Learning Objective
Describe the major body fluid compartments of the body
Understand the major differences in composition of interstitial fluid & ICF
Understand that plasma membranes separate ECF from ICF
Understand that blood plasma, a special ECF compartment, influences interstitial fluid which in turn influences ICF
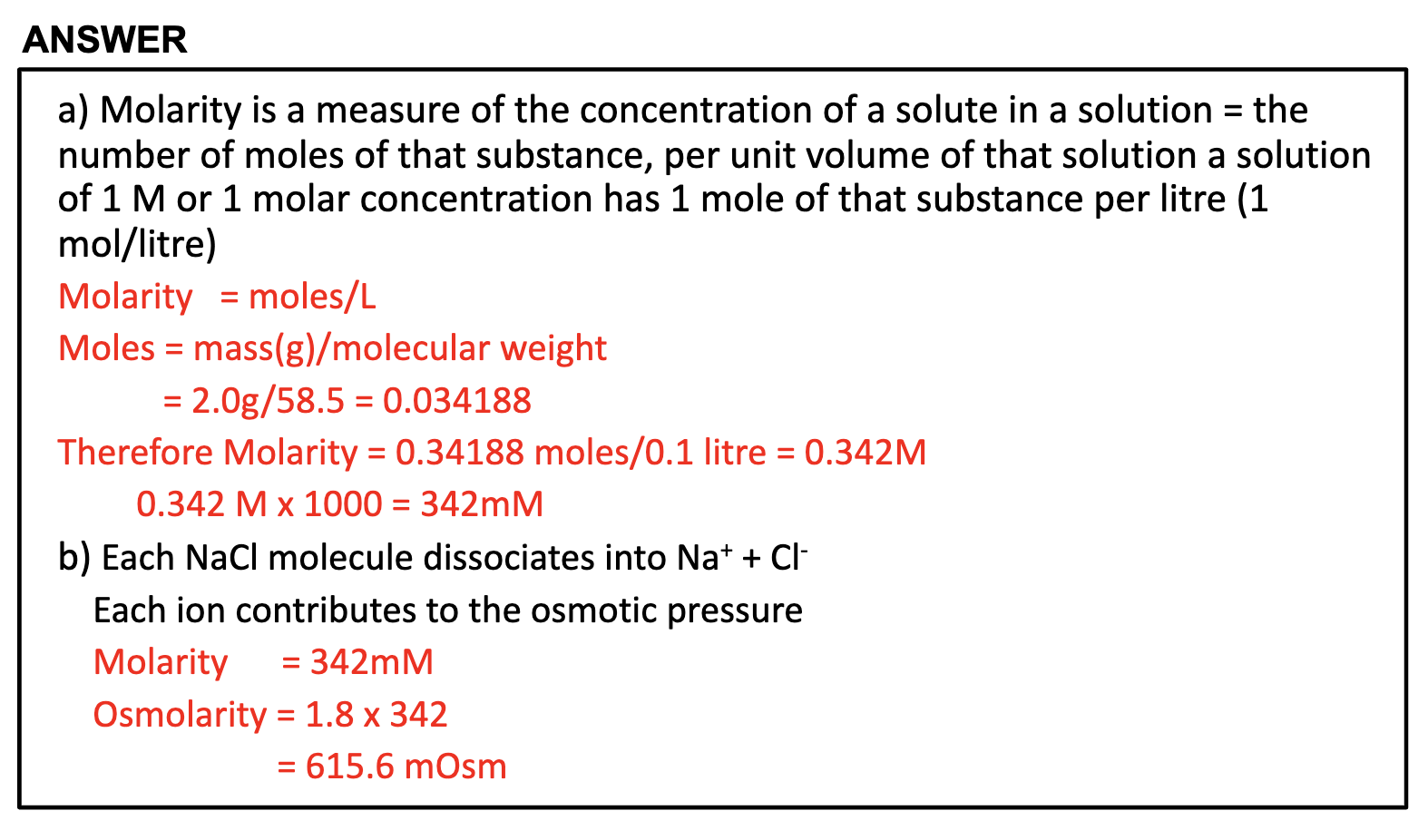
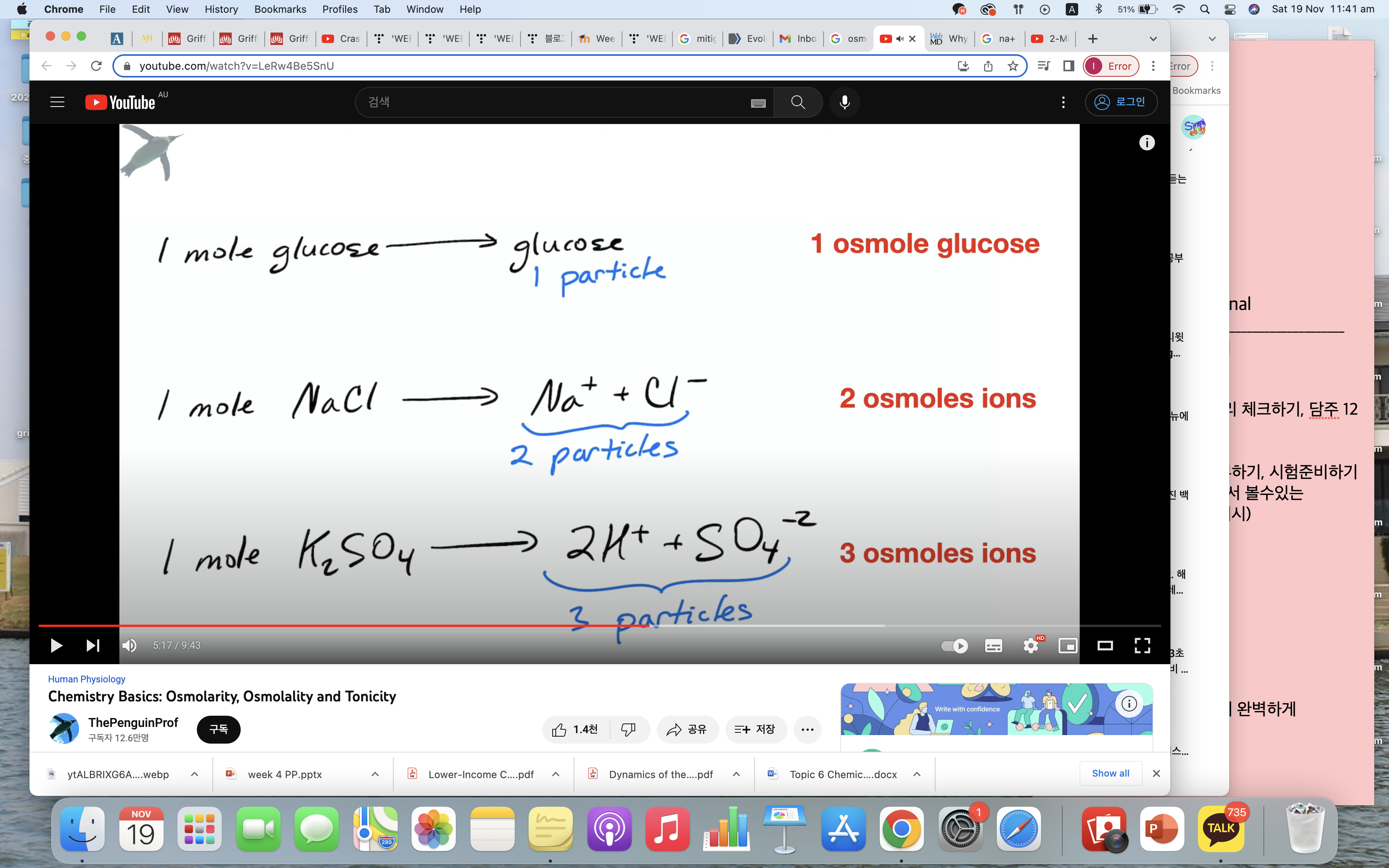
Be able to describe how it is that 1 M NaCl generates an approx. 2 Osmolar solution
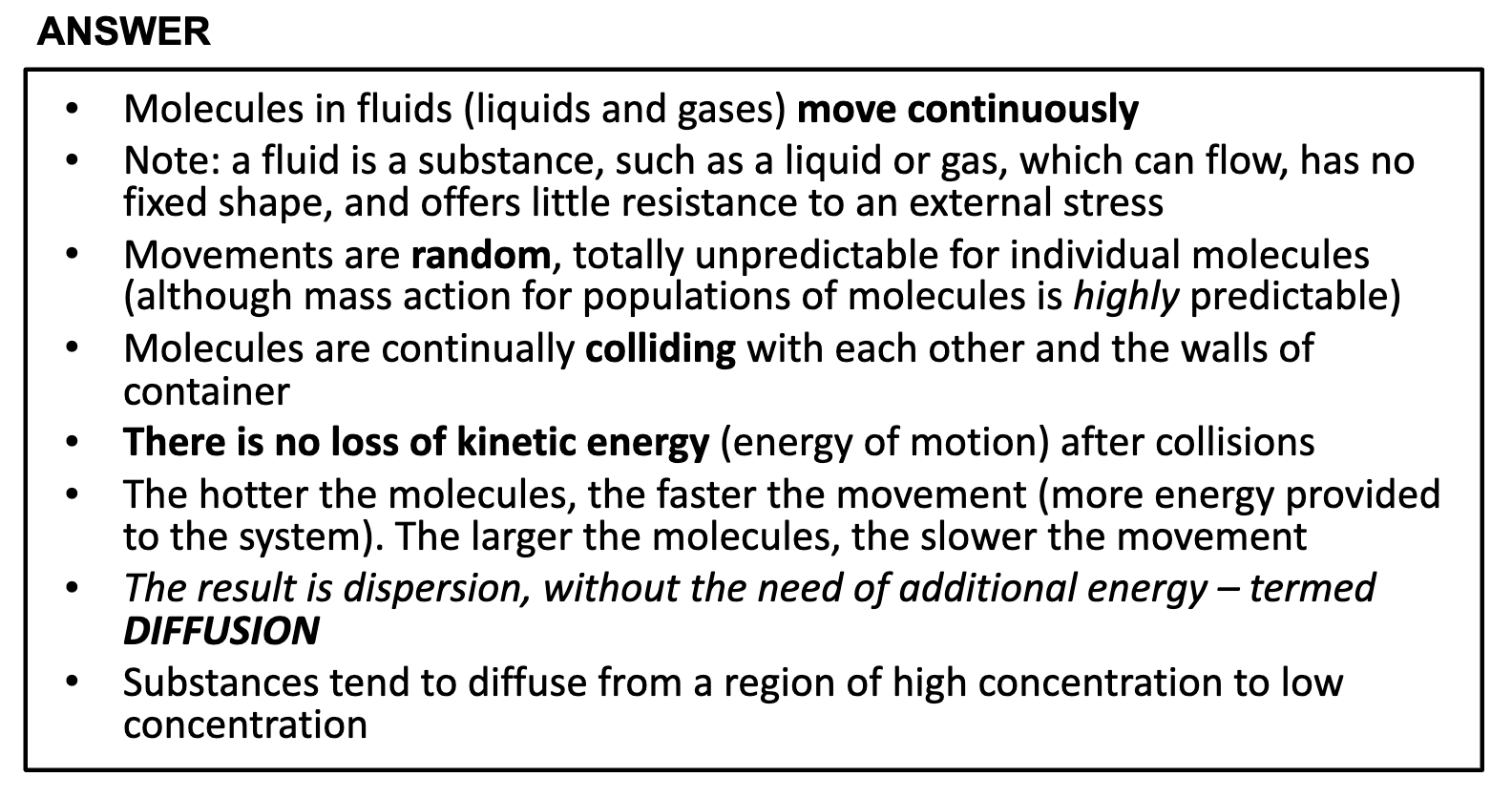
Understand the laws of diffusion, how these relate to biological function, with respect to movement of molecules in body fluids and respiratory gases
To fully appreciate the process of osmosis, and how this relates to the terms iso, hypo and hypertonicity
Describe the role of proteins in plasma, relating this to capillary permeability, COP, and oedema
Understand the process of facilitated diffusion, and what saturation and selectivity of carriers mean
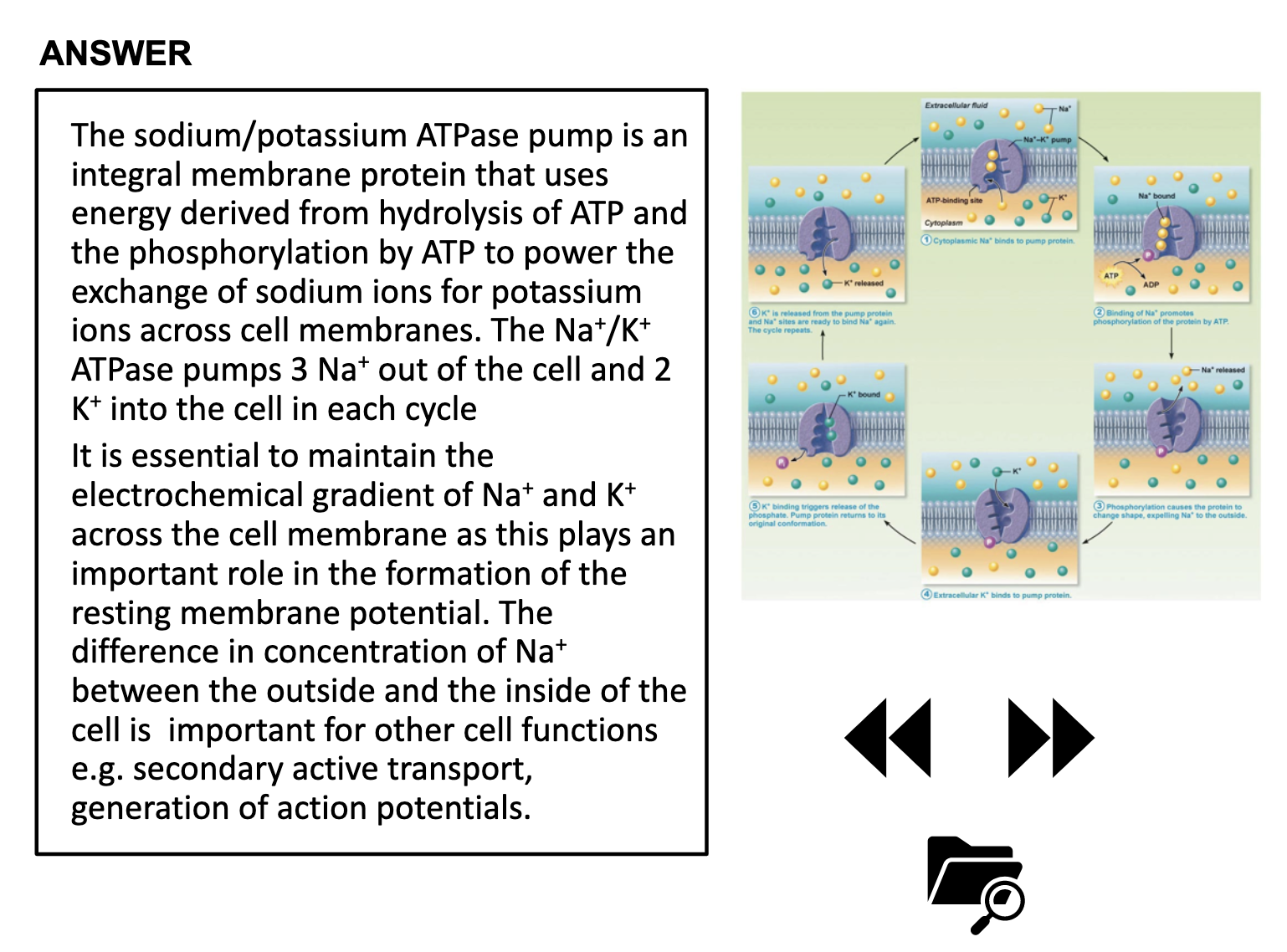
To be able to describe the process of active transport, using the NaK-ATPase pump as an example, and understand why this pump is ubiquitous
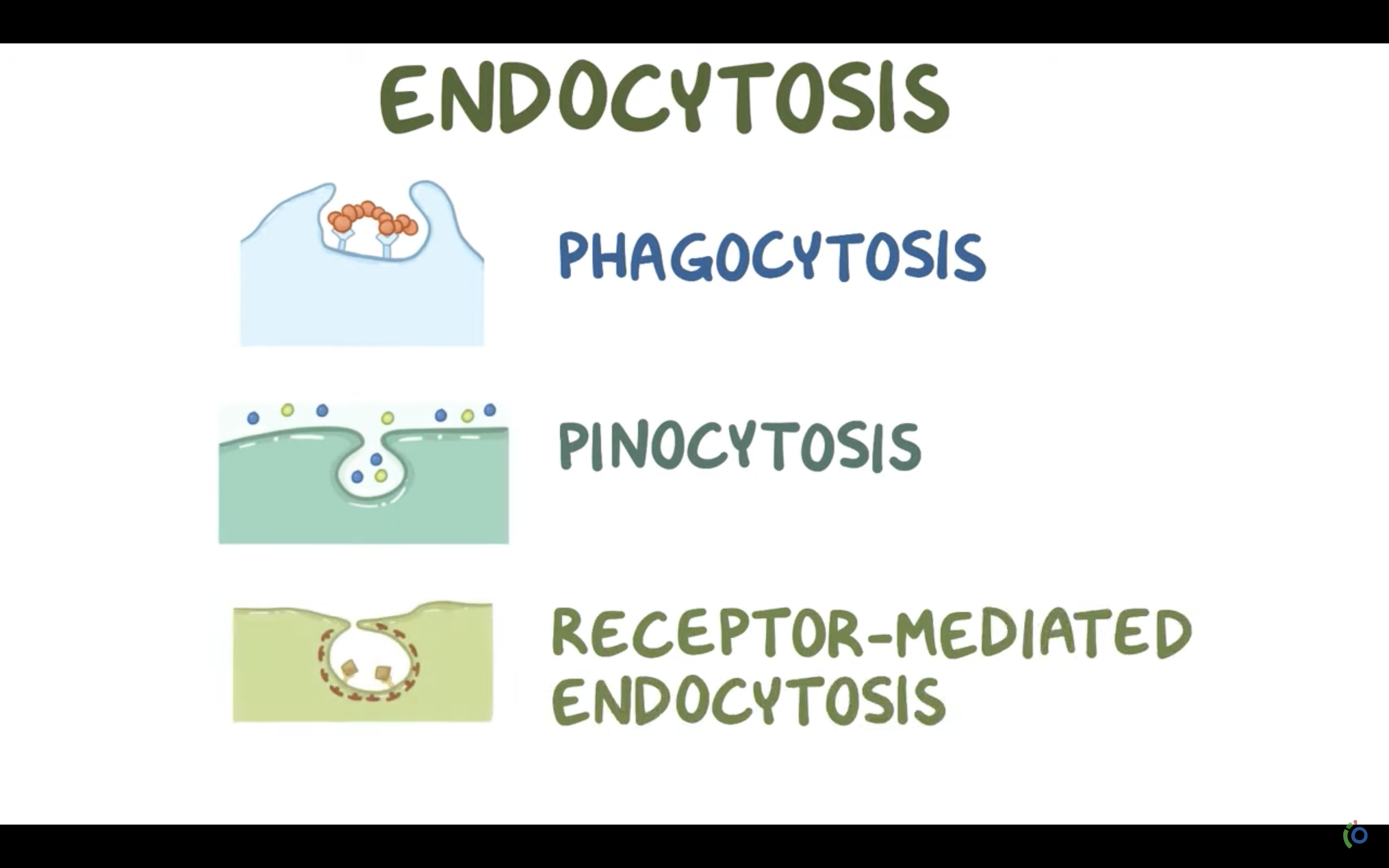
To differentiate between non-specific and receptor-mediated endocytosis, phagocytosis and pinocytosis
ppt

FLUID COMPARTMENTS


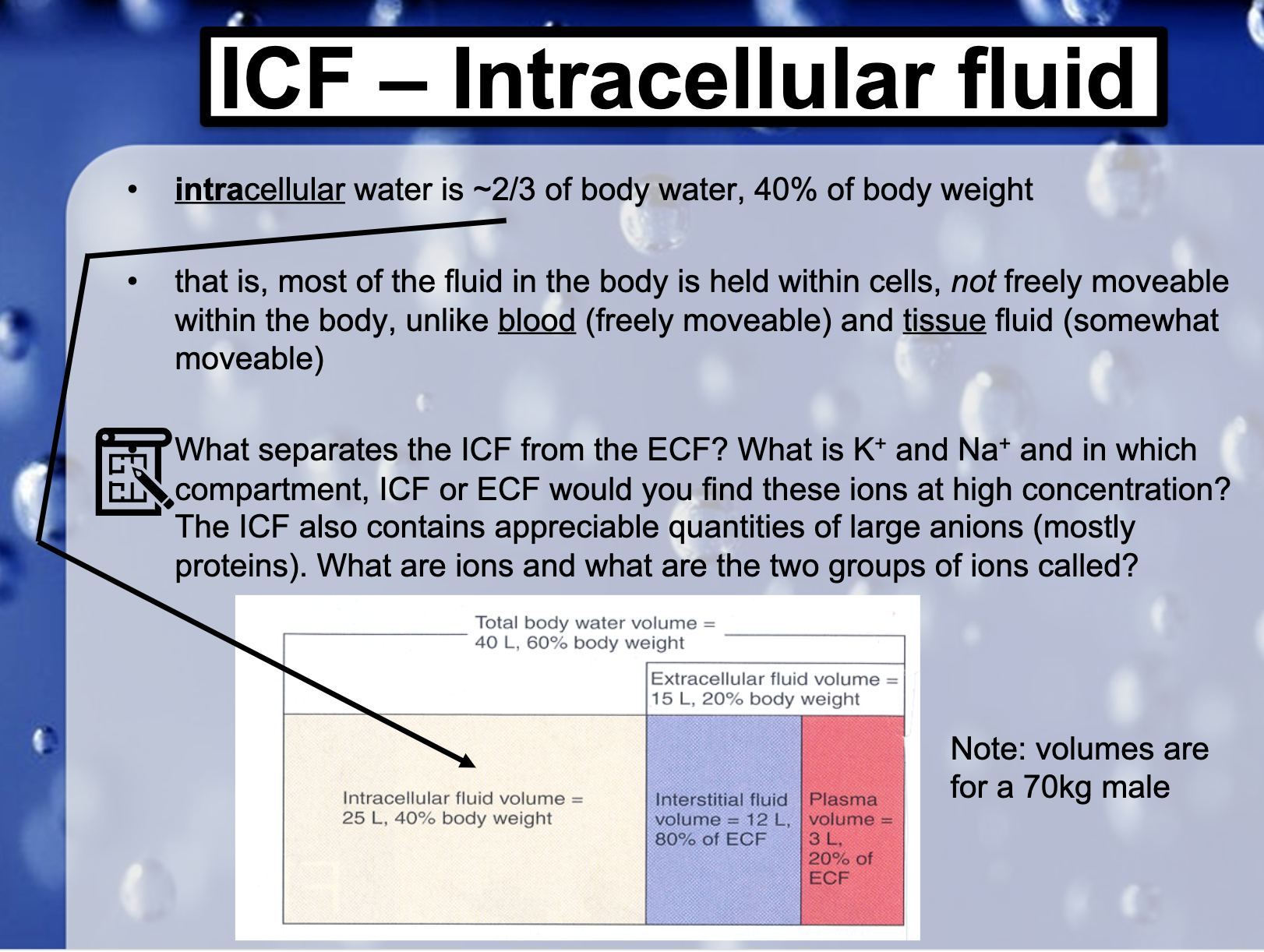
Interstitial Fluid, intercellular fluid is drained from the interstitum by lymphatic vessels
*What seperates the ICF from the ECF ?
Cell membrane (semi-permeable : permeable to water but to most solutes including electrolytes and proteins, which generally need transport system to move across the membrane) seperates ICF and ECF.
Water can pass through biological membranes via two pathways: simple diffusion through the lipid bilayer, or water-selective facilitated diffusion through aquaporins (AQPs).
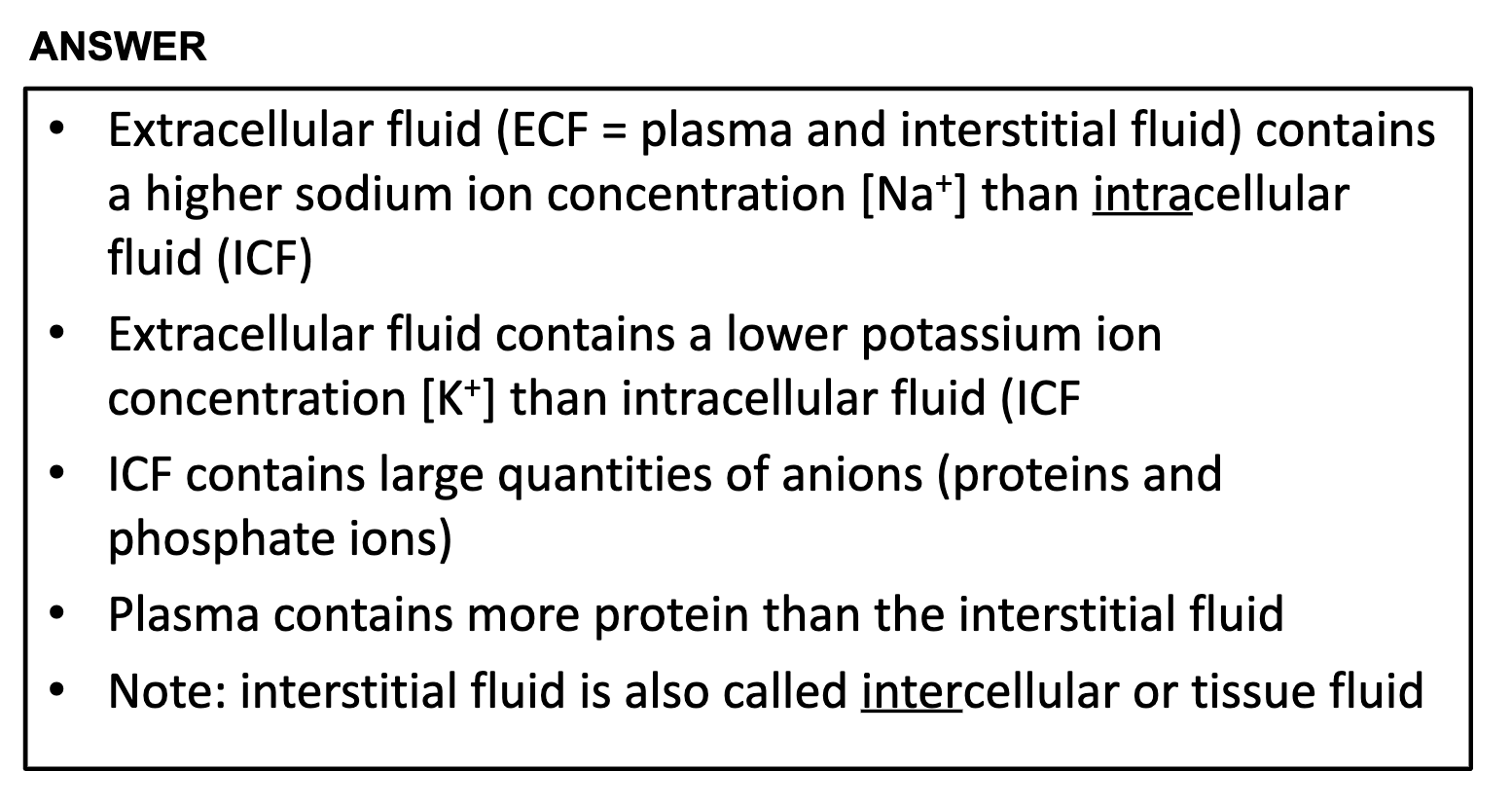
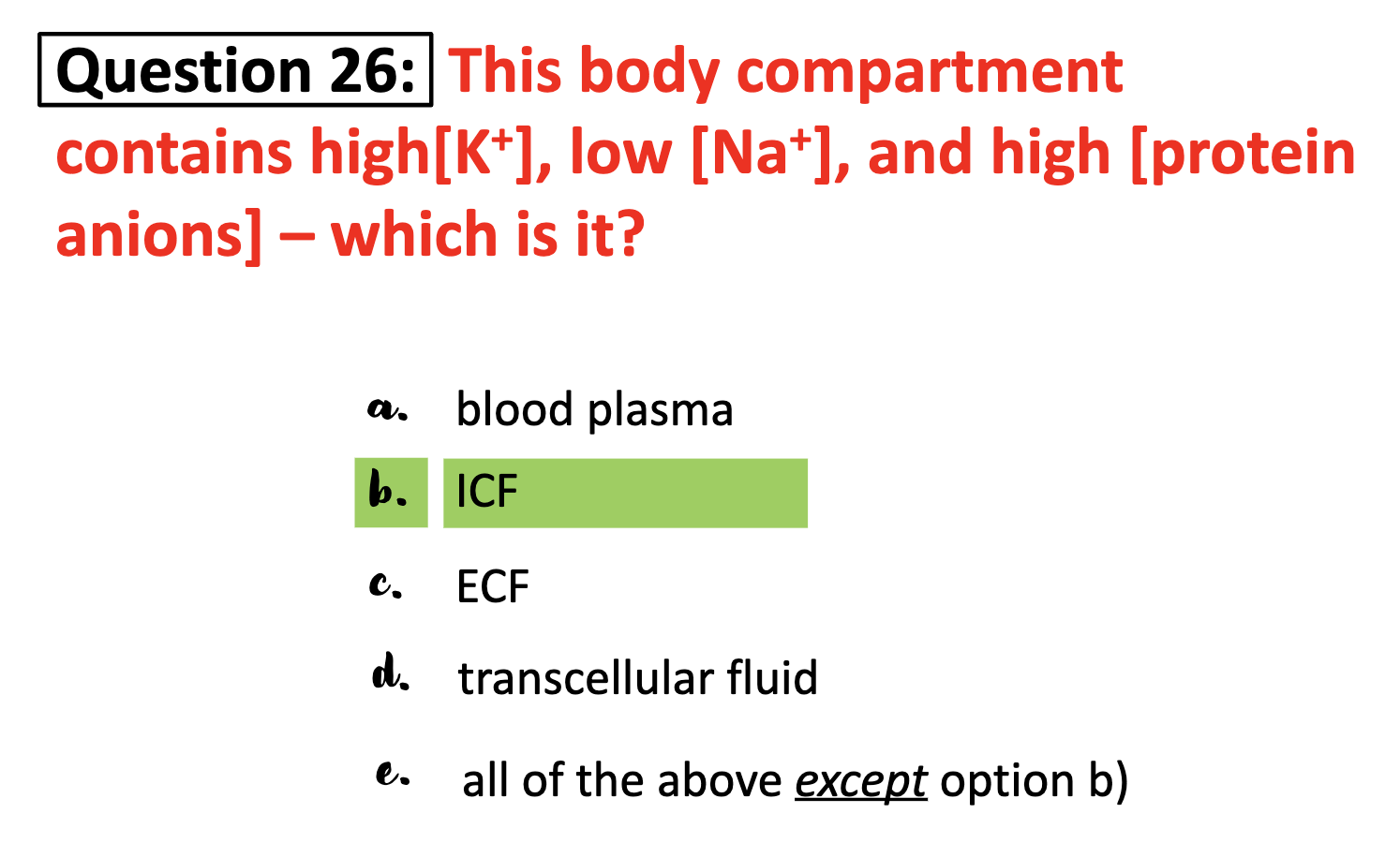
*What is K+ and Na+ and in which compartment, ICF or ECF would you find these ions at high concentration?
