This week you will be introduced to the nuclear atom model, properties of elements and naming inorganic compounds.
Topic 3 consists of 2 parts
Part 1: Dalton’s Model of the Atom, Subatomic Parts of the Atom, The Nuclear Atom (Rutherford model), Isotopes, Atomic mass.
Part 2: The Bohr Atom Model, Energy Level of Electrons, Electron Structures and the Periodic Table.
Topic 4: Nomenclature (Naming) of Inorganic Compounds.
TOPIC3 -part 1
The Development of the Atomic Model
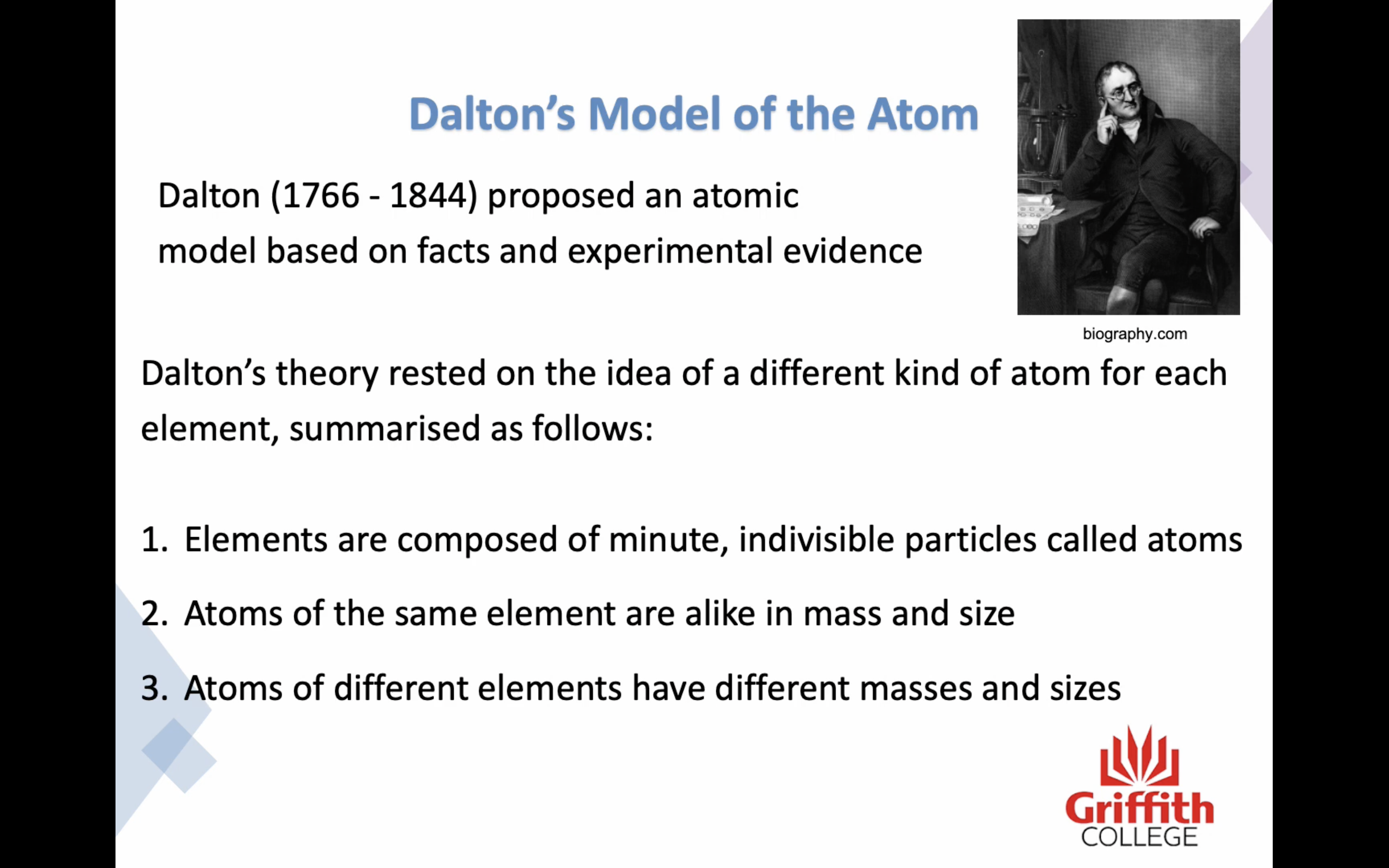
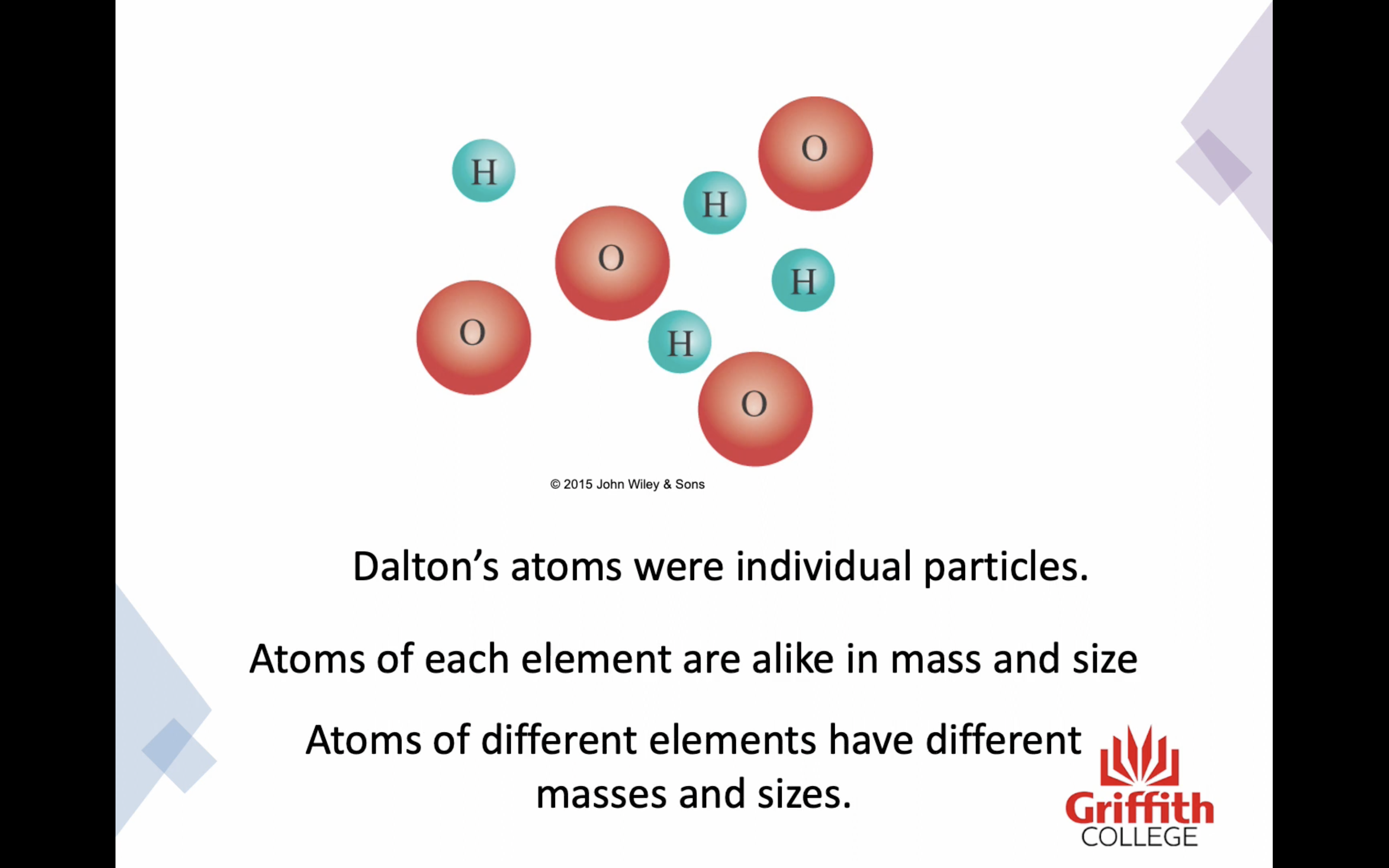
Dalton's model of the atom
1. Elements are composed of minute, indivisible particles called atoms
2. Atoms of the same elements are alike in mass and size
3. Atoms of different elements have different masses and sizes
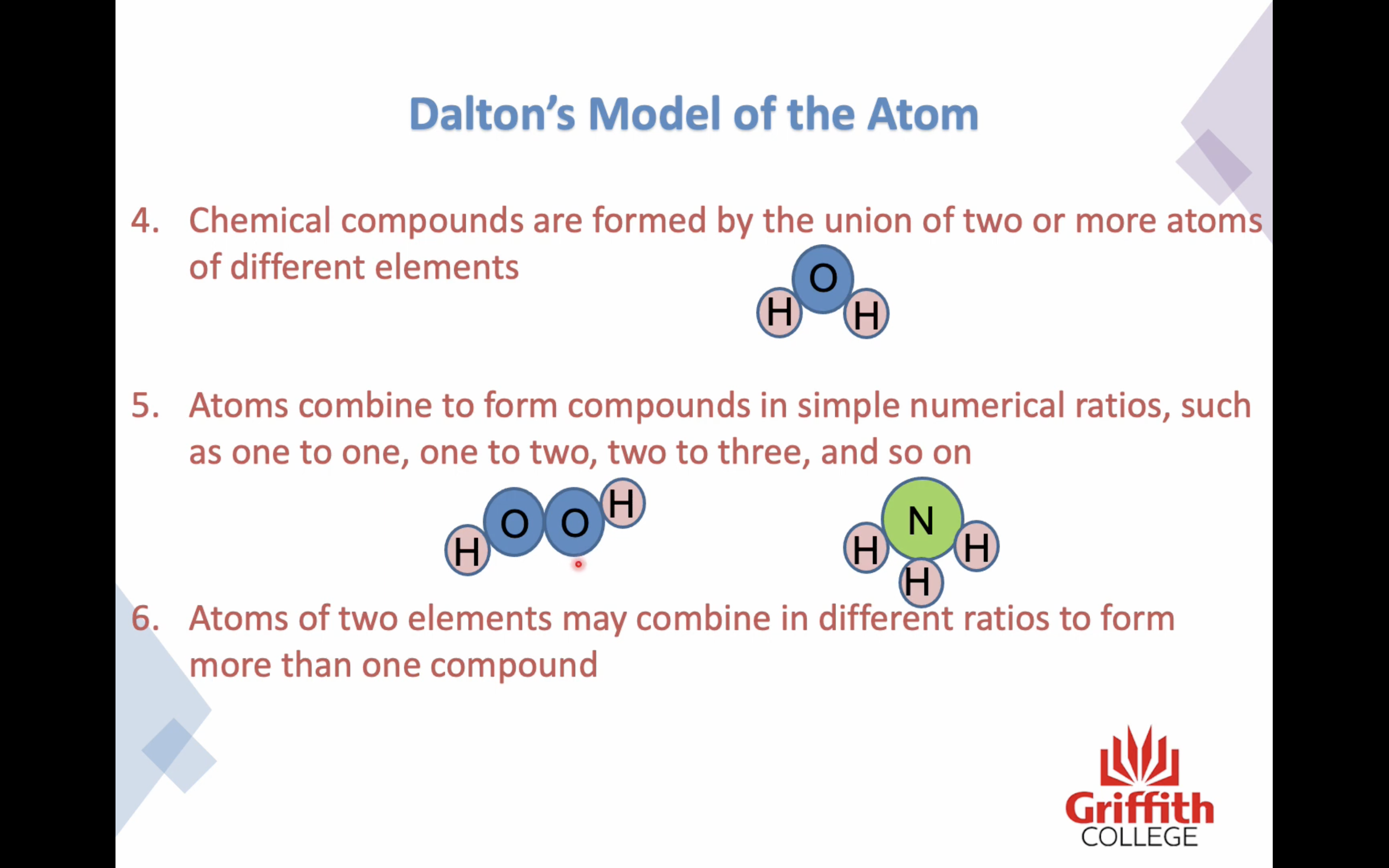
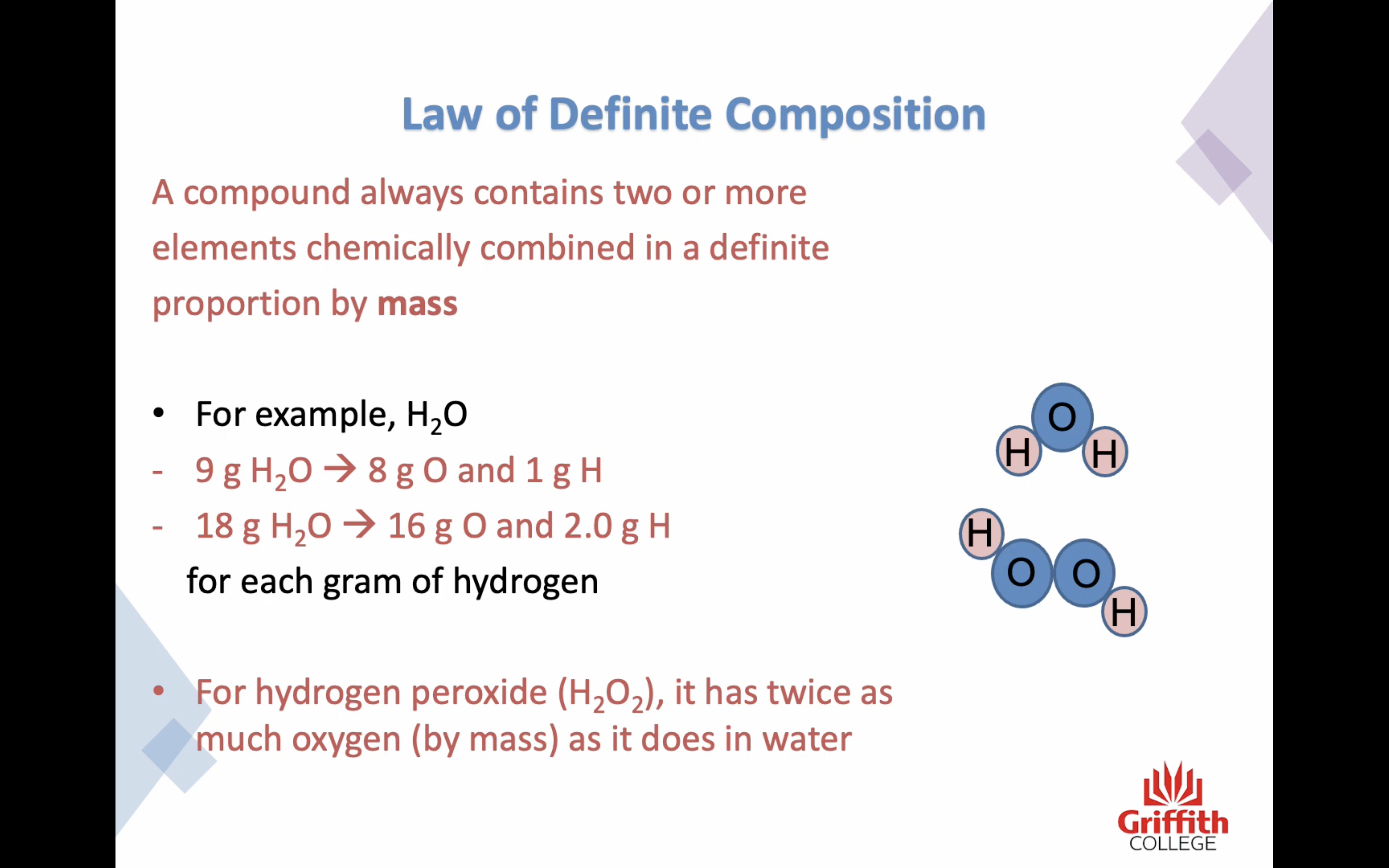
4. Chemical compounds are formed by the union of two or more atoms
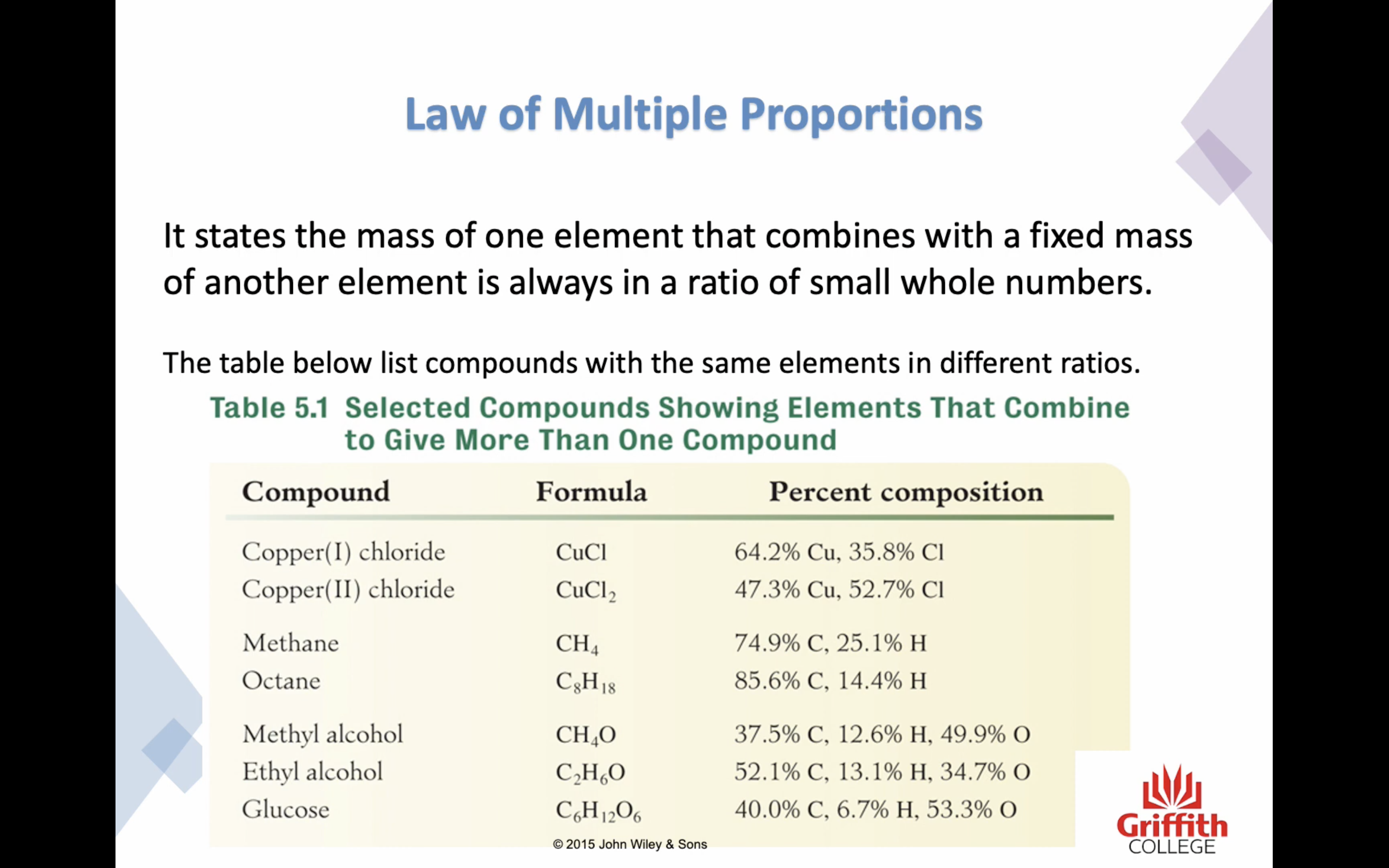
5. Atoms combine to form compounds in simple numerical ratios, such as one to one, one to two, two to three and so on
6. Atoms of two elements may combine in different ratios to form more than one compound

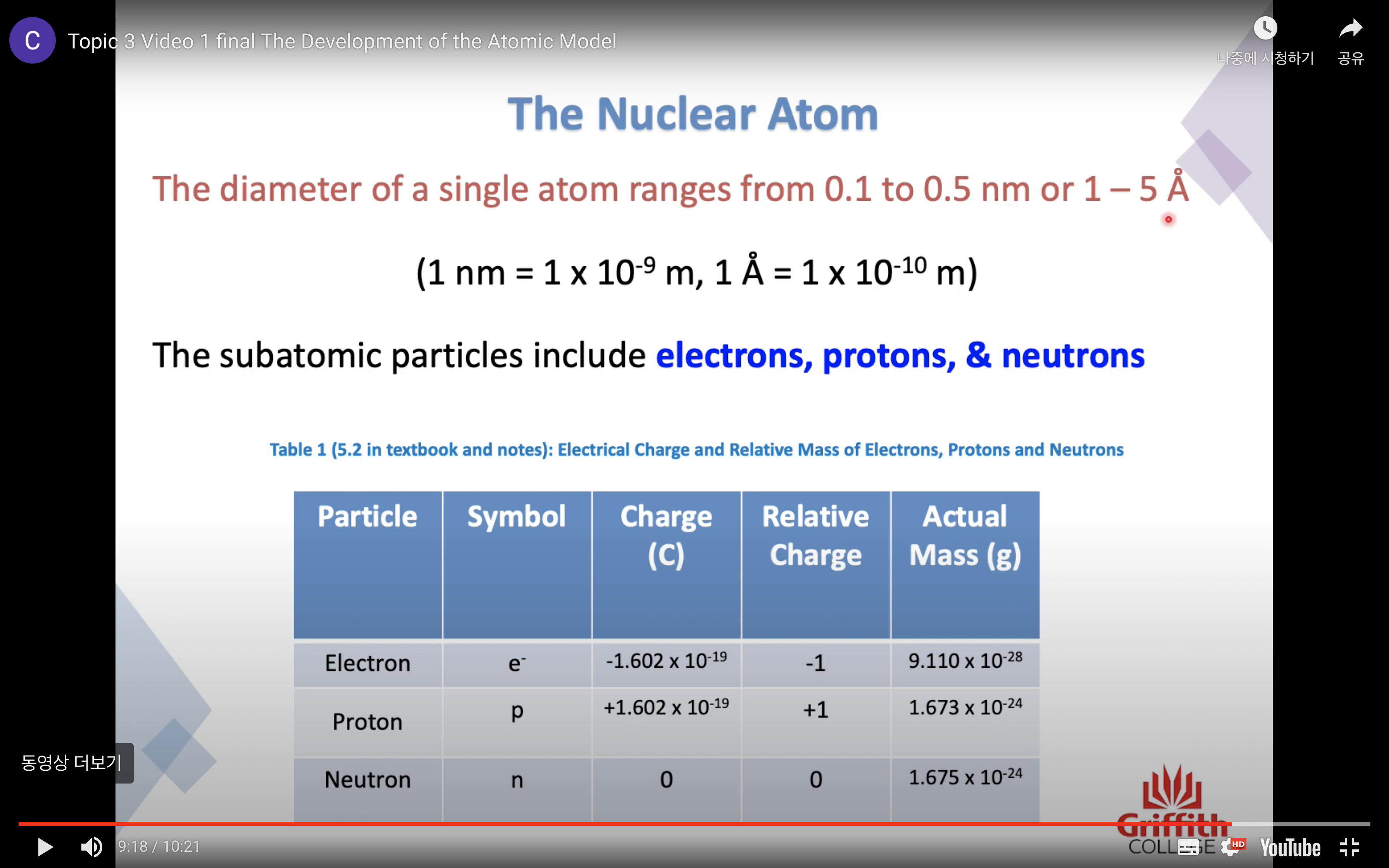
Rutherford's atomic model
