Articulate Lesson

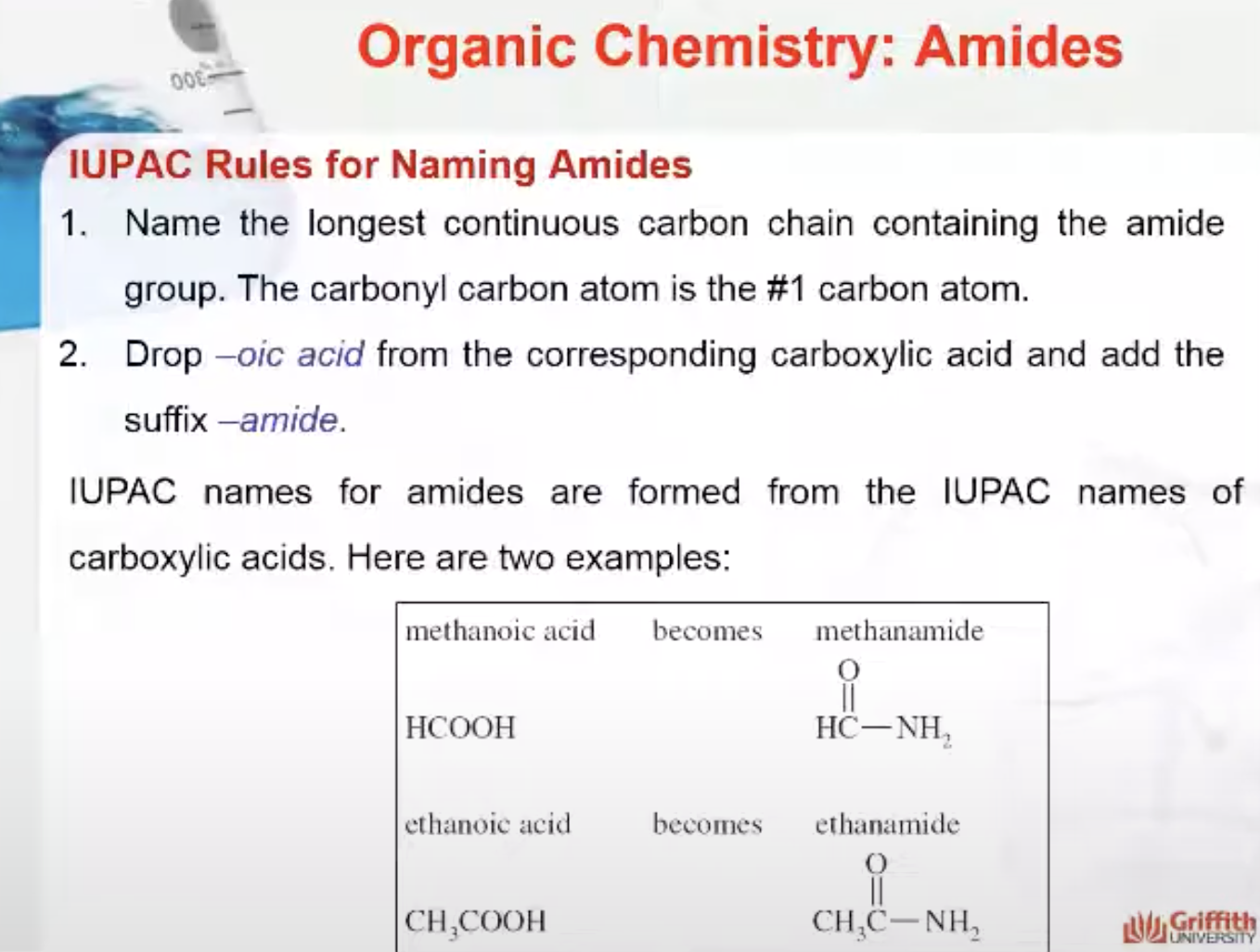
The main differenc e between amine and amide is the presence of a carbonyl group in their structure;
- amines have no carbonyl groups attached to the nitrogen atom; whereas
- amides have a carbonyl group attached to a nitrogen atom.


Amides
Amides are neutral nitrogen-containing compounds. They are not acidic or basic (they are neutral) and exist as molecules both in aqueous solutions and as pure substances. These compounds contain carbonyl groups. The carbonyl group is directly connected to a nitrogen atom.
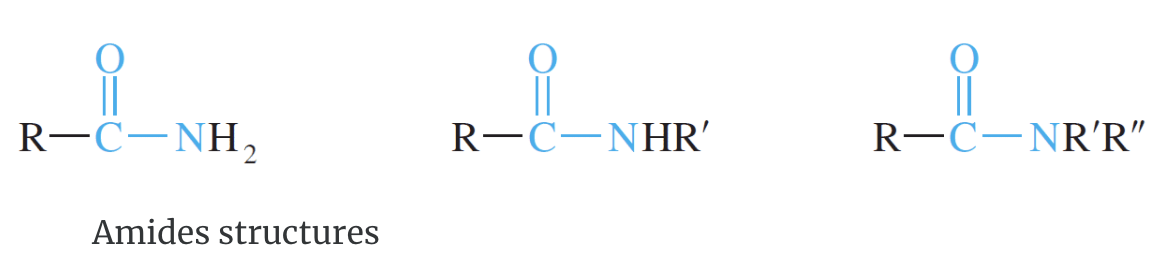
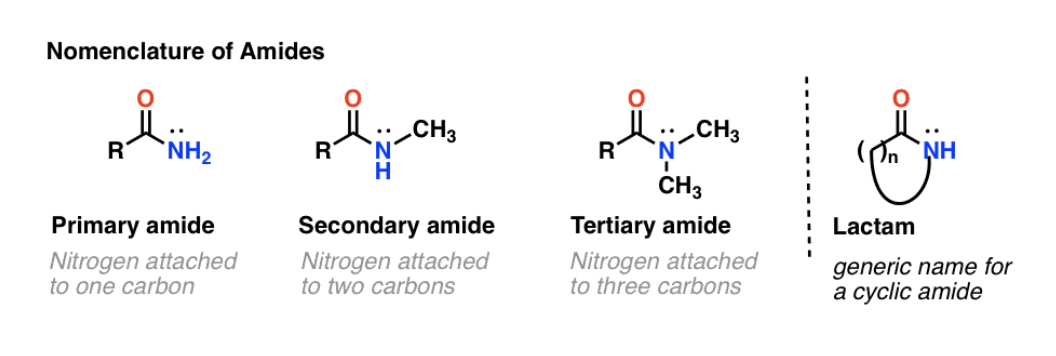
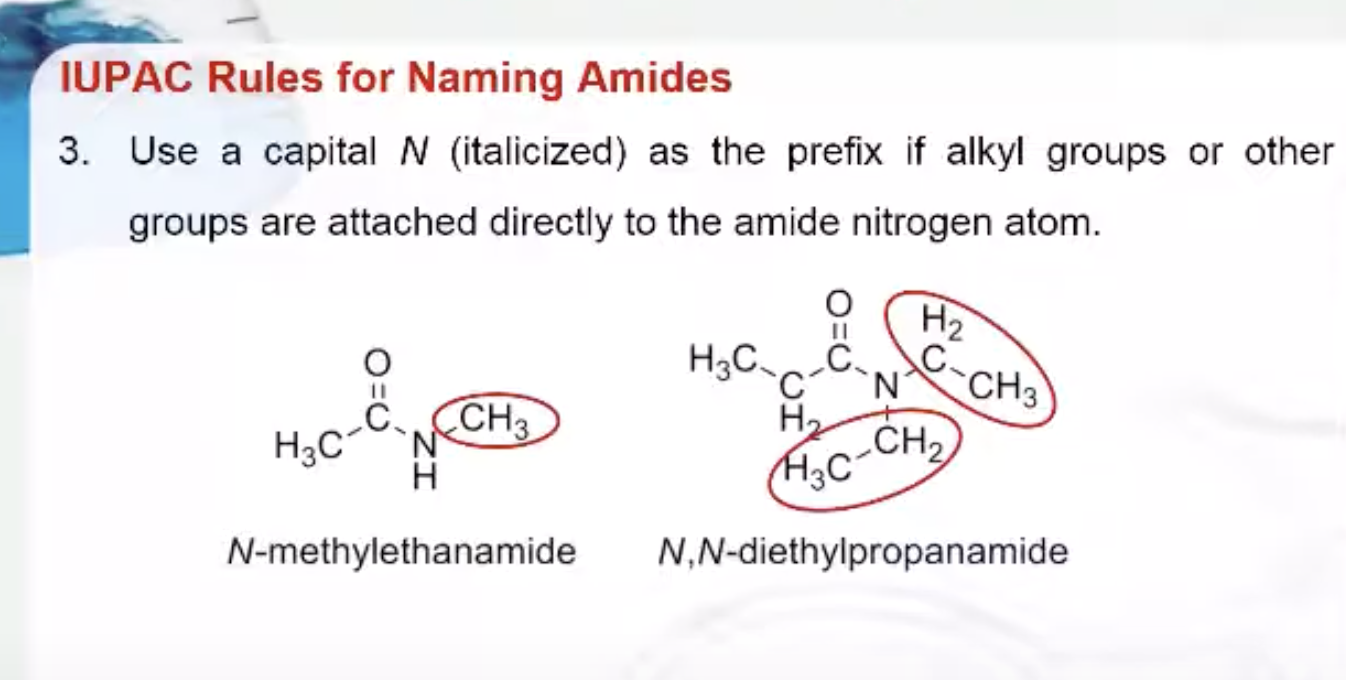
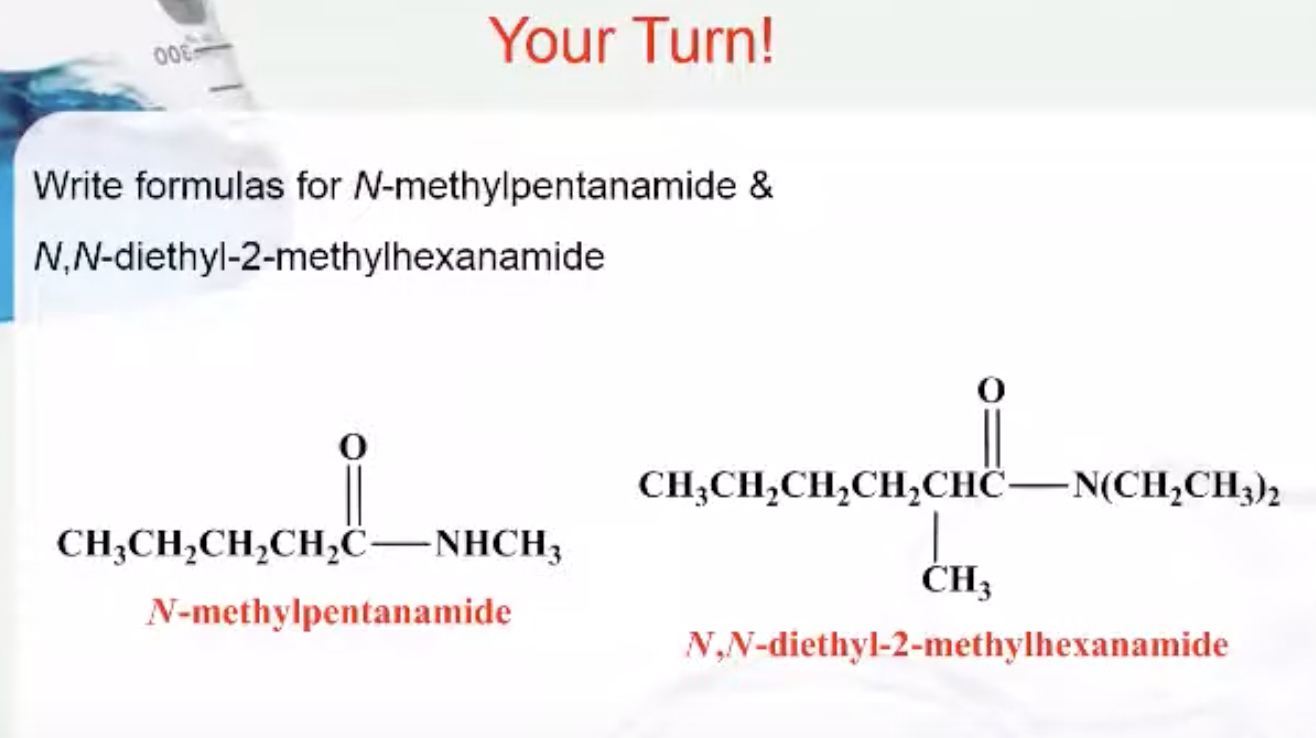
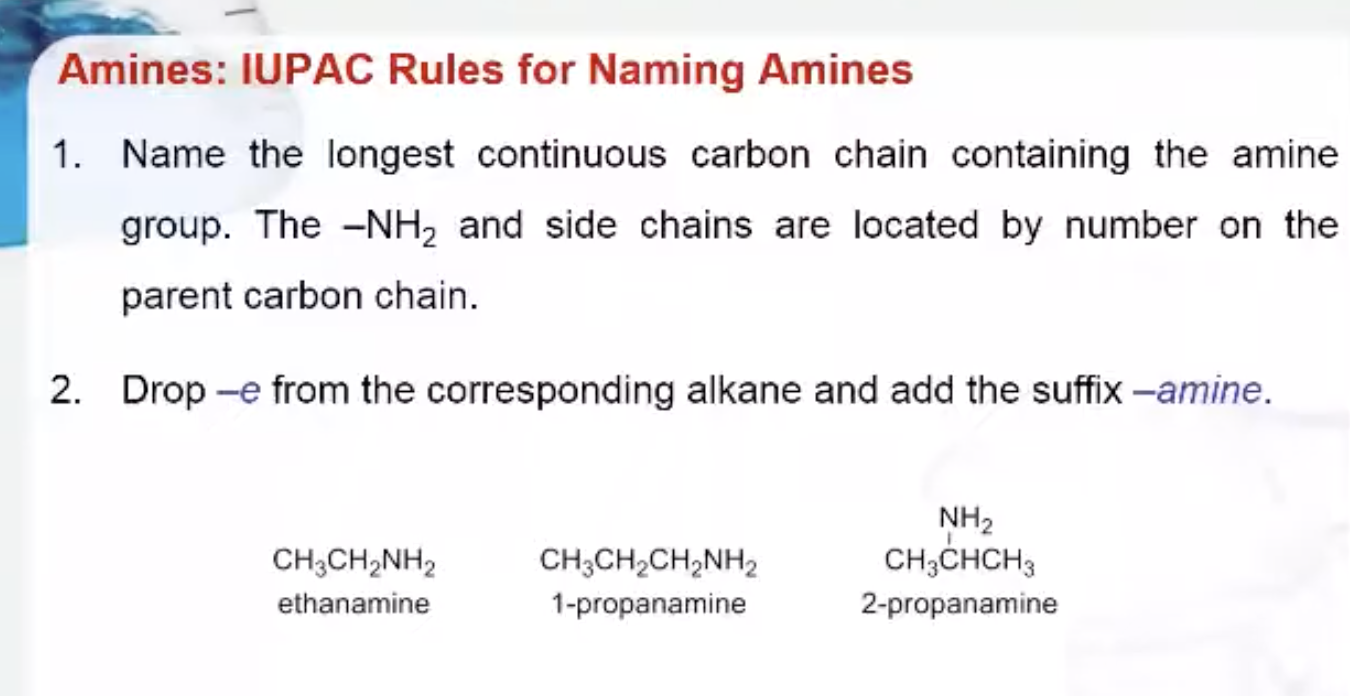
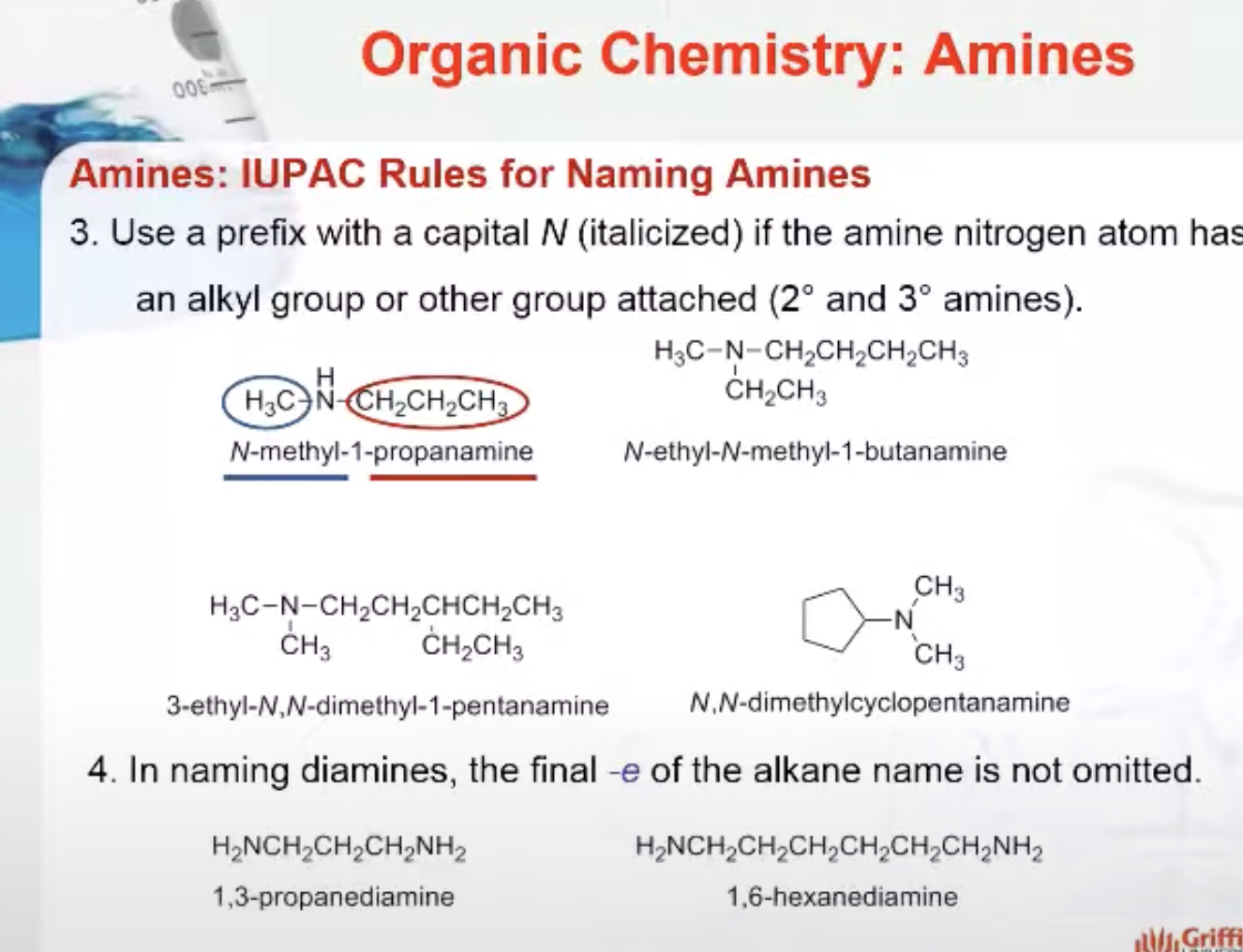
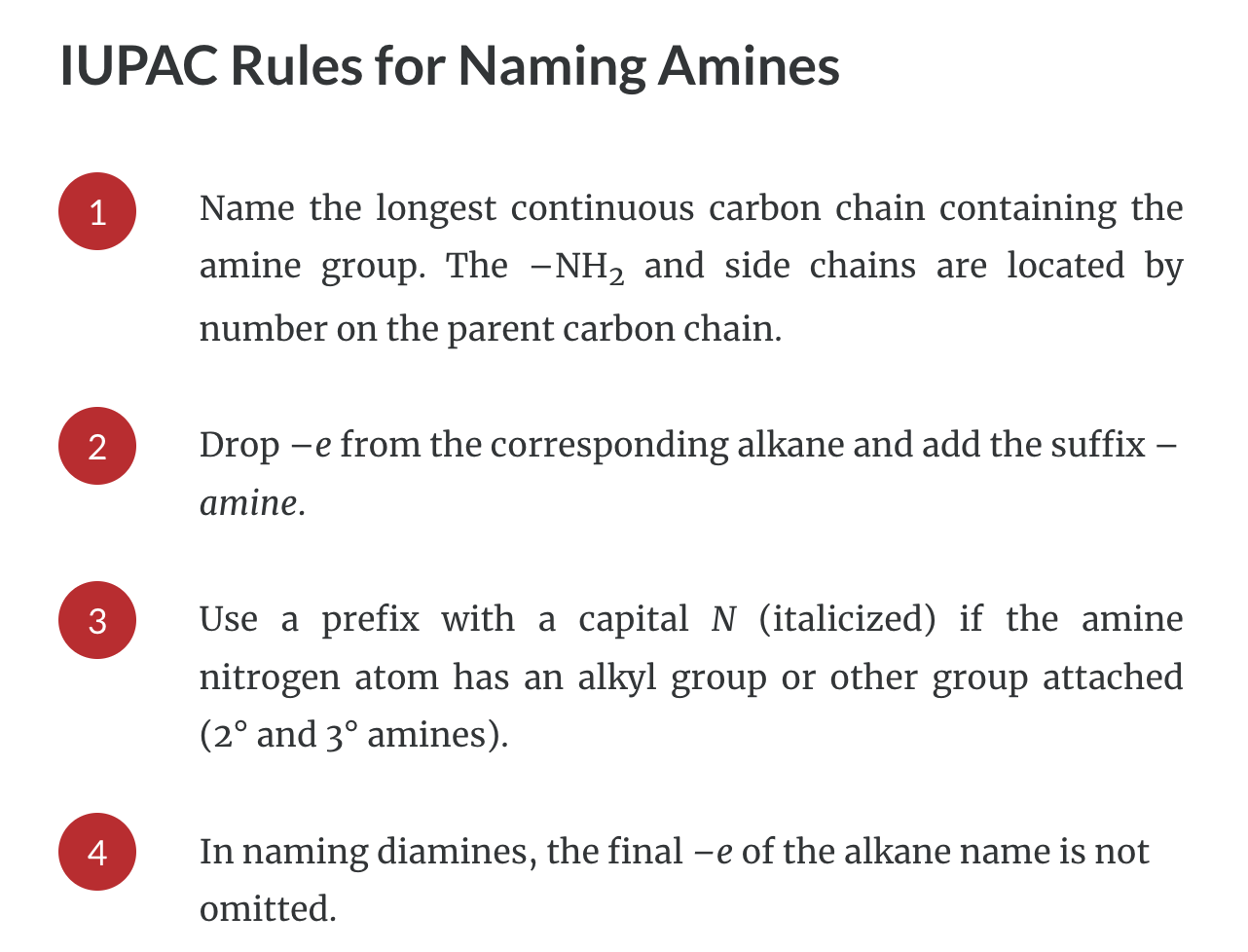
IUPAC Rules for Naming Amides
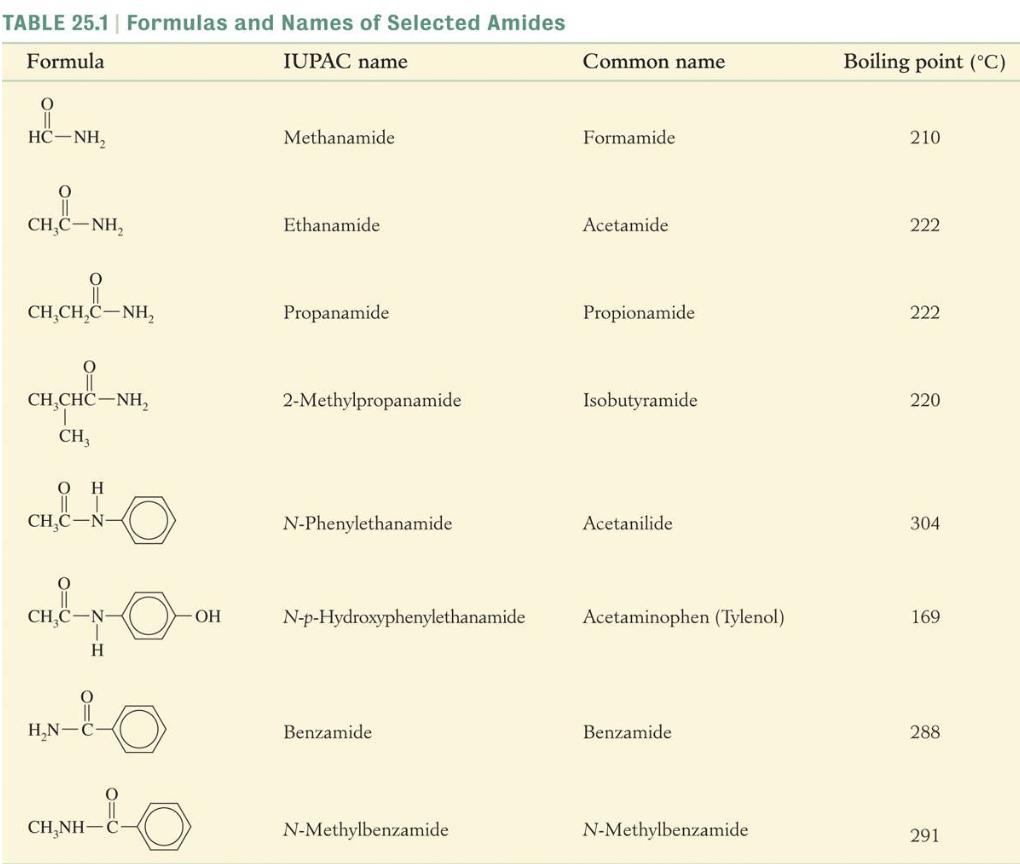
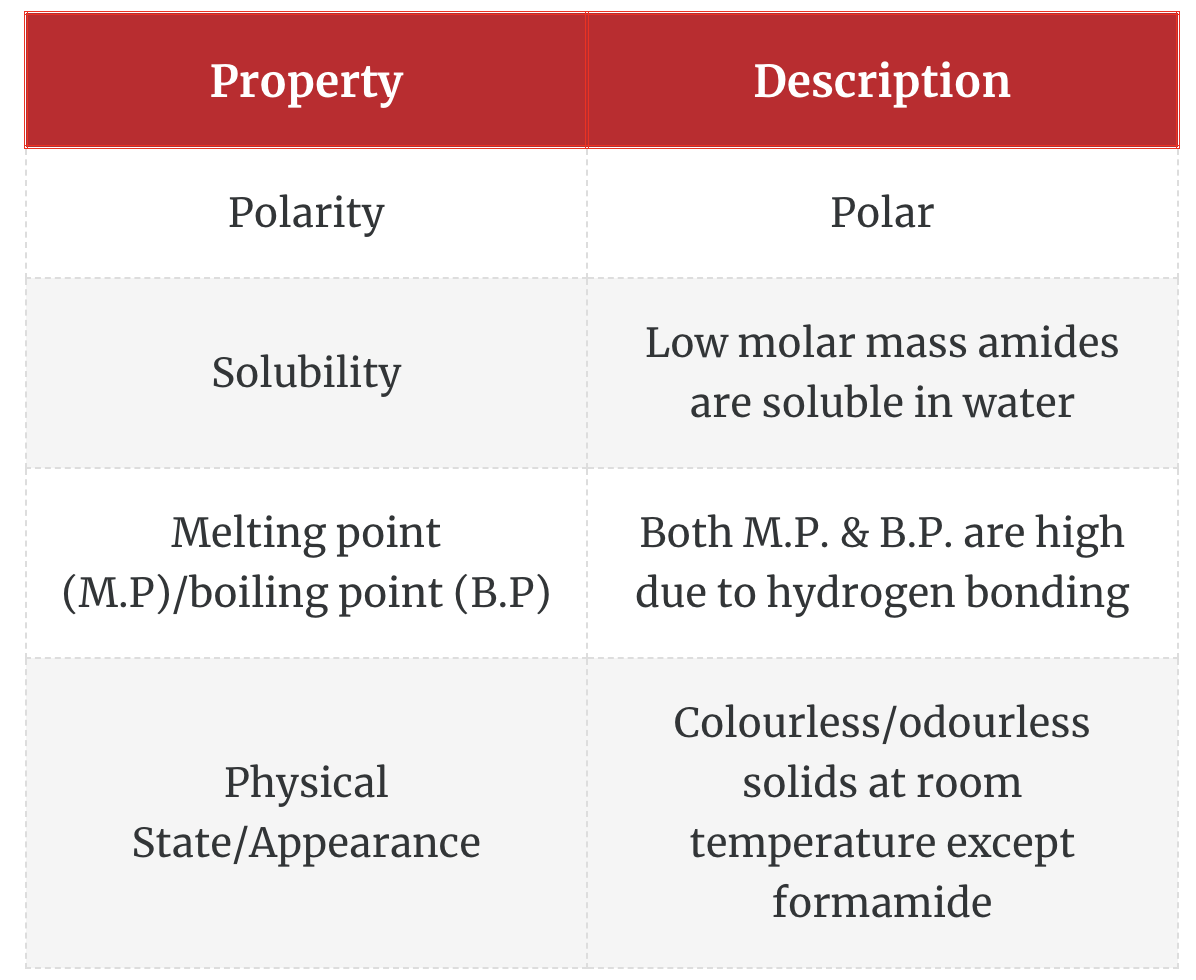
Physical Properties of Amides
The physical properties of amides are determined largely by hydrogen bonding. High water solubility and high melting and boiling points are the result of hydrogen bonding.