K+ is abundant in intercellular fluid and Na+ is at high concentration in extracellular space.
*The ICF also contains appreciable quantities of large anions (mostly proteins)
What are ions and what are the two groups of ions called?
Ions are the the state of charged atoms. An atom can lose electrons and become cation or gain electrons and become anion.
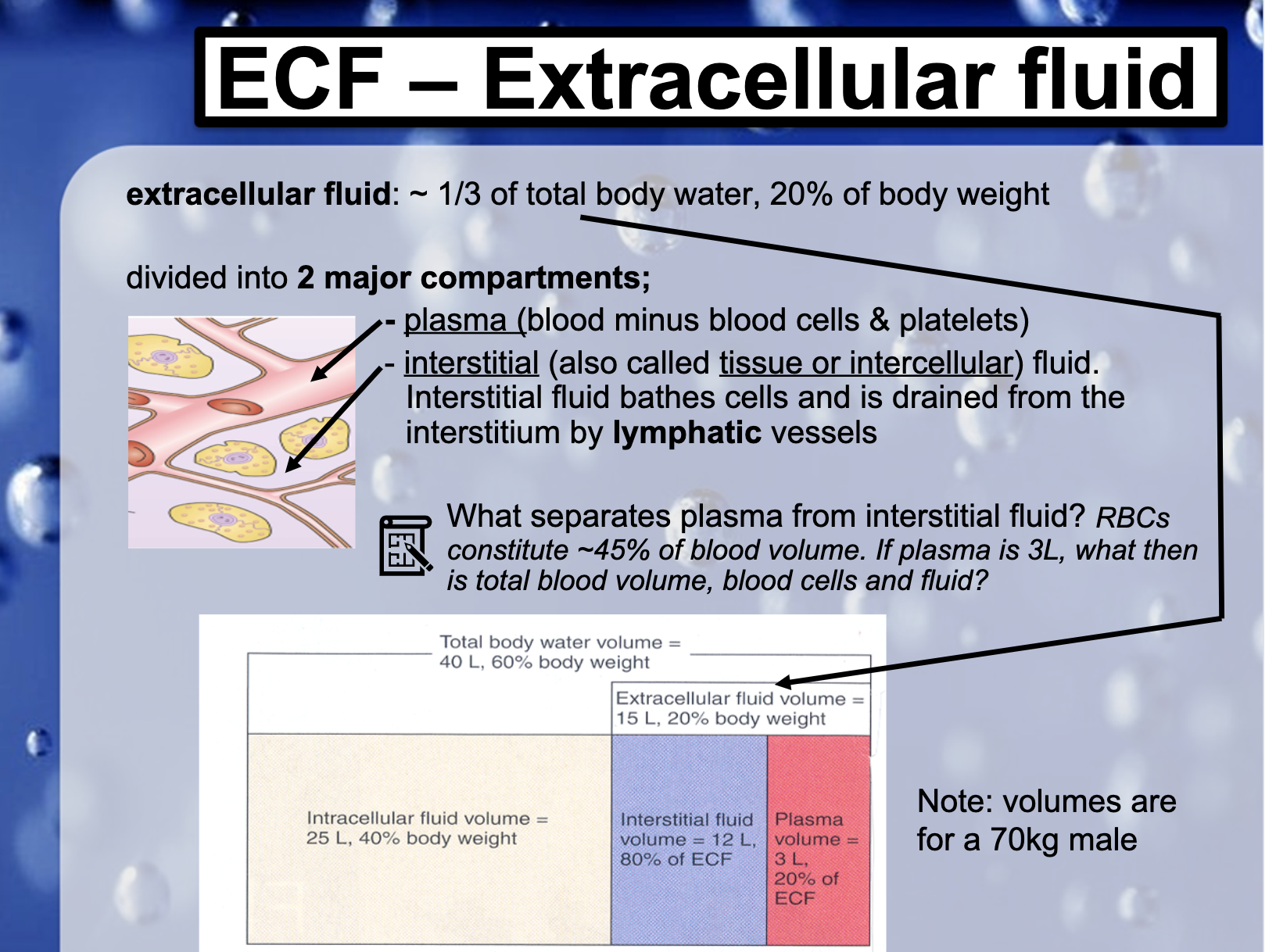
*What seperates plasma from interstial fluid?
Capillary endothelium (-Epithelial tissue) seperates intercellular space and the blood vessels.
*RBCs constitute ~45% of blood volume. If plasma is 3L, what then is total blood volume, blood cells and fluid?
About 5.5L is the total volume of blood.
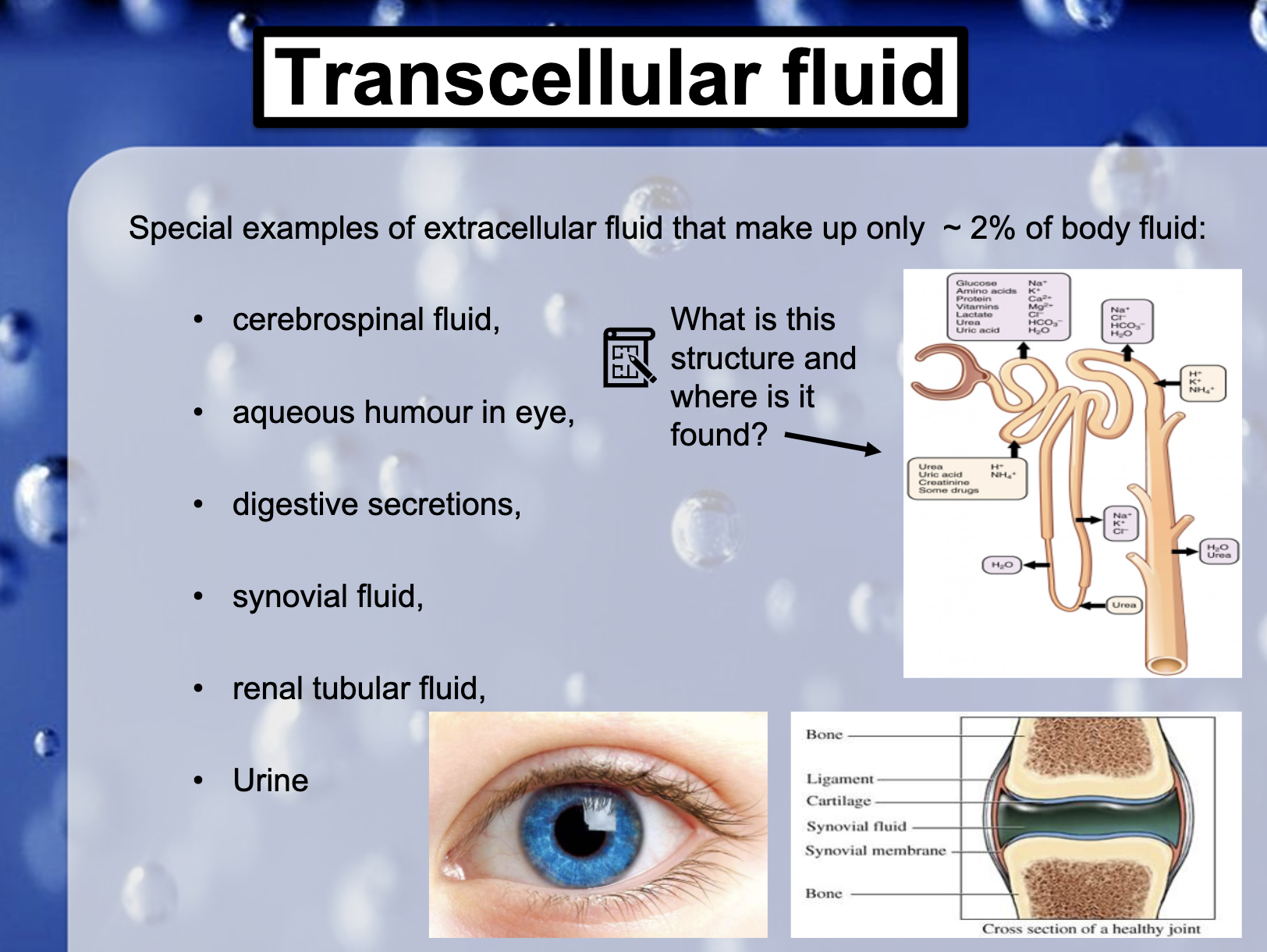
*What is this structure and where is it found?
It is the filtering unit of urinary system in kidney and it is called 'nephron'.
*Transcellular fluid = the portion of total body water contained within the epithelial spaces (2%)
-cerebrospinal fluid (뇌척수액, 뇌와 척수 및 뇌실을 채우고 있는 액체)
-aqueous humor in eye (우리 눈의 각막과 수정체 사이에 있는 공간에서 순환하는 맑은 액체)
-digestive secretions (소화액)
-synovial fluid (관절윤활액)
-renal tubular fluid (신장 관에 있는 액체)
-urine (오줌)
BODY FLUIDS AND ELECTROLYTES https://www.youtube.com/watch?v=JGF6ry0SWPs
❤️65% of mass is body fluids.
The percentage may differ by physiological characteristics.
(Female, individuals having more fat-> tend to have less body fluid)
(Fat carries a lot less water compared to muscle)
(Infants have higher BF percentage than the elderly)
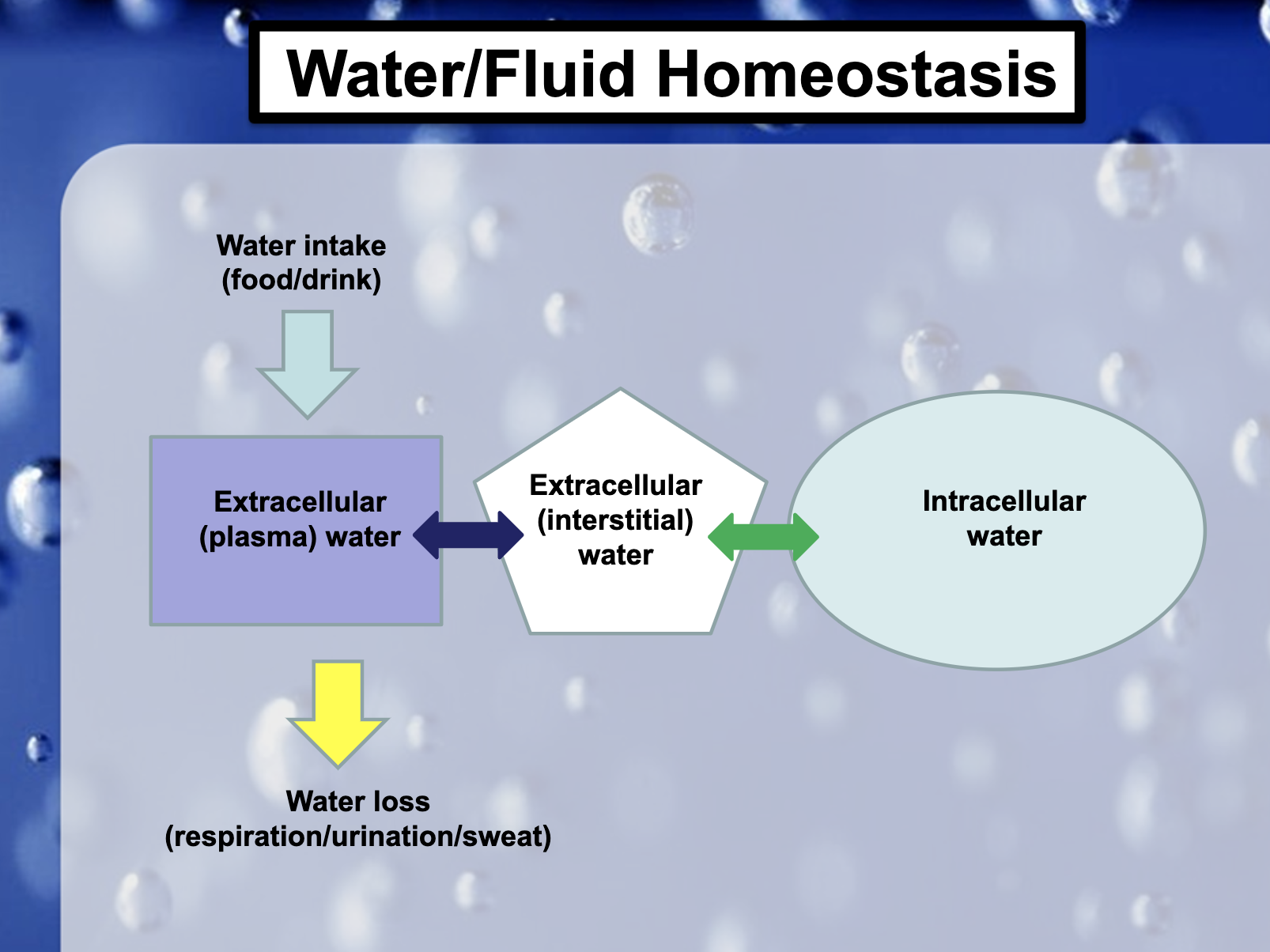
❤️BF -> Three different places to sit
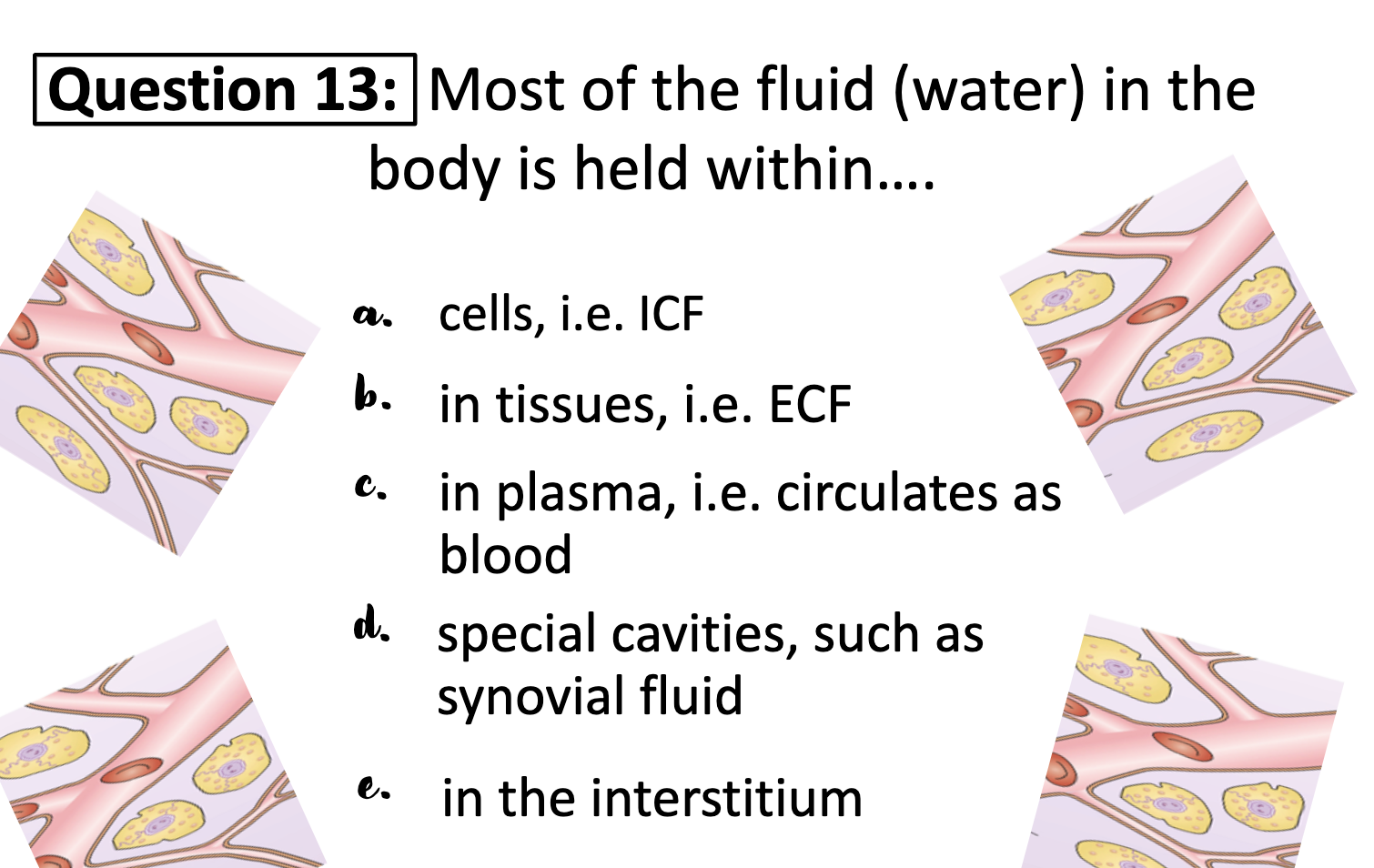
1. Within the cell (Intracellular Fluid ICF)
2. Within the blood vessels (=blood plasma) (Extracellular fluid ECF)
3.Between the cell and the blood vessels (= interstitial fluid) (ECF)
❤️Holes in the blood vessels allow interchanging ions with interstitial fluid
(Ions, solute통과가능/ RBC,WBC, proteins can’t pass through)
❤️Ions in Interstitial fluid = Na+, Ca2+, Cl-, HCO3-
❤️Ions in intracellular fluid = K+, Mg2+, PO43-,anion proteins
❤️Cell <-> interstitial fluid
Ions can’t permeate cell membranes by themselves ,Channels enable them to interchange
Water can be transferred between the two
Concentration of two compartments are different
But 290mOsm of concentration should be matched by each compartment
❤️Interstitial fluid <-> blood vessels
Blood vessels allow interchanging ions with interstitial fluid
Concentration is same in ISF and BP
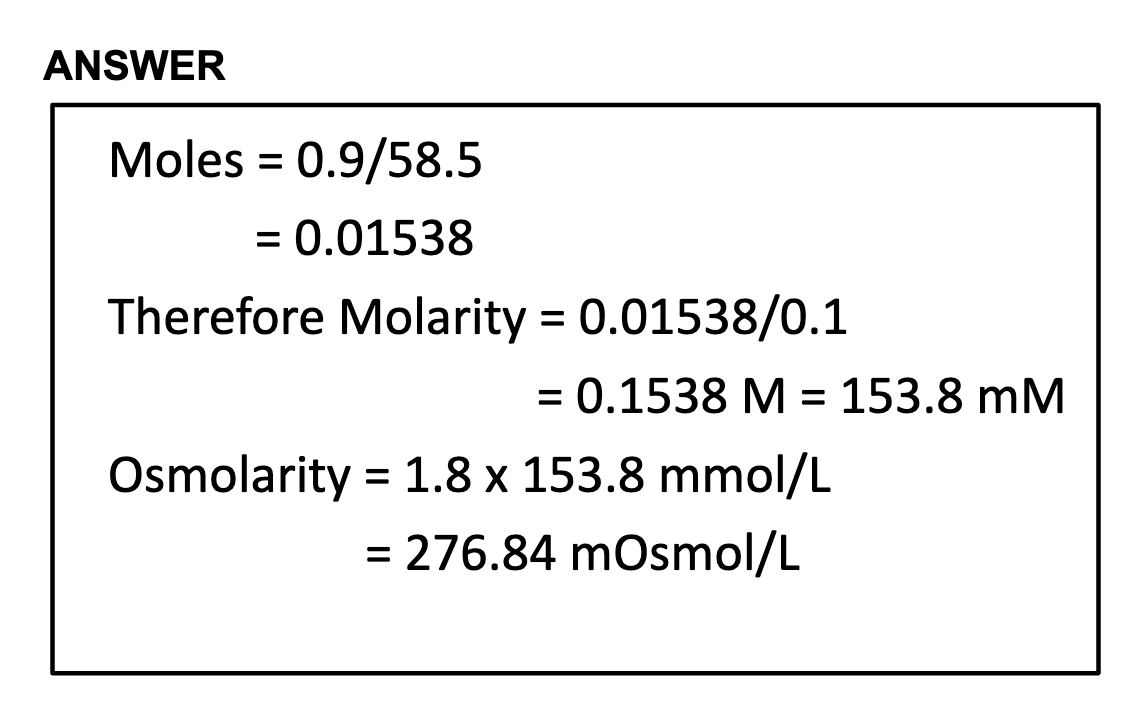
Osmolarity calculation
= 2(Na+) + 2(K+) + Glucose + urea
= 2(135mmol/L) +2(5mmol/L) + 5+ 5
= 290mOsm
❤️Don’t drink water for so long-> ECF drops
Water level dropped but the solute are still same amount -> increase in osmolarity (290mOsm->320mOsm)-> water,ICF comes out (Water moves to the high concentration of the solute) -> cell shrinks
FLUID COMPOSITION

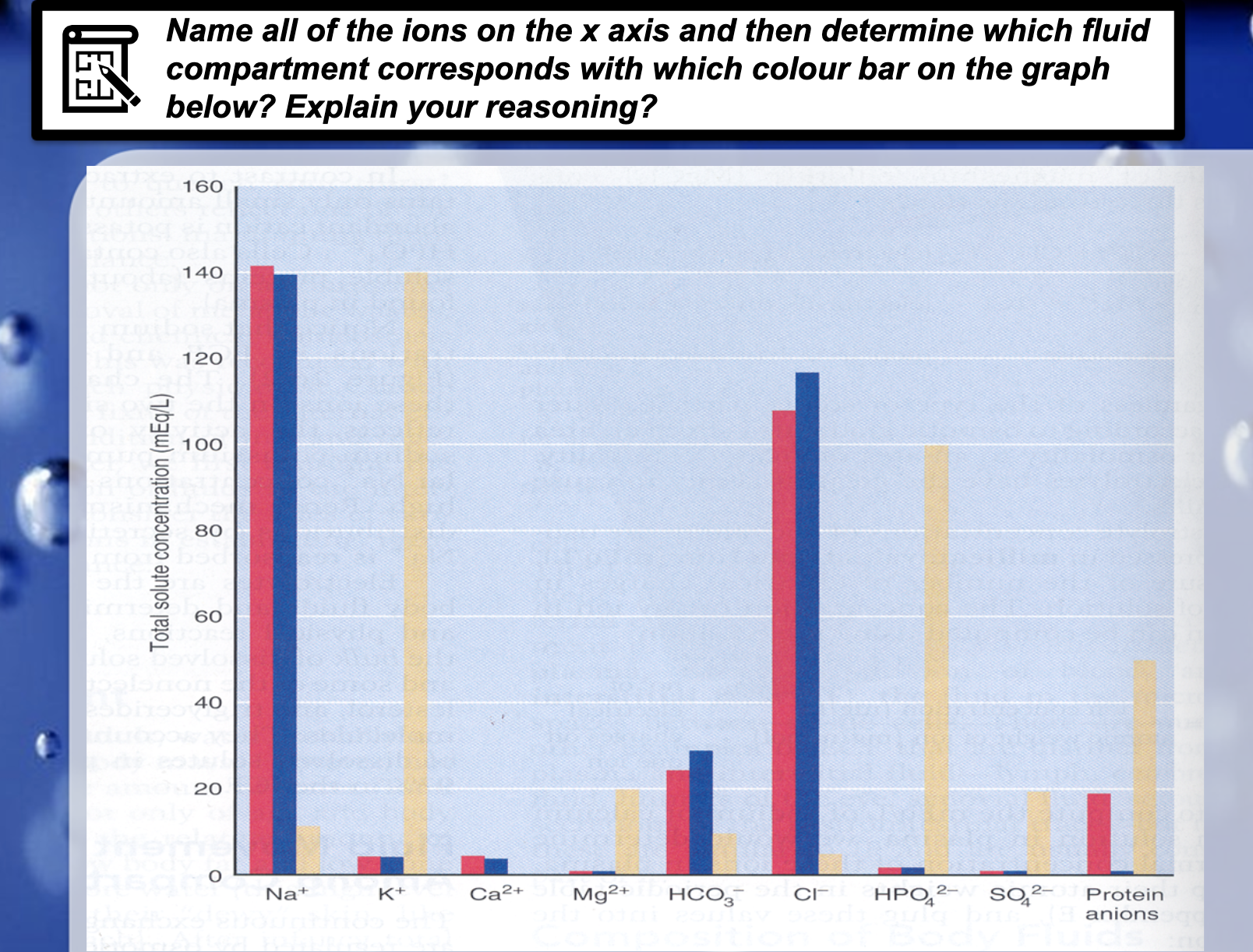
*Concentration of the electrolytes decide the movement of the water across the cell membrane, which is osmosis.
*Electrolytes : 전해질이란 화학적으로 물 또는 극성용매 속에서, 양이온과 음이온으로 전리하여, 그 용액이 전도성을 나타내는 물질을 말합니다. 그러나 생리적 또는 영양적으로는 나트륨, 칼륨, 칼슘, 마그네슘과 같이 알칼리금속, 알칼리토금속에 속하는 미네랄로, 체내, 특히 혈액 속에서 전리하여 생리적 작용을 나타내는 것을 말합니다.
*K+, Mg2+, PO42-,SO42-, protein anions are at high concentration in intracellular fluid.
Na+, Ca2+, HCO3-, Cl- are the electrolytes which are abundant in extracellular fluid, which has plasma and intracellular fluid compartments.
Na+ sodium ion, K+ potassium ion, Ca2+ calcium ion, Mg2+ magnesium ion, HCO3- Bicarbonate,
HPO42- Hydrogenphosphate, SO42- sulfate, Protein anions
-simple diffusion
-facilitated diffusion (requires protein transporters)
-active transport (requires protein transporters and consumption of energy)
DIFFUSION

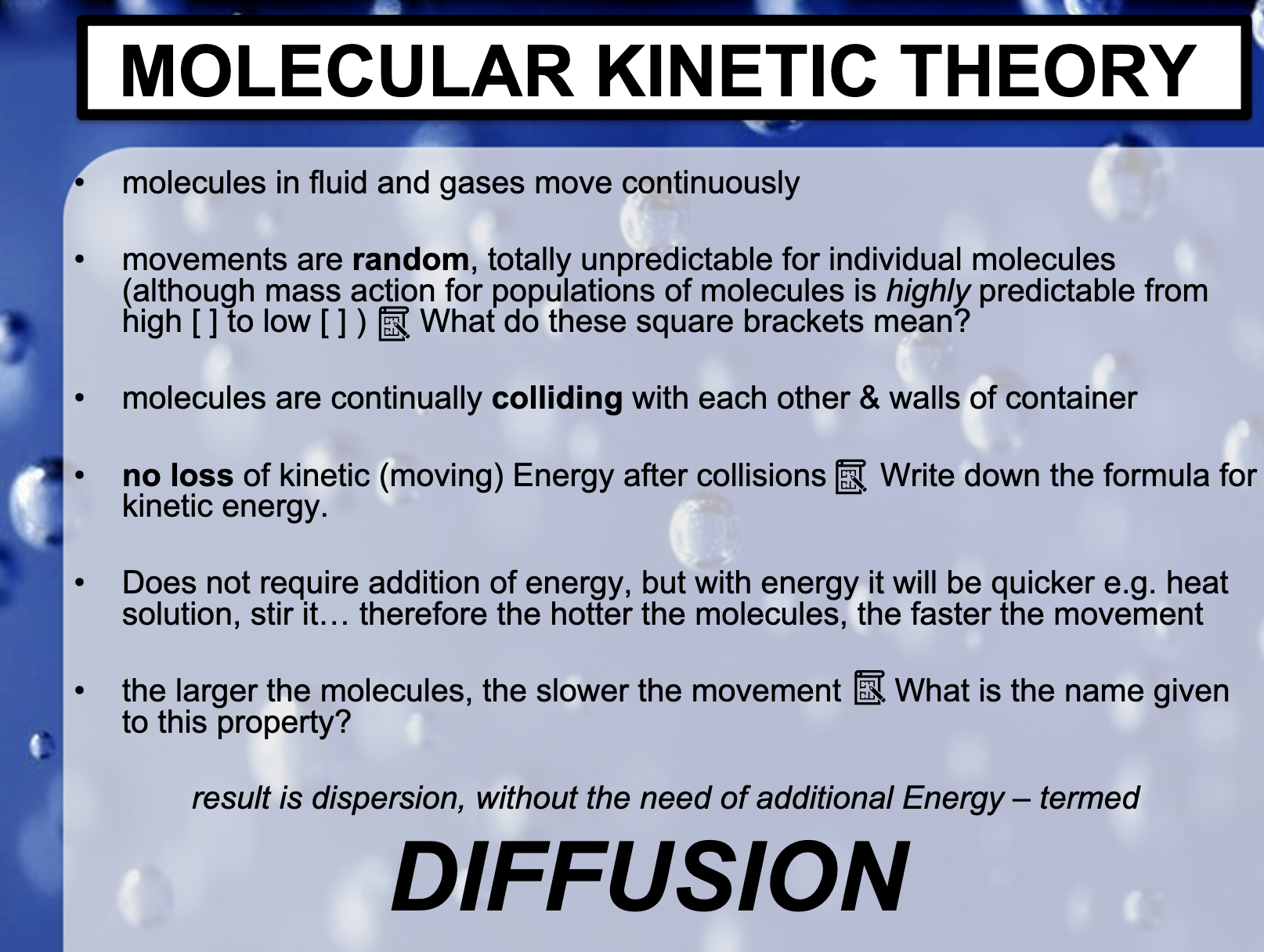
-> 1. The sugar dissolves without any extra effort.(Diffusion) 2. No it isn't, It needs much more energy to reconsitute the sugar cube. 3. No.