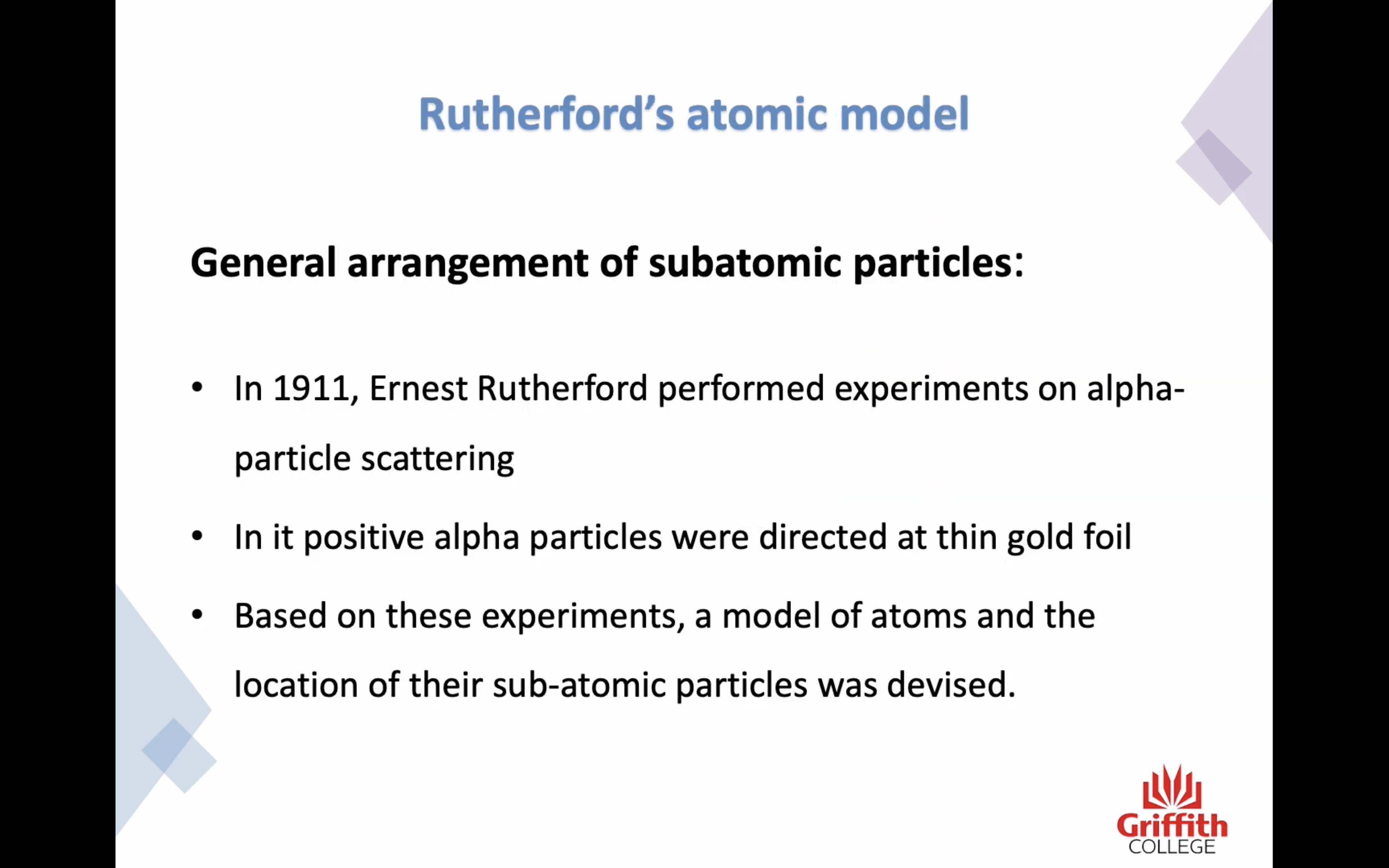
'General arrangement of subatomic particles'
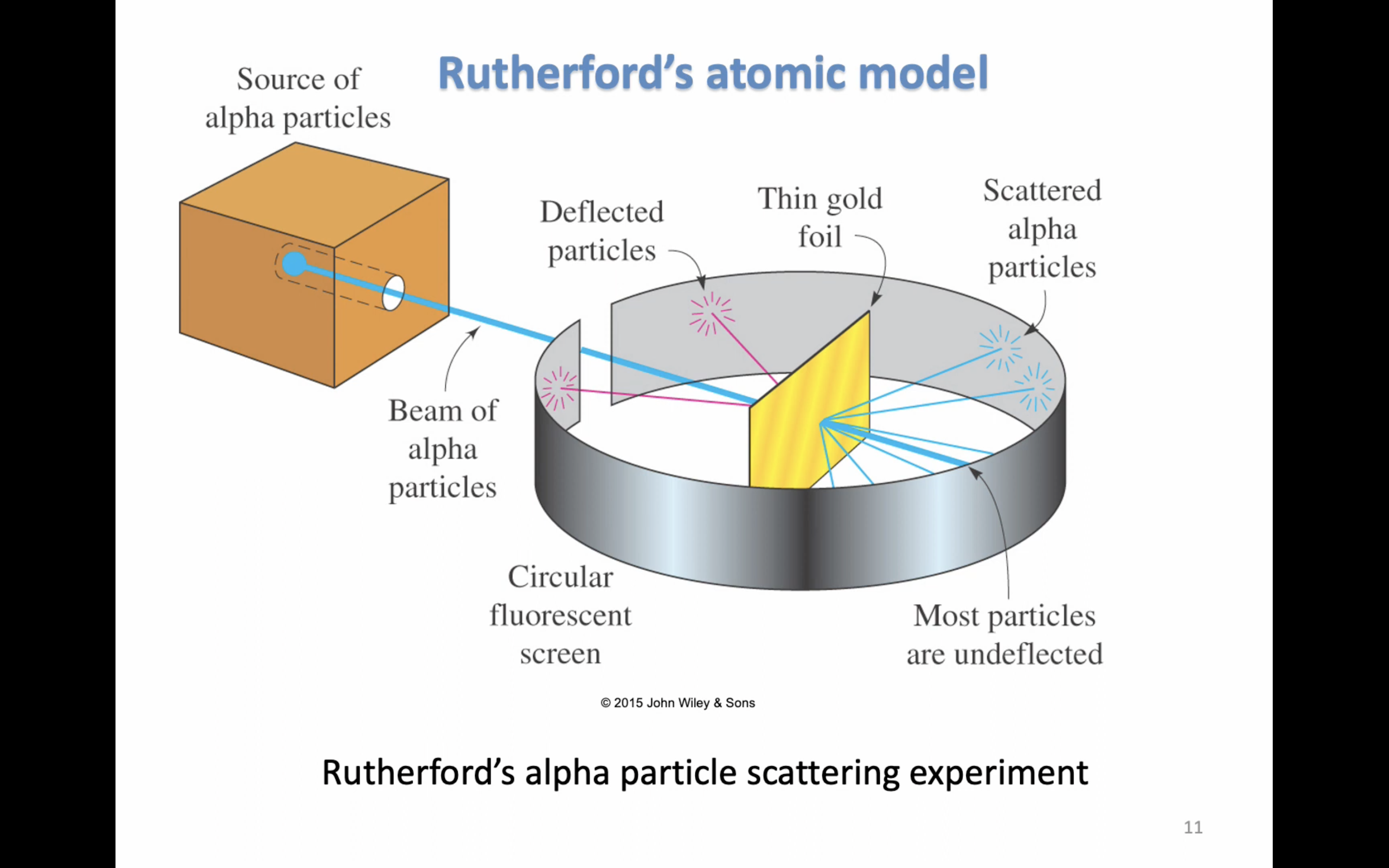
-In 1911, Ernest Rutherford performed experiments on alpha-particle scattering
-In it positive alpha particles were directed at thin gold foil
-Based on these experiments, a model of atoms and the location of their subatomic particles was devised
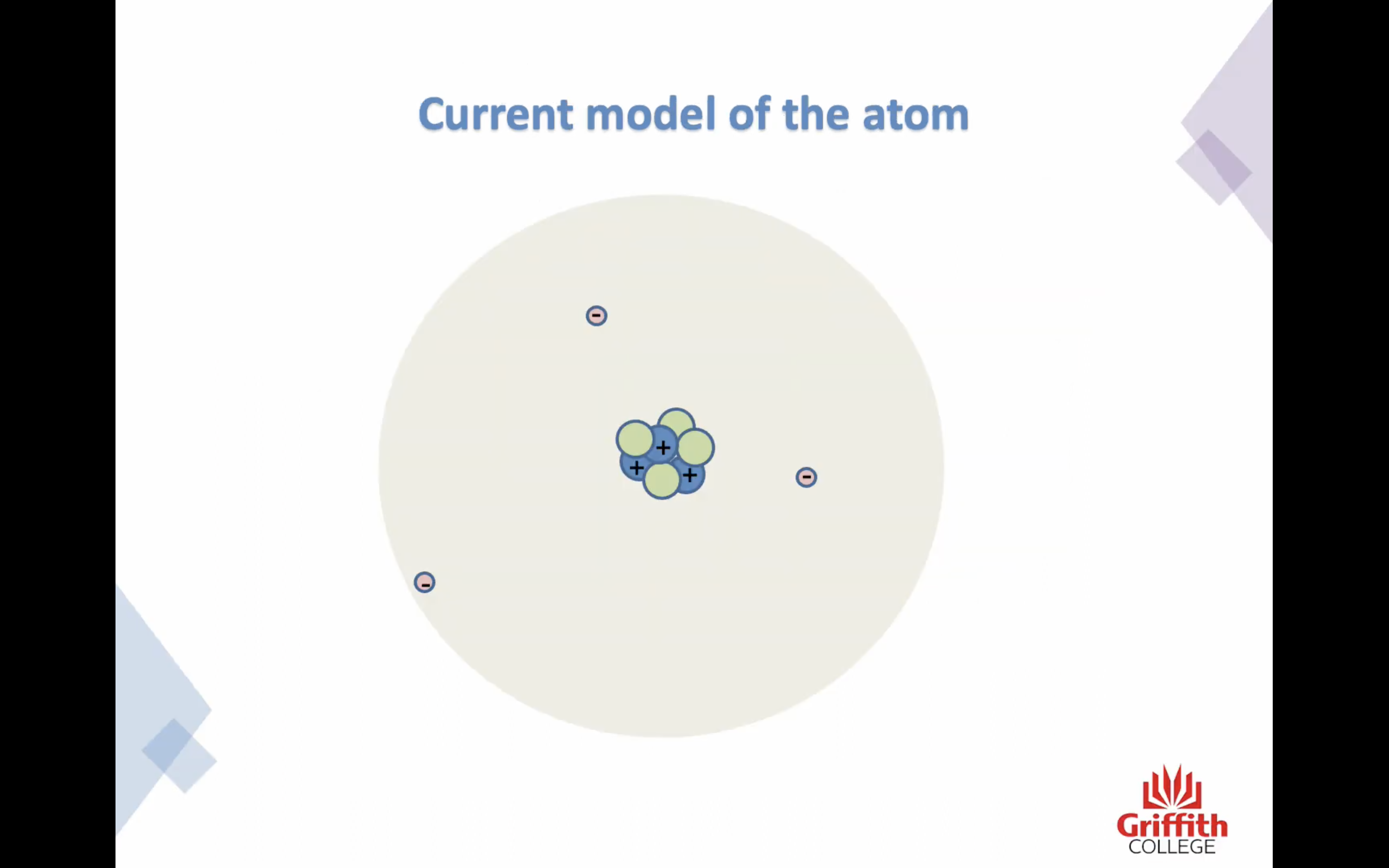
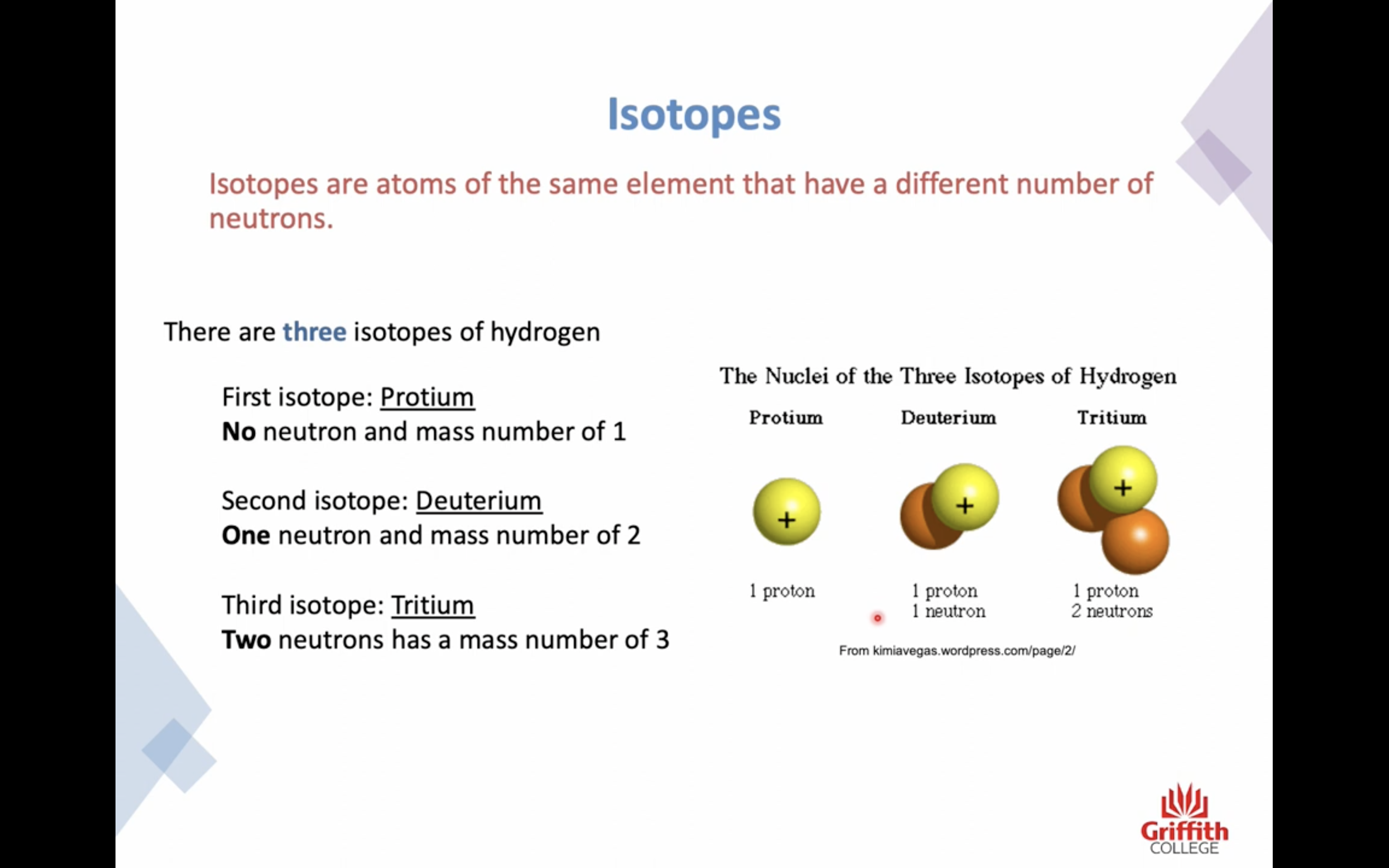
Isotopes
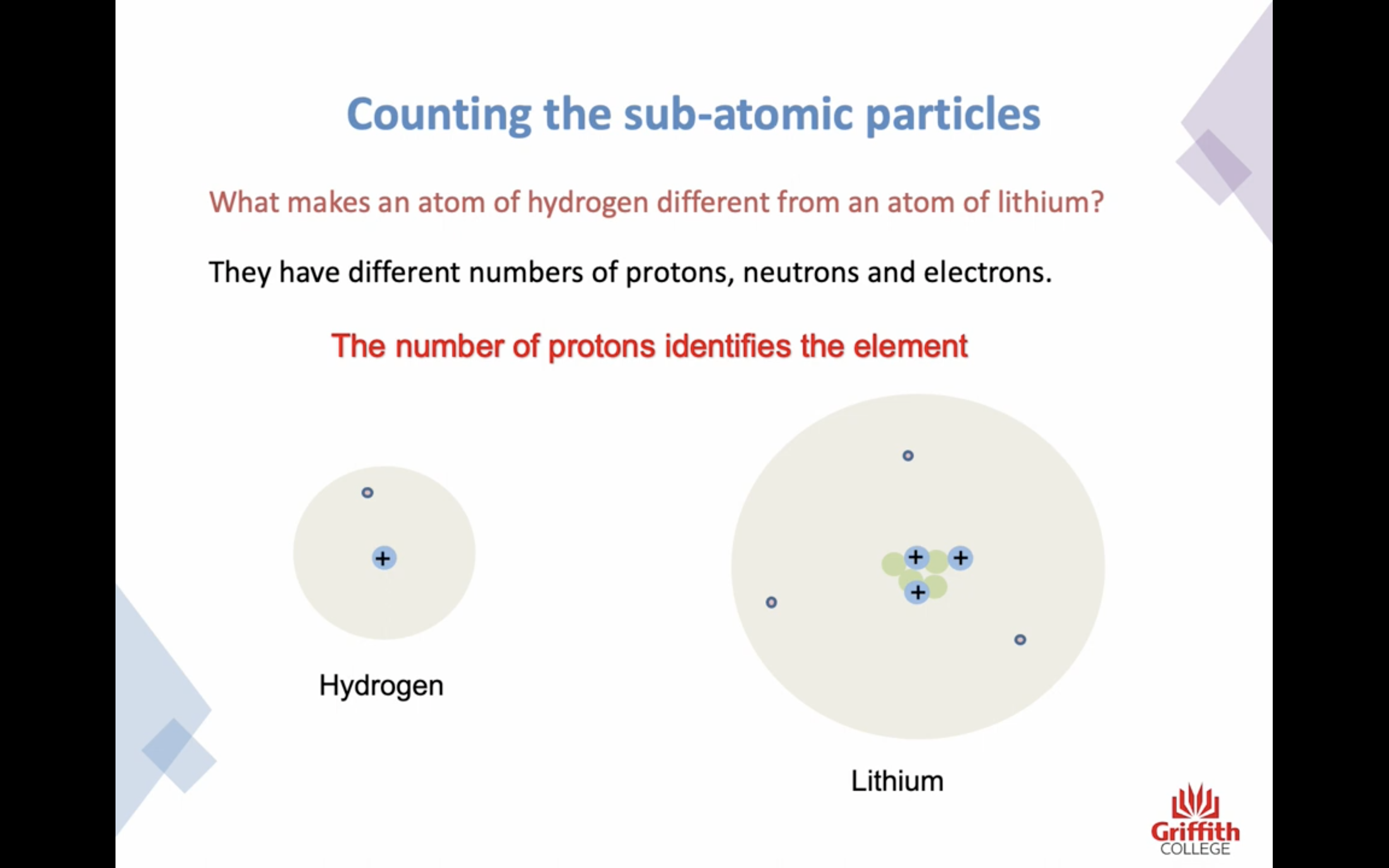
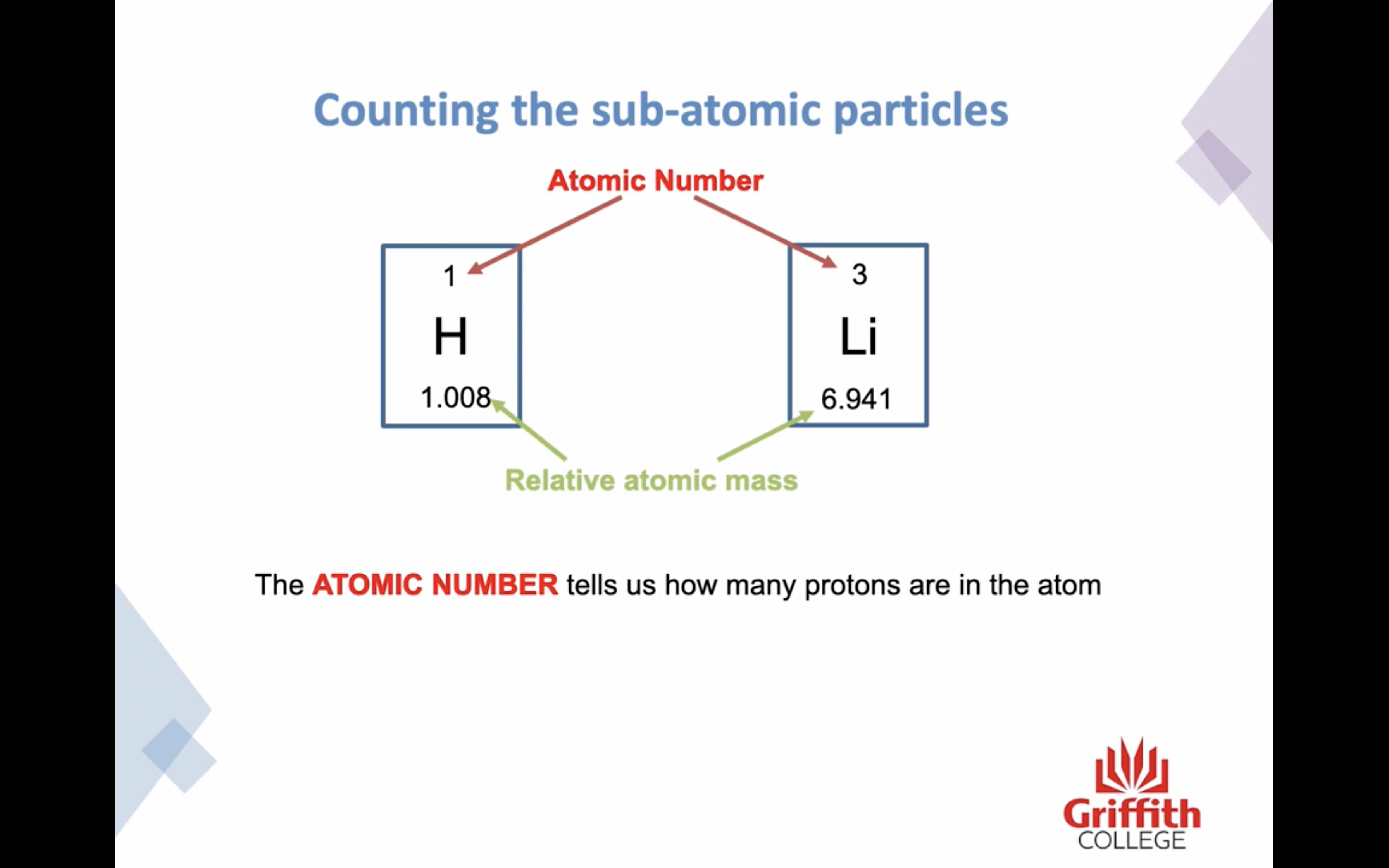
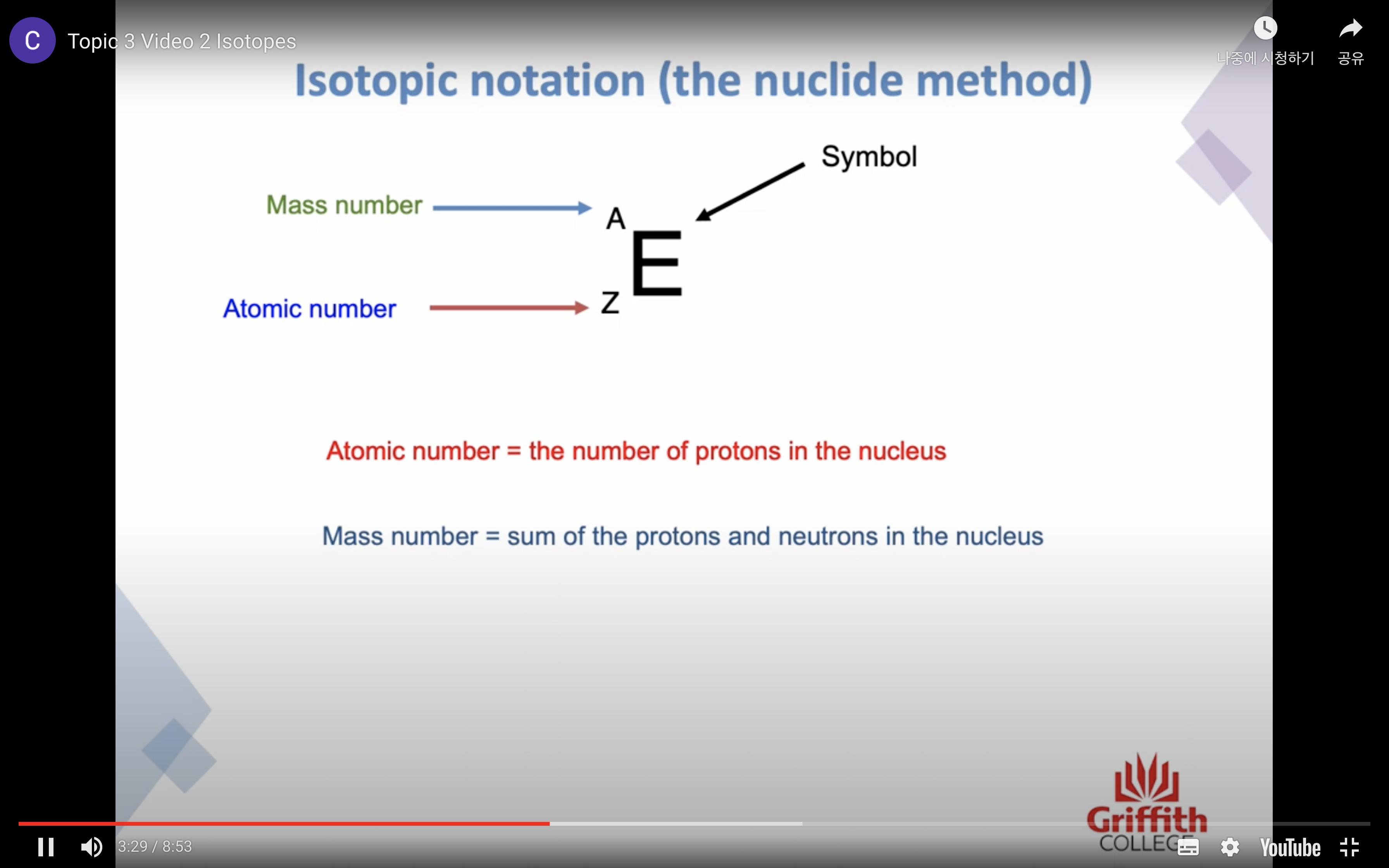
The number of protons identifies the element !
Isotypes are atoms of the same element that have a different number of neutrons
The atomic number tells us how many protons are in the atom
Mass number is the sum of the protons and neutrons in the nucleus
Atomic Mass
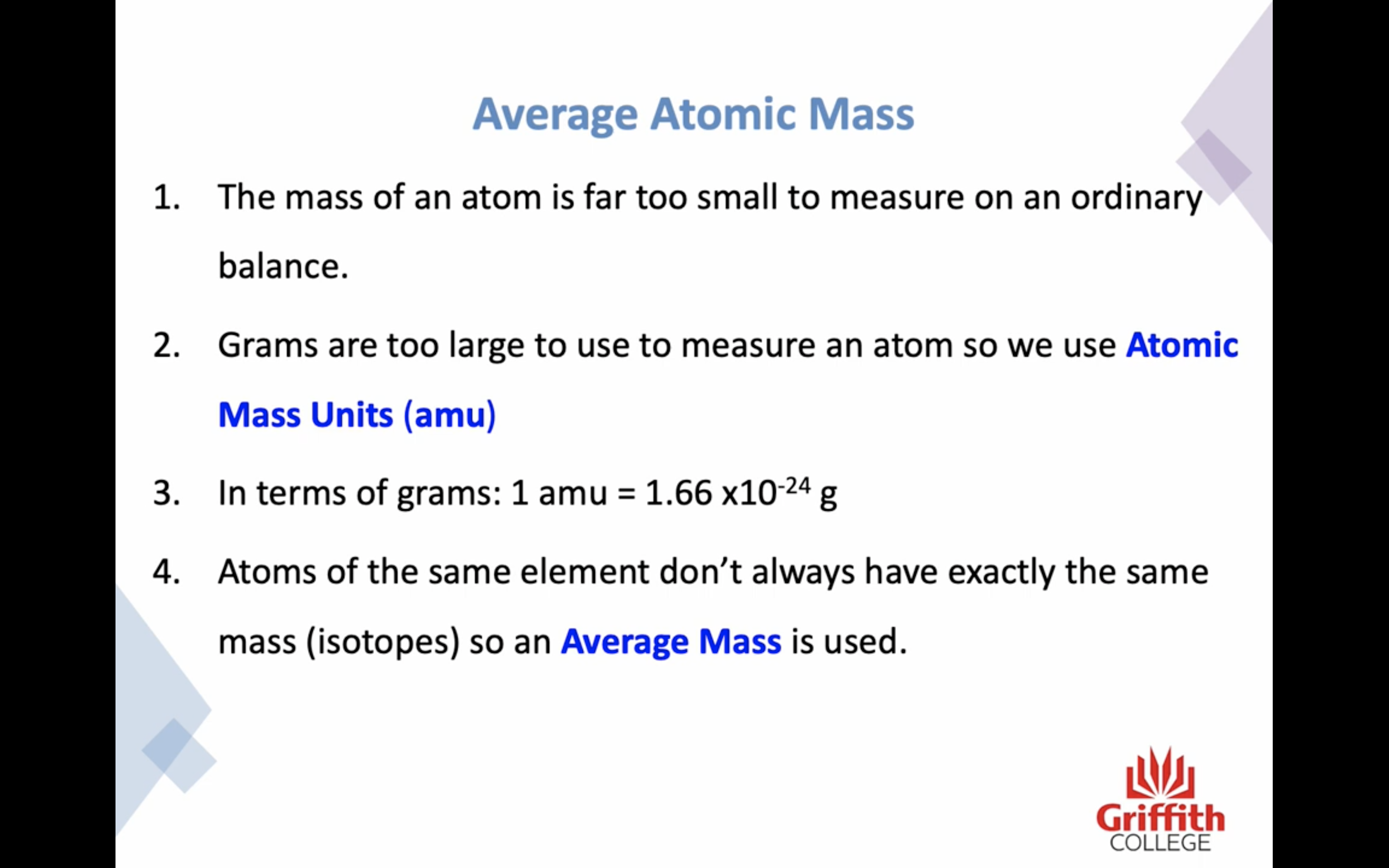
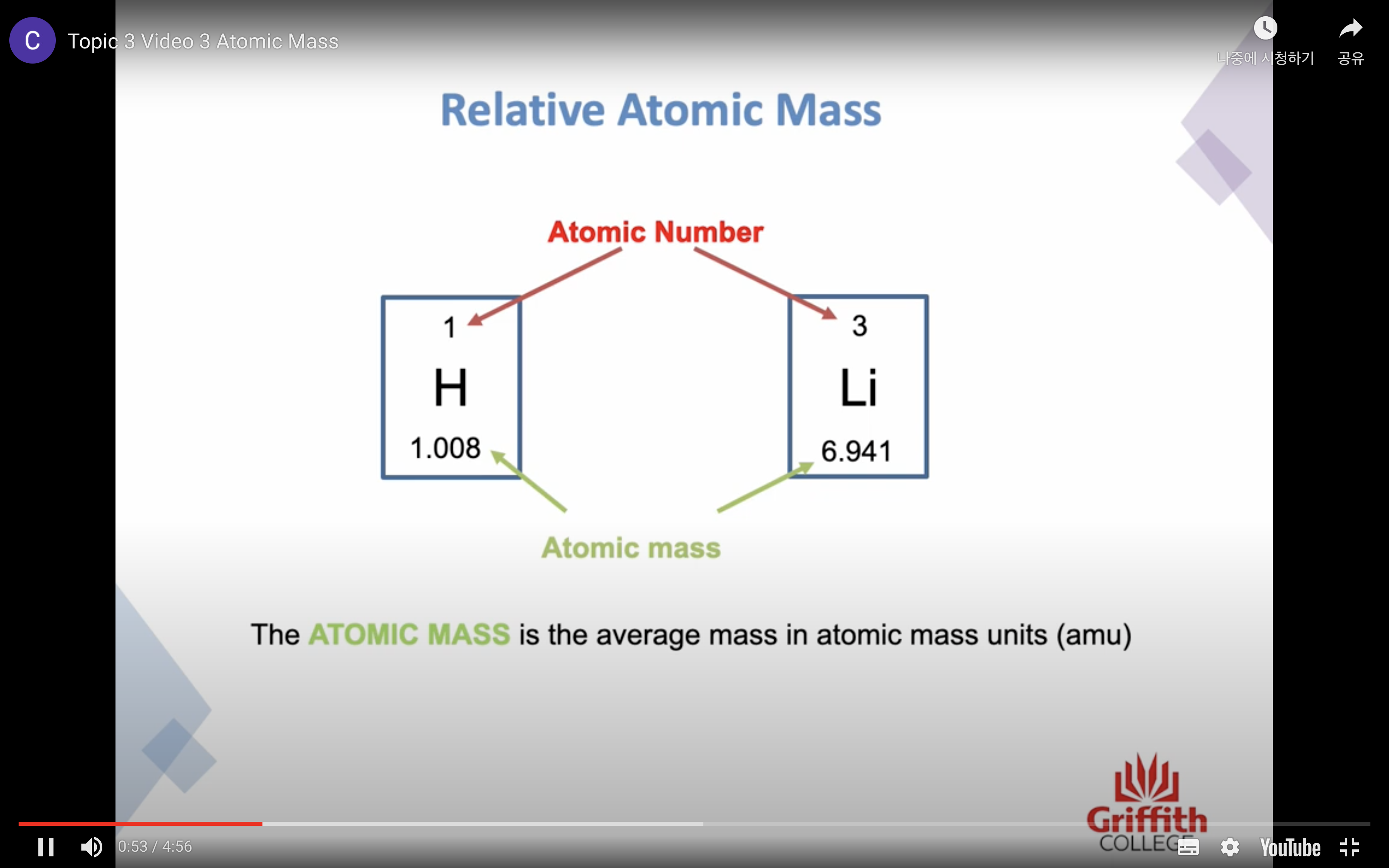
The mass of an atom is far too small to measure -> atomic mass units (amu)/ 1 amu = 1.66 x 10^(-24) g
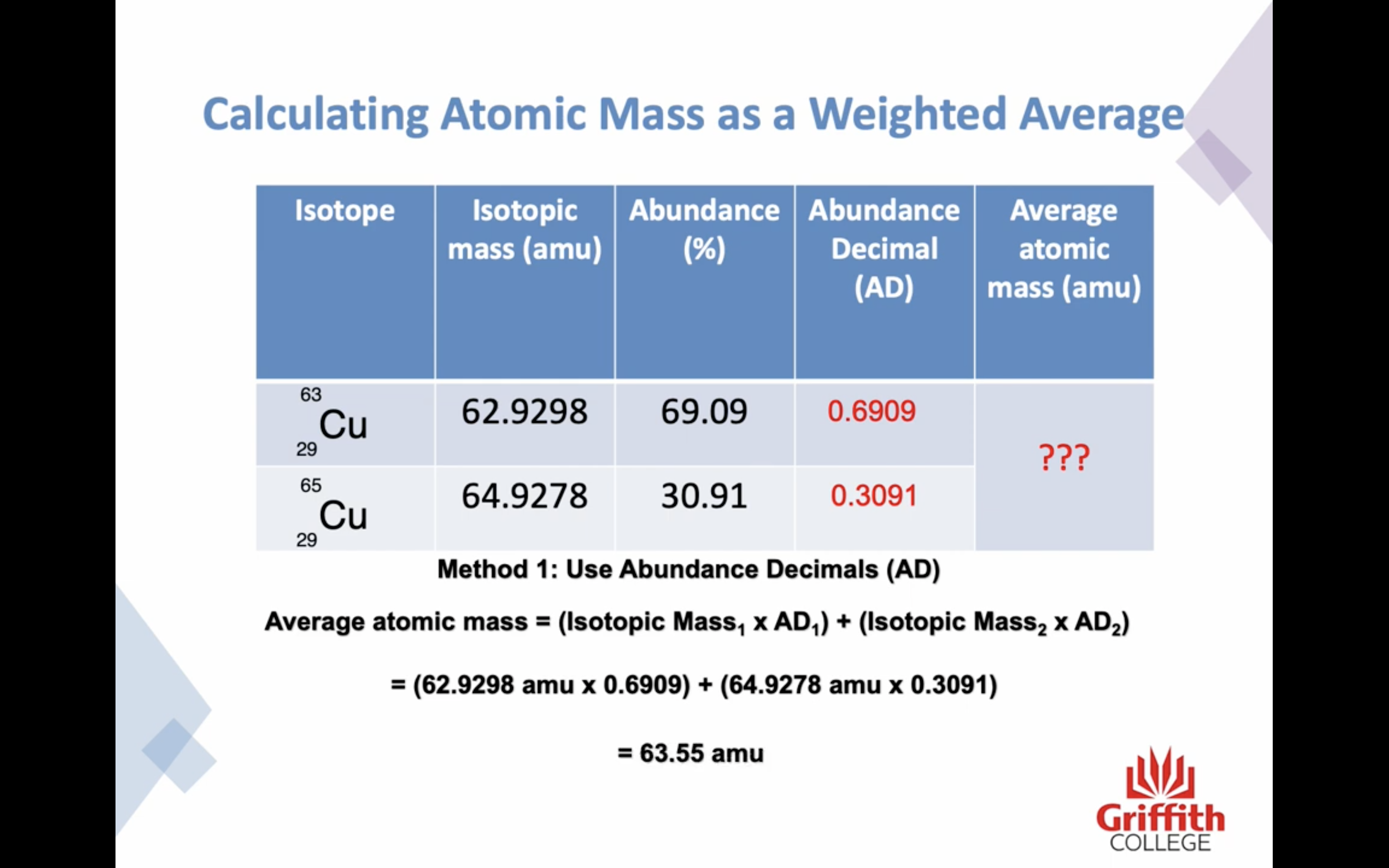
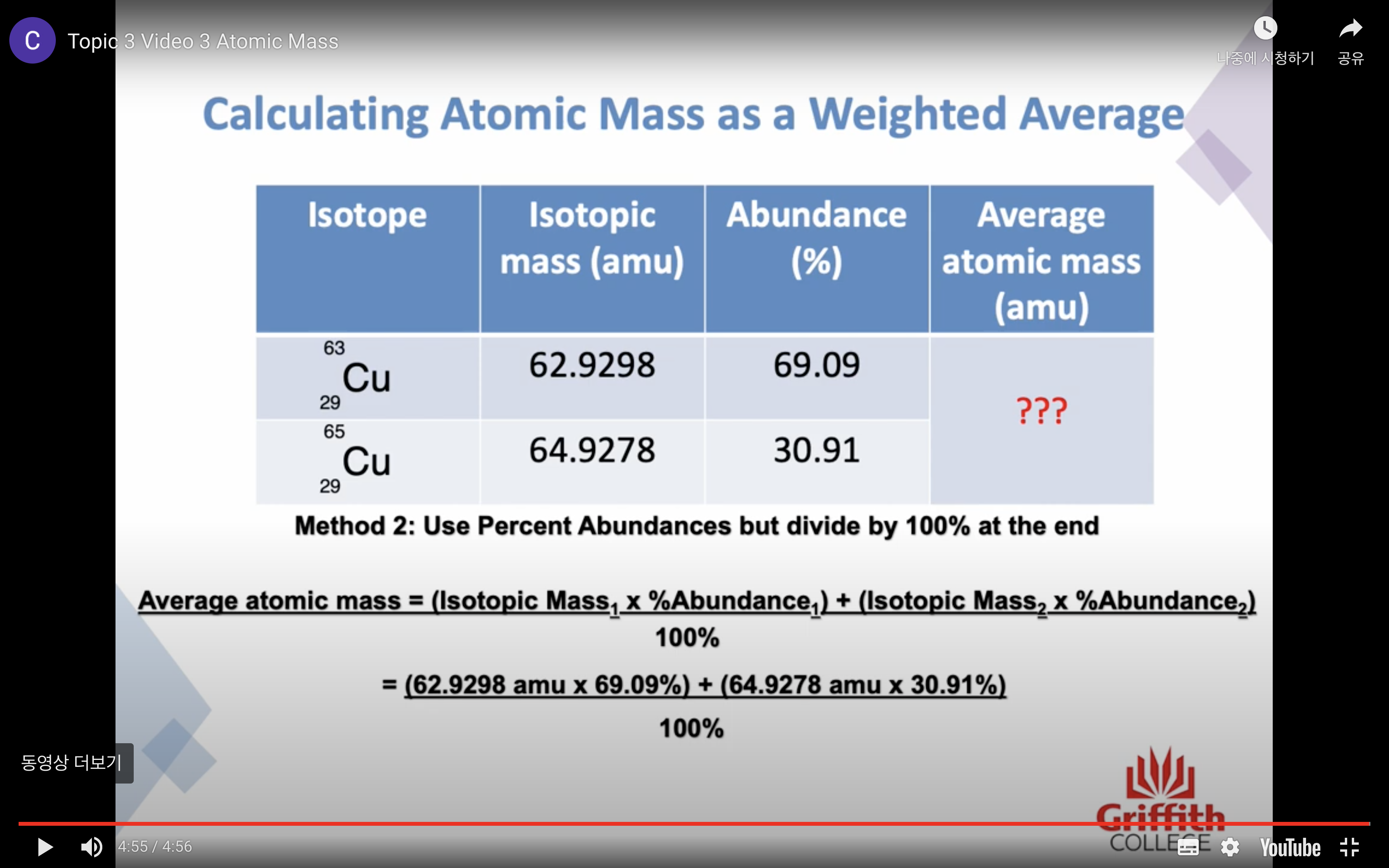
Because many elements exist as a mixture of isotopes AVERAGE MASS is used
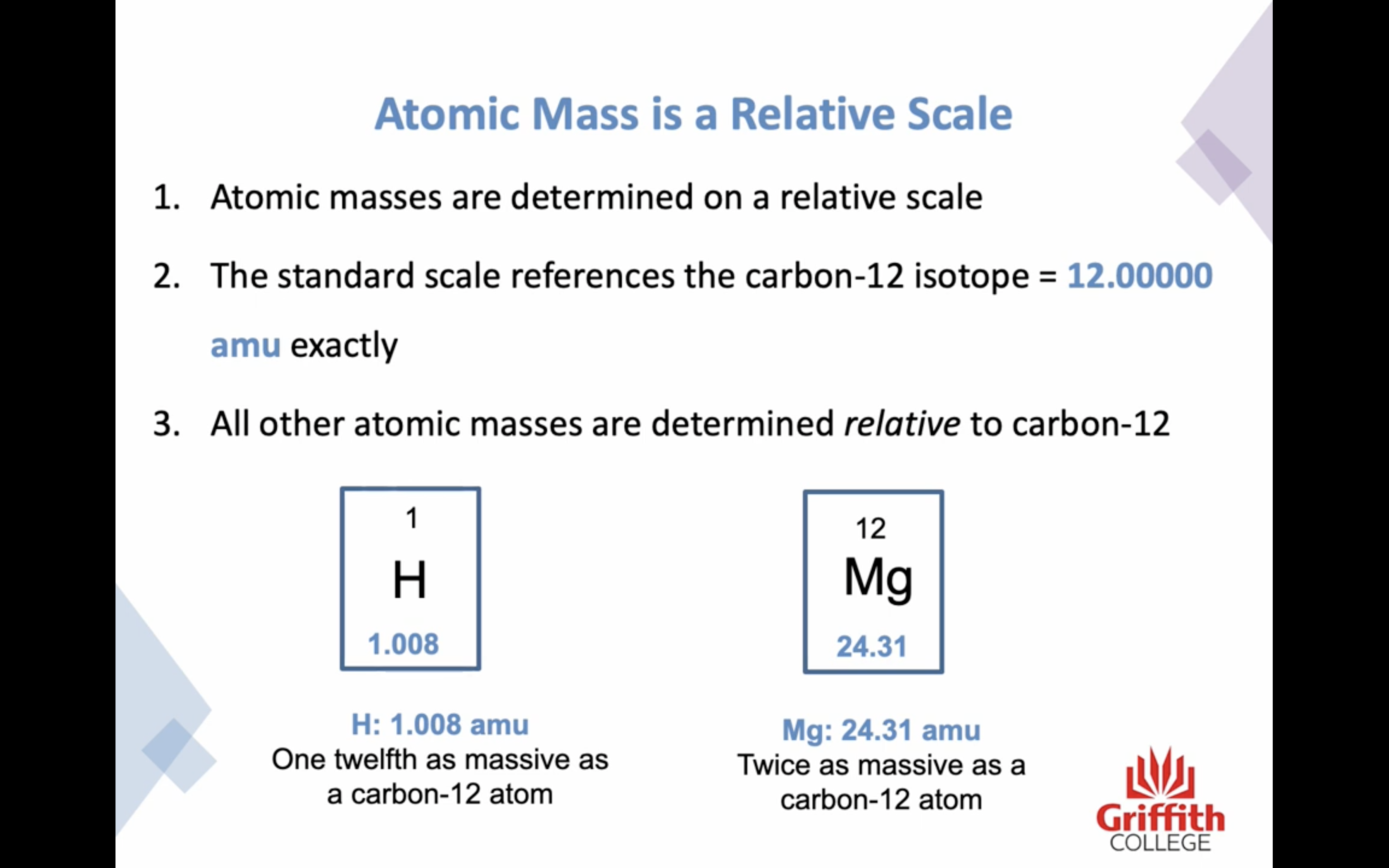
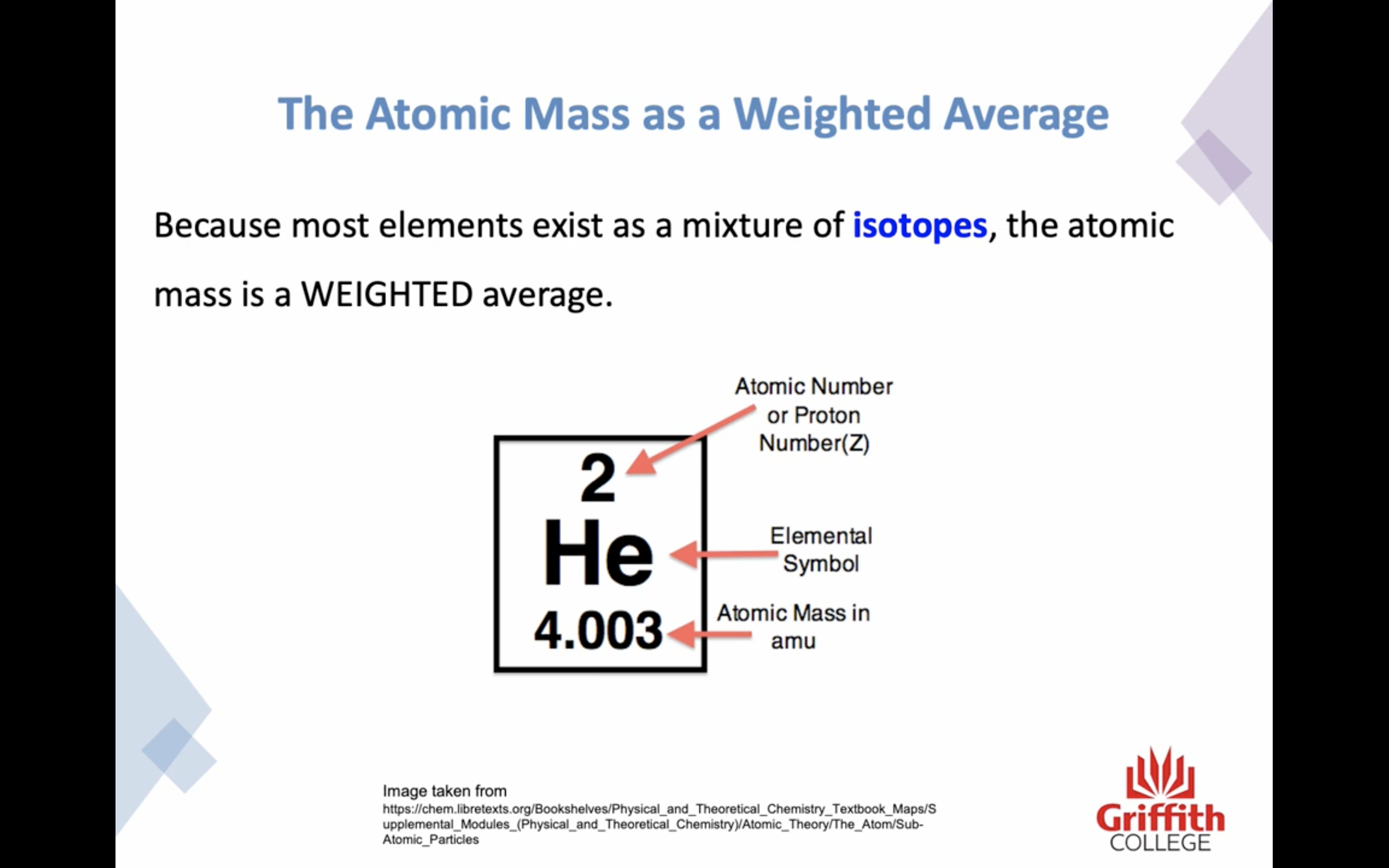