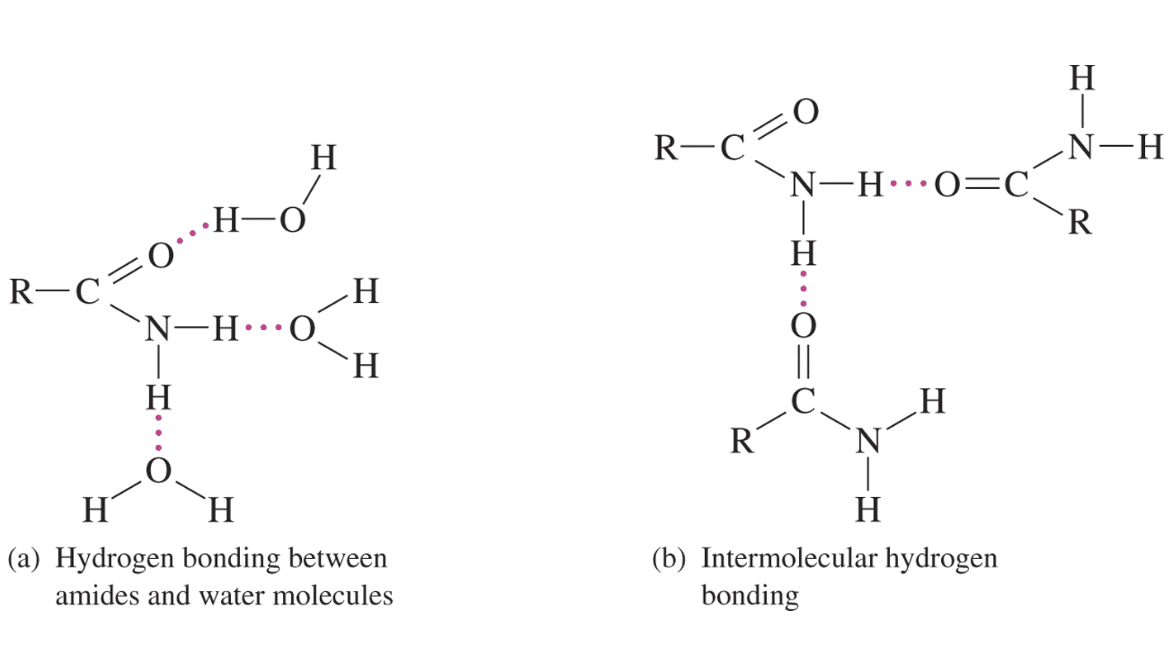
Chemical Properties of Amides
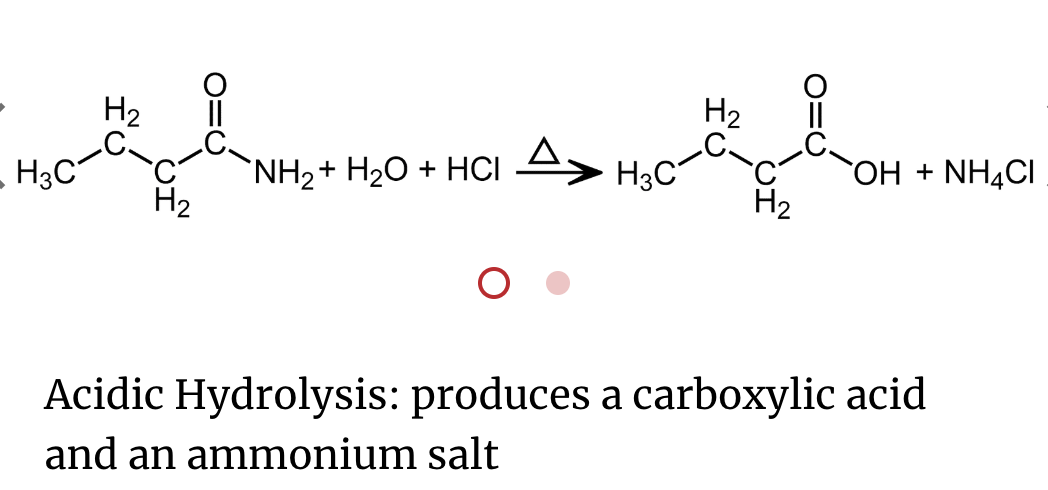
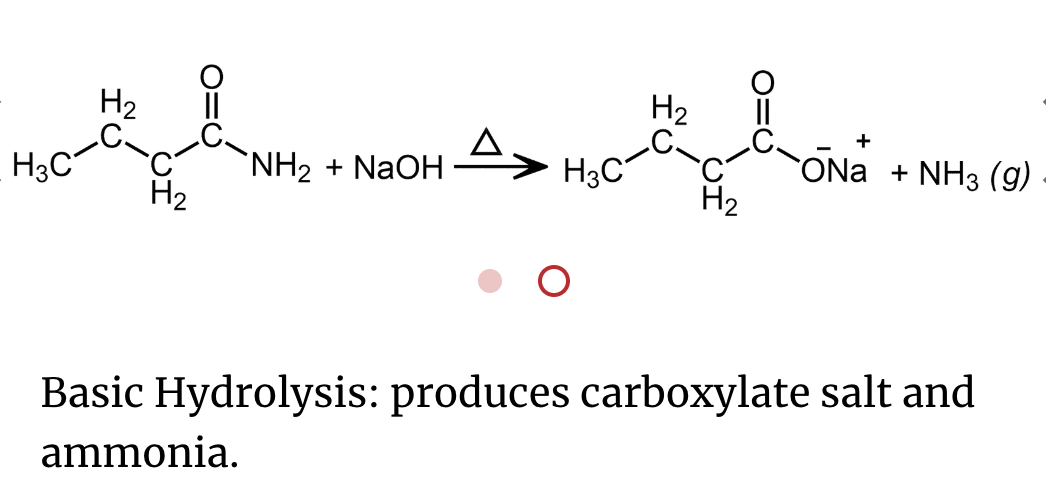
An important reaction of amides is hydrolysis. Amides undergo acidic and basic hydrolysis producing a carboxylic acid or carboxylate salt.
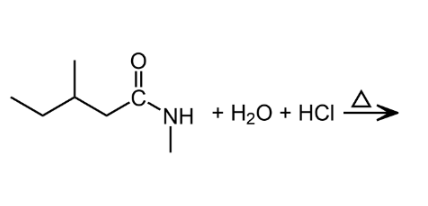
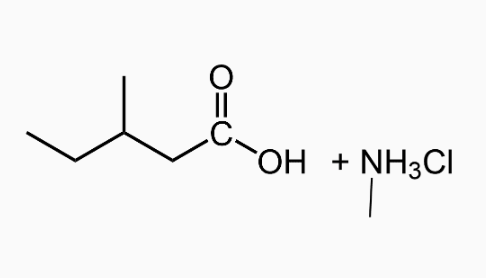
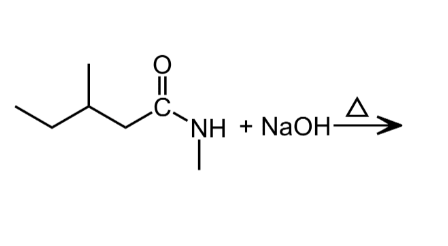
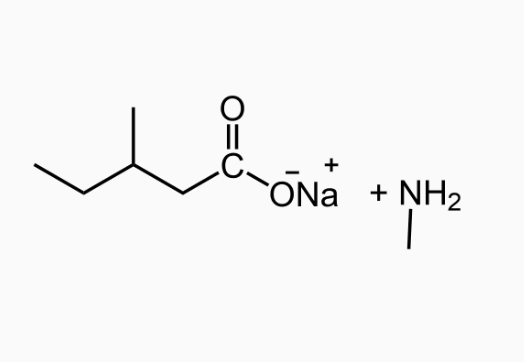
Polyamides: Condensation Polymers
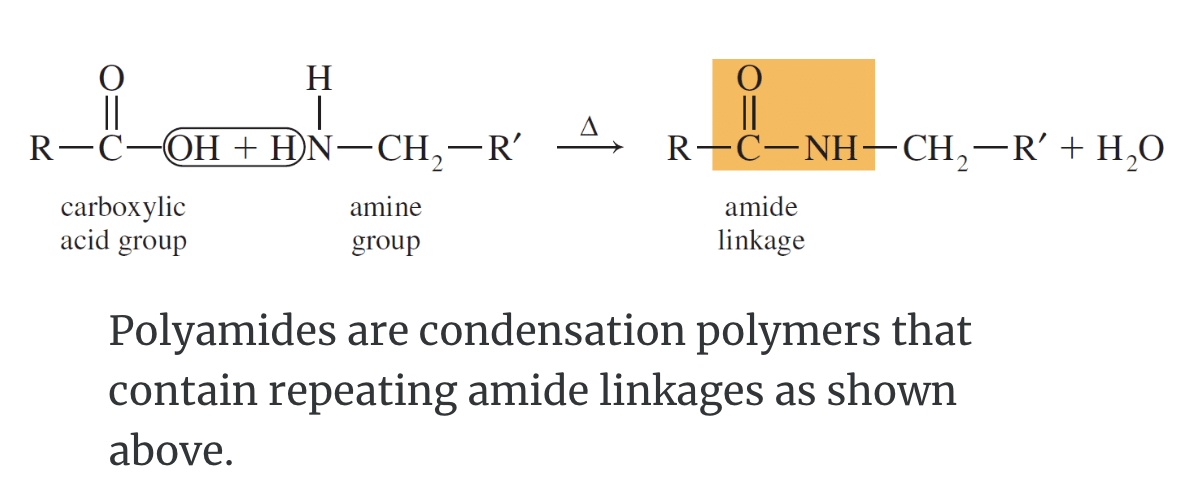
Polyamides are either synthetic like Nylon–66 or are biological like the protein chymotrypsin. You can see the similarity between the structures of chymotrypsin and synthetic Nylon–66 on the right.
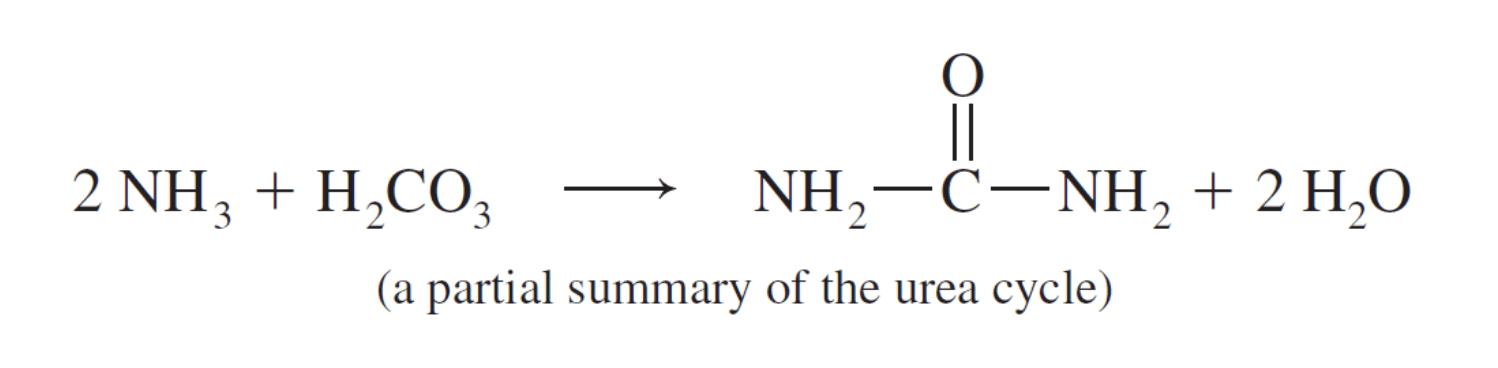
Urea
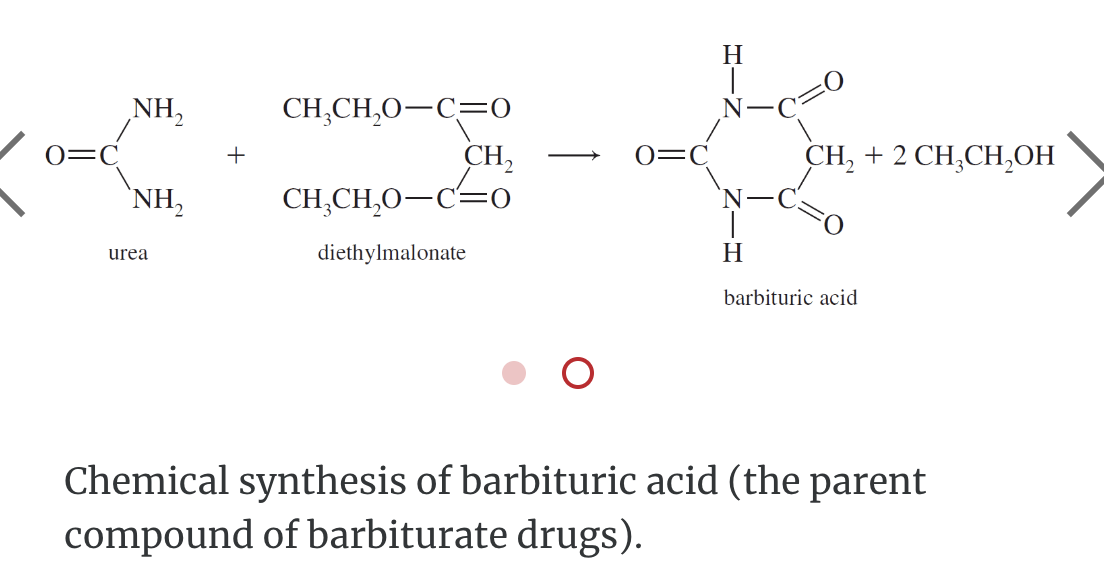
Urea is a simple diamide that is excreted in urine. Urea is the metabolite the body uses to excrete toxic ammonia. Urea is a common commercial product. It is widely used in fertilizers to add nitrogen to the soil and as a starting material in the production of plastics and barbiturates like barbituric acid.