*Diffusion occurs by the gradient of the solutes and doesn't need any energy.
*What do these square brackets mean? [] shows the concentration of the solution.
*Write down the formula for kinetic energy.
The amount of kinetic energy in a moving object depends directly on its mass and velocity. It can be calculated with the equation: KE = {(0.5) x mass} × velocity ^2
*Diffusion doesn't require addition of energy, but with energy it will be quicker
(heat solution, therefore the hotter the molecules, the faster the movement.)
Factors that affect the rate of diffusion !!!
1. concentration gradient
2. mass of molecules
3. temperature
4. permeability of membrane
5. density of solvent
6. solubility
7. surface area
8. distance travelled
*Since all gases have the same average kinetic energy at the same temperature, lighter molecules move faster and heavier molecules move slower on average.
*Diffusion becomes faster when the molecule is lighter and at higher temperature.
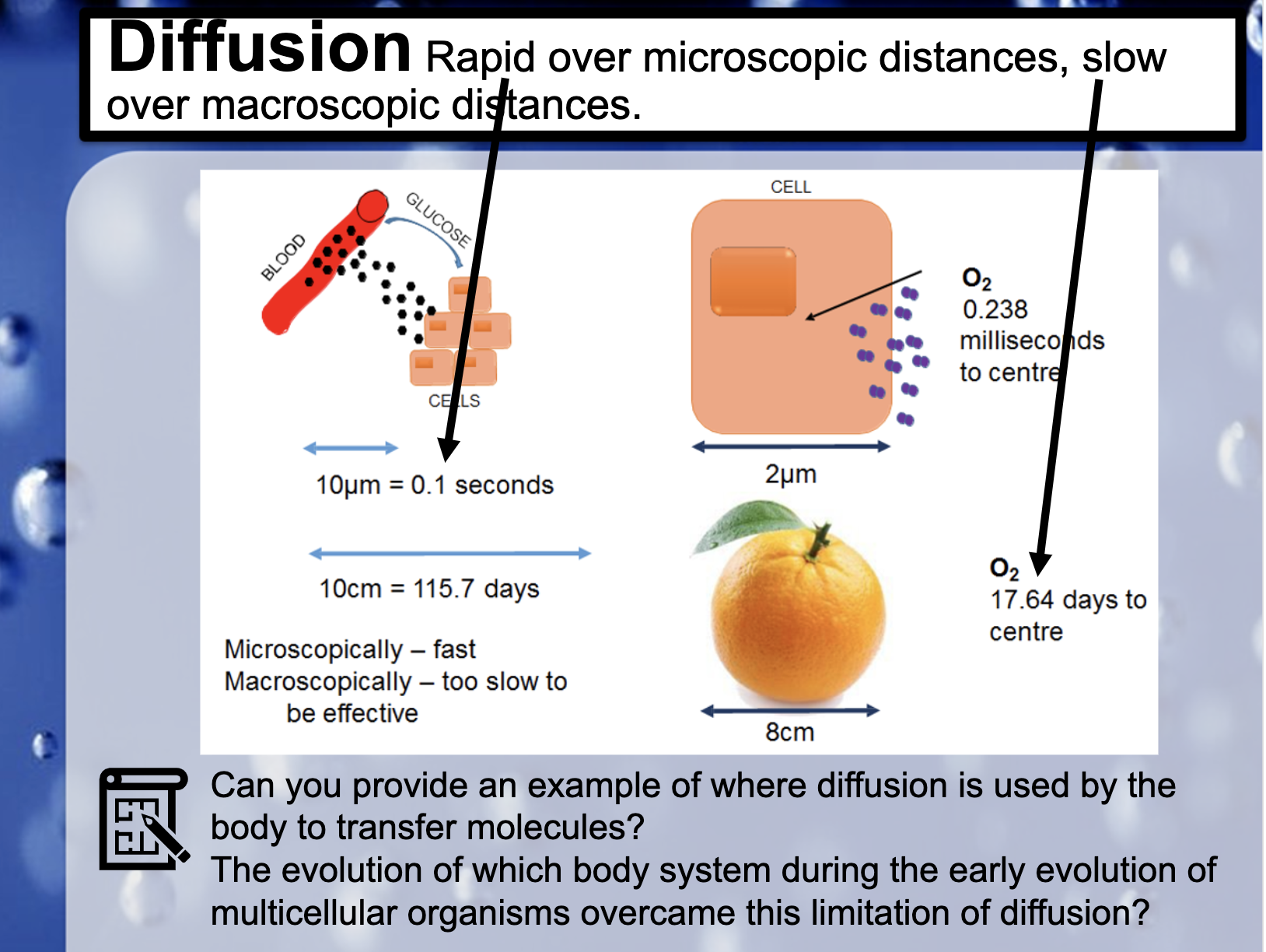
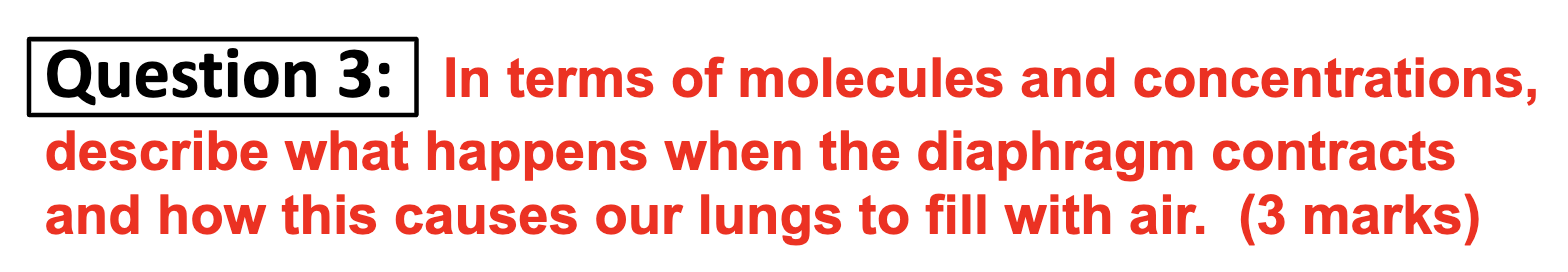
*Can you provide an example of where diffusion is used by the body the body to transfer molecules?
Within the lung and blood vessels, O2 diffuse into the blood vessel from the lungs and CO2 is diffused into the lungs to get out of the body.
*The evolution of which body system during the early evolution of multicellular organisms overcame this limitation of diffusion?
Ciculatory system ?? The endothelium evolved in an ancestral vertebrate some 540–510 million years ago to optimize flow dynamics and barrier function, and/or to localize immune and coagulation functions. Finally, we emphasize that endothelial heterogeneity evolved as a core feature of the endothelium from the outset, reflecting its role in meeting the diverse needs of body tissues.
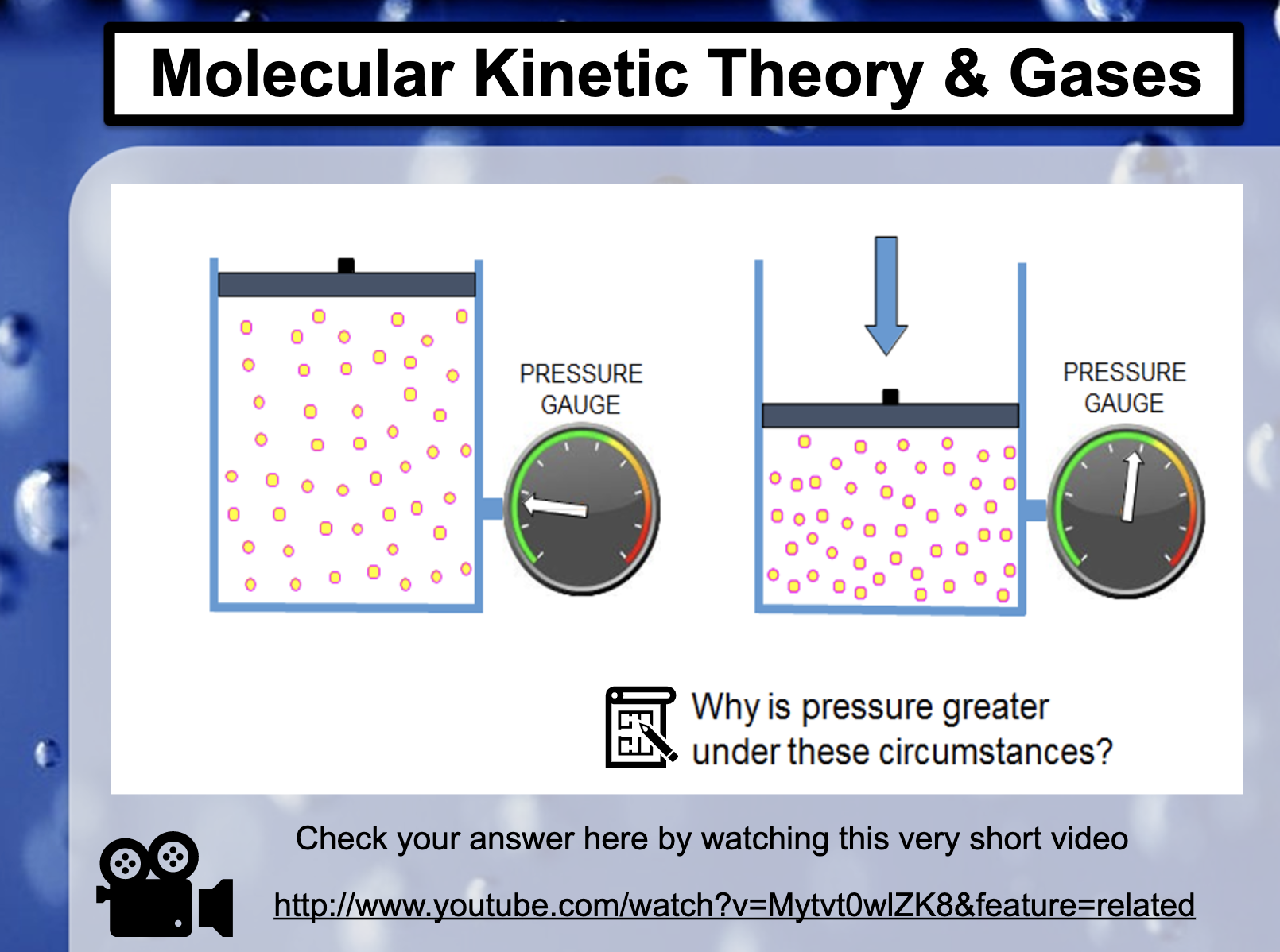
*Why is pressure greater under these circumstances?
The pressure increases when the same amount of atoms are trapped in a smaller volume. It increases rate of the collisions against the wall of the cylinder.
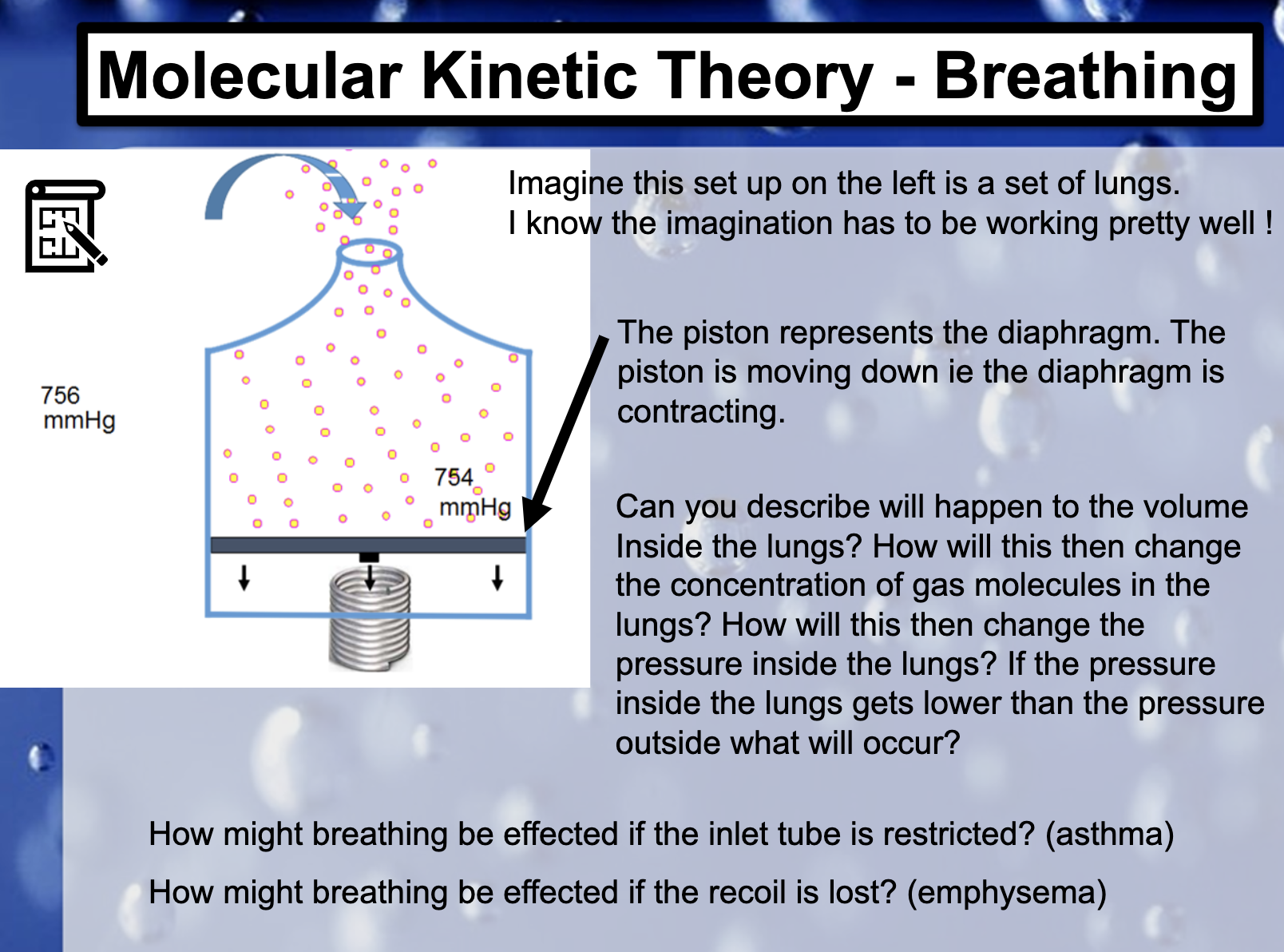
*asthma (inlet tube is restricted)
A condition in which a person's airways become inflamed, narrow and swell and produce extra mucus, which makes it difficult to breathe.
*emphysema (the recoil is lost)
Emphysema is a lung condition that causes shortness of breath. In people with emphysema, the air sacs in the lungs (alveoli) are damaged. Over time, the inner walls of the air sacs weaken and rupture — creating larger air spaces instead of many small ones.
폐를 이루고 있는 허파꽈리가 파괴되어 산소 접촉 표면적이 줄어들고 폐의 탄력성이 저하되어 영구적인 기도폐쇄를 일으키는 질환이다.
IDEAL GAS LAW https://www.youtube.com/watch?v=8SRAkXMu3d0
❤️temperature/pressure/number of atoms/volume
❤️Volume of the cylinder decreases under constant temperature and number of atoms
The pressure increases due to the increased rate of the collisions against the wall of the cylinder
❤️Constant volume and number of atoms
Increasing the temperature result in increased kinetic energy for the atoms -> increase in both temperature and pressure/ Removing the heat source -> allow kinetic energy to dissipate atoms to surroundings , decrease in pressure
❤️With constant Pressure and temperature, Additional atoms can be added -> increased in volume
/Double the atom, Doubled the volume
OSMOSIS
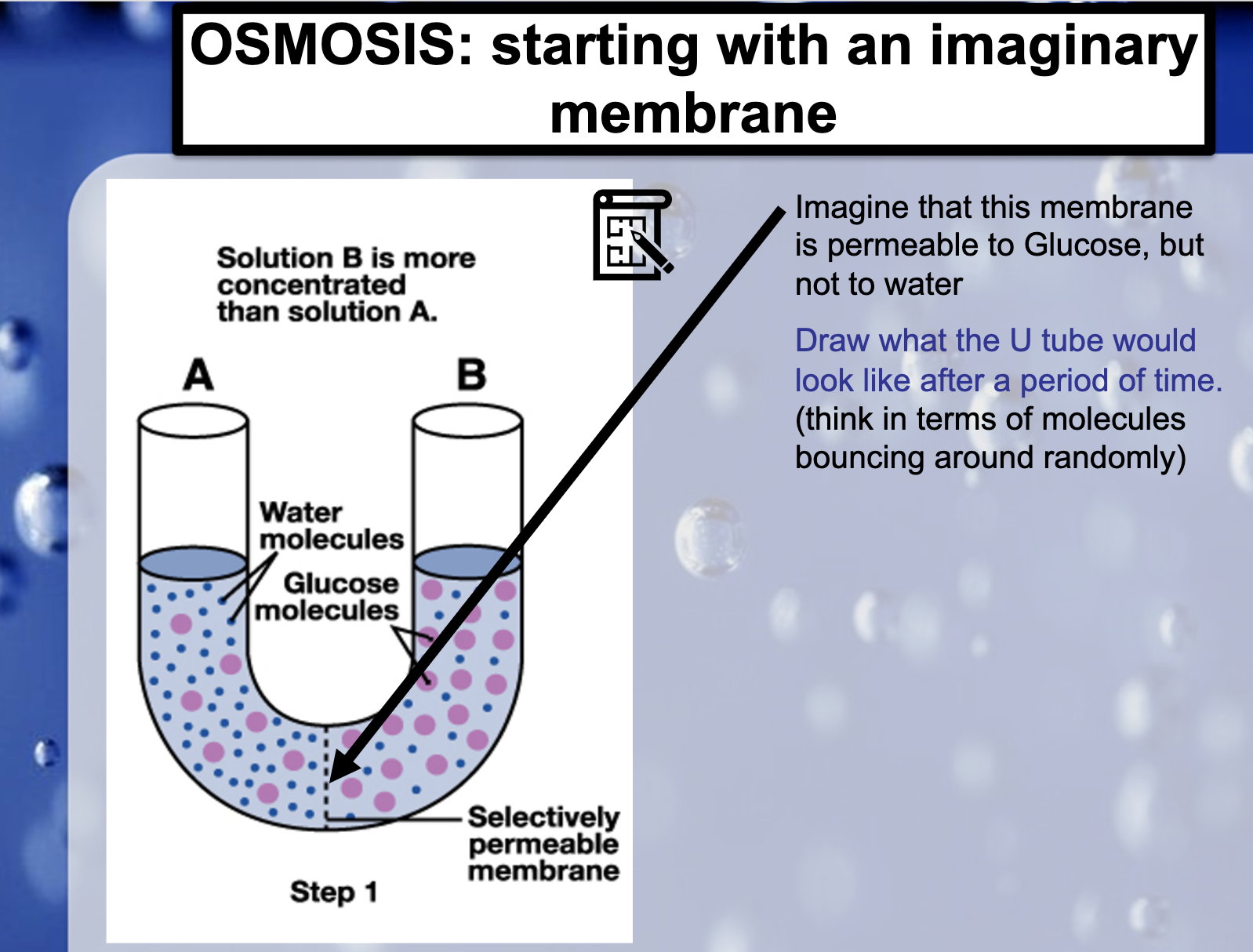
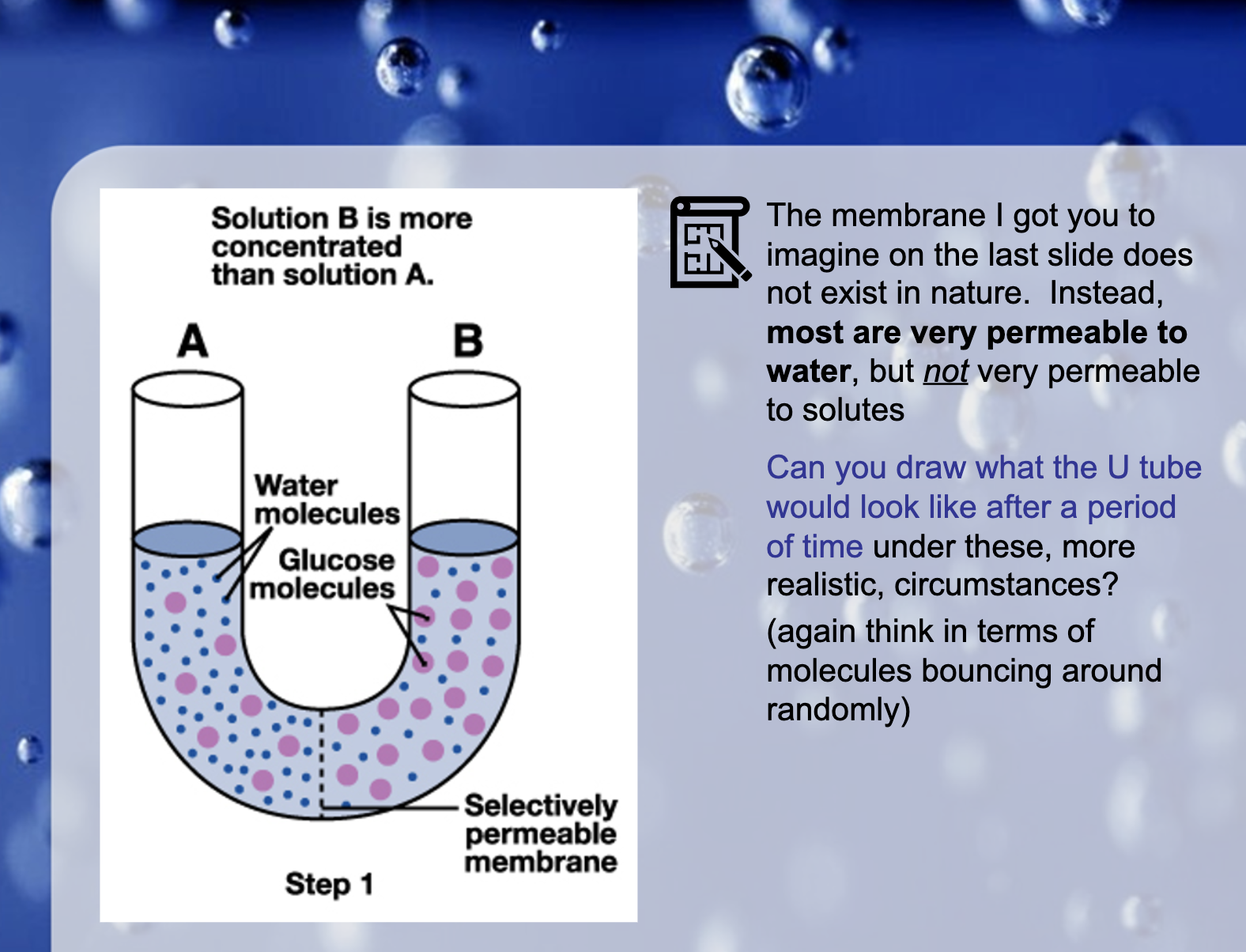
cell membrane 에는 CO2, O2 같이 무극성 작은 분자들은 통과 가능/ 작은 극성분자 H2O는 통과 가능/ 큰 극성 분자 포도당같은 애들은 통과 불가.
Large molecules like glucose cannot pass through the membrane, so the water moves to the higher concentration.
Osmosis occurs. It is the movement of the water from high concentration solution to the lower concentration.
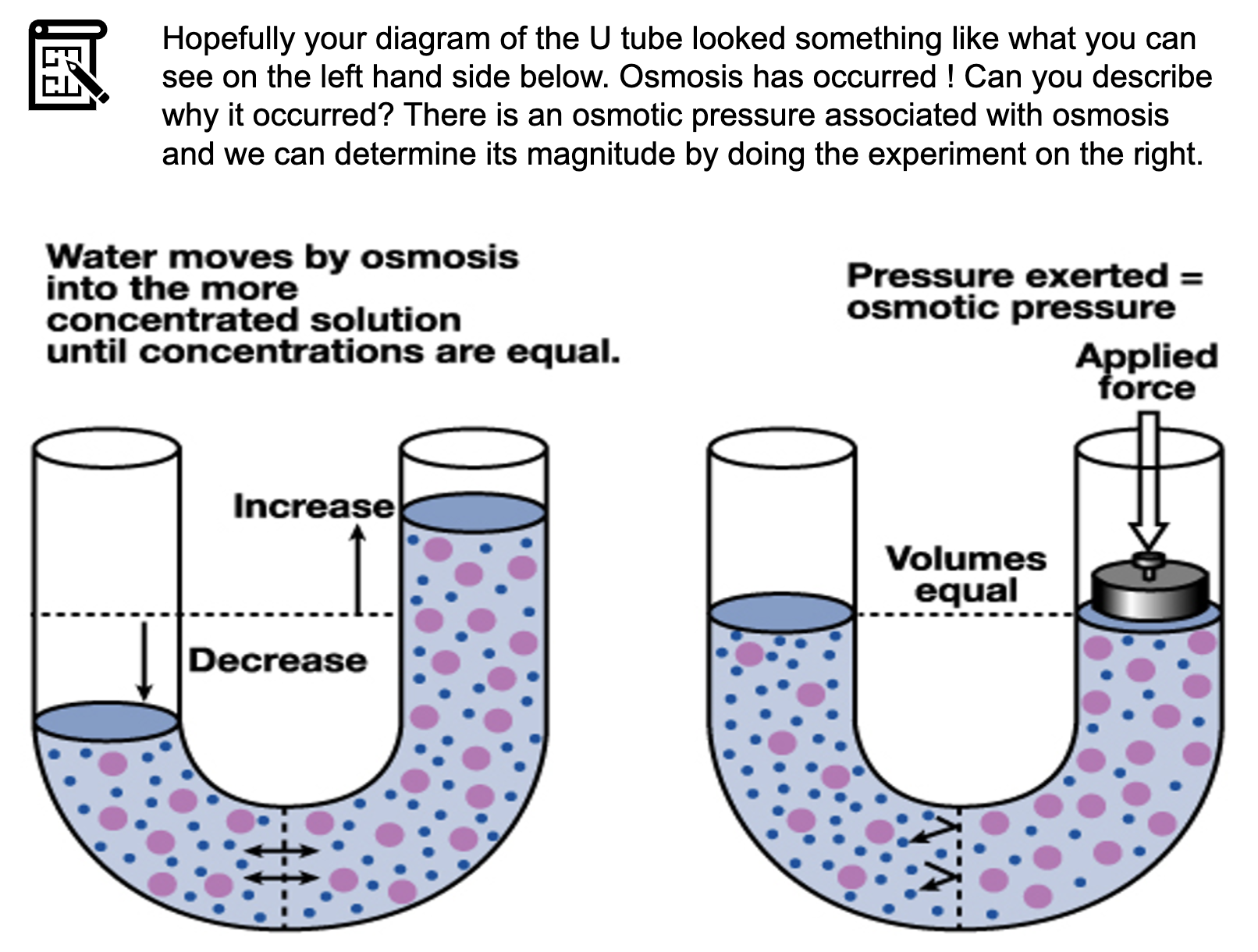
*Osmotic pressure
the pressure needed to stop osmosis
Osmotic pressure is a measure of the tendency of water to move into one solution from another by osmosis

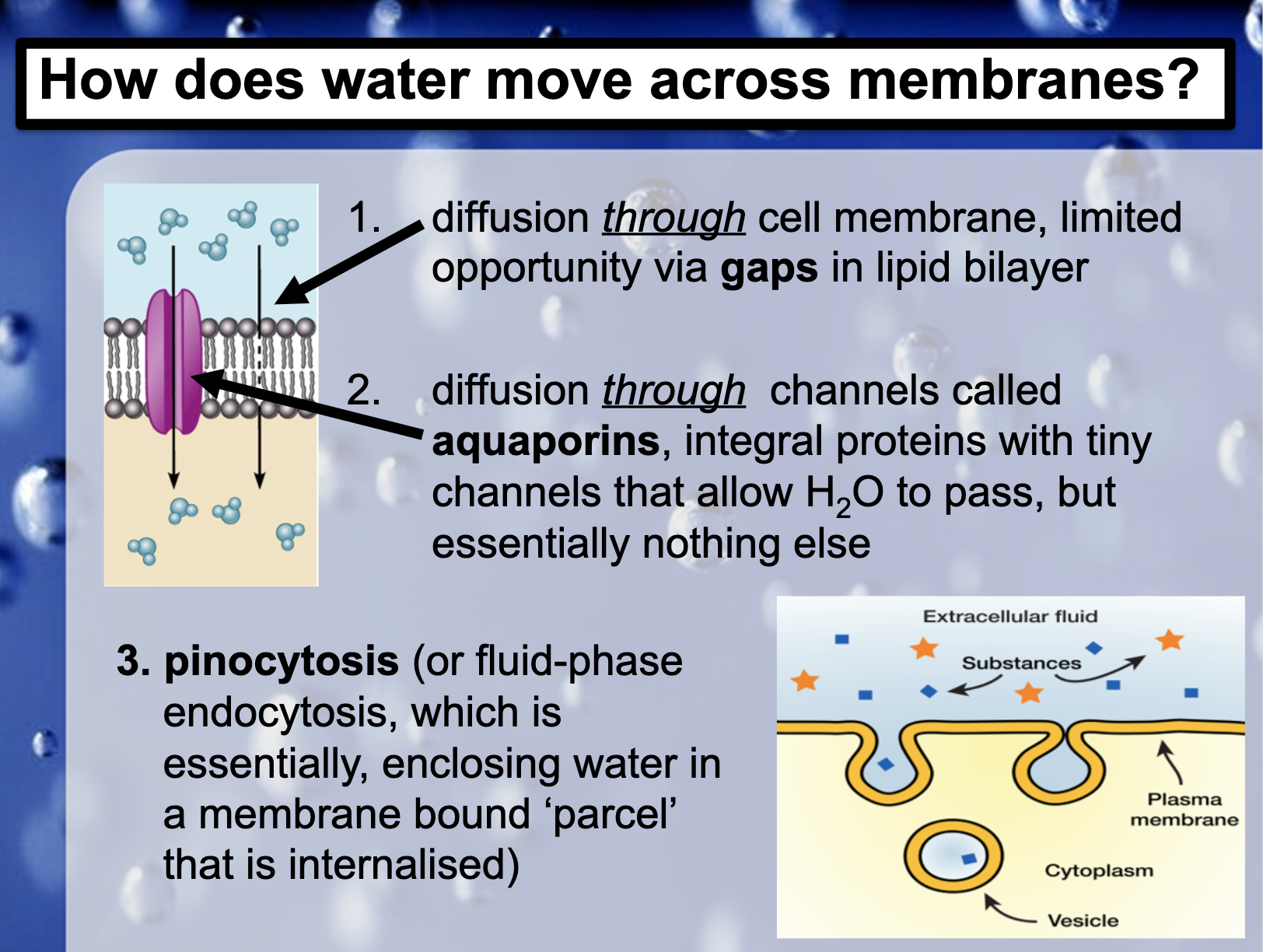
Water moves across membranes by..