Atomic mass is a RELATIVE SCALE
The standard scale references the carbon -12 isotope is 12.000000 amu exactly
TOPIC3 -part 2
The Bohr Atom and Development of the Quantum Physics Theory
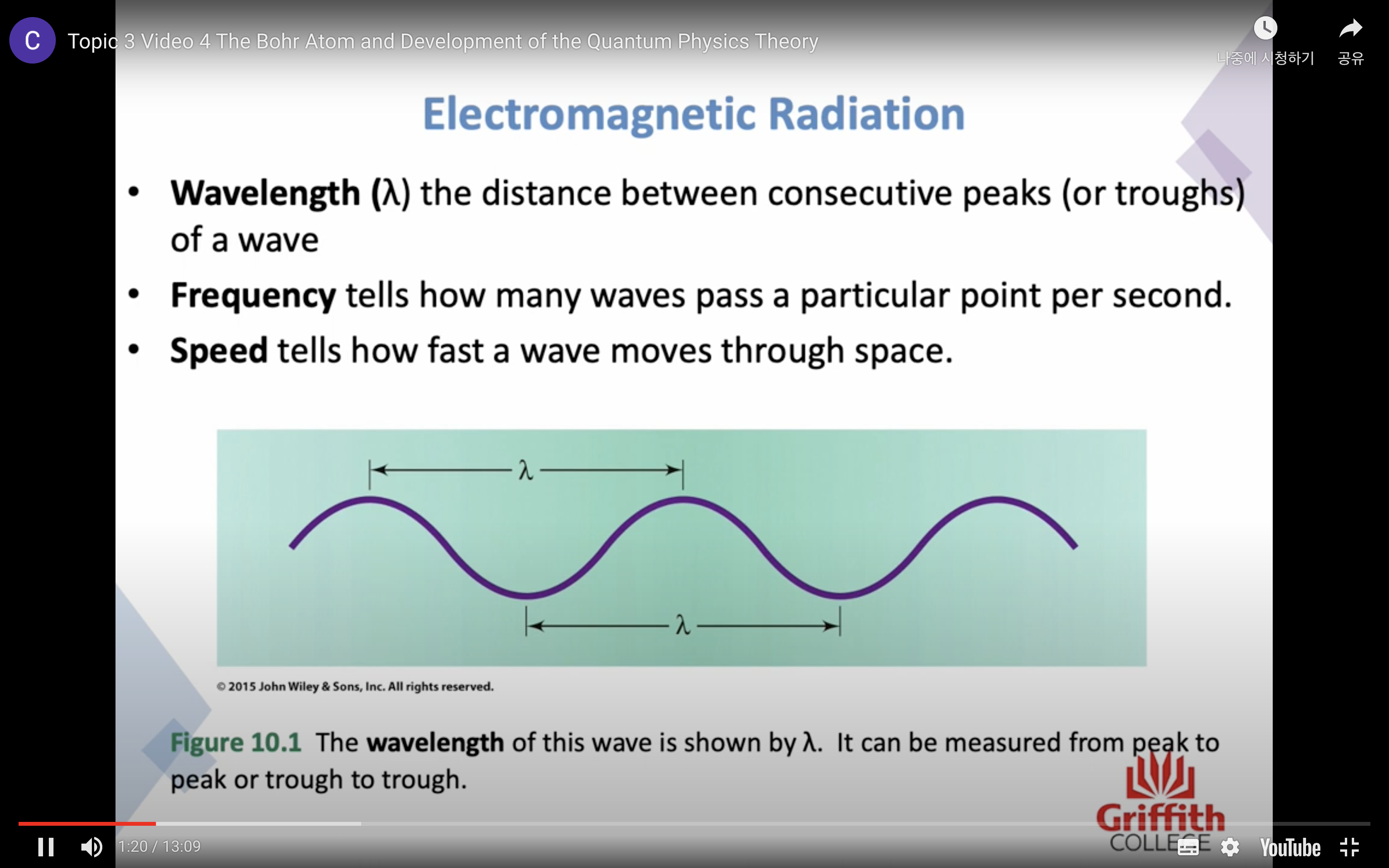
electromagnetic radiation = 전자기파
햇빛, 엑스레이, 전자레인지파, 라디오 파, 다 전자기 파임
-wavelength : 파장 the distance between consecutive peaks / troughs
-frequency : 주파수 tells how many waves pass a particular point per second
-speed : tells how fast a wave moves through space
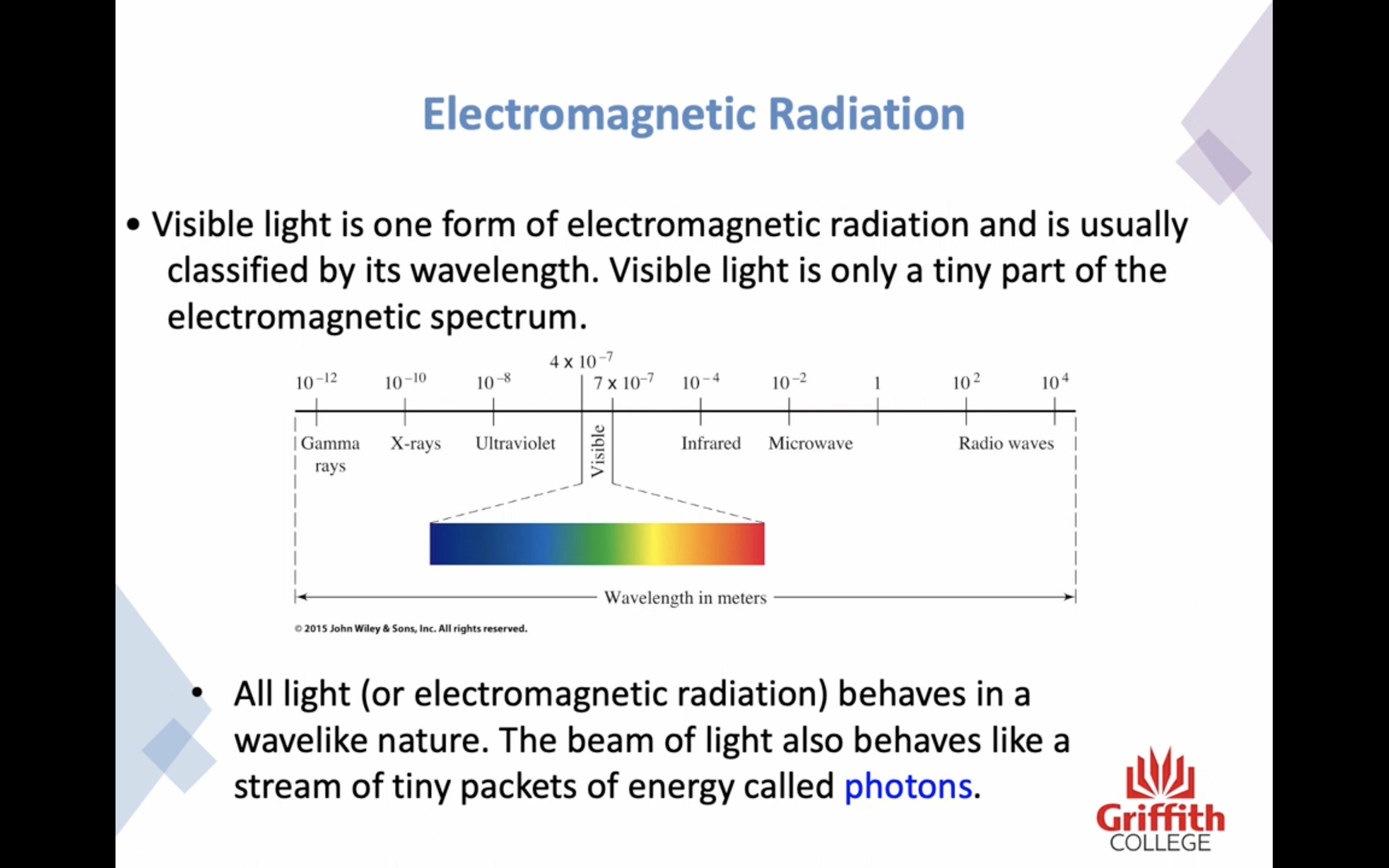
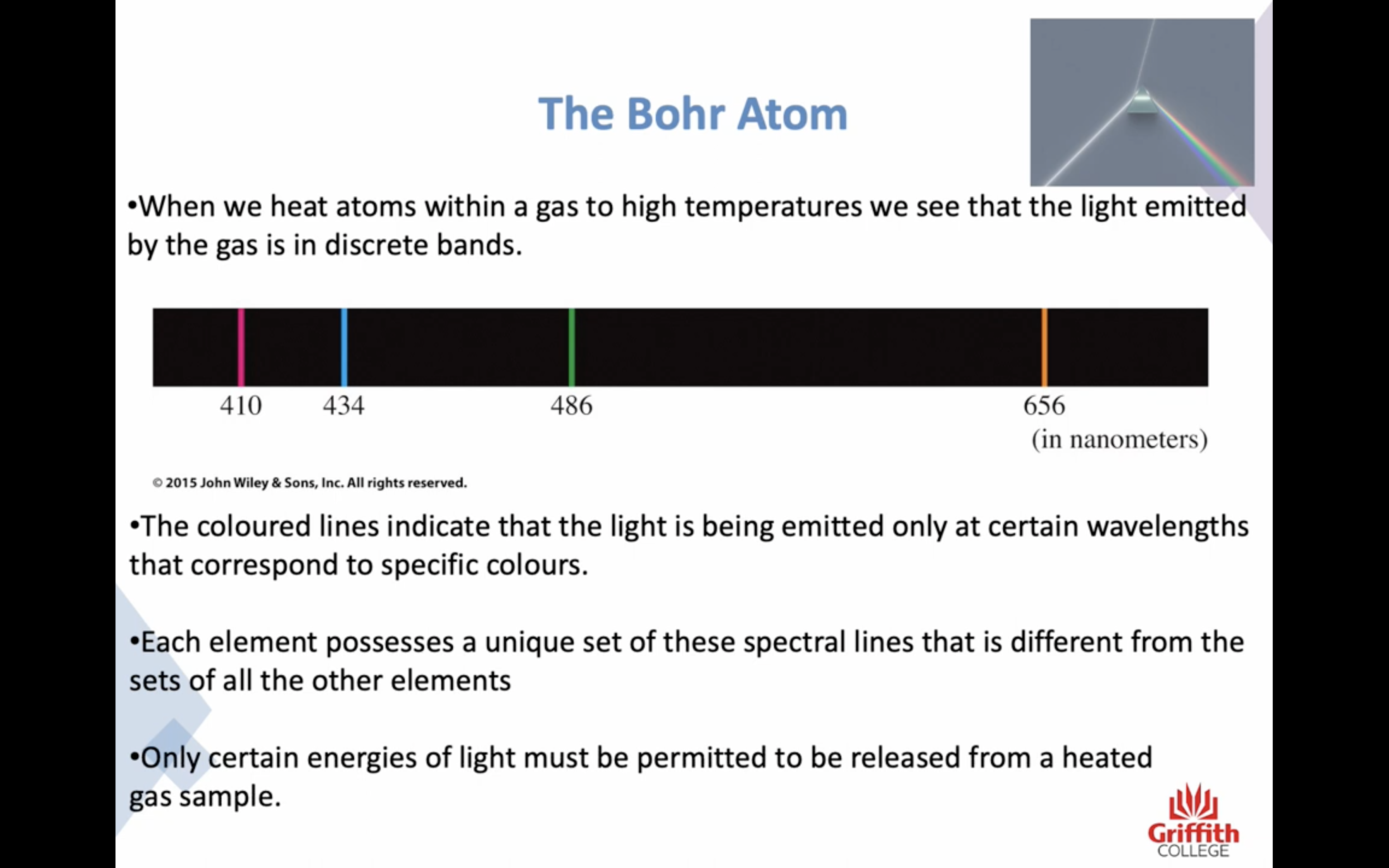
-All electromagnetic radiation behaves in a wavelike nature. The beam of light also behaves like a stream of tiny packets of energy called photons 광자
-Each element possess a unique set of these spectral lines that are different from the other elements
-Only certain energies of light must be permitted to be released from as heated gas sample
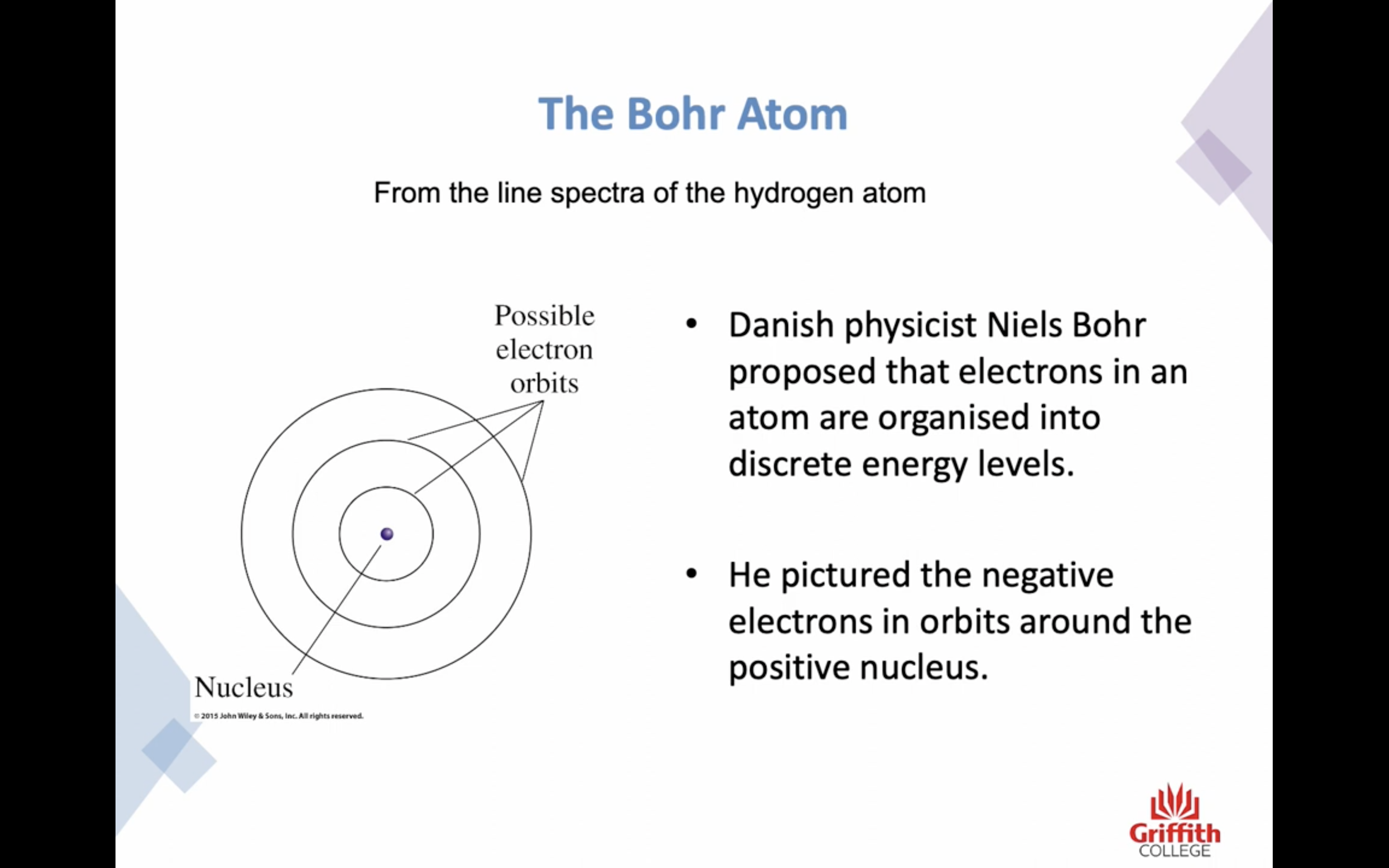
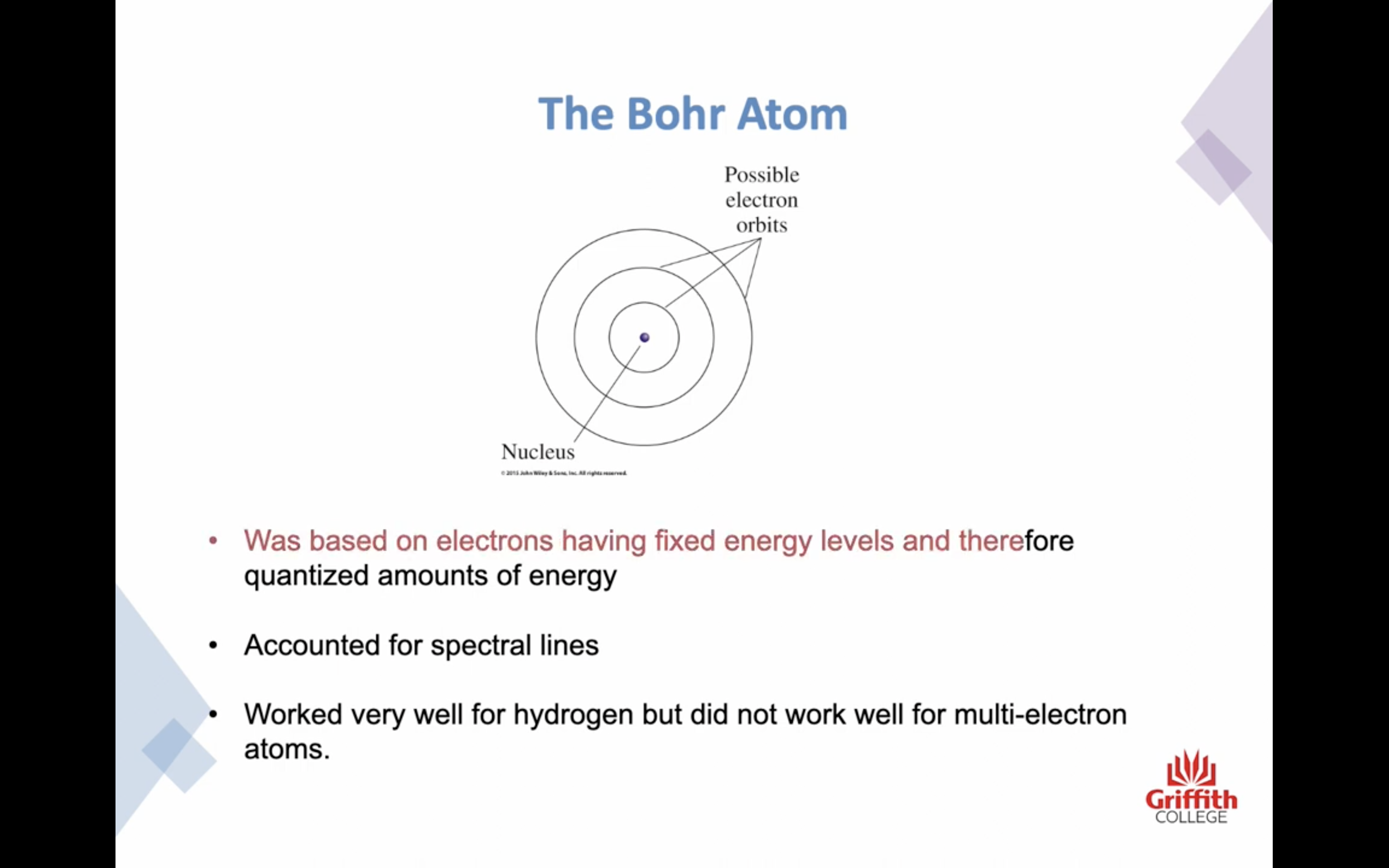
BOHS ATOM ( worked very well limited with hydrogen )
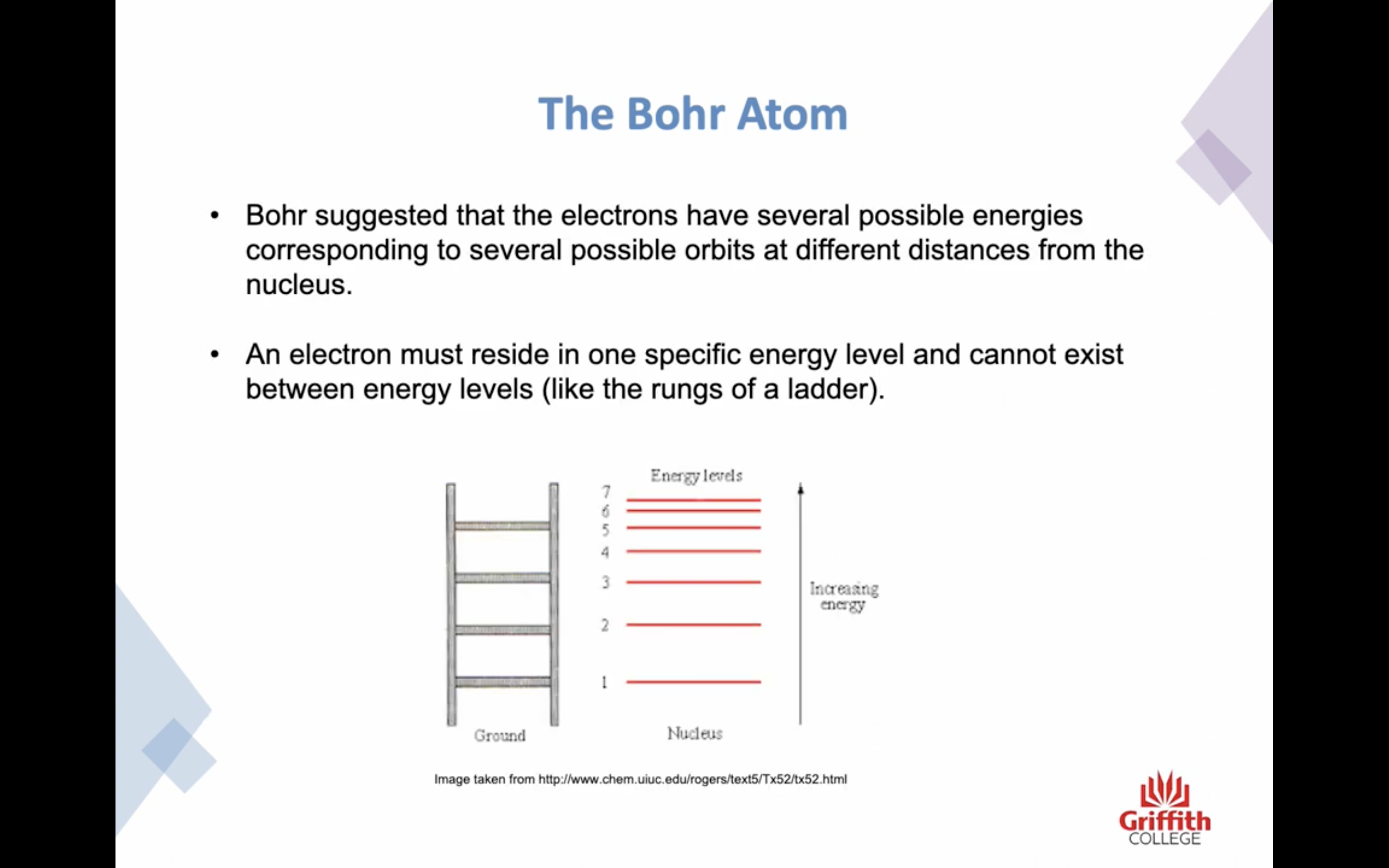
- Atoms are organised into discrete energy levels
- He pictured the negative electrons in orbits around the positive nucleus
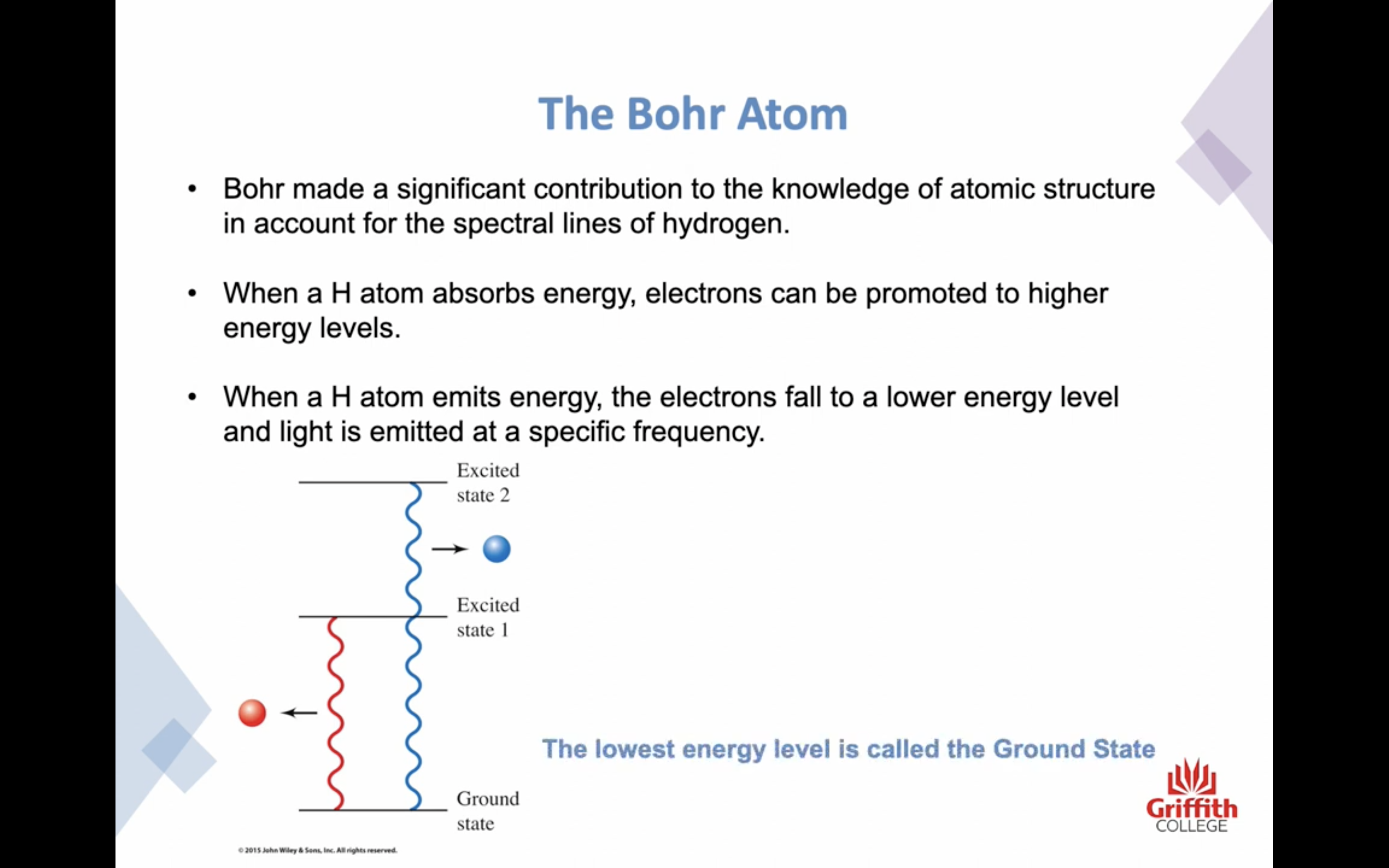
