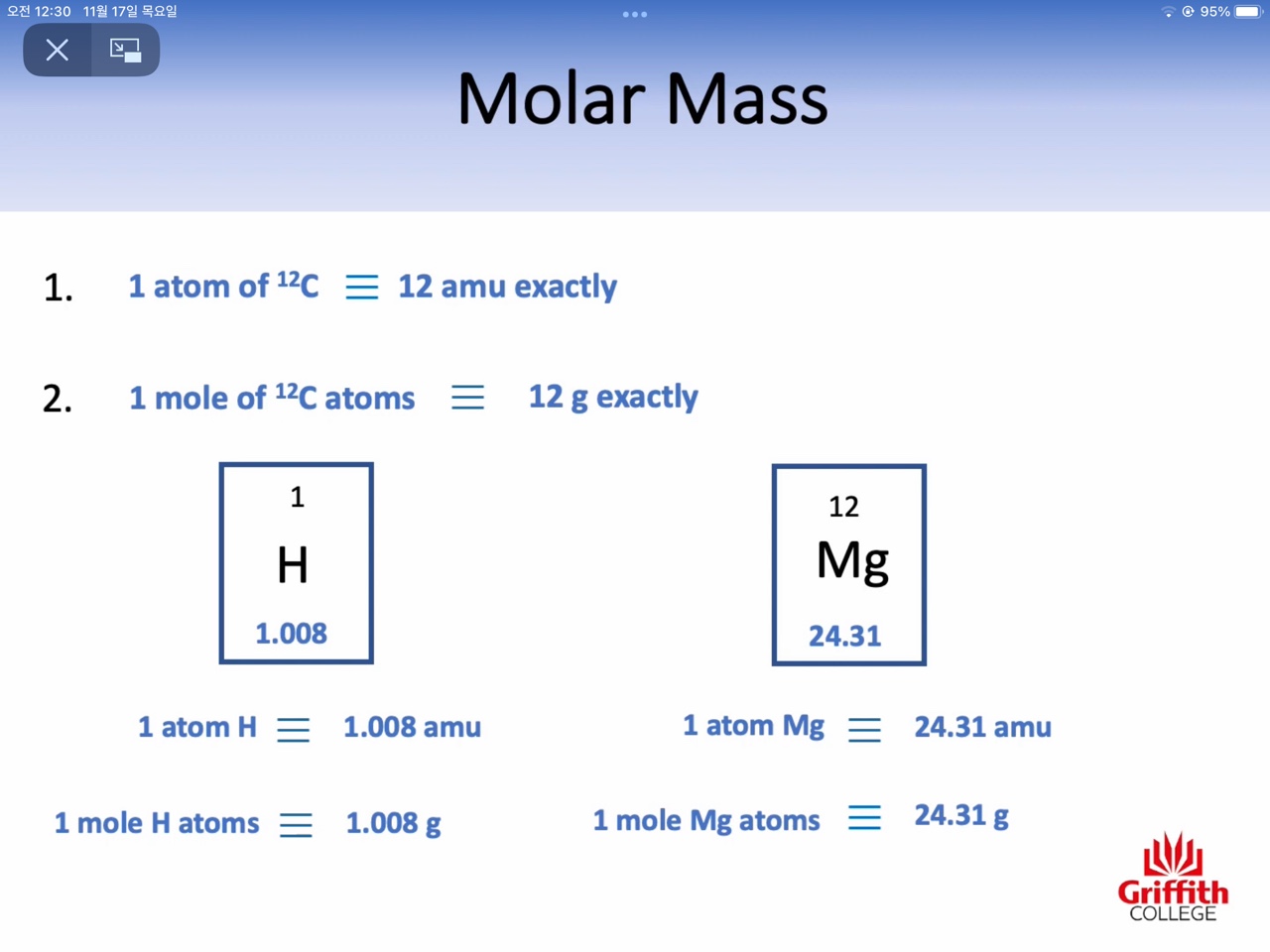
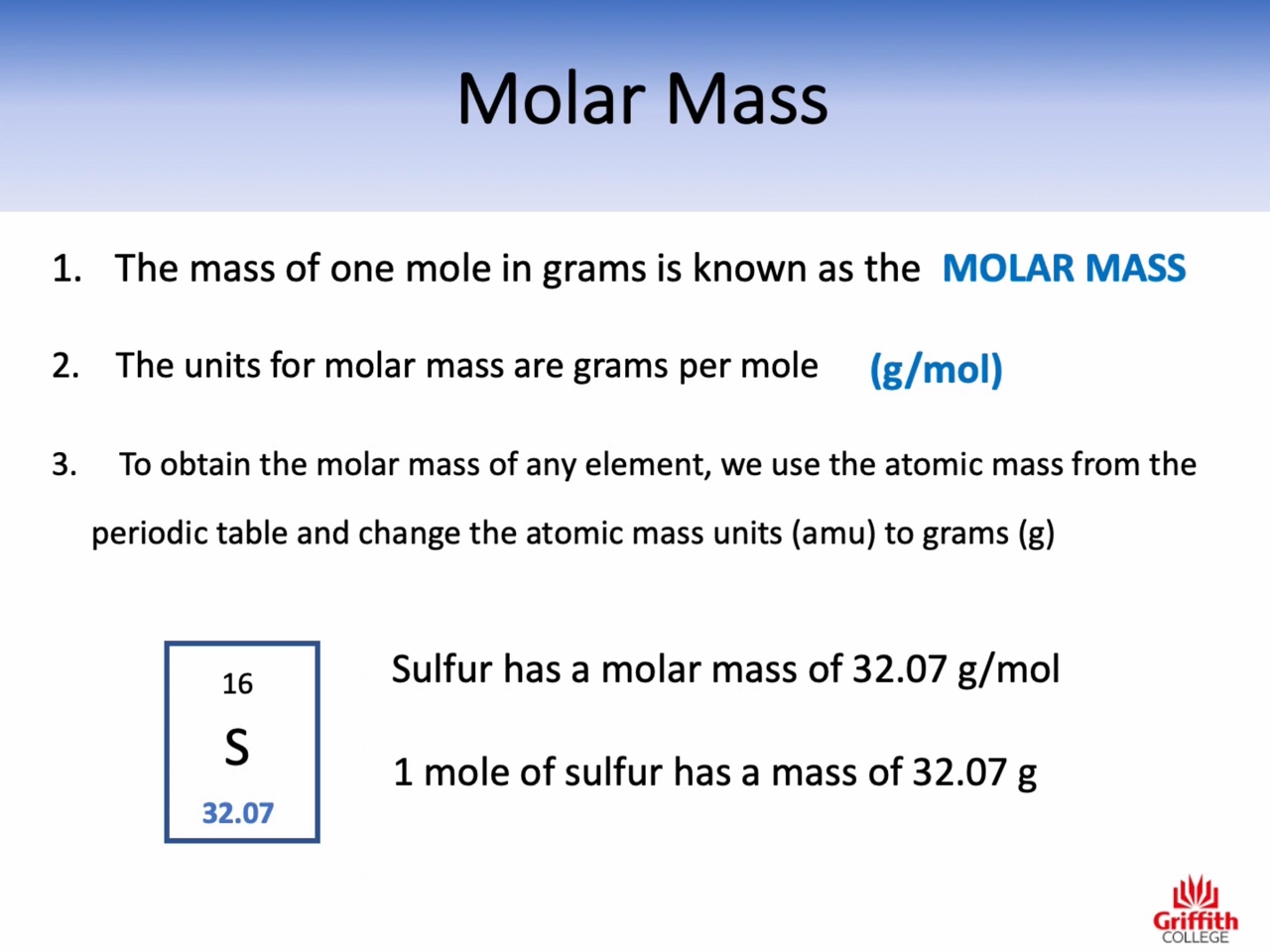
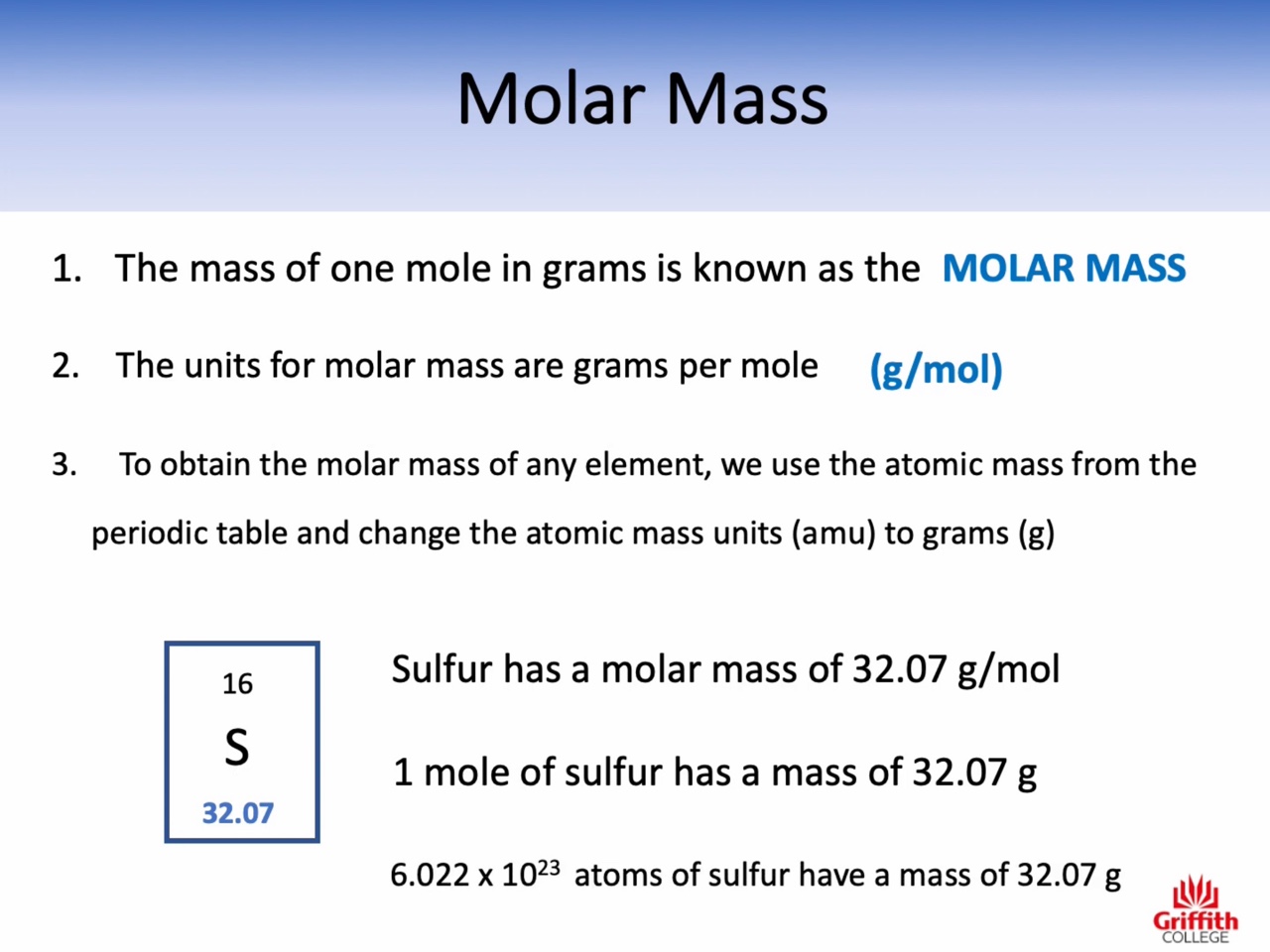