(No ATP)
1. Simple diffusion by the small gaps in lipid bilayer (simple diffusion)
2. Aquaporin(integral proteins with tiny channel) (facilitated diffusion)
(ATP)
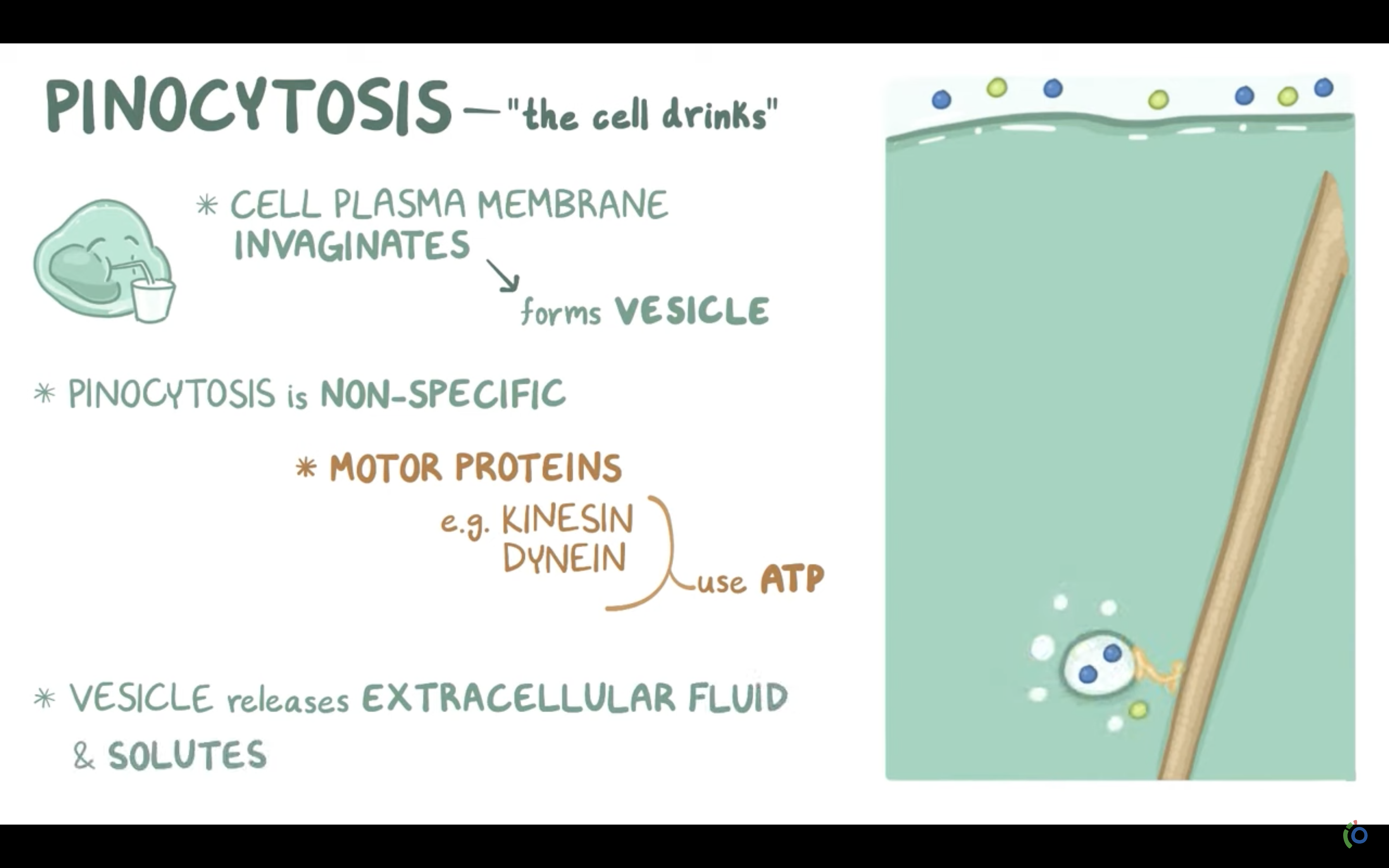
3. Pinocytosis(endocytosis of fluid, enclosing water in the membrane bound parcel) (endocytosis)

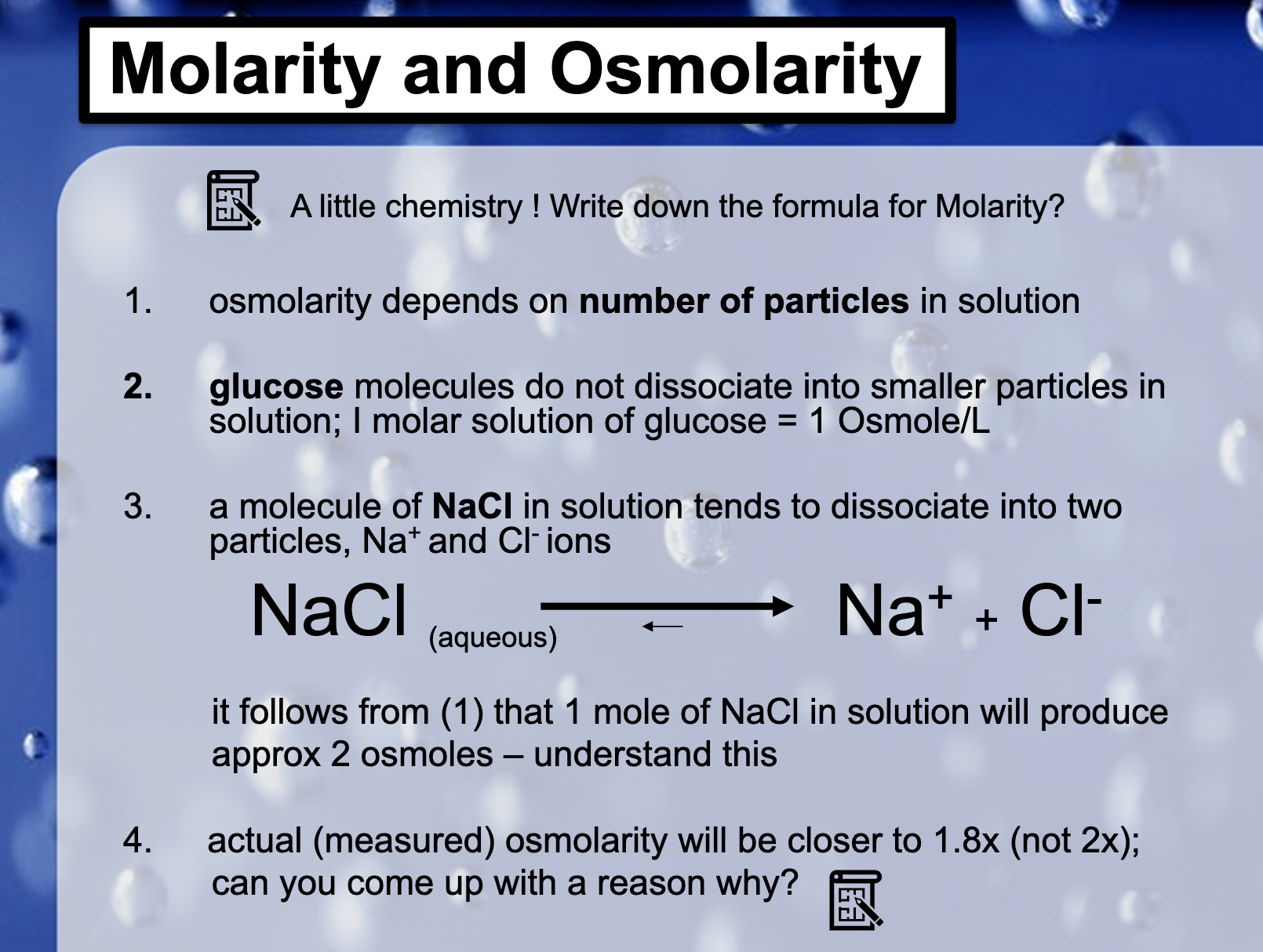
Solute is what is dissolved in a solution. (Not in molecules, DISSOLVED PARTICLES)
Osmolarity is decided by the concentration of the total solute. [solute]
Osmosis depends on the osmolarity (water moves from the high osmolarity to low osmolarity)
Osmolarity : Osmol, mOsm
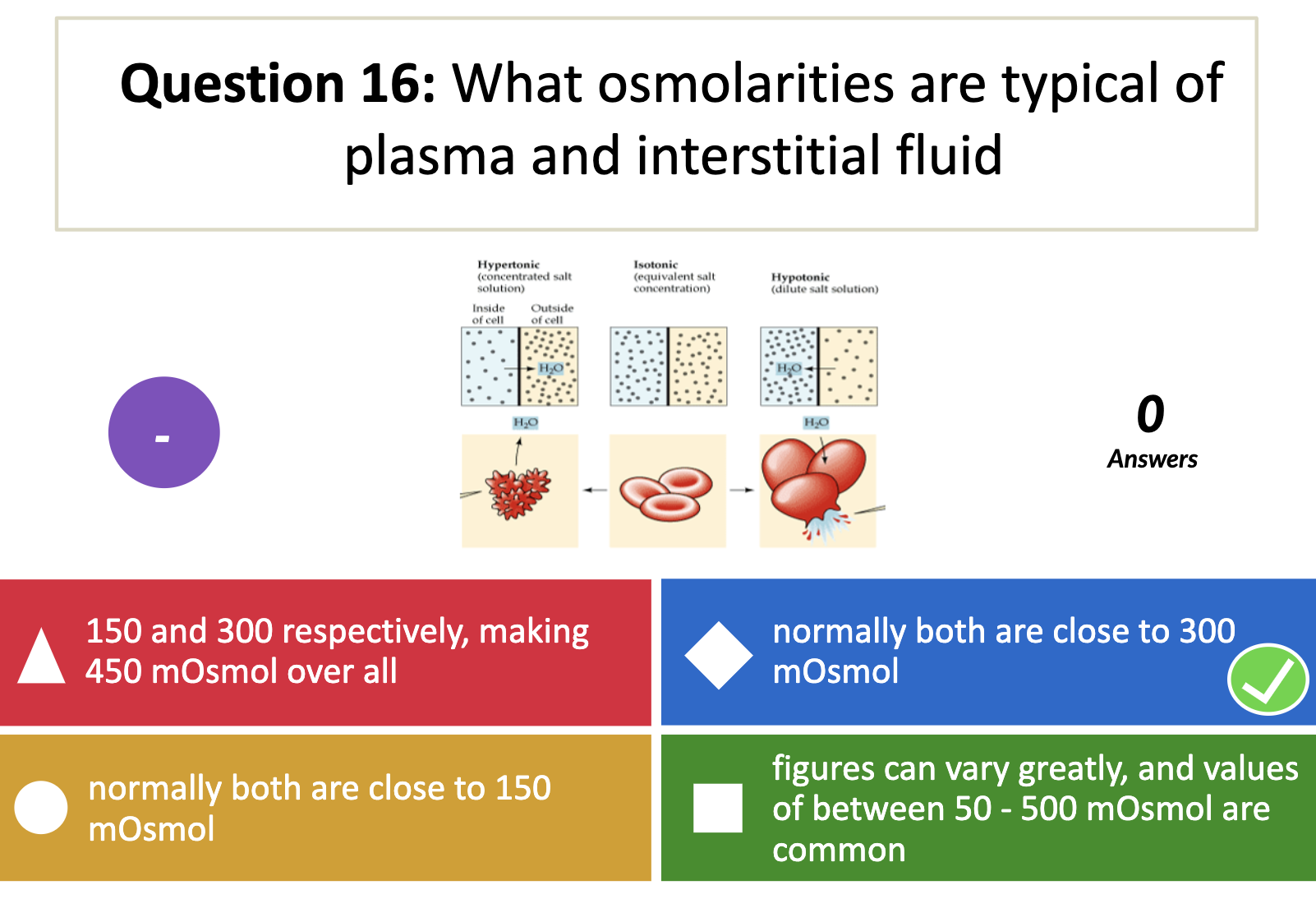
Intracellular fluid has the osmolarity at around 300mOsm
*Actual osmolarity is smaller than the expected osmolarity.
Because the total solvent weight (the divisor used for osmolality) excludes the weight of any solutes, whereas the total solution volume (used for osmolarity) includes solute content.
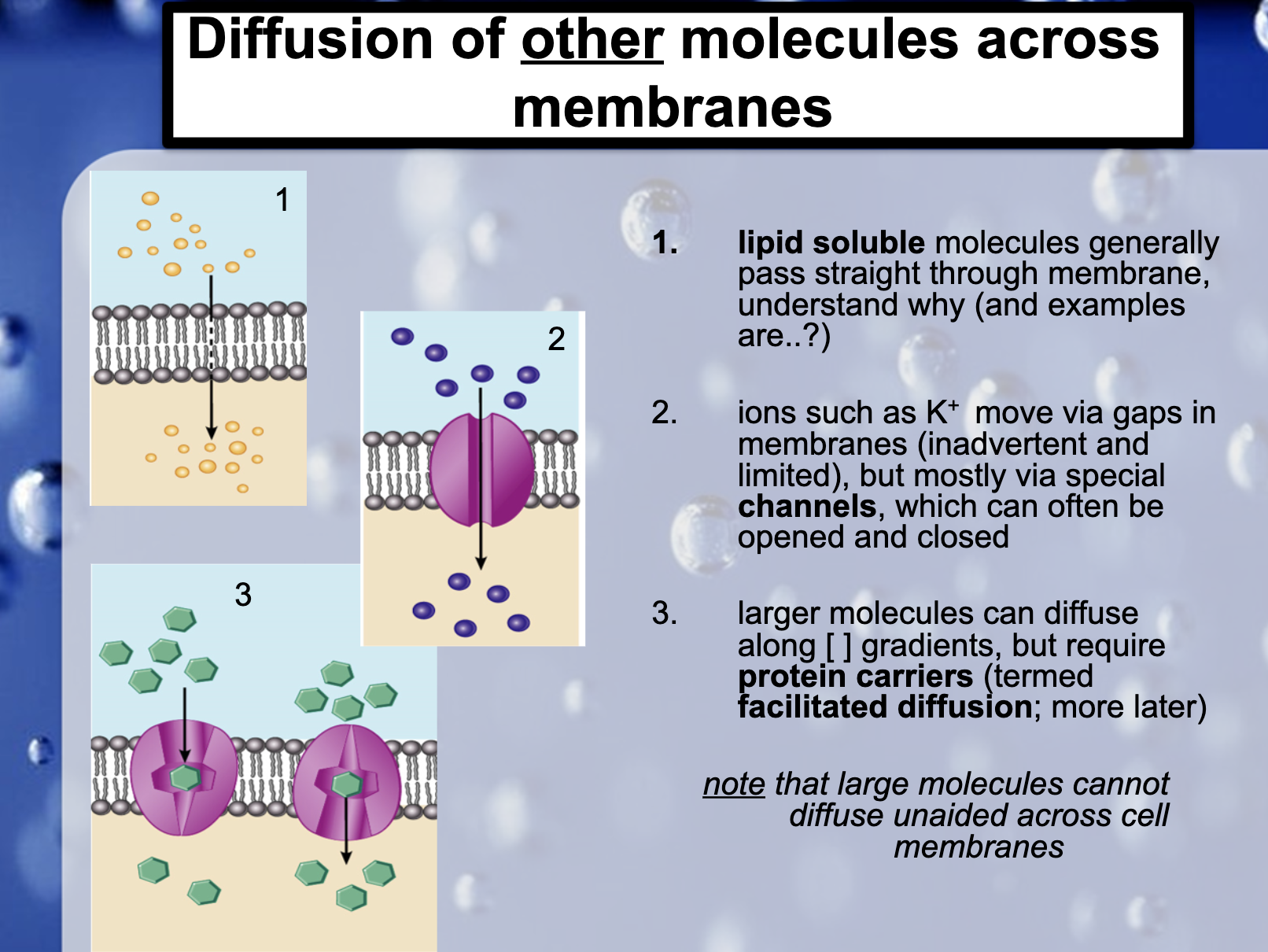
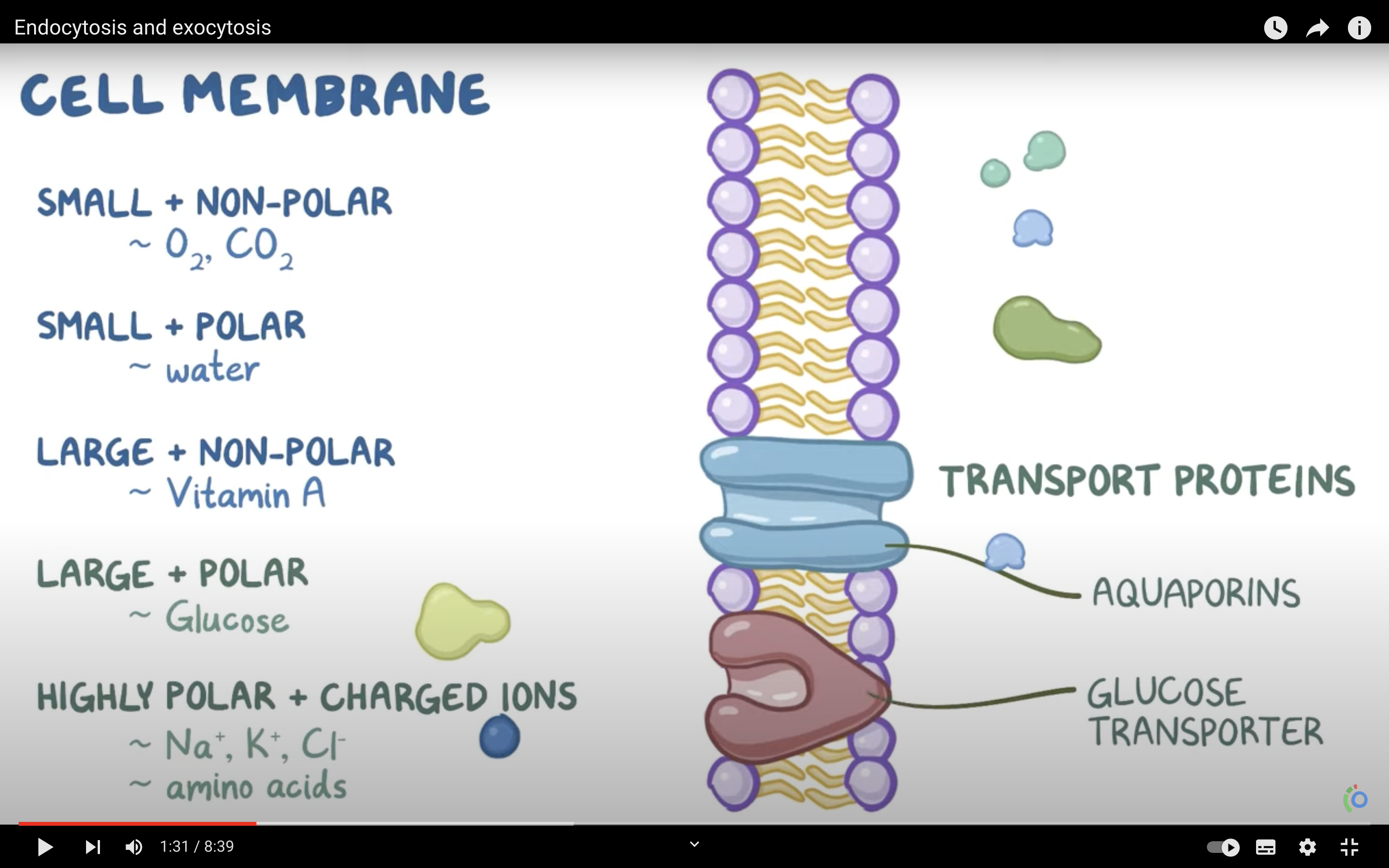
1. Lipid soluble molecules generally straight through membrane ,why? ( and example are..?)
Cell membrane is consist of phospholipid bilayers, where hydrophibic heads toward the outside of the cell.
As lipids are hydrophobic, they escape from the surface of the cell membrane and pass through the membrane.
2. Ions such as K+ move via gaps in membranes (inadvertent and limited), but mostly vias special channels, which can often be opened and closed
3. Larger molecules can diffuse along [] gradients, but require protein carriers (termed facilitated diffusion)
(Note that large molecules cannot diffuse unaided across cell membranes)
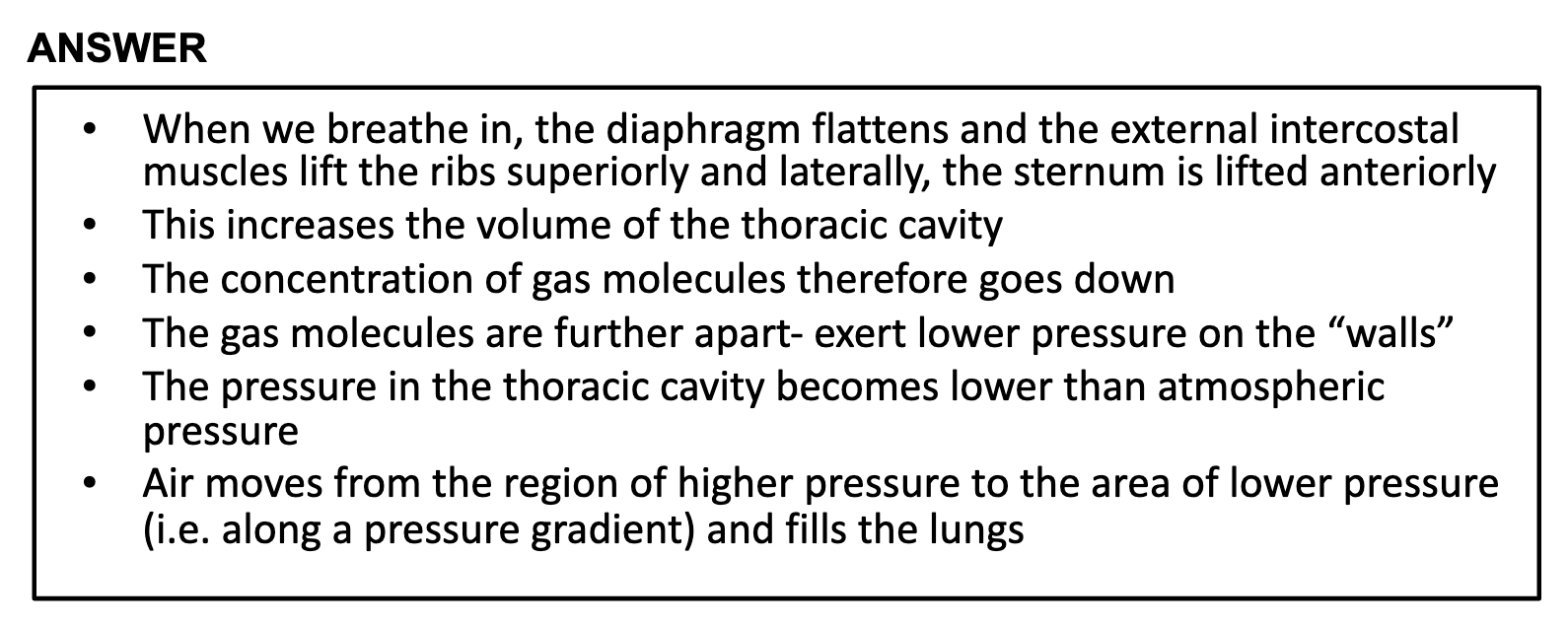
COP
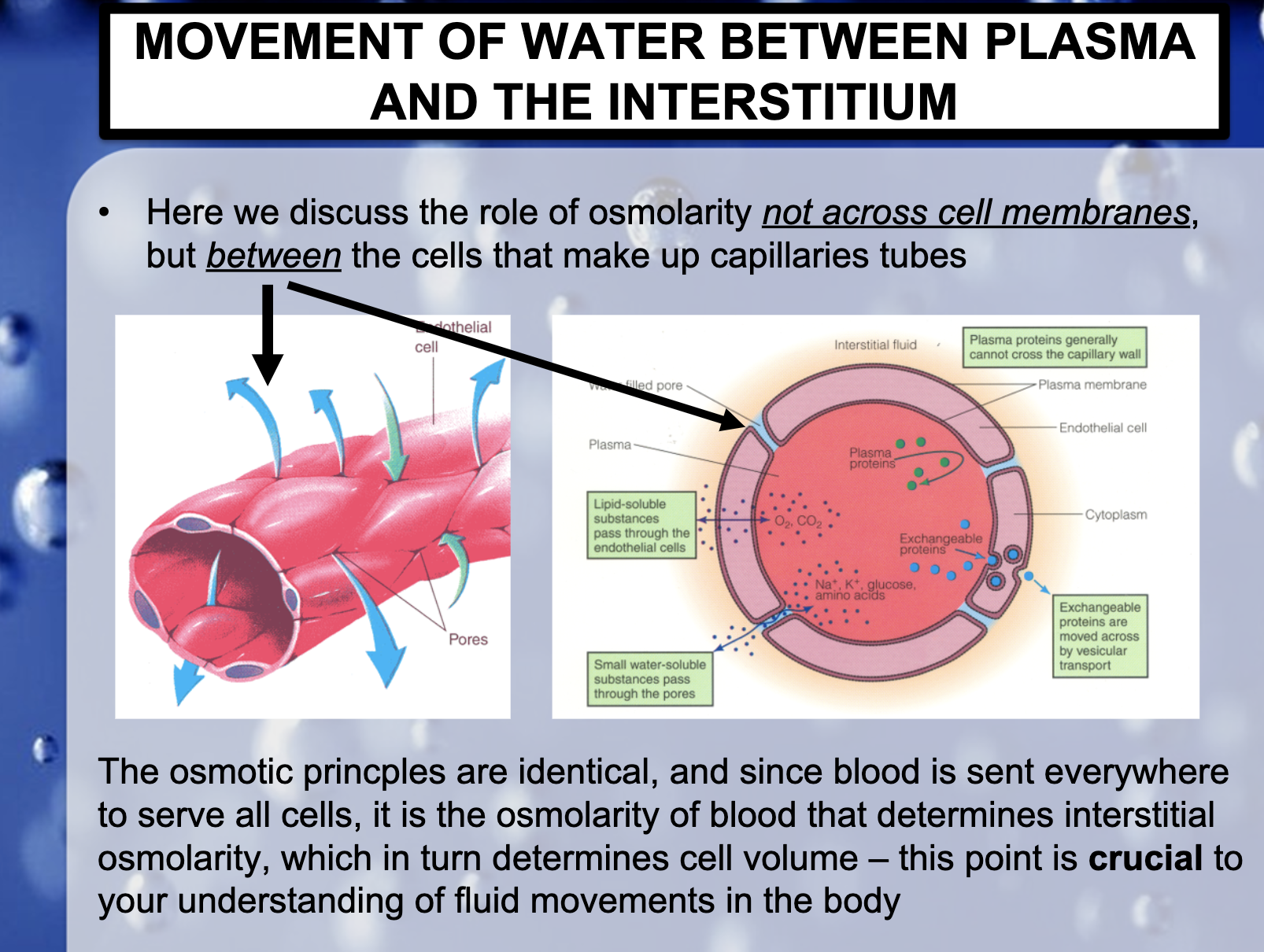
<Capillary permeability>
-Lipid- soluble substances pass through the endothelial cells
-Small water-soluble substances pass through the pores
-Plasma proteins generally cannot cross the capillary wall
-Exchangeable proteins are moved across by vescular transport

*low blood [protein], which is caused by malnutrition or proteinurea or liver failure, decreases the volume of the blood plasma.