-An electron must reside in one specific energy level and cannot exist between energy levels
-Ground state / excited state : the lowest energy level is called the ground state
-The bohr atom was based on electrons having fixed energy levels
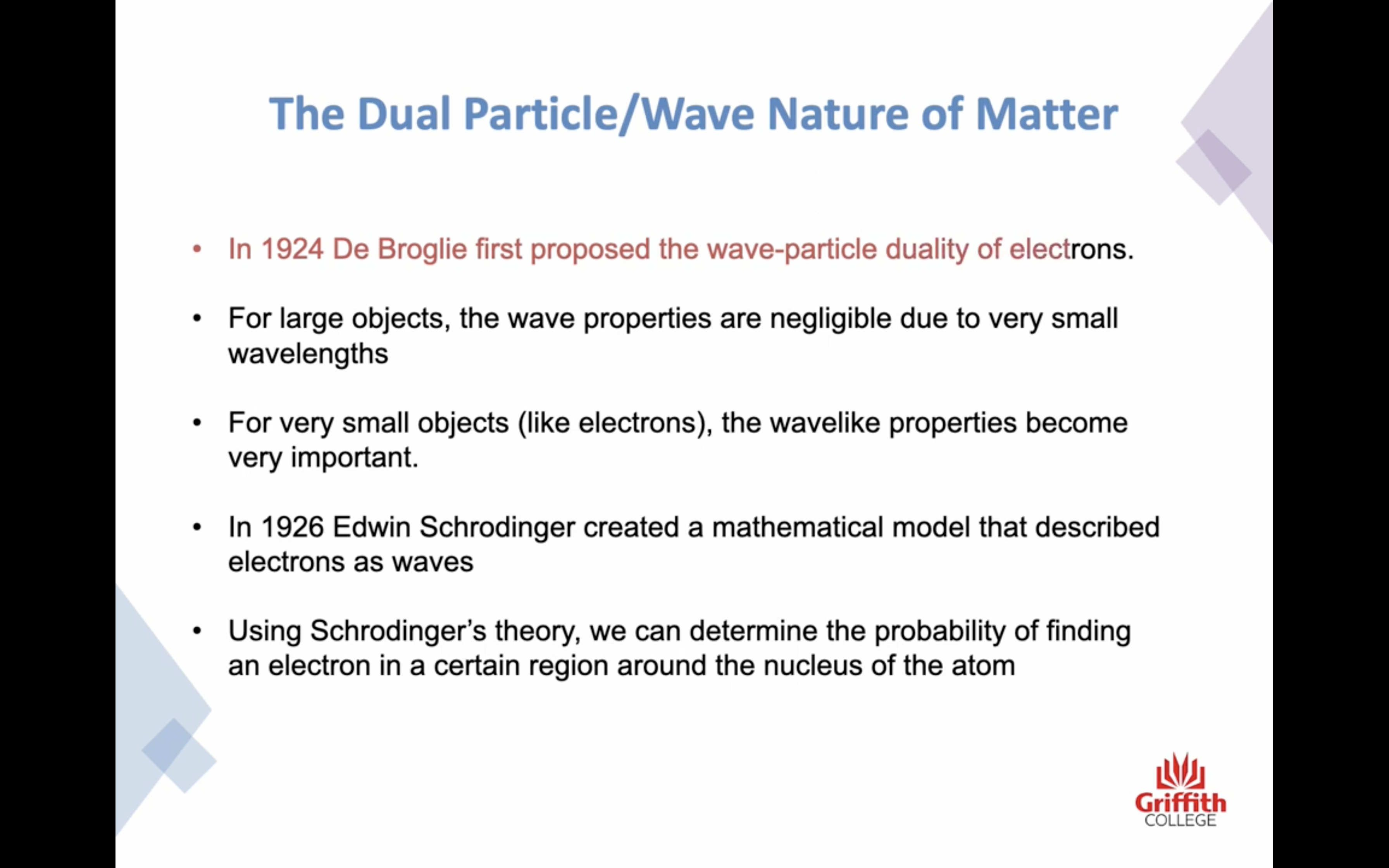
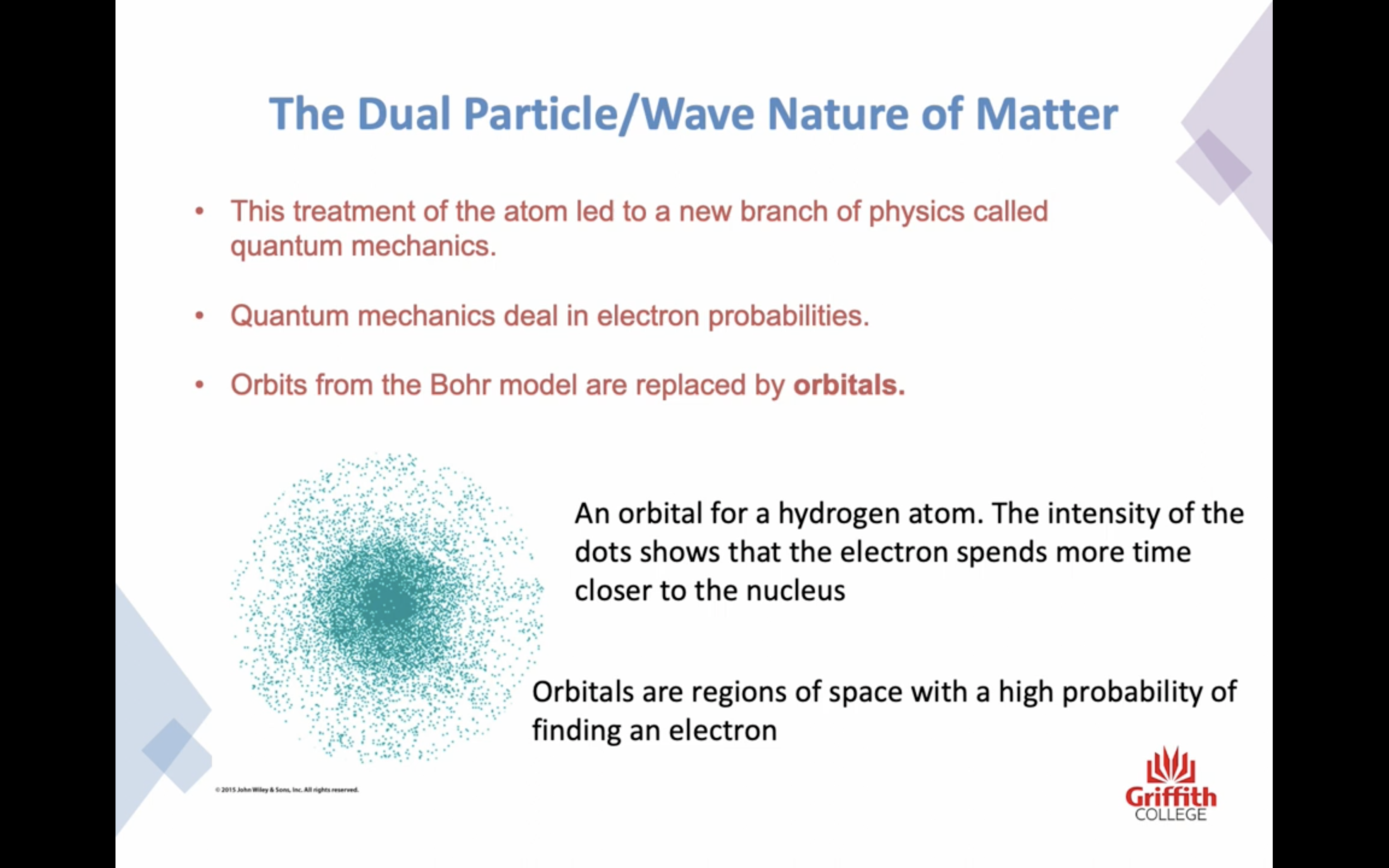
-Schrodinger's theory : we can determine the probability of finding an electron in a certain region around the nucleus of the atom
-Quantum mechanics : deal in electron probabilities 양자역학
-Orbits from the Bohr model are replaced by ORBITALS
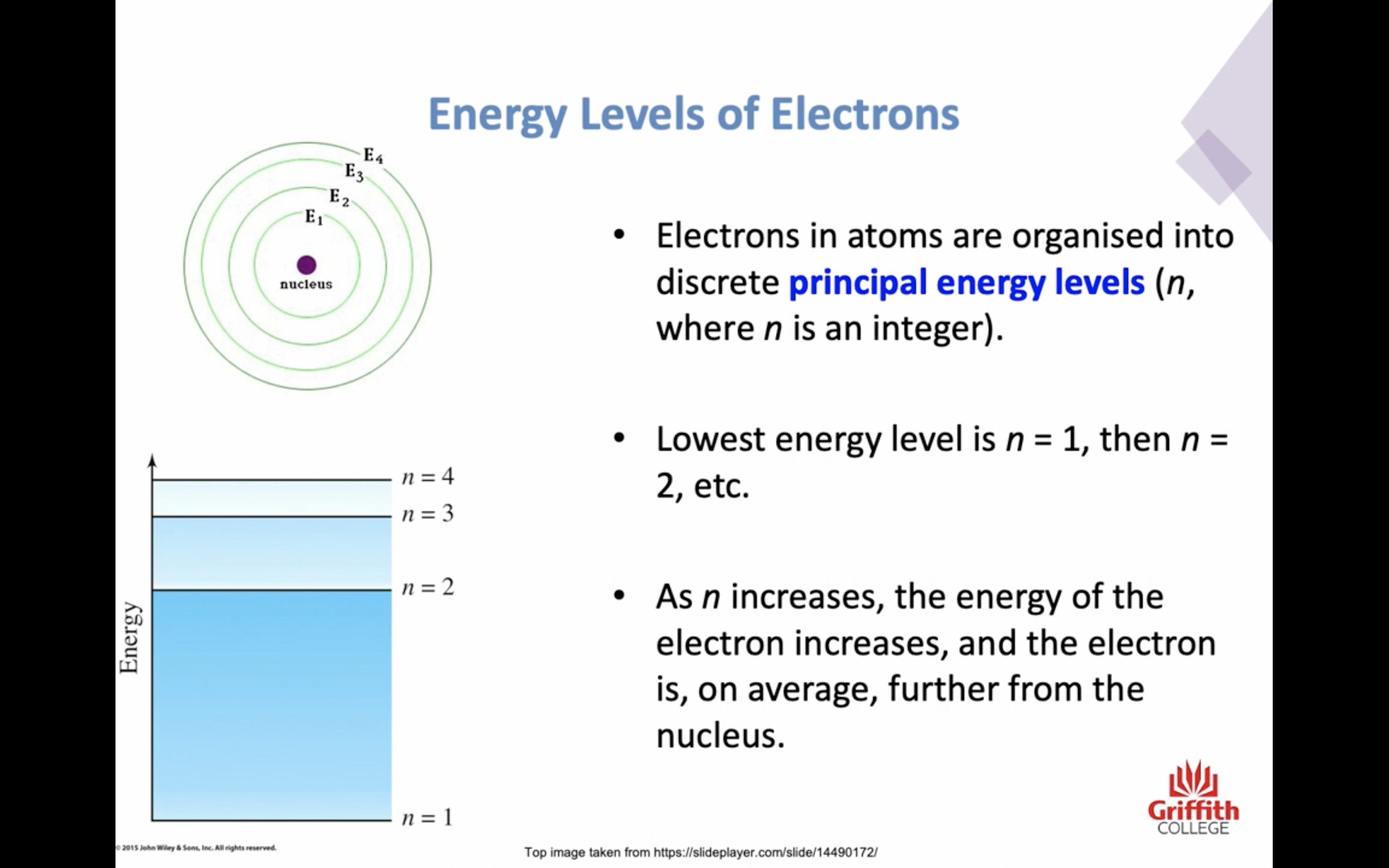
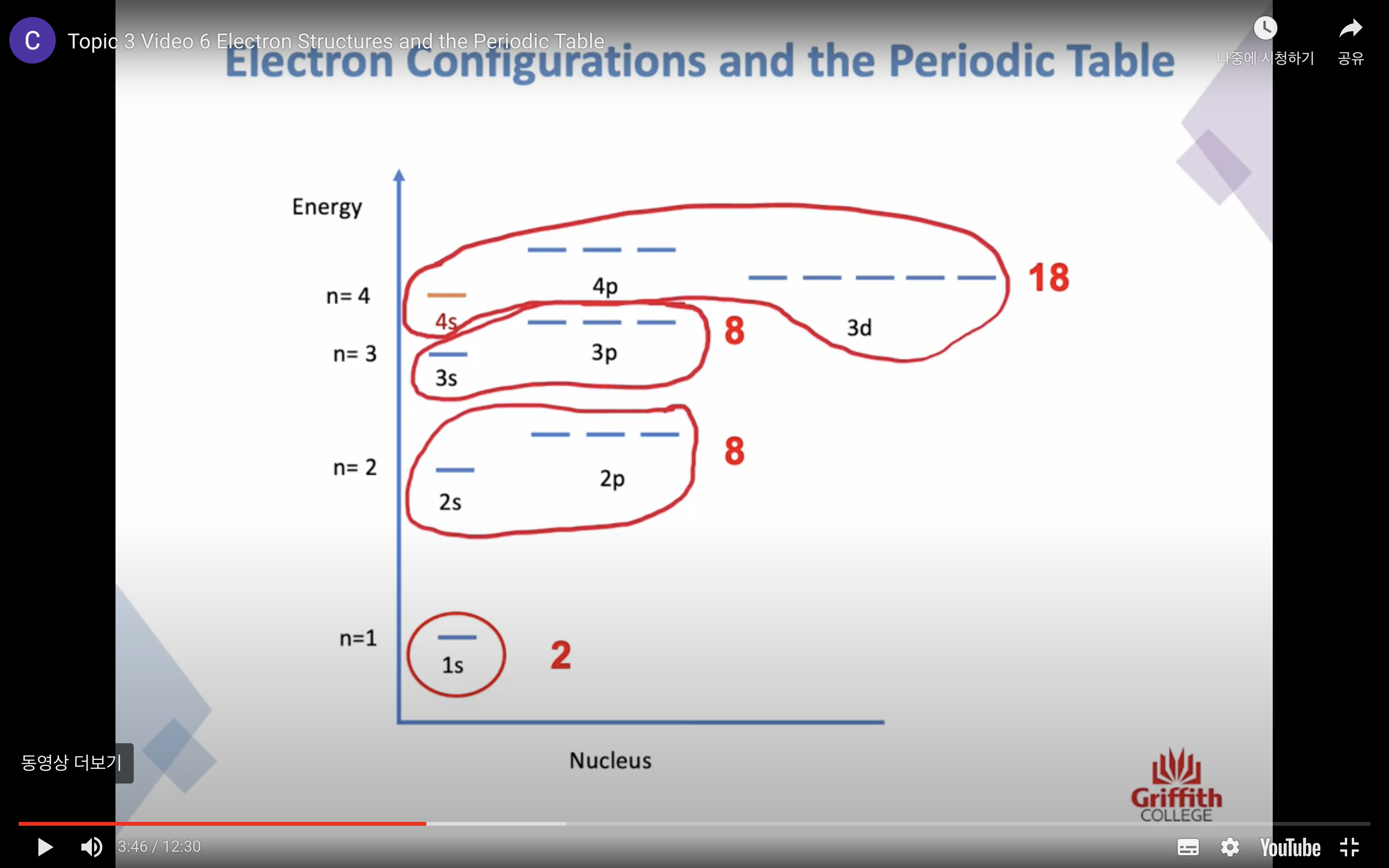
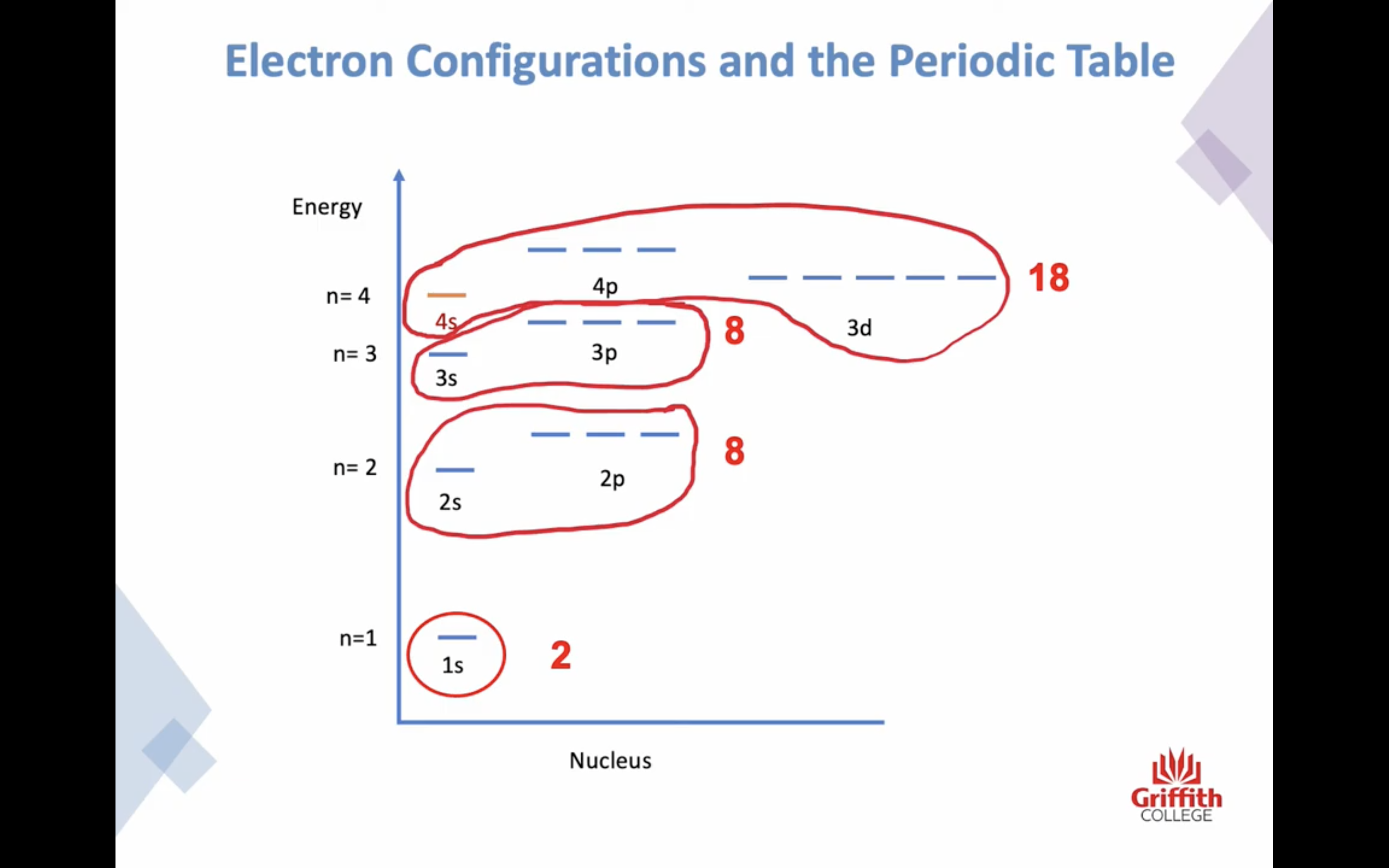
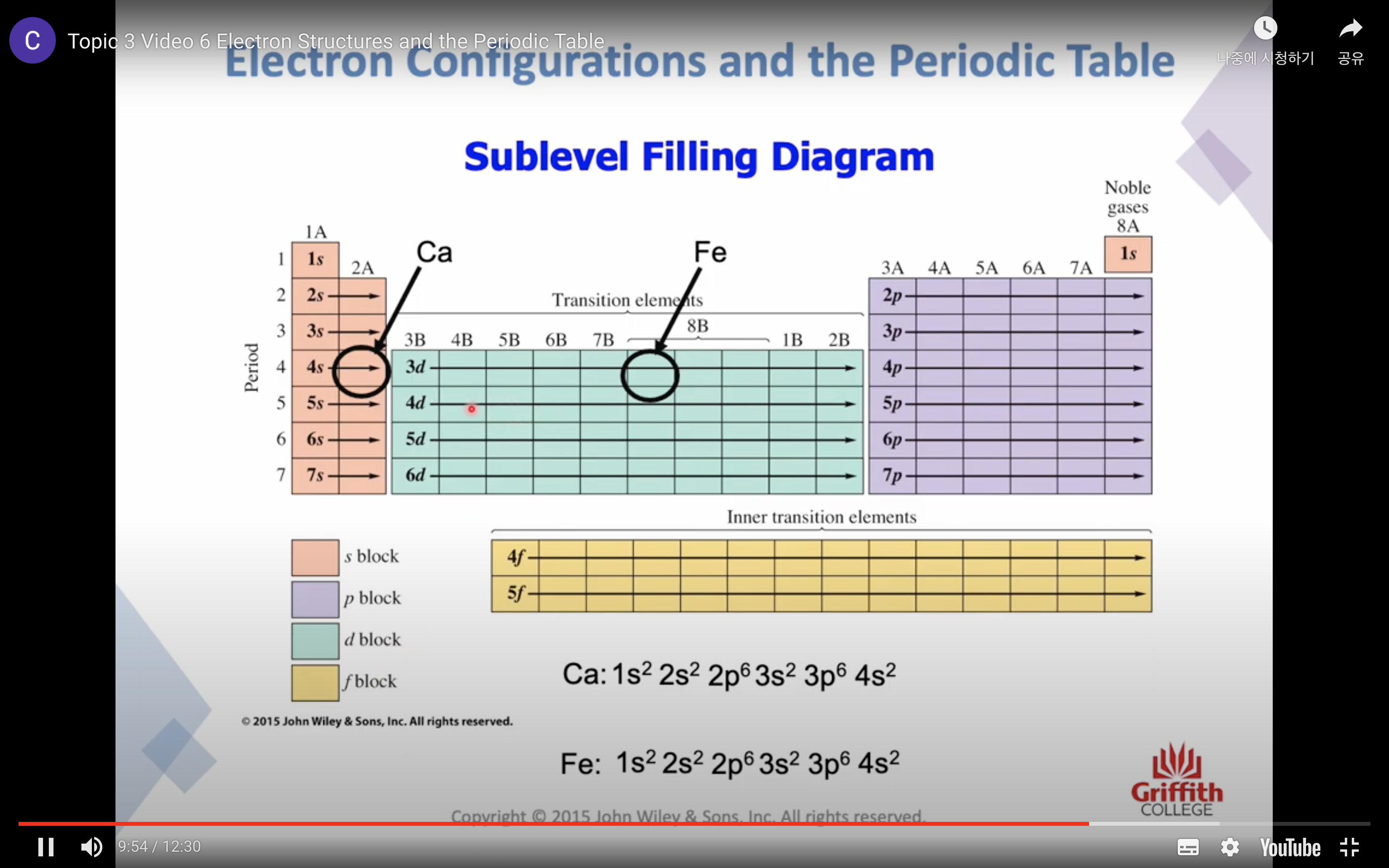
PRINCIPAL ENERGY LEVEL n increases, the energy of the electron increases
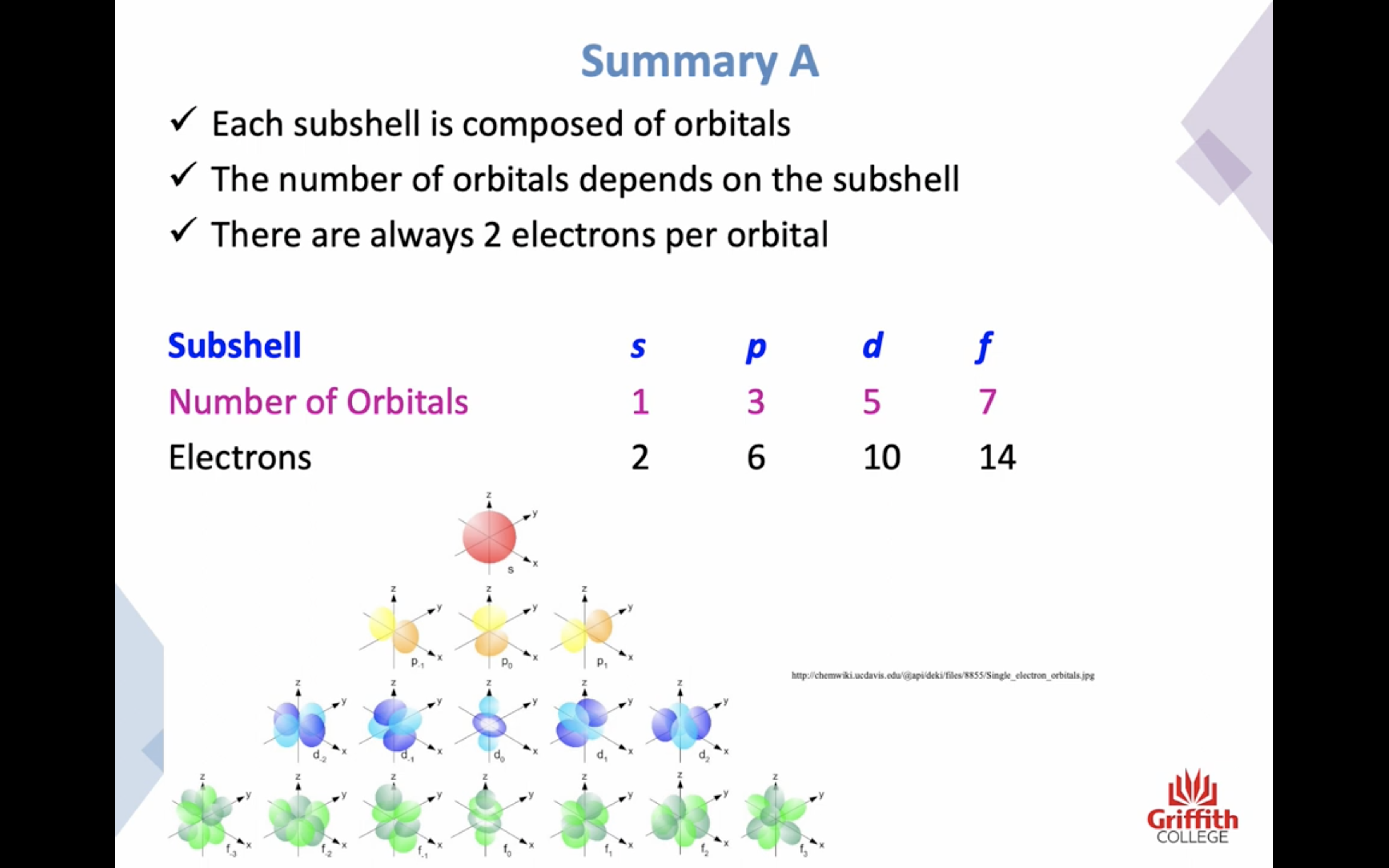
SUBSHELLS / SUBLEVELS s, p, d, f...
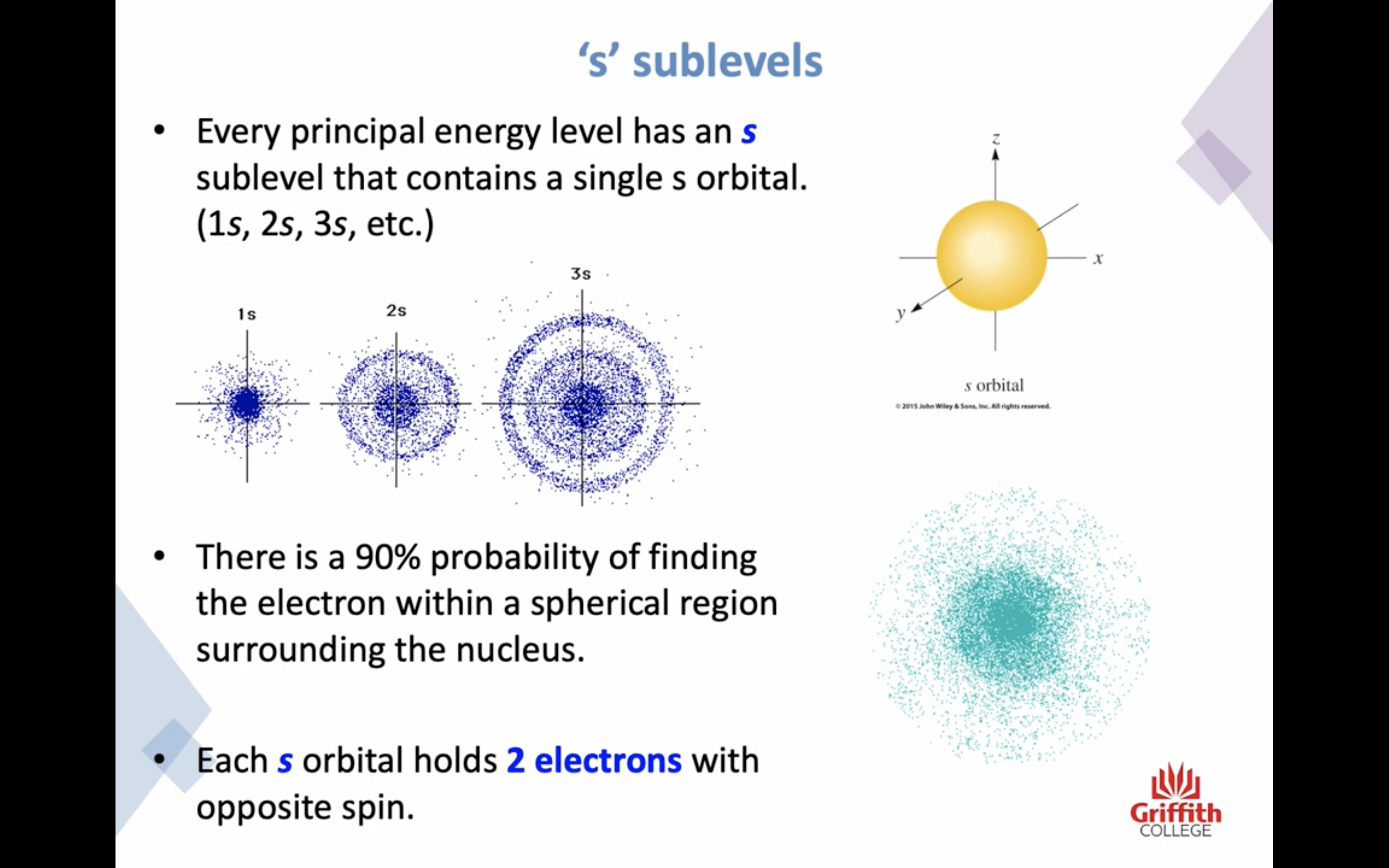
-s subshells have a single S ORBITAL , 90% probability of finding the electron within a spherical region surrounding the nucleus (2 electrons)
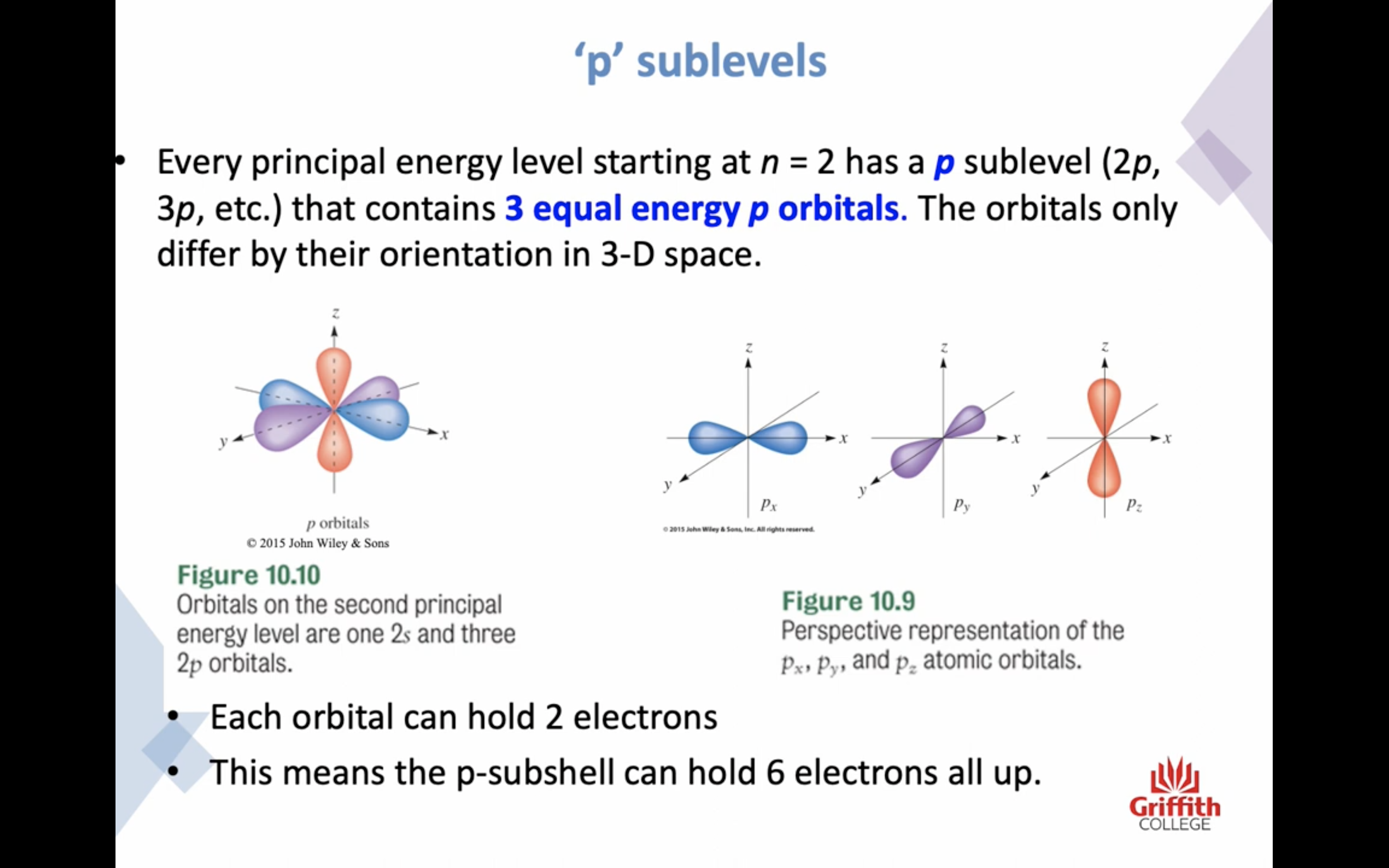
-p subshells have 3 equal ORBITALS (6 electrons)
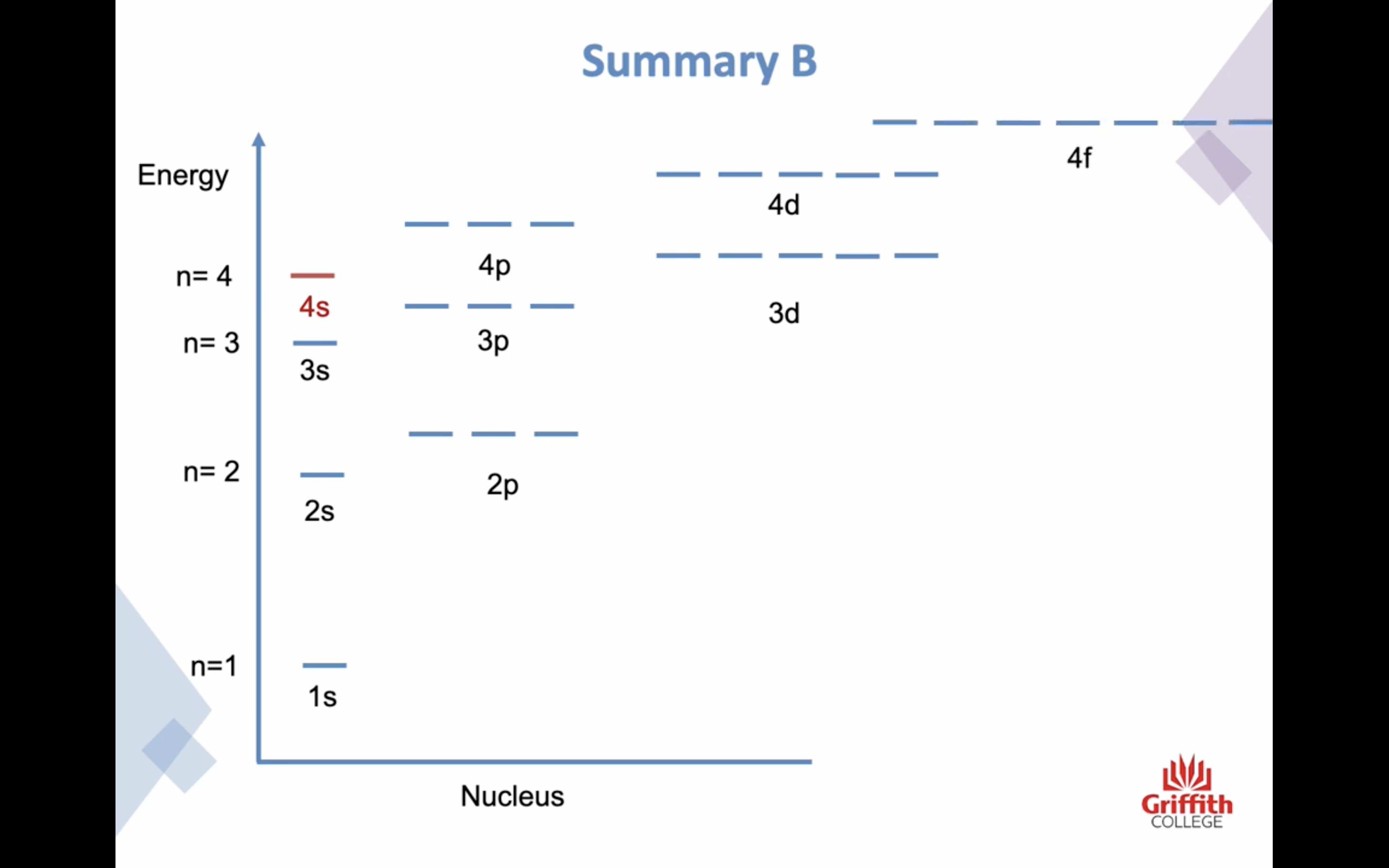
PRINCIPAL ENERGY LEVEL
n=1
n=2
n=3
n=4 ..