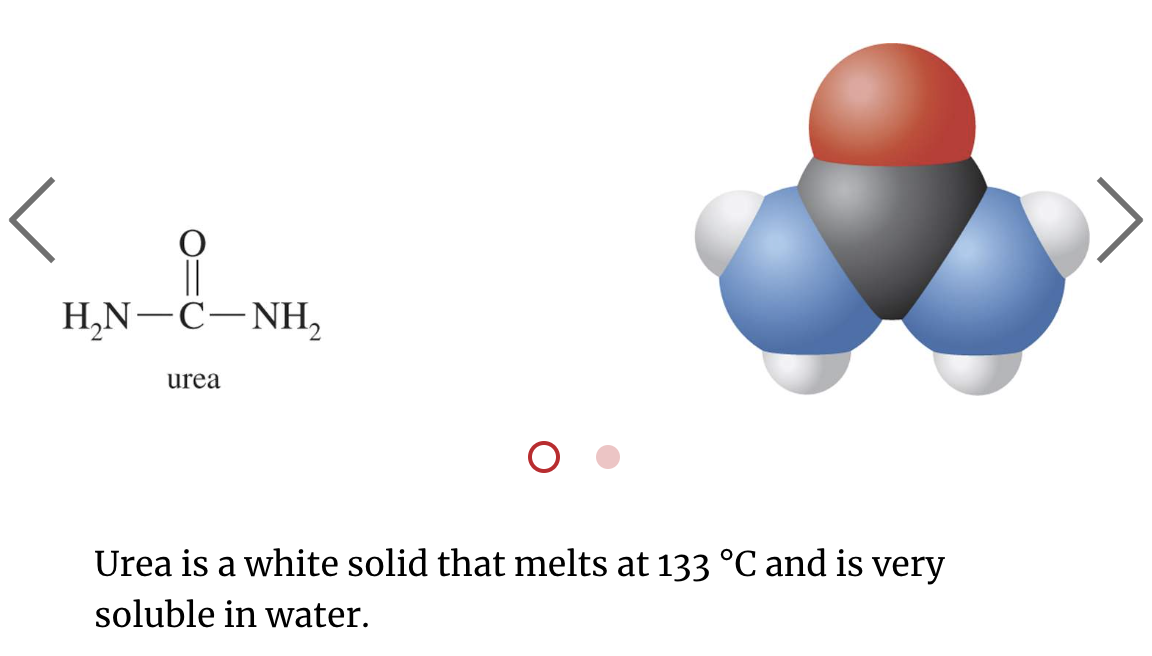
Amines
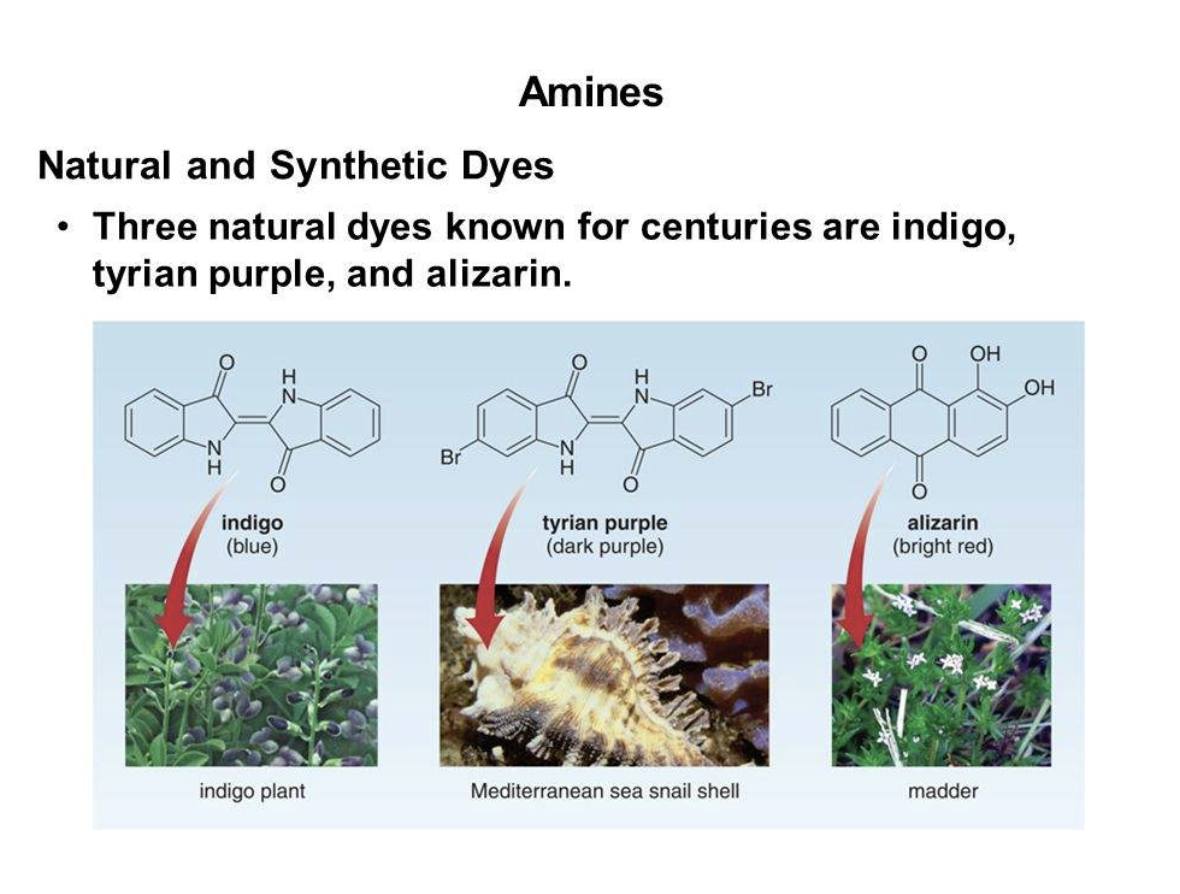
Amines are used in making azo-dyes and nylon apart from medicines and drugs. They are widely used in developing chemicals for crop protection, medication and water purification. They also find use in products of personal care. Ethanol amines are the most common type of amine used in the global market.
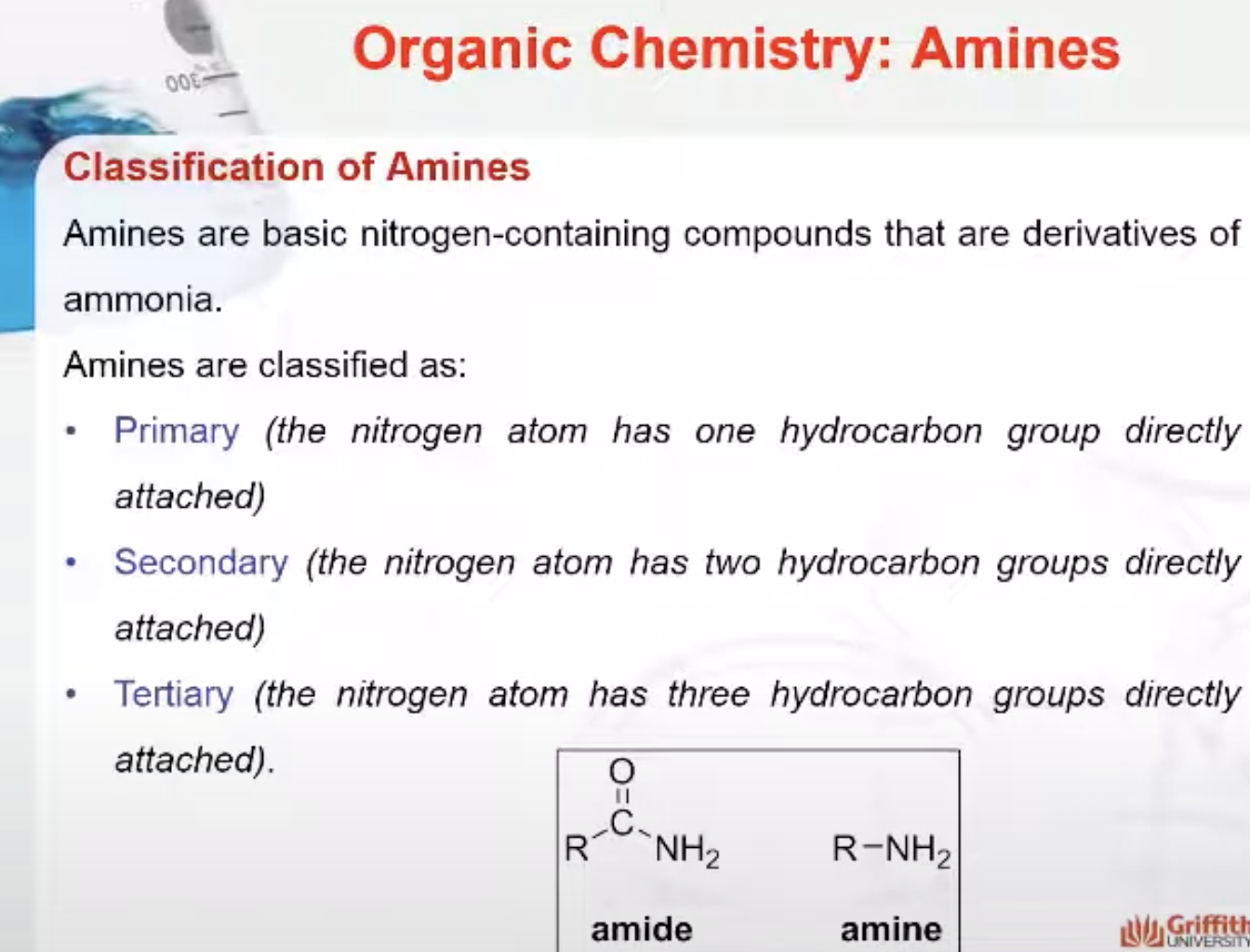
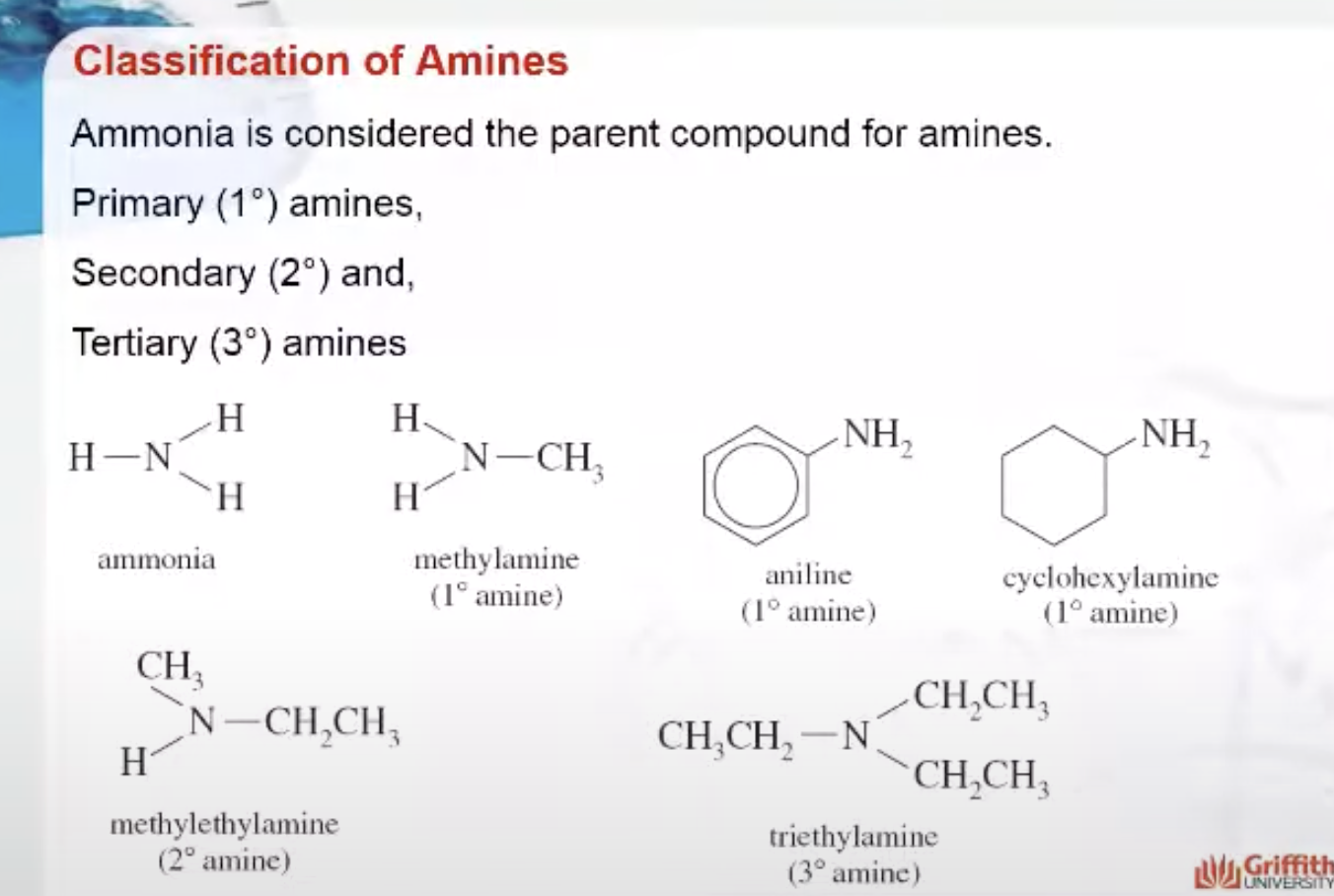

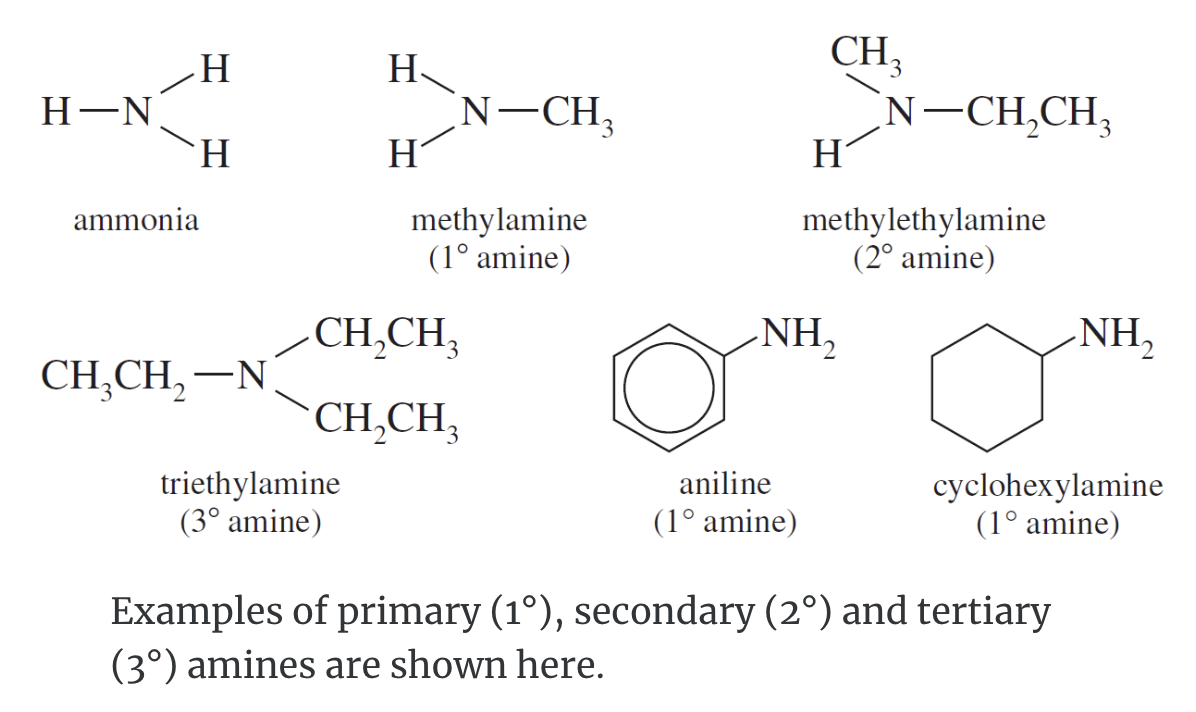
The smell of fertilizers or decaying plant matter is characteristic of nitrogen-containing compounds such as those found in mulch. Amines are basic nitrogen-containing compounds that are derivatives of ammonia. Amines are classified as:
- Primary (the nitrogen atom has one hydrocarbon group directly attached)
- Secondary (the nitrogen atom has two hydrocarbon groups directly attached)
- Tertiary (the nitrogen atom has three hydrocarbon groups directly attached).
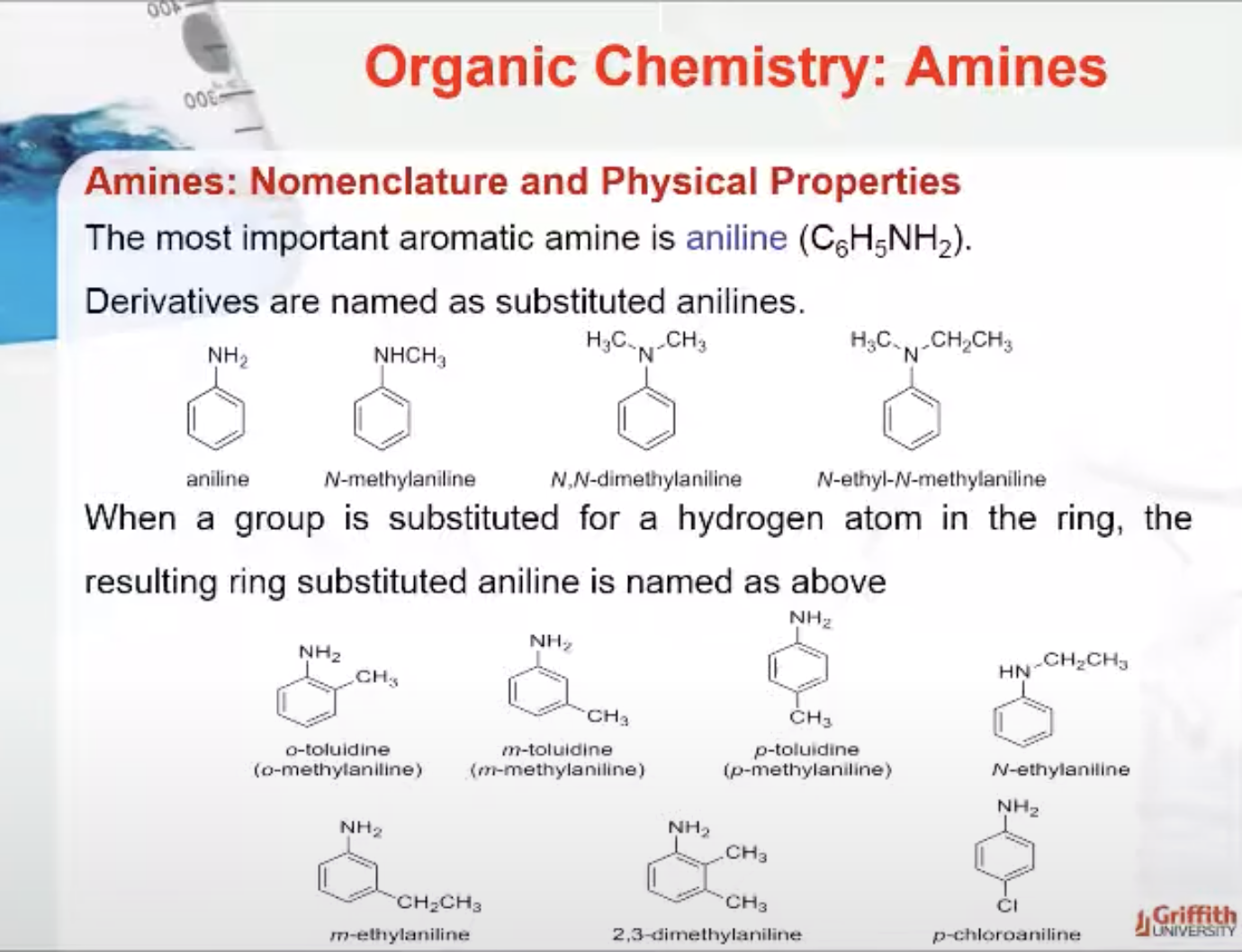
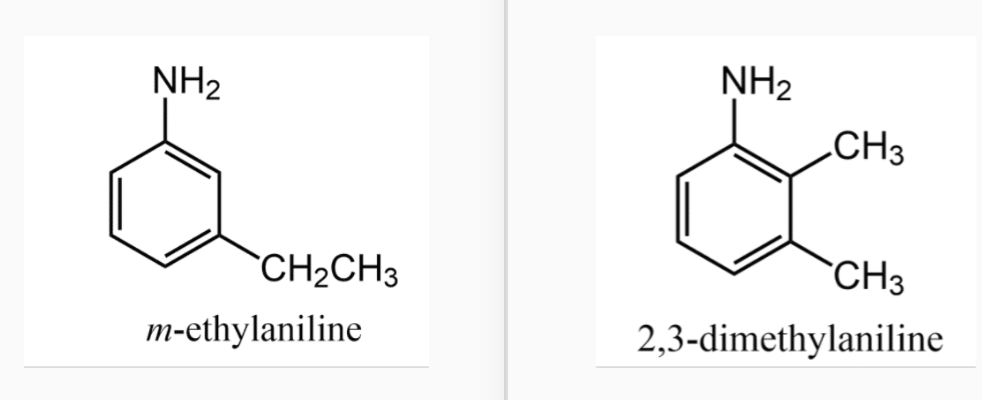
Aromatic amines
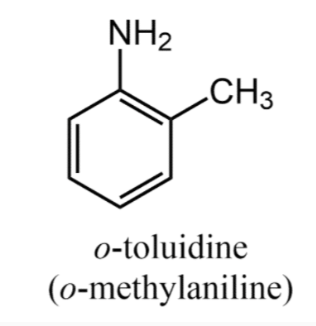
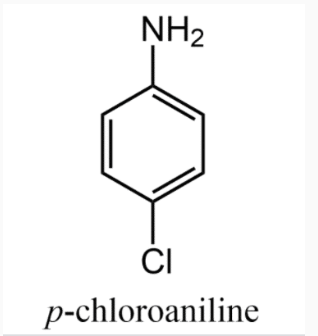
The most important aromatic amine is aniline (C6H5NH2). Derivatives are named as substituted anilines. When a group is substituted for a hydrogen atom in the ring, the resulting ring substituted aniline is named as we have previously done with aromatic compounds.
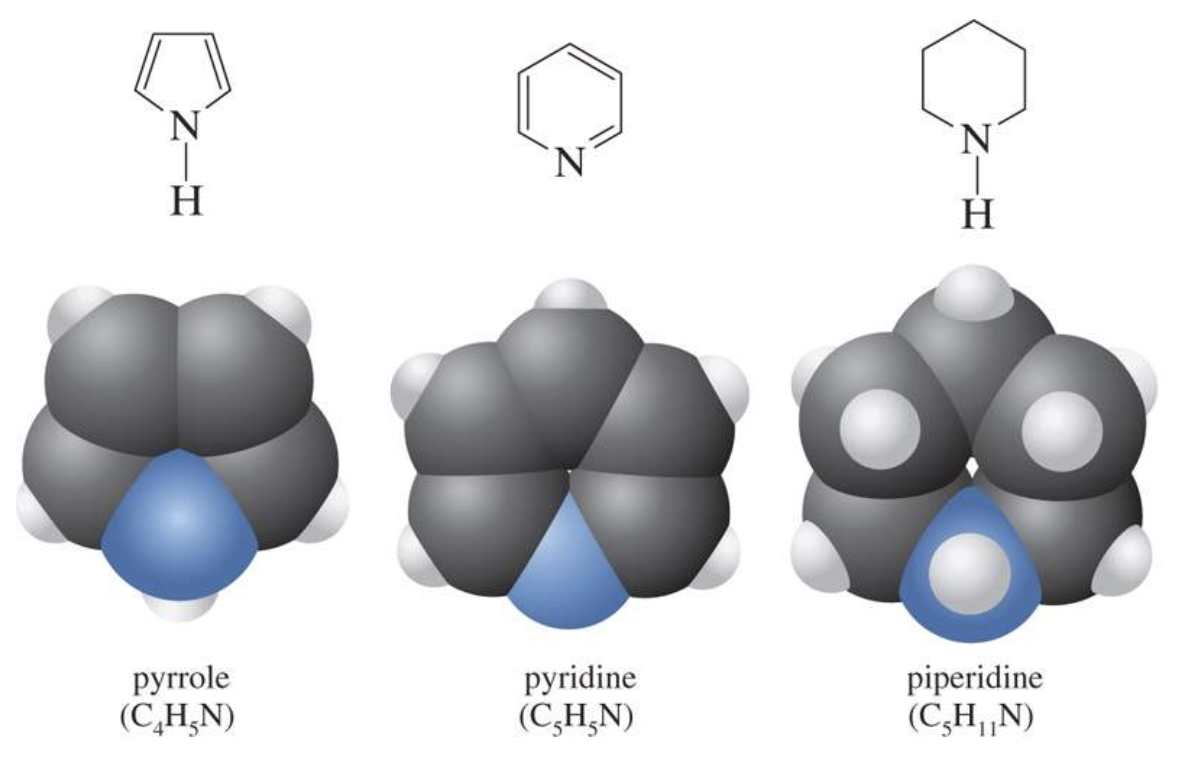
Heterocyclic Compounds
Heterocyclic compounds are ring compounds which have two or more atoms in the ring that are different. O, N, and S are common heteroatoms found in heterocyclic compounds. Nitrogen-based heterocyclic compounds like those shown right are found in DNA.