It is important to have maintain the blood volume because low volume of blood prevent blood from circulating the body and maintaining adequate perfusion to all of the tissues in the body. Nearly all cells in the body require replenishment of nutrients and a removal system for waste, both of which the blood provides.
*After a salty meal, water comes out from the epipthelial cells covering the gastronemius system by osmosis.
The volume of ICF decreases and the osmolarity increases. ISF then passes through the cell. The volume of ISF is decreased, and this consquently decreases the blood volume.
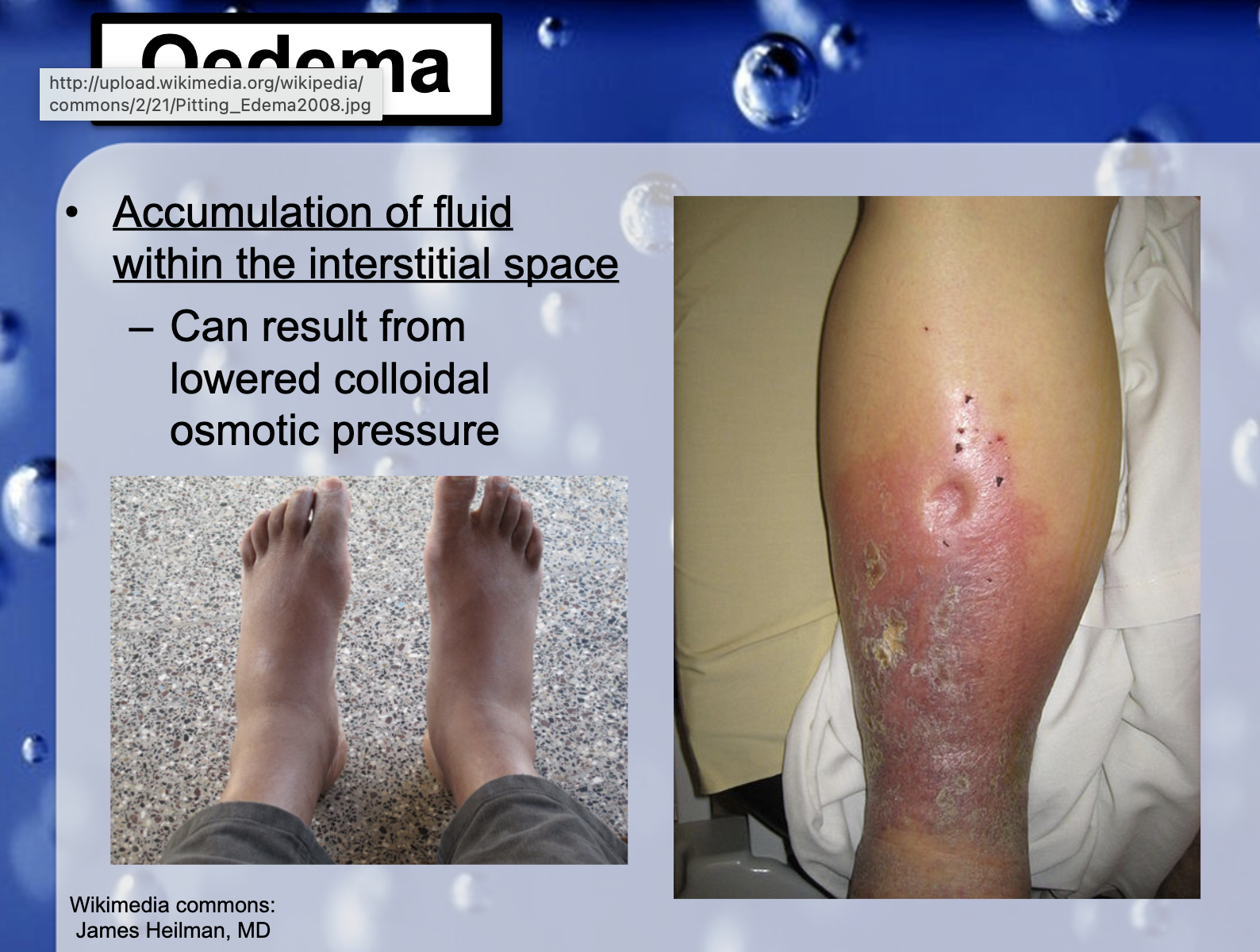

*A diuretic is any substance that promotes diuresis, the increased production of urine.
*Drinking a lot of alchol results in diuresis and the volume of ISF in the CNS is decreased.
If the loss of CSF is great enough, spontaneous intracranial hypotension (SIH) may occur. SIH most often presents with a positional headache caused by downward displacement of the brain due to loss of buoyancy previously provided by the CSF.