SUBSHELLS/ SUBLEVELS
s,p,d,f...
orbitals in each subshell
each orbital can contain 2 electrons
s subshell has 1 orbital
p subshell has 3 orbital
d subshell has 5 orbital
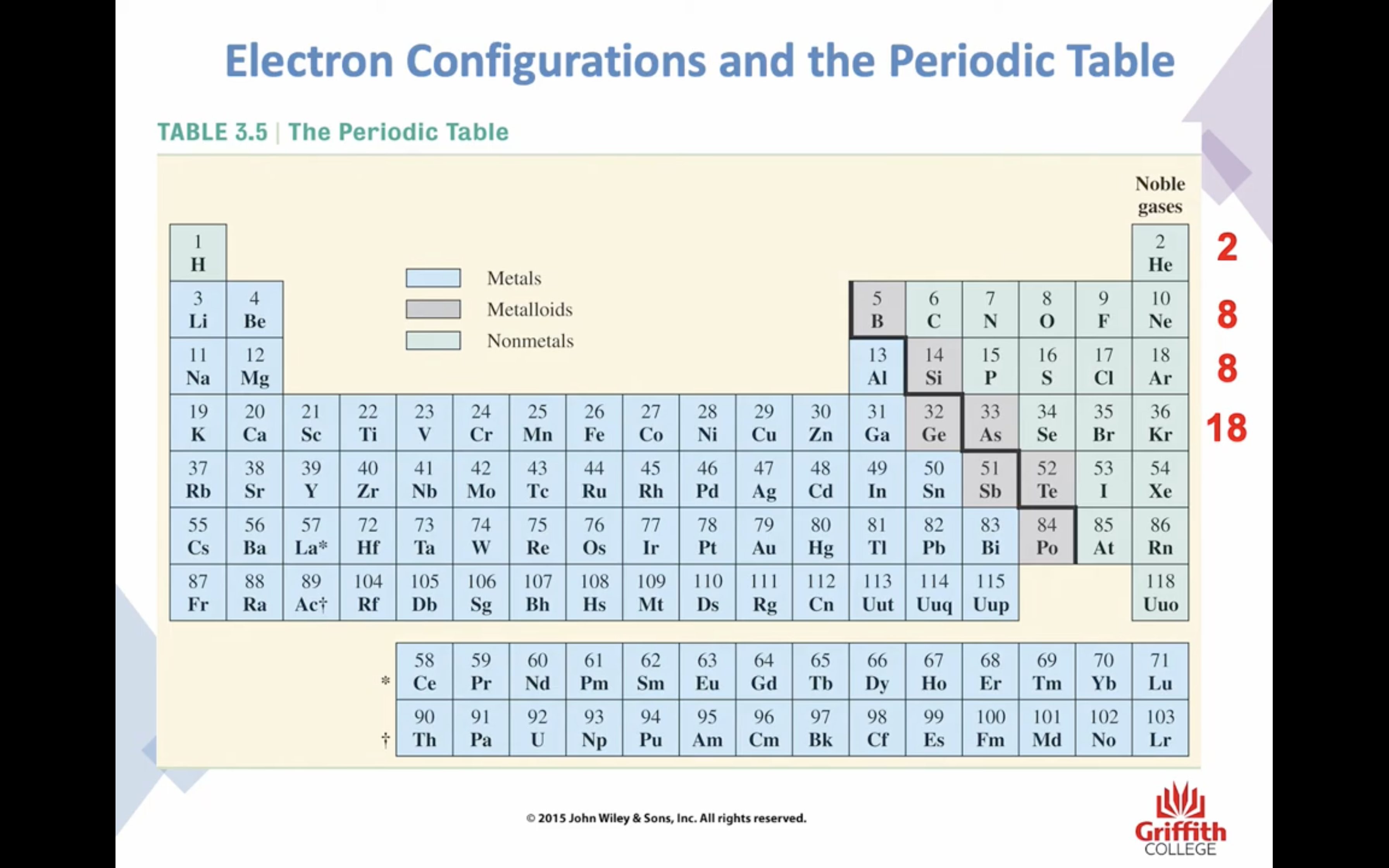
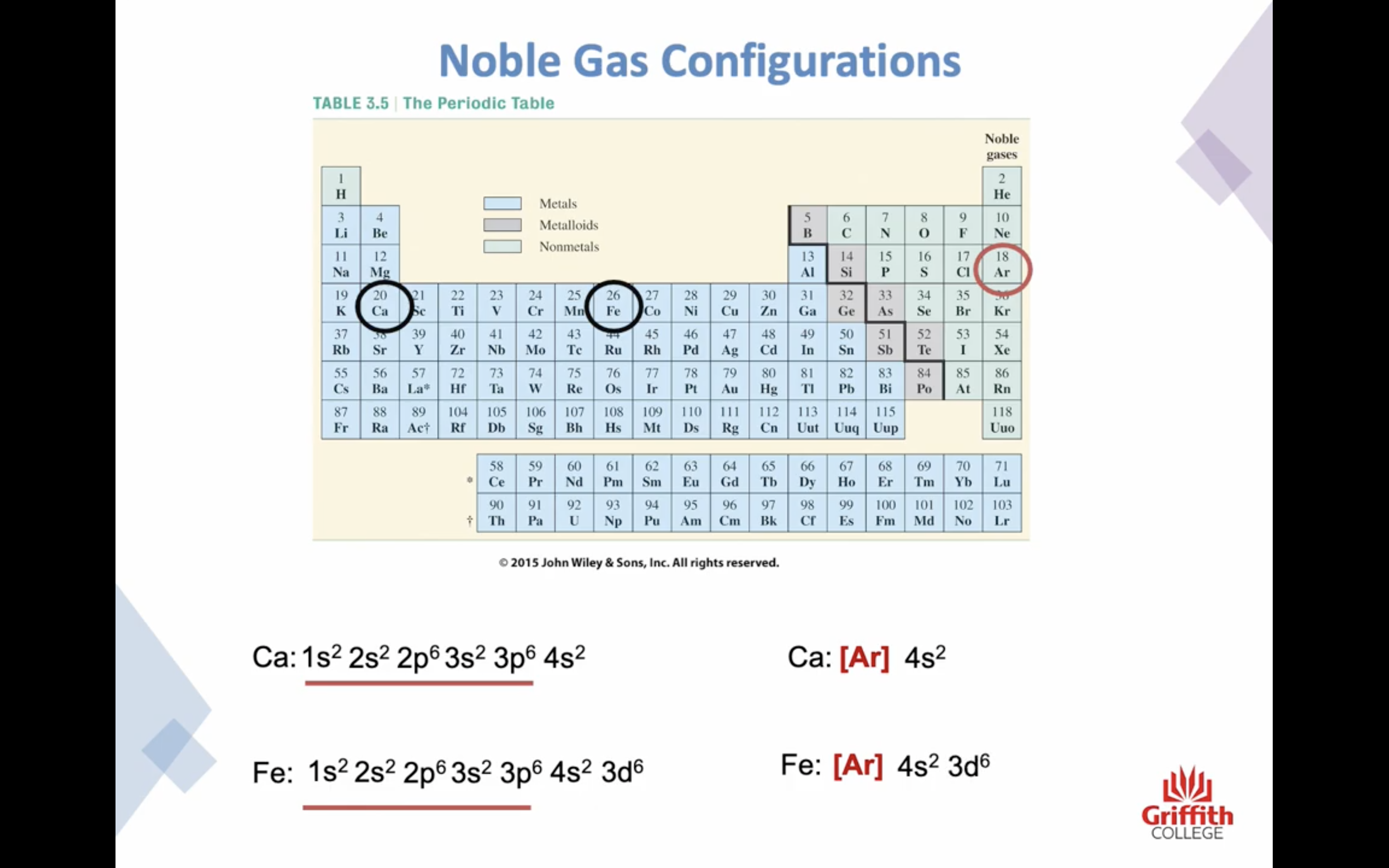
Electron Structures
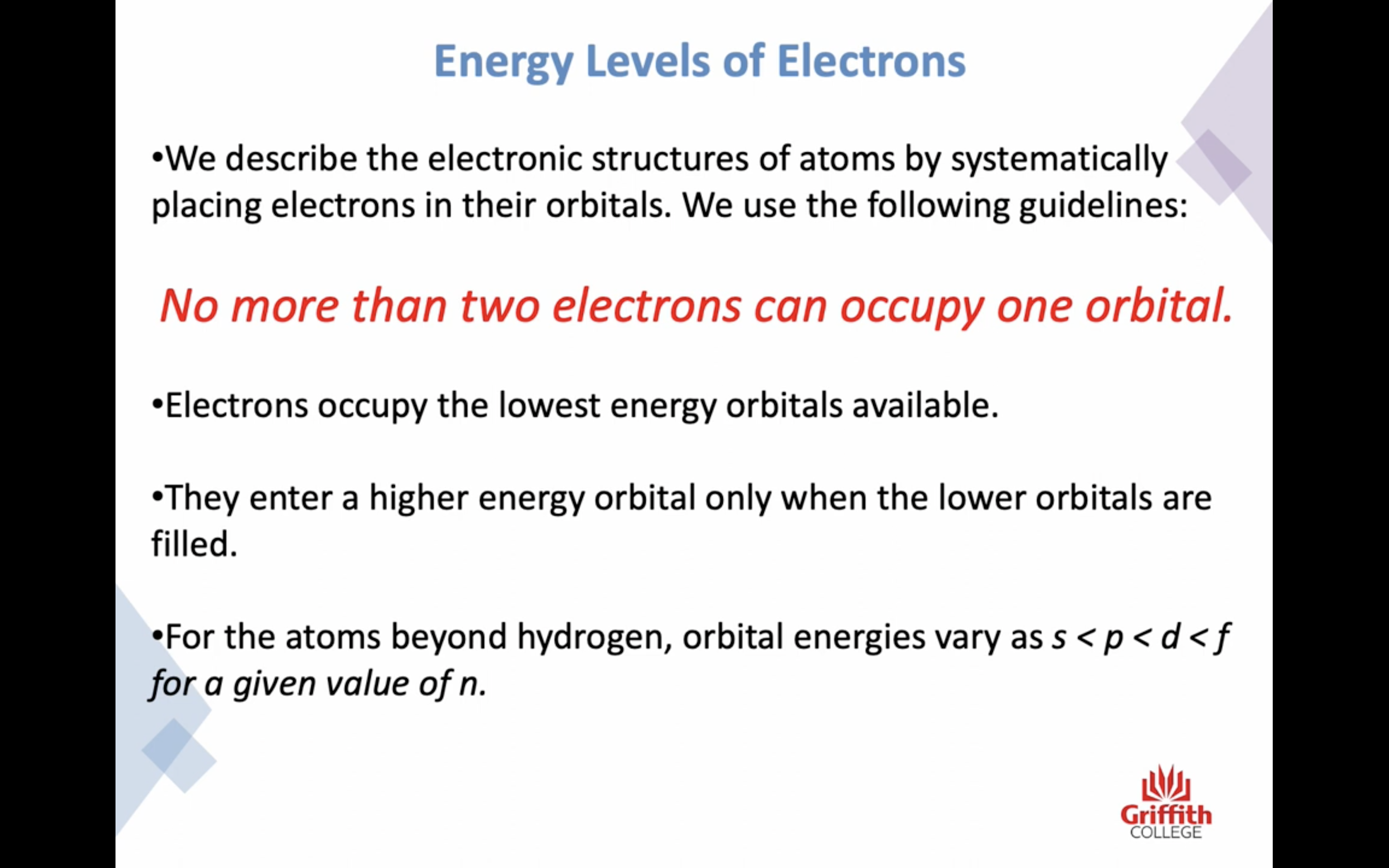
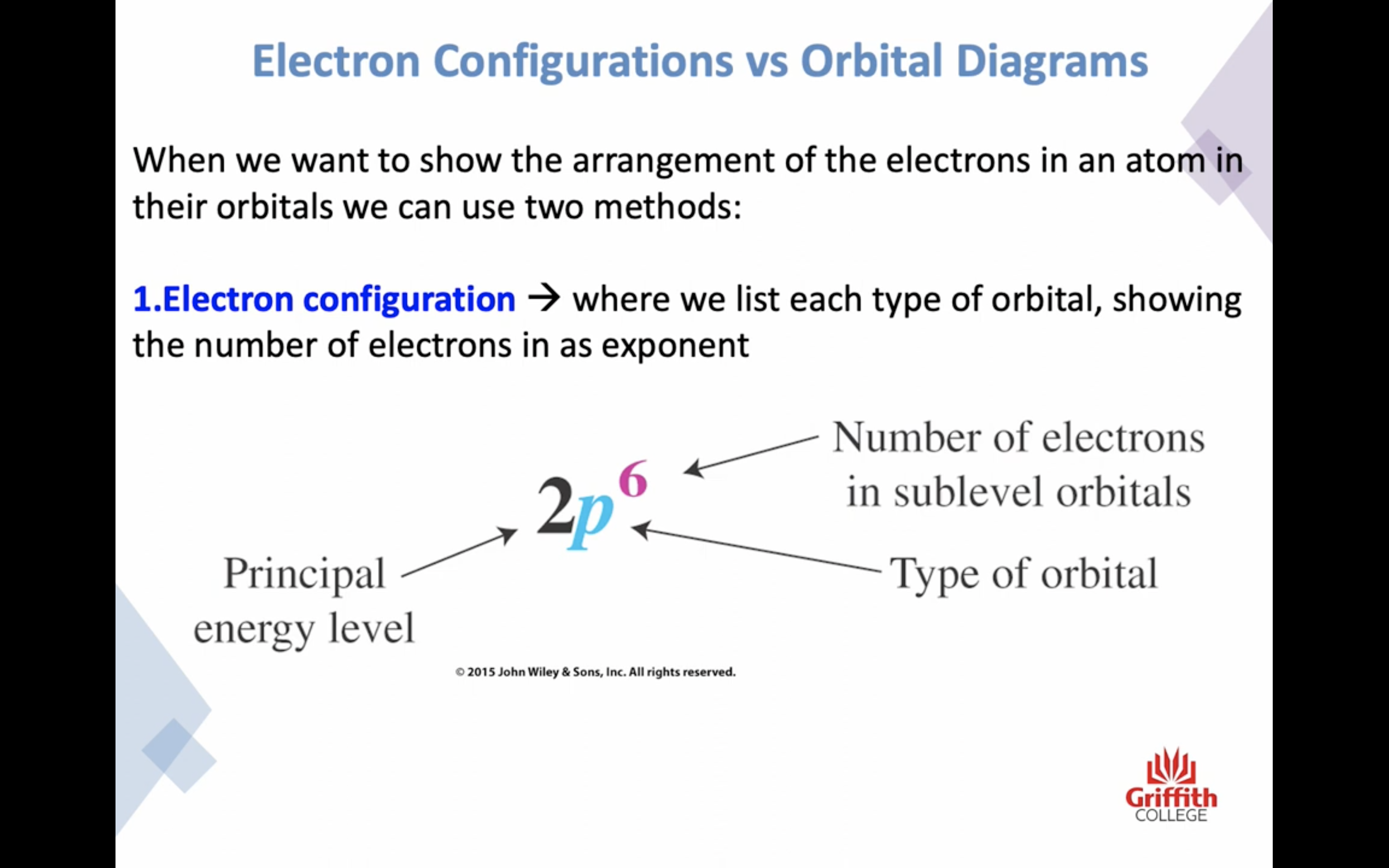


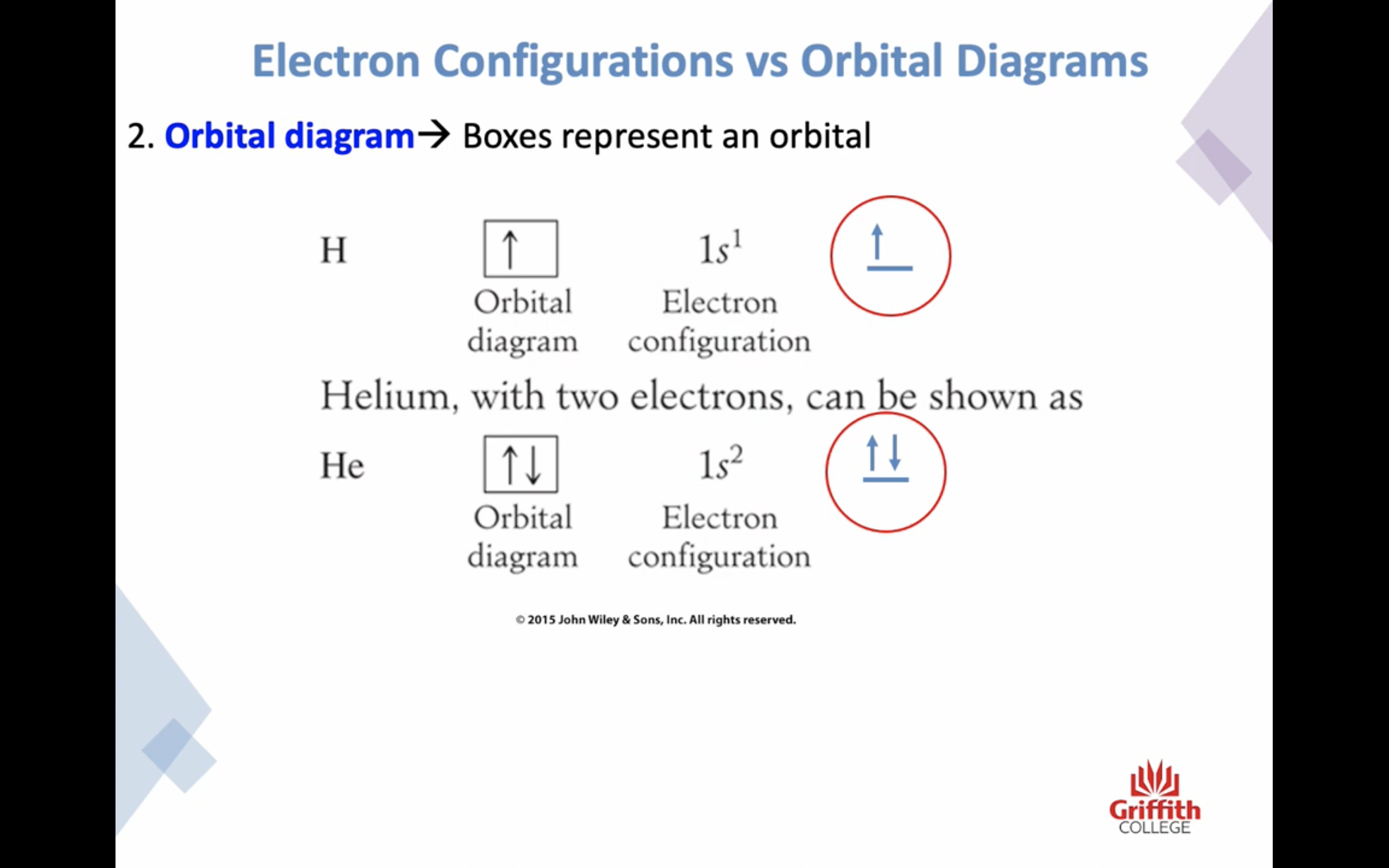
Electron Structures and the Periodic Table
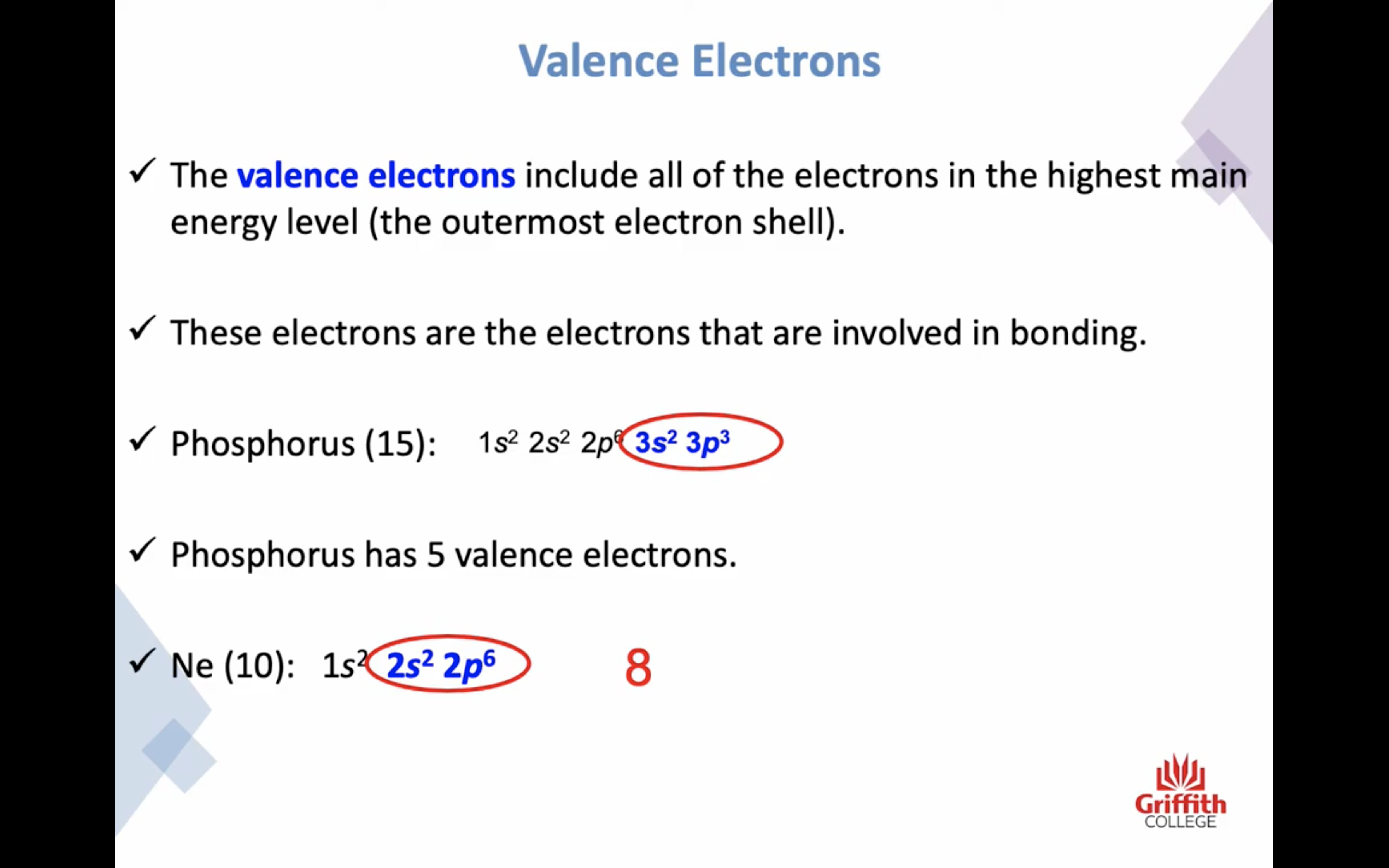
원자가 전자 2 8 8 10 !!!!!!!!
TOPIC4
Elements, Ions and Ionic Compounds
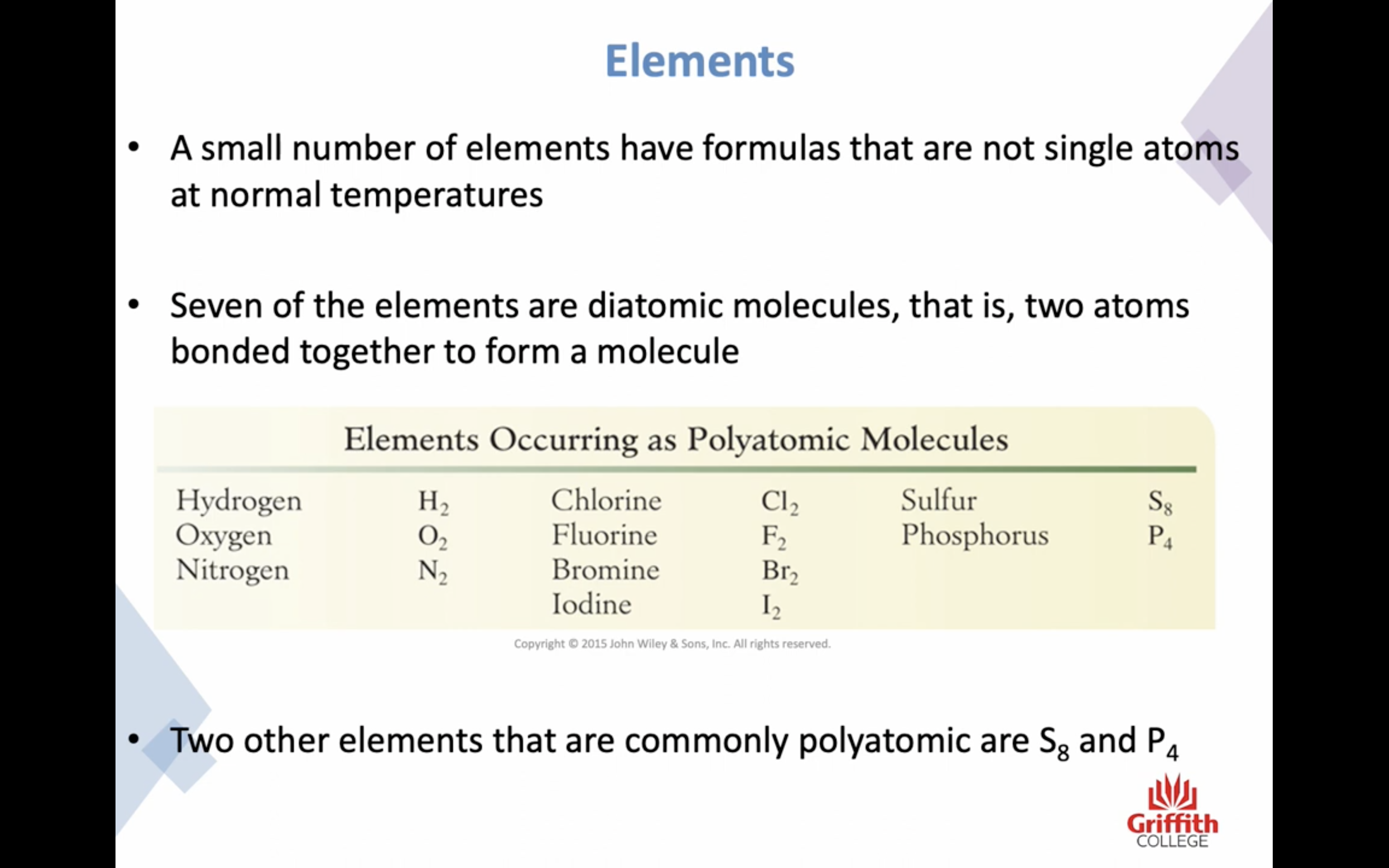

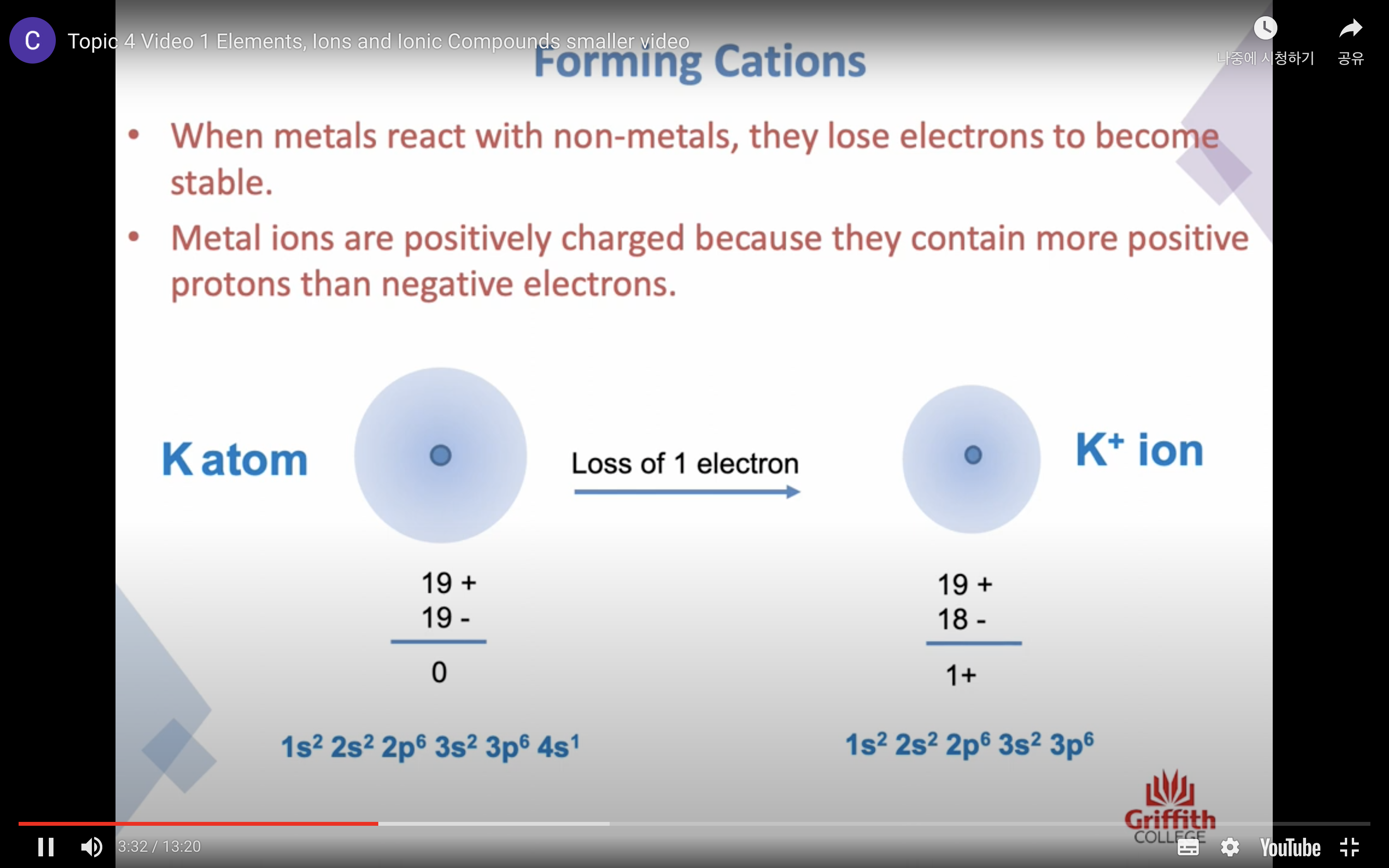
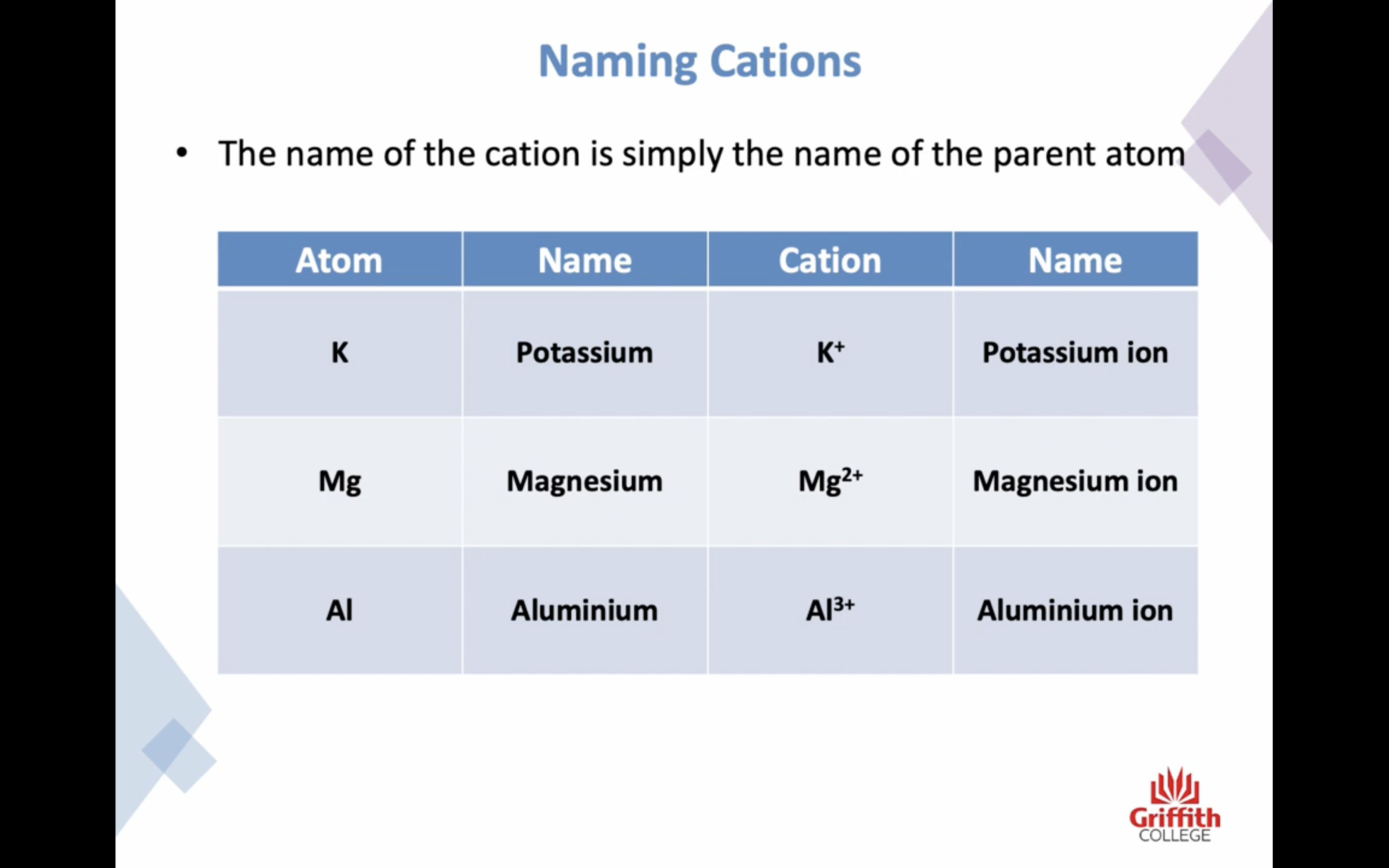