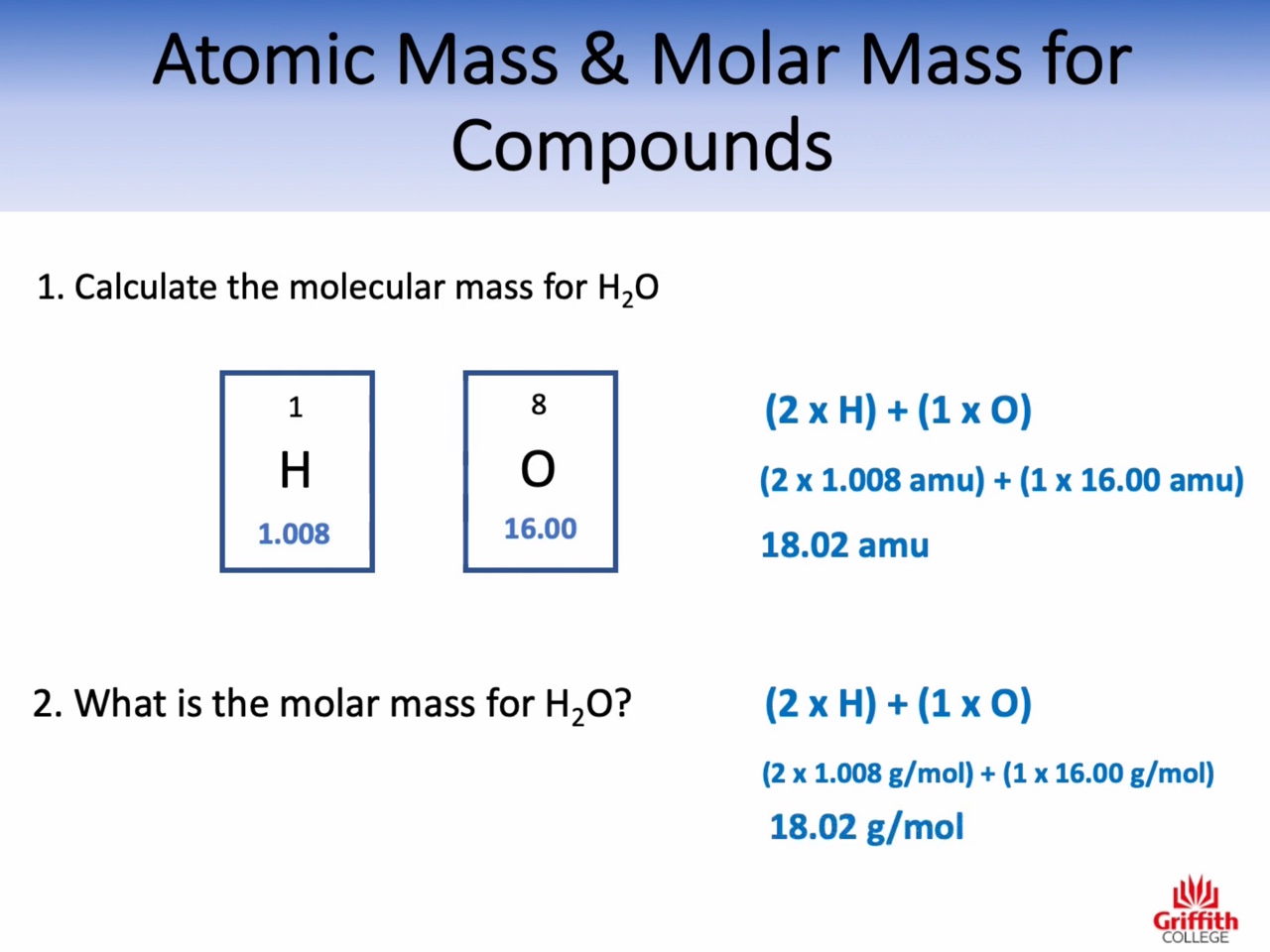
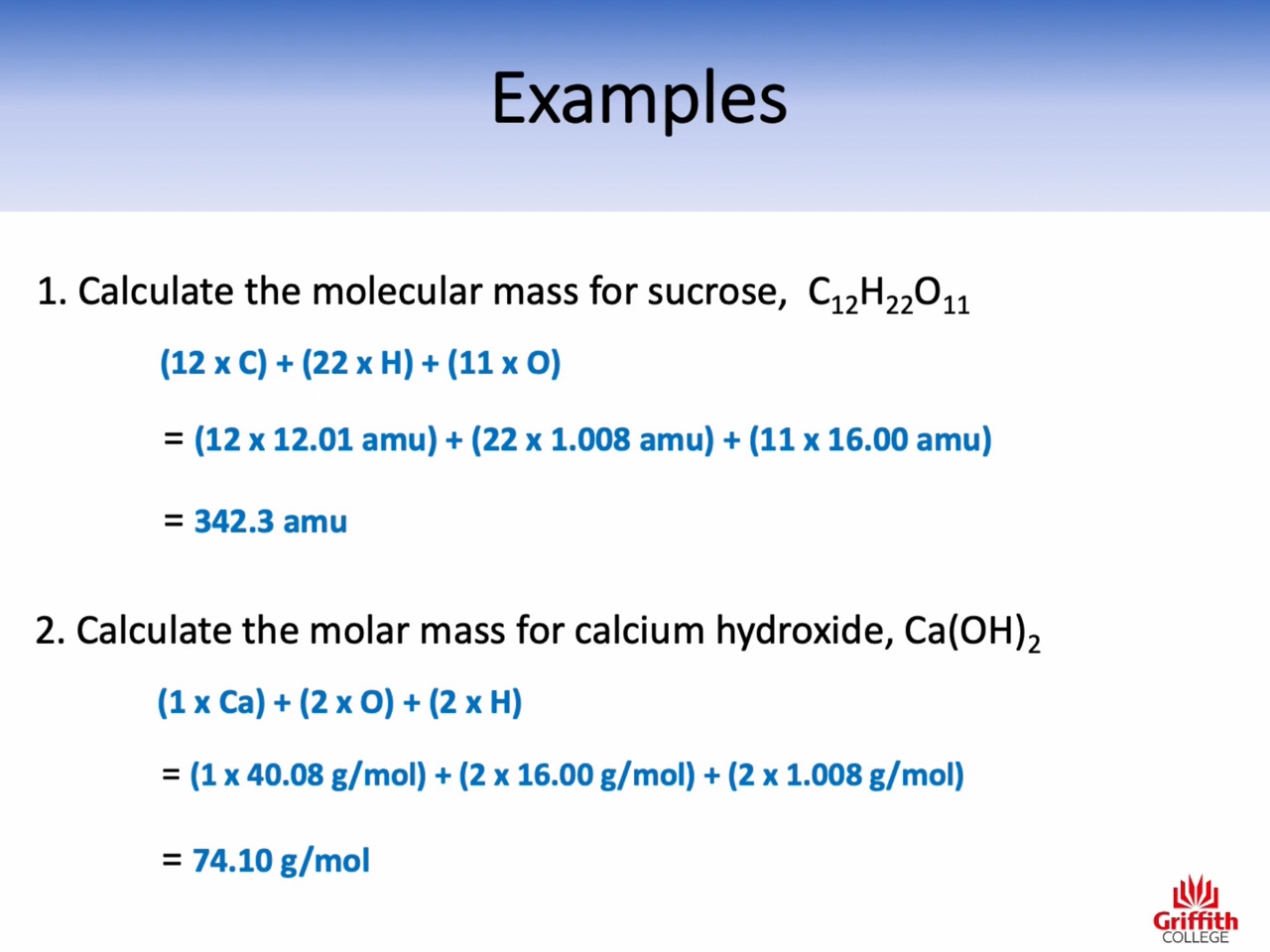
Atomic mass : amu (the relative mass of one atom)
Molecular mass : amu (the relative mass of one molecule)
Formula mass : amu (the relative mass of one unit of an ionic compound)
Molar mass : g/mol (the mass of one mole)
반응형
'Griffith college Tri3 2022 > 1001GRC (Chem)' 카테고리의 다른 글
| WEEK7 - Topic 9&10 (0) | 2022.12.03 |
|---|---|
| WEEK6- Topic 8&9 (0) | 2022.11.23 |
| WEEK4 - Topic 6 (0) | 2022.11.09 |
| WEEK3 - Topic 5 (0) | 2022.11.04 |
| WEEK2 - Topic 3&4 (0) | 2022.10.29 |