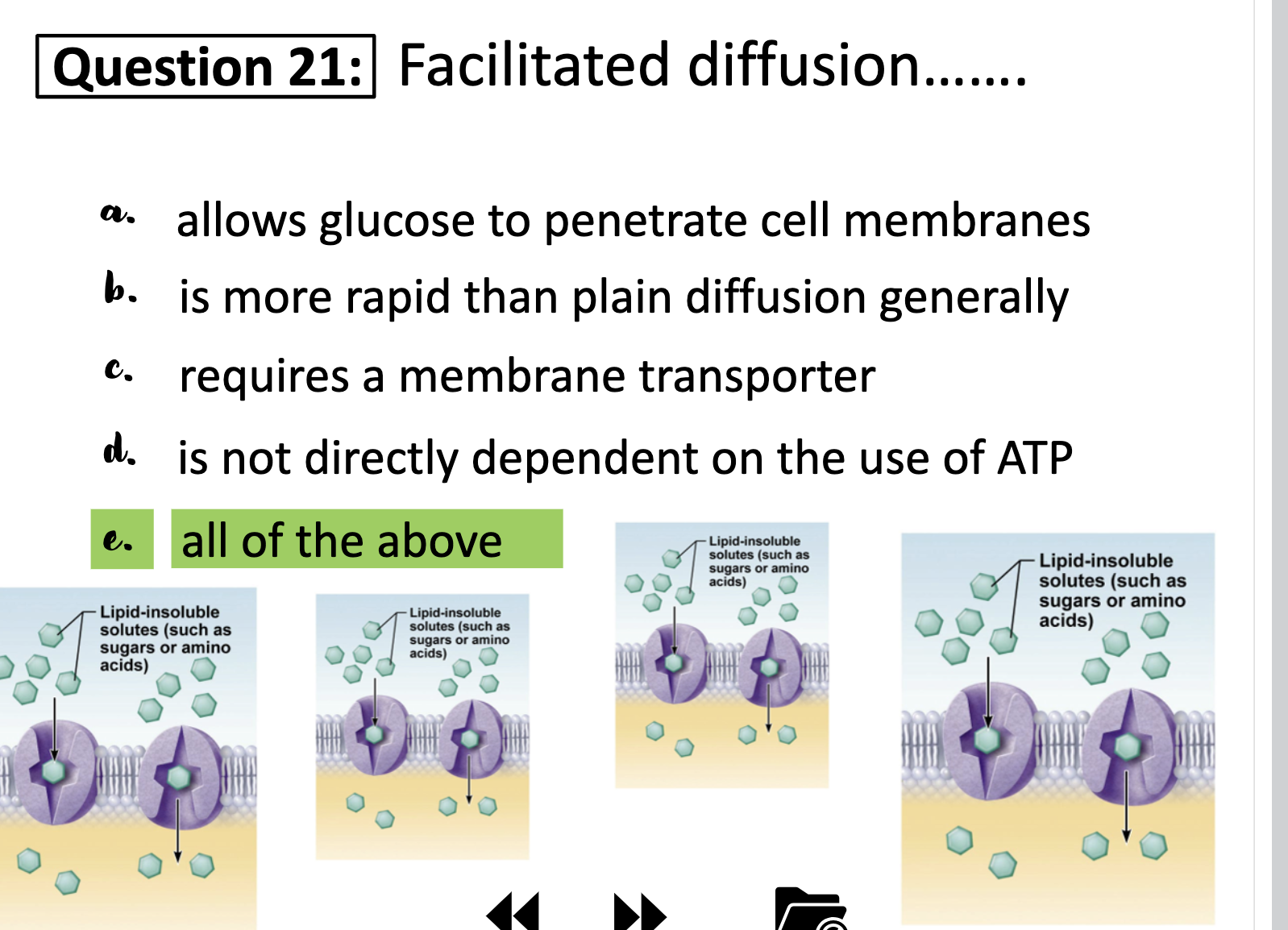
FACILITATED DIFFUSION

Construction of facilitated diffusion carried by protein carriers
Water molecules and ions move through channel proteins. Other ions or molecules are also carried across th e cell membrane by carrier proteins.
The ion or molecule binds to the active site of a carrier protein. The carrier protein changes shape, and releases the ion or molecule on the other side of the membrane.
Carriers are saturated : Unlike plain diffusion, carriers can be saturated and limits are imposed.
When the molecule binds to the carrier protein, gates are closed.
Carriers are selective : Protein carrier allows certain molecules to move into a cell along [] gradients , while doesn't allowing movement of other molecules.
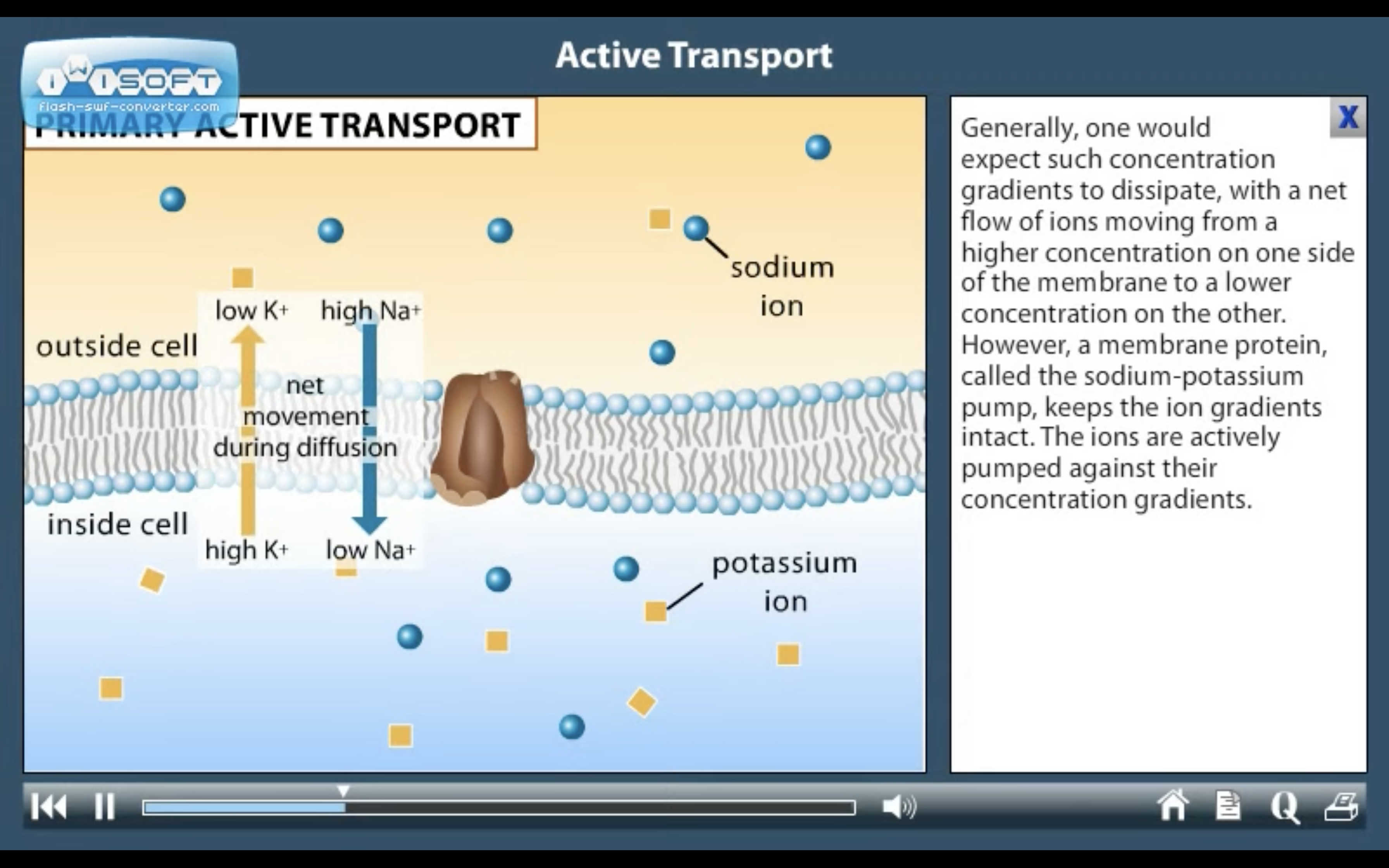
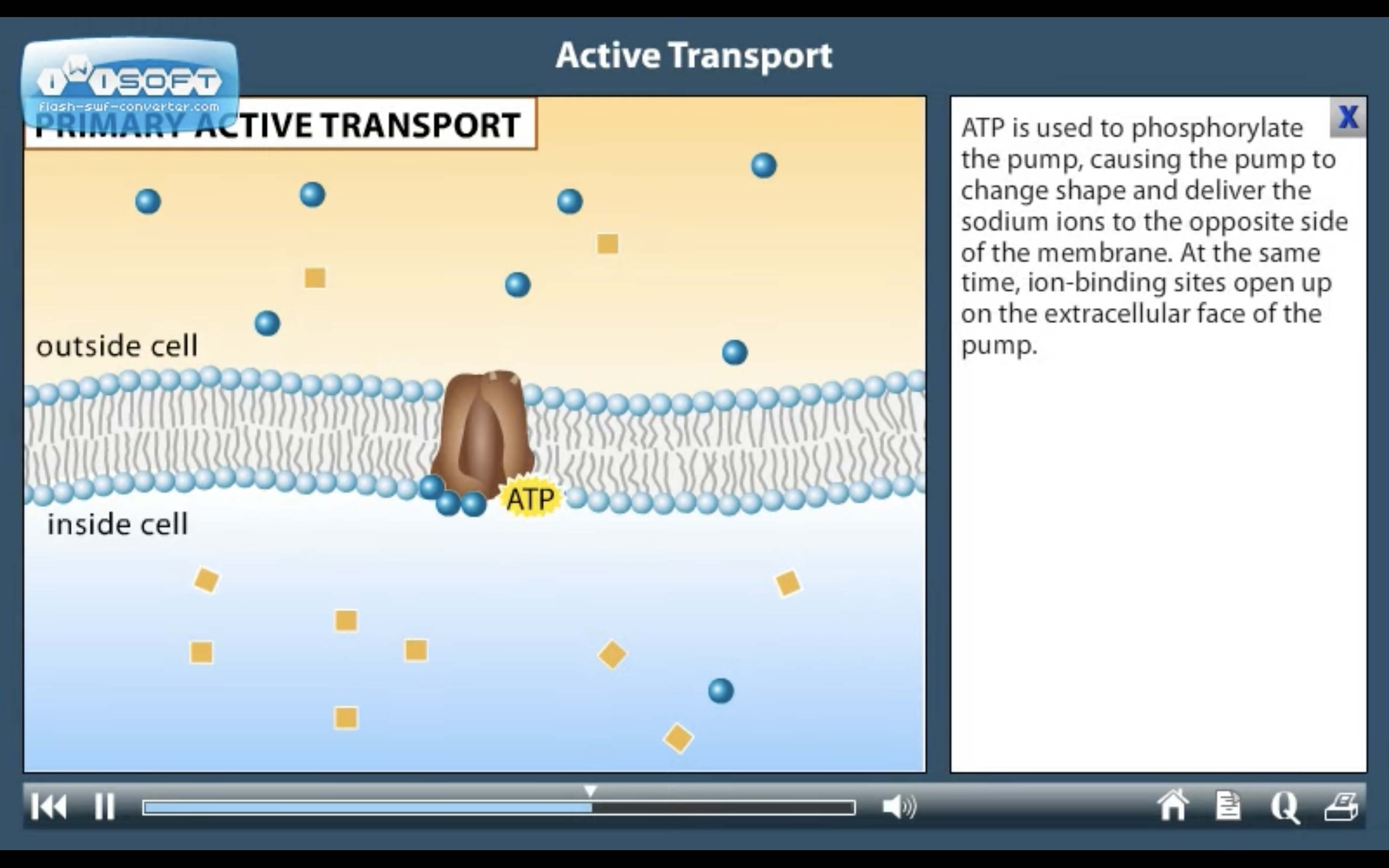
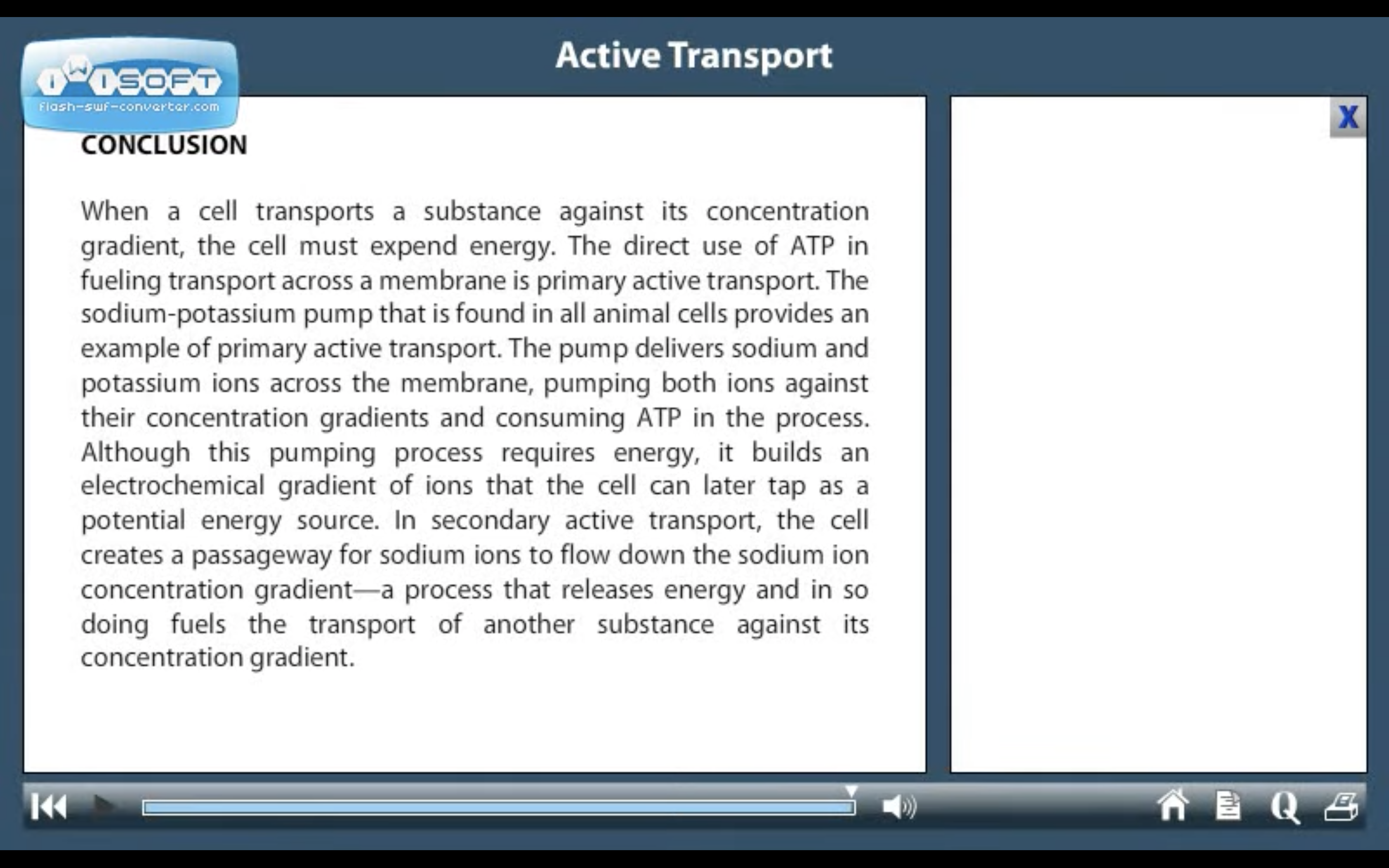
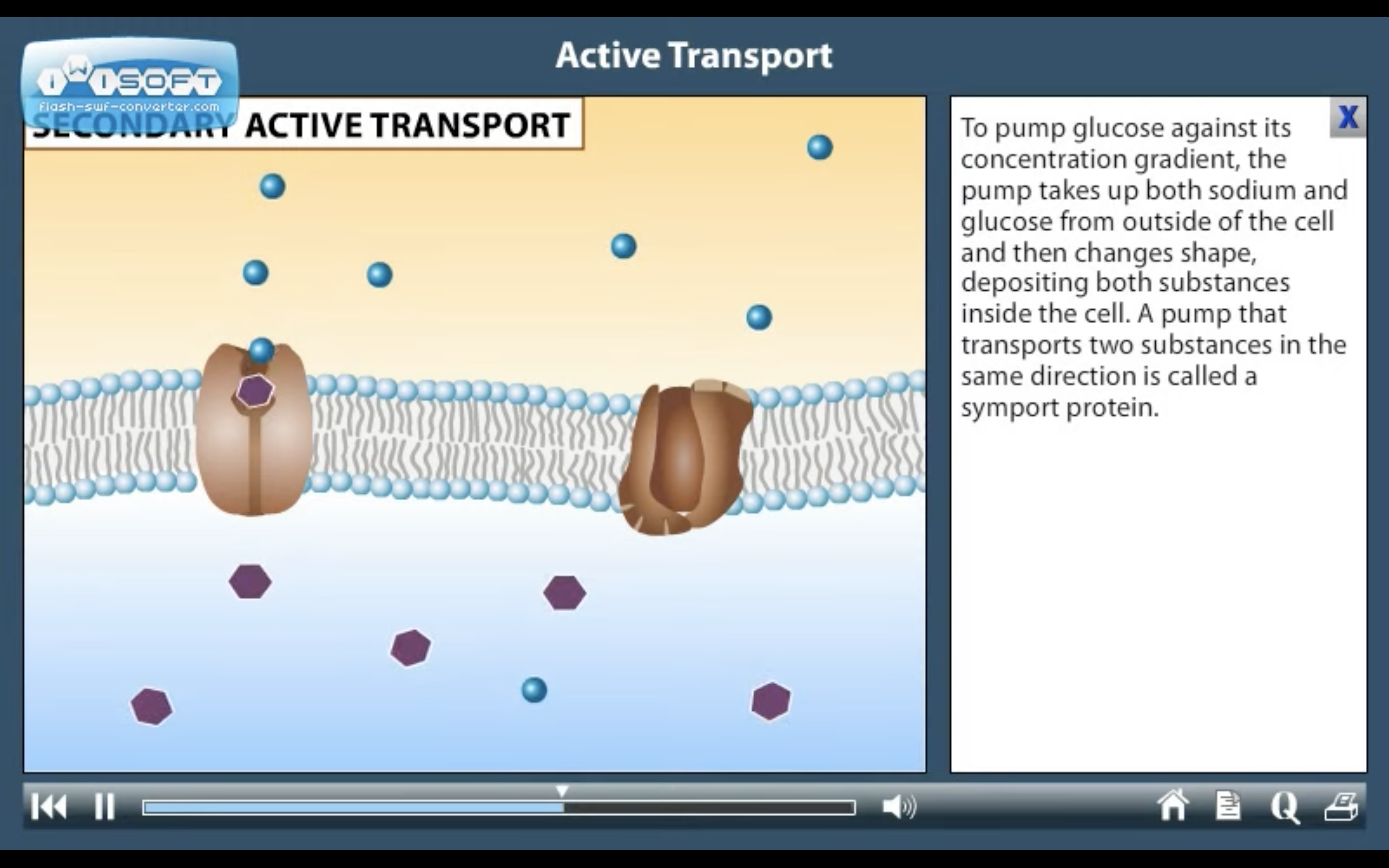
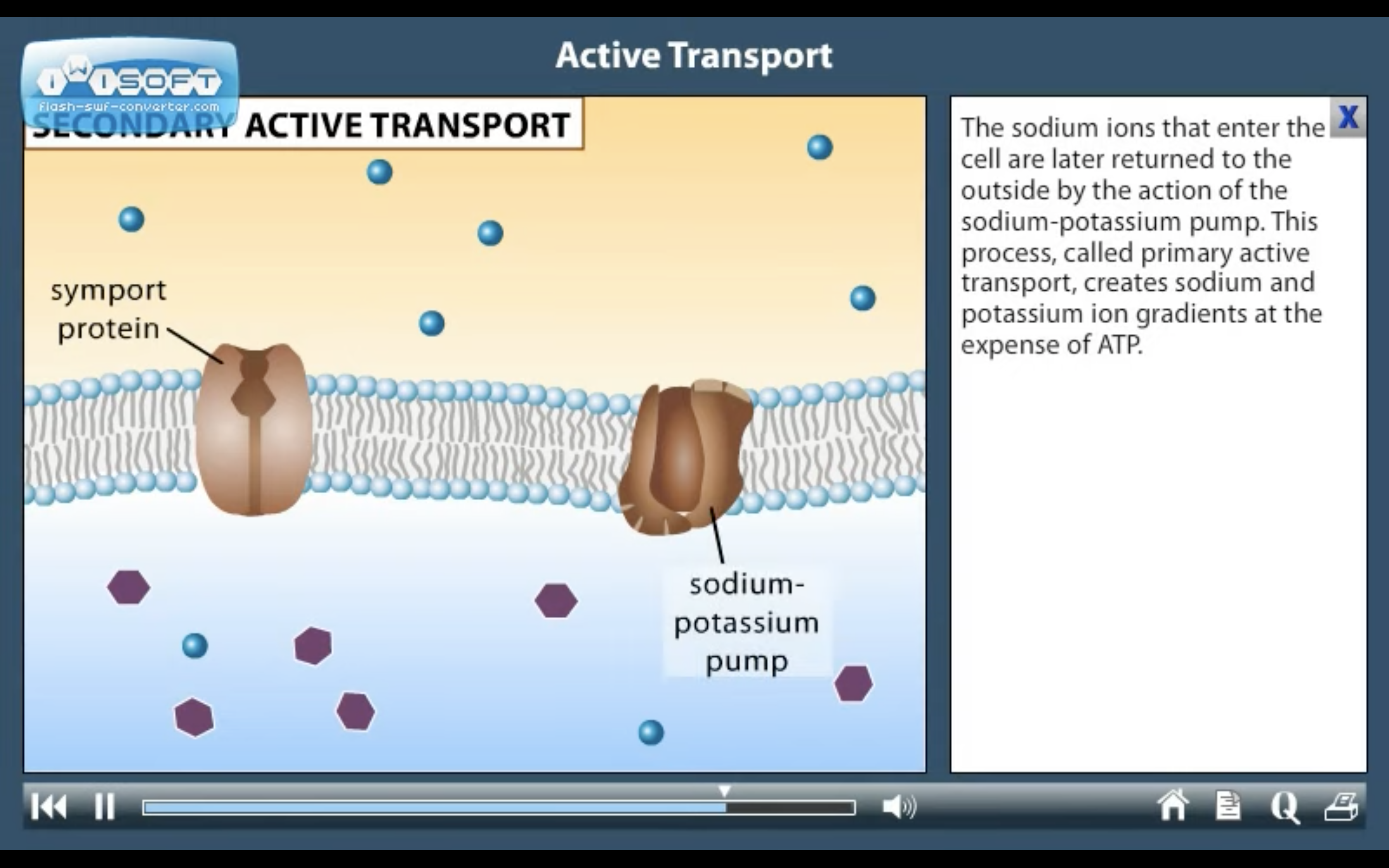
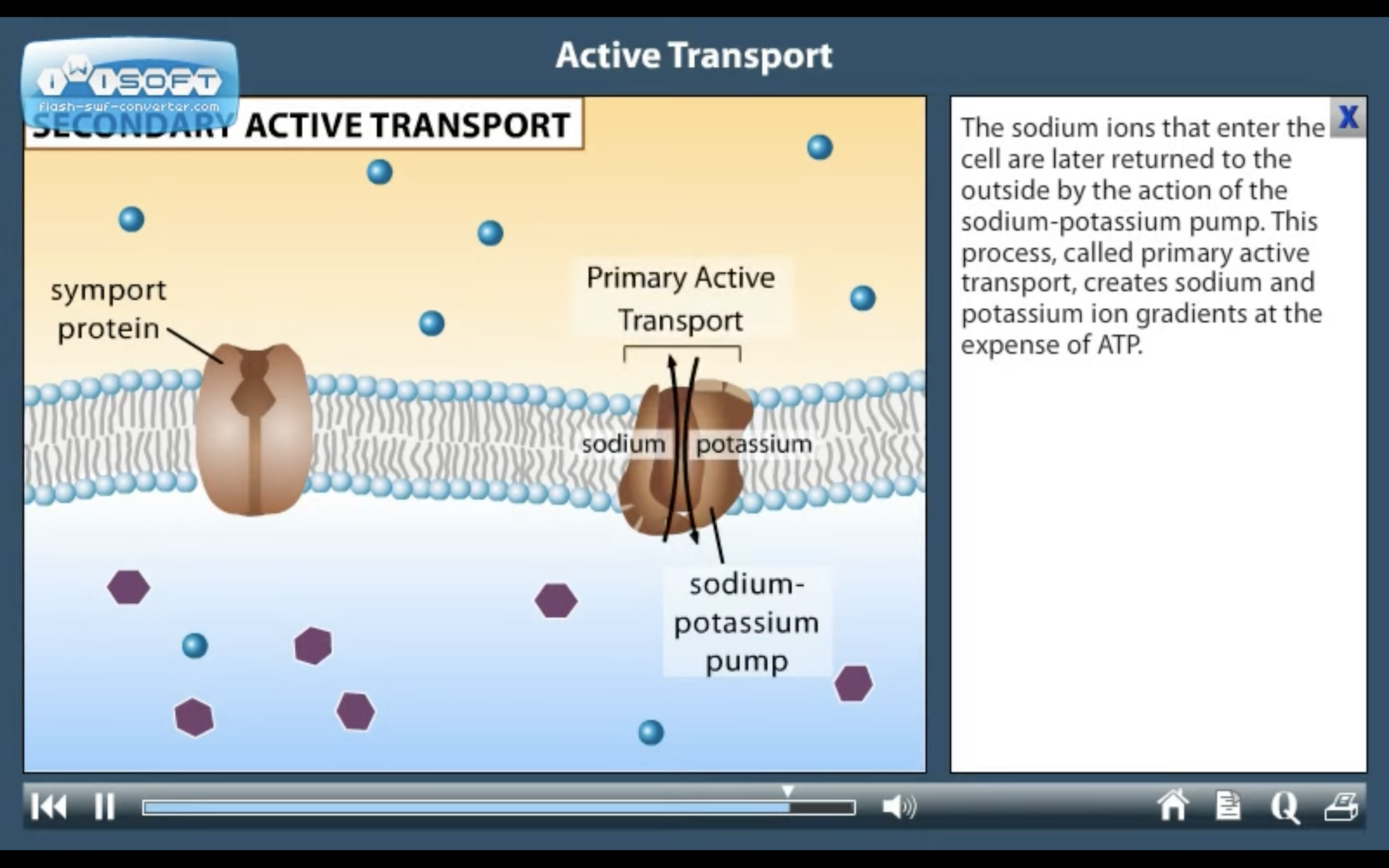
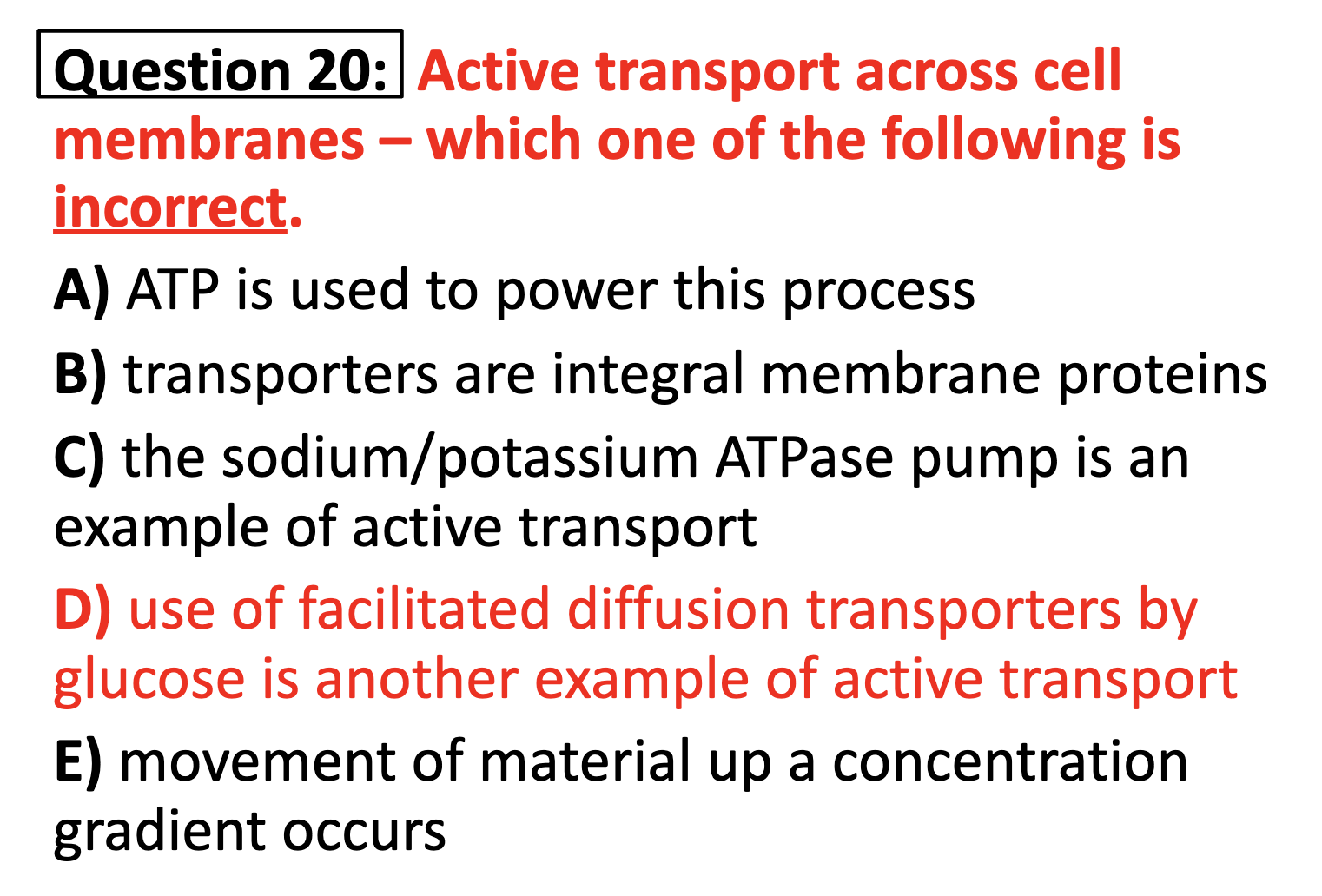