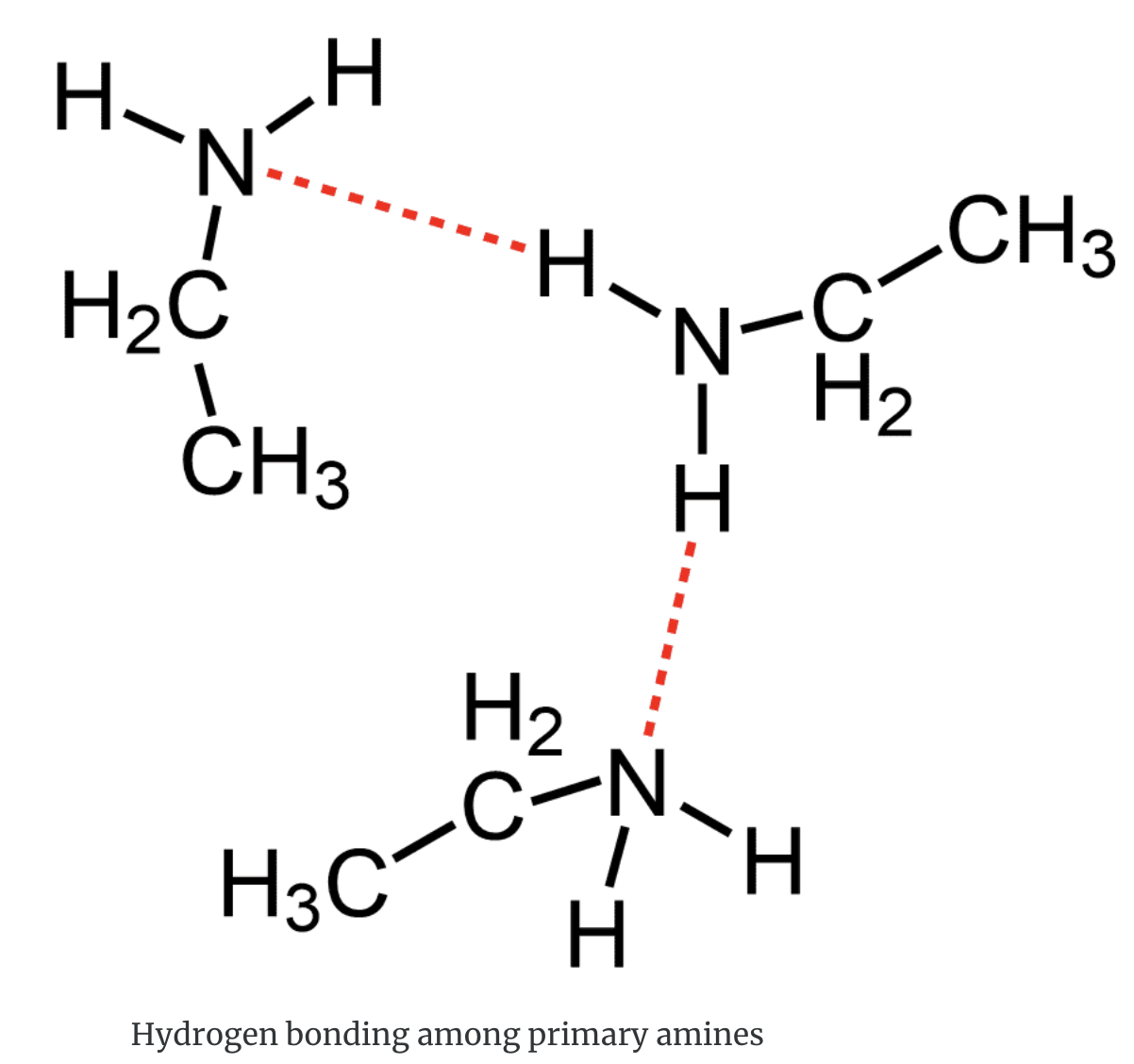
Amines are capable of hydrogen bonding with water. As a result, the aliphatic amines with up to six carbons are quite soluble in water. Methylamine and ethylamine are flammable gases with a strong ammoniacal odour.
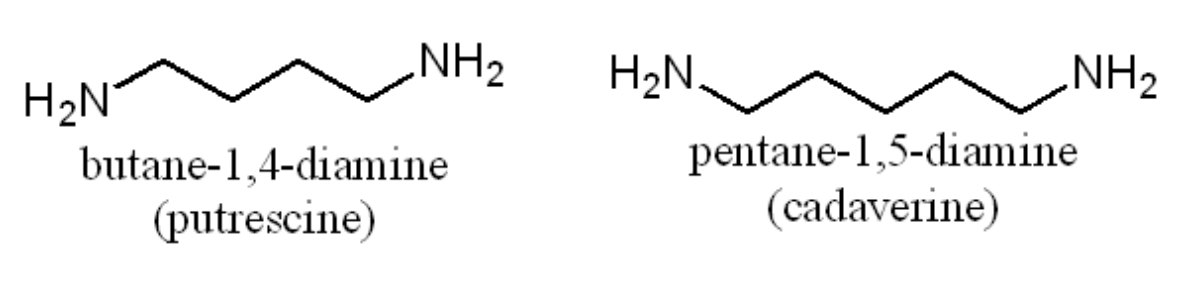
Trimethylamine has a “fishy” odour. Amines are responsible for the strong odours of decaying flesh which are produced by bacterial decomposition. Two of these compounds are actually diamines as shown here.
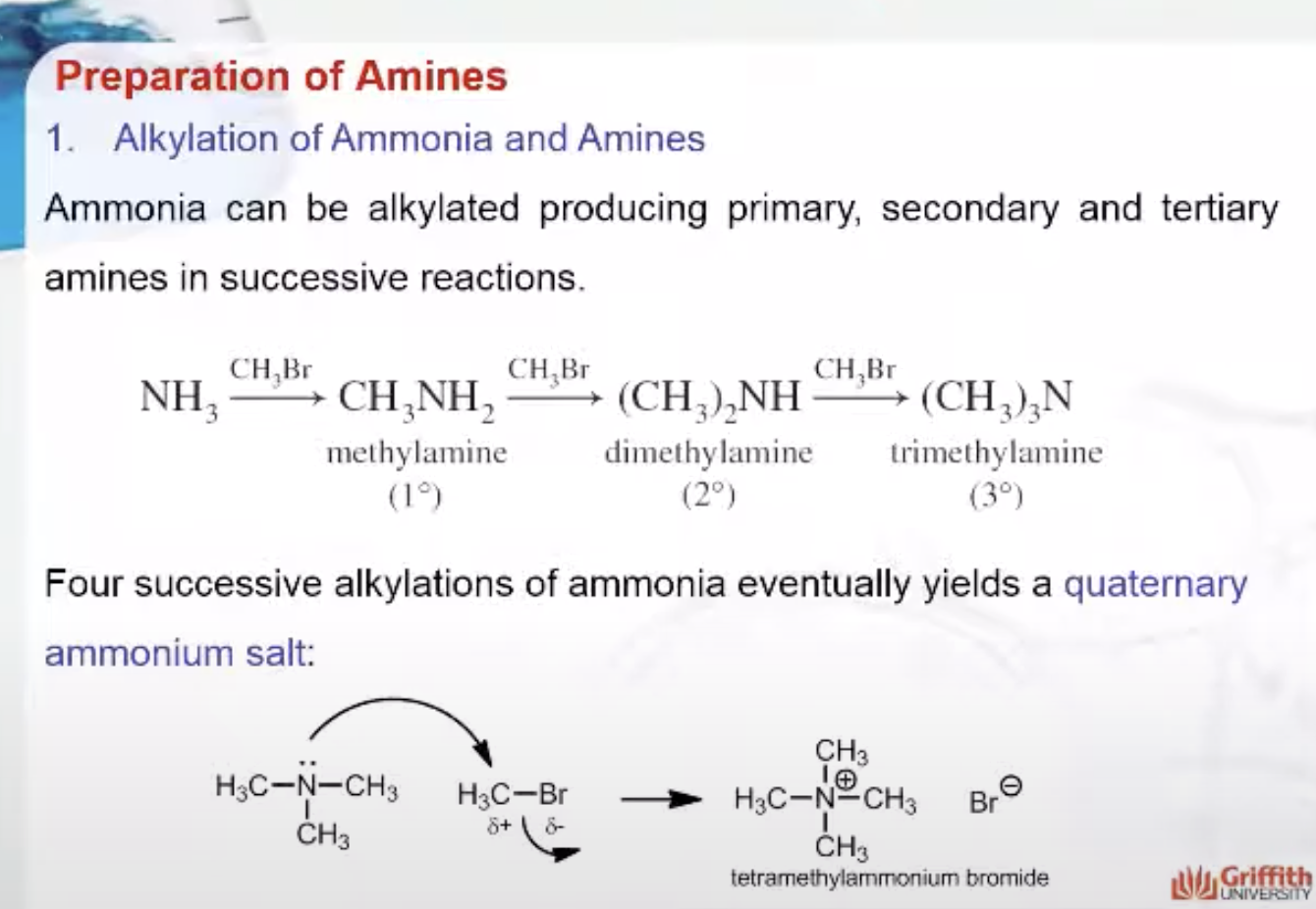
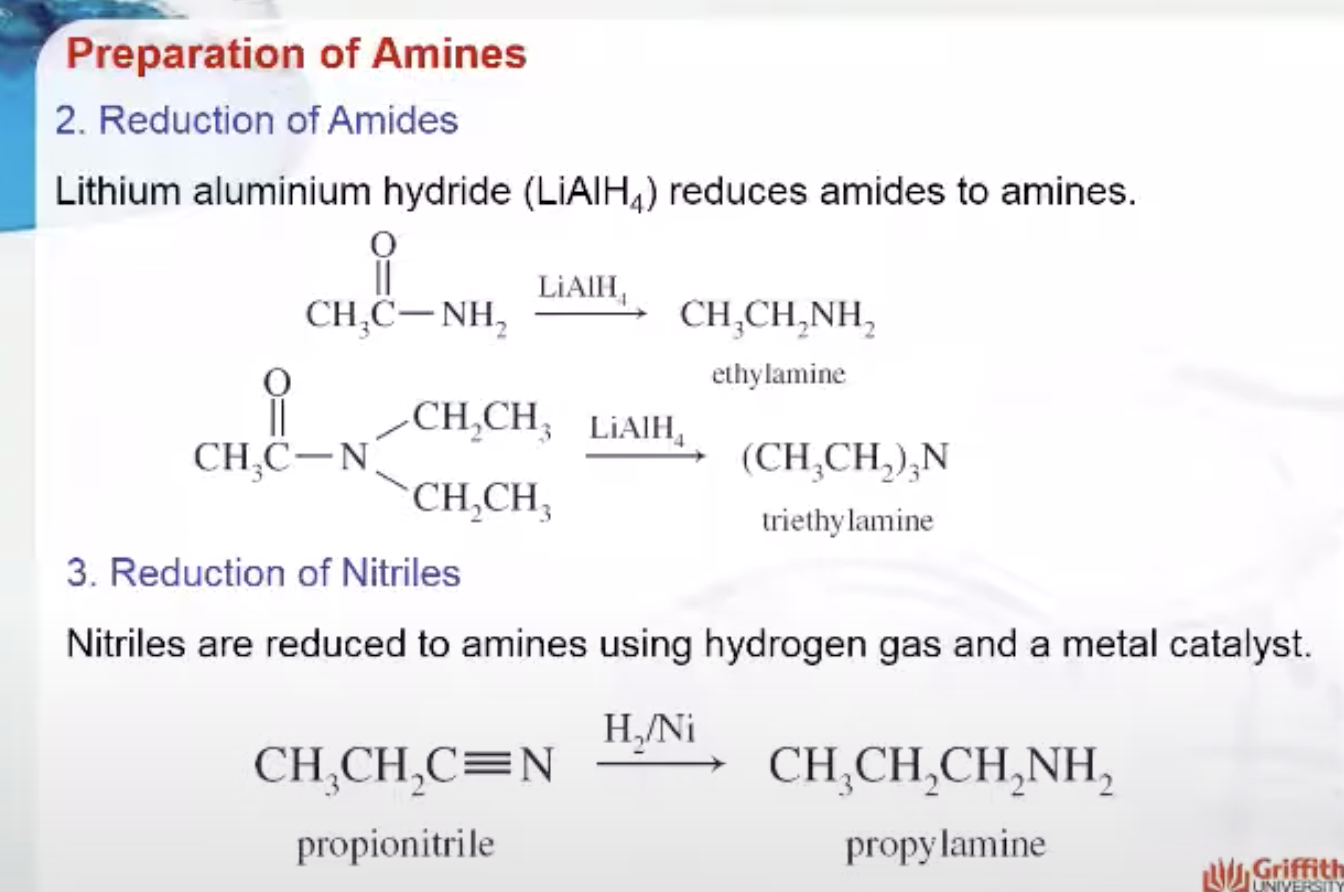
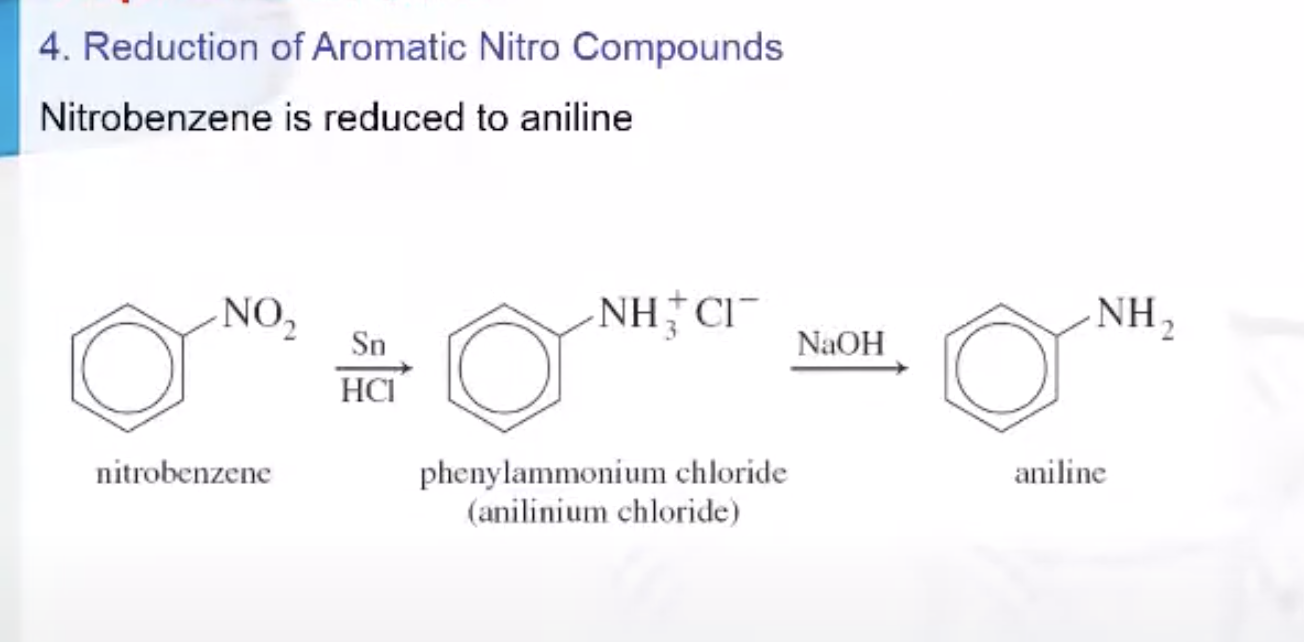
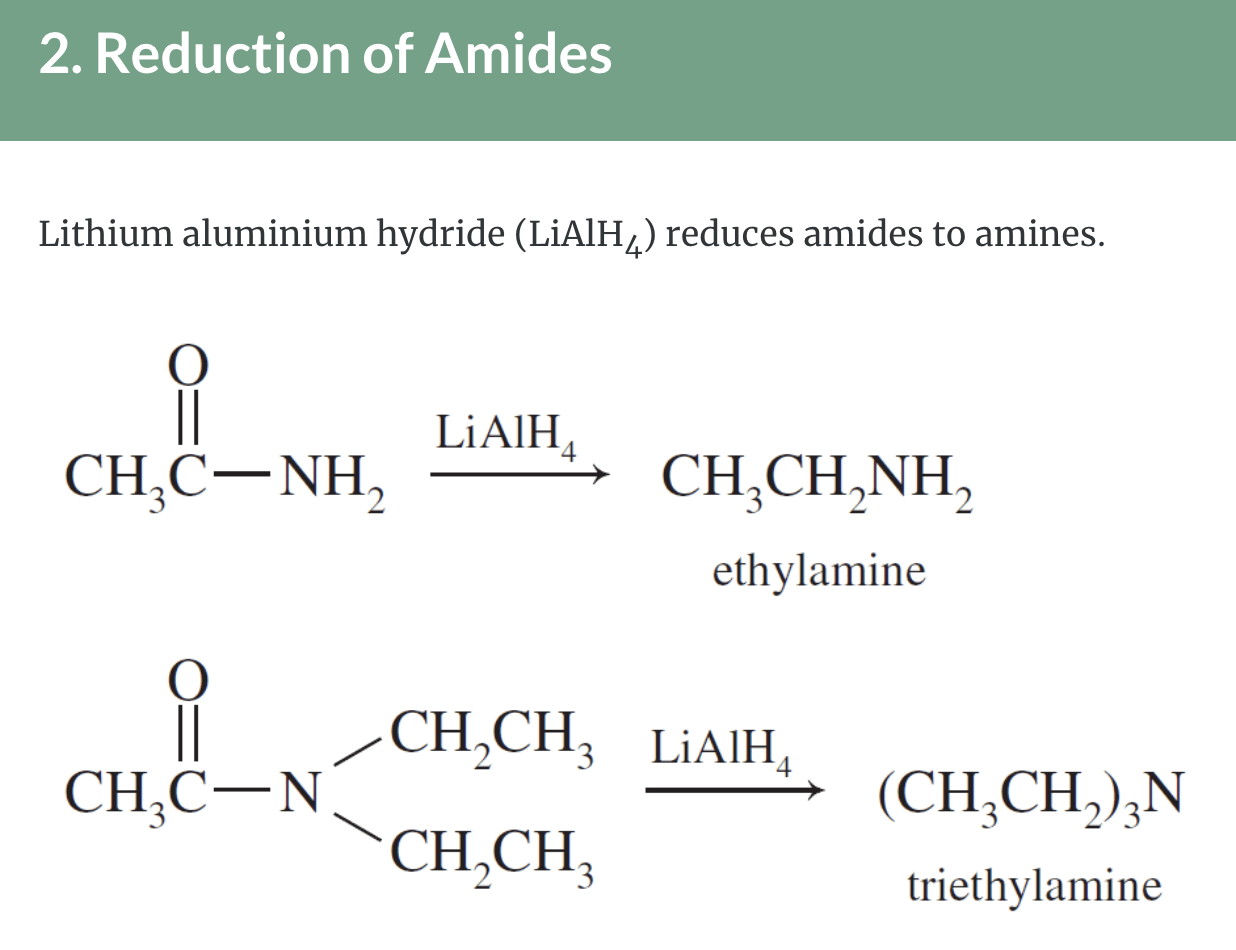
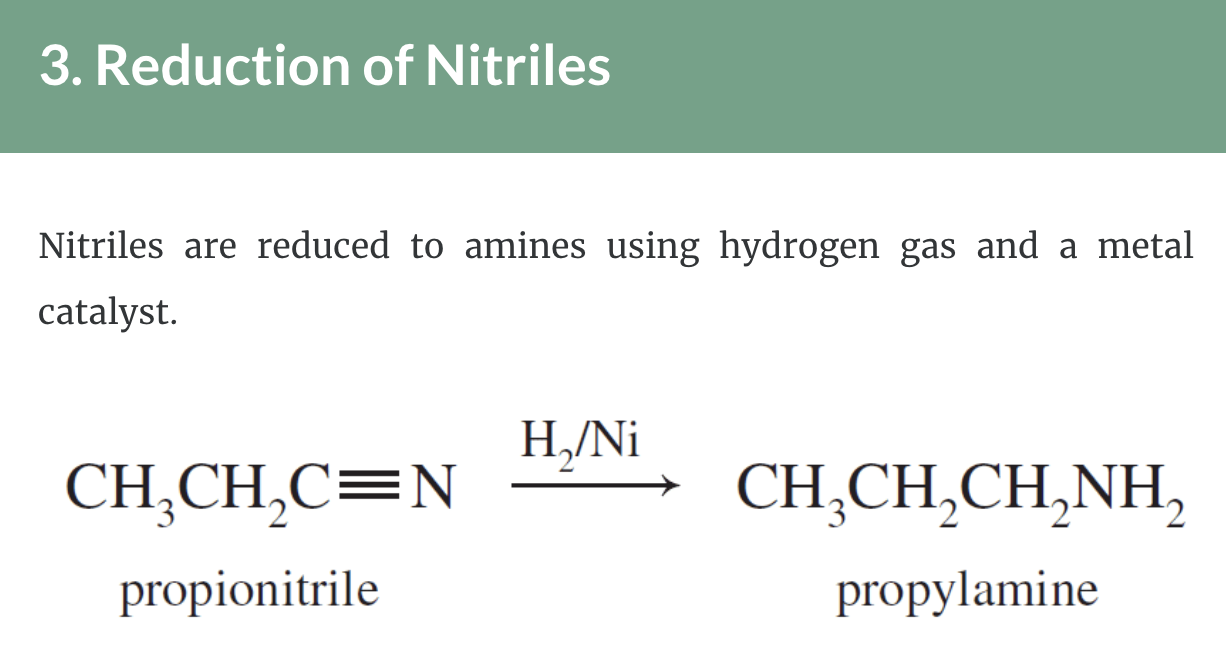
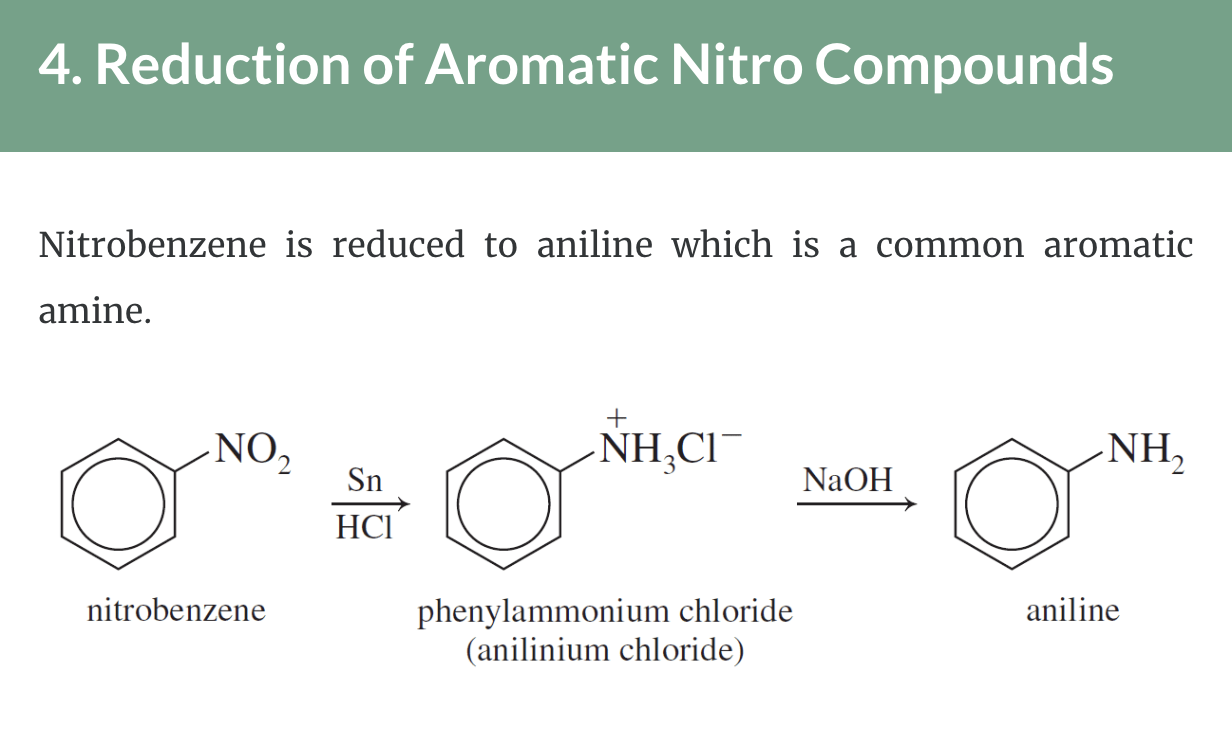
Preparation of Amines

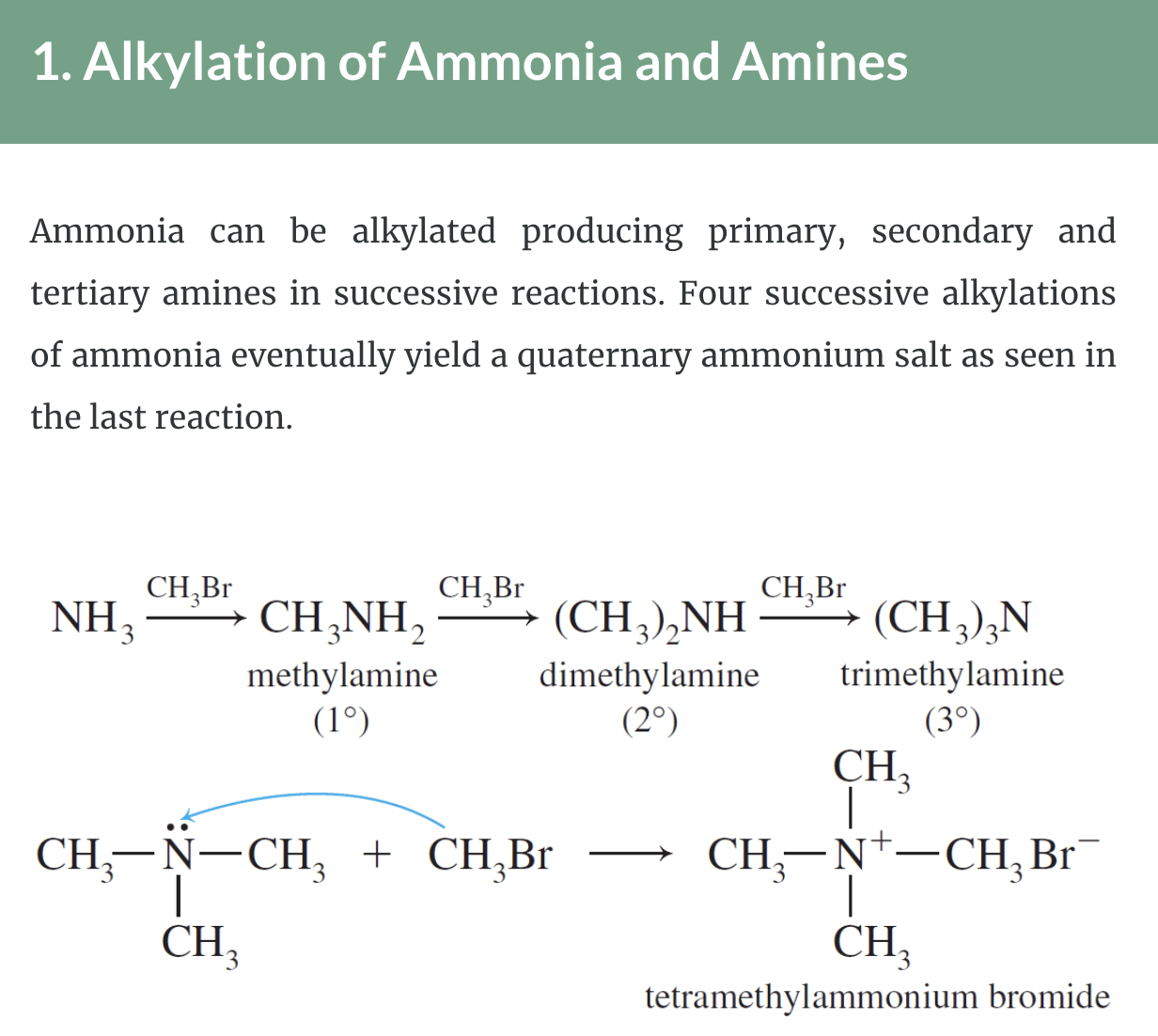
1.1. Alkylation of Ammonia and Amines