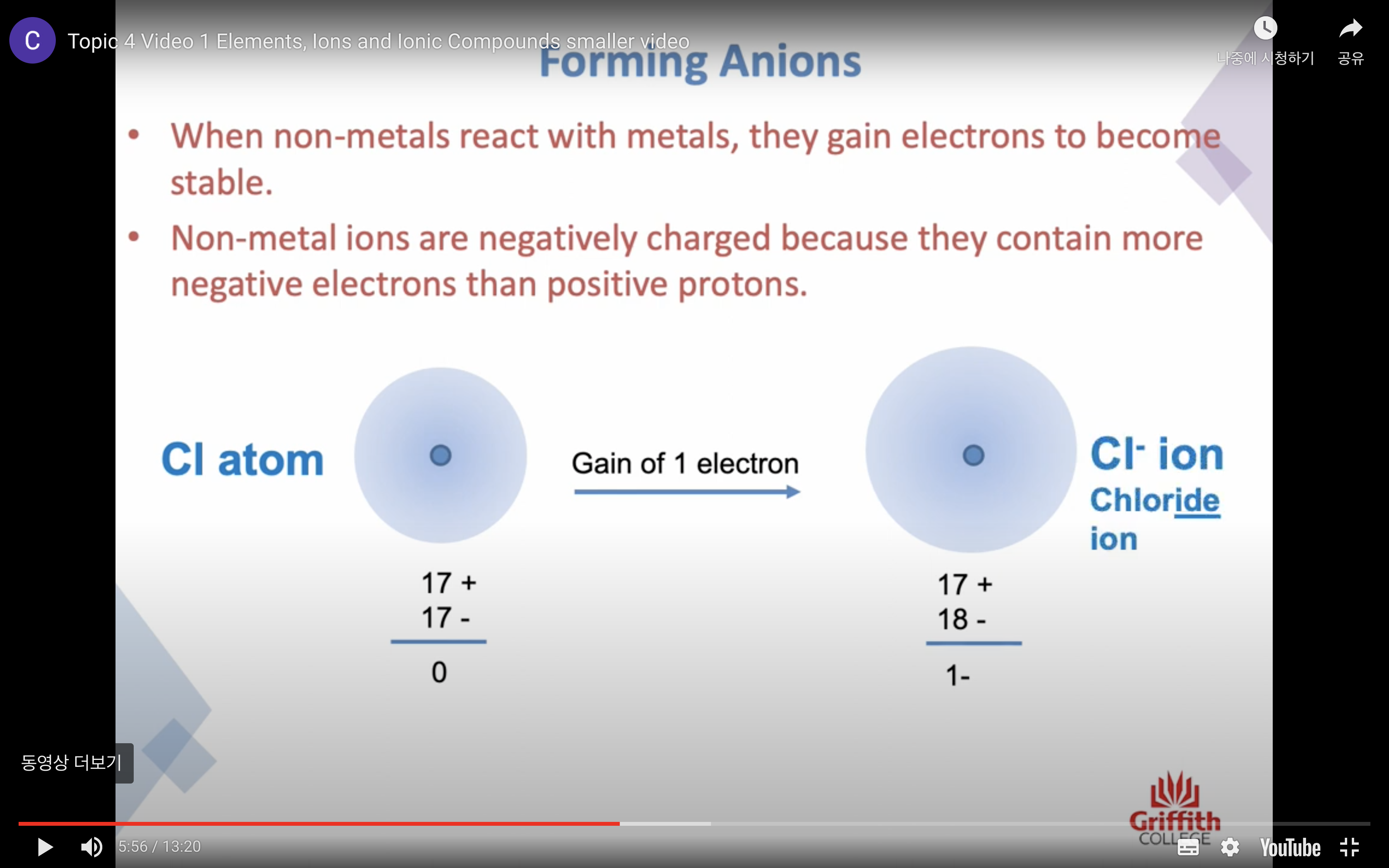
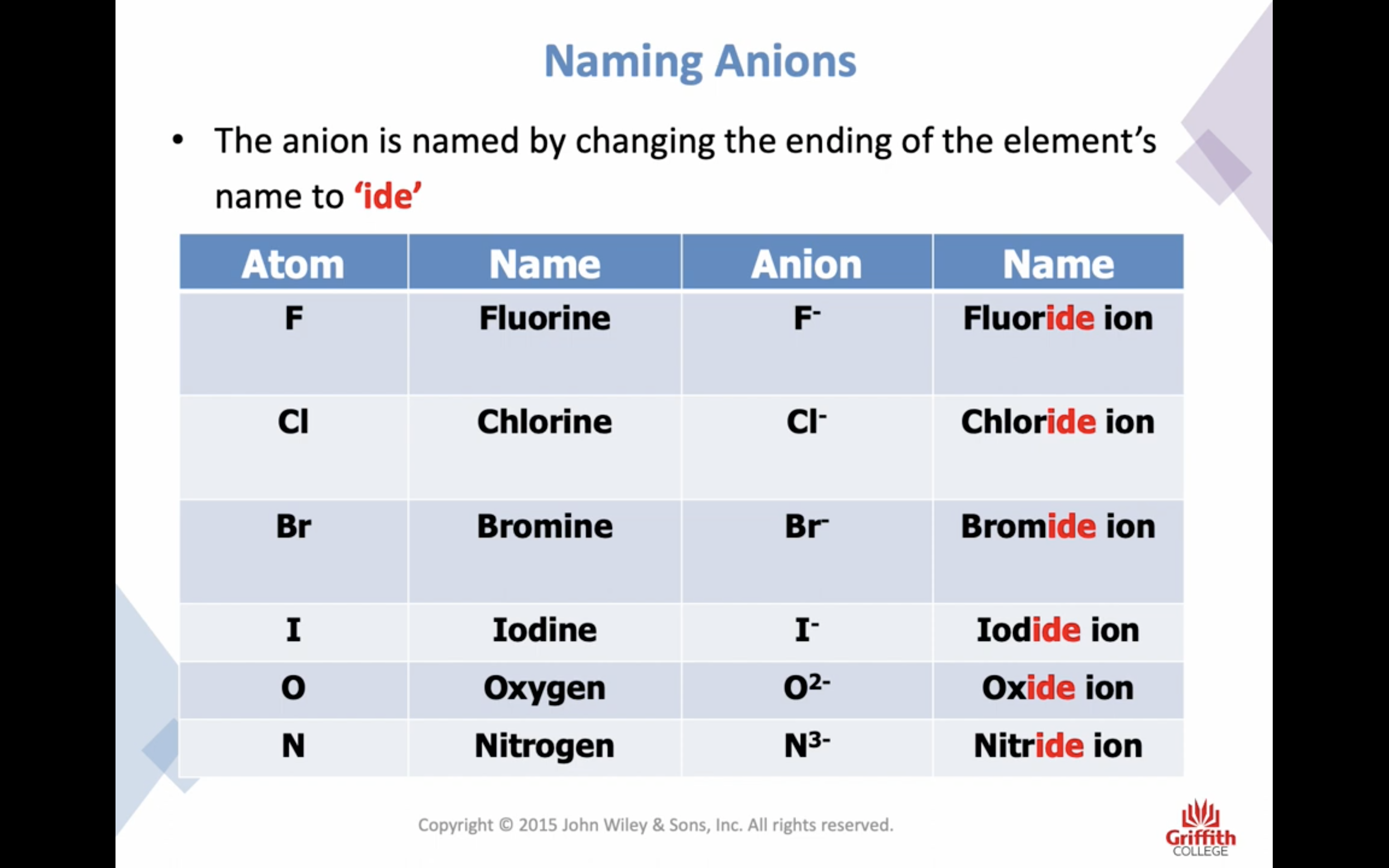
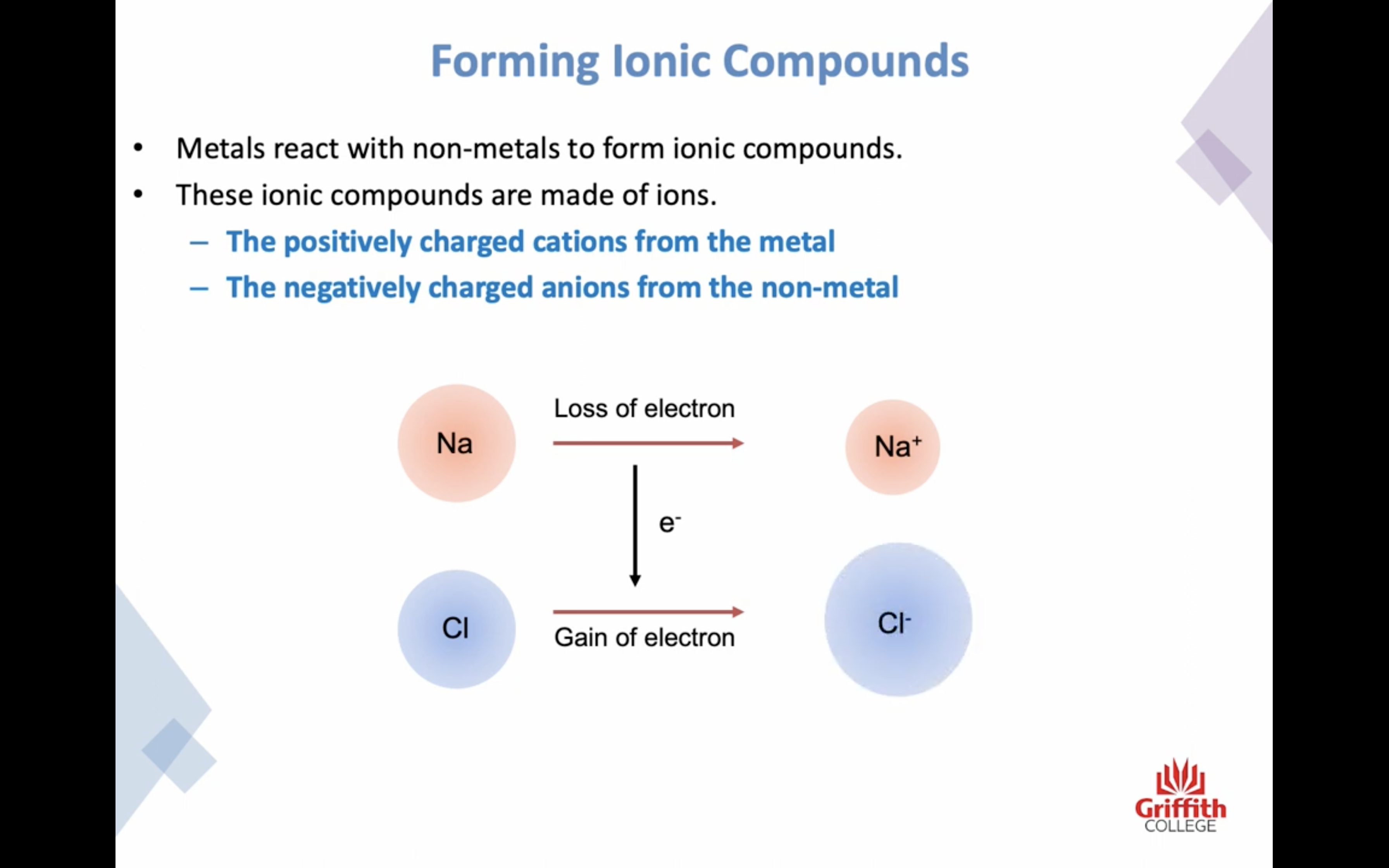
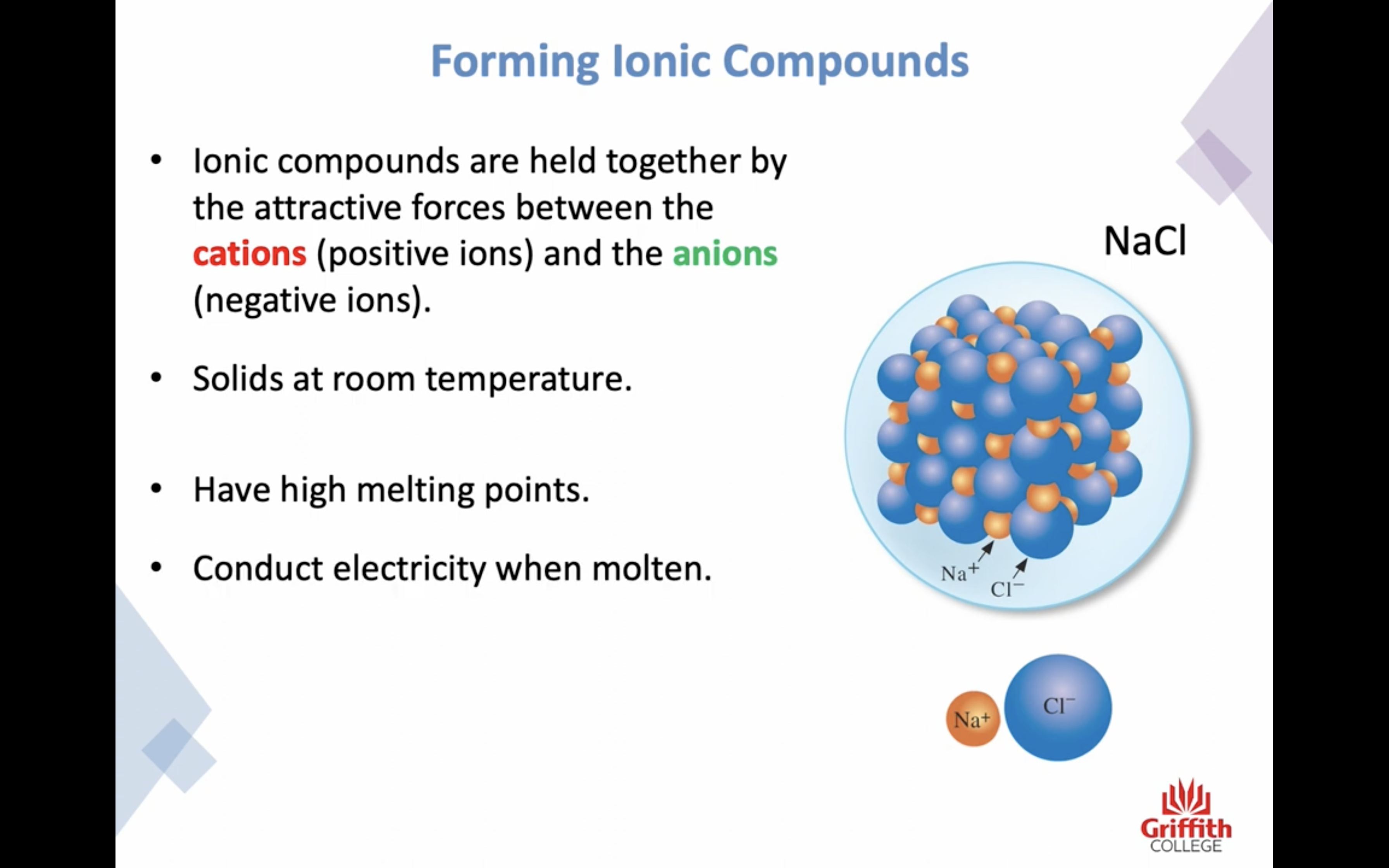
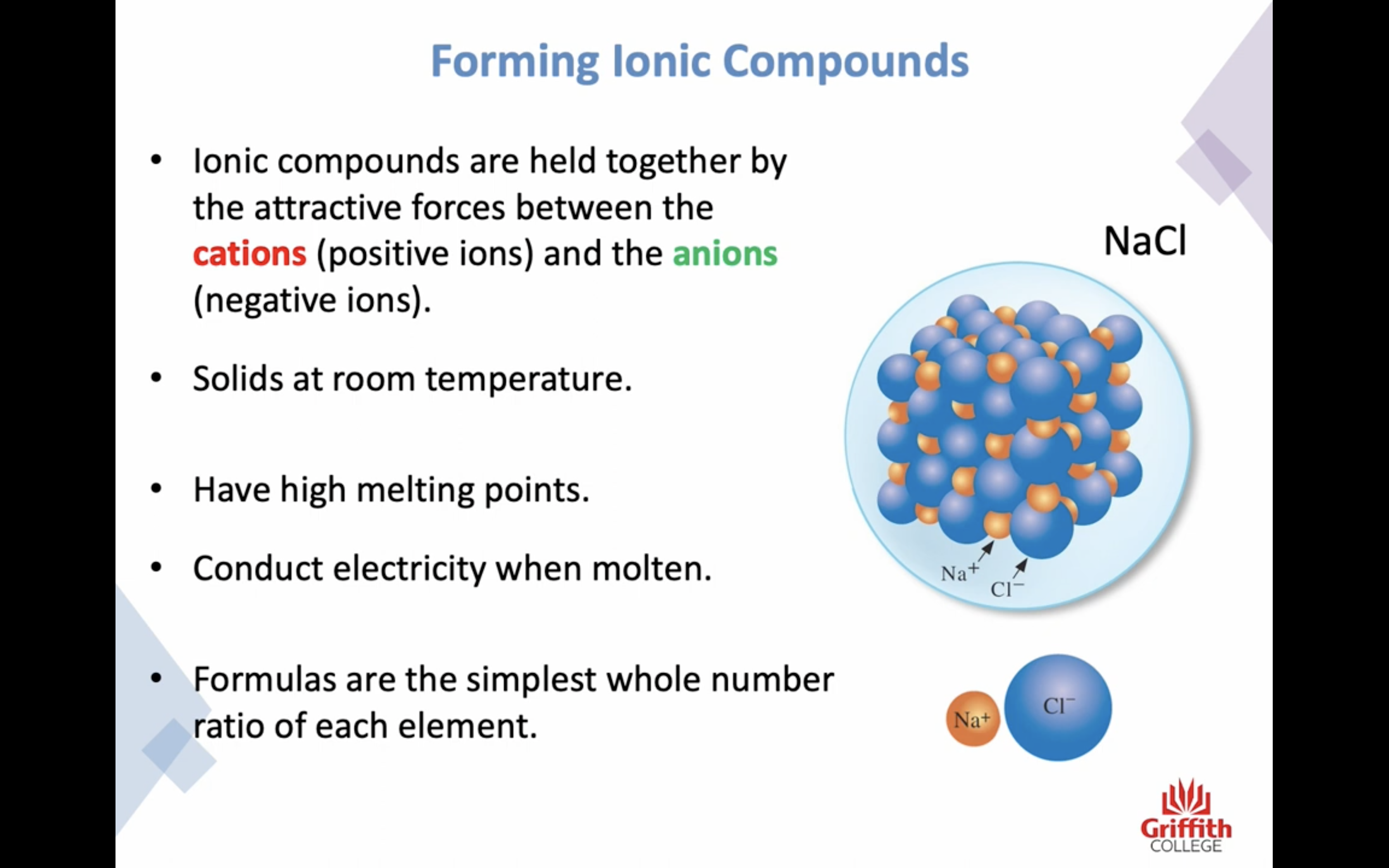
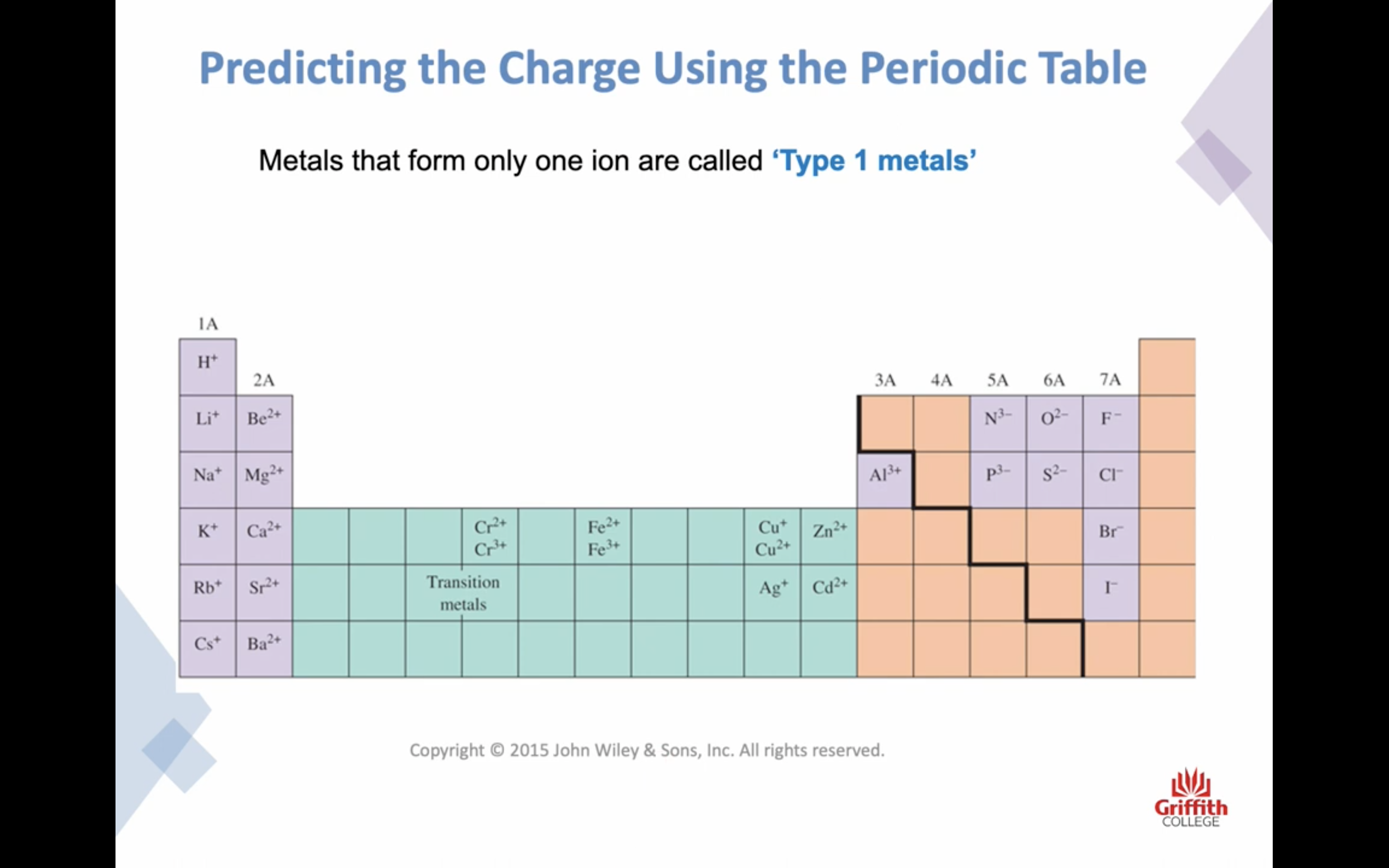
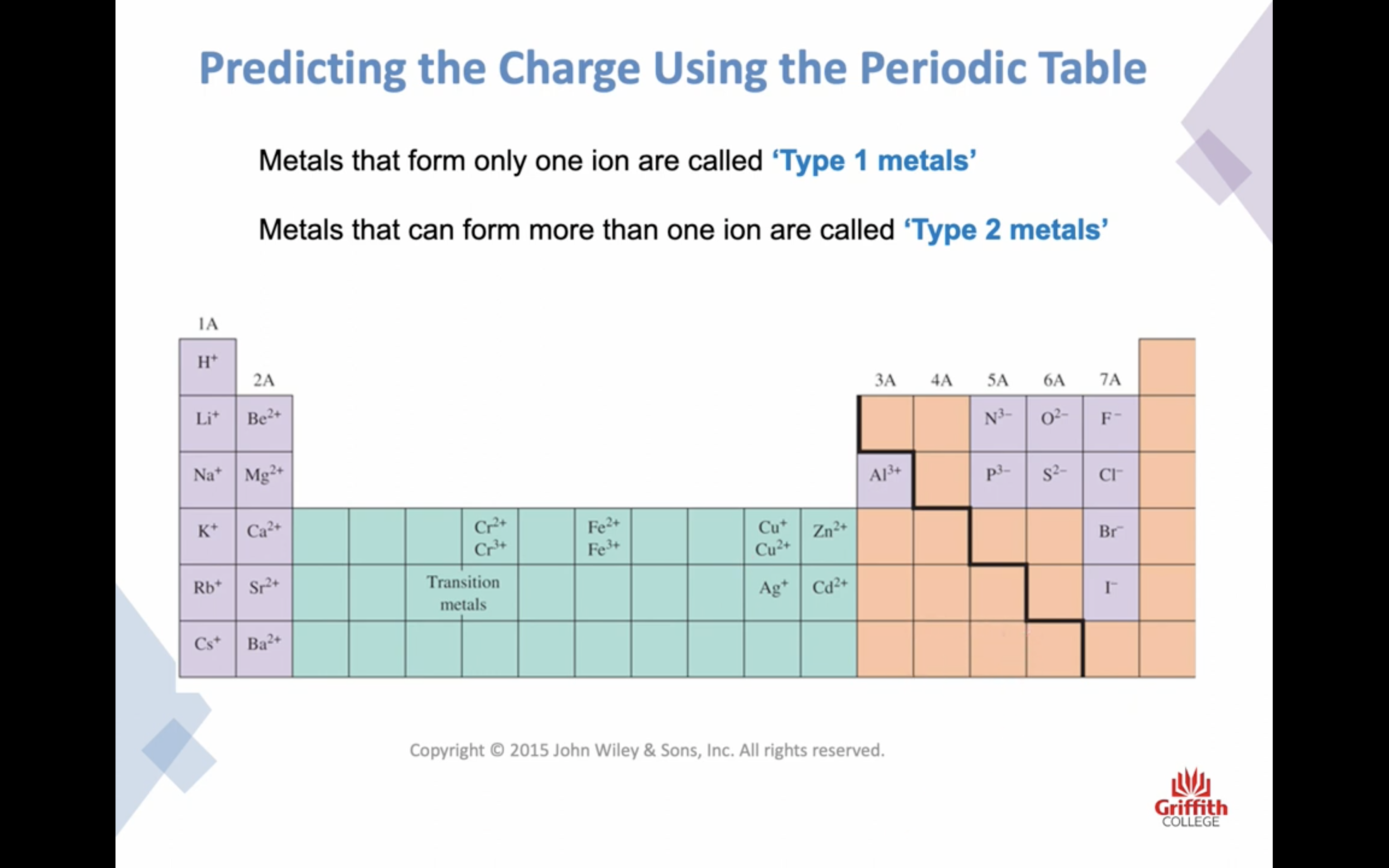
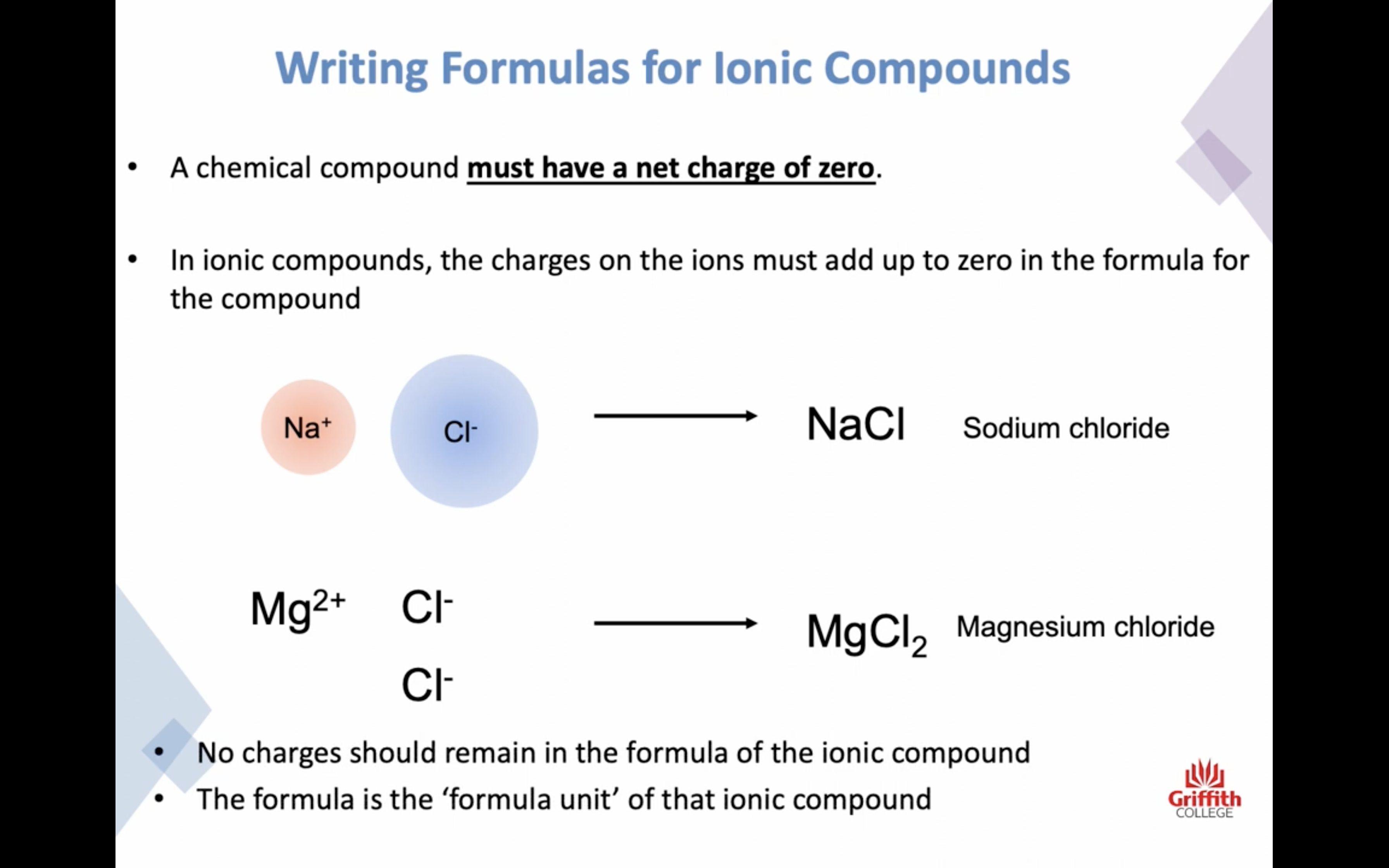
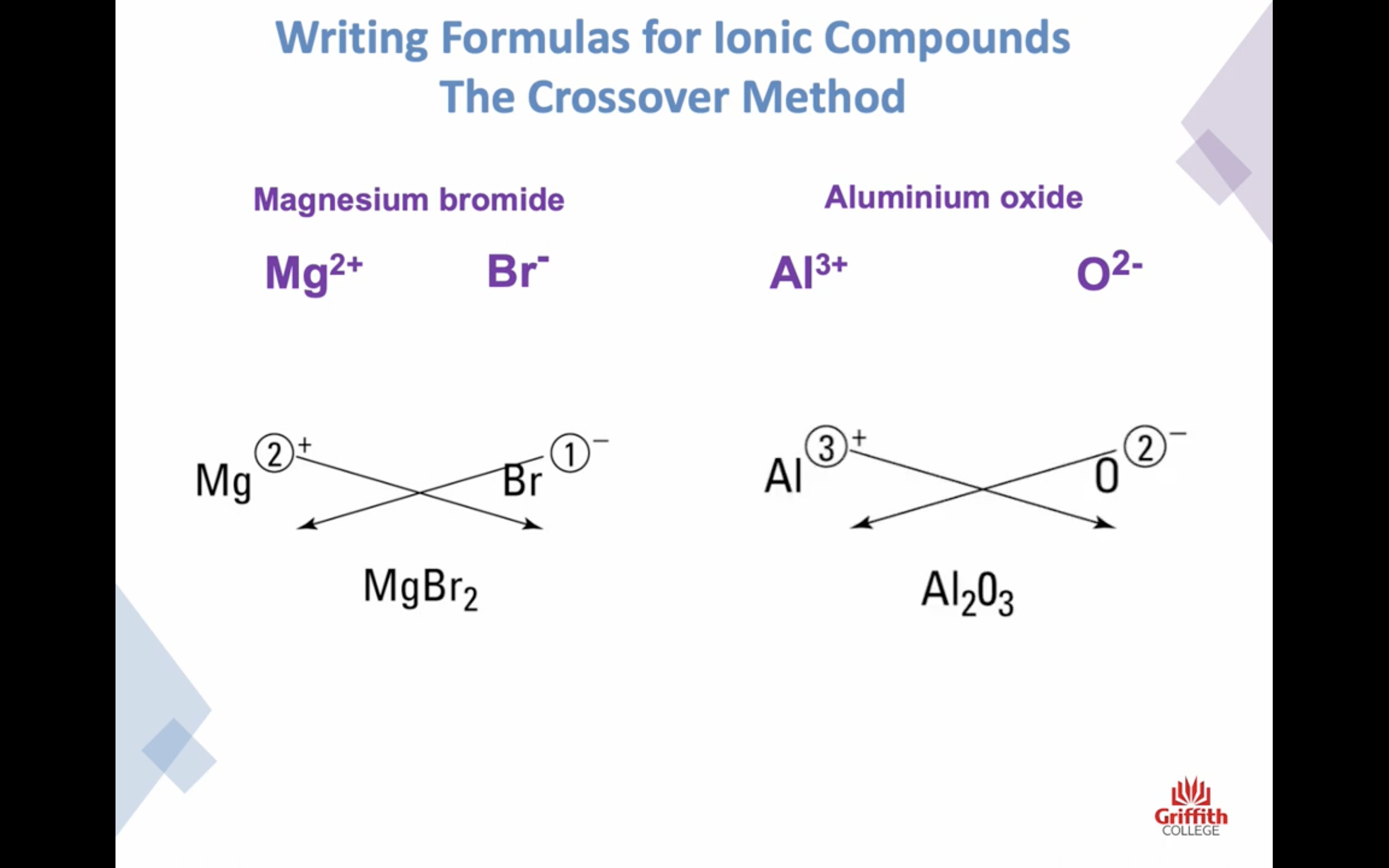
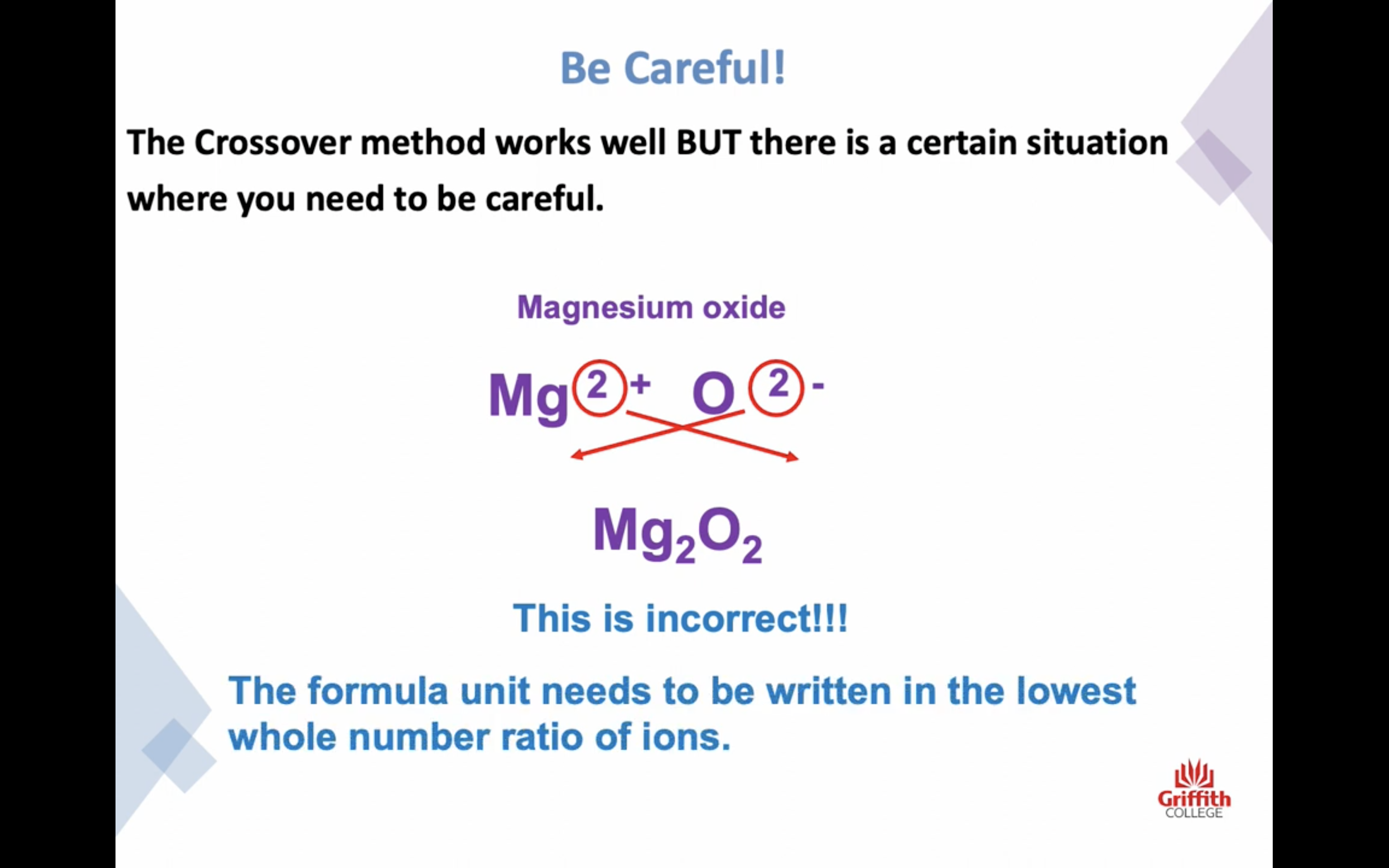
Naming Ionic Compounds
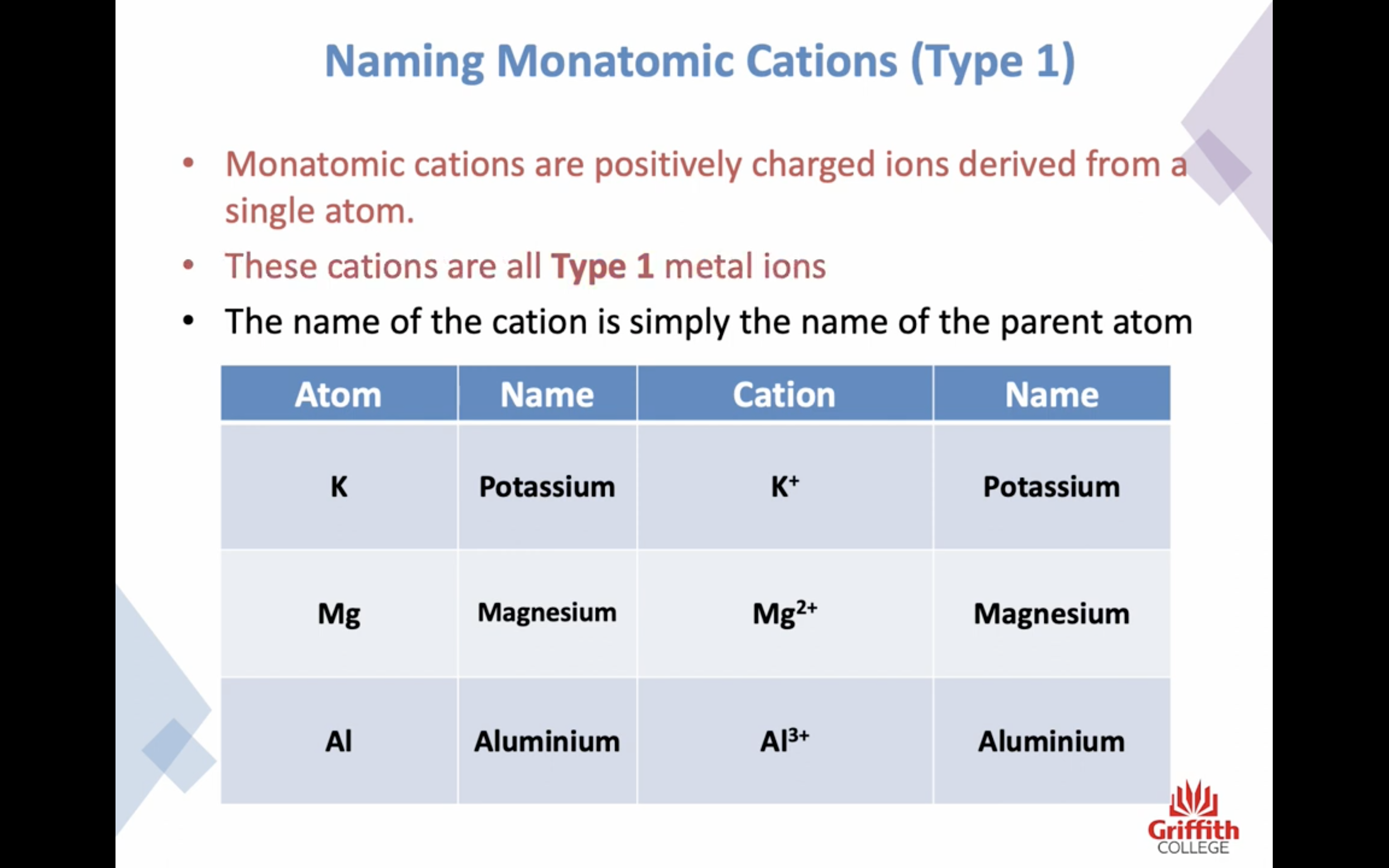
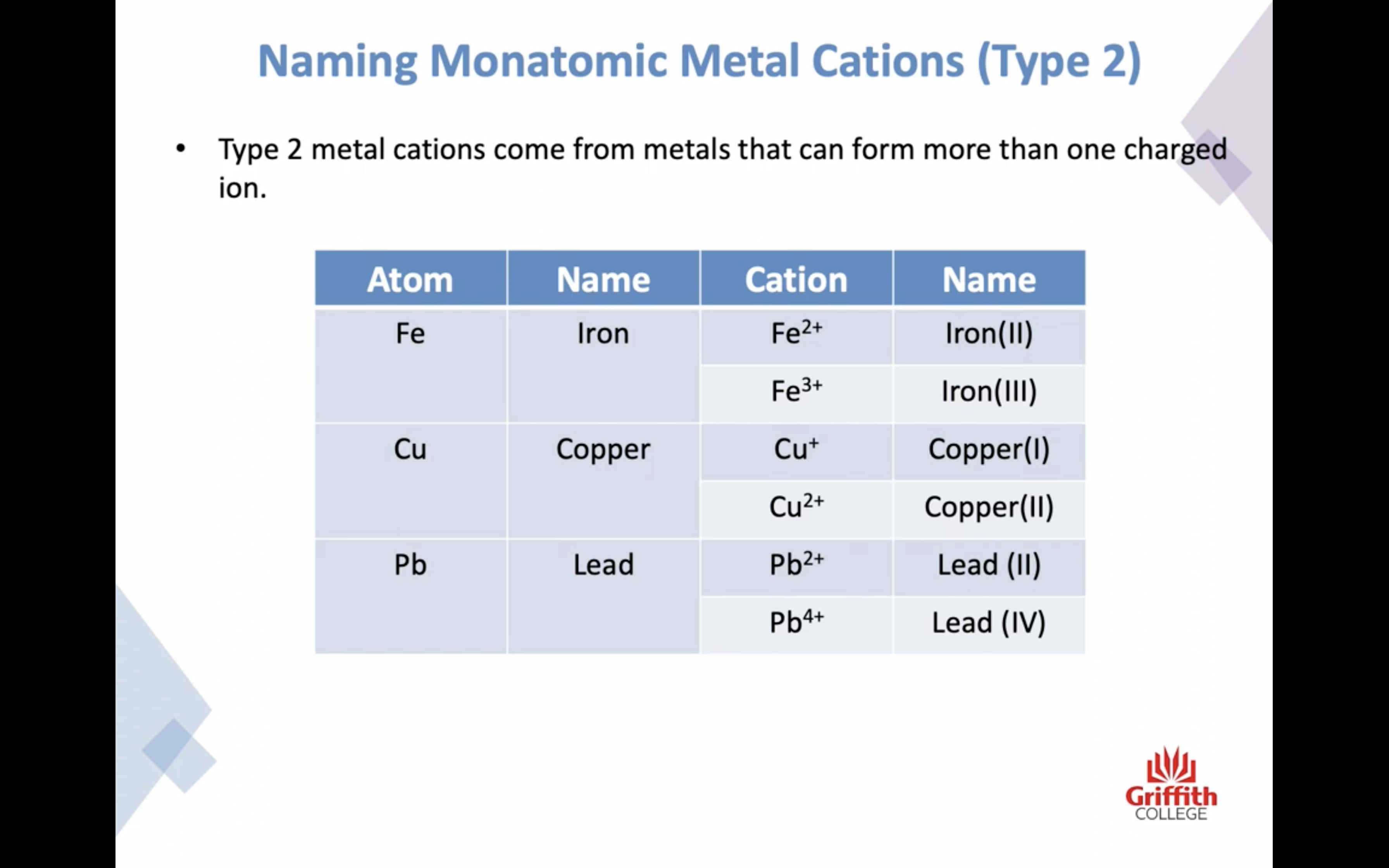
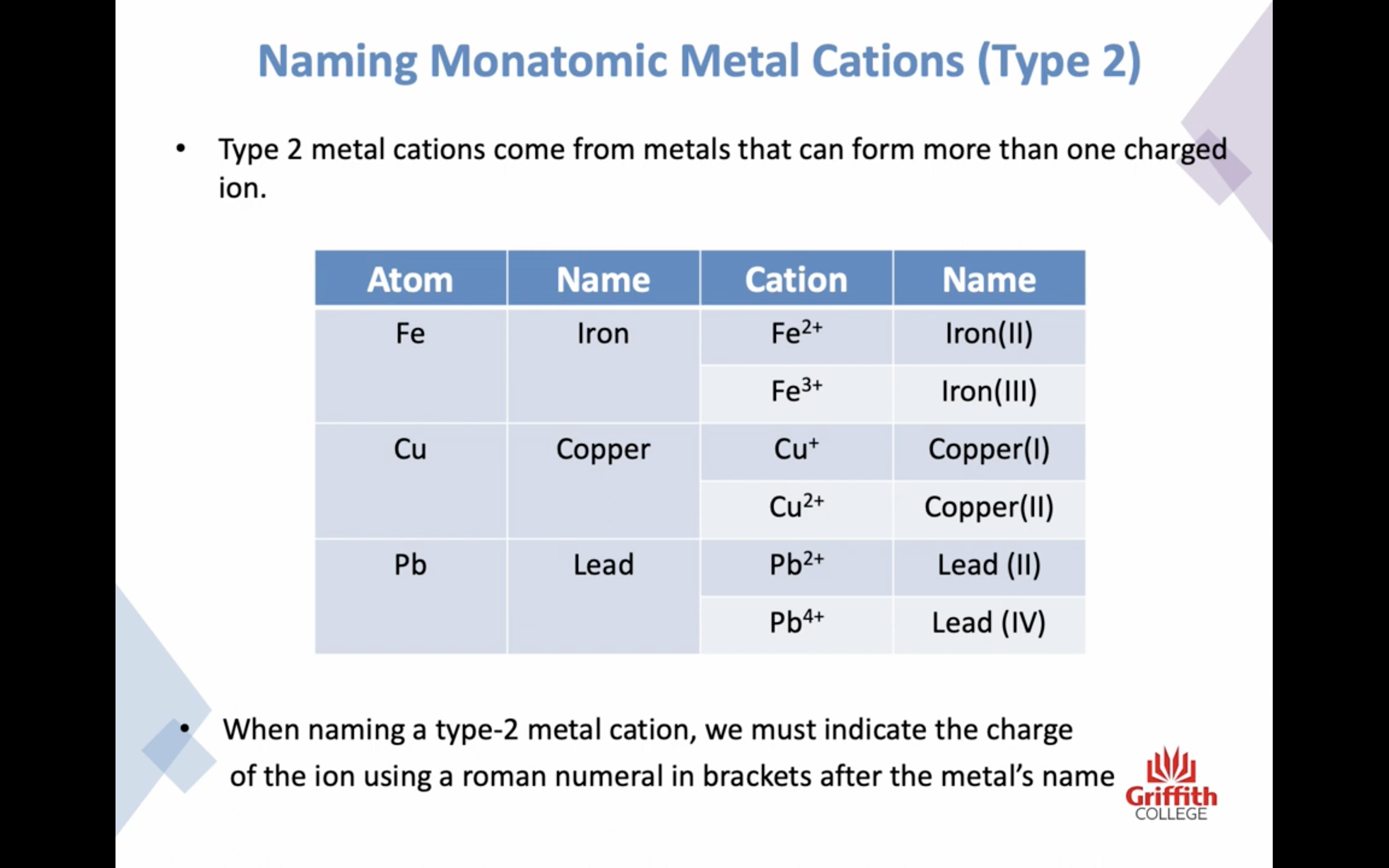
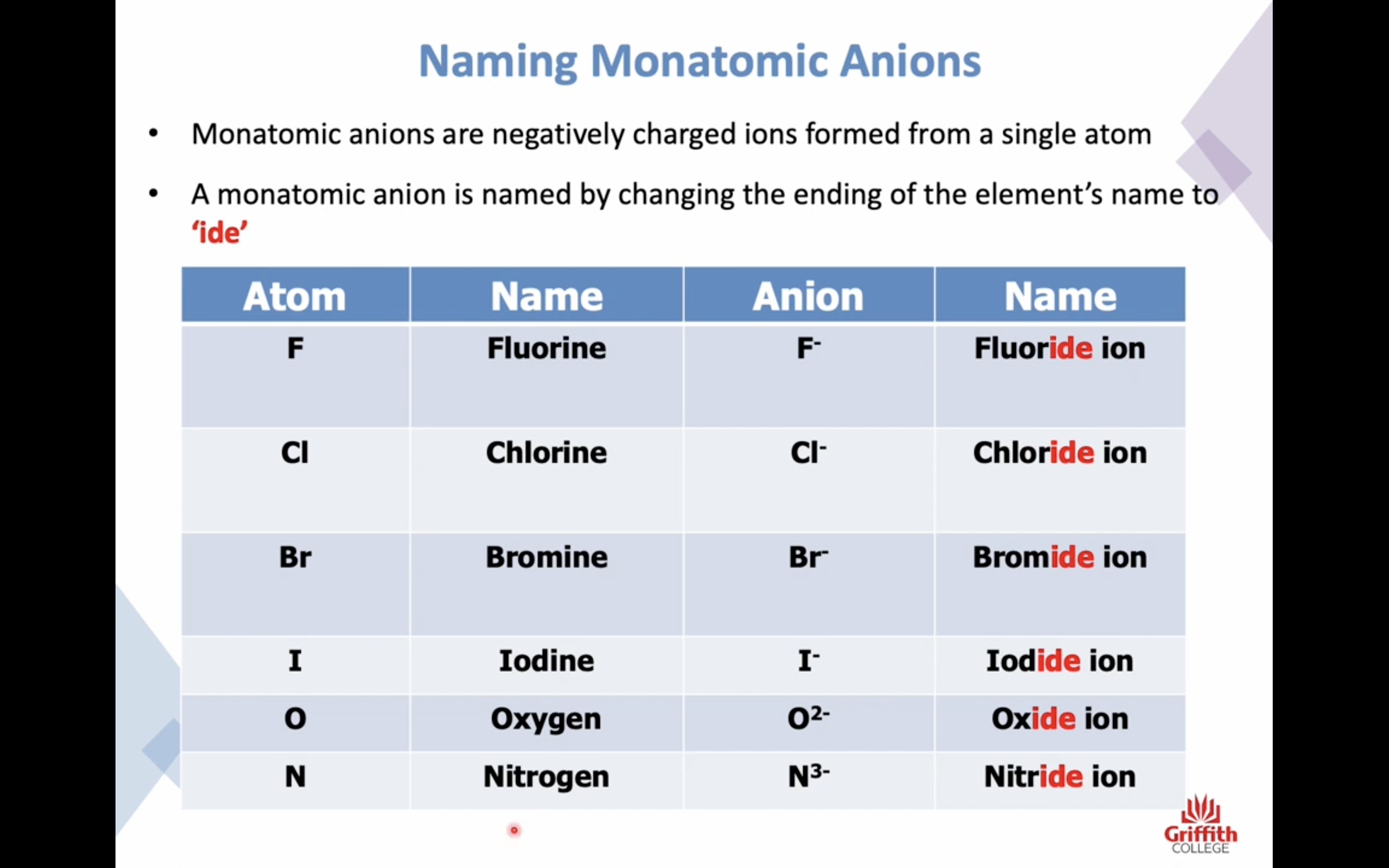

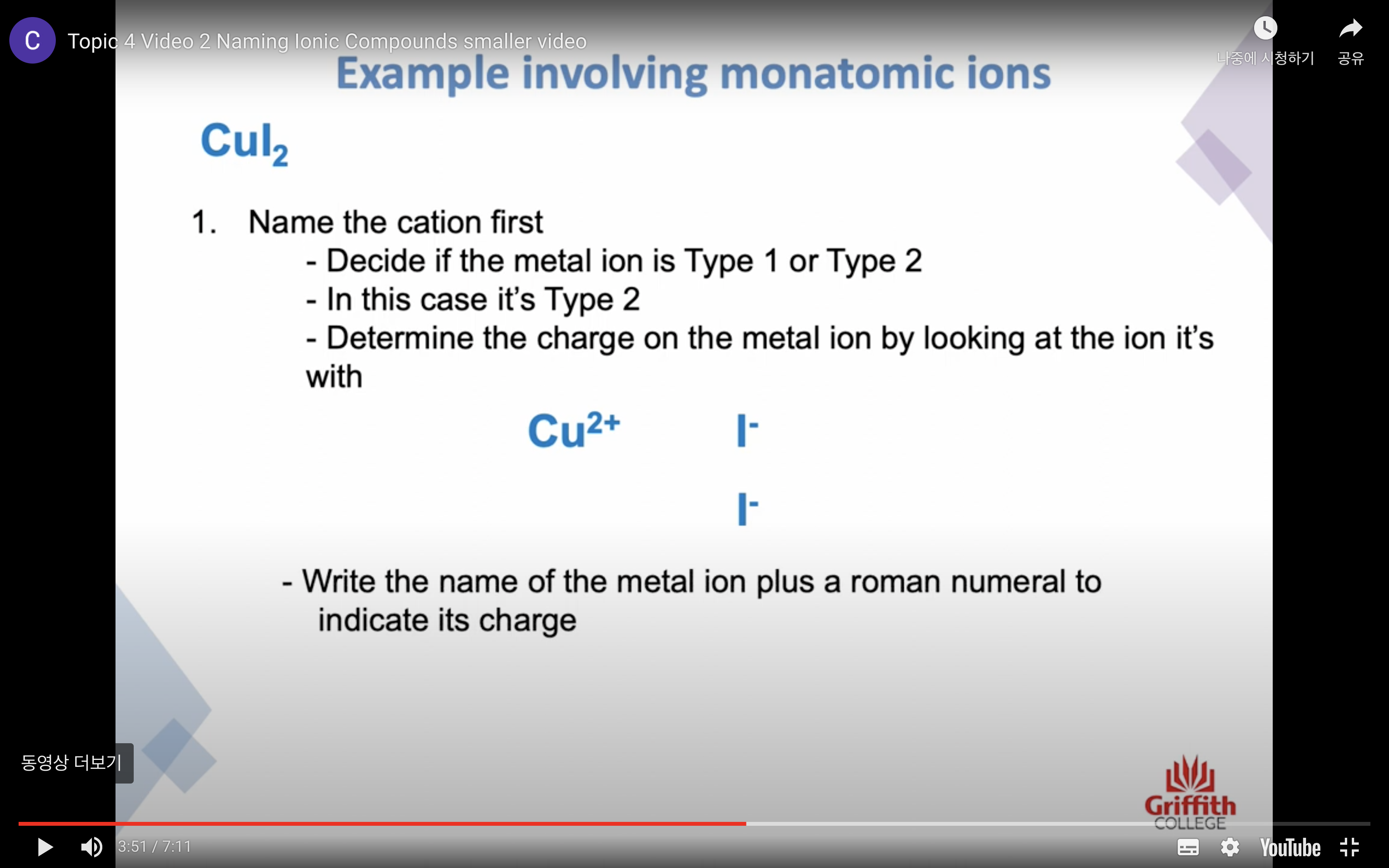
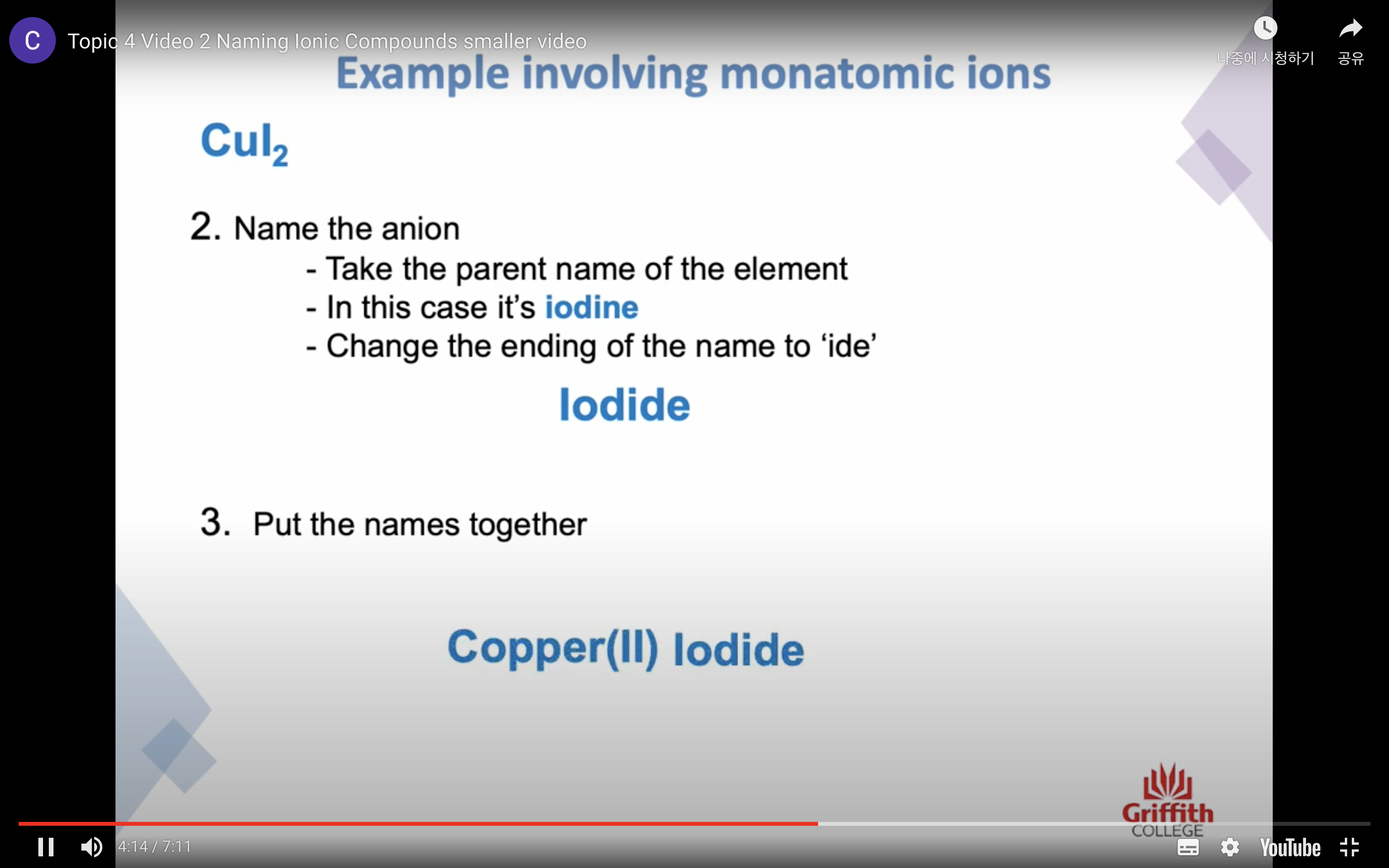