ACTIVE TRANSPORT
<Active Transport>
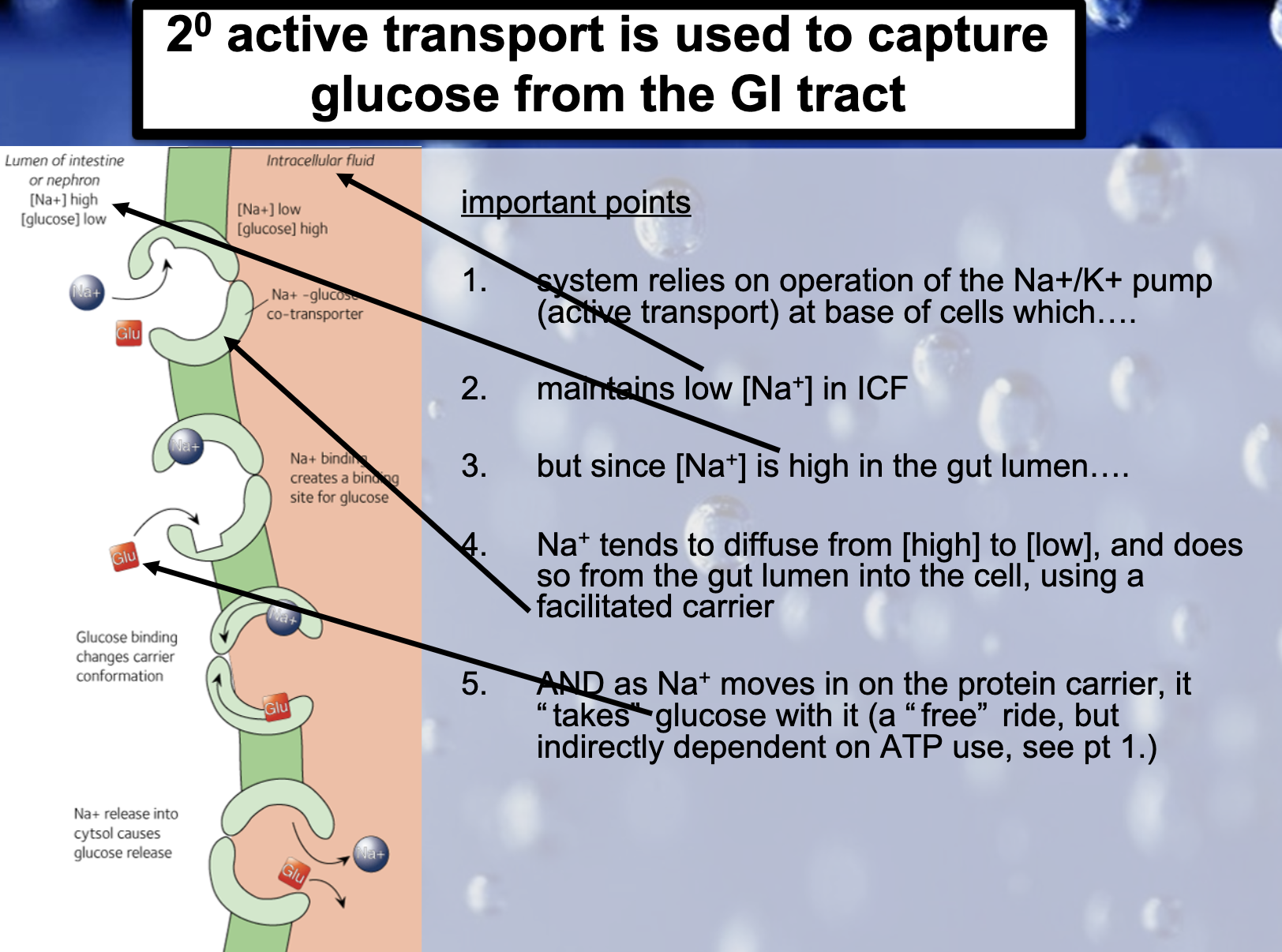
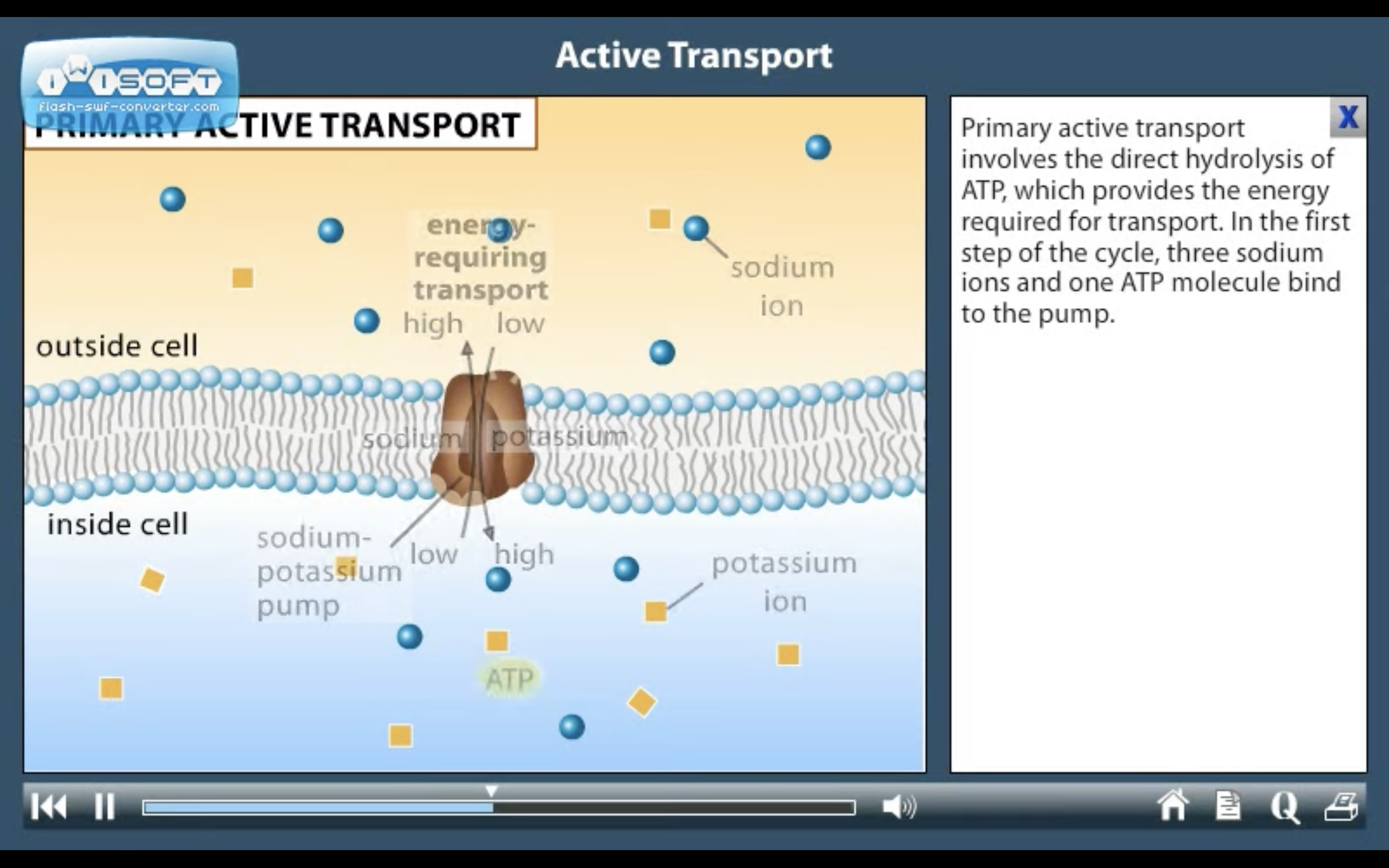
1. Primary active transport : directly uses ATP (Na+-K+ ATPase Pump)
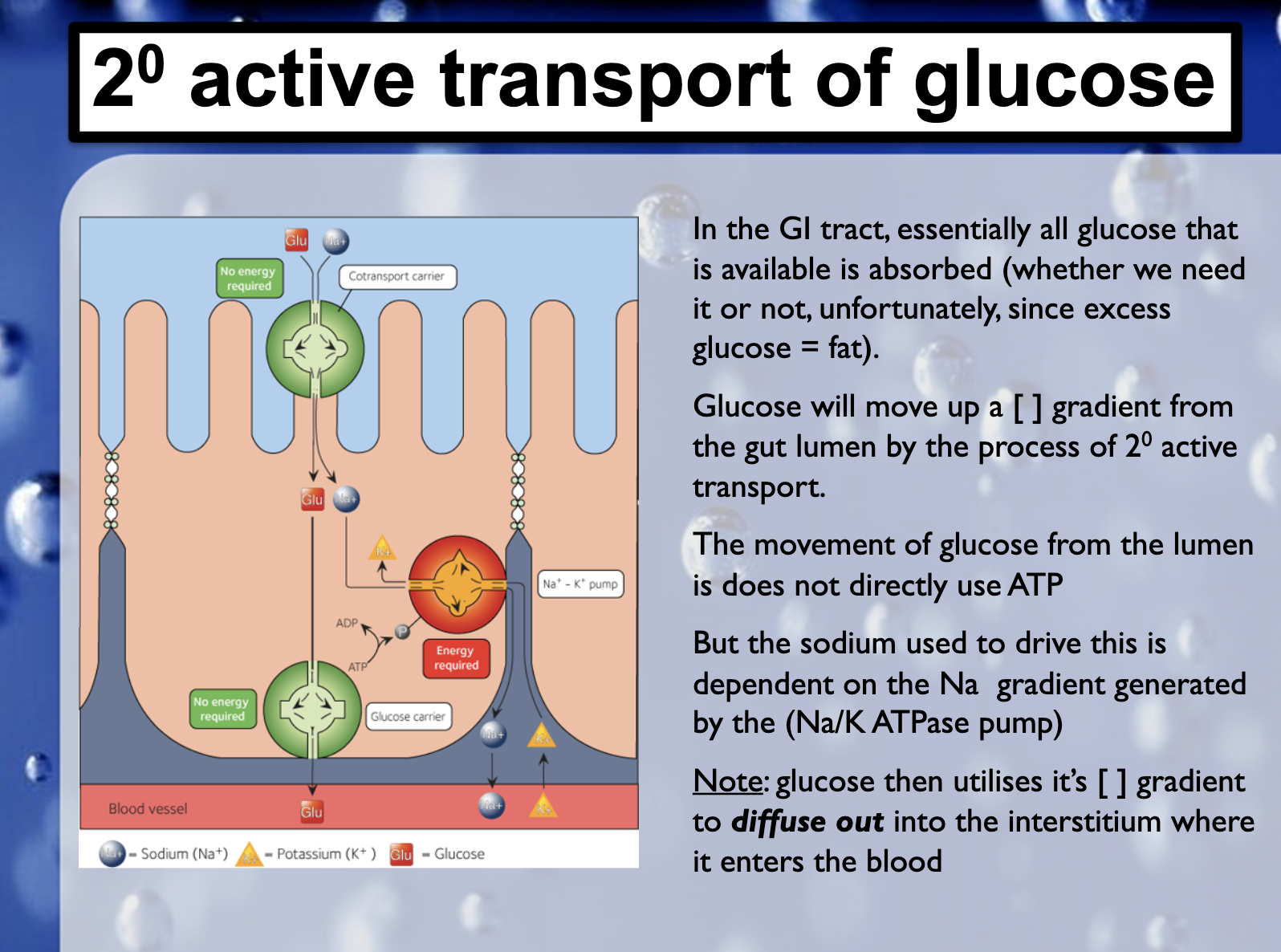
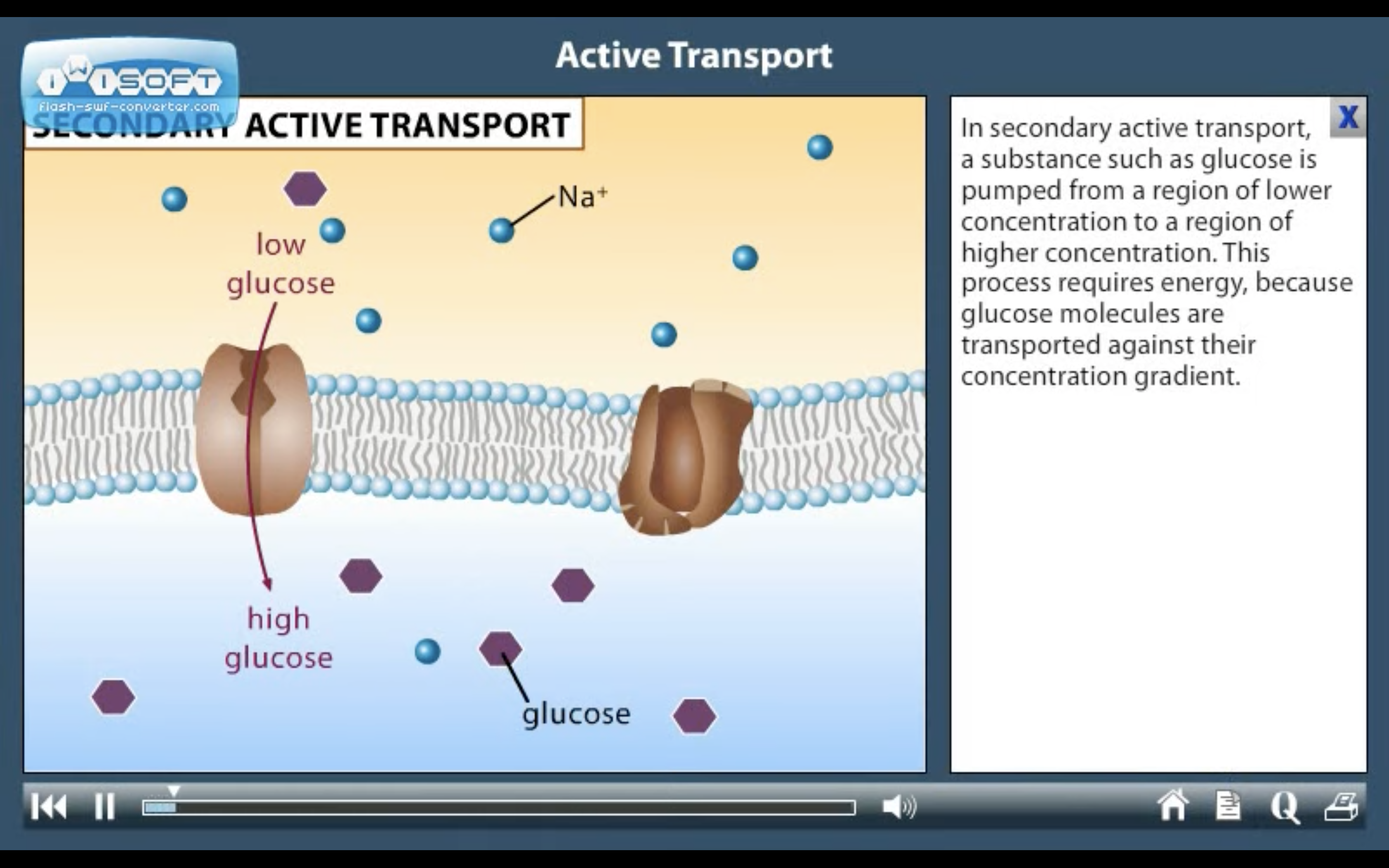
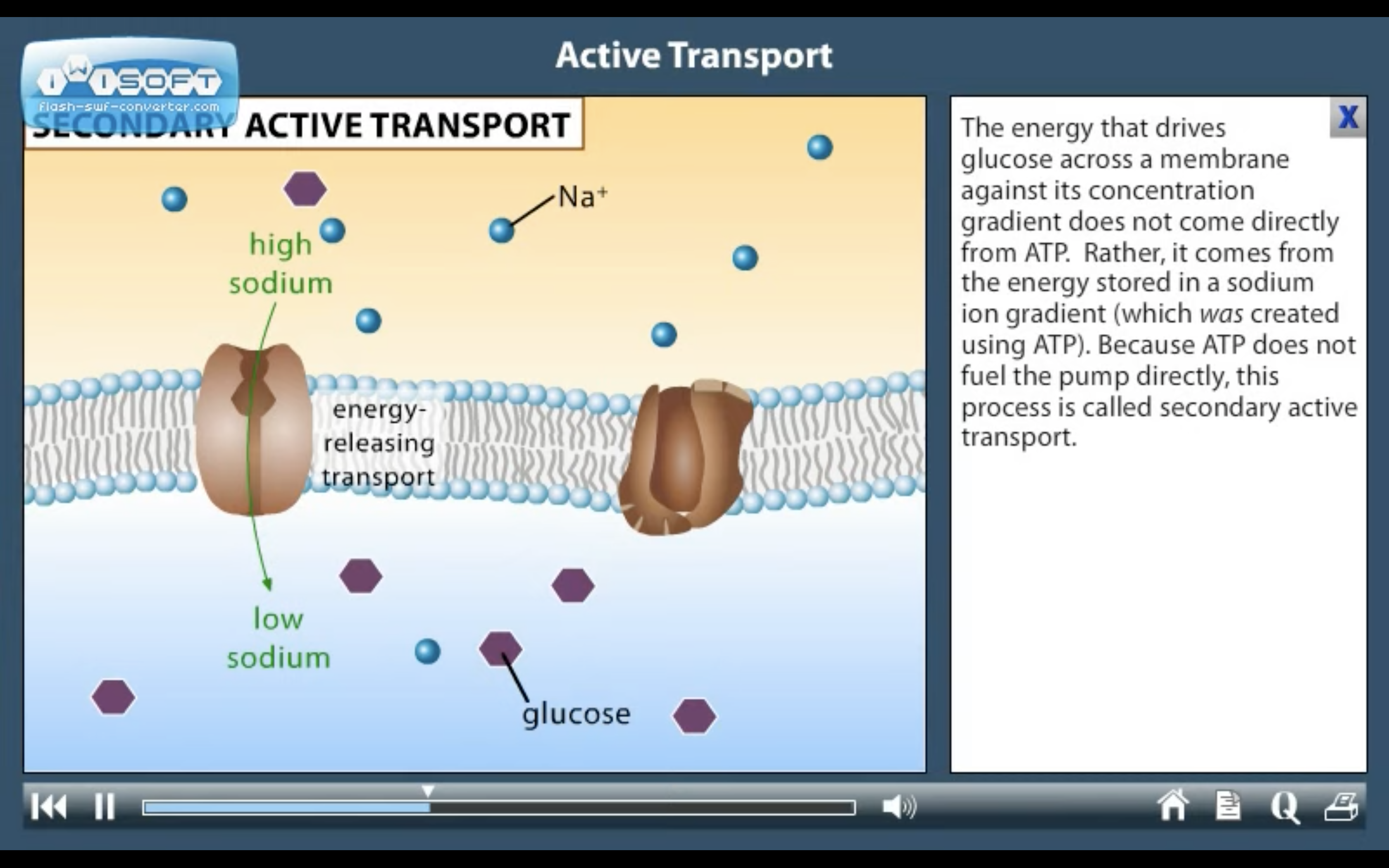
2. Secondary active transport : uses energy derived from primary active transport of a substance for the co-transport its concentration gradient ( Na+-Glucose transporter)
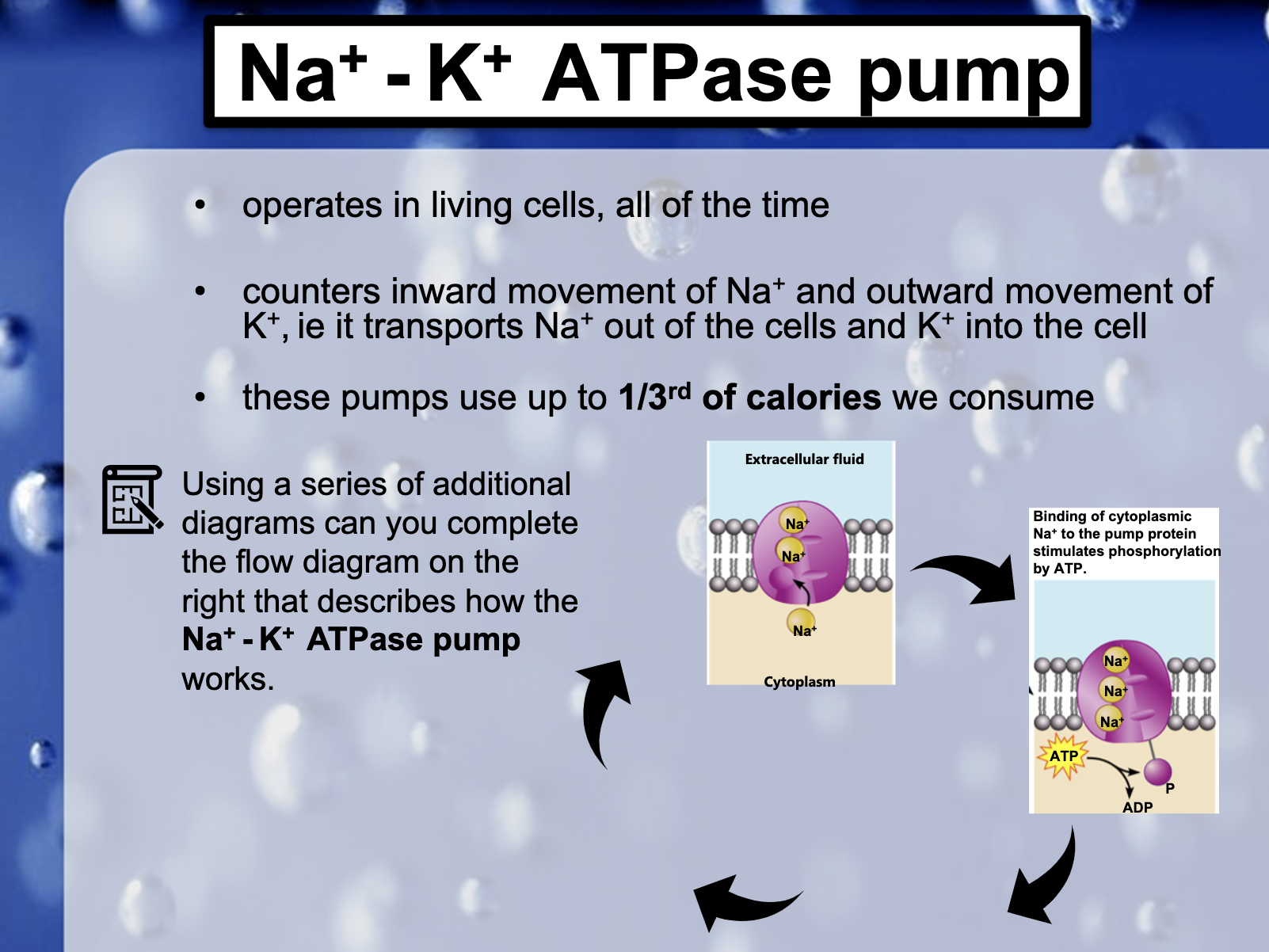
<Na+-K+ ATPase Pump>
3Na+ out, 2K+ in
<GI tract>
ACTIVE TRANSPORT https://www.youtube.com/watch?v=JGF6ry0SWPs
❤️Primary active transport
❤️Secondary active transport
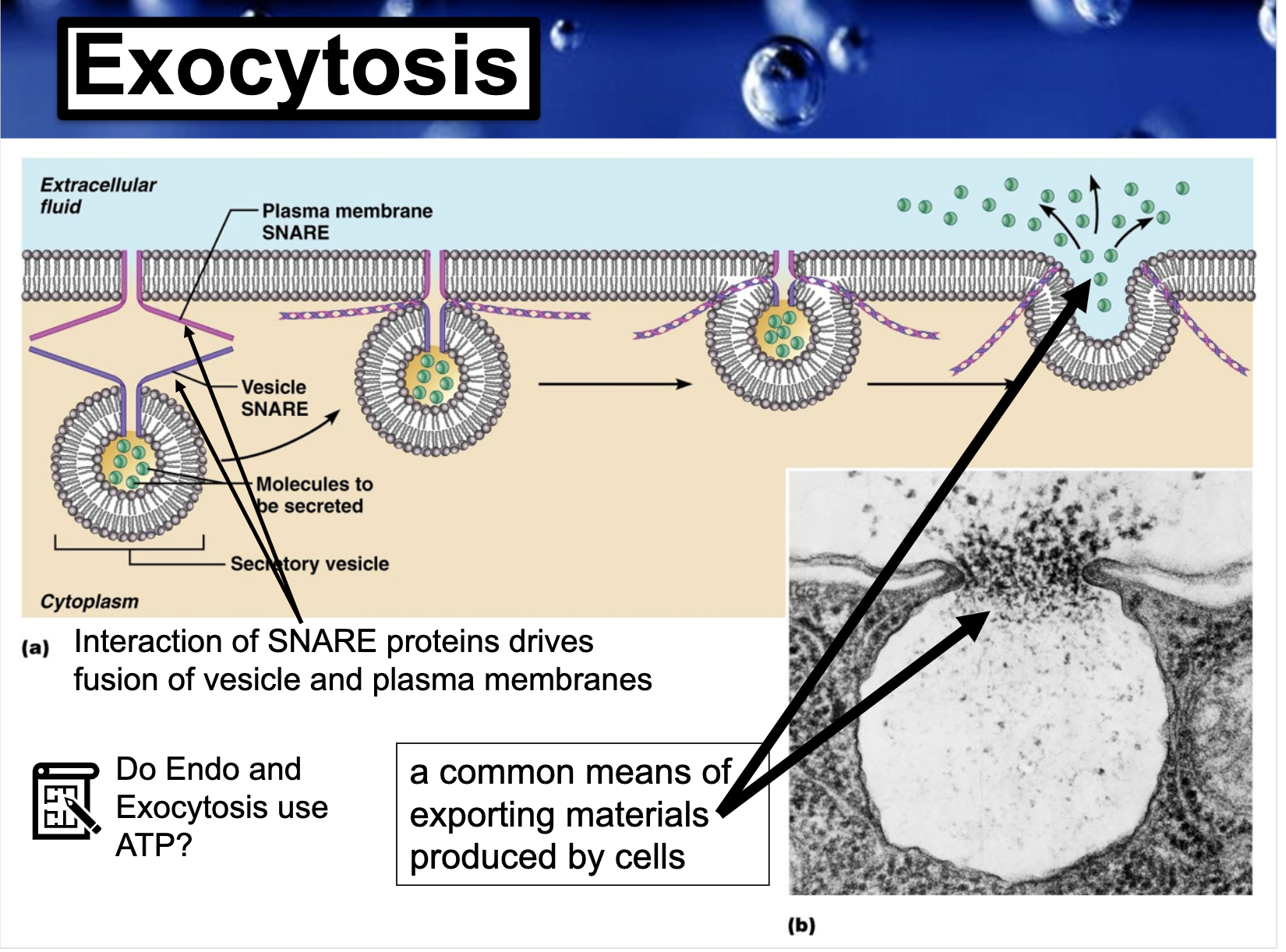
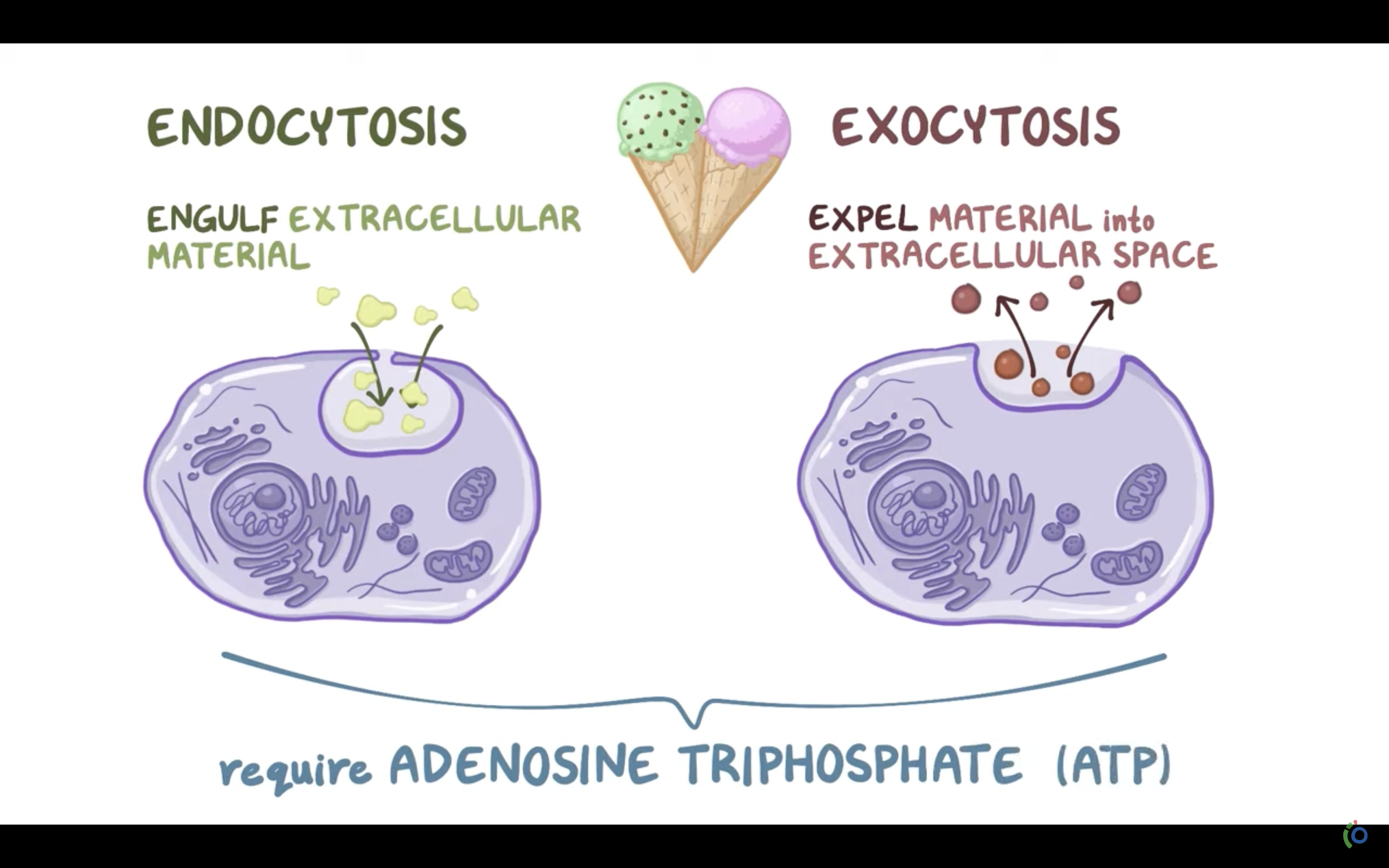
ENDO AND EXOCYTOSIS
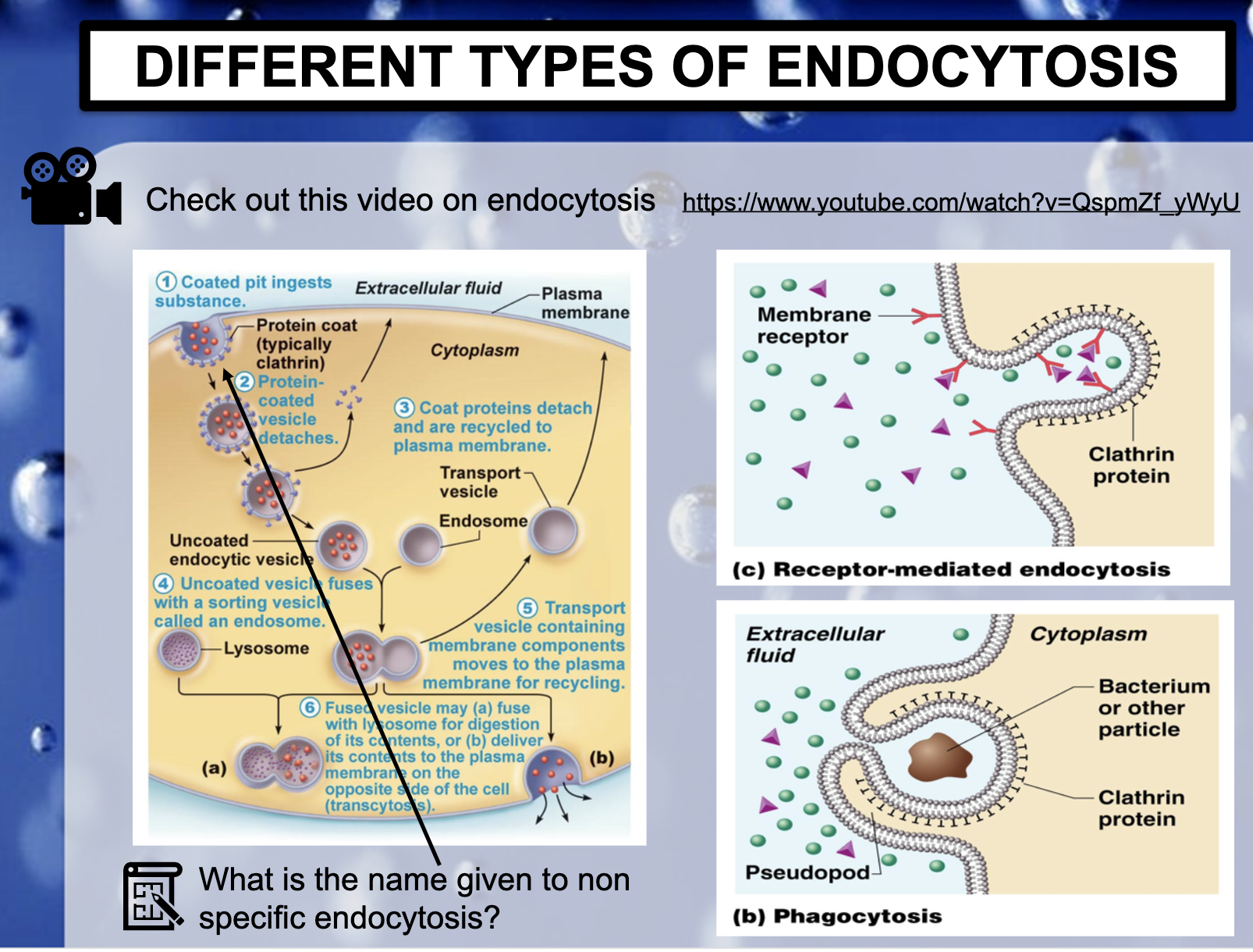
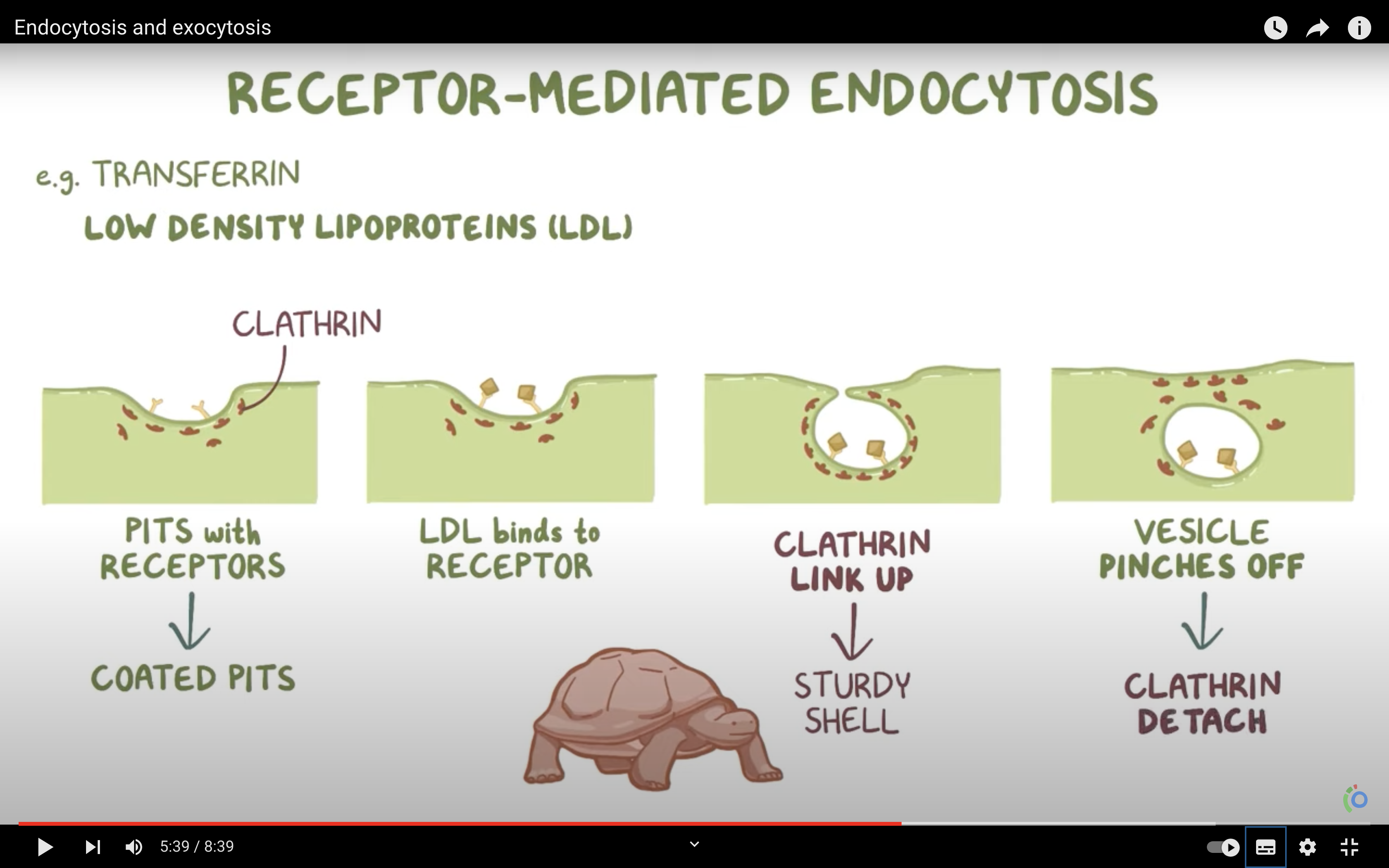
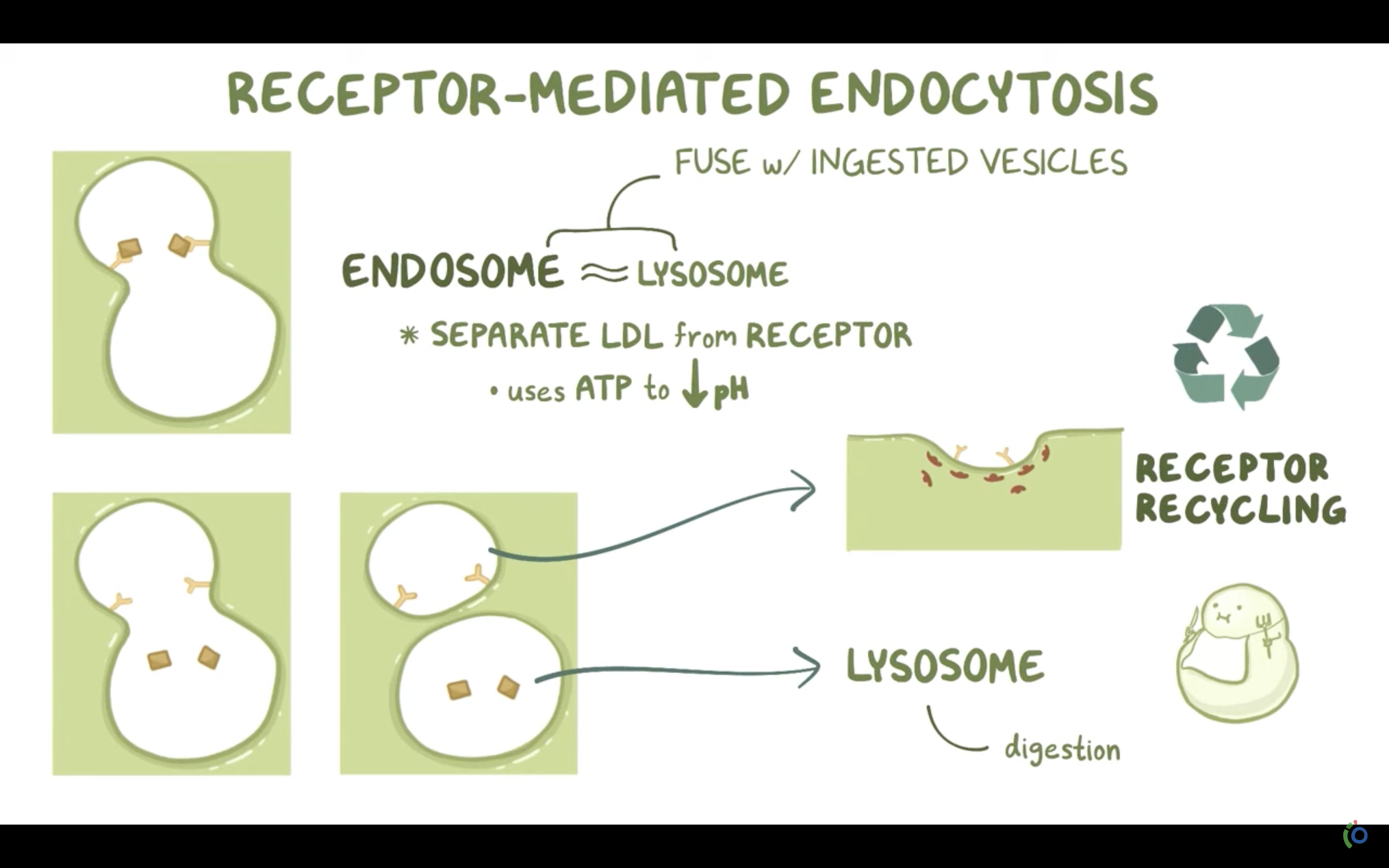
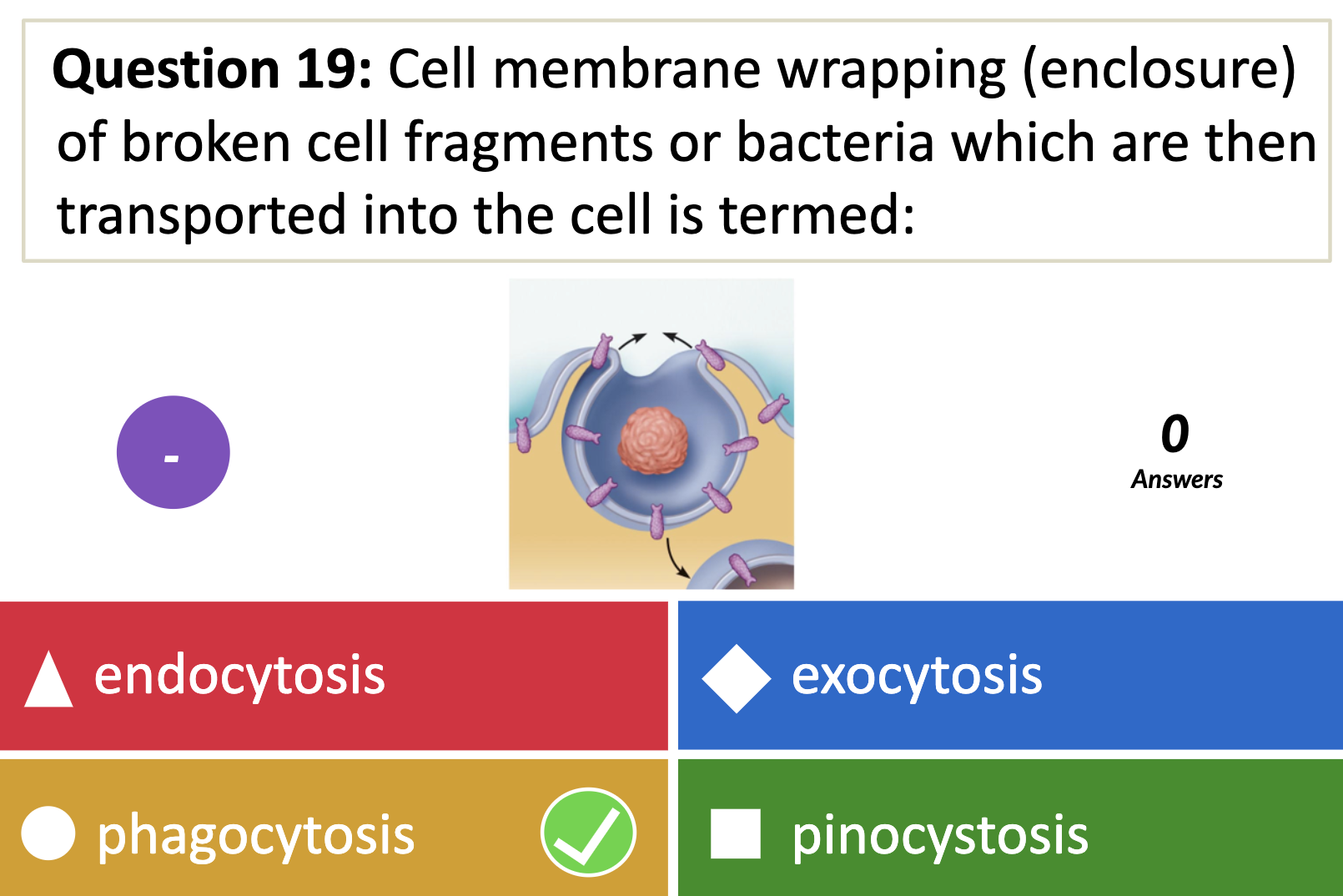
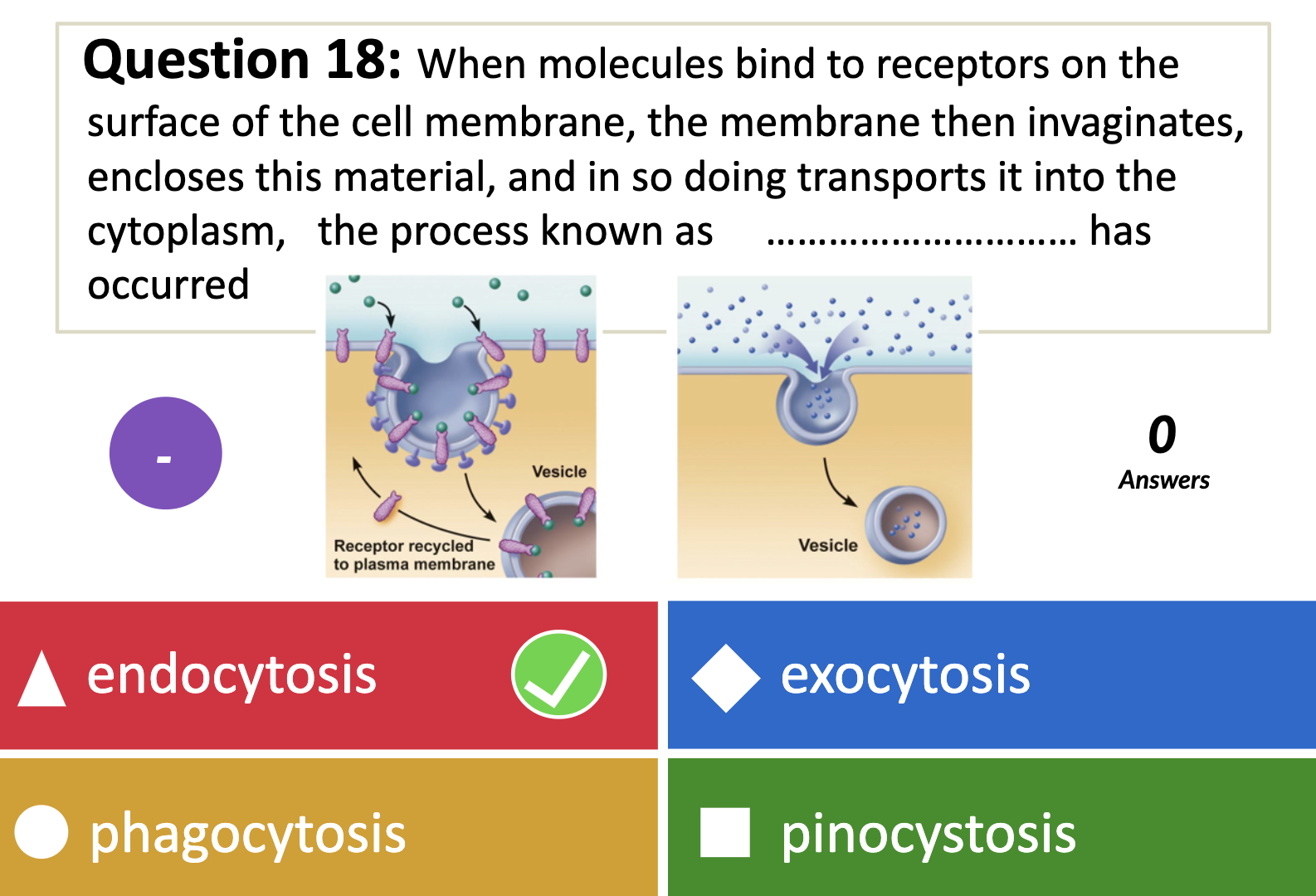
*What is the name given to non specific endocytosis?