1. Alkylation of Ammonia and Amines
1,
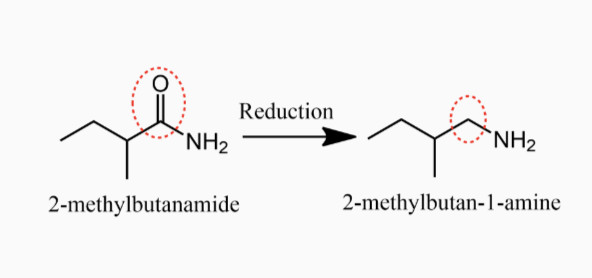
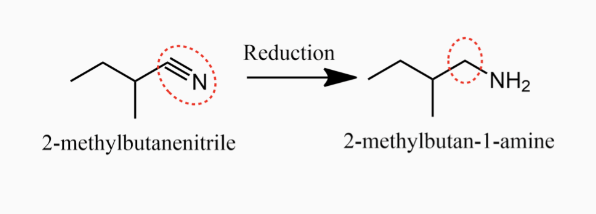
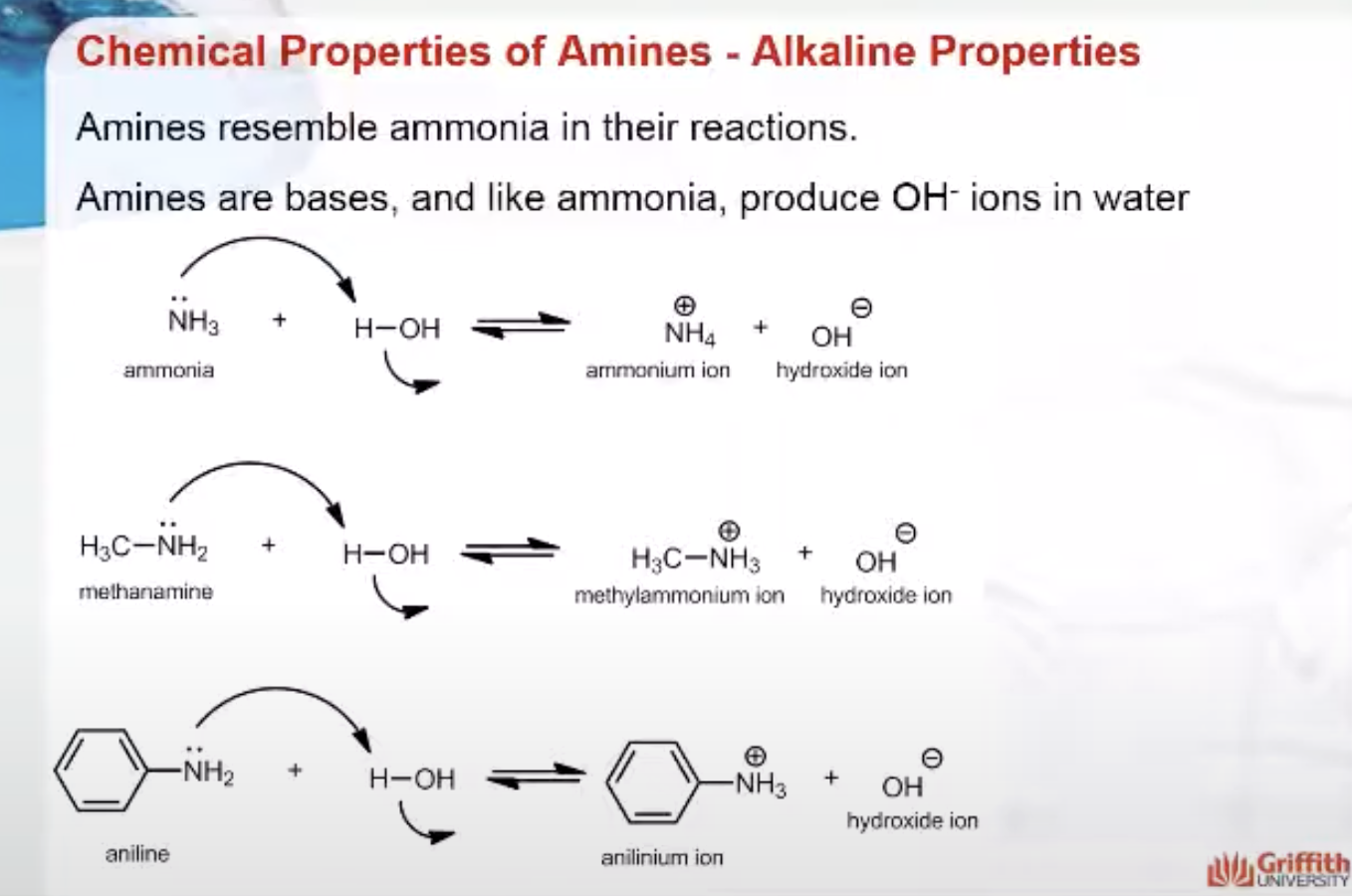
Chemical Properties of Amines
Amines resemble ammonia in their reactions. Amines are bases, and like ammonia, produce OH– ions in water. Amines are the bases of the organic world.
전자쌍 제공 => 염기
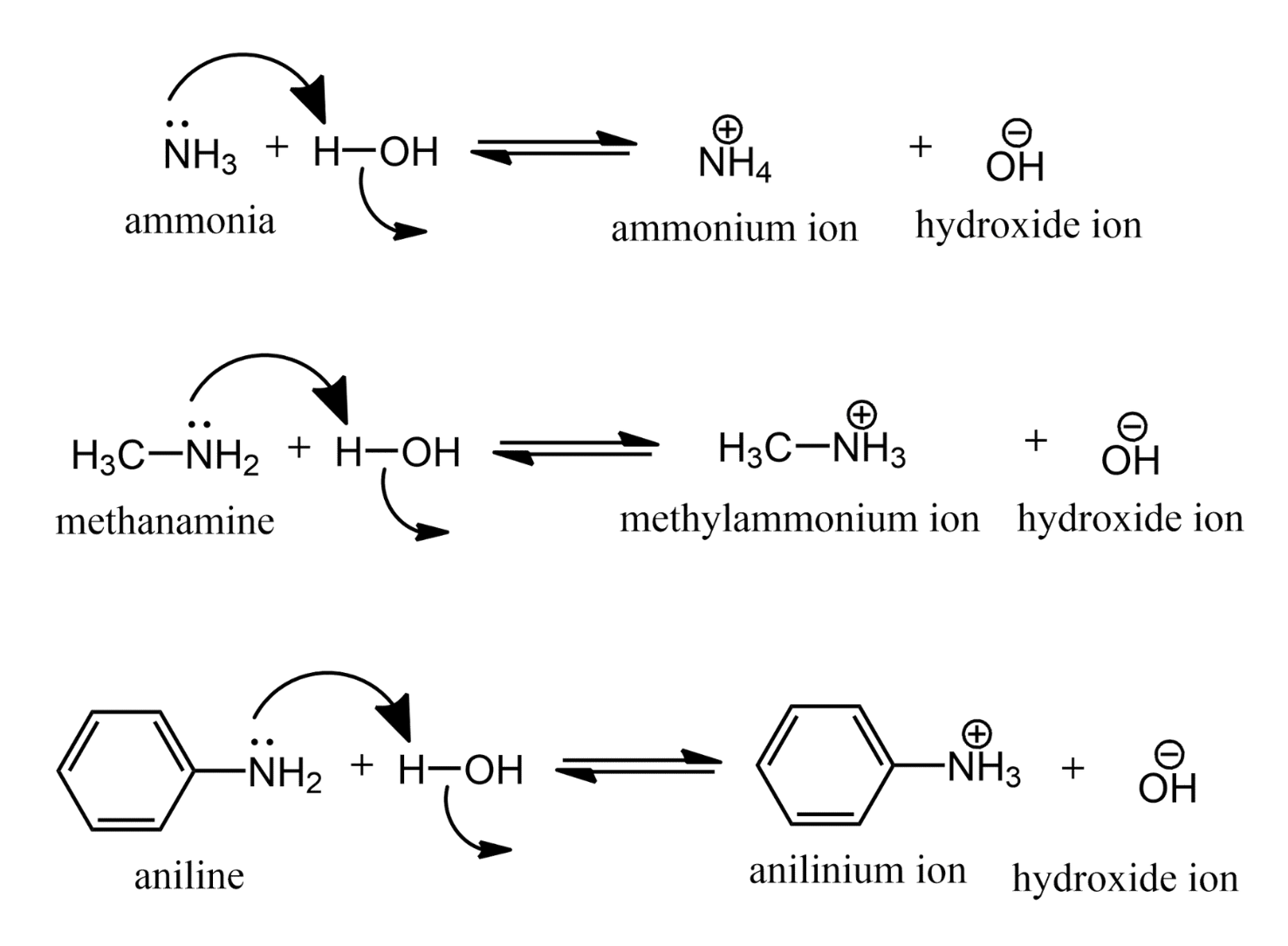
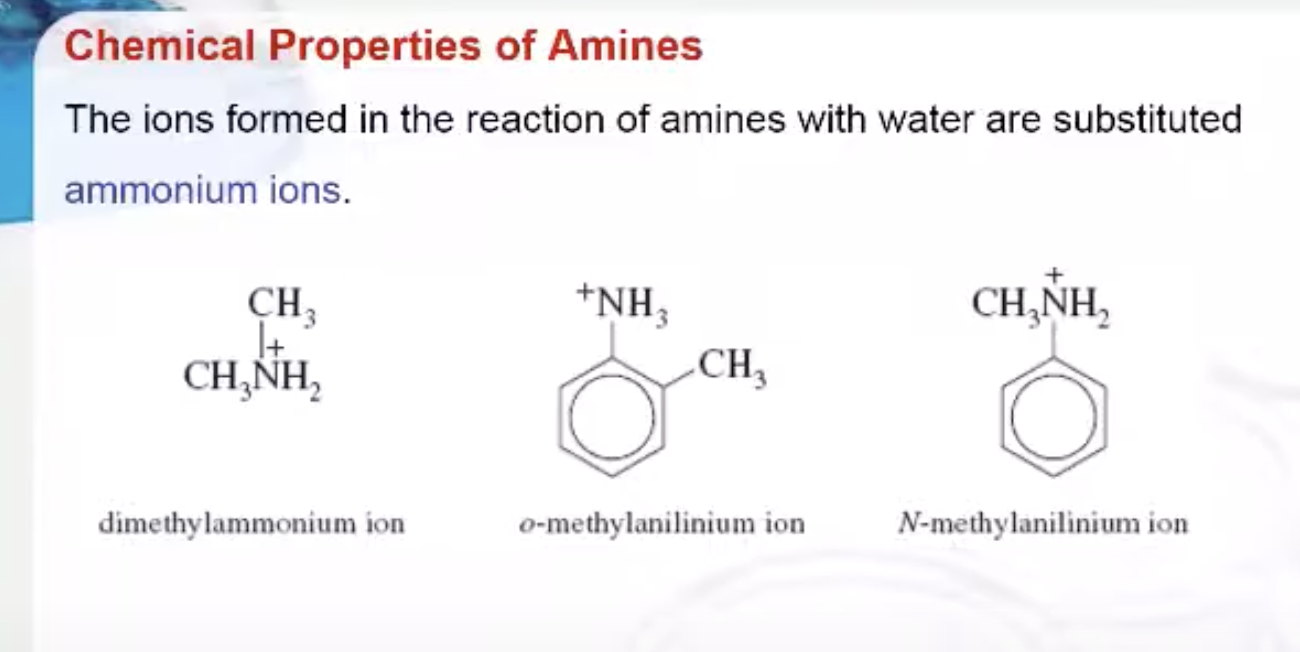
The ions formed in the reaction of amines with water are substituted ammonium ions. They are named by replacing the-amine ending by -ammonium and, for the aromatic amines, by replacing the -anilinename by -anilinium.
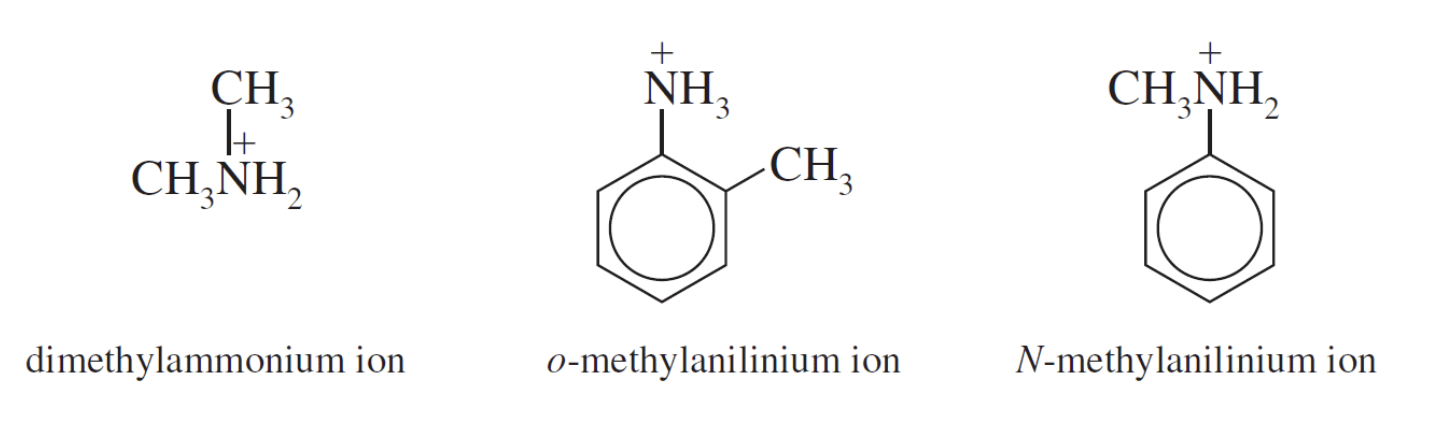
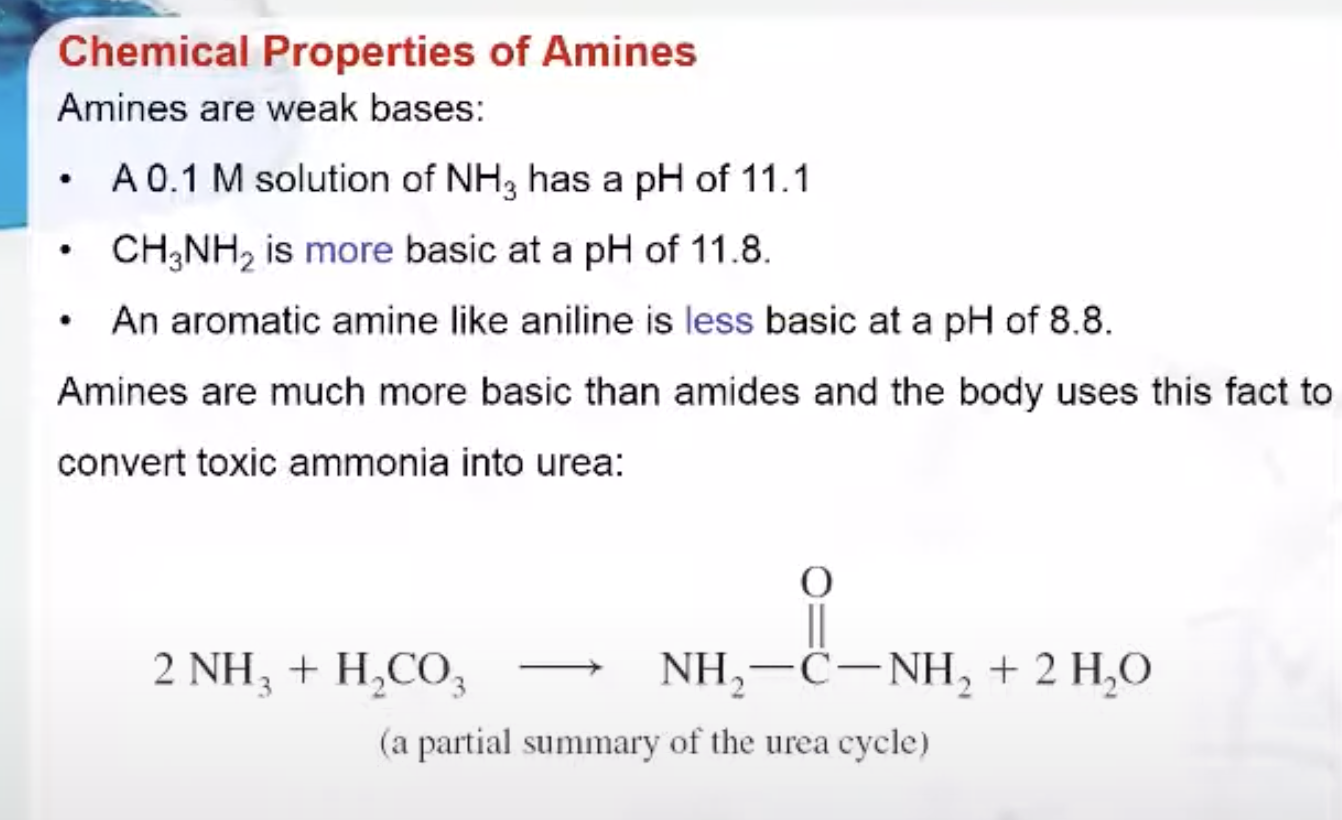
Amines are weak bases. Compare the pH of the following amines with the pH of ammonia.
