Griffith college Tri3 2022/1001GRC (Chem)
WEEK11 - Topic 13
황누누
2023. 1. 7. 12:10
Gas Laws
1: The kinetic-molecular theory
2: Measurement of pressure of gases
3: Empirical laws of gases
4: Ideal gas law
K = °C + 273
1 atm = 760 torr = 760 mm Hg
STP: standard Temperature (273 K) and pressure (1 atm).
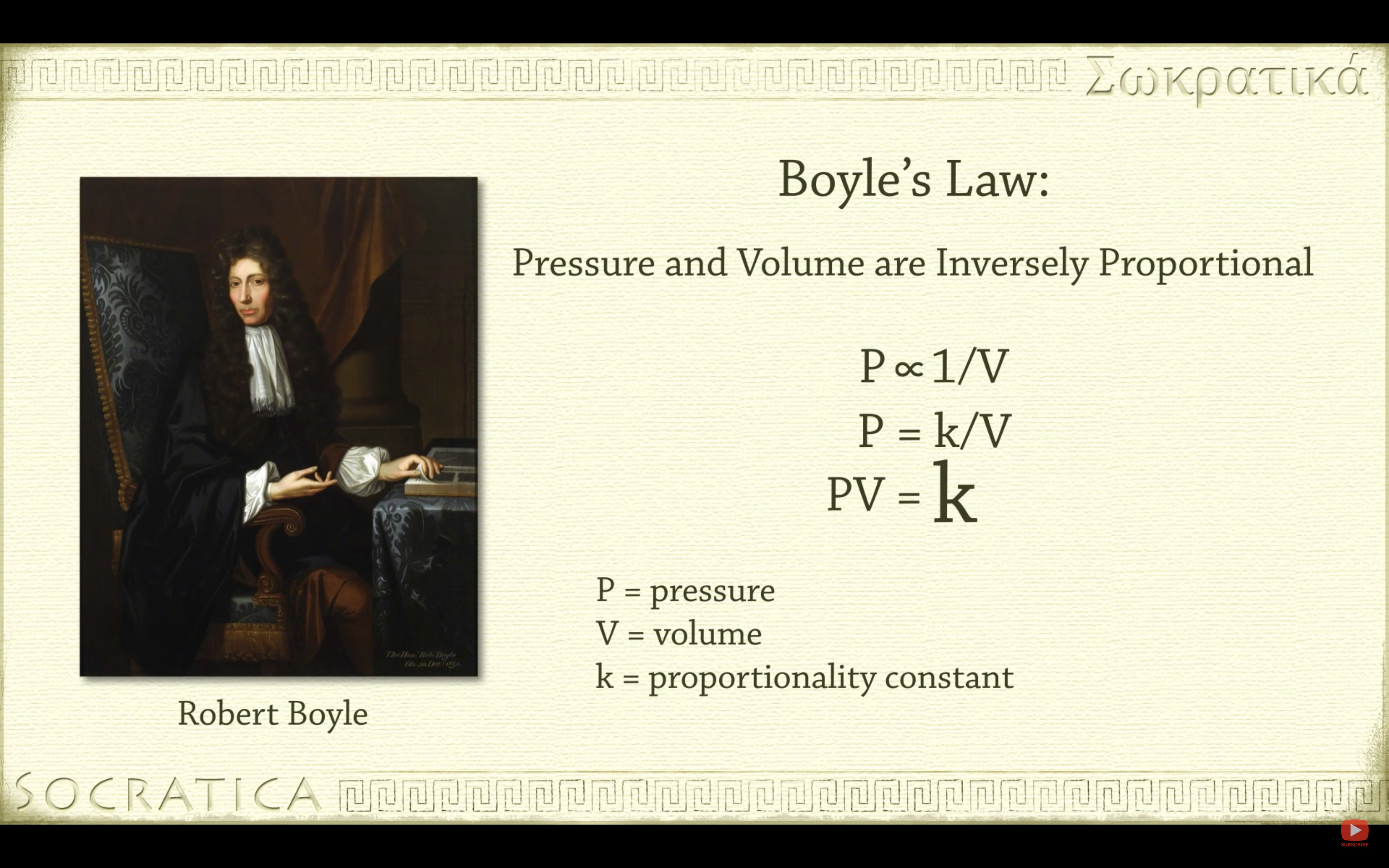
Boyle's Law



Charles's Law



Gay-Lussac's Law


Use the Combined gas law to deduce the equation you need based on what is provided in the question. e.g. if the question gives you P and V and T is constant, cover up the T's and you are left with P1V1 = P2V2






How to Use the Ideal Gas Law in Two Easy Steps

Ideal gas Law & Gas Stoichiometry Worked examples




반응형