ENDOCYTOSIS, PHAGOCYTOSIS, AND PINOCYTOSIS
https://www.youtube.com/watch?v=QspmZf_yWyU
❤️ENDOCYTOSIS
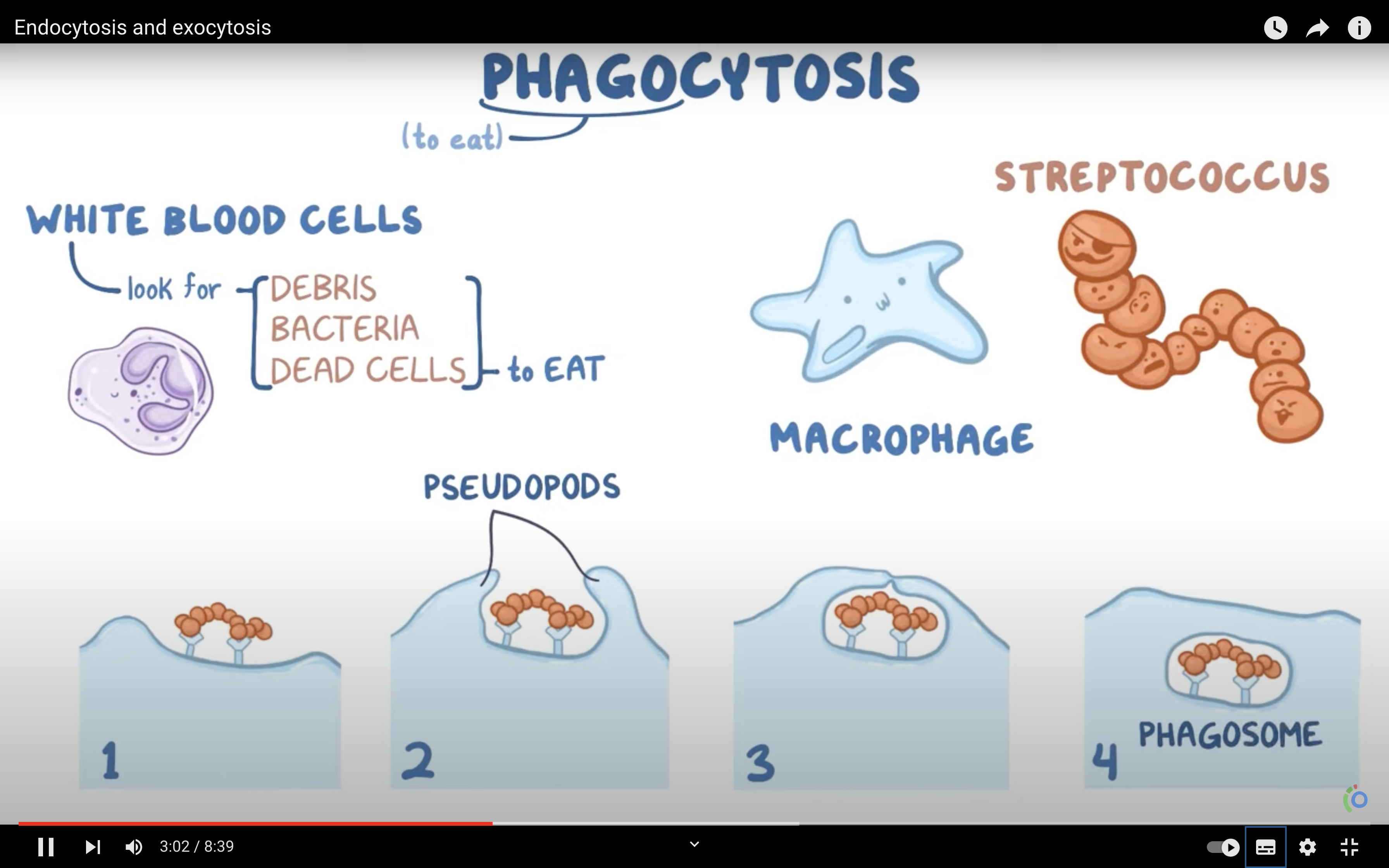
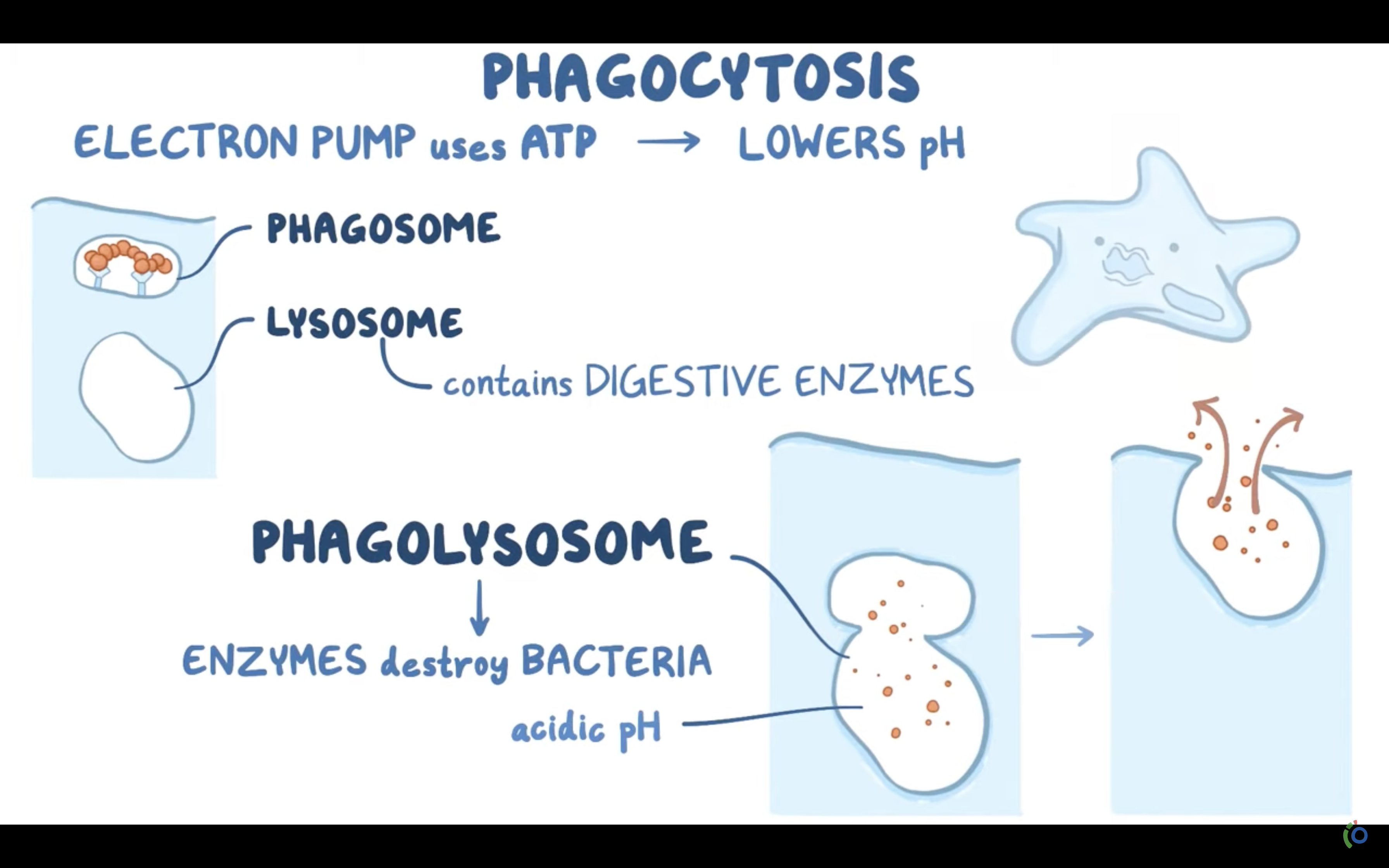
-> Phagocytosis (to engulf something like bacteria by wraping them up with cell membrane, pinching off, and make food vacuole inside the cell/ lysosome may digest after)
-> Pinocytosis (to engulf some fluid by wraping , pinching them with cell membrane and bring them inside the cell, making vesicle. vesicle is the membrane bound compartment inside the cell)
SELF PACED QUIZ














SUMMARY






*Describe lipid bilayer, role of cholesterol, composition and role of the glycocalyx
Cell membranes are consist of lipid bilayer which seperate water soulble substances from the cell.
Two layers of hydrophibic head outward and hydrophobic tails inward make up the lipid bilayer.
They are slightly movable so that they can maintain the cell system even in the low temperature.
Cholesterol gives the flexibility to the cell membrane by being penetrated in the lipid bilayers.
Glycocalyx prevents the entry of pathogens into the cell.
Glycoprotein and glycolipids,often called as fuzzy coat, are responsible for recognizing cells and attachment between cells.
*Be able to describe why a drop of perfume released at the lectern will be smelled almost immediately by those in the front seats, and over time, by those toward the back. (Molecular kinetic theory and diffusion)
Small particles of the perfume have a kinetic energy to move in random direction, which cause diffusion. Those particles move toward to low concentration area from the high concentration area.
The perfume drops were high concentrated in the front seats, so the particles move to the back seat.
*How movement of water across semi-permeable membrane will occur from low solute[] to high solute[]
Water will move from low solute[] to the high solute[]. Water always move from the high molarity(osmolarity) to the low molarity(osmolarity)
*This can also be described as movement from high [water] to low [water], which is osmosis
Osmosis is the movement of water molecules from the high concentration of water molecules to a solution with lower concentration of water molecules through a cell's partially permeable membrane.
*COP
Explain why cells are “faut”and will burst if pricked.
COP 는 혈관 속에서 물을 가지고 있도록 하게 하는 삼투압이다, 주로 큰 분자가 관여하며 보통 알부민이라는 혈장단백질이 COP 를 작동한다. COP is the osmotic pressure exerted by large molecules, serves to hold water within the vascular space. It is normally created by plasma proteins, named albulmin.
Cells burst due to osmotic imbalance which can cause too much water diffuse into the cell.
*What would happen to water movement if protein was lost from blood to accumulate in the tissue?
If the protein(albumin) which holds water into the blood is lost, cop decreases and it results in water and solutes escaping into the interstitial space from the capillaries. This is often called as edema.
*What might happen to the balance of fluid in blood vs the interstitium in someone suffering a protein malnutrition?
Insufficient protein causes fluid to shift to areas of the body that it should not be in, where it accumulates in the tissues. A fluid imbalance across the walls of capillaries can lead to fluid retention, or edema.
-Diffusion down a concentration gradient
Particle has a kinetic energy to randomly move from the high concentration to low concentrartion area, which is called diffusion.
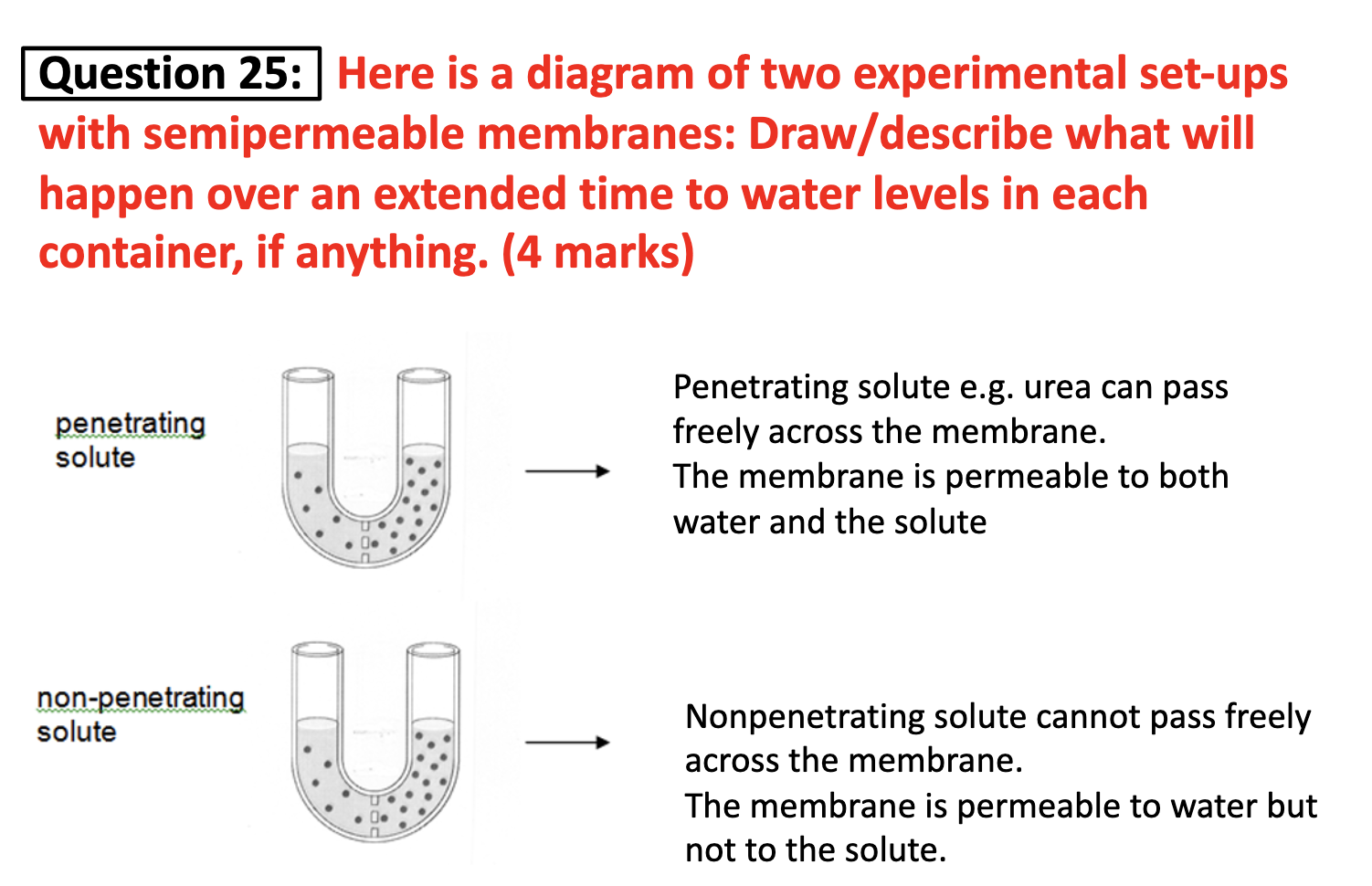
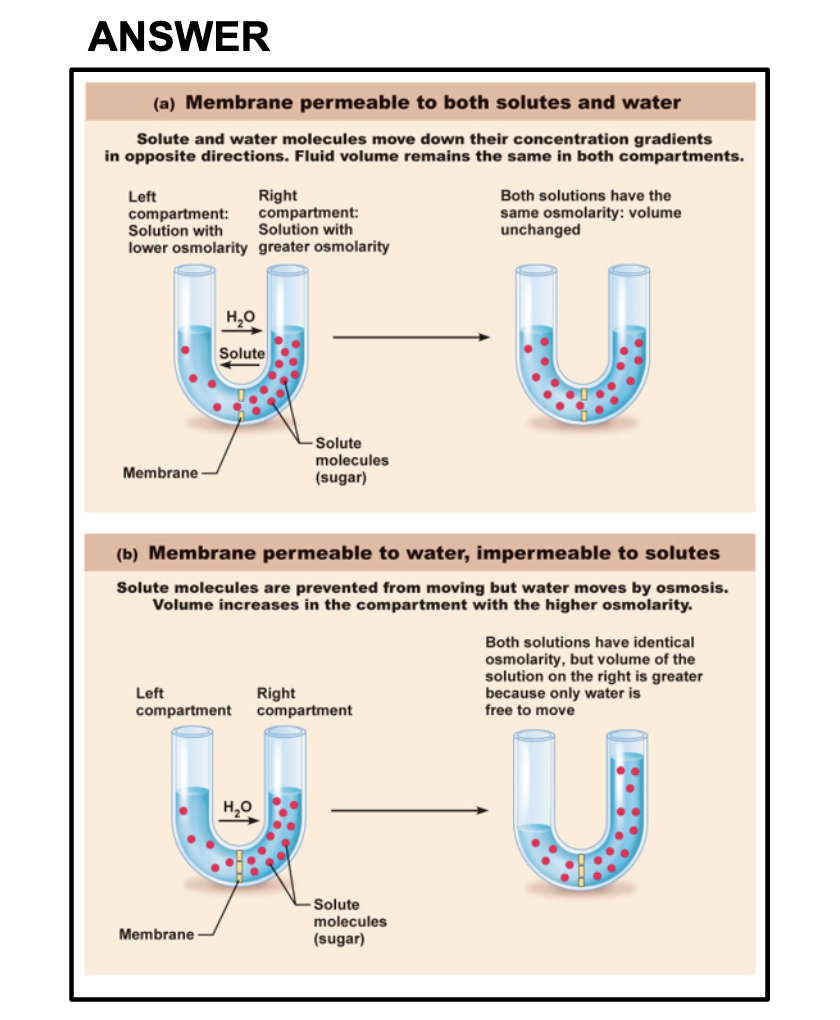
-penetrating vs non-penetrating solutes
Water soluble molecules cannot penetrate the cell membrane because of the hypophibic head of phospholipid bilayer block them not to get inside. In contrast fatty substances can penetrate. Also a large size of molecules cannot pass through the membrane, they need to enter the cell by a transporter.
-aquaporins
aquaporins are protein channel attached on the cell membrane which transport water molecules into the cell based on the osmotic gradients. (which created by active solute transport)
-lack of barrier to lipid soluble substances
Lipid soluble substances can pass through the plasma membrane easily if their sizes are small enough, as the membrane is consist of hydophibic phospholipid towarding outside and hydropphobic inward.
-osmosis
osmosis is the movement of water molecules from high concentration of the solution to the low concentration thorough the semi-permeable membrane.
-colloid osmotic pressure
cop is the osmosis exerted by the large molecules in the blood plasma to hold water inside the blood vessels.
-selectively permeable membrane(real life) vs semi-permeable membrane (artificial)
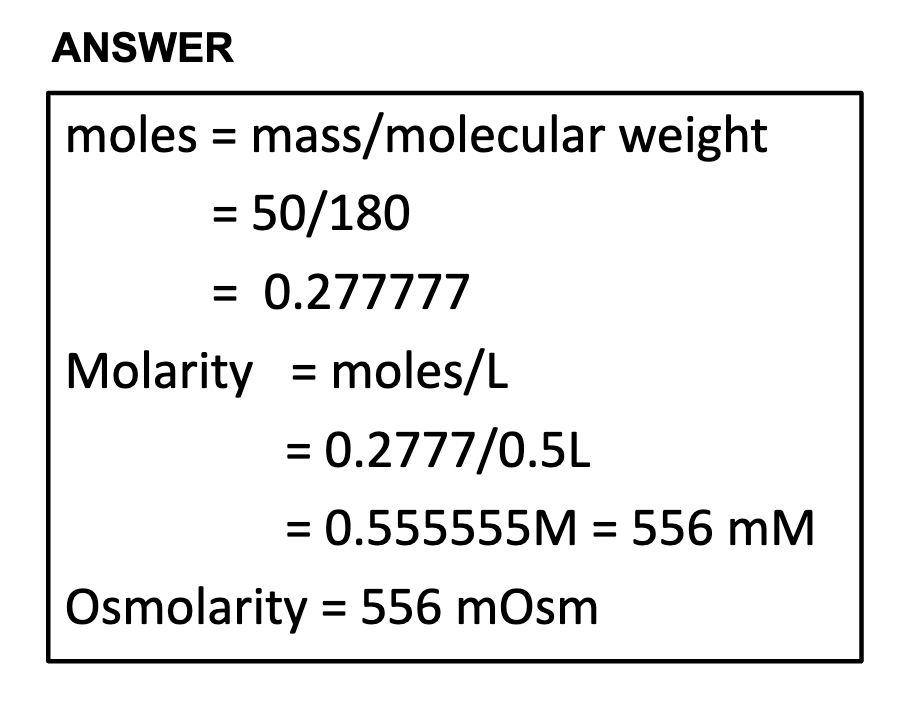
-osmolarity vs molarity
Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of osmoles of solute particles per unit volume of solution.
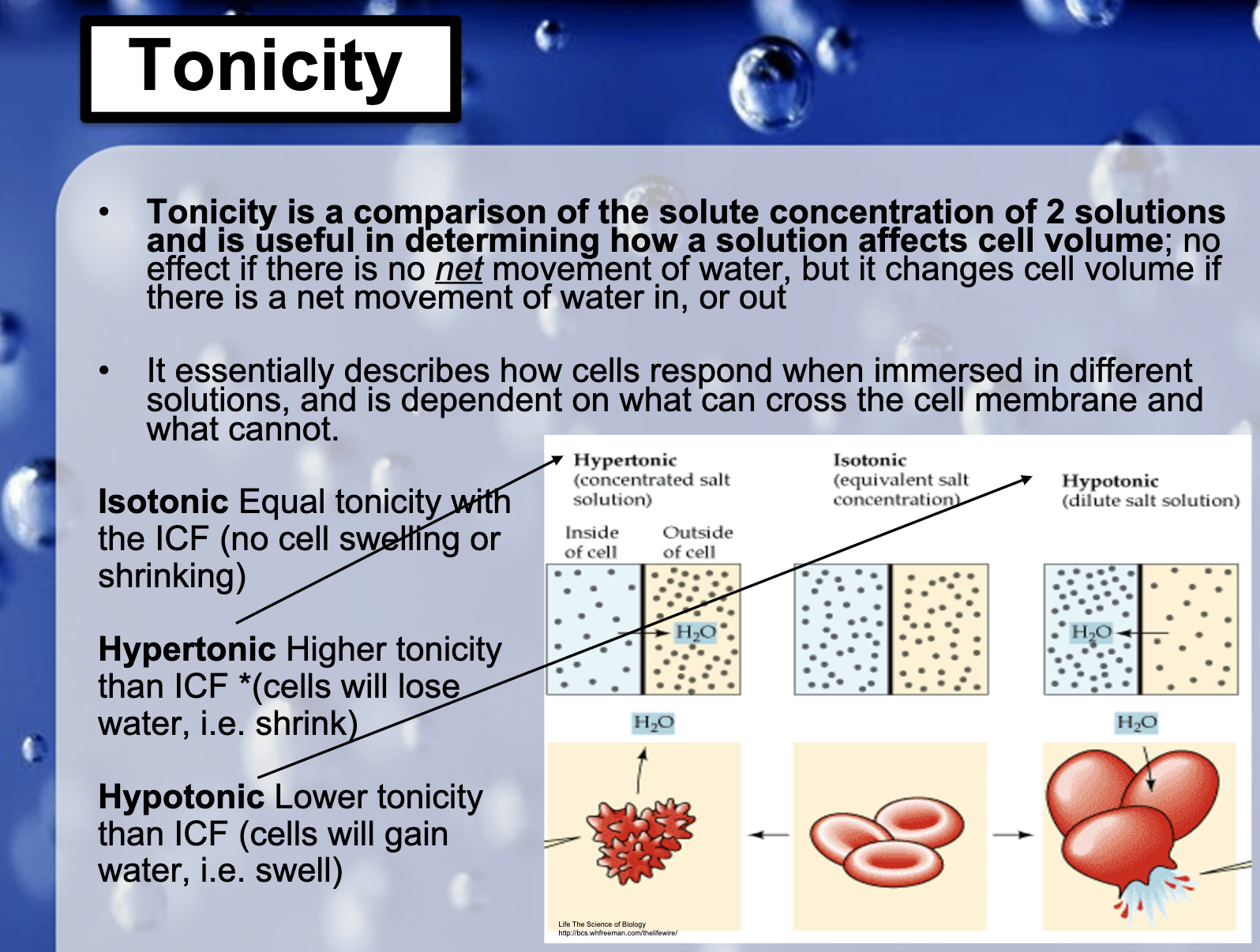
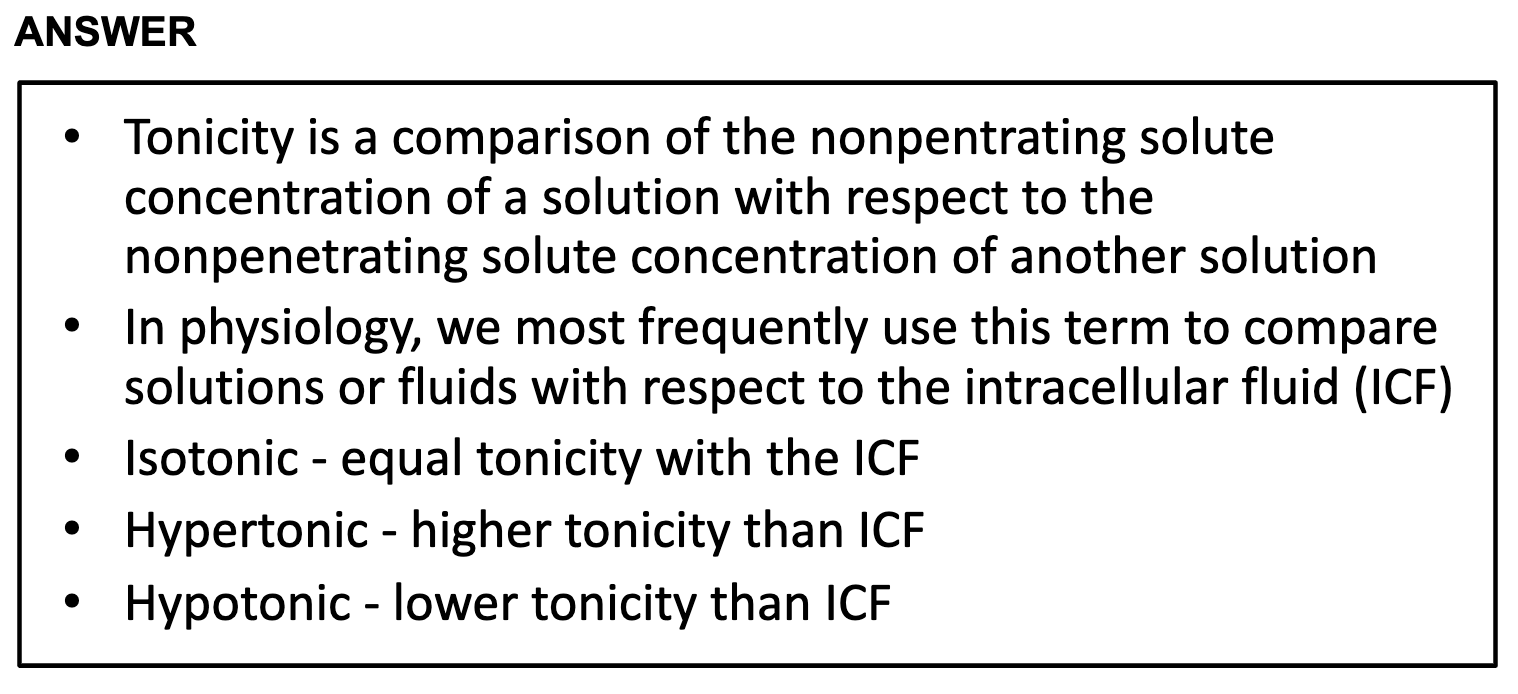
-isotonicity
Having same concentration inside and outside so that the volume of cell doesn't change
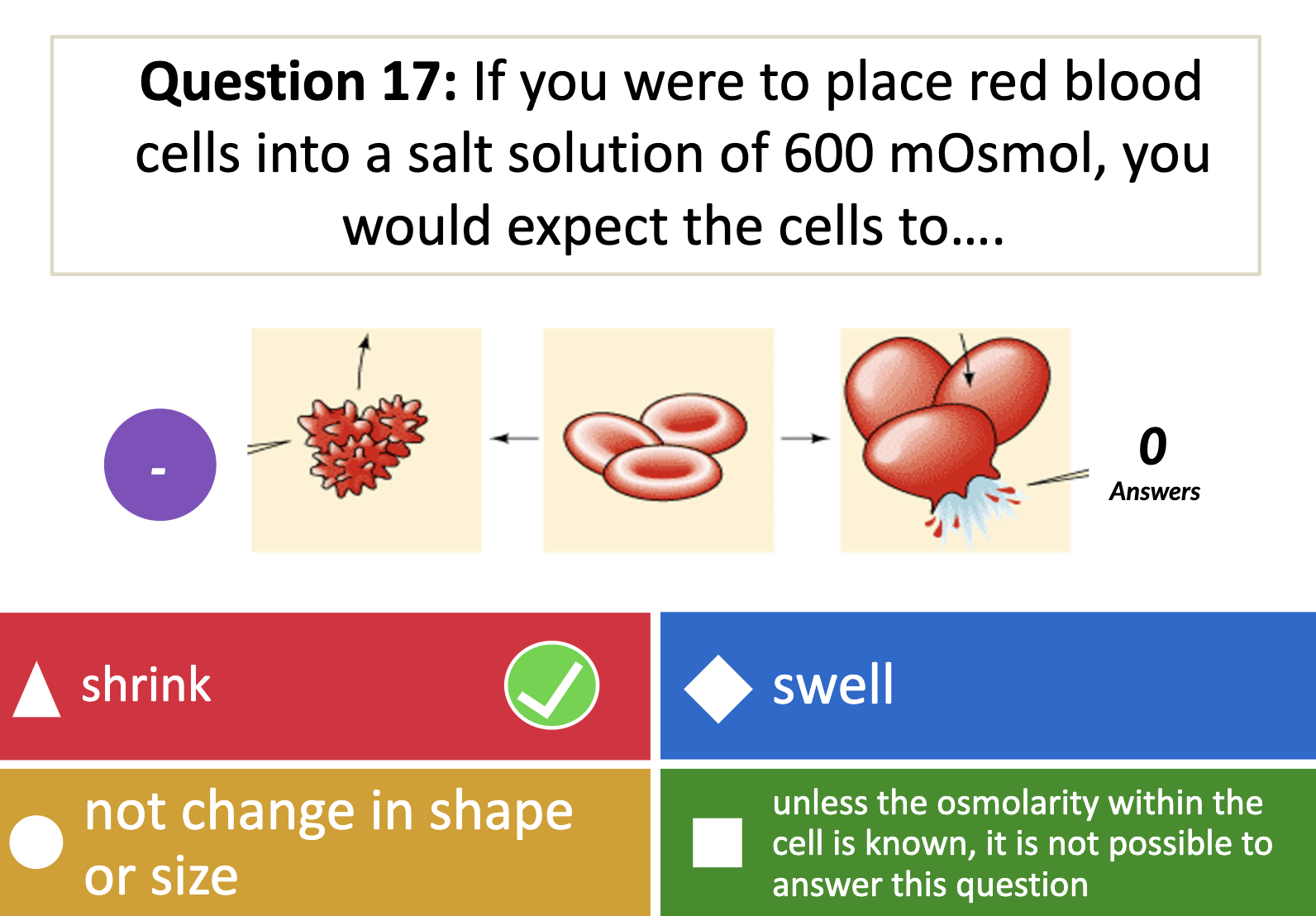
-hypotonicity
Having low concentration inside the cell than outside so it causes cells to swell.
-hypertonicity
Having high concentration outside than inside the cell in results, cells shrink
-High osmolarity has many applications. Why does salting meat or vegetables preserve them and why can cordial (especially strong cordial) help “purify” water?
When we place meat or vegetables within a high concentration of salt, water concentration is far less outside than inside the meat or vegetables so that water molecules escape from meat/vegetables to outside, which prevents baceteria or fungi growing.
-Understand the basic difference between facilitated diffusion and active transport
Facilitated diffusion is carried by integral protein carriers and they don't need ATP,
Active transport needs ATP to move molecules through the membrane.
-Phagocytosis/vescicular transport and receptor mediated vesicular transport/endocytosis
-Since ECF[Na+] is high, what might happen to
Water balance in a cell that was suffering a “power failure” and could generate sufficient ATP?
This happens, for example, to cells suffering a. interrupted blood supply and thus insufficient oxygen-this condition is termed hypoxia저산소증. A complete absence of oxygen is termed anoxia무산소증, by the way.
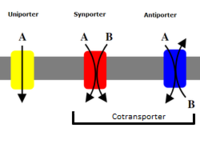
-There is another situation/region where 2nd active transport of glucose and amino acids occurs. ExplainIn the intestine and renal proximal tubule, glucose is transported against a concentration gradient by a secondary active transport mechanism in which glucose is cotransported with sodium ions.
-Do not confuse this co-transport with GLUT-transporters
Cotransporters are a subcategory of membrane transport proteins that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient.
'Griffith college Tri3 2022 > 1014MSC (CTR)' 카테고리의 다른 글
| LAB EXAM - ppt3,4 (0) | 2022.11.23 |
|---|---|
| LAB EXAM - ppt1,2 (0) | 2022.11.23 |
| WEEK4 - module2. The integumentary system (0) | 2022.11.14 |
| WEEK3 - module2. From cells to tissues (0) | 2022.11.06 |
| WEEK2 - module 1. Introduction to Microbiology (0) | 2022.10.29 |