- A 0.1 M solution of NH3 has a pH of 11.1
- CH3NH2 is more basic at a pH of 11.8.
- An aromatic amine like aniline is less basic at a pH of 8.8.
Amines are much more basic than amides and the body uses this fact to convert toxic ammonia into urea as shown here.
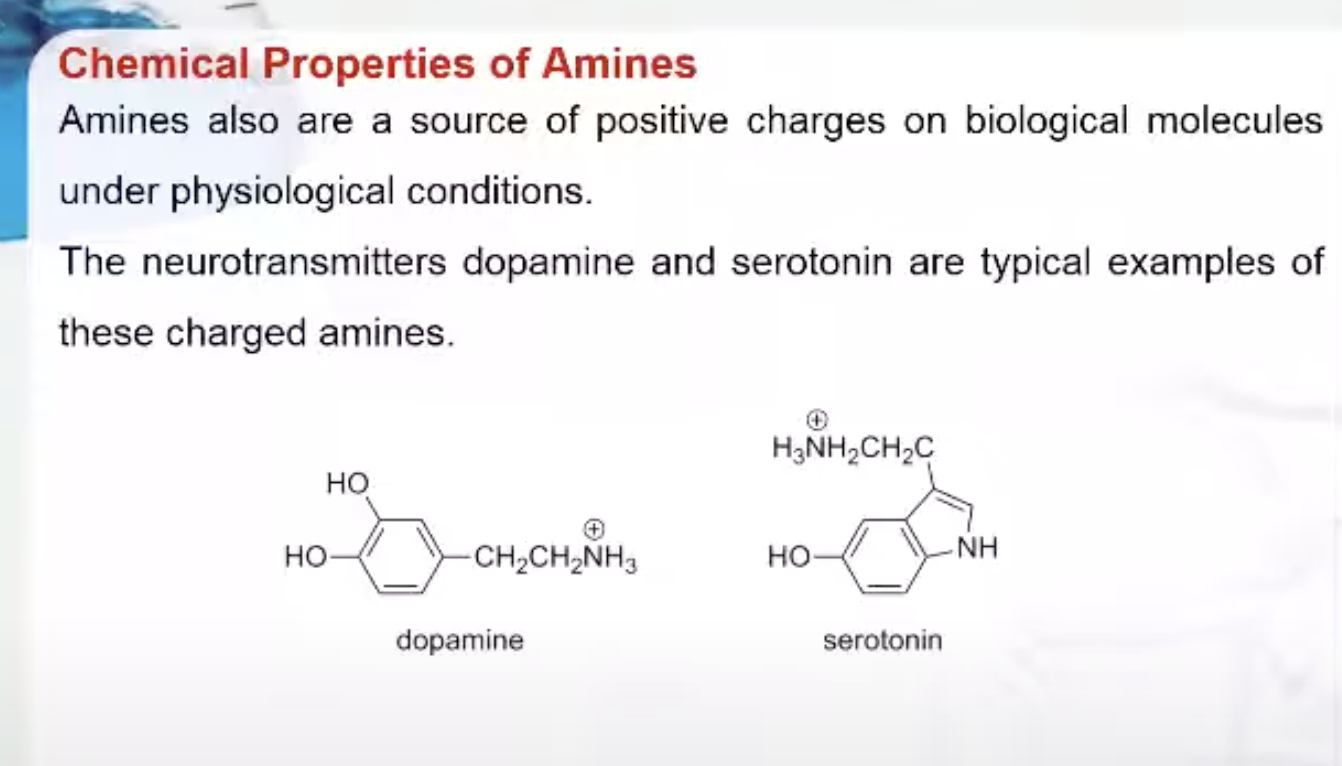
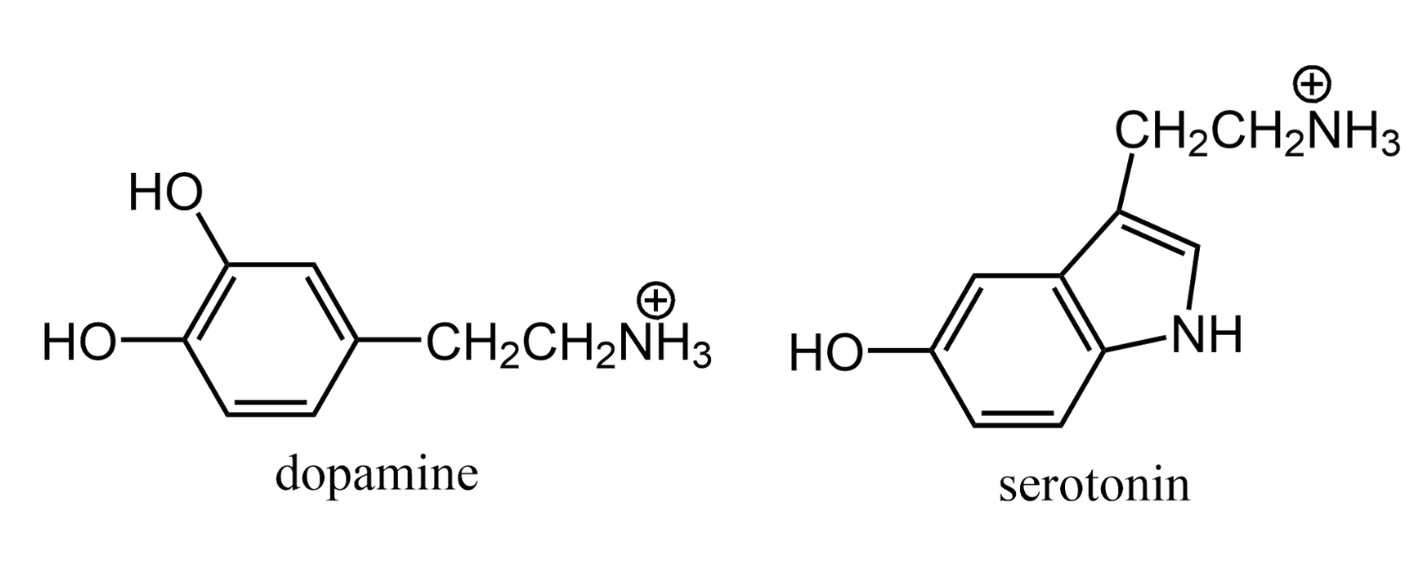
Amines also are a source of positive charges on biological molecules under physiological conditions. The neurotransmitters dopamine and serotonin are typical examples of these charged amines.
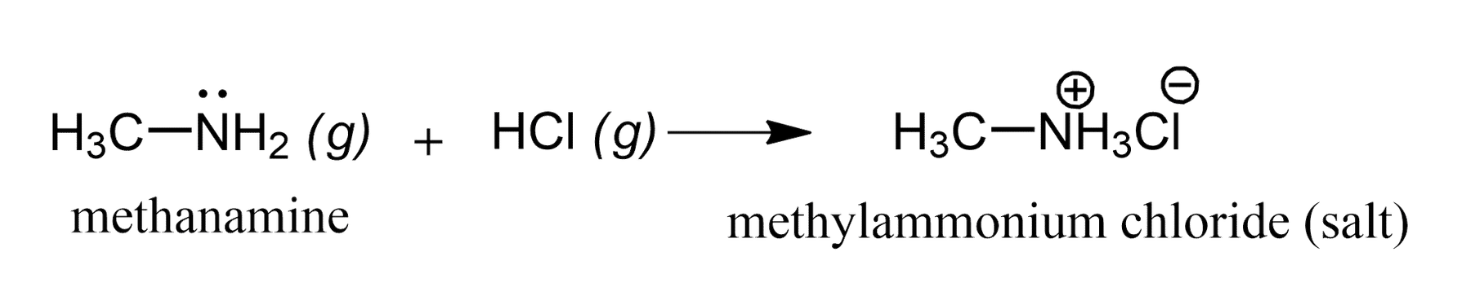
Salt Formation
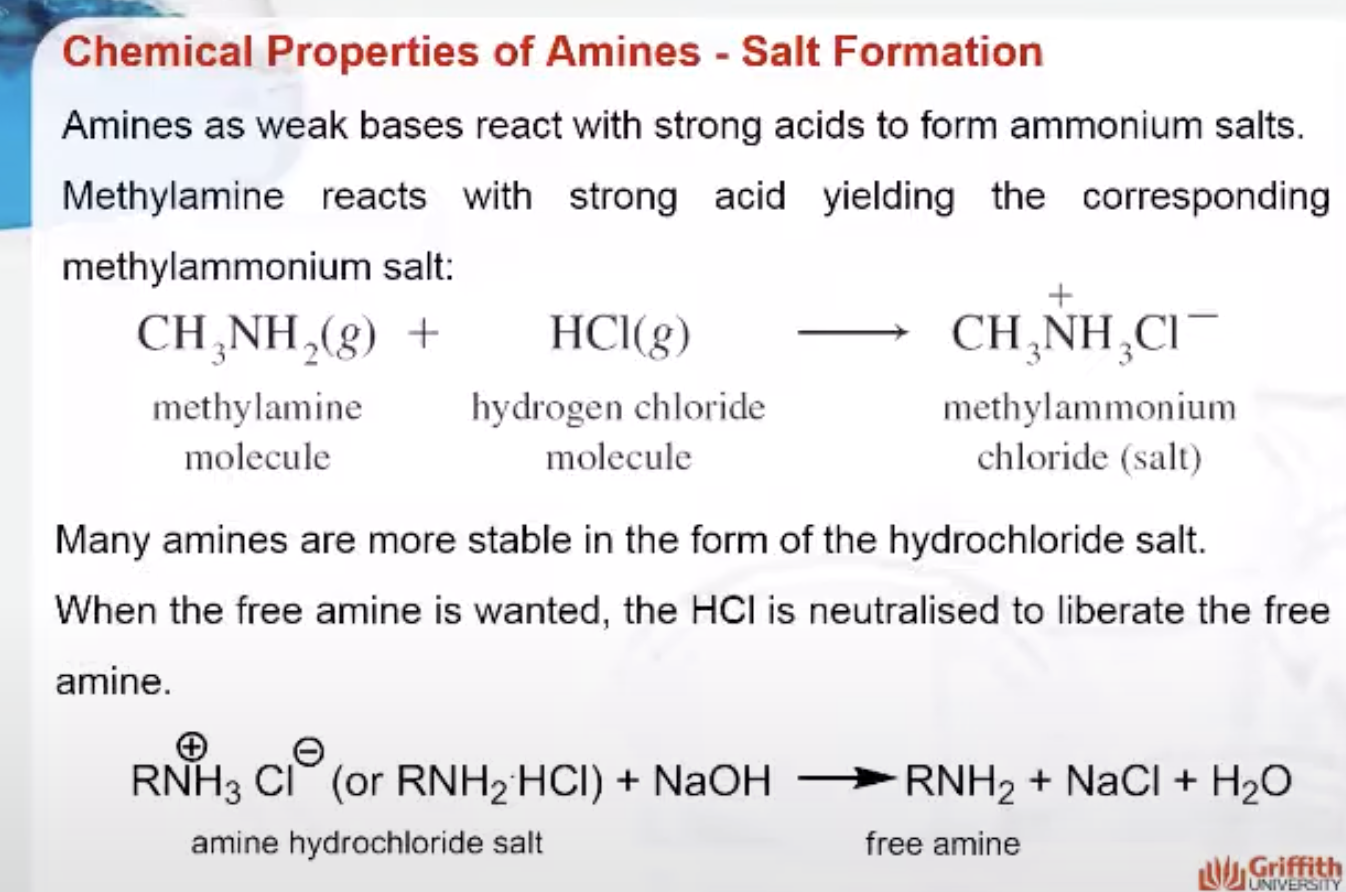
Because amines are weak bases, they react with strong acids to form ammonium salts. For example, methylamine reacts with strong acid yielding the corresponding methylammonium salt. Methylammonium chloride is a white crystalline solid made up of methylammonium ions, CH3NH3+, and chloride ions, Cl–.