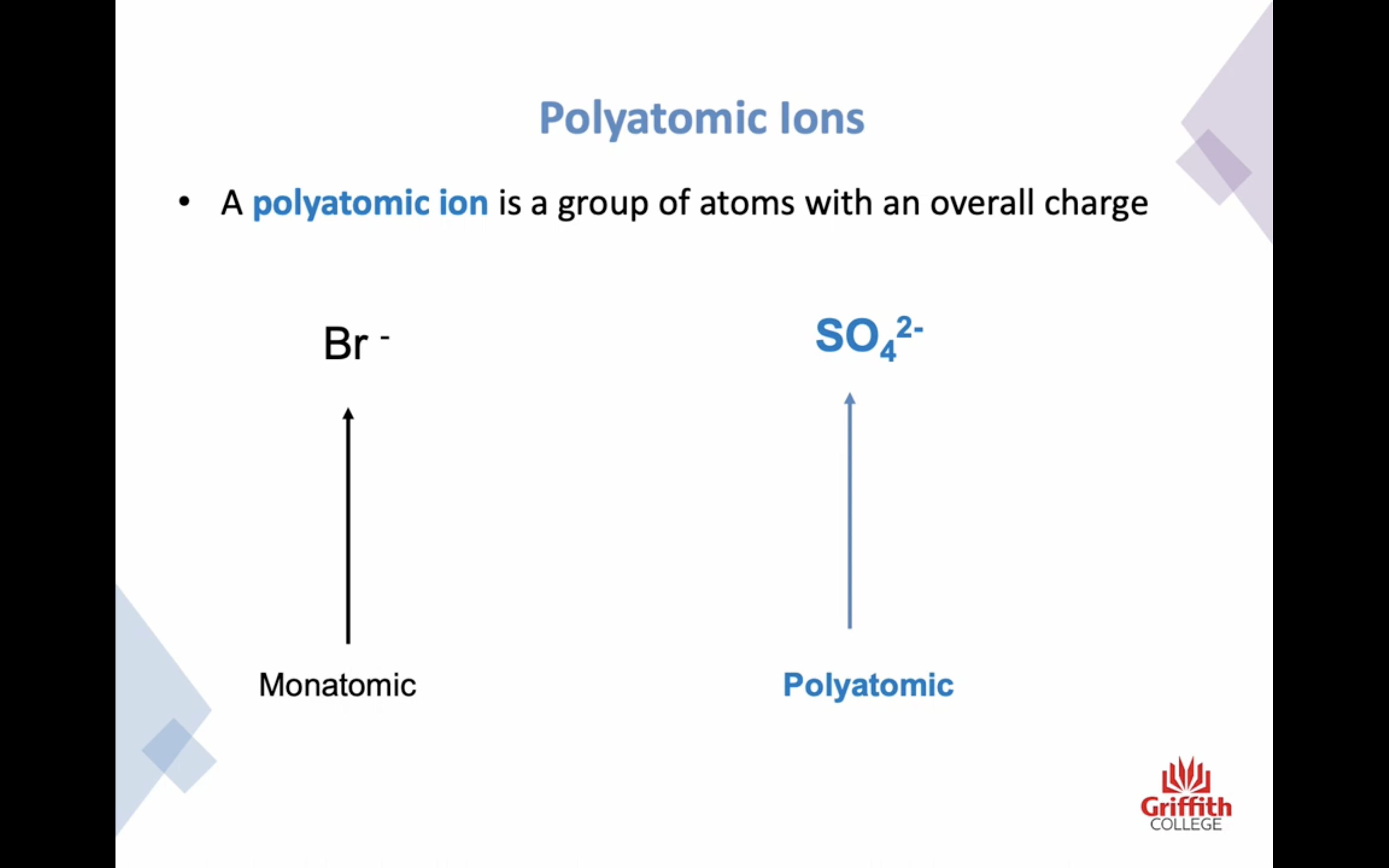
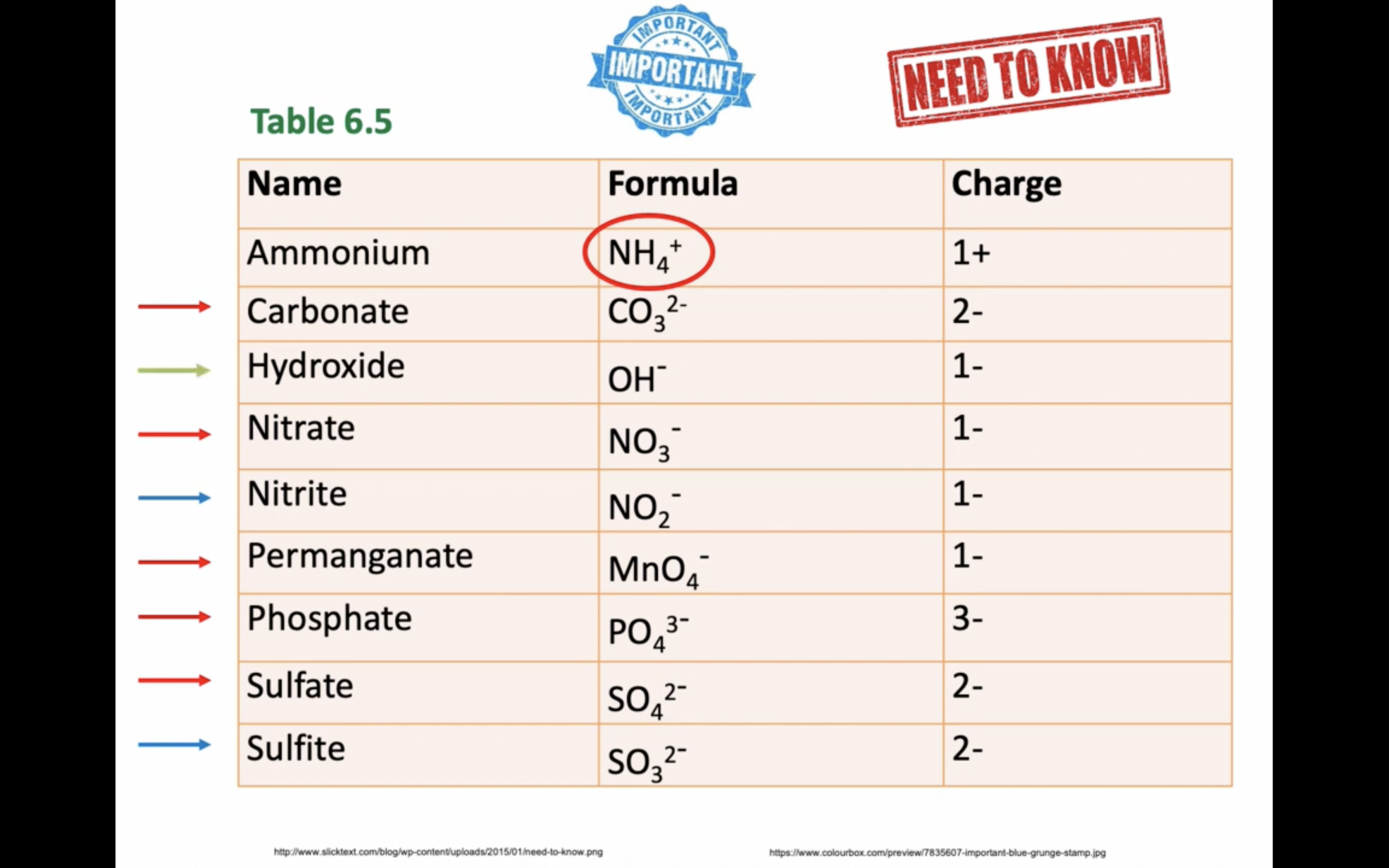
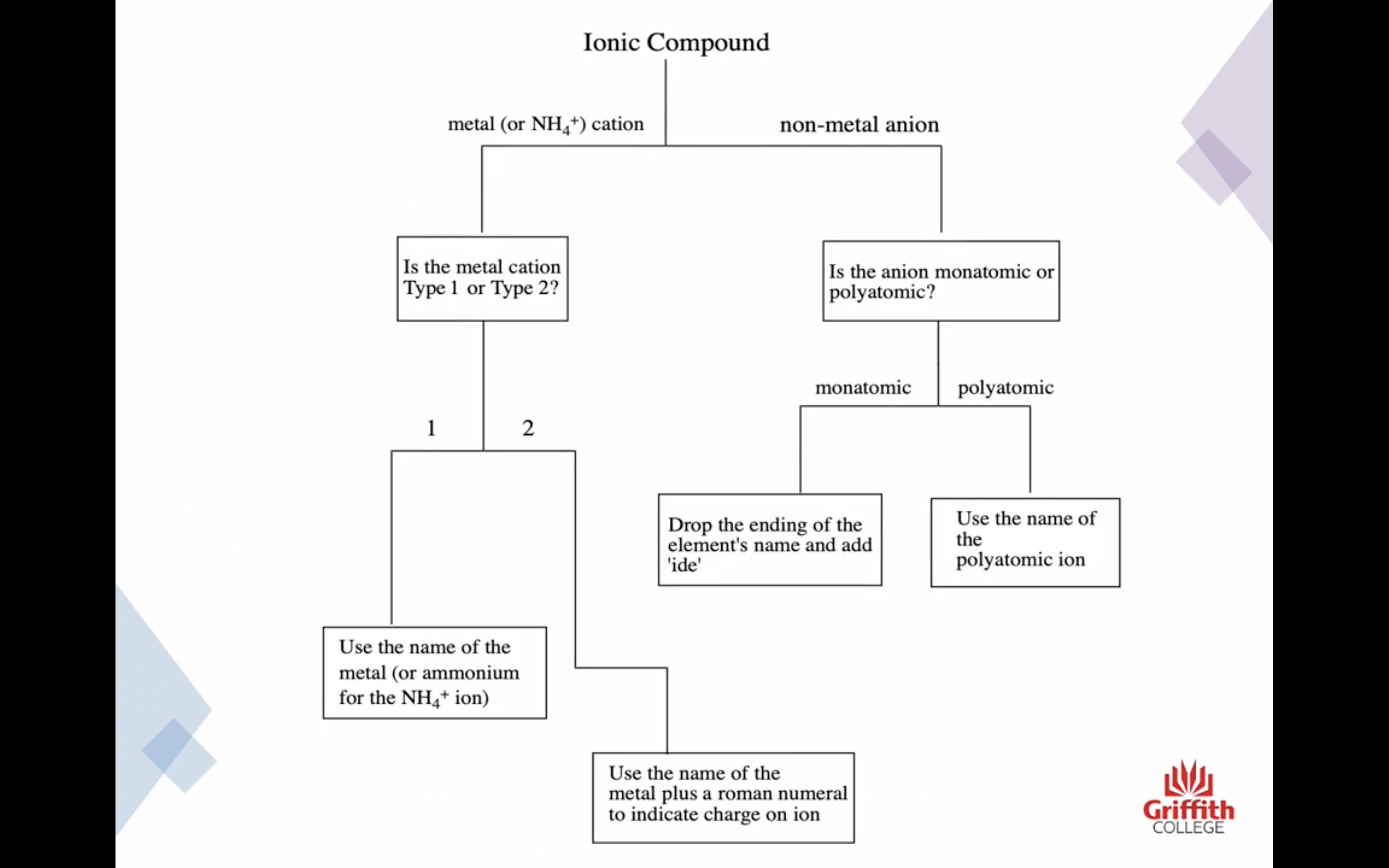
이온 결합 네이밍
-금속 1+ 이면 그냥 쓰고 2+, 3+ ..ㅇ 이면 (II) (III) 이런식으로 이름뒤에 쓴닷
-비금속은 ide 로 바꾸고 polyatomic 인 경우는 그 이름 쓰고
끝
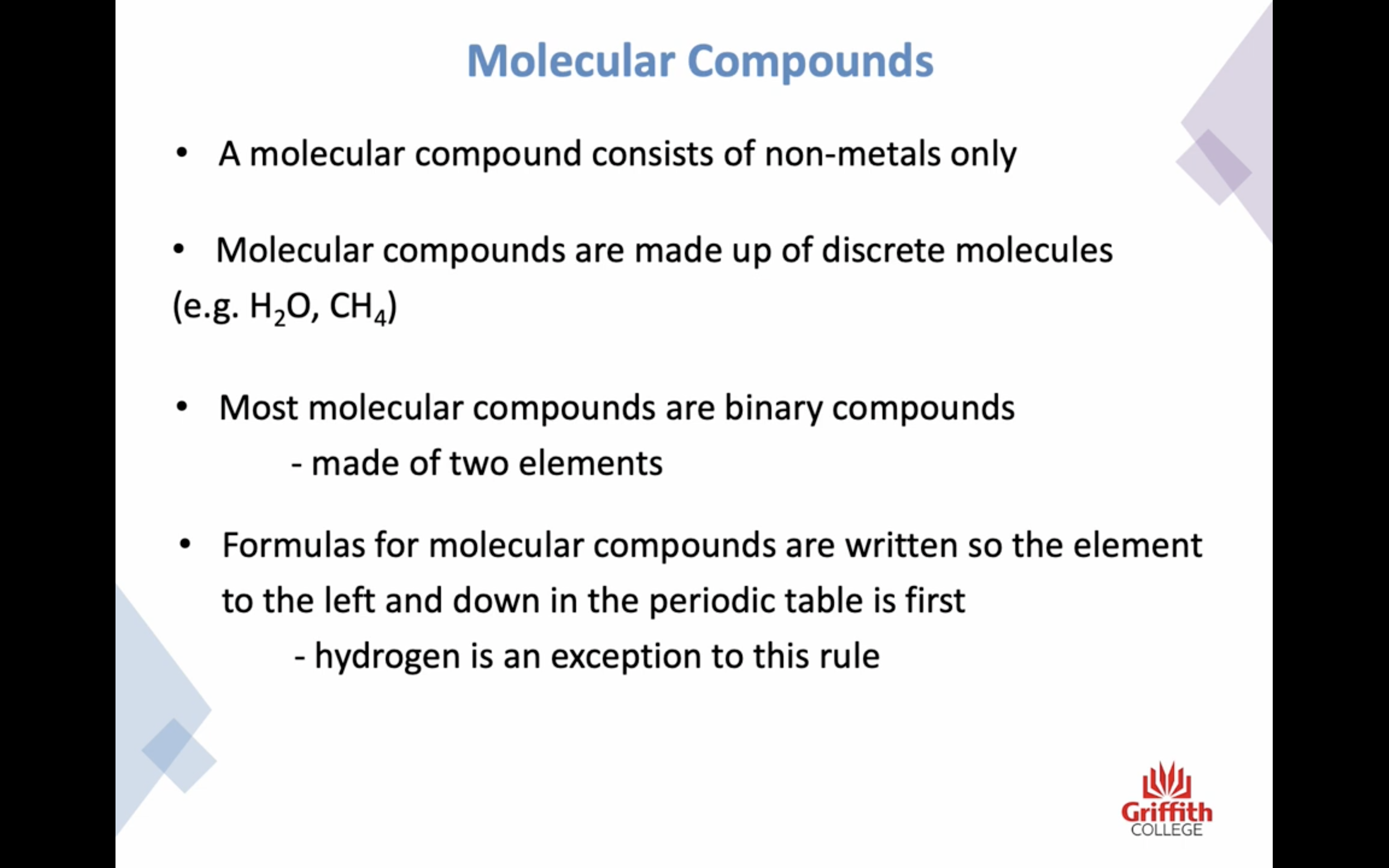
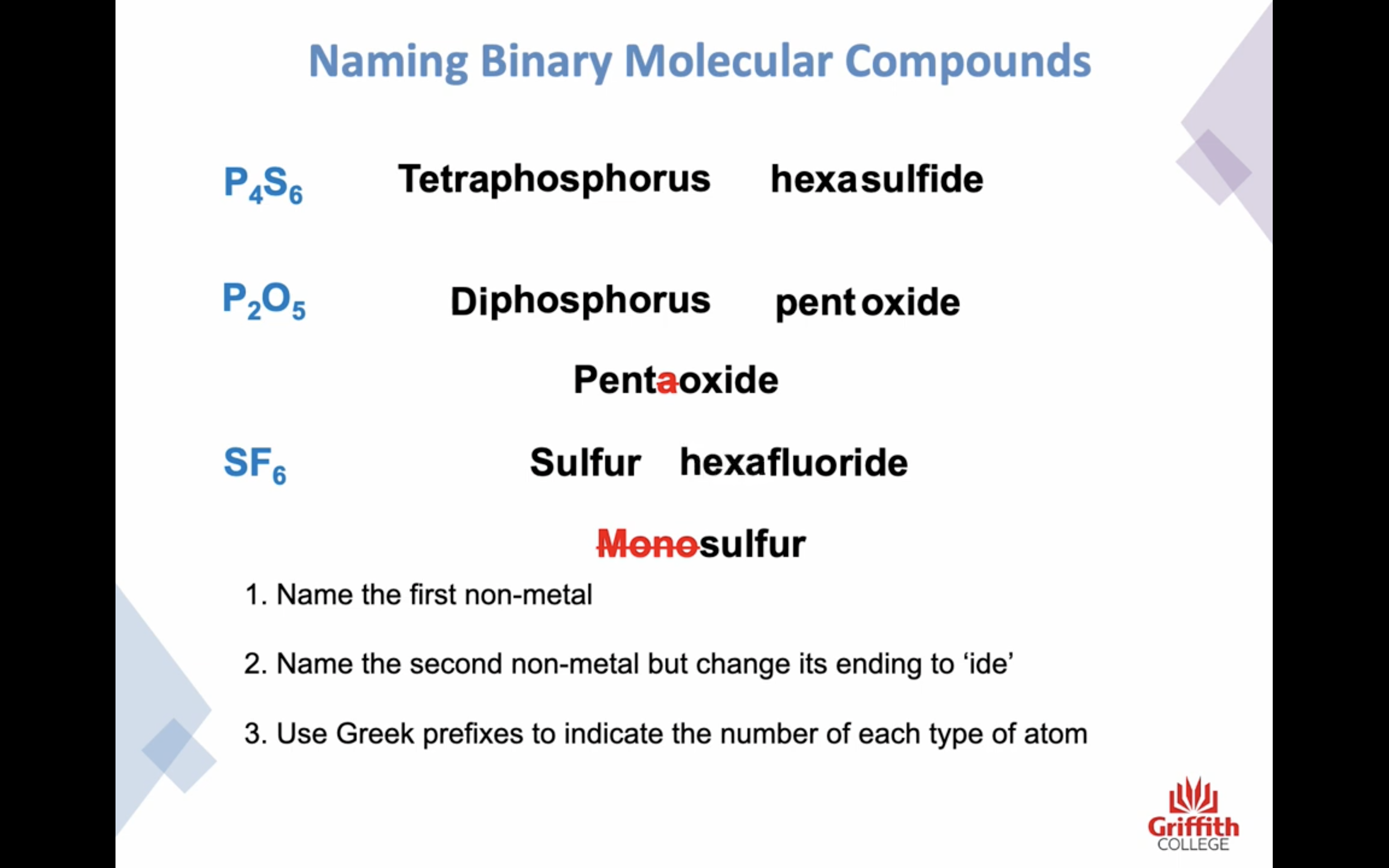
Naming Binary Molecular Compounds
공유 결합 네이밍은 좀 다르닷
-앞부분 비금속은 그냥 이름 그대로 쓰고
-뒷부분 비금속은 ide 로 바꾸거나 이름 있는 경우는 그대로 쓴다
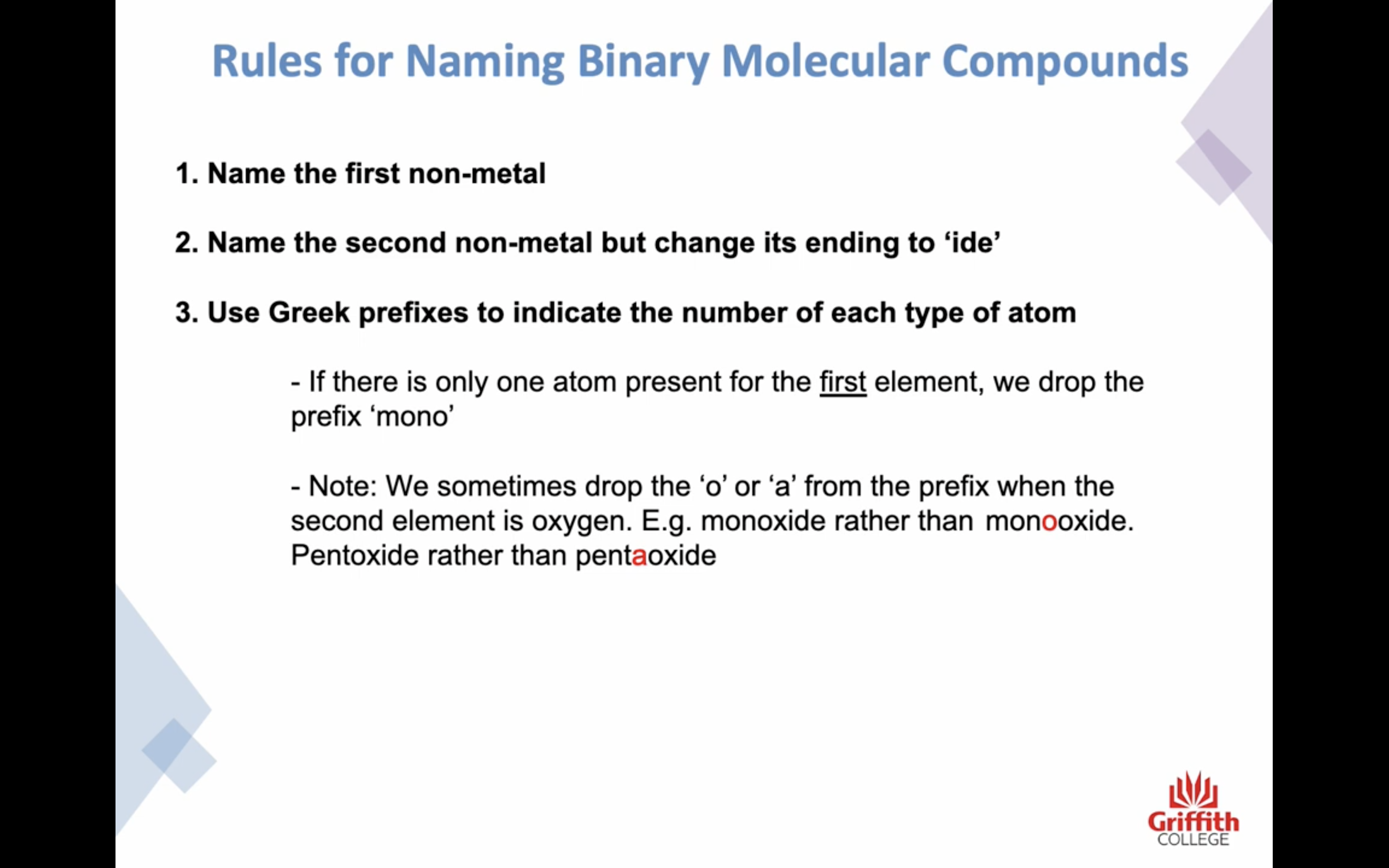
-하지만 ! 이온결합과는 좀 다르게 갯수도 명시해야한다!!!!! 결합의 경우의 수가 엄청 많기때문
prefixes를 이름 앞에 써주는데,
-H 의 경우는 prefixes 사용 안한닷 H 는 결합하는 방식이 한가지 뿐임
-앞부분 비금속이 하나인 경우는 mono 생략한닷
-o,a + o 인경우 앞부분 o,a 생략한닷
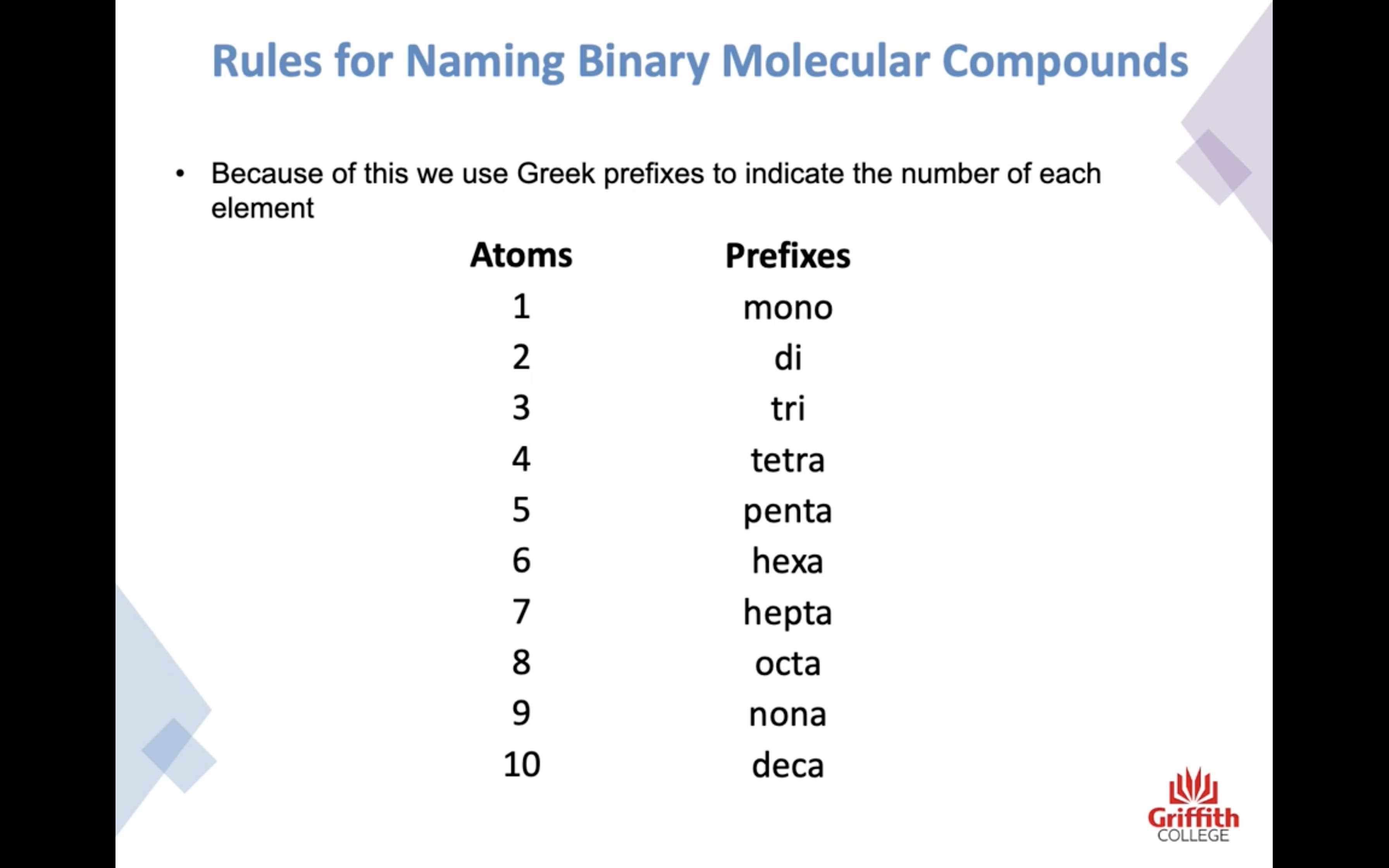
1 mono
2 di
3 tri
4 tetra
5 penta
6 hexa
7 hepta
8 octa
9 nona
10 deca
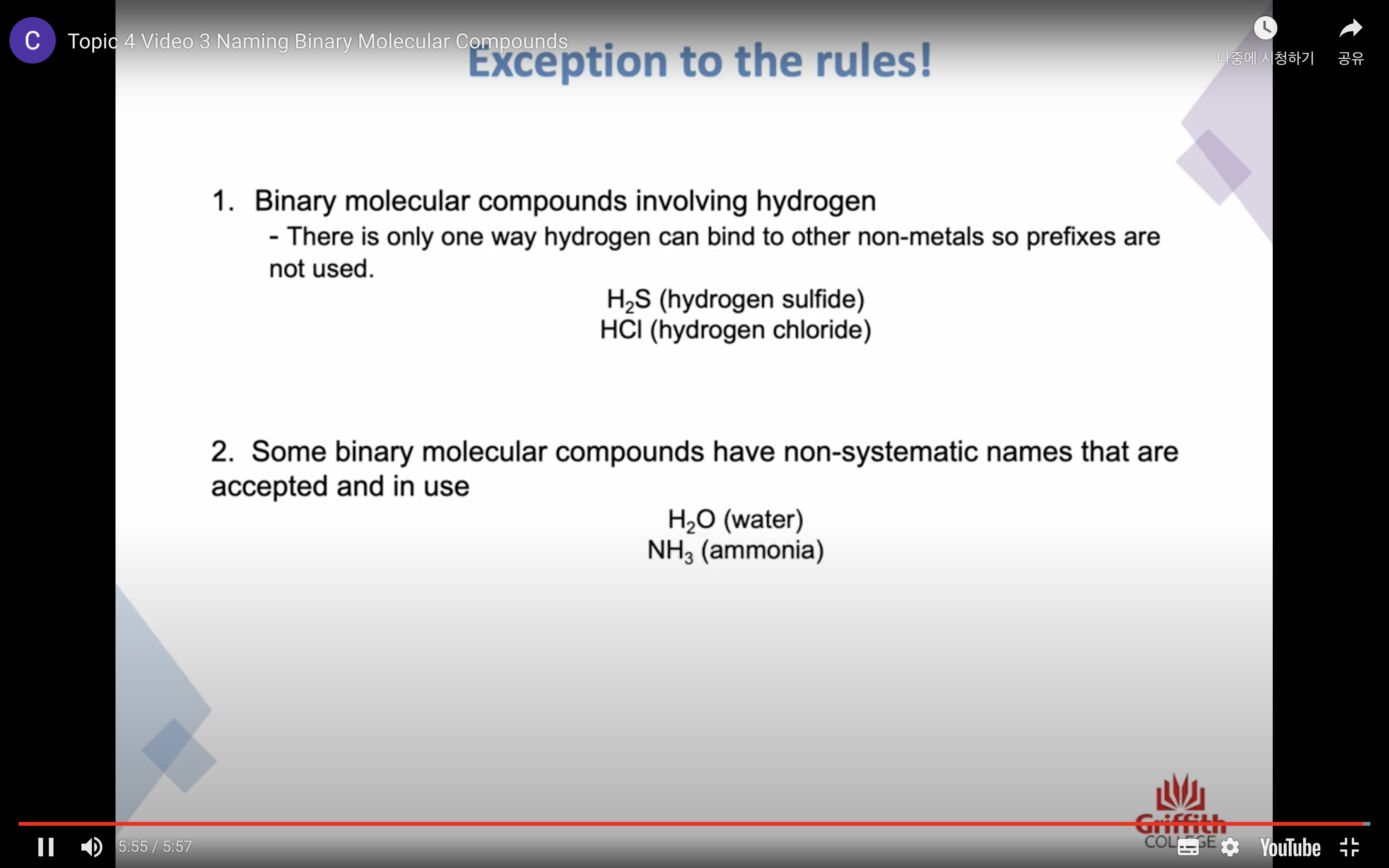
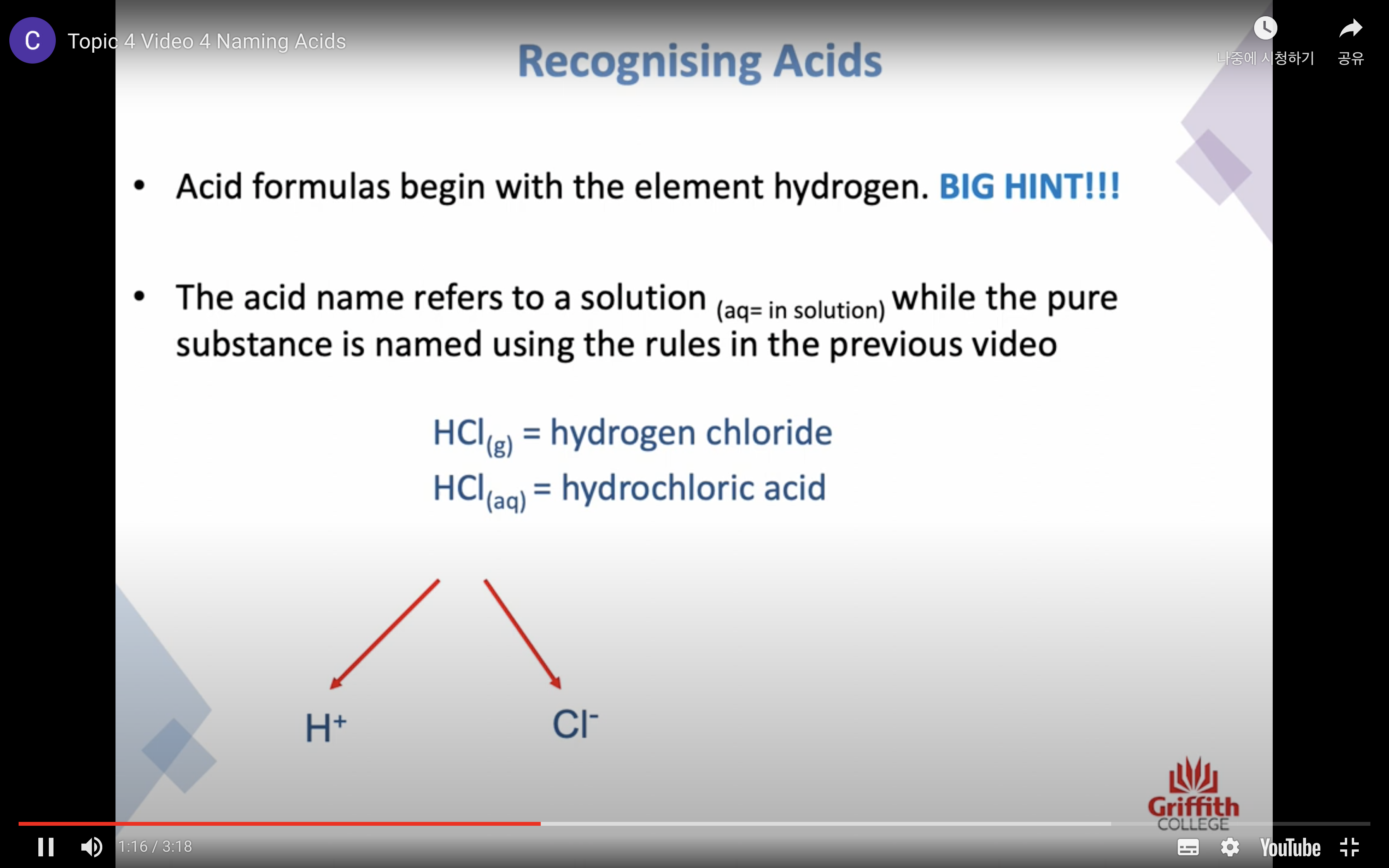
Naming Inorganic Acids

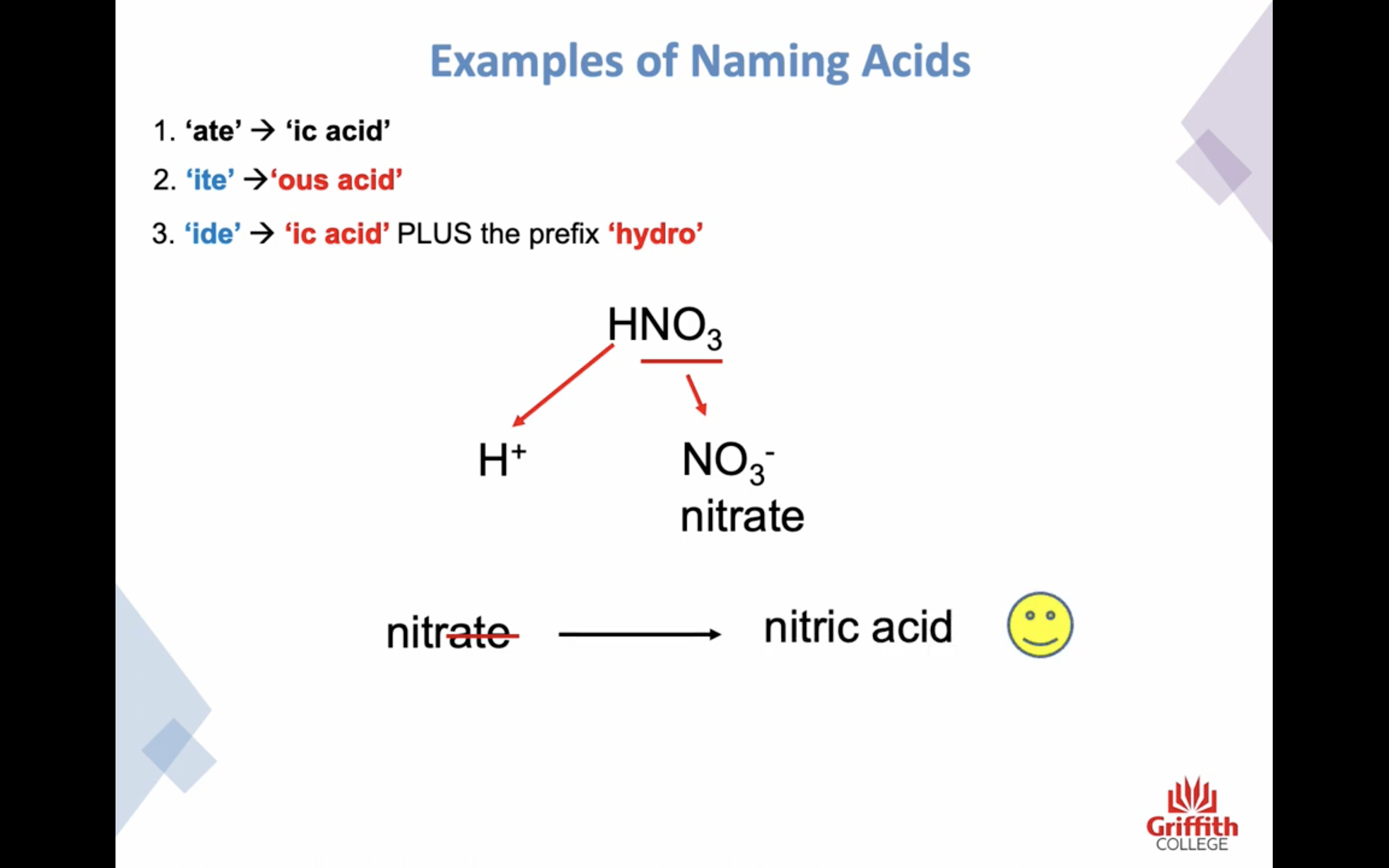
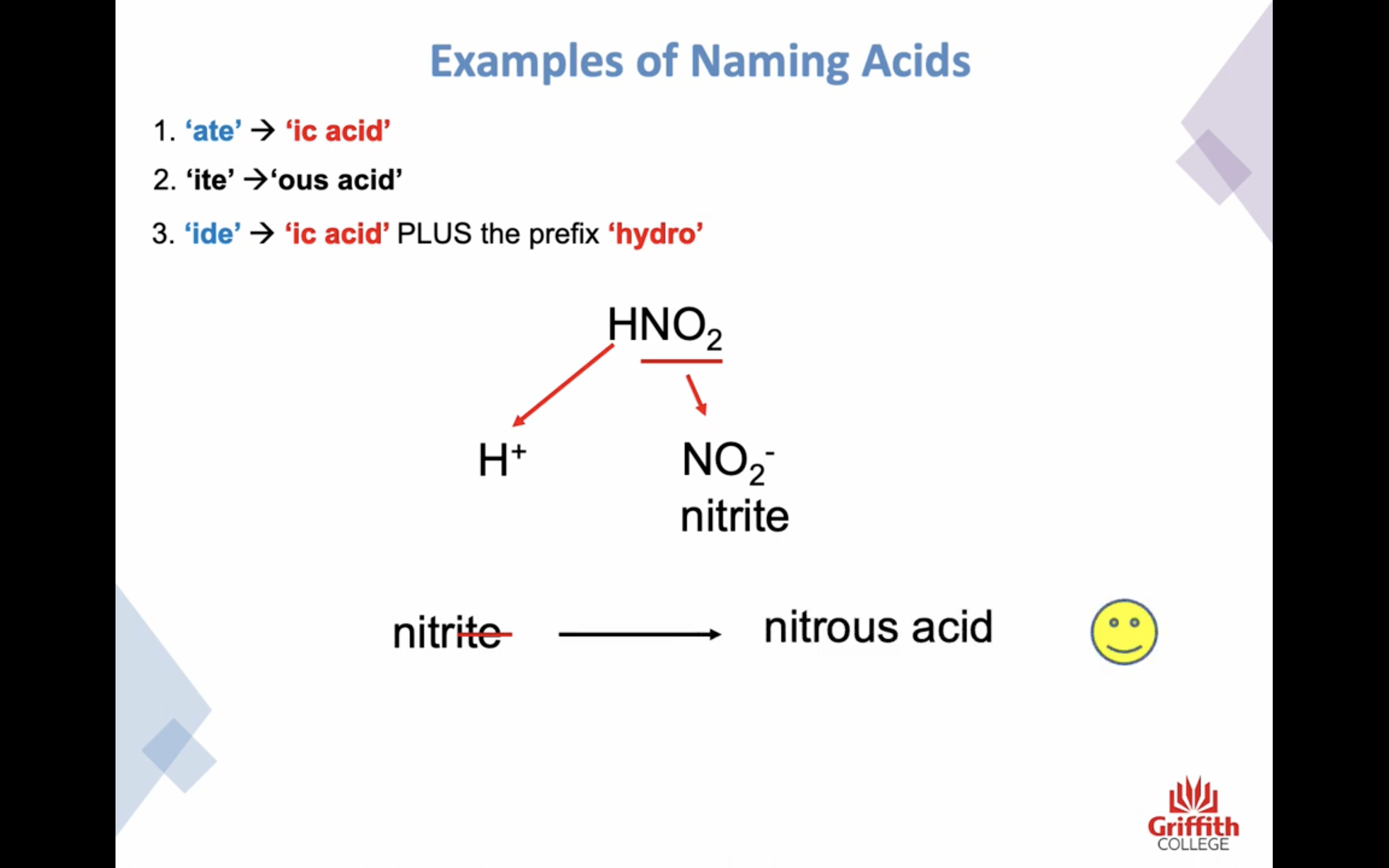
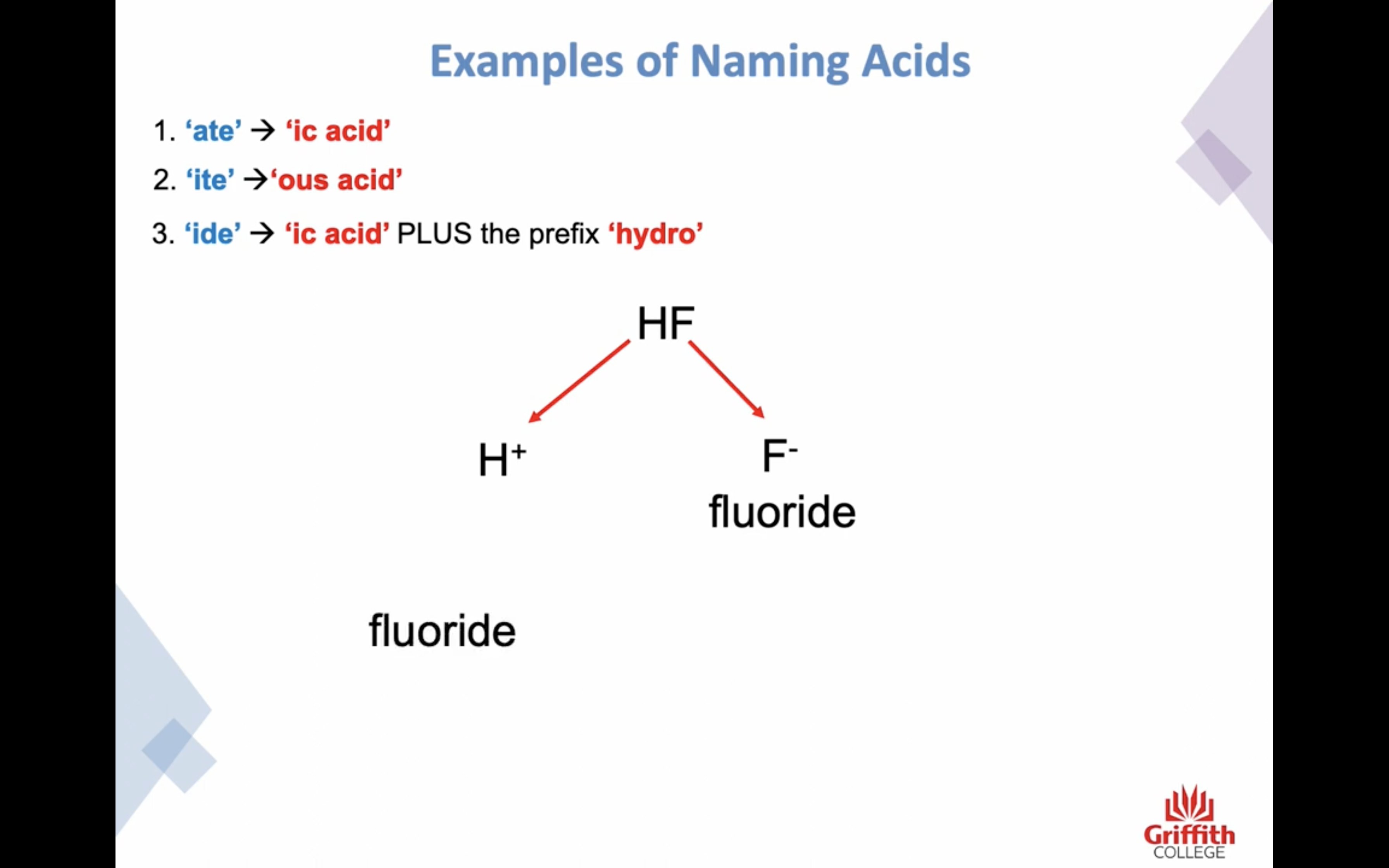
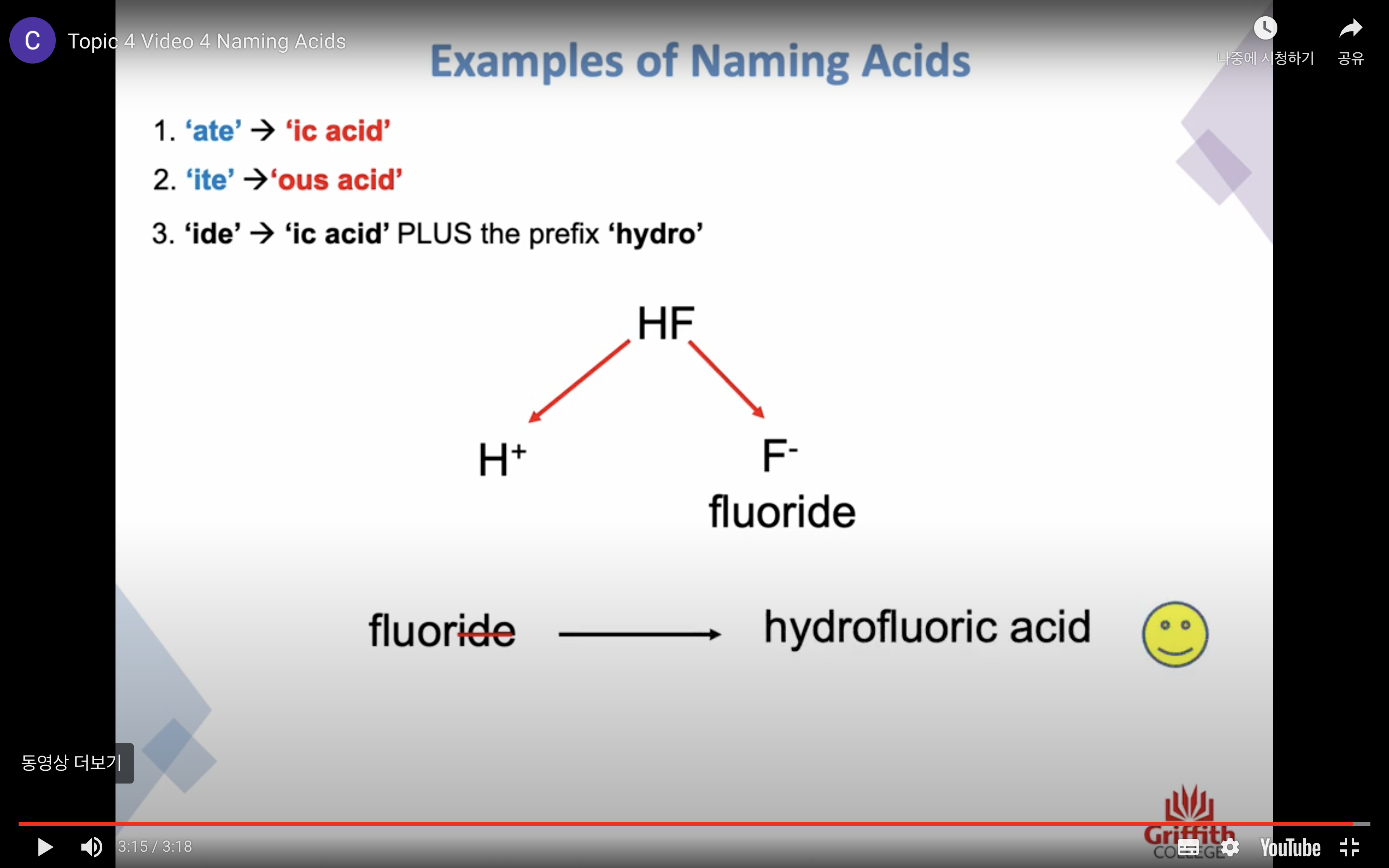
ate -> ic acid
ide -> ic + (앞에)hydo
ite -> ous acid
'Griffith college Tri3 2022 > 1001GRC (Chem)' 카테고리의 다른 글
| WEEK6- Topic 8&9 (0) | 2022.11.23 |
|---|---|
| WEEK5 - Topic 7 (0) | 2022.11.17 |
| WEEK4 - Topic 6 (0) | 2022.11.09 |
| WEEK3 - Topic 5 (0) | 2022.11.04 |
| WEEK1 - Topic 1&2 (0) | 2022.10.23 |