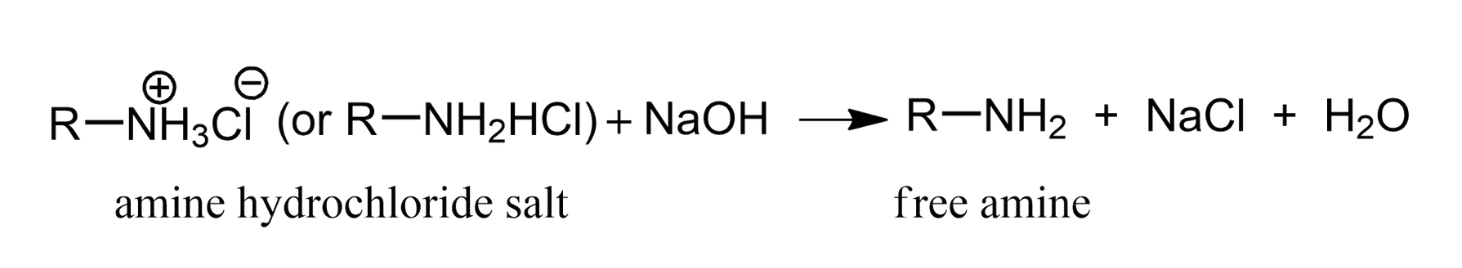
Many amines or amino compounds are more stable in the form of the hydrochloride salt. When the free amine is wanted, the HCl is neutralised to liberate the free amine.
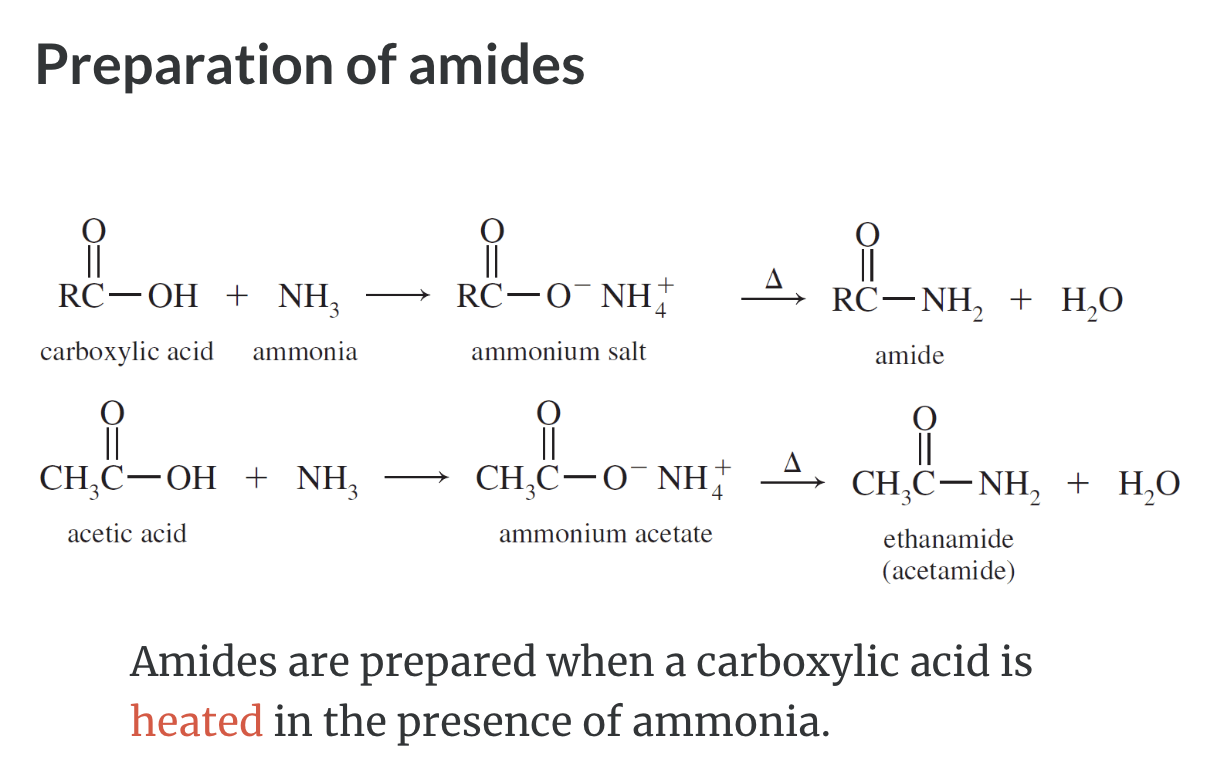
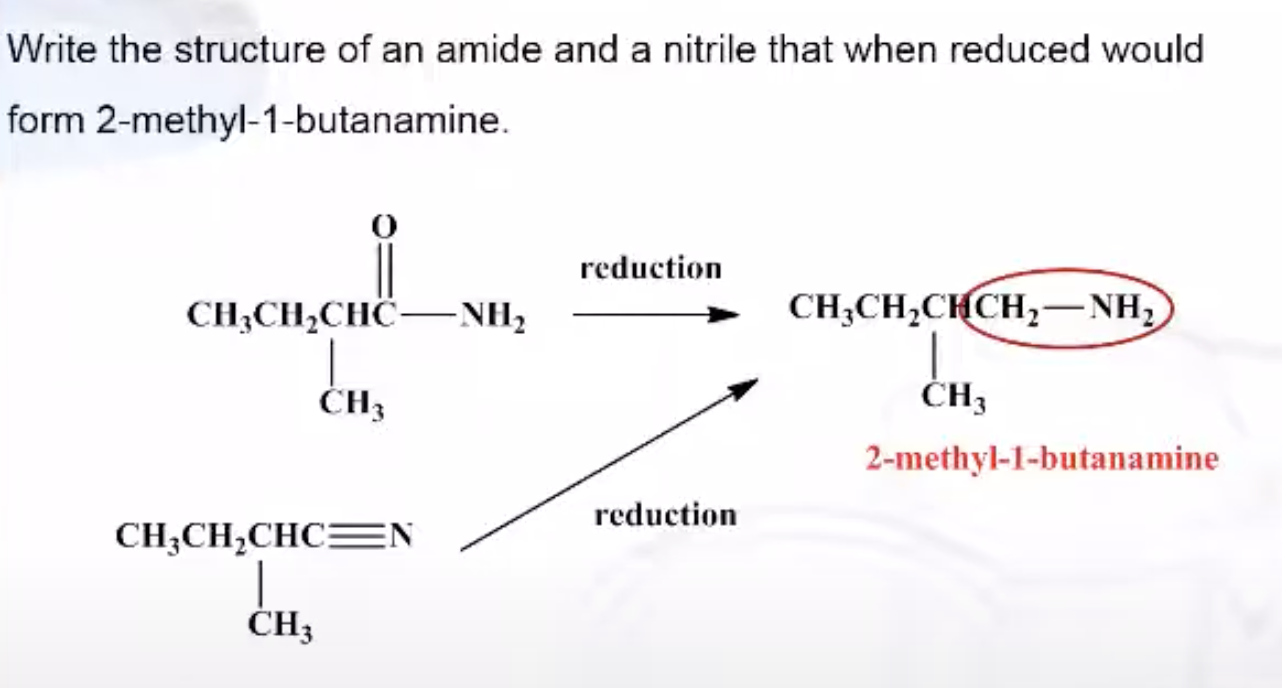
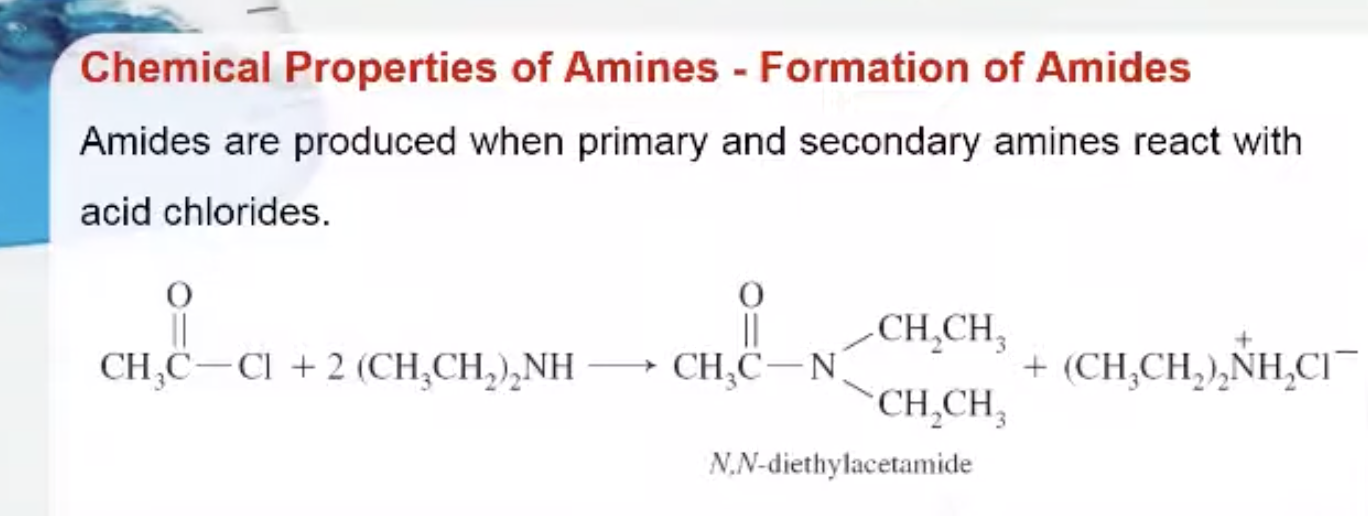
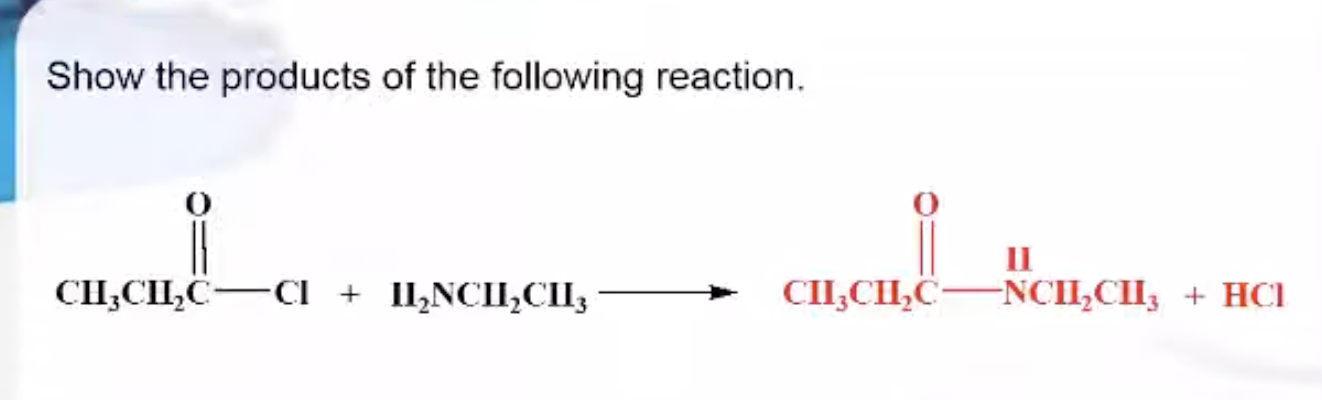
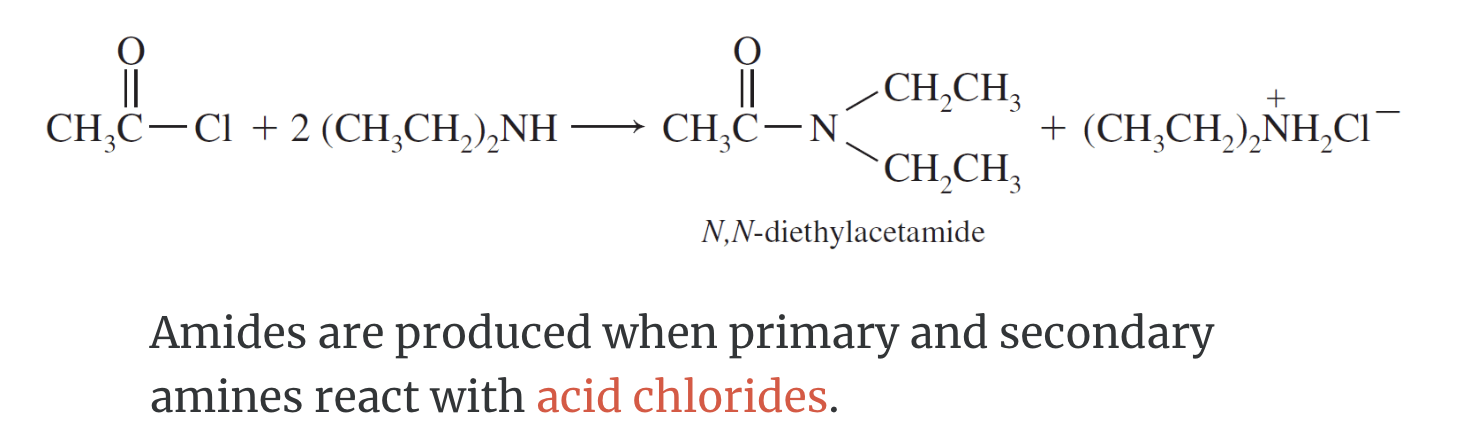
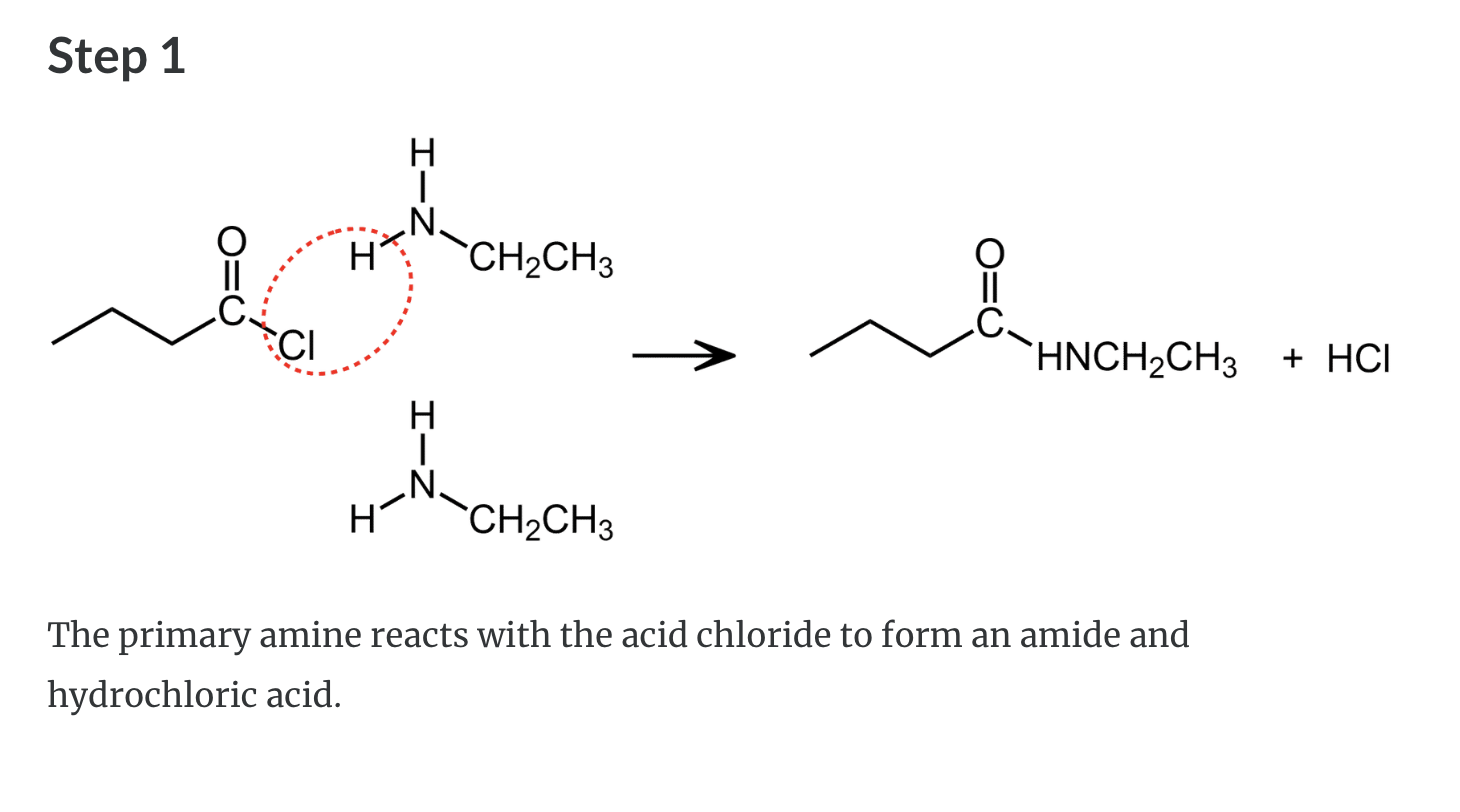
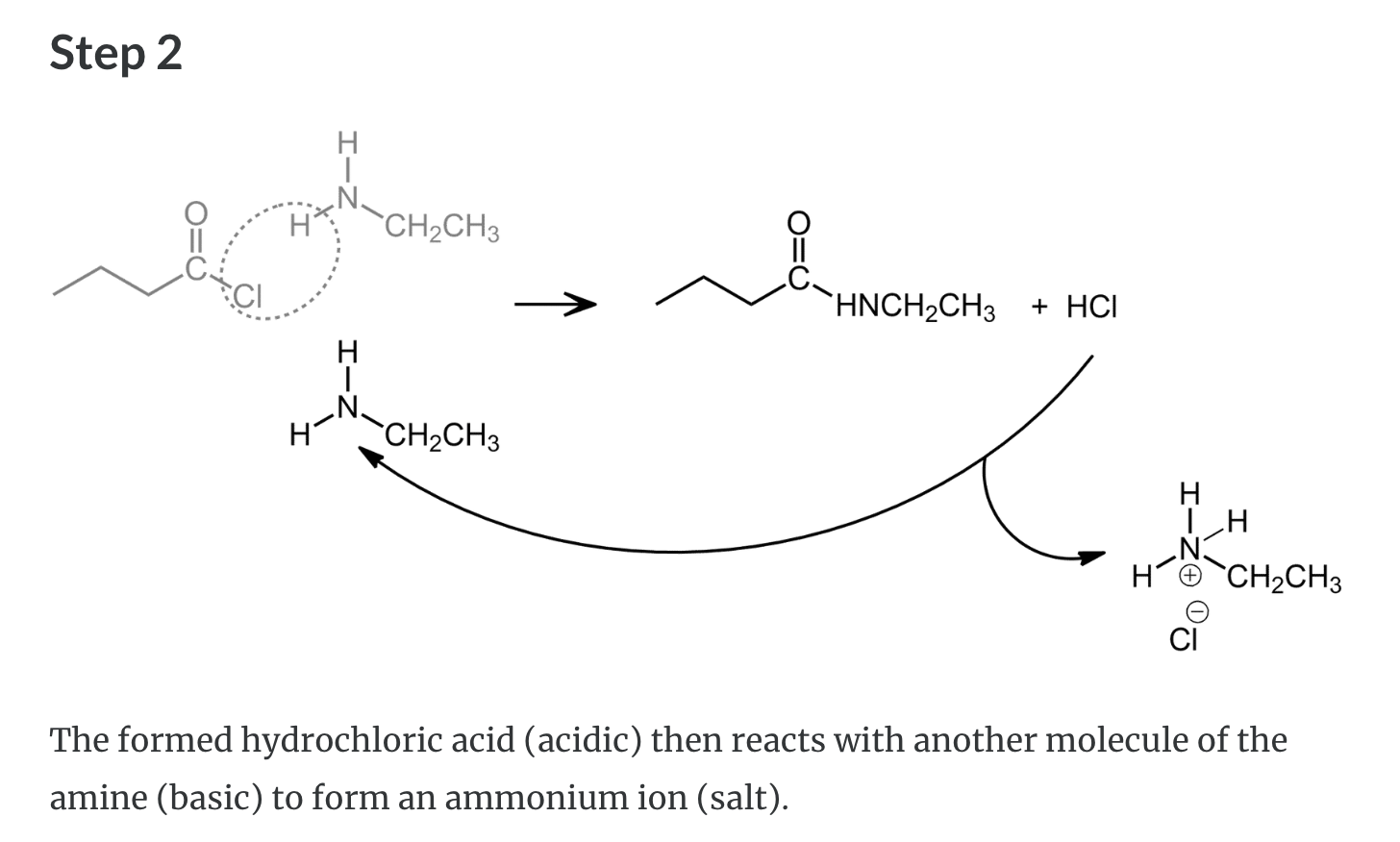
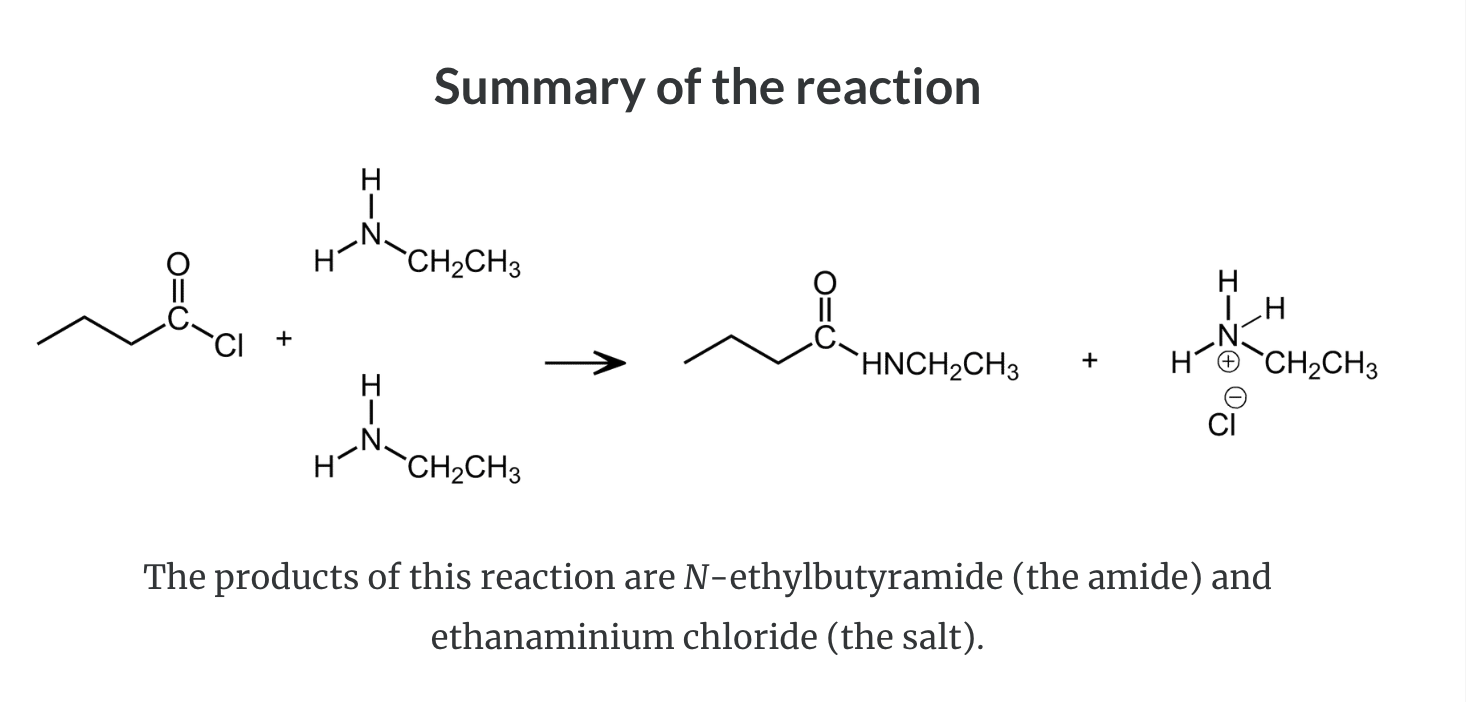
Formation of Amides
Sources and Uses of Selected Amines
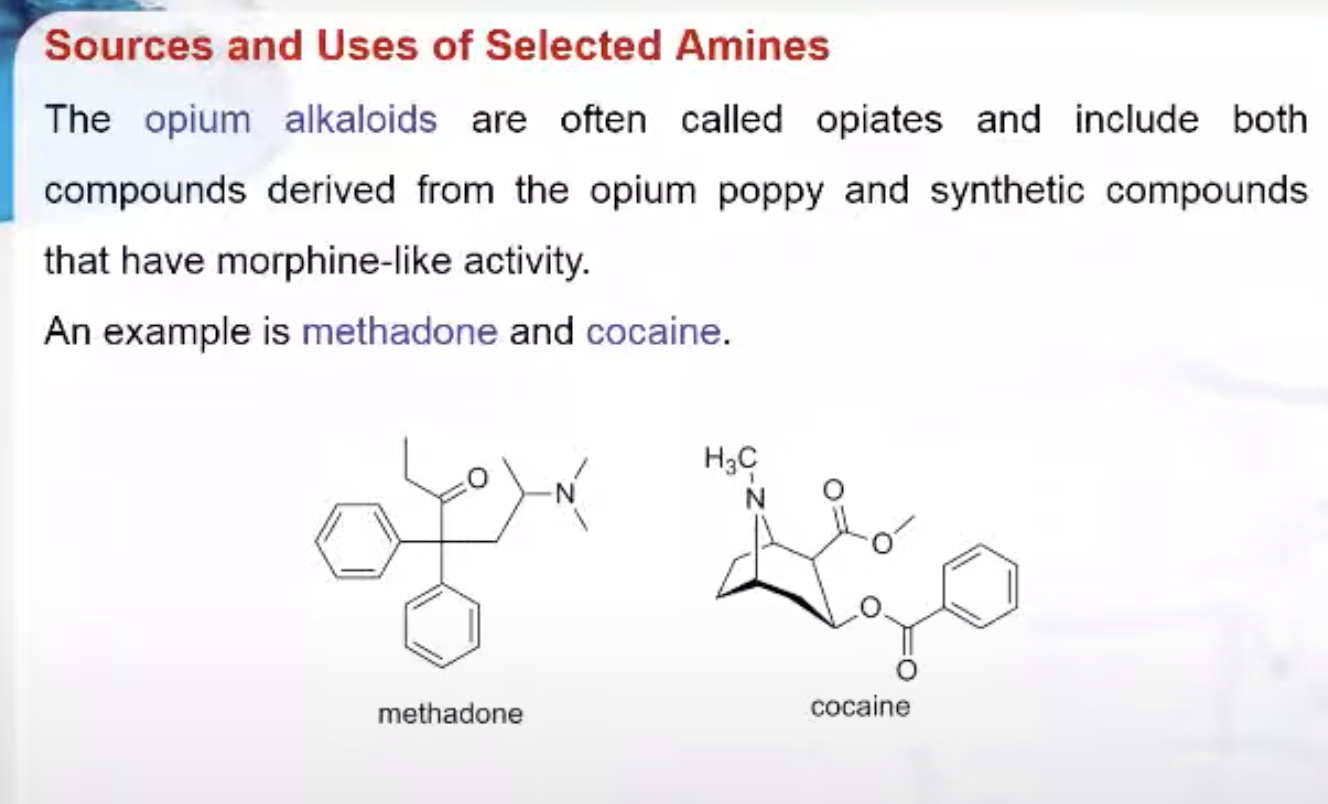
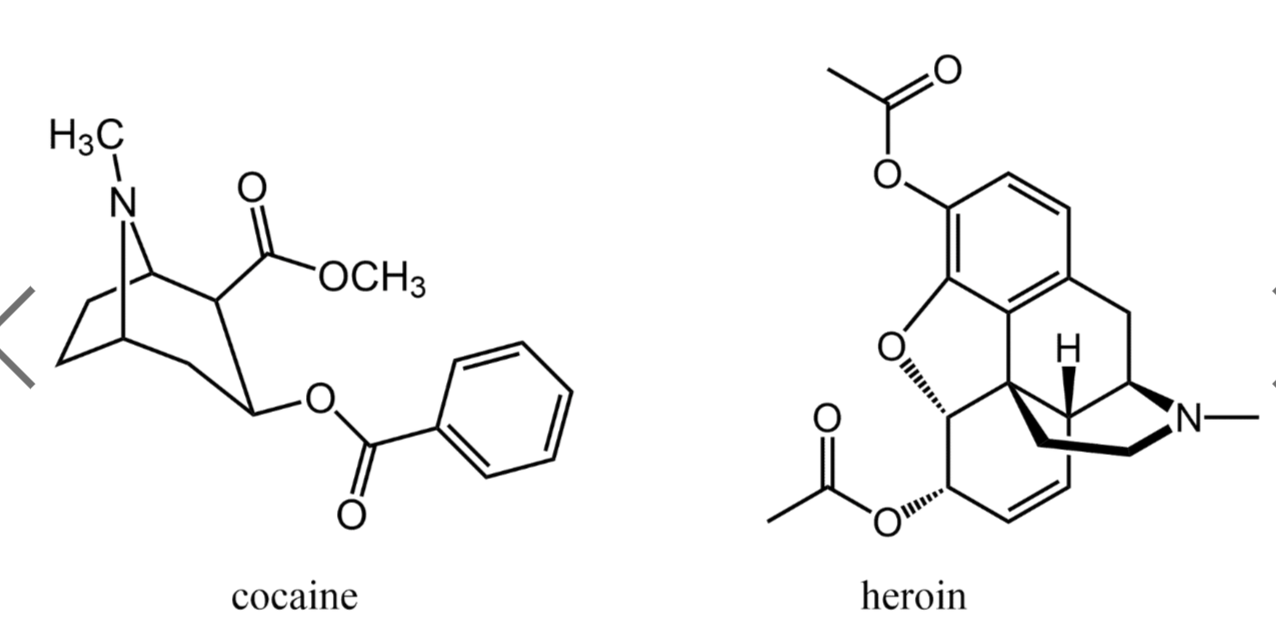
Coca crop in Colombia used to make the alkaloid, cocaine.
Nitrogen compounds are found throughout the plant and animal kingdoms. Amines, substituted amines, and amides occur in every living cell. Many of these compounds have important physiological effects.



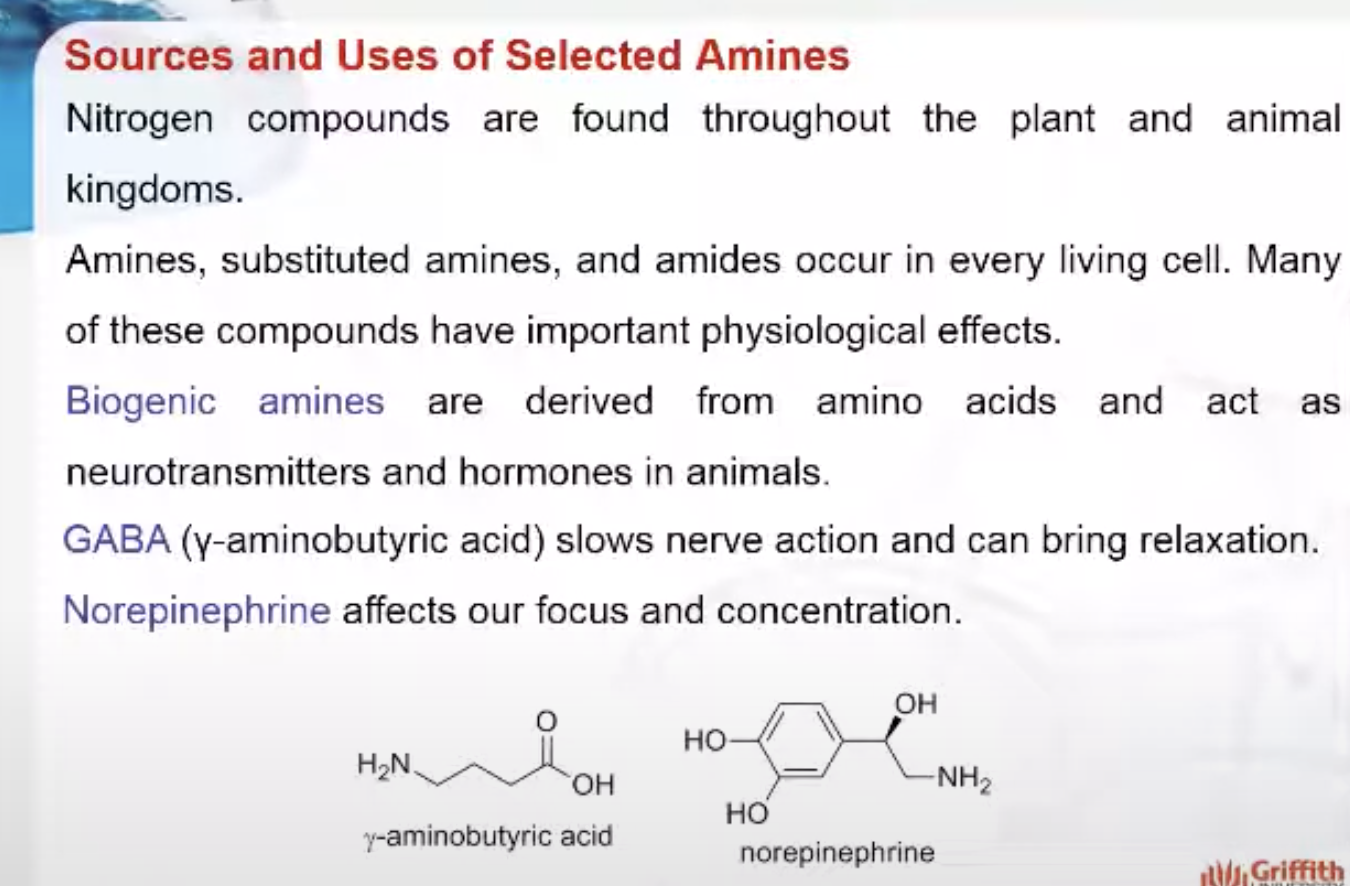
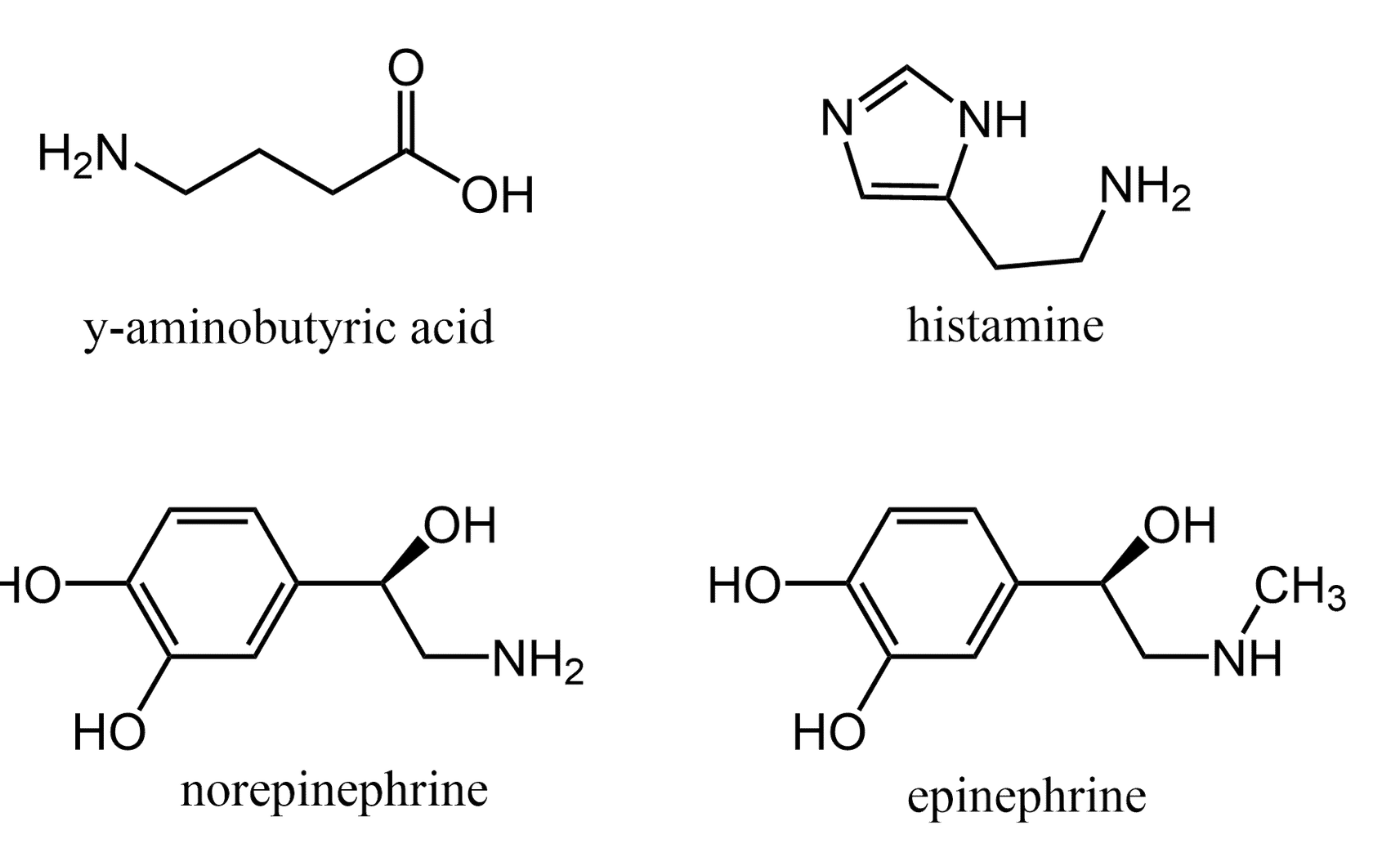
Biogenic amines are derived from amino acids and act as neurotransmitters and hormones in animals. Some examples are shown below.
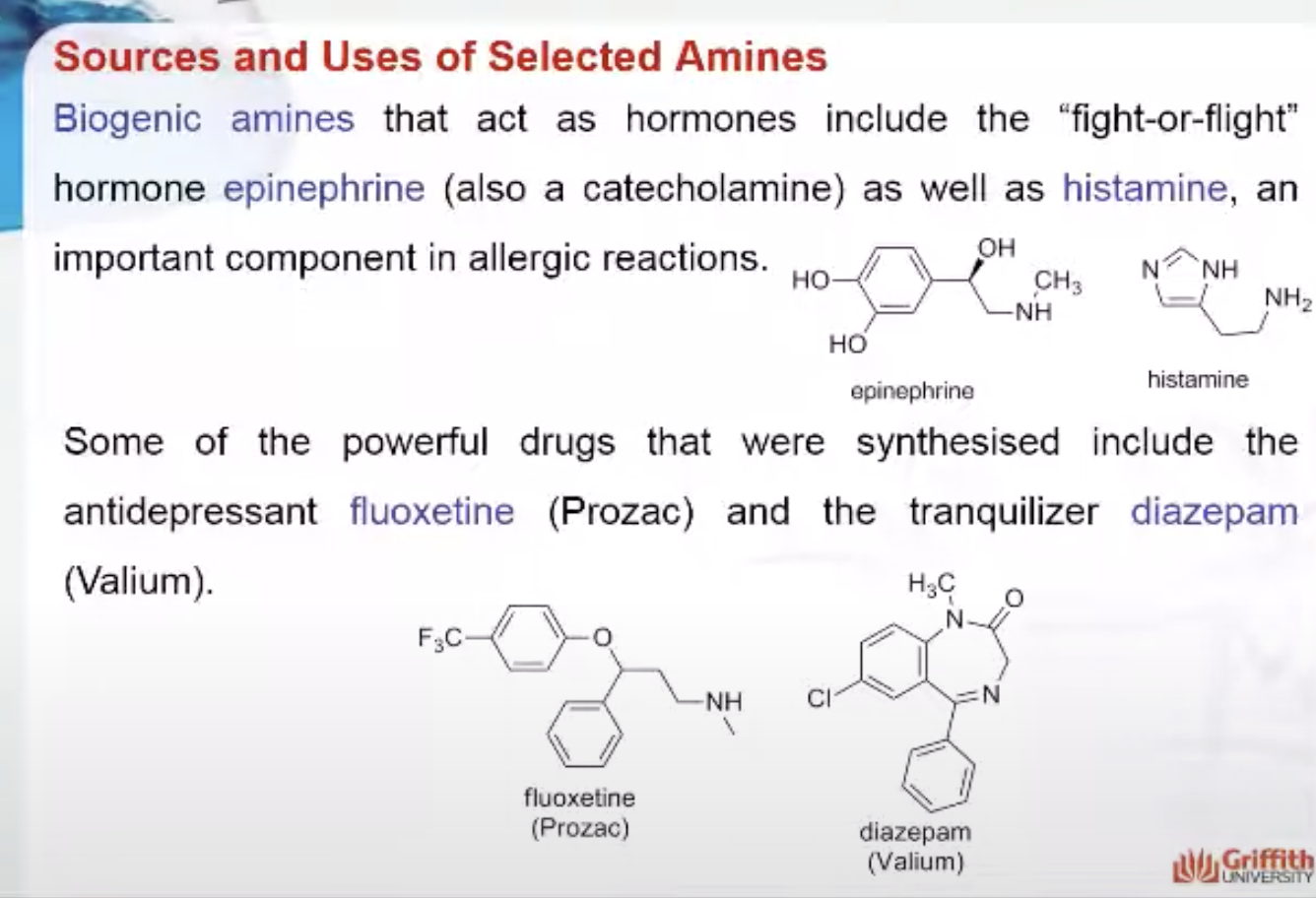
GABA (γ–aminobutyric acid) slows nerve action and can bring relaxation, while norepinephrine affects our focus and concentration. Biogenic amines that act as hormones include the “fight–or–flight” hormone epinephrine as well as histamine, an important component in allergic reactions. As these examples illustrate, biogenic amines are central to coordinating many of our body’s processes.
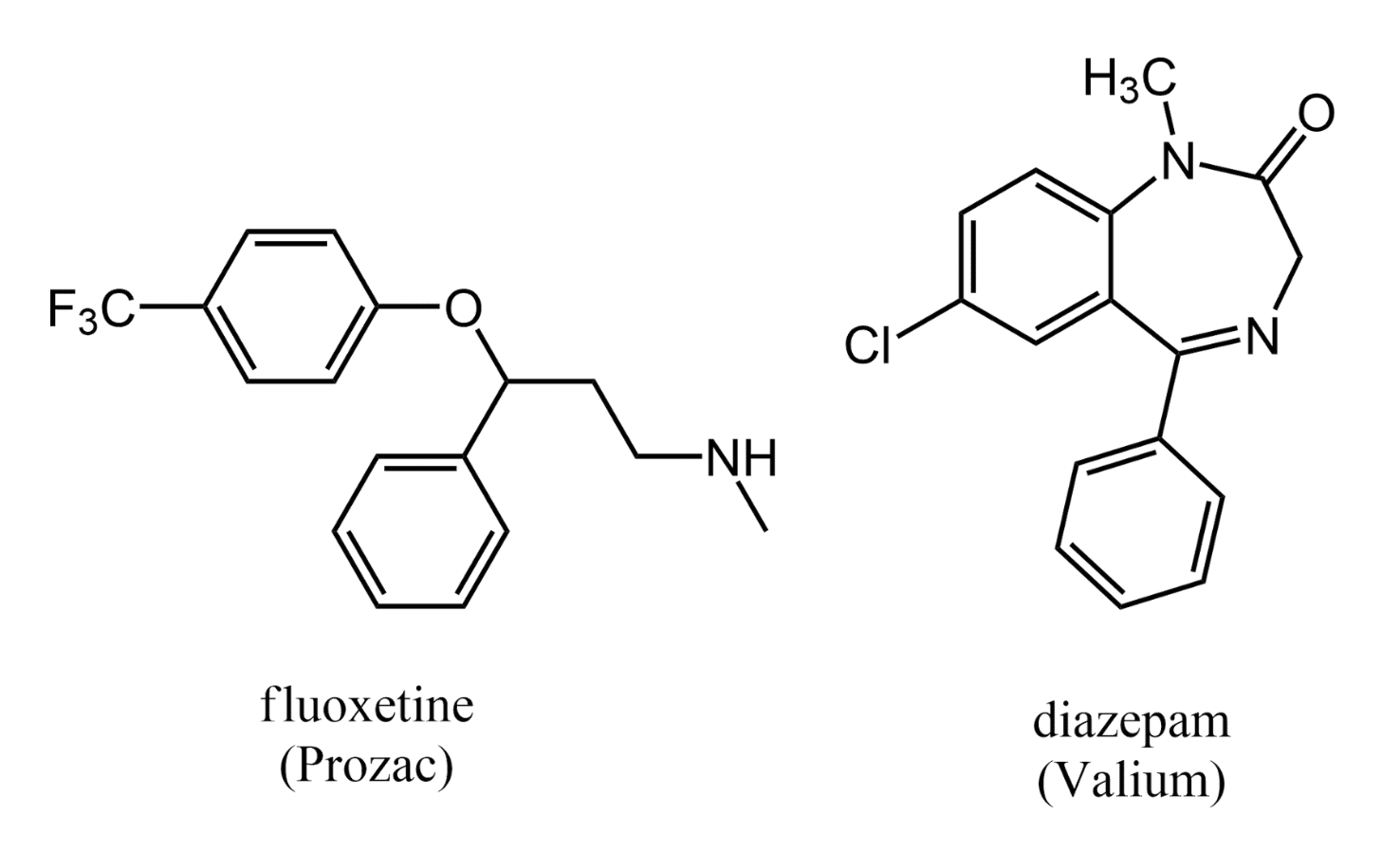
Pharmaceutical chemists have used their knowledge of biogenic amines to design many powerful drugs. Some examples include the antidepressant fluoxetine (Prozac) and the tranquilizer diazepam (Valium).
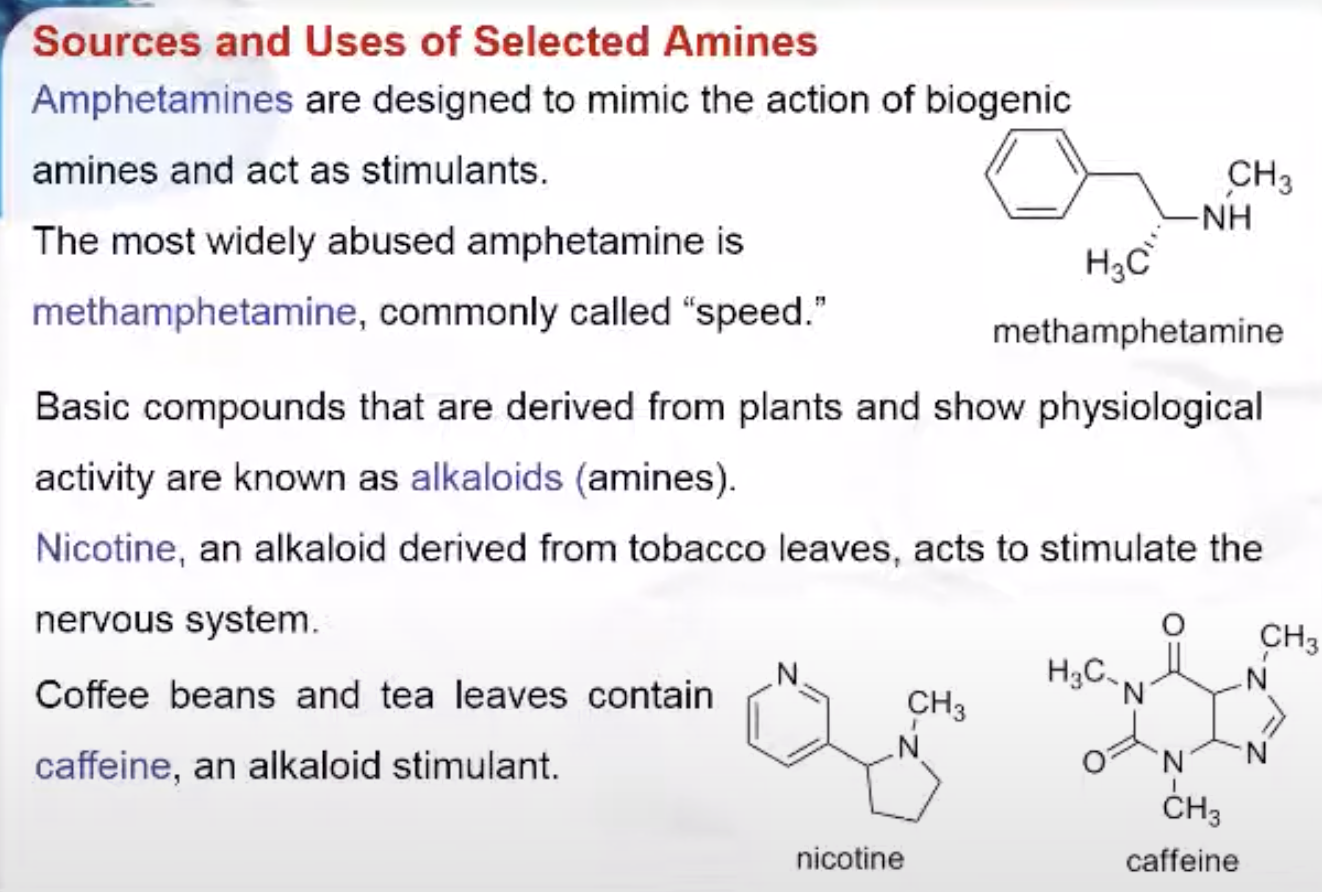
Amphetamines are designed to mimic the action of biogenic amines and act as stimulants. They are used to treat depression, narcolepsy, and obesity. The most widely abused amphetamine is methamphetamine, commonly called “speed.”
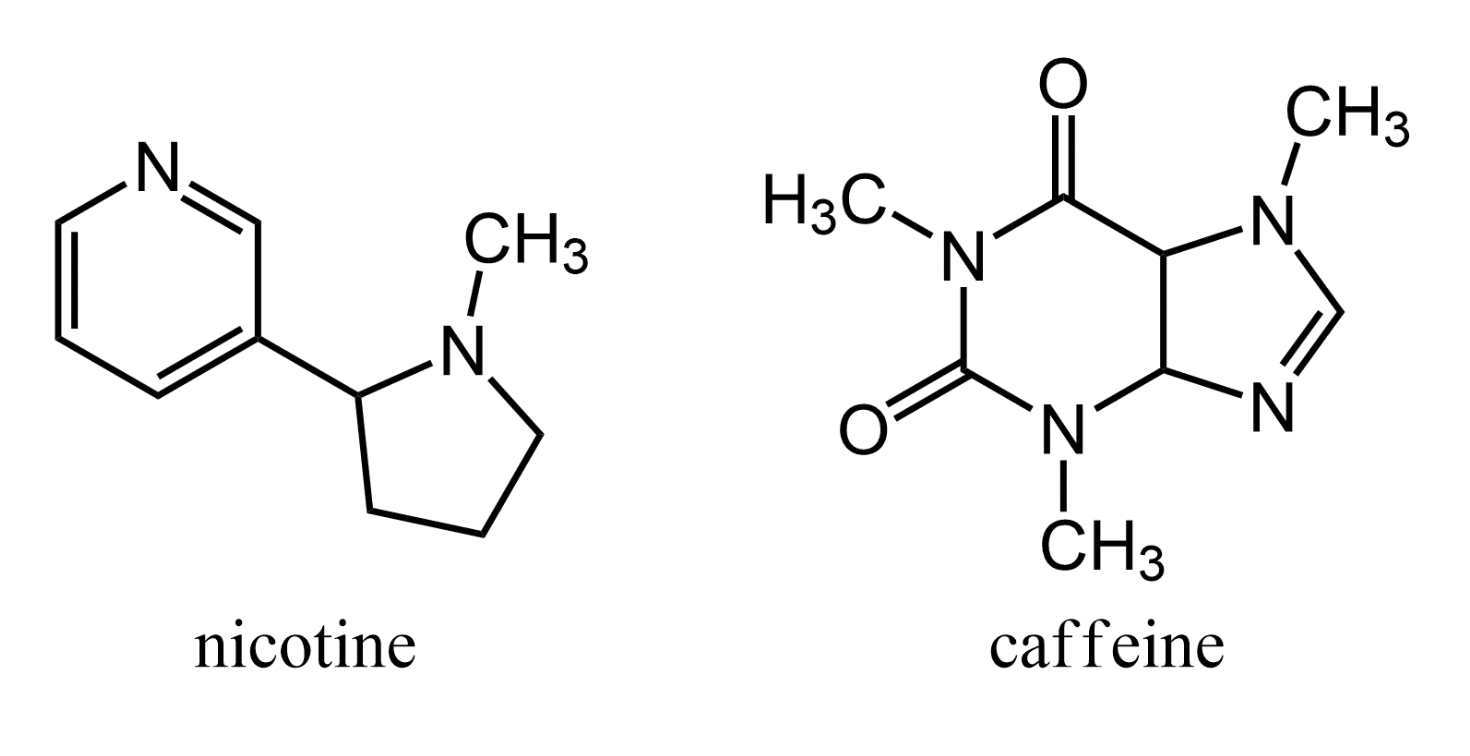
Basic compounds that are derived from plants and show physiological activity are known as alkaloids. These substances are usually amines. Nicotine, an alkaloid derived from tobacco leaves, acts to stimulate the nervous system. Coffee beans and tea leaves contain caffeine, an alkaloid stimulant.